Open Access Article
Open Access ArticleA new process for the recovery of palladium from a spent Pd/TiO2 catalyst through a combination of mild acidic leaching and photodeposition on ZnO nanoparticles†
Marica
Muscetta
 *a,
Giulio
Pota
a,
Giuseppe
Vitiello
*a,
Giulio
Pota
a,
Giuseppe
Vitiello
 a,
Samar
Al Jitan
b,
Giovanni
Palmisano
b,
Roberto
Andreozzi
a,
Raffaele
Marotta
a and
Ilaria
Di Somma
*c
a,
Samar
Al Jitan
b,
Giovanni
Palmisano
b,
Roberto
Andreozzi
a,
Raffaele
Marotta
a and
Ilaria
Di Somma
*c
aDipartimento di Ingegneria Chimica, dei Materiali e della Produzione Industriale, Università di Napoli “Federico II”, Italy. E-mail: marica.muscetta@unina.it
bDepartment of Chemical Engineering, Center for Membranes and Advanced Water Technology (CMAT Center) and, Research and Innovation Center on CO2 and Hydrogen (RICH Center), Khalifa University, P.O. Box 127788, Abu Dhabi, United Arab Emirates
cIstituto di Scienze e Tecnologie per l'Energia e la Mobilità Sostenibili (CNR), Napoli, Italy. E-mail: ilaria.disomma@stems.cnr.it
First published on 18th November 2022
Abstract
Currently, palladium represents an expensive and scarce element in the Earth's crust, with the mineral resources of platinum group metals (PGMs) predominately localized in South Africa and Russia. The growing demand for this material necessitates the development of suitable strategies to recover it from end-of-life devices. The recovery of palladium by combining leaching and sacrificial photocatalytic deposition in a low-waste process was proposed here as a possible alternative to the current technologies. The effect of the species involved in the single processes in the recirculated stream was evaluated, where a negligible influence of the sacrificial species (ethanol) in the leaching unit was noticed. Different home-prepared ZnO photocatalysts were tested, and the best performance in terms of recovery of palladium was obtained in the presence of ZnO nanoparticles prepared through sol–gel synthesis. The proposed procedure is a viable route for the recovery of Pd from spent catalysts: by combining the processes, the complete oxidation of palladium was observed in about 15 minutes, while the recovery of Pd through photodeposition on ZnO occurred within 120 minutes of irradiation.
1. Introduction
Nowadays, the depletion of resources and demographic growth, the increasing prices of raw materials, climate change and environmental problems related to production processes lead to recognition of the urgency to promote the development of a circular economy, through the study and the adoption of near-zero waste processes.1–3 Metals are the best candidates to take a similar approach, being indefinitely recyclable, without any problem related to downcycling.2,4,5 Among the recyclable materials, PGMs, and, in particular palladium, are the most interesting,6,7 due to the great number of applications in which they are used,7–14 such as catalytic converters, electronic devices and chemical production (see Fig. 1 for the percentage demand in different industrial applications). The European Union inserted platinum group metals in the list of 14 strategic materials, due to their key role in several industrial applications.15 Looking specifically at palladium, it is certainly an expensive and relatively scarce element in the Earth's crust, with the mineral resources of PGMs predominately localized in South Africa and Russia.16 A huge gap was recorded in the last few years between the growing demand for this material and its limited supply which requires the development of appropriate strategies to be developed.7 Recently, to overcome the shortage in palladium supply, several authors proposed and discussed the recycling of metals from end-of-life devices.17–26 | ||
| Fig. 1 Application demand for palladium in different industrial applications since 2016.7 | ||
At present, hydrometallurgical processes represent the most widely used approach for metal recovery,27–32 despite the numerous operating units, the strong acidity and the high-temperature conditions required, and the polluting streams resulting from those treatments. In a previous study,33 some of the authors proposed and discussed an alternative route for the dissolution of zero-valent palladium nanoparticles from solid wastes by means of NaCl and CuCl2 under mildly acidic conditions, identifying the best chloride and cupric ion concentrations to optimize the leaching process.
Nowadays, leaching solutions containing dissolved palladium are generally treated by means of different processes such as precipitation, membrane separation and electrodeposition, for which low selectivities are recorded and numerous operating units are required.22,34–39 An alternative was proposed by some of the authors in a recent study,40 in which the recovery of palladium from the leaching solution was achieved through its photocatalytic deposition in the presence of ZnO under visible and UV-visible light irradiation.
Starting from a spent palladium catalyst, the effective recovery of the metal may be thus obtained from the adoption of two separate stages (leaching and photodeposition), although this approach leads to the generation of wastewater streams that need proper treatment before discharge into the environment (see below). In the following sections, a detailed description of a new process combining leaching and photodeposition stages is presented and discussed.
2. Combination of the leaching and photodeposition processes
In Fig. 2, the block diagrams of the processes for the palladium recovery are reported according to the results of previous studies.33,40 The recovery of metallic palladium takes place from a spent catalyst in which the catalytic activity is based only on the presence of this metal. Referring to palladium, as reported previously and shown in Fig. 2(A), a solution containing NaCl/CuCl2 (1) is used to oxidize Pd(0) to Pd(II), obtaining a waste not containing palladium species. The solution derived from the leaching unit (2) is successively treated, in the presence of ZnO nanoparticles in a photocatalytic reactor; in particular, as previously reported and shown in Fig. 2(B), the photodeposition process takes place in the presence of ethanol as a sacrificial agent, under UV-A/visible light irradiation. The resulting Pd/ZnO composite material is separated from the solution by filtration and washed, obtaining a waste solution containing NaCl, Cu1+ and ethanol. As for the effective palladium recovery, the dissolution of zinc oxide nanoparticles, by adjusting the pH of the suspension below 6.30, was studied in a previous study.41 Therefore, metallic palladium precipitates are recovered, while a stream containing zinc ions and nitrates is generated (4). | ||
| Fig. 2 Block diagram for the recovery of palladium from waste materials. (A) Leaching stage (the unit reported in green colour was studied in a previous study33), (B) photodeposition stage and recovery of metallic palladium (the units reported in green colour were analysed in a previous study40), (C) combined process with recycling (studied in the current work). Leaching and photodeposition units (red dashed box); photodeposition unit and preparation of the photocatalyst (black dashed box). | ||
Table 1 shows the theoretical composition of the spent streams generated within the two processes, calculated according to the indications reported in previous papers33,40 when the total recovery of palladium was achieved starting from a synthetic waste containing about 100 milligrams of the metal. It is evident from the scheme in Fig. 2(B) that the streams (3) and (4) have to be considered as waste for which a proper treatment has to be arranged to allow their disposal. However, as shown in the Table 1, despite the presence of ethanol, being stream (3) mainly composed of chloride and copper species, it could be possible to reuse it in a new leaching stage, thus reducing the produced wastes. Meanwhile, stream (4), coming from the dissolution of zinc oxide and containing zinc ions and nitrates, could be used to newly synthesize fresh zinc oxide particles to be recycled and used as a photocatalyst in the photodeposition stage.
| Species | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| Ethanol (M) | — | — | ≈1.7 | — |
| Cu2+ (mM) | 2.5 | — | — | — |
| Cu+ (mM) | — | 2.5 | 2.5 | — |
| Cl− (M) | 4.0 | 4.0 | 4.0 | — |
| Na+ (M) | 4.0 | 4.0 | 4.0 | — |
| Zn2+ (mM) | — | — | — | 30.0 |
| NO3− (mM) | — | — | — | 60.0 |
| H2O (l) | 1 | 1 | 1 | 1 |
According to the facts reported above, the present work evaluates the possibility of effectively recovering palladium from spent catalysts containing it, combining processes A and B (Fig. 2) in a single two-stage process (leaching + photodeposition) in which streams (3) and (4) are recycled from the outlet of the photoreactor to the leaching unit, focusing the attention on the influence of the recycled streams on the kinetics and yield of palladium recovery. In particular, the effect of ethanol (used as a sacrificial agent) on the leaching process is analysed, along with the need to re-oxidize the Cu(I) ions produced during the leaching stage and the makeup of these species required at the end of the sequence of the two stages to maintain a fixed recovery efficiency in the successive treatments. Finally, a procedure is developed for the preparation of ZnO photocatalyst particles from exhaust effluents – generated during the photodeposition stage – and tested as an effective way of recycling zinc in the overall process.
3. Materials and methods
3.1. Materials
Copper chloride (CuCl2, powder, purity = 99%), sodium chloride (NaCl, purity ≥99%), zinc oxide (ZnO, nanopowder, <50 nm particle size (BET), purity >97% – used as a reference), palladium chloride (PdCl2, purity = 99%), titanium dioxide nanopowder (TiO2, commercial grade, Aeroxide P25, average particle size 21 nm, specific surface area 50 ± 15 m2 g−1, 80/20 anatase/rutile), metallic palladium (Pd, powder, <1 μm, purity ≥99.9% – used as a reference), zinc acetate dihydrate (ZAD, purity ≥99.0%) and triethylamine (TEA, purity ≥99.0%) were purchased from Aldrich Chemistry. Ethanol (EtOH, purity ≥99.8%) was purchased from Fluka. Sodium hydroxide pellets (NaOH), hydrochloric acid (HCl, 37%), and nitric acid (HNO3, 69%) were purchased from AnalaR BDH. Doubly glass-distilled water was used throughout this study.3.2. Experimental procedures
In the current work, ZnO nanoparticles were synthesized following different procedures:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 15 min and washed 3 times with double-distilled water.
500 rpm for 15 min and washed 3 times with double-distilled water.
4. Results and discussion
Starting from the simplified schematics of processes A and B reported in Fig. 2 and moving towards a combining scheme (C in the same figure) some problems may be expected due to a different composition of the streams concerning those obtained when leaching and photodeposition were carried out as separate processes. In the following subsections, the effects of this difference in the composition of the streams will be analysed in detail.4.1. Leaching process
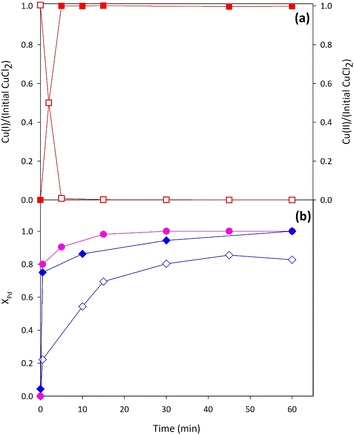
Following the approach described above, it can be easily understood that the leaching stage requires evaluating (i) the effect of the presence of ethanol and (ii) the effect of the presence of cuprous ions (instead of cupric ones). | (r.1) |
Moreover, an evaluation of the total copper and chloride amounts eventually lost during the separation and the washing of the solid phase from the suspension (about 15% with respect to the feed) allowed establishment of the suitable makeup of these species at the leaching unit.
4.2. Photodeposition process
In the previous paper,40 the recovery of palladium coming from the leaching solution through a photodeposition process was proposed, focusing attention on the effect of the radiation intensity, the sacrificial agent concentration, and the catalyst load. Moreover, considering the properties of ZnO to dissolve at certain pH values, an assessment of the best choice of the suspension pH was performed. In the next subsections, the influence of some operative variables on the photodeposition stage has been investigated, focusing on the possibility to recover the solutions as well as the photocatalytic material used.The behaviour of the different materials was analysed, focusing on (i) the photocatalytic activity and (ii) the preparation methods.
In Fig. 6, the palladium evolution versus reaction time was reported in the presence of different zinc oxide photocatalysts. As clearly shown by the trend lines, the highest photocatalytic reactivity was recorded in the presence of air calcined photocatalysts. All the materials prepared through the sol–gel method highlighted a trend typical for such processes, indicating an adsorption phenomenon at the initial stage of the photodeposition, as reported also for the benchmark photocatalyst (commercial ZnO). Moreover, the increase of the calcination temperature from 200 °C to 500 °C appears to have only a small influence on the photocatalytic deposition of palladium. As for the ZnO photocatalyst prepared through hydrothermal synthesis, a lower activity was recorded, despite a complete photoreduction observed after 80 minutes of irradiation. The lower reactivity can be probably ascribed to the different morphology of the material, for which initial adsorption was not observed.
It is clear that, in the latter case, the dissolution of zinc oxide – proposed in section 2 to recover the metallic Pd by adjusting the pH of the suspension below 6.30 – should be performed with acetic acid, thus obtaining a fresh precursor for the hydrothermal synthesis. This alternative preparation method was herein proposed because, from an environmental point of view, the use of organic acids might be preferred to that of nitric acid. Nevertheless, based on the above-reported considerations on the photocatalytic activity, the material prepared through the sol–gel method and calcination in air at a temperature of 200 °C was chosen as a photocatalyst for further deposition of palladium.
