Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Conversion of glycerol to acrylic acid: a review of strategies, recent developments and prospects
Umar C.
Abubakar
ab,
Yash
Bansod
 a,
Luke
Forster
a,
Luke
Forster
 a,
Vincenzo
Spallina
a,
Vincenzo
Spallina
 a and
Carmine
D'Agostino
a and
Carmine
D'Agostino
 *ac
*ac
aDepartment of Chemical Engineering, University of Manchester, Manchester M13 9PL, UK. E-mail: carmine.dagostino@manchester.ac.uk
bDepartment of Pure and Environmental Chemistry, Usmanu Danfodiyo University, Sokoto 840104, Nigeria
cDipartimento di Ingegneria Civile, Chimica, Ambientale e dei Materiali (DICAM), Alma Mater Studiorum – Università di Bologna, Via Terracini, 28, 40131 Bologna, Italy
First published on 23rd June 2023
Abstract
Acrylic acid is an essential chemical and a vital intermediate used in the production of various commodities and industrial chemicals. However, continued reliance on petroleum-based feedstocks for the production of acrylic acid is considered unsustainable and even counterproductive to the goal of achieving net zero carbon emissions. Glycerol waste generated from biodiesel production via transesterification processes has been identified as a viable alternative feedstock. However, commercial-scale adoption is yet to be realised due to inherent challenges associated with the conversion of glycerol to acrylic acid. Herein, we review the latest strategies, challenges and prospects for the utilisation of waste glycerol as a feedstock for the production of acrylic acid. Biochemical, electrochemical, photochemical, electrocatalytic and thermocatalytic conversion routes are discussed to provide insights into recent developments made in the field. Sustainable pathways that can be potentially implemented to transform readily available waste glycerol to acrylic acid at minimal costs are also considered. Biochemical conversion routes are the most promising from an environmental perspective as they have minimal energy requirements and low global warming potentials. However, higher acrylic acid yields have been reported from thermocatalytic conversion routes.
1. Introduction
Acrylic acid is an essential industrial chemical that can be used both as a precursor for other important commodity chemicals and as an ingredient to produce many commonly used consumer products. Acrylic acid or its derivatives are used in the manufacture of polymers, adhesives, paints, detergents and personal care products, demonstrating the wide utility of acrylic acid as an ingredient or platform chemical. Reports show that the global annual demand for acrylic acid stood at 6.2 million metric tons1 in 2020, while the estimated market size was $12 billion2 in 2021 and is expected to reach $19.5 billion3 by 2030. The increasing demand for energy owing to rapid population growth and industrialisation is already having a substantial effect on the price of crude oil. Crude oil is the main source of propylene, currently used as the raw material to produce acrylic acid industrially. Moreover, the depletion of available petroleum reserves coupled with the emission problems linked to the exploration and utilisation of petroleum resources is causing a major shift towards the renewable energy sector and sustainable chemical manufacturing.4As such, investment in renewable energy and green sources of raw materials with minimal or no environmental footprint5,6 is higher than ever. Investments in green technologies have become a global priority, owing to more stringent environmental regulations against emissions and incentives offered by various governments to encourage industries to adopt more sustainable practices. Hence, there has been a major surge in the production of biofuels and more intensive research efforts aimed towards the utilisation of biomass as alternative feedstocks for the production of important platform chemicals. Indeed, utilising renewable, sustainable bioresources as industrial feedstocks could significantly contribute towards the target of net zero emissions. Additional benefits could be derived from biodiesel production by converting the large amounts of waste crude glycerol generated into acrylic acid.
Recent developments in the energy sector are expected to stimulate further increases in biodiesel production, concomitant to this is the generation of excessive amounts of waste glycerol. Waste glycerol produced from biodiesel plants already accounts for more than 60% of the global production.7,8 Thus, more valorisation options are required to utilize the surplus glycerol generated, thereby averting a glycerol glut and further stimulating the production of biodiesell.9–12
Incineration of waste glycerol is not considered a sustainable option owing to its low heating value and the potential of exacerbating emission problems, such as the release of acrolein and other toxic chemicals. Additionally, the presence of impurities such as methanol, soap, free fatty glycerides, ash, biodiesel and catalyst residues often found in crude glycerol, make it unsuitable for conventional applications where higher grades of glycerol are required.13 Hence, there is more emphasis on converting accumulated glycerol to value-added chemicals, particularly acrylic acid and intermediates such as acrolein, allyl alcohol and lactic acid.14,15
Despite the vast potential for conversion to valuable chemicals, challenges associated with conversion processes have made commercial-scale utilisation of crude glycerol waste unattractive industrially. However, the conversion of glycerol to acrylic acid remains an attractive option considering the demand and diverse range of applications of this valuable chemical, as well as the potential positive environmental impacts. Scheme 1 shows the processes leading to the production of biodiesel and crude glycerol, as well as the inherent potential for the sustainable production of acrylic acid and the expected benefits to the environment.
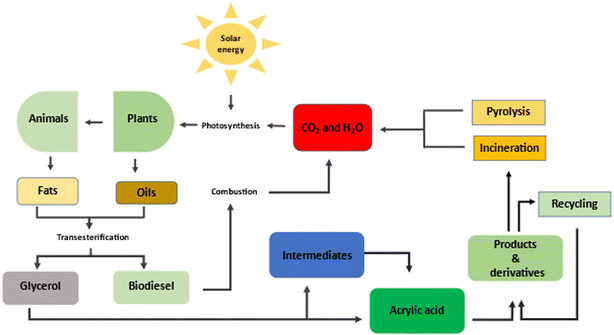 | ||
| Scheme 1 Origin of crude glycerol and its potential as a feedstock for the sustainable production of acrylic acid. | ||
Research in this area has attracted tremendous attention because of the economic relevance of acrylic acid and its derivatives. Global forecasts suggest that the market volume for acrylic acid could reach 11.3 million metric tons by 2029.3 Thus, various glycerol-to-acrylic acid conversion routes have been identified and several patents have been issued for processes developed for this transformation.16–20 However, commercial-scale utilisation of crude glycerol to produce acrylic acid is yet to be realised due to various problems associated with this transformation. Such problems include the lack of suitable catalysts, the requirement for multiple conversion steps, high associated energy demands and emission problems. Thus, further development of processes that will enable the large-scale conversion of waste glycerol to acrylic acid at competitive costs and with minimal negative environmental impacts has become imperative. Herein, we review the strategies, recent developments and prospects for the utilisation of crude glycerol as an alternative feedstock for the commercial-scale production of acrylic acid.
2. Routes to sustainable production of acrylic acid from glycerol
Conventionally, acrylic acid is produced from propylene through successive partial oxidation reactions. Initially, propylene is partially oxidised to acrolein, which is then converted to acrylic acid through a subsequent further oxidation step.21–24 Both reactions rely on mixed metal oxide catalysts to achieve desired levels of conversion and product yield. >90% acrylic acid yields have been reported using Bi–Mo-22 and MoVO-based21,24 mixed oxide catalysts for the first and second oxidation reactions, respectively. Different catalytic materials and processes are required to transform glycerol into acrylic acid. Some strategies under consideration include electrochemical, biocatalytic, photochemical and thermocatalytic reactions, in which glycerol is used as feed to produce acrylic acid or any related intermediate that can then be converted to the desired product.The main feature of the conventional routes for the production of acrylic acid and the sustainable alternatives to this commercial route have been summarised in Fig. 1 for ease of comparison. Considering the various indices, including the status of their developments, the relative availabilities of the raw materials required and the overall process requirements, the above-mentioned sustainable strategies have the potential to significantly reduce the cost of producing acrylic acid and any related value-added products, particularly now that petrochemical companies are becoming more reluctant towards investment in new oil exploration activities while seeking green alternatives.
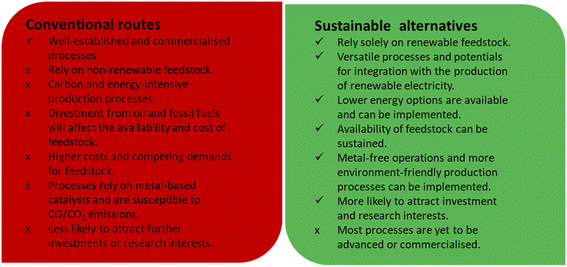 | ||
| Fig. 1 Comparison between the conventional routes to the production of acrylic acid and the sustainable alternative routes. | ||
Indeed, in 2010, the price of refined high grade (99.5%) glycerol dropped to about €450 per tonne and projections showed that the price could fall markedly towards much lower figures due to oversupply in the European Union.25 In contrast, the prices of ethylene and propylene hovered between $1000 to $1400 (€932 to €1503) per tonne.26 Even though the initial cost of implementing the glycerol to acrylic acid conversion routes might be higher in some cases, options for ambient and mild operation conditions to be used, lower emissions produced and greater protection for the environment are attractive incentives with long-term benefits.
2.1. Electrochemical conversion routes
The electrochemical conversion route is essentially a redox process that results in the oxidation of glycerol to acrylic acid intermediate chemicals, including 1,3-dihydroxyacetone, 1,3-propanediol27 and lactic acid.28 1,3-Propanediol and 1,3-dihydroxyacetone are converted to lactic acid through selective oxidation29–31 and isomerisation,32–35 respectively. The conversion of lactic acid to acrylic acid then occurs via dehydration over suitable catalysts (e.g. Na+ and K+ exchanged zeolites).36–38 Although multiple conversion steps are involved and integration with conversion routes is required to produce acrylic acid, the reactions are conducted under relatively mild conditions.31 Most electrochemical reactions used for the conversion of glycerol to acrylic acid are conducted at temperatures below the boiling point of the electrolyte, which is usually a basic or acidified aqueous glycerol solution, without relying on high gas pressures or complex equipment. For instance, electrochemical conversion of glycerol conducted in the galvanostatic mode (that is, maintaining the electrode at a constant current in an electrolyte) at 25 °C and 101 kPa produced various products including acrolein, 1,2-propanediol and 1,3-propanediol.39 The product selectivity of such reactions is controlled through the applied current, voltage, pH and reaction time. Thus, the electrochemical process is considered simple and sustainable.40,41A proposed mechanism for the electrochemical conversion of glycerol to lactic acid41 is shown in Scheme 2 and indicates that various reactions may occur simultaneously. However, the progress of these reactions can be controlled either through voltage and current modulation, or by changing the composition of the reaction media (e.g. by changing the pH).12,42
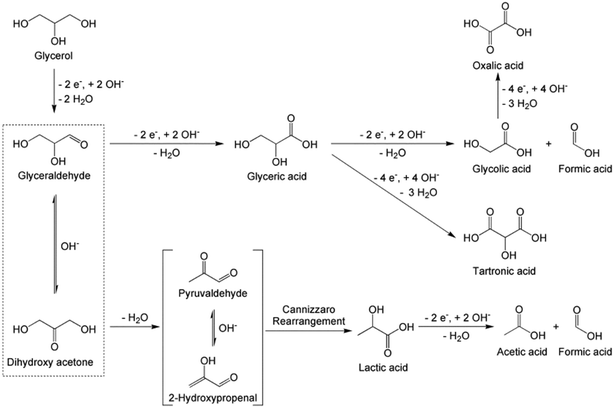 | ||
| Scheme 2 Proposed reaction pathways for glycerol oxidation in alkaline solution on AuPt catalysts. Products are determined from NMR and HPLC analysis of the reaction mixtures.41 Reproduced with permission from ref. 41 Copyright 2017, Elsevier. | ||
Noble metals such as Pt, Pd and Au have been reported as functional electrodes for the electrochemical oxidation of glycerol.43–45 In fact, earlier studies have relied on these noble metal-based electrodes, especially Pt electrodes, to achieve substantial feed conversion and high product selectivity.42,46 However, recent studies are more focused on the development of cheaper alternatives to replace or minimise reliance on expensive noble metal electrodes.41,45,47 For instance, Anastas et al. have reported the selective conversion of glycerol to lactic acid over a Co-based electrocatalyst in a basic medium at different temperatures ranging from 20 to 60 °C.48 The electrocatalyst was prepared by depositing Co-based nanoparticulates on a fluorine–tin–oxide electrode, which was used to achieve 34% glycerol conversion with a 37% selectivity to lactic acid. The production of lactic acid was favoured by reactions conducted at higher temperatures, higher current densities and in more strongly alkaline media. A recent study involving spinel Co3O4 has shown that cheaper electrodes can be made effective through doping with less expensive metals that offer synergetic interactions beneficial to glycerol oxidation.49 However, the active single-atom-bismuth-doped spinel Co3O4 electrode was more selective towards the formation of formic acid due to the generation of ˙OH radicals. Lee et al. have attempted using mixed carbon-black activated carbon composite as electrode and achieved a selectivity of 21% to lactic acid.50
Despite much effort dedicated to fabricating alternative and less expensive electrocatalysts, Pt-based electrocatalysts remain the most effective choice for glycerol oxidation. For example, Xu et al. used bimetallic AuPt nanoparticles dispersed on a carbon support to achieve 73% lactic acid selectivity at room temperature and atmospheric pressure.41 In this work, it was observed that the catalytic performance varied depending on the surface composition of the electrocatalysts, applied potentials, pH, glycerol concentration and reaction time. For instance, electrodes with Pt-enriched surfaces, and reactions in which lower oxidation potentials and higher base concentrations were used, favoured the selective conversion of glycerol to lactic acid conducted in a single-pot.41 More detail on the development of electrodes and electrocatalysts can be found elsewhere.28,51,52
2.2. Photochemical conversion routes
Photochemical conversion routes aim to take advantage of the immense opportunity of utilising solar energy, as opposed to relying on thermal energy often generated from carbon-intensive fuels, to facilitate the conversion of glycerol to acrylic acid. Photocatalysts capable of absorbing the energy of incident photons to generate charge carriers are required to facilitate the transfer of energy to adsorbed glycerol molecules for their conversion to target products. This strategy has been used to convert glycerol to various oxidation products such as 1,3-dihydroxyacetone, glyceraldehyde and hydrogen.53–55 1,3-Dihydroxyacetone and glyceraldehyde are isomers easily converted to acrylic acid via isomerisation to lactic acid,32–35,56,57 which is converted to acrylic acid via dehydration.58–60 The photochemical process is considered sustainable, less energy intensive and easy to implement.61 However, additional reaction steps and integration with chemical conversion routes are required to transform the intermediates produced to acrylic acid.Research on photochemical conversion processes includes the partial oxidation of glycerol over TiO2, which resulted in the formation of 1,3-dihydroxyacetone, glyceraldehyde and side products such as formic acid and CO2.62 This study showed that TiO2 is ineffective for this reaction, as selectivity towards 1,3-dihydroxyacetone was less than 10% and the maximum glycerol conversion was less than 40% even after 12 h of reaction.
Other notable contributions include the studies in which Chalermsinsuwan et al. compared the performance of different photocatalysts, Bi2O3, SiC, TiO2 and ZnO2.63 Results show that complete glycerol conversion can be achieved using H2O2 as an electron acceptor. Bi2O3, SiC and ZnO2 were more active than TiO2, but the selectivity towards 1,3-dihydroxyacetone was generally low (<10%) over all the photocatalysts tested. A similar observation was made by Payormhorm and Idem when they studied C-doped TiO2 designed to absorb photons in the visible region of the spectrum. This photocatalyst offered up to 67.5% glycerol conversion and was more selective towards transforming glycerol to formic acid than all other C3 molecules formed combined.64
A recent study by Xu et al. showed that more selective conversions could be achieved by tuning the photocatalyst's properties and using a suitable electron acceptor.65 Over 90% glycerol conversion and up to 95% 1,3-dihydroxyacetone selectivity was achieved after 12 h of irradiation using visible light-active Bi2WO6. The performance of the catalysts was attributed to the non-involvement of hydroxyl (˙OH) radicals and the mild oxidation power of Bi2WO6. ˙OH radicals are highly reactive and are known to facilitate C–C bond cleavage, often resulting in the formation of formic acid at the expense of the desired C3 compound.49 In the proposed mechanism for the reaction (Fig. 2), O2 serves as the electron acceptor of the photogenerated electrons and precursor to ˙O2− super radicals, which prevent the recombination of the generated electron–hole pairs. The Bi2WO6 photocatalysts with a flower-like superstructure were found to be the most active when compared with similar materials with different morphologies. Moreover, the authors also suggested that enhanced product selectivity was due to regioselective oxidation and weak adsorption of the products on the surface of Bi2WO6.49
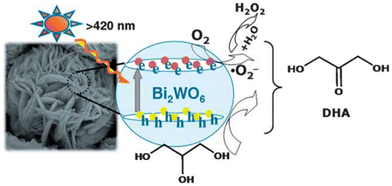 | ||
| Fig. 2 Suggested mechanism for selective oxidation of glycerol to 1,3-dihydroxyacetone (DHA) over the Bi2WO6 catalyst in water under visible-light irradiation.65 | ||
2.3. Biochemical conversion routes
Some microorganisms can transform glycerol to intermediates such as lactic acid66,67 and 3-hydroxypropionic acid68 which can then be subsequently converted to acrylic acid. However, the conversion of glycerol to 3-hydroxypropionic acid has currently attracted the most research interest. Modern biotechnology tools can be applied to introduce desired enzymes into other cells thereby enabling them to selectively transform glycerol into selected products through engineered metabolic pathways.69–72 Thus, recombinant strains of well-known microorganisms like Escherichia coli (E. coli) can implement the desired biochemical transformation.73Both 3-hydroxypropionic acid and lactic acid are produced through glycerol fermentation with different enzymes directing the formation of specific products. These two intermediates are isomers that are readily converted to acrylic acid via a subsequent dehydration step. For instance, Dishisha et al. have demonstrated that integrated systems comprising biochemical and chemical transformation processes can be used to convert glycerol to acrylic acid.68 In their work, a cascade system consisting of two biocatalysts and TiO2 was used to demonstrate the feasibility of integrating microbial and chemical synthesis for the sustainable production of acrylic acid from glycerol (Scheme 3).68 Over 95% acrylic acid yield was obtained from a series of reactions starting with 100 g L−1 aqueous glycerol feed, and the resting cells of Lactobacillus reuteri (L. reuteri) and Gluconobacter oxydans (G. oxydans) used as biocatalysts in fed-batch reactions conducted at 37 °C. While the L. reuteri cells converted the glycerol feed to an equimolar mixture of 3-hydroxypropionic acid and 1,3-propanediol, the G. oxydans acted on the 1,3-propanediol obtained from the first step to produce more 3-hydroxypropionic acid. Eventually, the 3-hydroxypropionic acid from the two biochemical conversion steps was converted to acrylic acid via dehydration over TiO2 as shown in Scheme 3. Up to 14 g L−1 of 3-hydroxypropionic acid and 14 g L−1 of 1,3-propanediol were produced from the first reaction that lasted for 55 h. Higher 3-hydroxypropionic acid yields have been reported through other metabolic pathways utilizing glycerol and glucose.70,74 Additionally, co-production of multiple acrylic acid intermediates such as hydroxypropionaldehyde and 1,3-propanediol have been investigated as attempts were made to improve glycerol utilisation.75
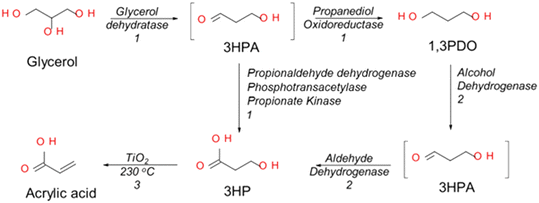 | ||
| Scheme 3 Schematic representation of the three-step process for conversion of glycerol to acrylic acid and 3-hydroxypropionic (3HP). Step numbers are indicated on the arrows. 1 Indicates reactions catalysed by L. reuteri, 2 indicates reactions catalysed by G. oxydans and 3 indicates reactions catalysed by TiO2.68 Adapted with permission from ref. 68 Copyright 2017, Springer Nature. | ||
Recent developments in the area have shown that product yield and selectivity can be improved by utilising available genetic modification or metabolic engineering tools.2,70,74,76,77 These tools have been employed to overexpress genes which promote the formation of the desired product or block pathways leading to the formation of by-products or inhibitors. For instance, enzymes capable of degrading 3-hydroxypropionic acid can be disrupted and genes directing catabolic reactions (breaking down glycerol into smaller molecules) could be removed to create more efficient and selective biocatalysts.78 In fact, genetic modification tools were instrumental in the recent study by Wei et al. whereby they engineered recombinant strains of E. coli capable of converting glycerol to acrylic acid by relying solely on the action of biocatalysts. This study was important as it showed that the biochemical processes can be relied upon for the direct conversion of acrylic acid.79 It was also reported that the enzymes from K. pneumoniae and G. oxydans could effectively convert 1,3-propanediol to acrylic acid via 3-hydroxypropionaldehyde and acrolein. Thus, these enzymes were co-expressed in E. coli to implement a new pathway, converting glycerol to acrylic acid via the intermediates 3-hydroxypropionic acid and acrolein as shown in Fig. 3. Only two of the recombinant E. coli strain were effective and up to 150 mg L−1 of acrylic acid was produced.
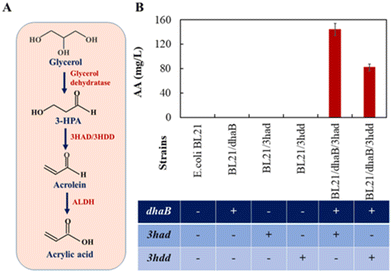 | ||
| Fig. 3 Construction of genetically engineered E. coli strains for acrylic acid (AA) production from glycerol. (A) Synthetic pathway of glycerol conversion to AA. (B) Production of AA with recombinant E. coli strains. 3-Hydroxypropionaldehyde (3-HPA); aldehyde dehydrogenase (ALDH); 3-hydroxyacyl-(ACP) dehydratase (3HAD); 3-hydroxydecanoyl-(ACP) dehydratase (3HDD); glycerol dehydratase encoding gene (dhaB); 3HAD encoding gene (3had); 3HDD encoding gene (3hdd). Error bars represent the standard deviation of the mean of three batches.79 Reproduced with permission from ref. 79 Copyright 2022, Elsevier. | ||
Some advantages of the biochemical conversion processes include minimal energy input requirements, lower operation costs and a lower global warming potential.80 Moreover, it could cost less to produce 3-hydroxypropionic acid or even lactic acid from crude glycerol than from sugars, considering that glycerol from biodiesel plants is a non-toxic by-product produced in large quantities. Moreover, the targeted intermediates, 3-hydroxypropionic acid and lactic acid, are readily converted to acrylic acid under ambient conditions using cheap and readily available catalysts e.g. zeolites. However, major drawbacks associated with biocatalytic systems include the dilute feed81,82 and low product concentrations used.2 Some viable species of bacteria, for example, Lactobacillus plantarumare have been rendered catalytically inactive in aqueous solutions with glycerol concentrations greater than 5%.81 Moreover, some impurities in crude glycerol could also inhibit the performance of biocatalysts.76,77
2.4. Thermocatalytic conversion routes
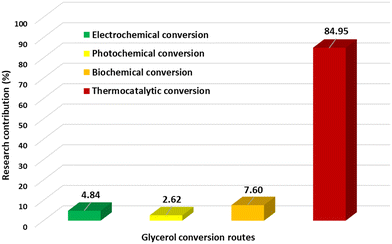
Thermocatalytic routes rely on the use of thermal energy and the action of catalysts to facilitate the conversion of glycerol to acrylic acid or related intermediates such as lactic acid, allyl alcohol and acrolein. Such routes are relatively versatile because they offer diverse pathways and reactions that can be exploited to convert glycerol to acrylic acid. A survey of the Scopus database (Fig. 4) shows that over 80% of research papers related to the conversion of glycerol to acrylic acid in the literature were based on thermocatalytic conversion. This is expected, as glycerol can be converted to a wider range of acrylic acid intermediates such as acrolein, allyl alcohol, propylene, acrylonitrile and lactic acid, which can be produced for subsequent conversion to the desired product. In fact, the direct conversion of glycerol to acrylic acid has been implemented using either multifunctional/tandem catalysts or through the integration of multiple thermocatalytic conversion processes.8,83–85Catalyst development plays a crucial role in the implementation of many conversion processes including those reactions conducted under sub and supercritical conditions.86,87 Thus, a diverse range of materials, including mineral acids, bases, organometallic complexes, zeolites, MOFs and metal oxides, have all been investigated as potential catalysts for the different glycerol to acrylic acid conversion routes and processes.85,88–91 Both homogeneous and heterogeneous catalysts have been shown to be effective, but more examples using heterogeneous catalysts have been reported. Heterogeneous catalysts have attracted more attention due to their many advantages, including the ease of separation and catalyst recovery and the potential to formulate cheaper and even metal-free catalysts.
