Open Access Article
Open Access ArticleA domino reaction for the synthesis of pyrrolo[2,1-a]isoquinolines from 2-aryl-pyrrolidines and alkynes promoted by a four-component catalytic system under aerobic conditions†
Zheng Luoab,
Huayou Hu *a,
Chao Wangb,
Zhen Yang
*a,
Chao Wangb,
Zhen Yang cd and
Yefei Wangcd
cd and
Yefei Wangcd
aJiangsu Key Laboratory for Chemistry of Low-Dimensional Materials, School of Chemistry and Chemical Engineering, Huaiyin Normal University, Huaian 223300, P. R. China. E-mail: HuayouHu@HYTC.EDU.CN
bSchool of Material Science and Engineering, Southwest University of Science and Technology, Mianyang 621010, P. R. China
cKey Laboratory of Unconventional Oil & Gas Development, China University of Petroleum (East China), Qingdao 266580, P. R. China
dShandong Key Laboratory of Oilfield Chemistry, School of Petroleum Engineering, China University of Petroleum (East China), Qingdao 266580, P. R. China
First published on 6th December 2023
Abstract
Pyrrolo[2,1-a]isoquinoline derivatives were synthesized from 2-aryl-pyrrolidines and alkynes via an oxidative dehydrogenation/cyclization coupling/dehydrogenative aromatization domino process. This reaction was promoted by a four-component catalytic system which included [RuCl2(p-cymene)]2, CuCl, copper acetate monohydrate and TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) under aerobic conditions.
In recent decades, domino reactions have received widespread attention and have been applied in the construction of complex organic molecules, such as materials, drugs, and other molecules.1 The advantages of domino reactions include the ability to construct complex molecules from easily available raw materials without the need to separate intermediates, which saves time and cost greatly. Recently, a large number of review articles on domino reactions have been published.2 However, developing more efficient and green domino reactions remains one of the challenges currently faced by organic chemists.
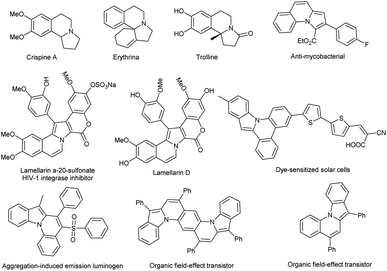
Pyrrolo[2,1-a]isoquinoline is a skeleton of diverse natural alkaloids whose biological activities were discovered by Mikhailovskii and Shklyaev in 1997.3 These biological activities include antitumor, antiviral, anticancer, anti-HIV, antibacterial, antidepressant etc.3,4 Furthermore, their excellent optoelectronic performance has also attracted the attention of material scientists in recent years (Fig. 1).5
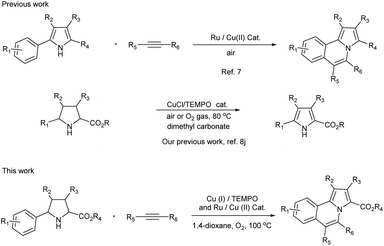
Therefore, the development of new synthetic methods for pyrrolo[2,1-a]isoquinoline is still a hot topic in organic chemistry.6 In most cases, 2-aryl-indoles were used as the starting materials to synthesize pyrrolo[2,1-a]isoquinolines. However, 2-aryl-pyrroles were rarely reported as a starting material due to lack of availability.7 As we known, pyrroles could be synthesized from pyrrolines through oxidative dehydrogenative aromatization reaction.8 Therefore, the development of a domino reaction which using the easily available 2-aryl-pyrrolidines9 as starting materials instead of 2-aryl-pyrroles to synthesize pyrrolo[2,1-a]isoquinolines would be very attractive.
Herein, a domino reaction for the synthesis of pyrrolo[2,1-a]isoquinolines from 2-aryl-pyrrolidines and alkynes under aerobic oxidation conditions was reported (Scheme 1).
In order to optimize the reaction conditions, we chose 5-phenylpyrrolidine-2,3,4-tricarboxylate (1a) and diphenylacetylene (2a) as the starting materials. The best isolated yield (86%) was achieved under the standard reaction conditions: 1a (0.20 mmol), 2a (0.26 mmol), CuCl (10 mol%), TEMPO (30 mol%), copper acetate monohydrate (10 mol%), [RuCl2(p-cymene)]2 (5 mol%) and 1,4-dioxane (1.0 mL) as the solvent under O2 atmosphere (balloon) at 100 °C for 18 h (Table 1, entry 8). Using other solvent instead of 1,4-dioxane significantly reduce the reaction yields (entry 1, see details in ESI†). Using 20% copper acetate monohydrate as the catalyst without any CuCl added reduce the yield of 3a to 74% (entry 2). Surprisingly, when using 20% CuCl as the catalyst and without any copper acetate monohydrate added, 4 was isolated in 54% yield and no any 3a was formed (entry 3). Also no 3a was formed and 4 was isolated in 90% yield when no any [RuCl2(p-cymene)]2 was added (entry 4). 3a and 4 were isolated in 8% yield and 33% yield without TEMPO (entry 5). Reducing the amount of TEMPO from 30% to 20% and prolonging the reaction time to 36 h would greatly reduce the yield of 3a to 24% (entry 6). 3a and 4 were isolated in 22% and 24% yields respectively when the reaction was running under air instead of oxygen gas (entry 7).
| Entry | Variation from the standard condition | Yielda (%) |
|---|---|---|
| a Isolated yield of 3a.b Isolated yield of 4.c 36 h. | ||
| 1 | Other solvent instead of 1,4-dioxane | 59–68 |
| 2 | With 20 mol% Cu(OAc)2·H2O without CuCl | 74 |
| 3 | With 20 mol% CuCl without Cu(OAc)2·H2O | (54b) |
| 4 | Without [RuCl2(p-cymene)]2 | (90b) |
| 5 | Without TEMPO | 8 + (33b) |
| 6 | With 20 mol% TEMPO | 24c |
| 7 | Replace O2 with air | 22 + (24b) |
| 8 | No | 86 |
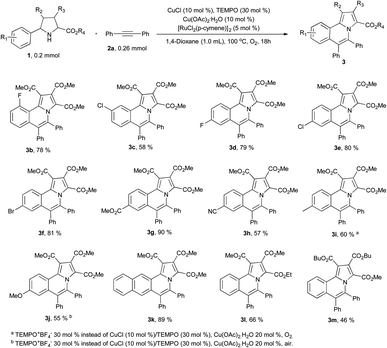
With the optimized conditions in hand, we firstly studied the scope of pyrrolidines (Scheme 2). When pyrrolidines substituted with electron-withdrawing groups on the phenyl ring were used as the starting materials, the corresponding products (3b–3h) were isolated in moderate to excellent yields (57–90%). Only a single isomer 3c was isolated in 58% yield due to steric effect when there was substituent in the meta position of the phenyl ring. However, when electron donating groups were bearing in the phenyl ring of pyrrolidines, the corresponding products were isolated in extremely low yields under the optimized conditions. Further optimization of the reaction conditions revealed that using TEMPO+BF4− (30%) as the catalyst to replace the CuCl and TEMPO and increasing the amount of copper acetate monohydrate to 20 mol%, 3i was isolated in 60% yield under O2 atmosphere and 3j was isolated in 55% yield under air. Only one isomer 3k was isolated in excellent yield (89%) when 2-naphthyl was bearing at the pyrrolidine. 3l and 3m also were isolated in 66% and 46% yields under standard reaction conditions respectively.
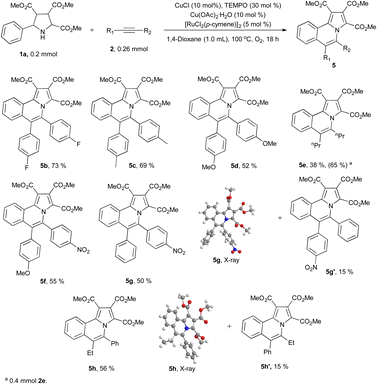
We next explored the substrate scopes of alkynes (Scheme 3). Diphenylacetylene derivatives with electron withdrawing or electron donating groups on the phenyl group could produce designed products (5b–5d) smoothly under standard reaction conditions. 4-Octyne (2e) also works well and the corresponding product 5e is isolated in 38% yield. When the amount of 2e is increased from 1.3 equivalent to 2.0 equivalent, the isolated yield of 5e also increased to 65%. Asymmetric alkynes show moderate region-selectivity (around 55% vs. 15%). The more electron deficient side of asymmetric alkyne tends to react with the nitrogen of pyrrolidine (5g vs. 5g′ and 5h vs. 5h′). The structure of 5g10 and 5h11 were confirmed by X-ray.
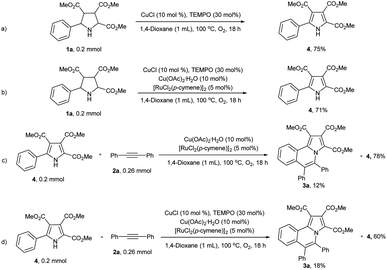
To reveal the reaction mechanism, a series of control experiments were conducted. When the alkyne was absence, pyrrolidines 1a was reacted under the standard reaction conditions and yield the corresponding pyrrole 4 in 71% yield (Scheme 4b). Which was similar to the yield (75%) in the absence of copper acetate monohydrate and [RuCl2(p-cymene)]2 (Scheme 4a). To our surprise, when pyrrole 4 reacted with diphenylacetylene (2a) under standard reaction conditions, 3a was only isolated in 18% yield and 60% of 4 was remain untouched (Scheme 4d). The yield of 3a even lower (12%) in the absence of CuCl and TEMPO (Scheme 4c).
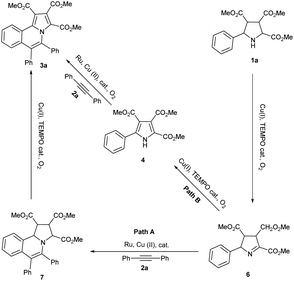
Based on the above results and our previous results, the mechanism of this domino reaction was proposed (Scheme 5). Under the catalytic system of Cu(I)/TEMPO/O2, pyrrolidine 1a was oxidized to the intermediate 6 via oxidative dehydrogenation firstly.12 Then 6 could transformed to the final product pyrrolo[2,1-a]isoquinoline 3a through two pathways. The major pathway is 6 reacted with alkyne 2a to produce intermediate 7 which was catalyzed by Ru and Cu(II). Then 7 was transformed to 3a through oxidative dehydrogenative aromatization which was catalyzed by Cu(I) and TEMPO with O2 as the terminal oxidant (path A).13 The minor pathway is that pyrrole 4 was formed firstly catalyzed by Cu(I) and TEMPO under oxygen gas. Then 4 reacted with alkyne 2a to form the final product 3a via Ru and Cu(II) catalyzed aerobic oxidative coupling reaction.7
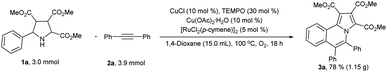
In order to demonstrate the practicality of the reaction, we successfully increased the reaction scale to gram-scale (Scheme 6). Under the standard reaction conditions, 1.15 grams of 3a was isolated in reasonable yield (78%) which was slightly lower than the yield of small scale reaction.
In summary, we have reported on the first of a domino reaction for the synthesis of pyrrolo[2,1-a]isoquinolines from 2-aryl-pyrrolidines and alkynes. This process was promoted by a four-component catalytic system which included [RuCl2(p-cymene)]2, CuCl, copper acetate monohydrate and TEMPO. Control experiments show all four catalysts were important for this reaction and pyrrole was not the key intermediate. Using oxygen gas as a solo oxidant made this transformation green. In short, this method of synthesizing pyrrolo[2,1-a]isoquinolines is green, simple, and practical, which may provide assistance for the application of such compounds in fields of biomedicine and materials.
Author contributions
Z. Luo and H. Hu conceived the project and performed the investigation on the scope. Z. Luo and Z. Yang optimized the reaction and prepared the experimental parts and first draft of the manuscript. H. Hu supervised the project, H. Hu, C. Wang and Y. Wang edited the manuscript and proofread the experimental part.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Key Projects of China National Key Research and Development Plan (2021YFE0107000) and the National Natural Science Foundation in China (Grant No. 52074339). Professor Zaichao Zhang (HYTC, Huaian, P. R. China) is acknowledged for the X-ray analysis.Notes and references
- (a) Domino Reactions: Concepts for Efficient Organic Synthesis, ed. L. F. Tietze, Wiley-VCH Verlag GmbH & Co. KGaA, 2014 Search PubMed; (b) L. F. Tietze, G. Brasche and K. M. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2006 CrossRef.
- For recent reviews: (a) H. Pellissier, Adv. Synth. Catal., 2023, 365, 620 CrossRef CAS; (b) A. Pounder, E. Neufeld, P. Myler and W. Tam, Beilstein J. Org. Chem., 2023, 19, 487 CrossRef CAS; (c) F. Doraghi, F. Mohaghegh, O. H. Qareaghaj, B. Larijani and M. Mahdavi, RSC Adv., 2023, 13, 13947 RSC.
- A. G. Mikhailovskii and V. S. Shklyaev, Chem. Heterocycl. Compd., 1997, 33, 243 CrossRef CAS.
- (a) Q. Zhang, G. Tu, Y. Zhao and T. Cheng, Tetrahedron, 2002, 58, 6795 CrossRef CAS; (b) R. Wang, X. Yang, C. Ma, S. Cai, J. Li and Y. Shoyama, Heterocycles, 2004, 63, 1443 CrossRef CAS; (c) B. Su, C. Cai, M. Deng, D. Liang, L. Wang and Q. Wang, Bioorg. Med. Chem. Lett., 2014, 24, 2881 CrossRef CAS PubMed; (d) C. Ballot, J. Kluza, A. Martoriati, U. Nyman, P. Formstecher, B. Joseph, C. Bailly and P. Marchetti, Mol. Cancer Ther., 2009, 8, 3307 CrossRef CAS PubMed; (e) M. V. R. Reddy, M. R. Rao, D. Rhodes, M. S. T. Hansen, K. Rubins, F. D. Bushman, Y. Venkateswarlu and D. J. Faulkner, J. Med. Chem., 1999, 42, 1901 CrossRef CAS; (f) S. Muthusaravanan, S. Perumal, P. Yogeeswari and D. Sriram, Tetrahedron Lett., 2010, 51, 6439 CrossRef CAS; (g) B. E. Maryanoff, J. L. Vaught, R. P. Shank, D. F. McComsey, M. J. Costanzo and S. O. Nortey, J. Med. Chem., 1990, 33, 2793 CrossRef CAS.
- (a) C. Baik, D. Kim, M. Kang, K. Song, S. O. Kang and J. Ko, Tetrahedron, 2009, 65, 5302 CrossRef CAS; (b) K. Sun, Y. Zhang, X. Chen, H. Su, Q. Peng, B. Yu, L. Qu and K. Li, ACS Appl. Bio Mater., 2020, 3, 505 CrossRef CAS; (c) E. Ahmed, A. L. Briseno, Y. Xia and S. A. Jenekhe, J. Am. Chem. Soc., 2008, 130, 1118 CrossRef CAS PubMed; (d) L. Zhu, E.-G. Kim, Y. Yi, E. Ahmed, S. A. Jenekhe, V. Coropceanu and J.-L. Brédas, J. Phys. Chem. C, 2010, 114, 20401 CrossRef CAS; (e) H. H. Choi, H. Najafov, N. Kharlamov, D. V. Kuznetsov, S. I. Didenko, K. Cho, A. L. Briseno and V. Podzorov, ACS Appl. Mater. Interfaces, 2017, 9, 34153 CrossRef CAS.
- (a) S. Kumarab and A. K. Singh, Chem. Commun., 2022, 58, 11268 RSC; (b) R. Michikita, Y. Usuki and T. Satoh, Eur. J. Org Chem., 2022, e202200550 CrossRef CAS; (c) D. Das and A. R. Das, J. Org. Chem., 2022, 87, 11443 CrossRef CAS PubMed; (d) R. Heckershoff, G. May, J. Däumer, L. Eberle, P. Krämer, F. Rominger, M. Rudolph, F. F. Mulks and A. S. K. Hashmi, Chem. – Eur. J., 2022, 28, e202201816 CrossRef CAS PubMed; (e) S. Kumarab and A. K. Singh, Chem. Commun., 2022, 58, 11268 RSC; (f) S. Thavaselvan and K. Parthasarathy, Org. Lett., 2020, 22, 3810 CrossRef CAS; (g) Y. Liu, Z. Yang, R. Chauvin, W. Fu, Z. Yao, L. Wang and X. Cui, Org. Lett., 2020, 22, 5140 CrossRef CAS PubMed; (h) A. Obata, A. Sasagawa, K. Yamazaki, Y. Ano and N. Chatani, Chem. Sci., 2019, 10, 3242 RSC; (i) K. Morimoto, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2010, 12, 2068 CrossRef CAS.
- (a) L. Ackermann, L. Wang and A. V. Lygin, Chem. Sci., 2012, 3, 177 RSC; (b) X. Tang, L. Huang, C. Qi, W. Wu and H. Jiang, Chem. Commun., 2013, 49, 9597 RSC.
- (a) A. Arrieta, D. Otaegui, A. Zubia, F. P. Cossío, A. Díaz-Ortiz, A. de la Hoz, M. A. Herrero, P. Prieto, C. Foces-Foces, J. L. Pizarro and M. I. Arriortua, J. Org. Chem., 2007, 72, 4313 CrossRef CAS; (b) T. Das, P. Saha and V. K. Singh, Org. Lett., 2015, 17, 5088 CrossRef CAS; (c) H. Liu, H. Tao, H. Cong and C. Wang, J. Org. Chem., 2016, 81, 3752 CrossRef CAS; (d) H. Liu, K. Liu, Z. Xue, Z. He and C. Wang, Org. Lett., 2015, 17, 5440 CrossRef CAS; (e) S. N. Murthy and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 4481 CrossRef; (f) I. Fejes, L. Toke, G. Blasko, M. Nyerges and C. S. Pak, Tetrahedron, 2000, 56, 8545 CrossRef CAS; (g) B. Oussaid, B. Garrigues and M. Soufiaoui, Can. J. Chem., 1994, 72, 2483 CrossRef CAS; (h) M. Baumann, I. R. Baxendale, A. Kirschning, S. V. Ley and J. Wegner, Heterocycles, 2011, 82, 1297 CAS; (i) Y. Liu, H. Hu, X. Wang, S. Zhi, Y. Kan and C. Wang, J. Org. Chem., 2017, 82, 4194 CrossRef CAS PubMed; (j) Z. Luo, Y. Liu, C. Wang, D. Fang, J. Zhou and H. Hu, Green Chem., 2019, 21, 4609 RSC.
- (a) A. Cayuelas, O. Larranaga, C. Najera, J. M. Sansano, A. de Cozar and F. P. Cossio, Tetrahedron, 2016, 72, 6043 CrossRef CAS; (b) E. M. Arpa, M. Gonzalez-Esguevillas, A. Pascual-Escudero, J. Adrio and J. C. Carretero, J. Org. Chem., 2016, 81, 6128 CrossRef CAS; (c) H. Xu, C. Golz, C. Strohmann, A. P. Antonchick and H. Waldmann, Angew. Chem., Int. Ed., 2016, 55, 7761 CrossRef CAS; (d) X. Bai, L. Li, Z. Xu, Z. Zheng, C. Xia, Y. Cui and L. Xu, Chem. – Eur. J., 2016, 22, 10399 CrossRef CAS PubMed; (e) Z. Zhang, B. Xu, S. Xu, H. Wu and J. Zhang, Angew. Chem., Int. Ed., 2016, 55, 6324 CrossRef CAS PubMed.
- CCDC no. 2299581.
- CCDC no. 2299583.
- H. Richter and O. G. Mancheño, Eur. J. Org Chem., 2010, 4460 CrossRef CAS.
- (a) J. Xu, H. Hu, Y. Liu, X. Wang, Y. Kan and C. Wang, Eur. J. Org Chem., 2017, 257 CrossRef; (b) F. Shi, Y. Zhang, Z. Lu, X. Zhu, W. Kan, X. Wang and H. Hu, Synthesis, 2016, 48, 413 CAS; (c) W. Wang, J. Han, J. Sun and Y. Liu, J. Org. Chem., 2017, 82, 2835 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2299581 and 2299583. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra07653a |
| This journal is © The Royal Society of Chemistry 2023 |