Open Access Article
Open Access ArticlePreparation of barium sulfate chelating agent DTPA-5Na and molecular dynamics simulation of chelating mechanism
Chao Ma*abc,
Xin Liu *a,
Cheng Wanga,
Shengtian Gaoa and
Xiaoyi Huanga
*a,
Cheng Wanga,
Shengtian Gaoa and
Xiaoyi Huanga
aSchool of Petroleum Engineering, Yangtze University, Wuhan 434100, China. E-mail: 500526@yangtzeu.edu.cn
bKey Laboratory of Oil and Gas Drilling and Production Engineering, Wuhan 434100, China
cNational Engineering Research Center for Oil & Gas Drilling and Completion Technology, Wuhan 434100, China
First published on 24th November 2023
Abstract
Barium sulfate (BaSO4) scale is dense and hard, making it difficult to remove using conventional acid and alkali treatments. Diethylenetriaminepentaacetic acid (DTPA) and its complexes have been identified as important chelating agents for the removal of BaSO4 scale. However, DTPA has good solubility only under strong alkali conditions, which in turn exacerbate scaling. To improve the solubility and chelation effectiveness of DTPA, penta sodium diethylenetriamine-pentaacetate (DTPA-5Na) was synthesized using chloroacetic acid, diethylenetriamine, sodium carbonate, and sodium hydroxide as raw materials. The structure of DTPA-5Na was characterized by infrared spectroscopy and 1H-NMR, and its chelation effectiveness was evaluated. Experimentation demonstrated that under conditions of 50 °C and with a molar ratio of chloroacetic acid (ClCH2COOH), sodium carbonate (Na2CO3), sodium hydroxide (NaOH), and diethylenetriamine (DETA) of 5.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.50
2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.25
5.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00, the reaction for 6 hours resulted in the optimal chelation value of DTPA-5Na at 76.8 mg CaCO3·per g. Analysis of the chelation and dissolution of BaSO4 scale using DTPA-5Na and microstructural scanning electron microscopy of the BaSO4 crystal indicate that DTPA-5Na functions through solubilization, lattice distortion, and flaking dispersion to remove BaSO4. Molecular dynamics simulation software was used to simulate the chelation mechanism of DTPA-5Na, where the results indicated strong adsorption of DTPA-5Na to the surface of BaSO4. The adsorption energy follows the order of (120) surface > (001) surface > (100) surface > (210) surface. The adsorption is mainly a result of the interaction between the carboxylic “O” atom in DTPA-5Na and the (001), (100), and (120) surfaces of BaSO4 scale, while N atoms in DTPA-5Na structure primarily interact with the (210) surface. The adsorption of “O” atoms is stronger than that of “N” atoms in the DTPA-5Na structure.
1.00, the reaction for 6 hours resulted in the optimal chelation value of DTPA-5Na at 76.8 mg CaCO3·per g. Analysis of the chelation and dissolution of BaSO4 scale using DTPA-5Na and microstructural scanning electron microscopy of the BaSO4 crystal indicate that DTPA-5Na functions through solubilization, lattice distortion, and flaking dispersion to remove BaSO4. Molecular dynamics simulation software was used to simulate the chelation mechanism of DTPA-5Na, where the results indicated strong adsorption of DTPA-5Na to the surface of BaSO4. The adsorption energy follows the order of (120) surface > (001) surface > (100) surface > (210) surface. The adsorption is mainly a result of the interaction between the carboxylic “O” atom in DTPA-5Na and the (001), (100), and (120) surfaces of BaSO4 scale, while N atoms in DTPA-5Na structure primarily interact with the (210) surface. The adsorption of “O” atoms is stronger than that of “N” atoms in the DTPA-5Na structure.
1 Introduction
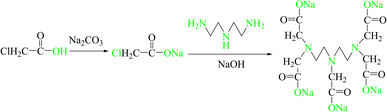
During oilfield production, the injection of water that is incompatible with the formation water or the existence of reservoirs with high levels of SO42− and Ba2+,1–5 and even the introduction of BaSO4 weighting material into the reservoir during drilling operations,6–8 can all generate varying degrees of BaSO4 scaling. BaSO4 scale possesses stable physicochemical properties, including hardness, crystalline density, and strong adhesion, resulting in its adsorption and deposition in the reservoir, wellbore, and pipelines, leading to reservoir contamination and pipeline constriction. Due to the low solubility of BaSO4 in water and its difficulty in dissolution in common acid/base solutions, chelating agents are commonly used for removal.9–12 Currently used chelating agents include hydroxycarboxylic acid salts, organophosphorus acid salts, amino carboxylic acid salts, macrocyclic polyethers, polyamines, etc. Among these, the amino carboxylic acid salt diethylenetriaminepentaacetic acid (DTPA) exhibits strong chelating ability for heavy metals such as barium and strontium; it wets, disperses, and breaks apart hard scale, transforming it into loose particles for further removal.13,14 Research shows that the chelating ability of DTPA is mainly due to the involvement of COO− groups in DTPA, which can only be smoothly dissociated in a strong alkaline environment, exhibiting excellent chelating ability. However, COO− groups are difficult to dissociate under acidic, neutral, and weakly alkaline conditions, severely limiting their chelating performance. DTPA-5Na is an amino carboxylic acid salt chelating agent that exhibits similar chelating ability to DTPA and has easily dissociable COO− groups. Therefore, the synthesis of a DTPA-5Na salt with good solubility and suitability for a wider range of acid/base environments has significant significance for the chelation removal of BaSO4.There are two main methods for synthesizing DTPA-5Na, namely, the sodium cyanide method and the chloroacetic acid method.15 The former involves the use of sodium cyanide, formaldehyde, and diethylenetriamine as raw materials, but its reliance on the highly toxic cyanide and difficulties in controlling and managing its production have caused it to be discarded. Instead, the latter method utilizes chloroacetic acid (ClCH2COOH), diethylenetriamine (DETA), sodium hydroxide (NaOH), or sodium carbonate (Na2CO3) to synthesize DTPA-5Na, which has raw materials that are readily available and a relatively simple process, making it a commonly used method in laboratories for the preparation of DTPA-5Na. For example, Lin et al.16 monitored and regulated the pH during the synthesis process to stabilize it between 11 to 11.5, DTPA-5Na was prepared by keeping the reaction mixture at 30 °C for 1 hour, and the chelation value was measured to be between 40 to 50 mg CaCO3·per g. Similarly, Wang et al.17 stabilized the pH at 11.5 during the synthesis process and prepared DTPA-5Na by keeping the reaction mixture at 50 °C for 1 hour, the chelation value was measured to be ≥ 60 mg CaCO3 per·g. Xu et al.18 also stabilized the pH at 11.5 during the synthesis process and prepared DTPA-5Na by keeping the reaction mixture at 45 °C for 8 hours, the chelation value was measured to be 71.04 mg CaCO3·per g. Therefore, the chelation value of DTPA-5Na is closely related to its synthetic conditions. In this study, we drew inspiration from the chloroacetic acid method and used diethylenetriamine, chloroacetic acid, sodium carbonate, and sodium hydroxide as raw materials to improve the synthesis conditions and enhance the chelation performance of DTPA-5Na. We analyzed the dissolution phenomena of barium sulfate scale and the microscopic changes of barium sulfate scale crystals before and after chelation. Furthermore, we used Materials Studio 5.0 molecular simulation software to simulate the scale removal mechanism of DTPA-5Na from a molecular dynamics perspective,19 these studies provide an effective approach for the development and design of barium sulfate scale chelating agents.
2 Materials and methods
2.1 Materials
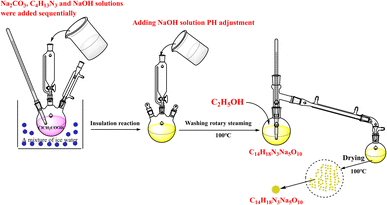
Anhydrous sodium hydrogen carbonate, chloroacetic acid, sodium hydroxide, and diethylenetriamine were obtained in high purity (99.9%) from Shanghai Maclin Biochemical Technology Co., Ltd. Anhydrous ethanol was purchased from Tianjin Bohua TONG Chemical Product Sales Center. Ammonium chloride, sodium oxalate, analyte pure, Xilong Technology Co., LTD; ammonia, analytically pure, Shanghai Suyi Chemical Reagent Co., LTD; calcium acetate, analytically pure, Tianjin Kemi Ou Chemical Reagent Co., LTD DTPA, EDTA-2Na and NTA were obtained in high purity (99.9%) from Chengdu Kelong chemical reagent factory. Barium sulfate powder (purity: 99%) was obtained from Tianjin Beichen Fangzheng, and barium sulfate scale was collected from the Changqing oilfield Ji Yuan Oil Zone gathering pipeline. The instruments used in this study included a Nicolet IS 50R Fourier transform infrared spectrophotometer (Nicolet, USA), Varian 500 MHz high-resolution NMR instrument (Varian in American). SU8000 scanning electron microscope (Hitachi High Technologies Ltd, Japan), RE-2000B rotary evaporator (Shanghai Yarong Biochemical Instrument Factory), DF-101S heat-collecting constant temperature magnetic stirrer (Gongyi Zihua Instrument Co., Ltd), FA2004 electronic balance (Shanghai Anting Electronic Instrument Factory), PHS-3c pH meter (Shanghai Leici Instrument Co., Ltd), and Materials Studio 5.0 molecular simulation software.2.2 Experimental and characterization methods
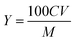 | (1) |
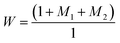 | (2) |
| V = M × 1 | (3) |
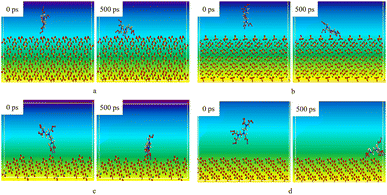
2.3 Molecular dynamics simulation of chelation performance of DTPA-5Na
3 Results and discussion
3.1 Synthesis and characterization of DTPA-5Na
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2CO3
Na2CO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH
NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DETA ratio of 5.00
DETA ratio of 5.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.50
2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.25
5.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00, a system pH of 11.5, a reaction temperature of 50 °C, and a reaction time of 6 hours.
1.00, a system pH of 11.5, a reaction temperature of 50 °C, and a reaction time of 6 hours.
| Level | a | b | c | d |
|---|---|---|---|---|
| ClCH2COOH/Na2CO3/NaOH/DETA (molar ratio) | System pH | Reaction temperature (°C) | Reaction temperature (°C) | |
| 1 | 4.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.00 2.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.00 5.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00 |
10.5 | 30 | 6 |
| 2 | 5.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.50 2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.25 5.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00 |
11.5 | 40 | 7 |
| 3 | 5.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.00 3.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.50 5.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00 |
12.5 | 50 | 8 |
| Experiment number | a | b | c | d | Y |
|---|---|---|---|---|---|
| 1 | a1 | b1 | c1 | d1 | 32.5 |
| 2 | a1 | b2 | c2 | d2 | 41.8 |
| 3 | a1 | b3 | c3 | d3 | 30.7 |
| 4 | a2 | b1 | c2 | d3 | 62.9 |
| 5 | a2 | b2 | c3 | d1 | 76.8 |
| 6 | a2 | b3 | c1 | d2 | 56.8 |
| 7 | a3 | b1 | c3 | d2 | 46.4 |
| 8 | a3 | b2 | c1 | d3 | 50.6 |
| 9 | a3 | b3 | c2 | d1 | 45.2 |
| K1 | 35.0 | 47.3 | 46.6 | 51.5 | |
| K2 | 65.5 | 56.4 | 50.0 | 48.3 | |
| K3 | 47.4 | 44.2 | 51.3 | 48.1 | |
| R | 30.5 | 12.2 | 4.7 | 3.4 |
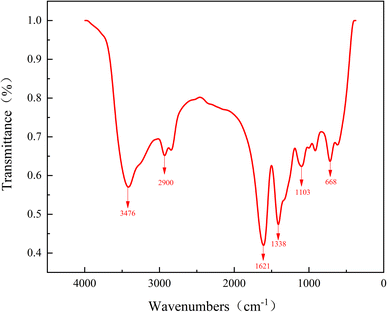
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, while the peak at 1103 cm−1 corresponds to the stretching vibration of the C–O bond. The peak at 2900 cm−1 corresponds to the stretching vibration of –CH2, and the peak at 1338 cm−1 corresponds to the stretching vibration of the amine group C–N. The peaks at 3476 cm−1 and 668 cm−1 correspond to the stretching vibration and out-of-surface bending vibration of the –OH group in alcohols, respectively, which can be attributed to residual impurities from the anhydrous ethanol washing step. Furthermore, there was no observed carboxylate O–H stretching vibration absorption peak centered at 3000 cm−1 within the 2500–3400 cm−1 range, nor was there an absorption peak of the COOH bending vibration at 1395–1440 cm−1. These findings indicate that COOH was not present in the synthesized product and that the monomer reaction fully took place. The results of the infrared spectrum tests demonstrate that all characteristic functional groups of DTPA-5Na are present within the synthesized product.
O, while the peak at 1103 cm−1 corresponds to the stretching vibration of the C–O bond. The peak at 2900 cm−1 corresponds to the stretching vibration of –CH2, and the peak at 1338 cm−1 corresponds to the stretching vibration of the amine group C–N. The peaks at 3476 cm−1 and 668 cm−1 correspond to the stretching vibration and out-of-surface bending vibration of the –OH group in alcohols, respectively, which can be attributed to residual impurities from the anhydrous ethanol washing step. Furthermore, there was no observed carboxylate O–H stretching vibration absorption peak centered at 3000 cm−1 within the 2500–3400 cm−1 range, nor was there an absorption peak of the COOH bending vibration at 1395–1440 cm−1. These findings indicate that COOH was not present in the synthesized product and that the monomer reaction fully took place. The results of the infrared spectrum tests demonstrate that all characteristic functional groups of DTPA-5Na are present within the synthesized product.
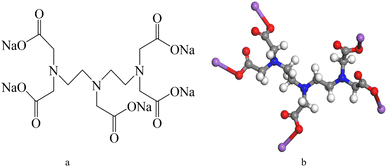
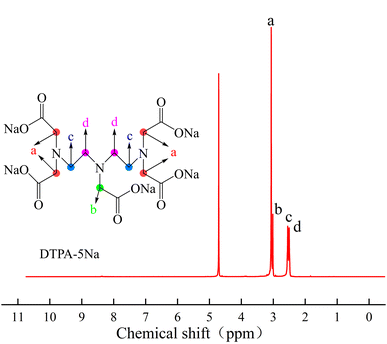
The proton nuclear magnetic resonance (1H-NMR) spectrum of DTPA-5Na is shown in Fig. 8. In this spectrum, the chemical shifts are as follows: at δ = 4.79, there is a solvent peak corresponding to D2O; at δ = 3.06, there is a peak corresponding to the methylene protons connected to the amino and carboxyl groups at both ends; at δ = 3.01, there is a peak corresponding to the methylene protons connected to the central amino and carboxyl groups; at δ = 2.55, there is a peak corresponding to the methylene protons connected to the amino groups at both ends; and at δ = 2.51, there is a peak corresponding to the methylene protons connected to the central amino group. From the above analysis, it can be concluded that the synthesized product contains all the characteristic hydrogen atoms of DTPA-5Na.
3.2 Crystal morphology
Three equal parts of BaSO4 were weighed. The first part was placed into a conical flask containing 100 ml of deionized water, the second part was placed into a conical flask containing 100 ml of a solution with the same pH as the working condition of DTPA-5Na, and the third part was placed into a conical flask containing 100 ml of DTPA-5Na solution. The conical flasks were then placed in a thermostatic water bath at 80 °C and allowed to react for 24 hours. After removal, they were dried. The crystal morphology of BaSO4 was characterized using a SU8000 scanning electron microscope. As shown in Fig. 9a, the BaSO4 crystals in deionized water had a smooth and irregular crystalline form. In this form, BaSO4 was hard and strongly adhered, facilitating adsorption and accumulation. Based on Fig. 9b, BaSO4 crystals placed in a water solution with the same working pH conditions as the DTPA-5Na solution exhibited a similar morphology to Fig. 9a. Both showed smooth and irregular crystalline structures, indicating that BaSO4 retained its hardness and strong adhesion properties, facilitating adsorption and deposition. Fig. 9c revealed that the BaSO4 crystals in the DTPA-5Na solution had a rough and porous granulated crystalline form. These irregular crystals were easily washed away by formation fluids and were difficult to adsorb and accumulate, possibly due to the introduction of DTPA-5Na into the BaSO4 crystal lattice, causing lattice dislocations and deformations, resulting in a change in the crystal structure and a decrease in density, making BaSO4 more prone to dispersion in the solvent.3.3 Evaluation of DTPA-5Na scale dissolving performance
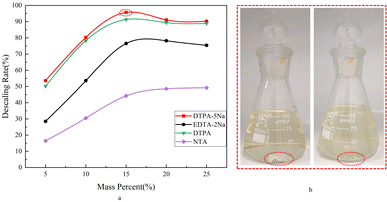
According to the 2.2.4 Testing of the descaling performance, a comparative study of the efficacy of DTPA-5Na versus several commonly used amino carboxylic acid chelating agents for the removal of BaSO4 scale was conducted while adjusting the solution's working pH conditions. As shown in Fig. 10a, DTPA-5Na demonstrated better scale dissolution than EDTA-2Na and NTA, attributed to DTPA-5Na's higher number of coordination atoms (8 coordination atoms from 3 amino and 5 carboxylate groups) compared to EDTA-2Na (6 coordination atoms from 2 amino and 4 carboxylate groups) and NTA (4 coordination atoms from 1 amino and 3 carboxylate groups), leading to stronger chelating ability23 with Ba2+. Compared to DTPA, DTPA-5Na exhibited a higher scale dissolution rate, possibly due to its stronger salt effect.23 According to the scale dissolution curve at different DTPA-5Na concentrations, the dissolution rate increased first, then decreased with increasing DTPA-5Na concentration, reaching the maximum at 15% concentration. This is because at low concentrations, the increase in DTPA-5Na concentration promotes the right shift of the equilibrium between Ba2+ + DTPA5− → Ba-DTPA3-, leading to an increase in dissolution rate. However, when the concentration of DTPA-5Na becomes too high, the chelating agent molecules tend to adsorb on the surface of BaSO4, resulting in steric hindrance.24 This weakens the chelation effect and consequently decreases the dissolution rate. Therefore, increasing the amount of DTPA-5Na does not indefinitely enhance the dissolution capacity.25 As shown in Fig. 10b, significant changes in the morphology of BaSO4 scale were observed before and after adding DTPA-5Na, likely due to DTPA-5Na diffusing to the BaSO4 surface, forming complexes, and then dispersing into the solution.3.4 Analysis of the interaction between DTPA-5Na and BaSO4
The interaction energy between different molecules is used to determine the strength and stability of the intermolecular interactions within the reaction system. If the interaction energy is negative, it indicates that the adsorption process can release energy and the adsorption system is more stable, making the adsorption easier to occur. Conversely, if the interaction energy is positive, it indicates that the adsorption process requires energy absorption, making the adsorption more difficult to occur.26–29 The magnitude of the negative interaction energy reflects the strength of the adsorption, with a larger negative value indicating a stronger adsorption. Energy analysis was performed on the DTPA-5Na and BaSO4 models after dynamic simulation, and the last ten frames of the simulation were used to analyze the adsorption configurations. The calculation formula for the interaction energy between DTPA-5Na and the BaSO4 crystals on each surface is shown in formula (4):| Einteract = Etotal − EDTPA-5Na − Esurface | (4) |
As shown in Table 3, the interaction energy between DTPA-5Na and different crystal planes of BaSO4 is negative, indicating that they have a certain adsorption capacity and DTPA-5Na is easily adsorbed on the surface of BaSO4, which impedes the accumulation of Ba2+ on the surface of BaSO4, making it difficult for the crystal to grow. The magnitude of the interaction energy between DTPA-5Na and the various crystal planes of BaSO4 is not uniform, indicating that the strength of the adsorption varies depending on the growth crystal plane. Based on the sorting of the interaction energy between the two, the strength of the adsorption of DTPA-5Na on the various crystal surfaces of BaSO4 is as follows: (120) surface > (001) surface > (100) surface > (210) surface.
| Surface | Etotal kcal mol−1 | EDTPA-5Na kcal mol−1 | Esurface kcal mol−1 | Einteract kcal mol−1 |
|---|---|---|---|---|
| (001) | −122.82 | 129.70 | 0 | −252.52 |
| (100) | −47.59 | 182.25 | 0 | −229.84 |
| (120) | −157.57 | 136.78 | 0 | −294.35 |
| (210) | 2.29 | 155.43 | 0 | −153.14 |
3.5 Radial distribution of adsorbed atoms in DTPA-5Na
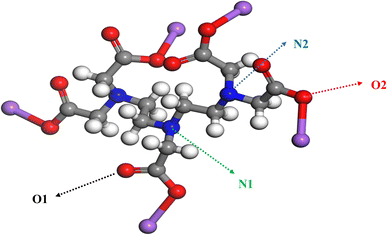
The radial distribution function (RDF) is used to represent the distance distribution between a specific atom (or molecule) and its surrounding atoms (or molecules).30 Therefore, RDF is commonly used to reflect the correlation of electrons and the orderliness of matter. The RDF can be expressed by g(r,r′), where g(r,r′) predominantly reflects the distance between atoms and the packing state of atoms when |r − r′| is relatively small, when |r − r′| is large, the probability of finding particles within a given distance almost no longer changes, so g(r,r′) gradually becomes flat and ultimately tends to a constant value.31 Radial Distribution Functions (RDF) of O1, O2, N1, and N2 in DTPA-5Na with different surfaces of BaSO4 are shown in Fig. 10.As shown in Fig. 11a, there are significant differences in the first peak radius and peak width among the four curves, indicating that the O1 and O2 atoms of DTPA-5Na have a shorter interaction radius and closer contact with BaSO4 compared to N1 and N2 atoms. Specifically, the first peak position of O1 atom is 2.86 Å with a frequency of 83.56%, and that of O2 atom is 2.79 Å with a frequency of 11.68%. Therefore, it can be concluded that both carboxyl O1 and O2 atoms of DTPA-5Na possess strong adsorption on BaSO4 (001) surface, with O2 slightly stronger than O1. Similarly, Fig. 11b and c show that the O1 and O2 atoms have a shorter interaction radius and closer contact with BaSO4 (100) and (120) surfaces, respectively. The first peak position of O1 atom on (100) surface is 3.13 Å with a frequency of 49.33%, and that of O2 atom is 2.93 Å with a frequency of 38.15%; while on (120) surface, the first peak position of O1 atom is 2.61 Å with a frequency of 40.95%, and that of O2 atom is 2.55 Å with a frequency of 18.02%. Therefore, it can be concluded that both carboxyl O1 and O2 atoms of DTPA-5Na possess strong adsorption on BaSO4 (100) and (120) surfaces, with O2 slightly stronger than O1. Regarding the adsorption on BaSO4 (210) surface, Fig. 11d shows that the central amino N2 atom of DTPA-5Na possesses the dominant role, as evidenced by its shorter interaction radius and closer contact with BaSO4. Specifically, the first peak position of N2 atom on (210) surface is 6.86 Å with a frequency of 14.02%.
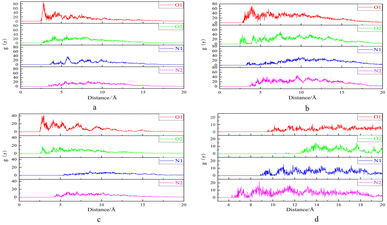 | ||
| Fig. 11 Radial distribution function plots of O1, O2, N1, and N2 in DTPA-5Na and BaSO4 surfaces. (a) (001) surface. (b) (100) surface. (c) (120) surface. (d) (210) surface. | ||
In summary, according to the predicted bonding distances of DTPA-5Na on different crystal surfaces of BaSO4, the dynamic adsorption capacity is ranked as (120) surface > (001) surface > (100) surface > (210) surface. Among them, the carboxyl O1 and O2 atoms play a major role on (001), (100) and (120) surfaces, with O2 having the shortest bonding distance and the strongest dynamic adsorption capacity, mainly for adsorption purposes; while O1 has a higher frequency and greater adsorption stability, mainly for chelating purposes. On (210) surface, the central amino N2 atom plays a main role, which has a longer bonding distance. Therefore, the adsorption of O atoms is stronger than that of N atoms in the structure of DTPA-5Na.
4 Conclusions
(1) By optimizing the synthesis conditions through orthogonal experiments, the molar ratio of chloroacetic acid, sodium carbonate, sodium hydroxide, and diethylenetriamine was set at 5.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.50
2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.25
5.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00, the reaction endpoint pH was 11.5, and the reaction was held at 50 °C for 6 hours. The resulting product exhibited good chelation performance, with a chelation value of 76.8 mg CaCO3·per g.
1.00, the reaction endpoint pH was 11.5, and the reaction was held at 50 °C for 6 hours. The resulting product exhibited good chelation performance, with a chelation value of 76.8 mg CaCO3·per g.
(2) Based on the macro-scale descaling morphology of DTPA-5Na, chelation and solubilization as well as peeling and dispersion are considered as the mechanisms by which DTPA-5Na removes scale, while the micro-scale descaling morphology suggests that the mechanism of DTPA-5Na in removing scale is induced lattice distortion.
(3) Evaluation of the synthesized DTPA-5Na was carried out through a descaling experiment, which demonstrated that, with an increase in the amount of DTPA-5Na, the descaling rate initially increased and then decreased. At a concentration of 15%, the descaling rate reached 95.7%, with a dissolution amount of 0.957 g.
(4) Molecular dynamics simulations showed that the adsorption strength of DTPA-5Na varied on different growth surfaces of BaSO4, with the (120) surface > (001) surface > (100) surface > (210) surface. Through radial functions calculation of DTPA-5Na's functional groups, it was found that the carboxyl atoms O1 and O2 played the main role in the (001), (100), and (120) surfaces, with O2 having the shortest bond distance and the strongest dynamic adsorption ability, mainly for adsorption; O1 had a high frequency and greater adsorption stability, mainly for chelation. On the (210) surface, the amino atom N2 played the main role, with a longer bond distance than the “O” atoms in the DTPA-5Na structure, suggesting that the adsorption ability of the “O” atoms was stronger than that of the “N” atoms.
Author contributions
Chao Ma, proposed the ideas, steps and details of the experiment, most of the experiments were done by Xin Liu, C. W., S. T. G., X. Y. H., where Xin Liu was instrumental in the proper conduct of the experiments and wrote the article together with Chao Ma, contributed equally to this work and should be considered co-first authors, and all the authors analyzed the data, discussed the conclusions.Conflicts of interest
The authors declare that there are no competing interests regarding the publication of this article.Acknowledgements
We acknowledge the Foreign Experts Bureau of the Ministry of Science and Technology for the funding of the Key support program project of Foreign Experts – Surface modified nano-silica/thermosensitive polymer composites development and application in unconventional oil and gas stimulation fluids (wgxz2022057).References
- A. Sun, J. He, L. Fei, L. Wang, L. Qiang and M. Fang, Extrusion system of barium strontium anti-scale agent in western oilfield of South China Sea, Sci. Tech. Eng., 2017, 17(18), 198–202 Search PubMed.
- H. Li and J. Zhang, Progress in oilfield anti scaling technology and its application, Chem. Ind. Eng. Technol., 2012, 33(04), 40–43 CAS.
- L. Xuejiao, Research on the Factors Influencing Barium Sulfate Scaling and Chemical Scale Inhibition Experiments, Southwest Petroleum University, 2015 Search PubMed.
- W. Yang, Y. Wang, C. Fu, et al., Experimental Investigation of the Minimum Inhibitor Concentration in Porous Media[C]//SPE International Oilfield Scale Conference and Exhibition, OnePetro, 2020 Search PubMed.
- Y. Wang, X. Li and J. Lu, Physicochemical modeling of barium and sulfate transport in porous media and its application in seawater-breakthrough monitoring, SPE J., 2021, 26(06), 3855–3876 CrossRef CAS.
- F. Lin, F. You, S. Wang and P. Yang, Anti-barium sulfate sedimentation technology of weighted drilling fluid, Drill. Fluid Completion Fluid, 2015, 32(03), 27–29 Search PubMed.
- Y. Dong, D. Chang and L. Hu, et al., Research and Application of Complex Method for Removing Barium Sulfate from Oilfield, Drill. Fluid Completion Fluid, 2017, 34(03), 122–126 CAS.
- Z. Tariq, M. S. Kamal and M. Mahmoud, et al., Self-destructive barite filter cake in water-based and oil-based drilling fluids, J. Pet. Sci. Eng., 2021, 197, 107963 CrossRef CAS.
- H. Li, L. Ran and T. Liu, et al., Study on the Effect of Scale Inhibitors on the Kinetic Parameters of Barium Sulfate Crystallization, J. Southwest Pet. Univ., 2022, 44(05), 175–184 CAS.
- L. Cheng, Y. Liang and Y. Li, et al., Research and application of barium sulfate strontium scale control technology in the west oilfield of South China Sea, Drill. Prod. Technol., 2020, 43(01), 61–64 Search PubMed.
- H. Sun, Y. Chen and H. Qian, Oilfield descaling technology research progress, J. Chem. Reagents, 2012,(11), 991–994, DOI:10.13822/j.cnki.hxsj.2012.11.026.
- S. Wu, L. Cheng and X. Wan, et al., Evaluation of an efficient and economical barium strontium scale remover system for offshore oil wells and research on its scale removal process, China Surfactant Deterg. Cosmet., 2022, 52(10), 1062–1071 CAS.
- F. M. Alissa, N. W. Aljuryyed, S. A. Balharth, et al., Calcium Sulfate Scale Dissolution Efficiency by Various Chemicals Additives[C]//SPE International Conference and Exhibition on Formation Damage Control, OnePetro, 2022 Search PubMed.
- M. Fu, Experimental study on the dissolution of barium sulfate scale with DTPA, Drill. Prod. Technol., 1999,(01), 61–62 Search PubMed.
- Ai Fei, Improvement of DTPA pentasodium salt synthesis process, Yunnan Chem. Ind., 2019, 46(05), 93–95 Search PubMed.
- Z. Lin, DTPA chelating agent and its manufacturing, Pap. Papermaking, 2005,(03), 63–64 Search PubMed.
- J. Wang, Study on the synthesis process of sodium diethylenetriamine pentaacetate chelating agent, Leather Chem. Ind., 1998,(04), 26–27 Search PubMed.
- Y. Xu, Z. Chen and Y. Gao, et al., Synthesis of DTPA and its application in oxygen bleaching, Print. Dyeing, 2021, 47(10), 16–20 CAS.
- C. Zhuang and H. Yue, Zhang Huijun Application of Molecular Simulation Methods and Simulation Software Ma materials Studio in Polymer Materials, Plastics, 2010, 39(4), 81–84 Search PubMed.
- X. Geng, R. D. Sosa and M. A. Reynolds, et al., Alginate as a green inhibitor of barite nucleation and crystal growth, Mol. Syst. Des. Eng., 2021, 6(7), 508–519 RSC.
- A. G. Stack, Molecular dynamics simulations of solvation and Kink Site formation at the {001} Barite – Water interface, J. Phys. Chem. C, 2009, 113(6), 2104–2110 CrossRef CAS.
- R. D. Sosa, X. Geng and A. Agarwal, et al., Acidic polysaccharides as green alternatives for barite scale dissolution, ACS Appl. Mater. Interfaces, 2020, 12(49), 55434–55443 CrossRef CAS.
- B. Xu, Research on Chemical Cleaning of Barium Strontium Sulfate Scale, Southwest Petroleum University, 2016 Search PubMed.
- T. Almubarak, J. H. Ng, and H. Nasr-El-Din, Oilfield Scale Removal by Chelating Agents: An Aminopolycarboxylic Acids Review, Paper presented at the SPE Western Regional Meeting, Bakersfield, California, 2017 Search PubMed.
- W. Zhongjin, F. Zhou and T. Xu, Review on the mechanism of barium sulfate filter cake blockage and the decision-making technology of chelation blockage removal, Drill. Fluid Completion Fluid, 2020, 37(06), 685–693 Search PubMed.
- Y. M. Tang, F. Zhang and Z. Y. Cao, et al., Crystallization of CaCO3 in the presence of sulfate and additives: Experimental and molecular dynamics simulation studies, J. Colloid Interface Sci., 2012, 377, 430–437 CrossRef CAS.
- W. Y. Shi, C. Ding and J. L. Yan, et al., Molecular dynamics simulation for interaction of PESA and acrylic copolymers with calcite crystal surfaces, Desalination, 2012, 291, 8–14 CrossRef CAS.
- E. Hadicke, J. Rieger and I. U. Rau, et al., Molecular dynamics simulations of the incrustation inhibition by polymeric additives, Phys. Chem. Chem. Phys., 1999, 1(17), 3891–3898 RSC.
- F. Jones, W. R. Richmond and A. L. Rohl, Molecular modeling of phosphonate molecules onto barium sulfate terraced surfaces, J. Phys. Chem. B, 2006, 110(14), 7414–7424 CrossRef CAS.
- W. Shi, F. Wang and M. Xia, et al., Molecular dynamics simulation of interaction between carboxylate copolymer and calcite crystal, Acta Chim. Sin., 2006, 64(17), 1817 CAS.
- C. Chen, W. Lei and M. Xia, et al., Molecular modeling of several phosphonates onto the stepped calcite (011) surface, Desalination, 2013, 309, 208–212 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |