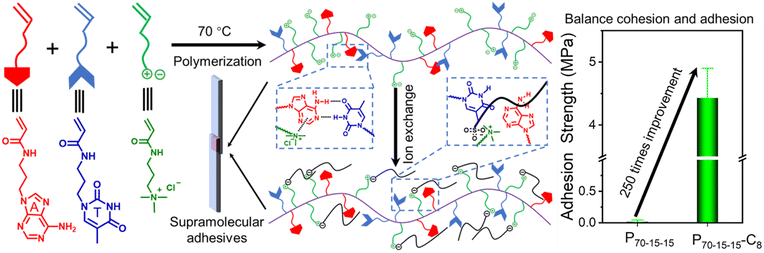
Open Access Article
Open Access ArticleBioinspired nucleobase-containing polyelectrolytes as robust and tunable adhesives by balancing the adhesive and cohesive properties†
Zhi
Dong
a,
Jiang
Wu
a,
Xinyi
Shen
a,
Zan
Hua
 *b and
Guangming
Liu
*b and
Guangming
Liu
 *a
*a
aDepartment of Chemical Physics, Key Laboratory of Surface and Interface Chemistry and Energy Catalysis of Anhui Higher Education Institutes, Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui 230026, China. E-mail: gml@ustc.edu.cn
bBiomass Molecular Engineering Center and Department of Materials Science and Engineering, Anhui Agricultural University, Hefei, Anhui 230036, China. E-mail: z.hua@ahau.edu.cn
First published on 17th March 2023
Abstract
Supramolecular polymeric adhesives inspired by nature have been strongly pursued by scientists, since they possess strong but dynamic reversible adhesive behaviors concurrently. Optimizing the adhesive and cohesive properties is of vital importance for the fabrication of strong supramolecular polymeric adhesives, but common strategies often strengthen one property at the expense of another. Herein, counterion exchange of nucleobase-containing polyelectrolyte adhesives was utilized to boost the interfacial adhesion without compromising the intermolecular cohesion, achieving high adhesion strengths. By employing the cationic polyelectrolyte poly(3-acrylamidopropyltrimethylammonium chloride), the slightly enhanced intermolecular cohesion of the polyelectrolyte with hydrophobic sulfonates is capable of enhancing the adhesion strength. Intriguingly, by introducing bioinspired complementary nucleobases within adhesives, the loss of interfacial adhesion was observed for adhesives containing high supramolecular hydrogen-bonding crosslinking densities. By optimizing the cohesive and adhesive properties of nucleobase-containing polyelectrolyte adhesives using sulfonates with suitable chain lengths, 60 to 250 times improvement of adhesion strengths can be attained over that of initial supramolecular polymeric adhesives. Additionally, nucleobase-containing supramolecular polymeric adhesives tolerate different external conditions, maintaining robust adhesion strengths. This work offers us an efficient and feasible way to optimize the cohesive and adhesive properties for constructing robust and tunable supramolecular adhesives.
Introduction
Adhesives are essential and important for various applications involving industrial and medical products.1–6 Based on the adhesive bonds, typical adhesives could be separated into three major categories. The first category of adhesives usually has strong adhesion strengths, depending on irreversible covalent bonds at the interface between the substrate and the adhesive. These adhesives include cyanoacrylates,7 epoxy resins,8,9 and so on. Although high adhesion strengths are obtained, the in situ formation of covalent bonds for efficient adhesion and cohesion often requires a long curing time. By contrast, the second kind of adhesives are capable of achieving relatively weak adhesion by employing reversible and non-covalent bonds based on the viscoelastic properties of polymers.10–12 Their unique properties result in the wetting of the substrate with the hydrophobic interaction between the adhesive and the substrate, giving rise to fast and reversible adhesion. Inspired by nature, supramolecular polymeric adhesives have been intensely researched, which possess outstanding merits of both conventional adhesives.13–17 They can bond to distinct substrates with high adhesion strength and good reversibility by combining multiple supramolecular interactions such as hydrogen bonds, metal-coordination bonds, π–π stacking, and hydrophobic interactions.18–20Actually, catechol-based supramolecular adhesives have been widely developed after unraveling the molecular structures of adhesive proteins (Mfps-3 and 5) secreted by mussels.21–27 Besides the catechol functionality, the cooperativity of catechols and positively charged amino acids in the adhesives is useful to improve the adhesion performance.28–30 The cationic groups not only improve the interfacial adhesion by providing efficient surface binding, but also enhance cohesive properties by achieving the cation–π interactions.28,31 Notably, the potential oxidation of catechol groups in the adhesives inevitably leads to the formation of covalent networks, damaging the reversible adhesive behaviors. Recently, nucleobases are also regarded as important moieties for fabricating robust supramolecular adhesives.32–38 Similar to catechols, nucleobases especially adenine and thymine in supramolecular adhesives have remarkable capacity for forming hydrogen bonds, metal-coordination bonds, π–π stacking and hydrophobic interactions.39,40 More importantly, the specific hydrogen bonding between complementary nucleobases enables the elegant modulation of the adhesive properties.20,40
To fabricate strong supramolecular polymeric adhesives, optimizing the adhesive and cohesive properties is of great significance. However, it is highly challenging to achieve the balanced adhesion and cohesion. For example, high supramolecular crosslinking for supramolecular polymeric adhesives potentially gives rise to improved intermolecular cohesion. As a consequence, the lower exposed functional groups at the interface decrease the interfacial adhesion dramatically. Therefore, common strategies often enhance one property at the expense of another.22,41 The key issue is attributed to that the supramolecular interactions for interfacial adhesion and intermolecular cohesion are highly coupled. Hence, developing a straightforward and feasible strategy to decouple interfacial adhesion and intermolecular cohesion is highly desired, potentially achieving the balanced adhesion and cohesion for enhanced properties and significantly expanding the applicability of supramolecular adhesives.
Herein, we have developed an efficient approach to enhance the adhesion strength without drastically destructing the intermolecular cohesion through the counterion exchange of polyelectrolytes (Fig. 1). The cationic polyelectrolyte poly(3-acrylamidopropyltrimethylammonium chloride) (PTMPA-Cl), which can undergo efficient counterion exchanges, was successfully prepared through free radical polymerization. After exchanging to sulfonates with different hydrophobic chain lengths, the slightly enhanced intermolecular cohesion of the polyelectrolyte improves the adhesion strength. To further reinforce the intermolecular cohesion, bioinspired complementary nucleobases were introduced. Unexpectedly, the loss of interfacial adhesion was observed for adhesives with high supramolecular hydrogen-bonding crosslinking densities. The cohesive and adhesive properties of nucleobase-containing polyelectrolyte adhesives can be balanced through counterion exchange, achieving 60 to 250 times improvement over that of initial supramolecular polymeric adhesives. More importantly, the robust nucleobase-containing supramolecular polymeric adhesives display outstanding tolerance to different external conditions, manifesting excellent applicable potentials. Therefore, this work provides an efficient method to fabricate robust and tunable supramolecular adhesives by balancing the cohesive and adhesive properties.
Results and discussion
Synthesis and properties of poly(3-acrylamidopropyltrimethylammonium chloride) (PTMPA-Cl) exchanged with different counterions
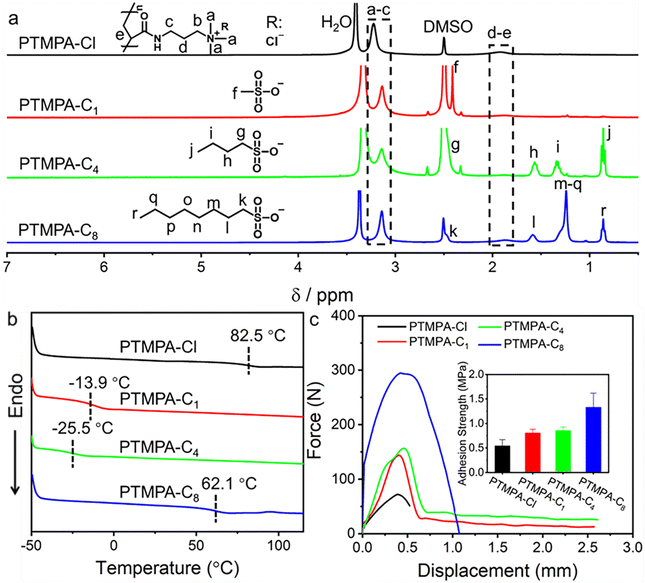
Poly(3-acrylamidopropyltrimethylammonium chloride) (PTMPA-Cl) as one of the typical cationic polyelectrolytes was prepared through conventional free radical polymerization in water at 70 °C (Scheme S1†). 2,2′-Azobis[2-(2-imidazolin-2-yl) propane] dihydrochloride (VA-044) was used as a water soluble initiator and high conversion (99%) was obtained after polymerization for 2 h. Size exclusion chromatography (SEC) analysis shows that PTMPA-Cl with a number-average molecular weight of 35.5 kDa and polydispersity (Đ) of 2.71 was yielded (Fig. S1†). We choose sodium methanesulfonate, sodium 1-butanesulfonate, and sodium 1-octanesulfonate, which have different lengths of hydrophobic chains for counterion exchanges. The PTMPA-Cl aqueous solution was mixed with aqueous solutions containing different sulfonates. The obtained mixtures were further dialyzed against water to yield the targeted product. PTMPA-C1, PTMPA-C4, and PTMPA-C8 were obtained when the counterion of PTMPA-Cl was exchanged with sodium methanesulfonate, sodium 1-butanesulfonate, and sodium 1-octanesulfonate, respectively (Scheme S1†).According to the Law of Matching Water Affinities (LMWA), the weakly hydrated quaternary ammonium groups attached to the polymer PTMPA-Cl are inclined to bind weakly to the chloride ion (Cl−).42–46 In contrast, stronger binding was formed between the weakly hydrated alkyl sulfonate anions and the quaternary ammonium groups.42–46 The unique properties enable us to exchange the chloride counterion with different sulfonates. The successful exchange of counterions was confirmed by 1H NMR spectra, FT-IR spectra, and surface wettability (Fig. 2a and S2, S3†). As shown in Fig. 2a, a significant shift of the peak at 3.22 ppm to 3.14 ppm was observed, which is assigned to protons in the alkyl group attached to the ammonium. Considering that the interaction between Cl− and the ammonium group is different from that between the sulfonate and the ammonium group, the peak for protons in the alkyl group connected to the ammonium shifted to the high field in all PTMPA-C1, PTMPA-C4, and PTMPA-C8. In addition, other new peaks for the sulfonates have emerged, suggesting the successful exchange of counterions (Fig. 2a). Furthermore, X-ray photoelectron spectroscopy (XPS) analyses show that the peak for Cl2p fully disappears and the peak for S2p emerges for PTMPA-C1, PTMPA-C4, and PTMPA-C8 (Fig. S4†). These results have validated that the counterion exchange between Cl− and the sulfonates is nearly complete.
Fig. 2b shows that the exchange of the counterions was further verified by DSC analyses. The polymer PTMPA-Cl has a glass transition temperature (Tg) of 82.5 °C, which was due to the rigid polymer chain of the polyelectrolyte. In stark contrast, Tgs of PTMPA-C1 and PTMPA-C4 were observed to decrease to −13.9 °C and −25.5 °C, respectively. Compared with PTMPA-Cl, the significant drop of Tg can be attributed to the efficient electrostatic shield owing to the stronger interaction between the sulfonate and the ammonium group. Intriguingly, PTMPA-C8 presents a high Tg of 62.1 °C. For PTMPA-Cl, PTMPA-C1, PTMPA-C4, and PTMPA-C8, they consist of the cationic polyelectrolyte poly(3-acrylamidopropyltrimethylammonium) with different counterions. As a result, the dominant intermolecular interaction is the hydrophobic interaction. We suppose that the hydrophobic interaction of the long alkyl chain in PTMPA-C8 leads to the decrease of segment mobility. Further increase of the chain length of the counterion to 1-dodecanesulfonate gives a similar result, suggesting that the alkyl group with the carbon number as long as 8 is essential for the strong interaction (Fig. S5†). Additionally, all these polymers have good thermal stability with the decomposition temperature over 268 °C, demonstrating good application potential (Fig. S6†).
The adhesion strengths of PTMPA-Cl, PTMPA-C1, PTMPA-C4, and PTMPA-C8 were evaluated through shear tests by using stainless steel as the substrate (Fig. 2c). A gradual increase of the maximum adhesive force was observed from 72 N for PTMPA-Cl to 295 N for PTMPA-C8. The inset in Fig. 2c shows that the adhesion strength of PTMPA-Cl is 0.54 ± 0.12 MPa. Further, the adhesion strengths of PTMPA-C1, PTMPA-C4, and PTMPA-C8 are observed to increase to 0.81 ± 0.07 MPa, 0.86 ± 0.07 MPa, and 1.33 ± 0.28 MPa, respectively. Although PTMPA-Cl is inclined to have strong interfacial adhesion with the substrate, the low intermolecular cohesion generates weak adhesion strength. After exchanging to the sulfonates, the intermolecular cohesion within PTMPA-C1, PTMPA-C4, and PTMPA-C8 was enhanced through the additional hydrophobic interaction of counterions, resulting in the improvement of adhesive properties.
Syntheses and adhesive properties of nucleobase-containing polyelectrolytes
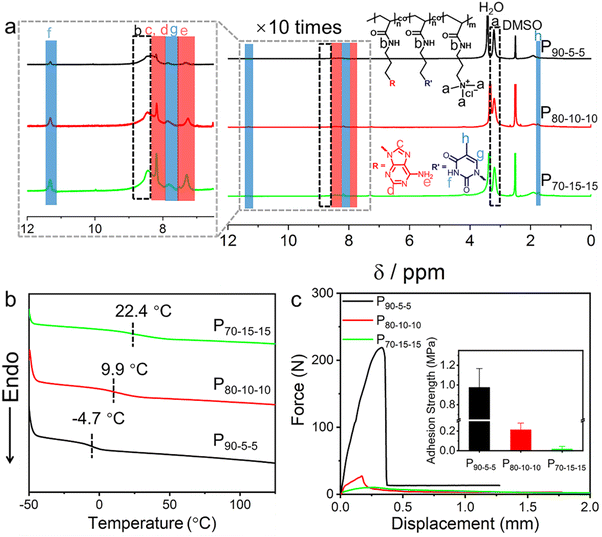
Since all the PTMPA polymers with different counterions undergo cohesive failures upon the shear test, the adhesion strengths of these polymers are expected to further be improved by increasing the intermolecular cohesion. A series of nucleobase-containing polyelectrolytes P(100-2x, x, x)s were synthesized through the ternary copolymerization of 3-(adenine-9-yl) propyl acrylamide (AAm), 3-(thymin-1-yl) propyl acrylamide (TAm), and TMPA-Cl, in which x represents the molar percent ratio of nucleobase-containing monomers in the copolymers (Scheme S2† and Fig. S7 and S8†). 1H NMR spectroscopy was used to obtain the final conversion of the nucleobase-containing copolymers by analysing the mixture after polymerization without purification (Fig. S9†). The integration of residual double bonds in the mixture suggests over 99% conversion of monomers was achieved for all P90-5-5, P80-10-10, and P70-15-15. Both the adenine and thymine molar ratios in the copolymer can be obtained from the initial feeding and the monomer conversion. As shown in Fig. S9,† nearly full conversion of feeding monomers was observed for the ternary copolymers. Therefore, the final attained polymers are supposed to have the same molar percentage as the initial target. The intermolecular hydrogen bonding interaction between AAm and TAm is capable of forming supramolecular crosslinking, generating enhanced cohesion. Of note, when the molar percent ratios of AAm and TAm are over 20 mol%, high supramolecular crosslinking leads to the formation of hydrogels, hampering the purification and probable applications as supramolecular polymeric adhesives. Therefore, copolymers P90-5-5, P80-10-10, and P70-15-15 were prepared and further investigated as supramolecular polymeric adhesives.As shown in Fig. 3a, 1H NMR spectra manifest the successful copolymerization of the three monomers. The peak at 3.21 ppm can be assigned to the methyl group appended to the ammonium. Meanwhile, the peaks at 8.20 and 7.36 ppm are assigned to adenine and the peaks at 11.33 and 7.87 ppm are assigned to thymine. Obviously, the peaks for both adenine and thymine gradually increase as the contents of them are improved in the copolymers. DSC analyses show that a single Tg was observed for the copolymers P90-5-5, P80-10-10, and P70-15-15, illustrating the random copolymerization of the comonomers (Fig. 3b). Interestingly, compared with PTMPA-Cl with a Tg of 82.5 °C, the Tg of P90-5-5 is as low as −4.7 °C after introducing 10 mol% of nucleobase-containing monomers. In addition to the perturbation of electrostatic interaction in PTMPA-Cl, the cation–π interaction should play a dominant role in the copolymer. Both the purine and pyrimidine structures of nucleobases facilitate the formation of cation–π interactions, further decreasing the repulsion between different chain segments. Considering the increase of supramolecular crosslinking between complementary nucleobases, the Tgs of the copolymers were observed to present an increasing trend with the further increase of nucleobase-containing monomers (Fig. 3b). Additionally, all these nucleobase-containing polyelectrolytes have good thermal stability with the decomposition temperature over 264 °C, demonstrating good applicability (Fig. S10†).
Interestingly, the adhesion strengths of the nucleobase-containing polyelectrolytes show a dramatic decrease from 0.98 ± 0.19 MPa for P90-5-5 to 0.21 ± 0.06 MPa for P80-10-10 (inset in Fig. 3c). Further increase of nucleobase-containing moieties in the adhesive leads to the loss of efficient adhesion (18 ± 16 kPa). As expected, the introduction of nucleobases should be beneficial to the adhesion owing to the existence of functional aromatic groups. Indeed, a higher adhesion strength was observed for P90-5-5, in contrast to the adhesion strength of 0.54 ± 0.12 MPa for PTMPA-Cl. Nonetheless, the formed hydrogen bonding and cation–π interactions cannot be neglected in the adhesives with high nucleobase contents, bringing about strong cohesion. As a result, the adhesives P80-10-10 and P70-15-15 have strong cohesion but weak interfacial adhesion, resulting in low adhesion strengths.
Enhanced adhesive properties of nucleobase-containing polyelectrolytes modulated through counterion exchange
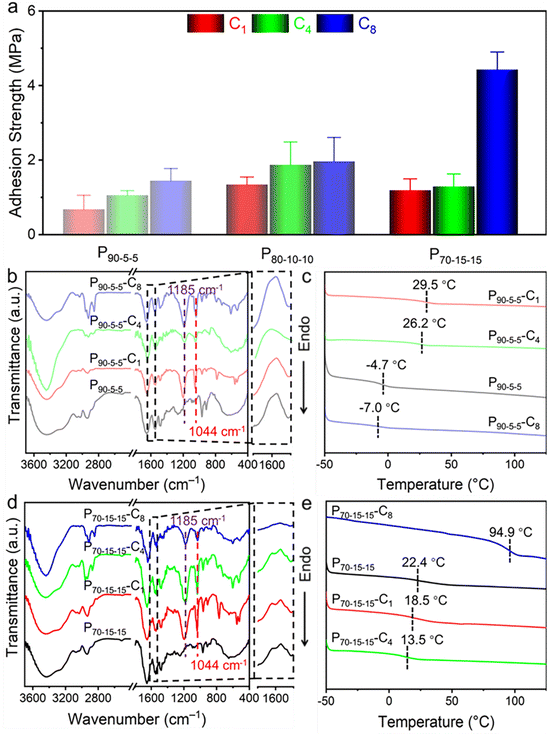
For nucleobase-containing polyelectrolytes, the unbalanced cohesion and adhesion generate relatively weak adhesion strengths. Notably, the charged parts in nucleobase-containing polyelectrolytes provide an additional handle to modulate the cohesive and adhesive properties of adhesives, which might not interrupt the supramolecular hydrogen bonding. Therefore, the adhesive properties of nucleobase-containing polyelectrolytes can be boosted through counterion exchange (Scheme S2 and Fig. S11–S13†). Indeed, a gradual increase of the adhesion strengths was observed when the length of counterion alkyl chains was increased from 1 to 4, and then to 8 for all P90-5-5, P80-10-10, and P70-15-15 (Fig. 4a). The adhesion strength of P70-15-15-C8 is as high as 4.43 ± 0.47 MPa, which is over 250 times higher than that of P70-15-15. The bioinspired nucleobase-containing polyelectrolyte P70-15-15-C8 reported in this work outperforms most of the common PSAs, supramolecular adhesives, and epoxy resins (Fig. S14†). Meanwhile, the adhesion strength of the bioinspired nucleobase-containing polyelectrolyte is on par with the strong mussel-inspired adhesives, showing good potential applicability. Additionally, the adhesion strengths of both P80-10-10-C8 and P80-10-10-C4 also represent over 10 times enhancement over P80-10-10. Hence, the counterion exchange provides a straightforward and feasible approach to alter the properties of adhesives over a wide range.To further explore the enhanced adhesive properties of nucleobase-containing polyelectrolytes with different counterions, FT-IR and DSC analyses were utilized to unveil the underlying mechanism (Fig. 4b–e). FT-IR spectra display that new peaks at 1044 and 1085 cm−1 emerged after exchanging with different counterions for P90-5-5 (Fig. 4b). These new peaks can be assigned to the vibration absorbance of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. A slight shift from 1201 cm−1 for methanesulfonate to 1185 cm−1 for sulfonates with long alkyl chains was observed, illustrating the stronger interaction with the ammonium group for methanesulfonate than that for 1-butanesulfonate and 1-octanesulfonate. Meanwhile, no significant change of the absorbance at 1600 cm−1 was discerned, which was attributed to the bending vibration peak of N–H
O bond. A slight shift from 1201 cm−1 for methanesulfonate to 1185 cm−1 for sulfonates with long alkyl chains was observed, illustrating the stronger interaction with the ammonium group for methanesulfonate than that for 1-butanesulfonate and 1-octanesulfonate. Meanwhile, no significant change of the absorbance at 1600 cm−1 was discerned, which was attributed to the bending vibration peak of N–H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) involving the hydrogen bonding between complementary nucleobases.
involving the hydrogen bonding between complementary nucleobases.
DSC curves show that the P90-5-5-C1 and P90-5-5-C4 have Tg values of 29.5 and 26.2 °C, which are much higher than that of P90-5-5 (Fig. 4c). Interestingly, P90-5-5-C8 has a lower Tg of −7.0 °C than that of P90-5-5. Considering the low supramolecular crosslinking for P90-5-5, the counterion exchange is unable to disturb the supramolecular hydrogen-bonding interaction, which was confirmed by FT-IR analyses (Fig. 4b). Additionally, the cation–π and hydrophobic interactions should play an important role in tuning the properties of the adhesives. The short alkyl chains of counterions in P90-5-5-C1 and P90-5-5-C4 did not influence the cation–π interactions dramatically, but the hydrophobic interaction was enhanced within the adhesives. Consequently, the Tgs of both P90-5-5-C1 and P90-5-5-C4 present a significant increase. In contrast, P90-5-5-C8 with a long alkyl chain can possibly disrupt the cation–π interaction, giving rise to a high chain mobility. Although the octane chain in P90-5-5-C8 might be able to form hydrophobic aggregation during the counterion exchange, the disruption of the cation–π interaction affects the chain mobility more dramatically. Taken together, the counterion exchange for P90-5-5 did not significantly change the supramolecular hydrogen bonding related to cohesion. Yet, the disruption of cation–π interaction and the formation of hydrophobic interaction are able to enhance the interfacial adhesion. Similar phenomena were observed for P80-10-10 with relatively low supramolecular crosslinking (Fig. S15 and S16†). Therefore, the adhesion strengths were improved for P90-5-5 and P80-10-10 after exchanging with the counterions by improving the adhesion without compromising the cohesion.
For P70-15-15 with a high supramolecular crosslinking, FT-IR analyses show that the absorbance at 1600 cm−1 for the hydrogen bonding interaction was disrupted when exchanging with 1-octanesulfonate (Fig. 4d). In contrast to P90-5-5 and P80-10-10, the Tg of P70-15-15 with different sulfonates presents a distinct trend. In addition to stronger supramolecular crosslinking, the higher nucleobase monomers and TMPA ratio in P70-15-15 generate more efficient cation–π interaction. For P70-15-15-C1 and P70-15-15-C4, the weakened cation–π interaction by the exchanged counterions leads to a higher chain mobility. Interestingly, P70-15-15-C8 has a high Tg of 94.9 °C. This result should be caused by the weakened cation–π interaction and the significantly enhanced hydrophobic interactions between long alkyl chains and nucleobases. Notably, the disruption of complementary hydrogen bonding is vital for strong interfacial adhesion, which enables the exposure of nucleobase functionalities. As a result, P70-15-15-C8 is capable of achieving an ultrastrong adhesion strength by balancing the adhesion and cohesion interaction.
Robust adhesive properties of nucleobase-containing polyelectrolytes
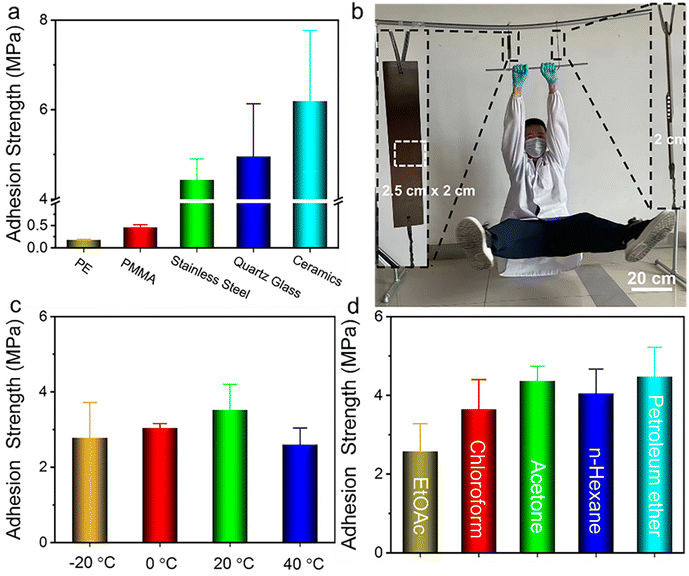
The nucleobase-containing polyelectrolyte P70-15-15-C8 shows an adhesion strength of as high as 4.43 MPa with stainless steel as the substrate after having 1-octanesulfonate as the counterion. We wondered whether the robust adhesive properties are universal to different substrates. In addition to stainless steel, several substrates such as polyethylene (PE), polymethylmethacrylate (PMMA), quartz glass, and ceramics are also employed to investigate the adhesive properties (Fig. 5a). Low adhesion strengths of 0.18 MPa and 0.46 MPa were observed for PE and PMMA respectively, indicating the importance of electrostatic interaction for the efficient adhesion of positively charged polyelectrolyte adhesives. The polymeric substrates PE and PMMA hinder the formation of strong interfacial adhesion. In contrast, the adhesive P70-15-15-C8 is able to achieve strong adhesion for quartz glass and ceramics with adhesion strengths of 4.95 ± 1.18 MPa and 6.19 ± 1.58 MPa, considering the additional electrostatic interaction with the substrate. It is noted that the quartz glass bonded with P70-15-15-C8 cannot be separated prior to the breakage of the glass substrate with the contact area of 1.50 cm × 1.25 cm. Therefore, we utilized a small contact area of 0.50 cm × 0.50 cm for measuring the adhesion strength of glass substrates bonded with P70-15-15-C8 (Fig. S17†).As shown in Fig. 5b a person with a weight over 70 kg can be easily held by two P70-15-15-C8 bonded steel substrates, demonstrating robust adhesion strength (Movie S1†). Meanwhile, the strongly adhesive P70-15-15-C8 shows good environmental tolerance towards different environmental conditions. High adhesion strengths over 2.60 MPa are observed for the adhesive P70-15-15-C8 from −20 °C to 40 °C, illustrating the widespread application temperature window (Fig. 5c). In addition, the adhesive presents good tolerance to different organic solvents such as ethyl acetate (EtOAc), chloroform, acetone, n-hexane, and petroleum ether. High adhesion strengths over 2.58 MPa were retained after immersing into the solvents for over 2 h (Fig. 5d). It is possible for highly polar solvents to swell the adhesive, inducing a decrease of adhesion strengths. More importantly, the supramolecular adhesive P70-15-15-C8 can be harnessed for repeated adhesion. No obvious decrease of the adhesion strength after several adhesion–shear–adhesion cycles was observed with the adhesion strength over 4.08 MPa continuously (Fig. S18†). Collectively, the nucleobase-containing polyelectrolyte adhesives have strong adhesive properties under distinct environmental conditions, showing tremendous applicability. Both adenine and thymine-containing polymers are confirmed to be biocompatible, and the cationic polyelectrolyte poly(3-acrylamidopropyltrimethylammonium chloride) is supposed to be biocompatible and antibacterial. Therefore, the strong adhesives developed in this work are potential biomedical adhesives for wound dressing or implantable biomedical devices.
Experimental
Materials
2,2′-Azobis[2-(2-imidazolin-2-yl) propane] dihydrochloride (VA-044) was obtained from Aladdin and recrystallized from methanol three times before use. N,N-Dimethylformamide (DMF, 99.9%) was purified by using a solvent purification system (MB-SPS-5) before use. 3-Acrylamidepropyltrimethylammonium chloride (TMPA-Cl, 75 wt% in water) was purified by filtering through an alkaline alumina column to remove the polymerization inhibitor. The water used was purified by filtration through a Millipore Gradient system, giving a resistivity of 18.2 MΩ cm. Other chemicals were purchased from Aladdin and used directly without further purification. 3-(Adenine-9-yl) propyl acrylamide (AAm) and 3-(thymin-1-yl) propyl acrylamide (TAm) were prepared according to the previous publication.Methods
All 1H NMR spectra were recorded on an Avance III HD400 spectrometer (Bruker) with DMSO-d6 as the solvent. Fourier transform infrared (FT-IR) spectra were obtained on a Nicolet 6700 spectrometer (Thermo Scientific). The molecular weight and molecular weight distribution of cationic polymer PTMPA-Cl and nucleobase-containing polymers were measured by size exclusion chromatography (SEC) at 20 °C with aqueous solution (100 mM NaCl, 25 mM NaH2PO4, and 25 mM Na2HPO4) as the eluent at a flow rate of 1 mL min−1. SEC data were analysed with Agilent SEC software calibrated with PEO standards prior to use. Thermogravimetric analysis (TGA) was carried out on a TA Q5000IR TGA instrument under a nitrogen atmosphere to obtain the decomposition temperature (Td, 5%) at 5% degradation of the polymers. Polymers with a weight of about 5–10 mg were slowly heated up from 40 to 700 °C with a rate of 10 °C min−1 to explore the thermal stability. Glass transition temperatures (Tgs) of obtained polymers were characterized by differential scanning calorimetry (DSC) using a PerkinElmer DSC 8500 instrument under a nitrogen atmosphere. The dried sample was first heated from room temperature to 150 °C at a rate of 10 °C min−1, and then cooled down to −50 °C at the same rate. Following this, the sample was reheated to 150 °C at a rate of 10 °C min−1. The Tg value was attained from the second heating scan. For the adhesive test, the typical preparation process of the sample is as follows. The P70-15-15-C8 polymer was put on stainless steel (1.50 × 1.25 cm2) at 80 °C on a heating plate. Then another piece of stainless steel sheet was pasted on it, and pressed on the stainless steel sheet with 500 g weight for 5 min. The bonded sample was placed in a vacuum oven at 60 °C for 24 h, and then the sample was kept at 25 °C and 50% RH for 8 h before the adhesion strength was tested. Although the sample preparation temperature is slightly lower than the Tg of P70-15-15-C8, enough heating and annealing time guarantees efficient interfacial wetting. Characterization of adhesive properties of nucleobase-containing adhesives with sulfonate ions of different alkyl chain lengths was performed on a SANS TS7104 electronic testing instrument (SANS, China) at 10 mm min−1. Each polymer sample was measured at least five times to ensure repeatability. Based on the same procedures, the adhesion strengths were tested when bonding with different substrates, immersed into organic solvents and exposed under different temperatures. The water contact angle (WCA) was determined by using a contact angle goniometer (CAM 200, KSV). The surface chemical element components of PTMPA-Cl and PTMPAs with different counterions were analysed by using X-ray photoelectron spectroscopy (XPS, Kratos Axis supra+). The instrument was equipped with a monochromatic Al Kα radiation source at 1486.6 eV, which detected the characteristic binding energies relevant to each element of sample.Synthetic procedures
Conclusions
In summary, bioinspired nucleobase-containing cationic polyelectrolytes can be harnessed as robust adhesives by balancing the interfacial adhesion and intermolecular cohesion through counterion exchange. First, it is found that the cationic polyelectrolyte PTMPA-Cl prepared through free radical polymerization can be effectively exchanged with different sulfonates. The enhanced intermolecular cohesion provides the polyelectrolytes containing different counterions with improved adhesion strengths. The cohesive failure for the polyelectrolyte adhesive prompts us to fabricate nucleobase-containing polyelectrolytes with high supramolecular crosslinking for robust adhesive behaviors. Interestingly, the dense supramolecular hydrogen-bonding crosslinking in the adhesive gives rise to the loss of interfacial adhesion. Further counterion exchange of the nucleobase-containing polyelectrolytes enables the boost of interfacial adhesion without significantly compromising the intermolecular cohesion. By balancing the cohesive and adhesive properties through counterion exchange, the adhesion strengths of nucleobase-containing polyelectrolytes can be improved as high as 250 times. In addition, the nucleobase-containing supramolecular polymeric adhesive manifests strong adhesion to distinct inorganic substrates. Meanwhile, the robust adhesive can tolerate the dramatic change of temperatures and various organic solvents, showcasing tremendous potential applicability. This work reports an efficient route for modulating the properties of supramolecular polymeric adhesives by achieving balanced adhesion and cohesion.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Z. H. and G. L. conceived the study. Z. D. and J. W. carried out most of the experiments and analyzed the related data. X. S. participated in the experiments for monomers and polymer syntheses.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22273098, 22103002, 52033001, and 21873091), Anhui Province Natural Science Funds (2008085QE249), the Fundamental Research Funds for the Central Universities (WK2480000007), and Anhui Provincial Innovation and Entrepreneurship Support Plan for Overseas Returnees (2019LCX023).References
- C. Ghobril and M. W. Grinstaff, The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial, Chem. Soc. Rev., 2015, 44, 1820–1835 RSC.
- C. Cui and W. Liu, Recent advances in wet adhesives: Adhesion mechanism, design principle and applications, Prog. Polym. Sci., 2021, 116, 101388 CrossRef CAS.
- M. Chen, Y. Wu, B. Chen, A. M. Tucker, A. Jagota and S. Yang, Fast, strong, and reversible adhesives with dynamic covalent bonds for potential use in wound dressing, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2203074119 CrossRef CAS PubMed.
- S. Rose, A. Prevoteau, P. Elzière, D. Hourdet, A. Marcellan and L. Leibler, Nanoparticle solutions as adhesives for gels and biological tissues, Nature, 2014, 505, 382–385 CrossRef CAS PubMed.
- J. Sun, L. Xiao, B. Li, K. Zhao, Z. Wang, Y. Zhou, C. Ma, J. Li, H. Zhang, A. Herrmann and K. Liu, Genetically engineered polypeptide adhesive coacervates for surgical applications, Angew. Chem., Int. Ed., 2021, 60, 23687–23694 CrossRef CAS PubMed.
- L. Zhang, M. Liu, Y. Zhang and R. Pei, Recent Progress of Highly Adhesive Hydrogels as Wound Dressings, Biomacromolecules, 2020, 21, 3966–3983 CrossRef CAS PubMed.
- S. M. Lim, J. Ryu, E. H. Sohn, S. G. Lee, I. J. Park, J. Hong and H. S. Kang, Flexible, Elastic, and Superhydrophobic/Superoleophilic Adhesive for Reusable and Durable Water/Oil Separation Coating, ACS Appl. Mater. Interfaces, 2022, 14, 10825–10835 CrossRef CAS PubMed.
- D. Ratna, Modification of epoxy resins for improvement of adhesion: a critical review, J. Adhes. Sci. Technol., 2003, 17, 1655–1668 CrossRef CAS.
- D. W. R. Balkenende, R. A. Olson, S. Balog, C. Weder and L. Montero de Espinosa, Epoxy Resin-Inspired Reconfigurable Supramolecular Networks, Macromolecules, 2016, 49, 7877–7885 CrossRef CAS.
- M. A. Droesbeke, R. Aksakal, A. Simula, J. M. Asua and F. E. Du Prez, Biobased acrylic pressure-sensitive adhesives, Prog. Polym. Sci., 2021, 117, 101396 CrossRef CAS.
- Q. Wang, W. B. Griffith, M. Einsla, S. Zhang, M. L. Pacholski and K. R. Shull, Bulk and Interfacial Contributions to the Adhesion of Acrylic Emulsion-Based Pressure-Sensitive Adhesives, Macromolecules, 2020, 53, 6975–6983 CrossRef CAS.
- S. Noppalit, A. Simula, L. Billon and J. M. Asua, Paving the Way to Sustainable Waterborne Pressure-Sensitive Adhesives Using Terpene-Based Triblock Copolymers, ACS Sustainable Chem. Eng., 2019, 7, 17990–17998 CrossRef CAS.
- J. Li, S. Luo, F. Li and S. Dong, Supramolecular Polymeric Pressure-Sensitive Adhesive That Can Be Directly Operated at Low Temperatures, ACS Appl. Mater. Interfaces, 2022, 14, 27476–27483 CrossRef CAS PubMed.
- S. Chen, Z. Li, Y. Wu, N. Mahmood, F. Lortie, J. Bernard, W. H. Binder and J. Zhu, Hydrogen-Bonded Supramolecular Polymer Adhesives: Straightforward Synthesis and Strong Substrate Interaction, Angew. Chem., Int. Ed., 2022, 61, e202203876 CAS.
- Q. Zhang, T. Li, A. Duan, S. Dong, W. Zhao and P. J. Stang, Formation of a Supramolecular Polymeric Adhesive via Water-Participant Hydrogen Bond Formation, J. Am. Chem. Soc., 2019, 141, 8058–8063 CrossRef CAS PubMed.
- H. Liu, S. Qin, J. Liu, C. Zhou, Y. Zhu, Y. Yuan, D. a. Fu, Q. Lv, Y. Song, M. Zou, Z. Wang and L. Wang, Bio-Inspired Self-Hydrophobized Sericin Adhesive with Tough Underwater Adhesion Enables Wound Healing and Fluid Leakage Sealing, Adv. Funct. Mater., 2022, 32, 2201108 CrossRef CAS.
- L. Liu, Z. Liu, Y. Ren, X. Zou, W. Peng, W. Li, Y. Wu, S. Zheng, X. Wang and F. Yan, A Superstrong and Reversible Ionic Crystal-based Adhesive Inspired by Ice Adhesion, Angew. Chem., Int. Ed., 2021, 60, 8948–8959 CrossRef CAS PubMed.
- X. Ji, M. Ahmed, L. Long, N. M. Khashab, F. Huang and J. L. Sessler, Adhesive supramolecular polymeric materials constructed from macrocycle-based host–guest interactions, Chem. Soc. Rev., 2019, 48, 2682–2697 RSC.
- C. Heinzmann, C. Weder and L. M. de Espinosa, Supramolecular Polymer Adhesives: Advanced Materials Inspired by Nature, Chem. Soc. Rev., 2016, 45, 342–358 RSC.
- Y. Tian, J. Wu, X. Fang, L. Guan, N. Yao, G. Yang, Z. Wang, Z. Hua and G. Liu, Rational Design of Bioinspired Nucleobase-Containing Polymers as Tough Bioplastics and Ultra-Strong Adhesives, Adv. Funct. Mater., 2022, 32, 2112741 CrossRef CAS.
- A. H. Hofman, I. A. van Hees, J. Yang and M. Kamperman, Bioinspired underwater adhesives by using the supramolecular toolbox, Adv. Mater., 2018, 30, 1704640 CrossRef PubMed.
- B. D. B. Tiu, P. Delparastan, M. R. Ney, M. Gerst and P. B. Messersmith, Enhanced Adhesion and Cohesion of Bioinspired Dry/Wet Pressure-Sensitive Adhesives, ACS Appl. Mater. Interfaces, 2019, 11, 28296–28306 CrossRef CAS PubMed.
- D. Pei, S. Yu, P. Liu, Y. Wu, X. Zhang, Y. Chen, M. Li and C. Li, Reversible Wet-Adhesive and Self-Healing Conductive Composite Elastomer of Liquid Metal, Adv. Funct. Mater., 2022, 32, 2204257 CrossRef CAS.
- O. Berger, C. Battistella, Y. Chen, J. Oktawiec, Z. E. Siwicka, D. Tullman-Ercek, M. Wang and N. C. Gianneschi, Mussel Adhesive-Inspired Proteomimetic Polymer, J. Am. Chem. Soc., 2022, 144, 4383–4392 CrossRef CAS PubMed.
- Y. Wang, G. Xia, H. Yu, B. Qian, Y. H. Cheung, L. H. Wong and J. H. Xin, Mussel-Inspired Design of a Self-Adhesive Agent for Durable Moisture Management and Bacterial Inhibition on PET Fabric, Adv. Mater., 2021, 33, 2100140 CrossRef CAS PubMed.
- Q. Lin, D. Gourdon, C. J. Sun, N. Holten-Andersen, T. H. Anderson, J. H. Waite and J. N. Israelachvili, Adhesion mechanisms of the mussel foot proteins mfp-1 and mfp-3, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 3782–3786 CrossRef CAS PubMed.
- H. Lee, N. F. Scherer and P. B. Messersmith, Single-molecule mechanics of mussel adhesion, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- B. D. B. Tiu, P. Delparastan, M. R. Ney, M. Gerst and P. B. Messersmith, Cooperativity of Catechols and Amines in High-Performance Dry/Wet Adhesives, Angew. Chem., Int. Ed., 2020, 59, 16616–16624 CrossRef CAS PubMed.
- S. Das, G. Vasilyev, P. Martin and E. Zussman, Bioinspired Cationic-Aromatic Copolymer for Strong and Reversible Underwater Adhesion, ACS Appl. Mater. Interfaces, 2022, 14, 26287–26294 CrossRef CAS PubMed.
- G. P. Maier, M. V. Rapp, J. H. Waite, J. N. Israelachvili and A. Butler, Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement, Science, 2015, 349, 628–632 CrossRef CAS PubMed.
- W. Wei, L. Petrone, Y. Tan, H. Cai, J. N. Israelachvili, A. Miserez and J. H. Waite, An Underwater Surface-Drying Peptide Inspired by a Mussel Adhesive Protein, Adv. Funct. Mater., 2016, 26, 3496–3507 CrossRef CAS PubMed.
- J. Li, Z. Wang, Z. Hua and C. Tang, Supramolecular Nucleobase-Functionalized Polymers: Synthesis and Potential Biological Applications, J. Mater. Chem. B, 2020, 8, 1576–1588 RSC.
- W. Wang, S. Liu, B. Chen, X. Yan, S. Li, X. Ma and X. Yu, DNA-Inspired Adhesive Hydrogels Based on the Biodegradable Polyphosphoesters Tackified by a Nucleobase, Biomacromolecules, 2019, 20, 3672–3683 CrossRef CAS PubMed.
- X. Liu, Q. Zhang, L. Duan and G. Gao, Tough adhesion of nucleobase-tackifed gels in diverse solvents, Adv. Funct. Mater., 2019, 29, 1900450 CrossRef.
- X. Liu, Q. Zhang and G. Gao, Bioinspired Adhesive Hydrogels Tackified by Nucleobases, Adv. Funct. Mater., 2017, 27, 1703132 CrossRef.
- J. Wu, H. Lei, J. Li, Z. Zhang, G. Zhu, G. Yang, Z. Wang and Z. Hua, Nucleobase-Tackified Renewable Plant Oil-Based Supramolecular Adhesives with Robust Properties Both under Ambient Conditions and Underwater, Chem. Eng. J., 2021, 405, 126976 CrossRef CAS.
- X. Liu, Q. Zhang, L. Duan and G. Gao, Bioinspired nucleobase-driven nonswellable adhesive and tough gel with excellent underwater adhesion, ACS Appl. Mater. Interfaces, 2019, 11, 6644–6651 CrossRef CAS PubMed.
- S. Cheng, M. Zhang, N. Dixit, R. B. Moore and T. E. Long, Nucleobase Self-Assembly in Supramolecular Adhesives, Macromolecules, 2012, 45, 805–812 CrossRef CAS.
- X. Liu, Q. Zhang, Z. Gao, R. Hou and G. Gao, Bioinspired adhesive hydrogel driven by adenine and thymine, ACS Appl. Mater. Interfaces, 2017, 9, 17645–17652 CrossRef CAS PubMed.
- J. Wu, H. Lei, X. Fang, B. Wang, G. Yang, R. K. O'Reilly, Z. Wang, Z. Hua and G. Liu, Instant Strong and Responsive Underwater Adhesion Manifested by Bioinspired Supramolecular Polymeric Adhesives, Macromolecules, 2022, 55, 2003–2013 CrossRef CAS.
- A. L. Dobson, N. J. Bongiardina and C. N. Bowman, Combined Dynamic Network and Filler Interface Approach for Improved Adhesion and Toughness in Pressure-Sensitive Adhesives, ACS Appl. Polym. Mater., 2020, 2, 1053–1060 CrossRef CAS PubMed.
- K. D. Collins, Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process, Methods, 2004, 34, 300–311 CrossRef CAS PubMed.
- N. Vlachy, B. Jagoda-Cwiklik, R. Vacha, D. Touraud, P. Jungwirth and W. Kunz, Hofmeister series and specific interactions of charged headgroups with aqueous ions, Adv. Colloid Interface Sci., 2009, 146, 42–47 CrossRef CAS PubMed.
- L. Liu, R. Kou and G. Liu, Ion specificities of artificial macromolecules, Soft Matter, 2016, 13, 68–80 RSC.
- G. Liu, Tuning the Properties of Charged Polymers at the Solid/Liquid Interface with Ions, Langmuir, 2019, 35, 3232–3247 CrossRef CAS PubMed.
- H. Yuan and G. Liu, Ionic effects on synthetic polymers: from solutions to brushes and gels, Soft Matter, 2020, 16, 4087–4104 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, 1H NMR spectra, SEC traces, surface wettability, FT-IR spectra, XPS, and thermal and adhesive properties of nucleobase-containing polyelectrolytes with different counterions. See DOI: https://doi.org/10.1039/d3sc00697b |
| This journal is © The Royal Society of Chemistry 2023 |