Developing green chemistry educational principles by exploring the pedagogical content knowledge of secondary and pre-secondary school teachers
Philip
Nahlik
 *,
Lauren
Kempf
,
Jayke
Giese
,
Elizabeth
Kojak
and
Patrick L.
Daubenmire
*,
Lauren
Kempf
,
Jayke
Giese
,
Elizabeth
Kojak
and
Patrick L.
Daubenmire
Department of Chemistry and Biochemistry, Loyola University Chicago, Chicago, IL 60660, USA. E-mail: pnahlik@luc.edu
First published on 19th October 2022
Abstract
Green chemistry developed historically from twelve industrial principles for research chemists. Recently, interest has grown to begin introducing these principles in science classrooms even at the secondary and pre-secondary school levels. However, teachers must do significant work to adapt and translate green chemistry from the industrial or manufacturing perspective into one more appropriate to students at younger ages. This research project explores how a group of current teachers in the US and Canada have been developing their language and understanding of green chemistry through Beyond Benign's Lead Teacher Program. Transcripts from phone interviews with program participants are analyzed to propose a classroom-based definition of green chemistry and its justification as an approach at the secondary and pre-secondary school levels. This pedagogical understanding provides a foundation to solidify green chemistry as a standard practice in science education. Then classroom observations and case studies of four teachers are developed into a framework for green chemistry education at the K-12 level.
Introduction
Green chemistry education: from industrial principles to educational values
Green chemistry originally developed from an industrial perspective and includes twelve principles to conceptualize and use chemicals in a more sustainable way (Anastas and Warner, 1998; Anastas and Eghbali, 2010). A list of the twelve principles is featured in Table 2 along with a classroom case study. Groups like the American National Standards Institute and the American Chemical Society's Green Chemistry Institute worked to develop voluntary standards for green chemistry practices in industry (Taylor, 2010). In some ways, chemistry had developed a bad reputation as being unnatural or inherently harmful. Green chemistry sought to redefine this view. Aubrecht et al. (2019) give this definition: “the practice of green chemistry stresses thoughtful design of molecules, materials, and processes to minimize adverse outcomes in humans and the environment through identification of the origin, transformation, and fate of atoms” (p. 2873). For chemists, the perspective that all manipulation of matter involved the use of chemistry did not translate well to a public that had seen the negative impacts of some chemical processes.Chemistry education also needed to contend with this negative image. As Haack and Hutchinson note, “the strategies of green chemistry provided a new context for teaching students the concepts and skills of chemistry that cast the discipline in a more positive light while better preparing students to discover and develop sustainable chemistries to meet society's needs” (2016, p. 5890). Green chemistry was both a public relations shift for the field and a call to action for chemists to consider the broader impacts of chemical processes all the way from resource extraction to waste processing.
Historically, chemistry applications to the environment had often been seen as supplementary or even as a less-rigorous approach to chemistry appropriate for non-majors (such as Chemistry in the Community, see Sutman and Bruce, 1992; American Chemical Society, 2011). A major accomplishment of green chemistry has been to shift this perspective to recognize the challenge and necessity of considering environmental impact as a core part of chemistry (Sharma and Mudhoo, 2011).
It will be helpful to frame this discussion of green chemistry with the educational outcomes expected from this approach. In order to define educational outcomes for green chemistry, several core competencies for the undergraduate chemistry curriculum have been developed by the American Chemical Society Green Chemistry Institute (MacKellar et al., 2020). These broad competencies hearken back to the optimistic perspective of science as a productive discipline that can actively improve human society and the planet, rather than only minimizing harm by restricting chemical activity.
The lack of K-12 green chemistry resources
Green chemistry has become an active research area over the past forty years, in both industry and education. Several recently edited journals and books bring together advances in green chemistry education, especially lab activities for the undergraduate curriculum (Bastin and Dicks, 2019; Benvenuto and Kolopajlo, 2019; Mahaffy et al., 2019; Goode et al., 2021). These volumes show the energy and engagement with green chemistry topics from a variety of subdisciplines. The focus of green chemistry research on undergraduate curricula also makes sense because the twelve industrial principles (as written) would be most useful to someone going into a chemistry profession rather than as introductory concepts (Grieger and Leontyev, 2021).However, not many K-12 teachers are using the principles directly in their classrooms (Anastas and Eghbali, 2010). The role of green chemistry in K-12 classrooms has not been well-documented yet, although this lack of published research could reflect the absence of publication culture among K-12 educators rather than the absence of green chemistry. Among the available K-12 perspectives, one secondary school teacher describes the principles of “prevent waste” and “atom economy” as important for instruction, as well as “benign solvents and auxiliaries” and “inherently benign chemistry for accident prevention” as important for choosing chemicals for demonstrations (Ause, 2018, pp. 186–187). Additionally, green chemistry education research has largely focused on labs and chemical practices in classrooms rather than content or pedagogy (Haack and Hutchison, 2016). There is a need for further integration of these principles throughout K-12 classrooms.
Since green chemistry helps to address the material dimensions of sustainability, there should be some minimum level of understanding we would want most citizens of an industrialized country to have.† The K-12 level is a particularly important time to teach students about green chemistry because it might be the last chemistry or science course many of the students take. For a scientifically literate society, we need students to learn about sustainability before the college level. These should be foundational topics, not advanced or niche contexts. To achieve that goal, we need better research about how K-12 teachers can begin preparing students for this work and ensure that students who will not pursue science further have a basis for understanding the molecular nature of sustainability.
Finally, a green chemistry teaching approach must be connected to specific student learning outcomes to justify its use. Without a clear pedagogical justification, “green chemistry” risks being (or being seen as) simply a Trojan horse to bring any trendy educational practice into science classrooms, similar to criticisms of environmental education (Sanera and Shaw, 1997). Teachers, administrators, families, and students will want to know if “green chemistry” is simply a marketing tool or another attempt at “green-washing.” Other authors have specified the need “to delineate the differences and overlap between green and sustainable chemistry because not all green chemistry is sustainable chemistry” (Green Chemistry Institute, 2020, p. 4, italics in original). For green chemistry to succeed in shifting scientific literacy towards more sustainable considerations, there must be coherent content and clear benefits to students, not just an ideological framework. Fortunately, there are signs that green chemistry is better equipped to address these concerns than previous environmental movements in chemistry.
Is it really ‘Chemistry?’ The place of traditional content knowledge
A major criticism or hesitancy around green and sustainable approaches to chemistry is that it sacrifices traditional content knowledge. Skeptics might complain about a lack of in-depth engagement with chemistry concepts in favor of a cursory engagement that makes room for social issues. A similar practical challenge is how to incorporate additional themes into classes that are already full. Teachers might think that students need to understand the content first before considering more complex applications. The solution must include some amount of cutting or condensing of material. Understandably, some teachers are reluctant to do so. Many science reform efforts have attempted to identify the main chemistry concepts in part to allow for deeper engagement and application to world issues (see especially: NGSS Lead States, 2013).Some teachers have justified the use of green and sustainable chemistry as a motivating force for traditional content learning. Aubrecht et al. (2019) write that “though the primary focus in general and organic chemistry courses is instruction of fundamental chemistry concepts, raising student awareness on the potential of the chemistry enterprise to address global issues involving sustainability can both inspire them and challenge them” (p. 2877). These authors also include charts for the general and organic chemistry curricula to show the overlap of traditional content (“enduring understanding”) and green chemistry connections (Aubrecht et al., 2019, pp. 2874–2875). Another study confirmed the positive change in high school students’ interest in chemistry and its perceived relevance to their lives through inquiry-based, life-cycle thinking approaches to sustainable chemistry (Juntunen and Aksela, 2013). When used well, environmental problems can provide a motivating context for student learning of content. Such a benefit might convince skeptical teachers that a sacrifice of some conventional content is worthwhile.
Finally, green chemistry also supports an optimistic and economic perspective of chemistry that can actively solve problems in the world rather than simply describing issues. In this way, it is a clear response to concerns about environmental education that convey a pessimistic image of the state of the world. Green chemistry provides an opportunity and framework for students and industrial chemists to invent new solutions that work better for people, the planet, and a community's economic well-being.
With this overview of environmental curricula, a case has been made that green chemistry can address significant concerns in current science education to motivate traditional content learning and foster skills that prepare students to consider the chemistry involved in environmental issues. The next section introduces the research background for this project designed to develop an actionable understanding of green chemistry principles and classroom practices for K-12 teachers through the lens of the Beyond Benign Lead Teacher Program. This understanding will provide a basis for further research and practice in the development of the field of green chemistry education.
