 Open Access Article
Open Access ArticleIsocyanide chemistry enabled by continuous flow technology†
Bruno
Cerra
 a,
Cecile
Blondeau
a,
Rolando
Cannalire
b,
Mariateresa
Giustiniano
a,
Cecile
Blondeau
a,
Rolando
Cannalire
b,
Mariateresa
Giustiniano
 b,
Shiva
Tali Shandiz
a and
Antimo
Gioiello
b,
Shiva
Tali Shandiz
a and
Antimo
Gioiello
 *a
*a
aLaboratory of Medicinal and Advanced Synthetic Chemistry (Lab MASC), Department of Pharmaceutical Sciences, University of Perugia, Via del Liceo 1, Perugia, 06123, Italy. E-mail: antimo.gioiello@unipg.it
bDepartment of Pharmacy, University of Naples Federico II, Via D. Montesano 49, 80131, Napoli, Italy
First published on 29th November 2022
Abstract
Isocyanides are valuable compounds for organic synthesis. However, the poor stability and distressing odour have often limited their widespread applications in common laboratory practice and industrial settings. Herein, a continuous flow approach to enable the synthesis, purification and in-line reaction of isocyanides is presented.
Isocyanides are important and versatile compounds with a long history in organic synthesis and production of chemicals.1 Currently, the global isocyanide market surges to more than 5% per year, underlining its relevance in agrochemicals and pharmaceuticals. Their divalent nature and ability to form multiple bonds on the terminal carbon make them a multifaceted group for organic synthesis with broad applications in medicinal chemistry.2 Indeed, the potential of isocyanide-based chemistry and related post-modification reactions has been demonstrated in both target- and diversity-oriented synthesis. However, several issues have barred their wide use in both academia and industry. Some isocyanides, such as the volatile ones, are characterized by a) strong foul odour, b) sensitivity to moisture, c) poor stability and d) potential toxicity.
Isocyanides are produced industrially using phosgenation of alkyl or aromatic amines.3 However, the high toxicity of the gas raises safety concerns and requires special equipment. Other methods such as the Curtius rearrangement, and the Lieke–Meyer, Hoffmann, and Ugi reactions are generally employed in common laboratory practise.1,2 Although many other options exist, the vast majority of isocyanide syntheses still suffer from one or more issues including tedious protocols and work-up operations, expensive reagents, waste generation, and exposure to potentially hazardous fumes.
In the last few years, continuous flow chemistry has greatly facilitated the development of safe and efficient approaches for conducting forbidden chemical reactions.4 Adoption of flow synthesizers has been a determinant in moving towards green chemistry and more sustainable practices.5 In addition to improved process safety and quality, flow chemistry guarantees the fine-tuning of the reaction conditions, an efficient heat and mass transfer, and an extended operative window.6 Furthermore, the possibility to perform telescoped reactions and to integrate downstream operations avoids intermediate isolation, minimizing the exposure to toxic and hazardous reagents with a great improvement to the safety and environmental impacts.4 The merger of these benefits with automation promises to accelerate the development of new synthetic routes and compound throughput relieving expert chemists of routine tasks.7
In a previous work, Kim and colleagues reported the preparation of isocyanides in a microfluidic system with short reaction times and high yield.8a However, the method suffered from solubility and tube-plugging issues that were partially solved using ultrasound irradiation. In 2015, Baxendale and co-workers reported the telescoped flow synthesis of different heterocyclic building blocks, including 1,2,4-triazole and pyrrolo[1,2-c]pyrimidine scaffolds, using ethyl isocyanoacetate that was generated in situ by the dehydration reaction of N-formylglycine in the presence of triphosgene.8b
In this work, we present a streamlined method based on reactor technology for the serial synthesis, purification, scale-up and in-line reaction of isocyanides. Such an approach would solve major drawbacks, enable the progress and expand the scope of isocyanide chemistry.9 Combination with various reagents is conducted in a continuous fashion to provide diverse building blocks and important products without requiring the isolation of highly unstable and foul odour volatile isocyanides.
In order to set a benchmark with which our flow approach would be compared, we opted to conduct the Ugi synthesis of t-butyl isocyanide (1a) from the corresponding formamide 2a, in the presence of phosphorus oxychloride (POCl3) (1 equiv.) (Scheme 1A). Initially, diverse solvents (CHCl3, CH2Cl2, THF, 2-Me-THF) and organic bases (Et3N, DIPEA, pyridine, DIPA, TBA, DBU, DBN, proton sponge) were tested in batch modality to evaluate possible solubility issues and reactor clogging (data not shown). Most of the solvents and bases resulted in suspensions or in-line precipitation. Nevertheless, the desired t-butyl isocyanide (1a) was obtained in moderate to high yields. The best outcome was achieved with the use of CHCl3 and Et3N (5 equiv.) at 0 °C which ensured a clear solution (up to 0.6 M) and a high yield (82%). The aqueous work-up required careful hydrolysis to destroy the possible excess of POCl3, keeping basic pH conditions to avoid isocyanide decomposition. The crude isocyanide should be then purified, increasing the exposure to smelly odour over a rather long period.
Next, the reaction was repeated under flow conditions. As depicted in Scheme 1B, the flow set-up consisted of two syringe pumps to combine a solution of 2a (0.6 M solution in CHCl3) and Et3N with a stream of POCl3 (0.6 M solution in CHCl3) via a PEEK T-piece. A 20 mL PFA coil was used as the reactor, which was followed by a silica packed column for the in-line removal of salts and excess Et3N.9 In order to avoid aqueous work-up in favour of a simple alternative, the collected reaction mixture was analysed by quantitative 1H-NMR, using dimethyl sulfone as an internal standard. The procedure allowed a simpler set-up in addition to enabling rapid flow processing of materials and post-modification reactions. It was found that running the reaction for 20 min at 25 °C rendered the expected product 1a in excellent yield (89%) (Table 1, entry 1). A partial conversion (65%) was obtained by reducing the Et3N equiv. from 5 to 3. Using the best conditions, the reaction was proven effective in the multi-gram preparation of t-butyl isocyanide (1a) with a throughput of 3 mmol h−1 (Scheme 1B).
| Entry | Isocyanide | Yielda | Literature yield |
|---|---|---|---|
| Reactions were performed according to Scheme 1. (−) = No literature available from formamide to isocyanide via dehydration under standard conditions.a Yield determined by calibrated 1H-NMR analysis of the crude product in the presence of dimethyl sulfone as the internal standard.b DMSO (5%, v/v in CHCl3) was used as the co-solvent, [2g] = 0.15 M, [2i] = 0.36 M.c Isolated yield.d Conducted by reaction of 1a with POCl3 (1 equiv.) and of Et3N (5 equiv.) in CHCl3 at 0 °C until starting material consumption. | |||
| 1 |
 1a
1a
|
89% | 82%d |
| 2 |
 1b
1b
|
87% | 95%962%10 |
| 3 |
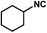 1c
1c
|
79% | 67%10 |
| 4 |
 1d
1d
|
69% | (−) |
| 5 |
 1e
1e
|
Traces | (−) |
| 6 |
 1f
1f
|
87%11 | 91%12 |
| 7 |
 1g
1g
|
44%b,c | 93%9 |
| 8 |
 1h
1h
|
77% | 97%13 |
| 9 |
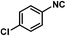 1i
1i
|
79%b | 81%9 |
| 10 |
 1j
1j
|
80%c | 97%14 |
The method was applied to other N-substituted formamides 2b–j, and the formation of isocyanides was nearly completed within 20 min (Table 1). The criteria for substrate selection include the extensive usage, high volatility, foul odour, and toxicity of the resulting isocyanides.9 As a general trend, the reaction allowed the formation of the desired isocyanides with high purity and in good to high yields, except for formamides 2e and 2g (Table 1, entries 5 and 7). While allyl isocyanide 1e was formed in traces, the preparation of 2,6-dimethylphenyl isocyanide (1g) required the addition of 5% DMSO to ensure the complete solubility of the reaction mixture. Unfortunately, the use of DMSO caused the formation of solvent-derived by-products leading to a moderate reaction yield (44%) (Table S1, ESI†). In the case of aryl isocyanides, both electron-donating and -withdrawing groups work similarly well (entries 8 and 9). Comparison of our results with the ones reported in the literature under standard conditions has evidenced similar yields with a few exceptions; however, it should be pointed out that for these cases, the preparation required a long reaction time (24 h) and tedious protocols.
Having established a reliable flow method for the preparation and purification of pure isocyanides, our efforts were then focussed on the development of a flow system enabling in-line capture. The flow set-up has been designed to allow the serial synthesis of relevant scaffolds in medicinal chemistry (Scheme 2). The synthesizer was composed of a pump system, switching/mixing devices, two 10 mL coil reactors, back pressure regulators (BPRs), an autosampler, a UV detector, an automated flash chromatograph, and a fraction collector. Three sets of compounds, including 5-aminoimidazoles, 1,3-oxazoles, and β-amino amides such as the anaesthetic lidocaine, were prepared to showcase the feasibility of the flow system, using standard, non-optimized process conditions. At this stage, we aim to demonstrate the merging and compatibility of the reaction conditions and solvents between steps. This reaction scoping also served to assess the robustness of the processing conditions against the propensity for blockage formation.8 Notably, at the concentration studied for the flow set-up, no blockages occurred across the screened substrates and reactions. By not performing all steps simultaneously, the flow rates, concentrations, and temperatures can be easily adjusted without changing the equipment. Moreover, to permit reaction monitoring, and product detection and collection, an aliquot of the exit stream was analysed using a UV detector.
1,3-Oxazoles
1,3-Oxazoles are an important class of pharmaceutically interesting heterocycles, and consequently, many synthetic protocols have been reported to access their structures.15 In-flow examples include a three-step method from vinyl azides16 and a fully automated flow synthesis by the reaction of alkyl isocyanides with acyl chloride followed by intramolecular cyclization.17 In our case, we sought to explore the Passerini-cyclization reaction sequence to obtain trisubstituted 1,3-oxazoles (Scheme 3). Thus, isocyanide 1a or 1c, prepared as previously described, was collected into an Erlenmeyer flask with a screw cap, pumped at 0.18 mL min−1 and mixed in a four-way connector with a solution of benzoic acid (3) and phenylglyoxal (4) The reaction mixture was reacted into a 10 mL coil reactor at 25 °C (τ = 33 min) affording the corresponding intermediates 5 and 6 in high yield (85% and 87% yield, respectively) after out-flow crystallization (Scheme 3). Cyclisation of the Passerini adducts 5 and 6 was performed using NH4OAc, in AcOH (5 M) under reflux for 45 min. Simple filtration of the solid products afforded the desired oxazole derivatives 7 and 8 in 75% and 52% yield, respectively, over two steps (Scheme 3). Notably, batch synthesis using the same reaction conditions provided 8 in 28% overall yield.185-Aminoimidazoles
Despite their potential in the synthesis of bioactive compounds, only a few methods have been reported for the preparation of 5-aminoimidazoles.19,20 We recently described the ytterbium-promoted isocyanide insertion/5-exo-dig cyclization of Strecker adducts towards 4-substituted 5-aminoimidazoles.21 In the course of the investigation, we noticed a certain compound instability that may limit their exploitation to further chemical manipulations. This could be overcome with the recourse to continuous flow technology. Thus, a CHCl3 solution of isocyanide 1a from step 1 (Scheme 1B, Table 1) was first mixed with a CHCl3 solution of α-aryl-α′-amino-acetonitriles 9a–c (1 equiv.) with a total flow rate of 0.5 mL min−1. The resulting solution was then mixed through a T-junction with Yb(OTf)3 dissolved in CHCl3. The reaction took place in a 10 mL coil reactor heated at 130 °C (τ = 10 min) (Scheme 4). The reactor outcome was collected, readily purified by automated chromatography, and converted into the corresponding hydrochloride salt for improving product stability. The reactions gave the expected 4-aryl-5-aminoimidazoles 9a–c in 34–51% yields.Lidocaine
Lidocaine (12), also known as xylocaine, is a commonly used local anesthetic and antiarrhythmic drug. Seeking to avoid the elevated reaction temperatures and chloroacetyl chloride employed in the traditional N-acylation/N-alkylation approach, we sought to adapt the Ugi multicomponent synthesis of lidocaine (12) to our flow method. Thus, the CHCl3 solution of 1g (0.1 M) collected from the previous step into a sealed Erlenmeyer flask was mixed with a solution of Et2NH (1 equiv.) and 2-hydroxymethylbenzoic acid (11) (1 equiv.) premixed with formaldehyde (1 equiv.) (Scheme 5). The mixture was reacted into a 10 mL coil reactor (τ = 66 min) at 80 °C. The outflow was collected and readily purified by automated flash chromatography to give pure lidocaine (12) in 69% yield (0.1 mmol h−1) (Scheme 5). Compared to conventional multi-step approaches for preparing lidocaine,22 this multicomponent telescoped protocol offers superior atom- and step-economy, eliminates the need for hazardous heating sources and solvent intensive extraction steps, employs readily starting materials and is easy to perform. Moreover, our approach is amenable for automation and can be employed for the rapid, diverse, and parallel synthesis of commercial and novel local anesthetics based on the amide-caine scaffold.Conclusions
New methods are needed for maximizing resource-accelerated synthesis and medicinal chemistry, which are areas where continuous flow chemistry can demonstrate distinct benefits.23 Enabling repetitive and trivial reaction sequences accelerates processing method development, especially when integrated with diverse units and downstream operations, telescoping approaches and automation.7 In this work, we have developed a reliable make-and-use flow system for the synthesis, analysis, and purification of isocyanides in regular laboratory practice. To demonstrate the utility of the approach, the reaction was applied to formamides bearing aromatic, aliphatic, and cyclic groups. The strategy described is one in which isocyanides are synthesized in flow mode and readily employed for post-modification reactions. This avoids the separation, purification, and storage of isocyanides and solves drawbacks of isocyanide chemistry including exposure to toxic and foul odour compounds and isocyanide decomposition. Despite some of the exemplified applications giving moderate to good yields, the designed flow set-up allows a facile access to valuable building blocks and could be important for the screening and discovery of bioactive molecules. Indeed, the system can be easily reconfigured to accommodate new reaction combinations. The complexity of the equipment described herein is one step away from automation and could be therefore further advanced depending on the laboratory capability and budget. Future efforts in this direction are ongoing in our laboratory and will be reported in due course.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) For selected reviews, see: (a) C. Six and F. Richter, Isocyanates, Organic, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, Germany, 2003 Search PubMed; (b) A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed; (c) M. Suginome and Y. Ito, in Science of Synthesis, Thieme, Stuttgart, 2004, pp. 445–530 Search PubMed; (d) M. A. Mironov, in Isocyanide Chemistry, Wiley-VCH, Weinheim, Germany, 2012, pp. 35–73 Search PubMed; (e) A. Massarotti, F. Brunelli, S. Aprile, M. Giustiniano and G. C. Tron, Chem. Rev., 2021, 121, 10742–10788 Search PubMed.
- (a) A. Dömling, Comb. Chem. High Throughput Screening, 1998, 1, 1–22 CrossRef; (b) C. Hulme and T. Nixey, Curr. Opin. Chem. Biol., 2003, 6, 921–929 CAS; (c) A. Massarotti, F. Brunelli, S. Aprile, M. Giustiniano and G. C. Tron, Chem. Rev., 2021, 121, 10742–10788 CrossRef CAS PubMed.
- R. J. Slocombe, E. E. Hardy, J. H. Saunders and R. L. Jenkins, J. Am. Chem. Soc., 1950, 72, 1888–1891 CrossRef CAS.
- (a) A. Bonner, A. Loftus, A. C. Padgham and M. Baumann, Org. Biomol. Chem., 2021, 19, 7737–7753 RSC; (b) B. Gutmann, D. Cantillo and O. Kappe, Am. Ethnol., 2015, 54, 6688–6728 CAS.
- L. Rogers and K. F. Jensen, Green Chem., 2019, 21, 3481–3498 Search PubMed.
- M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796–11893 Search PubMed.
- C. P. Breen, A. M. K. Nambiar, T. F. Jamison and K. F. Jensen, Trends Chem., 2021, 3, 373–386 CrossRef.
- (a) S. Sharma, R. A. Maurya, K. Min, G. Y. Jeong and D. Kim, Angew. Chem., Int. Ed., 2013, 52, 7564–7568 CrossRef CAS PubMed; (b) M. Baumann, A. M. Rodriguez Garcia and I. R. Baxendale, Org. Biomol. Chem., 2015, 13, 4231–4239 RSC.
- P. Patil, M. Ahmadian-Moghaddam and A. Dömling, Green Chem., 2020, 22, 6902–6911 Search PubMed.
- K. A. Waibel, R. Nickisch, N. Möhl, R. Seimb and M. A. R. Meier, Green Chem., 2020, 22, 933–941 RSC.
- Yield determination by 1H-NMR analysis was unfeasible due to the overlap of phenyl proton signals with CHCl3. The formation of phenylisocyanide (1f) was monitored by TLC analysis and quantified as reported in the ESI.
- S. M. Creedon, H. K. Crowley and D. G. McCarthy, J. Chem. Soc., Perkin Trans. 1, 1998, 1015–1018 RSC.
- K. Khamphaijun, P. Namnouad, A. Docker, A. Ruengsuk, J. Tantirungrotechai, R. D. Torres, D. J. Harding and T. Bunchuay, Chem. Commun., 2022, 58, 7253–7256 Search PubMed.
- T. Matsuo, A. Hayashi, M. Abe, T. Matsuda, Y. Hisaeda and T. Hayashi, J. Am. Chem. Soc., 2009, 131, 15124–15125 CrossRef CAS PubMed.
- (a) For reviews on classical methods of preparing oxazoles, see: (a) I. J. Turchi, Oxazoles, in Heterocyclic Compounds, Wiley, New York, 1986, pp. 1–342 Search PubMed; (b) F. W. Hartner, Oxazoles, in Comprehensive Heterocyclic Chemistry II, Pergamon Press, Oxford, 1996, p. 262, and references cited therein Search PubMed.
- T. A. Rossa, N. S. Suveges, M. M. Sà, D. Cantillo and C. O. Kappe, Beilstein J. Org. Chem., 2018, 14, 506–514 Search PubMed.
- M. Baumann, I. R. Baxendale, S. V. Ley, C. D. Smith and G. K. Tranmer, Org. Lett., 2006, 8, 5213–5234 Search PubMed.
- R. Bossio, S. Marcaccini and R. Pepino, Liebigs Ann. Chem., 1991, 10, 1107–1108 CrossRef.
- (a) L. Svec, L. Furiassi, C. G. Skibinski, T. M. Fan, G. J. Riggins and P. J. Hergenrother, ACS Chem. Biol., 2018, 13, 3206–3216 CrossRef PubMed; (b) J. Hergenrother, T. M. Fan and R. L. Svec, US Pat., US20210002286, 2021 Search PubMed.
- (a) M. McLaughlin, R. M. Mohareb and H. Rapoport, J. Org. Chem., 2003, 68, 50–54 Search PubMed; (b) C. E. Bell, A. Y. Shaw, F. De Moliner and C. Hulme, Tetrahedron, 2014, 70, 54–59 Search PubMed; (c) J. O. Strelnikova, N. V. Rostovskii, G. L. Starova, A. F. Khlebnikov and M. S. Novikov, J. Org. Chem., 2018, 83, 11232–11244 Search PubMed; (d) C. H. Soh, W. K. Chui and Y. Lam, J. Comb. Chem., 2006, 8, 464–468 Search PubMed; (e) X. Wang, J.-P. Fu, J.-H. Mo, Y.-H. Tian, C.-Y. Liu, H.-T. Tang, Z.-J. Sun and Y.-M. Pan, Adv. Synth. Catal., 2021, 363, 2762–2766 CrossRef CAS.
- R. Cannalire, C. Russo, B. Cerra, A. Gioiello, F. Brunelli, G. C. Tron and M. Giustiniano, Mol. Diversity, 2022 DOI:10.1007/s11030-022-10418-4.
- (a) P. Josephson, V. Nykvist, W. Qasim, B. Blomkvist and P. Diner, J. Chem. Educ., 2019, 96, 1389–1394 CrossRef CAS; (b) T. J. Reilly, J. Chem. Educ., 1999, 76, 1557 CrossRef CAS.
- A. Gioiello, A. Piccinno, A. M. Lozza and B. Cerra, J. Med. Chem., 2020, 63, 6624–6647 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details on experimental procedures, compound characterization and equipment are provided. See DOI: https://doi.org/10.1039/d2re00454b |
| This journal is © The Royal Society of Chemistry 2023 |





