Change in students’ explanation of the shape of snowflakes after collaborative immersive virtual reality
Henry
Matovu
 *a,
Mihye
Won
*a,
Mihye
Won
 a,
David Franklin
Treagust
a,
David Franklin
Treagust
 a,
Dewi Ayu Kencana
Ungu
a,
Dewi Ayu Kencana
Ungu
 a,
Mauro
Mocerino
a,
Mauro
Mocerino
 b,
Chin-Chung
Tsai
b,
Chin-Chung
Tsai
 c and
Roy
Tasker
c and
Roy
Tasker
 ad
ad
aSchool of Education, Curtin University, Perth, Australia. E-mail: henry.matovu@postgrad.curtin.edu.au
bSchool of Molecular and Life Sciences, Curtin University, Perth, Australia
cProgram of Learning Sciences, and Institute for Research Excellence in Learning Sciences, National Taiwan Normal University, Taipei, Taiwan
dSchool of Science, Western Sydney University, Penrith, Australia
First published on 6th December 2022
Abstract
In recent years, chemistry educators are increasingly adopting immersive virtual reality (IVR) technology to help learners visualise molecular interactions. However, educational studies on IVR mostly investigated its usability and user perceptions leaving out its impact on improving conceptual understanding. If they evaluated students’ knowledge gains, they tended to use information recall tests to assess knowledge gains. Employing interviews and diagram-drawing tasks, this study explored how students’ conceptual understanding of the nature of hydrogen bonds and the shape of snowflakes changed through a collaborative IVR experience on snowflakes. Participants were 68 undergraduate chemistry students. Videos of pre-/post-interviews and student-generated diagrams were analysed. The results indicated a marked improvement in students’ conceptual understanding of the nature of hydrogen bonds among water molecules in snowflakes. After IVR, 57 students provided scientifically acceptable explanations of the nature of hydrogen bonds. Improvements in students’ understanding were related to the intermolecular nature of hydrogen bonds, the role of lone pairs of electrons in forming hydrogen bonds, and molecular interactions in 3D space. This study suggests that collaborative IVR could be a powerful way for students to visualise molecular interactions, examine their alternative conceptions, and build more coherent understanding. Implications for the design and implementation of IVR activities for science learning are discussed.
Introduction
In recent years, science educators are increasingly adopting immersive virtual reality (IVR) as an advanced visualization technology to help students visualize and learn abstract science concepts (e.g., Bennie et al., 2019; Ferrell et al., 2019). Immersive virtual reality makes use of a head-mounted display unit to shut out the view of the physical world and ‘immerse’ the learner in an interactive three-dimensional (3D) computer-generated environment (Fombona-Pascual et al., 2022). Using handheld controllers in IVR, learners can manipulate 3D virtual molecules by dragging, pulling, or rotating the structures to improve their understanding of spatial relationships in simple and complex molecules (e.g., Won et al., 2019; Seritan et al., 2021). Engaging learners’ bodies in IVR can reinforce learning of abstract concepts by activating memory traces through embodied cognition (Johnson-Glenberg, 2018). Moreover, learners can also have opportunities to share IVR learning environments to negotiate meanings and build conceptual understanding together (Won et al., 2019).Despite the promising benefits of IVR for science teaching and learning, researchers and educators are still divided on whether investment in IVR for science learning is justified (Radianti et al., 2020). For instance, some studies have found IVR to be more motivating, but also more distracting compared to alternative learning modes (e.g., Parong and Mayer, 2021). In several other studies, learners using IVR did not have better science knowledge gains compared to those who used alternative learning modes such as 2D computer platforms or physical models. These observations span across different science education fields such as physics, biology, and chemistry (Wu et al., 2020b; Hamilton et al., 2021; Coban et al., 2022).
In chemistry learning, the reasons for the lack of conclusive evidence for the conceptual benefits of IVR may be varied. One reason is that many chemistry educators focus on investigating usability of IVR and learners’ affective outcomes (such as motivation and engagement) but not conceptual understanding (e.g., Edwards et al., 2019; Rychkova et al., 2020; Elford et al., 2021; Reeves et al., 2021; Zhao et al., 2022). Conceptual understanding involves the knowledge of basic concepts and the ability to use this knowledge in different contexts to solve problems, translate freely across different levels of scale, and to predict and explain observable phenomena (Holme et al., 2015). Yet, most studies that have attempted to investigate knowledge gains in IVR for chemistry learning rely on multiple-choice and short-answer pre- and post-tests (e.g., Webster, 2016; Ferrell et al., 2019). Such multiple-choice questions have limitations on assessing the level of conceptual understanding as they tend to assess the ability for information recall (Treagust, 1988; Martinez, 1999).
Many science educators and researchers contend that IVR learning environments provide opportunities for constructivist science learning (Winn, 1993; Dede et al., 2017). According to constructivism, learning involves construction of knowledge through active interactions with material resources in the learning environment and with peers or knowledgeable others (Vygotsky, 1978). Through these interactions, students continuously integrate new experiences with already assimilated knowledge (Jonassen, 1994). During learning, students gradually restructure disjointed, intuitive conceptions to build more coherent and scientific ideas (Duit and Treagust, 2003). In this view, researchers (e.g., Dede, 2009; Dede et al., 2017; Won et al., 2019; Matovu et al., 2022a) have suggested various ways of taking advantage of the technical and pedagogical features of IVR through sensory, actional, narrative and social considerations to promote science learning in constructivist ways.
Sensory considerations using IVR to reify abstract science concepts in the form of high-quality 3D displays allow the students to explore these concepts in concrete forms (Won et al., 2019; Matovu et al., 2022a). Actional considerations with the smooth interactivity and motion-tracking features of IVR allow learners to interact with virtual objects in embodied ways (Johnson-Glenberg, 2018) and observe the consequences of their actions (Dede et al., 2017). For instance, in IVR, students can interact with molecules, apply their prior knowledge, and test their ideas to reach new understanding (Zhang et al., 2019; Fombona-Pascual et al., 2022). Narrative considerations describe how engaging students in challenging but manageable tasks in IVR can increase the learners’ engagement and motivation to complete the learning tasks (Matovu et al., 2022a). Moreover, social considerations provide opportunities for students to complete learning tasks with their peers in IVR and can increase the students’ sense of belonging in IVR and engagement on the learning tasks (Krämer, 2017). Therefore, based on the existing literature on IVR for science learning, the position that the present research is taking is that, when students are provided with clear goals to achieve, interactive and collaborative IVR may help students to explore chemistry concepts in more engaging and productive ways. However, due to the unique nature of the students’ roles in learning while in IVR, assessment of learning using traditional information-recall questions provides limited evidence of how IVR benefits students’ conceptual understanding (Hamilton et al., 2021).
An alternative way to investigate students’ conceptual benefits from IVR is to analyse student-generated diagrams which can act as a ‘window’ into students’ conceptions of science phenomena (Ainsworth et al., 2011; Tippett, 2016; Chang et al., 2020). For example, through drawings, students can elaborate and clarify ideas that they would otherwise not be able to communicate explicitly (Ainsworth et al., 2011). Drawing activities also provide opportunities for students to develop an integrated understanding of science by representing, linking, and communicating ideas coherently (Gobert and Clement, 1999; Treagust et al., 2017). By analysing students’ diagrams, educators can identify patterns in students’ conceptions of science phenomena and their learning difficulties (McLure et al., 2021b). Moreover, learner's prior knowledge greatly influences their subsequent learning (Taber, 2017). Therefore, analysing patterns of conceptual understanding in student-generated diagrams before and after IVR may provide insights into how students of differing preconceptions benefit from IVR.
In chemistry education, several studies have used student-generated diagrams to assess the level of students’ conceptual understanding of key chemistry concepts. The concepts investigated included the particle theory of matter (e.g., Andrade et al., 2021; McLure et al., 2021a), atomic structure (e.g., Derman et al., 2019), and intermolecular forces (e.g., Cooper et al., 2015; Noyes and Cooper, 2019; Matovu et al., 2022b). In IVR research, however, few studies have used drawing tasks to assess students’ science conceptual understanding. Thompson et al. (2020) reported that student-generated diagrams of human cells after IVR were more complex and depicted a higher density of organelles compared to pre-IVR diagrams, suggesting an improvement in students’ conceptions of cells. However, the study provided no context for students to integrate and apply their improved understanding to explain science phenomena. Therefore, it remains unclear whether IVR would help students to build coherent understanding of abstract science concepts.
In the present study, we investigated how IVR would change university students’ conceptual understanding of the nature of hydrogen bonds among water molecules and the shape of snowflakes. For this purpose, we analysed diagrams and verbal explanations created by students before and after experiencing an interactive and collaborative IVR program on snowflakes. The nature of hydrogen bonds, the target concept in this study, is a fundamental concept in chemistry. The concept can be used to explain a wide range of macroscopic properties in substances, such as phase changes, boiling points, and solubilities. Yet, previous studies have identified a wide range of students’ alternative conceptions about the nature of hydrogen bonds (e.g., Henderleiter et al., 2001; Schmidt et al., 2009; Cooper et al., 2015). Therefore, investigating the benefits of IVR has the potential to provide information on whether the medium can help science educators to confidently deal with this abstract yet important chemistry concept.
This study was designed to answer the following research questions:
1. How do university students change their conceptions of the nature of hydrogen bonds among water molecules in snowflakes after exploring the concept in collaborative IVR?
2. How do university students change their explanations of the shape of snowflakes after exploring the concept in collaborative IVR?
Materials and methods
The collaborative snowflakes IVR program
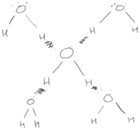
The IVR program was designed to help students build a coherent understanding of the nature of hydrogen bonds among water molecules in snowflakes by integrating their prior knowledge of structure and polarity, hydrogen bonds, and scale of water molecules. The IVR program was developed primarily with Unity®. While in IVR, learners’ bodies were represented as generic ‘avatars’ in the form of floating heads and hands (e.g., Fig. 1a). In each session, two students in the same physical room also shared the virtual space in IVR through a network. Since the students were in the same physical and virtual spaces, they could observe each other's avatar, communicate in real-time, and collaboratively manipulate the virtual objects. During the IVR learning activity, learners could also walk around the physical space in the room (approximately 3 m × 3 m of virtual space) to change their perspectives of the molecular structures and use their handheld controllers to move and connect virtual molecules. In addition, the students would receive haptic feedback in the form of vibrations from their IVR controllers when the molecular structures connected in the right orientations.Students completed multiple learning tasks involving water molecule structures in IVR. At every step in IVR, the students were prompted to manipulate the structures to explore molecular interactions. Students were also explicitly prompted to discuss ideas with one another to reach consensus before moving on to the next task. Instructions were provided to the students in the form of audio and text. The students had the freedom to experiment with their ideas and the time the students spent on each IVR task depended on the pace of each pair of students. The IVR learning tasks were presented in the order of increasing complexity and an increasing number of water molecule structures was provided as the students progressed through the learning tasks.
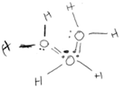
At the initial stage of the IVR activity, students were provided with two water molecules. Each molecule had the electron density regions highlighted – blue for electron poor and red for electron rich regions (Fig. 1a). A prompt was given to the students – “What are the features of water molecules? Discuss the features of the water molecules in front of you. When you are happy with your answer click the Submit button”. When the students pressed the submit button, a new prompt would appear – “How do you make a hydrogen bond? Make a hydrogen bond between two water molecules and discuss what you notice about the colour and thickness of the hydrogen bond. When you are happy with your answer, click the submit button.” The students manipulated the molecules to create a hydrogen bond and discussed their observations in terms of the angle and distance between the molecules.
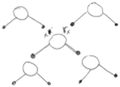
After exploring the interaction between two water molecules, the students were provided with more water molecules. The students were prompted to predict, and then explore by connecting multiple water molecules to a single molecule, the maximum number of hydrogen bonds a central water molecule could form and to explain why this would be the maximum number. By connecting many clusters of water molecules through hydrogen bonds, the students subsequently constructed layers of water molecules, and finally a lattice structure of molecules (Fig. 1b). Constructing the layers of molecules and the lattice structure required the students to apply their knowledge of hydrogen bonds, and different problem-solving and pattern recognition skills. The students were also prompted to use the lattice structure of water molecules to explain the macroscopic features of snowflakes. The collaborative IVR experience concluded with a short video explaining how variations in environmental conditions can influence the way in which snowflakes crystals grow. Short videos of some of the IVR learning tasks can be accessed online (https://tinyurl.com/je52p6pm).
Participants and study design
The study adopted a design-based research approach (Anderson and Shattuck, 2012). The approach follows an iterative cycle of designing and implementing a learning intervention and using mixed research methods to evaluate learning. Design-based research is useful in building a theory of how an intervention, such as IVR, works in the intended context, and to improve the design and implementation of the intervention (Wang and Hannafin, 2005). In the present study, we explored how a collaborative IVR experience would change students’ conceptions of the nature of hydrogen bonds among water molecules and the shape of snowflakes. The IVR program used in this study was first developed in 2020 and was refined iteratively following several testing sessions. The present study reports findings from the iteration of our IVR program in 2021. The findings from this study will be used to inform future design and implementation of our IVR programs.A total of 70 first- and second-year undergraduate chemistry students participated in the collaborative IVR learning activity. The students were those majoring in chemistry or chemistry related courses such as Chemical Engineering, Food science, and Nutrition at a large public university in Australia. The students had passed high school chemistry and at least one university level chemistry unit. The learning activity was integrated into two of the undergraduate chemistry units offered in the first half of the year 2021. The two chemistry units included a focus on the concept of intermolecular forces. The activity was, therefore, mandatory for all the students enrolled in the chemistry units. However, participation in the research study was voluntary. Approval to conduct the study was granted by the institutional Human Research Ethics Committee (HRE2020-0081) and all the students signed consent forms for their data to be used in the study.
The study involved a pre-interview, a collaborative IVR session, and a post-interview. The participating students were requested to complete the series of IVR learning tasks in pairs. The pairing of the students was managed using an online meeting scheduling tool (doodle.com) with time slots from which students could choose. The students were provided with a link where they could log in and select the most convenient time for them to participate in the IVR learning activities. Each slot could be selected by only two students. Because the students selected the time slots based on convenience, some students were paired with their friends while others were not.
The purpose and design of this study were discussed over several meetings among all the authors. Based on these initial discussions, two authors (HM and DU) developed the potential interview prompts. Three authors (HM, DU, and MW) then met to compare, discuss, and refine the interview prompts until consensus was reached amongst these authors. The prompts agreed upon by these three authors were further checked for validity and confirmed by all the authors. The interview prompts employed in this study are provided in the Appendix.
Pre-interview prompts were designed to elicit the students’ ideas about the nature of hydrogen bonds among water molecules in snowflakes before the IVR session. However, to avoid the students reproducing textbook definitions of a hydrogen bond, the interview prompts did not include the term ‘hydrogen bond’. Instead, the participants were asked to describe their understanding of the features of a water molecule and water molecule interactions in snowflakes, and to use a pen and paper to draw diagrams to illustrate their ideas. After illustrating the molecular interactions, the students were shown magnified images of snowflakes (such as those in Fig. 2) and were asked to use the concept of the molecular interactions among water molecules to explain the shape of snowflakes. Each pre-interview lasted 15–25 minutes.
 | ||
| Fig. 2 Examples of images of snowflakes used in the pre-interview (source: Adobe Creative Cloud, Adobe Inc., 2021). | ||
After the pre-interview, the researchers trained the participants on the use of the IVR headsets and controllers. Participants were also encouraged to talk to their partners while completing the IVR learning tasks, and to immediately inform the researchers in case they felt dizziness or any form of discomfort with the IVR headsets. Each student donned an HTC Vive pro Eye headset and two handheld controllers for interacting with the virtual molecules. The IVR session lasted 40 minutes on average for each pair of learners. During this time, the students collaborated with their peers to complete the IVR learning tasks and did not receive any guidance or feedback from the researchers.
In the post-interview (20–30 minutes), learners were assessed again. The learners were prompted to improve their drawings illustrating the nature of molecular interactions in snowflakes and to provide reasons for any changes in their diagrams. Students were also asked to revise their explanations of the six-fold symmetry in snowflakes and to draw diagrams to show their improved understanding.
Like the IVR learning tasks, the interview tasks were initially designed to be collaborative in nature. Students were paired and asked to discuss their ideas with peers to create shared diagrams illustrating the nature of hydrogen bonds among water molecules in snowflakes. Unfortunately, in many cases, students preferred to provide individual responses and draw individual diagrams. When the students showed reluctance to collaborate with peers in the interviews, the interviewer did not insist that they create shared diagrams and explanations. Instead, he asked the students to confirm whether they were happy with their individual diagrams. For the explanation of the nature of hydrogen bonds among water molecules, students generally worked individually (n = 60) in the pre-interview, but a small number of students switched to respond to interview prompts collaboratively in the post-interview (n = 12). For the explanations of the shape of snowflakes, the students preferred to provide individual explanations in both the pre- and post-interviews; the exceptions were two students in two pairs who stated that they were each happy with their peers’ explanations in the pre-interview. Data in the form of audios, videos, and students’ diagrams was collected. Audio and video data were transcribed before analysis.
Analysis
Out of the 70 students recruited, two students’ data were excluded on the bases of insufficient information (a student not completing the diagrams) and eligibility (another student not being in the target chemistry class). Therefore, audios, videos, and diagrams of the remaining 68 students were analysed. All student-generated diagrams (pre and post) and accompanying verbal explanations were analysed inductively using a constant comparison method (Merriam and Tisdell, 2015). This was done to generate categories relating to the students’ conceptual understanding of the nature of hydrogen bonds among water molecules and the shape of snowflakes.Analysis of the students’ diagrams and verbal explanations generated four conceptual categories (A–D) related to the students’ conceptual understanding of the nature of hydrogen bonds among water molecules in snowflakes. Category A related to students’ difficulties in drawing the structure of a water molecule, recognising polarity in water molecules, and distinguishing the nature of hydrogen bonds as intermolecular interactions from intramolecular interactions. Category B related to students’ difficulties in recognising the role of lone pairs of electrons in water molecules in the formation of hydrogen bonds. For categories C and D, the students recognised hydrogen bonds among water molecules as electrostatic interactions between hydrogen atoms and lone pairs of electrons in different molecules. For category C, hydrogen bonds were discussed as interactions in 2D space, while for category D, the students recognised hydrogen bonds as molecular interactions in 3D space. The descriptors of the conceptual categories, together with exemplar verbal explanations are summarised in Table 1. The detailed analysis of the student-generated diagrams has been discussed in a separate manuscript (Matovu et al., 2022b).
As mentioned earlier, some students collaborated with their peers in the interviews to construct shared diagrams illustrating the nature of hydrogen bonds among water molecules in snowflakes. Therefore, after coding the combined data, the first two authors (HM and MW) separated the diagrams and transcripts for the students who worked individually from the diagrams and transcripts of the students who collaborated with their peers. This was done to investigate whether the students’ ideas or depth of the discussions were different between the two groups of students. Students’ collaboration did not appear to have much influence on the depth of the conceptual discussions or to generate any new conceptual categories. Therefore, the authors of this research applied the same analysis scheme to all the data.
The transcripts of the students’ explanations (supplemented with the gestures the students used while explaining their understanding) were first coded inductively. Before and after IVR, there were wide variations in students’ explanations both in terms of the ideas the students presented and the comprehensiveness of the explanations. To reflect the differences in the students’ explanations, the authors (HM and MW) adopted a combination of both inductive and deductive approaches and identified seven key topics that students would incorporate in their explanations. These topics were: molecules spread out, hydrogen bonds among molecules, tetrahedral units of molecules, 3D lattice structures, hexagonal shapes among molecules interacting in 2D, hexagonal shapes among molecules in 3D, and variations in snowflakes patterns. The authors then coded each student's explanation based on the key topics identified in their explanation to identify common patterns. From these patterns, broader categories were then generated. In each category of explanations, there were variations in the level of complexity or sophistication in the students’ explanations. The categories of the students’ explanations and their descriptors are shown in Table 2.
| Category | Subcategory | Descriptor |
|---|---|---|
| Not sure | Student makes no attempt to explain the shape of snowflakes | |
| Incoherent or hard to categorise | It is not clear what feature of snowflakes' shape the student is explaining, or the student's' explanation includes contradicting ideas | |
| Focus only on the appearance | Flat structure | Student's focus is on explaining why snowflakes appear flat |
| Variation in patterns | Student focuses on environmental factors or randomness in molecular interactions to explain why there are different patterns of snowflakes but does not explain the hexagonal symmetry | |
| Explain molecular interactions in 3D | In terms of hydrogen bonds | Student recognises that molecules in snowflakes interact in 3D but does not explain other features of snowflakes shapes |
| + 3D lattices and the variations in patterns | Student recognises that molecules in snowflakes interact in 3D and attempts to explain the different variations in snowflakes patterns | |
| Explain hexagonal symmetry | In terms of hydrogen bonds | Students’ explanation focuses on the hexagonal symmetry in snowflakes only but there is no mention of tetrahedral units as the building blocks of the structure |
| + the variation in patterns | Student explains the hexagonal symmetry and variations in snowflakes patterns but there is no mention of tetrahedral units as the building blocks of the structure | |
| Recognise tetrahedral unit as the building block of snowflakes | In terms of hydrogen bonds or 3D lattice | Student recognises that molecules in snowflakes interact in 3D to form tetrahedral units but does not explain the hexagonal symmetry or variations in patterns |
| + The hexagonal symmetry | Student recognises that molecules in snowflakes interact in 3D and form tetrahedral units which result in hexagonal patterns amongst water molecules to explain the hexagonal symmetry in snowflakes | |
| + The variation in patterns | Student recognises that molecules in snowflakes form tetrahedral units and explains variations in snowflakes shapes but not the hexagonal symmetry | |
| + The hexagonal symmetry and the variation in patterns | Student recognises that molecules in snowflakes interact in 3D and form tetrahedral units, explains the hexagonal symmetry, as well as variations of patterns in snowflakes |
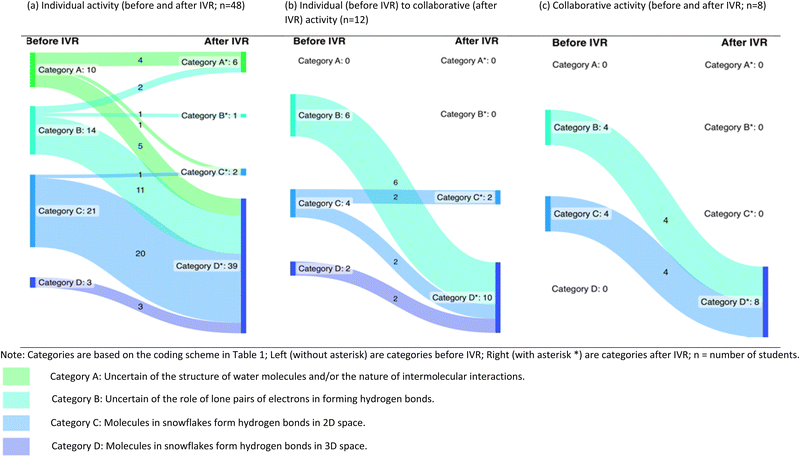
After coding the students’ pre-and post-responses and diagrams, changes in students’ conceptual understanding of the nature of hydrogen bonds among water molecules in snowflakes were illustrated using Sankey diagrams (Schmidt, 2008). A Sankey diagram is a flow diagram showing changes in quantities undergoing a given process or transformation. In this study, Sankey diagrams were used to illustrate the number of students with each category of preconceptions of the nature of hydrogen bonds among water molecules before IVR and how many of these students changed to different conceptual categories after IVR (Table 3). The width of each band (flow) is proportional to the number of students changing from one conceptual category to another. Sankey diagrams have been used in previous science education research (e.g., Williams et al., 2015) for purposes similar to the present study. In this study, the students approached the task differently (most worked individually while some collaborated to draw diagrams in one or both interview sessions). Therefore, we created different Sankey diagrams depending on whether the students worked individually or collaborated on the tasks. However, the changes in students’ explanations of the shape of snowflakes could not be illustrated using a Sankey diagram because of the big number of categories.
Findings
Research question 1: changes in students’ conceptions of the nature of hydrogen bonds among water molecules in snowflakes after exploring the concept in collaborative IVR
Before IVR, students had varying conceptions of the nature of hydrogen bonds among water molecules in snowflakes, with most students exhibiting alternative conceptions of some form. For example, 10 students had difficulties drawing the structure of a water molecule and/or recognising that hydrogen bonds were non-covalent interactions between molecules (category A). Twenty-four students recognised that hydrogen bonds were electrostatic interactions between oxygen and hydrogen atoms in different molecules, but the students had alternative conceptions about the role of lone pairs in the formation of hydrogen bonds (category B). Other students (n = 34) explained that one water molecule in snowflakes would form multiple hydrogen bonds with other water molecules using individual lone pairs of electrons and hydrogen atoms. However, most of these students (n = 29) explained hydrogen bonds as interactions in a flat plane (category C).After IVR, there was a marked improvement in the students’ conceptual understanding of the nature of hydrogen bonds among water molecules in snowflakes. Improvements in students’ conceptual understanding were related to the intermolecular nature of hydrogen bonds, the role of lone pairs in the formation of hydrogen bonds, and molecular interactions in 3D space. In their post-interview diagrams and explanations, more students (61 after IVR vs. 34 before IVR) explained that a hydrogen bond was formed between a lone pair of electrons in one water molecule and a hydrogen atom in a neighbouring water molecule (categories C* and D*). In addition, after IVR, more students (57 students in category D*) provided scientifically acceptable explanations of the nature of hydrogen bonds among water molecules. The 57 students described a hydrogen bond as an electrostatic force of attraction between molecules and recognised that each water molecule formed four hydrogen bonds with other molecules in 3D space. This number was exceptionally higher than the number of students who recognised the 3D nature of molecular interactions before IVR (5 students in category D).
As shown in Table 3, the changes in students’ conceptual understanding of the nature of hydrogen bonds were partly influenced by the nature of students’ preconceptions and the students’ tendencies to collaborate with peers on the interview tasks. About half of the students who had difficulties with recognising the non-covalent nature of intermolecular interactions retained their preconceptions after IVR. On the other hand, students from other categories of preconceptions generally improved their understanding of the nature of hydrogen bonds after IVR. Also, students who collaborated with peers on the interview tasks tended to change their conceptual understanding more consistently than those who worked individually on the interview tasks. Below we elaborate on these findings:
Changes in students’ understanding of the nature of hydrogen bonds among water molecules in snowflakes based on the nature of students’ preconceptions
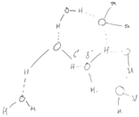
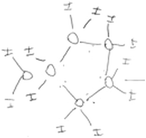
Although some of the students initially in category A had an idea of the bent shape and the presence of lone pairs of electrons in water molecules, the students had difficulty in recognising the atoms between which hydrogen bonds form and the non-covalent nature of hydrogen bonds. In attempting to illustrate intermolecular interactions, the students connected oxygen atoms of different water molecules with solid lines, reasoning that the oxygen atoms would form bonds by sharing their nonbonding electrons. After IVR, among the students initially in category A, six of them recognised a hydrogen bond as an attractive force between a lone pair of electrons on the oxygen atom in one molecule and a hydrogen atom of another water molecule, rather than as a covalent bond between molecules. Five of these six students discussed and/or illustrated that each water molecule in a snowflake formed hydrogen bonds with four other water molecules in 3D space. However, about half (n = 4) of the students initially in category A retained most of their preconceptions. For example, in his diagram before IVR, Ross connected oxygen atoms of different molecules with solid lines (Table 4) and reasoned that water molecules would use the lone pairs of electrons on the oxygen atoms to bond covalently. Through IVR, Ross noticed that each water molecule in snowflakes formed four hydrogen bonds with neighbouring molecules in 3D space. However, after IVR, Ross connected hydrogen atoms of different molecules with a solid line and placed oxygen atoms of different molecules close to each other (Table 4). Ross's drawing showed that the student was still not able to discern how exactly the hydrogen bonds were formed between molecules.The 24 students who initially recognised that hydrogen bonds were electrostatic in nature but had alternative conceptions about the role of lone pairs in the formation of hydrogen bonds (category B) moved to different categories after IVR, such as category A or category D. After IVR, most of the students initially in category B (21 out of 24) recognised that a hydrogen bond is formed between a lone pair of electrons in one molecule and a hydrogen atom of a neighbouring water molecule. These students also recognised that each water molecule in snowflakes formed four hydrogen bonds in 3D space. For example, before IVR, Collin recognised polarity in a water molecule but imagined a water molecule as having only two partially charged regions. Collin reasoned that both lone pairs of electrons created a single negatively charged region and both hydrogen atoms of a water molecule collectively formed a partially positive region. Therefore, a water molecule would form multiple hydrogen bonds without directionality. To represent the nature of hydrogen bonds among water molecules, Collin drew other water molecules stacked around the central one (Table 5). After IVR, Collin improved his diagram and explanation to emphasise that, to form hydrogen bonds, individual hydrogen atoms were attracted to individual lone pairs of electrons (Table 5). While diagrammatically representing the molecular interactions after IVR, Collin also emphasised verbally that the molecules interacted in 3D space.
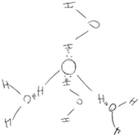
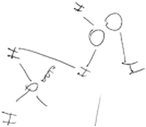
Most of the students (26 out of 29) who initially recognised hydrogen bonds among water molecules as electrostatic interactions between lone pairs of electrons and hydrogen atoms but drew the diagrams as if molecules were interacting in 2D (category C) realized the 3D nature of molecular interactions after IVR. Attempting to illustrate the 3D molecular interactions on paper after IVR, most of the students explained that it was too difficult to draw in 3D. They instead explained verbally and with gestures that the molecules interacted in 3D. For example, even though Brian had a good understanding of the nature of hydrogen bonds in snowflakes before IVR, his diagram and explanation did not represent the molecules interacting in 3D. However, from IVR, Brian noticed that the molecules were interacting in 3D. Therefore, after IVR, Brian attempted to represent these interactions with dashed and wedged lines. He also verbally emphasised the 3D nature of molecular interactions (Table 6).
Comparison of the changes in students’ individual and collaborative explanations of the nature of hydrogen bonds among water molecules in snowflakes
To assess students’ learning through IVR, the students drew and explained their understanding in pre- and post-interviews. As shown in Table 3, most students (n = 48) preferred to draw individual diagrams and provide individual explanations in the pre- and post-interviews. Some students (n = 12) worked individually before IVR but collaborated on the drawing tasks after IVR. Only eight students collaborated on the drawing tasks to create shared diagrams before and after IVR. Generally, students who struggled with the structure of a water molecule or had difficulties recognising the non-covalent nature of hydrogen bonds (category A) did not collaborate with peers. Similarly, only eight of the 29 students who already had a reasonable understanding of the nature of hydrogen bonds (category C) collaborated with peers in at least one interview session. Students who were initially in category B were more willing to collaborate with peers compared to those in other conceptual categories.When students collaborated with peers on the diagram-drawing tasks, their understanding of the nature of hydrogen bonds improved more consistently after IVR compared to those who worked individually. For instance, three of the 14 students who were initially uncertain of the role of lone pairs (category B) and did not collaborate with the peers both before and after IVR either remained in the same category or moved to a category of lower conceptual understanding (Table 3a). On the other hand, all the 10 students who were initially in category B but collaborated on the diagram-drawing tasks in at least one of the interview sessions moved to category D of conceptual understanding after IVR (Table 3b and c). These students recognised hydrogen bonds as interactions between lone pairs and hydrogen atoms and in 3D space. However, collaboration did not generate any new conceptual categories. In addition, the nature of the diagrams or verbal explanations created during the pre- and post-interviews did not differ much between the students who collaborated with peers and those who worked individually. For example, before IVR, the students Samson, Dom, and Sandra all exhibited alternative conceptions of the role of lone pairs of electrons in the formation of hydrogen bonds. After IVR, the changes in conceptions of the nature of hydrogen bonds among water molecules that were held by Dom and Sandra (collaborative pair before and after IVR) also did not differ much from those held by Samson who worked individually on the diagram-drawing tasks before and after IVR (Table 7). After IVR, each of the students (Samson, Dom, and Sandra) recognised that a hydrogen bond was formed between a lone pair of electrons in one water molecule and a hydrogen atom of another water molecule. Each water molecule in snowflakes formed a maximum of four hydrogen bonds and that the interactions were in 3D space.
Research question 2: changes in students’ explanations of the shape of snowflakes after exploring the concept in collaborative IVR.
Before IVR, the students had a wide range of ideas related to the formation of snowflakes. Some student participants (n = 11) were not sure how they could explain the shape of snowflakes and did not attempt to provide explanations. Some of the students focused on explaining the overall flat appearance of snowflakes (n = 17) and reasoned that molecules spread out in 2D, while some (n = 12) focused on the macroscopic variations in the patterns of snowflakes. Other students (n = 14) made efforts to explain the hexagonal symmetry in snowflakes but imagined that molecules were forming hexagonal shapes in 2D. Very few students recognised that the water molecules in snowflakes formed 3D lattices (n = 4) or that tetrahedral units of molecules were the building blocks of the structures of snowflakes (n = 3).After IVR, the student participants were more confident to provide explanations for the shape of snowflakes; only three students did not attempt to provide explanations after IVR compared to 11 who did not provide explanations before IVR. After IVR, none of the students discussed molecules spreading out in a flat plane. Most students (n = 50) explained that the submicroscopic structure of snowflakes was not 2D as many had initially imagined; therefore, many students shifted the focus from explaining the flat appearance of snowflakes to explaining other aspects of snowflakes such as the hexagonal symmetry and the variations in patterns (Table 8). In addition, after IVR, students tended to integrate multiple topics to explain the shape of snowflakes, instead of explaining individual features in the shape of snowflakes (such as the flat appearance of snowflakes only, the hexagonal symmetry only, or only the variations in the patterns in snowflakes). After IVR, students integrated topics such as hydrogen bonds, hexagonal shapes in 3D, and variations in patterns of snowflakes (n = 16) to explain the shape of snowflakes. Other students integrated topics like hydrogen bonds, tetrahedral units, and hexagonal shapes in 3D (n = 9); or hydrogen bonds, tetrahedral units, hexagonal shapes in 3D, and variations in snowflakes patterns (n = 8) in their explanations.
| Category | Subcategory | Number of students | |
|---|---|---|---|
| Before IVR | After IVR | ||
| Not sure | 11 | 3 | |
| Incoherent or hard to categorise | 7 | 7 | |
| Focus only on the appearance | Flat structure | 17 | 0 |
| Variation in patterns | 12 | 7 | |
| Explain molecular interactions in 3D | In terms of hydrogen bonds | 1 | 2 |
| + 3D lattices and the variations in patterns | 3 | 3 | |
| Explain hexagonal symmetry | In terms of hydrogen bonds | 13 | 13 |
| + the variation in patterns | 1 | 16 | |
| Recognise tetrahedral unit as the building block of snowflakes | In terms of hydrogen bonds or 3D lattice | 1 | 0 |
| + the hexagonal symmetry | 0 | 9 | |
| + the variation in patterns | 2 | 0 | |
| + the hexagonal symmetry and the variation in patterns | 0 | 8 | |
The 11 students who were initially not sure of the explanation of the shape of snowflakes moved to different levels of conceptual understanding after IVR. Some of the students did not attempt to explain the shape of snowflakes (n = 3), gave incoherent explanations (n = 1), or only explained the variations in snowflakes patterns (n = 1). After IVR, more than half of the students in this category (6 out of 11) recognised that molecules interacted in 3D to form hexagonal patterns. For example, after IVR, Jill explained:
“It [the central molecule] doesn't connect to six [other molecules]. [Before IVR], I wasn't sure how the lattice forms but… the hydrogen bonding between molecules allows the formation of a hexagonal lattice structure which forms the crystalline snowflakes, and the actual patterns and shapes are formed by the variations in temperature and humidity as the water gets drawn from the moisture, I guess”
Before IVR, some students (n = 17) focused on the overall flat appearance of snowflakes. These students reasoned that molecules would spread out from a central molecule to form flat structures and often used gestures to illustrate molecules branching out in a flat plane, as if the thickness of a snowflake would be one layer of water molecules. However, after IVR, 12 of the 17 students in this category (e.g., Lucas) recognised that the molecules did not interact in 2D. Instead, the molecules interacted in 3D to form lattice structures and hexagonal patterns among molecules. These 12 students also explained variations in the patterns of snowflakes. The excerpts below show the changes in the explanation of the shape of snowflakes made by Lucas:
Before IVR
“I guess as it kind of cools down, they all (pointing at his diagram), the water [molecules] can compact a little bit and the bonds will be closer, and they will pack in a kind of shape like this (points at the diagram) so that they are spreading out as much as they can and sort of, evening out a little bit (gestures molecules spreading out from the central molecule).”
After IVR
“When the water molecules are kind of packed and you fit four on one, they end up forming that hexagonal kind of shape but then when that kind of builds on (gestures water molecules building on the already existing chunk), they can only fully pack when the temperature and humidity are right, so when there's low humidity and there's less water it tends to branch out a bit more because the lattices are not packing as much, I suppose, and back into the molecules, you form that hexagonal kind of shape…”
Those students who focused on the macroscopic variations in the patterns of snowflakes before IVR (n = 12) used ideas such as differences in environmental conditions, differences in numbers of water molecules available or probabilities to explain the differences in snowflakes patterns. After IVR, some of the students in this category (n = 4) not only explained the differences in snowflakes patterns but also recognised that, through hydrogen bonds, water molecules formed hexagonal shapes in 3D space. Moreover, about half of the students in this category (5 out of 12) recognised the tetrahedral unit as the basis for the snowflakes structure. For instance, before IVR, Kevin reasoned that water molecules may have different chances to interact; these different chances may explain why there are so many different patterns of snowflakes. After IVR, Kevin integrated several topics (hydrogen bonds, tetrahedral units, hexagonal shapes, and variation in patterns) to explain both the hexagonal symmetry in snowflakes and the variations in patterns of snowflakes. The excerpts below show the explanations provided by Kevin before IVR and after IVR.
Before IVR
“(Looking at the images of snowflakes) Yeah, when they [water molecules] interact with each other, like, come close enough and they get cold, like, it forms in every single angle, and it can form differently. For every time, it's a different chance of something else happening.”
After IVR
“… So, the initial water molecules… bond to the hydrogen atoms and then the lone pairs and then it forms the tetrahedral, and then those tetrahedral shapes bond to another tetrahedral shape, and bond to another one… and it forms a hexagonal structure, and then it continues to expand. And then when different conditions hit the, to each point of that, it expands out slowly and forms different types of shapes. They're all very similar because they are experiencing the same conditions, as was said in the video.”
Fourteen students explained the hexagonal symmetry in snowflakes before IVR but with different levels of success. Most of these students reasoned that molecules branched out in 2D from a central molecule to form flat hexagons, while some (e.g., Nelson) reasoned that six molecules could connect to one central molecule to result in the six branches that appear in each snowflake. After IVR, 13 of 14 the students in this category recognised that, by forming hydrogen bonds in 3D space, water molecules formed hexagonal shapes which explains the six-fold symmetry in snowflakes. Moreover, six of these 13 students also recognised that tetrahedral units were the basis of the snowflakes structure. The excerpts below show the explanations of the hexagonal symmetry given by Nelson before and after IVR:
Before IVR
“I think this middle one here (points at the central water molecule in his drawing), will be the middle of the snowflake and that… like branches out. So, like different hydrogens will connect to the valence electrons forming a stem, like six stems off each one.”
After IVR
“… hydrogen bonding occurring between… the valence electrons of the oxygen and the hydrogens… and then there's all that bonding, we end up forming like the hexagonal shape, which kind of helps explain that [the hexagonal shape of snowflakes]… they [molecules] will bond together with other clusters at the correct orientation to form more hydrogen bonds and kind of just expand in a lattice structure… and that lattice structure will still go out. And then it kind of comes to our external factors that affect that final bit… because the actual flake is so small… the individual snowflakes [branches] experience like the same conditions which affect how the branch goes out…”
The IVR program was designed to help students recognise the link between the macroscopic shapes of snowflakes and the interactions among water molecules. After IVR, most students made efforts to use their knowledge of hydrogen bond interactions among water molecules in 3D space to coherently explain the shape of snowflakes. However, some students (e.g., Harriet) provided very superficial explanations based on what they saw in IVR but did not clearly link their explanations to hydrogen bonds among molecules. Other students (n = 7; e.g., Craig) simply described what they had seen/heard in the video at the end of the IVR activity regarding of the factors that affect the way snowflakes grow. The excerpts below show explanations provided by Harriet and Craig after IVR:
Harriet: “The basic structure is the hexagon to begin with. And then hexagons, hexagons together, like they have the pointy edges… and hexagons, hexagons together, planes are together… there's a plane, even the angles were different, like it wasn't like flat hexagons, it gives [the hexagonal] shape of snowflakes…”
Craig: “Snowflakes are made up of millions and millions of water molecules, and that's how they get their shape. They grow in different environments which affects how they grow”
When designing the collaborative IVR program on snowflakes, we included multiple ‘hands-on’ tasks for students to complete, such as connecting many water molecules to form a 3D lattice structure. We anticipated that, as the students constructed larger molecular structures in IVR, they would improve their knowledge of the nature of hydrogen bonds and use it to develop a coherent understanding of the macroscopic scale snowflakes. Although most of the participating students achieved a much better understanding through the IVR activities (for example, 50 out of 68 students recognised the hexagonal arrangements among water molecules in 3D space as the reason for the hexagonal shape of snowflakes), the students explained the shape of snowflakes to different extents. Some of the students did not successfully integrate the idea of hydrogen bonds in their explanations after IVR. These students might have had difficulty demonstrating what they learnt or describing what they saw in the 3D virtual space. For other students, the connection between the molecular interactions and the shape of snowflakes might not have fully registered in their minds in the limited time available.
Discussion
In this study, we investigated how undergraduate chemistry students changed their conceptions of the nature of hydrogen bonds among water molecules after experiencing an IVR program on snowflakes. Firstly, the study found that the collaborative IVR experience helped most of the students to move towards a more scientifically accepted understanding of the nature of hydrogen bonds among water molecules in snowflakes. After IVR, more students (n = 61) recognised hydrogen bonds as electrostatic, directional, and involving individual lone pairs of electrons compared to those (n = 34) who recognised this before IVR. The number of students who verbally discussed and/or represented molecular interactions in 3D also improved from five (5) before the IVR experience to 57 after the IVR experience.Secondly, our findings showed that, through the collaborative IVR experience, most students built a coherent understanding of the shape of snowflakes. Before IVR, 11 students were not sure how to explain the shape of snowflake. Twenty-six students imagined that six water molecules connected to one molecule at the centre of a snowflake or that molecules spread out in a two-dimensional plane to form snowflakes. These students focused on the ideas that snowflakes had six branches and looked flat. Therefore, the students concluded that six molecules must be connecting to a central one to form hexagons, and/or that molecules branch out in a two-dimensional plane to form flat shapes. This finding is consistent with previous studies which reported that, when asked to explain complex macroscopic properties of substances, many students tend to rely on simple pattern recognition, superficial features, and heuristics rather than mechanistic reasoning (e.g., McClary and Talanquer, 2011; Cooper et al., 2013). After exploring the molecular structures in IVR, many of the students were able to integrate concepts of hydrogen bonds, 3D molecular interactions, and hexagonal patterns among water molecules in 3D space in their explanations of the shape of snowflakes.
The collaborative IVR experience may have supported students’ understanding of the nature of hydrogen bonds among water molecules in several ways. For example, IVR showed the virtual water molecule models in three dimensions and provided visual cues to represent hydrogen bonds forming between lone pairs of electrons and hydrogen atoms. The students were also able to walk around the 3D structures to change perspectives and interact with the molecular structures by moving, flipping, rotating, and connecting them while discussing ideas with their partners in the same environment. These features may have helped the students to test their ideas in IVR, examine their preconceptions, and recognise how molecules interacted with each other and the arrangements of molecules in the structures formed. Consequently, most students were able to make positive changes to their diagrams and verbal explanations. Our findings are consistent with the argument that IVR not only supports development of practical skill-oriented knowledge as reported by Jensen and Konradsen (2018) but also the learning of abstract science concepts (Wu et al., 2020b). The present study lends support to previous studies which reported that IVR can improve students’ understanding of abstract science concepts (Dede et al., 1997; Kozhevnikov et al., 2013; Tsivitanidou et al., 2021).
The study found that, amongst the 10 students who initially had difficulties with drawing the structure of a water molecule or distinguishing intermolecular from intramolecular interactions, four of them retained most of their preconceptions whereas the other six improved their understanding. Studies have reported that students’ alternative conceptions may persist even after explicit instruction, and that changing these conceptions may be a gradual rather than a sudden process (Treagust and Duit, 2008). For meaningful learning to occur, learners need to make a connection between the new information and the existing prior knowledge (Taber, 2017). In the present study, some students may have failed to reconcile the new information provided in IVR with their own ideas or to interpret the relevant features in the molecular structures provided in IVR. This outcome is probably because the students had difficulties visualising basic concepts such as bonding and structure or polarity of water molecules.
Even though most students provided explanations that linked the nature of hydrogen bonds among molecules in 3D space to the shape of snowflakes after IVR, some students (n = 3) were still unable to successfully explain the shape of snowflakes after the IVR experience. Some students identified relevant features such as the large number of molecules in snowflakes, hexagonal patterns of molecules, and the 3D nature of the molecular lattice but did not provide elaborate explanations in relation to hydrogen bonds or polarity of molecules. This result was unexpected. However, using the concept of hydrogen bonds to explain the shape of snowflakes is a complex task which requires integration of many chemistry concepts. Students’ difficulties in coherently explaining structure-property relationships have been widely reported in previous studies (e.g., Cooper et al., 2013). Our findings suggest that a single exposure was not enough to help some of the students to make a clear link between the macroscopic and submicroscopic levels of snowflakes.
Implications
The intuitive nature of interactions and 3D visualisation capabilities of IVR hold promise in helping students visualise molecular interactions and improve their conceptual understanding of chemistry. In this study, we investigated undergraduate chemistry students’ conceptions of the nature of hydrogen bonds in snowflakes before and after a collaborative IVR session. Before the IVR session, many students did not demonstrate a good grasp of the nature of hydrogen bonds among water molecules. However, by engaging in a series of hands-on activities with the models of water molecules in a collaborative IVR environment, many students tested their preconceptions and built a more scientific understanding of the nature of hydrogen bonds and the shape of snowflakes. For example, after IVR, about half of the students who initially had difficulties recognising the nature of hydrogen bonds as intermolecular interactions recognized that hydrogen bonds are not covalent in nature. Instead, hydrogen bonds were electrostatic forces of attraction between lone pairs of electrons and hydrogen atoms in neighbouring molecules. This research investigation with IVR has demonstrated the potential of this medium to challenge and address students’ alternative conceptions in chemistry, such as those related to the concept of hydrogen bonds. Therefore, we recommend that IVR be further investigated with different chemistry content and in different contexts to further understand its potential.Our findings also showed that learners may not benefit equally from IVR interventions. For instance, about half of the students who initially struggled with drawing the structure of a water molecule or recognising the nature of intermolecular interactions retained their preconceptions. In future studies, educators may wish to experiment with different strategies to support low prior knowledge learners in gaining the benefit of this novel technology when learning different science concepts. For example, purposefully designed activities to orient students to the target concepts in preparation for more independent learning in IVR have been reported to support science knowledge gains (e.g., Wu et al., 2020a). The benefit of other strategies such as scaffolding or explicit guidance to focus students’ attention on the target features in the learning environment while they learn in IVR should also be explored further. In addition, as educators design to systematically investigate the educational benefits of IVR in future studies, they may wish to explore whether repeated use of IVR may further improve conceptual understanding.
In the present study, not many students collaborated on the pre- and post-interview tasks. However, students who collaborated on the diagram-drawing tasks tended to change their conceptions to scientifically accurate conceptions in a more consistent manner compared to students who worked individually on the tasks. Collaborating with peers may have allowed students to reflect on their learning from IVR or reinforce their understanding through discussion. This observation from our study is consistent with the findings of Klingenberg et al. (2020) who investigated students’ learning of biology concepts with IVR. The authors reported that students who were asked to teach peers after individual play in IVR had higher learning outcome benefits compared to students who did not engage in the peer-teaching task after IVR (Klingenberg et al., 2020). In fact, peer-teaching has been identified as one of the strategies that can enhance students’ learning (Fiorella and Mayer, 2016). In the present study, collaboration on post-IVR tasks may be considered as a form of reflection or peer-teaching; therefore, its role in students’ learning with IVR needs to be investigated further in future studies. For example, future studies may want to experiment with different designs (for example, collaborative versus individual IVR designs) to explore whether collaboration in IVR leads to better learning gains.
Limitations
This study had some limitations. Firstly, learners were paired to complete the IVR learning tasks and the assessment tasks in pre- and post-interviews together. This was done to foster collaborative learning and to provide an opportunity for the students to consider and reflect on their understanding while explaining their ideas to the peers. However, during interviews, most students showed reluctance to collaborate with their peers and, instead, completed the diagrams and explanations individually. A small number of students switched from individual work in the pre-interview to collaborative work in the post-interview. Due to the variety in students’ collaborative tendencies, the interviewer adjusted the assessment prompts from seeking collaborative responses to accommodating individual students’ preferences. These variations made the interpretation of students’ learning or the changes in their conceptions more complex than anticipated. Moreover, since the learners were paired, the verbal responses from each learner working individually during the pre- and post-interviews may have been influenced either directly or indirectly by the partners who were seated next to them. Secondly, although all the interactions were videotaped, this study did not investigate how learners interacted with the IVR environment to derive the learning benefits. In our future work, we intend to explore how each pair of learners constructed their understanding during the collaborative IVR experience. Thirdly, this study presents results from a single exposure of students to IVR. Educators warn that learning benefits may be partly influenced by the novelty of the learning technology (Clark, 1983). Based on the findings from this study, investigating conceptual benefits from IVR over multiple sessions is likely to demonstrate the power of IVR in supporting students’ learning of science concepts. Lastly, in this study, we aimed to investigate how IVR changed the students’ conceptual understanding rather than evaluate the effectiveness of IVR compared to other learning modes. In future studies, researchers may wish to include comparison groups to evaluate the learning benefits of IVR against alternative media.Conclusions
The huge investment in technology development by major tech companies has made IVR technology more accessible for use in science classes. However, the evaluation of the educational benefits of IVR has been limited to motivation, engagement, and recall knowledge, therefore, providing limited justification for the huge investment in IVR. By analysing student-generated diagrams and verbal explanations before and after IVR, this study provides evidence that IVR can support students’ conceptual understanding of abstract chemistry concepts such as the nature of hydrogen bonds among water molecules and the shape of snowflakes. The impact of IVR on students’ conceptual understanding needs to be investigated further in different chemistry learning contexts and with different concepts. Educators may also want to experiment with the implementation of different scaffolding strategies to maximise the learning benefits of IVR. Such strategies may include pre-activities to orient students to the target concepts before exploring them in IVR or highlighting the important features in molecular structures in IVR designs to focus students’ attention while exploring the science concepts in IVR.Conflicts of interest
The authors have no known conflict of interest to declare.Appendices
Pre-interview prompts
The prompts were adjusted depending on the willingness of the students to collaborate with peers.1. We are going to explore how we can use the chemistry concepts we have learnt to explain the unique shape of snowflakes. Have you seen snow before? Where?
2. Here I have some images of snowflakes (the interviewer displays pictures of some of the shapes of snowflakes). What do you notice about the shapes of snowflakes? Discuss your ideas.
3. To explain the shape of snowflakes, let's start with the chemistry concepts we have learned so far and see how we can build on those. Imagine I am a year 11 (high school) student, what can you tell me about a water molecule?
4. Please draw the water molecule you have described at the centre of the piece of paper. [Feel free to work together to create a combined diagram if you wish].
Why is the water molecule shaped like that? [Explain to your partner]
5. Now, let's imagine that this water molecule is at a very low temperature, say close to its freezing point, and we have another water molecule coming close to the first one to interact with it, how would you draw the interaction between those two water molecules?
Why would the molecules interact like that? [Explain to your partner]
6. We have another molecule coming close to the first one. Is it still possible for it to interact with the first molecule? How would you draw the interaction between the third water molecule and the first one? [Please explain to your partner why the molecules would interact like this.]
(The students were prompted to add more water molecules to the central water molecule until the students said that no more water molecules would interact with the central molecule, with reasons)
7. How do you think the interactions you have illustrated in the diagram would be translated into the shape of snowflakes?
Post-interview prompts
1. What do you think you have learned from the IVR activity?2. Now that you have explored the nature of interactions amongst water molecules, please look at your initial diagram.
Are you still happy with the diagram? Please explain.
How would you improve the diagram? Please explain the changes you have made.
3. Imagine that I am a year 11 student who knows about the structure and polarity of a water molecule. Starting with the structure and polarity of a water molecule, how would you explain to me why snowflakes are shaped the way they are? You may also use a diagram, if you can, to explain to me.
Acknowledgements
This study was supported by Australian Research Council Discovery Projects, Drawing science diagrams to enhance students’ scientific creativity (DP180100143) and Using immersive virtual reality to enhance students’ scientific visualisation (DP190100160).References
- Ainsworth S., Prain V. and Tytler R., (2011), Drawing to learn in science, Science, 333(6046), 1096–1097.
- Anderson T. and Shattuck J., (2012), Design-based research: a decade of progress in education research? Educ. Res., 41(1), 16–25.
- Andrade D. V. F., Freire S. and Baptista M., (2021), Constructing scientific explanations for chemical phenomena through drawings among 8th-grade students, Eurasia J. Math. Sci. Technol., 17(1), em1937.
- Bennie S. J., Ranaghan K. E., Deeks H., Goldsmith H. E., O’Connor M. B., Mulholland A. J. and Glowacki D. R., (2019), Teaching enzyme catalysis using interactive molecular dynamics in virtual reality, J. Chem. Educ., 96(11), 2488–2496.
- Chang H.-Y., Lin T.-J., Lee M.-H., Lee S. W.-Y., Lin T.-C., Tan A.-L. and Tsai C.-C., (2020), A systematic review of trends and findings in research employing drawing assessment in science education, Stud. Sci. Educ., 56(1), 77–110.
- Clark R. E., (1983), Reconsidering research on learning from media, Rev. Edu. Res., 53(4), 445–459.
- Coban M., Bolat Y. I. and Goksu I., (2022), The potential of immersive virtual reality to enhance learning: a meta-analysis, Educ. Res. Rev., 36, 100452.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An investigation of college chemistry students' understanding of structure–property relationships, J. Res. Sci. Teachnol., 50(6), 699–721.
- Cooper M. M., Williams L. C. and Underwood S. M., (2015), Student understanding of intermolecular forces: a multimodal study, J. Chem. Educ., 92(8), 1288–1298.
- Dede C., (2009), Immersive interfaces for engagement and learning, Science, 323(5910), 66–69.
- Dede C., Salzman M., Loftin R. B. and Ash K., (1997), Using virtual reality technology to convey abstract scientific concepts, in Jacobson M. J., Kozma R. B. and Erlbaum L. (ed.), Learning the sciences of the 21st century: Research, design, and implementing advanced technology learning environments, Hillsdale, NJ: Lawrence Erlbaum, pp. 1–44.
- Dede C. J., Jacobson J. and Richards J., (2017), Introduction: Virtual, augmented, and mixed realities in education, in Liu D., Dede C., Huang R. and Richards J. (ed.), Virtual, augmented, and mixed realities in education, Singapore: Springer, pp. 1–16.
- Derman A., Koçak N. and Eilks I., (2019), Insights into components of prospective science teachers’ mental models and their preferred visual representations of atoms, Educ. Sci., 9(2), 154.
- Duit R. and Treagust D. F., (2003), Conceptual change: a powerful framework for improving science teaching and learning, Int. J. Sci. Educ., 25(6), 671–688.
- Edwards B. I., Bielawski K. S., Prada R. and Cheok A. D., (2019), Haptic virtual reality and immersive learning for enhanced organic chemistry instruction, Virtual Real., 23, 363–373.
- Elford D., Lancaster S. J. and Jones G. A., (2021), Stereoisomers, not stereo enigmas: A stereochemistry escape activity incorporating augmented and immersive virtual reality, J. Chem. Educ., 98(5), 1691–1704.
- Ferrell J. B., Campbell J. P., McCarthy D. R., McKay K. T., Hensinger M., Srinivasan R., Zhao X., Wurthmann A., Li J. and Schneebeli S. T., (2019), Chemical exploration with virtual reality in organic teaching laboratories, J. Chem. Educ., 96(9), 1961–1966.
- Fiorella L. and Mayer R. E., (2016), Eight ways to promote generative learning, Educ. Psychol. Rev., 28(4), 717–741.
- Fombona-Pascual A., Fombona J. and Vázquez-Cano E., (2022), VR in chemistry, a review of scientific research on advanced atomic/molecular visualization, Chem. Educ. Res. Prac., 23(2), 300–312.
- Gobert J. D. and Clement J. J., (1999), Effects of student-generated diagrams versus student-generated summaries on conceptual understanding of causal and dynamic knowledge in plate tectonics, J. Res. Sci. Teach., 36(1), 39–53.
- Hamilton D., McKechnie J., Edgerton E. and Wilson C., (2021), Immersive virtual reality as a pedagogical tool in education: a systematic literature review of quantitative learning outcomes and experimental design, J. Comput. Educ., 8(1), 1–32.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How do organic chemistry students understand and apply hydrogen bonding? J. Chem. Educ., 78(8), 1126.
- Holme T. A., Luxford C. J. and Brandriet A., (2015), Defining conceptual understanding in general chemistry, J. Chem. Educ., 92(9), 1477–1483.
- Jensen L. and Konradsen F., (2018), A review of the use of virtual reality head-mounted displays in education and training, Educ. Inf. Technol., 23(4), 1515–1529.
- Jewitt C., (2013), Multimodal methods for researching digital technologies, in Price S., Jewitt C. and Brown B. (ed.), The SAGE handbook of digital technology research, London: SAGE, pp. 250–265.
- Johnson-Glenberg M. C., (2018), Immersive VR and education: Embodied design principles that include gesture and hand controls, Front. Robot. AI, 5, 81.
- Jonassen D. H., (1994), Thinking technology: Toward a constructivist design model, Educ. Technol., 34(4), 34–37.
- Klingenberg S., Jørgensen M. L., Dandanell G., Skriver K., Mottelson A. and Makransky G., (2020), Investigating the effect of teaching as a generative learning strategy when learning through desktop and immersive VR: a media and methods experiment, Br. J. Educ. Technol., 51(6), 2115–2138.
- Kozhevnikov M., Gurlitt J. and Kozhevnikov M., (2013), Learning relative motion concepts in immersive and non-immersive virtual environments, J. Sci. Educ. Technol., 22(6), 952–962.
- Krämer N. C., (2017), The Immersive power of social interaction, in Liu D., Dede C., Huang R. and Richards J. (ed.), Virtual, augmented, and mixed realities in education, Singapore: Springer, pp. 55–70.
- Martinez M. E., (1999), Cognition and the question of test item format, Educ. Psychol., 34(4), 207–218.
- Matovu H., Ungu D. A. K., Won M., Tsai C.-C., Treagust D. F., Mocerino M. and Tasker R., (2022a), Immersive virtual reality for science learning: Design, implementation, and evaluation, Stud. Sci. Educ., 1–40.
- Matovu H., Won M., Treagust D. F., Mocerino M., Ungu D. A. K., Tsai C.-C. and Tasker R., (2022b), Analysis of students’ diagrams of water molecules in snowflakes to reveal their conceptual understanding of hydrogen bonds, Chem. Educ. Res. Prac., 1–16.
- McClary L. and Talanquer V., (2011), College chemistry students' mental models of acids and acid strength, J. Res. Sci. Technol., 48(4), 396–413.
- McLure F., Won M. and Treagust D. F., (2021a), Analysis of students’ diagrams explaining scientific phenomena, Res. Sci. Educ., 52(4), 1225–1241.
- McLure F., Won M. and Treagust D. F., (2021b), What students’ diagrams reveal about their sense-making of plate tectonics in lower secondary science, Int. J. Sci. Educ., 43(16), 2684–2705.
- Merriam S. B. and Tisdell E. J., (2015), Qualitative research: A guide to design and implementation, San Francisco: John Wiley & Sons.
- Noyes K. and Cooper M. M., (2019), Investigating student understanding of London dispersion forces: a longitudinal study, J. Chem. Educ., 96(9), 1821–1832.
- Parong J. and Mayer R. E., (2021), Cognitive and affective processes for learning science in immersive virtual reality, J. Comput. Assist. Learn., 37(1), 226–241.
- Radianti J., Majchrzak T. A., Fromm J. and Wohlgenannt I., (2020), A systematic review of immersive virtual reality applications for higher education: design elements, lessons learned, and research agenda, Comput. Educ., 147, 103778.
- Reeves S. M., Crippen K. J. and McCray E. D., (2021), The varied experience of undergraduate students learning chemistry in virtual reality laboratories, Comput. Educ., 175, 104320.
- Rychkova A., Korotkikh A., Mironov A., Smolin A., Maksimenko N. and Kurushkin M., (2020), Orbital battleship: a multiplayer guessing game in immersive virtual reality, J. Chem. Educ., 97(11), 4184–4188.
- Schmidt M., (2008), The Sankey diagram in energy and material flow management, J. Ind. Ecol., 12(2), 173–185.
- Schmidt H.-J., Kaufmann B. and Treagust D. F., (2009), Students’ understanding of boiling points and intermolecular forces, Chem. Educ. Res. Prac., 10(4), 265–272.
- Seritan S., Wang Y., Ford J. E., Valentini A., Gold T. and Martínez T. J., (2021), InteraChem: Virtual reality visualizer for reactive interactive molecular dynamics, J. Chem. Educ., 98(11), 3486–3492.
- Taber K. S., (2017), The nature of student conceptions in science, in Taber S. K. and Akpan B. (ed), Science education. New directions in mathematics and science education, Rotterdam: Sense Publishers, pp. 119–131.
- Thompson M. M., Wang A., Bilgin C. U., Anteneh M., Roy D., Tan P., Eberhart R. and Klopfer E., (2020), Influence of virtual reality on high school students' conceptions of cells, J. Univers. Comput. Sci., 26(8), 929–946.
- Tippett C. D., (2016), What recent research on diagrams suggests about learning with rather than learning from visual representations in science, Int. J. Sci. Educ., 38(5), 725–746.
- Treagust D. F., (1988), Development and use of diagnostic tests to evaluate students’ misconceptions in science, Int. J. Sci. Educ., 10(2), 159–169.
- Treagust D. F. and Duit R., (2008), Conceptual change: a discussion of theoretical, methodological and practical challenges for science education, Cult. Stud. Sci. Educ., 3(2), 297–328.
- Treagust D. F., Won M. and McLure F., (2017), Multiple representations and students’ conceptual change in science, in Amin G. T. and Levrini O. (ed.), Converging perspectives on conceptual change: Mapping an emerging paradigm in the learning sciences, London: Routledge, pp. 121–128.
- Tsivitanidou O. E., Georgiou Y. and Ioannou A., (2021), A Learning experience in inquiry-based physics with immersive virtual reality: student perceptions and an interaction effect between conceptual gains and attitudinal profiles, J. Sci. Educ. Technol., 30(6), 841–861.
- Vygotsky L. S., (1978), Mind in society: The development of higher psychological processes, Harvard University Press.
- Wang F. and Hannafin M. J., (2005), Design-based research and technology-enhanced learning environments, Educ. Technol. Res. Dev., 53(4), 5–23.
- Webster R., (2016), Declarative knowledge acquisition in immersive virtual learning environments, Interact. Learn. Environ., 24(6), 1319–1333.
- Williams L. C., Underwood S. M., Klymkowsky M. W. and Cooper M. M., (2015), Are noncovalent interactions an Achilles heel in chemistry education? A comparison of instructional approaches, J. Chem. Educ., 92(12), 1979–1987.
- Winn W., (1993), A conceptual basis for educational applications of virtual reality: Technical Publication R-93-9, Human Interface Technology Laboratory of the Washington Technology Center, Seattle: University of Washington.
- Won M., Mocerino M., Tang K.-S., Treagust D. F. and Tasker R., (2019), Interactive immersive virtual reality to enhance students’ visualisation of complex molecules, in Schultz M., Schmid S. and Lawrie G. A. (ed.), Research and Practice in Chemistry Education, Singapore: Springer, pp. 51–64.
- Wu B., Hu Y. and Wang M., (2020a), How do head-mounted displays and planning strategy influence problem-solving-based learning in introductory electrical circuit design? Educ. Technol. Soc., 23(3), 40–52.
- Wu B., Yu X. and Gu X., (2020b), Effectiveness of immersive virtual reality using head-mounted displays on learning performance: a meta-analysis, Br. J. Educ. Technol., 51(6), 1991–2005.
- Zhang L., Bowman D. A. and Jones C. N., (2019), Exploring effects of interactivity on learning with interactive storytelling in immersive virtual reality, Vienna, Austria.
- Zhao R., Chu Q. and Chen D., (2022), Exploring chemical reactions in virtual reality, J. Chem. Educ., 99(4), 1635–1641.
| This journal is © The Royal Society of Chemistry 2023 |