Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A social semiotic lens to capture meaning-making of polymeric concepts during modelling in chemistry education
Lizette
Widing
 *a,
Pernilla
Nilsson
*a,
Pernilla
Nilsson
 a and
Pernilla
Granklint Enochson
a and
Pernilla
Granklint Enochson
 b
b
aHalmstad University, Sweden. E-mail: lizette.widing@hh.se; pernilla.nilsson@hh.se
bHalmstad and Malmö University, Sweden. E-mail: pernilla.granklint-enochson@mau.se
First published on 16th January 2023
Abstract
This study investigated students’ meaning-making of polymeric concepts during modelling and discuss students’ creation of visible representations in chemistry. The analysis combines a phenomenographic and social semiotic approach and leads to the finding and description of 21 different meaning-making processes. We refer to meaning-making as the outcome of translative communication through representations, discerned by students, where the collective meaning of created representations that build on each other constitutes the meaning as a whole. The study took place in three Swedish upper secondary chemistry classes. Data were collected from eight groups of 3–4 students (n = 30). Video, audio recordings and photos taken during modelling were analysed to investigate students’ meaning-making during the modelling process. The results show translative changes between and within semiotic resources, indicating meaning for students’ learning of polymeric concepts. Additionally, the representations produced during modelling were essential resources connecting the submicro and macro levels by creating a ‘bridge’ between levels. The results show that the modelling activities practised by all groups were multimodal. The study acknowledges that teachers can use the social semiotic lens as a tool to evaluate students’ modelling in addition to the importance of translative processes during modelling.
Introduction
It is well documented that learning science, especially chemistry, is experienced as difficult by many students (Osborne et al., 2003; Bennett and Hogarth, 2009; Gilbert and Justi, 2016). One way to increase students’ positive attitudes towards science can, according to Osborne et al. (2003), be conducting classroom activities to engage students to discuss and contextualise content in terms of their own experience and knowledge. In this study, students’ participation in modelling activities, i.e., creating visual representations, is considered a student-active approach within the context of chemistry education. Despite recognising the importance of student active approaches to science, studies have indicated (Duschl and Osborne, 2002; Mortimer et al., 2003; Berland and Reiser, 2009) that these kinds of activities are rare in science classrooms. It might be assumed that chemistry education lacks tools that teachers can use to evaluate and analyse the modelling process during the teaching context. This study contributes to the existing literature by investigating and discussing a social semiotic perspective's contribution to students’ meaning-making of polymeric concepts during modelling. We define meaning-making in line with Tang et al. (2014), where meaning is made through multiple representations of polymeric concepts where each representation forms a part of a sequence and where the sequences form a meaning as a whole. In agreement with Marton (2015), we also consider meaning-making when students discern and highlight aspects in representations and explain how these representations are sequenced and built on each other. According to Kress (2009) and Kress and Bezemer (2015), social semiotics offers a perspective for investigating meaning-making through socially shaped cultural resources used in communication in multimodal contexts.In chemistry education, there is a distinction between model-based teaching and modelling-based teaching, MBT. Model-based teaching concerns how students use existing models, while modelling-based teaching is an educational process of creating, testing, and communicating models (Gilbert and Justi, 2016). There is not one way to create models. However, other scholars have discussed the general steps from which models are created (e.g., Clement, 2000; Justi and Gilbert, 2002; Gilbert and Justi, 2016). In this study, the Model of Modelling v2 (version 2), suggested by Gilbert and Justi (2016), has been a central framework when planning and conducting modelling activities.
In this study, we refer to models created by students as visual representations. Visual representations are here understood as representations of concepts, objects, processes, and ideas made visible by semiotic resources. Visual representations can be expressed in text, mathematical formulas, sketches, drawings, diagrams, graphs, photographs, physical objects, animations, simulations and many more ways (Lemke, 1998). The use and creation of visual representations are essential when learning the abstract and the invisible since visual representations can mediate between the learner and an abstract concept or phenomenon (Jong et al., 1998).
According to, e.g., Johnstone (1991) and Jong and Taber (2014), students may also perceive chemistry as challenging since chemistry is taught at three different organisation levels; the macroscopic level (observable and visible), the submicroscopic level (unobservable atoms and molecules) and the representational level (symbols, concepts and representations used to describe particles existing at the microscopic level and their relationship). To understand chemistry, students need to understand how these levels are connected (Johnstone, 2009), something which is experienced difficult by many students (Harrison and Treagust, 2003; Arroio and Campos Santos, 2016). Understanding how the properties of materials (macro level) depend on the structure and interaction within the material (submicro level) is of great importance but also challenging for many students (Schmidt et al., 2009). Jong and Taber (2014) highlight that difficulties in learning chemistry through representational levels can also depend on students’ lack of knowledge of representations and modelling, which according to Gilbert and Treagust (2009), requires the practice of visualisation. Therefore, it is reasonable to suggest that chemistry education not only needs to present representations and sequences of representations to students but to construct teaching activities to learn about visual representations and the creation of representations, i.e., modelling.
This article investigates if and how the social semiotic perspective can be a valuable tool for teachers to analyse students’ meaning-making of polymeric concepts during modelling. The study builds on the assumption that meaning can be created and extended through translative processes during modelling, namely transductions and transformations (Kress, 2009; Kress and Bezemer, 2015) using semiotic resources.
Aim and research questions
The study investigates students’ semiotic work during modelling activities in an upper secondary chemistry course when learning about polymers. We aim to describe and further discuss students’ meaning-making of polymeric concepts from a social semiotic learning perspective (Kress, 2009; Kress and Bezemer, 2015). The research questions this study intends to answer are:• What social semiotic practices are observed during a modelling activity in a chemistry unit of polymers?
• How do these practices indicate students’ meaning-making of created representations crucial for understanding polymeric concepts?
Theoretical frameworks
This study has combined the frameworks of phenomenography and social semiotics to investigate students’ semiotic practices and meaning-making during a modelling activity in a chemistry unit of polymers. The phenomenographic framework is used to structure learning during modelling-based teaching, guiding the data collection and analysis. The social semiotic framework offers a perspective of how meaning-making is developed in multimodal contexts and is used as an analytical tool to investigate meaning-making during students’ modelling. Both perspectives contribute to aspects of learning relevant to the modelling activity. The phenomenographic and social semiotics approaches have been successfully combined in chemistry education research, e.g., Patron et al. (2021).In this article, we draw on Kroksmark's (1987) derivation on phenomenography: What appears or is manifested in an activity that depicts the phenomenon studied. According to phenomenography, people's thoughts and experiences are not seen as separate entities. Thoughts and experiences can be communicated through representations when learning to discern new things or in new ways (Marton and Booth, 1997). From this perspective, students communicate phenomena by how it is experienced.
Putting phenomenolography in an educational context, the object of learning becomes central. The object of learning refers to ‘What is to be learned’, and handling the object of learning determines the learning possibilities (Marton and Tsui, 2004; Marton, 2015). Marton and Tsui (2004) categorise the object of learning by the intended, enacted and meaning-making. According to Marton (2015), the intended object of learning refers to what critical aspects teachers consider essential and expect students to discern in relation to the curriculum content. A critical aspect can be exemplified through the concept of monomer. The students must discern that a monomer is a molecule, not an atom. Thus, the way students discern and highlight critical aspects while creating sequences of representations during modelling. In this study, the intended object of learning is presented in the Method section. We refer to students undertaking modelling as the enacted object of learning. Marton (2015) emphasises that what is considered a critical aspect depends on particular learners. An aspect can be critical to some but not to everyone, and states it needs to be searched for and found. However, the critical aspects in this article are based on the reasoning of Fredlund et al. (2015) and Lo (2012), stating that by interpreting the intended object of learning, teachers can define some critical aspects of concepts or phenomena being studied.
According to phenomenography, a person has learned something about a phenomenon when a person can be focused and aware of other or more aspects connected to a phenomenon than before the learning situation (Marton and Booth, 1997). From a learning perspective, we must discern critical aspects simultaneously, which is a challenge for the students.
Phenomena are only exposed to us as parts, and the parts are assembled into a whole. Parts that are not directly exposed during the learning activity of an abstract phenomenon may not be experienced by the students. From this perspective, students must know what lies behind the created representation to understand the whole, i.e., to appresent awareness (Marton and Booth, 1997). For example, students need to have a prior understanding of the concept of electronegativity to discuss and create a representation to visualise the polarity of a molecule. From this aspect, appresent awareness refers to the prior understanding and knowledge of chemical concepts. In addition, prior understanding of concepts is assumed to be essential for the students to interpret, recognise and react to created representations. Finally, the phenomenographic perspective highlights primarily what is to be learned, and the social semiotic perspective highlights primarily how learning can be communicated. However, the two perspectives are intertwined in this study. What is to be learned depends on how the students use the potential of produced representations and various semiotic resources to make meaning.
A multimodal social semiotic approach to learning
Social semiotics offers a perspective for investigating how meaning-making is developed in multimodal contexts (Kress, 2009; Kress and Bezemer, 2015). The multimodal context refers to different modes used in communication, such as speech, gestures, text, pictures, animations, and representations that work together to create meaning in a particular situation. According to Kress (2009, p. 79), “Mode is a socially shaped and culturally given semiotic resource for making meaning”. Meaning is created when combining the “messages” provided by all modes; thus, each mode contributes to the overall meaning (Kress and Bezemer, 2015). Within the theory of social semiotics, all modes are given the same priority, meaning that speech and writing are not prioritised but are two resources, among others, for making meaning (Kress, 2001; Leijon and Lindstrand, 2012; Kress and Bezemer, 2015). However, research indicates that discussion among students is an abundant modal resource for meaning-making science content (Chin and Osborne, 2010; Allchin, 2011). Tversky (2019) also states that combining words and images, in addition to visual representations Widing et al. (2022), is the most effective way to communicate.In social semiotics, meaning-making is the outcome of communication and sign-making (Kress, 2009; Kress and Bezemer, 2015). When a student creates sign(s) during the sequencing of representations, meaning can be translated between different modes or within the same mode (Kress, 2009; Kress and Bezemer, 2015). Rearrangement between modes is described as intermodal, i.e., transductions, and within a mode as intramodal, i.e., transformations. A student that listens to his/her teacher and draws a representation of what the teacher is telling (speech to picture) exemplifies a transduction (intermodal). Transformation (intramodal) can be used to explain the process when a student redraws the picture by changing or adding components, constituting a new meaning. Central in social semiotics is that any change, inter- or intramodal and sign-making, leads to meaning-making and constitutes learning (Kress and Bezemer, 2015). We believe the semiotic perspective on learning can be applied to different learning activities involving different modalities. For instance, Danckwardt-Lillieström et al. (2018) used a social semiotic perspective when investigating a drama activity in chemistry where students explored chemical bonding. The results show that the drama activity stimulated different transductions and transformations. Processes that, in interaction with different semiotic resources, influenced students’ meaning-making and exploration of intermolecular forces in new ways (Danckwardt-Lillieström et al., 2018, 2020).
Furthermore, central to social semiotics is that one shapes and constructs one's knowledge (Kress, 2009). Thus, it is not a matter of acquiring knowledge or transferring information from one person to another but a constant work of interpreting and creating understanding based on available resources. A representation can thus illustrate how one understands something in the world and what appears interesting and meaningful due to which aspects are emphasised (Kress, 2009).
This study contributes to the existing literature by applying phenomenographic and social semiotic frameworks to investigate how translative processes (transductions and transformations) indicate students’ meaning-making during modelling-based teaching in chemistry. How do these translative processes indicate students’ meaning-making of sequences of created representations crucial for understanding polymeric concepts.
Models and modelling in chemistry education
Models in chemistry education can be considered simplified representations of an object, process, or phenomenon, explaining an entity (Maia and Justi, 2009). Thus, a model can be used as a tool for thinking with and for making sense of an experience (Passmore et al., 2017).As stated, this article refers to models created by students as visual representations. Visual representations enable chemists to visualise and sequence the invisible. The chemists’ submicro world of atoms, ions and molecules is unobservable and, according to Bucat and Mocerino (2009), only accessible by imagination. Imagination is addressed as a critical component to understand chemistry, and a way to raise students’ chemical understanding is to develop their visualisation abilities (Clement et al., 2008). Another scholar, Tversky (2019), expresses that spatial reasoning, i.e., the ability to think with, process and communicate images, is essential to practice and to develop. In a study, Tversky (2019) showed that students who draw visual explanations instead of writing in words or using numbers showed better results on subsequent tests. However, on its own, the imagined world of the submicro level presents serious challenges for students and teachers (Bucat and Mocerino, 2009).
In chemistry education, students are often expected to deal with all three representational levels simultaneously (Jong and Taber, 2014) instead of focusing on one representational level at a time. Switching perception between the macro and submicro levels is, according to Hussein and Reid (2009), experienced difficult by many students and, according to Gilbert and Treagust (2009), requires the practice of visualisation. The success of learning chemistry involves the production of mental images of chemical phenomena expressed by different modes of representation (Cheng and Gilbert, 2009). According to Tversky (2019), we are helped by images and gestures to think, clarify, and communicate our thoughts. One way for students to understand chemical phenomena at the macro level can be to create models at the submicro level (Oversby, 2000; Harrison and Treagust, 2003). The macro-submicro thinking using property–structure relations is essential when learning chemistry (Justi and Gilbert, 2002). However, according to Meijer et al. (2009), learning to relate macroscopic phenomena to sub-microscopic is difficult and often implies several relations and sequences, so-called intermediate sequences or meso levels. By breaking up the macro-submicro thinking into sequences, students’ cognitive demands could be less stressful (Meijer et al., 2009).
Modelling can provide a teaching activity beyond memorising facts and offers a tool for students to reason and use facts to account for phenomena (Passmore et al., 2017). We consider modelling-based teaching (Gilbert and Justi, 2016) an educational approach where integrating and using knowledge in interaction with different semiotic resources constitute a more comprehensive approach to learning. Tversky (2019) also states that initially, one starts from unclear images, which during the process, are clarified more and more and adapted as a tool of communication. During modelling, the expression of a representation often develops through gradual sequencing from the first representation to the final representation (Gilbert and Justi, 2016). Modelling-based teaching aims to contribute to students’ active involvement in their learning process and enables students to discuss chemistry when creating, questioning, and evaluating representations. Other studies have shown that when students fail to express their chemical understandings verbally, representation can support argumentation (Gilbert and Justi, 2016; Widing et al., 2022).
Method
In Sweden, it is common for chemistry education at the upper secondary level to be conducted in multilingual classroom settings. This study is part of a context in which modelling-based teaching in a multilingual context is investigated to improve second language learners’ descriptions of chemistry concepts; for more information, see Widing et al. (2022). Data were collected from eight multilingual groups. A total of 30 students participated, originating from three upper secondary classes at a Natural Science Program in Sweden. Participating students had studied chemistry for one and a half years at the upper secondary level, equivalent to 2,5 hours a week.Intended object of learning
The modelling activity with polymers was designed in collaboration with three teachers. The curriculum and associated educational objects for learning through the modelling activity were:• The students should learn about different polymerisation processes, such as condensation and radical reactions.
• The students should understand how the properties of materials, i.e., polymers, are related to the structure of polymer chains and chemical bonding.
Students should develop representations of:
• What is a polymer?
• How are polymers formed?
• What is the chemistry behind the properties of polymers?
From a learning perspective, the teachers stated that to develop an understanding of polymers following critical aspects were essential for students to decern:
• The concept of monomer
• The concept of polymer
• The concept of polymerisation
• The concept of radical
• Structure of polymer chains
• Chemical attraction
Learning resources offered to students before the modelling activity
The teachers introduced polymers by conducting a lecture two days before the modelling-based teaching activity, using PowerPoint (to which students had access) and dealing with the critical aspects listed above. Below, we summarise sequences in the lecture that focus on the learning goals.What is a polymer?
Using a picture, the teachers introduced monomer, polymer, and polymerisation concepts. The teachers described polymer as a long chain of monomers and that the process where monomers are joined together is called polymerisation. Fig. 1 illustrates how monomers form a polymer.How are polymers formed?
During the lecture, the students were introduced to two polymerisation processes: condensation reaction and radical polymerisation. Free radical polymerisation was a new process for the students (condensation reaction was not), and the teachers explained the reaction mechanism using an animation downloaded from YouTube (Nouryon, 2019).What is the chemistry behind the properties of polymers?
During the lecture, teachers stated that the properties of polymers (materials) are due to the structure, i.e., amorphous and crystalline. The teachers also highlighted that different strengths in interactions between polymer chains are of great significance for understanding the properties of polymeric materials.Data collection
The modelling activities were distributed on 3–4 occasions of around 50 minutes each, over a week, and were film- and audio recorded. A video camera was placed with each group in a fixed position, and an audio recorder was placed in the middle of the student group. A photo camera was used to document the students’ representations.First, the teachers instructed the students to develop one or several representation(s) to visualise: What is a polymer? How are polymers formed? and What is the chemistry behind the properties of polymers? For 15–20 minutes, students individually considered possible representation(s). Then the students were grouped into groups of 3–4 students. In total, data were collected from eight groups of students. In each group, the students compared and discussed their different ideas. These discussions resulted in some initial ideas being rejected since they were not considered to visualise what was intended. Not rejected representations followed the parts: creation, expression, test, and evaluation by the framework of MBT (Gilbert and Justi, 2016). The students’ created representations of concepts and processes were first presented and developed by the students in the group during the modelling activity. The representation's meaning and form were discussed and altered until a consensus was reached. Secondly, the students presented their representation(s) to the teacher and finally to the whole class. Discussing the representation(s) strengths and limitations were conducted throughout the activity. The students worked independently in their groups with less teacher support during the modelling activity.
Data analysis
Data analysis was composed of several analytical steps. Initially, we started with an overview of the audio-visual material, searching for social semiotic practices, i.e., transductions (students changing between semiotic resources) and transformations (students making rearrangements within a representation) (Kress, 2009) relating to critical aspects of the intended object of learning. The deductive data analysis was composed of audio-visual data, including speech and other events (Heath et al., 2010) and was conducted on one student group at a time. As illustrated in Appendix 1, modelling sequences were documented to provide an overview of each group's translative processes. The next step was to analyse each part in the modelling sequence. The findings were documented in matrixes describing the translative process, created representation, examples from the transcript of speech, organisation level(s), semiotic resources, the enacted and intended objects of learning, critical aspects and meaning-making from students' modelling, as illustrated in Appendix 2. As the focus was on the translative processes illustrating meaning-making, only processes where other semiotic resources than the created representation (e.g., speech and gestures) contributed to meaning-making were selected. The created representations that did not meet this criterion were not included in the analysis. Student discussions were transcribed verbatim. Validity is addressed by the triangulation of several semiotic resources and their contribution to meaning-making during a modelling sequence. In addition, iterative steps in the analytic process were conducted, meaning returning to the data and matrices and recurring discussions about students’ meaning-making until a consensus between the three researchers was reached. Finally, we returned to the data to examine how the findings represented all groups.The submicro level was divided into three subcategories to clarify the organisation level analysis: molecular, macromolecular, and multiparticle. The subcategories were used to clarify the relationship and the organisation levels between created representations. The molecule level refers to small molecules (monomers). The macromolecule level refers to large molecules (polymer chain). The multiparticle level refers to several macromolecules.
The primary forms of validity for knowledge-based research are, according to Newton and Burgess (2008), outcome and process validity. The main action to ensure outcome and process validity has been the critical and reflective dialogue between the three researchers, i.e., the authors. Through the study, the researchers have discussed and reflected upon the implementation of the method, data analysis, and documentation of results. During the analysis, we constantly compared similarities and differences of aspects in the data, whether and how the identified social semiotic practices contributed to students’ meaning-making. To ensure external validity, we have analysed content-rich material forming our results (Robson, 2011). However, the analytical outcomes of the researchers’ analysis must be considered as the researchers’ ways of interpreting how others experience something (Marton and Booth, 1997). In this study, the researchers have various knowledge of chemistry, teaching chemistry and research. The variation of different knowledge can provide strength, such as different perspectives, during the analysis. Although reliability might be a limiting factor in this study, will another group of students constitute the same result? The study can only draw conclusions based on this group of students and does not provide any basis for generalising results to a broader population. Analysed data consists of group discussions, filmed sequences and produced representations. Thus, we can only analyse what these particular students make present to us. We cannot go beyond what is out in the ‘public space’ (Taber, 2015), which might be a limiting factor in this study.
Ethical considerations
This research project follows the ethical guidelines stated by the Swedish Research Council (2017). Before data collection, teachers and students were informed about the purpose of the study and chosen methods. Participating teachers and students (aged 17–20) gave their written consent to be part of the study and film-audio recorded and photographed. No individual personal data is stored, nor are privacy-invasive issues addressed. Ethical guidelines explicitly state that all participants must have the opportunity to approve or decline to participate in research at any time during data collection, of which everyone was informed. All data has been anonymised without distorting the scholarly meaning.Results
A social semiotic lens based on a phenomenographic framework has been used to analyse students’ meaning-making of polymers during modelling to answer our research questions: RQ1: What social semiotic practices are observed during a modelling activity in a chemistry unit of polymers? and RQ2: How do these practices indicate students’ meaning-making of created representations crucial for understanding polymeric concepts?To clarify meaning-making due to translative processes, we have chosen to present students’ modelling processes through examples from two different groups to enable the reader to follow students’ modelling- and translative processes. Results for RQ1 and RQ2 are summarised in Tables 1–3. The result is presented in three sections according to the intended objects of learning, i.e., What is a polymer? How are polymers foamed? and Properties of polymers. The analysis of meaning-making and translative processes shows three identified social semiotic practices in the first section. The second and third sections identified nine social semiotic practices, respectively. In each section, social semiotic practices identified meaning-making relating to critical aspects, and the number of groups has been summarised in Tables 1–3. The social semiotic practices are numbered, and the numbers are reproduced in the text, e.g., (1.2), to clarify the result. The Result section identifies each student by the code (SX), where X represents an individual number of students. To clarify presented comments or discussions (i.e., excerpts), we have inserted a clarification using a straight square [ ]. Finally, all quotations are presented in italics, and to increase clarity in presented dialogues, minor linguistic clarifications have been made, but without changing the content.
| Social semiotic practice (RQ1) | Critical aspect | Meaning-making (RQ2) | Student groups |
|---|---|---|---|
| 1.1 Transduction from picture(s) to physical representation | The concept of monomer | Molecular level | 8 |
| 1.2 Transduction from picture(s) to physical representation | The concept of polymer | Macromolecule level | 8 |
| 1.3 Transformation of representation | Multiparticle level | 8 |
| Social semiotic practice (RQ1) | Critical aspect | Meaning-making (RQ2) | Student groups |
|---|---|---|---|
| 2.1 Transduction from picture(s) to representation | The concept of polymerisation | Monomers connecting | 8 |
| 2.2 Transduction of initial representation | Intra-molecular bonding | 5 | |
| 2.3 Transformation | The same molecule reacting twice | 3 | |
| 2.4 Transformation | Reaction between dipolar molecules | 2 | |
| 2.5. Transduction of initial representation | Water molecules as by-products | 3 | |
| 2.6 Transduction from animation to transformation of representation | The concept radical | Creation of a radical | 4 |
| 2.7 Transduction from animation to stickball-model to transformation of representation | The reaction of a radical | 3 | |
| 2.8 Transduction from notes to stick-ball model to drawing | The concept of electron density | 2 | |
| 2.9 Transduction from animation and gesture to transformation of representation | End of radical reaction | 4 |
| Social semiotic practice (RQ1) | Critical aspect: | Meaning-making (RQ2) | Student groups |
|---|---|---|---|
| 3.1 Transduction from initial representation | Structure of polymer chains | Representation did not show intended | 5 |
| 3.2 Transformation | Amorphous structure | 8 | |
| 3.3 Transformation | Crystalline structure | 8 | |
| 3.4 Transformation | Extensibility | 4 | |
| 3.5 Transformation | Chemical attractions | Chemical attractions due to structure | 7 |
| 3.6 Transformation | Chemical attractions due to length of polymer chains | 2 | |
| 3.7 Transformation | Chemical attractions due to polymeric side chains | 2 | |
| 3.8. Transformation | Inter- and intramolecular attractions | 7 | |
| 3.9. Transformation | Difference in intramolecular attractions | 4 |
Intended object of learning: What is a polymer?
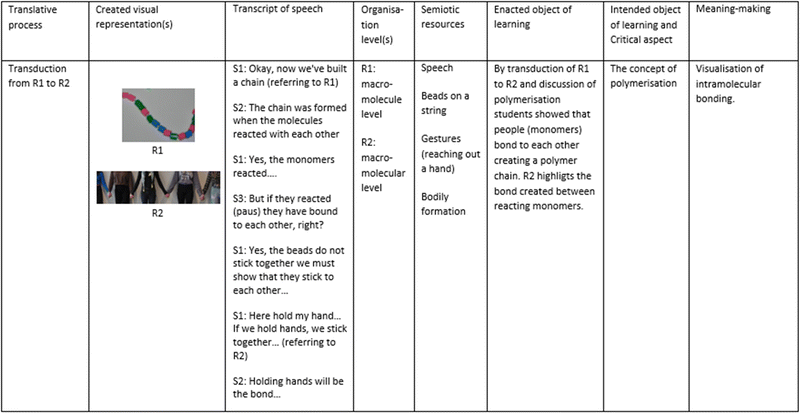
The first section presents the meaning-making of critical aspects of the monomer and polymer concepts. The result is summarised in Table 1.The reported examples illustrate modelling by group 1. To visualise a monomer, the students used different materials like beads, macaroni, grapes, trains, stick-ball representations, and their bodies. In all groups, the concept of monomer was discussed. Students’ discussions clarified that a monomer is a molecule, not just an atom, and a polymer chain consists of many monomers put together (1.1 and 1.2). The students exemplified the concepts of monomer and polymer: “this bead is a monomer, together they make up a chain, a polymer”. A polymer chain was visualised by putting beads or macaroni on a string, grapes were put together with toothpicks, and trains were connected, exemplified in Fig. 2 and 3.
In the modelling process described above, students distinguished between the concepts of a monomer (molecular level) and a polymer chain (macromolecule) level (1.2).
Further, meaning-making and enacted object of learning was registered as a transformative process where all groups transformed created representations to clarify the concept of the polymer at both macromolecular level and multiparticle level. During students’ discussions, the concept of the polymer was discussed from the aspect of the polymer as a double-meaning word, a polymer chain (macromolecule level) and as polymer chains (multiparticle level) within a material (1.3). The clarification of the polymeric concept is exemplified by a group, first creating a representation to visualise the macromolecule level and then a representation to visualise the multiparticle level, as illustrated in Fig. 2.
 | ||
| Fig. 2 Created representations to visualise the concept of polymer at the macromolecule and multiparticle level. (a) Macromolecule level (b) multiparticle level. | ||
Intended object of learning: How are polymers formed?
The second section presents the meaning-making of critical aspects of polymerisation and radical concepts. The result is summarised in Table 2. The reported examples illustrate the modelling process from group 2.The concept of polymerisation
As previously reported visualising the concept of polymerisation was done by putting beads or macaroni on a string, grapes were put together with toothpicks, and trains were connected (2.1). However, in five groups, further transduction(s) were observed. The transductions constituted meaning-making based on the students’ appresent awareness that an intramolecular bonding is created between reacting molecules, exemplified by the modelling process in group 2. (S1) clarifies that the beads on the string “do not stick together”. The first created representations did not visualise the intramolecular bonding formed during the polymerisation, which the students considered essential to visualise, leading to transduction of the initial representation, e.g., beads were exchanged to bodies (monomers). Each student used the other hand to illustrate the chemical bonding between monomers in polymerisation (2.2), illustrated in Fig. 3.While the teachers lectured, the students were presented with two different ways polymerisation could occur; condensation reaction and radical polymerisation. Analysed data show that several groups only discussed condensation reaction and radical polymerisation, not creating representations to visualise the processes. Translative processes were documented in three groups concerning condensation reaction and in four groups radical polymerisation.
Three groups of students discussed the process of condensation reaction as a polymerisation reaction, referring to appresent awareness that an ester synthesis occurs when two molecules react. Nevertheless, the students discussed how only two reacting molecules could make up polymerisation. The groups’ discussions led to the statement that condensation reaction as polymerisation reaction must be a molecule reacting in two ends instead of one (2.3) visualised in Fig. 4. Furthermore, the students also discussed why the reaction happened. Once again, students’ appresent awareness of ester synthesis guided the discussion. (S2) said, “a catalysator creates molecules with polar ends that can attract other polar molecules”. The students thus transformed their representation by visualising the reaction between molecules with two polar groups (2.4), where the same molecule reacts twice to create a polymer chain.
In addition, visualising the condensation reaction process as polymerisation, meaning-making through transduction, was observed as three groups created water molecules from stick-ball representations to visualise water as a by-product during the reaction (2.4).
The concept of radical
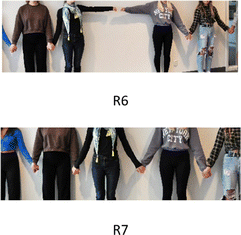
Four groups created representations to visualise the process of radical polymerisation by transduction from the animation presented by the teachers. Two groups used bodily formation, and two groups stick-ball models to visualise the process of radical polymerisation.Meaning-making of the creation of a radical (the initiation process) was observed through transduction from animation to representation(s) and transformations of representations in addition to students’ discussions. The process is exemplified by group 2. Based on the animation, (S2) stated that “a radical is a particle with a single, unpaired electron created by breaking a bond if energy is added”. (S3) said: “A radical can be one arm, making two arms a bonding”. The student referred to appresent awareness of covalent bonding consisting of two electrons (single-bond). Two students held hands to visualise the creation of a radical (2.6), and a third student used gestures to illustrate energy breaking the bond between the atoms. The two students let go of each other's hands, where one arm represents one unpaired electron, a radical visualised in Fig. 5.
When watching the animation, (S2) states that “a radical is a very reactive element”. (S1) asks, “why”? (S2) answers that “it is reactive as it has one unpaired electron and wants to bind to another molecule”. Transduction from the animation to a stick-ball model was observed to clarify the process. (S2) builds a stickball representation of ethylene. Discussing the reaction of a radical (the propagation process) by interacting with the stickball model (S2) showed that the double bonding was broken by a radical and that the former ethylene molecule forms a new radical that continues to react (2.7). Using the stick-ball model during the discussion, illustrated in excerpts 1 and 2, led to a transformation of representation, illustrated in Fig. 6.
Excerpt 1:
(S2): If we hold hands and you send UV-light, then I become a radical … and… I have to bond with someone.
(S1): It bonds so that there are two electrons again?
(S2): Yes, it bonds and bonds so that it just gets longer, a longer radical that can continue to react.
Furthermore, why the electrons attract the radical in the double bonding (ethylene molecule) was discussed and visualised in two groups. The example from group 2 shows transduction from notes to the stickball model and a drawing. By acting on the created stick-ball model of ethylene (pointing), students visualised their perceptions, enabling them to explain and discuss the concept of high electron density (2.8). The process is illustrated in excerpt 2. However, none of the groups included the concept of high electron density in their representation of visualising the reaction of a radical.
Excerpt 2:
(S3): When a radical bond here, it opens [the student points to the double bond in the ethylene molecule].
(S2): … a radical comes here… then the double bonding is broken and becomes a single bond and now this whole is a radical that can bond to another ethylene molecule [Fig. 6].
(S3): And so, it just goes on like this.
(S1): But why?
(S2): In my notes, it says that the double bond has a high electron density.
(S3): Yes, there are more electrons in the double bond and the radical lack an electron, look here [draws on paper].
In all four groups, transductions from animation to representations were observed to visualise how a radical polymerisation stops (the termination process), exemplified by group 2. When watching the animation, (S1) asks the others, “How do we make the process stop?” (S3) refers to the animation and answers, “it stops when two radicals react”. The student returned to the animation, clarified the process with the help of gestures by putting thumbs together, and stated: “When the radicals meet, the reaction ends”, and (S2) said: “Nice, then there are two electrons again” (2.9).
The transduction from animation to gestures and to created representation led to a transformation of (R6) to (R7) to visualise two radicals reacting (R6). (R7) visualises the created bond, consisting of 2 electrons, illustrated in Fig. 7.
Intended object of learning: properties of polymeric material
The third section presents the meaning-making of critical aspects of the structure of polymer chains and chemical attractions. The result is summarised in Table 3. The reported examples illustrate the modelling process from group 1.Structure of polymer chain
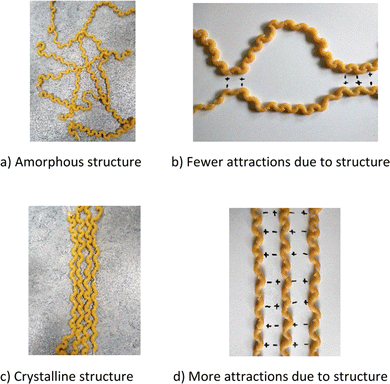
The result shows that all eight groups created representations to visualise the structure of polymer chains. In the first stage, five groups transduced their representations, e.g., humans were switched to paper clips or macaroni since the students did not think it would be possible to visualise the intended with initial representation(s). The observed transductive processes indicate the meaning-making of the polymer at the multiparticle level (3.1). After the first transduction stage, only transformation processes were observed for all eight groups. All eight groups illustrated the significance of the polymer chains’ structure linked to the material's properties by visualising amorphous and crystalline structures. They transformed their representations by placing the polymer chains in different patterns, i.e., amorphous (3.2) and crystalline (3.3), as exemplified by representations created by group 2 in Fig. 8a and c. Four groups further expanded their reasoning by discussing how the polymer chains’ structure, amorphous or crystalline, affects extensibility. Meaning-making was shown as the students interacted with their representations by pulling out a polymer chain from an amorphous to a crystalline structure (3.4). The students concluded that the amorphous polymer chain could be pulled out to a more crystalline chain structure before it broke when subjected to a tensile force.Chemical attractions
Furthermore, during modelling, seven out of eight groups discussed and created representations to visualise the importance of the amorphous and crystalline structure for the attraction possibilities between the polymer chains. In all seven groups, the students stated that an amorphous structure meant fewer opportunities for attraction, and a crystalline structure meant more (3.5). Amorphous structure, crystalline structure, and the significance of the structure for the number of attraction opportunities are exemplified by created representations from group 2 in Fig. 8.The interaction between chains was visualised by drawing plus (+) and minus (−) on paper.
In addition, by transforming their representations, two groups further created representations to visualise the significance of the length of the polymer chains and side chains for the number of interaction possibilities between them. By creating longer and shorter polymer chains, the students stated that “longer chains led to more attractions than shorter chains” (3.6). By creating polymer chains with side chains, the students stated that “side chains lead to fewer opportunities for attraction between the polymer chains” (3.7). The result shows that during discussions, the students linked the polymer chain structure and the interaction between chains to the properties of polymeric material at the macro level by stating that “fewer bonds result in a weaker material and more attraction to a stronger material”.
Seven groups further transformed their representations to visualise the difference between inter- and intramolecular forces. For example, intermolecular interaction was visualised with plus and minus (Fig. 8b and d), while linking polymer chains with string visualised intermolecular interaction (3.8). In all seven groups, the students stated that “intramolecular attraction is stronger than intermolecular attraction”, linking it to the strength of a material (thus integrating the macromolecule, multiparticle and macro level). Finally, four groups transformed their representations to visualise differences in strength in intermolecular forces. For example, in group 2, this was done by drawing plus (+) and minus (−) in different sizes, i.e., weak attractions were marked with small plus or minus and strong attractions with more significant plus and minus (3.9). These transformative processes indicate meaning-making concerning how chemical attraction influences the properties of polymers.
In summary, using a social semiotic lens when investigating students’ modelling, 21 different meaning-making processes were documented related to polymeric concepts, processes, and properties of polymeric materials. Translative changes were observed, scaffolded by chemistry discussions, to scaffold chemistry discussions, to visualise the invisible and shortcomings in created representations.
Discussion and conclusions
This study highlights that translative processes, i.e., transductions and transformations can be used as tools by teachers in chemistry to evaluate and analyse students’ modelling. This study's awareness of translative processes can direct teachers’ attention to situations where the meaning-making of chemistry concepts and processes occurs. The social semiotic lens offers a tool to recognise and study the different stages during students’ learning process. An analytical tool that pays attention to existing and complementary meanings within and between modalities. Since students need to understand similarities within and between modalities to create meaning. As such social semiotics offers a tool that highlights students’ modelling process and aspects influencing and indicating meaning-making.This study highlights the importance of students’ practice visualisation to raise chemical understanding, as noted by other researchers (e.g., Clement et al., 2008; Bucat and Mocerino, 2009). According to Kress (2009), social semiotic practices expressed by semiotic resources describe how someone perceives and understands something. Marton (2015) states that meaning-making is what is discerned by students in addition to the aspects they highlight. From those aspects, meaning-making becomes central from an educational perspective. Our results show that when the students create and transform representations, they transfer meaning between or within modalities, expressing their perceptions. The created representations not only make perceptions visible but also scaffold chemistry discussions and highlight certain aspects, such as conceptual clarification, misconceptions, or lack of chemistry knowledge. In example (3.8), students transduced their representation to visualise intermolecular (visualised with +/−) and intramolecular (visualised with string) bonding. Here, an opportunity was uncovered for the teacher to ask students to explain their representations and then further discuss the chemistry of chemical bonding. As such, teachers should respond to translative processes to discover meaning-making during the creation, interpretation, and discussion of visual representations shared and communicated within the learning context. The power of modelling based-teaching in chemistry is that the process opens up further discussion.
Meaning-making, although limited, could be considered from one isolated created visual representation. An example is beads on a string (Fig. 3) which clarifies the concept of polymer (macromolecule level). However, in line with Tang et al. (2014), we argue that more profound meaning is made through the interaction and sequencing of multiple representations, where each representation forms a part of a sequence and where the sequences as a whole form meaning shown by the examples in the result. As such, teachers should encourage translative processes (transductions and transformations) since visualising can help students discover new aspects of a concept or chemical process. Thus meaning-making during the creation, interpretation, and discussion of visual representations shared and communicated within the learning context.
According to social semiotics, all modes are treated equal (e.g., Kress, 2001; Kress and Bezemer, 2015), and the linguistic modes are not always central. However, this study clearly suggests that speech is an important mode for meaning-making during modelling. In this context, it is essential to highlight the importance of students’ discussions addressing students’ prior verbal knowledge, conceptual use and inferential reasoning, aspects critical for creating and developing representations. The study also highlights the importance of students discussing chemistry from a teaching perspective. Participation in and evaluation of the students’ discussions offers the teacher a tool to assess the student's level of chemical knowledge.
An important finding was that created representations scaffolded and constituted a ‘bridge’ between the submicro and macro levels. As mentioned earlier, one way for students to develop insight into chemical phenomena at the macro level is to create representations at the submicro level (Oversby, 2000; Harrison and Treagust, 2003). We argue that the analysis of all groups showed that the students interacted with their representations when exploring polymer concepts and phenomena at the submicro level in a context discussing the macro level. Moreover, in line with Passmore et al. (2017), integrating chemistry facts in the learning process. The produced representations constituted mediating artefacts between organisation levels.
Research points to risks associated with using simplified representations in chemistry (Taber and Coll, 2003; Bergqvist, 2017) in that such representations may hinder students from learning higher-level chemistry. As such, chemistry teachers need to be sensitive to the limitations of representations to avoid the risk of learning from incorrect or too limited representations. Therefore, teachers must provide students with opportunities to discuss and correct incorrect and limited representations. In line with Tversky (2019) and Gilbert and Justi (2016), we agree that teachers must allow the modelling process to evolve from a less detailed and less abstract to a more developed and abstract representation.
Some students’ created representations can be categorised as intermediate or meso levels. The result shows that some groups broke up the submicro-macro thinking into minor step(s). We agree with Meijer et al. (2009) that students’ created representations do not have to go directly from the macro to the atomic level of the submicro level or vice versa. Thus, chemical understanding can be indicated through meaning-making interacting with representations related to intermediate levels. Finally, there is a study focuses on transductive and transformative processes in drama in chemistry education (Danckwardt-Lillieström et al., 2018). However, this study makes an important contribution to the existing literature by discussing transductive and transformative processes during modelling-based teaching in polymer chemistry.
Implications
Above mentioned findings have important implications for chemistry education by providing tools for teachers to indicate students’ meaning-making during modelling. As stated in the introduction, it might be assumed that chemistry teachers lack the tools to evaluate and analyse the modelling process during the teaching context. If translative processes are identified, it opens up for teachers and students to discuss chemistry. The result of this study provides teachers with a tool to support a teaching situation that includes modelling using different modalities and a learning environment where students are stimulated to create, discuss, and translate representations. We believe that increased awareness of how these translative processes may lead to meaning-making offers new approaches to the teaching and learning of chemistry.This study presents data from eight groups modelling (in total 30 students), discussed by two examples. As such, the study can only draw conclusions based on this group of students and does not provide any basis for generalising results to a broader population. This might be a limitation, but profound studies of students’ communication and activities are essential. Much can be learned from descriptions of particular groups of students in particular learning contexts. Despite few investigated students, conclusions and implications for teachers can be drawn from using a semiotic lens on modelling to extend meaning-making and learning of chemistry. Conclusions that can be used in broader contexts within chemistry education.
This study does not focus on the role of the teachers in the students’ modelling process. Further research needs to be done to investigate how teachers can support students in translative processes during modelling; see Tytler and Prain (2022) for research describing a teacher's role in supporting students’ transductions in physics. In addition, more research must be conducted to investigate the ongoing development of knowledge and skills that semiotic work and modelling may contribute to.
Author contributions
Three researchers have authored this paper. The first author has been responsible for conceptualization, investigation, data curation, project administration, and writing of the original draft. All researchers have been involved in the methodology, analysis process, concluded the results, validation, and writing, thus reviewing and editing the original draft.Conflicts of interest
The ethical considerations in the way the project was conducted are described in the Methods section. The ethical considerations were reviewed and further supported by the ethical committee at the university. The authors declare that there is no conflict of interest.Appendices
Appendix 1
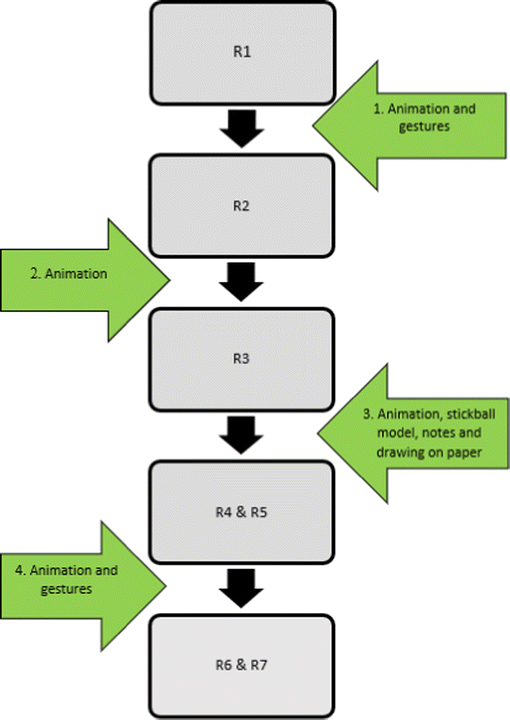
Students’ modelling sequences of created representations when visualising radical polymerisation. The green arrows represent semiotic resources and transductive processes. The black arrows represent transformative processes. R1 represents the first representation produced by the students, and R7 the last representation. Speech was a semiotic resource influencing the whole modelling process.Appendix 2
Matrix used for the description of a modelling sequence; the translative process, created representation, transcript of speech, organisation level(s), semiotic resources, the enacted object of learning, the intended object of learning, critical aspects, and meaning-making.Acknowledgements
The research was funded by the Swedish Research Council.References
- Allchin D., (2011), Evaluating Knowledge of the Nature of (Whole) Science, Sci. Educ., 95(3), 518–542 DOI:10.1002/sce.20432.
- Arroio A. and Campos Santos V., (2016), The representational levels: influences and contributions to research in chemical education. J. Turkish Sci. Educ., 13(1), 3–18 DOI:10.12973/tused.10153a.
- Bennett J. and Hogarth S., (2009), Would You Want to Talk to a Scientist at a Party? High school students’ attitudes to school science and to science, Int. J. Sci. Educ., 31(14), 1975–1998 DOI:10.1080/09500690802425581.
- Bergqvist A., (2017), Teaching and learning of chemical bonding models: Aspects of textbooks, students’ understanding and teachers’ professional knowledge, Doctoral thesis, Karlstad University, Faculty of Health, Science and Technology, Retrieved from https://login.e.bibl.liu.se/login?url=https://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,uid&db=cat00115a&AN=lkp.929547&lang=sv&site=eds-live&scope=site.
- Berland L. K. and Reiser B. J., (2009), Making sense of argumentation and explanation, Sci. Educ., 93(1), 26–55 DOI:10.1002/sce.20286.
- Bucat B. and Mocerino M., (2009), Learning at the Sub-micro Level: Structural Representations, in Gilbert J. K. and Treagust D. (ed.) Multiple Representations in Chemical Education, Netherlands: Springer, pp. 11–29 DOI:10.1007/978-1-4020-8872-8_2.
- Cheng M. and Gilbert J. K., (2009), Towards a Better Utilization of Diagrams in Research into the Use of Representative Levels in Chemical Education, in Gilbert J. K. and Treagust D. (ed.) Multiple Representations in Chemical Education, Netherlands: Springer, pp. 55–73 DOI:10.1007/978-1-4020-8872-8_4.
- Chin C. and Osborne J., (2010), Students’ questions and discursive interaction: their impact on argumentation during collaborative group discussions in science, J. Res. Sci. Teach., 47(7), 883–908 DOI:10.1002/tea.20385.
- Clement J., (2000), Model based learning as a key research area for science education, Int. J. Sci. Educ., 22(9), 1041–1053 DOI:10.1080/095006900416901.
- Clement J., Rea-Ramirez M. A. and van Driel J. H., (2008), Model Based Learning and Instruction in Science, 1st edn, Springer DOI:10.1007/978-1-4020-6494-4.
- Danckwardt-Lillieström K., Andrée M. and Enghag M., (2018), Creative drama in chemistry education: a social semiotic approach. Nordic. Stud. Sci. Educ., 14(3), 250–266.
- Danckwardt-Lillieström K., Andrée M. and Enghag M., (2020), The drama of chemistry – supporting student explorations of electronegativity and chemical bonding through creative drama in upper secondary school, Int. J. Sci. Educ., 42(11), 1862–1894 DOI:10.1080/09500693.2020.1792578.
- Duschl R. A. and Osborne J., (2002), Supporting and Promoting Argumentation Discourse in Science Education, Stud. Sci. Educ., 38(1), 39–72 DOI:10.1080/03057260208560187.
- Fredlund T., Linder C. and Airey J., (2015), A social semiotic approach to identifying critical aspects, Int. J. Lesson Learn. Stud., 4(3), 302–316 DOI:10.1108/IJLLS-01-2015-0005.
- Gilbert J. K. and Justi R., (2016), Modelling-based Teaching in Science Education, Springer DOI:10.1007/978-3-319-29039-3.
- Gilbert J. K. and Treagust D. F., (2009), Introduction: Macro, Submicro and Symbolic Representations and the Relationship Between Them: Key Models in Chemical Education, in Gilbert J. K. and Treagust D. (ed.) Multiple Representations in Chemical Education, Netherlands: Springer, pp. 1–8 DOI:10.1007/978-1-4020-8872-8_1.
- Harrison A. G. and Treagust D. F., (2003), The Particulate Nature of Matter: Challenges in Understanding the Submicroscopic World, in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.) Chemical Education: Towards Research-based Practice, Netherlands: Springer, pp. 189–212 DOI:10.1007/0-306-47977-X_9.
- Heath C., Hindmarsh J. and Luff P., (2010), Video in Qualitative Research: Analysing Social Interaction in Everyday Life, SAGE DOI:10.4135/9781526435385.
- Hussein F. and Reid N., (2009), Working memory and difficulties in school chemistry, Res. Sci. Technol. Educ., 27(2), 161–185 DOI:10.1080/02635140902853632.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assisted Learn., 7(2), 75–83 DOI:10.1111/j.1365-2729.1991.tb00230.x.
- Johnstone A. H., (2009), Multiple Representations in Chemical Education, Int. J. Sci. Educ., 31(16), 2271–2273 DOI:10.1080/09500690903211393.
- Jong O. and Taber K., (2014), The many faces of high school chemistry, in Lederman N. G. and Abell S. K. (ed.), Handbook of Research on Sci. Educ., 1st edn, Routledge, pp. 457–480 DOI:10.4324/9780203097267.
- Jong T., Ainsworth S., Dobson M., Hulst A. V. D., Levonen J., Reimann P., Sime J., Someren M., Spada H. and Swaak J., (1998), Acquiring knowledge in science and mathematics: the use of multiple representations in technology based learning environments, in van Someren M. W., Reimann P. and Boshuizen H. P. A. (ed.), Learning with multiple representations, Advances in learning and instruction series, Vol 1, Pergamin: Elsevier, pp. 9–40.
- Justi R. S. and Gilbert J. K., (2002), Modelling, teachers’ views on the nature of modelling, and implications for the education of modellers, Int. J. Sci. Educ., 24(4), 369–387 DOI:10.1080/09500690110110142.
- Kress G., (2001), Multimodal teaching and learning the rhetorics of the science classroom, Continuum.
- Kress G., (2009), Multimodality: A Social Semiotic Approach to Contemporary Communication, 1st edn, Routledge DOI:10.4324/9780203970034.
- Kress G. and Bezemer J., (2015), The SAGE Handbook of Learning DOI:10.4135/9781473915213.
- Kroksmark T. (1987), Fenomenografisk didaktik. [Phenomenographic didactics], Göteborg: Acta Universitatis Gothoburgensis.
- Leijon M. and Lindstrand F., (2012), Socialsemiotik och design för lärande, 2012, 17(3–4), 22. [Social Semiotics and Design for Learning]. Pedagogisk forskning i Sverige. [Educational research in Sweden]. 17(3–4), 171-192.
- Lemke J., (1998), Teaching All the Languages of Science: Words, Symbols, Images, and Actions, La Caixa Conference on Science Education DOI:10.13140/2.1.4022.5608.
- Lo M. L., (2012), Variation theory and the improvement of teaching and learning, Acta universitatis Gothoburgensis. https://gupea.ub.gu.se/bitstream/2077/29645/5/gupea_2077_29645_5.pdf.
- Maia P. F. and Justi R., (2009), Learning of Chemical Equilibrium through Modelling-based Teaching, Int. J. Sci. Educ., 31(5), 603–630 DOI:10.1080/09500690802538045.
- Marton F., (2015), Necessary conditions of learning, Routledge.
- Marton F. and Booth S., (1997), Learning and awareness, Erlbaum.
- Marton F. and Tsui A., (2004), Classroom discourse and the space of learning, Lawrence Erlbaum.
- Meijer M. R., Bulte A. M. W. and Pilot A., (2009), Structure-Property Relations Between Macro and Micro Representations: relevant Meso-levels I Authentic Tasks, in Gilbert J. K. and Treagust D. (ed.) Multilple representations in Chemical Education, Netherlands: Springer, pp. 195–213 DOI:10.1007/978-1-4020-8872-8_10.
- Mortimer E., Scott P. and Wertsch J. V., (2003), Meaning Making in Secondary Science Classrooms, McGraw-Hill Education.
- Newton P. and Burgess D., (2008), Exploring Types of Educational Action Research: Implications for Research Validity, Int. J. Qualitative Methods, 7(4), 18–30 DOI:10.1177/160940690800700402.
- Nouryon, (2019), Free radical polymerization. Retrieved from https://www.youtube.com/watch?v=HiEzlDLlcu4.
- Osborne J., Simon S. and Collins S., (2003), Attitudes towards science: a review of the literature and its implications, Int. J. Sci. Educ., 25(9), 1049–1079 DOI:10.1080/0950069032000032199.
- Oversby J., (2000), Models in Explanations of Chemistry: The Case of Acidity, in Gilbert J. K. and Boulter C. J. (ed.) Developing Models in Science Education, Netherlands: Springer, pp. 227–251 DOI:10.1007/978-94-010-0876-1_12.
- Passmore C. M., Schwarz C. V. and Mankowski J., (2017), Helping Students Make Sense of the World Using Next Generation Science and Engineering Practices, National Science Teachers Association DOI:10.2505/9781938946042.
- Patron E., Linder C. and Wikman S., (2021), Qualitatively different ways of unpacking visual representations when teaching intermolecular forces in upper secondary school, Sci. Educ., 105(6), 1173–1201 DOI:10.1002/sce.21662.
- Robson C., (2011), Real world research: A resource for users of social research methods in applied settings, 3rd edn, Wiley.
- Schmidt H.-J., Kaufmann B. and Treagust D. F., (2009), Students’ understanding of boiling points and intermolecular forces, Chem. Educ. Res. Pract., 10(4), 265–272 10.1039/B920829C.
- Swedish Research Council, (2017), Good research practice. Retrieved from https://www.vr.se/english/analysis/reports/our-reports/2017-08-31-good-researchpractice.html.
- Taber K. S., (2015), Exploring the language(s) of chemistry education, Chem. Educ. Res. Pract., 16(2), 193–197 10.1039/C5RP90003D.
- Taber K. S. and Coll R. K., (2003), Bonding, in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.) Chemical Education: Towards Research-based Practice, Netherlands: Springer, pp. 213–234 DOI:10.1007/0-306-47977-X_10.
- Tang K.-S., Delgado C. and Moje E. B., (2014), An Integrative Framework for the Analysis of Multiple and Multimodal Representations for Meaning-Making in Science Education: integrative framework for analyzing representation, Sci. Educ., 98(2), 305–326 DOI:10.1002/sce.21099.
- Tversky B. G., (2019), Mind in motion: how action shapes thought, 1st edn, New York, NY: Basics Books.
- Tytler R. and Prain V., (2022), Supporting Student Transduction of Meanings Across Modes in Primary School Astronomy, Front. Commun., 7 DOI:10.3389/fcomm.2022.863591.
- Widing L., Nilsson P. and Enochson P. G., (2022), Modelling as a Tool to Improve Second Language Learners’ Descriptions of Non-Spontaneous Chemistry Concepts. Sci. Educ. Int., 33(2), 181–191 DOI:10.33828/sei.v33.i2.6.
| This journal is © The Royal Society of Chemistry 2023 |