Open Access Article
Open Access ArticleSynthesis of P-stereogenic 1-phosphanorbornane-derived phosphine–phosphite ligands and application in asymmetric catalysis†‡
Kyzgaldak Ramazanova§
 a,
Soumyadeep Chakrabortty§
a,
Soumyadeep Chakrabortty§ b,
Bernd H. Müllerb,
Peter Lönnecke
b,
Bernd H. Müllerb,
Peter Lönnecke a,
Johannes G. de Vries
a,
Johannes G. de Vries *b and
Evamarie Hey-Hawkins
*b and
Evamarie Hey-Hawkins *a
*a
aInstitute of Inorganic Chemistry, Universität Leipzig, Johannisallee 29, D-04103 Leipzig, Germany. E-mail: hey@uni-leipzig.de
bLeibniz Institute for Catalysis e.V., Albert-Einstein-Strasse 29a, 18059 Rostock, Germany. E-mail: Johannes.deVries@catalysis.de
First published on 24th November 2023
Abstract
A convenient synthesis of enantiopure mixed donor phosphine–phosphite ligands has been developed incorporating P-stereogenic phosphanorbornane and axially chiral bisnaphthols into one ligand structure. The ligands were applied in Pd-catalyzed asymmetric allylic substitution of diphenylallyl acetate, Rh-catalyzed asymmetric hydroformylation of styrene and Rh-catalyzed asymmetric hydrogenation of an acetylated dehydroamino ester. Excellent branched selectivity was observed in the hydroformylation although low ee was found. Moderate ee's of up to 60% in allylic substitution and 50% in hydrogenation were obtained using bisnaphthol-derived ligands.
Introduction
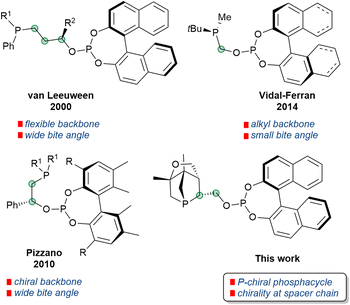
Phosphines have been the cornerstone of homogeneous catalysis for the last six decades.1 Especially, chiral phosphines are still the dominating class of ligands used in asymmetric catalysis.2,3 A plethora of P-stereogenic phosphines have already been reported; however, the search for better, more efficient and sustainable ligands is still ongoing as the topic remains demanding even in the present day. The discovery of the well-known cyclohexyl(o-anisyl)methylphosphine (CAMP) and (ethane-1,2-diyl)bis[(2-methoxyphenyl)(phenyl)phosphine] (DIPAMP) used in Rh-catalyzed asymmetric hydrogenation4 boosted the scientific interest in P-stereogenic5–9 (or P-chiral, P-chirogenic, P*-) ligands. Despite the challenging synthesis of P*- phosphines, a number of methods has been developed with pioneering contributions from Mislow,10 Knowles,4 Horner,11 Börner,12 Jugé,13 van Leeuwen and Kamer14 and many more.1,3 Very often resolution of secondary or tertiary phosphine oxides,15 use of bifunctional chiral auxiliaries ((−)-ephedrine or amino-alcohols),13 chiral base ((−)-sparteine) mediated enantioselective deprotonation,16 or chiral amine ((R/S)-1-phenylethylamine) as modifier17,18 are used for the preparation of P*-synthons. The P*-phosphine-borane adduct reported by Imamoto et al. has opened the way to a number of benchmark P*- phosphines which induce high enantioselectivity and can be used in various transition metal catalysed asymmetric transformations.19,20 However, in most cases, acyclic P*-synthons or ligands are used. Bidentate ligands based on P*- phosphacycles are scarce in the literature. At the same time, mixed donor bidentate phosphines are quite successful in many enantioselective transformations.21,22 The phosphine–phosphite ligand class developed by van Leeuwen et al.,23 Pizzano et al.,24 Vidal-Ferran et al.,25 and others were successfully applied in several hydrofunctionalization reactions (selected examples of ligands are shown in Fig. 1). Hey-Hawkins and co-workers reported the first phospha-aza-Diels–Alder cycloaddition reactions to give P*-phosphacycles (1-phospha-2-azanorbornenes)26 and could show that the products undergo P–N bond cleavage to afford racemic and enantiopure 1-alkoxy-2,3-dihydrophosphole derivatives27,28 or reduction to seven-membered phosphacycles.29 Moreover, in 2012 we reported an unprecedented asymmetric phospha-Diels–Alder reaction between 2H-phosphole and (5R)-(L-menthyloxy)-2(5H)-furanone (MOxF) to give P-stereogenic 1-phosphanorbornenes in high diastereoselectivity (Scheme 1).26 The diastereomers were separated via crystallization. Further functionalizations gave enantiomerically pure P*-1-phosphanorbornane alcohol30 (PNA; Scheme 1, 7) which was converted to silyl ethers via reaction with chlorosilanes. The resulting 1-phosphanorbornane silyl ethers were applied as ligands in asymmetric hydrogenations.31 We therefore envisioned that further functionalization of the P*-1-phosphanorbornane alcohol 7 could serve as the key step in the synthesis of various bidentate ligands.30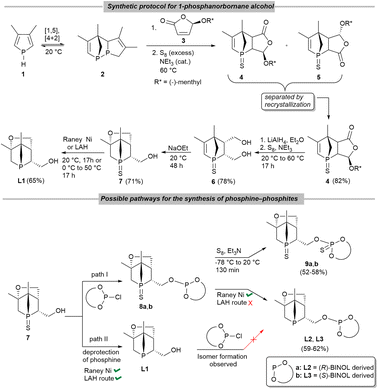 | ||
| Scheme 1 Synthetic protocol for 1-phosphanorbornane alcohol L1 (top) and pathway for the synthesis of phosphine–phosphite ligands 9a, b and L2, L3 (bottom). | ||
Herein, we report the synthesis of 1-phosphanorbornane-based P-stereogenic mixed donor bidentate ligands and their application in Pd-catalyzed asymmetric allylic substitution of benchmark diphenylallyl acetate, Rh-catalyzed asymmetric hydrogenation of methyl (Z)-2-acetamido-3-phenylacrylate and Rh-catalyzed asymmetric hydroformylation of styrene.
Results and discussion
Synthesis of mixed donor P-stereogenic phosphine–phosphites
The sulfur-protected P-stereogenic 1-phosphanorbornane alcohol (Scheme 1, 7) was readily synthesized following the reported procedure in good yields (ESI, Fig. S1‡).26,32,33 The unprotected compound L1 can be obtained by reaction of 7 with either excess of freshly activated Raney nickel (Raney Ni) or with 4–5 eq. of LiAlH4 at elevated temperatures for 17 h in THF (Scheme 1).For the synthesis of bidentate (R)- or (S)-BINOL-based mixed donor ligands from 7, two possible pathways were studied (Scheme 1, bottom). Path I involves the reaction of enantiopure BINOL-based chlorophosphites, which were prepared employing a general procedure for chlorophosphites,34 with sulfur-protected PNA 7 in the presence of a mild base (such as NEt3), followed by deprotection to give L2, L3. The mild reducing agent Raney Ni was used as desulfurizing reagent in this case in view of its high selectivity and facile work-up, as lithium aluminum hydride (LAH) also attacks the phosphite P(OR)3 motif in 8a, b. Path II started with deprotection of 7 to afford L1 by either Raney Ni or LAH, followed by reaction with the corresponding chlorophosphites. However, the 31P{1H} NMR spectrum of the reaction mixture showed several signals for unidentified products, rendering this pathway unsuitable.
P-stereogenic 1-phosphanorbornane alcohol L1 and the phosphine–phosphites L2, L3 were fully characterized by NMR spectroscopy and high-resolution mass spectrometry. In the 31P{1H} NMR spectra (CDCl3), L1 exhibits a singlet at −45.9 ppm, while two singlets are observed for L2 (135.4 and −44.7 ppm) and L3 (139.4 and −44.7 ppm) which are characteristic signals for phosphites and phosphines, respectively.
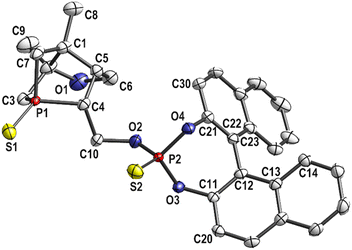
Intermediates 8a, b were sulfurized to obtain the double sulfur-protected air- and moisture-stable compounds 9a, b (Scheme 1, bottom). The compounds were isolated by slowly cooling down a hot iPrOH solution and storing at −25 °C overnight. The structures of 9a, b were confirmed by NMR spectroscopy and HRMS. Single crystals of 9b (S-protected analogue of L3) suitable for X-ray crystal structure determination were obtained by slow evaporation of solvent from an ethyl acetate solution of 9b; any other method resulted in formation of powder only due to rapid nucleation. Excellent chemical (98–100%) and optical purities of 9a, b were verified by chiral HPLC (ESI, Fig. S23 and S24‡), thus also indirectly confirming the optical purities of L2 and L3.
As shown in Fig. 2, both phosphorus atoms in compound 9b have a distorted tetrahedral environment. The P–O bond lengths of the phosphite moiety are in the range of 156.6–159.8 pm in agreement with the literature.35 Moreover, both P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond lengths (188.9 pm and 194.0 pm) are comparable to those reported previously.36
S bond lengths (188.9 pm and 194.0 pm) are comparable to those reported previously.36
Application of P-chiral ligands in asymmetric catalysis
Monodentate PNA L1 and bidentate BINOL-derived mixed donor phosphine–phosphites L2, L3 were tested in several benchmark transformations in asymmetric catalysis.The Pd-catalyzed enantioselective allylation37 is an important method for C–C or C–heteroatom bond formation, where hybrid bidentate phosphines (P,P′), bulky monodentate phosphines (mostly phosphoramidites) and P,N ligands (P, oxazoline) have been employed successfully. The PNA (L1) and phosphine–phosphite ligands (L2, L3) were tested in the Pd-catalyzed asymmetric allylic alkylation of diphenylallyl acetate using dimethyl malonate as C-based nucleophile. Low conversion of diphenylallyl acetate was observed employing monodentate PNA (L1) (Pd/L1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) resulting in only 15% ee (entry 1, Table 1). Higher conversions (up to 60% ee in CH2Cl2) were obtained with the BINOL-derived ligands L2 and L3 (entries 2 and 3, Table 1).
2) resulting in only 15% ee (entry 1, Table 1). Higher conversions (up to 60% ee in CH2Cl2) were obtained with the BINOL-derived ligands L2 and L3 (entries 2 and 3, Table 1).
As mixed donor phosphine–phosphite ligands have been used in the Rh-catalyzed asymmetric hydroformylation38 of styrene, we also tested ligands L1–L3 in this reaction. Excellent branch selectivity was observed for all ligands (Table 2), but poor ee values were obtained. The ee could be slightly improved by changing the Rh/L3 ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and increasing the syngas pressure to 30 bar. However, higher L3/Rh ratios did not afford higher ee values (Table 2).
2 and increasing the syngas pressure to 30 bar. However, higher L3/Rh ratios did not afford higher ee values (Table 2).
Ligands L1–L3 were also employed in the Rh-catalyzed asymmetric hydrogenation of the trisubstituted functionalized olefin methyl (Z)-2-acetamido-3-phenylacrylate as benchmark substrate (Table 3). Full conversion was achieved with [Rh(COD)2]BF4 and 2 eq. L1 in CH2Cl2, albeit no ee was observed. However, a slight increase in ee (up to 50%, entry 6, Table 3) was observed with L3 as bidentate ligand in THF.
Conclusions
The P-stereogenic enantiopure 1-phosphanorbornane-based mono- (L1) and bidentate ligands (L2, L3) combine different types of chiral information, namely P-chirality, backbone and axial chirality (for the BINOL derivatives). While monodentate L1 gave up to 15% ee in the Pd-catalyzed allylic substitution of diphenylallyl acetate with dimethyl malonate as nucleophile, the BINOL-based backbone in L2 and L3 indeed improved the activity and enantioselectivity up to 60%. High branched selectivity was observed in the Rh-catalyzed asymmetric hydroformylation of styrene for L1–L3, albeit poor ee was observed. In the Rh-catalyzed asymmetric hydrogenation of a dehydroamino ester 50% ee was obtained with the mixed donor phosphine–phosphite ligand L3 in THF. These results already show the potential of these ligands in asymmetric catalysis.Experimental details
General consideration
The synthetic work was conducted using standard Schlenk and glovebox techniques. All reagents were purchased from commercial sources and used as received, unless stated otherwise. (R)- and (S)-BINOL were bought from Merck and used as received. Compound 7 was prepared according to the literature.30 NEt3 and CDCl3 were dried over CaH2, distilled prior to use, and degassed with three freeze–pump–thaw cycles. THF was distilled from sodium/benzophenone and stored over activated 4 Å molecular sieves. Toluene and CH2Cl2 were bought from Acros Organics with 99.85% purity, <0.005% water content and stored under inert atmosphere over molecular sieves (3 Å). C6D6 was dried over Na/benzophenone and distilled prior to use. Samples for mass spectrometry were prepared in the glovebox and measured on a Finnigan MAT 95-XP (Thermo Electron) or Kratos MS-50 spectrometer in HRMS (ESI-TOF) mode. Fragment signals are given in mass per charge number (m/z). NMR spectra were recorded on Bruker Avance 300 (1H: 300.13, 13C: 75.46, 31P: 121.49 MHz), Bruker Fourier 300 (1H: 300.13, 13C: 75.46, 31P: 121.49 MHz) or Avance 400 (1H: 400.13, 13C: 100.63, 31P: 161.98 MHz) instruments operating at the denoted spectrometer frequency given in Megahertz (MHz) for the specified nucleus.Synthesis of phosphanorbornane alcohol (L1). LAH route: A suspension of 4–6 eq. of lithium aluminium hydride in 5 ml THF was slowly portion-wise transferred at 0 °C to a solution of 7 (100 mg, 0.43 mmol) in 2 ml THF. The reaction mixture was stirred for 10 min at 0 °C then warmed to room temperature. After stirring the mixture overnight, the excess of LAH was quenched with an aqueous KOH solution (20%, 5 ml) at 0 °C with vigorous stirring. After stirring for an additional 15 min at 0 °C, the reaction mixture was heated to 50 °C for 1 h (this compacts the precipitate and facilitates faster and better filtration in the next step). The precipitate is isolated by filtration and washed 2–3 times with 10 ml THF each. Then the solvent was removed under reduced pressure to give a light beige oil.
Raney nickel route. Raney nickel was activated and destroyed according to the literature.31 S-protected alcohol 7 (43 mg, 0.185 mmol) was added to a suspension of freshly activated Raney nickel (ca. 0.5 g, excess) in 2 ml THF and stirred for 17 h at room temperature. The clear solution was filtered and the black solid was washed four times with 3 ml THF each. The solution was concentrated to give 24 mg of L1 as a beige oil (65%).
1H NMR (400 MHz, CDCl3): δ 3.92–3.72 (m, 3H), 2.58–2.43 (m, 1H), 2.28–2.21 (m, 1H), 1.73–1.69 (m, 1H), 1.60–1.52 (m, 1H), 1.44–1.38 (m, 1H), 1.20 (s, 3H), 1.15 (s, 3H) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 88.9, 64.4, 61.3, 51.5, 45.0, 38.6, 36.7, 24.6, 17.2 ppm. 31P{1H} NMR (161 MHz, CDCl3): δ −45.9 (s) ppm. HRMS (ESI-TOF): m/z calculated for C10H18O2P: 201.2367 [M + H]+; observed 201.2249.
Synthesis of L2 and L3. Triethylamine (0.33 mmol) was added at −78 °C to a THF solution of (R)- or (S)-BINOL chlorophosphite (0.3 mmol). Optically pure sulfur-protected 7 (0.3 mmol, 70 mg) was dissolved in 10 ml THF and added dropwise to the NEt3–chlorophosphite mixture maintaining the low temperature. The reaction mixture was stirred at this temperature for 5 min and then warmed to room temperature and stirred overnight. The THF solution was filtered to remove the ammonium salt and the solvent evaporated under vacuum yielding an off-white solid. The solid was dissolved in 10 ml THF again and added to a THF suspension of freshly activated Raney Ni (1 g for 50 mg of 8a, b) and stirred for 25 h at room temperature. The THF solution was filtered off and the black solid was washed 2–3 times with THF (5 ml). The combined solutions were evaporated under vacuum yielding L2, L3 as off-white solid in 59–62% yield. The products were characterized by NMR spectroscopy and HRMS.
L2 (R-BINOL-derived): 1H NMR (400 MHz, CDCl3): δ 8.08–7.91 (m, 4H), 7.59–7.27 (m, 8H), 4.61–4.50 (m, 1H), 3.93–3.90 (m, 1H), 3.78–3.73 (m, 2H), 2.79–2.58 (m, 1H), 2.49–2.40 (m, 1H), 2.20–1.97 (m, 2H), 1.90–1.82 (m, 2H), 1.26 (s, 3H), 1.19 (s, 3H). 13C{1H} (75 MHz, CDCl3): δ 130.6, 128.5, 128.3, 128.0, 127.6, 127.2, 126.4, 125.2, 87.8, 68.1, 64.5, 45.9, 43.5, 38.5, 25.8, 24.7, 18.5. 31P{1H} NMR (122 MHz, CDCl3): δ 135.4 (s) ppm, −44.7 (s) ppm. HRMS (ESI-TOF): m/z calculated for C30H29O4P2: 515.1270 [M + H]+; observed 515.1255. EA: calcd. for C30H28O4P2: C: 70.05, H: 5.50. Found: C: 70.23, H: 5.47.
L3 (S-BINOL-derived): 1H NMR (300 MHz, CDCl3): δ 7.99–7.96 (m, 4H), 7.47–7.32 (m, 8H), 4.38–4.28 (m, 1H), 3.88–3.73 (m, 2H), 3.03 (br, 1H), 2.61–2.54 (m, 1H), 2.32–2.24 (m, 1H), 1.90–1.86 (m, 2H), 1.66–1.52 (m, 2H), 1.22 (s, 3H), 1.17 (s, 3H). 13C{1H} (75 MHz, CDCl3) δ 130.7, 128.3, 128.0, 127.6, 126.4, 87.8, 68.1, 63.1, 45.5, 44.0, 35.3, 25.9, 24.1, 19.3. 31P{1H} NMR (122 MHz, CDCl3): δ 139.4 (s) ppm, −44.7 (s) ppm. HRMS (ESI-TOF): m/z calculated for C30H29O4P2: 515.1271 [M + H]+; observed 515.1193. EA: calcd. for C30H28O4P2: C: 70.05, H: 5.50. Found: C: 70.31, H: 5.37.
Synthesis of 9a. Triethylamine (0.48 mmol, 67 μl) was added at −78 °C to a solution of (R)-1,1′-binaphthyl-2,2′-diyl chlorophosphite (0.48 mmol, 169.5 mg) in 10 ml THF. Optically pure sulfur-protected 7 (0.44 mmol, 102 mg) was dissolved in THF and added dropwise to the NEt3–chlorophosphite mixture maintaining the low temperature. The reaction mixture was stirred at this temperature for 10 min and then for 1 h at room temperature. Elemental sulfur (21 mg, 0.66 mmol) and NEt3 (5 drops) were added to the reaction mixture and stirred for 1 h at 20 °C. The solvent was evaporated to give a white solid. The crude product was washed 3 times with 5 ml Et2O each and then dissolved in 10 ml EtOAc followed by washing with 6 ml saturated aq. NH4Cl solution. The organic phase was separated and further washed 3 times with 5 ml H2O each. The organic phase was dried over MgSO4 and then the solvent was evaporated. The crude product was dissolved in boiling iPrOH (ca. 40 ml). After standing overnight at room temperature, 9a was obtained as a white powder (133 mg, 52%).
1H NMR (400 MHz, CDCl3): δ 8.09–7.93 (m, 4H, H-aryl), 7.58 (m, 1H, H-aryl), 7.55–7.44 (m, 3H, H-aryl), 7.44–7.28 (m, 4H, H-aryl), 4.98 (m, 1H, H-6a), 4.54 (m, 1H, H-6a), 4.11–4.05 (m, 1H, H-5a), 3.90 (m, 1H, H-5a), 2.84 (m, 1H, H-6), 2.40 (m, 1H, H-5), 2.19 (m, 1H, H-2 or 7), 2.10–2.02 (m, 4H, H-2 or 7), 1.97–1.84 (m, 2H, H-2 and 7), 1.25 (s, 3H, H-3a or 4a), 1.18 (s, 3H, H-3a or 4a) ppm; 13C{1H} NMR (101 MHz, CDCl3): δ 132.3 (s, C-quart. aryl), 131.9 (s, C-quart. aryl), 131.6 (s, C-quart. aryl), 131.2 (s, C-aryl), 130.9 (s, C-aryl), 128.5 (s, C-aryl), 128.4 (s, C-aryl), 127.2 (s, C-aryl), 127.0 (s, C-aryl), 126.8 (s, C-aryl), 126.6 (s, C-aryl), 125.8 (s, C-aryl), 121.1 (s, C-aryl), 120.3 (s, C-aryl), 86.1 (d, J = 1.3 Hz, C-quart.), 51.2 (d, J = 18.7 Hz, C-quart.), 66.1 (s, C-5a), 65.6 (dd, C-6a), 46.8 (s, C-5), 42.9 (dd, C-6), 41.6 (d, J = 44.6 Hz, C-2 or 7), 40.3 (d, J = 52.4 Hz, C-2 or 7), 23.8 (d, J = 7.6 Hz, C-3a or 4a), 18.1 (d, J = 16.5 Hz, C-3a or 4a) ppm; 31P{1H} NMR (162 MHz, CDCl3): δ 73.3 (d, J = 2.4 Hz, P-exocyclic), 43.0 (d, J = 2.4 Hz, P-endocyclic) ppm; 31P NMR (162 MHz, CDCl3): δ 73.3 (m, P-exocyclic), 43.0 (m, P-endocyclic) ppm; HRMS (ESI(+), MeCN/CH2Cl2), m/z: found: 579.0986, calculated for [M + H]+: 579.0977; found: 596.1242, calculated for [M + NH4]+: 596.1243; found: 601.0799, calculated for [M + Na]+: 601.0797; found: 1174.2156, calculated for [2 M + NH4]+: 1174.2147; infrared spectrum (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 3061 (w, C–H aryl), 2923 (w, C–H alkyl), 2855 (w, C–H alkyl), 1740 (w), 1620 (w), 1588 (w), 1505 (w), 1462 (w), 1433 (w), 1375 (w), 1322 (w), 1218 (m), 1199 (m), 1143 (m), 1084 (s), 1068 (s), 1026 (s, C–O stretching in P–O–CH2–C fragment), 1007 (s), 980 (s), 957 (s, P–O stretching in PV–O–Ar fragment), 900 (s), 866 (s), 812 (s), 787 (s), 772 (s), 749 (s), 720 (s), 706 (s), 695 (s), 682 (s), 671 (s, possibly P
= 3061 (w, C–H aryl), 2923 (w, C–H alkyl), 2855 (w, C–H alkyl), 1740 (w), 1620 (w), 1588 (w), 1505 (w), 1462 (w), 1433 (w), 1375 (w), 1322 (w), 1218 (m), 1199 (m), 1143 (m), 1084 (s), 1068 (s), 1026 (s, C–O stretching in P–O–CH2–C fragment), 1007 (s), 980 (s), 957 (s, P–O stretching in PV–O–Ar fragment), 900 (s), 866 (s), 812 (s), 787 (s), 772 (s), 749 (s), 720 (s), 706 (s), 695 (s), 682 (s), 671 (s, possibly P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S exocyclic), 652 (s, possibly P
S exocyclic), 652 (s, possibly P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S exocyclic), 629 (s, possibly P
S exocyclic), 629 (s, possibly P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S exocyclic), 566 (s), 548 (s), 527 (s), 470 (s), 447 (s) cm−1.
S exocyclic), 566 (s), 548 (s), 527 (s), 470 (s), 447 (s) cm−1.
Synthesis of 9b. The same procedure was employed as for 9a with triethylamine (0.57 mmol, 80 μl), (S)-1,1′-binaphthyl-2,2′-diyl chlorophosphite (0.57 mmol, 201 mg) in 8 ml THF, 7 (0.52 mmol, 120 mg) in 5 ml THF, elemental sulfur (25 mg, 0.78 mmol) and NEt3 (5 drops).
Yield 9b: 172 mg (58%).
1H NMR (400 MHz, CDCl3): δ 8.05 (m, 2H, H-aryl), 7.97 (m, 2H, H-aryl), 7.62–7.52 (m, 1H, H-aryl), 7.51–7.48 (m, 3H, H-aryl), 7.45–7.28 (m, 4H, H-aryl), 4.91 (m, 1H, H-6a), 4.57 (m, 1H, H-6a), 4.01 (m, 1H, H-5a), 3.83 (m, 1H, H-5a), 2.82 (m, 1H, H-6), 2.42 (m, 1H, H-5), 2.30–2.16 (m, 1H, H-2 or 7), 2.13–2.04 (m, 1H, H-2 or 7), 1.99–1.88 (m, 2H, H-2 and 7), 1.26 (s, 3H, H-3a or 4a), 1.19 (s, 3H, H-3a or 4a) ppm; 13C{1H} NMR (101 MHz, CDCl3): δ 132.3 (s, C-quart. aryl), 131.9 (s, C-quart. aryl), 131.6 (s, C-quart. aryl), 131.2 (s, C-aryl), 131.0 (s, C-aryl), 128.5 (s, C-aryl), 128.4 (s, C-aryl), 127.2 (s, C-aryl), 127.0 (s, C-aryl), 126.8 (s, C-aryl), 126.7 (s, C-aryl), 125.8 (s, C-aryl), 122.0 (s, C-aryl), 121.0 (d, J = 3.0 Hz), 120.2 (d, J = 2.7 Hz), 86.1 (s, C-quart.), 65.8 (s, C-5a), 65.3 (dd, C-6a), 51.3 (d, J = 18.5 Hz, C-quart.), 46.8 (s, C-5), 43.0 (dd, C-6), 41.5 (d, J = 44.9 Hz, C-2 or 7), 40.2 (d, J = 52.3 Hz, C-2 or 7), 23.8 (d, J = 7.4 Hz, C-3a or 4a), 18.1 (d, J = 16.1 Hz, C-3a or 4a) ppm; 31P{1H} NMR (162 MHz, CDCl3): δ 73.1 (d, J = 3.5 Hz), 43.2 (d, J = 3.5 Hz) ppm; 31P NMR (162 MHz, CDCl3): δ 73.1 (m, P-endocyclic), 43.2 (m, P-exocyclic) ppm; HRMS (ESI(+), MeCN), m/z: found: 579.0983, calculated for [M + H]+: 579.0977; found: 596.1241, calculated for [M + NH4]+: 596.1243; found: 601.0809, calculated for [M + Na]+: 601.0797; found: 1174.2142, calculated for [2 M + NH4]+: 1174.2147; infrared spectrum (KBr): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 3047 (w, C–H aryl), 2964 (w, C–H alkyl), 2876 (w, C–H alkyl), 1729 (m), 1589 (m), 1508 (m), 1462 (m), 1434 (w), 1371 (w), 1322 (w), 1222 (m), 1198 (w), 1156 (w), 1128 (w), 1072 (m), 1023 (m, C–O stretching in P–O–CH2–C fragment), 980 (s), 954 (s, P–O stretching in PV–O–Ar fragment), 865 (s), 845 (s), 813 (s), 784 (s), 772 (s), 748 (s), 717 (s), 680 (s), 670 (s, possibly P
= 3047 (w, C–H aryl), 2964 (w, C–H alkyl), 2876 (w, C–H alkyl), 1729 (m), 1589 (m), 1508 (m), 1462 (m), 1434 (w), 1371 (w), 1322 (w), 1222 (m), 1198 (w), 1156 (w), 1128 (w), 1072 (m), 1023 (m, C–O stretching in P–O–CH2–C fragment), 980 (s), 954 (s, P–O stretching in PV–O–Ar fragment), 865 (s), 845 (s), 813 (s), 784 (s), 772 (s), 748 (s), 717 (s), 680 (s), 670 (s, possibly P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S exocyclic), 650 (s, possibly P
S exocyclic), 650 (s, possibly P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S exocyclic), 630 (s, possibly P
S exocyclic), 630 (s, possibly P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S exocyclic), 567 (s), 546 (s), 527 (s), 495 (s), 470 (s) cm−1.
S exocyclic), 567 (s), 546 (s), 527 (s), 495 (s), 470 (s) cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 1
L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x ratio (x = 2 for monodentate L1, 1 for bidentate L2 and L3) and degassed. (E)-1,3-Diphenylprop-2-ene-1-yl acetate (1 mmol, 1 eq., C/S = 1/100), was added and stirred for 10 min followed by the addition of dimethyl malonate (1.5 mmol, 3 eq.), BSA (N,O-bis(trimethylsilyl)acetamide) (1.5 mmol, 3 eq.) and a catalytic amount of KOAc (0.024 mmol). The reaction mixture was stirred at room temperature for 14 h. When TLC indicated no further conversion, the reaction was quenched by dilution with Et2O (10 ml); the organic layer was washed twice with saturated NH4Cl solution (8–10 ml each) and dried over Na2SO4. Filtration and removal of solvent left a red oil, which was chromatographed (SiO2; petroleum ether/CH2Cl2 = 1
x ratio (x = 2 for monodentate L1, 1 for bidentate L2 and L3) and degassed. (E)-1,3-Diphenylprop-2-ene-1-yl acetate (1 mmol, 1 eq., C/S = 1/100), was added and stirred for 10 min followed by the addition of dimethyl malonate (1.5 mmol, 3 eq.), BSA (N,O-bis(trimethylsilyl)acetamide) (1.5 mmol, 3 eq.) and a catalytic amount of KOAc (0.024 mmol). The reaction mixture was stirred at room temperature for 14 h. When TLC indicated no further conversion, the reaction was quenched by dilution with Et2O (10 ml); the organic layer was washed twice with saturated NH4Cl solution (8–10 ml each) and dried over Na2SO4. Filtration and removal of solvent left a red oil, which was chromatographed (SiO2; petroleum ether/CH2Cl2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give analytically pure products. The enantioselectivity was determined by chiral HPLC. Absolute configuration of the products was determined by the known elution order. The analytical data of the product were in agreement with literature reports.39
1) to give analytically pure products. The enantioselectivity was determined by chiral HPLC. Absolute configuration of the products was determined by the known elution order. The analytical data of the product were in agreement with literature reports.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 1
L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and stirred for 10–15 min in the appropriate solvent (2 ml). The substrate methyl (Z)-2-acetamido-3-phenylacrylate (0.5 mmol) was added to the reaction vessels maintaining the inert atmosphere and the vessels were placed in the autoclave. The autoclave was purged two times with nitrogen and three times with hydrogen. Finally, it was pressurized at the desired H2 pressure at 25 °C for the desired reaction time. After the required reaction time, the autoclave was depressurized, and the contents of the reaction vessels were diluted with EtOAc (5 ml) and filtered through a short pad of silica. The conversion was determined by GC, GC-MS and NMR measurements, and the enantiomeric excess was determined by GC or HPLC using a chiral column. Absolute configuration of the products was determined by the known elution order. The analytical data were in agreement with literature reports.39
1) and stirred for 10–15 min in the appropriate solvent (2 ml). The substrate methyl (Z)-2-acetamido-3-phenylacrylate (0.5 mmol) was added to the reaction vessels maintaining the inert atmosphere and the vessels were placed in the autoclave. The autoclave was purged two times with nitrogen and three times with hydrogen. Finally, it was pressurized at the desired H2 pressure at 25 °C for the desired reaction time. After the required reaction time, the autoclave was depressurized, and the contents of the reaction vessels were diluted with EtOAc (5 ml) and filtered through a short pad of silica. The conversion was determined by GC, GC-MS and NMR measurements, and the enantiomeric excess was determined by GC or HPLC using a chiral column. Absolute configuration of the products was determined by the known elution order. The analytical data were in agreement with literature reports.39Author contributions
Conceptualization: E. H. H., (late) Prof. Paul C. J. Kamer, J. G. d. V.; Synthetic and catalytic work: K. R., S. C.; B. M. assisted with result analysis; manuscript preparation and correction: S. C., K. R., E. H. H., J. G. d. V.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the valuable contribution of (late) Prof. Dr Paul C. J. Kamer in the start-up phase of this project and research. We thank the Deutsche Forschungsgemeinschaft (DFG) for financial support (project number HE 1376/46-1 and 411421782). The analytical facilities at LIKAT are highly acknowledged. We thank Mara Wolniewicz for her help with chiral HPLC measurements. Funded by the Open Access Publishing Fund of Leipzig University, supported by the German Research Foundation within the program Open Access Publication Funding.References
- Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis, ed. P. C. Kamer and P. W. van Leeuwen, John Wiley & Sons, 2012 Search PubMed.
- Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer Science & Business Media, Berlin, Heidelberg, 2003 Search PubMed.
- Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Applications, ed. A. Börner, Wiley-VCH, Weinheim, vol. 1–3, 2008 Search PubMed.
- W. S. Knowles, M. J. Sabacky, B. D. Vineyard and D. J. Weinkauff, J. Am. Chem. Soc., 1975, 97, 2567–2568 CrossRef CAS.
- J. D. Gbubele and T. K. Olszewski, Org. Biomol. Chem., 2021, 19, 2823–2846 RSC.
- D. S. Glueck, Synthesis, 2021, 54, 271–280 CrossRef.
- S. Lemouzy, L. Giordano, D. Hérault and G. Buono, Eur. J. Org Chem., 2020, 2020, 3351–3366 CrossRef CAS.
- T. Murai, Chem. Lett., 2023, 52, 703–714 CrossRef CAS.
- T. K. Olszewski, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2023, DOI:10.1016/b978-0-32-390644-9.00115-3.
- O. Korpiun and K. Mislow, J. Am. Chem. Soc., 1967, 89, 4784–4786 CrossRef CAS.
- L. Horner and M. Jordan, Phosphorus Sulfur Relat. Elem., 1980, 8, 225–234 CrossRef CAS.
- N. V. Dubrovina and A. Börner, Angew. Chem., Int. Ed., 2004, 43, 5883–5886 CrossRef CAS PubMed.
- S. Juge, M. Stephan, J. A. Laffitte and J. P. Genet, Tetrahedron Lett., 1990, 31, 6357–6360 CrossRef CAS.
- U. Nettekoven, P. C. J. Kamer, M. Widhalm and P. W. N. M. V. Leeuwen, Organometallics, 2000, 19, 4596–4607 CrossRef CAS.
- O. Korpiun, R. A. Lewis, J. Chickos and K. Mislow, J. Am. Chem. Soc., 1968, 90, 4842–4846 CrossRef CAS.
- A. R. Muci, K. R. Campos and D. A. Evans, J. Am. Chem. Soc., 1995, 117, 9075–9076 CrossRef CAS.
- J.-O. Moritz, S. Chakrabortty, B. H. Müller, A. Spannenberg and P. C. J. Kamer, J. Org. Chem., 2020, 85, 14537–14544 CrossRef CAS PubMed.
- S. Chakrabortty, K. Konieczny, J.-O. Moritz, S. Zheng, S. Tin, B. H. Müller and J. G. de Vries, ACS Catal., 2023, 13, 12030–12040 CrossRef CAS.
- T. Imamoto, Proc. Jpn. Acad., Ser. B, 2021, 97, 520–542 CrossRef CAS PubMed.
- T. Imamoto, Chem. Rec., 2016, 16, 2659–2673 CrossRef PubMed.
- P. W. N. M. van Leeuwen, P. C. J. Kamer, C. Claver, O. Pàmies and M. Diéguez, Chem. Rev., 2011, 111, 2077–2118 CrossRef CAS PubMed.
- H. Fernández-Pérez, P. Etayo, A. Panossian and A. Vidal-Ferran, Chem. Rev., 2011, 111, 2119–2176 CrossRef PubMed.
- S. Deerenberg, P. C. J. Kamer and P. W. N. M. V. Leeuwen, Organometallics, 2000, 19, 2065–2072 CrossRef CAS.
- I. Arribas, S. Vargas, M. Rubio, A. S. Suárez, C. Domene, E. Álvarez and A. Pizzano, Organometallics, 2010, 29, 5791–5804 CrossRef CAS.
- H. Fernandez-Perez, J. Benet-Buchholz and A. Vidal-Ferran, Chem.–Eur. J., 2014, 20, 15375–15384 CrossRef CAS PubMed.
- T. Möller, M. B. Sárosi and E. Hey-Hawkins, Chem.–Eur. J., 2012, 18, 16604–16607 CrossRef PubMed.
- P. Wonneberger, N. König, F. B. Kraft, M. B. Sárosi and E. Hey-Hawkins, Angew. Chem., Int. Ed., 2019, 58, 3208–3211 CrossRef CAS PubMed.
- K. Ramazanova, P. Lönnecke and E. Hey-Hawkins, Chem.–Eur. J., 2023, 29, e202300790 CrossRef CAS PubMed.
- P. Wonneberger, N. König, M. B. Sárosi and E. Hey-Hawkins, Chem.–Eur. J., 2021, 27, 7847–7852 CrossRef CAS PubMed.
- T. Möller, P. Wonneberger, M. B. Sárosi, P. Coburger and E. Hey-Hawkins, Dalton Trans., 2016, 45, 1904–1917 RSC.
- K. Ramazanova, S. Chakrabortty, F. Kallmeier, N. Kretzschmar, S. Tin, P. Lönnecke, J. G. de Vries and E. Hey-Hawkins, Molecules, 2023, 28, 6210 CrossRef CAS PubMed.
- T. Möller, P. Wonneberger, M. B. Sarosi, P. Coburger and E. Hey-Hawkins, Dalton Trans., 2016, 45, 1904–1917 RSC.
- A. A. Zagidullin, E. S. Oshchepkova, I. V. Chuchelkin, S. A. Kondrashova, V. A. Miluykov, S. K. Latypov, K. N. Gavrilov and E. Hey-Hawkins, Dalton Trans., 2019, 48, 4677–4684 RSC.
- J. Scherer, G. Huttner, M. Büchner and J. Bakos, J. Organomet. Chem., 1996, 520, 45–58 CrossRef CAS.
- T. L. Cottrell, The Strengths of Chemical Bonds, Butterworth, London, 1958 Search PubMed.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19 RSC.
- O. Pàmies, J. Margalef, S. Cañellas, J. James, E. Judge, P. J. Guiry, C. Moberg, J.-E. Bäckvall, A. Pfaltz, M. A. Pericàs and M. Diéguez, Chem. Rev., 2021, 121, 4373–4505 CrossRef PubMed.
- S. Chakrabortty, A. A. Almasalma and J. G. de Vries, Catal. Sci. Technol., 2021, 11, 5388–5411 RSC.
- S. Chakrabortty, K. Konieczny, B. H. Müller, A. Spannenberg, P. C. J. Kamer and J. G. de Vries, Catal. Sci. Technol., 2022, 12, 1392–1399 RSC.
Footnotes |
| † In memory of Prof. Paul C. J. Kamer. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2302227. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra07630j |
| § These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |