Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Looking for solutions: students’ use of infrared cameras in calorimetry labs
Christopher Robin
Samuelsson
 *a,
Felix M.
Ho
*a,
Felix M.
Ho
 b,
Maja
Elmgren
b,
Maja
Elmgren
 b and
Jesper
Haglund
b and
Jesper
Haglund
 c
c
aDepartment of Physics and Astronomy, Uppsala University, Box 516, 751 20 Uppsala, Sweden. E-mail: Robin.samuelsson@physics.uu.se
bDepartment of Chemistry, Ångström, Uppsala University, Box 523, 751 20 Uppsala, Sweden
cDepartment of Engineering and Physics, Karlstad University, 651 88 Karlstad, Sweden
First published on 24th October 2022
Abstract
This study adds to the growing body of research on laboratory work. The study involves four pairs of students in a university introductory calorimetry lab of which two pairs, the IR-pairs (infrared camera-pairs), were given access to infrared cameras to use however they liked during their course lab work. Two other pairs, the T-pairs (thermometer-pairs), were not given access to infrared cameras during their course lab work. The IR-pairs were video recorded when they chose to use the IR cameras and the T-pairs were video-recorded during the corresponding sequences. Additionally, all pairs participated in a modified lab after their course lab, in which the pairs had access to IR cameras and were presented with the same phenomena although with equipment modified to better accommodate for the use of IR cameras (thin plastic cups were used instead of calorimeters). Students’ practice, communication and reasoning was studied to explore how the IR cameras affect students’ activity. The results show that the access to IR cameras led to a reasoning focused on a macroscopic level of chemistry knowledge, involving heat transfer to the surrounding and measurement errors, and that the lab practice of most of the students was continuous (rather than intermittent) when they had access to IR cameras. We conclude by arguing that the access to IR cameras affects students’ conceptual and epistemological framing of the lab, i.e. that the students perceive the lab activity differently when they get access to IR cameras (both in a conceptual and epistemological sense). As an implication for teaching, we suggest that giving students access to IR cameras in a chemistry lab may be a way to introduce flexibility in the degree of openness of the lab.
Introduction
Four decades ago, Hofstein and Lunetta (1982) came to the conclusion that there is a lack of research on how laboratory work, compared to other educational approaches, affects students’ learning. In a response to this, Tobin (1990, p. 414) proposed that meaningful learning can be achieved “if all students are provided with opportunities to manipulate equipment and materials while working cooperatively with peers in an environment in which they are free to pursue solutions to problems which interest them”.The ways students work and act in laboratory activities, for example how they manipulate laboratory equipment such as the thermometer, can be described through their framing of the activity. The notion of framing has been suggested in educational research to address the context-dependent nature of students’ learning (Hammer, 2000; Redish, 2003). In the present study, we have used this lens to discuss how the introduction of an infrared (IR) camera in an undergraduate calorimetry lab influences the students’ reasoning, practice and communication.
Inquiry and instruction
Whether people learn best through unguided or instructed environments is a long-going dispute (Kirschner et al., 2006; Hmelo-Silver et al., 2007). Whilst some researchers suggest ideas on learning related to a constructivist epistemology in which learning is achieved through experience and discovery (e.g.Bruner, 1961; Piaget, 1971; Papert, 1980), others provide empirical results suggesting that direct instructional guidance is more advantageous (e.g.Sweller, 2003; Klahr and Nigam, 2004; Kirschner et al., 2006).However, what initially appears to be a discrepancy between the two views on learning may not be as clear (Kirschner et al., 2006). Publications from both sides focus on a variety of expertise and put different meanings into words such as instruction and discovery. Perhaps a more nuanced description of learning and instruction, and a clarification of what inquiry really means, are needed: Buck et al. (2008) investigated the types of openness, or inquiry, in labs that have been proposed in previous research (e.g. guided inquiry, open inquiry or inquiry learning). They found that a wide variety of modifiers have been used when talking about inquiry, modifiers such as open inquiry, structured inquiry and partial inquiry. Katchevich et al. (2013), in turn, suggest that “experiments can be classified into four types: confirmatory, inquiry, discovery, and conducting an experiment around a specific problem” (Katchevich et al., 2013, p. 318). The authors studied students’ construction of arguments during chemistry labs and found that inquiry experiments can enhance argumentation. A comparison between inquiry-type and confirmatory-type experiments showed that the latter were more sparse in arguments than the former type of experiments. However, as Andersson and Enghag (2017) argue, cumulative sequences where students “just” confirm and repeat what they observe, i.e. sequences that lack the construction of arguments, have an important role in students’ laboratory work as it seems to keep the students on track with the task, for example when making measurements, and guide them toward the goal of the lab. A more recent study on students’ communication in laboratory activities (Petritis et al., 2021) argues that we need to investigate how students frame an activity and how this impacts the argumentation during the activity.
What is gaining in support by research (e.g.Deslauriers et al., 2011, 2019; Wieman, 2014), however, is that it is important for students to engage in active learning, i.e. that they are required to continuously apply and process their knowledge in different ways. But what is active engagement during a lab? In line with Tobin's (1990) proposal of providing students with equipment that they can use to pursue explorations out of their own interest, this could be explored by giving students access to a tool that promotes active learning but at the same time provides the students with some basic instruction so that they are not completely left on their own. Such a tool could be the IR camera as IR cameras have been shown to be an apt tool for keeping students active (see “Infrared cameras in science education”).
In our study, in an effort to contribute to a reconciliation between direct instruction and more open-ended approaches, we explore the possibilities of giving students access to additional equipment (an IR camera), so that the students have the potential to investigate additional points of interest during their laboratory work as extra equipment may add more stimuli to the investigation process.
Infrared cameras in science education
Concepts like heat and temperature are difficult for students to understand (e.g.Erickson, 1979; Brookes and Etkina, 2015) but the difficulties have mainly been studied from a perspective of conceptual change rather than from whether students can identify instances in which the concepts apply.The concepts of heat and temperature are especially important in the domain of calorimetry, a topic that has not been explored much in past education research. However, there are a few publications that touch upon the subject (Xie, 2011; Xu et al., 2019; Green et al., 2020) and that include activities in calorimetry where IR cameras support students’ investigations, a tool that has the potential to support students in identifying instances where they can apply the concepts. Additionally, it has been shown that IR cameras engage students in activities and keep them active in their investigations (e.g.Haglund et al., 2016).
Chemistry educators have suggested a range of phenomena where students’ laboratory work may benefit from the introduction of infrared (IR) cameras (Xie, 2011; Bohrmann-Linde and Kleefeld, 2019; Wong and Subramaniam, 2019; Xu et al., 2019). For example, Xie (2011) describes how IR cameras enable the study of temperature change of a sheet of paper due to condensation and evaporation as the paper is placed above a water surface at room temperature and later removed. He also brings up how the technology can be used to observe convection as the melting water from an ice cube that is placed in fresh water sinks to the bottom, whereas such convection does not occur when an ice cube is placed in a saturated solution of table salt, due to the higher density of the salt solution.
IR cameras may also enrich students’ experiences of exothermic and endothermic reactions. In contrast to the regular procedure of measuring the temperature of a solvent before and after a solute is added, an IR camera enables students to investigate the process of solution reactions in a more dynamic way (Xie, 2011). Xu et al. (2019) describe the surprising effect of dropping concentrated sulphuric acid in water: a warm spot appears on the surface with a delay of 13–15 seconds and then diffuses explosively. Similarly, Bohrmann-Linde and Kleefeld (2019) suggest adding sodium hydroxide dropwise into a Petri dish of hydrochloric acid for students to see whirls with increased temperature due to the exothermic neutralization reaction.
