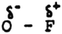Understanding covalent bonding – a scan across the Croatian education system
R.
Vladusic
 *a,
R. B.
Bucat
*a,
R. B.
Bucat
 b and
M.
Ozic
b and
M.
Ozic
 c
c
aFaculty of Science, University of Split, Croatia. E-mail: vladusic@pmfst.hr
bSchool of Molecular Sciences, The University of Western Australia, Nedlands, WA, Australia. E-mail: bob.bucat@uwa.edu.au
cEducation and Teacher Training Agency, Split, Croatia. E-mail: mirjana-mia.ozic@azoo.hr
First published on 13th September 2022
Abstract
This article reports on a study of understanding of key sub-topics of the curricular model of covalent bonding among participants at all levels of the chemical education system in Croatia, including among secondary school students, university students (some of whom are pre-service teachers), and secondary school chemistry teachers. The diagnostic instrument, specially designed for that purpose, was a three-tiered conceptual test. It was administered to 739 high school students, 284 university science students, and 62 secondary chemistry teachers. The study focuses on the sub-topics of bond polarity, molecular polarity, the octet rule, shapes of molecules, intermolecular forces and covalent networks. The aim is to investigate (a) the extent to which the understanding of participants from each group corresponds to curricular models, (b) which deficits in understanding can be classified as misconceptions, and which are due to lack of knowledge, and (c) the misconceptions exhibited by the participants in each category. The results highlight many cases of lack of correspondence between participants’ knowledge and scientifically accepted models, especially among students. Serious misconceptions, even among teachers, were found in each sub-topic area. Many students demonstrated lack of knowledge of concepts (rather than misconceptions). The results underline the need for better understanding of the teaching and learning demands of the six sub-topics of covalent bonding at all levels of the educational system in Croatia. Special attention needs to be paid to the sub-topics shapes of molecules and molecular polarity. This scan of understanding across the Croatian educational system, showing extensive lack of understanding of fundamental chemistry topics, reveals serious systemic problems. These findings are a call to chemistry teachers, curriculum developers and education administrators to take action in all domains and realms of pedagogical content knowledge (PCK) related to covalent bonding.
Introduction
Chemical bonding is one of the central topics in chemistry curricula (Nahum et al., 2010). Sub-microscopic models of chemical bonding are used to rationalise macroscopic phenomena; that is, the observable or measurable physical and chemical properties of substances. To understand almost any advanced topic in chemistry, such as reactivity, spectroscopy, thermodynamics, chemical equilibrium and organic chemistry concepts, students need to have understanding of chemical bonding (Taber and Coll, 2002; Ünal et al., 2006; Pabuccu and Geban, 2012).In this paper we distinguish between understandings of bonding concepts shared by research chemists and the simplified models specified in secondary and tertiary chemistry curricula. It is unreasonable to expect students to attain the true scientific understandings. While the curricular models are necessarily simplified versions of the scientific understandings, they should not be inconsistent with them. This study investigates correspondences between students’ understandings and curricular models of concepts.
It is generally conceded that coming to a functional level of understanding of chemical models, as well as using them to rationalise properties, present high learning demands on students. For example, Tsaparlis et al. (2018) comment:
Understanding the topic of chemical bonding requires the appreciation of many critical details and some sophisticated reasoning, making it complicated and therefore difficult for a large majority of the general student population.
The concepts associated with common curricular models of bonding are abstract (Nahum et al., 2007; Kumpha et al., 2014; Nimmermark et al., 2016; Prodjosantoso et al., 2019), far removed from daily experiences (Tan and Treagust, 1999) and everyday discourse (Taber and Coll, 2002). We cannot see atoms nor molecules, so the only sources of students’ understandings are drawings and animations in texts and electronic resources, as well as teachers’ descriptions. Bonding models refer to components of atoms (protons and electrons), their charges, and the interactions between the charged species – none of which can be seen, nor experienced. The fluidity of electrons in atoms (different orientations in different circumstances) is a mind-boggling notion. Concepts such as ionisation, electronegativity, shared electrons, unequally shared electrons, and polarity of molecules (as distinct from polarity of bonds) all require relatively sophisticated thought, even at the simplified curricular level.
Superimposed upon the conceptualisation difficulties is the highly reported failure to distinguish between the sub-microscopic level of models and the macroscopic level of observable and measurable substance behaviours (Johnstone, 1991; Taber, 2013). And yet the only defensible reason for inclusion of the former in curricula is to rationalise the latter.
Over and above all of these issues is the necessity of properly interpreting and using symbols, as expressed by Taber and Coll (2002, p. 213):
“…learners are expected to interpret a disparate range of symbolic representations standing for chemical bonds.”
In the light of this brief analysis, it is perhaps no wonder that previous research, presented below, has identified common student conceptions about covalent bonding that are not consistent with the models of covalent bonding used in curricula at the secondary level of schooling, in several countries.
Review of previous research papers related to understanding of covalent bonding
A diagnostic instrument for probing conceptual understanding and misconceptions about covalent compounds has been designed by Peterson (1986). Consisting of 15 two-tiered multiple-choice items, this instrument was administered to high school students by various researchers (Peterson et al., 1986; Treagust, 1988; Peterson and Treagust, 1989; Peterson et al., 1989; Goh et al., 1993), as well as college and university students (Birk and Kurtz, 1999, Dhindsa and Treagust, 2009). Beside weaknesses in general comprehension of covalent bonding and structure, especially at the level of high school students, many specific misconceptions related to bond polarity, molecular polarity, the octet rule, shapes of molecules, intermolecular forces and lattices were recognised by one or more of the research studies. It was found, for example, that a number of students perceive equal sharing of electron pairs between atoms in all bonds in molecules. Many believe that the atoms in non-polar molecules must have similar electronegativities, and do not appreciate the role of the shapes of molecules in their polarity. Some students attribute the shape of molecules to repulsion between bonding electron pairs only, without consideration of repulsion from the central atom's non-bonding pairs. A common misconception is that application of the “octet rule” means that the number of covalent bonds formed by a non-metal atom is the same as the number of electrons in its valence shell. Some students believe that intramolecular bonding is weaker than intermolecular forces of attraction.Butts and Smith (1987) conducted an interview study about chemistry students’ understandings of the structure and properties of molecular and ionic compounds and found deficiencies. Some students who referred to ionic bonding in sodium chloride nonetheless perceived NaCl molecules, sometimes even invoking covalent bonds to account for the forces between ions in the solid.
Taber (1995) conducted a case study investigating progressive development of understanding covalent bonding by student Annie. At the beginning of her course, Annie saw covalent bonding as the type of bonding between non-metallic atoms, whereas she seemed to consider all bonds involved sharing of electrons. During the course, although Annie made some progression in her understanding of bonding concepts, she did not develop a single integrating framework for understanding bonding interactions that could subsume the familiar classes of covalent and ionic bonding.
In a study aimed to identify high school students’ understandings about chemical bonds and energetics, Boo (1998) found numerous indications that covalent and ionic bonds were confused with each other and with other kinds of bonds. For example, the following misconceptions were observed: a covalent bond was seen as the result of the transference of electrons and sharing of one electron between two atoms; metals such as magnesium and copper form covalent bonds with non-metals such as chlorine and oxygen; ionic bonds and metallic bonds are “not real bonds, in the sense of covalent bonds.” For a large number of the students, the chemical bond was seen as a physical entity. Some of them thought that breaking a bond requires energy input to begin the process, but that during the bond breaking process, energy is released.
Barker and Millar (2000) conducted a longitudinal study probing students’ ideas about exothermicity of bond formation, as well as about covalent, ionic and intermolecular bonding. They found that students consider covalent bonds weaker than ionic bonds and so they break more easily. Some of them perceived interactions in sodium chloride as the sharing of electrons. One student attributed low boiling points of substances to weak covalent bonds, rather than to weak intermolecular bonding. Similar thinking is identified by Othman et al. (2008) in a sample of secondary school students in Singapore. This attribution of physical properties of substances to the strengths of intramolecular covalent bonds is consistent with a perception that molecules are decomposed during evaporation, and re-form during condensation back to a liquid. These findings are also diagnosed by Ogden (2017).
Although undergraduate students seemed to know about the concept of bond polarity, Nicoll (2001) found that some didn’t associate polarity with the electronegativities of the bonded atoms. Nicoll also identified student beliefs that bond polarity influences the shape of molecules. Molecular geometry frequently was argued incorrectly: for example, in her explanation of the V-shaped geometry of water molecules, freshman Bridgette focused not on lone pair-lone pair and lone pair-bonded pair repulsions, but on the imaginary idea that two lone pairs of electrons have higher energy levels, and, that is why, stronger, and need more space, so they push the energetically weaker bonded pairs (bonds) down. Further, some students were confused with the origins of covalent and ionic bonding. For example, one student thought that covalent bonding is due to attractions between positive and negative ends of atoms.
Similar confusions about chemical bonding, among both undergraduate and postgraduate students, were identified by Coll and Taylor (2001). It was found that some students conceived of covalent bonding as weak bonding. Polar covalent compounds, and even homonuclear diatomics such as molecular iodine, were seen as structures built up of charged species.
Taber and Coll (2002) discussed the impediments to learning about chemical bonding, and proposed principles to overcome them. They pointed out that learners’ sense-making about bonding is often influenced by a common alternative conceptual framework which has been labelled the ‘octet’ framework. It is based on the idea that atoms forms bonds to achieve an octet: a state with a full valence shell of electrons. This is consistent with findings of Eymur and Geban (2016). Taber and Coll (2002) also discussed sources of other misconceptions, which they labelled bond as physical entity, shared electron pair as the bond, and sharing of electrons as something more than an image or metaphor.
Luxford and Bretz (2014) devised The Bonding Representation Inventory (BRI) test to quantify the prevalence of misconception about multiple representations of covalent and ionic bonding among 725 USA high school and general chemistry students. It was found that some students cannot classify bonds as covalent or ionic because of the representations not including the labelling of atoms. The octet rule was often used as an explanatory principle for why bonding occurs. Sodium chloride was considered covalent, with sodium and chlorine atoms sharing electrons. Some students thought that spacing of dots between atoms indicates equal sharing and that slightly different electronegativities means equal sharing. Later, a translated version of BRI was used in Slovakia with 330 high school students (Vrabec and Prokša, 2016). The findings in the Slovakian education system have many commonalities with those in the USA system.
In a study aimed to identify some perceptions of 77 Indonesian graduate students’ regarding covalent bonds, Erman (2016) used a semi-open two-tiered diagnostic test and interviews. Eight primary misconceptions were recognised: (i) a covalent bond is formed between two atoms, each with a pair of free electrons; (ii) each atom in a stable molecule must follow the octet rule; (iii) a covalent bond is polar if the electron affinity of two bonded atoms is different; (iv) the shape of the molecule depends on the number of atoms bonded to the central atom; (v) non-polar molecules have a dipole moment; (vi) all bonds in polar molecules are polar, while all bonds in non-polar molecules are non-polar; (vii) the number of bonds depends on the electronegativity of the atoms; and (viii) bond length depends on the type of bond.
It is apparent from the above that the topic of chemical bonding presents considerable challenges for students to understand. It follows that the teaching of this topic presents high demands on teachers, as well as on the curriculum.
Motivation for the research, and overview
The research reported here is an investigation into the understandings of the key sub-topics of the specified curricular model of covalent bonding amongst selected students and teachers across Croatia.One of the authors (R.V.), who has considerable experience related to the teaching and learning of Chemistry in Croatia, particularly in the field of teacher preparation, has also commonly observed misconceptions about chemical bonding in the classroom.
With some awareness of research findings in other countries, and bearing in mind that language can be a substantial moderator of meaning (Taber, 2015; Markic and Childs, 2016; Vladušić et al., 2016b), one objective of this research study was to explore common strengths and weaknesses of understanding about chemical bonding in Croatia, where the language of instruction and testing is Croatian, rather than English. Such knowledge can obviously be important in the design of programs for education of future chemistry teachers – always with the intention of expanding their pedagogical content knowledge in this topic, and with the long-term aim of improving the design of the school curricula.
Although there is much information in the research literature about students’ misconceptions of chemical bonding, data on how well chemistry teachers understand chemical bonding concepts are rare. Pondering upon whether particular deficiencies in understanding about covalent bonding are endemic in the Croatian system, perhaps re-cycling from generation to generation, a decision was made to conduct a system-wide study that probed, at a rather general level, the quality of understandings of secondary school students, tertiary-level students (some of whom intend to be chemistry teachers), as well as chemistry teachers in the secondary schools.
Through this study, it was hoped that a picture of understandings at each of the levels of education could point to the most problematic issues in the system and suggest origins of identified deficiencies of understanding.
During planning of the research study, a question that increasingly presented itself was whether deficiencies of understanding were really misconceptions (student knowledge inconsistent with the accepted science), or perhaps simply lack of knowledge (ignorance) about the concept being tested. A probing instrument was designed to distinguish between these possibilities.
This research study complements our previously published research on the understandings of ionic bonding by high school students, tertiary students, and school teachers in Croatia (Vladušić et al., 2016a). Findings from both studies may serve as a base for development of new instructional strategies in the area of chemical bonding.
