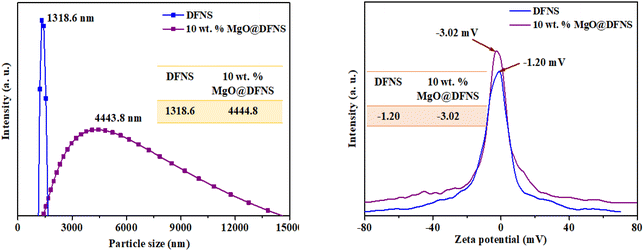
Open Access Article
Open Access ArticleFacile one pot synthesis of 2-substituted benzimidazole derivatives under mild conditions by using engineered MgO@DFNS as heterogeneous catalyst
Suman Kusumaab,
Dipak B. Bawiskara,
Chob Singha,
Pratheep Panneerselvam a,
Pradipta Sinhab,
Akshaya K. Samal
a,
Pradipta Sinhab,
Akshaya K. Samal a and
Arvind H. Jadhav
a and
Arvind H. Jadhav *a
*a
aCentre for Nano and Material Sciences, JAIN University, Global Campus, Bengaluru 562112, Karnataka, India. E-mail: j.arvind@jainuniversity.ac.in; jadhav.ah@gmail.com
bAragen Life Science Pvt. Ltd., Plot No. 284-A (Part), Bommasandra, Bengaluru 562106, India
First published on 1st November 2023
Abstract
Benzimidazole derivatives are considered as important heterocyclic motifs that show a wide range of pharmaceutical applications. In view of their wide-ranging bioactivities, it is imperative to direct research on the sustainable catalytic synthesis of benzimidazole. Therefore, herein, we report a novel approach for the synthesis of benzimidazole and its derivatives with engineered MgO supported on dendritic fibrous nano silica (MgO@DFNS) as a sustainable heterogeneous catalyst. The catalyst MgO@DFNS was thoroughly characterized to understand its physio-chemical properties using XRD, FE-SEM, XPS, FT-IR, zeta potential, HR-TEM, TGA, TPR and TPD. The obtained results suggested that the catalyst MgO@DFNS prepared well and have the desired characteristics in it. After the successful characterisation of the prepared catalyst MgO@DFNS, it was applied in the synthesis of benzimidazole derivatives via condensation of o-phenylenediamine, and various aromatic and aliphatic aldehydes under ambient temperature. The catalyst produced a clean reaction profile with excellent yields in a shorter time under the umbrella of green chemistry. The effect of reaction parameters such as the effect of time, catalyst dosage, loading of MgO, effect of solvents and effect of different homo and heterogeneous catalyst were also tested. Furthermore, to understand the scope of the catalyst different substituted diamines and substituted aldehydes were reacted and obtained desired products in good to efficient yield. In addition, a recyclability study was also conducted and it was observed that the catalyst could be recycled for up to six cycles without noticeable changes in the morphology and activity. We believe that the present methodology gave several advantages such as an eco-friendly method, easy work-up, good selectivity, high yields and quick recovery of catalyst. MgO@DFNS is highly stable for several cycles without significant loss of its activity, which possibly demonstrates its applicability at the industrial scale.
1. Introduction
Benzimidazole is a hetero bicyclic aromatic compound which is a fusion of benzene and imidazole rings.1 Benzimidazole and its derivatives have many applications in therapeutic areas such as antimicrobial agents,2 antiviral agents against several viruses such as HIV,3 influenza,4 antitumor,5 anti-inflammatory6 as well as antiprotozoal agents.7 Benzimidazole moieties play a crucial role in the treatment of ulcers, as an antihelminth and antihistamine agent. The benzimidazole ring is an essential pharmacophore in current drug discovery. Synthesis of novel benzimidazole and its derivatives are the main focus of medicinal research. Recently, researchers are focusing on substituted benzimidazole, which shows easy interactions with the biopolymers, and possesses potential activity with lower toxicities in the chemotherapeutic approach in humans (Haugwitz et al., 1982). Benzimidazole and its derivatives are important intermediates because of their pharmacology properties. Moreover, various synthetic methodologies have been reported from different reactants and reaction conditions such as the reaction between o-phenylenediamine and carboxylic acid,8,9 condensation between o-phenylenediamine and aldehydes catalyzed by metal triflates such as Sc(OTf)3,10 H2O2/HCl,11 different oxidizing agents12–14 and lanthanides such as Lewis acid catalysts for the synthesis of benzimidazole derivatives.15Nanomaterials have emerged as superior alternatives to conventional materials for various organic transformations. Their advanced applicability in heterogeneous catalysis and as catalyst supports is attributed to their higher surface area, unique textural and structural properties.16 Supported metal nanoparticles have garnered more attention than unsupported ones due to their large surface and volume ratio, intramolecular bonding, surface interaction, and interaction between metal–support.16
In addition, SiO2 as a solid support17 and its provides additional characteristics in the reactions such as high surface area, high active sites uniform distribution, catalyst strength and easy of recyclability. For the particular silica supported polyphosphoric acid has been used as a catalyst under MW irradiation18 many more catalytic process have been developed with the SiO2 supported system and showed efficient to good yield.17,18 However, many of these methodologies have some disadvantages such as low yields, prolonged reaction time, high temperature, the excess requirement of catalysts, harsh reaction conditions, difficulty in extraction of product, difficulty in recovery of catalyst, formation of byproduct etc. Therefore, it is necessary to adopt a simpler and more efficient protocol for the synthesis of benzimidazole derivatives.
Our research group has previously successfully reported the multiple heterogeneous nanocatalysts for the synthesis of various organic scaffolds such as benzimidazole,19,20 Sustainable fixation of CO2 into epoxides to form cyclic carbonates,21 and chemical conversion of fructose into 5-hydroxymethylfurfural (HMF).22 Herein we introduce a novel engineered MgO@DFNS catalyst for the one pot synthesis of 2-substituted benzimidazole derivatives under greener conditions. The catalyst MgO@DFNS has thoroughly characterized to understand its physio-chemical properties using XRD, FE-SEM, XPS, FT-IR, zeta potential, HR-TEM, TGA, TPR and TPD. The obtained material has found to be steady under the employed reaction conditions and easily recovered and recycled up to six times without any considerable loss in the yield.
2. Materials and methods
2.1. Materials
Cetyl trimethyl ammonium bromide (CTAB, purity 99%), tetraethylorthosilicate (TEOS, 99%) were purchased from Sigma-Aldrich, magnesium oxide (MgO, purity 99%), Mg(NO3)2·6H2O, pentanol (99%), urea were procured from Sigma-Aldrich, India. Acetonitrile (99%), methanol (99%), tetrahydrofuran (99%), n-hexane (99%), ethanol (99%), diethyl ether (99%), cyclohexane (99%), and chloroform (purity 99%) solvents were supplied by Sd Fine Chem. Limited. All substituted o-phenylenediamines (OPDA) and different substituted aldehydes were obtained from Sigma-Aldrich, India (purity 99%).2.2. Synthesis of MgO@DFNS catalyst
2.3. Characterization techniques
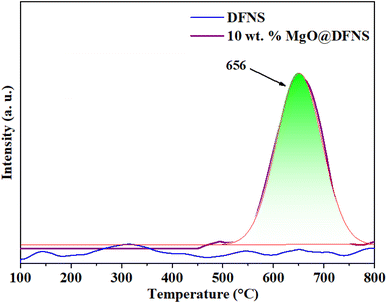
The physicochemical features of the synthesized catalyst were studied by using several advanced characterization techniques. The presence of functional groups in the prepared samples was analyzed by using FT-IR spectroscopy. PerkinElmer FT-IR spectrophotometer (Spectrum Two) instrument was used to record FT-IR spectra by using KBr (IR grade) pellet method. The information on crystalline nature and phase development were obtained from their respective powder X-ray diffraction (XRD) patterns recorded on an X-ray diffractometer (XRD; Rigaku Japan) with Cu Kα radiation source (λ = 1.5406 Å). The spectra were recorded between the 10 and 80° range and a scan rate of 2° min−1.The TGA analysis of prepared catalysts was performed to understand their decomposition process. The thermal analysis was performed from room temperature to 800 °C at a heating rate of 10 °C min−1 under N2 flow on a Scinco TGA N-100 instrument. Field emission scanning electron microscopy (FE-SEM), was used to gain morphological and topographical information. Prior to the analysis, the samples were evenly coated on the carbon tape placed on an aluminium metal stub. The sample on the stub was then sputtered by gold nanoparticles for 120 s and the analysis was executed by using FE-SEM (JEOL Model-JSM7). X-ray photoelectron spectroscopy (XPS) analysis was employed to understand elemental composition as well as the electronic state of the elements in the samples. The analysis were performed on an X-ray photoelectron spectrometer (PerkinElmer PHI1257) at 4 × 10−10 torr pressure with Al Kα X-ray as the excitation source (1486.7 eV).
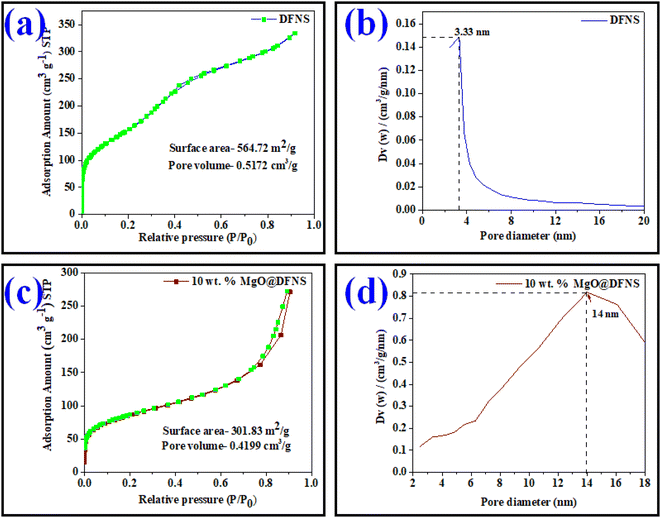
Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods were used, respectively to evaluate the specific surface area and pore size distribution of the materials. The analysis was performed on the Belsorp MAX instrument (BEL Japan) at the temperature of liquid nitrogen. The materials were degassed at 100 °C for 2 h in a high vacuum before the analysis. Additionally, the acidic sites in the samples were quantified by using NH3-TPD analysis using pure ammonia gas (99.999% purity). The TPD instrument was designed by handmade set-up, the quartz tube (length: 300 mm, inner diameter: 6 mm) was connected to a six-port valve with thermal conductivity detector (M/s. Mayura Analytical Pvt. Ltd., India).
1H-NMR spectra were recorded on Varian Gemini (400 MHz) spectrometer using DMSO-d6 as a solvent and tetramethylsilane (TMS) as an internal standard. 13C-NMR spectra were also recorded on 100 MHz in the same solvent. The reactions were monitored by Thin Layer Chromatography (TLC) on silica gel plates using pet ether and ethyl acetate as a solvent system. The melting points of the respective samples were recorded using the Sigma Scientific instrument.
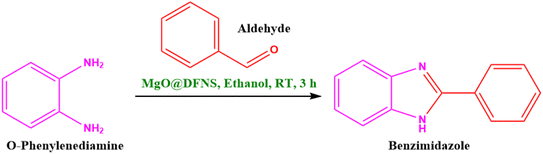
2.4. General procedure for the synthesis of benzimidazole derivatives over synthesized MgO@DFNS catalyst
A mixture of o-phenylenediamine (OPD) (1 mmol) and aldehyde (1.1 mmol) in ethanol was placed in a two-neck round bottom flask and the required amount of 10 wt% MgO@DFNS catalyst was added to the reaction mixture at room temperature (RT) and stirred for 3 h. After completion of the reaction (monitored by TLC), the reaction mixture was filtered through the filter paper to separate the catalyst. The filtrate was evaporated under reduced pressure to afford crude product. The obtained crude product was purified by silica gel (100–200 mesh) column chromatography using ethyl acetate/pet ether as an eluent in a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 ratio to obtain pure compounds. The obtained compounds were characterized by 1H NMR, 13C-NMR and mass spectroscopy and also compared with the literature values. The filtered catalyst was dried overnight at 80 °C and reused for the further cycles.21,26
80 ratio to obtain pure compounds. The obtained compounds were characterized by 1H NMR, 13C-NMR and mass spectroscopy and also compared with the literature values. The filtered catalyst was dried overnight at 80 °C and reused for the further cycles.21,26
3. Results and discussion
3.1. MgO@DFNS catalyst characterization
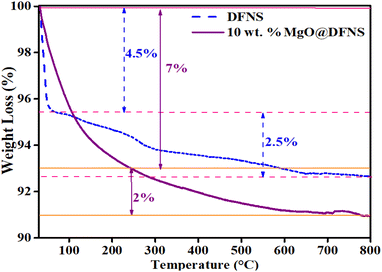
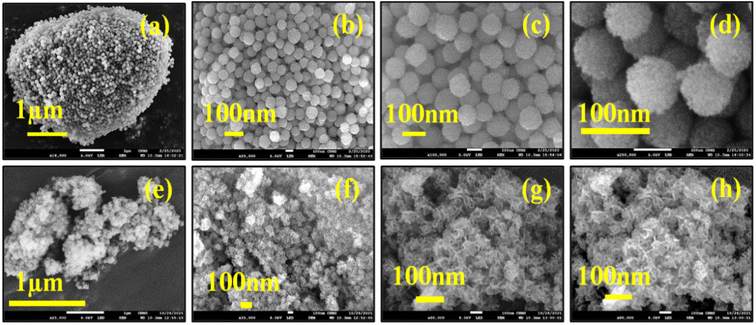
Further, EDAX analysis (Fig. 5) of DFNS and 10 wt% MgO@DFNS suggested the presence of all the expected elements in the prepared catalyst in the stoichiometric amount. Additionally, MgO particles were uniformly distributed over the DFNS surface as could be observed from mapping analysis.
| Entry | Sample | Specific surface area (m2 g−1) | Mean pore diameter (nm) | Mean pore volume (cm3 g−1) |
|---|---|---|---|---|
| 1 | DFNS | 564.72 | 3.33 | 0.5172 |
| 2 | 10 wt% MgO@DFNS | 301.83 | 14.0 | 0.4199 |
3.2. Catalytic activity of MgO@DFNS towards the synthesis of benzimidazole derivatives
| Entry | Reactants | Catalyst dosage (wt%) | Time (h) | bConv./sel. (%) | bYield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: all reactions proceeded with 1 mmol o-phenylenediamine (OPDA), 1.2 mmol aldehyde.b Yield refers to an isolated product which characterized by 1H NMR, 13C NMR. | |||||
| 1 | OPDA, aldehyde | 0 | 4 | 45/38 | 32 |
| 2 | OPDA, aldehyde | 0 | 16 | 72/54 | 60 |
| 3 | OPDA, aldehyde | 5.0 | 3 | 93/80 | 85 |
| 4 | OPDA, aldehyde | 10.0 | 3 | 100/90 | 95 |
| 5 | OPDA, aldehyde | 15.0 | 3 | 100/85 | 92 |
| 6 | OPDA, aldehyde | 20.0 | 3 | 100/82 | 88 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio were also investigated (entries 11–14) and it is observed that ethanol was excellent solvent and gave good to excellent yield for the synthesis of benzimidazole among other solvents. The mixed solvents system were also fail to give efficient results as compared with the pure ethanol. Therefore, ethanol was chosen as a solvent that can be considered as an optimized condition for further applications in this manuscript.
1 ratio were also investigated (entries 11–14) and it is observed that ethanol was excellent solvent and gave good to excellent yield for the synthesis of benzimidazole among other solvents. The mixed solvents system were also fail to give efficient results as compared with the pure ethanol. Therefore, ethanol was chosen as a solvent that can be considered as an optimized condition for further applications in this manuscript.
| Entry | Solvent (10 mL) | Time (h) | bConv./sel. (%) | bYield (%) |
|---|---|---|---|---|
| a Reaction conditions: all reactions proceeded with 1 mmol o-phenylenediamine (OPDA), and 1.2 mmol aldehyde with MgO@DFNS catalyst (amount of catalyst-5 wt%).b Yield refers to an isolated product which characterized by 1H NMR, 13C NMR. | ||||
| 1 | Neat | 3 | 100/45 | 35 |
| 2 | Acetonitrile | 3 | 97/81 | 82 |
| 3 | THF | 5 | 97/84 | 88 |
| 4 | 1,4-Dioxane | 8 | 85/79 | 76 |
| 5 | Chloroform | 8 | 85/72 | 80 |
| 6 | n-Hexane | 6 | 88/82 | 72 |
| 7 | Ethanol | 3 | 100/90 | 95 |
| 8 | DMF | 3 | 75/82 | 60 |
| 9 | Methanol | 4 | 90/85 | 85 |
| 10 | EtOAc | 4 | 80/79 | 75 |
| 11 | Ethanol/acetonitrile | 4 | 90/72 | 70 |
| 12 | Ethanol/THF | 4 | 74/51 | 56 |
| 13 | Ethanol/dioxane | 4 | 83/60 | 65 |
| 14 | Ethanol/DMF | 4 | 78/38 | 41 |
3.3. Comparison of the present catalytic system with known catalysts for benzimidazole synthesis
To know the efficiency of the prepared MgO@DFNS catalyst towards the synthesis of benzimidazole derivatives with other known homogeneous and heterogeneous catalysts we conducted the reaction. As per the available literature, the catalysts for the synthesis of benzimidazole derivatives have different reaction conditions and parameters. To compare the results and for uniformity, we have performed the same reaction under an optimized condition in the presence of different available catalysts from the literature. We have conducted the reaction with available catalysts from the literature and also with the prepared catalyst. The results are summarized in Table 4. Initially, we tried with CeCl3·3H2O taking optimized catalyst dosage and observed 85% conversion with moderate yield (Table 4, entry 1) subsequently we performed with MgCl2, NiCl2, CoCl2·7H2O simultaneously and observed that increasing the conversion towards di amine and also decreasing towards the selectivity (Table 4, entries 2–4). However, we have tried with Mg(NO3)2, MgO gave almost the same as earlier results, then also tried with pristine DFNS observing poor conversion concerning diamine. Finally concluded that MgO@DFNS behaved as a good selective heterogeneous catalyst under optimized conditions.| Entry | Catalyst | bConv./sel. (%) | bYield (%) |
|---|---|---|---|
| a Reaction conditions: all reactions proceeded with 1 mmol o-phenylenediamine (OPDA), 1.2 mmol aldehyde, in ethanol for 3 h.b Yield refers to an isolated product which characterized by 1H NMR, 13C NMR. | |||
| 1 | CeCl3·3H2O | 85/72 | 60 |
| 2 | MgCl2 | 90/71 | 75 |
| 3 | NiCl2 | 98/65 | 62 |
| 4 | CoCl2·7H2O | 100/68 | 71 |
| 5 | Mg(NO3)2 | 85/70 | 67 |
| 6 | MgO | 87/80 | 70 |
| 7 | DFNS | 75/55 | 45 |
| 8 | MgO–CeO2@SiO2 | 92/88 | 85 |
| 9 | MgO@DFNS | 100/90 | 95 |
| 10 | CeCl3·3H2O | 85/72 | 60 |
3.4. Synthesis of different benzimidazole derivatives from substituted OPDA and aldehyde by using MgO@DFNS catalyst
To investigate the catalytic activity towards benzimidazole derivatives we have explored the different reactants under optimized conditions and characterized their structures by 1H NMR and 13C NMR. The results are summarized in Table 5. In this, we have screened aromatic, aliphatic and also heterocyclic aldehydes, we studied the substrate scope with various sets of substituted aliphatic and aromatic aldehydes. It was found that all the substrates were viable in this transformation, providing benzimidazole derivatives in moderate to good yields, which has a pharmacological and biological interest.| Entry | Reactant-1 | Reactant-2 | Product | Conversion (%) | bYield/selectivity (%) |
|---|---|---|---|---|---|
| a Reaction conditions: all reactions proceeded with 1 mmol substituted o-phenylenediamine (OPDA), 1.2 mmol aldehyde, 5 wt% FC/AC catalyst.b Yield refers to isolated products which characterized by 1H NMR, and 13C NMR. | |||||
| 01 |  |
 |
 |
100 | 93/90 |
| 02 |  |
 |
 |
100 | 90/88 |
| 03 |  |
 |
 |
100 | 91/85 |
| 04 |  |
 |
 |
100 | 94/91 |
| 05 |  |
 |
 |
100 | 92/90 |
| 06 |  |
 |
 |
99 | 88/85 |
| 07 |  |
 |
 |
100 | 90/87 |
| 08 |  |
 |
 |
95 | 80/75 |
| 09 | 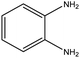 |
 |
 |
100 | 89/83 |
| 10 | 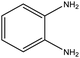 |
 |
 |
100 | 92/86 |
| 11 |  |
 |
 |
97 | 85/82 |
| 12 |  |
 |
 |
98 | 88/82 |
| 13 |  |
 |
 |
95 | 85/80 |
| 14 |  |
 |
 |
96 | 82/75 |
| 15 |  |
 |
 |
94 | 82/78 |
3.5. Proposed plausible mechanism for MgO@DFNS catalyzed synthesis of benzimidazole
Fig. 12 illustrates the plausible reaction mechanism pathway for the 2-phenyl-benzimidazole product synthesis with the help of model reagents such as benzaldehyde and o-phenylenediamine (OPD) molecules over Lewis acidic sites containing 10% MgO@DFNS catalyst, with the support of characterization results and earlier reports.45,46 Initially, the carbonyl group of the benzaldehyde molecule was interacted and activated with the aid of the Lewis acidic site of Mg2+ present the catalyst (step 1). The activated aldehyde molecule enhanced the electrophilic nature of the carbonyl carbon atom, which ultimately helps to decrease the transition state energy and attack the amino group of the OPD on the carbonyl carbon atom (step 2).45,46 As a result of the transfer of the proton in step 3 and the elimination of the water molecule as side product, the stable imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) bond was formed in step 4. Furthermore, the another free amino group (NH2) of the OPD molecule interacted with the carbon atom of the imine group. In next step, the transfer of proton was happen (step 5) and the dehydrogenation process occurred in presence of air (step 6).47,48 Finally, the stable desired product 2-phenyl-benzimidazole was formed by the successful elimination of the catalyst surface in the reaction medium. The eradicated catalyst surface will again involve as an effective active surface to achieve the desired product in the reaction medium for the next round.
N) bond was formed in step 4. Furthermore, the another free amino group (NH2) of the OPD molecule interacted with the carbon atom of the imine group. In next step, the transfer of proton was happen (step 5) and the dehydrogenation process occurred in presence of air (step 6).47,48 Finally, the stable desired product 2-phenyl-benzimidazole was formed by the successful elimination of the catalyst surface in the reaction medium. The eradicated catalyst surface will again involve as an effective active surface to achieve the desired product in the reaction medium for the next round.
 | ||
| Fig. 12 Plausible reaction mechanism for 2-phenyl-benzimidazole synthesis over 10% MgO@DFNS catalyst. | ||
3.6. Recyclability studies
The heterogeneous catalyst is of extreme importance from an industrial viewpoint as they can be reused for a number of catalytic cycles.21,22,24,25 To investigate the reusability performance of the present catalyst, we have performed a recyclability study of the MgO@DFNS catalyst by filtering the solid catalyst from the reaction mixture, giving an excessive amount of solvent washing several times and finally drying it under vacuum for 6 h. The dried MgO@DFNS catalyst was then used in the required amount and the reaction was performed under optimized reaction conditions. The recyclability study results are shown in Fig. 13, which revealed that the MgO@DFNS catalyst could effectively produce consistent conversion and yield towards the desired product for up to six consecutive recycles without losing noticeable activity. Additionally we have also carried our the characterisation of recycled catalyst such as XRD, FE-SEM and EDX. These recycled catalyst characterisation data revealed that, the catalyst was consistant in terms of morphology and as well as physicochemical properties up to six cycles.4. Conclusions
In summary, we developed a mild and efficient protocol for the synthesis of benzimidazole derivatives by condensation of o-phenylenediamine and aldehydes using the prepared engineered MgO supported on dendritic fibrous nano-silica (MgO@DFNS) as an efficient sustainable catalyst. The prepared catalyst was thoroughly examined and characterized and it was successfully confirmed by several spectrochemical and analytical techniques such as using XRD, XPS, FT-IR, FE-SEM, HR-TEM, TPR, and NH3-TPD analysis. The 10 wt% of MgO@DFNS catalyst was explored for its activity towards the one-pot synthesis of benzimidazole derivatives and showed remarkable results. The MgO@DFNS catalyst expressed 100% conversion and 90% selectivity and 90% yields of desired products under mild and eco-friendly reaction conditions. Furthermore, the prepared catalyst was studied for the effect of reaction parameters such as reaction time, the effect of solvents, the effect of catalyst dosage, the effect of reaction temperature, and the effect of different homogeneous and heterogeneous catalysts. To understand the substrate scope 15 different types of benzimidazole derivatives were prepared by using the different substituted diamines and substituted aldehydes under optimized reaction conditions. Additionally, the recyclability study of the catalyst was done up to 6 cycles and it was observed that, almost consistent conversion and selectivity towards desired product up to six subsequent cycles without significant loss of catalytic activity and morphology of the MgO@DFNS catalyst. Hence, the present work highlights a sustainable approach for the synthesis of benzimidazole derivatives in high yields with the help of 10 wt% MgO@DFNS as a heterogeneous catalyst under ambient temperature. We believed that these particular findings of the new protocol may open a new green pathway for innovative synthesis of benzimidazole and its derivatives and also valuable scale-up processes of the same.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the Science and Engineering Research Board (SERB), Government of India, for financial support through Core Research Grant (CRG) file no. CRG/2021/000656.References
- W. A. Zoubi, Int. J. Org Chem., 2013, 2013, 73–95 CrossRef.
- T. Fonseca, B. Gigante and T. L. Gilchrist, Tetrahedron, 2001, 57, 1793–1799 CrossRef CAS.
- T. Roth, M. L. Morningstar, P. L. Boyer, S. H. Hughes, R. W. Buckheit and C. J. Michejda, J. Med. Chem., 1997, 40, 4199–4207 CrossRef CAS PubMed.
- M. T. Migawa, J.-L. Girardet, J. A. Walker, G. W. Koszalka, S. D. Chamberlain, J. C. Drach and L. B. Townsend, J. Med. Chem., 1998, 41, 1242–1251 CrossRef CAS PubMed.
- W. A. Denny, G. W. Rewcastle and B. C. Baguley, J. Med. Chem., 1990, 33, 814–819 CrossRef CAS PubMed.
- S. M. Sondhi, N. Singh, A. Kumar, O. Lozach and L. Meijer, Bioorg. Med. Chem., 2006, 14, 3758–3765 CrossRef CAS PubMed.
- D. Valdez-Padilla, S. Rodríguez-Morales, A. Hernández-Campos, F. Hernández-Luis, L. Yépez-Mulia, A. Tapia-Contreras and R. Castillo, Bioorg. Med. Chem., 2009, 17, 1724–1730 CrossRef CAS PubMed.
- J. D. Geratz, F. M. Stevens, K. L. Polakoski, R. F. Parrish and R. R. Tidwell, Arch. Biochem. Biophys., 1979, 197, 551–559 CrossRef CAS PubMed.
- R. W. Middleton and D. G. Wibberley, J. Heterocycl. Chem., 1980, 17, 1757–1760 CrossRef CAS.
- L. Fan, W. Chen and L. Kong, Heterocycles, 2015, 91, 2306 CrossRef CAS.
- K. Bahrami, M. M. Khodaei and I. Kavianinia, J. Chem. Res., 2006, 2006, 783–784 CrossRef.
- L.-H. Du and Y.-G. Wang, Synthesis, 2007, 2007, 675–678 CrossRef.
- M. Faheem, A. Rathaur, A. Pandey, V. Kumar Singh and A. K. Tiwari, ChemistrySelect, 2020, 5(13), 3981–3994 CrossRef CAS.
- K. R. Kumar, P. V. V. Satyanarayana and B. S. Reddy, Arch. Appl. Sci. Res., 2012, 4, 1517–1521 Search PubMed.
- Y. Venkateswarlu, S. R. Kumar and P. Leelavathi, Org. Med. Chem. Lett., 2013, 3, 7 CrossRef PubMed.
- G. R. S. Kohli, S. Hooda and R. Chandra, Dalton Trans., 2021, 50, 7750–7758 RSC.
- A. Ben-Alloum, S. Bakkas and M. Soufiaoui, Tetrahedron Lett., 1998, 39, 4481–4484 CrossRef CAS.
- J. Lu, B. Yang and Y. Bai, Synth. Commun., 2002, 32, 3703–3709 CrossRef CAS.
- M. B. Swami, S. G. Patil, S. R. Mathapati, H. G. Ghuge and A. H. Jadhav, Der Pharma Chem., 2015, 7, 533–535 CAS.
- M. B. Swami, A. H. Jadhav, S. R. Mathpati, H. G. Ghuge and S. G. Patil, Res. Chem. Intermed., 2017, 43, 2033–2053 CrossRef CAS.
- D. Prasad, K. N. Patil, R. B. Dateer, H. Kim, B. M. Nagaraja and A. H. Jadhav, Chem. Eng. J., 2021, 405, 126907 CrossRef CAS.
- D. Prasad, K. N. Patil, V. K. Manoorkar, R. B. Dateer, B. M. Nagaraja and A. H. Jadhav, Mater. Manuf. Processes, 2021, 36, 1571–1578 CrossRef CAS.
- A. Maity, R. Belgamwar and V. Polshettiwar, Nat. Protoc., 2019, 14, 2177–2204 CrossRef CAS PubMed.
- A. H. Jadhav, A. C. Lim, G. M. Thorat, H. S. Jadhav and J. G. Seo, RSC Adv., 2016, 6, 31675–31686 RSC.
- K. N. Patil, P. Manikanta, R. R. Nikam, P. M. Srinivasappa, A. H. Jadhav, H. P. Aytam, K. S. R. Rao and B. M. Nagaraja, Results Eng., 2023, 17, 100851 CrossRef CAS.
- A. S. Patki, K. N. Patil, S. Kusuma, D. B. Muley and A. H. Jadhav, Res. Chem. Intermed., 2021, 47, 2751–2773 CrossRef CAS.
- A. H. Jadhav, A. Chinnappan, R. H. Patil, S. V. Kostjuk and H. Kim, Chem. Eng. J., 2014, 240, 228–234 CrossRef CAS.
- J. Shabir, S. Rani, M. Sharma, C. Garkoti, Surabhi and S. Mozumdar, RSC Adv., 2020, 10, 8140–8151 RSC.
- S. Arunmetha, A. Karthik, S. R. Srither, M. Vinoth, R. Suriyaprabha, P. Manivasakan and V. Rajendran, RSC Adv., 2015, 5, 47390–47397 RSC.
- I. M. El-Nahhal, F. S. Kodeh, J. K. Salem, T. Hammad, S. Kuhn, R. Hempelmann and S. Al Bhaisi, Chem. Afr., 2019, 2, 267–276 CrossRef CAS.
- Z. Wang, X. Li, L. Feng, B. Liu and F. Shamsa, Catal. Lett., 2021, 151, 1911–1922 CrossRef CAS.
- M. Moradi, N. Rastakhiz, M. Ghaedi and R. Zhiani, Catal. Lett., 2021, 151, 1653–1662 CrossRef CAS.
- M. Wu, Y. Fu, W. Zhan, Y. Guo, Y. Guo, Y. Wang and G. Lu, Catalysts, 2017, 7, 155 CrossRef.
- X. Li, P. Xu, Y. Zhou, Y. Chen, H. Jia, H. Yu and X. Li, Anal. Chem., 2022, 94, 16502–16509 CrossRef CAS PubMed.
- A. P. Jakhade, M. V. Biware and R. C. Chikate, ACS Omega, 2017, 2, 7219–7229 CrossRef CAS PubMed.
- Y. Fang, X. Wang, Q. Pan, Y. Chen and L. Dai, Chin. Chem. Lett., 2021, 32, 3976–3979 CrossRef CAS.
- X. Sun, H. T. Liu and H. F. Cheng, RSC Adv., 2017, 7, 47833–47839 RSC.
- D. Arl, V. Roge, N. Adjeroud, B. R. Pistillo, M. Sarr, N. Bahlawane and D. Lenoble, RSC Adv., 2020, 10, 18073–18081 RSC.
- S. E.-D. Hassan, A. Fouda, E. Saied, M. M. S. Farag, A. M. Eid, M. G. Barghoth, M. A. Awad, M. F. Hamza and M. F. Awad, J. Fungi, 2021, 7, 372 CrossRef PubMed.
- J. Moulder, W. Stickle, W. Sobol and K. D. Bomben, Handbook of X-Ray Photoelectron Spectroscopy, PerkinElmer Corporatioon, Physical Electronics Division, 1992 Search PubMed.
- S. Hu, Y. Zhou, C. Yuan, W. Wang, J. Hu, Q. Li and J. He, High Volt., 2020, 5, 249–255 CrossRef.
- Z. Mohammadbagheri and N. Chermahini, Renewable Energy, 2020, 147, 2229–2237 CrossRef CAS.
- S. Cheng, L. E. Metzger and S. I. Martinez-Monteagudo, Sci. Rep., 2020, 10, 2730 CrossRef CAS PubMed.
- L. Wang, M. Ammar, P. He, Y. Li, Y. Cao, F. Li, X. Han and H. Li, Catal. Today, 2017, 281, 360–370 CrossRef CAS.
- K. Bahrami and Z. Karami, J. Exp. Nanosci., 2018, 13, 272–283 CrossRef CAS.
- A. H. Cahyana, B. Ardiansah and N. A. Asrianti, AIP Conf. Proc., 2018, 2023, 020061 CrossRef.
- B. Li, R. Tayebee, E. Esmaeili, M. S. Namaghi and B. Maleki, RSC Adv., 2020, 10, 40725–40738 RSC.
- P. L. Reddy, R. Arundhathi, M. Tripathi, P. Chauhan, N. Yan and D. S. Rawat, ChemistrySelect, 2017, 2, 3889–3895 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |