Investigation of students’ cognitive structures concerning the topic of physical and chemical changes: a cross-level study
Canan
Nakiboğlu

Division of Chemistry Education, Necatibey Education Faculty, Balıkesir University, Balıkesir, Turkey. E-mail: nakiboglu2002@yahoo.com
First published on 7th September 2022
Abstract
The topic of physical and chemical changes is one of the basic and essential issues of both the lower-secondary school science curriculum and the upper-secondary school chemistry curriculum in many countries. The focus of the present study is to investigate the students' cognitive structures on the topic of physical and chemical changes at different grade levels and to compare how the aspects of students' cognitive structures differ at 8th, 9th, and 10th-grade levels. The data were collected from a total of 388 students (126 8th-graders, 129 9th-graders, and 133 10th-graders) through using a word association test (WAT) which contains eight stimulus words. Both the relatedness coefficient (RC) method and the response frequency mapping method were used in the analysis of the data and mapping of the group cognitive structures of the students. The variables related to the concepts or ideas contained, the connections between the concepts, and the information processing skills, which are the three main aspects in defining the cognitive structures, are compared according to the grade level. It has been concluded that the cognitive structure of the 9th-grade students is the most comprehensive, and integrated, and has a higher information retrieval rate. On the other hand, when the data obtained from both the RC values and the response frequency mappings were examined, it was found that there were also several similarities in the cognitive structures of the three grade levels. For instance, the relationship level of the stimulus word 'energy' with other stimulus words is very weak at all grade levels.
Introduction
The role of prior knowledge in science learning is largely based on theories from cognitive science. Many cognitive scientists are concerned with the possible mechanisms by which new and previously acquired information is modified to make it more suitable for storage in memory (Anderson, 1992). According to Johnstone's (1997) information processing model, successful learning requires an efficient perception filter, working memory, and long-term memory. Baddeley (2012) indicated that the term “working memory” evolved from the earlier concept of short-term memory (STM), and the two are still on occasion used interchangeably. On the other hand, Baddeley and Hitch (1974) used the term working memory to refer to a system comprising multiple components and emphasized the functional importance of this system as opposed to simple storage capacity (cited in Baddeley, 2002).Fisher (2004) has pointed out that all learners construct knowledge in their conscious working memory and store this knowledge in long-term memory. Long-term memory is the large store where facts are stored, concepts are developed, and which informs the perception filter (Johnstone et al., 1994). Fisher and Lipson (1985) stated that although conscious thought and working memory processes are sometimes different, activity in working memory is sometimes considered synonymous with conscious thought. They indicated that “emotions and the limitations of the information processing system interact with sensory information, memories, and intentions to determine what we pay attention to, what we remember, and what actions we undertake (p. 53)”. Miller et al. (2018) also indicated that working memory is the “sketchbook of conscious thought” and forms the basis of the organization of goal-directed behaviour.
There are several models of how information is stored in learners' long-term memory (St Clair-Thompson et al., 2010). The knowledge acquired by students in science classes is stored in their long-term memory in a hierarchically organised form and can be represented as a cognitive structure in their memory (Tsai, 2001; Kalyuga, 2006). The cognitive structure includes the learners' existing experiences and knowledge that lead to their reconstruction and information processing of incoming stimuli (Nakiboglu, 2008). Various terms are used to describe the cognitive structure, e.g. structural knowledge (Jonassen et al., (1993) cited in Tsai and Huang (2002)) or knowledge structure (Champagne et al., 1981; Novak and Cañas, 2008).
The cognitive structure is a considerable building block for meaningful learning and retention of instructional materials (Ifenthaler et al., 2011). It is also a significant factor in both remembering previous knowledge and learning new knowledge (Gorodetsky and Hoz, 1985). Therefore, determining students' cognitive structure is useful in assessing both subject knowledge and prior knowledge. Snow (1989) pointed out that the uncovered cognitive structure can serve as a topographical map to identify key areas of learning difficulties and facilitate instructional interventions (cited in Ifenthaler et al., 2011). Thus, exposing students' cognitive structure can also help teachers develop appropriate instructional strategies.
The present study investigates how the cognitive structures of Turkish students at different grade levels on the topic of physical and chemical changes differ in the learning process. The Turkish education system is divided into three stages of 12 years of compulsory education. The first level is a four-year elementary education (which comprises grades 1, 2, 3, and 4), and the second level is a four-year lower-secondary education (which comprises grades 5, 6, 7, and 8). The third stage is a four-year upper-secondary education (which comprises grades 9, 10, 11, and 12).
The Science course starts at the 3rd-grade level and there is one unit related to the matter at each grade. The unit at the 3rd-grade level is “Let's Get to Know the Matter” and in this unit, the matter is introduced and the states of matter are explained. Examples of states of matter are given from daily life, but their structures (fluidity, the distance between particles, etc.) are not mentioned. At the 4th grade level, there is the unit “Properties of Matter”. In this unit, it is aimed to enable the students to define the concept of matter within the scope of mass and volume concepts. It is aimed to explain the melting and freezing events from state changes, classify the substance into two groups as pure and mixture, and separate various mixtures by sieving, filtering and magnetic attraction without the particular structure of matter being mentioned. In the 5th grade, the “Matter and Change” unit aims to explain melting, freezing, boiling, condensation, evaporation, sublimation and defrosting phenomena on the basis of heat exchange. The particulate nature of matter is introduced for the first time in the 6th grade. The “Matter and Heat” unit in the 6th grade aims to calculate the changes in matter and the density of matter. In the unit of “Pure Substances and Mixtures” in the 7th grade, students are taught the structure of an atom. This unit aims to classify elements, compounds and mixtures on the basis of pure and impure substances. In addition, it is aimed to learn some separation techniques used in the separation of mixtures, the symbols of elements and the formulae of compounds, and to explain the dissolution phenomenon with the relationship between solvent and solute molecules. Besides, energy and its types are examined in more detail in the 7th grade. The topic of “physical and chemical changes” is taught for the first time in the 8th grade in the unit of “Matter and Industry”. In this unit, students learn the classification of elements, changes in matter, and classification of physical and chemical changes. It is also taught that compounds are formed as a result of chemical reactions.
In the Turkish 8th-grade Science Curriculum, there are two learning outcomes related to physical and chemical changes, which are given below.
“(1) To explain the differences between physical and chemical changes by observing various events.
(2) To know that compounds are formed as a result of chemical reactions.”
Students re-learn the topic of physical and chemical changes and, accordingly, chemical reactions, in the first year of their upper-secondary school education, that is, in the 9th grade. Physical and chemical changes are included as a topic in the 9th-grade unit “Chemical Interactions Between Species”. In this unit, after explaining chemical bonds and intermolecular interactions, physical and chemical changes are taught. In the Turkish 9th-grade Chemistry Curriculum, there is one learning outcome related to physical and chemical changes and this is given below.
“To be able to distinguish physical and chemical changes on the basis of the magnitude of the bond energies that are broken and formed.”
As seen from the aforementioned explanations, the topic of physical and chemical changes is one of the basic and essential topics in both the lower-secondary school science curriculum and the upper-secondary school chemistry curriculum in Turkey. In this study, I didn't attempt to develop a conceptually modified teaching approach to the topic of physical and chemical changes. Instead, I was interested in the learning phase, i.e. I was interested in how students' cognitive structures were affected by traditional teaching, which teachers mostly use in schools in Turkey. The traditional teaching method is a teacher-centric method. In the traditional ways of teaching, the teacher's opinion dominates and the students are only compelled to memorize and reproduce knowledge (Vlassi and Karaliota, 2013). Nevertheless, whether the traditional ways of teaching or student-centred teaching approaches are used, it's clear that in each learning phase there's a major or minor change in the minds of the students. While the major changes lead to a radical transformation of the learners' knowledge, the minor changes are assimilations and lead to a weak reconstruction of knowledge. In the traditional teaching approach, it was hoped that learners' cognitive structures would change only piecemeal, i.e. to a small extent. From this point of view, the focus of the present study is to investigate the cognitive structures of students at different grade levels regarding the topic of physical and chemical changes after instruction. The literature on science education has found that students have learning difficulties and misunderstandings in identifying the physical and chemical changes. On the other hand, many studies have been reported in the literature on specific learning problems and misconceptions of students regarding the physical and chemical changes, but no study has been found that compares students' cognitive structures on this topic with a cross-grade study.
Theoretical framework
Cognitive development is one of the widely studied areas. One of the influential theories of cognitive development that can be accepted as Post-Piagetian theory is the information-processing approach, which provides new insights into how the human mind receives, stores, retrieves, and uses information. The most widely used model of information processing is the stage theory model, based on the work of Atkinson and Shiffrin (cited in Lutz and Huitt, 2003). The major elements of this model are that it views learning and memory as discontinuous and multistage. According to this model, human memory comprises a sequence of three stages: sensory memory, short-term memory, and long-term memory. It's assumed that new information that's taken in is processed in some way before it is stored. The multi-store model accepts that different types of memory are used for different tasks. Long-term memory is a large store where facts are retained, concepts are developed and attitudes are formed (Johnstone et al., 1994), and it also provides the lasting retention of information from minutes to a lifetime. Long-term memory is considered a crucial area that influences learning and performance (St Clair-Thompson et al., 2010). Tulving (1972) pointed out that long-term memory consists of different components, namely episodic and semantic memory. He suggested that “episodic memory receives and stores information about temporally dated episodes or events and temporal–spatial relationships between these events” (Tulving, 1972, p. 385). Tulving (1972) also defined semantic memory as “the memory necessary for the use of language”. He explained that semantic memory is “a mental thesaurus, an organised knowledge that a person possesses about words and other verbal symbols, their meanings, and references, about relationships between them, and the rules, formulas, and algorithms for handling these symbols, concepts, and relationships (p. 386).”The knowledge of the language, the rules, and the concepts are stored in the semantic memory. In other words, semantic memory is the memory for meanings and can be more directly relevant to the practice of teaching science (Preece, 1978). Schwartz (2020) used the associative model of semantic memory and the hierarchical network model to explain how knowledge is symbolized in the cognitive structure. He described the associative model as the information in semantic memory represented as connections between information units. According to the hierarchical network model, “semantic memory is organised according to levels or hierarchies in which particular nodes are associated with characteristics associated with that level, but not with higher or lower levels (p. 220)”.
The term “association” describes the connection or relationship between ideas, concepts, or words that exist in the human mind, and manifests itself in the fact that the appearance of one entity entails the appearance of the other in the mind (Sinopalnikova, 2004). As can be understood from these explanations, revealing cognitive structures generally using diagrams and networks can provide a better understanding of the organization of cognitive structures. For this purpose, some techniques have been proposed by researchers to use to gain insights into cognitive structures.
Some of these techniques explore students' cognitive structures individually, while others reveal group cognitive structures. While techniques such as concept maps and flow charts are used to form individual cognitive structures, the word association test (Nakiboglu, 2008) can be used to obtain the cognitive structures of a group of students, and knowledge space theory can be utilized to investigate the cognitive organization of information characteristics of a group of students (Tóth, 2007). The word association test (WAT) is one of the most commonly used methods for mapping cognitive structures (Cachapuz and Maskill, 1987; Nakiboglu, 2008). Shavelson (1972) expressed that the underlying assumption of the WAT is that the order of retrieval of responses from long-term memory reflects at least a substantial part of the structure within semantic memory and between concepts. A measure of the semantic proximity of stimulus words in a WAT is related to the degree of overlap between response hierarchies.
Tsai and Huang (2002) pointed out that there are three main aspects in the description of cognitive structures, including the concepts or ideas contained, the connections between the concepts, and the information processing skills, and that these aspects of cognitive structures consist of five existing variables: extent, correctness, integration, availability, and information processing strategy analysis. The extent and correctness are used to evaluate the concepts identified. The extent indicates the number of elicited concepts contained in the cognitive structure, while the correctness concerns the number of alternative conceptions shown in the cognitive structures or the number of correct conceptions. The second aspect of the cognitive structure is the connection between the concepts in the cognitive structures and the connection indicates the degree of integration between the concepts. Information processing skills refer to the availability and analysis of information processing strategies. Tsai and Huang (2002) said that the availability of cognitive structures can be represented by the information retrieval rate and the information processing strategies, such as the use of defining, describing, inferring, or explaining (Tsai, 1999), revealed during information recall. According to Tsai and Huang (2002), the WAT examines only three variables of the cognitive structure, namely extent, integration, and availability.
Students’ conceptions about physical and chemical change topic
Gensler (1970) handled the discussions in books and lectures on physical and chemical changes with a critical perspective. He stated that he did not consider the distinctions between ‘physical’ and ‘chemical’ to be either guaranteed or wise. Thus, he put a powerful argument for abandoning the distinction between the physical and the chemical changes because it is empty of content. However, Strong (1970) stated that although he agrees with Gensler's view, it is still not right to abandon the distinction completely, and he put forward criteria for making this distinction. Palmer (1994) also stated that although great efforts were made in the ways of defining physical and chemical changes, none of these attempts led to clear explanations of what physical and chemical changes are, and therefore he described this situation as paradoxical.Researchers have argued that the reason for both students not having trouble making a distinction between physical and chemical changes and successfully understanding chemical changes and subsequent chemical reactions is based on the concept of the particulate nature of matter (Strong, 1970; Stavridou and Solomonidou, 1989; Ahtee and Varjola, 1998; Johnson, 2000; Merinoa and Sanmartí, 2009; Christian and Yezierski, 2012). Christian and Yezierski (2012) have indicated that this situation could encourage a deeper conceptual understanding. The problematic situation is related to the student's inability to establish a relationship between the properties of matter at the atomic scale as compared with the macro scale. Regarding this situation, Palmer (1994) stated that since physical properties are bulk properties, there will be no physical properties at the atomic scale, whereas chemical properties can be properties of a comparatively small number of atoms or molecules. Besides these difficulties, it has been stated that it is not only the abstract nature of the particular nature of matter that causes difficulties in understanding physical–chemical changes but also students' scientifically incorrect ideas about matter and their epistemological understandings of scientific models (Wiser and Smith (2008) cited in Christian and Yezierski (2012)).
Rationale and research questions
Based on the aforementioned explanations, it can be said that different understandings of physical and chemical changes should be expected from students of different ages, and also it would not be realistic to expect a single understanding of physical and chemical changes common to students of all ages. In particular when studies on chemical change are examined, it is seen that there was a differentiation in the students' opinions according to their age (Stavridou and Solomonidou, 1998; Papageorgiou et al., 2010). Studies have indicated that the idea of chemical change is particularly problematic for younger students to grasp (Stavridou and Solomonidou, 1998; Papageorgiou et al., 2010; Kypraios et al., 2014) and that the student's connection ability to establish a relationship between the properties of matter at the atomic scale varies from student to student. However, Kypraios et al. (2014) indicated that although this is a challenge for all ages, individual differences play also an important role. Thus Kypraios et al. (2014) investigated whether logical thinking has a similar effect on the understanding of chemical changes, with a study including student age, since cognitive variables have a significant impact on understanding the particulate nature of matter. At the end of their studies, they first determined that understanding chemical change is a challenge for all ages, and at this point, they pointed out that they could agree with those who support this idea (e.g.Stavridou and Solomonidou, 1998; Brosnan and Reynolds, 2001). However, they still couldn't help but ask the question “is ‘age’ really a key factor?”Kypraios et al. (2014) concluded that although a gradual increase in the mean of all cognitive variables was determined as the age increases; this is not observed in the case of students' understanding of the chemical change. They revealed that only in the 12th grade, that is, only in the group of those who are specialized, the effect of teaching on learning chemical change manifests itself. Therefore, they discussed that understanding chemical changes as the cause of this situation may mean that students at higher grade levels have more opportunity to acquire knowledge and thus be more able to answer relevant questions, rather than a knowledge development due to the age factor itself. Thus, they stated that the source of the problem related to chemical changes at every grade level can be attributed to the curriculum and teaching. Accordingly, they emphasized that an age group would represent the grade level and would essentially be an indirect evaluation of the curriculum and teaching efficiency.
Cognitive variables, which are also seen in the findings of the study by Kypraios et al. (2014), increase with age. However, the knowledge development of students about chemical changes is also related to the amount of knowledge they acquire during teaching. For this reason, it can be noteworthy to investigate the explanatory role of age or the grade level on students' cognitive structures on the topic of physical and chemical changes. From this point of departure, this study aimed to determine and compare the cognitive structures of students at different levels in terms of the main aspects of cognitive structures. For this purpose, the following three research questions were addressed.
• How do 8th, 9th, and 10th-grade students relate a given stimulus to another regarding the topic of physical and chemical changes, and what is the level of interconnectedness of these stimulus words?
• How are the power and the direction of associations 8th, 9th, and 10th-grade students establish between stimulus and response concepts regarding the topic of physical and chemical changes?
• How do aspects of the students' cognitive structures on the topic of physical and chemical changes differ at the 8th, 9th, and 10th-grade levels?
Method
Design, participants, and procedure
This study was conducted as a cross-level study. Cross-level studies are useful for describing students' understanding of a particular concept across grades and for identifying differences and similarities in their understanding at different age levels. Markic and Eilks (2013) have indicated that educational cross-level studies focus insights into different levels within a certain educational program and the advantage of it is that data can be collected in a manageable period. However, it has been shown as a limitation of such studies that investigating different groups at different levels of education may cause some limitations and that cross-level studies will not allow defining individual developments.The data were collected from a total of 388 students (126 8th-graders, 129 9th-grades, and 133 10th-grades) attending four different schools (three upper-secondary schools and two lower-secondary schools) in the same province in western Turkey. All of the students in this study were educated in the same educational system since Turkey uses a strict nationwide curriculum for every course in elementary, lower and upper secondary schools. From the interviews with the course teachers, it was determined that all of them used the traditional lecture method which is the regular instruction type in most courses in Turkey, and occasionally employed the question and answer method in teaching the subject. For this reason, it can be accepted that the students had similar academic experiences on the topic of physical and chemical changes although they come from different schools and levels.
While the subjects of 8th and 9th-grade were asked to complete the WAT after the instruction on the topic of physical and chemical changes, data were collected from 10th-grade students towards the end of the first semester. Participation in the study was done voluntarily and ethical principles were fully taken into account. Ethical permission for the study was obtained from Balikesir University Science and Engineering Disciplines Ethics Committee. Students were provided with information about the study before applying for the WAT and no data were collected from students who did not want to complete the WAT.
Development and content validity of the WAT
To provide the content validity of the WAT, the 8th-grade science textbooks, 8th-grade science curriculum, 9th-grade chemistry textbooks, and the high school chemistry curriculum were examined by the author. Johnson (1967) who examined the relationship between teaching and cognitive structures in the area of mechanics determined that mechanics concept words with a high frequency of occurrence in the students’ textbook tended to elicit more responses in a continued word association test than low-frequency words. Therefore, at the step of determining the stimulus words to be included in the WAT, common concepts that are most frequently used for both classes and that can be a model for the subject structure were determined. While determining the common concepts, at first a list of the concepts and how many times they were used in the teaching of the topic of physical and chemical changes in the 9th and 8th-grade books was created. After the list was created, concepts with a frequency value of one and concepts related to physical or chemical change examples (melting, evaporation, etc.) were extracted and the concept list was rearranged for both grade levels. When the concepts in this draft list were examined, seven common concepts were seen for both 8th and 9th graders. These are chemical changes, physical changes, chemical reactions, chemical properties, physical properties, matter, and reaction equations.The difference between 8th-grade and 9th-grade textbooks in the presentation of the topic of physical and chemical changes in the textbooks is as follows. While the topic is explained depending on the changes in the structure of the matter by presenting the physical and chemical change examples, and without emphasizing the energy changes in the 8th-grade textbook, the topic is presented depending on the changes in the structure of the matter and chemical changes are also explained according to bond breaking and formation and the energy changes in the 9th-grade textbook. As can be seen, the concept of energy is an important concept for the 9th-grade in teaching the topic of physical and chemical changes, and also the frequency of the energy concept was ranked 4th in the 9th-grade concept list. For this reason, it was decided to add the energy concept to the seven concepts in the draft list, and the number of concepts to draft the WAT was eight. Furthermore, some chemical phenomena and energy relations are taught to 8th-grade students during their 7th-grade education. It was thought that it would be appropriate to add the concept of energy to the WAT in order to see how these differences in the presentation of the topic of physical and chemical changes in the 8th and 9th-grade textbooks might cause a difference or similarity between the cognitive structure of the 8th-grade students and the students at other grade levels.
Finally, an online interview was conducted with a chemistry teacher, who teaches chemistry courses in upper secondary schools, and with a science teacher, who teaches science courses in lower secondary schools. The science teacher was asked which concepts they used most when teaching physical and chemical changes in the 8th-grade science lesson, and the chemistry teacher was asked which concepts they used most when teaching physical and chemical changes in the 9th-grade chemistry lesson. It was seen that these concepts overlapped with the determined concepts to a large extent for both 8th and 9th grades. In addition, the opinion of the chemistry and science teachers was consulted about the fact that eight concepts cover all the concepts in the teaching of the topic of physical and chemical changes and that they are suitable for the level of the student.
From the aforementioned process, it was decided that all concepts are plausible candidates for the basic concepts which define the dimensions of semantic space and other concepts could form the ostensive base on which the structure of relational concepts is built. Afterwards, pilot applications were made and as a result of data analysis, it was concluded that it would be appropriate to enclose eight concepts in the WAT as the stimulus concepts (chemical changes, physical changes, chemical reactions, chemical properties, physical properties, matter, and reaction equations).
Data collection and analysis
The following procedure was followed during the implementation of the WAT. The students were provided with a booklet, each page of which contained one of the eight stimulus words. To prevent distraction from the stimulus word it was rewritten along the page leaving space for associations (Shavelson, 1974). First of all, an explanation was given to the students about how they would answer the WAT. It has been reported that during the test, the time will be controlled and they will be given 30 seconds for each stimulus word. This optimum time span was preferred because it is a time used in many studies (Bahar and Hansell, 2000; Cordellini and Bahar, 2000). The students were also told that the response words should be primarily from the field of chemistry and not to write sentences. After that students were asked to write down within 30 seconds as many response words as they could think of in association with each stimulus word in the WAT. The data were collected under the control of the course teachers. After the data about the WAT were collected, the tests of the students who did not complete the test were cancelled and were not included in the data analysis.There are several ways to score data provided by a WAT and use it to graph cognitive structures. In this study, both ways were chosen in the analysis of the data, since the graphical interpretations obtained from both ways serve different purposes. One of them is the relatedness coefficient method suggested by Garskof and Houston (Stewart, 1979) and has been used by many researchers (Bahar et al., 1999; Nakiboglu, 2008). Thus, to get a determination of how the group of students relates a given stimulus to another, the relatedness coefficients (RC) were calculated for each grade (Bahar et al., 1999). Then, a mapping representing the cognitive structure was made from the RC values for stimuli concepts (Waern, 1972). Using this technique, a graph was produced showing concepts as points and proximity as lines. These were also used to measure the proximity or relatedness of the stimulus used in the WAT. In this analysis suitable cut-off points are chosen while drawing graphs and it is started from the strongest RC while this structure occurred (Bahar et al., 1999).
The strongest cut-off point for the graph was taken as 0.8 since it was 0.8 and above, which is the highest cut-off point for the data obtained in this study at all grade levels. Then the cut-off point was successively lowered until all stimulus words are appearing. The cut-off point at which all stimulus words join the graph corresponds to the lowest cut-off point on the graph. However, in this study, the cut-off point at which all the other seven stimulus words were added to the graph was taken as the lowest cut-off point since the stimulus word ‘energy’ at all grade levels came out at a very low cut-off point (0.1000). While the lowest cut-off point was 0.500 for 8th and 9th grades, it was determined as 0.400 for 10th grades. In the conclusion part of the study, to make a comparison between three grades, it was decided to take 0.400 for all grades as the lowest cut-off point, and graphs were created in this way. Finally, the thickness of the lines used to show the associations at each cut-off point level was drawn differently. The line thicknesses indicate the relative strengths of the associations.
The second way of obtaining students' cognitive structures is to draw a map using response frequencies instead of RC values (Bahar et al., 1999; Nakiboglu, 2008). In this way, from all the students’ responses to every stimulus word, a frequency table is obtained. The words used in the count are taken to be valid if they are meaningful and acceptable in terms of content. After creating a frequency table in this way, a ‘map’ is drawn using the frequency of response words to each stimulus word. Maps representing the cognitive structure of the student group were created by using the method developed by Nakiboglu (2008) from two different methods in map drawing. This map helps to determine the strength and direction of the relationships the students establish between the concepts of stimulus and response. In Nakiboğlu's method, the direction of the arrows and the strength of associations are determined by using frequency tables. Since the frequency tables obtained in this study contain a huge amount of data, the sample pattern of the frequency tables is given before the figures to correctly interpret how the student cognitive structures from Fig. 4 to Fig. 6 are drawn. When constructing a map representing cognitive structures using such a frequency table, a stimulus word obtained as a response word is framed, but a new response word is not framed. The thickness of the frames and arrows is determined by the frequency value of a stimulus word, and the thickness of the lines indicates the strength of the associations. While drawing the map, starting from the highest frequency value, it is decreased to the frequency at which all stimulus words are output. In this study, since the highest and lowest frequency values at each grade level are different from each other, the start and end frequency ranges for 8th, 9th, and 10th-grade levels are taken as different.
The reliability of the analysis was ensured in the form of both inter-referee reliability and intra-referee reliability. The first step of WAT analysis is to create a frequency table by counting the words given to each answer word. At this stage, the frequency tables were prepared by both the author and two graduate students. The author also made the analysis process final by repeating each analysis herself and correcting the different situations. Then, to get a determination of how the group of students relates a given stimulus to another, Garskoff and Houston relatedness coefficients (RC) were calculated for each grade (Bahar and Hansell, 2000;). Excel was used for these calculations, and the author repeated these calculations twice at different times, ensuring the reliability of the analysis. Besides, another lecturer did some of the calculations again and the results were compared. For this purpose, 15 randomly selected procedures were repeated and the results were compared. The results were determined to be completely compatible with this work.
Findings
Findings concerning the interconnectedness of the stimulus words
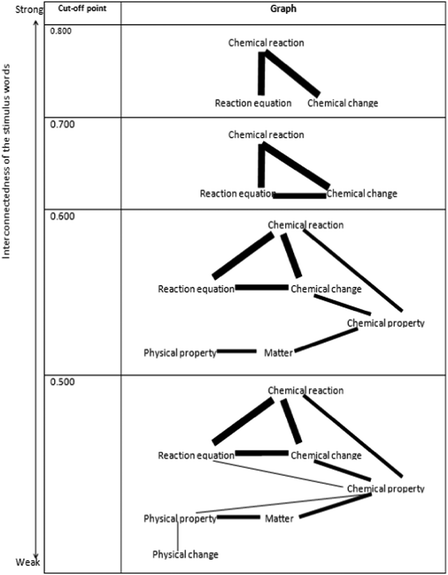
The first research problem focuses on the level of interconnectedness of the stimulus words which was determined by using the relatedness coefficients. To acquire associations of how 8th-grade students relate a given stimulus to another regarding physical and chemical changes, the relatedness coefficients were calculated, and the RCs computed are given in Table 1. The cognitive structures of the students were mapped and the graph is shown in Fig. 1 by using the relatedness coefficients in Table 1.| Stimulus words | Physical change | Chemical reaction | Energy | Chemical property | Physical property | Matter | Reaction equation |
|---|---|---|---|---|---|---|---|
| Chemical change | 0.481 | 0.809 | 0.075 | 0.689 | 0.387 | 0.423 | 0.730 |
| Physical change | — | 0.259 | 0.057 | 0.346 | 0.533 | 0.346 | 0.263 |
| Chemical reaction | — | — | 0.102 | 0.661 | 0.276 | 0.334 | 0.880 |
| Energy | — | — | — | 0.094 | 0.068 | 0.182 | 0.103 |
| Chemical property | — | — | — | — | 0.500 | 0.622 | 0.524 |
| Physical property | — | — | — | — | — | 0.627 | 0.223 |
| Matter | — | — | — | — | — | — | 0.296 |
As can be seen from Table 1 and Fig. 1, the most closely related stimulus concepts are ‘chemical reaction’ and ‘reaction equation’ (RC = 0.880), and ‘chemical change’ and ‘chemical reaction’ (RC = 0.809). The three stimulus words formed an interrelated island in the students' cognitive structures at a very high cut-off point which is 0.800, and these strong associations are from ‘chemical reaction’ to ‘reaction equation’, and ‘chemical change’ to ‘chemical reaction’ as presented in the first cell of Fig. 1. It can also be seen from Table 1 that when the cut-off point was lowered to 0.500, it is seen that all stimulus words except energy appeared on the map in cell four in Fig. 1. When the relationship level of the stimulus word ‘energy’ with other stimulus words is examined, it is seen that there are 3 relationships with relatedness coefficient values above 0.1. These associations are ‘chemical reaction’ and ‘energy’ (RC= 0.102), ‘energy’ and ‘matter’ (RC = 0.182), and ‘energy’ and ‘reaction equation’ (RC = 0.103).
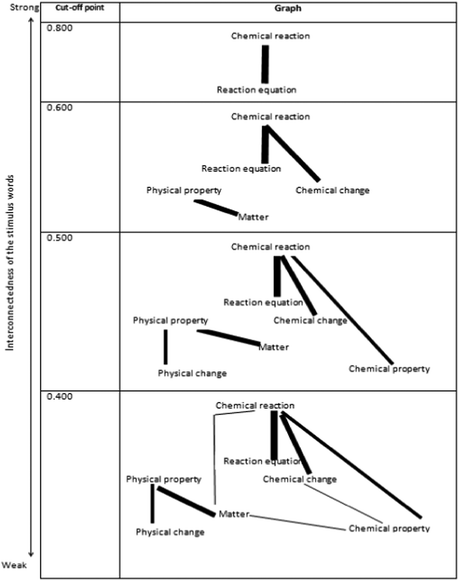
To acquire associations of how 9th-grade students relate a given stimulus to another regarding physical and chemical changes, the relatedness coefficients were calculated, and the RCs computed are given in Table 2. The cognitive structures of the 9th-grade students were mapped and the graph is shown in Fig. 2 by using the relatedness coefficients in Table 2.
| Stimulus words | Physical change | Chemical reaction | Energy | Chemical property | Physical property | Matter | Reaction equation |
|---|---|---|---|---|---|---|---|
| Chemical change | 0.221 | 0.654 | 0.084 | 0.498 | 0.129 | 0.314 | 0.311 |
| Physical change | — | 0.185 | 0.054 | 0.218 | 0.543 | 0.379 | 0.147 |
| Chemical reaction | — | — | 0.134 | 0.566 | 0.142 | 0.428 | 0.841 |
| Energy | — | — | — | 0.092 | 0.040 | 0.091 | 0.089 |
| Chemical property | — | — | — | — | 0.263 | 0.488 | 0.357 |
| Physical property | — | — | — | — | — | 0.656 | 0.098 |
| Matter | — | — | — | — | — | — | 0.320 |
As can be seen from Table 2, the most closely related stimulus concepts are ‘chemical reaction’ and ‘reaction equation’ (RC = 0.841). This association formed an interrelated island in the students' cognitive structures at a very high cut-off point which is 0.800, and this strong association is from ‘chemical reaction’ to ‘reaction equation’ as presented in the first cell of Fig. 2. It can be seen from Table 2 and Fig. 2 that at the 0.500 relatedness level all the stimulus words except ‘energy’ link to each other. When the relationship level of the stimulus word ‘energy’ with other stimulus words is examined, it is seen that there is only one relationship with a relatedness coefficient value above 0.1. These related stimulus concepts are ‘chemical reaction’ and ‘energy’ and the RC value for this association is 0.134.
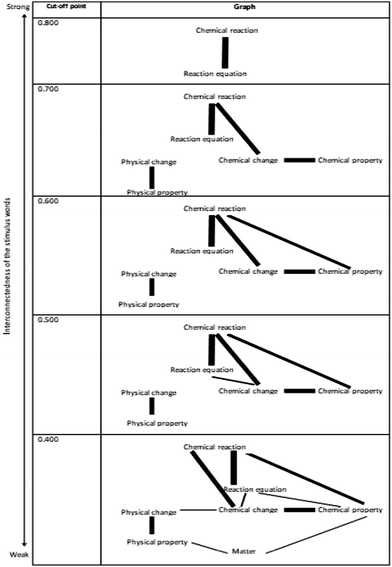
To acquire associations of how 10th-grade students relate a given stimulus to another regarding physical and chemical changes, the relatedness coefficients were calculated, and the RCs computed are given in Table 3. The cognitive structures of the 10th-grade students were mapped and the graph is shown in Fig. 3 by using the relatedness coefficients in Table 3.
| Stimulus words | Physical change | Chemical reaction | Energy | Chemical property | Physical property | Matter | Reaction equation |
|---|---|---|---|---|---|---|---|
| Chemical change | 0.411 | 0.790 | 0.143 | 0.725 | 0.252 | 0.177 | 0.532 |
| Physical change | — | 0.269 | 0.040 | 0.333 | 0.717 | 0.199 | 0.176 |
| Chemical reaction | — | — | 0.198 | 0.647 | 0.174 | 0.297 | 0.862 |
| Energy | — | — | — | 0.132 | 0.046 | 0.103 | 0.216 |
| Chemical property | — | — | — | — | 0.320 | 0.450 | 0.428 |
| Physical property | — | — | — | — | — | 0.441 | 0.137 |
| Matter | — | — | — | — | — | — | 0.275 |
As can be seen from Table 3 and Fig. 3, the strongest connected stimulus concepts for 10th-grade students are ‘chemical reaction’ and ‘reaction equation’ (RC = 0.862). When the cut-off point of 0.700 is examined, it is seen that the new associations between the stimulus words in the form of two separate islands are included in the map. When the cut-off point is reduced to 0.600, a new association from ‘chemical reaction’ to ‘chemical property’ is added to the first island, while when the cut-off point is reduced to 0.500, a new association appears between ‘chemical reaction’ and ‘reaction equation’ in the first island. The cut-off point was reduced to 0.400 because not all stimulus words appeared on the map.
At the cut-off point of 0.400, it is seen that all stimulus words appear except energy and a complete network is formed by connecting the two islands via the stimulus word ‘matter’. When the relationship level of the stimulus word ‘energy’ with other stimulus words is examined, it is seen that there is only one relationship with a relatedness coefficient value above 0.200. These related stimulus concepts are ‘energy’ and ‘reaction equation’, and the RC value for this association is 0.216. On the other hand, the total number of different response words for the stimulus word ‘energy’ is very high.
Findings concerning the associations between stimulus and response concepts
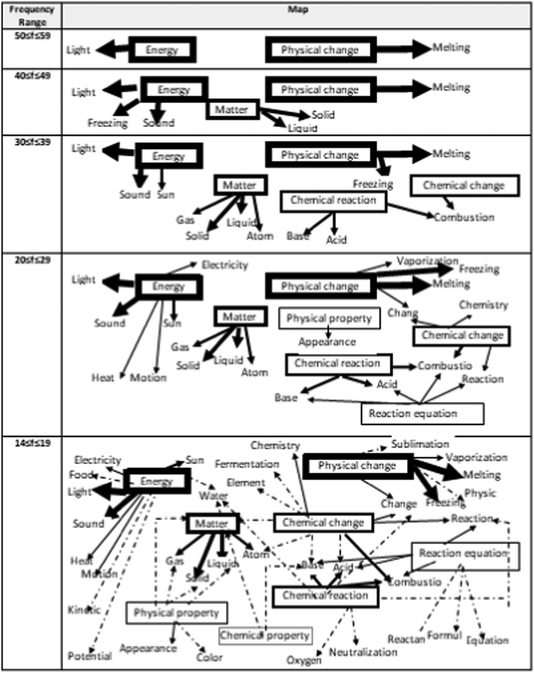
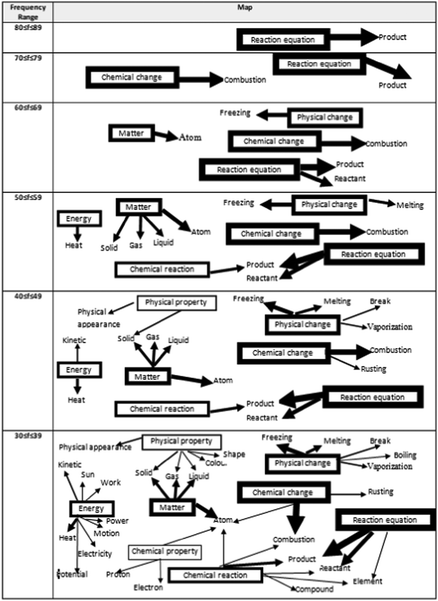
The second research problem focuses on the power and the direction of associations of both between the stimulus words and also between stimulus and response concepts regarding physical and chemical changes which were determined by using the frequency tables. First, the sample pattern of the frequency table of WAT analysis for 8th-grade students is given in Table 4. As seen in Table 4, the highest frequency value is 58, and this value belongs to the response word 'light' that emerges in conjunction with the stimulus word ‘energy’. For this reason, the drawing of the map representing the cognitive structures of 8th-grade students was started from the frequency range 50 ≤ f ≤ 59, and the map was continued to be created until all stimulus words appeared. Since it is the frequency at which the stimulus word ‘chemical property’ appears for the 8th grade, the lowest frequency value on the map is 14 ≤ f ≤ 19. The map of the cognitive structures drawn in this way is shown in Fig. 4.| Response words | Stimulus words | |||||||
|---|---|---|---|---|---|---|---|---|
| Chemical change | Physical change | Chemical reaction | Energy | Chemical property | Physical property | Matter | Reaction equation | |
| Light | 1 | — | — | 59 | — | — | 5 | 1 |
| Melting | 11 | 51 | 3 | — | 4 | 8 | 5 | 5 |
| Sound | 1 | 1 | — | 48 | — | — | 6 | 1 |
| Freezing | 11 | 48 | 3 | — | 3 | 6 | 5 | 3 |
| Liquid | 9 | 11 | 3 | 2 | 10 | 17 | 42 | 1 |
| Solid | 9 | 10 | 3 | 2 | 10 | 19 | 41 | 1 |
| Combustion | 39 | 12 | 31 | 1 | 7 | 2 | 1 | 26 |
| Gas | 8 | 10 | 6 | 2 | 9 | 16 | 36 | 2 |
| Atom | 16 | 5 | 9 | 1 | 10 | 2 | 34 | 11 |
| Base | 18 | — | 33 | — | 14 | 3 | 4 | 24 |
| Acid | 17 | — | 31 | — | 11 | 3 | 4 | 24 |
| Sun | — | — | — | 31 | — | — | 1 | — |
| Electricity | — | — | — | 29 | — | — | 1 | 2 |
| Heat | — | 3 | 3 | 28 | 4 | 1 | 9 | 2 |
| Motion | — | 1 | 3 | 27 | 1 | 2 | 1 | 1 |
| Vaporization | 8 | 27 | 1 | 1 | — | 4 | 3 | 1 |
| Change | 26 | 21 | 16 | 2 | 8 | 6 | 3 | 10 |
| Chemistry | 24 | — | 11 | 2 | 11 | — | 2 | 4 |
| Reaction | 22 | 5 | 17 | 1 | 5 | — | 3 | 21 |
| Appearance | — | 5 | 1 | — | 1 | 21 | 3 | — |
| Water | 7 | 9 | 19 | 16 | 3 | 3 | 19 | 13 |
| Matter | 19 | 8 | 8 | 3 | 14 | 15 | 1 | 9 |
When Fig. 4 is examined, the stimulus words that appear at the highest frequency range, 50 ≤ f ≤ 59, are ‘energy’ and ‘physical change’, and these have emerged as two separate islands. In a lower frequency range of 40 ≤ f ≤ 49, while new response words are connected to these two islands, it is seen that a third island stimulus word ‘matter’ also emerges. In the frequency range 30 ≤ f ≤ 39, it is seen that a fourth island has emerged and this new island contains two stimulus words, ‘chemical reaction’ and ‘chemical change’ by connecting via the ‘combustion’ response word. In the fourth frequency range of 20 ≤ f ≤ 29, it is seen that the ‘physical change’ island and the ‘chemical change’ island are connected via the ‘change’ response word. However, although it is seen that other islands have expanded with the newly added response words, it is seen that these are still separate islands. In the map in the last cell, it is seen that all the islands are connected via the response word ‘atom’.
The sample pattern of the frequency table of WAT analysis for 9th-grade students is given in Table 5. As seen in Table 5, the highest frequency value is 86, and this frequency belongs to the response word ‘product’ that emerges in conjunction with the stimulus concept ‘reaction equation’. For this reason, the drawing of the map representing the cognitive structures of 9th-grade students was started from the frequency range of 80 ≤ f ≤ 89, and the map was continued to be created until all stimulus words appeared. Since it is the frequency at which the stimulus word ‘chemical property’ appears for the 9th grade, the lowest frequency value on the map is 30 ≤ f ≤ 39. The map of the cognitive structure drawn in this way is shown in Fig. 5.
| Response words | Stimulus words | |||||||
|---|---|---|---|---|---|---|---|---|
| Chemical change | Physical change | Chemical reaction | Energy | Chemical property | Physical property | Matter | Reaction equation | |
| Product | 8 | 2 | 53 | 3 | 8 | 86 | ||
| Combustion | 78 | 7 | 30 | — | 3 | — | — | — |
| Reactant | 1 | 1 | 34 | 1 | 7 | 66 | ||
| Atom | 36 | 11 | 36 | 10 | 34 | 7 | 60 | 20 |
| Freezing | 1 | 60 | 1 | 2 | 10 | 5 | ||
| Solid | 2 | 21 | 2 | 6 | 40 | 58 | 3 | |
| Melting | 7 | 57 | 1 | 2 | 11 | 6 | ||
| Liquid | 2 | 23 | 2 | 7 | 37 | 56 | 3 | |
| Gas | 2 | 21 | 6 | 7 | 37 | 55 | 3 | |
| Heat | 2 | 5 | 54 | 1 | ||||
| Vaporization | 3 | 48 | 1 | 1 | 10 | 4 | ||
| Kinetic | 46 | |||||||
| Rusting | 43 | 1 | 3 | 2 | 1 | 5 | ||
| Break | 42 | 1 | 7 | |||||
| Physical appearance | 15 | 40 | ||||||
| Potential | 38 | 2 | ||||||
| Element | 16 | 4 | 37 | 2 | 23 | 2 | 22 | 32 |
| Electron | 22 | 3 | 21 | 6 | 36 | 5 | 9 | 16 |
| Motion | 36 | 2 | ||||||
When Fig. 5 is examined, it is seen that the stimulus word ‘reaction equation’ and attached to it the response word ‘reactant’ appears at the highest frequency range, 80 ≤ f ≤ 89. In a lower frequency range of 70 ≤ f ≤ 79, it is seen that there are two separate islands. While the first of them is composed of the response word ‘product’ associated with the stimulus word ‘reaction equation’ in an upper-frequency range, the newly formed second island is formed by connecting the response word ‘combustion’ to the stimulus word ‘chemical change’. In the frequency range of 60 ≤ f ≤ 69, it is observed that four separate islands are formed around four stimulus words, while in the range of 50 ≤ f ≤ 59, five separate islands are formed around six stimulus words. In this frequency range, the stimulus words ‘chemical reaction’ and ‘reaction equation’ are linked by the response word ‘product’. When the frequency range is reduced to 40 ≤ f ≤ 49, it is seen that seven of the stimulus words appear and although new response words are added to the existing islands, it is seen that there are still five separate islands. Since the last stimulus word did not appear in the frequency range of 40 ≤ f ≤ 49, the frequency range was reduced once again and the mapping was continued in the frequency range of 30 ≤ f ≤ 39.
When the last cell in Fig. 5 is examined, it is observed that in the frequency range of 30 ≤ f ≤ 39 all stimulus words appear on the map. There are three separate islands in the last frequency range, the first of which consists of the stimulus word ‘energy’ and its associated response concepts, while the second island consists of the stimulus word 'physical change' and its associated response concepts. When the third island, located in the last frequency range of 30 ≤ f ≤ 39, is examined, it is seen that the islands formed separately by the six stimulus words combine to form a complete network. The stimulus words ‘matter’, ‘chemical property’, ‘chemical reaction’, and ‘chemical change’ are connected via the response word ‘atom’, and the stimulus words ‘chemical reaction’ and ‘reaction equation’ are linked to each other by the response words ‘product’, ‘reactant’ and ‘element’.
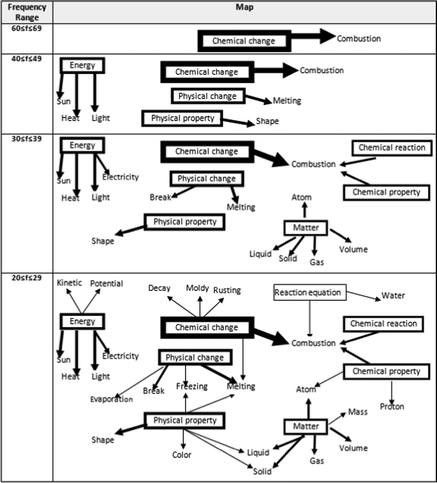
The sample pattern of the frequency table of WAT analysis for 10th-grade students is given in Table 6. As seen in Table 6, the highest frequency value is 66, and this frequency belongs to the response word ‘combustion’ that emerges in conjunction with the stimulus concept ‘chemical change’. For this reason, the drawing of the map representing the cognitive structures of 10th-grade students was started from the frequency range of 60 ≤ f ≤ 69, and the map was continued to be created until all stimulus words appeared. Since it is the frequency at which the stimulus word ‘reaction equation’ appears for the 10th grade, the lowest frequency value on the map is 20 ≤ f ≤ 29. The map of the cognitive structure drawn in this way is shown in Fig. 6.
| Response words | Stimulus words | |||||||
|---|---|---|---|---|---|---|---|---|
| Chemical change | Physical change | Chemical reaction | Energy | Chemical property | Physical property | Matter | Reaction equation | |
| Combustion | 66 | 7 | 38 | 2 | 31 | 3 | — | 20 |
| Heat | 9 | — | 9 | 45 | 3 | — | 1 | 9 |
| Melting | 27 | 43 | 7 | — | 9 | 24 | 4 | 3 |
| Shape | 4 | 18 | — | 1 | 2 | 41 | 2 | — |
| Light | 2 | — | 1 | 40 | — | 1 | 1 | — |
| Sun | — | 1 | — | 40 | — | — | — | — |
| Electricity | — | — | — | 39 | — | — | — | — |
| Atom | 14 | 1 | 12 | 9 | 20 | 1 | 39 | 7 |
| Break | 2 | 38 | — | — | 1 | 16 | — | — |
| Liquid | 1 | 6 | 3 | — | 7 | 20 | 34 | 1 |
| Gas | 1 | 5 | 3 | 1 | 7 | 18 | 33 | 3 |
| Solid | 1 | 6 | 2 | — | 7 | 21 | 32 | — |
| Volume | 1 | 2 | — | 2 | 1 | 6 | 31 | 3 |
| Mass | 1 | 1 | 1 | 2 | 2 | 5 | 29 | 4 |
| Evaporation | 11 | 29 | 5 | — | 6 | 6 | 1 | 1 |
| Potential | — | — | 28 | — | 1 | — | — | |
| Freezing | 6 | 27 | 4 | — | 6 | 20 | 3 | 3 |
| Color | 1 | 10 | — | — | 2 | 27 | 4 | — |
| Kinetic | — | — | — | 26 | — | — | — | — |
| Decay | 24 | 2 | 2 | — | 11 | — | — | — |
| Water | 8 | 4 | 16 | 10 | — | 4 | 7 | 23 |
| Rusting | 22 | — | 7 | — | 1 | — | — | — |
| Moldy | 21 | 2 | 4 | 1 | 2 | — | — | 2 |
| Proton | 7 | 1 | 7 | 5 | 20 | — | 7 | 1 |
When Fig. 6 is examined, it is seen that the stimulus word ‘chemical change’ and attached to it the response word ‘combustion’ appear in the highest frequency range, 60 ≤ f ≤ 69. In the lower frequency range of 40 ≤ f ≤ 49, it is seen that there are four separate islands. While the first of them is composed of the response word ‘combustion’ associated with the stimulus word ‘chemical change’ in the upper-frequency range, the newly formed second island is formed by connecting the response word ‘melting’ to the stimulus word ‘physical change’, the third island is formed by connecting the response word ‘shape’ to the stimulus word ‘physical property’, and the fourth island is produced by linking the response words ‘sun’, ‘heat’, and ‘light’ to the stimulus word ‘energy’.
From Fig. 6, it is seen that five separate islands are formed in the frequency range of 30 ≤ f ≤ 39. It is seen that three of these islands related to the stimulus words ‘energy’, ‘physical property’, and ‘physical change’ are the same islands that emerge at a higher frequency, although new response words are added to them. The fourth island was formed by connecting two new stimulus words ‘chemical reaction’ and ‘chemical property’ to the island, which was previously formed by connecting the stimulus word ‘chemical change’ to the response word ‘combustion’ in the first frequency range. The fifth island newly emerged in this frequency range and is formed by connecting the response words ‘liquid, solid, gas, atom, and volume’ to the stimulus word ‘matter’. When the last cell in Fig. 6 is examined, it is seen that all stimulus words emerge on the map in the frequency range of 20 ≤ f ≤ 29 and there are two separate islands in the last cell of the figure. While the first island consists of the stimulus word ‘energy’ and the related response concepts, it is seen that the second island is formed by connecting seven stimulant words to form a complete network.
Findings on the differences in terms of aspects of cognitive structures at 8th, 9th, and 10th-grade levels
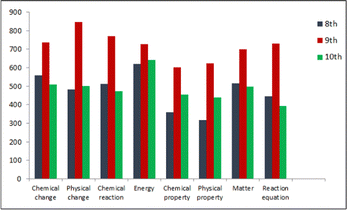
Based on the findings that answered the first and second research questions, the comparison of class levels related to the concepts or ideas contained, the connections between the concepts, and the information processing skills, which are the three main aspects in defining cognitive structures, is presented below separately.One of the criteria used to evaluate the concepts or ideas contained, which is the first aspect in describing cognitive structures, is “extent”. Fig. 7 and 8 show a comparison of the three-grade levels regarding “extent”. The comparison of the total number of different response words given to each stimulus word in the word association test according to grade levels is given in Fig. 7.
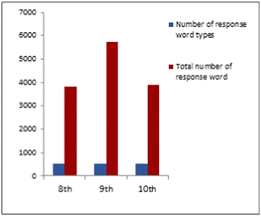
When Fig. 7 is examined, it is seen that the highest number of response words for each stimulus word is at the 9th-grade level, and the number of response words for the three grade levels for the stimulus word energy is close to each other. The comparison of the three grade levels of the total number of responses to all stimulus words and the number of response word types is shown in Fig. 8.
When Fig. 8 is examined, the total number of response words for all stimulus words is 3812 for 8th grade and 5738 for 9th grade, and 3908 for 10th grade. Looking at the number of response word types for each grade, it is seen that these values are 530, 513, and 515 for 8th, 9th and 10th grades, respectively.
The second aspect of describing cognitive structures is “integration” and to determine the integration between concepts, the degree of relations between concepts in the cognitive structure maps and the number of stimulus words that remained unconnected in the cognitive structure were determined. The data obtained are shown in Table 7.
| Grade level | Height frequency | Lowest frequency | Number of strong associations (f ≤ 40) | Number of middle associations (39≤ f ≤ 30) | Number of weak associations (29 ≤ f ≤ 14) | Unrelated stimulus words |
|---|---|---|---|---|---|---|
| 8th | 59 | 14 | 6 | 7 | 43 | — |
| 9th | 86 | 36 | 17 | 24 | 75 | 1 |
| 10th | 66 | 23 | 6 | 9 | 36 | 1 |
The association ranges in Table 7 were determined by considering the highest and lowest frequency values found for the three grades. Since the highest frequency value is 59 for the 8th grade, the range (f ≤ 40) is taken for high associations; this range is (39 ≤ f ≤ 30) for medium associations, and the lowest frequency value is for the 8th grade for the weak association. Since it is 14, the range (29 ≤ f ≤ 14) was taken as the weak association. The stimulus word remaining unconnected to the cognitive structure at the lowest frequency was shown as the number of unrelated stimulus words.
When Table 7 is examined, while the number of strong associations is 17 for the 9th grade, this value is 6 for the 8th and 10th grades. While the number of medium associations is 7 for the 8th grade, 24 for the 9th grade, and 9 for the 10th grade, the weak association value is 43 for the 8th grade, 75 for the 9th grade, and 36 for the 10th grade.
The last variable of cognitive structures is the availability and data on availability were obtained by considering the highest and lowest frequency values found for stimulus words in the same response time given to students for each grade level and are shown in Table 7. When Table 7 is examined, the highest frequency value for grade 8 is 59, while the lowest frequency is 14. For the 9th grade, the highest frequency is 86 and the lowest frequency is 36, while the highest frequency is 66 and the lowest frequency is 23 for the 10th grade.
Conclusions and implications
This study investigated students' cognitive structures concerning physical and chemical changes at different grade levels and tried to reveal how aspects of students' cognitive structures were affected by traditional teaching and how they differed at 8th, 9th, and 10th-grade levels. For this purpose, the level of interconnectedness of the stimulus words, which is among the most prominent stimulus words in teaching the subject about both the topic of physical and chemical changes, the power and the direction of associations of both between the stimulus words and also between stimulus and response concepts and aspects of students' cognitive structures were investigated.The conclusion for all stimulus words in the cognitive structures are drawn depending on the frequency values determined for the whole grade level and the response word relations for each stimulus word are as follows. It was determined that students at all grade levels established the strongest association with “combustion” for the first stimulus word, “chemical change”. It can be said that this strong association between chemical changes and combustion in the cognitive structures of students in all grades is very appropriate. Combustion is one important type of chemical change and one of the most important oxidation–reduction reactions in general chemistry (Gillespie, 1997), significant because historically much of the understanding of chemical change has emerged through the study of combustion reactions. The topic of chemical change, especially combustion, has attracted the attention of many researchers and many studies have been conducted on students' understanding of this subject (Hesse III and Anderson, 1992; Prieto, et al., 1992; Watson et al., 1997; Kypraios et al., 2014; Basheer et al., 2018; Sesto and García-Rodeja, 2021). Watson et al. (1997) investigated 14–15 year-old students’ ideas about combustion and produced three categories of students’ explanations of combustion. These categories were modification, transmutation, and chemical reactions. Modification is the simplest and requires the lowest level of cognitive processing. They noted that “transformation” and “modification” use a certain type of reasoning, are limited in scope, and include concepts that are incorrectly placed in ontological categories.
It was determined that while students at all grade levels established the strongest association with “melting” for the second stimulus word, “physical change”, 8th and 9th-grade students also found another strong association with “freezing” for the “physical change”. These correctly constructed associations by students of all grades show that students easily identify examples of physical change. Studies show that although students are not very good at explaining why the changes are physical or why they are chemical, they are quite good at identifying examples of chemical or physical changes (Demircioğlu et al., 2012).
Regarding the third stimulus word “chemical reaction”, it was determined that the strongest association response word was “combustion” for 8th and 10th grades, while the strongest association was established with “chemical reaction” for the 8th grade, with the response word “product”. This result indicates that although 8th and 9th-grade students made a correct connection with an example of a chemical reaction type, it can be said that 10th-grade students made a more significant connection. When the map of cognitive structures was examined, it was determined that 9th-grade students weakly linked the stimulus word “chemical reaction” with the response word “combustion”, while 8th and 9th-grade students did not establish an association between the “chemical reaction” and “product”. In addition, it was determined that 9th-grade students formed a highly related relationship with the “chemical reaction” such as “reactant” in their cognitive structures. Another conclusion is that only 9th-grade students made a connection between the “chemical reaction” and “atom”. The concept of chemical reactions, which has an essential role in understanding the difference between chemical and physical changes, is also important to understand at the atomic level. Studies have shown that students of all levels have problems understanding the concept of chemical reactions (Andersson, 1986; Barker and Millar, 1999). Ahtee and Varjola (1998) were asked to describe the concept of a chemical reaction from the 7th and 8th grades, first-year senior secondary school, and first-year university students. They found that the students had difficulties in the usage of terms such as substance and atom and very few of the senior secondary and university students were able to describe properly the meaning of chemical reactions. Hesse and Anderson (1992) also indicated that although students used the term reaction in their explanations of natural phenomena, they did not understand that chemical reactions involved the rearrangement of atoms.
Another important result is related to the concept of energy, which is the fourth stimulus word. Although the stimulus word “energy” is located at high or medium cut-off points in the cognitive structure at all grade levels and the total number of different response words is the highest stimulus word at the 8th-grade level, it was concluded that the stimulus word “energy” was the weakest associated with all other stimulus words (RC value 0.01) at all grade levels. In addition, it was found that while “energy” remained as a separate island without being connected to the cognitive structure map for the 9th and 10th grades, “energy” was connected at the lowest cut-off point in the cognitive structure map for the 8th grade. One of the important criteria in distinguishing physical and chemical changes is energy change, and physical changes include modest energy changes, while most chemical changes involve large energy changes. On the other hand, research has shown that there are problems in understanding the concept of energy at all student levels, from middle school to university (Watts, 1983; Hirça et al., 2008; Güneş and Taştan Akdağ, 2016; Opitz et al., 2017). The concept of energy is interdisciplinary in biology, chemistry, physics, and environmental science and it is also used to explain many events and situations in everyday life. Considering the concept of energy from the perspective of that field within the scope of a different discipline may cause some confusion in students' minds regarding the understanding of the concept of energy (Duit, 1984; Eisenkraft et al., 2014). In addition, the concept of energy is seen as a challenging concept due to its abstract and unobservable nature (Ryoo et al., 2018). Park and Liu (2016) developed an interdisciplinary energy concept assessment to assess students' understanding of the energy concept within and across different science disciplines. They found that students' difficulties in understanding the concept of energy were combined with specific science content. When the response words for the stimulus word “energy” were examined, it was revealed that a wide variety of response words (such as kinetic, potential, light, power, sound, ATP, photosynthesis, cell, respiration, renewable, non-renewable, savings, living, carbohydrate, oil, factory, lightning, and work) related to both all disciplines and daily life were written by students of all grade levels. This finding can be attributed to the fact that the concept of energy is interdisciplinary and relevant to daily life, and also students associated energy change only with energy types rather than chemical or physical changes.
The fourth and fifth stimulus words of the WAT were physical and chemical properties and they are the characteristics that enable us to distinguish substances from each other and are directly related to chemical and physical changes. While the physical properties can be measured and observed without changing the composition or identity of a substance, the chemical properties are a characteristic or behaviour of a substance that may be observed when it undergoes a chemical change or reaction. Regarding this, some researchers have shown that there is a relationship between problems in distinguishing physical and chemical changes and associating them with physical and chemical properties. For example, Hanson et al. (2016) assessed first-year high school students’ conceptual understanding of changes in matter, with interpretive underpinnings. Findings from the study revealed that only a few students had difficulty distinguishing between physical and chemical changes and that these students did not associate the changes in states with associated physical and chemical properties. Kypraios et al. (2014) also stated that because students usually fail to connect the identity of a substance and its properties, their problems in understanding the nature of substances will lead to the inability to distinguish between chemical and physical phenomena. In this study, the fact that the stimulus words “chemical property” and “chemical change” and “physical property” and “physical change” were associated with a high cut-off point value at each grade level can be accepted as an indication that the students can structure this relationship in their minds appropriately. Chemical properties are seen either during or following a reaction and knowing the properties can help to make predictions about the type of reaction to expect. For this reason, it is also necessary that students construct a high value of the relationship between stimulus words “chemical property” and “chemical reaction”, and in this study, it was determined that this relationship was at high cut-off point values at all grade levels. Ultimately, when the response words that were written by the students for the stimulus word “chemical property” are examined, it is seen that the answer words with the highest frequency are matter and base for the 8th grade, electron and atom for the 9th grade, and combustion for the 10th grade. Chemical properties are also associated with the particulate nature of the matter. At this point, it can be said that 9th-grade students associate the stimulus word “chemical property” with the particulate nature of matter.
The stimulus word “matter” is another essential concept to understand chemical and physical changes. It has been determined that students at all three grade levels primarily associate matter with its states, namely solid, liquid and gas. When the cognitive structure maps were examined, it was concluded that the response words for the stimulus word “matter” at all three grade levels were correctly connected with the concepts of solid, liquid, and gas, and the stimulus word “physical properties”. In addition, it contributes to the integration of the pattern of the cognitive structure by connecting the stimulus word “matter”, the response word “atom” and other stimulus words in cognitive structures at every grade level. On the other hand, it has been revealed that the most comprehensive and strongest relationship among these relationships is established in the cognitive structures of 9-grade students. In many studies, it has been stated that understanding the particulate structure of matter is a prerequisite for understanding chemical changes (Papageorgious et al., 2010; Kypraios et al., 2014).
Comparing the findings regarding the three main dimensions of the definition of cognitive structures according to grade levels obtained in the third research problem, the following conclusions are reached. When we look at the results related to the “extent” showing the quantity of elicited concepts within the cognitive structure, it was concluded that the highest frequency value of both the number of response words for each stimulus word and the total number of responses to all stimulus words was at the 9th-grade level. Based on these results, it is shown that the cognitive structure of 9th-grade students about physical and chemical changes is more extensive than in other classes. The second aspect of describing cognitive structures which is the connection among concepts in cognitive structures is another important issue and it indicates the degree of integration among concepts (Tsai and Huang, 2002). Considering the number of strong, medium, and weak associations of the cognitive structure concerning the topic of physical and chemical changes, it was determined that the highest frequency values for all associations were at the 9th-grade level. Based on this result, it can be said that the degree of integration among concepts in the cognitive structure related to physical and chemical changes is the highest at the 9th-grade level. On the other hand, it was determined that while all stimulus words were connected to each other in the cognitive structure at the 8th-grade level, only the stimulus word energy was not connected in the cognitive structure at the 9th and 10th-grade levels. The last aspect of describing cognitive structures is the availability which is related to how facile the respondent is in retrieving information within a given task context and it can be represented by the information retrieval rate. Concerning this, it was determined that the highest frequency value was at the 9th-grade level.
These results regarding the variables of the cognitive structure show that the cognitive structure for the 9th-grade level is the most comprehensive and integrated, and has a higher information retrieval rate. The term “association” describes the connection or relationship between ideas, concepts, or words that exist in the human mind, and manifests itself in the fact that the appearance of one entity entails the appearance of the other in the mind (Sinopalnikova, 2004).
Kypraios et al. (2014) found that although a gradual increase in the mean of all cognitive variables was determined as the age increases, this is not observed in the case of Greek students' understanding of the chemical change. They expressed that they agree with those who argue that understanding chemical changes is a challenge for all ages. Although they found that only 12th-grade students were better at dealing with chemical changes, they interpreted this to mean that the understanding of chemical changes is not related to a knowledge development due to age, but rather to students at higher grade levels having the opportunity to acquire more knowledge and thus be able to answer more relevant questions. In their study, Tóth and Kiss (2006) indicated that Hungarian students have serious problems with the physical and chemical compositions of matter but they could not find long-lasting changes in the cognitive structure of students of different ages. They revealed that only in the 8th and 10th grades, slight and temporary changes could be observed in the understanding of the basic concepts of matter.
As can be seen in the studies carried out, there are no great differences in the cognitive structures of students in basic subjects such as states of matter, physical and chemical changes at different grade levels, or it seems to be a very difficult subject for all ages to understand chemical change. Although this study did not focus on students' understanding levels of physical and chemical changes, the cognitive structure maps and various variables of cognitive structures that emerged in the study reached similar results to previous studies that age is not of great importance for this subject.
The fact that students' cognitive structures on the subject of physical and chemical changes do not differ greatly in the learning process may also mean that students' cognitive structures are not affected much by traditional teaching. It has been stated that traditional teaching strategies and teacher-centered teaching approaches are some of the main sources of difficulty in learning students' science concepts (Kolomuc et al., 2012). For this reason, it may be suggested to teach the topic of physical and chemical changes with student-centered teaching approaches or approaches based on constructivist learning theory, which have been shown in science education studies to be effective for better learning the topic of physical and chemical changes or eliminating misconceptions (Kolomuc et al., 2012; Çayan and Karsli, 2015).
Depending on the results of the study, another suggestion for teaching the topic of physical and chemical changes is related to teaching the concept of energy. In addition to understanding the difference between physical and chemical changes, the concept of energy, which is a crucial concept for the interpretation of these changes, should be explained in relation to other concepts. On the other hand, the fact that especially high school students take physics and biology courses at the same time as chemistry courses and that energy is handled from a different perspective in each of the disciplines may prevent students from fully understanding how to relate the concept of energy to chemical events. For this reason, it can be suggested that not only chemistry teachers but also teachers in other disciplines be given integrated teaching for the concept of energy.
Conflicts of interest
There are no conflicts to declare.References
- Ahtee M. and Varjola I., (1998), Students’ understanding of chemical reaction, Int. J. Sci. Educ., 20(3), 305–316.
- Anderson O. R., (1992), Some interrelationships between constructivist models of learning and current neurobiological theory, with implications for science education, Res. Sci. Technol. Educ., 29(10), 1037–1058.
- Andersson B., (1986), Pupils' explanations of some aspects of chemical reactions, Sci. Educ., 70(5), 549–63.
- Baddeley A. D., (2002), Is working memory still working? Eur. Psychol., 7(2), 85–97.
- Baddeley A. D. (2012). Working memory: Theories, models, and controversies, Annu. Rev. Psychol., 63, 1–29.
- Bahar M. and Hansell M. H., (2000), The relationship between some psychological factors and their effect on the performance of grid questions and word association tests, Educ. Psychol., 20, 349–364.
- Bahar M., Johnstone A. H. and Sutcliffe R. G., (1999), Investigation of students' cognitive structure in elementary genetics through word association tests, J. Biol. Educ., 33(3), 134–141.
- Barker V. and Millar R., (1999), Students' reasoning about chemical reactions: what changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 21(6), 645–665.
- Basheer A., Kortam N., Zahran N., Hofestein A. and Hugerat M., (2018), Misconceptions among middle school students regarding the conservation of mass during combustion, Eurasia J. Math. Sci. Technol., 14(7), 3109–3122.
- Brosnan T. and Reynolds Y., (2001), Student's explanations of chemical phenomena: Macro and micro differences, Res. Sci. Tech. Educ., 19(1), 69–78.
- Cachapuz A. F. C. and Maskill R., (1987), Detecting changes with learning in the organization of knowledge: Use of word association tests to follow the learning of collision theory, Int. J. Sci. Educ., 9(4), 491–504.
- Çayan Y. and Karsli F., (2015), The effects of the problem based teaching learning approach to overcome students’ misconceptions on physical and chemical change, Kastamonu Educ. J., 23(4), 1437–1452.
- Champagne A. B., Klopfer L. E., Desena A. T. and Squires D. A., (1981), Structural representations of students’ knowledge before and after science instruction, J. Res. Sci. Teach., 18, 97–111.
- Christian B. N. and Yezierski E. J., (2012), Development and validation of an instrument to measure student knowledge gains for chemical and physical change for grades 6–8, Chem. Educ. Res. Pract., 13(3), 384–393.
- Cordellini L. and Bahar M., (2000), Monitoring the learning of chemistry through word association test, in Fogliani C. L. (ed.), Australian Chemistry Resource Book, The Royal Australian Chemical Institute, vol. 19, pp. 59–69.
- Demircioğlu H., Demircioğlu G., Ayas A. and Kongur S., (2012), A comparison of 10th grade students’ theoretical and applied knowledge about the concepts of physical and chemical change, J. Turkish Sci. Educ., 9, 162–181.
- Duit R., (1984), Learning the energy concept in school – empirical results from the Philippines and West Germany, Phys Educ., 19(2), 59–66.
- Eisenkraft A., Nordine J., Chen R. F., Fortus D., Krajcik J., Neumann K. and Scheff A., (2014), Introduction: Why focus on energy instruction?, in Teaching and Learning of Energy in K-12 Education, Cham: Springer, pp. 1–11 Search PubMed.
- Fisher K. M., (2004), The importance of prior knowledge in college science instruction, in Sunal D. W., Bland Day J. and Wright E. L. (ed.), Reform in undergraduate science teaching for the 21st century, Information Age Publishing Inc., ch. 5, pp. 69–83.
- Fisher K. M. and Lipson J. I., (1985), Information processing interpretation of errors in college science learning, Inst. Sci., 14(1), 49–74.
- Gensler W. J., (1970), Physical versus chemical change, J. Chem. Educ., 47(2), 154–155.
- Gorodetsky M. and Hoz R., (1985), Changes in the group cognitive structure of some chemical equilibrium concept following a university course in general chemistry, Sci. Educ., 69, 185–199.
- Güneş T. and Taştan Akdağ F., (2016), Determination of perceptions of science high school students on energy and their levels of interdisciplinary association, Int. J Soc. Sci. Educ. Res., 2(2), 625–635.
- Hanson R., Twumasi A. K., Aryeetey C., Sam A. and Adukpo G., (2016), Secondary school students’ conceptual understanding of physical and chemical changes, Asian J. Educ. Train., 2(2), 44–52.
- Hesse III J. J. and Anderson C. W., (1992), Students’ conceptions of chemical change, J. Res. Sci. Teach., 29(3), 277–299.
- Hirça N., Çalik M. and Akdeniz F., (2008), Investigating grade 8 students’ conceptions of ‘energy’ and related concepts, J. Turkish Sci. Educ., 5(1), 75–88.
- Ifenthaler D., Masduki I. and Seel N. M., (2011), The mystery of cognitive structure and how we can detect it: Tracking the development of cognitive structures over time, Instr. Sci., 39(1), 41–61.
- Johnson P. E., (1967), Some psychological aspects of subject matter structure, J. Educ. Psychol., 58, 75–83.
- Johnson P., (2000), Children's understanding of substances, part 1: Recognizing chemical change, Int. J. Sci. Educ., 22(7), 719–737.
- Johnstone A. H., (1997), Chemistry teaching-science or alchemy? J. Chem. Educ., 74(3), 262.
- Johnstone A. H., Sleet R. J. and Vianna J. F., (1994), An information processing model of learning: Its application to an undergraduate laboratory course in chemistry, Stud. High. Educ., 19(1), 77–87.
- Kalyuga S., (2006), Rapid cognitive assessment of learners’ knowledge structures, Learn. Instr., 16, 1–11.
- Kolomuc A., Ozmen H., Metin M. and Acisli S., (2012), The effect of animation enhanced worksheets prepared based on 5E model for the grade 9 students on alternative conceptions of physical and chemical changes, Procedia – Soc. Behav. Sci., 46, 1761–1765.
- Kypraios N., Papageorgiou G. and Stamovlasis D., (2014), The role of some individual differences in understanding chemical changes: A study in secondary education, Int. J. Environ. Sci., 9(4), 413–427.
- Lutz S. and Huitt W., (2003), Information processing and memory: Theory and applications. Educational Psychology Interactive, Valdosta, GA: Valdosta State University, from http://www.edpsycinteractive.org/papers/infoproc.pdf.
- Markic S. and Eilks I., (2013), Potentıal changes ın prospectıve chemıstry teachers’ beliefs about teachıng and learnıng—A cross-level study, Int. J. Sci. Math. Educ., 11(4), 979–998.
- Merinoa C. and Sanmartí N., (2009), How young children model chemical change, Chem. Educ. Res. Pract., 9(3), 196–207.
- Miller E. K., Lundqvist M. and Bastos A. M., (2018), Working memory 2.0, Neuron, 100(2), 463–475.
- Nakiboglu C., (2008), Using word associations for assessing non major science students’ knowledge structure before and after general chemistry instruction: the case of atomic structure, Chem. Educ. Res. Pract., 9(4), 309–322.
- Novak J. D. and Cañas A. J., (2008), The Theory Underlying Concept Maps and How to Construct and Use Them, Technical Report IHMC CmapTools 2006-01 Rev 01-2008, Florida Institute for Human and Machine Cognition, available at: http://cmap.ihmc.us/Publications/ResearchPapers/TheoryUnderlyingConceptMaps.pdf.
- Opitz S. T., Neumann K., Bernholt S. and Harms U., (2017), How do students understand energy in biology, chemistry, and physics? Development and validation of an assessment instrument, Eurasia J. Math. Sci. Technol. Educ., 13(7), 3019–3042.
- Palmer W. P., (1994), Physical and Chemical Change as Viewed Through School Textbooks: An Initial View, AARE Conference at the University of Newcastle at 12.30 pm on 29 November, Room V 102. Ref no. for paper PALMB94.417.
- Papageorgiou G., Grammaticopoulou M. and Johnson P. M., (2010), Should we teach primary pupils about chemical change? Int. J. Sci. Educ., 32(12), 1647–1664.
- Park M. and Liu X., (2016), Assessing understanding of the energy concept in different science disciplines, Advance online publication, Sci. Educ., 100(3), 483–516.
- Preece P. F. W., (1978), Exploration of semantic spaces: Review of research on the organization of scientific concepts in semantic memory, Sci. Educ., 62, 547–562.
- Prieto T., Watson R. and Dillon J., (1992), Pupils’ understanding of combustion, Res. Sci. Educ., 22, 331–340.
- Ryoo K., Toutkoushian E. and Bedell K., (2018), Exploring different types of assessment items to measure linguistically diverse students’ understanding of energy and matter in chemistry, Chem. Educ. Res. Pract., 19(1), 149–166.
- Schwartz B. L., (2020), Memory: Foundations and Applications, SAGE Publications.
- Sesto V. and García-Rodeja I., (2021), How do five- to six-year-old children interpret a burning candle? Educ. Sci., 11(5), 213.
- Shavelson R. J., (1972), Some aspects of the correspondence between content structure and cognitive structure in physics instruction, J. Educ. Psychol., 63(3), 225.
- Shavelson R. J., (1974), Methods for examining representations of a subject matter structure in a student's memory, J. Res. Sci. Teach., 11, 231–249.
- Sinopalnikova A., (2004), Word association thesaurus as a resource for building wordnet, in Proceedings of the 2nd International WordNet Conference, pp. 199–205.
- Stavridou H. and Solomonidou C., (1989), Physical phenomena–chemical phenomena: do pupils make the distinction? Int. J. Sci. Educ., 11(1), 83–92.
- Stavridou H. and Solomonidou C., (1998), Conceptual reorganization and the construction of the chemical reaction concept during secondary education, Int. J. Sci. Educ., 20(2), 205–221.
- Stewart J., (1979), Content and cognitive structure: critique of assessment and representation techniques used by science education researchers, Sci. Educ., 63, 395–405.
- St Clair-Thompson H., Overton T. and Botton C., (2010), Information processing: A review of implications of Johnstone's model for science education, Res. Sci. Technol. Educ., 28(2), 131–148.
- Strong L. E., (1970), Differentiating physical and chemical changes, J. Chem. Educ., 47(10), 689.
- Tóth Z., (2007), Mapping students’ knowledge structure in understanding density, mass percent, molar mass, molar volume and their application in calculations by the use of the knowledge space theory, Chem. Educ. Res. Pract., 8(4), 376–389.
- Tóth Z. and Kiss E., (2006), Using particulate drawings to study 13-17 year olds’ understanding of physical and chemical composition of matter as well as the state of matter, Pract. Theory Syst. Educ., 1(1), 109–125.
- Tsai C. C., (1999), Content analysis of Taiwanese 14 year olds' information processing operations shown in cognitive structures following physics instruction, with relation to science attainment and scientific epistemological beliefs, Res. Sci. Technol. Educ., 17, 125–138.
- Tsai C. C., (2001), Probing students’ cognitive structures in science: the use of a flow map method coupled with a meta listening technique, Stud. Educ. Eval., 27, 257–268.
- Tsai C. C. and Huang C.-M., (2002), Exploring students’ cognitive structures in learning science: A review of relevant methods, J. Biol. Educ., 36(4), 163–169.
- Tulving E., (1972), Episodic and semantic memory, in Tulving E. and Donaldson W. (ed), Organization of Memory, New York, NY: Academic Press, pp. 381–403.
- Vlassi M. and Karaliota A., (2013), The comparison between guided inquiry and traditional teaching method. A case study for the teaching of the structure of matter to 8th grade Greek students, Procedia – Soc. Behav. Sci., 93, 494–497.
- Waern Y., (1972), Structure in similarity matrices, Scand. J. Psychol., 13(1), 5–16.
- Watson J. R., Prieto T. and Dillon J. S., (1997), Consistency of students' explanations about combustion, Sci. Educ., 81(4), 425–443.
- Watts D. M., (1983), Some alternative views of energy, Phys. Educ., 18(5), 213–217.
| This journal is © The Royal Society of Chemistry 2023 |