Research questions
Consistent with the motivating factors described above, three research questions were designed to guide this study:(1) To what extent do the understandings of Croatian high school students, university students and school teachers compare with curricular models of the covalent bonding sub-topics bond polarity, molecular polarity, the octet rule, shapes of molecules, intermolecular forces, and covalent network substances?
(2) To what extent can deficiencies of understanding about the chosen covalent bonding sub-topics be classified as misconceptions, and to what extent can deficiencies be attributed to lack of knowledge?
(3) What are the most serious misconceptions of the designated covalent bonding sub-topics among Croatian high school students, university students and school teachers?
Defining the subject matter of interest
The models of covalent bonding presented in high schools and introductory tertiary courses are simplified versions of those understood by academic theoretical chemists. Many levels of simplification are possible, and each is open to diverse interpretations. Our intention was to identify whether the understandings of students and teachers are consistent with the simplified models of covalent bonding appropriate to the secondary school curriculum in Croatia – which has much in common with that used in schools around the world.The appropriate knowledge set of this model is defined by a set of propositional statements listed in Appendix A. Propositional statements are created by one of the authors (R.B.B.) and validated by triangulation with the other two authors. They were then reviewed and discussed by three experienced high school chemistry teachers for validity and compatibility with a chemistry curriculum. The propositional statements are categorized into six sub-topics, the first five of which concern covalent molecular substances: (1) bond polarity, (2) molecular polarity, (3) the octet rule, (4) shapes of molecules, (5) intermolecular forces, and (6) covalent network substances.
Deciding on a term to describe understanding deficiencies
In this study, we also look for strongly held conceptualizations by participants about covalent bonding which are inconsistent with scientifically acceptable understandings. Many different terms in the literature, such as alternative conceptions (Helm, 1980), alternative frameworks (Driver, 1981), children's science (Gilbert et al., 1982) and misunderstandings (Wandersee et al., 1994) have been used to describe such conceptualizations. These terms differ from each other in subtle ways, depending on the research context. For this study, we chose to use the term misconception because it evokes comparison to expert thinking (Luxford and Bretz, 2014).A participant's conceptualization in this study is classified as a misconception only if: (i) it is in disharmony with scientifically acceptable meaning and (ii) the participant was convinced that the conceptualization is valid.
The above does not apply to deficiencies of understanding that can be categorised as lack of knowledge.
Methodology
The diagnostic instrument
A diagnostic instrument used in this study comprised fifteen three-tiered items. The first two tiers of each item were tests of knowledge based on a two-tier instrument developed by Peterson (1986) and translated into Croatian. Participants were first required to select the correct answer from a multiple-choice set, and then asked to choose the reason for their answer from another multiple-choice set. The third tier items give participants the opportunity to express their level of self-confidence in their answers.Peterson's instrument was used as a base because (i) it was designed specifically to identify misconceptions related to covalent bonding and structure, (ii) it had already been used in various studies (Peterson et al., 1986; Treagust, 1988; Peterson and Treagust, 1989; Peterson et al., 1989; Goh et al., 1993, Birk and Kurtz, 1999; Dhindsa and Treagust, 2009), (iii) its reliability coefficient is acceptable (Peterson et al., 1989), and (iv) the subject-matter level generally corresponds with the curricula of gymnasiums and undergraduate studies in Croatia, and the list of propositional statements in Appendix A.
Two of the Croatian authors (R.V. and M.O.), fluent in English and with expertise in chemistry education, independently translated the Peterson's test into Croatian. The translations were analysed and discussed with the third author (R.B.B.), who is an expert in the field of chemistry education, fluent in English (native language), and has knowledge of the Croatian language. Although we were satisfied that the translation was as close as possible, it is recognised that linguistic and cultural factors will affect the interpretation of items by the Croatian participants. Nevertheless, what is important are the answers to the probes presented to the students in their language.
One item in Peterson's test (number 11) was not fully appropriate to the Croatian curriculum, so a substitute item, taken from extended list of 27 questions (number 17, Peterson, 1986), was used. Other minor changes were made to improve clarity of the task, or to render chemical expression more precise.
After collecting the data, the instrument was analysed to check its reliability and discriminatory power. For this purpose, different methods were used, such as the Kuder–Richardson 20 (KR – 20) formula for dichotomous variables (the criterion used was that the answers must be correct for both tiers of the test items), the difficulty level (DL) and the discrimination index (DI).
The reliability index for both tiers of the test items measured by the formula KR – 20 is 0.765, indicating good reliability. The average DL of the items (P) is 0.332. DI for individual test items (TI) are: TI1, 0.661; TI2, 0.803; TI3, 0.740; TI4, 0.205; TI5, 0.307; TI6, 0.539; TI7, 0.335; TI8, 0.736; TI9, 0.354; TI10, 0.390; TI11, 0.496; TI12, TI13, 0.665; TI14, 0.457; TI15, 0.551. The average DI is 0.532.
The test presented to participants is shown in Appendix B. The Peterson's (1986) two-tiered tasks which served as a base for this study diagnostic instrument are displayed in Appendix C.
Data collection and analysis
The high school students and university students, in the presence of teachers, anonymously responded to a printed version of the diagnostic instrument. Chemistry teachers responded to a web questionnaire (online) because it was not possible to test the understanding of teachers from all administrative regions on site. Teachers had received an email with the linked test instrument and a detailed description of the procedure and the conditions to be met. Considering that teachers participate voluntarily and anonymously (like students), we assumed that the different answering modes would not affect the results. Finally, when analysing the data, we could not detect any differences in response patterns between the groups that had responded in different ways (printed or electronic).Each item was designed to test understanding of one or more of the propositional statements (see Appendix A), although no items tested understanding of statements 1, 6, 7, 8, 9, 10, 11, 12, 15, 20, 21, and 22.
Table 1 shows, for each item, the correct answer key for first two tiers, the propositional statement(s) tested, and the appropriate sub-topic category.
| Item | Answer | Propositional statement | Sub-topic |
|---|---|---|---|
| 1 | 1 (C) | 2 | Bond polarity |
| 2 | 2 (B) | 17, 18 | Molecular shape |
| 3 | 2 (C) | 5 | Bond polarity |
| 4 | 2 (D) | 26, 27 | Covalent network substances |
| 5 | 1 (A) | 18, 19 | Molecular shape |
| 6 | 2 (B) | 12, 13 | Molecule polarity |
| 7 | 1 (C) | 14 and 23, 24 | Molecule polarity |
| Intermolecular forces | |||
| 8 | 2 (D) | 18, 19 | Molecular shape |
| 9 | 1 (C) | 13 | Molecular polarity |
| 10 | 2 (A) | 15, 16 | Octet rule |
| 11 | 1 (B) | 25 and 27 | Intermolecular forces |
| Covalent network substances | |||
| 12 | 1 (D) | 13, 14 and 23 | Molecular polarity |
| Intermolecular forces | |||
| 13 | 2 (B) | 18, 19 | Molecular shape |
| 14 | 1 (D) | 2, 3, 4, 5 | Bond polarity |
| 15 | 3 (C) | 16 | Octet rule |
Table 2 shows at a glance which items address each of the six sub-topic areas.
| Sub-topic | Item numbers |
|---|---|
| Bond polarity | 1, 3, 14 |
| Molecular polarity | 6, 7, 9, 12 |
| Octet rule | 10, 15 |
| Shapes of molecules | 2, 5, 8, 13 |
| Intermolecular forces | 7, 11, 12 |
| Covalent network substances | 4, 11 |
The answer sheet also contained a third multiple-choice answer set. Correct answers do not necessarily reflect good understanding: the participant may have guessed the correct choice. Similarly, incorrect answers do not necessarily indicate particular misconceptions held by the participants: it is also possible that a participant has no significant knowledge about concept being tested, and guessed the answer. To diagnose between these possibilities, participants were asked to indicate, in a third multiple-choice answer set, their level of self-confidence in their response to each item, among the options: (i) I am sure; (ii) I am not sure; and (iii) I am just guessing.
This allowed us to reasonably categorise the knowledge status of each participant with regard to any item as either (a) acceptable conception, (b) misconception, or (c) lack of conception (that is, insufficient knowledge, rather than incorrect knowledge) which we hereafter label as “lack of knowledge”. For example, even in those cases when a participant answers both first and second-tier questions correctly, if they acknowledge that they were “just guessing”, we cannot conclude that they have an acceptable level of understanding. And we can distinguish the knowledge levels of participants who answered incorrectly to one or both of the knowledge questions: we can conclude that those who choose “I am sure” have some misconception, while those who choose “I am not sure” or “I am just guessing” have insufficient knowledge of the conceptions being tested.
Table 3 shows the twelve different possible answer sets for each item, and, for each combination, the judgement made about the student's knowledge level.
| Tier 1 response | Tier 2 | Tier 3 response | Judgement made |
|---|---|---|---|
| Correct | Correct | I am sure | Acceptable |
| Correct | Correct | I am not sure | Acceptable |
| Correct | Correct | I am just guessing | Lack of knowledge |
| Correct | Incorrect | I am just guessing | Lack of knowledge |
| Correct | Incorrect | I am not sure | Lack of knowledge |
| Correct | Incorrect | I am sure | Misconception |
| Incorrect | Incorrect | I am not sure | Lack of knowledge |
| Incorrect | Incorrect | I am just guessing | Lack of knowledge |
| Incorrect | Correct | I am not sure | Lack of knowledge |
| Incorrect | Correct | I am just guessing | Lack of knowledge |
| Incorrect | Correct | I am sure | Misconception |
| Incorrect | Incorrect | I am sure | Misconception |
Participants and research context
The diagnostic instrument was administered to 739 high school students, 284 students in the Faculties of Science and 62 secondary school teachers of chemistry.The high school students were from all four administrative regions of Croatia's education system. The number of participating students in each region were not necessarily in proportion to the number of students in each region, because the latter information could not be obtained. More of the participating students were in the first year of high school – the year in which the topic of chemical bonding is presented in the high school curriculum – than in higher grades.
Of the 284 university students, 200 were undergraduates enrolled in programs leading to degrees in chemistry, or chemistry and biology: 109 at the first-year level, 66 at second year, and 25 at third year. There were 84 graduate participants, 43 in their first year and 41 in their second year, all of whom were intending to be chemistry teachers.
The instrument was administered about a month after the first year of high school and undergraduate students had finished their chemical bonding class.
The 62 chemistry teachers who accepted the invitation to participate were from all four administrative regions.
Limitations of the study
There are several limitations to the certainty of the findings. First, we are limited in our ability to interpret the thinking processes that lead to the responses. We cannot know what people think: we can only design questions to provide indicators. So, our findings should be taken strictly as observation of participants’ responses, and evidences of their thinking, rather than proof of their conceptions.It is possible that in some cases where we needed to linguistically reshape participant's response in aim to provide simpler, clearer or more general message, especially regarding misconceptions, our interpretation was not fully compatible with thinking that participant was led by responding the item.
Some test items probe understanding of more than one sub-topic. In case of incorrect responses to those items, all of the relevant concepts are taken to be not understood. There was no way to know if one of the concepts was understood.
Finally, students responded in more constrained situations than the teachers because they were allocated a limited time, under controlled classroom conditions.
Ethical considerations
The research was conducted at the Faculty of Science, University of Split, as a part of research toward a PhD degree. Everything was performed in compliance with the relevant laws and institutional guidelines. The approval for the research was provided by the Ethics Committee of Faculty of Science, University of Split, Croatia (classification mark 641-01/13-01/00009) and by Ministry of Science, Education and Sport (classification mark: 602-01/14-01/00326). Special attention was paid to ethical considerations about investigating with children. The surveys were answered anonymously, and only by participants who agreed to participate.Results and discussion
The findings from this study are presented here in the following order: (a) for each sub-topic, the percentages of teachers, university and high school students, respectively, whose level of knowledge was judged to be acceptable; (b) the frequency of the main misconceptions identified in each sub-topic; (c) the performances of participants in each of the items in the diagnostic instrument.(a) Knowledge level of participant groups in each of the sub-topics
For each participant in the study, their level of knowledge across any one sub-topic was judged to be “acceptable” only if answers to the all sub-topic test items were correct. We deemed that otherwise, participants could not be said to understand the sub-topic.Table 4 displays the percentages of participants in each of the groups (i) teachers, (ii) university students, and (iii) high school students, whose knowledge level was deemed to be acceptable in each of the sub-topics.
| Sub-topic | Teachers | University students | High school students |
|---|---|---|---|
| Bond polarity | 62.90 | 31.85 | 8.48 |
| Molecular polarity | 35.48 | 4.43 | 1.52 |
| Octet rule | 40.32 | 7.33 | 7.10 |
| Shapes of molecules | 22.58 | 1.11 | 1.11 |
| Intermolecular forces | 66.13 | 8.49 | 3.88 |
| Covalent network substances | 41.94 | 7.33 | 0.69 |
Table 4 shows a worrying picture of the level of knowledge about covalent bonding across the educational system in Croatia. The results clearly show that in every sub-topic, the knowledge displayed by the school teachers is more aligned with the curricular models than that of the university students, and even more so than that of the high school students. Even so, it can be argued that the knowledge levels demonstrated by the school teachers is below expectations.
In most sub-topics, students at both university and school levels performed disappointingly. In particular, the performance across all groups with respect to the shapes of molecules is very poor indeed. In discussions of these findings, teachers generally expressed surprise at the low level of student performance. It seems that the diagnostic questions in this instrument expose deficiencies not detected in commonly used testing procedures.
(b) The frequency of misconceptions identified in each sub-topic
From analysis of the responses of all participants across all six sub-topics, 129 different misconceptions were identified. Among the high school students, a total of 124 misconceptions were identified. Among university students, the existence of 91 different misconceptions were evidenced, and teachers displayed 60 of them.Table 5 shows percentages of participants in each group with at least one misconception identified in each sub-topic. For example, according to the teachers’ responses to the 3 test items which question understanding of bond polarity, 25.81% of teachers (16 teachers) hold at least one misconception.
| Sub-topic | Teachers | University students | High school students |
|---|---|---|---|
| Bond polarity | 25.81 | 27.41 | 36.86 |
| Molecular polarity | 50.00 | 52.40 | 59.17 |
| Octet rule | 27.42 | 20.88 | 25.91 |
| Shapes of molecules | 58.06 | 50.18 | 52.50 |
| Intermolecular forces | 20.97 | 33.58 | 39.81 |
| Covalent network substances | 24.19 | 41.39 | 54.23 |
Significant percentages of participants in all groups displayed at least one misconception in each sub-topic. In general, but not in every case, the percentages are higher for high school students than for university students, and are lowest for teachers.
Shape of molecules and molecular polarity are the sub-topics in which the highest percentages of participants in all groups have at least one misconception. It is interesting that the percentage of teachers with a misconception related to the shapes of molecules is higher than the percentages of students.
A list of the most common misconceptions identified in this study can be found in Appendix D.
Analysis of the results, and discussion related to the findings in each of the sub-topics follows.
(c) Item-by-item analysis
Table 3 displays how the answer choices made by the respondents were used to classify their knowledge about a particular item as “acceptable”, “lack of knowledge”, or “misconception” (knowledge not in accordance with the curricular model).The findings are presented here sub-topic by sub-topic, looking in turn at each of the items within each sub-topic. Data related to levels of knowledge annotated in the text are shown in bold in the tables for ease of reading.
| Test item | Level of knowledge | Groups of participants | |||||
|---|---|---|---|---|---|---|---|
| Teachers | University students | High school students | |||||
| N | % | N | % | N | % | ||
| 1 | Acceptable | 53 | 85.5 | 193 | 70.4 | 317 | 43.1 |
| Lack of knowledge | 0 | 0.0 | 46 | 16.8 | 270 | 36.7 | |
| Misconception | 9 | 14.5 | 35 | 12.8 | 149 | 20.2 | |
| Total | 62 | 100.0 | 274 | 100.0 | 736 | 100.0 | |
| 3 | Acceptable | 53 | 85.4 | 136 | 50.0 | 193 | 26.4 |
| Lack of knowledge | 4 | 6.5 | 105 | 38.6 | 439 | 60.1 | |
| Misconception | 5 | 8.1 | 31 | 11.4 | 99 | 13.5 | |
| Total | 62 | 100.0 | 272 | 100.0 | 731 | 100.0 | |
| 14 | Acceptable | 52 | 83.8 | 150 | 55.6 | 166 | 23.1 |
| Lack of knowledge | 4 | 6.5 | 102 | 37.7 | 453 | 63.0 | |
| Misconception | 6 | 9.7 | 18 | 6.7 | 100 | 13.9 | |
| Total | 62 | 100.0 | 270 | 100.0 | 719 | 100.0 | |
Test item 1 is designed to expose whether participants understand that the shared pair of electrons in a covalent bond is not centrally located between atoms of different elements because of a difference in the strength of attraction by the atoms for the shared pair. The term electronegativity is not used. The correct tier 1 and tier 2 answers are 1 and C.
Results show, as expected, and as is generally the case for all questions, that a higher percentage of the school teachers demonstrated an acceptable level of knowledge than did university students, and (fewer still) high school students. The percentages of respondents with misconceptions in each group were low and not very different, because considerable numbers of student responses were classified as “lack of knowledge”.
Inspection of the distracter answers shows that the most common misconception among teachers (4.8%), university (6.9%) and high school students (15.9%), is demonstrated by answer set 2, B: that the shared electron pair must be centrally located. It seems that these respondents see covalent bonds only as non-polar bonds. This is consistent with findings of Peterson et al. (1989) and Birk and Kurtz (1999).
The second most common misconception (teachers 4.8% and university students 4.4%), identified by answer set 2, C, shows awareness that the fluorine atom has a stronger attraction for the shared electron pair, but, looking in the context of molecule, shows non-understanding how that attraction influences the position of the shared electron pair in each HF molecule. 2.7% of high school students believe that in diatomic molecules, the bigger atom exerts greater control over the shared electron pair, so the shared electron pair is closer to it (Answer set 1, D).
Test item 3. This question checks that the respondents can recognise the appropriate partial charges on the atoms in an O–F bond (Choice 2), and the reason that the bonding pair is closer to the fluorine atom (Choice C).
The low percentages of students exhibiting a misconception, despite low numbers classified as “acceptable”, are attributable to the relatively large percentages classified as “lack of knowledge”.
The most common misconception of teachers (3.2%) and university students (4.0%) was evidenced by correct tier 1 choice (2), but incorrect tier 2 choice (A), which posits that the bond polarity is determined by the non-bonding pairs on the bonded atoms. Among high school students, the most common misconception (4.5%), identified also in 3.3% of university students, is indicated by choice of incorrect reason (B): the bond polarity is because oxygen atoms have fewer valence electrons than fluorine atoms.
Although the term electronegativity is not used in the item, we can speculate that respondents with either of these two misconceptions tried to rationalise the origin of the different electronegativities, rather than the consequence of the difference. Burrows and Mooring (2015) have previously attributed misunderstanding about polar covalent bonding to lack of comprehension of the concept of electronegativity.
Test item 14. Item 14 probes understanding that the more electronegative atom in a molecule will influence the shared electron pair to be closer and, hence, have a partial negative charge.
The correct answers are that in a molecule of SCl2, the S atoms would have a partial positive charge (Choice 1), for the reason that the shared electron pair reside closer to the Cl atom because it is the more electronegative (Choice D).
The data (Table 6) give cause for confidence in the methodology because questions 3 and 14 test the same knowledge, in different contexts, and there is no significant difference in the quantitative data. This is even though question 14 uses the concept of electronegativity, although question 3 does not.
As for question 3, the performance of teachers is much better than that of the students. Although the percentage of high school students deemed to be acceptable was low (23.1%), so was the percentage who indicated existence of a misconception (13.9%). This was because so many of them (63.0%) were deemed “lack of knowledge”.
The most common misconception, held by 4.8% of teachers, 2.2% of university students, and 4.3% of high school students is that the atom with partial positive charge is the one with a higher electronegativity – indicated by incorrect tier 1 answer (2) and correct tier 2 answer (D). It seems probable that these respondents either do not understand the concept of electronegativity, or are confused about the residual charge when nuclear charge and the charge on electrons is not equal.
The second most common misconception in all samples (3.2% of teachers; 1.5% of university students and 3.9% of high school students) is identified by answer set 1, A. It states The SCl2molecule has a polar covalent bonds between the sulfur atom and the chlorine atoms. The atom assigned the partial positive charge (δ+) in these bonds would be sulfur (not chlorine) because sulfur donation of one electron to the chlorine atom results in the formation of S+and Cl−ions. This finding could be seen as an example of perceiving covalent bonding as a model based on transfer of electrons rather than on sharing of electrons as well as seeing atoms with partial charges as ions. This is consistent with findings of Luxford and Bretz (2014) who reported about student's thinking that covalent CCl4and covalent PCl5has a transfer of electrons.
| Test item | Level of knowledge | Groups of participants | |||||
|---|---|---|---|---|---|---|---|
| Teachers | University students | High school students | |||||
| N | % | N | % | N | % | ||
| 6 | Acceptable | 30 | 48.4 | 67 | 24.6 | 92 | 12.6 |
| Lack of knowledge | 12 | 19.4 | 144 | 52.7 | 382 | 52.1 | |
| Misconception | 20 | 32.2 | 62 | 22.7 | 259 | 35.3 | |
| Total | 62 | 100.0 | 273 | 100.0 | 733 | 100.0 | |
| 7 | Acceptable | 47 | 75.8 | 65 | 23.8 | 120 | 16.5 |
| Lack of knowledge | 5 | 8.1 | 142 | 52.0 | 397 | 54.5 | |
| Misconception | 10 | 16.1 | 66 | 24.2 | 212 | 29.0 | |
| Total | 62 | 100.0 | 273 | 100.0 | 729 | 100.0 | |
| 9 | Acceptable | 40 | 64.5 | 79 | 29.0 | 251 | 34.5 |
| Lack of knowledge | 14 | 22.6 | 144 | 52.9 | 369 | 50.7 | |
| Misconception | 8 | 12.9 | 49 | 18.1 | 108 | 14.8 | |
| Total | 62 | 100.0 | 272 | 100.0 | 728 | 100.0 | |
| 12 | Acceptable | 46 | 74.2 | 82 | 30.3 | 164 | 22.6 |
| Lack of knowledge | 9 | 14.5 | 163 | 60.1 | 474 | 65.4 | |
| Misconception | 7 | 11.3 | 26 | 9.6 | 87 | 12.0 | |
| Total | 62 | 100.0 | 271 | 100.0 | 725 | 100.0 | |
In general, the percentage of teachers classified as “acceptable” is highest, and the percentage of respondents with misconceptions is highest for high school students. In item 9 there is some deviation from these generalities. Again, large numbers of students were allocated to the category “lack of knowledge”.
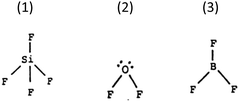
Test item 6. This item checks whether respondents can correctly decide that OF2 molecules are polar, while SiF4 and BF3 molecules are not (Choice 2). The most appropriate rationalisation is that non-symmetrical molecules are polar (Choice B).
Less than half of the teachers made the correct choices. We surmise that this may be due to a language issue that constitutes a weakness in the diagnostic instrument. An OF2 molecule is symmetrical in the sense that it has a plane of symmetry. More precisely, an OF2 molecule lacks sufficient symmetry that the dipole moments due to bond polarities cancel out.
Misconceptions were common among all groups. The most common misconception identified (9.7% of teachers, 14.7% of university students and 26.2% of high school students) was that an OF2 molecule is polar (Correct choice 2) because of non-bonding electrons on an atom (Reason C).
A second, less frequent, misconception (6.5% of teachers, 2.2% of university students and 2.3% of high school students) was that an OF2 molecule is polar because of the high electronegativity of fluorine (Reason A).
Common to both misconceptions is failure to recognise that the shape of a molecule is a factor that determines molecular polarity. The same misconceptions were identified in all studies based on Peterson's (1986) test for probing understanding covalent bonding and structure. These findings are also consistent with those of Prodjosantoso et al. (2019).
Test item 7. Respondents were asked to consider why water is a liquid and hydrogen sulfide is a gas, even though they have similar formulas and similar molecular shapes.
75.8% of teachers correctly attributed the difference of states to the presence of stronger intermolecular forces between water molecules (Choice 1), and that this is due to the difference in polarity of the molecules (Reason C). Only 23.8% of university students and 16.5% of high school students made these choices. More than a half of both student groups were classified as “lack of knowledge”.
The most common teachers’ misconception (8.1%), identified by correct first tier answer 1, and wrong reason C, is based on judgement that hydrogen sulfide molecules are polar.
Significant numbers of respondents (6.5%, 13.2% and 7.4% respectively; Choice set 1, A) attributed the different substance properties to differences in the strengths of the intramolecular O–H and S–H covalent bonds. Even more high school students (16.0%; Choice set 1, B) believe that the different states of the two substances are due to the fact that the covalent bonds in hydrogen sulfide are more easily broken than those in water. These findings clearly show that significant numbers of participants in each group attribute the state of a substance, and the strength of intermolecular forces, to the strength of the intramolecular bonds. This is consistent with findings of Peterson and Treagust (1989), Peterson et al. (1989), Othman et al. (2008) and Ogden (2017).
Test item 9. This item was designed to check if the influence on shape of a molecule on its polarity is understood. Shown the planar T-shaped structure of ClF3 molecules, it was expected that respondents would decide that these molecules are polar (Choice 1) because of the T-shaped arrangement of polar bonds (Reason C).
The responses of only 64.5% of teachers were deemed to be “acceptable”, although this was considerably more than for both groups of students. Unusually, the percentage of university students deemed “acceptable” was lower than for the school students.
The most frequent misconception among university and high school students (14.0% and 7.1%) is that ClF3 molecules are non-polar (Choice 2) because there is a very little difference between the electronegativity of chlorine and fluorine atoms (Reason D).
The most common misconception among teachers (8.1%) is that although they correctly decided that the ClF3 molecule is polar, the polarity was attributed to the high electronegativity of fluorine (reason B). Again we see that the shape of molecules is neglected as a factor that determines molecular polarity.
Test item 12. This item checks whether respondents understand that the intermolecular forces between OF2 molecules are greater than those between CF4 molecules (Choice 1), because CF4 molecules are symmetrical and non-polar (Choice D).