Pedagogical content knowledge: the research of teacher expertise
The main research construct and area of focus for this project is pedagogical content knowledge (PCK), which has remained an active area of research since it was proposed by Lee S. Shulman in 1986. Distinct from content knowledge and general pedagogical knowledge, PCK includes content-specific understandings of how students learn a subject, such as common misunderstandings or the progression of topics (Shulman, 1986). Originally, Shulman was concerned about the pendulum swing away from wide-ranging content knowledge as the test of teacher proficiency toward pedagogical knowledge devoid of any content. He defined PCK “for the most regularly taught topics in one's subject area, [as] the most useful forms of representation of those ideas, the most powerful analogies, illustrations, examples, explanations, and demonstrations—in a word, the ways of representing and formulating the subject that make it comprehensible to others” as well as “an understanding of what makes the learning of specific topics easy or difficult: the conceptions and preconceptions that students of different ages and backgrounds bring with them to the learning of those most frequently taught topics and lessons” (Shulman, 1986, p. 9). In this way, he reframed content and pedagogy as interrelated fields of teacher expertise rather than independent skillsets.The perspective of PCK emphasizes that an expert chemistry teacher is distinguished neither by advanced content knowledge nor by skilled use of educational strategies but by an area of expertise specific to teaching chemistry at a certain grade level. An experienced chemist cannot simply learn some educational techniques and become an expert teacher. Similarly, an experienced high school chemistry teacher would likely struggle to transition to teaching English even with deep familiarity with the content. Teachers in any discipline require a rationale for their pedagogy that is not transferable from other subject areas. Shulman gave the analogy that “a professional is capable not only of practicing and understanding his or her craft, but of communicating the reasons for professional decisions and actions to others” (1986, p. 13). A true test of teaching expertise would be this type of reflective reasoning as described by PCK.
Because PCK is linked with content, it must also be studied in content-specific ways that may require unique frameworks. Within science education research, Magnusson et al. (1999) defined the following five components of PCK for science teaching:
1. Orientations toward science teaching,
2. Knowledge and beliefs about science curriculum,
3. Knowledge and beliefs about students’ understanding of specific science topics,
4. Knowledge and beliefs about assessment in science, and
5. Knowledge and beliefs about instructional strategies for teaching science (p. 97).
These five components delineate the connections that Shulman (1986) implied among content, pedagogy, and PCK.
Importantly, PCK does not supplant content knowledge. As other researchers have noted, “the most effective teachers have deep knowledge of the subjects they teach, and when teachers’ knowledge falls below a certain level it is a significant impediment to students’ learning” (Coe et al., 2014, p. 2). As a defining level of teacher expertise, PCK draws from “the three base domains of teacher knowledge: subject matter, pedagogy, and context” (Magnusson et al., 1999). Expert teachers need deep knowledge in each of these three areas, yet this knowledge alone does not guarantee that a teacher has or will develop the necessary PCK to teach a topic at a certain grade level.
After the concept of PCK was better defined and accepted in the academic community, the practical research interest became: are there ways to teach PCK directly in order to prepare teachers better? Instead of each teacher learning independently, could PCK provide a shortcut to develop expert teachers more quickly within a community of teachers in training? Research began to focus on this practical dimension of PCK for teacher training (Neumann et al., 2019). For example, Demirdöğen et al. (2016) used PCK to structure and assess a course for pre-service chemistry teachers. As Magnusson et al., (1999) explained:
The practical value of pedagogical content knowledge as a construct has to do with its potential to define important dimensions of expertise in science teaching that can guide the focus and design of pre-service and in-service teacher education programs. Many science teachers and science teacher educators have a wealth of knowledge about how to help particular students understand ideas such as force, photosynthesis, or heat energy; they know the best analogies to use, the best demonstrations to include, and the best activities in which to involve students (p. 116).
Studying PCK is one way to access and share this wealth of knowledge with wider communities.
More recent research has identified PCK as a major indicator of student learning. In an extensive literature review on teaching effectiveness, Coe et al. (2014) identified PCK as one of six components of great teaching and one that has “strong evidence of impact on student outcomes” (p. 2). They also noted the positive impact of physics teachers’ PCK and motivation on students’ achievement and interest. Therefore, studying a teacher's PCK can be an indirect way of predicting student growth in a course.
However, researchers have been careful to note differences in types of PCK. Aydeniz and Kirbulut (2014) emphasized “that PCK must be understood and explored at two levels: (1) espoused/planned PCK and (2) enacted PCK” (p. 149). Although both areas are important, this subtle distinction clarifies that how a teacher describes their pedagogy might be different from how they enact that pedagogy. Even if a teacher has highly developed espoused PCK, there can still be barriers to integrating that knowledge into instructional practice (Barendsen and Henze, 2019).
Understandably, research on PCK has focused especially on pre-service training or individual administrative interventions (De Jong et al., 2005; Aydeniz and Kirbulut, 2014; Parga Lozano, 2015; Demirdöğen et al., 2016). However, more research is needed to understand how PCK is shared between in-service peers beyond one-time interventions and in specific contexts like green chemistry education (Baxter and Lederman, 1999; Loughran et al., 2012). Part of our research involved developing descriptions of pedagogical content knowledge specifically related to green chemistry, inspired by previous research with pre-service teachers. These descriptions will allow future research to more easily track and categorize green chemistry principles and associated teaching practices as a novel and valuable area of chemistry PCK.
The resource folios approach to pedagogical content knowledge
For this project, we use the methodology of Resource Folios as developed by Bernard Loughran and his colleagues to help teachers formulate their PCK and share that knowledge with others (Bertram and Loughran, 2012; Loughran et al., 2012). This approach provides both a reasoning and a structure for studying PCK and has been used successfully in science education contexts (Lederman and Gess-Newsome, 1999; De Jong et al., 2005).In their pivotal text, Loughran et al. (2012) reviewed the work on PCK and critiqued some developments that have become counterproductive. They highlighted the tension between the generalizing thrust of research and the specificity inherent in the concept of PCK, saying, “It seems as though the more that PCK is refined and/or redefined in a bid to make it more concrete, the less valuable it becomes as a descriptor of specialist or expert knowledge of practice” (Loughran et al., 2012, p. x). Furthermore, an overly prescriptive approach to PCK risks omitting some factors that support good teaching. As they argue, “although it is important to have some routines in teaching, when teaching becomes ‘routinized’, elements of quality teaching (e.g. engagement, enjoyment and intellectual challenge) can be dramatically diminished; or worse, absent all together” (Loughran et al., 2012, p. 2). Therefore, PCK research needed some clarification to avoid overly concretizing or routinizing teaching practice.
Loughran et al. (2012) also argued that research on PCK has not been tailored to the practical goal of supporting teacher practice. As they explain (Loughran et al., 2012),
The manner in which research into PCK has been conducted has created an impasse for teachers. The research literature on PCK is certainly extensive; however, the outcomes of such research appear to speak more to educational researchers and other such academics than to teachers who surely are not only the producers of such knowledge, but also important end users… much time and energy was expended evaluating PCK as opposed to exploring concrete examples of how teachers teach particular content topics in particular ways that promote understanding. Therefore, unfortunately, PCK has not been developed through the research literature in ways that necessarily directly correlate with enhancing the practice of science teaching (p. 11).
Some research on PCK became too theoretical and separated from classroom practice in a way that made it less meaningful.
The major value of PCK, then, is its role in clarifying teachers’ and researchers’ language about teacher practice. In other words, for PCK as a practical tool, “the value to teachers was in terms of encouraging reflection on practice, creating a shared language for discussing science teaching and learning, and offering insights into practice, all of which became a springboard for their own professional learning.” (Loughran et al., 2012, p. x). Developing teachers’ PCK does not guarantee good teaching practice, but it does provide one additional touchpoint for teachers to develop with each other. Loughran et al. (2012) refocused PCK research on teacher practice because,
Teachers are in fact producers, not just users, of sophisticated knowledge of teaching and learning. And, the complex ideas associated with exemplary practice are better able to be portrayed and shared in meaningful ways if labels and descriptors such as PCK are better understood and used. Therefore, a language that comprises aspects of professional practice is central to moving knowledge of practice out from the individual and into the professional community at large. For example, in many studies by teacher researchers, language (a shared vocabulary) has been central to the development and sharing of their sophisticated knowledge of practice (p. 12).
This perspective should inform both research and interventions about teacher practice. Importantly, other aspects of language, like content knowledge and pedagogical knowledge as distinct from PCK, can still contribute to teachers’ professional learning (Loughran et al., 2012, p. 5). Similar to other researchers, they noted that individual teachers naturally develop their own PCK. The goal is to use the language of PCK to facilitate the transfer and development of teaching practice for more teachers (Loughran et al., 2012, p. 13).
One challenge in this research is that such a depth of engagement required in developing PCK for individual topics means sacrificing the breadth of topics normally covered in a class. “The dilemma, then, is that although students’ conceptual understanding may well be richer, the amount of content covered is likely (at least, initially) to be much less than that which might normally be achieved” (Loughran et al., 2012, p. 16). Teachers and researchers might need to be convinced of the long-term value of developing PCK to overcome the inertia of existing curricular structures.
The structure of resource folios: CoRes and PaP-eRs
The Resource Folios approach includes two parallel structures for organizing and communicating PCK. The first structure is a Content Representation (CoRe) which is a table that delineates a teacher's knowledge and approach for certain content at their grade level. An adapted version of a CoRe is included in Table 1.| Grade level and topic for this CoRe: | Big idea or subunit topic |
|---|---|
| Title or description of the big idea. | |
| What you intend students to learn about this idea. | |
| Why it is important for students to know this idea. | |
| What else you know about this idea that you do not intend students to know yet. | |
| Difficulties, limitations, or misconceptions connected with teaching this idea. | |
| Knowledge about students’ thinking which influences your teaching about this idea. | |
| Other factors that influence your teaching of this idea. | |
| Teaching procedures (and particular reasons for using them to engage with this idea). | |
| Specific ways of ascertaining students’ understanding or confusion around this idea. |
The second structure is a Pedagogical and Professional-experience Repertoire (PaP-eR) which provides a contextualized example of teacher practice for a given topic through a range of formats, like a syllabus, annotated lesson plan, or stylized interview (Loughran et al., 2012, p. 17). In contrast to the overarching perspective of CoRes, PaP-eRs present a contextualized story of how one teacher approaches the content. These examples help support teacher development because, “in many ways, teachers’ stories actually carry most of the important information that helps other teachers to identify with, and therefore extract their own meaning from, a given description of a teaching and learning situation” (Loughran et al., 2012, p. 16). It might seem counter-intuitive that a more specific example is more easily transferable, but the contextual information of a real class often helps teachers to imagine the content in their own classroom.
After compiling CoRes and PaP-eRs for a given topic, these Resource Folios can then be used in professional development for teachers, providing the common language to move research into practice (Loughran et al., 2012, p. 20). This structure helps prompt teacher reflection on their often-unstated knowledge about teaching certain content. In these types of professional development experiences, “participants required an opportunity to work with a CoRe to develop a familiarity with the process in order to manage the demands inherent in completing the task” (Loughran et al., 2012, p. 217).
There are significant challenges to developing Resource Folios for any content. For many teachers, “in reflecting upon one's own experiences of teaching and learning in science, it can sometimes be difficult to look back and see the changes in practice (and the reasons for those changes) that led to the manner in which one teaches at the present point in time” (Loughran et al., 2012, p. 223). Researchers and facilitators still have a significant role to play in supporting and scaffolding this type of reflection with teachers, working together to construct accurate representations of PCK that can be shared with a wider community.
Methods
The overall purpose of this research is to explore how secondary and pre-secondary school teachers have been developing their language and understanding of green chemistry through the perspective of Beyond Benign's Lead Teacher Program (LTP). The LTP fosters a community of educators to promote teaching green chemistry in chemistry education. Teachers are provided tools and support to effectively integrate green chemistry into their classrooms.Research partner: Beyond Benign
Beyond Benign is a leader in green chemistry education. It was founded by Drs Amy Cannon and John Warner in 2007 and “is dedicated to fostering a green chemistry community that empowers educators to transform chemistry education for a sustainable future” (Beyond Benign, 2021). Their programming promotes green chemistry principles in K-12 schools, higher education, and industry to create “a world where the chemical building blocks of products used every day are healthy and safe for humans and the environment” (Beyond Benign, 2021).A group of staff and teachers started the Lead Teacher Program (LTP) in 2016 to help train K-12 educators in principles of green chemistry and empower them to share that expertise with other teachers. The program accepts a small group of two to eight teachers each year for a three-year, stipend position. Throughout this project, there were about 13 teachers in the program at a time across the three years. Within the cohorts, teachers had a wide variety of teaching experience, from new teachers (full time in the classroom for one to three years) to veteran teachers (full time in the classroom for more than twenty years). The teachers also varied in their familiarity with green chemistry, with some teachers having just completed an introductory series of two graduate level courses developed through Beyond Benign or with some teachers who had been heavily involved in green chemistry and resource development prior to their involvement with LTP. Because of these variations, the teachers’ years of involvement in LTP does not necessarily reflect their familiarity with green chemistry education. These teachers participate in a wide variety of activities, which include developing curriculum, presenting webinars, and joining monthly phone calls with other program participants. By working at the K-12 level, LTP seeks to integrate green chemistry principles into early levels of science education. They emphasize the importance of starting at this level to influence higher education, industry, and the whole planet.
Research questions
The research questions to be further explored in this paper are: (1) how do LTP participants understand green chemistry and its role in K-12 education? (2) What does the practice of green chemistry look like in some K-12 classrooms? And (3) how can teachers and researchers frame this green approach to chemistry education?To address these questions, the primary author first conducted twenty-eight semi-structured interviews of teachers and staff from LTP after receiving Institutional Review Board (IRB) approval to work with human subjects (project #3028). Informed consent was obtained from each participant before each interview following the approved protocol. All active teachers from two years of the program as well as the majority of former teachers from LTP were interviewed, for a total of twenty-six teachers from a wide variety of public and private schools throughout the US and Canada. Most of these teachers (n = 18) teach primarily at the secondary-level, and the rest (n = 8) teach primarily pre-secondary students, including three teachers from the elementary level. The two staff members directly involved with LTP were also interviewed in order to provide their direct programmatic reflections on the program and better shape research conclusions. These population sampling approaches help ensure that our research is reflective of all program perspectives.
As a research team, we transcribed each interview to collect qualitative data from subsequent coding. Each week, we discussed our initial impressions of the interviews as we would transcribe them and started noticing some commonalities amongst them. Once the interviews were transcribed, we were able to begin the qualitative coding process. This process involved assigning pairs (in rotation) to code each interview transcript. We constantly compared coding decisions and ultimately would come to an agreement on which code would fit a certain part of an interview best. If there was uncertainty, it could be brought up in the weekly team meeting to further discuss. This consensus coding process lessened biases and allowed for collaboration as a team. Once the interviews were coded, they were put into NVivo for further sub-coding.
Classroom observations
Because of the distinction between planned and enacted PCK (Aydeniz and Kirbulut, 2014), it was important for us to investigate teachers’ practice of green chemistry along with their self-described understanding. Originally, the research plan included in person classroom observations for six program participants as well as six control teachers over the two years of data collection. Because most of the teachers moved to online instruction during the years of the study, this plan had to be revised. Two teachers were observed in-person for half a day each in the Spring of 2021. These observations served as a pilot to consider future work. In Fall of 2021, the primary researcher coordinated with three additional teachers to observe a series of class periods virtually. Letters of cooperation were coordinated with each school, and a message for parents was shared through each of these teachers as approved in the IRB protocol. Field notes and audio recordings were taken for each observation and focused on the teacher's enacted PCK as related to green chemistry. The five areas of PCK provided the initial categories for these observations, namely the teacher's understanding of (1) orientations toward science, (2) science curriculum, (3) students’ understanding, (4) assessment, and (5) instructional strategies (see Magnusson et al., 1999, p. 97). Transcriptions of the recorded audio helped provide a basis for quotes and organizing these observations. The observations were mainly developed into textured examples of PaP-eRs to illustrate green chemistry PCK in action (Loughran et al., 2012), which are included the Results section.Field notes and content representations
The final source of data included field notes of various LTP activities, including several group video calls, two Summit events in the summers, and some online webinars conducted by teachers and program staff. Notes from these events helped to track the wider impact of the program and the variety of ways that teachers share PCK. Field notes draw from a grounded theory approach where themes are developed from the language of program participants in addition to the interviews (Weiss, 1995; Emerson et al., 2011; Charmaz, 2014). By actively participating in these events, our research also helped shape program activities and provide the direct benefit of an outsider perspective for the staff and teachers. The reflective process involved in field notes supported larger program reflection and flexibility when methods needed to be adapted to respond to a program in development.Additionally, the Resource Folio approach provided another method of investigating teacher's espoused PCK (Loughran et al., 2012). Before the LTP Summit from July 20–22 of 2020, each teacher was asked to work on a Content Representation (CoRe) worksheet, which prompted them to describe their classroom practices for specific content. See Table 1 for an example CoRe worksheet. This method is designed to make explicit the often implicit knowledge that teachers have about PCK. It provides a common structure and language for teachers to share their teaching expertise with each other and with a wider community. At the Summit, one session was reserved for teachers of similar content areas to talk about their initial CoRe. These conversations and the worksheets helped shape interview questions and later research plans.
Results
The field of K-12 green chemistry education
Apart from specifically programmatic considerations about LTP during the interviews,‡ participants described the broader field of green chemistry especially in K-12 educational settings. The green chemistry expertise within LTP as exemplified in the CoRe worksheets and extensive presentations by the participants could be shared more widely if it were systemized similar to other areas of PCK to form an initial understanding of how green chemistry functions in K-12 classrooms. Following Loughran et al. (2012), we used the interviews with teachers and the classroom observations to construct an initial Content Representation (CoRe) for green chemistry in K-12 education in this article. First, we sketch out several themes from interview quotes to explore participants’ understandings of green chemistry as a context for the CoRe.Definition
A major motivation for this project is that green chemistry has not been well studied or defined in education. The twelve principles lend themselves well to industrial and research contexts (Anastas and Eghbali, 2010) but not as easily to K-12 classrooms (Haack and Hutchison, 2016). Teachers in LTP have direct experience in adapting green chemistry to their classrooms and with other teachers. Like other areas of PCK, this information is often implicit until directly assessed (Loughran et al., 2012).To investigate this area of expertise, the interview script asked participants directly to define green chemistry as they understand it in their classroom. Based on interview data, here is our working definition as a synthesis of participants’ descriptions:
Green chemistry is practicing sustainability; it is a healthier, safer, and more cost-effective approach to studying chemistry. It is a lens that allows for the understanding that sustainability at all levels can change our surroundings. As a worldview, it demonstrates the need to shift towards safer production methods, ultimately reducing waste and toxicity in the environment.
Some teachers described green chemistry with a more industrial or manufacturing understanding, such as one participant who said, “green chemistry is a way of making stuff safely and smartly, a way that improves everyone's lives without messing with your own health or negatively impacting others that you don't necessarily think about. It's the safest and smartest way to make stuff to help improve the world.” Many teachers retained some connection with the manufacturing roots of green chemistry and convey that message to students.
Other teachers described green chemistry in terms of the educational benefits it brings, such as “emphasizing that chemistry doesn't need to be taught using things that make explosions, that uses chemicals that have higher risk, whether it's reactivity, flammability, things like that, but it can be done in a safe way and actually… when you use materials that are more benign, kids [sic] are typically more familiar with them and can make better connections to the concepts we're trying to teach anyway.” For many teachers, green chemistry necessarily included these types of connections to students’ lives.
The variety of descriptions from these teachers helps form a basis for an educational understanding of green chemistry. This type of description can help support the program goals of empowering and equipping other teachers to implement green chemistry. However, the diverse ways of defining green chemistry also imply a need for making these teachers’ PCK more explicit to share with wider audiences. The CoRe worksheets will be an additional source of data to understand how green chemistry is incorporated to these teachers’ classrooms and share that information more widely.
Justification
A related theme included the justifications that teachers use for including green chemistry in their classrooms. Teachers often have to make the case to administrators or other teachers for taking on the atypical approach of green chemistry, so they have developed clear reasoning and evidence for their justifications. Beyond a definition, this theme captured several common reasons that teachers cite for using this approach with their students, ranging from educational benefits to moral commitments to life skills that students develop.A large set of justifications covered the educational benefits of green chemistry. Teachers noted many observations from their own classrooms involving student motivation and learning which confirmed their teaching approach. One teacher understood their classroom in this way:
And having made that switch [to be ‘more hands-on’] what a teacher always fears are accidents in the classroom and so you tend to do many demonstrations with students. And of course, if the students don't understand you kind of give them the answer. But having the green chemistry part and having students take ownership of it… it actually opens up a whole spectrum for these students.
This teacher highlighted both the paired benefits of allowing students to be “more hands-on” and to take more “ownership” of their work in the classroom, which allowed students more freedom for inquiry in a lab, pursuing questions that interested them within a safe context as guided by their teacher. Green chemistry is not the only educational approach that includes these types of goals. However, for teachers in LTP, green chemistry seemed to answer many of their wider questions about modern science education, which became a powerful justification for taking that approach.
Another benefit of designing labs to be safer and more environmentally friendly is creating more opportunities within special education. As one teacher said:
But with green chemistry, I think there is a market there where we could target… that's my goal for this year: target special ed. If you have a child who can't behaviorally, academically, physically, or if you have a kid that flails, if you have CP or things like that, and there are body motion issues. If they have issues with that, using the harsh chemicals with the goggles, they may not want to wear them. Just because it's a tactile issue. They may not want gloves on. But we might have replacement labs that they can learn the same skill in a safe manner that maybe the special ed teacher could even do with them, because they don't have to worry about the harsh chemicals.
This teacher brings up an important aspect of green chemistry in education which is inclusivity. Students with disabilities can be more safely taught and involved in hands-on experiences through using green chemistry.
Several teachers spoke passionately about their moral commitment to green chemistry and sustainable science education. As one of them explained:
It's the only way that we can kind of save this planet, and I think that it's exciting for kids. It's empowering for kids, and it's a different way of thinking about things. And we have to change the way we think about things.
These teachers viewed science education as part of their broader commitment to the planet and to their students. For them, green chemistry is a vital tool for addressing environmental issues and, therefore, imperative to prepare “conscious citizens and not just scientists.”
Teachers also explained the benefits of green chemistry to foster life skills that extend beyond their classrooms. Collaboration was a key skill that one teacher highlighted by saying, “We want our students to be scientifically literate once they’re out in the community. But if they can't collaborate well together, that's not going to change anything.” Another teacher extended some of the twelve principles of green chemistry into life skills beyond chemistry, saying:
I had students going on and not only going into chemistry, which I was excited for, but if they were going into any other field, they're taking those skills with them and they’re really branching out and again, utilizing those skills that they're learning, whether it be as simple as waste reduction or toxicity, or you name it, any principle that we're really looking at.
The ethical framework embedded in green chemistry offered these teachers something different than other science curricula. In the interviews, these justifications built upon the motivations to explain why participants stayed involved in LTP and continued using green chemistry principles in their classroom beyond their initial interest.
Green chemistry as chemistry
Another common theme was that green chemistry could be described more broadly as simply chemistry or sustainability. According to another teacher, “we shouldn't call it ‘green chemistry.’ It should just be ‘chemistry.’” Rather than separating green chemistry, all of chemistry should be green, and, therefore, there is no need for the term “green chemistry.” One teacher expressed his hope for the future, saying, “I want to go to a big conference and do a workshop and not have to say it's ‘green.’” Many teachers shared this sentiment that all chemistry should be done in a green way to such an extent that the entire field of chemistry is replaced or reconsidered. Some teachers, especially at younger grade levels, extended this understanding to science education more generally and argued for the use of the term “sustainability” instead of “green chemistry.” One teacher said, “the term I like to use with my students is it's ‘kind by design.’” Another teacher explained further:You don't have to be a chemist to implement green chemistry. [Teachers] don’t have to be teaching chemistry to implement green chemistry. Let's think about the sustainable practices or changing the name slightly so you feel more comfortable with that implementation in the classroom, but outside of more teaching pedagogy and just general outreach to some younger grades or younger teachers as well.
These teachers struggled to decide on the best language for their collective classroom approach, but green chemistry served as a common entry point or hook for all of them.
For these teachers, green chemistry added to and supported their existing approach to science education. One of the teachers gave this definition: “Green chemistry really is chemistry but done in a more thoughtful manner with the health and safety of not just your students but the environment at the forefront.” For some teachers, green chemistry became the predominant lens through which they saw and described their own teaching practice, such as one teacher who said, “Green chemistry can be found in every single unit that I do.” By viewing green chemistry more as sustainability rather than chemistry, the term can reduce the intimidation many K-12 students feel towards chemistry as a subject. Another teacher added that they had been using green chemistry even before using the term, explaining, “as an elementary teacher, I’ve always done sustainable science, and we always do green chemistry. Everything in elementary is kitchen science.” Their efforts to translate this commitment to other teachers seemed to require a reconsideration of their language and led to these distinctions around “sustainability” or “green chemistry” simply as “chemistry.”
Summary of green chemistry in education
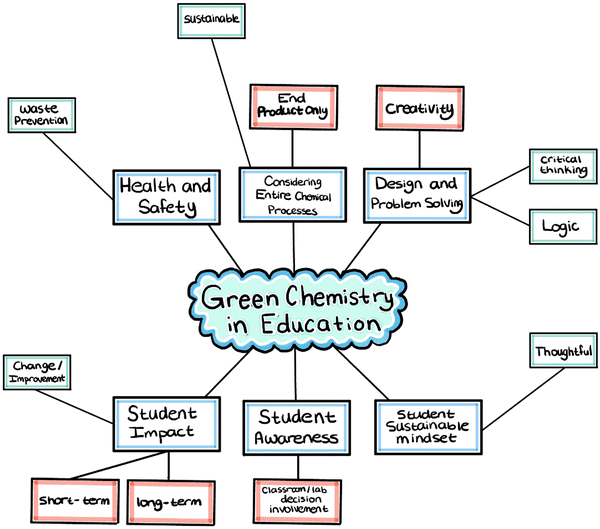
Green chemistry involves the development of safe and effective solutions to a variety of problems that can extend into a classroom setting. A visual summary of these results is included in Fig. 1. Many K-12 chemistry teachers actively involve their students and make them aware of the lab procedural decisions intended to eliminate waste products and foster a safe environment. Through design, creativity, and critical thinking, students begin to develop a sustainable mindset and discover their own individual impacts they have in the world, both short and long-term. It focuses on entire chemical processes rather than just an end product to lead with the intention to do no harm to human health and the environment.Health and safety were described by many teachers as a way to develop safe and effective solutions to problems in the classroom. With an emphasis on the word “safe,” labs can be designed to foster a safe environment for the instructor and the students. A direct result of this is waste prevention, specifically regarding the labs done in the classroom. A teacher said “green chemistry is not just looking at the immediate effect, it's looking at the long-term effect and how do we make it better and safer for all of us.” It is important to think about the entire chemical processes when it comes to green chemistry in education, not just the end products and cleaning up in response. By teaching green chemistry in the classroom, students can begin to think about the risks that are involved with every decision they make. One teacher described this as “looking at the very beginning of the creation of those molecules all the way through their end of life.” Then asking ourselves, “how can we make things greener and make them so that they have the least amount of impact on the environment and have the lowest risk involved to people?” The goal is to move forward with sustainability enforcing the prevention of harm to human health and the environment.
Through designing and problem solving in the classroom, students learn to think creatively. This requires the use of critical thinking and logic to arrive to the best possible solutions with green chemistry as the basis. One teacher said that “green chemistry is an important way to advance chemistry education by increasing sustainability and also increasing creativity for students.” As a direct result of designing and problem-solving, students can develop a more sustainable mindset. They can also become more thoughtful in the decisions they make both in and outside of the classroom. Teachers say this can further develop with students as they are made aware of the classroom and lab decisions being made behind the scenes. A teacher described an example where students “learn how to read an MSDS sheet because they need to do that in order to evaluate whether they're going to use iron or they're going to use nickel.” The students also “have to figure out which one is the safer option they’re going to use, and which one produces a safer byproduct.” This way of teaching chemistry was described as “the green chemistry way”. For similar reasons, another teacher promotes “students to look at the toxicity as well as different chemicals [they] are using in the classroom.” Rather than designing a safer, more environmentally friendly lab for the students and having them do it, the teachers mentioned involving or explaining to the students the decisions that were made and why.
In addition to students becoming more aware and involved in classroom lab decisions, students can be impacted by green chemistry beyond the classroom setting in both short and long-term ways. They can become more conscious of their daily life decisions beyond their K-12 education, as echoed by one teacher who named the goal of “preparing conscious citizens and not just scientists.”
Pedagogical and professional experience repertoires as classroom case studies
During the conversations with teachers and in the classroom observations, we focused on potential case studies to include as Pedagogical and Professional-experience Repertoires (PaP-eRs, as identified by Loughran et al., 2012). The goal of these case studies is to provide accessible examples to support teachers’ development of their own PCK. Developing PaP-eRs based on these teachers’ stories is meant to help other teachers understand better what it means to integrate green chemistry into their PCK. To use a green chemistry metaphor, PaP-eRs act as catalysts that provide an alternate reaction pathway with lower energetic barriers than traditional teaching approaches. If used in pre-service teacher formation (De Jong et al., 2005; Aydeniz and Kirbulut, 2014; Parga Lozano, 2015; Demirdöğen et al., 2016), PaP-eRs can illustrate parts of the reaction pathways of a teacher moving toward more sophisticated and explicit PCK around green chemistry principles. They can be used within LTP to support internal participant conversation or with outside groups to facilitate the diffusion and transformation of PCK between communities of teachers.To accomplish these goals, four case studies are presented below with further commentary developed into a Content Representation (CoRe) for green chemistry in K-12 classrooms. We chose to present these PaP-eRs first to build our grounded theory starting from the classroom experiences of teachers before generalizing an overall CoRe. As Loughran et al. (2012) state:
PaP-eRs bring the CoRe to life and shed new light on the complex nature of PCK. They help create ways to better understand and value the specialist knowledge, skills and ability of teachers thus making that which is so often tacit, explicit for others (p. 25).
These case studies highlight the expertise of four exceptional teachers of green chemistry at the K-12 level.
In one of the interviews, a teacher described a full lesson to illustrate how green chemistry works on different levels between teachers and students to encourage ownership of safety in the classroom.
One of my favorite things to do with Beyond Benign is… a types of reactions lab. Ultimately my curriculum said students have to be able to identify different types of reactions, and they should conduct experimentation with those different reactions… They’re told they have to evaluate both of them using the 12 principles of green chemistry.
So one of those synthesis reactions will maybe be you have to heat things using the Bunsen burner. The other one you don't have to heat it, and so those two reactions they evaluate which one they're going to do, and they have to explain based on the 12 principles, which activity adheres best, or it's either safest or occurs at an ambient temperature, or, you know, whichever one of the 12 principles it hits. Explain why, and then they do that for reaction type one, and then reaction type two, which is a decomposition reaction and then a single displacement. Then a double displacement. So, there are eight potential reactions, and they gotta pick which four they're going to do.
And they do that by researching the toxicity of the chemicals by looking at, you know, the waste products that may be produced by looking at the safety aspect of which ones do I have to use PPE for? Which ones do I only need safety glasses for? Which ones will I need gloves for? And also, which ones you’ve done at ambient temperature? Which ones have to be heated?
And they go through, and they pick the four that they're going to do, and then they justify them, and all of that is done in pre-lab, so that might be done in the period before the lab and then the next day they actually conduct the experiment where they do the four different experiments, they make their observations, they go through, they write the chemical reaction, they balance the chemical reaction, they classify the reaction.
So that is them meeting the curriculum expectations. But the pre-work is where they actually had to evaluate the safety of the molecules, of materials, safety of the process as well as the waste stream. That's the green chemistry part. That's the sustainability piece, that's where the Lead Teacher Program leads them through. So where in my class students have to evaluate which reaction they do, another class in another school the teacher might prescribe them the four reactions, but those four reactions may not have even been the four safe ones, and even if they were the safe ones, that's the teacher doing green chemistry, not the students doing the green chemistry.
If the teacher does the green chemistry. That's good. You save the materials you save the cost, you are doing it safe for the students, but the students aren't getting the opportunity to evaluate the different procedures, and so that's where that topic goes from being just a very cursory cookbook lesson: mix these chemicals, write the reactions… which still they should hit the expectation.
But by doing it the green chemistry way, by doing it through the lesson plan developed through the Lead Teacher Program, then you actually have students being thoughtful about choosing them. They learn how to read an MSDS sheet because they need to do that in order to evaluate whether they're going to use iron or they're going to use nickel. And they have to figure out which one is the safer options they’re going to use and which one produces a more safe byproduct.
This teacher integrated an understanding of green chemistry that includes teachers making design decisions for their students while also enabling their students to consider chemical safety throughout a lab. This approach to safety represents the first component of PCK from Magnusson et al. (1999): “orientations toward science teaching.” For this teacher, science education involves students actively in the process of planning and carrying out investigations safely.
As exemplified in this case study, a green chemistry approach enables teachers to make decisions that minimize risks and include students actively in the safety process with appropriate levels of support. Students’ understandings and abilities differ across grade levels, but even younger students can be guided to think through potential chemical or physical risks involved in an experiment. Therefore, green chemistry is not only a behind-the-scenes method for teachers. It can also support a direct approach to involve students in planning and carrying out investigations.
The teacher first reminded students that they had previously talked about green chemistry. As a reminder, he summarized three “criteria” of green chemistry: materials that are safer to make and clean, just as effective, and, on an industrial level, cheaper. He then told the students that they would be making a green glue, which refers “not to the color” but to it being “made of household chemicals.” As further emphasis, he added that “you could eat it. It would taste nasty, but it wouldn’t hurt you.” Finally, he noted that other glues can contain many harmful chemicals, in contrast to the green glue which the students will make.
Throughout the procedure, the teacher guided students to work in pairs by talking through each step. First, he asked them to pick up materials from lab benches including baking soda, vinegar, and powdered milk which were already measured out into plastic cups. Then he had students label the cups on their own, noting that they can easily “find the vinegar with your nose, even with a mask on.” He reminded them of several safety rules when they are in the lab like being aware of the space around them and cleaning up any spills immediately. Next, he had them begin adding ingredients together. The teacher brought hot water to each table to mix in with the powdered milk and dissolve it. Then students needed to add the vinegar to the milk and mix until a curd formed, which they removed and put on a paper towel. The teacher collected the remaining liquid whey. Then students broke up the curd with a fork in the plastic cup and added some hot water as needed. Finally, they added some pinches of baking soda to neutralize the remaining solution and finish their green glue.
After finishing the procedure and cleaning up briefly, the teacher returned to the green chemistry criteria to decide if this glue fit. He emphasized that the materials were safer going in, since the students did not wear aprons or goggles, and going out, since they are able to pour everything down the drain at the end. He connected the importance of waste that goes down the drain because it can impact well water and aquifers in the local area. Then he suggested that they could calculate how cost-effective the glue was by adding up the ingredients. Finally, he asked: “does it work?” He continued by saying, “If it's not effective, then there's no point in doing it.” So, he suggested that students use their green glue to create a collage out of torn up construction paper in the last ten minutes of class. Then they could check the next day to see how their glue worked. Several students were proud to show me their collages as they finished.
Overall, this lesson exemplifies the second component of PCK from Magnusson et al. (1999): “knowledge and beliefs about science curriculum.” For elementary students, making glue from ingredients that they knew was an experience of green chemistry as a creative activity. The curriculum for this lesson and this teacher's specific approach guided students through the process safely and effectively. Students of any age being able to hold something in their hands that they helped make is an empowering tool for learning. These students might not be able to restate the three criteria of green chemistry, but they could certainly show off the collages they created and know they had a hands-on role in creating something useful.
In one of the classroom observations for a high school chemistry class, the teacher introduced the twelve principles as part of a unit on plastics. This case study provides an annotated outline of the principles as presented in this class, including the examples the teacher used for each, to provide a basis for other teachers to consider how or if they can integrate the principles directly (Table 2).
| Green chemistry principle | Formal description and teacher connections |
|---|---|
| 1. Waste prevention | Formal description: prioritize the prevention of waste, rather than cleaning up and treating waste after it has been created. Plan ahead to minimize waste at every step. |
| Simplified description: “Whenever you’re doing a chemical reaction, it ends up producing some waste… maybe not everything you have in that reaction actually gets used, so the whole point is to limit that waste.” | |
| 2. Atom economy | Formal description: reduce waste at the molecular level by maximizing the number of atoms from all reagents that are incorporated into the final product. Use atom economy to evaluate efficiency. |
| Simplified description: “Whatever atoms you start with and put into the reaction should be what you’re taking out of the reaction.” | |
| Connection to previous material: “If we remember working with our molymods [molecular modeling kits], we were making all of those different chemicals. We added a bunch of chemicals together, and those atoms are what ended up in our product… Basically: do you have atoms that are hanging out, or are they all present in your products?” | |
| 3. Less hazardous chemical synthesis | Formal description: design chemical reactions and synthetic routes to be as safe as possible. Consider the hazards of all substances during the reaction including waste. |
| Simplified description: “Basically that means that we’re designing safe reactions.” | |
| Real-life examples: “If I’m talking about a safe reaction, what are we not going to be doing during a safe reaction? What would that not look like?… We wouldn’t be lighting things on fire. We wouldn’t be causing things to explode… This actually happened to me when I was in undergraduate. I was working in a research lab, and someone was working with a reaction that was very sensitive to water. And they actually ended up having a bunch of glassware explode on them that day… They were doing everything correct; it was just humid that day.” | |
| 4. Designing safer chemicals | Formal description: minimize toxicity directly by molecular design. Predict and evaluate aspects such as physical properties, toxicity, and environmental fate throughout the design process. |
| Simplified description: “This is going more into the toxicity of it. So how toxic is that chemical compared to others?” | |
| Real-life examples: “A big area you can kind of think about designing safer chemicals are actually with your skincare and healthcare products. So are there any skin or healthcare things that you try to avoid?… Botox might be one of them. That's quite literally putting something poisonous in it so that it freezes that area… Lead, yeah, so pencils are now made of graphite, so we’re not ingesting a lot of lead… Asbestos, that is pretty common with a lot of household materials.” | |
| 5. Safe solvents and auxiliaries | Formal description: choose the safest solvent available for any step. Minimize the total amount of solvents and auxiliary substances used, as these make up a large percentage of the total waste created. |
| Real-life examples: “This one is a little bit tricky to understand… One of the safest solvents you can actually use is water. Water is a nice, safe solvent that we use in most chemical reactions to mix a lot of those powders and things.” | |
| 6. Designed for energy and efficiency | Formal description: choose the least energy-invasive chemical route. Avoid heating and cooling, as well as pressurized and vacuum conditions (i.e., ambient temperature and pressure are optimal). |
| Real-life examples: “What would it look like to not be energy-efficient? If we’re not energy-efficient, what are we using?… Fossil fuels, yeah, so that's going into more the renewability of it. But in terms of energy, maybe we’re using fossil fuels to power up a gas generator that we’re pulling energy from. Anytime you have to heat up a reaction, you’re using electricity. That is not energy efficient. Anytime you have to cool it down. Anytime you have to add pressure to it. All of those situations require energy that you don’t need. If you could do a reaction just on your tabletop without doing anything, that is a nice, energy-efficient reaction.” | |
| 7. Use of renewable feedstock | Formal description: use of chemicals which are made from renewable (i.e., plant based) sources, rather than other, equivalent chemicals originating from petrochemical sources. |
| Real-life examples: “That's basically anything that's not oil-based, so like corn, potato, tapioca. All of those feedstocks we can grow again and again, so those are all considered renewable resources.” | |
| 8. Reduce derivatives | Formal description: minimize the use of temporary derivatives such as protecting groups. Avoid derivatives to reduce reactive steps, resources required, and waste created. |
| Simplified description: “We’re trying to minimize how many side products that get made.” | |
| Connection to previous material: “A lot of the time, the way that I’ve taught you a reaction,” teacher says while drawing an example reaction on the board, “is you start with your reactants, and all of that goes together to make your product. In real life, that's not what happens. You get some side products. You get a whole lot of different things happening. Maybe you get like part of A mixed with part of B. So you might be able to get a lot of derivatives from that reaction, and this is all about how efficient your reaction is. The whole goal is that you don’t get a lot of these derivatives.” | |
| 9. Catalysis | Formal description: use catalytic instead of stoichiometric reagents in reactions. Choose catalysts to help increase selectivity, minimize waste, and reduce reaction times and energy demands. |
| Simplified description: “We’re using a catalyst to help speed up that reaction, and it also reduces waste and reduces the reaction time. Basically, we’re speeding it up.” | |
| Real-life example: “What is a catalyst that got you to school today?… Your car, okay, so your car got you to school. Is your car getting the education it needs right now? No, so your car helped get you here. It got you here quicker so that you can learn and then you can go home. That is like a catalyst. It's not part of the reaction. It's not part of your learning, but it's helping you make that learning possible.” | |
| 10. Design for degradation | Formal description: design chemicals that degrade and can be discarded easily. Ensure that both chemicals and their degradation products are not toxic, bio accumulative, or environmentally persistent. |
| Simplified description: “That basically means: can it degrade and dissolve in whatever material? Is it going to break down and not just make a little microplastic? Ideally when it degrades, it degrades into things that are not toxic, because a lot of times things degrade into things that are toxic. So how can we prevent that toxicity?” | |
| 11. Real-time prevention | Formal description: monitor chemical reactions in real-time as they occur to prevent the formation and release of potentially hazardous and polluting substances. |
| Simplified description: “That's all about preventing hazardous pollutants. That one's pretty self-explanatory.” | |
| 12. Safer chemistry for accident prevention | Formal description: choose and develop chemical procedures that are safer and inherently minimize the risk of accidents. Know the possible risks and assess them beforehand. |
| Simplified description: “You don’t want to have accidents, especially if we want to bring this to a mass scale. So that's really taking in all of the risk factors and seeing ‘are those risk factors going to affect us?’” | |
In the first part of this observation, the class watched videos about recycling and ocean plastic clean-up as initial context to motivate the lesson. The teacher then asked students to consider examples, benefits, and drawbacks of plastics from their experience. To introduce green chemistry, the teacher explained that creating a biodegradable plastic would be one solution to the recycling issues. She gave a brief history of the development of green chemistry and stated this initial definition: “green chemistry is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances.” Then students were asked to match up formal descriptions of each of the twelve principles with the titles, as shown below. They had time in pairs to work on that matching exercise on their computers. After about ten minutes, the teacher went through each principle and gave examples or annotations to make the ideas more relatable to the students. Connections to previous material, simplified descriptions, and real-life examples were used for different principles to explain them more fully. The examples serve as initial entry points and providing them here can allow teachers to consider their own examples or descriptions more easily.
These initial examples were intended as an overview of the principles before continuing the unit on plastics where the students would apply the principles more directly. After these examples, the teacher also acknowledged that “if you didn’t get all of these, that's okay. A lot of these definitions are very technical. We’re going to continue working with these throughout the year as we’re learning these different principles.” Finally, the teacher assigned one principle each to pairs of students for them to explore more deeply by watching a video and answering a series of prompts. In subsequent days of this unit, students created their own plastics based on polylactic acid with a variety of starting materials and additives.
Overall, this case study helps to address one of the central issues of green chemistry education at the K-12 level, which is how to adapt a system that was developed for an industrial and research context into educationally appropriate and valuable content. Teachers often adapt green chemistry by providing examples that make sense developmentally and culturally to their students. The expertise needed to make these kinds of connections was one of the main ways that PCK was initially described by Shulman (1986, p. 9). This expertise will necessarily differ at other levels of education like in university classrooms (Grieger et al., 2022). This classroom provides just one glimpse into that process from a high school teacher's perspective.
The supplemental examples that teachers used for green chemistry represent another subset of expertise that varies depending on local context and how familiar the teacher is with the material. Knowing appropriate examples would fit into the third component of PCK from Magnusson et al. (1999): “knowledge and beliefs about students’ understanding of specific science topics.” Naturally these examples would differ based on classrooms, local regions, cultural background of students, and age range. These types of connections are similar to the approach of place-based education (Sobel, 2013), and they help to capture the PCK that teachers develop for their individual classrooms.
The teacher's role in this classroom was largely to propose questions for the students and ask them to explain their reasoning or to give evidence for their conclusions, a method heavily reflective of the NGSS Lead States (2013). The types of questions varied based on the abilities of the students and where they were in the experiment. At one point, the teacher warned a student to think through their experiment thoroughly, saying, “I’m going to ask you a bunch of questions before you finish, so make sure you understand.” In fact, questions were the vast majority of ways that the teacher spoke with students and provide insight into how this teacher views the experimental process.
Some of the questions that the teacher asked included:
(1) “What are you going to do? How are you going to do that?”
(2) “Is this making sense?”
(3) “What is a rule of good chemistry? What is important to remember when you are doing chemistry?”
(4) “What do you mean by ‘precautions?’ What would be an example of a safety precaution?”
(5) “How will you be safe? What kinds of things will you do to stay safe?”
(6) “What are you going to do next? You get to decide.”
(7) “What are you doing for your second iteration?”
(8) “What did we learn?”
(9) “How is [the soap] going to take away the germs? And how is homemade soap different from other types, like Dove?”
(10) “What scents are you using [for your soap]? What additives are you using? What are you putting in yours?”
(11) “What did you notice about the pictures you took before and after washing your hands?”
(12) “Other groups had pictures that looked like this, but yours looked like that. Why do you think that is?”
The teacher rarely gave direct answers or instructions to students and instead focused on prompting their own reflection on the experiment and their conclusions. At times, the teacher gave advice to students about the experimental procedure to limit the range of options, such as adding essential oils one drop at a time. The procedural scaffolding supported students to focus on the scientific process of investigation and explaining their reasoning.
Because of the inherent safety of green chemistry, this teacher was better able to construct the classroom in a way that supported this type of open and self-paced inquiry. This approach exemplifies the fourth component of PCK from Magnusson et al. (1999): “knowledge and beliefs about assessment in science.” Verbal assessments gave this teacher direct information about students’ understanding in addition to the written assignments included in the unit. For a green chemistry approach that can tend to be more variable and student-directed, this type of assessment works well. Labs and activities are built around scientific explanations more than accurate results or correct answers. Green chemistry does not require this assessment approach, but it does allow for significant flexibility and explicit scaffolding from the twelve principles to enable students to test and defend their ideas safely and thoughtfully.
Green chemistry content representation
Based on the interview summaries and classroom observations, we propose an initial framework for a Content Representation (CoRe) for green chemistry education in K-12 classrooms. As Loughran et al. (2012) caution before each CoRe,The CoRe outlines some of the aspects of PCK “most attached to that content,” but it is not the only representation. It is a necessary, but incomplete, generalization that helps to make the complexity accessible and manageable; it is neither complete nor absolute (p. 25).
The descriptions in Table 3 explain the main components of this CoRe connected with the relevant components of PCK from Magnusson et al. (1999): (1) orientations toward science teaching, (2) knowledge and beliefs about science curriculum, (3) knowledge and beliefs about students’ understanding of specific science topics, (4) knowledge and beliefs about assessment in science, and (5) knowledge and beliefs about instructional strategies for teaching science (p. 97).
| CoRe area | Description | Relevant PCK components(s) from Magnusson et al. (1999) |
|---|---|---|
| Chemical safety | Green chemistry is inherently safer, smaller scale, and more cost effective compared to traditional curricula. It encourages teacher and student ownership of chemical safety throughout the classroom. | 1 |
| Practice of chemistry | Green chemistry provides an empowering experience of making and using chemicals, helping students to imagine creative possibilities of the future. | 1, 2 |
| Teacher-facilitated connections | Teachers support their students’ engagement with green chemistry by making connections with industrial, community-based, or real-life examples. | 3, 5 |
| Experimentation mindset | Green chemistry supports classrooms to be exploratory, design-oriented, and based in problems and solutions. Students are supported to make claims and cite evidence to support their conclusions. | 4, 5 |
The four CoRe areas reflect the four case studies above but are generalized to be applicable to more classrooms. Overall, the CoRe summarizes the expertise of teachers in LTP beyond traditional chemistry content and classroom management strategies. These four areas speak to the unique contribution that green chemistry makes to K-12 science education and can serve as a basis for further reflection and development by teachers and researchers. Some of the areas overlap, but each one highlights an emphasis that green chemistry brings compared to more traditional chemistry curricula.
First, green chemistry makes chemical safety more explicit for teachers and students. By using less hazardous chemicals and smaller scales, experiments are inherently safer for students and for the environment. Everyone in the classroom can take ownership for safety through open conversations and applying the twelve principles to their experiments. Teachers who choose to incorporate green chemistry view learning as a shared process with students in their classroom. An added benefit of chemical safety also includes cost savings from materials, waste disposal, and addressing risks of injuries.
Second, green chemistry supports an empowering experience of chemistry as a creative science of solutions. K-12 green chemistry curricula should be designed to encourage engagement with this practice of chemistry rather than simply a science of following procedures. Through green chemistry, teachers and students can develop a perspective of science that is capable of confronting future issues in ways that protect people and the planet.
Third, green chemistry requires teachers to develop a wide range of connections to real-life and their local region in order to connect students with the content. A contextual approach to chemistry engages students and motivates their learning far beyond the classroom. Simplified descriptions and connections to previous material also serve to weave green chemistry into existing curricula.
Finally, green chemistry includes an experimentation mindset where students are assessed based on their ability to cite evidence from experience to justify their claims. These classrooms are exploratory, design-oriented, and based in finding solutions to real world problems. Both instruction and assessment must be adapted to this type of environment.
Limitations
The results of this project reflect the perspectives of twenty-eight educators from a highly self-selected group of green chemistry leaders. Their views would not represent an average K-12 teacher, and the perspectives of participants in LTP also differed from each other based on factors like location and time in the program. However, the depth of this qualitative research with a narrow subset of teachers provides an appropriate basis to consider future work in green chemistry. Rich case studies are an important source of data and reflection for both the research and practice of green chemistry. Furthermore, these experienced teachers provide an expertise based on their work with green chemistry that can be accessible and transferable to larger groups of science educators.Conclusions
Implications for K-12 green chemistry education
The teachers involved in this project described and justified their use of green chemistry for K-12 classrooms in overlapping ways. They reported that green chemistry can promote health and safety through waste reduction and student ownership. Similarly, some teachers justified the importance of teaching green chemistry because it allows for safer and more environmentally-friendly practices in their classroom. As a result, more hands-on activities can be done with minimized concerns about harsh chemicals, especially with younger students.The value of teaching green chemistry to K-12 students was also very prevalent throughout this research. There are many benefits for the students to learn green chemistry regardless of their intent to pursue a science education or not. Teachers described using green chemistry to engage their students and develop a sustainable mindset. Students can think about the consequences of their daily life decisions and how they can impact human health and the environment.
Teachers often highlighted the idea that all chemistry should be seen through the lens of green chemistry. However, from a research standpoint, they are separate for a reason (MacKellar et al., 2020). Yes, it is beneficial for educators to teach chemistry through a green chemistry lens across many topics. However, the point of green chemistry is ultimately to make our current and new chemistry practices safer for the environment and human health (Mahaffy et al., 2019). If all chemistry was green chemistry, chemists may be less likely to use this sustainable mindset in re-evaluating current practices or in designing new ones.
One important take-away from this research is the need for more K-12 green chemistry resources. K-12 teachers have difficulty integrating the twelve principles of green chemistry directly because they are not as applicable to the curriculum of younger students (Ause, 2018). However, given the demands of their job and work-load as teachers, the green chemistry educational community could benefit from having access to more resources to allow greater integration of green chemistry in their classrooms.
Future research directions
The ultimate success of this research will be in prompting conversations among K-12 teachers, teacher trainers, and academic researchers to build on the foundation of green chemistry education. The examples of PCK in the CoRe and PaP-eRs are designed to facilitate these conversations as a starting point and common place for comparison (Loughran et al., 2012).A critical component of any educational program is its effects on students in the short and long term. With a more well-defined foundation for green chemistry in K-12 classrooms, further work will be needed to study how this approach affects student learning (Bransford et al., 1999; Grieger et al., 2022). With a basis for defining PCK for green chemistry, connections could be investigated more thoroughly between teachers’ levels of expertise and student outcomes (Coe et al., 2014). Future studies can consider the longer term impact of learning about green chemistry for students in later university classes or careers in industry. These types of connections would be critical to advancing green chemistry education.
Finally, we return to the purpose for this project. The future cultivation of teacher expertise in the current landscape of green chemistry can benefit from the results and discussion covered here. Developing pedagogical content knowledge among K-12 teachers in the Lead Teacher Program has served to advance to prominence and understanding of green chemistry. And if continued to be developed in ways that are sustainable for participants and staff, LTP and other work by Beyond Benign can continue to bear fruit for many years to come.
The reaction equation of exceptional chemistry teaching
A major concern in research on chemistry education could be phrased as: what makes an exceptional chemistry teacher? We might conceptualize a reaction equation for that process where the right amount of content knowledge in chemistry is mixed with enough pedagogical knowledge to produce the ideal teaching and, therefore, student learning. However, the reality of this process is much more complicated. There is a highly variable yield of student outcomes. The perfect conditions of a classroom are difficult to maintain. And any teacher knows that there are some unproductive combinations of content and pedagogy that take away from the desired results. In short, this approach to exceptional teaching is unsustainable.What is needed is a green approach to K-12 education that more directly supports exceptional teaching and learning. The Pedagogical Content Knowledge developed with teachers in LTP through this project can act as a catalyst to provide alternate kinetic patterns and help teachers to identify the most sustainable and productive educational pathways for their own classroom. The approach of green chemistry more broadly can act as a renewable energy source to drive the reaction thermodynamically. Together, the knowledge and practices explored in this project can make exceptional chemistry teaching more sustainable and attainable for K-12 teachers. As one Lead Teacher shared, that vision will truly be “something that's valuable to humankind at the end of the day.”
Author contributions
Philip Nahlik: conceptualization, methodology, investigation, writing – original draft, review and editing, project administration. Lauren Kempf: investigation, data curation, visualization, writing – original draft. Jayke Giese: investigation, data curation. Elizabeth Kojak: investigation, data curation. Patrick L. Daubenmire: supervision, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This article draws significantly from the dissertation work of the primary author.Notes and references
- American Chemical Society, (2011), Chemistry in the community (ChemCom), New York: W. H. Freeman.
- Anastas, P., and Eghbali, N., (2010), Green chemistry: principles and practice, Chem. Soc. Rev., 39(1), 301–312.
- Anastas, P. T. and Warner, J. C., (1998), Green chemistry: theory and practice. Oxford University Press.
- Aubrecht, K. B., Bourgeois, M., Brush, E. J., MacKellar, J. and Wissinger, J. E., (2019), Integrating green chemistry in the curriculum: building student skills in systems thinking, safety, and sustainability, J. Chem. Educ., 96(12), 2872–2880 DOI:10.1021/acs.jchemed.9b00354.
- Ause, R., (2018), Green chemistry in secondary school, in Benvenuto M. A. and Kolopajlo L. (ed.), Green chemistry education: recent developments, De Gruyter, pp. 185–195.
- Aydeniz, M. and Kirbulut, Z., (2014), Exploring challenges of assessing pre-service science teachers' pedagogical content knowledge (PCK), Asia-Pacific Journal of Teacher Education, 42(2), 147–166.
- Barendsen, E. and Henze, I., (2019), Relating teacher PCK and teacher practice using classroom observation, Res. Sci. Education, 49(5), 1141–1175.
- Bastin, L. and Dicks, A., (2019), Advances in green chemistry education, Green Chem. Lett. Rev., 12(2), 101.
- Baxter, J. A. and Lederman, N. G., (1999), Assessment and measurement of pedagogical content knowledge, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge: the construct and its implications for science education, Dordrecht, London: Kluwer Academic, pp. 147–161.
- Benvenuto, M. A. and Kolopajlo, L., (2019), Green chemistry education: recent developments, De Gruyter DOI:10.1515/9783110566499.
- Bertram, A. and Loughran, J., (2012), Science teachers’ views on CoRes and PaP-eRs as a framework for articulating and developing pedagogical content knowledge, Res. Sci. Education, 42(6), 1027–1047 DOI:10.1007/s11165-011-9227-4.
- Beyond Benign, (2021), About us: Mission and vision. Retrieved fromhttps://www.beyondbenign.org/about-mission-vision/.
- Bransford, J., Brown, A. L., Cocking, R. R. and National Research Council (US) Committee on Developments in the Science of Learning, (1999), How people learn: brain, mind, experience, and school, Washington, DC: National Academy Press.
- Charmaz, K., (2014), Constructing grounded theory: a practical guide through qualitative analysis, 2nd edn, Sage.
- Coe, R., Aloisi, C., Higgins, S. and Major, L. E., (2014) What makes great teaching? Review of the underpinning research. Project Report, London: Sutton Trust.
- De Jong, O., Van Driel, J. and Verloop, N., (2005), Preservice teachers' pedagogical content knowledge of using particle models in teaching chemistry, J. Res. Sci. Teach., 42(8), 947–964.
- Demirdöğen, B., Hanuscin, D., Uzuntiryaki-Kondakci, L. and Köseoğlu, E., (2016), Development and Nature of Preservice Chemistry Teachers’ Pedagogical Content Knowledge for Nature of Science, Res. Sci. Educ., 46(4), 575–612.
- Emerson, R., Fretz, R. and Shaw, L., (2011), Writing ethnographic fieldnotes, 2nd edn, The University of Chicago Press.
- Goode, S. R., Wissinger, J. E. and Wood-Black, F., (2021), Special issue on chemical safety education: methods, culture, and green chemistry, J. Chem. Educ., 98(1), 1–6 DOI:10.1021/acs.jchemed.0c01459.
- Green Chemistry Institute, (2020), Connections between green and sustainable chemistry, systems thinking and existing chemistry curricula, American Chemical Society, https://www.acs.org/content/dam/acsorg/greenchemistry/redesign/students-educators/education-roadmap/gci-guidance-for-developing-education-materials-2020.pdf.
- Grieger, K. and Leontyev, A., (2021), Student-Generated Infographics for Learning Green Chemistry and Developing Professional Skills, J. Chem. Educ., 98(9), 2881–2891 DOI:10.1021/acs.jchemed.1c00446.
- Grieger, K., Schiro, A. and Leontyev, A., (2022), Development of the Assessment of Student Knowledge of Green Chemistry Principles (ASK-GCP), Chem. Educ. Res. Pract., 23, 531–544 10.1039/D1RP00291K.
- Haack, J. and Hutchison, J., (2016), Green chemistry education: 25 years of progress and 25 years ahead, ACS Sustainable Chem. Eng., 4(11), 5889–5896.
- Juntunen, M. and Aksela, M., (2013), Life-cycle thinking in inquiry-based sustainability education – effects on students’ attitudes towards chemistry and environmental literacy, Center Educ. Policy Studies J., 3(2), 157–180.
- Lederman, N. G. and Gess-Newsome, J. (1999), Examining pedagogical content knowledge: the construct and its implications for science education, Kluwer Academic.
- Loughran, J., Berry, A. and Mulhall, P., (2012), Understanding and developing science teachers' pedagogical content knowledge, 2nd edn, Professional learning; v. 12, Rotterdam, the Netherlands: Sense.
- MacKellar, J. J., Constable, D. J. C., Kirchhoff, M. M., Hutchison, J. E. and Beckman, E., (2020), Toward a green and sustainable chemistry education road map, J. Chem. Educ., 97(8), 2104–2113 DOI:10.1021/acs.jchemed.0c00288.
- Magnusson, S., Krajcik, J. and Borko, H., (1999), Nature, sources, and development of pedagogical content knowledge for science teaching, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge: the construct and its implications for science education, Dordrecht, London: Kluwer Academic, pp. 95–132.
- Mahaffy, P. G., Ho, F. M., Haack, J. A. and Brush, E. J., (2019), Reimagining chemistry education: systems thinking, and green and sustainable chemistry, J. Chem. Educ., 96(12), 2679–2681 DOI:10.1021/acs.jchemed.9b00991.
- Neumann, K., Kind, V. and Harms, U., (2019), Probing the amalgam: the relationship between science teachers’ content, pedagogical and pedagogical content knowledge, Int. J. Sci. Educ., 41(7), 1–15.
- NGSS Lead States, (2013), Next generation science standards: for states, by states, Washington, DC: The National Academies Press.
- Parga Lozano, D. L., (2015), Pedagogical content knowledge about green chemistry: for university professors of chemistry, Revista de la Facultad de Ciencia y Tecnología - Tecné, Episteme y Didaxis, 167–182.
- Sanera, M. and Shaw, J. S., (1997), Environmental ‘science’ in the classroom, Consumers’ Research Magazine, 80(4), 15–18.
- Sharma, S. K. and Mudhoo, A., (2011), Green chemistry for environmental sustainability, Boca Raton: CRC Press.
- Shulman, L., (1986), Those who understand: knowledge growth in teaching, Educ. Res., 15(2), 4–14.
- Sobel, D., (2013), Place-based education: connecting classrooms and communities, 2nd edn, Orion.
- Sutman, F. X. and Bruce, M. H., (1992), Chemistry in the community - ChemCom: a five-year evaluation, J. Chem. Educ., 69(7), 564.
- Taylor, D., (2010), Principles into practice: setting the bar for green chemistry, Environ. Health Perspect., 118(6), A254–A257.
- Weiss, R., (1995), Learning from strangers: the art and method of qualitative interview studies, 1st Free Press pbk. edn, Free Press.
Footnotes |
| † See a recent set of reports from the Royal Society of Chemistry titled Green shoots: A sustainable chemistry curriculum for a sustainable planet, accessible at https://www.rsc.org/new-perspectives/sustainability/a-sustainable-chemistry-curriculum/. |
| ‡ Program evaluation results to be published separately in a forthcoming article. |
| This journal is © The Royal Society of Chemistry 2023 |