Ueda et al. have reviewed earlier contributions on the various thermocatalytic conversion processes that have been explored to convert glycerol to acrylic acid.6 Highlights of the review include discussions on the potential pathways, reaction conditions and the different types of catalysts involved. The identified pathways are mostly multistep processes involving the formation of stable intermediates and their subsequent conversion to acrylic acid. Examples include dehydration–oxidation, oxidation–dehydration and deoxydehydration–oxidation, which involves the formation of acrolein, lactic acid, 3-hydroxypropionic acid and allyl alcohol, respectively. The advantages and disadvantages of the thermocatalytic conversion routes and the other sustainable routes discussed have been highlighted in Table 1.
| Route | Key features | Advantages | Disadvantages |
|---|---|---|---|
| Electrochemical | • Conversion processes rely on electricity | • Mild operation conditions | • Most active electrodes are based on expensive precious metals |
| • Electrocatalysts are used to facilitate the conversion processes | • Integration with the generation of renewable electricity is feasible | • Low product yields and long reaction time | |
| • Key intermediates include 1,3-dihydroxyacetone. | • Easy control of reaction through current or electric potential modulation | • The electrodes and electrocatalysts are often susceptible to fouling | |
| • Direct conversion of glycerol to acrylic is not yet feasible | |||
| • Multiple reaction and separation steps might be required to transform glycerol into acrylic acid | |||
| Photochemical | • Conversion processes rely on photo irradiation | • Solar radiation can be harnessed in some cases | • Most of the available photocatalysts rely on UV irradiation for activation |
| • Photocatalysts are used to facilitate the conversion processes | • Mild operation conditions | • Relative low product selectivities compared to other routes | |
| • Key intermediates include 1,3-dihydroxyacetone | • Cheap materials can be used to formulate photocatalysts | • Multiple reaction steps and photocatalysts are required | |
| • Integration with other conversion processes is still required to produce acrylic acid | |||
| Biochemical | • Rely mainly on the action of microorganisms, especially bacteria | • High feed conversions and effective control over product selectivity | • Microorganisms are sensitive to changes in feed concentration and temperature, pH, and other reaction conditions |
| • Genetic modification tools are often exploited to make recombinant strains of microorganisms to improve productivity | • Reactions are conducted under ambient conditions | • Multiple reaction and separation steps are often required | |
| • Useful intermediates include lactic acid, 3-hydroxypropionic acid and acrolein | • Low energy requirements compared to the thermal processes | • Multiple biocatalysts or integration with other conversion routes are required to convert glycerol to acrylic acid | |
| • The direct conversion of glycerol to acrylic acid is feasible | |||
| • Integration with other conversion routes is also possible | |||
| Thermocatalytic | • Rely on heat and action of homogenous/heterogeneous catalysts | • High glycerol conversions and product yields | • High reaction temperatures might be required |
| • Versatile options and ease of integration | • Catalyst deactivation | ||
| • Direct conversion feasible with multifunctional catalysts or integrated systems | • High energy consumption |
3. Conversion of glycerol to acrylic acid via acrolein, allyl alcohol, lactic acid and other intermediates
Acrolein
Acrolein is produced from glycerol generally via a dehydration reaction facilitated by acid catalysts. The reaction generally proceeds via selective protonation of the hydroxyl groups and subsequent elimination of water molecules, as shown in Scheme 4.92 No additional reagents are required to conduct this reaction, which has been implemented in both the liquid and gas phase.83,85 Complete glycerol conversion and high acrolein selectivities have been demonstrated over a diverse range of catalysts, particularly those that are zeolite-based. However, preventing rapid catalyst deactivation remains a major challenge. Most reported catalysts are susceptible to rapid deactivation due to coking, even though reactions are mostly conducted at relatively low temperatures between 250–400 °C.93–95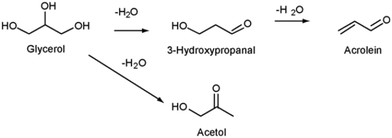 | ||
| Scheme 4 Dehydration of glycerol over acid catalysts.92 Reproduced with permission from ref. 92 Copyright (2010), Elsevier. | ||
Substantial progress has been made towards developing more effective catalysts and optimising reaction conditions. For instance, grafting zirconia onto alumina (ZrO2–Al2O3) resulted in improved catalytic performance of the supported silicotungstic acid-based catalysts in terms of activity, stability and selectivity towards the formation of acrolein via glycerol dehydration reactions conducted at 300 °C.96 Complete glycerol conversion and 85% acrolein selectivity were achieved and sustained for up to 10 h over the STA/ZrO2–Al2O3 supported catalysts while the STA supported on ceria grafted alumina (CeO2–Al2O3) and unmodified Al2O3 supports were less effective. Instead of relying on conventional acidic zeolites such as ZSM-5. Zhu et al. synthesised nanosheets of the MFI zeolite with different Si/Al ratios, which were found to be more active, selective and stable than the conventional MFI zeolite due to their improved mass transport properties, tuned acid properties and morphologies.97 Wang et al. showed that product selectivity and catalyst deactivation can be controlled by adjusting the thickness and channel length of crystalline zeolites.98–100 In this work, H-ZSM-5 nanosheets with different channel lengths along the b-axis (one of the three axes of the coordinate system) were synthesised and their catalytic activities were compared in the gas-phase dehydration of glycerol at 320 °C.101 The zeolites with shorter channel lengths in the b-axis were found to be more active, selective and stable when compared to conventional H-ZSM-5 zeolites.100 The superior performance of these catalysts was attributed to enhanced mass transport properties and easier access to catalytically active sites. More extended b-axis channels lead to slower internal diffusion, making the catalysts more susceptible to coking due to the increased chances of polymerisation and other side reactions inside the pores. Mijoin et al. recorded significant improvements in glycerol conversion and selectivity through incorporating Fe into the framework of an MFI zeolite.102 The Fe-MFI zeolite offered up to 80% acrolein selectivity and over 95% glycerol conversion. The catalyst remained effective even after 8 h on stream, while the unmodified conventional zeolite underwent rapid deactivation, as the zeolite acidity is effectively weakened, thereby reducing the formation of carbonaceous species on the catalyst surface. More details about developments in this area can be found elsewhere.88,90
Partial oxidation of acrolein is the second and final step, which leads to the formation of acrylic acid through the dehydration–oxidation pathway. Mechanistic studies have shown that the reaction proceeds via the Mars-van Krevelen mechanism.103Scheme 5 shows that the adsorption of acrolein, C–H bond activation, incorporation of lattice oxygen and reoxidation of the catalyst are crucial steps in this mechanism.103 The process is well-established and already in use for the oxidation of propylene-derived acrolein.104 It is evident from the first step of the mechanism that the reaction is susceptible to the generation of CO and CO2 due to over oxidation. Hence, highly effective catalysts are required to minimise the generation of these by-products that are likely to increase the global warming potential of the process.
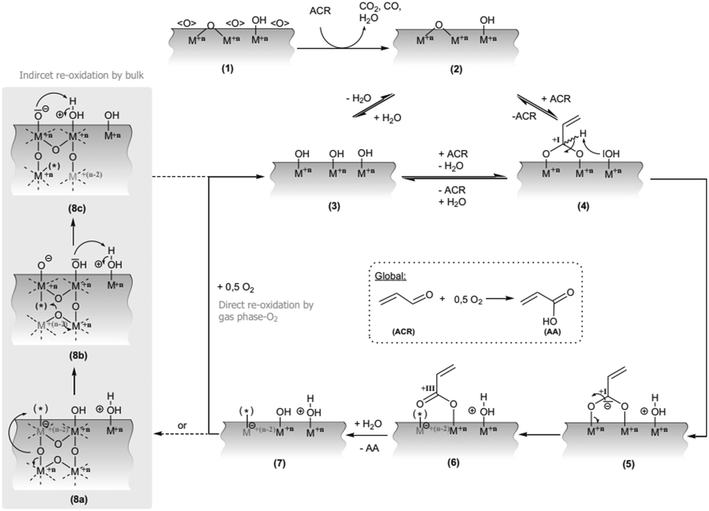 | ||
| Scheme 5 Extended mechanism of the ACR-oxidation on mixed oxides by Vogel et al. The key intermediate is a surface acetal (4) which is converted into a surface acrylate (6) (M: Mo or V, n: charge state, <O>: non-selective oxygen species or high surface degree of oxidation, (asterisk): oxygen vacancy).104 Reprinted with permission from ref. 104. Copyright 2016, Springer Science. | ||
The catalysts used for the oxidation reaction are usually mixed transition metal oxides, mainly consisting of Mo and V (ref. 105–107) with other metals often added as promoters.108 The performance of some representative catalysts are highlighted in Table 2.
| Catalyst | Temperature (°C) | TOS (h) | Acrolein conversion (%) | Acrylic acid selectivity (%) | References |
|---|---|---|---|---|---|
| Acronyms: (Tri = trigonal, Orth = orthogonal, and Amor = amorphous).a Indicates that the TOS information is not stated.b Contact time × 10−3 gcat. min mL−1. | |||||
| Orth-MoVO | 215 | 68.2 | 98.1 | 105 | |
| Tri-MoVO | 215 | 99.3 | 97.3 | 105 | |
| Amor-MoVO | 223 | 25.2 | 98.4 | 105 | |
| MoVOx | 230 | 71.5 | 92 | 108 | |
| MoVWO | 250 | 5b | 100 | 93.2 | 108 |
| MoVFeO | 250 | 5b | 100 | 96.2 | 108 |
| MoVCuO | 250 | 5b | 100 | 92.6 | 108 |
| MWCNTs | 300 | 14 | 15 | 85 | 110 |
| O-CNTs | 300 | 20 | 7.5 | 80 | 111 |
The performance of these catalyst varies depending on the nature and composition of the catalysts. However, the nature of the active phase, even amongst commercial formulations, is yet to be established.21 Recent studies involving these multi-metallic Mo3VOx catalysts showed that the presence of heptagonal channels is crucial to the overall catalytic performance.21,24,105,108,109 It was observed that product selectivity could be controlled by adjusting the size of these heptagonal micropore channels. No meaningful conversion could be achieved under ambient conditions on similar catalysts without these heptagonal micropores. Results show that the orthorhombic and trigonal forms of the crystalline Mo3VOx with abundant hexagonal pores were much more active and selective when compared to the other crystalline and amorphous forms of the catalyst,24 a clear indication that the crystal structure is vitally important. It was suggested that trigonal MoVWCuO (Tri-MoVWCuO) is the active structure even among similar industrial catalysts synthesized by the incorporation of selected metals (Cu Fe Sn and/or W). Attempts have also been made by other research groups to formulate metal-free catalysts as alternatives, however their performance could not match those obtained with the metal-based catalysts.110,111 Examples highlighted in Table 2 show that the metal-free catalysts exhibit much lower acrolein conversion (7.5–15%) and acrylic acid selectivity (80–85%) although they are cheaper and more ecofriendly.
Allyl alcohol
Allyl alcohol is obtained from glycerol through deoxydehydration, which is a reaction designed to produce alkenes from alcohols with two vicinal diols. The reaction is facilitated104 by catalysts and reductants such as formic acid, hydrogen, and alcohols including glycerol112 are often used as mediators to achieve high glycerol conversion and selectivity. The reductants used tend to have a significant impact on the reaction as evidenced from various studies which show that glycerol conversion and selectivity tend to depend on the nature of the catalysts and reductant involved. Catalysts that have been found to be effective in this reaction are mostly transition metal oxides.113–116 For instance, when α-Fe2O3 was used as catalyst for glycerol deoxydehydration, higher allyl alcohol yields could be obtained with formic acid acting as a reductant, compared to similar reactions carried out without any external reductant.117Attempts to convert glycerol to allyl alcohol in the gas-phase using Fe-supported ZSM-5 as catalyst and N2 as carrier gas resulted in low feed conversions and higher selectivity towards acrolein than the target product.118 However, up to 76% allyl alcohol yield was obtained using unsupported ReOx NPs and 3-octanol as catalyst and reductant, respectively, as shown in Scheme 6.119 Tazawa et al. reported 91% allyl alcohol yield using supported ReOx and Au NPs (ReOx–Au/CeO2) as catalysts and H2 as reductant.120 In this case, the addition of Au NPs was found to be responsible for H2 activation, as no significant amounts of allyl alcohol could be produced with similar catalysts that did not use Au NPs. Kon et al. have also reported over 90% allyl alcohol yield over ReOx supported on Al2O3 using 2-hexanol as a reductant, while other alcohols, including a series of primary alcohols and diols used as reductants, were not as effective as 2-hexanol.121
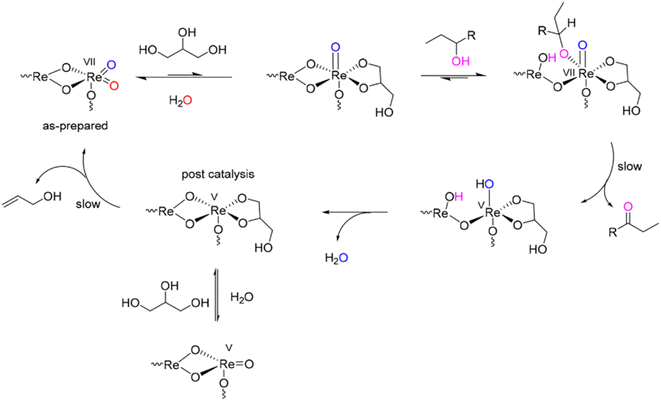 | ||
| Scheme 6 Proposed mechanism for the heterogeneous deoxydehydration (DODH) by ReOx NPs in the presence of alcohol reductant.119 Reprinted with permission from ref. 119. Copyright 2019, American Chemical Society. | ||
The selective oxidation of allyl alcohol is the second step of the conversion process (Scheme 7), which leads to the formation of acrylic acid. Mechanistic studies have shown that the reaction proceeds via the formation of allyl alcohol and its subsequent conversion to acrylic acid. Hence, similar materials used in industry for the partial oxidation of acrolein have been investigated as potential catalysts. Pramod et al. have reported 77% acrylic acid selectivity and ~90% allyl alcohol conversion from gas-phase reactions conducted at 225 °C using MoWVOx as a catalyst.80 This reaction has been implemented using a variety of catalysts including supported Au nanoparticles (NPs).122 Yang et al. reported complete allyl alcohol conversions and up to 51% acrylic acid yield over Fe/CeO2, which exhibited superior performance among other catalytic reactions conducted at 50 °C under basic conditions. Results from this study showed that catalysts performance can be enhanced by selecting the appropriate support and adjusting the synthesis method. CeO2 supported catalysts were the most effective compared to TiO2, ZnO, and Fe2O3 regardless of the preparation method used, Au nanoparticle size or even the Au oxidation states. It was also observed that the catalysts prepared through the deposition–precipitation method contained more oxidic Au and were found to be more active and selective towards the production of acrylic acid than supported catalysts prepared through the colloidal deposition method. More results from selected studies are highlighted on Table 3.
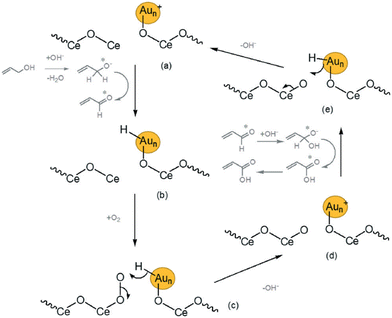 | ||
| Scheme 7 Suggested mechanism for the selective oxidation of allyl alcohol to acrylic acid using Au/ceria catalysts under basic conditions. The notation * indicates that the chemical is being adsorbed on the Au surface and steps (a)–(e) show the sequence of events involved.122 | ||
| Catalyst | Temperature (°C) | TOS (h) | Allyl alcohol conversion (%) | Acrylic acid selectivity (%) | References |
|---|---|---|---|---|---|
| a Contact time × 10−3 gcat. min mL−1. | |||||
| Au/CeO2 | 50 | 12 | 100 | 51.1 | 122 |
| Au/TiO2 | 50 | 12 | 100 | 31.2 | 122 |
| Au/ZnO2 | 50 | 12 | 100 | 33.4 | 122 |
| MoVO | 250 | 5a | 100 | Ca. 36 | 108 |
| MoVFeO | 250 | 5a | 100 | Ca. 47 | 108 |
| MoVCuO | 250 | 5a | 100 | Ca. 37 | 108 |
| Pd NP/C (sol) | 100 | 12 | 100 | 41.2 | 123 |
Lactic acid
Glycerol is converted to lactic acid and 3-hydroxypropionic acid through oxidative dehydrogenation.124–127Scheme 8 is one of the proposed mechanisms showing the various reaction steps leading to the formation of lactic acid and other intermediates including glyceraldehyde.128 Various catalyst formulations have been investigated with the aim of improving feed conversion and product selectivity. Even low-cost materials, such as CaO, can be used to facilitate the conversion of glycerol to lactic acid.129 However, higher yields have been reported from studies involving supported catalysts, especially when the reactions are conducted in basic media.128,130 Complete glycerol conversion and up to 83% lactic acid selectivity have been reported from reactions conducted in a basic medium at 100 °C and oxygen pressure of 3 bar for a duration of 4 h using AuPt/TiO2 as catalyst.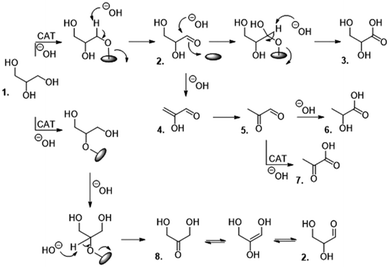 | ||
| Scheme 8 Proposed mechanisms for the transformation of glycerol under alkaline conditions. 1. Glycerol, 2. glyceraldehyde, 3. glyceric acid, 4. 2-hydroxypropenal, 5. pyruvaldehyde, 6. lactic acid, 7. pyruvic acid, and 8. dihydroxyacetone.128 Reproduced with permission from ref. 128 copyright 2020, AIP Publishing. | ||
Currently, emphasis is on the development of base-free conversion processes to avoid the need for product separation and possible corrosion problems.131–133 For instance, Feng et al. have reported achieving 79% lactic acid selectivity at about 54% glycerol conversion from a one-pot reaction involving a catalysts, Pt supported on layered Nb2O5 (Pt/L-Nb2O5), and aqueous glycerol without any additives.131 Similar attempts have been made using Ca and Ce mixed metal oxides supported on ZrO2 (CaCe/ZrO2).132 Ce and Zr mixed metal oxides supported on SBA-15 (SBA-15) have also been used as catalysts in a bid to replace the expensive noble metals.133
Lactic acid is converted to acrylic acid through a dehydration reaction. Results summarized in Table 4 show the performance of a range of materials that have been investigated as potential catalysts for this transformation. Complete lactic acid conversion and up to 70% acrylic acid yield have been reported from various studies.134,136,138 In all cases, aqueous lactic acid was used as the feed and reaction temperatures were in the range of 300–400 °C. For instance, up to 74% acrylic acid selectivity and 100% lactic acid conversion have been reported for a series of LaP catalysts with different La/P ratios and calcined at different temperatures to generate different morphologies and a range of acid–base properties.138 The catalyst with La/P ratio of 0.35 and calcined at 500 °C was found to be the most active and selective under optimized conditions due to a relative abundance of weak acid sites.
