Open Access Article
Open Access ArticleA DFT study towards dynamic structures of iron and iron carbide and their effects on the activity of the Fischer–Tropsch process†
Qiang Yinac,
Hanqing Wang*bc,
Jinping Zhaob,
Chengjun Lib and
Yu Mao *d
*d
aDepartment of Forestry Engineering, School of Materials Science and Engineering, Central South University of Forestry & Technology, Changsha, China
bSchool of Civil Engineering, Central South University of Forestry & Technology, Changsha, China. E-mail: hqwang@csuft.edu.cn
cHunan Engineering Research Centre of Full Life-cycle Energy-efficient Buildings and Environmental Health, Central South University of Forestry and Technology, Changsha, Hunan, China
dSchool of Chemical Sciences, University of Auckland, Auckland 1010, New Zealand. E-mail: yu.mao@auckland.ac.nz
First published on 22nd November 2023
Abstract
The Fe-based Fischer–Tropsch synthesis (FTS) catalyst shows a rich phase chemistry under pre-treatment and FTS conditions. The exact structural composition of the active site, whether iron or iron carbide (FeCx), is still controversial. Aiming to obtain an insight into the active sites and their role in affecting FTS activity, the swarm intelligence algorithm is implemented to search for the most stable Fe(100), Fe(110), Fe(210) surfaces with different carbon ratios. Then, ab initio atomistic thermodynamics and Wulffman construction were employed to evaluate the stability of these surfaces at different chemical potentials of carbon. Their FTS reactivity and selectivity were later assessed by semi-quantitative micro-kinetic equations. The results show that stability, reactivity, and selectivity of the iron are all affected by the carbonization process when the carbon ratio increases. Formation of the carbide, a rather natural process under experimental conditions, would moderately increase the turnover frequency (TOF), but both iron and iron carbide are active to the reaction.
Introduction
As one of the most important catalytic processes in industry, Fischer–Tropsch synthesis (FTS) converts synthesis gas (CO + H2) into a wide spectrum of long-chain hydrocarbons and oxygenates.1 Four transition metals, namely Fe, Co, Ni, and Ru, have been used in catalyzing the FTS; however, only Fe and Co are industrially feasible because the methane selectivity on Ni is undesirably high, and the resources of Ru are rather limited.2 Both Fe and Co are suitable for low-temperature FTS (473–513 K) while only Fe is used at high temperature (573–623 K).3 In recent years, Fe-based FTS catalysts have attracted increasing attention from researchers owing to their lower price, higher resistance to contaminants, and higher activity of the water–gas shift (WGS) reaction.4,5The Fe-based FTS catalyst shows a rich phase chemistry under pretreatment and FTS conditions.6 Industrially, small iron oxide crystallites (e.g. hematite, α-Fe2O3) are firstly reduced to magnetite (Fe3O4) irrespective with H2, CO, or their mixture during the pre-treatment, and the catalyst then converts to a mixture of carbides, oxides, and metallic iron depending on the pre-treatment conditions.2,7–11 Among the mixture, iron oxides, especially Fe3O4, are reported to be active for WGS reaction, while metallic iron and iron carbides are known for its ability to dissociate C–O bond at room temperature, thus reported to be active for FTS.2,12,13 Experimentally, it is found that FTS activity increases with the extent of Fe3O4 conversion into iron carbides;14–16 therefore, many researchers argue that the carbide is the active phase and responsible for high FTS activity.4,10,17–19 Among them, Hägg iron carbide (χ-Fe5C2) is the most common one and has been investigated extensively.7,10,11,15,20–23 In addition, other carbides surfaces, with the atomic ratio of C to Fe ranged from 0.33 to 0.50 (Fe2C, Fe2.2C, Fe3C, Fe7C3, etc.), have also been studied.14,24–27
However, most of these studies28–30 are based on comparing energy barriers of elementary steps on some facets of pure Fe and Fe carbide, without investigating their relative stability and overall micro-kinetics. In addition, the exact mechanism to produce hydrocarbons over iron carbide phases is largely unknown. Therefore, the exact structural composition of the active phase and the role of these phases are still controversial due to the limitations of in situ characterization of the catalyst.2,14 Claims of catalytic activity of Fe3O4,31–33 pure Fe,16,34,35 and iron carbides26,36,37 in FTS are all found in previous studies. From the Mössbauer emission spectroscopy (MES), Niemantsverdriet et al.34 found that the carbides can only be observed in coexistence with pure Fe or just after it disappeared. Therefore, the carbide during FTS may be a poorly defined intermediate between pure Fe and known carbide structures. The working catalyst is likely to be a mixture of amorphous regions, in which the surface composition may differ from the bulk.2,11,38
In addition to rich phase chemistry, FTS is also regarded as one of the most complicated catalytic system owing to two facts: (i) there are scores of – if not hundreds of – elementary reactions interconnected with each other in FTS mechanism; (ii) both reactivity and selectivity should be considered since the reaction is aiming for long-chain hydrocarbon, rather than by-product methane. Generally, there are three types of mechanisms proposed in the literature:3,39 the carbene mechanism,40 the hydroxyl-carbene mechanism,41 and the CO-insertion mechanism.42 Supported by plenty of theoretical and experimental evidences,43–45 the carbene mechanism is the most popular among them.1,46–49 In this mechanism, CO adsorbs dissociatively into C and O on the surface, C then sequentially hydrogenated to give C1 (CHx, x = 1–3) species, and O gets removed from the surface as water. After that, the C1 species can either be further hydrogenated to form methane, or couple with another C1, resulting in C2 species (CHx–CHy, x, y = 0–3). Similar hydrogenation and coupling processes continue to give long-chain hydrocarbons.50–52
In such a complicated catalytic reaction system, a lack of deep understanding of the active phase composition and corresponding reaction pathways greatly limits the design of better Fe-based FTS catalysts. Several questions remain to be addressed: (i) what is the influence of the reactions conditions on the dynamic structure of iron surfaces; (ii) what is the origin of the high activity, as claimed in many literature, of iron carbides;50,53 (iii) what is the actual composition of the active phase during FTS. In this study, swarm intelligence algorithm54 was implemented to search Fe(100), Fe(110), Fe(210) surfaces with different carbon ratios, which extends well beyond the current literature that focus on investigating a specific type of iron or carbide phase. It is perhaps more close to the realistic FTS system since the catalyst activity is determined by the nature of the surface phase rather than the bulk phase composition.38 Then, the stability of these surfaces was evaluated by ab initio atomistic thermodynamics and Wulffman construction55–57 at different reaction conditions. Their FTS reactivity and selectivity were later semi-quantitively assessed by a micro-kinetic model developed by Chen et al.46,58–60 and a general discussion was addressed on these results.
Methods
All calculations in the paper were carried out with the Perdew–Burke–Ernzerhof61 functional using Vienna ab initio simulation package (VASP).62,63 The project-augmented wave (PAW) method was used to represent the core–valence interaction.64,65 For the calculations of total energy, a cut-off energy of 450 eV was set for plane wave basis sets to expand the valence electronic states. Spin polarization was included for all calculations to obtain an accurate description of magnetic systems. The values of magnetic moments of each layer by our calculation agree well with previous results.66 For example, the magnetic moments of first and second layer atoms of Fe(110) are 2.61 and 2.36 μB, respectively, very close to 2.59 and 2.35 μB in the work of Kresse et al.67 The agreement verifies the rationality of our computational setting. Also, the PBE functional have been extensively used in similar studies.68–70 We have also tested the CO adsorption energy using different functionals, namely PBEsol and RPBE, and shows similar trends as that of PBE functional.To obtain the free energy of species, some standard formulas of statistical mechanics were used to calculate the zero point energy (ZPE), thermal energy, and entropy derived from partition functions71,72 (see Section SI-1 in the ESI† for detailed equations). The Brillouin zone was sampled with a k-point mesh of 4 × 4 × 1 for Fe(100), 4 × 3 × 1 for Fe(110), and 3 × 4 × 1 Fe(210) (see Section SI-2 in the ESI† for the illustrations of surface models and their cell parameters). Upper half of substrate layers and adsorbates were fully relaxed until the forces were lower than 0.05 eV Å−1 while the lower half of substrate layers were fixed. The adsorption energy of X is defined as:
| EXad = E(X/substrate) − E(substrate) − E(X) | (1) |
The most stable structures of Fe(100), Fe(110), and Fe(210) with different ratio of surface carbon are obtained using the swarm intelligence algorithm implemented in CALYPSO,75,76 which is an effective tool to search for the global minimum of structures for many different systems77–80 In CALYPSO, swarm intelligence, specifically the particle swarm optimization (PSO) algorithm, is employed to predict the crystal structures of materials based on their chemical compositions. The PSO algorithm is best-known for its ability to conquer large barriers of energy landscapes by making use of the swarm intelligence and by self-improving structures. This this study, it is used to search the potential energy landscape of the surface.81
For these surface models, 20 generations with 16 individuals in each generation are optimized during the structure evolution. Lower two layers are fixed as Fe bulk while top two layer are generated by CALYPSO by specifying the number of Fe and C atoms. All other parameters are default values of CALYPSO surface evolution. Furthermore, ab initio thermodynamics82–84 was employed to evaluate the surface energy γ of iron carbide surfaces at experimental conditions:7,14
| γ(T, P) = [Gsur(T, P, NFe, NC) − NFeμFe(T, P) − NCμC(T, P)]/2A | (2) |
The crystal morphology of iron carbide is obtained from Wulffman construction,55–57 which is the standard method for determining the equilibrium shape of bulk crystals by minimizing its orientation-dependent surface free energy for a given enclosed volume.85,86 It offers a simple and rigorous way to describe the shape of nanoparticles by the surface energies of some (hkl) faces and the point group of the material.87
Results and discussion
Carbide surfaces obtained from CALYPSO
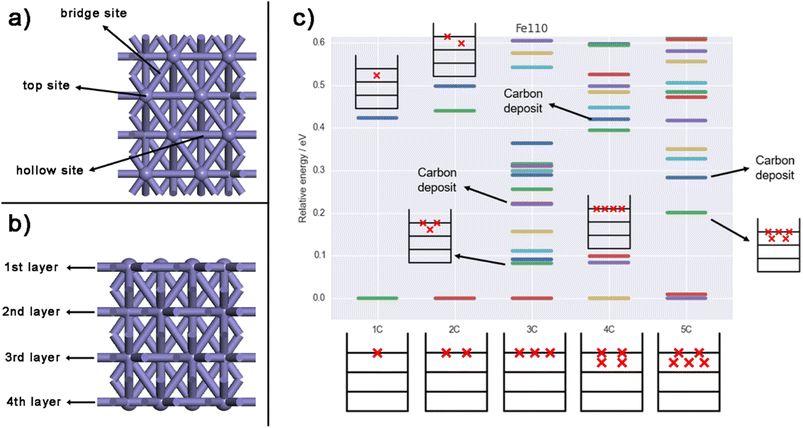
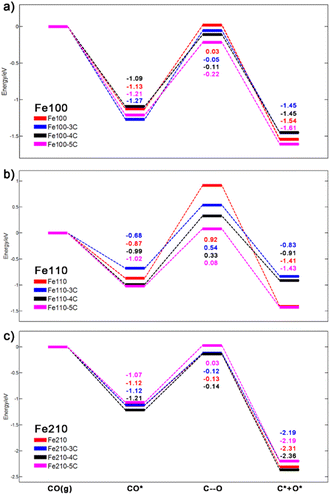
Fig. 1 illustrate the structures and relative energies of Fe(110) surface with different carbon ratio from 1 to 5 (results of Fe(100) and Fe(210) can be found in Section SI-3 in the ESI†). As shown in Fig. 1a and b a four-layer model was built for pure Fe(110) surface. In CALYPSO, the bottom two layers are fixed while the 2nd layer is allowed to relax. The first layer is generated randomly by swarm intelligence algorithm along with a certain number of C atoms (one to five). After optimization, the most stable structures and their relative energies are displayed in Fig. 2 and 1c, respectively. This simulation method aligns closely with experimental observations. After pre-treatment, iron carbides form on the exterior of Fe3O4 or Fe crystallites, resulting in an enveloping core structure.10,88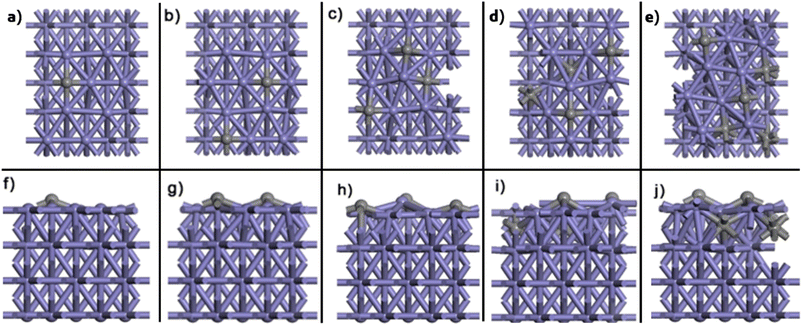 | ||
| Fig. 2 (a–e) Top and (f–j) front views of the most stable Fe(110) surfaces with one to five C, respectively. Black balls represent C and purple balls stand for Fe. | ||
In Fig. 2, the C atom tends to stay above the surface; for Fe(110)-1C, the energy of the structure with a surface C atom is ∼0.4 eV lower than that of the structure with a subsurface C. However, when there are more than three C atoms, two or three of them will diffuse to sub-surfaces due to the large repulsion between surface atoms. For Fe(110)-3C, the energy of the structure with all surface C is ∼0.08 eV lower than the second stable one; while the energy of the Fe(110)-4C with four surface C, on the other hand, is ∼0.1 eV higher than the most stable one. Similar conclusions can be made for Fe(100) and Fe(210) surfaces. In Fig. S1 and S2,† C atoms diffuse into sub-surfaces in 4C and 5C structures for both Fe(100) and Fe(210). It should be noted that for Fe(210)-5C, the most stable structure is the carbon deposit, which is reasonable since the deposit is easier to grow on the step facets.72 It is well recognized that the C deposits (e.g. coke, graphitic carbon, and amorphous carbon) on the surface will lead to the deactivation;2,34,37,89 therefore, in this study, any structure with two or more C atoms link together on the surface is regarded inactive, and the second most stable one of Fe(210)-5C is adopted for further activity analysis. It should be noted that those linking C atoms may be an artifact of the small size of the system. The more detailed examination of coke formation and its stability as the carbon ratio increases (e.g. employing large slab size for global optimization) is our future research endeavors.
Generally, iron surfaces show a variety of structures under different carbon concentration. Starting from the upper surface, the C would gradually penetrate the bulk into sub-surfaces when the number of C atoms accumulates, forming the carbide structure as observed in the experiments.90 For carbide with one or two C, structures with low energy are quite sparse and the most stable one prevails among all. When the number of C atoms increase, however, surface structures become more flexible, and many of them are with similar stability.
Stability of carbide surfaces in the reaction conditions
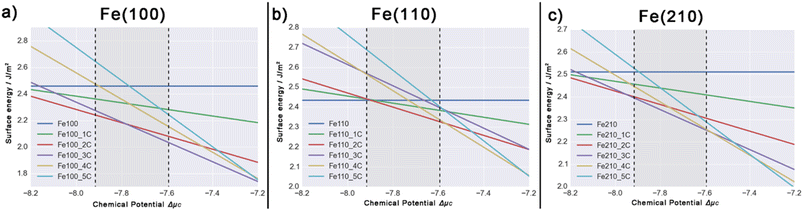
The pretreatment conditions such as the ratio of reduction gases (H2/CO), temperature, and pressure have significant effects on the stability of iron carbide surfaces.7,9 These complicated factors can be lumped to one variable μC,7 the chemical potential of carbon, which can be used to evaluate surface energy γ under experimental conditions.7,14,24,91 Using eqn (2), the ab initio thermodynamics stability for carbide surfaces of Fe(100), Fe(110), and Fe(210) from 1C to 5C are plotted in Fig. 3. In this figure and following discussion, we use the total energy of a carbon atom (EC) as the reference, i.e., ΔμC = μC − EC. Considering the carbide formation reaction involved in synthesis gas (CO + H2 → C(Fe) + H2O), μC is obtained by: μC = μCO + μH2 − μH2O, which can be expanded into:
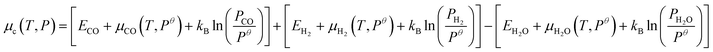 | (3) |
The partial pressures of the gas-phase constituents are determined by considering reaction conditions (more details in Table S9† of ref. 7). At low H2/CO ratio (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 2.5
10 to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ΔμC hardly changes and the maximum value is −7.594 eV when H2/CO = 2.5
1), ΔμC hardly changes and the maximum value is −7.594 eV when H2/CO = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. On the other hand, ΔμC decrease significantly as the ratio continue to rise; when H2/CO reaches 10
1. On the other hand, ΔμC decrease significantly as the ratio continue to rise; when H2/CO reaches 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ΔμC would be as small as −7.916 eV. The gray area in Fig. 3 represents these typical experimental ΔμC range (−7.916 to −7.594), and their corresponding γ values are listed in Table 1.
1, ΔμC would be as small as −7.916 eV. The gray area in Fig. 3 represents these typical experimental ΔμC range (−7.916 to −7.594), and their corresponding γ values are listed in Table 1.
| Surface | Fe(100) | Fe(110) | Fe(210) | |||
|---|---|---|---|---|---|---|
| ΔμC (eV) | −7.594 | −7.916 | −7.594 | −7.916 | −7.594 | −7.916 |
| Pure facet | 2.46 | 2.46 | 2.44 | 2.44 | 2.51 | 2.51 |
| 1C | 2.28 | 2.36 | 2.38 | 2.44 | 2.41 | 2.46 |
| 2C | 2.08 | 2.24 | 2.33 | 2.44 | 2.30 | 2.40 |
| 3C | 2.03 | 2.27 | 2.40 | 2.57 | 2.25 | 2.40 |
| 4C | 2.15 | 2.47 | 2.34 | 2.56 | 2.26 | 2.45 |
| 5C | 2.24 | 2.64 | 2.40 | 2.69 | 2.27 | 2.53 |
Taking Fe(100) (Fig. 3a) as an example, one can see that the surface energy of pure Fe(100) is a horizontal line, and Fe(100)-5C has the most steep slope; it is reasonable since more C on the surface means more influence by ΔμC; the higher the chemical potential of carbon is, the more stable the carbide with higher carbon ratio. At experimental conditions, Fe(100)-2C is the most stable one when ΔμC < −7.78 eV, while Fe(100)-3C become dominate after that. In Fig. 3b and c, Fe(110)-2C and Fe(210)-3C are the most stable surface, respectively. Therefore, under realistic conditions (H2/CO ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 2.5
10 to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 600 K), all three Fe surfaces are moderately carbonized (with two to three C atoms).
1 at 600 K), all three Fe surfaces are moderately carbonized (with two to three C atoms).
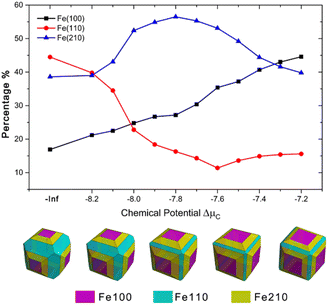
In addition to single facets, catalysts are more likely in a form of nanocrystals under realistic conditions. Wulff construction is a standard method to determine the equilibrium shape of bulk crystals by minimizing surface energy on given orientations. By Wulff construction, morphologies of iron crystal with Fe(100), Fe(110), and Fe(210) facets under five different ΔμC are presented in Fig. 4 (detailed results are listed in Section SI-4 in the ESI†). For pure Fe (ΔμC = −Inf), there are about 16.9% of Fe(100), 44.5% of Fe(110), and 38.6% of Fe(210). When ΔμC increase, Fe(100) expands monotonously and finally dominates the surface with a coverage of 44.6%. The percentage of Fe(110) firstly decreases to 11.4% at ΔμC = −7.6 eV and then starts to increase again. The percentage of Fe(210), on the contrary, increase to 56.5% until ΔμC reaches −7.8 eV and then decrease to 39.8% in the end. Five crystal structures (from left to right, ΔμC = −Inf, −8.1, −7.8, −7.6, −7.4 eV, respectively) are illustrated in the bottom of the figure to give a visual impression of this trend.
Reactivity and selectivity
In Chen et al.’s previous studies,46,58–60,92 key elementary reactions of FTS were investigated, and some micro-kinetic equations, considering both energy barrier and surface coverage, were derived to quantitively evaluate the activity of FTS (details in Section SI-5 in the ESI†). To obtain the required parameters for evaluating the FTS activity, we systematically calculated the intermediates and transition states of CO dissociative adsorption, CHx hydrogenation, and CHx + CHx coupling process on all surfaces of Fe(100), Fe(110), and Fe(210). Regarding the H2 dissociation, Fe is known to be a quite active metal towards H2 dissociation, according to previous research,93 the H2 dissociation barrier is as low as 0.16 eV at Fe100 surface; therefore, we assume that H2 dissociation is very fast in our study.For the selectivity of the FTS, by considering both energy barriers and surface coverage, the following equation can be derived (details in ESI Section SI-5†):
| rCH4/rC–C ∝ e−ΔEeff/RT | (4) |
Owing to the extremely complicated reaction network of the FTS (typically more than 40 elementary reactions even in a simplified reaction model), calculating the turnover frequency of the system becomes very hard and laborious. Fortunately, it is well-known that the activity of CO hydrogenation versus the binding strength of C and O atoms generally shows a volcano curve,94,95 and the rate of the FTS could be further qualitatively estimated from these descriptors.58,96 In this study, we use the dissociative adsorption barrier of CO, EC–O, as the indictor of the surface reactivity.19,58
For the reactivity and selectivity of the FTS, it should be noted that when the ratio of C to Fe is low, the C atoms are exclusively located on the surface (Fig. 2, S3, and S4†). Under steady state, surface carbon species will continuously exchange with gas phase CO flow to reach an equilibrium. Therefore, kinetically, there is no difference between clean Fe and carbides with only surface C when the coverage is low. It is the sub-surface C atoms that modify their electronic and geometrical properties. This treatment is supported by the isotope labelling experiments that the exchange of 13C carbon atoms in iron carbide with the reacting molecules was very slow.97,98 According to the study of Ordomsky et al.36 for FTS on Fe13Cx surface, 13C decrease with time for all hydrocarbons, and only one 13C appears on long chain products. It means that the surface C of the carbides, which were labelled by 13C, involved only in the first catalytic cycle, and all following C comes from gas phase CO which then reaches an equilibrium with the surface. Therefore, in this study, we deem the reactivity and selectivity of clean surface and carbide with one and two carbons are the same.
Finally, all EC–O and ΔEeff of Fe(100), Fe(110), Fe(210), and their carbides are calculated. Taking Fe(210)-1C and 2C for example, all structures and selective energies are show in Fig. 5 and Table 2.
| Ei,j | Ei | Ej | Ei,j + Ei + Ej | |
|---|---|---|---|---|
| C–C | 3.19 | 0.00 | 0.00 | 3.19 |
| C–CH | 2.47 | 0.00 | 0.49 | 2.96 |
| C–CH2 | 1.32 | 0.00 | 1.47 | 2.78 |
| C–CH3 | 1.19 | 0.00 | 1.56 | 2.75 |
| CH–CH | 2.37 | 0.49 | 0.49 | 3.35 |
| CH–CH2 | 1.68 | 0.49 | 1.47 | 3.64 |
| CH–CH3 | 1.71 | 0.49 | 1.56 | 3.76 |
| CH2–CH2 | 0.87 | 1.47 | 1.47 | 3.80 |
| CH2–CH3 | 1.43 | 1.47 | 1.56 | 4.46 |
| Eeff,C–C | — | — | — | 2.75 |
| Eeff,CH4 | — | — | — | 3.01 |
| ΔEeff | — | — | — | 0.25 |
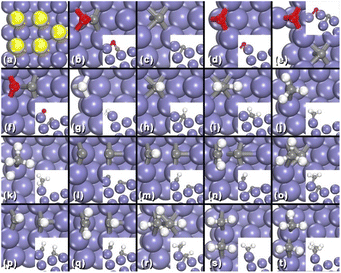
As we stated in last paragraph, under steady state, the surface carbons in Fe(210)-1C and 2C would reach an equilibrium with gas phase carbon species, and there is kinetically no difference between them and pure Fe(210) surface. There is a typical B-5 site in this step surface (Fig. 5a), in which most of elementary reactions happen. Fig. 5b–e represent CO, C, and O adsorption, C and O co-adsorption, respectively. In the C–O transition state (Fig. 5f), C locates on the 3-fold hollow site on the lower terrace, while the O atom is on the 2-fold edge-bridge site, with a stretched C–O distance of 2.01 Å. The energy profile of CO dissociative adsorption is shown by the red line in Fig. 6c; we can see that the energy barrier is 0.99 eV with respect to adsorbed CO and −0.13 eV with respect to CO in the gas phase.
After CO dissociation, carbon species will be hydrogenated gradually. The most stable sites for CH and CH2 are the corner sites, while the edge-bridge site is favoured by CH3 (Fig. 5h–j). Transition states of CH3 + H and nine possible C1 + C1 coupling pathways, C + C, C + CH, C + CH2, C + CH3, CH + CH, CH + CH2, CH + CH3, CH2 + CH2, and CH2 + CH3, are shown in Fig. 5k–t. With energies of these transition states, Eeff,C–C, Eeff,CH4, and their energy difference ΔEeff are listed in Table 2. As we mentioned before, Eeff,C–C is obtained from the minimal value of Ei,j + Ei + Ej of all nine C1 + C1 coupling process while Eeff,CH4 stands for the effective barrier of CH3 hydrogenation. For Fe(210), the difference of Eeff,C–C and Eeff,CH4, ΔEeff, is 0.25 eV, indicating that C1 + C1 coupling is moderately more feasible than CH3 hydrogenation.
Complete results of all carbide surfaces are listed in Table 3, and their energy profiles of the CO dissociative adsorption are illustrated in Fig. 6 (all relevant structures and energies are listed Section SI-6 in the ESI†). We can see that the EC–O of Fe(110), a close packed surface, are very high; while the EC–O of Fe(210) are the lowest among three, which is quite reasonable since the step surfaces are generally regarded more active than flat surfaces. When the number of C increases, the EC–O of Fe(100) and Fe(110) decrease considerably from 0.03 to −0.22 eV and 0.92 to 0.08 eV, respectively. The EC–O of Fe(210), on the other hand, remain at the same level of −0.13 eV except Fe(210)-5C. It means that the formation of iron carbide could significantly lower the energy barrier of CO dissociation adsorption of flat surfaces like Fe(100) and Fe(110), making FTS more reactive on these surfaces. For example, on pure Fe(110) surface, the FTS is nearly impossible to happen with a EC–O of 0.92 eV, the incorporation of C atoms into the Fe bulk phase lower the barrier to 0.54, 0.33, and finally 0.08 eV, which makes it a probably active phase for FTS.
| Surface | Fe(100) | Fe(110) | Fe(210) | |||
|---|---|---|---|---|---|---|
| EC–O | ΔEeff | EC–O | ΔEeff | EC–O | ΔEeff | |
| Pure facet | 0.03 | 0.39 | 0.92 | 1.53 | −0.13 | 0.25 |
| 1C | 0.03 | 0.39 | 0.92 | 1.53 | −0.13 | 0.25 |
| 2C | 0.03 | 0.39 | 0.92 | 1.53 | −0.13 | 0.25 |
| 3C | −0.05 | 0.57 | 0.54 | 1.26 | −0.12 | 0.59 |
| 4C | −0.11 | 0.53 | 0.33 | 0.83 | −0.14 | 0.55 |
| 5C | −0.22 | 0.76 | 0.08 | 1.01 | 0.03 | 0.03 |
Fe(110) surfaces have the highest selectivity towards long chain hydrocarbons because of their large ΔEeff values, although the values decrease moderately as the formation of carbide; however, that may not affect their overall activity much due to the low reactivity of Fe(110) surfaces. For both Fe(100) and Fe(210) surfaces (except Fe(210)-5C), on the other hand, ΔEeff increase significantly when the carbide are formed.
Discussion
Here we are in a stage to answer the following important question: what is the origin of the high activity, as claimed in many literature,50,53 of the iron carbide, and what is the actual active phase during FTS? There are mainly three factors that influence the activity of surfaces: stability, reactivity, and selectivity.99 In Table 1, it is quite evident that the stability of Fe(100) and Fe(210) surfaces increase as C atoms are introduced into the system; the stability of Fe(110), on the other hand, do not vary a lot (at ΔμC = −7.594 eV) and even decrease when more C atoms are incorporated in (at ΔμC = −7.916 eV). This trend becomes more evident in Fig. 4, the surface morphologies by Wulffman construction. Starting from pure Fe particles, the surface ratio of Fe(100) and Fe(210) increase while Fe(110) shrinks significantly; Fe(210) then becomes the dominant phase in a large range of ΔμC from −8.1 to 7.4 eV; it peaks around the working condition of FTS (ΔμC from −7.916 to −7.594 eV). These features of surface stability show that carbides are in favour of FTS activity; as shown in last sub-section, Fe(110), a close-packed surface, is quite inert while Fe(210) is active towards FTS as a step surface. Therefore, the FTS activity of Fe will benefit from the formation of carbides on the surface, because it will stabilise the FTS-active step surface rather than FTS-inert close packed surface.In Table 3, with respect to the selectivity, both Fe(100) and Fe(210) – except Fe(210)-5C – tend to produce more long-chain hydrocarbons when the surface are carbonized, whereas for Fe(110), on the other hand, the value of ΔEeff decrease, but they still remain highest among three surfaces. In Fig. 6, with respect to the reactivity, pure Fe(110) is almost inert with a high EC–O of 1.79 eV (the energy difference between C–O, 0.92 eV and CO*, −0.87 eV), but the barrier drops dramatically as the C ratio increase. Therefore, for Fe(110) facet, we can safely conclude that it is the carbide that make it active towards FTS. Fe(100) has a similar trend with Fe(110); however, EC–O do not vary a lot on Fe(210), which already has a relatively low EC–O of ∼0.99 eV. Overall, the carbonisation of Fe surfaces will significantly lower the EC–O of flat facet (Fe(100) and Fe(110)) and increase the selectivity of Fe(100) and Fe(210) towards long-chain hydrocarbons.
We can see that all three factors – stability, selectivity, and reactivity – contribute to a higher FTS activity of iron carbides. One question naturally arises here: why does this happen? Geometrically, it is obvious that the introduction of C alters the surface structure. For flat facet like Fe(100) and Fe(110) (Fig. 2 and S3†), the C atoms bend the plane and make it uneven, which provides them possible B-5-like sites to facilitate FTS process. We then analyse electronic effects of C in the system by calculating the location of the d-band center with respect to the Fermi energy, εd. εd was proposed by Nørskov and co-workers100–102 and has been widely used to evaluate surface reactivity. For CO adsorption on surface, a higher εd usually means a stronger adsorption and higher reactivity. We can see from Fig. 7a that as the number of carbon atoms increase, the d-band center (εd) of the carbide surfaces decrease monotonously. The result indicates that the introduced C will make the surface more inert, which is opposite to the reactivity trend we have gotten before. Computational results show that geometrical effects of the C play major roles in shaping the reactivity of Fe surfaces, since the trend of d-band center (electronic effects) does not account for the strengthened CO adsorption. It is quite reasonable since Fe(210), an originally stepped surface, do not benefit from the doping of C, and EC–O of Fe(210)-5C even increased, which is probably due to its electronic factors.
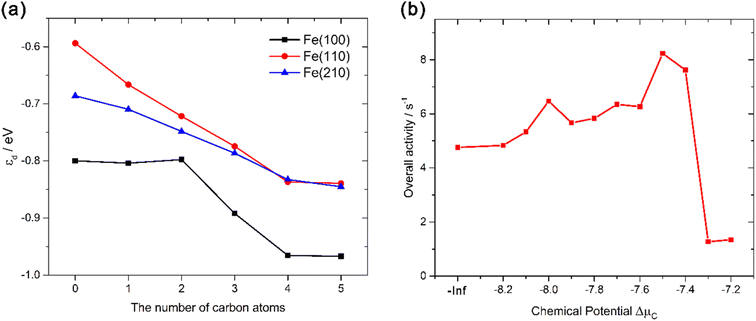 | ||
| Fig. 7 Line graph of (a) d-band centers of different carbide surfaces with respect to the number of carbon atoms in the carbide; (b) the estimated overall FTS activity of the carbide surfaces versus chemical potential of carbon ΔμC. It should be noted that since the density of state (DOS) of Fe, as a magnetic material, is not symmetrical along with the x-axis, the spin-up d-band center (εd↑) and the spin-down d-band center (εd↓) are not the same;106 εd used in (a) are the spin-averaged d-band center. All εd↑, εd↓, and εd values and their calculation methods are in Section SI-7 of the ESI.† | ||
Finally, to combine all three factors together, we conduct a rough kinetic estimation by deeming CO dissociative adsorption as the rate-determining step. We admit that the RDS in FTS can vary based on the catalyst used, the specific conditions under which the reaction occurs, and the particular mechanistic pathway considered. However, for many FTS catalysts, the dissociation of CO is indeed a critical step and has been proposed as the rate-determining step, especially for iron based catalysts.103–105 For the rough estimation for general discussion, the overall rate can be expressed as:
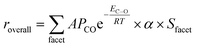 | (5) |
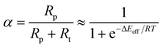 | (6) |
Therefore, from above qualitative analysis, we may conclude that both Fe and iron carbide are active to FTS process. Formation of the carbide during FTS would moderately increase the reaction rate and make some originally inert facets active, but it is not essential to the overall activity of the catalysts. Rather, as we can see from the ab initio thermodynamics analysis, the formation of the carbide is a natural process under realistic conditions. It is also experimentally reasonable since the activation barriers of carbon diffusion into the metallic Fe structure are similar to that of the hydrogenation and polymerization at the surface.111
Conclusion
In summary, this work is aimed at obtaining a computational understanding of the formation of the iron carbide and their roles in catalysing Fischer–Tropsch synthesis. Given the complexities of the experimental conditions and the complexity of FTS mechanism (>100 elementary reactions), we could not claim that our simulation exactly matches experimental conditions. However, we believe our computational results could inspire experimentalists and deepen the broader understanding of FTS over Fe/FeCx surfaces.Swarm intelligence algorithm was implemented to search most stable Fe(100), Fe(110), Fe(210) surfaces with different carbon ratios. Then, ab initio atomistic thermodynamics and Wulffman construction were employed to evaluate the stability of these surfaces at different chemical potentials of carbon ΔμC. Their FTS reactivity and selectivity were later assessed by some micro-kinetic equations derived in our previous studies. The results and conclusions regarding carbide structures and stability are summarized in the following:
(i) The introduced C atoms tend to stay on the surface of the Fe facets; they will not penetrate into sub-surfaces to form bulk carbide until a certain concentration of C is reached.
(ii) When C concentration is low (typical with one or two C atoms), structures with low energy are quite sparse and the most stable one prevails among all. In a 2 × 2 slab model used in this study (four adsorption sites), one or two C atoms could correspond to 0.25 and 0.50C coverage.112–114 When C coverage increase, however, surface structures become more flexible, and many of them are with similar stability.
(iii) Under realistic conditions (H2/CO ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 2.5
10 to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 600 K), all three Fe surfaces are moderately carbonized (with two to three C atoms in the model of this study, corresponding to ∼0.5C coverage).
1 at 600 K), all three Fe surfaces are moderately carbonized (with two to three C atoms in the model of this study, corresponding to ∼0.5C coverage).
(iv) According to Wulff construction of Fe crystal, Fe(100) expands monotonously and finally dominates the surface with a coverage of 44.6%. The percentage of Fe(110) firstly decreases to 11.4% at ΔμC = −7.6 eV and then starts to increase again. The percentage of Fe(210), on the contrary, increase to 56.5% until ΔμC reaches −7.8 eV and then decrease to 39.8% in the end. In most cases (ΔμC = −8.1 to 7.4 eV), Fe(210) dominates the surface.
Regarding to FTS activity, the following conclusions are obtained:
(i) The carbonisation of Fe surfaces will significantly lower the EC–O of flat facets – Fe(100) and Fe(110). Theoretically, with a reduced EC–O from 1.79 to 1.10 eV, Fe(110) surface transformed from a FTS inert surface to an active one.
(ii) Using a selectivity equation derived from simplified micro-kinetic derivation, we found that the selectivity of Fe(100) and Fe(210) towards long-chain hydrocarbons are improved when the carbon ratio increases, and Fe(110) always has a highest selectivity among all three facets.
(iii) By a rough overall kinetic estimation, we suggest that both Fe and iron carbide are active to FTS process. Formation of the carbide during FTS would moderately increase the reaction rate but is not essential to the overall activity of the catalysts; it is rather a natural process under realistic conditions.
Conflicts of interest
The authors declare that they have no known competing financial interests of personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. U1867221 and 52276094), the Natural Science Foundation of Hunan Province (2022JJ40038), the Changsha Science and Technology Project (kq2022141), and Joint research project of State Administration of Science, Technology and Industry for National Defense, KY20005.References
- R. A. van Santen, I. M. Ciobîcă, E. van Steen and M. M. Ghouri, in Advances in Catalysis, ed. C. G. Bruce and K. Helmut, Academic Press, 2011, vol. 54, pp. 127–187 Search PubMed.
- E. de Smit and B. M. Weckhuysen, Chem. Soc. Rev., 2008, 37, 2758–2781 RSC.
- J. Cheng, P. Hu, P. Ellis, S. French, G. Kelly and C. M. Lok, Top. Catal., 2010, 53, 326–337 CrossRef CAS.
- P. Thanh Hai, Y. Qi, J. Yang, X. Duan, G. Qian, X. Zhou, D. Chen and W. Yuan, ACS Catal., 2015, 5, 2203–2208 CrossRef.
- H. M. Torres Galvis, A. C. J. Koeken, J. H. Bitter, T. Davidian, M. Ruitenbeek, A. I. Dugulan and K. P. de Jong, J. Catal., 2013, 303, 22–30 CrossRef CAS.
- E. de Smit, I. Swart, J. F. Creemer, G. H. Hoveling, M. K. Gilles, T. Tyliszczak, P. J. Kooyman, H. W. Zandbergen, C. Morin, B. M. Weckhuysen and F. M. F. de Groot, Nature, 2008, 456, 222–225 CrossRef CAS PubMed.
- S. Zhao, X.-W. Liu, C.-F. Huo, Y.-W. Li, J. Wang and H. Jiao, J. Catal., 2012, 294, 47–53 CrossRef CAS.
- M. Ding, Y. Yang, B. Wu, J. Xu, C. Zhang, H. Xiang and Y. Li, J. Mol. Catal. A: Chem., 2009, 303, 65–71 CrossRef CAS.
- M. Luo, H. Hamdeh and B. H. Davis, Catal. Today, 2009, 140, 127–134 CrossRef CAS.
- T. Herranz, S. Rojas, F. J. Pérez-Alonso, M. Ojeda, P. Terreros and J. L. G. Fierro, J. Catal., 2006, 243, 199–211 CrossRef CAS.
- E. de Smit, A. M. Beale, S. Nikitenko and B. M. Weckhuysen, J. Catal., 2009, 262, 244–256 CrossRef CAS.
- M. A. Petersen, J.-A. van den Berg and W. J. van Rensburg, J. Phys. Chem. C, 2010, 114, 7863–7879 CrossRef CAS.
- J. M. Gracia, F. F. Prinsloo and J. W. Niemantsverdriet, Catal. Lett., 2009, 133, 257–261 CrossRef CAS.
- E. de Smit, F. Cinquini, A. M. Beale, O. V. Safonova, W. van Beek, P. Sautet and B. M. Weckhuysen, J. Am. Chem. Soc., 2010, 132, 14928–14941 CrossRef CAS PubMed.
- T. Riedel, H. Schulz, G. Schaub, K.-W. Jun, J.-S. Hwang and K.-W. Lee, Top. Catal., 2003, 26, 41–54 CrossRef CAS.
- M. D. Shroff, D. S. Kalakkad, K. E. Coulter, S. D. Kohler, M. S. Harrington, N. B. Jackson, A. G. Sault and A. K. Datye, J. Catal., 1995, 156, 185–207 CrossRef CAS.
- S. Li, W. Ding, G. D. Meitzner and E. Iglesia, J. Phys. Chem. B, 2002, 106, 85–91 CrossRef CAS.
- S. Li, G. D. Meitzner and E. Iglesia, J. Phys. Chem. B, 2001, 105, 5743–5750 CrossRef CAS.
- J. X. Liu and W. X. Li, Wiley Interdiscip. Rev. Comput. Mol. Sci., 2016, 6, 571–583 CrossRef CAS.
- S. Zhao, X.-W. Liu, C.-F. Huo, X.-D. Wen, W. Guo, D. Cao, Y. Yang, Y.-W. Li, J. Wang and H. Jiao, Catal. Today, 2015, 93–100, DOI:10.1016/j.cattod.2015.07.035.
- M. O. Ozbek and J. W. Niemantsverdriet, J. Catal., 2014, 317, 158–166 CrossRef CAS.
- P. Thanh Hai, X. Duan, G. Qian, X. Zhou and D. Chen, J. Phys. Chem. C, 2014, 118, 10170–10176 CrossRef.
- R. Gao, D.-B. Cao, S. Liu, Y. Yang, Y.-W. Li, J. Wang and H. Jiao, Appl. Catal., A, 2013, 468, 370–383 CrossRef CAS.
- S. Zhao, X.-W. Liu, C.-F. Huo, Y.-W. Li, J. Wang and H. Jiao, Catal., Struct. React., 2015, 1, 44–60 CrossRef.
- B. Wang, X. Yu, C. Huo, J. Wang and Y. Li, Chin. J. Catal., 2014, 35, 28–37 CrossRef CAS.
- K. Xu, B. Sun, J. Lin, W. Wen, Y. Pei, S. Yan, M. Qiao, X. Zhang and B. Zong, Nat. Commun., 2014, 5, 5783 CrossRef CAS PubMed.
- X.-Y. Liao, D.-B. Cao, S.-G. Wang, Z.-Y. Ma, Y.-W. Li, J. Wang and H. Jiao, J. Mol. Catal. A: Chem., 2007, 269, 169–178 CrossRef CAS.
- H. Zhao, J.-X. Liu, C. Yang, S. Yao, H.-Y. Su, Z. Gao, M. Dong, J. Wang, A. I. Rykov, J. Wang, Y. Hou, W.-X. Li and D. Ma, CCS Chem., 2021, 3, 2712–2724 CrossRef CAS.
- J. Cheng, P. Hu, P. Ellis, S. French, G. Kelly and C. M. Lok, J. Phys. Chem. C, 2010, 114, 1085–1093 CrossRef CAS.
- R. Gao, X. Liu, Z. Cao, X. Liu, K. Louis, D. Ma, Y. Yang, Y.-W. Li, R. Hoffmann and X.-D. Wen, Catal. Lett., 2019, 149, 1–20 CrossRef.
- C. S. Kuivila, P. C. Stair and J. B. Butt, J. Catal., 1989, 118, 299–311 CrossRef CAS.
- J. B. Butt, Catal. Lett., 1990, 7, 61–81 CrossRef CAS.
- D. Mahajan, P. Gütlich, J. Ensling, K. Pandya, U. Stumm and P. Vijayaraghavan, Energy Fuels, 2003, 17, 1210–1221 CrossRef CAS.
- J. W. Niemantsverdriet, A. M. Van der Kraan, W. L. Van Dijk and H. S. Van der Baan, J. Phys. Chem., 1980, 84, 3363–3370 CrossRef CAS.
- C.-K. Kuei and M.-D. Lee, J. Mol. Catal., 1991, 65, 293–305 CrossRef CAS.
- V. V. Ordomsky, B. Legras, K. Cheng, S. Paul and A. Y. Khodakov, Catal. Sci. Technol., 2015, 5, 1433–1437 RSC.
- D. B. Bukur, L. Nowicki, R. K. Manne and X. S. Lang, J. Catal., 1995, 155, 366–375 CrossRef CAS.
- N. Sirimanothan, H. H. Hamdeh, Y. Zhang and B. H. Davis, Catal. Lett., 2002, 82, 181–191 CrossRef CAS.
- R. A. van Santen, A. J. Markvoort, I. A. Filot, M. M. Ghouri and E. J. Hensen, Phys. Chem. Chem. Phys., 2013, 15, 17038–17063 RSC.
- F. Fischer and H. Tropsch, Brennst. Chem., 1926, 7, 97–104 CAS.
- J. T. Kummer and P. H. Emmett, J. Am. Chem. Soc., 1953, 75, 5177–5183 CrossRef CAS.
- H. Pichler and H. Schultz, Chem. Ing. Tech., 1970, 12, 1160–1174 Search PubMed.
- Z. Teimouri, N. Abatzoglou and A. K. Dalai, Catalysts, 2021, 11, 330 CrossRef CAS.
- L. Zhou, J. Gao, X. Hao, Y. Yang and Y. Li, Reactions, 2021, 2, 161–174 CrossRef.
- M. Martinelli, M. K. Gnanamani, S. LeViness, G. Jacobs and W. D. Shafer, Appl. Catal., A, 2020, 608, 117740 CrossRef CAS.
- J. Cheng, X. Q. Gong, P. Hu, C. M. Lok, P. Ellis and S. French, J. Catal., 2008, 254, 285–295 CrossRef CAS.
- Z. P. Liu and P. Hu, J. Am. Chem. Soc., 2002, 124, 11568–11569 CrossRef CAS PubMed.
- R. C. Brady and R. Pettit, J. Am. Chem. Soc., 1981, 103, 1287–1289 CrossRef CAS.
- R. A. van Santen, A. J. Markvoort, M. M. Ghouri, P. A. J. Hilbers and E. J. M. Hensen, J. Phys. Chem. C, 2013, 117, 4488–4504 CrossRef CAS.
- B. Chen, D. Wang, X. Duan, W. Liu, Y. Li, G. Qian, W. Yuan, A. Holmen, X. Zhou and D. Chen, ACS Catal., 2018, 8, 2709–2714 CrossRef CAS.
- T. H. Pham, Y. Qi, J. Yang, X. Duan, G. Qian, X. Zhou, D. Chen and W. Yuan, ACS Catal., 2015, 5, 2203–2208 CrossRef CAS.
- J. Ren, N. Ai and Y. Yu, RSC Adv., 2021, 11, 34533–34543 RSC.
- Y. Chen, L. Ma, R. Zhang, R. Ye, W. Liu, J. Wei, V. V. Ordomsky and J. Liu, Appl. Catal., B, 2022, 312, 121393 CrossRef CAS.
- A. Chakraborty and A. Kar, Nature-Inspired Computing and Optimization, 2017, pp. 475–494, DOI:10.1007/978-3-319-50920-4_19.
- A. Rajan, A. P. Pushkar, B. C. Dharmalingam and J. J. Varghese, iScience, 2023, 26, 107029 CrossRef PubMed.
- J. Cao, N. Song, W. Chen, Y. Cao, G. Qian, X. Duan and X. Zhou, Chem.–Asian J., 2020, 15, 4014–4022 CrossRef CAS PubMed.
- X. Wang, N. Liu, Q. Zhang, X. Liang, B. Chen and D. Mei, Particuology, 2020, 48, 2–12 CrossRef CAS.
- J. Cheng, P. Hu, P. Ellis, S. French, G. Kelly and C. M. Lok, J. Phys. Chem. C, 2010, 114, 1085–1093 CrossRef CAS.
- J. Cheng, P. Hu, P. Ellis, S. French, G. Kelly and C. M. Lok, J. Phys. Chem. C, 2009, 113, 8858–8863 CrossRef CAS.
- J. Cheng, P. Hu, P. Ellis, S. French, G. Kelly and C. M. Lok, J. Phys. Chem. C, 2008, 112, 6082–6086 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- P. E. Blöchl, O. Jepsen and O. K. Andersen, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 16223–16233 CrossRef PubMed.
- B. Liu, W. Li, J. Zheng, Q. Lin, X. Zhang, J. Zhang, F. Jiang, Y. Xu and X. Liu, Catal. Sci. Technol., 2018, 8, 5288–5301 RSC.
- A. Stibor, G. Kresse, A. Eichler and J. Hafner, Surf. Sci., 2002, 507–510, 99–102 CrossRef CAS.
- Z. Yao, C. Guo, Y. Mao and P. Hu, ACS Catal., 2019, 9, 5957–5973 CrossRef CAS.
- J.-F. Chen, Y. Mao, H.-F. Wang and P. Hu, ACS Catal., 2019, 9, 2633–2638 CrossRef CAS.
- Y. Liu, H. Liu, L. Luo, B. Lin, Y. Zhou, H. Wang, P. Wang and Y. Mao, ChemCatChem, 2023, 15, e202201544 CrossRef CAS.
- Y. Mao, Z. Wang, H.-F. Wang and P. Hu, ACS Catal., 2016, 7882–7891, DOI:10.1021/acscatal.6b01449.
- Z. Wang, X. M. Cao, J. Zhu and P. Hu, J. Catal., 2014, 311, 469–480 CrossRef CAS.
- A. Alavi, P. Hu, T. Deutsch, P. L. Silvestrelli and J. Hutter, Phys. Rev. Lett., 1998, 80, 3650–3653 CrossRef CAS.
- Z.-P. Liu and P. Hu, J. Am. Chem. Soc., 2003, 125, 1958–1967 CrossRef CAS PubMed.
- Y. Wang, J. Lv, L. Zhu and Y. Ma, Comput. Phys. Commun., 2012, 183, 2063–2070 CrossRef CAS.
- Y. Wang, J. Lv, L. Zhu and Y. Ma, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 094116 CrossRef.
- Y. Wang, F. Li, Y. Li and Z. Chen, Nat. Commun., 2016, 7, 11488 CrossRef CAS PubMed.
- S. Lu, Y. Wang, H. Liu, M. S. Miao and Y. Ma, Nat. Commun., 2014, 5, 3666 CrossRef CAS PubMed.
- X. Tian, S. Wang, Z.-j. Wang, H. Wang, Y. Zhou, H. Zhong and Y. Mao, ChemCatChem, 2020, 12, 3240–3248 CrossRef CAS.
- S. Lu, Y. Wang, H. Liu, M.-s. Miao and Y. Ma, Nat. Commun., 2014, 5, 3666 CrossRef CAS PubMed.
- Y. Wang, J. Lv, P. Gao and Y. Ma, Acc. Chem. Res., 2022, 55, 2068–2076 CrossRef CAS PubMed.
- K. Reuter and M. Scheffler, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 045407 CrossRef.
- K. Reuter and M. Scheffler, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 65, 035406 CrossRef.
- Y. Mao and P. Hu, Sci. China Chem., 2020, 63, 850–859 CrossRef CAS.
- R. V. Zucker, D. Chatain, U. Dahmen, S. Hagège and W. C. Carter, J. Mater. Sci., 2012, 47, 8290–8302 CrossRef CAS.
- A. R. Roosen, R. P. McCormack and W. C. Carter, Comput. Mater. Sci., 1998, 11, 16–26 CrossRef.
- G. D. Barmparis, Z. Lodziana, N. Lopez and I. N. Remediakis, Beilstein J. Nanotechnol., 2015, 6, 361–368 CrossRef PubMed.
- S. Li, R. J. O'Brien, G. D. Meitzner, H. Hamdeh, B. H. Davis and E. Iglesia, Appl. Catal., A, 2001, 219, 215–222 CrossRef CAS.
- M. E. Dry, T. Shingles, L. J. Boshoff and C. S. van H. Botha, J. Catal., 1970, 17, 347–354 CrossRef CAS.
- X. Liu, J. Liu, Y. Yang, Y.-W. Li and X. Wen, ACS Catal., 2021, 11, 2156–2181 CrossRef CAS.
- E. de Smit, M. M. van Schooneveld, F. Cinquini, H. Bluhm, P. Sautet, F. M. de Groot and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2011, 50, 1584–1588 CrossRef CAS PubMed.
- J. Cheng, T. Song, P. Hu, C. M. Lok, P. Ellis and S. French, J. Catal., 2008, 255, 20–28 CrossRef CAS.
- E. van Steen and P. van Helden, J. Phys. Chem. C, 2010, 114, 5932–5940 CrossRef CAS.
- J. Cheng, P. Hu, P. Ellis, S. French, G. Kelly and C. M. Lok, J. Phys. Chem. C, 2008, 112, 1308–1311 CrossRef CAS.
- T. Bligaard, J. K. Nørskov, S. Dahl, J. Matthiesen, C. H. Christensen and J. Sehested, J. Catal., 2004, 224, 206–217 CrossRef CAS.
- I. A. Filot, R. A. van Santen and E. J. Hensen, Angew. Chem., Int. Ed., 2014, 53, 12746–12750 CrossRef CAS PubMed.
- D. M. Stockwell, D. Bianchi and C. O. Bennett, J. Catal., 1988, 113, 13–24 CrossRef CAS.
- H. Matsumoto and C. O. Bennett, J. Catal., 1978, 53, 331–344 CrossRef CAS.
- G. P. Van Der Laan and A. A. C. M. Beenackers, Catal. Rev., 1999, 41, 255–318 CrossRef CAS.
- B. Hammer and J. K. Norskov, Nature, 1995, 376, 238–240 CrossRef CAS.
- J. K. Nørskov, F. Studt, F. Abild-Pedersen and T. Bligaard, Fundamental Concepts in Heterogeneous Catalysis, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014 Search PubMed.
- C.-F. Huo, Y.-W. Li, J. Wang and H. Jiao, J. Am. Chem. Soc., 2009, 131, 14713–14721 CrossRef CAS PubMed.
- Sonal, K. Kondamudi, K. K. Pant and S. Upadhyayula, Ind. Eng. Chem. Res., 2017, 56, 4659–4671 CrossRef CAS.
- J. Yang, Y. Qi, J. Zhu, Y.-A. Zhu, D. Chen and A. Holmen, J. Catal., 2013, 308, 37–49 CrossRef CAS.
- G. R. Johnson, S. Werner and A. T. Bell, ACS Catal., 2015, 5, 5888–5903 CrossRef CAS.
- S. Bhattacharjee, U. V. Waghmare and S. C. Lee, Sci. Rep., 2016, 6, 35916 CrossRef CAS PubMed.
- J. K. Nørskov, F. Studt, F. Abild-Pedersen and T. Bligaard, in Fundamental Concepts in Heterogeneous Catalysis, 2014, pp. 47–67 Search PubMed.
- R. B. Anderson, Catalysts for the Fischer–Tropsch Synthesis, Van Nostrand Reinhold, New York, 1956 Search PubMed.
- K. Honkala, A. Hellman, I. N. Remediakis, A. Logadottir, A. Carlsson, S. Dahl, C. H. Christensen and J. K. Nørskov, Science, 2005, 307, 555–558 CrossRef CAS PubMed.
- T. Wang, X.-X. Tian, Y.-W. Li, J. Wang, M. Beller and H. Jiao, ACS Catal., 2014, 4, 1991–2005 CrossRef CAS.
- J. W. Niemantsverdriet and A. M. van der Kraan, J. Catal., 1981, 72, 385–388 CrossRef CAS.
- T. Li, X. Wen, Y.-W. Li and H. Jiao, J. Phys. Chem. C, 2019, 123, 25657–25667 CrossRef CAS.
- I. V. Mutigullin, D. I. Bazhanov and A. S. Ilyushin, Phys. Solid State, 2011, 53, 599–605 CrossRef CAS.
- C. Guo, Y. Mao, Z. Yao, J. Chen and P. Hu, J. Catal., 2019, 379, 52–59 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06467k |
| This journal is © The Royal Society of Chemistry 2023 |