In common with most other items, the percentage of teachers with “acceptable” understanding (74.2%) is less than desirable, but higher than for both groups of students. In both groups of students, many more were classified as “lack of knowledge” than the number that could be reasonably confirmed to have misconceptions.
In the discussion of item 6, it was mooted that the description of OF2 molecules as non-symmetrical may have influenced sense-making. The same description is used in this item, but if it is a factor here, it is less influential than in item 6.
The most common misconception identified in the sample of teachers (4.8%) is indicated by choice of reason C: the CF4 molecule is polar because of a large difference of electronegativity between carbon and fluorine. This confirms previous evidence that some participants, even teachers, do not recognise the importance of molecular shape on polarity.
There is not a significant number of any particular misconception demonstrated in students’ samples. However, we think that it is worth reporting that 3.3% of university students misconceive that intermolecular forces are stronger between non-polar molecules, like CF4, than between polar molecules, like OF2 (Choice 2, Reason D). We can’t even speculate on the origin of that misconception, but we know that it is not attributable to the difference in the number of polar bonds in molecules. That misconception – the more polar bonds it forms, the stronger intermolecular forces molecule will affect – was identified in 2.6% of high school students by answer set: Choice 2, Reason A.
| Test item | Level of knowledge | Groups of participants | |||||
|---|---|---|---|---|---|---|---|
| Teachers | University students | High school students | |||||
| N | % | N | % | N | % | ||
| 10 | Acceptable | 51 | 82.3 | 156 | 56.9 | 448 | 61.6 |
| Lack of knowledge | 5 | 8.1 | 94 | 34.3 | 202 | 27.8 | |
| Misconception | 6 | 9.6 | 24 | 8.8 | 77 | 10.6 | |
| Total | 62 | 100.0 | 274 | 100.0 | 727 | 100.0 | |
| 15 | Acceptable | 29 | 46.7 | 31 | 11.4 | 68 | 9.5 |
| Lack of knowledge | 21 | 33.9 | 204 | 74.7 | 521 | 72.5 | |
| Misconception | 12 | 19.4 | 38 | 13.9 | 129 | 18.0 | |
| Total | 62 | 100.0 | 273 | 100.0 | 718 | 100.0 | |
Test item 10 is essentially about understanding the use of the octet rule to decide the number of bonds that atoms form: the “acceptable” responses were 2 and A. A common misconception exhibited by both groups of students (3.6% and 3.3%) was indicated by first-tier choice 1; that the primary purpose of the octet rule is to determine the shape of a molecule. Perhaps these students were thinking of the use of the octet rule to decide the number of electron pairs around an atom in order to apply the VSEPR theory of shape prediction. Another student misconception (3.4% and 3.6%) was indicated by correct choice 2, but incorrect reason B: the number of bonds formed equals the number of electrons in the outer shell.
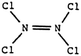
Test item 15 requires respondents to choose, from four options, the best representation of the structure of a N2Cl4 molecule. The acceptable choices are 3 and C. The percentages of participants deemed “acceptable” in all groups were low. More than 70% of students were classified as “lack of knowledge”. The most common misconception is identified in 6.5% of teachers and 5.1 and 5.3% of university and high school students who chose the best representation, but selected incorrect reason B which refers to 5 electron pairs on the N atom (rather than 4). We might speculate that they have considered only bonding electron pairs (and ignored non-bonding electron pairs).
| Test item | Level of knowledge | Groups of participants | |||||
|---|---|---|---|---|---|---|---|
| Teachers | University students | High school students | |||||
| N | % | N | % | N | % | ||
| 2 | Acceptable | 29 | 46.8 | 34 | 12.5 | 52 | 7.1 |
| Lack of knowledge | 17 | 27.4 | 182 | 67.2 | 524 | 71.3 | |
| Misconception | 16 | 25.8 | 55 | 20.3 | 159 | 21.6 | |
| Total | 62 | 100.0 | 271 | 100.0 | 735 | 100.0 | |
| 5 | Acceptable | 39 | 63.9 | 105 | 38.5 | 235 | 32.1 |
| Lack of knowledge | 2 | 3.3 | 106 | 38.8 | 371 | 50.7 | |
| Misconception | 20 | 32.8 | 62 | 22.7 | 126 | 17.2 | |
| Total | 61 | 100.0 | 273 | 100.0 | 732 | 100.0 | |
| 8 | Acceptable | 29 | 46.8 | 37 | 13.6 | 65 | 9.0 |
| Lack of knowledge | 19 | 30.6 | 179 | 65.8 | 509 | 70.1 | |
| Misconception | 14 | 22.6 | 56 | 20.6 | 152 | 20.9 | |
| Total | 62 | 100.0 | 272 | 100.0 | 726 | 100.0 | |
| 13 | Acceptable | 53 | 85.5 | 181 | 66.3 | 245 | 34.0 |
| Lack of knowledge | 6 | 9.7 | 65 | 23.8 | 374 | 51.9 | |
| Misconception | 3 | 4.8 | 27 | 9.9 | 101 | 14.1 | |
| Total | 62 | 100.0 | 273 | 100.0 | 720 | 100.0 | |
In each of the items 2, 5 and 8, the percentage of teachers classified as “acceptable” is worryingly low, and yet much higher than for either group of students. On the other hand, more misconceptions were diagnosed in teachers than in the students: most of the wrong choices made by students were attributed to lack of knowledge, rather than to particular misconceptions. This finding, for example, would not be diagnosed by usual two-tier testing.
Test item 2. Participants are classified as “acceptable” if they decide that molecules of the product of reaction between nitrogen and bromine (NBr3, although the substance is not named) have trigonal pyramidal shape (Choice 2) because of the tetrahedral arrangement of bonding and non-bonding electron pairs around the N atom (Reason B).
Less than half of the teachers, and only 7% of high school students made both correct choices. A majority of the students in both groups were classified as “lack of knowledge”. At least 20% of participants within each of the groups demonstrated misconceptions.
Among the teachers, 8.1% chose correctly that NBr3 molecules have trigonal pyramidal shape, but gave the reason (A) that nitrogen forms three bonds which equally repel each other. These choices are contradictory. Another 6.5% of teachers, as well as 7.5% of high school students believed that NBr3 molecules are trigonal planar because of repulsion between the three bonds around the nitrogen atom (Choices 1, A), demonstrating that they did not realise that there is a lone pair on the nitrogen atom – or did not realise its significance.
A different misconception was displayed by 12.5% of university students. They were confident of their responses 3 and B; that NBr3 molecules are tetrahedral because of the tetrahedral arrangement of bonding and non-bonding pairs around the N atom. These students realised the existence of the lone pair on the N atom, but appear to confuse the arrangement of electron pairs and the shape of the molecules (defined by the positions of the atoms).
Test item 5. Participants were classified as “acceptable” if they decided that a molecule of SCl2 is probably V-shaped (Choice 1) because of repulsion between bonding and non-bonding electron pairs (Choice A).
For all groups, the performance was higher than for item 2 – which was similar except that respondents had first to deduce the formula NBr3. Nevertheless, more participants displayed misconceptions in item 5 than in item 2 (corresponding with fewer expressing lack of confidence at tier 3). The most common misconception, indicated by 11.3% of teachers and 9.4% of high school students, was that a SCl2 molecule is linear because of repulsion of bonds between the atom of sulfur and the chlorine atoms (Choice 2, Reason C). Perhaps they made a correspondence with CO2 molecules, rather than draw a correct Lewis diagram.
Most of the university students allocated to the “misconception” class (13.2% of the total) correctly decided that a SCl2 molecule is V-shaped, but wrongly attributed this to repulsion between non-bonding pairs (Choice 1, Reason B). It is clear that there is confusion about how the shape of a molecule can be seen to depend on interactions among bonding and non-bonding electron pairs. This is, again, consistent with findings of Peterson et al. (1989) and Birk and Kurtz (1999).
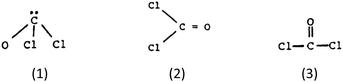
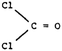
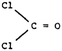
Test item 8. Like items 2 and 5, this item required prediction of the shape of a molecule – in this case, phosgene, COCl2. In item 8, however, the participants were given the formula as well as a choice of Lewis diagrammatic representations. Unlike the examples in times 2 and 5, a COCl2 molecule has both single and double bonds around the C atom, and no lone pairs. The “acceptable” classification was given to those who chose, with confidence, choices 2 and D: the molecule has planar shape because of equal repulsion between bonding regions.
Despite the fact that more relevant information was given in this item, in all groups the percentages classified as “acceptable” was low. The most common misconception (11.3%, 12.9%, and 11.2%, respectively) was the attribution of shape to the stronger polarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond. The fact that a significant number of participants confidently believe that bond polarity influences the shape of a molecule, is one more call for consideration of the curriculum. This misconception has been identified in various studies (Peterson et al., 1989; Birk and Kurtz, 1999; Nicol, 2001).
O double bond. The fact that a significant number of participants confidently believe that bond polarity influences the shape of a molecule, is one more call for consideration of the curriculum. This misconception has been identified in various studies (Peterson et al., 1989; Birk and Kurtz, 1999; Nicol, 2001).
Test item 13. The expected understanding is that VSEPR theory is used to determine the shape of a molecule (Choice 2), and an acceptable rationale for VSEPR theory (Choice B).
The performance of respondents is better in this item than in the other items probing understanding about shapes of molecules. Nevertheless, it is somewhat disappointing that 14.5% of teachers and 66% of high school students did not display acceptable knowledge.
The most common misconception among students in both groups (6.2% and 6.9%) was that the shape of a molecule is due to repulsion between the atoms in the molecule (Answer set 2, D).
In answers to items 7, 12 (Table 7) and 11 (Table 10), 75.8%, 74.2% and 91.9%, respectively, of teachers showed “acceptable” knowledge. More than 50% of students (except in case of university students’ answers to question 11) were classified as “lack of knowledge”.
| Test item | Level of knowledge | Groups of participants | |||||
|---|---|---|---|---|---|---|---|
| Teachers | University students | High school students | |||||
| N | % | N | % | N | % | ||
| 4 | Acceptable | 27 | 43.5 | 35 | 12.8 | 31 | 4.2 |
| Lack of knowledge | 21 | 33.9 | 136 | 49.8 | 334 | 45.6 | |
| Misconception | 14 | 22.6 | 102 | 37.4 | 367 | 50.2 | |
| Total | 62 | 100.0 | 273 | 100.0 | 732 | 100.0 | |
| 11 | Acceptable | 57 | 91.9 | 123 | 45.1 | 222 | 30.8 |
| Lack of knowledge | 1 | 1.6 | 123 | 45.1 | 431 | 59.8 | |
| Misconception | 4 | 6.5 | 27 | 9.8 | 68 | 9.4 | |
| Total | 62 | 100.0 | 273 | 100.0 | 721 | 100.0 | |
Misconceptions related to intermolecular forces are discussed earlier. In summary, some participants attribute the state of substances to intramolecular forces (rather than intermolecular forces), and some take polarity of bonds as a factor influencing intermolecular forces, without regard to the shape of the molecules.
Test item 4. This item probes understanding of the very essence of the curricular model of covalent network substances: that the bonds in the crystal are strong (Choice 2), and that the crystal is composed of covalently bonded atoms (Choice D). The percentage of teachers classified as “acceptable” is poor, given that they are the sources of understanding to so many chemistry students. The percentages of “acceptable” students is very low, and of great concern.
All respondent groups, especially the students, displayed high levels of misconceptions, distinct from lack of knowledge. By far the most common (12.9%, 31.9%, and 46.0%) were detected by the confident selection of choices 1 and B; that is, that the high melting point suggests that the bonds in SiC are strong, so a large amount of energy is required to break the intermolecular forces. It is apparent that many participants, including teachers, have strong perceptions about covalent network substances that do not align with the curricular model. 4.8% of teachers are convinced that high melting and boiling points of silicon carbide are due strong bonds between covalently bonded molecules.
Test item 11. Respondents classified in the “acceptable” category confidently declared that, on the basis of its property of sublimation, dry ice is a molecular crystal (Choice 1), and that its lattice is composed of carbon dioxide molecules interconnected by weak attractive forces.
There were more “acceptable” responses than for other items, and only a low frequency of misconceptions was diagnosed. The most frequent misconception (4.8% of teachers, and 3.5% of high school students) was evidenced by a declaration that dry ice is a covalent molecular substance because its crystal lattice is composed of covalently bonded carbon and oxygen atoms which together form a giant molecule (Choice 1, reason A). 3.7% of university students believe that dry ice is a covalent molecular substance because its crystal lattice is composed of carbon dioxide molecules interconnected by covalent bonds (Choice 1, Reason C).
Final discussion and considerations
The results show covalent bonding comprehension problems at all levels of the educational system in Croatia. In general, the percentage of teachers and especially university and high school students with sound conceptual knowledge about each sub-topic of covalent bonding (Table 4) is low. The number of participants, primarily students, classed as “lack of knowledge” (rather than having misconceptions) is concerning. Four factors have to be taken into account in consideration of what these findings mean:(a) Although covalent bonding is taught only in the first year of high school and undergraduate studies, participants were from all grades of high schools and faculties. Similarly, we did not distinguish between teachers who had recently been teaching covalent bonding and those who had not done so for some time. Therefore, the results are not indicators of knowledge immediately after participation in a course on bonding, but is a snapshot of knowledge of all teachers and students in the system.
(b) The results are a measure of the participants' conceptual knowledge levels, classified as “acceptable”, “misconceptions”, or “lack of knowledge”. We did not analyse participants' responses to the first-tier items (correct or incorrect) because this was not the goal of the research, and because the instrument was not designed for that purpose.
(c) We established the criterion that only participants who correctly answered all test items related to a sub-topic had “acceptable” knowledge about that sub-topic. This criterion might be perceived as rigorous, however, we see it as the only way for confident conclusion (based on the test instrument's results) about participants with “acceptable” knowledge.
(d) Learning outcomes of covalent bonding are clearly defined in the chemistry curriculum for the first grade of the high school and in first year general chemistry curriculum for the undergraduate studies. However, what students should know about covalent bonding in higher grades is not specified: there is only an expectation that students should understand fundamental topics, like covalent bonding, to understand further concepts.
That the concepts of covalent bonding are fundamental to chemistry understanding does not mean that they are easy to understand or teach. Using conceptual profile theory, Baltieri et al. (2021) found how a covalent bonding concept, apparently simple and well-defined by the great area of Chemistry, may be complex in the teaching and learning processes. The results of this study supported that conclusion showing that covalent bonding for students and teachers in Croatia is difficult topic. Instead of resonance between conceptualisation of different covalent bonding sub-topics, many students in this study exhibit different knowledge categories: “lack of knowledge” and misconceptions.
Misconceptions are considered as a big problem because they are stable (Taber, 2001; Ozmen, 2004; Luxford and Bretz, 2014; Erman, 2016; Eymur and Geban, 2016) and act as blocks to learning related content. Therefore, it is important that they be accurately identified. Peterson's (1986) two-tier conceptual test Covalent Bonding and Structure, which has been used in many studies in different educational systems and formed the basis for the instrument used in this study, provided lots of data on students' misconceptions. However, it lacks the third test tier, so could not distinguish between participants who demonstrated “lack of knowledge” and misconceptions. The instrument used in the present study allows this to be done. Moreover, apart from misconceptions, this instrument is more accurate in distinguishing between acceptable knowledge and “lack of knowledge” than some other three-tier tests that focus on misconceptions, such as that of Prodjosantoso et al. (2019), which lacks just guessing self-confidence rate options in the third test tier.
Consequently, it was possible to confidently rank the covalent bonding sub-topics in terms of difficulty. The results showed that bond polarity is the best understood sub-topic, while the shape of molecules and molecular polarity are the least understood. We can speculate that this is due to the different complexity of the sub-topics. Determining the shape or polarity of a molecule requires a deeper intellectual engagement than other covalent bonding sub-topics. Especially in the case of shape of molecules, solutions require more decision-making steps of logic, each dependent on previous steps. Alternatively, or additionally, understanding of the less well-mastered sub-topics may have more dependence on difficult conceptual knowledge. The results show that many participants poorly understand the VSEPR model, the understanding of which is a prerequisite for thinking about the shapes of molecules. The lack of understanding of the shape of molecules affects the understanding of the polarity of molecules. Therefore, the finding that many students consider the polarity of molecules only in terms of the polarity of bonds and ignore their shape is not surprising. Perhaps more importantly, the interdependence of knowledge among different sub-topics is clear.
Going further, it is known that students (and teachers) are expected to develop mental models – internal representations that one constructs in order to understand or provide a rational explanation for an experienced phenomenon – when working mentally on complex problems, such as the shape of molecules (Greca and Moreira, 2002). This takes time. In Croatia, a lesson on molecular shape in high school is regularly conducted in two teaching hours – almost without further opportunity for assessment, feedback and consolidation. It seems that this is not enough engagement time for the majority of students to develop adequate mental models that could improve their understanding.
The lack of teaching time could influence the lack of student's mental modelling ability, visualisation and spatial ability and metacognitive skills (Wang and Barrow, 2010), which result in students’ thinking flaws. Taking into account the complexity of the sub-topics molecular shape and polarity, their abstract and modelled nature, and the requirements for specific skills that students must have, Croatian teachers and curriculum developers should reconsider the level of harmonisation between the curriculum requirements and the abilities of 15–16 year old students.
Students' thinking flaws could develop into misconceptions, sometimes directly induced by textbooks or teachers. Although there is evidence in the literature of misconceptions about covalent bonding since the 1980s, this does not seem to have affected Croatian teachers' awareness of their existence. Moreover, some in-service and pre-service teachers (almost 30% of university students involved in this study are pre-service chemistry teachers) have been found to be carriers of certain misconceptions and thus a source of misconceptions for their students. This makes it obvious that some misconceptions about covalent bonding in Croatian educational system are re-cycling from generation to generation.
As described in the literature review, several studies have been conducted to determine the understanding of covalent bonding in different educational systems. Most of them are focused on misconceptions. All of the studies examined the understanding of secondary school students and/or university students (undergraduates and/or graduates), most of whom were assessed shortly after instruction. In addition, the studies differ in methodology, type of test instrument, and method of identifying misconceptions.
Among all the studies published to date on understanding covalent bonding, this study is specific because of (a) the fact that it was conducted in a Croatian context, (b) the fact that it covers the entire educational system and includes secondary school chemistry teachers in addition to high school and university students, (c) the timing of the use of the test instrument - for some participants, one year, two years, or even longer after instruction, and (d) the method used to determine misconceptions. Because of all these specifics, the quantitative data from this study cannot be compared with previous studies about covalent bonding understanding. However, the qualitative data on misconceptions about covalent bonding are comparable across different educational systems. The misconceptions found in this study were identified in Australian secondary schools more than 30 years ago. Some of them are also recognised in other educational systems (Coll and Taylor, 2001; Nicoll, 2001; Luxford and Bretz, 2014; Erman, 2016; Vrabec and Prokša, 2016). There is no doubt that many misconceptions exist, especially in relation to fundamental chemical topics such as chemical bonding.
Misconceptions aside, this study reports on the acceptable (conceptual) knowledge and lack of knowledge in the (Croatian) education system. This is not the case with previously published studies on covalent bonding. Therefore, the comparison with the results focused on the understanding of covalent bonding in other educational systems can only be general. Moreover, it must be guided by the studies that are based on similar test instruments. Taking this into account, it could be concluded that high school students in Croatia, as well as tested secondary school students from South Australia (Peterson et al., 1989) and the United States (Birk and Kurtz, 1999), have not acquired a satisfactory understanding of the topic of covalent bonding. The results of this study indicate that conceptual understanding in Croatia is increasing with educational level, although it still does not come close to meeting expectations. A similar trend was noted by Birk and Kurtz (1999), who concluded that the gap between retrieved knowledge and conceptual understanding has not closed completely even at the university level.
With the exception of the studies by Luxford and Bretz (2014) and Vrabec and Prokša (2016), there is a lack of recent data collected in different educational systems using the “same” instrument (in the original and in translation) to test understanding of covalent bonding. A comparison of such data with this study could reveal differences and attribute them to the specifics of the educational system, especially when understanding is examined at all levels. This approach, focusing on the Croatian educational system, seems to reveal linked problems of conceptual understanding among all groups of participants: some misconceptions are common to teachers and students, there is a consistency in the ratio of acceptable knowledge of teachers and students (the least understood sub-topics of teachers are also the least understood by students), and the dimension of the problem seems to be relevant to all its groups. Taking everything into account and recognising that teachers are a product of the educational system and implementers of educational policy, rather than perpetrators, it seems that the problem of understanding covalent bonding in Croatia is a problem of the system rather than a problem of the individual.
Implications for teaching practice and research
The results are a call for systemic action aimed at improving students' conceptual understanding of covalent bonding in Croatia. Chemistry education administrators in Croatia should plan and implement changes that target all five domains of pedagogical content knowledge (PCK) (Magnusson et al., 1999), directed to both in-service and pre-service teachers, with the intention of influencing all realms – personal, enacted and collective – of PCK (Carlson and Daehler, 2019) about covalent bonding.In addition to administrators, this is a call to chemistry teachers, as individuals and as a community, to analyse the identified problems of low conceptual knowledge, and the high percentage of students with misconceptions or lack of knowledge.
Incorrect, imprecise or incomplete teaching (Tsaparlis, 1997), inadequate representation of concepts in textbooks, ineffective communication between learners and teachers, exam-oriented pedagogy, simplistic teaching approaches, and incompetent teachers (Gudyanga and Madambi, 2014) may play an important role in causing students' misconceptions about chemical bonding and preventing understanding. Therefore, the first step might be to analyse and change teaching practices.
The impression that the scope and depth of high school chemistry content are not consistent with the number of hours of instruction (70 hours per year) should be investigated by experts and researchers. These study results could be a consequence of the teacher-centred approach regularly adopted by teachers who feel that the instructional time is not sufficient to “teach” the content in other ways. Therefore, the second step could be to analyse the high school chemistry curriculum and possibly intervene.
When planning instruction, teachers are expected to be aware of, and understand, common misconceptions in science at a certain educational level (Özmen, 2004; Ünal et al., 2006). In regard to that, a compulsory section in the lesson preparation sheets for pre-service chemistry teachers has been shown as a useful addition (Vladušić et al., 2020). In it, after analysis of the scientific literature, pre-service teachers have to consider: (a) what common misconceptions are related to the specific lesson they are preparing and (b) textbook analysis. This can raise their awareness of what misconceptions students might develop during their lessons. It also gives them the opportunity to (a) prevent the influence of the textbook and their own misconceptions on students, (b) use specific misconceptions as an impetus for class discussions, (c) select the best teaching approaches for dealing with certain misconceptions, and (d) enhance their own conceptual understanding. The same lesson sheet addition could be used by in-service teachers, some of whom, as this study showed, hold misconceptions.
There is no doubt that prevention is the best way to deal with misconceptions. However, in systems in which the state of knowledge is unsatisfactory, prevention should be organised simultaneously with curative actions. The set of misconceptions about covalent bonding found in this study, as well as the diagnostic instrument, could be useful in both respects. In addition, the triple-tier conceptual test and the knowledge classification criteria presented in this study may serve as a reliable format for identifying misconceptions.
Finally, there is a number of teaching and assessment strategies for dealing with problems of understanding, ranging from general to topic-specific strategies. Some of them are the drip-feed approach (Barker and Millar, 2000), Herein – a student-centred activity (Ogden, 2017), the Bonding Representations Inventory Tool (Luxford and Bretz, 2013) and the Flow Maps method (Temel and Özcan, 2016). However, there is little information in the literature about examples and effectiveness of remedial approaches. Considering this and the fact that misconceptions are difficult to change, there is a great need to develop and evaluate new teaching strategies that can help avoidance of student misconceptions. It is in this area that our further research will be directed.
Conclusions
A three-tiered diagnostic instrument, was used as a tool to assess the level of knowledge about covalent bonding at all levels of the educational system in Croatia. It enables the distinction between knowledge levels that we have classified as “acceptable”, “misconception” or “lack of knowledge”.The percentage of participants, especially students with deficiencies of understanding that can be described as lack of knowledge is high, especially considering that covalent bonding is a fundamental topic.
The scan of the system shows the low extent to which participants' conceptual knowledge matches the curricular models especially among the students. Various misconceptions are identified in each group of participants in each sub-topic of covalent bonding, some of which are particularly prevalent. Some misconceptions are common to both in-service and pre-service teachers and students. This supports the assumption that in Croatian educational system covalent bonding misconceptions are re-cycling.
The conceptual understanding of Croatian school teachers is more comparable to the curricular models of covalent bonding than the understanding of university students and especially high school students. Intermolecular forces is the best understood sub-topic by teachers, and bond polarity by university and high school students. The shapes of molecules is the least understood sub-topic across all participant groups. Misconceptions are most prevalent in the high school student group. The shape of molecules and molecular polarity are sub-topics where the largest number of participants from all groups had misconceptions. The scan of the Croatian educational system in relation to the sub-topics of covalent bonding seems to reveal a systemic (problem of the system) rather than an individual problem. This is a call for Croatian chemistry teachers, curriculum developers, and education officials to act on all domains and realms of covalent bonding PCK.
Conflicts of interest
There are no conflict to declare.Appendices
Appendix A: propositional statements concerning models of covalent compounds
Presumed prior knowledge
• In molecular substances, there are discrete aggregates of atoms, called molecules. All of the molecules in a sample of one substance are identical with each other, and different from those in every other molecular substance.• Adjacent atoms in molecules are held in place by forces of attraction called covalent bonds.
• According to the model, electrons are located between adjacent atoms, and can be considered to be shared by the atoms: that is, the electrons are in the valence shells of both of the atoms.
• The force of attraction between adjacent atoms in molecules is the net force arising from (i) attractions between the shared electrons (with negative electrical charge) and the nuclei of both atoms (with positive charges), and (ii) repulsions between the two nuclei.
• When the covalent bond between two atoms is due to sharing of two electrons, called a shared electron pair, it is said to be a single bond.
• If four electrons are shared between the nuclei, the bond is called a double bond.