| Temperature (°C) | TOS (h) | Lactic acid conversion (%) | Acrylic acid selectivity (%) | References | |
|---|---|---|---|---|---|
| LaP-4 | 350 | 30 | 72.9 | 46.9 | 134 |
| NH3–BaSO4 | 400 | 10 | 100 | 82 | 135 |
| BaSO4 | 350 | 100 | Ca. 79 | 58 | |
| Co-BEA | 370 | 4 | 99.7 | Ca. 62 | 136 |
| RbxNa1−xβ | 360 | 10 | 96 | Ca. 70 | 137 |
| K0.97Na0.03ZSM-5_27 | 360 | 1(80) | 70(81) | 36 | |
| LaP(0.35)[500] | 360 | 8 | 100 | 74 | 138 |
Scheme 9 provides some insight into the steps involved in the dehydration of lactic acid to acrylic acid over a LaPO4 catalyst. The performance of the conversion process depends largely on the properties and effectiveness of the catalysts involved. For instance, the incorporation of transition metal ions into BEA zeolite resulted in significant changes in the distribution of active sites which, in turn, reflected on the performance of the catalysts.136 Both the lactic acid conversion and acrylic acid selectivity varied among the transition metal-incorporated BEA zeolite catalysts depending on the type of metal and preparation method employed. Higher selectivity towards acrylic acid was achieved over Co–BEA and Cu–BEA catalysts prepared through sonication as opposed to similar catalysts prepared through ion exchange. The superior catalysts were more effective due to the formation and distribution of more metal–O–metal oligomeric species identified as binding sites, which are crucial for the selective elimination of hydrogen and hydroxyl groups. In addition, results from lactic acid dehydration over LaP have shown that controlling the pore structure and acid–base properties of the catalysts can have a significant impact on catalyst stability and product selectivity.134 The catalysts with higher Lewis acid site density were more effective, while catalysts with more basic site density were less stable due to fouling by the deposition of carbon on the catalyst surface. Furthermore, studies have shown that particle size, morphology and surface defects can be tuned to improve the catalytic performance of crystalline solids or nanoparticles.58,135 For instance, crystal defects in BaSO4 crystals generated through ultrasound treatment in ethanol were found to have substantially improved the performance of the catalysts for the dehydration of lactic acid to acrylic acid. The catalysts with substantial crystal defects showed enhanced surface acidity and resulted in a significant increase in selectivity towards the formation of acrylic acid.58
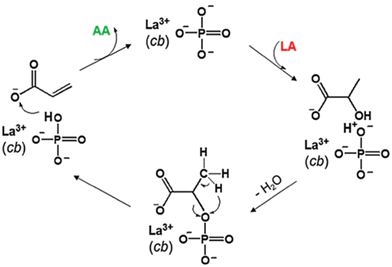 | ||
| Scheme 9 Proposed mechanism for the conversion of lactic acid to acrylic acid.134 | ||
Other acrylic acid intermediates
Propylene and acrylonitrile can also be obtained from glycerol thermocatalytic conversion processes as intermediates which can subsequently be converted to acrylic acid. Propylene is produced through glycerol hydrogenolysis, although the process often requires high hydrogen pressures and multiple catalysts to achieve reasonable feed conversion and high propylene selectivity.139–141 Propylene obtained from glycerol hydrogenolysis can then be converted to acrylic acid through conventional partial oxidation reactions. However, the need for multiple reaction steps, catalyst deactivation and low carbon economy are major disadvantages hindering further development of this pathway.Acrylonitrile is yet another acrylic acid intermediate obtainable from glycerol through ammoxidation (the production of nitrile compounds using NH3 and O2). In this case, glycerol is reacted with a mixture of ammonia and O2 to produce acrylonitrile, which can be converted to acrylic acid through a subsequent hydrolysis step.142–144 However, owing to the requirement for additional reagents, the use of relatively high temperatures and the low acrylonitrile selectivities reported, there are few studies available in the literature for this particular process.142,143
4. Direct conversion of glycerol to acrylic acid
The direct conversion of glycerol to acrylic acid can be achieved in either a one-reactor system or in integrated systems involving multiple reactors. Direct conversion in one reactor has attracted significant attention as it eliminates the need for the handling, separation or storage of reaction intermediates. Effective multifunctional catalysts are required to implement different reactions simultaneously in a single-bed. Oxydehydration (a combination of dehydration and oxidation reactions) is the only thermocatalytic conversion route that has been implemented with multifunctional catalysts for the direct conversion of glycerol to acrylic acid.145–148 It is clear from the results summarized in Table 5 that the process is feasible but not yet economically viable due to the low acrylic acid selectivities reported. The low acrylic acid selectivities reported are largely attributed to the competitive adsorption of reactants and intermediates on the active sites resulting in the blocking of catalytically active sites thereby inhibiting catalytic activity.149 As such, the development of effective multifunctional catalysts with optimised distributions of acid and redox sites are required to facilitate the dehydration and oxidation reactions.| Catalyst | Temperature (°C) | TOS (h) | Glycerol conversion (%) | Acrylic acid selectivity (%) | References |
|---|---|---|---|---|---|
| MFI(HZSM-5)/V | 320 | 10 | 95.9 | 12.5 | 150 |
| MWW(MCM-22)/V | 320 | 10 | 76.2 | 21.0 | 150 |
| BEA(Beta)/V | 320 | 10 | 87.6 | 20.2 | 150 |
| Al(AlPO4)/Co(Co3(PO4)2) | 280 | 3 | >90 | 2 | 151 |
| V2O5/MFI | 350 | 1–8 | 100(80) | 12(17) | 147 |
| Fe-MFI | 320 | 0.5(8) | 96(58) | 24(47) | 145 |
| Mo4.65V0.35O14 | 320 | 1(6) | 100(90) | 17(33.5) | 152 |
| MoVo/ZSM-5 | 320 | 1 | >90 | 18 | 153 |
The second alternative is using tandem catalysts in separate sequential beds, which are typically more effective when compared to multifunctional catalysts used in a single bed system.154 Results in Fig. 5 show how catalysts in separate sequential beds outperform mixed tandem catalysts in a single bed. Complete glycerol conversion and more than 75% acrylic acid yield was achieved with the dehydration and oxidation catalysts in separate sequential beds.149 In contrast, complete glycerol conversion could not be achieved, and the maximum acrylic acid yield obtained even after a prolonged period was less than 30% from a similar reaction with the catalysts packed in a single-bed. Additionally, higher amounts of by-products and unreacted intermediate were obtained from the reaction conducted in a single-bed reactor due to multiple side reactions and competition for active sites. To prevent this, separate sequential beds can be assembled within a single reactor and separate heating zones can even be used to improve selectivity in single-reactor systems as the dehydration and oxidation reactions may have different optimal operation temperatures.
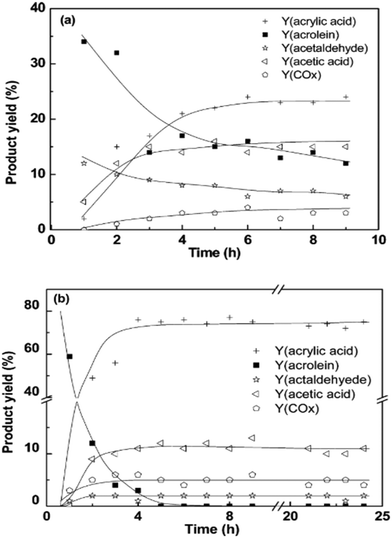 | ||
| Fig. 5 Comparison of (a) the single-bed system and (b) two-bed system. Conditions: 300 °C, 0.50 g 20CsPW–Nb, 0.5 g VMo–SiC, 40 mL min−1 gas flow rate (O2 = 6 mL min−1), 20 wt% glycerol solution fed at 0.6 mL h−1 (0.24 h−1 glycerol WHSV).149 Reprinted with permission from ref. 149. Copyright 2014, American Chemical Society. | ||
Higher acrylic acid yields than those reported in Table 5 have been reported from studies involving integrated systems in which reactions are conducted in separate sequential reactors. Compared to multiple reactions in a single reactor, the integrated multiple reactor systems are generally more flexible than using multiple reactions in a single bed as various reaction scan be conducted and the operation conditions in each reactor can be optimized independently. Li and Zhang reported an integrated system comprising formic acid-mediated deoxydehydration and allyl alcohol oxidation reactions occurring in two separate sequential reactors and obtained up to 80% acrylic acid yield.155 The deoxydehydration reaction was conducted in one reactor at 230 °C and subsequent oxidation of the allyl alcohol intermediate was carried out in a second reactor packed with a supported mixed metal oxide catalyst (Mo–V–W–O) operating at 340 °C with a continuous flow of air or oxygen. Indeed, this demonstrates that innovative reactor designs can be used to enhance the performance of the catalysts used in various conversion processes, instead of relying on conventional batch or fixed-bed flow reactors. Some possible reactor configurations are illustrated in Fig. 6. However, it should be considered that design and manufacture of such reactors can be laborious, complex and expensive, thereby limiting their wider use. As such, a more efficient system design, in which all processes are integrated for optimal production of acrylic acid is highly desirable. The choice of reactors and the opportunity of coupling separation with conversion processes is an additional benefit of integrated systems involving multiple reactors. A recent study by Dimian and Bildea has demonstrated how conversion and separation steps can be linked to produce high purity acrylic acid from glycerol through sequential dehydration and oxidation reactions.156 They proposed using different system configurations including those involving turbulent fluidized bed (TFB) and circulating turbulent fluidized bed (CTFB) reactors which allow for periodic and continuous catalyst regeneration, respectively. In fact, conceptual designs have the potential for net-zero energy inputs as heat exchanger pipes and combined heat and power (CHP) systems can be used to enable energy harvesting from the exothermic reactions conducted and reactor effluents produced. Such energy could be channeled to the heating, pumping and compression needs of the system. Therefore, coupling the two reaction steps together within such a system could result in considerable energy savings, as glycerol dehydration is an endothermic process while acrolein oxidation is exothermic.90,156
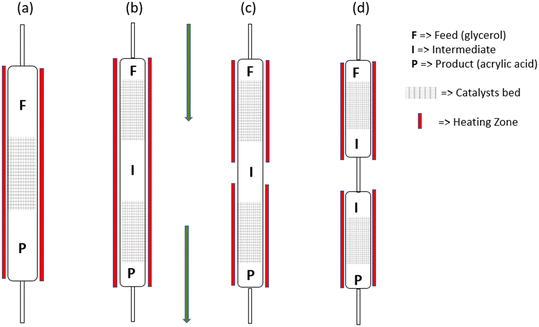 | ||
| Fig. 6 (a) Single-bed, (b) separate sequential beds with a single heating zone, (c) separate sequential beds with two heating zones and (d) integrated reactor configurations. | ||
5. Challenges and prospects
Substantial progress has been made in the development of processes that can be used for the conversion of glycerol to acrylic acid. However, no such processes have been adopted industrially due to several issues, i.e., low acrylic acid yields, rapid catalyst deactivation and high costs of operation. For instance, photochemical and electrochemical conversion routes generally exhibit relatively low product selectivities, while biochemical conversion routes require long reaction times and the biocatalysts used can be susceptible to deactivation at high glycerol, intermediates, or even acrylic acid concentrations. Besides, photochemical and electrochemical conversion routes mostly lead to the formation of intermediates, requiring further transformation to acrylic acid. Even though the direct conversion of glycerol to acrylic acid is feasible via thermocatalytic conversion processes, reactions are conducted at higher temperatures and some of the reactions produce significant amounts of CO and CO2.Complete glycerol conversions and high product selectivities (>90%) have been reported using thermocatalytic conversion processes. However, the catalysts often used in glycerol dehydration reactions tend to undergo rapid deactivation due to coking, especially those with a high concentration of strong acid sites or restricted pores.97,98,157,158 Besides, most multifunctional catalysts designed for implementation in single-pot direct conversions of glycerol to acrylic acid exhibit only low to moderate product selectivity (about 2–35%). In some cases, the nature of the active phase and its interactions with reactants and intermediates are not clearly understood.21,108 Thus, more research is required to make the suggested conversion processes more robust and competitive when compared to the conventional thermocatalytic production process relying on petroleum-derived feedstocks.
Nonetheless, various strategies can be implemented to overcome some of these challenges and an overview of such strategies is detailed within this review. Such strategies include the development of novel catalytic materials and the modification of existing catalysts to introduce desirable textural and chemical properties, which can be beneficial towards improving the acrylic acid yield and selectivity. In fact, most of the improvements recorded in recent studies were achieved through the fine tuning of the textural and chemical properties of certain catalytic materials. For example, by changing the catalyst composition through doping or component substitution, which can be favourable towards improving the acrylic acid yield and selectivity. This can be done using ion exchange or dispersion of a catalytically active phase on compatible supports to induce enhanced synergetic interactions between the active phase and support beneficial for the catalytic activity, i.e., the creation of oxygen vacancies or selective binding of substrates and activation of target bonds.
More effective catalysts can be made by exploring new materials such as catalysts with immobilised enzymes as active sites159–162 or even isolated single-atom-site catalysts.163–165 Immobilised enzymes could make biochemical routes more efficient, while the isolated single-atom site catalysts could ensure maximum utilisation of metal atoms and the metal–support interface compared to bulk materials with a lower surface area and lower distribution of active sites.166 Besides, instead of relying on rare metals, such as expensive Au and Pt, cheaper alternatives like Fe have been considered to achieve the desired conversions at lower costs. The incorporation of Fe species has been found to be effective in improving the catalytic performance of ZSM-5 zeolites102 in glycerol dehydration reactions while carbon nano tubes (CNTs) have shown promising results in the selective oxidation of acrolein to acrylic acid and could be used to replace Mo–V mixed metal oxide catalysts which are commonly used in the production of acrylic acid.110,111,167,168 Additionally, unravelling the nature of the active sites of different catalytic materials and systems and how these sites evolve/change during reactions, will provide further insights into the reaction mechanisms and deactivation patterns. This can, in turn, allow for a greater control of the above discussed catalytic systems leading to a greater optimisation and increased acrylic acid yield. To achieve this, in situ and operando characterisation techniques are required to reveal more details about the catalytically active sites and their evolution in real time, instead of attributing activity or selectivity to the few features of the catalysts which are easily observable. Techniques such as in situ Raman have been successfully used to identify and monitor the active sites of various heterogeneous catalysts used in thermochemical conversion of CO.169 High-throughput simulation/computational tools such as density-functional theory (DFT) could be used to further help inform catalyst design and synthesis, rather than relying on a trial-and-error approach for screening catalytic materials.
Another possible way to optimise the sustainable production of acrylic acid is through the integration of different routes, especially when renewable energy or low-cost operations can be implemented. Dishisha et al. have shown that such strategies are feasible by utilising biocatalysts to convert glycerol to 3-hydroxypropionic acid and also by using relatively cheap heterogeneous catalysts to convert the 3-hydroxypropionic acid to acrylic acid through thermocatalytic routes under mild operation conditions.68 Such strategies can significantly reduce the cost of acrylic acid production, decrease the risk of handling toxic intermediates (e.g. acrolein) and even eliminate COx emissions. Similar schemes, involving photochemical and electrochemical routes can also be explored, especially if complementary intermediates can be formed for subsequent conversion to acrylic acid. Additional requirements may include separation, pH adjustment and concentration steps.
In general, more research on catalyst development, in addition to process optimisation and reactor technology, will fast-track the transition to commercial scale production of acrylic acid from glycerol. Furthermore, alternative heating sources such as the less energy intensive microwave-assisted systems and ergonomic integration of conversion processes for maximum utilization of energy could reduce the cost of production and emission problems.170
6. Conclusions
The conversion of glycerol to acrylic acid can be achieved to some extent by implementing any of the various pathways, ranging from the biochemical, electrochemical, photochemical and thermocatalytic conversion routes. Substantial progress has been made in the development of glycerol to acrylic acid conversion processes, with the thermocatalytic conversion route appearing to be the most versatile in terms of the different types of reactions that can be implemented. The higher glycerol feed conversions, and the product yields/selectivities reported from these reactions make the thermocatalytic routes seem more viable when compared to the other glycerol to acrylic acid conversion routes. Although the biochemical, electrochemical and photochemical routes offer milder operation conditions, they are characterised with longer reaction times and lower yields of products, which are mostly intermediates requiring further transformation to obtain acrylic acid. Moreover, the intermediates produced from the biochemical, electrochemical and photochemical routes are readily converted to acrylic acid via thermocatalytic routes. Thus, acrylic acid can be produced through the integration of multiple conversion routes.The direct conversion of glycerol to acrylic acid can be implemented through thermocatalytic or biochemical conversion routes. However, multiple enzymes and biocatalysts are required to implement the biochemical reactions, while thermocatalytic reactions can be implemented using a single multifunctional catalyst. Higher acrylic acid yields can be obtained when separate catalysts are used to implement the different reactions. So far, thermocatalytic routes involving tandem catalysts in separate sequential beds or multiple reactors have been more effective than multifunctional catalysts used in single-pot reactions. However, none of the conversion processes has yet been adopted industrially due to several challenges, including high costs of operation and inadequate catalyst performance. Further research, especially towards the development of more efficient and robust catalysts, is required to convert glycerol to acrylic acid at competitive costs.
Nonetheless, the prospect of adopting crude glycerol as an alternative feedstock for the sustainable production of acrylic acid is of high potential since there are available tools that can be explored to produce more effective catalysts and further improve the efficiency of the glycerol to acrylic acid conversion processes discussed. Successful scale-up and commercialisation of improved conversion processes will not only encourage a paradigm shift to sourcing industrial chemicals from renewable resources, but also incentivize the production of biodiesel as the major waste being produced, that is, gycerol, will have a higher economic value.
Author contributions
Umar C. Abubakar: conceptualisation, data curation, investigation, writing – original draft, writing – review and editing; Yash Bansod: data curation, investigation, writing – original draft, review, and editing; Luke Forster: data curation, review, and editing; Vincenzo Spallina: review, editing, funding acquisition, resources, and project administration; and Carmine D'Agostino: review, editing, funding acquisition, resources, project administration, supervision and validation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Carmine D'Agostino, Luke Forster and Vincenzo Spallina would like to acknowledge the EPSRC, grant no. EP/V026089/1, for supporting their research activities. Umar C. Abubakar would like to acknowledge the Petroleum Technology Development Trust Fund (PTDF), for sponsoring his PhD program. Yash Bansod would like to acknowledge the Social Welfare Department, Government of Maharashtra, India.References
- S. S. Bhagwat, Y. Li, Y. R. Cortés-Peña, E. C. Brace, T. A. Martin, H. Zhao and J. S. Guest, ACS Sustainable Chem. Eng., 2021, 9, 16659–16669 CrossRef CAS.
- J. L. Rodrigues, SynBio, 2022, 1, 3–32 CrossRef.
- L. Fernández, Market volume of acrylic acid worldwide from 2015 to 2021, with a forecast for 2022 to 2029, https://www.statista.com/statistics/1245262/acrylic-acid-market-volume-worldwide/, (accessed 20 December 2022) Search PubMed.
- P. Wattanapaphawong, P. Reubroycharoen and A. Yamaguchi, Catal. Today, 2017, 18561–18568 CAS.
- R. Sun, Y. Liao, S. T. Bai, M. Zheng, C. Zhou, T. Zhang and B. F. Sels, Energy Environ. Sci., 2021, 14, 1247–1285 RSC.
- D. Sun, Y. Yamada, S. Sato and W. Ueda, Green Chem., 2017, 19, 3186–3213 RSC.
- R. Gérardy, D. P. Debecker, J. Estager, P. Luis and J. C. M. Monbaliu, Chem. Rev., 2020, 120, 7219–7347 CrossRef PubMed.
- Y. Wang, Y. Xiao and G. Xiao, Chin. J. Chem. Eng., 2019, 27, 1536–1542 CrossRef CAS.
- C. H. Zhou, J. N. Beltramini and G. Q. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC.
- C. A. G. Quispe, C. J. R. Coronado and J. A. Carvalho, Renewable Sustainable Energy Rev., 2013, 27, 475–493 CrossRef CAS.
- M. R. Monteiro, C. L. Kugelmeier, R. S. Pinheiro, M. O. Batalha and A. da Silva César, Renewable Sustainable Energy Rev., 2018, 88, 109–122 CrossRef CAS.
- S. A. N. M. Rahim, C. S. Lee, F. Abnisa, M. K. Aroua, W. A. W. Daud, P. Cognet and Y. Pérès, Sci. Total Environ., 2020, 705, 135137 CrossRef CAS.
- M. Ayoub and A. Z. Abdullah, Renewable Sustainable Energy Rev., 2012, 16, 2671–2686 CrossRef CAS.
- B. Katryniok, S. Paul, V. Bellière-Baca, P. Rey and F. Dumeignil, Green Chem., 2010, 12, 2079–2098 RSC.
- B. Katryniok, S. Paul and F. Dumeignil, ACS Catal., 2013, 3, 1819–1834 CrossRef CAS.
- World Intellectual Property Organization, WO2006/087083A2, 2006.
- Arkema France, Colombes (FR), US Pat., US9206110B2, 2015 Search PubMed.
- LG Chem, Ltd., Seoul (KR), US Pat., US9546124B2, 2017 Search PubMed.
- Arkema France, Colombes (FR), US Pat., US8212070B2, 2012 Search PubMed.
- Arkema France, Colombes (FR), US Pat., US8748545B2, 2014 Search PubMed.
- S. Ishikawa, Y. Yamada, N. Kashio, N. Noda, K. Shimoda, M. Hayashi, T. Murayama and W. Ueda, ACS Catal., 2021, 11, 10294–10307 CrossRef CAS.
- G. I. Panov, E. V. Starokon, M. V. Parfenov, B. Wei, V. I. Sobolev and L. V. Pirutko, ACS Catal., 2018, 8, 1173–1177 CrossRef CAS.
- J. L. Dubois, Main industrial processes using metal oxides as catalysts or support and future trends in heterogeneous catalysis, 2018 Search PubMed.
- S. Ishikawa, Y. Yamada, C. Qiu, Y. Kawahara, N. Hiyoshi, A. Yoshida and W. Ueda, Chem. Mater., 2019, 31, 1408–1417 CrossRef CAS.
- R. Ciriminna, C. Della Pina, M. Rossi and M. Pagliaro, Eur. J. Lipid Sci. Technol., 2014, 116, 1432–1439 CrossRef CAS.
- S. Kim, S. Jeong and E. Heo, Energy Sources, Part B, 2019, 14(3), 49–66 CrossRef CAS.
- O. O. James, W. Sauter and U. Schröder, RSC Adv., 2018, 8, 10818–10827 RSC.
- L. Fan, B. Liu, X. Liu, N. Senthilkumar, G. Wang and Z. Wen, Energy Technol., 2021, 9, 1–17 Search PubMed.
- Y. Ryabenkova, Q. He, P. J. Miedziak, N. F. Dummer, S. H. Taylor, A. F. Carley, D. J. Morgan, N. Dimitratos, D. J. Willock, D. Bethell, D. W. Knight, D. Chadwick, C. J. Kiely and G. J. Hutchings, Catal. Today, 2013, 203, 139–145 CrossRef CAS.
- Y. Feng, W. Xue, H. Yin, M. Meng, A. Wang and S. Liu, RSC Adv., 2015, 5, 106918–106929 RSC.
- Y. Feng, C. Lu, H. Wang, M. Meng, Y. Zhang, D. Rao, L. Liu and H. Yin, Catal. Sci. Technol., 2020, 10, 8094–8107 RSC.
- K. D. Kim, Z. Wang, Y. Jiang, M. Hunger and J. Huang, Green Chem., 2019, 21, 3383–3393 RSC.
- E. Jolimaitre, D. Delcroix, N. Essayem, C. Pinel and M. Besson, Catal. Sci. Technol., 2018, 8, 1349–1356 RSC.
- X. Wang, F. Liang, C. Huang, Y. Li and B. Chen, Catal. Sci. Technol., 2016, 6, 6551–6560 RSC.
- S. Lux and M. Siebenhofer, Catal. Sci. Technol., 2013, 3, 1380–1385 RSC.
- B. Yan, L. Z. Tao, A. Mahmood, Y. Liang and B. Q. Xu, ACS Catal., 2017, 7, 538–550 CrossRef CAS.
- X. Zhang, L. Lin, T. Zhang, H. Liu and X. Zhang, Chem. Eng. J., 2016, 284, 934–941 CrossRef CAS.
- M. M. Buitelaar, E. Van Daatselaar, D. G. Van Teijlingen, H. I. Stokvis, J. D. Wendt, R. J. De Sousa Ribeiro, A. M. M. Brooks, E. C. Kamphuis, S. Lopez Montoya, J. C. Van Putten, A. G. J. Van Der Ham, H. Van Den Berg and J. P. Lange, Ind. Eng. Chem. Res., 2020, 59, 1183–1192 CrossRef CAS.
- M. Hunsom and P. Saila, Renewable Energy, 2015, 74, 227–236 CrossRef CAS.
- A. Khosravanipour Mostafazadeh, M. S. De La Torre, Y. Padilla, P. Drogui, S. K. Brar, R. D. Tyagi, Y. Le Bihan, G. Buelna and P. G. Moroyoqui, Renewable Energy, 2021, 172, 130–144 CrossRef CAS.
- C. Dai, L. Sun, H. Liao, B. Khezri, R. D. Webster, A. C. Fisher and Z. J. Xu, J. Catal., 2017, 356, 14–21 CrossRef CAS.
- J. F. Gomes, F. B. C. De Paula, L. H. S. Gasparotto and G. Tremiliosi-Filho, Electrochim. Acta, 2012, 76, 88–93 CrossRef CAS.
- Y. Dong, W. Wang, Y. Wang, P. Wang, L. Zhang and Z. Lei, J. Taiwan Inst. Chem. Eng., 2018, 93, 500–508 CrossRef CAS.
- J. Maya-Cornejo, M. Guerra-Balcázar, N. Arjona, L. Álvarez-Contreras, F. J. Rodríguez Valadez, M. P. Gurrola, J. Ledesma-García and L. G. Arriaga, Fuel, 2016, 183, 195–205 CrossRef CAS.
- B. Rezaei, E. Havakeshian and A. A. Ensafi, J. Electroanal. Chem., 2016, 782, 108–116 CrossRef CAS.
- Y. Li, S. Chen, H. Zhang, Y. Zhao, Y. Wang and Z. Liu, CEAN-Soil, Air, Water, 2014, 25, 1140–1144 CrossRef.
- J. Han, Y. Kim, H. W. Kim, D. H. K. Jackson, D. Lee, H. Chang, H. J. Chae, K. Y. Lee and H. J. Kim, Electrochem. Commun., 2017, 83, 46–50 CrossRef CAS.
- C. H. Lam, A. J. Bloomfield and P. T. Anastas, Green Chem., 2017, 19, 1958–1968 RSC.
- Y. Wang, Y.-Q. Zhu, Z. Xie, S.-M. Xu, M. Xu, Z. Li, L. Ma, R. Ge, H. Zhou, Z. Li, X. Kong, L. Zheng, J. Zhou and H. Duan, ACS Catal., 2022, 12432–12443 CrossRef CAS.
- C. S. Lee, M. K. Aroua, W. A. Wan Daud, P. Cognet, Y. Pérès and M. A. Ajeel, Front. Chem., 2019, 7, 1–11 CrossRef CAS PubMed.
- Z. Fan, W. Zhang, L. Li, Y. Wang, Y. Zou, S. Wang and Z. Chen, Green Chem., 2022, 7818–7868 RSC.
- P. N. A. M. Othman, N. A. Karim and S. K. Kamarudin, Int. J. Energy Res., 2021, 45, 12693–12727 CrossRef CAS.
- M. R. Karimi Estahbanati, M. Feilizadeh, F. Attar and M. C. Iliuta, Ind. Eng. Chem. Res., 2020, 59, 22330–22352 CrossRef CAS.
- G. Carraro, C. MacCato, A. Gasparotto, T. Montini, S. Turner, O. I. Lebedev, V. Gombac, G. Adami, G. Van Tendeloo, D. Barreca and P. Fornasiero, Adv. Funct. Mater., 2014, 24, 372–378 CrossRef CAS.
- M. Cheng, Q. Zhang, C. Yang, B. Zhang and K. Deng, Catal. Sci. Technol., 2019, 9, 6909–6919 RSC.
- G. Innocenti, E. Papadopoulos, G. Fornasari, F. Cavani, A. J. Medford and C. Sievers, ACS Catal., 2020, 10, 11936–11950 CrossRef CAS.
- P. Y. Dapsens, C. Mondelli and J. Pérez-Ramírez, ChemSusChem, 2013, 6, 831–839 CrossRef CAS PubMed.
- S. Lyu and T. Wang, RSC Adv., 2017, 7, 10278–10286 RSC.
- G. M. Lari, B. Puértolas, M. S. Frei, C. Mondelli and J. Pérez-Ramírez, ChemCatChem, 2016, 8, 1507–1514 CrossRef CAS.
- J. Peng, X. Li, C. Tang and W. Bai, Green Chem., 2014, 16, 108–111 RSC.
- T. W. P. Seadira, G. Sadanandam, T. Ntho, C. M. Masuku and M. S. Scurrell, Appl. Catal., B, 2018, 222, 133–145 CrossRef CAS.
- V. Augugliaro, H. A. H. El Nazer, V. Loddo, A. Mele, G. Palmisano, L. Palmisano and S. Yurdakal, Catal. Today, 2010, 151, 21–28 CrossRef CAS.
- P. Limpachanangkul, T. Jedsukontorn, G. Zhang, L. Liu, M. Hunsom and B. Chalermsinsuwan, Korean J. Chem. Eng., 2019, 36, 1527–1535 CrossRef CAS.
- J. Payormhorm and R. Idem, Appl. Catal., A, 2020, 590, 117362 CrossRef CAS.
- Y. Zhang, N. Zhang, Z. R. Tang and Y. J. Xu, Chem. Sci., 2013, 4, 1820–1824 RSC.
- K. Tian, X. Chen, W. Shen, B. A. Prior, G. Shi and Z. Wang, Afr. J. Biotechnol., 2012, 11, 4860–4867 CAS.
- N. Murakami, M. Oba, M. Iwamoto, Y. Tashiro, T. Noguchi, K. Bonkohara, M. A. Abdel-rahman, T. Zendo, M. Shimoda, K. Sakai and K. Sonomoto, J. Biosci. Bioeng., 2016, 121, 89–95 CrossRef CAS PubMed.
- T. Dishisha, S. H. Pyo and R. Hatti-Kaul, Microb. Cell Fact., 2015, 14, 1–11 CrossRef PubMed.
- S. Mazumdar, J. M. Clomburg and R. Gonzalez, Appl. Environ. Microbiol., 2010, 76, 4327–4336 CrossRef CAS PubMed.
- T. Lee, W. Min, H. Jin and J. Seo, Bioresour. Technol., 2020, 299, 122600 CrossRef CAS.
- E. Fokum, H. M. Zabed, J. Yun, G. Zhang and X. Qi, Int. J. Environ. Sci. Technol., 2021, 18, 2467–2490 CrossRef CAS.
- A. D. da Silva Ruy, R. M. de Brito Alves, T. L. Reis Hewer, D. de Aguiar Pontes, L. S. Gomes Teixeira and L. A. Magalhães Pontes, Catal. Today, 2021, 381(1), 243–253 CrossRef CAS.
- A. Hong, K. Cheng, F. Peng, S. Zhou, Y. Sun, C. Liu and D. Liu, J. Chem. Technol. Biotechnol., 2009, 84, 1576–1581 CrossRef CAS.
- M. Sankaranarayanan, A. Somasundar, E. Seol and A. Singh, J. Biotechnol., 2017, 259, 140–147 CrossRef CAS PubMed.
- O. Zaushitsyna, T. Dishisha, R. Hatti-kaul and B. Mattiasson, J. Biotechnol., 2017, 241, 22–32 CrossRef CAS PubMed.
- J. W. K, Y. S. Ko, T. U. Chae and S. Y. Lee, Biotechnol. Bioeng., 2020, 117, 2139–2152 CrossRef.
- P. Zhao, C. Ma, L. Xu and P. Tian, Appl. Microbiol. Biotechnol., 2019, 103, 4017–4031 CrossRef PubMed.
- Y. Zhang, H. M. Zabed, J. Yun, G. Zhang, Y. Wang and X. Qi, ACS Sustainable Chem. Eng., 2021, 9, 4625–4637 CrossRef CAS.
- L. Zhao, J. Zhu, K. S. Ro, J. Xie and D. Wei, Process Biochem., 2022, 118, 182–189 CrossRef CAS.
- C. V. Pramod, R. Fauziah, K. Seshan and J. P. Lange, Catal. Sci. Technol., 2018, 8, 289–296 RSC.
- G. S. Jadtowski and E. Strzelec, Open Chem., 2021, 19, 998–1008 CrossRef.
- R. A. Sheldon, J. Mol. Catal. A: Chem., 2016, 422, 3–12 CrossRef CAS.
- A. Abdullah, A. Z. Abdullah, M. Ahmed, P. U. Okoye and M. Shahadat, Can. J. Chem. Eng., 2021, 1–30 CAS.
- Y. Wang, J. Zhou and X. Guo, RSC Adv., 2015, 5, 74611–74628 RSC.
- M. Y. Ahmad, N. I. Basir and A. Z. Abdullah, J. Ind. Eng. Chem., 2021, 93, 216–227 CrossRef CAS.
- S. Ramayya, A. Brittain, C. DeAlmeida, W. Mok and M. J. Antal, Fuel, 1987, 66, 1364–1371 CrossRef CAS.
- M. Watanabe, T. Iida, Y. Aizawa, T. M. Aida and H. Inomata, Bioresour. Technol., 2007, 98, 1285–1290 CrossRef CAS PubMed.
- A. Galadima and O. Muraza, J. Taiwan Inst. Chem. Eng., 2016, 67, 29–44 CrossRef CAS.
- M. Checa, S. Nogales-Delgado, V. Montes and J. M. Encinar, Catalysts, 2020, 10, 1–41 CrossRef.
- A. Abdullah, A. Zuhairi Abdullah, M. Ahmed, J. Khan, M. Shahadat, K. Umar and M. A. Alim, J. Cleaner Prod., 2022, 341, 130876 CrossRef CAS.
- A. Abdullah, A. Z. Abdullah, M. Ahmed, P. U. Okoye and M. Shahadat, Can. J. Chem. Eng., 2022, 100(10), 2956–2985 CrossRef CAS.
- A. Alhanash, E. F. Kozhevnikova and I. V. Kozhevnikov, Appl. Catal., A, 2010, 378, 11–18 CrossRef CAS.
- A. S. Belousov, ChemistrySelect, 2021, 6, 9191–9198 CrossRef CAS.
- L. Liu, X. P. Ye, B. Katryniok, M. Capron, S. Paul and F. Dumeignil, Front. Chem., 2019, 7, 1–13 CrossRef.
- X. Li, L. Huang, A. Kochubei, J. Huang, W. Shen, H. Xu and Q. Li, ChemSusChem, 2020, 13, 5073–5079 CrossRef CAS PubMed.
- M. H. Haider, C. D'Agostino, N. F. Dummer, M. D. Mantle, L. F. Gladden, D. W. Knight, D. J. Willock, D. J. Morgan, S. H. Taylor and G. J. Hutchings, Chem. – Eur. J., 2014, 20, 1743–1752 CrossRef CAS PubMed.
- J. Shan, Z. Li, S. Zhu, H. Liu, J. Li, J. Wang and W. Fan, Catalysts, 2019, 9, 13–27 CrossRef.
- B. Ali, X. Lan, M. T. Arslan, H. Wang, S. Z. A. Gilani, S. Wang and T. Wang, ACS Appl. Nano Mater., 2020, 3, 10966–10977 CrossRef CAS.
- J. Zhang, L. Ren, A. Zhou, W. Li, S. Shang, Y. Liu, Z. Jia, W. Liu, A. Zhang, X. Guo and C. Song, Chem. Mater., 2022, 34, 3217–3226 CrossRef CAS.
- B. A. Qureshi, X. Lan, M. T. Arslan and T. Wang, Ind. Eng. Chem. Res., 2019, 58, 12611–12622 CrossRef CAS.
- B. Ali, X. Lan, M. T. Arslan, S. Z. A. Gilani, H. Wang and T. Wang, J. Ind. Eng. Chem., 2020, 88, 127–136 CrossRef CAS.
- M. M. Diallo, S. Laforge, Y. Pouilloux and J. Mijoin, Catal. Lett., 2018, 148, 2283–2303 CrossRef CAS.
- P. Mars and D. W. van Krevelen, Chem. Eng. Sci., 1954, 3, 41–59 CrossRef CAS.
- A. Drochner, D. Ohlig, S. Knoche, N. Gora, M. Heid, N. Menning, T. Petzold and H. Vogel, Top. Catal., 2016, 59, 1518–1532 CrossRef CAS.
- C. Qiu, C. Chen, S. Ishikawa, T. Murayama and W. Ueda, Top. Catal., 2014, 57, 1163–1170 CrossRef CAS.
- C. Chen, N. Kosuke, T. Murayama and W. Ueda, ChemCatChem, 2013, 5, 2869–2873 CrossRef CAS.
- T. V. Andrushkevich, Catal. Rev.: Sci. Eng., 1993, 35, 213–259 CrossRef CAS.
- S. Ishikawa, T. Murayama, B. Katryniok, F. Dumeignil, M. Araque, S. Heyte, S. Paul, Y. Yamada, M. Iwazaki, N. Noda and W. Ueda, Appl. Catal., A, 2019, 584, 117151 CrossRef.
- S. Ishikawa and W. Ueda, Catal. Sci. Technol., 2016, 6, 617–629 RSC.
- B. Frank, R. Blume, A. Rinaldi, A. Trunschke and R. Schlögl, Angew. Chem., Int. Ed., 2011, 50, 10226–10230 CrossRef CAS PubMed.
- B. Zhong, H. Liu, X. Gu and D. S. Su, ChemCatChem, 2014, 6, 1553–1557 CrossRef CAS.
- A. R. Petersen, L. B. Nielsen, J. R. Dethlefsen and P. Fristrup, ChemCatChem, 2018, 10, 769–778 CrossRef CAS.
- A. Fernandes, M. F. Ribeiro and J. P. Lourenço, Microporous Mesoporous Mater., 2022, 329, 111536 CrossRef CAS.
- H. Lan, Q. Yao, Y. Zhou, B. Zhang and Y. Jiang, Mol. Catal., 2020, 498, 111279 CrossRef CAS.
- A. Kostyniuk, D. Bajec, P. Djinović and B. Likozar, Chem. Eng. J., 2020, 397, 125430 CrossRef CAS.
- R. Almeida, M. F. Ribeiro, A. Fernandes and J. P. Lourenço, Catal. Commun., 2019, 127, 20–24 CrossRef CAS.
- H. Fujitsuka, K. Terai, M. Hayashi, T. Yoshikawa, Y. Nakasaka, T. Masuda and T. Tago, J. Jpn. Pet. Inst., 2019, 62, 319–328 CrossRef CAS.
- G. Sánchez, B. Z. Dlugogorski, E. M. Kennedy and M. Stockenhuber, Appl. Catal., A, 2016, 509, 130–142 CrossRef.
- J. H. Jang, H. Sohn, J. Camacho-Bunquin, D. Yang, C. Y. Park, M. Delferro and M. M. Abu-Omar, ACS Sustainable Chem. Eng., 2019, 7, 11438–11447 CrossRef CAS.
- S. Tazawa, N. Ota, M. Tamura, Y. Nakagawa, K. Okumura and K. Tomishige, ACS Catal., 2016, 6, 6393–6397 CrossRef CAS.
- Y. Kon, M. Araque, T. Nakashima, S. Paul, F. Dumeignil and B. Katryniok, ChemistrySelect, 2017, 2, 9864–9868 CrossRef CAS.
- S. Yang, M. Kim, S. Yang, D. S. Kim, W. J. Lee and H. Lee, Catal. Sci. Technol., 2016, 6, 3616–3622 RSC.
- M. Kim and H. Lee, ChemistrySelect, 2017, 2, 2420–2425 CrossRef CAS.
- Z. Xiu, H. Wang, C. Cai, C. Li, L. Yan, C. Wang, W. Li, H. Xin, C. Zhu, Q. Zhang, Q. Liu and L. Ma, Ind. Eng. Chem. Res., 2020, 59, 9912–9925 CrossRef CAS.
- R. Palacio, S. Torres, D. Lopez and D. Hernandez, Catal. Today, 2018, 302, 196–202 CrossRef CAS.
- N. Mimura, N. Muramatsu, N. Hiyoshi, O. Sato and A. Yamaguchi, Catal. Today, 2021, 375, 191–196 CrossRef CAS.
- R. Palacio, Á. A. Amaya, D. Blach, S. Torres, D. Hernández, D. López and F. Martinez, ChemistrySelect, 2020, 5, 7789–7796 CrossRef CAS.
- C. D. Evans, M. Douthwaite, J. H. Carter, S. Pattisson, S. A. Kondrat, D. Bethell, D. W. Knight, S. H. Taylor and G. J. Hutchings, J. Chem. Phys., 2020, 152(13), 134705 CrossRef CAS PubMed.
- L. Chen, S. Ren and X. P. Ye, Fuel Process. Technol., 2014, 120, 40–47 CrossRef CAS.
- A. M. Bruno, T. D. R. Simões, M. M. V. M. Souza and R. L. Manfro, RSC Adv., 2020, 10, 31123–31138 RSC.
- S. Feng, K. Takahashi, H. Miura and T. Shishido, Fuel Process. Technol., 2020, 197, 106202 CrossRef CAS.
- N. Razali and A. Z. Abdullah, Indones. J. Chem., 2020, 20, 608–615 CrossRef CAS.
- S. N. M. Saleh and A. Z. Abdullah, Waste Biomass Valoriz., 2021, 12, 2565–2578 CrossRef CAS.
- Z. Guo, D. S. Theng, K. Y. Tang, L. Zhang, L. Huang, A. Borgna and C. Wang, Phys. Chem. Chem. Phys., 2016, 18, 23746–23754 RSC.
- X. Li, Z. Chen, P. Cao, W. Pu, W. Zou, C. Tang and L. Dong, RSC Adv., 2017, 7, 54696–54705 RSC.
- N. Sobuś, B. Michorczyk, M. Piotrowski, Ł. Kuterasiński, D. K. Chlebda, J. Łojewska, R. J. Jędrzejczyk, P. Jodłowski, P. Kuśtrowski and I. Czekaj, Catal. Lett., 2019, 149, 3349–3360 CrossRef.
- B. Yan, A. Mahmood, Y. Liang and B. Q. Xu, Catal. Today, 2016, 269, 65–73 CrossRef CAS.
- N. Nekkala, P. Balla, S. R. Ginjupalli, P. K. Seelam, S. K. Hussain, B. Ponnala and V. R. C. Komandur, Biomass Convers. Biorefin., 2020, 12, 3535–3546 CrossRef.
- D. Sun, Y. Yamada, S. Sato and W. Ueda, Appl. Catal., B, 2016, 193, 75–92 CrossRef CAS.
- Z. Wu, K. Zhao, S. Ge, Z. Qiao, J. Gao, T. Dou, A. C. K. Yip and M. Zhang, ACS Sustainable Chem. Eng., 2016, 4, 4192–4207 CrossRef CAS.
- C. J. A. Mota, V. L. C. Gonçalves, J. E. Mellizo, A. M. Rocco, J. C. Fadigas and R. Gambetta, J. Mol. Catal. A: Chem., 2016, 422, 158–164 CrossRef CAS.
- C. Liebig, S. Paul, B. Katryniok, C. Guillon, J. L. Couturier, J. L. Dubois, F. Dumeignil and W. F. Hoelderich, Appl. Catal., B, 2013, 132–133, 170–182 CrossRef CAS.
- L. D. da Silva, R. C. Santos, J. G. A. B. Silva, E. de Paiva Alves, R. T. F. Fréty and L. A. M. Pontes, React. Kinet., Mech. Catal., 2022, 135, 271–285 CrossRef.
- M. O. Guerrero-Pérez and M. A. Bañares, ChemSusChem, 2008, 1, 511–513 CrossRef PubMed.
- M. M. Diallo, S. Laforge, Y. Pouilloux and J. Mijoin, Catal. Commun., 2019, 126, 21–25 CrossRef CAS.
- A. Chieregato, C. Bandinelli, P. Concepción, M. D. Soriano, F. Puzzo, F. Basile, F. Cavani and J. M. L. Nieto, ChemSusChem, 2017, 10, 234–244 CrossRef CAS.
- L. G. Possato, W. H. Cassinelli, T. Garetto, S. H. Pulcinelli, C. V. Santilli and L. Martins, Appl. Catal., A, 2015, 492, 243–251 CrossRef CAS.
- A. S. Paula, L. G. Possato, D. R. Ratero, J. Contro, K. Keinan-Adamsky, R. R. Soares, G. Goobes, L. Martins and J. G. Nery, Microporous Mesoporous Mater., 2016, 232, 151–160 CrossRef CAS.
- R. Liu, T. Wang, D. Cai and Y. Jin, Ind. Eng. Chem. Res., 2014, 53, 8667–8674 CrossRef CAS.
- T. Q. Silva, M. B. dos Santos, A. A. C. Santiago, D. O. Santana, F. T. Cruz, H. M. C. Andrade and A. J. S. Mascarenhas, Catal. Today, 2017, 289, 38–46 CrossRef CAS.
- S. Lopez-Pedrajas, R. Estevez, J. Schnee, E. M. Gaigneaux, D. Luna and F. M. Bautista, Mol. Catal., 2018, 455, 68–77 CrossRef CAS.
- L. F. Rasteiro, L. H. Vieira, L. G. Possato, S. H. Pulcinelli, C. V. Santilli and L. Martins, Catal. Today, 2017, 296, 10–18 CrossRef CAS.
- L. G. Possato, M. D. Acevedo, C. L. Padró, V. Briois, A. R. Passos, S. H. Pulcinelli, C. V. Santilli and L. Martins, Mol. Catal., 2020, 481, 110158 CrossRef CAS.
- A. Witsuthammakul and T. Sooknoi, Appl. Catal., A, 2012, 413–414, 109–116 CrossRef CAS.
- X. Li and Y. Zhang, ACS Catal., 2016, 6, 143–150 CrossRef CAS.
- A. C. Dimian and C. S. Bildea, Chem. Eng. Res. Des., 2021, 166, 121–134 CrossRef CAS.
- M. Dalil, M. Edake, C. Sudeau, J. L. Dubois and G. S. Patience, Appl. Catal., A, 2016, 522, 80–89 CrossRef CAS.
- W. Suprun, M. Lutecki, T. Haber and H. Papp, J. Mol. Catal. A: Chem., 2009, 309, 71–78 CrossRef CAS.
- V. Campisciano, M. Gruttadauria and F. Giacalone, Modified nanocarbons as catalysts in organic processes, 2019 Search PubMed.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS.
- N. R. Mohamad, N. H. C. Marzuki, N. A. Buang, F. Huyop and R. A. Wahab, Biotechnol. Biotechnol. Equip., 2015, 29, 205–220 CrossRef CAS PubMed.
- R. A. Wahab, N. Elias, F. Abdullah and S. K. Ghoshal, React. Funct. Polym., 2020, 152, 104613 CrossRef CAS.
- S. Weon, D. Huang, K. Rigby, C. Chu, X. Wu and J.-H. Kim, ACS ES&T Engg, 2021, 1, 157–172 Search PubMed.
- M. B. Gawande, K. Ariga and Y. Yamauchi, Small, 2021, 17, 1–4 CrossRef.
- X. Yang, A. Wang, B. Qiao, J. Li, J. Liu and T. Zhang, Acc. Chem. Res., 2013, 46(8), 1740–1748 CrossRef CAS PubMed.
- Z. Li, S. Ji, Y. Liu, X. Cao, S. Tian, Y. Chen, Z. Niu and Y. Li, Chem. Rev., 2020, 120, 623–682 CrossRef CAS.
- B. Frank, A. Rinaldi, R. Blume, R. Schlögl and D. S. Su, Chem. Mater., 2010, 22, 4462–4470 CrossRef CAS.
- B. Zhong, R. Huang, D. S. Su and H. Liu, Catal. Today, 2019, 330, 142–148 CrossRef CAS.
- K. Feng, Y. Wang, M. Guo, J. Zhang, Z. Li, T. Deng, Z. Zhang and B. Yan, J. Energy Chem., 2021, 62, 153–171 CrossRef CAS.
- Y. R. Herrero and A. Ullah, ACS Sustainable Chem. Eng., 2021, 9, 9474–9485 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |