Open Access Article
Open Access ArticleOrganocatalyzed enantio- and diastereoselective isomerization of prochiral 1,3-cyclohexanediones into nonalactones bearing distant stereocenters†
Antoine
d’Aleman
,
Oscar
Gayraud
,
Catherine
Fressigné
,
Emilie
Petit
,
Laetitia
Bailly
,
Jacques
Maddaluno
 and
Michaël
De Paolis
and
Michaël
De Paolis
 *
*
COBRA, Normandie University, 76000 Rouen, France. E-mail: Michael.depaolis@univ-rouen.fr
First published on 27th January 2023
Abstract
The lactonization of 2-(2-nitrophenyl)-1,3-cyclohexanediones containing an alcohol side chain and up to three distant prochiral elements is reported by isomerization under the mediation of simple organocatalysts such as quinidine. Through a process of ring expansion, strained nonalactones and decalactone are produced with up to three stereocenters in high er and dr (up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Distant groups, including alkyl, aryl, carboxylate and carboxamide moieties, were examined.
1). Distant groups, including alkyl, aryl, carboxylate and carboxamide moieties, were examined.
Introduction
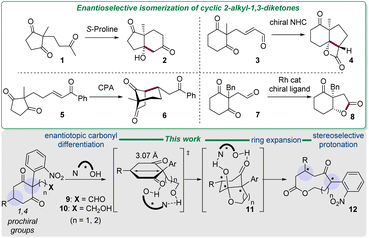
Desymmetrization of prochiral reagents is an ambitious but rewarding strategy, as the chiral products feature one or more stereocenters, after a single-step reaction in which the catalyst binds in the vicinity of the enantiotopic groups for an effective transfer of the chiral information.1 Applied to cyclic 2,2-disubstituted-1,3-diketones with enantiotopic carbonyl groups, this strategy was illustrated by Hajos and Parrish with the organocatalyzed enantioselective isomerization reaction of triketone 1 into ketol 2 (Scheme 1).2 Even though the desymmetrization of 2,2-disubstituted-1,3-diones has been richly investigated since then,3 few reports actually showcase an enantioselective isomerization of these substrates. For example, Scheidt reported the conversion of enal 3, tethered to 2-methyl-1,3-cyclohexanedione scaffold, to β-lactone 4 in the presence of chiral N-heterocyclic carbene (NHC).4 Through noncovalent interactions, enone 5 was isomerized to bicyclic diketones 6 by exposure to chiral phosphoric acid (CPA), as described by Lam.5 Later, Dong demonstrated the enantioselective transformation of 2-acetaldehyde-1,3-cyclohexanedione 7 to bicyclic γ-lactone 8 by Rh-catalyzed ketone hydroacylation reactions.6 Note that the enantioselective isomerization of cyclic 1,3-diones by a process effecting a ring enlargement is unknown.Nine-membered ring compounds have important applications in medicinal chemistry,7 but are challenging to synthesise due to ring strain,8 and their tendency to undergo transannular reactions to form bicyclic products.9
Amidst the enantioselective ones leading to medium-sized lactones from racemic or prochiral materials, a general strategy presented itself based on the in situ generation of electrophiles, binding to transition metal-chiral ligands or organocatalysts, reacting with enolates or olefins.10 To our knowledge, though, a single example of enantioselective synthesis of nonalactones from prochiral reagents was reported,10a and it is hitherto unknown with distant stereocenters. Herein is reported the distal enantio- and diastereoselective ring expansion of 2-aryl-1,3-cyclohexanediones, into nonalactones with up to three distal stereocenters through an organocatalyzed isomerization.
Results and discussion
Our interest in the topic stems from our previous report of a domino sequence starting from aldehyde 9 (R = H), containing the motif 2-(2-nitrophenyl)-1,3-cyclohexanedione, which was converted into nonalactones when treated with carbanions (Scheme 1).11 The resulting alcoholate initiated a series of transformations, eventually affording racemic nonalactones.In a further step, the reactivity of the alcoholate holds promise for a enantioselective access to nonalactones 12 from alcohol 10 bearing two prochiral groups (R ≠ H). In the presence of a chiral bidentate organocatalyst, we envisaged that the hydroxyl and the carbonyl moieties of 10 would be engaged in a chiral environment allowing the enantioselective formation of the fused bicyclic lactol 11 preceding the ring expansion by retro-Claisen condensation into 12.12 Notably, our blueprint implies a spatial discrimination of prochiral elements through noncovalent interactions.
The desymmetrization of meso cyclic acid anhydrides well illustrates the concept of activation of carbonyl groups for the addition of a nucleophile, effecting thereby the discrimination of vicinal enantiotopic groups. To that end, Oda13 and Aitken14 independently identified Cinchona alkaloids as efficient organocatalysts. If it was thus conceivable to promote the enantioselective isomerization of 10 with these alkaloids, whether the process of desymmetrization could establish distant stereocenters was uncertain. For that matter, different strategies were developed requiring catalysts/ligands with large structures.15
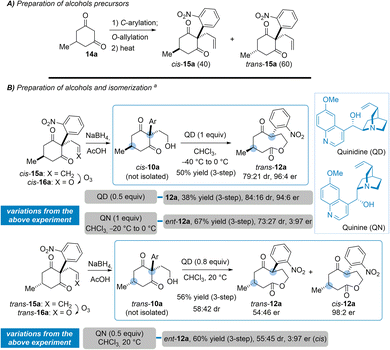
To test our hypothesis, the alcohols trans- and cis-10a were prepared from the corresponding isomers of the olefins 15a after a sequence of reactions, without purification, including ozonolysis into aldehydes 16a followed by a careful reduction (Scheme 2A). Starting from 5-methyl-1,3-cyclohexanedione 14a, the construction of 15a began with one-pot C-arylation and O-allylation steps, preceding a Claisen rearrangement for which the stereochemical outcome, the ratio trans/cis-15a (60/40), is rectifiable under Tsuji-Trost conditions.16 This is an important aspect as the configuration of alcohol 10a had an interesting bearing on the selectivity of the process (see the ESI for the optimization†).17
When exposed to quinidine (QD (1 equiv.), CHCl3, 17 h, −40 to 0 °C), cis-10a was converted into trans-12a (79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 diastereoisomers ratio (dr)), the relative configuration being determined by NOESY experiments, with enantioselectivity (96
21 diastereoisomers ratio (dr)), the relative configuration being determined by NOESY experiments, with enantioselectivity (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 enantiomers ratio (er), Scheme 2B) and with a yield of 50% yield over 3-step amounting to a theoretical 85% yield for each step. Note that operating instead with 0.5 equivalent of QD gave trans-12a with close selectivity (86
4 enantiomers ratio (er), Scheme 2B) and with a yield of 50% yield over 3-step amounting to a theoretical 85% yield for each step. Note that operating instead with 0.5 equivalent of QD gave trans-12a with close selectivity (86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 dr, 94
14 dr, 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er) after a longer reaction time (40 h, 38% yield). On the other hand, trans-10a was lactonized into 12a (56% yield, 58
6 er) after a longer reaction time (40 h, 38% yield). On the other hand, trans-10a was lactonized into 12a (56% yield, 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 dr, trans/cis) upon exposure to QD (0.8 equiv.) in CHCl3 at room temperature (16 h) and while trans-12a was formed without enantioselectivity, high enantiopurity (98
42 dr, trans/cis) upon exposure to QD (0.8 equiv.) in CHCl3 at room temperature (16 h) and while trans-12a was formed without enantioselectivity, high enantiopurity (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er) was measured for cis-12a.
2 er) was measured for cis-12a.
Moreover, the treatment of cis-10a with quinine QN (1 equiv., −40 to 0 °C)—a natural pseudo enantiomer of QD—led to trans-12a (67% yield, 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 dr) having the attendant opposite enantioselectivity (3
27 dr) having the attendant opposite enantioselectivity (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97 er). Similarly, exposure of trans-10a to QN (0.5 equiv., rt) led to 12a (70% yield, 55
97 er). Similarly, exposure of trans-10a to QN (0.5 equiv., rt) led to 12a (70% yield, 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 dr) and, while the formation of trans-12a occurred without enantioselectivity, the enantiopurity of cis-12a was excellent (3
45 dr) and, while the formation of trans-12a occurred without enantioselectivity, the enantiopurity of cis-12a was excellent (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97 er).
97 er).
To sum up, both alcohols trans-10a and cis-10a were simply lactonized into 12a by an enantioselective ring expansion process upon exposure to readily available organocatalysts, that are easily recovered.
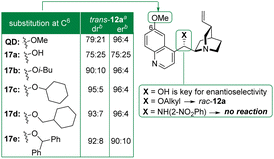
We then sought to identify the catalyst functions influencing the selectivity and, among them, the secondary benzylic alcohol appeared crucial for the enantioselectivity (Fig. 1). Bearing instead an alkyl ether or the acidic 2-nitroaniline, the corresponding catalysts induced the formation of rac-12a or were inactive.18
While no improvement was noted with cupreidine 17a (trans-12a: 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 er), increasing the steric hindrance of the quinolin-6-ol scaffold was rewarding. Simply obtained by O-alkylation of 17a with i-butyl bromide (47% yield), 17b isomerized cis-10a to trans-12a (70 h, 51% yield) with better selectivity (90
25 er), increasing the steric hindrance of the quinolin-6-ol scaffold was rewarding. Simply obtained by O-alkylation of 17a with i-butyl bromide (47% yield), 17b isomerized cis-10a to trans-12a (70 h, 51% yield) with better selectivity (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr, 96
10 dr, 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er). Prepared by O-alkylation of 17a with bromocyclohexane (15% yield), 17c induced the formation of trans-12a (120 h, 63% yield) with high values of 95
4 er). Prepared by O-alkylation of 17a with bromocyclohexane (15% yield), 17c induced the formation of trans-12a (120 h, 63% yield) with high values of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr and 96
5 dr and 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er. For a slight modification of the ether appendage, 17d was synthesized by O-alkylation of 17a with (bromomethyl)cyclohexane in excellent yield (92%). Gratifyingly, the lactonization of cis-10a catalyzed by 17d was completed within a shorter timeframe (96 h, 60% yield) and with excellent selectivity (93
4 er. For a slight modification of the ether appendage, 17d was synthesized by O-alkylation of 17a with (bromomethyl)cyclohexane in excellent yield (92%). Gratifyingly, the lactonization of cis-10a catalyzed by 17d was completed within a shorter timeframe (96 h, 60% yield) and with excellent selectivity (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr, 96
7 dr, 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er). As noted with 17e, a bulkier group at this position was detrimental to trans-12a enantiopurity (90
4 er). As noted with 17e, a bulkier group at this position was detrimental to trans-12a enantiopurity (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 er).
10 er).
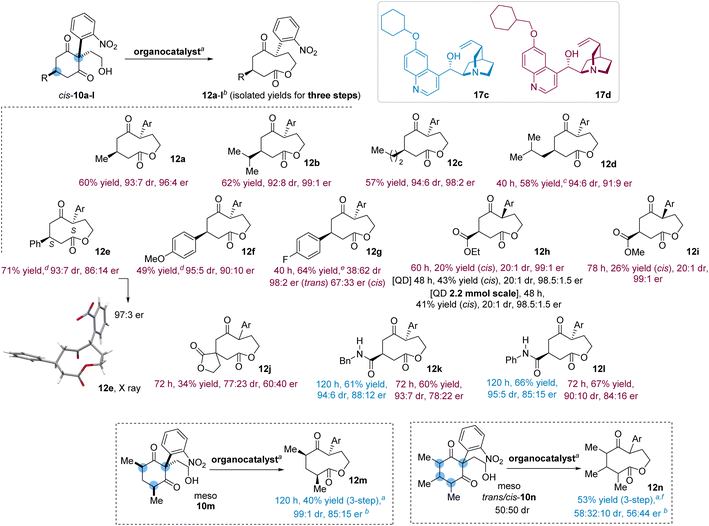
Selecting catalysts 17c and 17d, the scope of the process was first examined with prochiral 5-alkyl-2-(2-nitrophenyl)-1,3-cyclohexanediones (Scheme 3). Substituted with i-propyl group, trans-12b was isolated (62% yield—over three steps as every other given yields) with 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dr and 99
8 dr and 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er after exposure of cis-10b to 17d (results obtained with 17c are provided in the ESI†). Substituted with the n-propyl group, cis-10c was lactonized with excellent selectivity (trans-12c: 94
1 er after exposure of cis-10b to 17d (results obtained with 17c are provided in the ESI†). Substituted with the n-propyl group, cis-10c was lactonized with excellent selectivity (trans-12c: 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr, 98
6 dr, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er). The i-butyl derivative cis-10d was transformed into trans-12d with 94
2 er). The i-butyl derivative cis-10d was transformed into trans-12d with 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr and 91
6 dr and 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 er, values obtained at −10 °C due to a solubility issue.
9 er, values obtained at −10 °C due to a solubility issue.
We then investigated the desymmetrization of 5-aryl-2-(2-nitrophenyl)-1,3-cyclohexanediones. Starting with the lactonization of phenyl derivative cis-10e, trans-12e (71% yield, 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr) was isolated with moderate enantiopurity (86
7 dr) was isolated with moderate enantiopurity (86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 er). A suitable crystal for X-ray crystallography was obtained with enhanced values (99
14 er). A suitable crystal for X-ray crystallography was obtained with enhanced values (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 97
1 dr, 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er) allowing the determination of the absolute (S, S)-configuration of the major isomer. The isomerization of the 4-methoxyphenyl derivative cis-10f led to trans-12f (49% yield) with high selectivity (95
3 er) allowing the determination of the absolute (S, S)-configuration of the major isomer. The isomerization of the 4-methoxyphenyl derivative cis-10f led to trans-12f (49% yield) with high selectivity (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, 90
5 dr, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 er). Lactonization of the 4-fluorophenyl derivative cis-10g into 12g (64% yield, 40 h) occurred with an intriguing switch of diastereoselectivity. While the cis-lactone was so far formed in traces, cis-12g became slightly predominant (38
10 er). Lactonization of the 4-fluorophenyl derivative cis-10g into 12g (64% yield, 40 h) occurred with an intriguing switch of diastereoselectivity. While the cis-lactone was so far formed in traces, cis-12g became slightly predominant (38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 dr, trans/cis), the relative configuration being determined by NOESY experiments, and although the enantiopurity of cis-12g was low (67
62 dr, trans/cis), the relative configuration being determined by NOESY experiments, and although the enantiopurity of cis-12g was low (67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 er), trans-12g was enantioenriched (98
33 er), trans-12g was enantioenriched (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er).
2 er).
With derivatives of 5-alkylcarboxylate-2-(2-nitrophenyl)-1,3-cyclohexanedione such as 10h, the selectivity of the process was worth examining. Unlike the previous pattern, cis-12h was isolated enantiopure (20% yield, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er) while trans-12h was obtained with lower enantiopurity (57% yield, 61
1 er) while trans-12h was obtained with lower enantiopurity (57% yield, 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 er), the relative configuration being determined by NOESY experiments. As noted at the beginning of the study, QD induced the lactonization of cis-10a with high enantioselectivity but with moderate diastereoselectivity. This proved to be advantageous, though, in this context as the treatment of cis-10h with QD led to an equal amount of trans- and cis-12h, the latter being isolated with an exquisite enantiopurity (43% yield, 98.5
39 er), the relative configuration being determined by NOESY experiments. As noted at the beginning of the study, QD induced the lactonization of cis-10a with high enantioselectivity but with moderate diastereoselectivity. This proved to be advantageous, though, in this context as the treatment of cis-10h with QD led to an equal amount of trans- and cis-12h, the latter being isolated with an exquisite enantiopurity (43% yield, 98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 er), values that remain consistent on a larger scale experiment (2.2 mmol, 41% yield, 98.5
1.5 er), values that remain consistent on a larger scale experiment (2.2 mmol, 41% yield, 98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 er).19
1.5 er).19
Decreasing the steric hindrance of the ester, as with cis-10i, was slightly beneficial since 17d catalyzed the formation of cis-12i in 26% yield and 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er, trans-12i (40% yield) being isolated with 65
1 er, trans-12i (40% yield) being isolated with 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 er values. The treatment of the hindered 10j (unknown relative configuration), bearing a spiro γ-lactone moiety, afforded 12j (34% yield) with modest selectivity (77
35 er values. The treatment of the hindered 10j (unknown relative configuration), bearing a spiro γ-lactone moiety, afforded 12j (34% yield) with modest selectivity (77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 dr, 60
23 dr, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 er).
40 er).
After examining hydrogen bond acceptors, the isomerization of scaffolds bearing also hydrogen bond donors was evaluated with the secondary benzylcarboxamide derivative cis-10k. In this setting, 17c performed best the lactonization into trans-12k (120 h, 61% yield) and, in contrast with the carboxylate esters, the trans-isomer was prevalent (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr) and enantioenriched (88
6 dr) and enantioenriched (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 er). On the other hand, 17d enabled the formation of trans-12k in shorter reaction time (72 h, 60% yield, 93
12 er). On the other hand, 17d enabled the formation of trans-12k in shorter reaction time (72 h, 60% yield, 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr) but with lower selectivity (78
7 dr) but with lower selectivity (78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 er). Embedding a more acidic N–H bond, the anilinecarboxamide cis-10l was isomerized into trans-12l (120 h, 66% yield, 95
22 er). Embedding a more acidic N–H bond, the anilinecarboxamide cis-10l was isomerized into trans-12l (120 h, 66% yield, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) by 17c without enhancement of the enantiopurity (85
5 dr) by 17c without enhancement of the enantiopurity (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 er). But this structural modification had more consequences when exposed to 17d, as the reaction of cis-10l (72 h, trans-12l: 67% yield, 90
15 er). But this structural modification had more consequences when exposed to 17d, as the reaction of cis-10l (72 h, trans-12l: 67% yield, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr) proceeded with higher enantioselectivity (84
10 dr) proceeded with higher enantioselectivity (84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 er) than with cis-10k (78
16 er) than with cis-10k (78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 er). When comparing the influence of alkyl carboxylate and secondary carboxamide functions on the selectivity, the latter steers the reaction pathway toward the enantioselective production of the trans-isomer.
22 er). When comparing the influence of alkyl carboxylate and secondary carboxamide functions on the selectivity, the latter steers the reaction pathway toward the enantioselective production of the trans-isomer.
The formation of a trisubstituted lactone was next studied from a meso reagent. Advantageously synthesized as a single isomer, cis-10m was converted by 17c into the trisubstituted lactone 12m (40% yield), isolated as a single diastereoisomer (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and with appreciable enantioselectivity (85
1 dr) and with appreciable enantioselectivity (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 er). In contrast, exposure of cis-10m to 17d gave 12m (99
15 er). In contrast, exposure of cis-10m to 17d gave 12m (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 55
1 dr, 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 er) with low enantioselectivity, demonstrating thus the superior affinity of 17c for the topography of meso compounds. A step further, we sought to prepare the persubstituted lactone 12n from cis/trans-10n (50
45 er) with low enantioselectivity, demonstrating thus the superior affinity of 17c for the topography of meso compounds. A step further, we sought to prepare the persubstituted lactone 12n from cis/trans-10n (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 dr). In this complex scenario however, 12n (53% yield, 58
50 dr). In this complex scenario however, 12n (53% yield, 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr) was obtained without enantioselectivity (56
10 dr) was obtained without enantioselectivity (56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 er).
44 er).
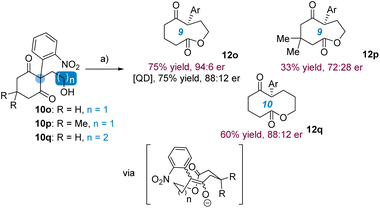
Subsequent to the isomerization of substrates with at least two prochiral elements, the strategy was applied to 10o bearing a single prochiral group (Scheme 4). Note that it was not part of our initial plan devised in Scheme 1 since it was unclear whether the chiral information of lactol 11o would be preserved during the ring expansion into enolate (12o)−.
We were therefore surprised to measure the high enantioselectivity of 12o (75% yield, 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er) obtained by treatment of 10o with 17d, which dropped to 88
6 er) obtained by treatment of 10o with 17d, which dropped to 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 er when QD was instead employed. Indicative that a quaternary carbon within the 1,3-cyclohexanedione ring remains moderately compatible with the chemistry, 10p was converted into 12p (33% yield) with 72
12 er when QD was instead employed. Indicative that a quaternary carbon within the 1,3-cyclohexanedione ring remains moderately compatible with the chemistry, 10p was converted into 12p (33% yield) with 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 er value. Pleasingly, bearing a longer alcohol appendage, 10q was converted into decalactone 12q with significant enantioselectivity (60% yield, 88
28 er value. Pleasingly, bearing a longer alcohol appendage, 10q was converted into decalactone 12q with significant enantioselectivity (60% yield, 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 er).20
12 er).20
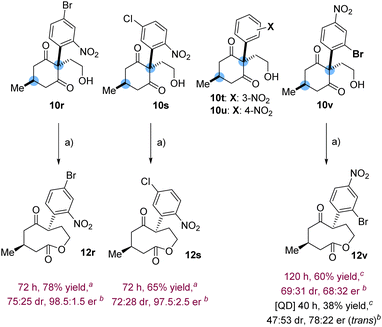
Amidst all the structural modulations examined within the study, the aromatic substitution of the prochiral scaffold was left to explore. To that end, alcohols cis-10r,s were synthesized, each one embedding a different halo-substituted 2-nitrophenyl ring (Fig. 2).
Compared to the case of cis-10a, 4-bromo-2-nitrophenyl derivative cis-10r was isomerized with less diastereoselectivity (12r: 78% yield, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 dr) but enhanced enantioselectivity (98.5
25 dr) but enhanced enantioselectivity (98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 er) when exposed to 17d. With a different pattern of substitution, 5-chloro-2-nitrophenyl derivative cis-10s was converted by 17d into 12s (65% yield, 72
1.5 er) when exposed to 17d. With a different pattern of substitution, 5-chloro-2-nitrophenyl derivative cis-10s was converted by 17d into 12s (65% yield, 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 dr) with excellent enantioselectivity (97.5
28 dr) with excellent enantioselectivity (97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 er).
2.5 er).
To modulate the position of the nitro group, we set about preparing 2-(3-nitrophenyl)-1,3-cyclohexanedione derivative 10t. But the instability of the precursor aldehyde 9t thwarted our plan and a similar outcome was noted with the 4-nitrophenyl analogue 9u. We surmised that a steric effect, induced by the ortho substitution of the aromatic ring, could prevent the degradation of the aldehyde. Lending credence to this hypothesis, 2-(2-bromo-4-nitrophenyl) derivative 9v was successfully obtained and converted into alcohol cis-10v. In the presence of 17d, the formation of trans-12v occurred slowly at −10 °C (120 h, 60% yield) with modest selectivity (69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 dr, 68
31 dr, 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 er). A slight enhancement was noted upon the catalysis of QD (40 h, −10 °C, 38% yield), trans-12v being formed with higher enantiopurity (78
32 er). A slight enhancement was noted upon the catalysis of QD (40 h, −10 °C, 38% yield), trans-12v being formed with higher enantiopurity (78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 er) but at the expense of the diastereoselectivity (47
22 er) but at the expense of the diastereoselectivity (47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 dr). The nitro group in the ortho-position of the aromatic ring seems therefore favorable to achieve high stereoselectivity.
53 dr). The nitro group in the ortho-position of the aromatic ring seems therefore favorable to achieve high stereoselectivity.
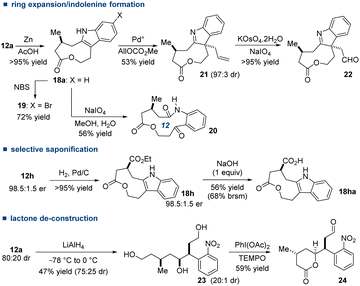
However, as illustrated in Scheme 5, this structural requirement advantageously paves the way to the synthesis of enantioriched alkaloids and derivatives. For instance, the indole 18a was prepared from 12a by reduction of the nitro group (Zn, AcOH, >95% yield) and the regioselective halogenation occurred in 6-position (19, 72% yield), offering a synthetic handle for the decoration of the aromatic ring. Underscoring the strain of the indolononalactone, the spontaneous air oxidation of the aromatic ring of 18a into hydroxylindolenine was noted (∼20% after two months of storage at rt, see the ESI for the details†). For that matter, we purposely performed the consecutive ring-expansion reaction of 18a by oxidative cleavage (NaIO4)21a delivering the 12-membered ring 20 (56% yield). Not only this demonstrated a short route to enantioenriched 12-membered ring from 6-membered ring 10avia12a,21b but 20 provides a platform for further ring expansion, as illustrated by Unsworth, to access macrocycles.21c
Palladium-catalyzed alkylative dearomatization of the indole 18a led to trans-allylic indolenine 21 (53% yield, non-optimized) after the distal diastereoselective formation of a quaternary carbon (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dr).22 Bearing an additional reactive function, the aldehyde 22 was smoothly obtained after oxidative cleavage of the allylic appendage (>95% yield). Prepared from 12h by hydrogenation (>95% yield), the indolic ethyl ester 18h was treated with NaOH (1 equiv., 1,4-dioxane, H2O) to give the carboxylic acid lactone 18ha (56% yield, 68% brsm), demonstrating thus the robustness of the strained but sterically hindered ring.23 To access new scaffolds, 12a (80
3 dr).22 Bearing an additional reactive function, the aldehyde 22 was smoothly obtained after oxidative cleavage of the allylic appendage (>95% yield). Prepared from 12h by hydrogenation (>95% yield), the indolic ethyl ester 18h was treated with NaOH (1 equiv., 1,4-dioxane, H2O) to give the carboxylic acid lactone 18ha (56% yield, 68% brsm), demonstrating thus the robustness of the strained but sterically hindered ring.23 To access new scaffolds, 12a (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 dr) was deconstructed with LiAlH4 into triol 23 (42% yield, 75
20 dr) was deconstructed with LiAlH4 into triol 23 (42% yield, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 dr) and next converted into δ-lactone 24 (PhI(OAc)2/TEMPO, 59% yield, relative configuration determined by NOESY experiments).24
25 dr) and next converted into δ-lactone 24 (PhI(OAc)2/TEMPO, 59% yield, relative configuration determined by NOESY experiments).24
Conclusions
While they are among the most difficult cyclic scaffolds to prepare for kinetic and thermodynamic reasons,8b nonalactones and decalactone with up to three non-vicinal stereocenters were prepared by ring expansion of prochiral alcohols (21 examples). Modulation of the configuration and substitution patterns of the prochiral material allowed the exploration of various steric and electronic scenarios (alkyl, aryl, carboxylate and carboxamide), picturing the perimeter and potential of the strategy with high values of dr and er (up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) owing to the 2-nitrophenyl function. Whether other electron-withdrawing groups may enable the enantioselective desymmetrization of the corresponding alcohol remains to be determined. However, this current limitation is counterbalanced by the enantio- and diastereoselective access to indoles, indolenines and derivatives thus provided, structural motifs notably encountered in many alkaloids (natural products or pharmaceuticals). From this investigation emerges a tool box in which readily available organocatalysts—QD or QN, 17c, 17d—effectively synthesized isomers of lactones 12 from cis-10 or trans-10. Bearing a quinolin-6-ol substituted with a methylenecyclohexyl ether, which increased the volume of the appendage, 17d offered a good balance between reactivity and stereoselectivity in most cases. Quinidine and quinine being commercially available, the two other derivatives are prepared in one step from cupreidine and are easily recovered after reaction, offsetting the catalytic load used to perform the energy-demanding ring expansion step. For that matter, the process of ring expansion of trans-10a into cis-12a required higher temperature to occur, without inducing lower enantioselectivity.
1) owing to the 2-nitrophenyl function. Whether other electron-withdrawing groups may enable the enantioselective desymmetrization of the corresponding alcohol remains to be determined. However, this current limitation is counterbalanced by the enantio- and diastereoselective access to indoles, indolenines and derivatives thus provided, structural motifs notably encountered in many alkaloids (natural products or pharmaceuticals). From this investigation emerges a tool box in which readily available organocatalysts—QD or QN, 17c, 17d—effectively synthesized isomers of lactones 12 from cis-10 or trans-10. Bearing a quinolin-6-ol substituted with a methylenecyclohexyl ether, which increased the volume of the appendage, 17d offered a good balance between reactivity and stereoselectivity in most cases. Quinidine and quinine being commercially available, the two other derivatives are prepared in one step from cupreidine and are easily recovered after reaction, offsetting the catalytic load used to perform the energy-demanding ring expansion step. For that matter, the process of ring expansion of trans-10a into cis-12a required higher temperature to occur, without inducing lower enantioselectivity.
Futures studies will be focused on some features and implications of this complex and yet simple to implement process, such as the inversion of selectivity observed with fluorophenyl and carboxylate ester derivatives, or the origins of the stereoselectivity induced by the alkyl ether of the quinolin-6-ol appendage of the catalyst.
Data availability
All experimental and characterization data including HPLC traces and NMR spectra are available in the ESI.† Crystallographic data for compound 12e has been deposited in the Cambridge Crystallographic Data Centre under accession number CCDC 2162117.Author contributions
A. A. and O. G. conducted the investigation and prepared the ESI.† C. F. conducted the DFT calculations. L. B. and E. P. contributed to the formal analysis of the results. J. M. reviewed the manuscript. M. D. P. conceptualized and supervised the research, wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AA thanks the Ministere de la Recherche et de l’Education Nationale for a PhD fellowship. This work has been partially supported by University of Rouen Normandy, INSA Rouen Normandy, the Centre National de la Recherche Scientifique (CNRS), European Regional Development Fund (ERDF), Labex SynOrg (ANR-11-LABX-0029), Carnot Institute I2C, the graduate school for research XL-Chem (ANR-18-EURE-0020 XL CHEM), and by Région Normandie. AA thanks Prof. Adam Daïch (University of Le Havre) for suggesting the oxidative ring expansion of compound 18a. We thank Dr Jean-Francois Brière and Dr Julien Legros for helpful input, Dr Baptiste Picard for preliminary experiments and Jean-François Lohier (LCMT) for X-ray analysis. The CRIANN is gratefully acknowledged for providing computation resources.Notes and references
- C. Nájera, F. Foubelo, J. M. Sansano and M. Yus, Tetrahedron, 2022, 106, 132629 CrossRef.
- Z. G. Hajos and D. R. Parrish, J. Org. Chem., 1974, 39, 1615–1621 CrossRef CAS.
- (a) X.-P. Zeng, Z.-Y. Cao, Y.-H. Wang, F. Zhou and J. Zhou, Chem. Rev., 2016, 116, 7330–7396 CrossRef CAS PubMed; (b) R. Chegondi, S. M. Patel, S. Maurya and A. Donthoju, Asian J. Org. Chem., 2021, 10, 1267–1281 CrossRef CAS; (c) Y.-S. Zhao, X.-Q. Tang, J.-C. Tao, P. Tian and G.-Q. Lin, Org. Biomol. Chem., 2016, 14, 4400–4404 RSC; (d) F. Leonelli, A. Trombetta, A. La Bella, G. Lucarelli, N. Demitri, D. Lamba, L. M. Migneco and R. Marini Bettolo, Eur. J. Org. Chem., 2019, 2019, 1594–1599 CrossRef CAS; (e) T. Yoshimura, Y. Enami and J. Matsuo, Synthesis, 2020, 52, 3667–3674 CrossRef CAS; (f) P. Zhou and T. Xu, Chem. Commun., 2020, 56, 8194–8197 RSC; (g) X. Han, L. Shan, J. Zhu, C. Zhang, X. Zhang, F. Zhang, H. Wang, Y. Tu, M. Yang and W. Zhang, Angew. Chem., Int. Ed., 2021, 60, 22688–22692 CrossRef CAS PubMed; (h) X.-L. Qin, A. Li and F.-S. Han, J. Am. Chem. Soc., 2021, 143, 2994–3002 CrossRef CAS PubMed; (i) B. Yang, J. Dai, Y. Luo, K. K. Lau, Y. Lan, Z. Shao and Y. Zhao, J. Am. Chem. Soc., 2021, 143, 4179–4186 CrossRef CAS PubMed; (j) L. Zhang and M. Oestreich, ACS Catal., 2021, 11, 3516–3522 CrossRef CAS; (k) J. Li, J. Sun, W. Ren, J. Lei, R. Shen and Y. Huang, Org. Lett., 2022, 24, 2420–2424 CrossRef CAS PubMed; (l) Z. Zhou, D. Xu, W. Jiang, J. chen, Y. Zhen, J. Huo, J. Yan, J. Gao and W. Xie, Org. Lett., 2022, 24, 9017–9022 CrossRef CAS PubMed; (m) M. Dajek, A. Pruszczyńska, K. A. Konieczny and R. Kowalczyk, Adv. Synth. Catal., 2020, 362, 3613–3620 CrossRef CAS; (n) Y. Kawamoto, D. Ozone, T. Kobayashi and H. Ito, Eur. J. Org. Chem., 2020, 4050–4058 CrossRef CAS.
- (a) M. Wadamoto, E. M. Phillips, T. E. Reynolds and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 10098–10099 CrossRef CAS PubMed; (b) T. Ema, K. Akihara, R. Obayashi and T. Sakai, Adv. Synth. Catal., 2012, 354, 3283–3290 CrossRef CAS.
- A. R. Burns, A. G. E. Madec, D. W. Low, I. D. Roy and H. W. Lam, Chem. Sci., 2015, 6, 3550–3555 RSC.
- X. Wu, Z. Chen, Y.-B. Bai and V. M. Dong, J. Am. Chem. Soc., 2016, 138, 12013–12016 CrossRef CAS PubMed.
- (a) Heterocyclic Chemistry III, eds. Katritzky, A. R., Ramsden, C. A., Scriven, E. F. V. and Taylor, R. J. K., Elsevier, Amsterdam, 2008, vol. 14, pp. 547–611 Search PubMed; (b) I. Shiina, Chem. Rev., 2007, 107, 239–273 CrossRef CAS PubMed; (c) T. Huber, R. E. Wildermuth and T. Magauer, Chem. – Eur. J., 2018, 24, 12107–12120 CrossRef CAS PubMed; (d) J. S. Clark, R. Berger, S. T. Hayes, L. H. Thomas, A. J. Morrison and L. Gobbi, Angew. Chem., Int. Ed., 2010, 49, 9867–9870 CrossRef CAS PubMed; (e) Z. Meng and A. Fürstner, J. Am. Chem. Soc., 2019, 141, 805–809 CrossRef CAS PubMed; (f) S. Shiomi, K. Wilailak, W. Soutome, H. Takayama, M. Kitajima and H. Ishikawa, J. Org. Chem., 2022, 87, 3730–3735 CrossRef CAS PubMed; (g) C. Liu, Y. Li, W.-Y. Shi, Y.-N. Ding, N. Zheng, H.-C. Liu and Y.-M. Liang, Org. Lett., 2021, 23, 4311–4316 CrossRef CAS PubMed; (h) A. Lawer, J. A. Rossi-Ashton, T. C. Stephens, B. J. Challis, R. G. Epton, J. M. Lynam and W. P. Unsworth, Angew. Chem., Int. Ed., 2019, 58, 13942–13947 CrossRef CAS PubMed; (i) G. Yang, Y. Ke and Y. Zhao, Angew. Chem., Int. Ed., 2021, 60, 12775–12780 CrossRef CAS PubMed; (j) J. R. Donald and W. P. Unsworth, Chem. – Eur. J., 2017, 23, 8780–8799 CrossRef CAS PubMed; (k) A. K. Clarke and W. P. Unsworth, Chem. Sci., 2020, 11, 2876–2881 RSC.
- (a) J. D. Dunitz and V. Prelog, Angew. Chem., 1960, 72, 896–902 CrossRef; (b) C. Galli, G. Illuminati, L. Mandolini and P. Tamborra, J. Am. Chem. Soc., 1977, 99, 2591–2597 CrossRef CAS.
- (a) Y. Ban, K. Yoshida, J. Goto and T. Oishi, J. Am. Chem. Soc., 1981, 103, 6990–6992 CrossRef CAS; (b) I. Shiina, Y. Takasuna, R. Suzuki, H. Oshiumi, Y. Komiyama, S. Hitomi and H. Fukui, Org. Lett., 2006, 8, 5279–5282 CrossRef CAS PubMed; (c) M. Mewald, J. W. Medley and M. Movassaghi, Angew. Chem., Int. Ed., 2014, 53, 11634–11639 CrossRef CAS PubMed; (d) K. L. White and M. Movassaghi, J. Am. Chem. Soc., 2016, 138, 11383–11389 CrossRef CAS PubMed; (e) For a review: E. Reyes, L. Prieto, L. Carrillo, U. Uria and J. L. Vicario, Synthesis, 2022, 54, 4167–4183 CrossRef CAS.
- (a) L. Ding, H. Song, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2022, 144, 4770–4775 CrossRef CAS PubMed; (b) J. L. Payne, Z. Deng, A. L. Flach and J. N. Johnston, Chem. Sci., 2022, 13, 7318–7324 RSC; (c) X. Jiang, X. Xu, W. Xu, P. Yu and Y.-Y. Yeung, Org. Lett., 2021, 23, 6316–6320 CrossRef CAS PubMed.
- (a) D. Reyes Loya, A. Jean, M. Cormier, C. Fressigné, S. Nejrotti, J. Blanchet, J. Maddaluno and M. De Paolis, Chem. – Eur. J., 2018, 24, 2080–2084 CrossRef PubMed; (b) D. Reyes Loya and M. De Paolis, Chem. – Eur. J., 2019, 25, 1842–1847 CrossRef CAS PubMed.
- For an example of enantioselective four-atom ring-expansion, see: Y. Zhou, M. Y.-L. Wei, J. Rodriguez and Y. Coquerel, Angew. Chem., Int. Ed., 2018, 58, 456–460 CrossRef PubMed.
- (a) J. Hiratake, Y. Yamamoto and J. Oda, J. Chem. Soc., Chem. Commun., 1985, 1717 RSC; (b) J. Hiratake, M. Inagaki, Y. Yamamoto and J. Oda, J. Chem. Soc., Perkin Trans., 1987, 1, 1053 RSC.
- (a) R. A. Aitken, J. Gopal and J. A. Hirst, J. Chem. Soc., Chem. Commun., 1988, 632 RSC; (b) R. A. Aitken and J. Gopal, Tetrahedron: asymmetry, 1990, 1, 517–520 CrossRef CAS.
- (a) C.-C. Hsiao, H.-H. Liao and M. Rueping, Angew. Chem., Int. Ed., 2014, 53, 13258–13263 CrossRef CAS PubMed; (b) A. J. Metrano and S. J. Miller, Acc. Chem. Res., 2019, 52, 199–215 CrossRef CAS PubMed; (c) W. Luo, L. Lin, Y. Zhang, X. Liu and X. Feng, Org. Lett., 2017, 19, 3374–3377 CrossRef CAS PubMed; (d) J. T. Payne, P. H. Butkovich, Y. Gu, K. N. Kunze, H. J. Park, D.-S. Wang and J. C. Lewis, J. Am. Chem. Soc., 2018, 140, 546–549 CrossRef CAS PubMed; (e) Y. Lou, J. Wei, M. Li and Y. Zhu, J. Am. Chem. Soc., 2022, 144, 123–129 CrossRef CAS PubMed.
- Along this study, the ratio trans/cis-15 varied with the substitution of the 1,3-cyclohexanedione ring (see ESI for the details†). An isomerization step was devised under Tsuji-Trost conditions (Pd(Ph3)4 cat., THF, rt) to convert pure trans-15 into an isomeric mixture from which cis-15 is isolated. The method was illustrated from trans-15b yielding a mixture of isomers (68
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32, trans/cis) in spite of the steric hindrance of the i-Pr group (see the ESI for the details†).
32, trans/cis) in spite of the steric hindrance of the i-Pr group (see the ESI for the details†). - In the absence of a catalyst, no formation of 12a was noted at a low temperature. Likely by acidic activation, the slow conversion of cis-10a into rac-12a occurred at room temperature in CDCl3 (after 1 h) or on silica gel, making a full conversion mandatory to achieve high enantioselectivity. Otherwise, oxidation of the unreacted alcohol into aldehyde was performed by addition of Dess-Martin periodinane.
- Several bifunctional organocatalysts were tested among the cinchona alkaloids including stronger H-bonding appendage as thiourea but lower conversion was noted (see ESI doi details†). Further, the Takemoto catalyst performed the isomerization with lower er values (see ESI†).
- We sought to confirm the absolute configuration of 12h,i, to no avail. While the absolute configuration of 12e was used as a basis of extrapolation to the other cases, the peculiar selectivity noted with 12h,i suggests a different mechanism with potential implication on this point. Moreover, performing the experiment at room temperature (4 h, 70% yield) revealed that the enantiopurity of cis-12h (18% yield) was only marginally decreased (96
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er), the stereoselectivity being more impacted (75
4 er), the stereoselectivity being more impacted (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 dr) with trans-12h isolated in 70
25 dr) with trans-12h isolated in 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 er. Incidentally, trans-12h was measured with reversed and higher, though still modest, enantiopurity when the reaction was conducted at room temperature than at −40 °C (45
30 er. Incidentally, trans-12h was measured with reversed and higher, though still modest, enantiopurity when the reaction was conducted at room temperature than at −40 °C (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 er), probably due to the epimerization of enantioenriched cis-12h into trans-12h occurring at room temperature.
55 er), probably due to the epimerization of enantioenriched cis-12h into trans-12h occurring at room temperature. - Since E-enolates potentially exhibit planar chirality when embedded in medium-sized rings, it may also be the case for enolate (12)−. See: K. Tomooka, T. Ezawa, H. Inoue, K. Uehara and K. Igawa, J. Am. Chem. Soc., 2011, 133, 1754–1756 CrossRef CAS PubMed Whether chirality of the transient enolate (12o–q)−, deprived of stereogenic carbons, could account for the enantioselective isomerization of 10o–q or be the result of an enantioselective protonation of (12o–q)− by the catalyst was not investigated..
- (a) L. J. Dolby and D. L. Booth, J. Am. Chem. Soc., 1966, 88, 1049–1051 CrossRef CAS; (b) T. C. Stephens and W. P. Unsworth, Synlett, 2020, 31, 133–146 CrossRef CAS; (c) Z. Yang, C. R. B. Swanson and W. P. Unsworth, Synlett, 2022 DOI:10.1055/a-1932-9717.
- N. Kagawa, J. P. Malerich and V. H. Rawal, Org. Lett., 2008, 10, 2381–2384 CrossRef CAS PubMed.
- Owing to a restriction of conformation, the formation of indolononalactone 12h incidentally revealed a point-to-planar chirality transfer, which was not observed, at room temperature, with the less hindered 12a (see the ESI for the details†). For a review: R. Lopez and C. Palomo, Angew. Chem., Int. Ed., 2022, 61, e202113504 CAS.
- T. M. Hansen, G. J. Florence, P. Lugo-Mas, P. Chen, J. N. Abrams and C. J. Forsyth, Tetrahedron Lett., 2003, 44, 57–59 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2162117. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc06842g |
| This journal is © The Royal Society of Chemistry 2023 |