• If six electrons are shared the bond is called a triple bond.
• Electrons on each atom that are not shared are called non-bonding electrons – or, since they are believed to form pairs, lone pairs.
Covalent molecular substances
(2) In covalent bonds joining two atoms of different elements, the shared bonding electrons are not located centrally between the atoms: they reside closer to one of the atoms.
(3) A measure of the ability of atoms of an element to attract the shared bonding electrons is called its electronegativity. In a covalent bond joining two atoms of different elements, the atom to which the bonding electrons are closer is the more electronegative.
(4) The electronegativity of every element is different from that of every other element.
(5) Unequal sharing of the bonding electrons (when the bonding electrons are closer to one of the bound atoms) results in charges on the atoms – a small negative charge (<1, labelled δ−) on the more electronegative atom, and an equal positive charge (<1, δ+) on the other atom.
(6) Bonds between atoms of different elements are said to be polar, because of the opposite charges on the two bound atoms. The greater is the difference of electronegativity of the atoms (and, therefore, the larger the charges on the atoms), the more polar is the bond.
(8) Non-polar bonds in a molecule have no influence on the polarity the molecule.
(9) Diatomic molecules composed of identical atoms (i.e., of the same element) do not have a dipole moment: they are non-polar.
(10) Because of the charges on the two atoms bound by a polar bond, polar bonds in a molecule tend to align the molecule in the direction of an applied electrical field.
(11) Diatomic molecules composed of atoms of different elements have a dipole moment: they are polar.
(12) A molecule whose structure can be represented by the formula MXn (in which all of the X atoms are joined to the same M atom) has n polar M–X bonds. Such a molecule has zero dipole moment (is non-polar) if the shape of the molecule has symmetry such that the forces of alignment from the polar bonds cancel out.
(13) A molecule whose structure can be represented by the formula MXn has a dipole moment (is polar) if the shape of the molecule is such that the forces of alignment from the polar bonds do not cancel out.
(14) Polar molecules can differ in the magnitude of their dipole moment (they are more or less polar), depending upon (i) the polarities of the bonds in the molecules, and (ii) the extent to which the spatial orientation of the bonds causes cancellation of the bond dipoles.
(16) According to the octet rule, each atom in a molecule (except H atoms) has eight electrons in its valence shell, comprising (i) a half-share of bonding electrons (one electron of each single bond, two electrons of each double bond, and three electrons of each triple bond), and (ii) lone (non-bonding) pairs.
(18) According to VSEPR model, electron regions (single bonds, double bonds, triple bonds, and lone pairs) in the valence shell of the central atom attain a spatial distribution that minimizes repulsion between them.
(19) The shape of a molecule of type MXn refers to the relative positions of the X atoms around the central M atom, and does not include the location of lone pairs.
(21) The strength of intermolecular forces varies from substance to substance.
(22) The intermolecular forces between polar molecules are stronger than those between non-polar molecules of similar size.
(23) Comparing different substances with polar molecules of similar size and shape, the larger the polarity of the molecules, the stronger are the intermolecular forces.
(24) The stronger the forces of attraction between the molecules of substances, the higher are their melting points and boiling points. In other words, whether a molecular substance is a solid, liquid, or gas at a specified temperature depends on the strength of the intermolecular forces.
(25) Generally, the melting points of covalent molecular substances are lower than those of covalent network substances. This is because melting of a solid molecular substance occurs when the energy of the molecules is sufficient to overcome the intermolecular forces, whereas melting of a solid network substances requires that covalent bonds are broken. [See also #27]
Covalent network substances
(26) In solid covalent network substances, the atoms are bound together in fixed positions by covalent bonds between adjacent atoms in a continuous lattice – as though one giant molecule.(27) For melting to occur, covalent bonds must be broken (in contrast to the melting of covalent molecular substances, during which intermolecular forces need to be overcome, without the breaking of covalent bonds).
Appendix B: diagnostic instrument in Croatian language
1. Koji od sljedećih prikaza bolje prikazuje položaj zajedničkog elektronskog para u molekuli fluorovodika (HF)?Razlog
(A) Elektroni nepodijeljenih elektronskih parova uvjetuju položaj zajedničkog elektronskog para.
(B) Budući da se vodik (H) i fluor (F) povezuju kovalentnom vezom, zajednički elektronski par treba biti smješten u sredini.
(C) Fluor (F) snažnije privlači zajednički elektronski par.
(D) Atom fluora (F) je veći od atoma vodika (H) pa snažnije djeluje na zajednički elektronski par.
2. Reakcijom dušika (element 15. skupine) i broma (element 17. skupine) nastat će molekule produkta. Te će molekule, najvjerojatnije, imati oblik koji najbolje opisujemo kao:
(1) Planaran (trokut) (2) Trostrana piramida (3) Tetraedar
Razlog
(A) Atom dušika ostvaruje tri veze koje se podjednako odbijaju jedne od drugih formirajući planarnu molekulu trokutasta oblika.
(B) Tetraedarski raspored zajedničkih i nepodijeljenih parova elektrona oko (jezgre) atoma dušika određuje oblik molekule.
(C) Polarnost veze između atoma dušika i broma određuje oblik molekule.
(D) Razlika u elektronegativnosti između atoma broma i dušika određuje oblik molekule.
3. Polarnost veze između kisika i fluora bolje prikazuje izraz:
Razlog
(A) Nepodijeljeni elektronski parovi na svakom od atoma uzrokuju polarnost veze.
(B) Veza među atomima kisika i fluora je polarna jer atom kisika ima 6 valentnih elektrona, a atom fluora 7.
(C) Zajednički elektronski par je bliže atomu fluora.
(D) Veza je polarna jer se iz atoma kisika formira O2− ion, a iz atoma flora F− ion.
4. Silicijev karbid ima visoko talište i vrelište. Ovi podatci sugeriraju da su veze u silicijevom karbidu:
(1) slabe (2) jake
Razlog
(A) Silicijev karbid je kristalna tvar sastavljena od kovalentno povezanih molekula.
(B) Velika količina energije je potrebna da bi se nadvladale međumolekulske sile u kristalnoj rešetci silicijeva karbida.
(C) Silicijev karbid je molekulski kristal.
(D) Silicijev karbid je kristalna tvar sastavljena od niz(ov)a kovalentno vezanih atoma. Možemo ga smatrati jednom velikom (makro)molekulom.
5. Molekula SCl2 najvjerojatnije ima:
(1) savijen (svinut ili “V”) oblik (2) ravan (linearan) oblik
Razlog
(A) Odbijanja između zajedničkih i nepodijeljenih elektronskih parova rezultiraju oblikom molekule.
(B) Odbijanja između nepodijeljenih elektronskih parova određuju oblik molekule.
(C) Dvije veze između atoma sumpora i klora se maksimalno odbijaju zauzimajući linearan položaj pa molekulu SCl2 možemo prikazati sljedećom strukturom:
(D) Visoka elektronegativnost klora u odnosu na sumpor je najznačajniji čimbenik koji utječe na oblik molekule.
6. Koja je, među prikazanim molekulama, polarna?
Razlog
(A) Molekula je polarna zbog visoke elektronegativnosti fluora.
(B) Nesimetrične molekule sastavljene od različitih atoma su polarne.
(C) Nepodijeljeni elektroni atoma u molekuli induciraju dipol i time molekulu čine polarnom.
(D) Velika razlika u elektronegativnosti atoma povezanih kemijskim vezama molekulu čini polarnom.
7. Voda (H2O) i sumporovodik (H2S) imaju sličnu kemijsku formulu i isti (savijen, “V”) oblik molekule. Pri sobnoj temperaturi, voda je tekućina, a sumporovodik plin. Ta razlika u agregacijskim stanjima posljedica je relativno jakih međumolekulskih sila između:
(1) H2O molekula (2) H2S molekula
Razlog
(A) Razlika u jakosti međumolekulskih sila je posljedica razlike u jakosti O-H i S-H kovalentnih veza.
(B) Veze u molekuli H2S lako pucaju, dok one u molekuli H2O nisu.
(C) Razlika u jakosti međumolekulskih sila je posljedica razlike u polarnosti molekula.
(D) Razlika u jakosti međumolekulskih sila je posljedica činjenice da je H2O polarna molekula dok je H2S nepolarna molekula.
8. Koja od sljedećih struktura najbolje prikazuje oblik COCl2 molekule?
Razlog
(A) Oblik molekule COCl2 ovisi o elektronegativnosti svakog od atoma.
(B) Oblik molekule COCl2 je posljedica približno jednakih odbijanja između zajedničkih i nepodijeljenih elektronskih parova ugljika.
(C) Oblik molekule COCl2 je posljedica jače polarnosti C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O dvostruke veze u molekuli, u odnosu na druge veze.
O dvostruke veze u molekuli, u odnosu na druge veze.
(D) Oblik molekule COCl2 je posljedica jednakih odbijanja između veznih područja nastalih povezivanjem atoma klora i kisika s atomom ugljika.
9. Molekula klorova trifluorida (ClF3) često se opisuje kao planarna molekula, “T” oblika. Strukturu joj prikazuje formula:
Na temelju ovih informacija, ClF3 je, najvjerojatnije:
(1) polarna molekula (2) nepolarna molekula
Razlog
(A) Molekula je polarna ako su veze među njenim atomima polarne.
(B) Budući da fluor ima visoku vrijednost elektronegativnosti, molekula je polarna.
(C) “T” oblik raspodjele polarnih veza uzrok je polarnosti molekule.
(D) Molekula nije polarna zbog male razlike u vrijednosti elektronegativnosti između atoma klora i fluora.
10. “Pravilo okteta” se koristi da bi se odredio:
(1) oblik molekule (2) broj veza koje atom ostvaruje
Razlog
(A) Pravilom okteta se utvrđuje da atom formira kovalentne veze dijeljenjem elektrona s ciljem popunjavanja valentne ljuske s 8 elektrona.
(B) Pravilom okteta se utvrđuje da je broj kovalentnih veza jednak broju elektrona u vanjskoj ljusci.
(C) Pravilom okteta se utvrđuje ovisnost oblika molekule o broju zajedničkih elektronskih parova.
(D) Pravilom okteta se definira oblik molekule kao posljedica usmjerenosti četiriju elektronskih parova u vrhove tetraedra.
11. Suhi je led čvrsta tvar sastavljena od molekula ugljikova dioksida. Koristi se kao sredstvo za hlađenje pojedinih vrsta namirnica. Tijekom tog procesa, suhi led sublimira. Na temelju ovih informacija suhi se led može svrstati u:
(1) molekulske kristale (2) atomske kristale
Razlog
(A) Kristalna rešetka suhog leda sastavljena je od niza kovalentno vezanih atoma ugljika i kisika koji zajedno čine veliku molekulu.
(B) Kristalna rešetka suhog leda sastavljena je od molekula ugljikova dioksida međusobno povezanih slabim privlačnim silama.
(C) Kristalna rešetka suhog leda sastavljena je od molekula ugljikova dioksida međusobno povezanih kovalentnim vezama.
(D) Kristalna rešetka suhog leda sastavljena je od molekula ugljikova dioksida međusobno povezanih jakim privlačnim silama.
12. Temeljem usporedbe tvari sastavljenih molekula OF2 i tvari sastavljenih od molekula CF4, najlogičnije je zaključiti kako će međumolekulske sile biti:
(1) snažnije među molekulama OF2
(2) snažnije među molekulama CF4
(3) jednako snažne među molekulama OF2 i CF4
Razlog
(A) Četiri su polarne veze u molekuli CF4, a samo dvije u molekuli OF2.
(B) Mala razlika u elektronegativnosti atoma kisika i fluora razlog je nepolarnosti molekule OF2.
(C) Velika razlika u elektronegativnosti atoma ugljika i fluora razlog je polarnosti molekule CF4.
(D) CF4 je simetrična, nepolarna molekula, dok je OF2 nesimetrična i polarna molekula.
13. Teorija odbijanja elektronskih parova valentne ljuske, VSEPR (valence shell electron pair repulsion), koristi se da bi odredili:
(1) polarnost molekula.
(2) oblik molekula.
Razlog
(A) Nepodijeljeni elektroni određuju polarnost molekula. Na primjer, nepodijeljeni elektroni atoma B u molekuli
uzrokuju djelomičnu negativnost (δ−) atoma B.
(B) VSEPR teorijom se utvrđuje kako je oblik molekule uvjetovan raspodjelom zajedničkih i nepodijeljenih elektronskih parova oko centralnog atoma s ciljem minimiziranja odbijanja elektrona.
(C) VSEPR teorijom se utvrđuje da je polarnost molekule ovisna o broju prisutnih (postojećih) polarnih veza.
(D) VSEPR teorijom se utvrđuje da je oblik molekule posljedica odbijanja atoma u molekuli.
14. Atomi sumpora i atom klora tvore polarne kovalentne veze u molekuli SCl2.
U ovim vezama, atom s djelomično pozitivnim nabojem (δ+) je:
(1) sumpor (2) klor
Razlog
(A) Sumpor donira jedan elektron atomu klora pri čemu nastaju S+ i Cl− ioni.
(B) Sumpor je djelomično negativan (δ−) i može formirati S2− ion, dok klor može formirati samo kloridni ion, Cl−.
(C) Broj valentnih elektrona sumpora i klora određuje polarnost veze.
(D) Klor ima visok koeficijent elektronegativnosti pa će se podijeljeni elektronski par nalaziti malo bliže njemu nego atomu sumpora.
15. Koji od sljedećih prikaza najbolje predstavlja strukturu N2Cl4?
Razlog
(A) Visoki koeficijent elektronegativnosti dušika uzrok je činjenici da se atom dušika uvijek veže barem jednom dvostrukom ili trostrukom vezom.
(B) Struktura je posljedica odbojnih sila koje djeluju između 5 elektronskih parova dušikova atoma (uključujući zajedničke i nepodijeljene).
(C) Struktura je posljedica odbojnih sila koje djeluju između 4 elektronska para dušikova atoma (uključujući zajedničke i nepodijeljene).
(D) Struktura je rezultat odbijanja među vezama u molekuli.
Appendix C: Peterson's two-tiered conceptual questions which were translated into Croatian language to serve in diagnostic instrument (Peterson, 1986)
1. Which of the following best represents the position of the shared electron pair in the HF molecule?Reason
(A) Non-bonding electrons influence the position of the bonding or shared electron pair.
(B) As hydrogen and fluorine form a covalent bond the electron pair must be centrally located.
(C) Fluorine has a stronger attraction for the shared electron pair.
(D) Fluorine is the larger of the two atoms and hence exerts greater control over the shared electron pair.
2. Nitrogen (a group 5 element) combines with bromine (a group 7 element) to form a molecule. This molecule is likely to have a shape which is best described as:
(1) trigonal planar (2) trigonal pyramidal (3) tetrahedral
Reason
(A) Nitrogen forms three bonds which equally repel each other to form a trigonal planar shape.
(B) The tetrahedral arrangement of the bonding and non-bonding electron pairs around nitrogen results in the shape of the molecule.
(C) The polarity of the nitrogen–bromine bonds determines the shape of the molecule.
(D) The difference in electronegativity values for bromine and nitrogen determine the shape of the molecule.
3. The po1arity of the oxygen–f1uorine bond would be best shown as:
Reason
(A) The non-bonding electron pairs p resent on each atom determine the polarity of the bond.
(B) A polar covalent bond forms as oxygen has s ix outer shell electrons and fluorine seven outer shell electrons
(C) The shared electron pair is closer to fluorine.
(D) The polarity of the bond is due to the oxygen atom forming O2− ion, whereas fluorine forms an F− ion.
4. Silicon Carbide has a high melting point and a high boiling point. This information suggests that the bonds in silicon carbide are:
(1) weak (2) strong
Reason
(A) Silicon carbide is a covalent network solid (continuous covalent lattice) composed of covalently bonded mo1ecules.
(B) A large amount of energy is required to break the intermolecular forces in the silicon carbide lattice.
(C) Silicon carbide is a covalent molecular solid.
(D) Silicon carbide is a covalent network solid (continuous covalent lattice) composed of covalently bonded atoms.
5. The molecule SCl2 is likely to be:
(1) V shaped (2) linear
Reason
(A) Repulsion between the bonding and non-bonding electron pairs results in the shape.
(B) Repulsion between the non-bonding electron pairs results in the shape.
(C) The two sulfur–chlorine bonds are equally repelled to linear positions structure shown as
(D) The high electronegativity of chlorine compared to Sulfur is the major factor influencing the shape of the molecule.
6. Which of the following molecules is polar:
Reason
(A) The polarity of the molecule is due to the high electronegativity of fluorine.
(B) Non-symmetrical molecules containing different atoms are polar.
(C) Non-bonding electrons on an atom in the molecule produce a dipole and hence a polar molecule.
(D) A large difference in the electronegativities of the atoms involved in bonding results in a polar molecule.
7. Water and hydrogen sulphide have similar chemical formulae and have V-shaped structures. At room temperature, water is a liquid and Hydrogen Sulphide a gas. The difference in state between water and Hydrogen Sulphide is due to the presence of strong intermolecular forces between:
(1) H2O molecules (2) H2S molecules
Reason
(A) The difference in strength of the intermolecular forces is due to the difference in the strength of the O–H and S–H covalent bonds.
(B) The bonds in H2S are easily broken whereas in H2O they are not.
(C) The difference in strength of the intermolecular forces is due the difference in polarity of the molecules.
(D) The difference in strength of the intermolecular forces is due the fact that H2O is a polar molecule, whereas H2S is a non-polar molecule.
8. Which of the following best indicates the shape of the COCl2 molecule?
Reason
(A) The shape of COCl2 is dependent on the electronegativity of each atom.
(B) The shape of COCl2 is due to approximately equal repulsion between the bonding and the non-bonding electron pairs on the carbon.
(C) The shape of COCl2 is due to the stronger polarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond in the molecule.
O double bond in the molecule.
(D) The shape of COCl2 is due to equal repulsion between the bonding regions formed by the atoms joining to the carbon.
9. The substance chlorine trifluoride (ClF3) is often described as a planar. T-shaped molecule, whose structure can be represented as
Based on this information ClF3 is most likely to be a:
(1) polar (2) non-polar molecule.
Reason
(A) The molecule is polar as it has polar bonds.
(B) As fluorine has a very high electronegativity the molecule is polar.
(C) The T-shaped arrangement of the polar bonds results in a polar molecule.
(D) The molecule is non-polar because there is very little difference between the electronegativity values of Cl and F.
10. The octet rule is used to determine the:
(1) shape of a molecule (2) number of bonds an atom forms.
Reason
(A) The octet rule states that an atom forms covalent bonds through the sharing of electrons in order to have 8 electrons in the valence shell.
(B) The octet rule states that the number of bonds formed equals the number of electrons in the outer shell.
(C) The octet rule states that the shape of a molecule is dependent on the number of shared electron pairs.
(D) The octet rule states that the shape of a molecule is due to 4 electron pairs being located in tetrahedral positions.
11. Dry ice has been used to cool foodstuffs for short periods of time. Gradually, during this process, the dry ice disappears. Chemically, dry ice is a solid, containing CO2 molecules. Based on this information, dry ice would be best classified as a:
(1) molecular solid (2) continuous covalent solid
Reason
(A) Dry Ice has a continuous lattice of carbon–oxygen bonds.
(B) Dry Ice has a lattice of weakly joined CO2 molecules.
(C) Dry Ice has a continuous lattice of weakly joined carbon–oxygen bonds.
(D) Dry Ice has a lattice of strongly joined CO2 molecules.
12. When comparing the molecules OF2 and CF4 it is most likely that the strength of the intermolecular forces will be:
(1) greater between OF2 molecules (2) greater between CF4 molecules (3) the same for each type of molecule
Reason
(A) There are four polar bonds in CF4 and only two in OF2.
(B) The similar electronegativities of oxygen and fluorine result in OF2 being non-polar.
(C) The large electronegativity difference between carbon and fluorine atoms results in CF4 being polar.
(D) CF4 is symmetrical and non-polar, whereas OF2 is non-symmetrical and polar.
13. The ‘electron pair repulsion theory’ is used to determine the:
(1) po1arity of a molecule (2) shape of a molecule.
Reason
(A) Non-bonding electrons determine the polarity of the molecule. For example, non-bonding electrons on the atom B in the molecule  cause B to become partially negative (δ−).
cause B to become partially negative (δ−).
(B) The theory states that the shape of a molecule is due to the arrangement of the bonding and non-bonding electron pairs around the central atom to minimize electron repulsion.
(C) The theory states that the polarity of a molecule is dependent on the number of polar bonds present.
(D) The theory states that the shape of a molecule is due to repulsion between the atoms in the molecule.
14. The molecule SCl2 has polar covalent bonds between the sulfur and the ch1orine atoms. The atom assigned the partial positive charge (δ+) in these bonds would be:
(1) sulfur (2) chlorine
Reason
(A) Sulfur donates one electron to the chlorine atom resulting in the formation of S+ and Cl− ions.
(B) Sulfur is partially negative (δ−) as it can form an S2− ion, whereas chorine can only form a C1− ion.
(C) The number of valence electrons on sulfur and chlorine determine the polarity of the bonds.
(D) Chlorine has a high electronegativity and the shared electron pair tends to be located slightly closer to it than the sulfur atom.
15. Which of the following best represents the structure of N2Cl4?
Reason
(A) The high electronegativity of nitrogen requires that a double or triple bond is always present.
(B) The structure is due to repulsion between the 5 electron pairs (including bonding and non-bonding pairs) on the nitrogen atom.
(C) The structure is due to repulsion between the 4 electron pairs (including bonding and non-bonding pairs) on the nitrogen atom.
(D) The structure is due to the repulsion between bonds in the molecule.
Appendix D: the list of misconceptions identified in the study
Following is a list of the most common misconceptions identified in this study. The misconceptions are tabulary presented in subsections depending on which sub-topic they relate to: bond polarity, molecular polarity, octet rule, shape of molecules, intermolecular forces, and covalent network and molecular substances.In each case, we present (a) the situation-specific misconception identified by a particular item, and (b) a generalisation of that finding, usually expressed as an incorrect version of one of the propositional statements.
In the left column is the situation-specific misconception: the key (Q1, 2B), for example (Table 1), indicates that this misconception was identified from item Question 1, in which the participant chose response 2 in the first tier, and response B in the second tier. In the right column is the deduced generalisation of that specific misconception, showing, in parentheses, the propositional statement that is not well understood.
In generalising participants' ideas identified as misconceptions, the authors attempted to interpret the “logic” of participants' thoughts from their responses to the test items – guided by the design of the items. It is, of course impossible to get inside the participants' heads, so the authors' interpretation of their thought processes has limitations and should be taken with caution (Tables 11–16).
| Specific misconception identified | Generalisation |
|---|---|
| (Q1, 2B) The representation of the HF molecule with the shared electron pair centrally placed is the best representation of its position because fluorine and hydrogen atoms forms covalent bond so shared electron pair must be centrally located | The shared electron pair between two covalently bonded atoms is always centrally located (PS 2) |
| (Q1, 2C) Despite the fact that fluorine atom has a stronger attraction for the shared electron pair, the best representation of the position of shared electron pair in HF molecule is when it is centrally located | Even in polar molecules, the best representation of the position of the shared electron pair is when it is centrally located (PS 2) |
| (Q1, 1D) The best representation of the position of the shared electron pair in an HF molecule is when it is drawn closer to the symbol of the fluorine atom because the fluorine atom is larger than the hydrogen atom, and hence exerts greater control over the shared electron pair | In diatomic molecules, the larger atom exerts greater control over the shared electron pair, so the shared electron pair is closer to it (PS 3 and 5) |
| (Q3, 2A) The polarity of the oxygen–fluorine bond would be best shown with a sign for partial positive charge (δ+) on the symbol for the fluorine atom (rather than on the symbol for the oxygen atom) because the (number of) non-bonding electron pairs on each atom determines the polarity of the bond | Between two atoms bonded by polar covalent bond, the atom with more non-bonding electron pairs is partially positive (PS 5) |
| (Q3, 2B) The polarity of the oxygen–fluorine bond are best shown with a sign for partial positive charge (δ+) on the oxygen atom (rather than on the fluorine atom) because the oxygen atom has six outer shell electrons, and the fluorine atom has seven | Whether the atoms joined by a covalent bond have a partial positive charge or a partial negative charge depends on the relative numbers of valence electrons in the bonded atoms (PS 5) |
| The molecule SCl2 has a polar covalent bonds between the sulfur atom and the chlorine atoms. The atom assigned the partial positive charge (δ+) is chlorine because chlorine has a high electronegativity and the shared electron pair tends to be located slightly closer to it than to the sulfur atom | Between two covalently bonded atoms, the one with a partial positive charge is the more electronegative one because it more strongly attracts the shared electron pair (PS 5) |
| (Q14, 1A) The molecule SCl2 has a polar covalent bonds between the sulfur atom and the chlorine atoms. The atom assigned the partial positive charge (δ+) in these bonds is sulfur because sulfur's donation of one electron to the chlorine atom results in the formation of S+ and Cl− ions | A polar covalent bond is a result of transfer of electron(s) between two atoms (PS 6) |
| Between two atoms covalently bonded, the one with a partial positive charge is less electronegative one because it donates electron(s) |
| Specific misconception identified | Generalisation |
|---|---|
| (Q6, 2C) The OF2 molecule is polar because non-bonding electrons of an atom in the molecule produce a dipole and hence a polar molecule | Non-bonding electrons of atoms in a molecule induce a dipole and make the molecule polar (PS 5) |
| (Q6, 2A) The polarity of the OF2 molecule is due to the high electronegativity of fluorine | If there is a fluorine atom in a heteroatomic molecule, the molecule must be polar (PS 13) |
| (Q9, 1B) The T-shaped molecule of chlorine trifluoride (ClF3) is a polar molecule because fluorine has a very high electronegativity | A bond between a fluorine atom and any other atom is polar, so all such molecules are polar (PS 13) |
| (Q9, 2D) The T-shaped molecule of chlorine trifluoride (ClF3) is non-polar because there is a very small difference between the electronegativity of chlorine and fluorine atoms | The difference in electronegativity of chlorine and fluorine atoms is too small to render the Cl–F bond polar |
| (Q9, 1A) The ClF3 molecule is polar because it has polar bonds | All molecules with polar bonds are polar (PS 13) |
| (Q12, 2C) The CF4 is polar molecule because there is a large electronegativity difference between carbon and fluorine atoms | All molecules with polar bonds are polar (PS 13) |
| (Q9, 1B) T-shaped molecule of chlorine trifluoride (ClF3) is a polar molecule because fluorine has a very high electronegativity | In heteroatomic molecules, fluorine atoms forms only polar bonds, so the molecules must be polar (PS 13) |
| Specific misconception identified | Generalisation |
|---|---|
| (Q7, 1A) Difference in strength of the intermolecular forces between water and hydrogen sulfide both substances consisted of triatomic V-shaped molecules (with general formula A2B) is due to the difference in the strength of the O–H and S–H covalent bonds | The strengths of the covalent bonds in molecules influence the strength of intermolecular forces in a substance |
| (Q7, 1B) Water and hydrogen sulfide, both consisted of molecules with similar formula and the same V-shaped molecular structure are occurring in different (water in liquid, and hydrogen sulfide in gaseous) state under the room conditions. That's because of different strength of intermolecular forces caused by the fact that covalent bonds in hydrogen sulphide are easily broken, whereas in water they are not | The state of a molecular substance depends on how easily covalent bonds in molecules can be broken (PS 24 and 27) |
| (Q12, 2D) Intermolecular forces are stronger between CF4 molecules than between OF2 molecules because CF4 is symmetrical and non-polar molecule, whereas OF2 is non-symmetrical and polar | Intermolecular forces are stronger between non-polar molecules, than between polar molecules (PS 23) |
| (Q12, 2A) Intermolecular forces are stronger between CF4 molecules than between OF2 molecules because there are four polar bonds in CF4 and only two in OF2 | The more polar bonds in a molecule, the stronger are the intermolecular forces (PS 23) |
| Specific misconception identified | Generalisation |
|---|---|
| (Q4, 2B) High melting and boiling points suggest that the bonds in silicon carbide are strong. That's why a large amount of energy is required to break the intermolecular forces in the silicon carbide lattice | Intermolecular forces are strong attractions which result in high melting and boiling points of covalent network substances |
| (Q4, 2A) High melting and boiling points suggest that the bonds in silicon carbide are strong. That's because silicon carbide is a covalent network solid composed of covalently bonded molecules | Covalent networks solids are composed of covalently bonded molecules (PS 26) |
| (Q4, 2C) High melting and boiling points suggest that the bonds in silicon carbide are strong. That's because silicon carbide is a covalent molecular solid | Because of strong bonds within the molecules, covalent molecular solids have high melting and boiling points (PS 27) |
| There are discrete molecules in covalent network substances | |
| (Q11, 1A) Dry ice is a covalent molecular substance because its crystal lattice is composed of covalently bonded carbon and oxygen atoms which forming, altogether, a giant molecule | Covalent molecular substances are composed of a lattice of atoms interconnected by covalent bonds (PS 27) |
| (Q11, 1C) Dry ice is a covalent molecular substance because its crystal lattice is composed of carbon dioxide molecules interconnected by covalent bonds | Molecules in molecular substances are interconnected by covalent bonds (PS 27) |
Acknowledgements
We thank the school teachers, tertiary students and high school students who participated in this study. A special thank deserve the secondary and university chemistry teachers who conducted the diagnostic instrument in their schools and faculties.References
- Baltieri R. S., Bego A. M. and Cebim M. A., (2021), Why the covalent bond is such a complex concept: A conceptual profile proposal, Int. J. Sci. Educ., 43(12), 1–18.
- Barker V. and Millar R., (2000), Students’ reasoning about basic chemical thermodynamics and chemical bonding: What changes occur during a context based post-16 chemistry course? Int. J. Sci. Educ., 22(11), 1171–1200.
- Birk J. P. and Kurtz M. J., (1999), Effect of experience on retention and elimination of misconceptions about molecular structure and bonding, J. Chem. Educ., 76(1), 124–128.
- Boo H. K., (1998), Students’ understanding of chemical bonds and the energetic of chemical reactions, J. Res. Sci. Teach., 35(5), 569–581.
- Burrows N. L. and Mooring S. R., (2015), Using concept mapping to uncover students’ knowledge structures of chemical bonding concepts, Chem. Educ. Res. Pract., 16(1), 53–66.
- Butts B. and Smith R., (1987), HSC chemistry students’ understanding of the structure and properties of molecular and ionic compounds, Res. Sci. Educ., 17, 192–201.
- Carlson J. and Daehler K. R., (2019), The refined consensus model of pedagogical content knowledge in science education, in Hume A., Cooper R. and Borowski A. (ed.), Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science, Singapore: Springer, pp. 77–92.
- Coll R. K. and Taylor N., (2001), Alternative conceptions of chemical bonding held by upper secondary and tertiary students, Res. Sci. Technol. Educ., 19(2), 171–191.
- Dhindsa H. S. and Treagust D. F., (2009), Conceptual understanding of Bruneian tertiary students: Chemical bonding and structure, Brunei Int. J. Sci. Math. Educ., 1(1), 33–51.
- Driver R., (1981), Pupils’ alternative frameworks in science, Eur. J. Sci. Educ., 3(1), 93–101.
- Erman E., (2016), Factors contributing to students’ misconceptions in learning covalent bonds, J. Res. Sci. Teach., 54(4), 520–537.
- Eymur G. and Geban Ö., (2016), The collaboration of cooperative learning and conceptual change: Enhancing the students’ understanding of chemical bonding concepts, Int. J. Sci. Math. Educ., 15(5), 853–871.
- Gilbert J. K., Osborne J. R. and Fensham P. J., (1982), Children's science and its consequences for teaching, Sci. Educ., 66(4), 623–633.
- Goh N. K., Khoo L. E. and Chia L. S., (1993), Some misconceptions in chemistry: Across-cultural comparison, and implications for teaching, Aust. Sci. Teach. J., 39(3), 65–68.
- Greca I. M. and Moreira M. A., (2002), Mental, physical, and mathematical models in the teaching and learning of physics, Sci. Educ., 86(1), 106–121.
- Gudyanga E. and Madambi T., (2014), Pedagogics of chemical bonding in chemistry; perspectives and potential for progress: The Case of Zimbabwe Secondary Education, Int. J. Second. Educ., 2(1), 11–19.
- Helm H., (1980), Alternative conceptions in physics amongst South African students, Phys. Educ., 15(2), 92–105.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7(2), 75–83.
- Kumpha P., Suwannoi P. and Treagust D. F., (2014), Thai grade 10 students conceptual understanding of chemical bonding, Procedia Soc. Behav. Sci., 143, 657–662.
- Luxford C. J. and Bretz S. L., (2013), Moving beyond definitions: What student-generated models reveal about their understanding of covalent bonding and ionic bonding, Chem. Educ. Res. Pract., 14(2), 214–222.
- Luxford C. J. and Bretz S. L., (2014), Development of the bonding representations inventory to identify student misconceptions about covalent and ionic bonding representations, J. Chem. Educ., 91(3), 312–320.
- Magnusson S., Krajcik J. and Borko H., (1999), Nature, sources, and development of pedagogical content knowledge for science teaching, in Gess-Newsome J. and Lederman (ed.), Examining predagogical content knowledge, Dordrect, The Netherlands: Kluwer Academic, pp. 95–132.
- Markic S. and Childs P. E., (2016), Language and the teaching and learning of chemistry, Chem. Educ. Res. Pract., 17(3), 434–438.
- Nahum T. L., Mamlok-Naaman R., Hofstein A. and Krajcik J., (2007), Developing a new teaching approach for the chemical bonding concept aligned with current scientific and pedagogical knowledge, Sci. Educ., 91(4), 579–603.
- Nahum T. L., Mamlok-Naaman R., Hofstein A. and Taber K. S., (2010), Teaching and learning the concept of chemical bonding, Res. Sci. Educ., 46(2), 179–207.
- Nicoll G., (2001), A report of undergraduates’ bonding misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Nimmermark A., Öhrström L., Mårtensson J. and Davidowitz B., (2016), Teaching of chemical bonding: a study of Swedish and South African students’ conceptions of bonding, Chem. Educ. Res. Pract., 17(4), 985–1005.
- Ogden M., (2017), An inquiry experience with high school students to develop an understanding of intermolecular forces by relating boiling point trends and molecular structure, J. Chem. Educ., 94(7), 897–902.
- Othman J., Treagust D. F. and Chandrasegaran A. L., (2008), An investigation into the relationship between students’ conceptions of the particulate nature of matter and their understanding of chemical bonding, Int. J. Sci. Educ., 30(11), 1531–1550.
- Özmen H., (2004), Some student misconceptions in chemistry: A literature review of chemical bonding, J. Sci. Educ. Technol., 13(2), 147–159.
- Pabuccu A. and Geban O., (2012), Students’ conceptual level of understanding on chemical bonding, Int. Online J. Educ. Sci., 4(3), 563–580.
- Peterson R. F., (1986), The development, validation and application of a diagnostic test instrument measuring year 11 and year 12 students' understanding of covalent bonding and structure, Unpublished Master's thesis, Perth, Australia: Curtin University of Technology.
- Peterson R. F. and Treagust D. F., (1989), Grade-12 students’ misconceptions of covalent bonding and structure, J. Chem. Educ., 66(6), 459–460.
- Peterson R. F., Treagust D. and Garnett P., (1986), Identification of secondary students’ misconceptions of covalent bonding and structure concepts using a diagnostic instrument, Res. Sci. Educ., 16(1), 40–48.
- Peterson R. F., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students’ concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26(4), 301– 314.
- Prodjosantoso A. K., Hertina A. M. and Irwanto (2019), The misconception diagnosis on ionic and covalent bonds concepts with three tier diagnostic test, Int. J. Instr., 12(1), 1477–1488.
- Taber K. S., (1995), Development of student understanding: A case study of stability and lability in cognitive structure, Res. Sci. Technol. Educ., 13(1), 89–99.
- Taber K. S., (2001), Constructing chemical concepts in the classroom? Using research to inform the practice, Chem. Educ. Res. Pract., 2(1), 43–51.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Taber K. S., (2015), Exploring the language(s) of chemistry education, Chem. Educ. Res. Pract., 16(2), 193–197.
- Taber K. S. and Coll R. K., (2002), Chemical bonding, in Gilbert J. K., de Jong O., Justi R., Treagust D. F. and van Driel J. H. (ed.), Chemical Education: Towards Research-based Practice, Dordrecht: Kluwer Academic Publishers BV, pp. 213–234.
- Tan K. C. D. and Treagust D. F., (1999), Evaluating students’ understanding of chemical bonding, Sch. Sci. Rev., 81(294), 75–83.
- Temel S. and Özcan,Ö., (2016), The analysis of prospective chemistry teachers’ cognitive structure: The subject of covalent and ionic bonding, Eurasia J. Math. Sci. Technol. Educ., 12(8), 1953–1969.
- Treagust D. F., (1988), Development and use of diagnostic tests to evaluate students’ misconceptions in science, Int. J. Sci. Educ., 10(2), 159–169.
- Tsaparlis G., (1997), Atomic and molecular structure in chemical education: A critical analysis from various perspectives of science education, J. Chem. Educ., 74(8), 922–925.
- Tsaparlis G., Pappa E. T. and Byers B., (2018), Teaching and learning chemical bonding: Research-based evidence for misconceptions and conceptual difficulties experienced by students in upper secondary schools and the effect of an enriched text, Chem. Educ. Res. Pract., 19(4), 1253–1269.
- Ünal S., Çalık M., Ayas A. and Coll R. K., (2006), A review of chemical bonding studies: Needs, aims, methods of exploring students’ conceptions, general knowledge claims and students’ alternative conceptions, Res. Sci. Technol. Educ., 24(2), 141–172.
- Vladušić R., Bucat R. B. and Ožić M., (2016a), Understanding ionic bonding – A scan across the Croatian education system, Chem. Educ. Res. Pract., 17(4), 685–699.
- Vladušić R., Bucat R. B. and Ožić M., (2016b), Understanding of words and symbols by chemistry university students in Croatia, Chem. Educ. Res. Pract., 17(3), 474–488.
- Vladušić R., Bucat R. B. and Ožić M., (2020), Evidence of the development of pedagogical content knowledge related to chemical bonding during a course for preservice chemistry teachers, Cent. Educ. Policy Stud. J., 10(1), 59–81.
- Vrabec M. and Prokša M., (2016), Identifying misconceptions related to chemical bonding concepts in the Slovak school system using the bonding representations inventory as a diagnostic tool, J. Chem. Educ., 93(8), 1364–1370.
- Wandersee J. H., Mintzes J. J. and Novak J. D., (1994), Research on alternative conceptions in science, in Gabel D. L. (ed.), Handbook of research on science teaching and learning, New York: Simon and Schuster and Prentice Hall International, pp. 177–210.
- Wang C.-Y. and Barrow L. H., (2010), Characteristics and levels of sophistication: An analysis of chemistry students’ ability to think with mental models, Res. Sci. Educ., 41(4), 561–586.
| This journal is © The Royal Society of Chemistry 2023 |