4.3. Materials characterization
In this subsection, a thorough characterization of the best photocatalyst tested (ZnO–S air 200 °C) will be analysed and discussed, along with the recovered Pd/ZnO composite, the recovered metal, and the newly prepared and recycled photocatalysts. As a comparison, the characterization of the ZnO nanoparticles prepared through the solvothermal approach (ZnO–H) will be investigated.Estimated Eg values are collected in Table S1.†Fig. 7 shows the [F(R)hν]2 function against photon energy for all the fresh photocatalysts. Tauc-plot linearization (inset in Fig. 7) points out that the 3.23 eV band gap is estimated for ZnO–S air 200 °C and a very similar value was obtained for ZnO–H, confirming the value found by Vitiello et al.43 for ZnO nanoparticles produced through a similar hydrothermal route. This Eg is associated with the direct transition between the O2p–Zn3d valence band and the Zn4s–Zn4p conduction band.46,47 A minor enhancement of the band gap is detected when calcination is carried out at a higher temperature (ZnO–S air 500 °C) or under a N2 flux (ZnO–S N2 200 °C). Similarly, Eg rises for photocatalysts obtained through single (ZnO–S air 200 °C-II) and double (ZnO–S air 200 °C-III) recycling of stream (4) with respect to freshly prepared ZnO–S air 200 °C (Fig. 8). As for commercial ZnO, it exhibits the lowest band gap, equal to 3.18 eV and significantly narrower than those reported in the literature.48–50 This could be due to the important Al doping (6%) of the reference materials used, which cause defects in the wurtzite lattice with the consequent shrinking of the band gap for Al doping higher than 1.5%.51
In Fig. 9, XRD diffraction patterns are reported for different materials (commercial ZnO and commercial Pd), ZnO prepared through the sol–gel method (ZnO–S air 200 °C), ZnO newly prepared after one (ZnO–S air 200 °C I) and two dissolutions (ZnO–S air 200 °C II), the Pd/ZnO composite material (Pd/ZnO (S air 200 °C)) and the recovered Pd. The peaks in all ZnO spectra can be indexed to the hexagonal phase of ZnO (wurtzite) and the face-centered cubic phase for Pd. The ZnO and Pd peaks agreed well with the reported literature diffraction peak values for ZnO (JCPDS no. 36-1451) and Pd (JCPDS no. 05-0681). In the case of the sol–gel synthesized ZnO, additional peaks were observed, indicating the likely presence of impurities, while the shifting in the diffraction peaks of the ZnO sample is typical for the home-prepared materials. The presence of metallic palladium was confirmed by the Pd/ZnO spectra, in which a peak corresponding to Pd(111) was observed. The crystallite size and the crystallinity of the sol–gel synthesized ZnO are larger than that of the commercial sample. The crystallite size and the crystallinity of the recovered Pd are smaller than that of the commercial sample. Hydrothermal synthesis conditions did not result in any changes in the diffraction pattern of ZnO–H if compared to the other presented photocatalysts (Fig. S1†), confirming the formation of a proper wurtzite lattice (JCPDS no. 36-1451).
The comparison between the SEM images of the commercial ZnO and that prepared through sol–gel synthesis, reported in Fig. 10, demonstrated a smaller size of agglomerates (ca. 100 nm), mostly spherical-like in shape in the first case, while the home-prepared samples show a larger size of agglomerates (ca. 500 nm), with shapes varying from spherical-like to plate-like to rod-like. The newly prepared samples (ZnO–S air 200 °C I and II) show more agglomerates than the fresh sample (ZnO–S air 200 °C), mostly spherical-like and plate-like in shape (see Fig. S2†). As concerns the Pd/ZnO sample, recovered after the photocatalytic deposition of palladium (see Fig. S3†), the size of the agglomerates (ca. 50 nm) is much smaller than that of the ZnO sample (ZnO–S air 200 °C), with a morphology more homogeneous than that of ZnO–S air 200 °C and most particle agglomerates are spherical-like in shape.
 | ||
| Fig. 10 SEM images of commercial ZnO (A and B) and ZnO samples (ZnO–S air 200 °C (C and D)) prepared through sol–gel synthesis. | ||
Moreover, in Fig. 11, the TEM micrographs of the recovered palladium are reported. As clearly shown by the figure, the recovered metal shows particle sizes ranging from 2 to 5 nm (see Fig. S4†).
Concerning the morphology of the ZnO samples, micrographs for the ZnO–H sample (Fig. 12A and B) display the presence of rod-like nanostructures with a diameter of about 10 nm and a heterogeneous length distribution ranging from 10 to 150 nm (see Fig. S5†). An alkaline pH allows for the enlargement of ZnO particles due to the migration of ZnO22− anions from the hydro-alcoholic reaction mixture to primary ZnO nuclei. Moreover, the anisotropic shape of rods is attributed to the presence of TEA, which acts as a chelating agent for Zn2+ cations, preventing ZnO nanostructures from radial expansion.52 Furthermore, micrographs of ZnO–S air 200 °C (Fig. 12C and D) show the presence of shapes varying from spherical-like to hexagonal-like to rod-like, with a larger size of particles (50–500 nm). The different morphology observed for ZnO–H and ZnO–S air 200 °C may be related to the different reactivity recorded in the presence of the two different photocatalysts, as reported in the previous section (see Fig. 6). Finally, the comparison of the TEM micrographs of the fresh ZnO (Fig. 12C and D) to the newly prepared samples (Fig. 12E–H, obtained through sol–gel synthesis) shows that the morphology of sol–gel ZnO particles did not change after being recycled, while the size of particles seems to decrease after every regeneration cycle.
4.4. Photocatalyst recycling
The chosen material was used to evaluate the possibility of effectively reusing the solution containing zinc ions and nitrates for the newly prepared photocatalyst. As clearly shown in Fig. 13, the photocatalytic activity in the presence of the newly prepared materials is comparable to that obtained with the fresh sample, thus indicating the possibility to recycle the stream (4). The comparison between the XRD patterns of the fresh samples and the samples prepared from zinc recovery (see Fig. 8) indicates a similar crystalline structure of the three materials, thus confirming the effectiveness of this choice, despite some impurities being observed in the fresh sample, which are probably not fully eliminated during the washing procedure. The moderate reduction in the photodeposition rate may be ascribed to the higher agglomeration of the particles of the newly prepared samples with respect to the fresh material, as clearly indicated in the SEM images reported in Fig. 9 and previously discussed.4.5. Reuse of the leaching solution
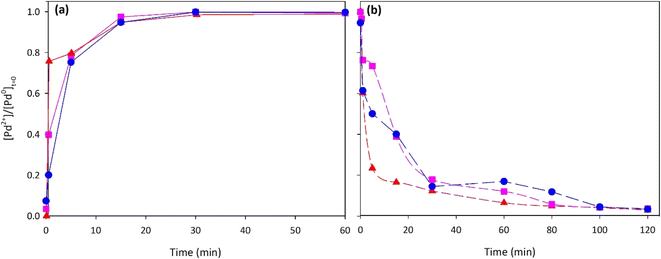
To conclude this investigation on the possibility to combine the leaching and photocatalytic deposition processes of palladium, some experimental runs were performed with more than one recycling of the solution coming from the photocatalytic unit. Starting from a spent Pd0/TiO2 catalyst, the leaching stage was carried out; then, the best-tested photocatalyst was used for the photodeposition (ZnO–S air 200 °C). Once the material Pd/ZnO has been separated, the solution from the photodeposition process has been treated with airflow, as the presence of cuprous ions requires the oxidation of copper(I) to copper(II), the latter being the active species in the leaching process. A second (and third) aliquot of the spent Pd0/TiO2 catalyst was thus used to carry out the successive leaching and photodeposition stages. In Fig. 14 the results (in terms of Pd2+ evolution vs. time, with respect to the initial Pd0) in the single “two-stage process” were reported. As indicated in the diagrams, regarding the leaching process, the effectiveness to recirculate the solution coming from the photodeposition stage was confirmed, obtaining the complete oxidation of palladium in about 15 minutes; moreover, in all photocatalytic stages, the photodeposition of palladium occurred within 120 minutes of irradiation.5. Proposed mechanism
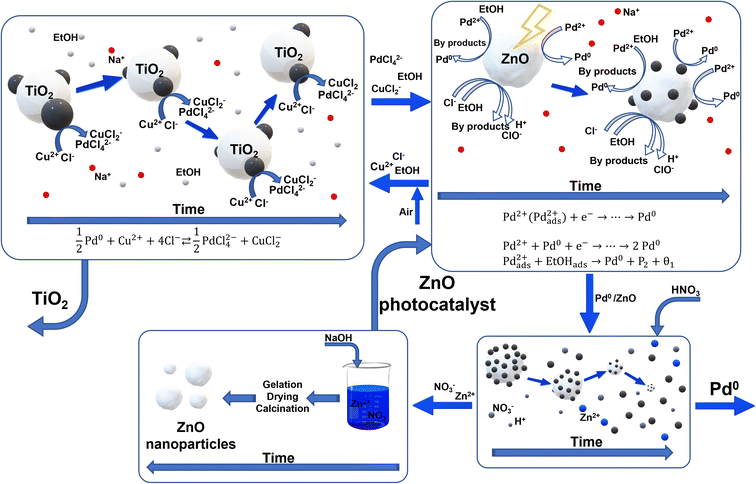
Fig. 15 shows the schematic illustration of the mechanism of the main stages of the proposed process. In the first block, the leaching of palladium occurs through the reaction (r.1); as previously discussed,33 the volume of the zero-valent palladium particles progressively decreases with time, as a result of the leaching reaction taking place at the palladium/solution interface. Then, the palladium ions, obtained from the leaching stage, can react under UV-visible light irradiation in the presence of ZnO nanoparticles (prepared through sol–gel synthesis): in particular, as discussed in a previous article40 and reported in Fig. 15, palladium ions can be reduced to metallic Pd through the reaction with the photogenerated electrons. Moreover, the formation of the metal catalyzes further palladium reduction. Finally, ethanol adsorbed on the catalyst surface may react with the adsorbed palladium, producing again metallic Pd and by-products.Conclusions
The recovery of palladium from spent catalysts containing palladium by combining leaching and photodeposition processes was investigated, focusing attention on the influence of the recycled streams on the kinetics and yield of palladium recovery. In particular, the presence of ethanol in the recirculated stream seems to not influence the leaching process, possibly because it does not react with the present species, and the recovery efficiency was not modified after varying the make-up of ethanol, probably due to the high concentration of the sacrificial species in a single photocatalytic step. Different home-prepared ZnO photocatalysts were synthesized and tested, focusing on (i) the photocatalytic activity and (ii) the preparation methods. Based on the results obtained, ZnO nanoparticles prepared through sol–gel synthesis, calcined at 200 °C, are suggested to be suitable for the recovery of palladium. The proposed procedure for the preparation of ZnO photocatalyst particles may be considered as an effective way of recycling zinc within the overall process. The effective combination of the leaching and photocatalytic deposition processes was confirmed, obtaining the complete oxidation of palladium after 15 minutes and the total photocatalytic deposition of palladium within 120 minutes of irradiation. Despite a mere comparison between the proposed process and those reported in the literature being difficult, the use of mild acidic conditions and the possibility to effectively reuse the solution containing zinc ions and nitrates to newly prepare photocatalyst make the process potentially attractive in the metal recovery field.Conflicts of interest
There are no conflicts to declare.References
- J. Spooren, K. Binnemans, J. Björkmalm, K. Breemersch, Y. Dams, K. Folens, M. González-Moya, L. Horckmans, K. Komnitsas, W. Kurylak, M. Lopez, J. Mäkinen, S. Onisei, K. Oorts, A. Peys, G. Pietek, Y. Pontikes, R. Snellings, M. Tripiana, J. Varia, K. Willquist, L. Yurramendi and P. Kinnunen, Resour., Conserv. Recycl., 2020, 160, 104919 CrossRef.
- D. Giurco, A. Littleboy, T. Boyle, J. Fyfe and S. White, Resources, 2014, 3, 432–453 CrossRef.
- Y. Kalmykova, M. Sadagopan and L. Rosado, Resour., Conserv. Recycl., 2018, 135, 190–201 CrossRef.
- C. Hagelüken, J. U. Lee-Shin, A. Carpentier and C. Heron, Recycling, 2016, 1, 242–253 CrossRef.
- M. Jackson, A. Lederwasch and D. Giurco, Resources, 2014, 3, 516–543 CrossRef.
- D. I. Kim, G. Gwak, P. Dorji, D. He, S. Phuntsho, S. Hong and H. Shon, ACS Sustainable Chem. Eng., 2018, 6(2), 1692–1701 CrossRef CAS.
- J. Matthey, PGM market report May 2021, 2021, pp. 1–48 Search PubMed.
- C. Saguru, S. Ndlovu and D. Moropeng, Hydrometallurgy, 2018, 182, 44–56 CrossRef CAS.
- Y. Lu and Z. Xu, Resour., Conserv. Recycl., 2016, 113, 28–39 CrossRef.
- I. Saldan, Y. Semenyuk, I. Marchuk and O. Reshetnyak, J. Mater. Sci., 2015, 50, 2337–2354 CrossRef CAS.
- Y. Li, C. Zhang, J. Ma, M. Chen, H. Deng and H. He, Appl. Catal., B, 2017, 217, 560–569 CrossRef CAS.
- O. F. Aldosari, S. Iqbal, P. J. Miedziak, G. L. Brett, D. R. Jones, X. Liu, J. K. Edwards, D. J. Morgan, D. K. Knight and G. J. Hutchings, Catal. Sci. Technol., 2016, 6, 234–242 RSC.
- W. Liang, X. Du, Y. Zhu, S. Ren and J. Li, Catalysts, 2020, 10(3), 347 CrossRef CAS.
- B. Tapin, F. Epron, C. Especel, B. K. Ly, C. Pinel and M. Besson, ACS Catal., 2013, 3, 2327–2335 CrossRef CAS.
- N. Florin, B. Madden, S. Sharpe, S. Benn, R. Agarwal, R. Perey and D. Giurco, Shifting Business Models for a Circular Economy: Metals Management for Multi-Product-Use Cycles, 2015, vol. 10 Search PubMed.
- Y. K. Taninouchi and T. H. Okabe, Metall. Mater. Trans. B, 2018, 49, 1781–1793 CrossRef CAS.
- M. H. Morcali, Resour., Conserv. Recycl., 2020, 159, 104891 CrossRef.
- H. Li, J. Eksteen and E. Oraby, Resour., Conserv. Recycl., 2018, 139, 122–139 CrossRef.
- C. H. Kim, S. I. Woo and S. H. Jeon, Ind. Eng. Chem. Res., 2000, 39, 1185–1192 CrossRef CAS.
- D. Xun, H. Hao, X. Sun, Z. Liu and F. Zhao, J. Cleaner Prod., 2020, 266, 121942 CrossRef.
- Y. Ding, S. Zhang, B. Liu, H. Zheng, C. C. Chang and C. Ekberg, Resour., Conserv. Recycl., 2019, 141, 284–298 CrossRef.
- A. M. Yousif, J. Chem., 2019, 1–7 Search PubMed.
- D. Bourgeois, V. Lacanau, R. Mastretta, C. Contino-Pépin and D. Meyer, Hydrometallurgy, 2020, 191, 105241 CrossRef CAS.
- V. T. Nguyen, S. Riaño, E. Aktan, C. Deferm, J. Fransaer and K. Binnemans, ACS Sustainable Chem. Eng., 2021, 9, 337–350 CrossRef CAS.
- Y. Chen, M. Xu, J. Wen, Y. Wan, Q. Zhao, X. Cao, Y. Ding, Z. L. Wang, H. Li and Z. Bian, Nat. Sustain., 2021, 4, 618–626 CrossRef.
- S. E. Can Sener, V. M. Thomas, D. E. Hogan, R. M. Maier, M. Carbajales-Dale, M. D. Barton, T. Karanfil, J. C. Crittenden and G. L. Amy, ACS Sustainable Chem. Eng., 2021, 9, 11616–11634 CrossRef CAS PubMed.
- D. Fontana, M. Pietrantonio, S. Pucciarmati, G. Nadia and T. Chiara, J. Mater. Cycles Waste Manage., 2018, 20, 1199–1206 CrossRef CAS.
- Y. Ding, H. Zheng, B. Liu and C. Ekberg, Materials, 2019, 12(8), 1205 CrossRef CAS PubMed.
- C. A. Nogueira, A. P. Paiva, M. C. Costa and A. M. R. da Costa, Environ. Technol., 2019, 1–11 CAS.
- M. K. Jha, J. C. Lee, M. S. Kim, J. Jeong, B. S. Kim and V. Kumar, Hydrometallurgy, 2013, 133, 23–32 CrossRef CAS.
- M. A. Barakat, G. A. EL-Mahdy, M. Hegazy and F. Zahran, Open Miner. Process. J., 2009, 2, 31–36 CrossRef CAS.
- U. Jadhav and H. Hocheng, Sci. Rep., 2015, 5, 1–10 Search PubMed.
- M. Muscetta, N. Minichino, R. Marotta, R. Andreozzi and I. Di, J. Hazard. Mater., 2021, 404, 124184 CrossRef CAS PubMed.
- A. Behnamfard, M. M. Salarirad and F. Veglio, Waste Manage., 2013, 33, 2354–2363 CrossRef CAS PubMed.
- D. Bourgeois, V. Lacanau, R. Mastretta, C. Contino-Pépin and D. Meyer, Hydrometallurgy, 2020, 191, 105241 CrossRef CAS.
- J. Y. Lee, B. Raju, B. N. Kumar, J. R. Kumar, H. K. Park and B. R. Reddy, Sep. Purif. Technol., 2010, 73, 213–218 CrossRef CAS.
- Ş. Sarioǧlan, Platinum Met. Rev., 2013, 57, 289–296 CrossRef.
- Z. Zhang and F. S. Zhang, J. Hazard. Mater., 2014, 279, 46–51 CrossRef CAS PubMed.
- Y. Chen, Q. Qiao, J. Cao, H. Li and Z. Bian, Joule, 2021, 5, 3097–3115 CrossRef CAS.
- M. Muscetta, R. Andreozzi, R. Marotta and I. di Somma, J. Environ. Chem. Eng., 2021, 9, 106523 CrossRef CAS.
- M. Muscetta, R. Andreozzi, R. Marotta and I. di Somma, J. Environ. Chem. Eng., 2021, 9, 106523 CrossRef CAS.
- A. Sangeetha, S. Jaya Seeli, K. P. Bhuvana, M. A. Kader and S. K. Nayak, J. Sol-Gel Sci. Technol., 2019, 91, 261–272 CrossRef CAS.
- G. Vitiello, G. Iervolino, C. Imparato, I. Rea, F. Borbone, L. de Stefano, A. Aronne and V. Vaiano, Sci. Total Environ., 2021, 762, 143066 CrossRef CAS PubMed.
- M. Muscetta, L. Clarizia, C. Garlisi, G. Palmisano, R. Marotta, R. Andreozzi, F. Ii, I. Chimica and P. Industriale, Int. J. Hydrogen Energy, 2020, 51, 26701–26715 CrossRef.
- A. R. Gahler, Bunseki Kagaku, 1954, 26, 577–579 CAS.
- M. Topsakal, S. Cahangirov, E. Bekaroglu and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 235119 CrossRef.
- Y. J. Choi, K. M. Kang and H. H. Park, Sol. Energy Mater. Sol. Cells, 2015, 132, 403–409 CrossRef CAS.
- J. Vishwakarma, B. Sabu, K. Bhotkar, S. Mehta and H. Muthurajan, Int. J. Chem. Phys. Sci., 2015, 5, 28–35 Search PubMed.
- F. K. Shan and Y. S. Yu, J. Eur. Ceram. Soc., 2004, 24, 1869–1872 CrossRef CAS.
- S. T. Tan, B. J. Chen, X. W. Sun, W. J. Fan, H. S. Kwok, X. H. Zhang and S. J. Chua, J. Appl. Phys., 2005, 98, 013505 CrossRef.
- R. Chen, P. Zhu, L. Deng, T. Zhao, R. Sun and C. Wong, ChemPlusChem, 2014, 79, 743–750 CrossRef CAS.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc., 2003, 125, 4430–4431 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2re00240j |
| This journal is © The Royal Society of Chemistry 2023 |