Open Access Article
Open Access ArticleIsolation and total synthesis of dysidone A: a new piperidone alkaloid from the marine sponge Dysidea sp.†
Yu Lei,
Boao Li,
Xiaojian Liao,
Xiwen Xing,
Pengju Feng *,
Bingxin Zhao
*,
Bingxin Zhao * and
Shihai Xu*
* and
Shihai Xu*
Department of Chemistry, College of Chemistry and Materials Science, Jinan University, Guangzhou, 510632, P. R. China. E-mail: pfeng@jnu.edu.cn; zbx840622@163.com; txush@jnu.edu.cn
First published on 6th October 2023
Abstract
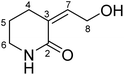
A new piperidone alkaloid, dysidone A (1), was isolated from the marine sponge Dysidea sp. The structure of 1 was elucidated by the method of spectroscopic analysis. Compound 1 represented the first example of piperidone alkaloid isolated from the sponge of the genus Dysidea with the exocyclic double bond. Furthermore, the total synthesis of 1 was also carried out, which was started with piperidine proceeding a PIDA/I2-mediated α and β-C (sp3) –H bond dual oxygenation to achieve a 5-steps synthesis in a total yield of 10.6%. In addition, the anti-inflammatory activities of 1 and its derivative dysidone B (1d) were evaluated, which suggested that 1 showed weak anti-inflammatory activity.
Introduction
Piperidine alkaloids have incomparable skeleton diversity and novelty, which often exhibit a wide range of biological activities including anticancer, antibacterial, anti-inflammatory, antiviral, antimalarial and antipsychotic effects, etc.1,2 Dozens of piperidine-containing drug molecules have been approved for marketing for the treatment of various diseases,3,4 such as Piperine and Febrifugine, which were isolated from Traditional Chinese herb Piper nigrum L. and Dichroa febrifuga Lour. for the treatment of convulsion and malaria,5–7 respectively. Biosynthetically, piperidines are often formed following lysine cyclization into 2-pipecolinic acid.8 In addition, piperidines can be used to synthesize antineoplastic, antidiabetic, anti-inflammatory, antiviral (including HIV), cardiovascular and anti-influenza drugs, etc.9,10, which makes piperidine a favoured dominant scaffold in drug synthesis. However, the synthesis strategies for this kind of compounds are very limited, most of them are traditional chemical methods,11,12 which have problems such as narrow application range, harsh reaction conditions, complicated steps, and low yield.13 Therefore, there is an urgent need to find new methods for efficient synthesis.The sponge of genus Dysidea, belonging to the family of Dysideidae, order Dictyoceratida, was widely distributed in the South China Sea. Abundant secondary metabolites had been isolated from this genus such as terpenes, alkaloids and sterols, which exhibited extensive biological activities such as cytotoxic, antioxidant, antibacterial and anti-HIV activities.14–17 It was worth noting that there was no piperidone alkaloids had been discovered from this genus before. During the course of chemical investigation on marine sponges,18–23 a new piperidone alkaloid, dysidone A (1), featuring an exocyclic double bond moiety, was isolated from Dysidea sp. (Fig. 1). Herein, we describe the isolation, structure elucidation, total synthesis, and biological evaluation of 1.
Results and discussion
Dysidone A (1) was obtained as light-yellow oil. Its molecular formula was determined as C7H11NO2 by HRESIMS at m/z 164.0684 [M + Na]+ (calcd for C7H11NO2Na: 164.0687), indicating three degrees of unsaturation. The UV absorption maximum was at 224 nm. The IR bands showed the presence of amino (3350 cm−1), hydroxyl (3263 cm−1) and carbonyl (1639 cm−1) groups. Analysis of NMR spectra revealed that 1 had one carbonyl [δC 167.3 (C-2)], a trisubstituted double bonds [δH 6.21 (m, H-7); δC 141.5 (C-7) and 130.9 (C-3)], four methylenes [δH 4.32 (d, J = 5.9 Hz, H-8), 3.37 (m, H-6), 2.52 (m, H-4) and 1.87 (m, H-5); δC 59.4 (C-8), 42.7 (C-6), 31.9 (C-4) and 22.9 (C-5)] as well as two active hydrogen signals [δH 6.23 (br s, –NH) and 5.03 (s, 8-OH)]. The above data (Table 1) were similar to those of valerolactam,24 suggesting that 1 might be a piperidone alkaloid.| No. | δH | δC |
|---|---|---|
| NH | 6.23 br s | — |
| 2 | — | 167.3 |
| 3 | — | 130.9 |
| 4 | 2.52 m | 31.9 |
| 5 | 1.87 m | 22.9 |
| 6 | 3.37 m | 42.7 |
| 7 | 6.21 m | 141.5 |
| 8 | 4.32 d (5.9) | 59.4 |
| 8-OH | 5.03 s | — |
The 1H–1H COSY spectrum of 1 revealed the presence of two spin coupling systems in bold as shown in Fig. 2. The HMBC correlations from H-4/H-6/H-7 to C-2, from H-4/H-5/H-8 to C-3, as well as from H-4 to C-7 indicated the basic skeleton of 1. In addition, according to the molecular formula information and the obvious downshift at C-8 revealed that the remaining hydroxyl group should be attached at C-8. Therefore, the planer structure of 1 was determined. In the NOESY spectrum, the NOE correlation between H-7 and H-4a suggested the Z configuration of the double bond.
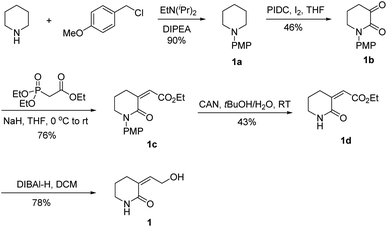
A total synthesis of 1 was also carried out to confirm its structure. Firstly, DIPEA was used to release EtN(iPr)2 to give compound 1a in 90% yield, and the PMP group could be readily removed under oxidative condition. By mixing compound 1a with iodobenzene diacetate (PIDC) and I2 in THF, α- and β-C (sp3)–H bond dual oxygenation of piperidine derivative 1b was achieved in 46% yield, which made fast installation of 1 possible.25 Subsequently, a HWE reaction was smoothly conducted between compound 1b and organophosphine reagent to deliver compound 1c, which was undertaken selective de-protection reaction with oxidant CAN to give compound 1d in 43% yield. DIBAl-H reagent was employed to selectively reduce ester to alcohol under mild condition for delivering 1, which was then fully characterized. The 1H and 13C NMR spectra of the synthesized 1 were nearly the same as those of the natural one (Fig. S10 and S11†). Eventually, the structure of 1 was unambiguously determined as shown in Scheme 1.
In fact, NO is an important pro-inflammatory mediator with an effect on the immune system. It can activate inflammatory pathways and induce the expression of iNOS by stimulating tumor necrosis factor (TNF-α), lipopolysaccharide (LPS), and interleukin 21 (IL-21), thereby causing an increase in NO synthesis. The previous study reported that many piperidines showed the anti-inflammatory activities, like (+)-homocrepidine A and homocrepidine B, which inhibited the production of NO with IC50 values of 3.6 and 27.6 μM, respectively.26 Therefore, we evaluated the NO inhibitory activity of 1 and 1d. The result indicated that compound 1 showed inhibitory activity (IC50 = 378.27 μM) comparable to that of SMT (IC50 = 117.59 μM), while compound 1d did not show anti-inflammatory activity in this assay.
Conclusions
In conclusion, a new piperidone alkaloid, dysidone A (1), was isolated from the marine sponge Dysidea sp. Compound 1 represented the first example of piperidone alkaloid isolated from the sponge of genus Dysidea with the moiety of exocyclic double bond. Meanwhile, compound 1 was also fast installed from commercially available piperidine via a PIDA/I2-mediated dual oxygenation reaction in five steps. Furthermore, the bioactivity evaluation results showed that 1 exhibited weak anti-inflammatory inhibitory activity. In the future, we will explore more bioactivities of 1, such as antimalarial, analgesic, anticancer and antibacterial activities, etc.Experimental section
General experimental procedures
Optical rotations were determined with a P-2000 Digital Polarimeter (JASCO International Co. Ltd, Hachioji, Japan). The UV spectra were recorded in MeOH on Shimadzu UV-2401PC spectrometer (Shimadzu, Kyoto, Japan) and the IR spectrums were measured on Thermo Nicolet iS50 FT-IR (Thermo Fisher Scientific, Waltham, MA USA). HR-ESI-MS was acquired from Agilent 6210 LC-ESI-Q/TOF mass spectrometer (Agilent Technologies Inc., California, America). The NMR spectra were obtained from Bruker Av 600/300 NMR (Bruker, Fällanden, Switzerland). HPLC was performed using YMC-Pack ODS-A [250 × 4.6 mm., D. 5 μm, 12 nm] was accomplished by an Agilent 1260 series apparatus (Agilent, Palo Alto, America). Column chromatography was performed with silica gel (300–400 mesh, Qingdao Marine Chemical Co., Qingdao, China) and GE Sephadex LH-20. The Thin-Layer Chromatography silica gel plate (0.2 ± 0.03 mm, HSGH 254) were purchased from the Qingdao Marine Chemical Factory, Qingdao, China.Materials
The sponge was collected from Xuwen County, Zhanjiang City, Guangdong Province, China, in May 2017, which was identified by the professor De-Xiang Wang as Dysidea sp. The specimen (2017-05) had been deposited in the Department of Chemistry, College of Chemistry and Materials Science, Jinan University.Extraction and isolation
The sponge Dysidea sp. (20.0 kg, wet weight) was powdered and exhaustively extracted with 95% EtOAc to give a crude extract (1.8 kg) at room temperature, which was suspended in 2 L H2O and partitioned by petroleum ether, EtOAc and n-butanol for five times, respectively. The EtOAc extract (40.0 g) was applied to silica gel column chromatography with gradient elution using CH2Cl2/CH3OH (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–0
0–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to yield 11 fractions (Frs 1–11). Fr. 8 (3.8 g) was subjected to silica gel column eluted with CH2Cl2/CH3OH to gain 9 fractions (Frs 8–1–8–9). Fr. 8–7 (1.6 g) were applied to Sephadex LH-20 column with CH2Cl2/CH3OH (1
100) to yield 11 fractions (Frs 1–11). Fr. 8 (3.8 g) was subjected to silica gel column eluted with CH2Cl2/CH3OH to gain 9 fractions (Frs 8–1–8–9). Fr. 8–7 (1.6 g) were applied to Sephadex LH-20 column with CH2Cl2/CH3OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 7 fractions (Frs 8–7–1–8–7–7). Frs 8–7–6 (289.5 mg) was purified by preparative reversed-phase HPLC (8 mL min−1, MeOH/H2O = 5
1) to yield 7 fractions (Frs 8–7–1–8–7–7). Frs 8–7–6 (289.5 mg) was purified by preparative reversed-phase HPLC (8 mL min−1, MeOH/H2O = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) to afford 1 (tR = 64.01 min, 5.4 mg).
95) to afford 1 (tR = 64.01 min, 5.4 mg).
NO production assay
The anti-inflammatory activity of each sample was tested by nitric oxide (NO) production assay. In brief, RAW264.7 cells were seeded at a density of 33 × 104 cells per mL in DMEM and incubated for 24 h. The seeded cells were treated with the compounds for 1 h, then treated with LPS and incubated for 24 h. After incubation for 24 h, 10 μL cells supernatant were incubated with an equal volume of Griess reagent at room temperature for 10 min, the optical density was then measured by detection of the absorbance at 540 nm on a microplate reader.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2022YFC2804100), the National Natural Science Foundation of China (No. 42376085), and the Fundamental Research Funds for the Central Universities (No. 21623210).References
- A. J. Freyer, A. D. Patil, L. Killmer, N. Troupe, M. Mentzer, B. Carte, L. Faucette and R. K. Johnson, Three new pseudodistomins, piperidine alkaloids from the Ascidian Pseudodistoma megalarva, J. Nat. Prod., 1997, 60, 986–990 CrossRef CAS PubMed.
- C. Viegas, Jr., V. d. S. Bolzani, M. Furlan, E. J. Barreiro, M. C. M. Young, D. Tomazela and M. N. Eberlin, Further bioactive piperidine alkaloids from the flowers and green fruits of Cassia spectabilis, J. Nat. Prod., 2004, 67, 908–910 CrossRef PubMed.
- E. Vitaku, D. T. Smith and J. T. Njardarson, Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- R. D. Taylor, M. Maccoss and A. D. Lawson, Rings in drugs, J. Med. Chem., 2014, 57, 5845 CrossRef CAS PubMed.
- L. Gorgani, M. Mohammadi, G. D. Najafpour and M. Nikzad, Piperine-the bioactive compound of black pepper: from isolation to medicinal formulations, Compr. Rev. Food Sci. Food Saf., 2017, 16, 124–140 CrossRef CAS PubMed.
- K. Srinivasan, Black pepper and its pungent principle-piperine: A review of diverse physiological effects, Crit. Rev. Food Sci. Nutr., 2007, 47, 735–748 CrossRef CAS PubMed.
- S. Jiang, Q. Zeng, M. Gettayacamin, A. Tungtaeng, S. Wannaying, A. Lim, P. Hansukjariya, C. O. Okunji, S. Zhu and D. Fang, Antimalarial activities and therapeutic properties of febrifugine analogs, Antimicrob. Agents Chemother., 2005, 49, 1169–1176 CrossRef CAS PubMed.
- M. W. Mullowney, R. A. McClure, M. T. Robey, N. L. Kelleher and R. J. Thomson, Natural products from thioester reductase containing biosynthetic pathways, Nat. Prod. Rep., 2018, 35, 847–878 RSC.
- U. Das, R. K. Sharma and J. R. Dimmock, 1,5-diaryl-3-oxo-1,4-pentadienes: a case for antineoplastics with multiple targets, Curr. Med. Chem., 2009, 16, 2001–2020 CrossRef CAS PubMed.
- M. S. M. Pearson, M. Mathe-Allainmat, V. Fargeas and J. Lebreton, Recent advances in the total synthesis of piperidine aza-sugars, Eur. J. Org Chem., 2005, 11, 2159–2191 CrossRef.
- Y. Zhu, L. D. Shao, Z. T. Deng, Y. Bao, X. Shi and Q. S. Zhao, PIDA/I2-Mediated α- and β-C(sp3)-H Bond Dual functionalization of tertiary amines, J. Org. Chem., 2018, 83, 10166–10174 CrossRef CAS PubMed.
- Y. He, J. Yang, X. Zhang and X. Fan, Selective cleavage and reconstruction of C-N/C-C bonds in saturated cyclic amines: tunable synthesis of lactams and functionalized acyclic amines, Org. Chem. Front., 2021, 8, 5118–5123 RSC.
- S. Park, G. Kang, C. Kim, D. Kim and S. Han, Collective total synthesis of C4-oxygenated securinine-type alkaloids via stereocontrolled diversifications on the piperidine core, Nat. Commun., 2022, 13, 5149 CrossRef CAS PubMed.
- S. Hirsch, A. Rudi, Y. Kashman and Y. Loya, New avarone and avarol derivatives from the marine sponge Dysidea cinerea, J. Nat. Prod., 1991, 54, 92–97 CrossRef CAS PubMed.
- W. E. Muller, A. Maidhof, R. K. Zahn, H. C. Schroder, M. J. Gasic, D. Heidemann, A. Bernd, B. Kurelec, E. Eich and G. Seibert, Potent antileukemic activity of the novel cytostatic agent avarone and its analogues in vitro and in vivo, Cancer Res., 1985, 45, 4822–4826 CAS.
- W. H. Jiao, J. Li, Q. Liu, T. T. Xu, G. H. Shi, H. B. Yu, F. Yang, B. N. Han, M. Li and H. W. Lin, Dysidinoid A, an unusual meroterpenoid with anti-MRSA activity from the South China sea sponge Dysidea sp, Molecules, 2014, 19, 18025–18032 CrossRef PubMed.
- W. H. Jiao, B. H. Cheng, G. D. Chen, G. H. Shi, J. Li, T. Y. Hu and H. W. Lin, Dysiarenone, a dimeric C21 meroterpenoid with inhibition of COX-2 expression from the marine sponge Dysidea arenaria, Org. Lett., 2018, 20, 3092–3095 CrossRef CAS PubMed.
- Y. Q. Liang, X. J. Liao, B. X. Zhao and S. H. Xu, Novel 3,4-seco-3,19-dinorspongian and 5,17-epoxy-19-norspongian diterpenes from the marine sponge Spongia sp, Org. Chem. Front., 2020, 7, 3253–3261 RSC.
- F. R. Jiao, B. B. Gu, H. R. Zhu, Y. Zhang and H. W. Lin, Asperfloketals A and B, the First Two ergostanes with rearranged A and D rings: from the sponge-associated Aspergillus flocculosus 16D-1, J. Org. Chem., 2021, 86, 10954–10961 CrossRef CAS PubMed.
- R. Zhou, X. J. Liao, H. Li, J. Li, P. J. Feng, B. X. Zhao and S. H. Xu, Isolation and synthesis of misszrtine A: a novel indole alkaloid from marine sponge-associated Aspergillus sp, Front. Chem., 2018, 6, 212 CrossRef PubMed.
- Z. C. Wang, Y. Wang, L. Y. Huang, X. J. Liao, Z. H. Jiang, S. H. Xu and B. X. Zhao, Two new halogenated metabolites from the red alga Laurencia sp, J. Asian Nat. Prod. Res., 2023, 25, 61–67 CrossRef CAS PubMed.
- P. Tang, D. Huang, K. X. Zheng, D. Hu, P. Dai, C. H. Li, S. Y. Qin, G. D. Chen, X. S. Yao and H. Gao, Thirteen new peptaibols with antimicrobial activities from Trichoderma sp, Chin. J. Nat. Med., 2023, 21, 1–12 Search PubMed.
- G. D. Chen, B. X. Zhao, M. J. Huang, J. Tang, Y. B. Li, L. D. Guo, R. R. He, D. Hu, X. S. Yao and H. Gao, Tripodalsporormielones A.-C., unprecedented cage-like polyketides with complex polyvdent bridged and fused ring systems, Acta Pharm. Sin. B, 2021, 11, 3648–3654 CrossRef CAS PubMed.
- L. Li, Y. M. Yan, X. N. Li and H. M. Zhong, Nitrogenous compounds from Holotrichia diomphalia bates, Asian J. Chem., 2013, 25, 329–332 CAS.
- Y. Zhu, L. D. Shao, Z. T. Deng, Y. Bao, X. Shi and Q. S. Zhao, J. Org. Chem., 2018, 83, 10166–10174 CrossRef CAS PubMed.
- Y. Hu, C. F. Zhang, X. Zhao, Y. Wang, D. Q. Feng, M. Zhang and H. F. Xie, (±)-Homocrepidine A, a pair of anti-inflammatory enantiomeric octahydroindolizine alkaloid dimers from Dendrobium crepidatum, J. Nat. Prod., 2016, 79, 252–256 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06115a |
| This journal is © The Royal Society of Chemistry 2023 |