In a teaching sequence on phase change (Samuelsson et al., 2019a), preservice teacher students connected the temperature decrease of a moist paper due to evaporation in IR-camera experiments with their experience of feeling cold when they walk out of a shower. This contributed to their explanation that energy is required for evaporation to occur. Furthermore, Samuelsson et al. (2019b) have previously shown that access to IR cameras after an exploratory sequence of hypothesis generation may stimulate a quick testing experiment of the generated hypotheses. A pair of students explored multiple explanations for why solid sodium hydroxide is moist after it has been exposed to air for some time. When they were presented with the same phenomenon again and given access to an IR camera, they could quickly confirm one of the hypotheses, i.e. that the salt reacted exothermally with the water in the air.
IR cameras seem to be useful in laboratory practice given that they fit with the purpose of the activity. However, it is still not known what it is that changes in terms of students’ behaviour when they get access to IR cameras for some experiment. We want to explore this by comparing groups of students that do not have access to IR cameras and students that do have access to IR cameras (during the same sequence of a lab).
Framing
Tobin (1990, p. 414) argues that studies on students’ participation in lab education should focus on “how students engage, construct understandings and negotiate meanings in cooperative groups.” A way of approaching the last point is to look for students’ framing of the lab activity.This paper will apply the concept of framing in order to analyze how the context influences students’ laboratory activity (more specifically the experimental part). Past knowledge is always used in making arguments about a present phenomenon. In addition, how reasoning is carried out is further influenced by the framing of the situation. Framing a situation “is to interpret it in terms of structures of expectations based on similar events. […] A student may frame a physics problem as an opportunity for sense making, or an occasion for rote use of formulas” (Hammer et al., 2004, p. 9). How a pair of students frame a situation will thus affect how they act and what they notice in the situation.
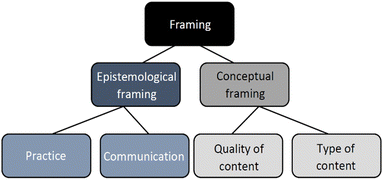
Some of the previous research has focused on categorizing different types of framing. For example, Hammer et al. (2004) describe epistemological (How should I build knowledge and answer questions?), social (Who should I interact with?) and affective (How should I feel?) framing. Additionally, van de Sande and Greeno (2012) used three types of framing: positional framing (how the students perceive themselves to be related to each other); epistemological framing (what kind of knowledge that is perceived as relevant for the activity); and, conceptual framing (how different aspects of information is perceived as related to each other), to study how students come to a mutual understanding (e.g. the aligning the framings). The authors refer to epistemological and conceptual framing collectively as cognitive framing. By contrast, Haglund et al. (2015) focused only on students’ epistemological and conceptual framing. Additionally, they argue that students answer the question “What knowledge should I use for the situation?” by applying a set of concepts and memories deemed appropriate to the situation. This may differ between students depending on what they attend to, i.e. how they conceptually frame a situation. This is also the categorization of framing that will inform our discussion in the study, where we choose to use conceptual framing as what the situation is about in terms of content (e.g. what knowledge do they use and do they know what they are talking about?).
The concept of framing has been used in previous research on infrared cameras as educational tools: Samuelsson et al. (2019a) showed how it is possible to design a teaching sequence that involves infrared cameras, so that students coordinate their knowledge in such a way that they find coherence across multiple tasks involving evaporation of water, i.e. the teaching sequence restricted “students’ frame of phase transitions” (Samuelsson et al., 2019a, p. 582) so that they focused on what is relevant in each task. Haglund et al. (2015) investigated students’ framing, in terms of their idea regarding what a situation is about, of exercises where they investigated thermal phenomena with IR cameras. They found that the students differed in how they framed what type of knowledge was deemed relevant – should they follow the instructions or explore the surroundings more freely? – but not in the framing of what information to foreground.
Research question
In light of this background, we want to learn more about how the use of IR cameras during a calorimetry lab session for undergraduate students affects the students’ activity, in terms of what they do, how they talk and what they say. The research question guiding this study is:How does access to IR cameras affect students’ reasoning, practice and communication during a calorimetry lab?
We answer this question by analyzing how IR cameras affect the students’ participation in terms of what they do, how they talk and what they say.
Methodology
Research context – the laboratory exercise in question
The laboratory exercise centred around the determination of the heats of solution of sodium hydroxide (NaOH) and sodium nitrate (NaNO3), with these dissolutions contrasting each other in being exothermic and endothermic, respectively. In terms of content knowledge, this exercise was connected to the course topic of thermodynamics in introductory university chemistry. The exercise covered content such as the system-surroundings distinction, reaction enthalpies, as well as specific heats and heat capacities. In addition, the dissolution reaction was considered at the molecular level, with the Born–Haber cycle and Hess’ Law being used both as tools for numerical analysis and for promoting conceptual insight into the energetic bases for the contrasting heat flows for the dissolution of these two salts. The participating students have all studied chemistry in upper secondary school, which includes topics such as thermodynamics and calorimetry.In terms of laboratory and experimental skills, the laboratory exercise had been recently designed by two of the authors, Ho and Elmgren, prior to (and independently of) this research study, as part of an educational development project to revise a series of three laboratory exercises in the first-year general chemistry courses. One overarching aim was to replace traditional exercises consisting of fully defined experimental design and procedural instructions (“cookbook labs”) with ones that would gradually include more open-ended elements of inquiry, decision-making and experimental design. As shown by Katchevich et al. (2013), inquiry design may leverage students’ argumentation process but we still want to keep room for the type of cumulative discussions that provide a focus for the activity (Andersson and Enghag, 2017).
As the second revised laboratory in the series, the content objectives of this exercise were well-defined, and pre-lab exercises were provided to prepare and guide the students through the general thermodynamics behind the determination of heats of solution. However, specific details in the experimental procedure, methods of analysis and reporting of results were deliberately excluded in order to leave room for own discussions and decision making by the students (e.g. the amounts of salt and water to be used during measurements in order to obtain observationally useful temperature changes; which glassware and measurement instruments were appropriate for the experiment; how the experiments should be repeated and how many times; how to minimize experimental errors and uncertainties; how the heats of solution should be calculated and reported to allow for chemically reasonable and standardized comparison between salts). Some guiding questions were instead included to draw the students’ attention to aspects that should be considered. Further discussion questions in the preparatory exercise and for the laboratory report were also designed to guide student thinking and understanding. In contrast to the rubric by Buck et al. (2008), in which different characteristics either was provided or not, here several characteristics, like lab design and result analysis, were partly opened with some expectations on student inquiry.
Students worked in pairs during the laboratory class, with ample opportunities for discussions with each other and with the laboratory instructor. The students were to dissolve two salts (sodium hydroxide and sodium nitrate) in water in a Styrofoam coffee cup calorimeter and measure the temperature changes during the reaction, and use the results obtained to determine the enthalpy change for the dissolution of the salts. The overarching purpose of the lab was stated in the instructions available to the students (the text is translated by the authors from Swedish):
“The purpose of the lab is, by using a coffee cup calorimeter, to determine the enthalpy change for the dissolution of some salts. During the lab session, the students will learn more about heat and heat capacity, the chemical principles that explain the obtained results and plan and carry through the experiment.”
Design of the study and data collection
This study is centred around the experiments in the lab described above (dissolving two salts in water and measuring the temperature change): two lab groups in a chemistry introduction course, in which none of the authors were involved as teachers in the lab, were chosen for the study. In these two groups, students were informed about the study, that if they enrolled they could withdraw from the study at any time, and that their identity would be protected. They were then asked if they were interested in participating in the study (for this, they were given movie tickets). Students that expressed an interest were handed consent forms to read and sign if they wanted to participate. As no sensitive personal data were gathered, an ethics committee approval was not required in line with local regulations. Eight students (N = 8) participated as pairs (i.e. four pairs). Each pair performed the experiment in two stages.In the first stage, the students performed the experiments together with the rest of their laboratory class (this stage is referred to as the “course lab” below). Two pairs of participants (pair IR1 and IR2) were provided with IR cameras and given brief oral instructions on how to use them (and some time to test them out), and had the cameras available to them during the lab experiments (each pair worked separately). They were then video-recorded when they chose to use the cameras. This happened during one of the experimental runs for each of the salts (sodium hydroxide and sodium nitrate).
The two other pairs of participants (pair T1 and T2), performed the lab experiments using only thermometers and without access to IR cameras. They were video-recorded when they measured the temperature change of dissolving the salts in water with a thermometer, which was the event corresponding to when the pairs IR1 and IR2 chose to use the IR camera.
In the second stage, the students repeated the experiments (this stage is referred to as the “modified lab” below). During this modified lab, thin, transparent plastic cups were used instead of the opaque Styrofoam coffee cup. Thermometers were not used simultaneously with the cameras and the mixture was not stirred when the salt was added. These modifications made the experiments more apt for investigating with IR cameras.
Additionally, during the modified lab, the students were asked to predict what would happen, to make observations and to explain the encountered observations (loosely based on the probing method of Prediction–Observation–Explanation (POE) (White and Gunstone, 1992)). The students were also asked what the purpose of the course lab was and what they had learnt from it.
Something should be noted about the authenticity of the modified lab compared to the course lab. The modified lab was carried out in the same environment as the course lab and during the same time slot as to keep it as authentic as possible to the students’ regular course work. However, as the modified lab included slightly different equipment and was structured through POE, it included different contextual cues than the course lab (more directed toward the use of IR cameras and direct instructions).
It is also worthwhile to note that this research was not aimed at comparing the efficacy of using a thermometer or an IR-camera for this laboratory exercise. Thus, while the lab instructor checked the reasonableness of the values measured by the students using the two methods, so that students would not be distracted by any erroneously large differences in results, the enthalpy values obtained were not recorded or analysed as part of our research.
All data were collected through video recording and subsequently manually transcribed using a spreadsheet software.
Data analysis
For data analysis, comparisons were made based on these contrasting conditions:– Lab pairs having access to IR cameras throughout the experiments of their course lab as well as during a subsequent modified lab: IR1 (Ike and Jill) and IR2 (Rolf and Soren).
– Lab pairs not having access to the cameras during the experiments of their course lab and then getting access to IR cameras during a subsequent modified lab: T1 (Mia and Mist) and T2 (Oscar and Anna).
Conversation analysis (Jewitt et al., 2016) was used a basis for guiding the analysis of the data (together with thematic coding of the transcribed data). Within conversation analysis it is a common practice to choose short excerpts and, through iterations, closely analyze those rather than to attempt to describe and analyze the full teaching activity (e.g. all hours of lab activity in our case). By selecting the sequences in which the students with access to IR cameras chose to use the IR cameras (and the corresponding sequence for the students that did not have access to IR cameras) we limited the amount of data to the parts that are purposeful for our study. Additionally, our data captured experimentation on both types of salts and reactions.
The data were analysed in phases: in general, every phase began with the first author analysing the data, followed by discussion of the analysis with some or all of the other authors (that received the material as preparation for the meeting). Some of the phases included additional parallel analysis by one or more of the other authors (to compare the analyses at the meeting). After the first meeting of the phase, the first author returned to the analysis with the discussion in mind. Each phase ended with at least one additional meeting with all the authors.
The first phase included a transcription of the video data and an inductive generative coding of the transcripts, which generated several codes (conceptual codes such as “Spontaneity/entropy” and epistemological codes such as “Spatial”). The first author did the coding, followed by discussion with two of the other authors while one author remained outside of the discussion and wrote their own comments to the transcript to compare with the coding of the first author at the later meeting in the phase.
For the second phase, the first author wrote a general summary on how the students worked, what concepts they talked about, what data they collected and how productive they were in terms of fulfilling the task at hand. One of the other authors wrote their own summaries to compare with the first author's summaries at the first meeting of the second phase.
We found that the final codes and summaries from the first two phases could be used for a further analysis of three aspects of the data: students’ reasoning, communication and practice. These aspects were subsequently analysed in new phases: the transcripts were structured as segments: the unit of analysis in the transcripts were segments of utterances and exchanges consisting of contiguous arguments or statements centred around a specific theme, concept or phenomenon (i.e. a change in topic results in a new unit of analysis). A segment would usually end when the students had come to a conclusion about something, when they could not come up with anything further to say about a topic or when they moved on with another activity in the experiment.
The partitioning of the transcripts into segments was carried out by the first author, followed by discussion with the other authors. However, note that the segments in themselves do not tell us anything about the quality, or type, of content within the talk. Another phase of analysis was therefore initiated after this. This time we looked for the type of content of each segment (macroscopic or submicroscopic), whether the reasoning was easy or difficult to follow and if it was relevant for the activity at hand (see the purpose of the lab under “Lab design”) (coherency and relevance). In particular, when analysing and comparing coherence between the different student pairs, only segments consisting of five or more arguments or statements were used. This was due to the difficulty of assessing coherence for segments with fewer arguments or statements than this.
The interaction of the students was analyzed in terms of how much the students were engaged with the experiment. The analysis was done separately by two of the authors, followed by discussion with the other two authors. Finally, the pairs were ranked in terms of coherency and relevance of reasoning, and interaction.
The final phase of analysis was on the communication of the students. We had, in the earlier phases, noticed how the students that used IR cameras seemed to come up with ideas and things to discuss more often than the students that did not have access to IR cameras so in the next phase we decided to focus on the initiation of the students’ communication. This was analysed through the segments: the segments were coded either as self-initiated or as responsive depending on whether the segment started out as something that the students had come up with themselves or if it started out as a response to the researcher or lab instructor.
To summarize the description of our analysis: students’ practice included how they moved around the experiment, what they interacted with and how they attended to the experiment. This was studied directly through the video data and summarized. Communication as an aspect of participation involved the initiation of the reasoning around a specific concept or idea: did the students initiate the reasoning based on their own observations or ideas, or did they respond to questions or prompts from the researcher or lab instructor? This was studied through coding of the segments in the transcripts. Reasoning was analyzed in terms of the level of knowledge used, and how relevant (compared to the purpose) and coherent the reasoning was for the activity (see Table 1). The level of knowledge used was analyzed in terms of the three levels of chemistry knowledge that students have to coordinate, described as “(a) the macro and tangible: what can be seen touched and smelt; (b) the submicro: atoms, molecules, ions and structures; and (c) the representational: symbols, formulae, equations, molarity, mathematical manipulation and graphs” (Johnstone, 2000, p. 11). This was also studied through coding of the segments in the transcripts, in addition to summaries.
| Lab | Aspect | IR1 | IR2 | T1 | T2 |
|---|---|---|---|---|---|
| Course lab | Reasoning (quality of content) | Coherent and relevant | Coherent and relevant | Coherent and relevant | Incoherent but relevant |
| Reasoning (type of content) | Macroscopic | Macroscopic | Macroscopic and submicroscopic | Macroscopic and submicroscopic | |
| Practice | Continuous | Continuous | Intermittent | Intermittent | |
| Communication | Self-initiated | Self-initiated | Responsive | Responsive | |
| Modified lab | Reasoning (quality of content) | Coherent and relevant | Coherent and relevant | Coherent and relevant | Incoherent but relevant |
| Reasoning (type of content) | Macroscopic | Macroscopic | Macroscopic | Macroscopic | |
| Practice | Continuous | Continuous | Continuous | Intermittent | |
| Communication | Responsive | Responsive | Responsive | Responsive | |
Results and analysis
Overview of the results
Each pair spent in total about 16–20 minutes on the course lab and the modified lab. The students’ reasoning, practice and communication varied between the pairs, and seemed partly to be influenced by the access to IR cameras.In general, the students with access to IR cameras during the course lab were more engaged in their investigation of the experiments than the pairs that only had access to thermometers. They exhibited a continuous practice, in the sense that they continuously focused on and interacted with the experiment, in contrast to the pairs with only thermometers, who exhibited an intermittent practice, where they interacted with the experiments more sporadically. The IR-pairs also investigated things that they noticed with the support of the IR camera, which formed starting points for interactions and discussions. There was no equivalent to such investigations in the T-pairs’ observations and measurements with thermometers.
The pairs with IR cameras also initiated an absolute majority of their reasoning during the course lab on their own; their communication was self-initiated. In contrast, the pairs without IR cameras initiated only about half of their reasoning, while the rest was initiated through questions from the researcher or instructor; their communication was responsive.
Both the reasoning of the IR-pairs and one of the T-pairs (T1) were coherent and relevant for the activity. However, when more closely comparing these pairs in terms of the content of their reasoning during the course lab, it becomes apparent that the pairs with IR cameras exclusively focused on macroscopic aspects, whilst the T-pairs reasoned both about the submicroscopic and the macroscopic level. When the T-pair was equipped with an IR camera in the modified lab and asked to predict, observe and explain the phenomena there, the discussion instead shifted to become exclusively about the macroscopic level. The reasoning of the fourth pair (T2) was incoherent and difficult to follow, both during the course lab and the modified lab, and the reasoning was mostly irrelevant for the activity.
In the sections below, we illustrate these differences and similarities of the pairs with analyses of selected dialogue excerpts.
IR cameras promote reasoning about macroscopic phenomena
In general, both IR1 and IR2 investigate the phenomenon mainly at a macroscopic level (they talk about heat both in a technical and everyday sense). At times, they use concepts like temperature and heat to bring the attention to the potential errors in the experiment. For example, at one occasion during the course lab, IR1 find that the temperature of the outer surface of the calorimeter increases when the sodium hydroxide is dissolved in the water and the following dialogue unfolds:Ike: “There is a large difference.”
Researcher: “What does that imply about the cup?”
Ike: “This means that the cup does not insulate since the cup becomes warm too, which means that there is a pretty large error in the experiment. In the best of worlds, the calorimeter would be completely insulating so that no heat is transferred out.”
[…]
Researcher: “What determines how long it takes before we observe the heat transfer now from the side when we look at the cup?”
Ike: “It should be the specific heat capacity of the water and the cup […] since the specific heat capacity of the water determines how fast the heat will spread in the water and the specific heat capacity of the cup determines how it is heated […] how large of a difference in temperature that is required for it to become warm.”
This reasoning is initiated through the observation made by the students and subsequently fed by the questions from the researcher. Although not acknowledging the role of the heat conductivity in explaining the phenomenon, they raise a concern about the cup not insulating enough as it is “warm” and heat is transferred out of the cup. The reasoning is coherent and relevant (in line with the purpose of the lab: to learn more about heat and heat capacity). See Table 1 for an overview of the students’ reasoning.
Although the reasoning of T1 during the course lab is also coherent and relevant – in fact their reasoning is on par with, or even more elaborate than, the reasoning of IR1 and IR2 – it is contrasted with the IR-pairs’ focus on the macroscopic level. A majority of the sustained reasoning of T1 was focused on the submicroscopic level (both the submicroscopic and macroscopic level appeared in their reasoning). The explanations of T1 are based on their prior conceptual understanding and are tied to some empirical understanding of the experiment in an intelligible way, for example in this sequence during the course lab:
Mia: “Because the salt [NaNO 3 ] has a very low entropy as there aren’t many ways it can exist in because it is very solid. While dissolved in the water it'd get many more…like ways it can be in. It is much more probable that it would like to break free and then what is formed…It is also an endothermic reaction so there has to be…a difference in entropy if it is going to spontaneously occur…or else you'd have to provide more energy. And I'd say that we don't do that.”
Here they argue that the observed reaction is spontaneous on the basis of a change in entropy. They justify this by arguing that a non-spontaneous reaction would require them to supply the reaction with energy somehow and that this is not the case here. Additionally, they know that the entropy is lower for the salt when in solid state than when it is dissolved in water and that this would be the reason for the change in entropy, which makes it possible for the endothermic reaction to be spontaneous. The concept of entropy seems to be associated to different configurations or “ways” the salt can be in, i.e. they connect the concept of entropy to microstates.
As T1 do not have access to the IR cameras they lack the perceptual cuing that IR cameras provide and thus instead have to rely on what they know from before. While the sustained reasoning of IR1 and IR2 in the course lab mainly focuses on a macroscopic level, involving the rate of heat flow, heat conduction and heat transfer, the sustained reasoning of T1 mainly concerns the submicroscopic level, e.g. chemical bonds forming, ions and the “ways” that the salt could be in. This does not mean that the lack of IR cameras exclusively leads to reasoning about the submicroscopic level (as the T-pairs’ reasoning also involves the macroscopic level). Rather, students are not cued towards the macroscopic level.
The sustained reasoning of T2 during the course lab is initiated by a question from the researcher and includes a conclusion about the type of reaction that they encountered (i.e. responsive communication). However, in contrast to T1, the reasoning of T2 is indecisive or incoherent: during the course lab, when asked whether the endothermic reaction is spontaneous or not, and if the reaction would occur even without using the magnetic stirrer, the students respond with:
Oscar: “The molecular charge of the water that does things…it is like, the molecular charges. Like, it is negative at the oxygen and positive at the hydrogen atoms and that makes the reaction spontaneous.”
The students are indecisive and have a difficult time formulating their arguments. The concepts that they use are quite broad and unexplained, i.e. the reasoning is incoherent (see Table 1). Additionally, they unsuccessfully attempt to employ some knowledge that concerns a submicroscopic level (molecular charges).
The IR camera gives access to spatial observations, both in the solutions and in the surroundings. One such possible observation that all student identified, with or without cues from the researcher, was the thermal convection resulting from the exothermic reaction between sodium hydroxide and water in the plastic cup during the modified lab (see Fig. 1).
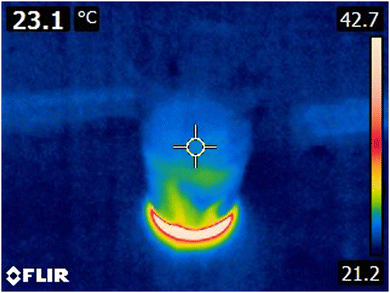 | ||
| Fig. 1 IR image of thermal convection in a (non-stirred) solution containing water and sodium hydroxide (notice the rising heat streaks due to convection). | ||
When observing the sodium hydroxide reacting with the water in a thin plastic cup during the modified lab, one of the students from IR1 remarks on the thermal convection observable with the IR camera and compares it with the observation of sodium nitrate reacting endothermically with water in which no convection strings are visible:
Ike: “You see that there are…strings of heat.”
[…]
Ike: “One did not see that with the cold [solution] it was only that…that it originated from cold. Almost all of the water is yellow soon.”
A similar spatial observation is made by T1 when having access to the IR camera during the modified lab:
Mist: “But it is rising.”
Mia: “If you look at the…sodium hydroxide, it is kind of rising. Considerably more.”
Researcher: “What is rising?”
Mist: “The heat.”
Mia: “The heat.”
Mia: “While that [referring to the endothermic reaction] doesn't really do that as it is cold water, it sinks to the bottom, so it is ehm water of 4 degrees Celsius that has the highest density so the cold water should sink so it should not rise without stirring some while doing it.”
Here T1 attend to the spatiality of the lines of convection, just like IR1. Their reasoning here is based on how the density of water changes with temperature. As the sodium hydroxide reacts exothermically with the water and forms a solution it expands and rises, i.e. the convection observed through the IR camera. The pairs’ practice is continuous and their reasoning is coherent and relevant (see Table 1).
As we see here, the students’ use of the IR cameras and what they choose to do affect what they find and talk about. As such, there is an exchange between what they do and what they talk about: the practice leads to a focus on some aspects that are associated to concepts that the students communicate to each other: looking at the side of the cup (through the IR camera) leads them to think of and communicate that there is thermal convection (strings of heat). The new observation leads to another statement, “But it is rising”, and a clarification, “If you look at the…sodium hydroxide, it is kind of rising”, which leads to the conclusion that it is heat that is rising.
Note that IR cameras provide macroscopic information and stimulate reasoning about the macroscopic level. This risks leading to students neglecting the submicroscopic aspects of the phenomenon. It is important to consider multiple levels of representation in the disciplines of science. This was one of the reasons that Johnstone (2000) proposed the triangle: to emphasize that the levels complement each other and are equally important.
IR cameras stimulate sustained interaction with the experiment and self-initiated discussions
When investigating with the help of IR cameras, IR1 and IR2 are continuously focused on the experiments, both during the course lab and the modified lab. The camera is constantly aimed at the experiments. In other words, their practice is continuous throughout the lab.During the course lab, all pairs’ reasoning focused on measurement, conclusion and explanation to some extent. However, the reasoning of IR1 and IR2 resulted from some exploration, i.e. self-initiated investigations based on some observation or hypothesis:
Rolf: “Shall we look at the…surface there?” [refers to the top of the cup]
Soren: “Yes [removes some of the aluminium foil covering the top of the cup, and looks at the surface of the solution with the IR camera]. The colour on the camera is quite dark which means that the temperature is low…or not that low…”
Rolf: “If you look to the right here… [points at the lower part of the scale on the display of the camera] the lowest is 18.”
Some measurements are stated and the students finally arrive at the conclusion that the reaction is endothermic.
A novel finding from the exploration, and following discussion, may lead the students to new findings that lead to new proposals on how to interact with the experiment and so on through the continuous interaction with the experiment that the access to IR cameras partly seems to result in:
Ike: “But we can do this [folds up the aluminium foil]… you should be able to see… the Styrofoam should become warm, right?”
Jill: “No, maybe it won't.”
Ike: “Look at it [with an IR camera].”
Jill: “We'll see. Or else we'll just have to carry out another experiment without the foil too.”
Ike: “Yes but…”
Jill: “It looks like the magnetic stirrer is warm…”
Ike: “Yes.”
Jill: “If you look at it like this.”
Ike: “But if you let it calibrate on the cup…”
Jill: “It seems to be quite cold here.”
Ike: “Yes, no, we will have to remove the aluminium to get a result there.”
The students test the possibility of observing a transfer of heat from the content of the calorimeter to the outside (through the walls of the calorimeter) and discover that it looks “cold” (close to room temperature). This leads them to take the decision of modifying the experiment to be more suitable for observations with IR cameras (in other words to make it possible to observe the result of a transfer of heat from and to the exothermic and endothermic reactions). Additionally, they make another discovery: there is a transfer of heat from the magnetic stirrer to the surroundings (possibly from the motor).
The subsequent attempt with a modified experiment (no foil) leads the students to discover that the thermometer and IR camera show different temperatures which is discussed in terms of how (a constant difference between the types of equipment) and why they differ (surface versus bulk temperature).
The sustained interaction thus generates multiple points of observation that lead to self-initiated discussions about how to modify the set-up, possible explanations for findings and instructive advice on how to use the IR cameras.
In contrast, the more talkative of the two pairs without IR cameras, T1, almost exclusively respond to questions posed by the researcher or the lab teacher whenever they are not stating the measurements gained from the thermometer. An even more apparent responsive type of communication and intermittent type of practice could be observed from the recordings of the other pair without IR cameras (T2): they often stand silent at the side of the fume hood waiting for the temperature change but respond to questions posed by the researcher.
As they do not engage closely in observation, T1's discussions during the course lab are mainly connected to questions posed by the researcher or teacher and are not centred around their own findings through exploration. In fact, a majority of the more sustained reasoning during the course lab is initiated through questions from the researcher or lab teacher (and about half of all reasoning in total during the course lab), and the lines of reasoning are not initiated from the students’ observation of the experiment (this type of communication is labelled as responsive in Table 1). Overall, they use the experiments to confirm temperature increases and decreases, respectively, but do not engage in the process of the reactions.
From the results above, we also notice a pattern in the students’ communication: while the T-pairs’ sustained reasoning (and about half of all the reasoning in total) tends to be responses to the lab instructor's or researcher's questions (waiting in silence for some measurement between these instances), the IR-pairs most often initiate the communication on their own. This self-initiated communication of the IR-pairs can for example be a result of a proposal by one of the students on how to use the equipment, which leads them to agree that they have to make several attempts, as seen in the following case:
Ike: “But if you let it [the IR camera] calibrate on the cup…”
Jill: “It seems to be quite cold here”
Ike: “Yes, no, we will have to remove the aluminium to get a result there. Should we say that we do that for this attempt?”
Jill: “I believe we should”
This then leads them to initiate a new line of reasoning about how to interact with the experiment and subsequently about the measured temperature. The IR-pairs have access to two modes of measurement, from the IR camera and a thermometer, so they receive more input in terms of perceptual cues than the T-pairs, i.e. they have more findings and potential interactions that they can discuss. Additionally, the IR camera provides multiple sources of information (a 2D screen that shows a full image of points of measurement shown as colours in addition to numerical values of the temperature). However, the communication of the IR-pairs shifted to responsive when they moved on to the modified lab:
Researcher: “What type of phenomena will we observe here?”
Ike: “One reaction is exothermic so it will become warm and the other is endothermic so it will become cold.”
Researcher: “Yes, is there anything else we will observe?”
Ike: “Perhaps transfer of heat from the cup to the table?”
The modified lab is structured through POE and not as integral to the course as the course lab, which seems to lead the students to interpret the situation to be more interview-like.
Access to two modes of measurements promotes thinking of experimental limitations/uncertainty
During the modified lab, the students are asked about the purpose of the lab and what they learnt during the lab: the students who used IR cameras are asked the questions at the end of the modified lab and the T-pairs are asked the questions before getting access to IR cameras. As such, this gives a contrasting case about what students thought about the purpose of the lab with, or without, IR cameras.There is a difference between the T-pairs and the IR-pairs in that the responses of the T-pairs are focused on the calculation of the solution enthalpy and the IR-pairs include more practice-oriented responses about limitations, errors, uncertainty and the IR cameras.
For example, IR2 explain how it was possible to find sources of error with the IR camera:
Rolf: “But then it was possible to see multiple sources of error. And that was possible to see through the IR camera, for example that the cup became warm. It showed that the cup conducted heat and then that the magnetic stirrer was warm and that shows that it can also affect the temperature of the cup and therefore the temperature of the content.”
IR1 reason in a similar way:
Jill: “Yes, actually. It was…it felt like you could see…I mean you saw quite clearly how the heat…I mean this…because…we said that you could see that there were multiple sources of error where the heat, this thing with the cup…”
Ike: “Exactly!”
Jill: “That the cup for example was not as closed as you would have thought and hoped for. And the same with this magnetic stirrer that it released heat, that could have affected quite a lot if…or at least it could have affected quite a lot if you wanted accurate values.”
Additionally, both IR-pairs mention the difference in measurement between the IR camera and thermometer and how the IR camera measures the temperature of surfaces:
Soren: “Like the same thing and like the difference if you use IR camera or thermometer. Because you get different temperatures. They are different, because the IR camera shows the temperature of the surface if you for example would take the water and meanwhile the thermometer measures the temperature of the water itself.”
The IR-pairs have had access to more perceptual cues during their lab work as they had both thermometer and IR camera. These two instruments give two types of information: one point of measure versus multiple points of measure and bulk temperature versus temperature of surfaces. As shown, the students are aware of this and had an intention of not only measuring the temperatures but also to compare the instruments, which, together with the observations of the heat transfers, led them to reason about potential errors of measurement. The thermometer is in this case used as a point of reference in regards to how the IR camera is used, i.e. the students’ reasoning about errors is in relation to the measurements of the thermometer (which is the instrument instructed to use for the lab).
The fact that the IR cameras provide a set of measurement points and thus access to spatiality in measurement, led the students to reason about errors in the experiment itself in terms of processes, such as how transfer of heat from the magnetic stirrer could affect the outcome of the experiment. So, not only did they investigate and reason about errors and measurement during the lab, they seem to have integrated this in how they thought of the lab on a practical level too (as seen from the responses to the questions about what they have learnt and what the purpose of the lab is).
Summary of the results
Reviewing our research question, “How does access to IR cameras affect students’ reasoning, practice and communication during a calorimetry lab?”, we can summarize our results according to these categories (Table 1). Our results show that given enough conceptual understanding, students may use the IR cameras in a continuous way towards the purpose of the lab, both within already set course instructions (the course lab) and instructions adapted for the IR cameras (the modified lab). With the cameras, they may find novel and relevant observations, indicated by the continuous focus with access to IR cameras.The results also show that with access to IR cameras the students’ reasoning had a predominantly macroscopic focus, on aspects like heat losses to the surrounding, and they engaged in a continuous practice. This stands in contrast to the reasoning of the pairs who did not use IR cameras during the course lab, which also involved submicroscopic reasoning, and an intermittent practice. It should be noted that none of the students relate to the representational level of symbols and formulae in their activities (this includes both the course lab and the sequence on POE during the modified lab).
We would also like to point out the differences in the communicative patterns. The students who used IR cameras during the course lab engaged in self-initiated communication, but changed to responsive communication driven by questions from the researcher during the modified lab. The students who used thermometers during the course lab never came to engage in self-initiated communication.
Discussion and conclusion
The research question for this study was to find out how the short-term access (one lab) to IR cameras during a chemistry lab activity would affect the participation of the students, which has been described through three aspects, i.e. students’ communication, practice and reasoning.The results show that coherent and relevant reasoning can be achieved without an IR camera, if students – as in the case of T2 – have sufficient understanding of the topic. However, the cameras do change the focus of the reasoning and allows for a more investigative and active practice. The communication, in turn, seems to be affected by both the students’ practice (if they take initiative to investigate something on their own) and the context of instruction (how the situation is framed by the students and the structure of the instructions). These themes can be tied to the concepts of conceptual and epistemological framing (see Fig. 2).
Types of framing: conceptual versus epistemological
Our results can be related to two of the previously mentioned types of framing: epistemological and conceptual framing. In our interpretation, epistemological framing concerns how one perceives a situation in terms of how to gather and share information (i.e. how we know, or epistemology). Conceptual framing is about what information is perceived as relevant (i.e. employed knowledge or concepts in the situation and how pieces of knowledge relate to each other). These are similar but not identical to the definitions described in previous work (cf.Haglund et al., 2015; van de Sande and Greeno, 2012).We construe students’ communication and practice as reflecting their epistemological framing and the type of content and quality of content, regarding the students’ reasoning, as reflecting their conceptual framing. The students’ conceptual framing has both a content (what knowledge or type of knowledge is deemed relevant) and a quality component (how well does the perceived purpose fit with the prescribed purpose of the lab and is it communicated well enough?), as reasoning relates to both what the reasoning is about and the relevance of the content in addition to how coherent the reasoning is (see Fig. 2). A conceptual framing that has a quality component which includes both coherence and relevance, will henceforth be referred to as productive (and one which lacks either one of the aspects will be referred to as unproductive).
Conceptual framing is reflected in reasoning
Our results indicate that IR cameras have a direct effect on what level of representation learners use (macro or submicro), i.e. the knowledge employed. In this way, IR cameras have an effect on the students’ conceptual framing. However, the access to an IR camera does not necessarily lead to a conceptual framing that is productive.IR cameras direct the content component of conceptual framing toward macroscopic aspects. In contrast to the T-pairs, the IR-pairs had a strong focus on the macroscopic level of the experiment during the course lab. The T-pairs did however shift their conceptual framing in the modified lab so that the content component was the same as that of the IR-pairs, namely the macroscopic level. It could be argued that the shift found in the T-pairs’ conceptual framing relates to the shift in setting, slight modification of equipment or the instructive nature of the activity (formal course instructions are replaced by POE instructions). However, as we see in the data, the IR-pairs kept the conceptual framing at the macroscopic level throughout both the course lab and the modified lab and did not shift their conceptual framing as equipment, room and instructions were modified or altered. In other words, access to IR cameras seems to lead to the shift and specific conceptual framing found in the course lab for the IR-pairs and the modified lab for the IR-pairs and T1 (T2 focused on the macroscopic level but did not reason productively about the phenomena). The overall performance of T2 may be an indication of the students’ lack of relevant knowledge, both in terms of the macroscopic and submicroscopic level (as seen in the data of the course lab).
We suggest that the strong steering effect of IR cameras towards the macroscopic level is a result of perceptual cues provided by the IR camera. The IR camera provides a specific set of perceptual cues, namely the colours, the numbers and the form of the tool itself (Samuelsson et al., 2019b; Samuelsson, 2020). These perceptual cues seem to both pull users’ associations to the macroscopic level and suppress associations to the submicroscopic level. Additionally, during the modified lab, the macroscopic focus is reinforced by the modifications to the experiment (thinner cup, no stirring, etc.) that makes the experiment even more apt for the IR camera (i.e. more perceptual cues through the camera).
In addition, we argue that conceptual framing has a quality of content component (productive/unproductive) apart from the type of content component (submicro/macro). Students employ the knowledge they believe to be most relevant for a situation based on their interpretation of the cues that they attend to in the situation. However, as we see in the results, a focus on the macroscopic or submicroscopic level alone does not tell us whether the conceptual framing is productive or not: despite the fact that both T-pairs employ the same type of content in their reasoning throughout the course lab, they differed in that the reasoning of T1 was more elaborate and more coherent than the reasoning of T2, i.e. the conceptual framing was more productive for T1. The quality is an important component of conceptual framing as it differentiates between students that have sufficient knowledge to deal with the activity and students that struggle with it. A pair like T2 may at first glance look like they know what they are doing when they are employing concepts in their reasoning about the phenomena. However, they may just as well be saying words that do not have any meaning to them (more than that they remember that these are words associated with the broader context of the activity), this is why it is important to also look at the coherence and how elaborate the reasoning is.
In our data set, the quality component of the students’ conceptual framing is unaffected by the use of IR cameras. The T-pairs keep the same quality of reasoning after getting access to the IR cameras. The IR-students productively conceptually framed both labs to be about the macroscopic level while the T-pairs only conceptually framed the modified lab to be about the macroscopic level. Even so, T1 was productive during the course lab. In other words, the type of content employed in reasoning alone does not tell us whether students’ conceptual framing will lead to progress in laboratory work.
Furthermore, the additional access of IR cameras expanded the students’ range of conceptual framing to include discussions about experimental uncertainties. While the T-pairs focused on measurement, the IR-pairs were more explicit with their discussions about limitations and error. This could possibly be due to the students “seeing” the transfer of heat to the surroundings, which makes them question the idealized model, or due to the fact that they are given two sources of measurement (the thermometer and the IR camera) that they can compare.
Epistemological framing is reflected in practice and communication
Our results indicate that IR cameras have a direct effect on the students’ epistemological framing, as the IR cameras influence what they perceive as a relevant way of gathering information. However, even though the IR-pairs had constant access to the IR cameras, they shifted their style of communication between the course lab and the modified lab. This indicates that some other difference between the two situations had an effect on the students’ epistemological framing as it also involves ways of sharing information, i.e. their communication.As a parallel to the explanation of differences in conceptual framing, we suggest that the fact that IR cameras provide additional (explicit) perceptual cues may also explain differences in epistemological framing. By moving the camera around the experiment, the colour pattern on the display changes according to how the experiment thermally develops: directing the camera to the stirrer reveals a red spot which leads the students to investigate the stirrer; removing the aluminium foil from the calorimeter reveals new colours that lead the students to investigate the inner surface of the water and cup, etc.: again, engagement with the IR camera leads to more possible perceptual cues, which leads to more engagement (the students are more directly prompted to move around). Engaging with the IR cameras could thus be said to lead to a self-feeding cycle that affects the students’ epistemological framing.
We further suggest that epistemological framing has a communication component (self-initiated vs. responsive communication) and a practice component (continuous vs. intermittent engagement). In other words, how students perceive an activity in terms of what to do, i.e. their epistemological framing, can be characterized by how they communicate with each other and their practice. A pair of students that does not engage in constructive discussions during their experimental work may be able to finish the task in a satisfactory way but they also miss opportunities in which they can test and share their ideas, connect learnt concepts to experience and elaborate on what they know. The same goes for students that do not engage more with the experiment than what is necessary for the required measurements prescribed in the written instructions: each engagement with the experiment may generate new perceptual cues that lead to more discussions and further engagement.
Furthermore, the epistemological framing interacts with conceptual framing. The cycle of the epistemological framing affects the students’ conceptual framing as more perceptual cues lead to additional points for discussion that direct the content component of the conceptual framing toward the macroscopic level (and limitations of the experiment), which leads to the students investigating for additional cues that involve the macroscopic level (and limitations of the experiment).
We note, however, that the fact that the modified lab was more interview-like is likely to have contributed to the shift in the students’ epistemological framing: an interview situation would prompt a more responsive type of communication (e.g. we are supposed to answer the questions of the interviewer). A similar result was found in a previous study (Samuelsson et al., 2019b) on students working in an identical type of chemistry lab on calorimetry in which the students shifted from exploratory to cumulative talk when shifting instructions from the course lab to a modified lab. In that study, the communication was less investigative when students observed the phenomenon in a setting less integrated in the course lab (and with modified instructions) than when observing it during the regular course lab, as an IR camera was used to confirm one of several alternative hypotheses. Again, the situation may be epistemologically framed by the students as a research interview that is led by the interviewing researcher, with limited room for investigation.
Implications for the teaching practice
Teachers may encounter a wide variety of students in their classroom, whether it be during lab work or during a seminar. Some students will always have a broader understanding of the subject at hand than other students. One of the challenges for the teacher is to individualize the instruction, and take as many students as possible into account when designing a teaching sequence. This is a difficult task but the results from our study show that there are ways of adding openness that may make the lab flexible in its instructive structure and thus make it possible to adapt the lab to different students. These added activities, or parallel instructions, can be thought of as layers: even if one layer of instructions is highly structured, another layer of less guided instructions (but that aligns with the same overall purpose) could be added to a lab.By giving some of the students access to IR cameras and allowing them to choose when to use them, another layer was added to the already somewhat open-ended instructions for the course lab that our students participated in. In such a way, the students had one layer of tasks that they could fall back to when they wanted to work with a more organized task and a completely open-ended layer that they could put effort in when they had time left from the regular tasks. In that way, the students were also in charge of how and when to spend time with each layer of instruction. This could contribute to a more complex way of describing inquiry or openness of a lab, like an extra layer of activity added to the structure proposed by Buck et al. (2008): for example, while a problem and a procedure may be provided in the first layer of instruction, a second layer may omit both of these characteristics. So, in our case (in the course lab), the students were provided with a problem (dissolve salts in water and study the enthalpy of solution) and procedures for doing this but the decision on how and when to use the IR cameras (the second layer) was left open for the students to decide on.
Each layer would also have the potential of highlighting some part of the overall purpose that sometimes may be overlooked by the students (or that they may be unaware of). By having an additional activity that focuses on one or two aspects of the overall purpose (like heat capacity and heat transfer), it can be easier to discern those aspects of the purpose for the students. For example, by providing T1 with an additional layer of instruction (i.e. using IR cameras) their sustained reasoning may also have included a broader discussion on the macroscopic level that then could have enrichened their understanding of the submicroscopic level. However, there is also a risk that they would have abandoned the submicroscopic perspective altogether.
Although both one T-pair and the IR-pairs reasoned in a coherent and elaborate way during the course lab, they differed in their conceptual framing. There is thus potential for improvement if the teacher wants the students to progress their understanding further and align the framings of the students. Since the macroscopic perceptual cues of the IR cameras are so strong, one option could be to stimulate students’ submicroscopic explanation of phenomena before IR cameras are introduced. Furthermore, connecting knowledge about the macroscopic level to knowledge about the submicroscopic level with the support of the teacher can be done through whole-class discussions: Becker et al. (2015) show that whole-class discussions can be fruitful for connecting the macroscopic, submicroscopic and symbolic ideas as the instructor could act as a facilitator by posing guiding questions to the students. This could be an additional design to consider in a lab, i.e. to specifically design the last part of the lab with a certain productive “pattern of instructor facilitation” (Becker et al., 2015, p. 783) in mind.
Our data show that it is possible to design a flexible openness or inquiry in labs (semi-open lab). Some students, like T2, will have to practice on the basic skills in the lab (e.g. how to gather data, recording the data and how to relate basic concepts to observations) and others (like IR1 and T1) will have sufficient knowledge about this to go further and “pursue solutions to problems which interest them” (Tobin, 1990, p. 414). Both these types of epistemological framings are important, they are both steps of the same staircase. However, it may seem difficult for the designer of a lab to fulfil the needs for both of these pairs, but a semi-open lab may do just that.
Our study may contribute to the discussion on the laboratory instruction and the role of laboratory in science education that Hofstein and Lunetta (1982) initiated. By offering additional “layers” of instructions within a lab, it could be possible to integrate the potential of a wider range of instructions in one laboratory activity.
Our study shows that it is possible to identify the needs of the students by looking at how they frame the activity, and that, through semi-open lab instructions, it is possible to fulfil the needs for students that have a stronger conceptual understanding of the phenomena in focus for the lab.
Conflicts of interest
There are no conflict of interest to declare.Acknowledgements
The authors would like to thank all the participants as well as the course instructors that allowed us to collect data in their lab classes. Additionally, we would like to thank the reviewers for their comments. This work was supported by funding from Vetenskapsrådet (project VR 2016-04113), with additional support from the Centre for Discipline-Based Education Research in Mathematics, Engineering, Science and Technology at Uppsala University.Notes and references
- Andersson J. and Enghag M., (2017), The relation between students’ communicative moves during laboratory work in physics and outcomes of their actions, Int. J. Sci. Educ., 39(2), 158–180.
- Becker N., Stanford C., Towns M. and Cole R., (2015), Translating across macroscopic, sub-microscopic, and symbolic levels: The role of instructor facilitation in an inquiry-oriented physical chemistry class, Chem. Educ. Res. Pract., 16(4), 769–785.
- Bohrmann-Linde C. and Kleefeld S., (2019), Can you see the heat? – Using a thermal imaging camera in the chemistry classroom, World J. Chem. Educ., 7(2), 179–184.
- Brookes D. T. and Etkina E., (2015), The importance of language in students’ reasoning about heat in thermodynamic processes, Int. J. Sci. Educ., 37(5–6), 759–779.
- Bruner J. S., (1961), The art of discovery, Harv. Educ. Rev., 31, 21–32.
- Buck L. B., Bretz S. L. and Towns M. H., (2008), Characterizing the level of inquiry in the undergraduate laboratory, J. Coll. Sci. Teach., 38(1), 52–58.
- Deslauriers L., Mccarty L. S., Miller K., Callaghan K. and Kestin G., (2019), Measuring actual learning versus feeling of learning in response to being actively engaged in the classroom, Proc. Natl. Acad. Sci. U. S. A., 116(39), 19251–19257.
- Deslauriers L., Schelew E. and Wieman C., (2011), Improved learning in a large-enrollment physics class, Science, 332, 862–865.
- Erickson, G., (1979), Children's conceptions of heat and temperature, Sci. Educ., 63(2), 221–230.
- Green T. C., Gresh R. H., Cochran D. A., Crobar K. A., Blass P. M., Ostrowski A. D., Campbell D. J., Xie C. and Torelli A. T., (2020), Invisibility cloaks and hot reactions: Applying infrared thermography in the chemistry education laboratory, J. Chem. Educ., 97(3), 710–718.
- Haglund J., Jeppsson F., Hedberg D. and Schönborn K. J., (2015), Students’ framing of laboratory exercises using infrared cameras, Phys. Rev. Phys. Educ. Res., 11(2), 1–22.
- Haglund J., Jeppsson F. and Schönborn K. J., (2016), Taking on the heat—a narrative account of how infrared cameras invite instant inquiry, Res. Sci. Educ., 46(5), 685–713.
- Hammer D., (2000), Student resources for learning introductory physics, Am. J. Phys., 68(S1), S52–S59.
- Hammer D., Elby A., Scherr R. E. and Redish E. F., (2004), Resources, framing, and transfer, in Mestre J. (ed.), Transfer of Learning: Research and Perspectives, Information Age Publishing, pp. 1–26.
- Hmelo-Silver C. E., Duncan R. G. and Chinn C. A., (2007), Scaffolding and achievement in problem-based and inquiry learning: A response to Kirschner, Sweller, and Clark, (2006), Educ. Psychol., 42(2), 99–107.
- Hofstein A. and Lunetta V. N., (1982), The role of the laboratory in science teaching: Neglected aspects of research, Rev. Educ. Res., 52(2), 201–217.
- Jewitt C., Bezemer J. and O’Halloran K., (2016), Introducing Multimodality. Routledge.
- Johnstone A., (2000), Teaching of chemistry – Logical or psychological? Chem. Educ. Res. Pract., 1(1), 9–15.
- Katchevich D., Hofstein A. and Mamlok-Naaman R., (2013), Argumentation in the chemistry laboratory: Inquiry and confirmatory experiments, Res. Sci. Educ., 43, 317–345.
- Kirschner P. A., Sweller J. and Clark R. E., (2006), Why minimal guidance during instruction does not work: An analysis of the failure of constructivist, discovery, problem-based, experiential, and inquiry-based teaching, Educ. Psychol., 41(2), 87–98.
- Klahr D. and Nigam M., (2004), The Equivalence of Learning Paths in Early Science Instruction: Effects of Direct Instruction and Discovery Learning, Psychol. Sci., 15(10), 661–667.
- Papert S., (1980), Mindstorms: Children, Computers and Powerful Ideas, Basic Books.
- Petritis S. J., Kelley C. and Talanquer V., (2021), Exploring the impact of the framing of a laboratory experiment on the nature of student argumentation, Chem. Educ. Res. Pract., 22, 105–121.
- Piaget J., (1971), The Child's Conception of the World, Routledge.
- Redish E., (2003), A theoretical framework for physics education research: Modeling student thinking, Proc. Int. Sch. Phys. “Enrico Fermi” Course CLVI Res. Phys. Educ., 156, 1–56.
- Samuelsson R., (2020), Reasoning with thermal cameras settings in higher education, Uppsala University.
- Samuelsson C. R., Elmgren M., Xie C. and Haglund J., (2019a), Going through a phase: Infrared cameras in a teaching sequence on evaporation and condensation, Am. J. Phys., 87(7), 577–582.
- Samuelsson C. R., Elmgren M. and Haglund J., (2019b), Hot vision: Affordances of infrared cameras in investigating thermal phenomena, Des. Learn., 11(1), 1–15.
- Sweller J., (2003), Evolution of human cognitive architecture, in Ross B. (ed.), The Psychology of Learning and Motivation, vol. 43, Academic, pp. 215–266.
- Tobin K., (1990), Research on science laboratory activities: In pursuit of better questions and answers to improve learning, Sch. Sci. Math., 90, 403–418.
- van de Sande C. C. and Greeno J. G., (2012), Achieving alignment of perspectival framings in problem-solving discourse, J. Learn. Sci., 21(1), 1–44.
- White R. and Gunstone R., (1992), Probing Understanding, Falmer Press.
- Wieman C. E., (2014), Large-scale comparison of science teaching methods sends clear message, Proc. Natl. Acad. Sci. U. S. A., 111(23), 8319–8320.
- Wong C. P. and Subramaniam R., (2019), Exploring thermal effects and behaviors of chemical substances using an infrared camera, J. Chem. Educ., 96(10), 2339–2344.
- Xie C., (2011), Visualizing chemistry with infrared imaging, J. Chem. Educ., 88(7), 881–885.
- Xu X., Wu M. and Wang X., (2019), Smartphone visualization of thermal phenomena with thermal imaging accessories, J. Chem. Educ., 96(11), 2545–2552.
| This journal is © The Royal Society of Chemistry 2023 |