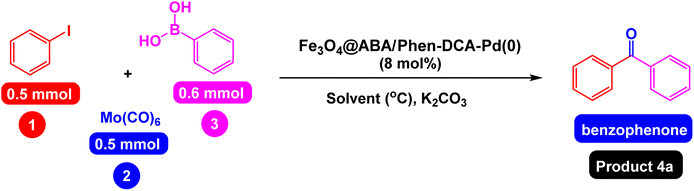
Open Access Article
Open Access ArticleRetracted Article: Palladium complex supported on the surface of magnetic Fe3O4 nanoparticles: an ecofriendly catalyst for carbonylative Suzuki-coupling reactions†
Shanshan Jiang *
*
Department of Chemistry and Chemical Engineering, Lvliang University, Lvliang, Shanxi 033000, PR China. E-mail: jiang_shanshan1@sina.com
First published on 1st December 2023
Abstract
Diaryl ketone derivatives include well-known compounds with important physiological and biological properties. In order to prepare diaryl ketone derivatives, we constructed a pallidum (0) complex immobilized on Fe3O4 nanoparticles modified with aminobenzoic acid and phenanthroline [Fe3O4@ABA/Phen-DCA-Pd(0)], and evaluated its catalytic performance for carbonylative Suzuki-coupling reactions of aryl iodides with aryl boronic acid in the presence of Mo(CO)6 as the CO source under mild conditions. FT-IR, SEM, TEM, EDX, VSM, TGA, XRD, ICP-OES and Elemental mapping techniques were employed to identify the structure of the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst. Different derivatives of aryl iodides and aryl boronic acids containing withdrawing and donating functional groups were studied for the preparation of diaryl ketones. Also, various derivatives of heteroaryl iodides and boronic acids were used and the desired products were prepared with high yields. The Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst was separated magnetically and reused 7 consecutive times without reducing its catalytic activity. VSM, TEM and ICP-OES spectroscopic techniques confirmed that the synthesized Fe3O4@ABA/Phen-DCA-Pd(0) catalyst was still stable and maintained its structure despite repeated reuse.
Introduction
Research on catalysis and the uses of catalysts is always the focus of many theoretical and applied science researchers.1,2 Heterogeneous catalysts are more useful than conventional homogeneous catalysts due to their easy removal from the reaction medium by simple separation and reusability, which is essential in green synthesis.3–7 Due to the high active surface and many active centers in nanocatalysts, these catalysts show improved reactivity.8 To increase the catalytic activity and reduce the number of valuable catalysts required, the ability to control the particles, the surface area and the effective distribution of nanoparticles are the key principles that should be considered.9–12 But it is not easy to separate these nano catalysts from the reaction mixture, and conventional separation methods such as filtering are not effective due to the nano size of these catalysts.13–16 Therefore, chemists in the catalysis field decided to use magnetic nanocatalysts because these catalysts are easily separated from the reaction environment only by an external magnetic field.17–20 Among the nanocatalyst materials, magnetic nanoparticles are widely used due to their better catalytic activity, high surface-to-volume ratio and high reusability.21–24 A large active surface area and excellent selectivity in magnetic nanocatalysts increase the speed and efficiency of the reaction.25–27 Magnetic nanocatalysts combine the merits of homogeneous (high level) and heterogeneous (separability) catalysts.28–30 The most research among magnetic nanoparticles has been done on Fe3O4 nanoparticles because the structures of magnetic nanocatalysts are very diverse, and it is easy to isolate and change their performance by chemical modification.31–33Ketone derivatives have well-known compounds with important physiological and biological properties.34–36 Molecules containing ketone scaffolds are found in several sugars and for medicinal use in compounds including natural and synthetic steroid hormones.37–39 For example, the molecules of the anti-inflammatory substance cortisone contain three ketone groups.40,41 Many ketones are known and many of them are of great importance in industry and biology.42 Ketones include many sugars (ketoses) and acetone (the smallest ketone).43 Ketones are mostly used as solvents, especially in explosives, varnish, paint and fabric production industries.43,44 Ketones are also used as preservatives and hydraulic fluids.45 Many complex organic and chemical compounds are synthesized using ketones as the structural intermediates or reagents.40 Several important molecules containing diaryl ketone scaffolds with pharmaceutical and biological applications are shown in Fig. 1.46–48
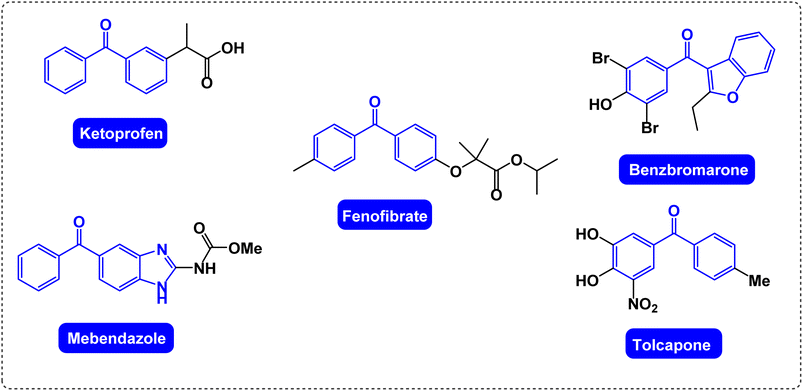 | ||
| Fig. 1 Several important molecules containing diaryl ketone scaffolds with pharmaceutical and biological applications. | ||
Due to the valuable applications of diaryl ketones, many methods and catalytic systems have been reported for the preparation of these compounds.36 Although these methods have good advantages, some of them still have disadvantages such as: performing the reaction under harsh conditions, synthesizing products with low or moderate yields, long time and high temperature for performing the reaction, performing the reaction under harmful conditions for the environment and laborious separation the catalyst of the reaction mixture. Therefore, research on new methods in line with the principles of green chemistry for the preparation of diaryl ketones can be a real challenge in organic synthesis.49 Now, in this article, we would like to report a very attractive, efficient and ideal method, especially from the point of view of green chemistry, for the preparation of diaryl ketones. In this research work, pallidum (0) complex immobilized on Fe3O4 nanoparticles modified with aminobenzoic acid and phenanthroline [Fe3O4@ABA/Phen-DCA-Pd(0)] was constructed and its catalytic performance was evaluated in carbonylative Suzuki-coupling reactions of aryl iodides with aryl boronic acid in the presence of Mo(CO)6 as CO source.
Result and discussion
As you can see in Scheme 1, by using phenanthroline immobilized on magnetic nanoparticles, we were able to prepare a good and attractive ligand to stabilize the palladium complex. First, the magnetic Fe3O4 nanoparticle was simply prepared and then its surface was coated by 4-aminobenzoic acid [Fe3O4@ABA]. Then, due to the reaction of the Fe3O4@ABA nanomaterial with 1,10-phenanthroline-2,9-dicarbaldehyde at refluxing ethanol, the Fe3O4@ABA/Phen-DCA ligand was well made to stabilize palladium metal. And finally, the PdCl2 was well fixed on the Fe3O4@ABA/Phen-DCA ligand and was converted into palladium (0) complex using NaBH4.Synthesis of Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst
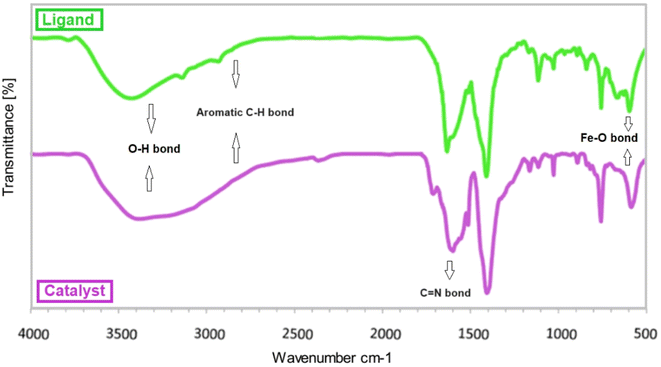
At the first step the Fe3O4 was prepared according to previous report.50 In the next step 2 g of Fe3O4 was dispersed in 50 mL of ethanol for 30 min and, then, 5 mmol of 4-aminobenzoic acid (ABA) was added to the reaction mixture and stirred under reflux conditions for 2 h. After the mixture was cooled, the synthesized Fe3O4@ABA MNPs were accumulated using an external magnet, washed with water and ethanol and dried at 80 °C. Afterwards, 2 g of Fe3O4@ABA was dispersed in 100 mL of ethanol for 30 minutes. Then, 2.5 mmol of 1,10-phenanthroline-2,9-dicarbaldehyde was added to the prepared suspension, and refluxed under vigorous stirring and N2 atmosphere for 10 h. Afterwards, the solid particles were collected magnetically, washed several times with ethanol and finally dried. Eventually, 1 gr of Fe3O4@ABA/Phen-DCA MNPs was dispersed in 50 mL of ethanol and, then, it was treated with 2.5 mmol of PdCl2 and stirred under reflux conditions for 24 h. The obtained [Fe3O4@AMNA-CuBr] complex was collected using an external magnet, washed using water and ethanol and, finally, dried at 80 °C (Scheme 1).In order to find out whether the functional groups are well placed on the surface of the magnetic nanoparticles or not, FT-IR analysis was taken from the as-constructed Fe3O4@ABA/Phen-DCA ligand and Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst (Fig. 2). The appearance of the absorption band with the wave number of about 570 cm−1 is related to stretching vibrations Fe–O bond, which is confirmed the formation of Fe3O4 NPs. The presence of absorption bands in the region of 1600 cm−1 corresponding to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds. Also, the broad absorption band in the region of about 3300 cm−1 is related to the stretching vibration of O–H groups. It is also possible to prove the existence of aromatic ring on the magnetic nanoparticles with stretching vibrations of C–H bond, which appear approximately in the region of 2800 cm−1. As you can see in the spectrum of the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst, due to imine coordination with Pd metal, the C
N bonds. Also, the broad absorption band in the region of about 3300 cm−1 is related to the stretching vibration of O–H groups. It is also possible to prove the existence of aromatic ring on the magnetic nanoparticles with stretching vibrations of C–H bond, which appear approximately in the region of 2800 cm−1. As you can see in the spectrum of the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst, due to imine coordination with Pd metal, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequency has shifted to a lower stretching frequency.51 Also, the presence of Pd was confirmed by ICP and EDX spectroscopic techniques.
N stretching frequency has shifted to a lower stretching frequency.51 Also, the presence of Pd was confirmed by ICP and EDX spectroscopic techniques.
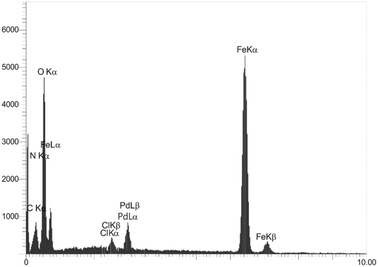
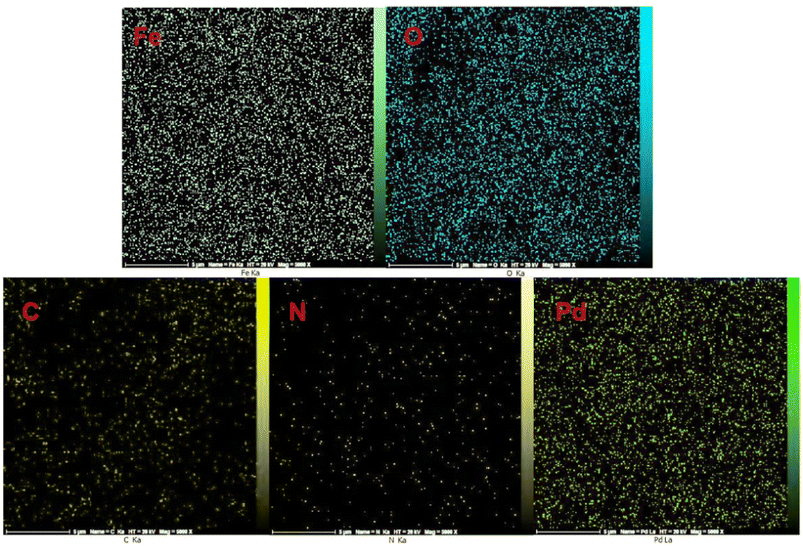
In order to qualitatively analyze the surface and identify the constituent elements of the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst, X-ray energy diffraction spectroscopy (EDX) and elemental mapping techniques were used. In both analyses (Fig. 3 and 4), the presence of iron (44.12 wt%), nitrogen (2.23 wt%), carbon (7.71 wt%), oxygen (44.18 wt%) and palladium (1.76 wt%) elements in the sample was confirmed, which indicates that the modification of the surface of magnetic nanoparticles and stabilization of the palladium complex has been done successfully. Also, in order to find the amount of Pd in the structure of Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst, ICP-OES analysis was token. This analysis confirmed that the amount of Pd is 14.21 × 10−5 mol g−1.
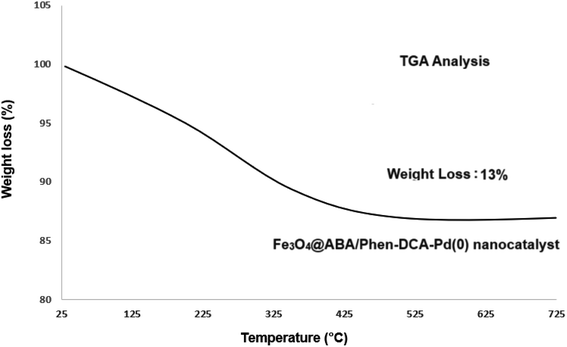
The graph of TGA analysis of the composition of the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst is shown in Fig. 5. The weight loss in the first stage up to the range of 220 °C can be related to the removal of a small amount of physically adsorbed water or by hydrogen bonding on the surface of the nanocatalyst. A higher mass reduction in the range of 220–600 °C is related to the decomposition of the organic groups placed on Fe3O4 nanoparticles modified with aminobenzoic acid and 1,10-phenanthroline-2,9-dicarbaldehyde groups.
The magnetic property of nanomaterials was measured using vibrating-sample magnetometer (VSM). The VSM analysis of Fe3O4 NPs, Fe3O4@ABA/Phen-DCA ligand and Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst are shown in Fig. 6. As the VSM analysis shows, the Fe3O4 nanoparticle with a magnetic property of 66.29 (emu g−1) was prepared, which shows the high magnetic property of this nanoparticle. Modification of the magnetic nanoparticle surface by 1,10-phenanthroline and stabilization of palladium metal decreased the value to 51.65 (emu g−1) and 47.15 (emu g−1), respectively. However, showing the number 47 by VSM analysis is a confirmation that the synthesized Fe3O4@ABA/Phen-DCA-Pd(0) catalyst has high magnetic properties.
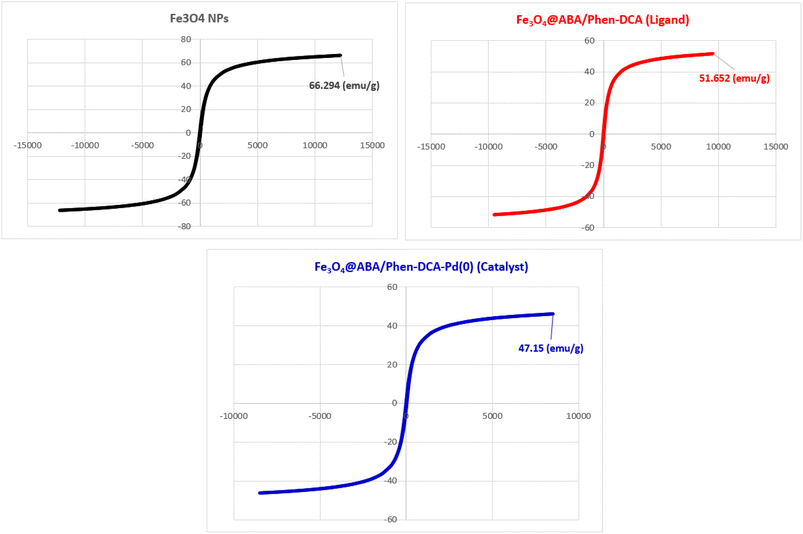 | ||
| Fig. 6 VSM analysis of Fe3O4 NPs, Fe3O4@ABA/Phen-DCA ligand and Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst. | ||
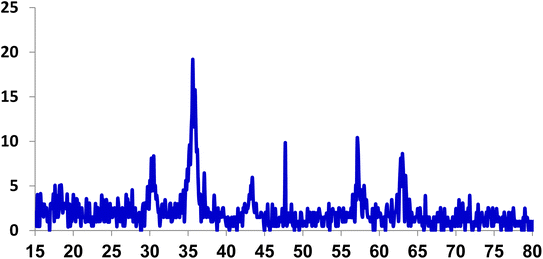
In order to check the purity of the phase, the XRD analysis of the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst was taken (Fig. 7). The analysis confirmed that the position and relative intensity of all peaks are in accordance with the standard XRD pattern for Fe3O4 magnetic nanoparticles reported in the literature.51 Therefore, the XRD analysis reveals that the magnetic core was stable during modification with aminobenzoic acid and 1,10-phenanthroline-2,9-dicarbaldehyde groups and immobilization of palladium complex.
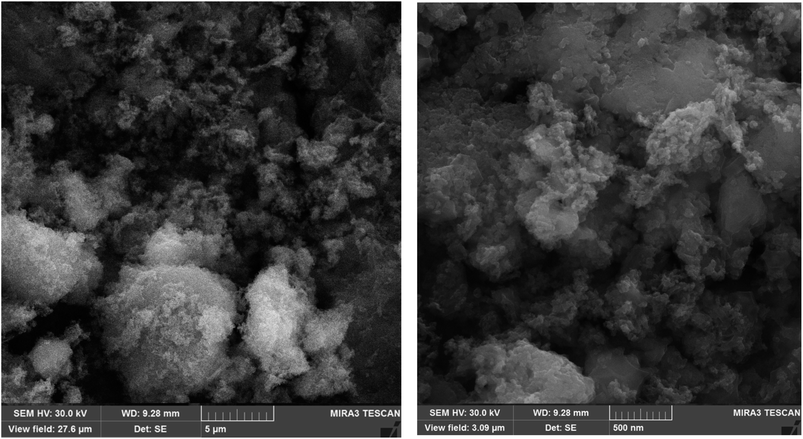
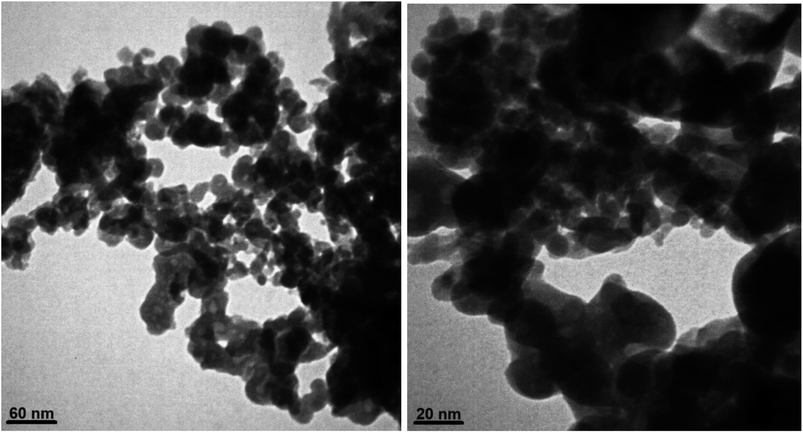
Surface morphology and particle size of Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst were evaluated using Scanning Electron Microscope (SEM) and Transmission Electron Microscopy (TEM) images at different magnifications (Fig. 8 and 9). The SEM images show a general picture of proper and relatively uniform granulation for particles. The images obtained from the TEM analysis show more correct and accurate information about the particle size and shape of Fe3O4@ABA/Phen-DCA-Pd(0) nanoparticles. According to the TEM images, the size of the particles is about 20 nm, which have an almost spherical shape, which is consistent with the value calculated through XRD analysis.
Catalytic investigation
First, the reaction of the iodobenzene model with phenylboronic acid and Mo(CO)6 as CO source was carried out in the absence of a catalyst under the conditions observed in Scheme 2, which was predictable that the reaction would not be carried out in the absence of a catalyst. In the next step, the effect of catalyst components including Fe3O4 catalyst and a single ABA/Phen-DCA-Pd(0) on reaction progress was compared to target Fe3O4@SiO2@ABA/Phen-DCA-Pd(0) catalyst and the best resalt was obtained in the presence of Fe3O4@SiO2@ABA/Phen-DCA-Pd(0) (Scheme 2). As when the model reaction was carried out in the presence of nanomagnetic palladium catalyst, the desired product was synthesized with relatively good efficiency (Scheme 3). Therefore, we decided to optimize the amount of Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst, the results of these tests are shown in Fig. 10. By increasing the amount of Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst, the yield of the obtained product also increased, but the amount above 8 mol% did not affect the reaction efficiency and the desired product was prepared with the same efficiency of 88%.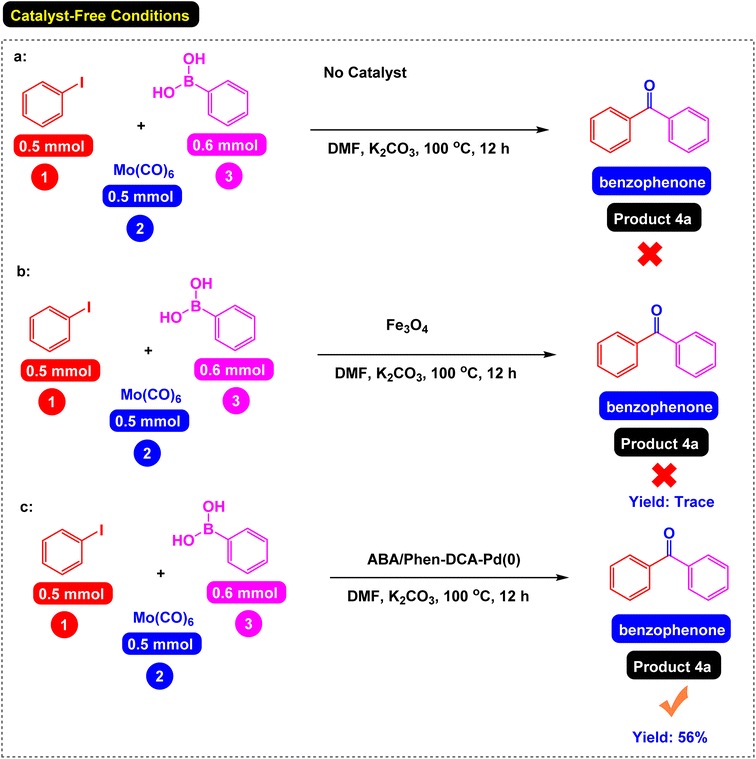 | ||
| Scheme 2 Carbonylative Suzuki-coupling reactions of iodobenzene with phenyl boronic acid and Mo (CO)6 under (a) catalyst-free conditions, (b) Fe3O4 and (c) ABA/Phen-DCA-Pd(0) catalysis. | ||
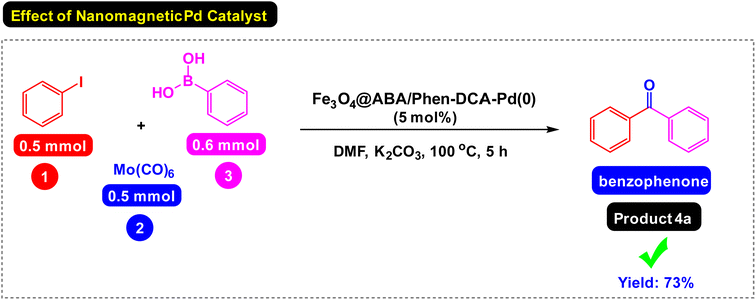 | ||
| Scheme 3 Carbonylative Suzuki-coupling reactions of iodobenzene with phenyl boronic acid and Mo (CO)6 catalyzed by Fe3O4@ABA/Phen-DCA-Pd(0) nanomaterial. | ||
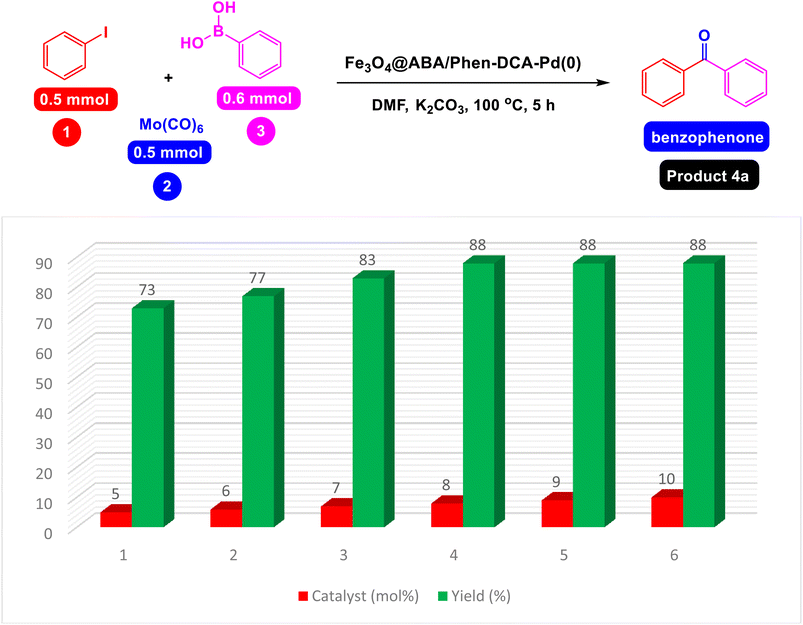 | ||
| Fig. 10 Effect of Nanomagnetic Pd Catalyst on Carbonylative Suzuki-coupling reactions of iodobenzene with phenyl boronic acid and Mo(CO)6. | ||
In the next step, we investigate the effect of different solvents on the reaction model in the presence of the optimal amount of the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst, which the results of these tests are summarized in Table 1. The results showed that protic solvents are more efficient than aprotic solvents. Although high yields were observed in DMSO, DMF and anisole solvents, but the highest yield was obtained when PEG was used as solvent (Table 1, entry 8). After PEG was chosen as a solvent, the model reaction was carried out at both higher and lower temperatures than 100 °C, and the results were not satisfactory. As the temperature decreased, the yield of the desired product decreased (Table 1, entry 11), and at a temperature higher than 100 °C, the yield remained constant (Table 1, entry 10).
| Entry | Solvent (temperature) | Time (h) | Yielda (%) |
|---|---|---|---|
| a Yields referred to isolated products. | |||
| 1 | DMF (100 °C) | 5 | 88% |
| 2 | EtOH (reflux) | 5 | 80% |
| 3 | Water (reflux) | 5 | 46% |
| 4 | DMSO (100 °C) | 5 | 85% |
| 5 | MeCN (reflux) | 5 | 79% |
| 6 | Toluene (100 °C) | 5 | 33% |
| 7 | Anisole (100 °C) | 5 | 87% |
| 8 | PEG (100 °C) | 5 | 90% |
| 9 | Solvent-free (100 °C) | 12 | 14% |
| 10 | PEG (110 °C) | 5 | 90% |
| 11 | PEG (90 °C) | 5 | 85% |
Then we decided to investigate the effect of base on the model reaction. First, the model reaction was studied in the presence of the optimal amount of catalyst in PEG solvent and in the absence of base, and the results confirmed that base is an important and key component in the synthesis of diaryl ketones because the desired product was not formed under these conditions (Scheme 4). The model reaction was carried out in the presence of different bases (Fig. 11). The results showed that potassium acetate (KOAc) is the best base for the synthesis of the diaryl ketones because the desired product was obtained with the highest yield (94%).
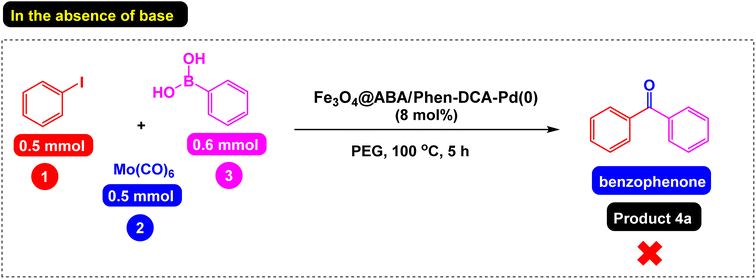 | ||
| Scheme 4 Carbonylative Suzuki-coupling reaction of iodobenzene with phenyl boronic acid and Mo(CO)6 catalyzed by Fe3O4@ABA/Phen-DCA-Pd(0) nanomaterial in the absence of base. | ||
 | ||
| Fig. 11 Effect of base on Carbonylative Suzuki-coupling reactions of iodobenzene with phenyl boronic acid and Mo(CO)6 catalyzed by Fe3O4@ABA/Phen-DCA-Pd(0) nanomaterial. | ||
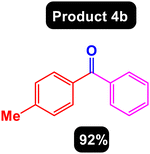
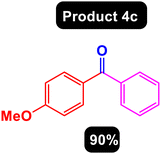
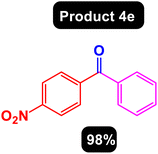
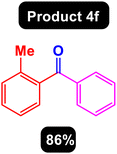
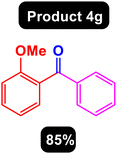
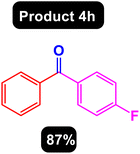
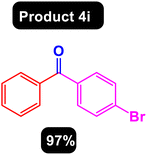
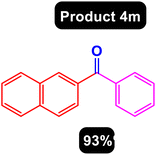
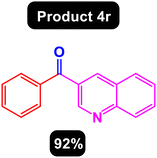
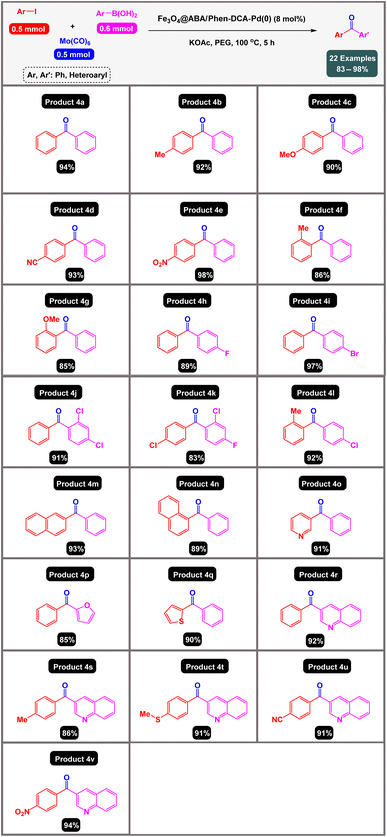
After obtaining the optimal conditions, using 8 mol% of the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst and KOAc in PEG at 100 °C for 5 h, we decided to evaluate the scope of the reaction in the presence of different derivatives of aryl iodides and aryl boronic acids. As can be seen in Table 2, different derivatives of iodobenzene and phenylboronic acid were used in which different functional groups (withdrawing and donating functional groups) are located on the ring, and the desired diaryl ketone products were prepared with high to excellent yields. Also, various derivatives of heteroaryl iodides and boronic acids were used and the desired products were prepared with high yields.
| a Reaction conditions: aryl iodides (0.5 mmol), Mo(CO)6 (0.5 mmol), aryl boronic acids (0.6 mmol), Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst (8 mol%), KOAc (1.5 equiv.), PEG (3 mL) at 100 °C for 5 h. Yields: referred to isolated products by column chromatography. |
|---|
 |
According to the previously reported methods on the literature for the synthesis of diaryl ketones in the presence of palladium catalysts, we proposed a plausible synthetic pathway for the Fe3O4@ABA/Phen-DCA-Pd(0) nanocomposite-catalyzed carbonylative Suzuki-coupling reactions of aryl iodides with aryl boronic acid in the presence of Mo(CO)6 as CO source as shown in Scheme 5.
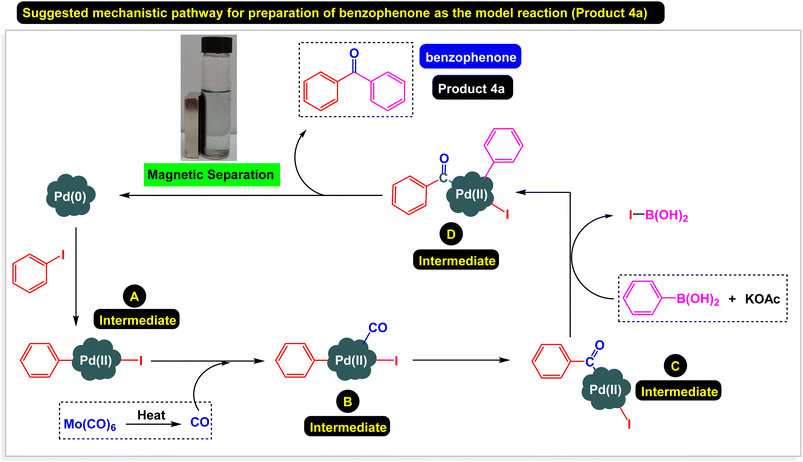 | ||
| Scheme 5 Suggested mechanistic pathway for carbonylative Suzuki-coupling reactions of aryl iodides with aryl boronic acid and Mo(CO)6 catalyzed by Fe3O4@ABA/Phen-DCA-Pd(0) nanomaterial. | ||
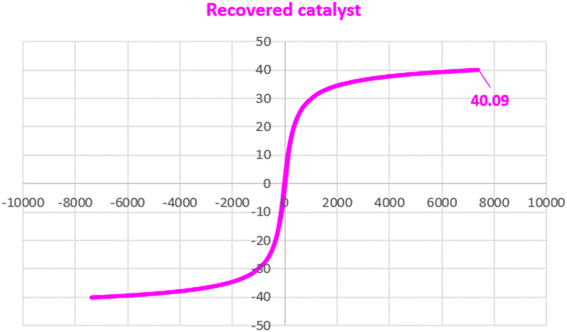
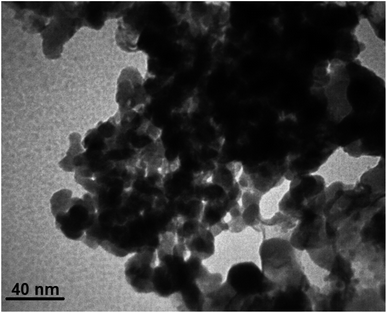
Separating the catalyst from the reaction mixture and reusing it in reactions is an important challenge in catalyst science. To this end, we decided to investigate the catalyst recovery in the model reaction of iodobenzene model with phenylboronic acid and Mo(CO)6 as CO source. After the reaction, the Fe3O4@ABA/Phen-DCA-Pd(0) catalyst was easily separated by an external magnetic field and reused for next reactions. As shown in Fig. 12, the catalyst was able to be used up to 7 consecutive times without reducing its catalytic activity. After performing the catalyst recovery-experiments, in order to find out how stable the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst is and whether its structure has changed or not, we decided to take a series of analyzes from the recovered catalyst (after 7 runs). As shown in Fig. 13, VSM analysis confirmed that the amount of magnetic property is 40.09 (emu g−1) that revealed that the recovered Fe3O4@ABA/Phen-DCA-Pd(0) catalyst (after 7 runs) still has high magnetic properties. TEM-analysis also confirmed that the structure of the recovered Fe3O4@ABA/Phen-DCA-Pd(0) catalyst (after 7 runs) catalyst has not changed significantly despite repeated reuse and has kept its shape (Fig. 14). ICP-OES analysis also confirmed that the amount of palladium (14.16 × 10−5 mol g−1) fixed on the nanoparticles (in the recovered Fe3O4@ABA/Phen-DCA-Pd(0) catalyst after 7 runs) did not change much and only a very small amount decreased that is in agreement of the EDAX analysis with iron (44.11 wt%), nitrogen (2.17 wt%), carbon (7.88 wt%), oxygen (44.09 wt%) and palladium (1.75 wt%) elements in the. All of these analysis confirmed that the synthesized Fe3O4@ABA/Phen-DCA-Pd(0) catalyst was still stable and maintained its structure despite repeated reuse.
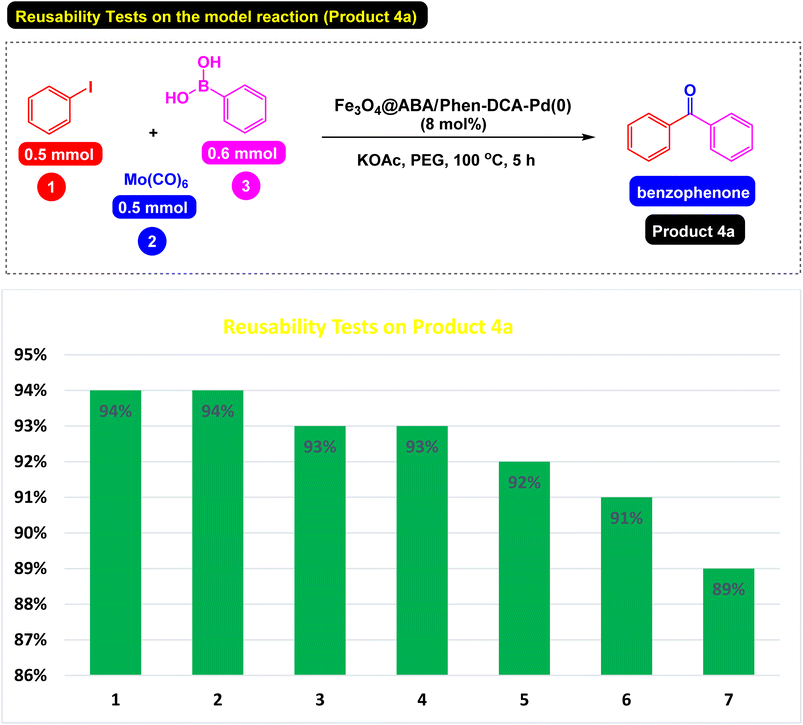 | ||
| Fig. 12 Reusability of Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst for carbonylative Suzuki-coupling reactions of iodobenzene with phenyl boronic acid and Mo(CO)6 (product 4a). | ||
In order to demonstrate the high activity of the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst compared to the catalytic systems reported in the literature, we decided to investigate the model reaction of iodobenzene model with phenylboronic acid and Mo(CO)6 as CO source. As you can see in Table 3, in most of the reported methods, the reaction was carried out for a long time under harsh conditions and the benzophenone product was prepared with moderate or good efficiency. But in this method, the model reaction was carried out in environmentally friendly solvent for 5 hours and the desired benzophenone product was prepared with excellent yield (94%). Also, the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst is completely stable and compatible with the principles of green chemistry.
| Entry | Catalytic system | Conditions | Yield (%) [ref] |
|---|---|---|---|
| 1 | PdCl2/([(PhNH)P2(NPh)2]2NPh) | KOAc/PEG, 90 °C, 10 h | 78% (ref. 52) |
| 2 | [(Cinnamyl)PdCl]2/DPEphos | DCC, THF, 120 °C, 24 h | 63% (ref. 46) |
| 3 | Fe3O4@SiO2–2P–PdCl2 | DCC, toluene, K2CO3, 100 °C, 12 h | 85% (ref. 47) |
| 4 | Pd-APDMS | Anisole, K2CO3, 100 °C, 5 h | 60% (ref. 45) |
| 5 | Pd(OAc)2, DMAP | KOH, toluene, 80 °C, 12 h | 75% (ref. 41) |
 |
|||
Conclusion
In summary, pallidum (0) complex immobilized on Fe3O4 nanoparticles modified with aminobenzoic acid and phenanthroline [Fe3O4@ABA/Phen-DCA-Pd(0)] was successfully constructed and its structure well characterized FT-IR, SEM, TEM, EDX, VSM, TGA, XRD, ICP-OES and elemental mapping spectroscopic techniques. This nanomagnetic palladium catalyst was performed for the carbonylative Suzuki-coupling reactions of aryl iodides with aryl boronic acid in the presence of Mo(CO)6 as CO source under mild conditions. Different derivatives of aryl iodides and aryl boronic acids containing withdrawing and donating functional groups were studied for the preparation of diaryl ketones, as seen above, a broad range of diaryl or heteroaryl ketones were synthesized with high to excellent yields. The Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst was separated magnetically and reused for 7 consecutive times without reducing its catalytic activity.Experimental
General procedure for Fe3O4@ABA/Phen-DCA-Pd(0) nanomaterial catalyzed carbonylative Suzuki-coupling reactions
A mixture of aryl iodides (0.5 mmol), Mo(CO)6 (0.5 mmol), aryl boronic acids (0.6 mmol), Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst (8 mol%), KOAc (1.5 equiv.) was stirred in PEG (3 mL) at 100 °C for 5 h. After the end of reaction, the Fe3O4@ABA/Phen-DCA-Pd(0) nanocatalyst was magnetically separated and the reaction mixture was cooled down to room temperature. The mixture was washed with 10 mL water, and the crude product was isolated using EtOAc (3 × 10 mL). The organic phases were combined and dried over Na2SO4, evaporated, and purified by column chromatography on silica gel (n-hexane/EtOAc) to deliver the diaryl ketones with high to excellent yields. All the products are well-known, however, the physical properties of the diaryl ketone products were compared with the reported samples and HNMR and CNMR techniques were used to better identify their structures.NMR data
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Lv Liang City High-level Talent Introduction Project for the year 2023. Shanxi Province Youth Science and Technology Research Fund for the year 2023.References
- Q. Li, L. Xu and D. Ma, Angew. Chem., 2022, 134(43), e202210483 CrossRef.
- T. Li, H. Pang, Q. Wu, M. Huang, J. Xu, L. Zheng, B. Wang and Y. Qiao, Int. J. Mol. Sci., 2022, 23, 6259 CrossRef CAS PubMed.
- M. Kazemi, Nanomater. Chem., 2023, 1(1), 1–11 Search PubMed.
- M. B. Chaudhari and B. Gnanaprakasam, Chem. – Asian J., 2019, 14, 76–93 CrossRef CAS PubMed.
- I. Fatimah, G. Fadillah, G. Purwiandono, I. Sahroni, D. Purwaningsih, H. Riantana, A. N. Avif and S. Sagadevan, Inorg. Chem. Commun., 2022, 137, 109213 CrossRef CAS.
- Y. Liang, J. Li, Y. Xue, T. Tan, Z. Jiang, Y. He, W. Shangguan, J. Yang and Y. Pan, J. Hazard. Mater., 2021, 420, 126584 CrossRef CAS PubMed.
- W. Liu, F. Huang, Y. Liao, J. Zhang, G. Ren, Z. Zhuang, J. Zhen, Z. Lin and C. Wang, Angew. Chem., Int. Ed., 2008, 47, 5619–5622 CrossRef CAS PubMed.
- M. R. Abdi, Biol. Mol. Chem., 2023, 1, 1–14 Search PubMed.
- A. M. Mustafa and A. Younes, Nanomater. Chem., 2023, 1, 12–23 Search PubMed.
- S. H. Gebre, Synth. Commun., 2021, 1–31 CrossRef.
- B. Zeynizadeh, E. Gholamiyan and M. Gilanizadeh, Curr. Chem. Lett., 2018, 121–130 CrossRef.
- Y. Dou, A. Wang, L. Zhao, X. Yang, Q. Wang, M. Shire Sudi, W. Zhu and D. Shang, J. Colloid Interface Sci., 2023, 650, 943–950 CrossRef CAS PubMed.
- M. Aqeel Ashraf, Z. Liu, Y. Yang, C. Li and D. Zhang, Synth. Commun., 2020, 50, 2629–2646 CrossRef CAS.
- Q. Zhang, X. Yang and J. Guan, ACS Appl. Nano Mater., 2019, 2, 4681–4697 CrossRef CAS.
- S. Gupta, J. Synth. Chem., 2022, 1, 37–41 Search PubMed.
- W. Li, X. Chu, F. Wang, Y. Dang, X. Liu, X. Wang and C. Wang, Appl. Catal., B, 2021, 288, 120034 CrossRef CAS.
- M. Lakshman, J. Synth. Chem., 2022, 1, 48–51 Search PubMed.
- R. K. Sharma, S. Dutta, S. Sharma, R. Zboril, R. S. Varma and M. B. Gawande, Green Chem., 2016, 18, 3184–3209 RSC.
- A. Wang, Y. Dou, X. Yang, Q. Wang, M. S. Sudi, L. Zhao, D. Shang, W. Zhu and J. Ren, Dalton Trans., 2023, 52, 11234–11242 RSC.
- Y. Zheng, Y. Liu, X. Guo, Z. Chen, W. Zhang, Y. Wang, X. Tang, Y. Zhang and Y. Zhao, J. Mater. Sci. Technol., 2020, 41, 117–126 CrossRef CAS.
- B. Thangaraj, B. Muniyandi, S. Ranganathan and H. Xin, Rev. Adv. Sci. Eng., 2015, 4, 106–119 CrossRef.
- R. Zhang, C. Miao, Z. Shen, S. Wang, C. Xia and W. Sun, ChemCatChem, 2012, 4, 824–830 CrossRef CAS.
- M. Kazemi, Synth. Commun., 2020, 50(14), 2095–2113 CrossRef CAS.
- T. Tang, M. Zhou, J. Lv, H. Cheng, H. Wang, D. Qin, G. Hu and X. Liu, Colloids Surf., B, 2022, 216, 112538 CrossRef CAS PubMed.
- M. Ghobadi, M. Kargar Razi, R. Javahershenas and M. Kazemi, Synth. Commun., 2021, 51(5), 647–669 CrossRef CAS.
- P. Rai and D. Gupta, Synth. Commun., 2021, 51, 3059–3083 CrossRef CAS.
- R. Dalpozzo, Green Chem., 2015, 17, 3671–3686 RSC.
- S. Payra, A. Saha and S. Banerjee, J. Nanosci. Nanotechnol., 2017, 17, 4432–4448 CrossRef CAS.
- M. Kazemi and M. Mohammadi, Appl. Organomet. Chem., 2020, 34(3), e5400 CrossRef CAS.
- Z. Kheilkordi, G. Mohammadi Ziarani, F. Mohajer, A. Badiei and M. Sillanpää, RSC Adv., 2022, 12, 12672–12701 RSC.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371 RSC.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS PubMed.
- J. Kurian, B. B. Lahiri, M. J. Mathew and J. Philip, J. Magn. Magn. Mater., 2021, 538, 168233 CrossRef CAS.
- G. K. Dhandabani, C.-L. Shih and J.-J. Wang, Org. Lett., 2020, 22, 1955–1960 CrossRef CAS PubMed.
- X. Zhou, Q. Huang, J. Guo, L. Dai and Y. Lu, Angew. Chem., 2023, 135(45), e202310078 CrossRef.
- P. Mehara, P. Sharma, A. K. Sharma, Shaifali and P. Das, Mol. Catal., 2023, 550, 113546 CrossRef CAS.
- D. Bhattacherjee, M. Rahman, S. Ghosh, A. K. Bagdi, G. V. Zyryanov, O. N. Chupakhin, P. Das and A. Hajra, Adv. Synth. Catal., 2021, 363, 1597–1624 CrossRef CAS.
- T. Xu, Q. Wang, Z. Yang, L. Yi, J. Wang, W. Lu, J. Ying and X. Wu, Chem. – Asian J., 2021, 16, 2027–2030 CrossRef CAS PubMed.
- H.-F. Liu, L. Long, Z.-Q. Zhu, T.-F. Wu, Y.-R. Zhang, H.-P. Pan, A.-J. Ma, J.-B. Peng, Y.-H. Wang, H. Gao and X.-Z. Zhang, Sci. Adv., 2023, 9(24), eadg7754 CrossRef CAS PubMed.
- T. Wakaki, T. Togo, D. Yoshidome, Y. Kuninobu and M. Kanai, ACS Catal., 2018, 8, 3123–3128 CrossRef CAS.
- P. Sharma, S. Rohilla and N. Jain, J. Org. Chem., 2017, 82, 1105–1113 CrossRef CAS PubMed.
- Z. Nie, T. Yang, M. Su, W. Luo, Q. Liu and C. Guo, Adv. Synth. Catal., 2022, 364, 2989–2995 CrossRef CAS.
- J. Zhang and X.-F. Wu, Org. Lett., 2023, 25, 2162–2166 CrossRef CAS PubMed.
- W. Zhang, C. Ai, K. Wang, J. Guo and B. Zhao, Asian J. Org. Chem., 2023, e202300413 CrossRef CAS.
- W. Zawartka, P. Pośpiech, M. Cypryk and A. M. Trzeciak, J. Mol. Catal. A: Chem., 2016, 417, 76–80 CrossRef CAS.
- F. Wu, J. Peng, X. Qi and X. Wu, ChemCatChem, 2018, 10, 173–177 CrossRef CAS.
- S. You, C. Yan, R. Zhang and M. Cai, Appl. Organomet. Chem., 2019, 33(2), e4650 CrossRef.
- X. Zeng, D. Xu, C. Miao, C. Xia and W. Sun, RSC Adv., 2014, 4, 46494–46497 RSC.
- X. Wu, Y. Zeng, L. Meng and X. Li, Mol. Catal., 2023, 541, 113098 CrossRef CAS.
- M. Aqeel Ashraf, Z. Liu, Y. Yang and D. Zhang, Synth. Commun., 2020, 50, 2885–2905 CrossRef CAS.
- J. Choi, A. Cho, J. H. Cho and B. M. Kim, Appl. Catal., A, 2022, 642, 118709 CrossRef CAS.
- E. Etemadi-Davan and N. Iranpoor, ChemistrySelect, 2016, 1, 4300–4304 CrossRef CAS.
- Q. Zhou, S. Wei and W. Han, J. Org. Chem., 2014, 79, 1454–1460 CrossRef CAS PubMed.
- M. L. N. Rao, V. Venkatesh and D. Banerjee, Tetrahedron, 2007, 63, 12917–12926 CrossRef CAS.
- K. Yan, M. Liu, J. Wen, X. Liu, X. Wang, X. Sui, W. Shang and X. Wang, New J. Chem., 2022, 46, 7329–7333 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06533b |
| This journal is © The Royal Society of Chemistry 2023 |