Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring the role of Mn2+ in the structure, magnetic properties, and radar absorption performance of MnxFe3−xO4–DEA/MWCNT nanocomposites
Wida Puteri Agistaa,
ST. Ulfawanti Intan Subadraa,
Ahmad Taufiq *a,
Arif Hidayata,
Erfan Handokob,
Mudrik Alaydrusc,
Tahta Amrillahd and
Itthipon Jeerapan
*a,
Arif Hidayata,
Erfan Handokob,
Mudrik Alaydrusc,
Tahta Amrillahd and
Itthipon Jeerapan e
e
aDepartment of Physics, Faculty of Mathematics and Natural Science, State University of Malang, Jl. Semarang 5, Malang, 65145, Indonesia. E-mail: ahmad.taufiq.fmipa@um.ac.id
bDepartment of Physics, Faculty of Mathematics and Natural Sciences, Universitas Negeri Jakarta, Jl. Rawamangun Muka 1, Jakarta 13220, Indonesia
cDepartment of Electrical Engineering, Universitas Mercu Buana, Jl. Meruya Selatan 1, Jakarta 11650, Indonesia
dNanotechnology Engineering, Faculty of Advanced Technology and Multidiscipline, Universitas Airlangga, Jl. Ir. Sukarno 1, Surabaya 60115, Indonesia
eDivision of Physical Science, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla 90110, Thailand
First published on 9th October 2023
Abstract
Iron oxide/carbon-based nanocomposites are known as an ideal combination of magnetic–conductive materials that were recently developed in radar absorption application; one example is the Fe3O4/multiwalled carbon nanotubes (MWCNTs). In this study, we try to boost their radar absorption ability by Mn-ion doping. Mn is an appropriate Fe substitute that is predicted to alter the magnetic properties and enhance the conductivity, which are crucial to developing their radar absorption properties. Diethylamine (DEA) is also used as a capping agent to improve the size and shape of the nanocomposite. In this study, a MnxFe3−xO4–DEA/MWCNT nanocomposite is successfully prepared by the coprecipitation method using a variation of x = 0, 0.25, 0.5, 0.75, and 1. We found that the sample's magnetic saturation (Ms) decreases, while the reflection loss (RL) increases with increasing the molar fraction of Mn. The enhancement of the radar wave absorption in the sample is dominated by dielectric losses due to the increase of electrical conductivity and interfacial polarization with the addition of Mn in the nanocomposites. We believe that our finding could shed light on the role of doping elements to develop the radar absorption properties, and further pave the way for the real implementation of iron oxides/graphene-based nanocomposite as radar-absorbing materials (RAMs).
1. Introduction
Recently, the escalating growth of communication technology, particularly wireless communication, has led to a significant rise in electromagnetic pollution, emerging as a critical concern.1 Electromagnetic pollution damages electronic devices, and has a detrimental effect on human health.2,3 Therefore, researchers are currently working on the development of microwave-absorbing materials, particularly radar-absorbing materials (RAMs). Apart from addressing the issue of electromagnetic pollution, RAMs have potential applications in the military sector to conceal military defense equipment, such as fighter planes and tanks, from adversaries.4 RAMs attenuate the energy of electromagnetic waves by converting the waves into heat energy through magnetic and dielectric losses. Nevertheless, the primary challenge in RAM design lies in the selection of materials. To maximize their capability to absorb electromagnetic waves, a better understanding of how to engineer materials composed of magnetic and dielectric elements is required.Fe3O4 nanoparticles (NPs) have garnered attention as potential microwave absorbents5,6 owing to their unique features, including outstanding magnetic properties,7,8 great permeability, excellent chemical and thermal stability,9 high biocompatibility, and nontoxicity.10 Despite these superiorities, Fe3O4 still has relatively low absorption8 and deficient dielectric properties. Fe3O4 also tends to agglomerate;11 therefore, the use of capping agents, such as PEG and PVP, as templates is essential.12–14 However, they fail to generate NPs with uniform size and shape. Diethylamine (DEA) is one of the capping agents serving as a soft template for improving the size and shape of NPs to minimize agglomeration, which is also important to boost the absorption ability.15,16
The performance of Fe3O4 NPs in radar absorption can be improved through compositing and substitution with other materials. Recently, carbon nanotubes (CNTs) have gained attention as composite materials with Fe3O4 NPs owing to their dielectric, mechanical, electrical, and thermal properties and extensive surface area.17 Multiwalled CNTs (MWCNTs) are one of the CNTs commonly composited with Fe3O4 NPs for numerous applications, including RAMs.18 A combination of carbon and magnetic materials is considered as an effective strategy to obtain high-performance RAMs.19 For RAM application, MWCNT offers excellent conductivity.20 Combining MWCNT with Fe3O4 enhances the absorbing intensity and bandwidth and reduces RAM density, which benefits the performance of the RAM.21 Fe3O4 as a magnetic component also could enrich the electromagnetic wave dissipation mechanism, which is important in wave absorption ability.22 Nevertheless, although the iron oxides/graphene-based nanocomposite is known as an ideal combination of magnetic–conducting materials that was recently developed in radar absorption applications, further development is required. Various attempts had been carried out, such as element/ion substitution, shape or morphology modification, and compositing strategies. Among them, we believe that element/ion substitution could substantially develop the RAM. Thus, an in-depth exploration of this strategy is very important.
In this study, we performed Mn2+ ion substitution to improve the radar absorption ability and performance of Fe3O4–DEA/MWCNT. Mn2+ ion substitution can alter the magnetic properties of iron oxides since it has the appropriate atomic radii with Fe.23 Magnetic properties are one of the vital parameters for Fe3O4 NP application in radar absorption application.24 Mn ion substitution can also increase the conductivity of a material, including Fe3O4, which affects the dielectric loss of the RAM.23,24 This study aims to identify the effects of Mn2+ ion substitution on the structure, morphology, and magnetic properties of Fe3O4–DEA/MWCNT nanocomposites. An in-depth study of the effect of Mn substitution is very important to pave the way for the real implementation of Fe3O4–DEA/MWCNT and other types of iron oxides/graphene-based nanocomposites as RAMs.
2. Experimental method
2.1. Synthesis of MnxFe3−xO4–DEA/MWCNT nanocomposites
Iron sand was used as the central precursor in the MnxFe3−xO4 preparation. MnCl2·6H2O, DEA, HCl (12 M, 99.9%), NH4OH (6.5 M, 999%), and HNO3 (37%) were purchased from Merck. MWCNTs were obtained from Sigma-Aldrich, and distilled water was acquired from Pro Analysis. MWCNTs were functionalized as previously described.25 First, 1 g of MWCNTs was mixed with 100 mL of HNO3, and sonicated for 2 h at 40 kHz and 50 °C. The solution was filtered, washed using distilled water until pH 7, and then dried in an oven at 100 °C for 5 h to produce functionalized MWCNT powder (F-MWCNT). The synthesis of nanocomposites began with the production of MnxFe3−xO4. First, iron sand was separated using a permanent magnet to select the magnetic powder with high purity.26 Then, 20 g of magnetic powder was reacted with 58 mL of HCl, followed by stirring for 30 min at room temperature to produce FeCl2 and FeCl3 solutions. MnCl2·6H2O with Mnx fraction variations of x = 0, 0.25, 0.5, 0.75, 1 was then added. We controlled the amount of molar fraction x with the calculated Mol divided with Mr, and reaction of MnxFe3−xO4 followed eqn (1).| xMn2+ + (1 − x)Fe2+ + 2Fe3+ + 8OH− → MnxFe3−xO4 + 4H2O | (1) |
The solution was then mixed for 20 min, and titrated with 6 mL of DEA solution previously dissolved into 9 mL of distilled water. The titration products were combined with 0.1 g of F-MWCNT while stirring at room temperature. A total of 19 mL of NH4OH was titrated until a black precipitate was obtained. The precipitate was washed using distilled water until pH 7, and then dried at 100 °C to attain a sample of the MnxFe3−xO4–DEA/MWCNT powder.
2.2. Characterizations
The structure, phase, crystallite size, and lattice parameter of the MnxFe3−xO4–DEA/MWCNT nanocomposites were investigated by X-ray diffraction (XRD) using X'Pert Pro test, Cu-Kα 1.540 Å Panalytical Merck with Cu-Kα 1.54060 Å radiation beam. The morphology and components of the nanocomposites were determined by scanning electron microscopy (SEM) combination of carb characterization type FEI, Inspect-S50. The functional groups within the sample were investigated using Fourier transform infrared (FTIR) spectroscopy type IRPrestige-21. The magnetic properties of the nanocomposites were characterized using a vibrating sample magnetometer (VSM) type PPMS VersaLab with a magnetic field ranging from −3 to 3 Tesla. Lastly, the radar absorption performance of the nanocomposites was characterized using vector network analyzer (VNA) type Rohde-Schwarz ZVA 67 to determine the complex permittivity, complex permeability, and reflection loss (RL).3. Results and discussion
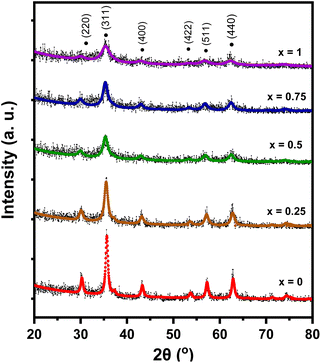
The XRD pattern of MnxFe3−xO4–DEA/MWCNT is presented in Fig. 1. Diffraction peaks with x = 0 code (Fig. 1) were detected at 2θ = 30.21°, 35.57°, 43.13°, 53.65°, 57.13°, and 62.69°. This diffraction pattern is similar to that reported for Fe3O4–DEA.15 The absence of new peaks following DEA addition suggests the successful utilization of DEA as a surfactant.16 Furthermore, the lack of MWCNT diffraction peaks at 2θ = 26° is caused by the lower mass of MWCNT relative to that of MnxFe3−xO4 at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 for MWCNT
30 for MWCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MnxFe3−xO4. The diffraction patterns of all samples also indicate that the addition of Mn as a dopant (x = 0.25–1) generates the same pattern as that of the x = 0 sample. Therefore, our results showed that Mn addition causes no new peak or phase, signifying that Mn has successfully penetrated Fe3O4. Li et al. demonstrated that the absence of new peaks on MnxFe3−xO4 indicates that Mn2+ successfully enters MnxFe3−xO4, replaces Fe3+, and does not form MnO2 deposits on the Fe3O4 surface.27
MnxFe3−xO4. The diffraction patterns of all samples also indicate that the addition of Mn as a dopant (x = 0.25–1) generates the same pattern as that of the x = 0 sample. Therefore, our results showed that Mn addition causes no new peak or phase, signifying that Mn has successfully penetrated Fe3O4. Li et al. demonstrated that the absence of new peaks on MnxFe3−xO4 indicates that Mn2+ successfully enters MnxFe3−xO4, replaces Fe3+, and does not form MnO2 deposits on the Fe3O4 surface.27
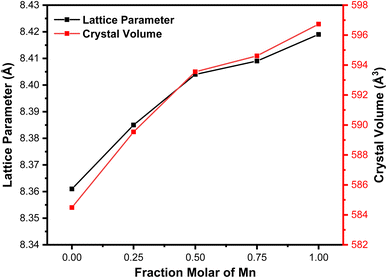
We performed a quantitative analysis by comparing the obtained data with the ICSD database number 30860. The results showed that the observed peaks are identical with the Miller index of (220), (311), (400), (422), (511), and (440), with the highest peak observed at ∼2θ = 35.5° on the hkl (311). This finding indicates the single phase of the sample with cubic spinel structure and the space group of Fd3m. The diffraction patterns at hkl (220) and (311) demonstrate a shift towards smaller 2θ values with Mn substitution. This shift is associated with the increase in the sample's lattice parameters (8.361–8.429 Å) due to Mn substitution, as summarized in Table 1. The observed increase in lattice parameter is attributed to the effects of ionic size because Mn2+ (0.81 Å) possesses a larger ionic radius compared to Fe3+ (0.77 Å).28 Therefore, Mn substitution on Fe3O4 drives the expansion of cell units, as proven by the expanded sample's crystal volume shown in Table 1 and Fig. 2. This finding also signifies that some Fe3+ ions on the tetrahedral structure have been substituted by Mn2+ ions.24 Our results are similar to those of a previous study reporting an increase in lattice parameters from 8.372 Å to 8.474 Å with Mn2+ substitution on x = 0.25 to x = 1 variations.29
| Samples | Crystallite size (nm) | Lattice parameter (Å) | Crystal volume (Å3) |
|---|---|---|---|
| x = 0 | 17.0 ± 0.2 | 8.361 ± 0.003 | 584.5 ± 0.3 |
| x = 0.25 | 11.0 ± 0.3 | 8.385 ± 0.001 | 589.5 ± 0.2 |
| x = 0.5 | 8.0 ± 0.1 | 8.404 ± 0.003 | 593.6 ± 0.1 |
| x = 0.75 | 7.9 ± 0.2 | 8.409 ± 0.002 | 594.6 ± 0.2 |
| x = 1 | 4.5 ± 0.3 | 8.419 ± 0.002 | 596.7 ± 0.5 |
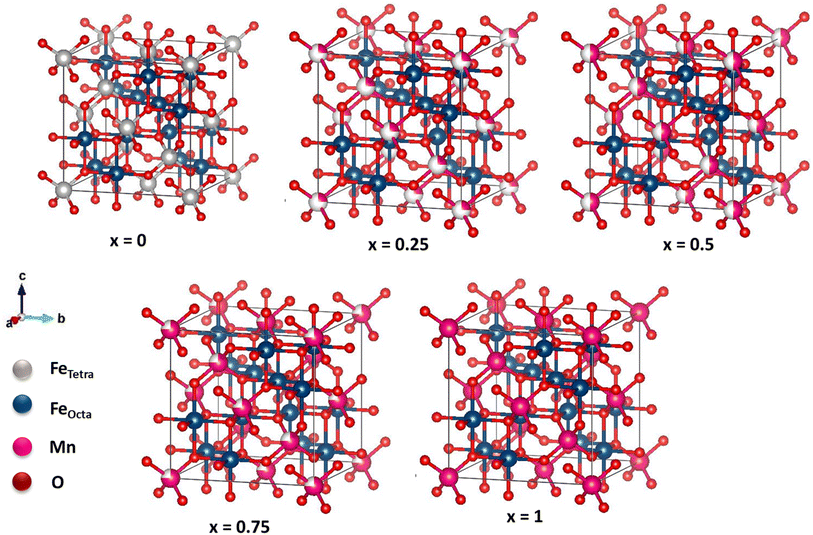
The crystal structure of MnxFe3−xO4 is illustrated in Fig. 2. The x = 0 sample structure has an inverse cubic spinel structure with eight Fe3+ ions in the tetrahedral site, and eight Fe2+ and Fe3+ ions in the octahedral site. The addition of Mn2+ has enlarged the cubic spinel crystal structure compared to that of the sample x = 0, signifying the expansion of the cell unit. In samples with x = 0.25, 0.5, and 0.75, Mn2+ ions replace some Fe3+ ions on the tetrahedral site. In sample x = 1, all Fe3+ ions are substituted by Mn2+ ions. Four and six O ions are observed surrounding the Fe2+, Fe3+, and Mn2+ ions on the tetrahedral and octahedral sites, respectively.
Fig. 3 displays the graph depicting variations in the lattice parameters and Mn molar fraction variations. The Mn molar fraction demonstrates an increasing trend with two distinct slopes: one for molar fractions ranging from 0 to 0.5, and another for molar fractions from 0.5 to 1. This observed trend closely resembles the increase in lattice parameters reported in a previous study of about ∼8.4 Å.30 Additionally, this trend is consistent with the Mössbauer results, which indicate that Mn2+ ions replace Fe3+ ions on the tetrahedral sites. This finding is supported by the Mössbauer spectroscopy, which shows that the iron ions on the MnFe2O4 system are in the 3+ state, allowing the substitution of Mn2+ to be written as (Fex−12+Fex+13+)octa(Mnx2+Fex−13+)tetra.
The crystallite size of the MnxFe3−xO4–DEA/MWCNT nanocomposites decreases with the increase in Mn substitution, which is consistent with previous results.23 The reduced crystallite size is caused by the addition of DEA as the controlling and reducing agent for the crystallite size. Taufiq et al. similarly reported that all synthesized Fe3O4 samples with the DEA template present a nanometric particle size, which decreases upon the addition of DEA at high concentrations.16 By using DEA, the particle size of the nanocomposite is easy to control. Thus, by controlling the particle size, we also can tune the absorbance ability of the nanocomposite.19
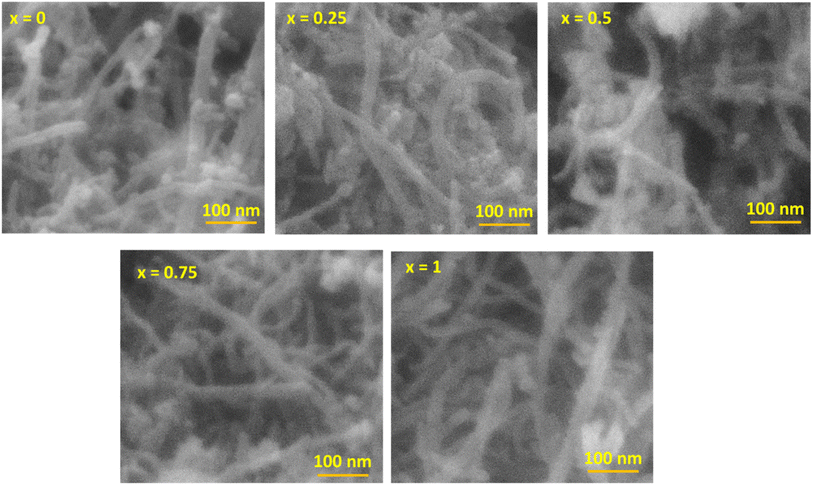
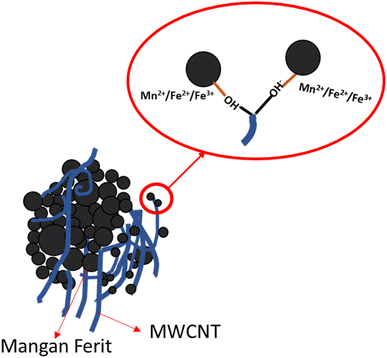
The morphology of the MnxFe3−xO4–DEA/MWCNT nanocomposites was assessed by SEM characterization. The SEM image with 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification is presented in Fig. 4. The MnxFe3−xO4 cluster is in the form of a chunk, and the MWCNTs exhibit a cylindrical shape. The MWCNTs in the cylinder form a shroud and bind to the spherical MnxFe3−xO4 chunk. The bond arises due to MWCNT functionalization, which introduces OH-functional groups on the MWCNT surface. These groups interact with the positive charge of MnxFe3−xO4, as depicted in Fig. 5. Theoretically, the positive ions of Fe3O4, such as Mn2+, Fe2+, or Fe3+, form bonds with the negative ions and the charged carboxyl group on the MWCNT surface, facilitated by the MWCNT functionalization using HNO3. The presence of the carboxyl group and damage in certain parts of MWCNT facilitates its binding to other compounds, in this case, MnxFe3−xO4–DEA.18
000× magnification is presented in Fig. 4. The MnxFe3−xO4 cluster is in the form of a chunk, and the MWCNTs exhibit a cylindrical shape. The MWCNTs in the cylinder form a shroud and bind to the spherical MnxFe3−xO4 chunk. The bond arises due to MWCNT functionalization, which introduces OH-functional groups on the MWCNT surface. These groups interact with the positive charge of MnxFe3−xO4, as depicted in Fig. 5. Theoretically, the positive ions of Fe3O4, such as Mn2+, Fe2+, or Fe3+, form bonds with the negative ions and the charged carboxyl group on the MWCNT surface, facilitated by the MWCNT functionalization using HNO3. The presence of the carboxyl group and damage in certain parts of MWCNT facilitates its binding to other compounds, in this case, MnxFe3−xO4–DEA.18
The carboxyl group with a negative charge will interact with MnxFe3−xO4 having positive ions (Mn2+, Fe2+, and Fe3+), which initiate an electrostatic attraction and increase the structural stability of the nanocomposites.31,32 Due to this fact, MnxFe3−xO4 is strongly bonded to the surface of MWCNT. MWCNT also serves as a barrier to suppress the aggregation tendency of MnxFe3−xO4, and thus further increases the structural stability.33 In addition, the morphology of MWCNTs that form a long tube shape tends to have better conductivity because they can form a more continuous conductive path. This agrees with previous reports indicating that RAMs with tubular shape and considerable anisotropy can form three-dimensional conduction networks to enhance its conductivity.34 This condition favors wave propagation through the material and its attenuation. Thus, an excellent wave attenuation in this proposed RAM could be induced due to the excellent interface between CNTs and MnxFe3−xO4, which creates an interface polarization.35 It is important to note that the interface polarization could induce excellent dielectric loss capacity for microwave absorption of the nanocomposite.34 Controlling the shapes and morphology of RAMs is important because the microwave absorption ability also depends on the degree of density, weight and dispersion of RAMs itself.36 The morphology of the nanocomposites after Mn addition, as depicted in Fig. 4, closely resembles the nanocomposites with a molar fraction of 0. Moreover, we observed a decrease in agglomeration with Mn addition. On the other hand, the further reduction of agglomeration upon inclusion of DEA was also indicated.18,37
Fig. 6 shows the FTIR spectrum of the MnxFe3−xO4 nanocomposites. At 3491–3290 cm−1, we identified a widened O–H vibration, possibly induced by the –OH groups on the surface areas. This functional group facilitates the combination of Mn–Fe2O4 with MWCNT.15,38 Our obtained vibrations are similar to the vibrational peak of O–H reported in previous studies, specifically those at 3321,25 3420,38 and 3430 cm−1.15 We also detected the C–O functional groups at 2325 and 1359 cm−1 that are possibly from the CO2 atmosphere15 and identified the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C functional group at 1438 cm−1, characterizing the graphite bonds compiled in the MWCNT. No functional groups of DEA are found, especially N–H, C–N, and N–H at 733, 1143, and 3288 cm−1, respectively. This finding suggests that the DEA used in nanoparticle preparation has not evaporated. The functional groups of metal oxide (M–O), namely, Fe–O and Mn–O, are detected at 412 and 673 cm−1. These two vibrations indicate that the manganese ferrite has a cubic spinel structure at the octahedral and tetrahedral sites. This finding is in line with a previous study reporting vibration peaks in the range of 430–482 cm−1 and 433–573 cm−1.39 As illustrated in Fig. 6, the absorbance intensity between 600 and 700 cm−1 increases with further addition of Mn molar fraction. This finding confirms the success of the Mn2+ ions in substituting Fe3+ ions in the tetrahedral sites. In addition, the increasing concentration of Mn leads to a shift of absorbance peaks towards lower wavenumbers. This phenomenon is correlated with the increase in the distance between two atoms, which occurs due to the increase in lattice parameters.
C functional group at 1438 cm−1, characterizing the graphite bonds compiled in the MWCNT. No functional groups of DEA are found, especially N–H, C–N, and N–H at 733, 1143, and 3288 cm−1, respectively. This finding suggests that the DEA used in nanoparticle preparation has not evaporated. The functional groups of metal oxide (M–O), namely, Fe–O and Mn–O, are detected at 412 and 673 cm−1. These two vibrations indicate that the manganese ferrite has a cubic spinel structure at the octahedral and tetrahedral sites. This finding is in line with a previous study reporting vibration peaks in the range of 430–482 cm−1 and 433–573 cm−1.39 As illustrated in Fig. 6, the absorbance intensity between 600 and 700 cm−1 increases with further addition of Mn molar fraction. This finding confirms the success of the Mn2+ ions in substituting Fe3+ ions in the tetrahedral sites. In addition, the increasing concentration of Mn leads to a shift of absorbance peaks towards lower wavenumbers. This phenomenon is correlated with the increase in the distance between two atoms, which occurs due to the increase in lattice parameters.
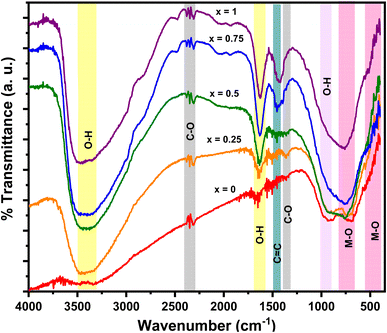 | ||
| Fig. 6 FTIR spectrum for the MnxFe3−xO4–DEA/MWCNT nanocomposites with different compositions of Mn substitution. | ||
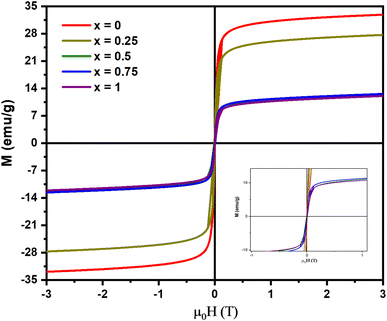
Fig. 7 shows the M–H hysteresis curve from the VSM analysis of the MnxFe3−xO4–DEA/MWCNT nanocomposites. All samples show superparamagnetic features as indicated by the obtained S curve. The attained hysteresis curve was then analyzed using the Langevin method with susceptibility,40 and the results are summarized in Table 2. The remanent magnetization and coercivity field are close to 0, characterizing the superparamagnetic components.30 The highest magnetization of 32.32 ± 0.03 emu g−1 is observed for MnxFe3−xO4–DEA/MWCNT at x = 0. This value is lower than the previously reported magnetization of Fe3O4–DEA at 35.76 emu g−1. Therefore, the obtained values can be correlated with the addition of MWCNT (nonmagnetic), i.e., MWCNT lowers the magnetic fraction of the samples and affects the Ms value. In our previous study, we uncovered that Fe3O4/MWCNT has Ms of 12.64 and 21.15 emu g−1, which are lower than the magnetization values obtained in the present work. The addition of DEA to the nanocomposites also affects the sample's magnetization. The magnetization values also decrease following the increase in the Mn molar fraction substituted for Fe3O4. This decrease can also be attributed to the low crystallite value, as demonstrated in Table 1. Nanosized particles typically have a single domain and exhibit paramagnetic behavior, resulting in an increased spin disorder on the nanoparticle's surface, which subsequently reduces the magnetic moment.41
| Samples | Ms (emu g−1) | Mr (emu g−1) | Hc (T) | χ |
|---|---|---|---|---|
| x = 0 | 32.32 ± 0.03 | −0.013 ± 0.012 | 0.520 ± 0.001 | 0.81 ± 0.02 |
| x = 0.25 | 27.12 ± 0.03 | −0.012 ± 0.011 | 0.510 ± 0.003 | 0.84 ± 0.02 |
| x = 0.5 | 12.07 ± 0.02 | −0.010 ± 0.002 | 0.066 ± 0.015 | 0.65 ± 0.01 |
| x = 0.75 | 11.91 ± 0.02 | −0.005 ± 0.001 | 0.052 ± 0.001 | 0.78 ± 0.01 |
| x = 1 | 11.51 ± 0.02 | −0.011 ± 0.004 | 0.010 ± 0.002 | 0.71 ± 0.01 |
The real and imaginary parameters of the electrical permittivity (real permittivity (ε′) and imaginary permittivity (ε′′)) and the magnetic permeability (real permeability (μ′) and imaginary permeability (μ′′)) shown in Fig. 8 are parameters used to determine the absorption mechanism of the MnxFe3−xO4–DEA/MWCNT nanocomposites for radar waves. ε′ and μ′ represent the ability to store electric and magnetic energy, respectively; and ε′′ and μ′′ are related to the dissipation and loss of electric and magnetic energy, respectively. As illustrated in Fig. 8(a), which is the ε′ curve, the real permittivity value decreases from 6.4 to 4.2 when the molar fraction of Mn increases. Meanwhile, the ε′′ value tends to be greater after Mn addition, which can be explained by eqn (2).
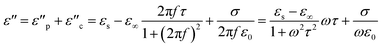 | (2) |
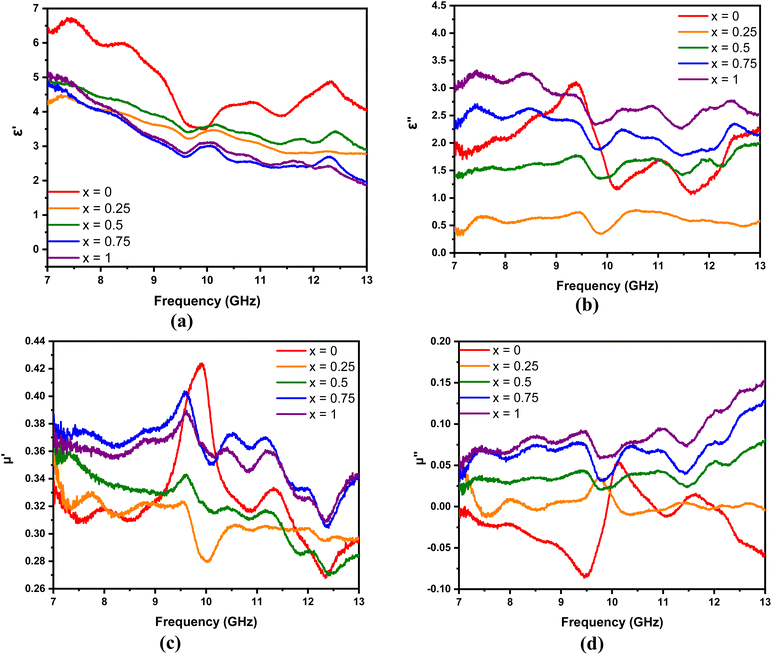 | ||
| Fig. 8 (a)–(c) Real and (b)–(d) imaginary parts of the permittivity and permeability of MnxFe3−xO4–DEA/MWCNT with different compositions of Mn substitution, respectively. | ||
According to eqn (2), an increase in ε′′ is related to electrical conductivity. Mn substitution increases the conductivity value of a material due to the production of holes during charge transfer.42 An increase in electrical conductivity also increases the loss and dissipation ability of the electric part, as indicated by the ε′′ value of the nanocomposites with x = 1 greater than x = 0. In addition to electrical conductivity, the ε′′ value is related to interfacial polarization, which is marked by several resonance peaks around the frequencies of 7.5, 8.5, 9.5, 11, and 12.5 GHz. These peaks are related to the relaxation stage when interfacial polarization occurs.2
The μ′ and μ′′ of MnxFe3−xO4–DEA/MWCNT are shown in Fig. 8(c) and (d), respectively. The μ′ of all samples tends to decrease gradually, and the μ′′ increases with the frequency. Fluctuations are observed in the μ′ and μ′′ curves, especially at frequencies above 9 GHz. The fluctuation peaks represent the resonance peaks, where the absorbance peaks at low frequencies originate from the wall resonance domain, and the peaks at high frequencies correspond to natural resonance. Furthermore, the μ′′ of the sample with Mn addition is greater than that of the sample without Mn substitution. This finding indicates that the magnetic loss of the nanocomposites increases with the molar fraction of Mn. The μ′′ in the sample x = 0 shows a negative value at several frequencies, implying that the magnetic energy from this sample is directly emitted without being absorbed at that frequency.43
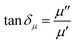 | (3) |
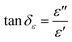 | (4) |
In general, the absorption capability of radar waves is described by magnetic loss and dielectric loss, which are represented by tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ and tan
δμ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε, respectively. tan
δε, respectively. tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ and tan
δμ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε describe the loss capacity of the radar absorption materials, and can be calculated using eqn (3) and (4), respectively. Fig. 9 shows the relationship of tan
δε describe the loss capacity of the radar absorption materials, and can be calculated using eqn (3) and (4), respectively. Fig. 9 shows the relationship of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ and tan
δμ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε with frequency. Comparison between Fig. 9(a) and (b) shows that the tan
δε with frequency. Comparison between Fig. 9(a) and (b) shows that the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε value is higher than the tan
δε value is higher than the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ value. These results explain that dielectric loss is the main contributor to the absorption of radar waves by the MnxFe3−xO4–DEA/MWCNT nanocomposites.44
δμ value. These results explain that dielectric loss is the main contributor to the absorption of radar waves by the MnxFe3−xO4–DEA/MWCNT nanocomposites.44
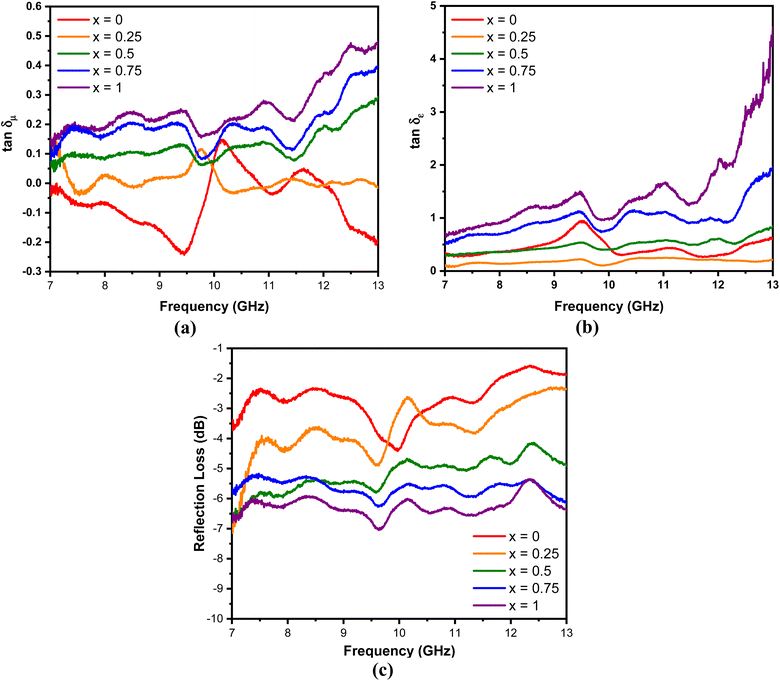 | ||
Fig. 9 (a) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ, (b) tan δμ, (b) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε, and (c) RL of MnxFe3−xO4–DEA/MWCNT with different compositions of Mn substitution. δε, and (c) RL of MnxFe3−xO4–DEA/MWCNT with different compositions of Mn substitution. | ||
The performance of the MnxFe3−xO4–DEA/MWCNT nanocomposites for the absorption of radar waves is shown as a graph of the relationship between the reflection loss (RL) and frequency depicted in Fig. 9(c). The RL values of MnxFe3−xO4–DEA/MWCNT for x = 0–1 are −4.39, −4.85, −5.77, −6.26, and −7.03 dB. The increase in RL is associated with a decrease in particle size as the molar fraction of Mn increases in the nanocomposites. Nanocomposites with a small size have a large proportion of surface atoms, which can be magnetized and polarized under an external electromagnetic field. This situation allows the radar wave energy to be converted into heat, resulting in high RL values.45 The highest RL of −7.03 dB is recorded for the sample with x = 1, which is consistent with the highest tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ and tan
δμ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε values (magnetic and dielectric loss) for this sample. In addition, x = 1 has a smaller crystallite size than other samples (Table 1). A low particle size is associated with a high dipole polarization that induces dielectric loss.46 In addition, the particle sizes also affect the degree of density, weight, and dispersion of nanocomposites, which further affect their absorption ability.36
δε values (magnetic and dielectric loss) for this sample. In addition, x = 1 has a smaller crystallite size than other samples (Table 1). A low particle size is associated with a high dipole polarization that induces dielectric loss.46 In addition, the particle sizes also affect the degree of density, weight, and dispersion of nanocomposites, which further affect their absorption ability.36
4. Conclusion
MnxFe3−xO4–DEA/MWCNT nanocomposites have been successfully synthesized by the coprecipitation method having Mn fraction variations of x = 0, 0.25, 0.5, 0.75, 1. The crystallite size decreases from 17 to 4.5 nm with the increase in substituted Mn substitution. The morphology of the nanocomposites contains spherical shapes and chunks of MnxFe3−xO4, as well as tubular shapes of the MWCNT, indicating they are physically contacted. All of the synthesized nanocomposites exhibit superparamagnetism with the tendency of decreasing saturation magnetization at around 32.32 ± 0.03 to 11.51 ± 0.02 emu g−1 due to Mn addition, which presumably enhances the spin disorder on the nanocomposite's surface. Furthermore, the performance of the nanocomposites in absorbing radar waves is shown by the RL value that increases from −4.39 to −7.03 dB with the increase in the molar fraction of Mn. It shows that the radar absorption performance of the MnxFe3−xO4–DEA/MWCNT nanocomposites is dominated by dielectric loss, owing to increased electrical conductivity and interfacial polarization with the addition of Mn. We believe that our findings may shed light on the role of substitution elements in developing the radar absorption properties not only for MnxFe3−xO4–DEA/MWCNT nanocomposites, but also for other iron oxides/graphene-based nanocomposites, which further pave the way for their real implementation as RAMs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by the Kerjasama Internasional research grant for A. T. under grant number 5.4.407/UN32.20.1/LT/2023.References
- H. Yao, J. Yang, H. Li, J. Xu and K. Bi, Optimal design of multilayer radar absorbing materials: a simulation-optimization approach, Adv. Compos. Hybrid Mater., 2023, 6(1), 43, DOI:10.1007/s42114-023-00626-3
.
- M. M. A. Yahya, et al., Investigation of the optical, magnetic, and radar absorption characteristics of CoxFe3-xO4/ZnO/graphite nanocomposites, Mater. Sci. Semicond. Process., 2023, 165, 107683, DOI:10.1016/j.mssp.2023.107683
.
- F. Meng, et al., Graphene-based microwave absorbing composites: a review and prospective, Composites, Part B, 2018, 137, 260–277, DOI:10.1016/j.compositesb.2017.11.023
.
- P. Sahoo, L. Saini and A. Dixit, Microwave-absorbing materials for stealth application: a holistic overview, Oxf. Open Mater. Sci., 2023, 3(1), itac012, DOI:10.1093/oxfmat/itac012
.
- Y. Xie, et al., Efficient electromagnetic wave absorption performances dominated by exchanged resonance of lightweight PC/Fe3O4@PDA hybrid nanocomposite, Chem. Eng. J., 2023, 457, 141205, DOI:10.1016/j.cej.2022.141205
.
- W. Xia, X. Wang, D. Liu, C. Li, J. Xie and C. Xiong, Enhanced microwave absorbing performance of epoxy composites filled with solvent-free and liquid-like Fe3O4 organic hybrid material, Appl. Surf. Sci., 2023, 615, 156376, DOI:10.1016/j.apsusc.2023.156376
.
- S. Wei, R. Yan, B. Shi and X. Chen, Characterization of flexible radar-absorbing materials based on ferromagnetic nickel micron-fibers, J. Ind. Text., 2019, 49(1), 58–70, DOI:10.1177/1528083718772304
.
- A. Taufiq, et al., Radar Absorption Performance of Fe3O4/AC/PANI Nanocomposites Prepared from Natural Iron Sand, Int. J. Eng., 2020, 33(2), 2 Search PubMed
.
- E. Bagheripour, A. Moghadassi and S. M. Hosseini, Incorporated poly acrylic acid-co- Fe3O4 nanoparticles mixed matrix polyethersulfone based nanofiltration membrane in desalination process, Int. J. Eng., 2017, 30(6), 821–829 CAS
.
- X.-H. Do, et al., High Biocompatibility, MRI Enhancement, and Dual Chemo- and Thermal-Therapy of Curcumin-Encapsulated Alginate/Fe3O4 Nanoparticles, Pharmaceutics, 2023, 15(5), 5, DOI:10.3390/pharmaceutics15051523
.
- L. Maldonado-Camargo, I. Torres-Díaz, A. Chiu-Lam, M. Hernández and C. Rinaldi, Estimating the contribution of Brownian and Néel relaxation in a magnetic fluid through dynamic magnetic susceptibility measurements, J. Magn. Magn. Mater., 2016, 412, 223–233, DOI:10.1016/j.jmmm.2016.03.087
.
- D. Arista, A. Rachmawati, N. Ramadhani, R. E. Saputro and A. Taufiq, Antibacterial performance of Fe3O4/PEG-4000 prepared by co-precipitation route, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 012085 CAS
.
- M. S. Jabir, U. M. Nayef, W. K. Abdulkadhim and G. M. Sulaiman, Supermagnetic Fe3O4-PEG nanoparticles combined with NIR laser and alternating magnetic field as potent anti-cancer agent against human ovarian cancer cells, Mater. Res. Express, 2019, 6(11), 115412, DOI:10.1088/2053-1591/ab50a0
.
- X. Wang, F. Pan, Z. Xiang, W. Jia and W. Lu, Magnetic Fe3O4@PVP nanotubes with high heating efficiency for MRI-guided magnetic hyperthermia applications, Mater. Lett., 2020, 262, 127187, DOI:10.1016/j.matlet.2019.127187
.
- A. Taufiq, et al., Preparation of Superparamagnetic Fe3O4 Nanoparticles from Iron Sand Mediated by Soft Template and Their Performance as Antibacterial Agent, J. Magn., 2018, 23(3), 3, DOI:10.4283/JMAG.2018.23.3.337
.
- S. U. I. Subadra, et al., Synthesis and characterisation of Fe3O4/MWCNT/ZnO nanocomposites covered by a soft template as a new antibacterial agent, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2022, 13(3), 035010, DOI:10.1088/2043-6262/ac8de8
.
- E. Sano and E. Akiba, Electromagnetic absorbing materials using nonwoven fabrics coated with multi-walled carbon nanotubes, Carbon, 2014, 78, 463–468, DOI:10.1016/j.carbon.2014.07.027
.
- A. Taufiq, et al., Eco-Friendly Fabrication of Fe3O4/MWCNT/ZnO Nanocomposites from Natural Sand for Radar Absorbing Materials, Int. J. Nanosci. Nanotechnol., 2021, 17(1), 41–53 CAS
.
- T. Hou, J. Wang, T. Zheng, Y. Liu, G. Wu and P. Yin, Anion Exchange of Metal Particles on Carbon-Based Skeletons for Promoting Dielectric Equilibrium and High-Efficiency Electromagnetic Wave Absorption, Small, 2023, 2303463, DOI:10.1002/smll.202303463
.
- P. Saini, V. Choudhary, B. P. Singh, R. B. Mathur and S. K. Dhawan, Polyaniline–MWCNT nanocomposites for microwave absorption and EMI shielding, Mater. Chem. Phys., 2009, 113(2–3), 919–926, DOI:10.1016/j.matchemphys.2008.08.065
.
- F. Ruiz-Perez, S. M. López-Estrada, R. V. Tolentino-Hernández and F. Caballero-Briones, Carbon-based radar absorbing materials: a critical review, J. Sci.: Adv. Mater. Devices, 2022, 7(3), 100454, DOI:10.1016/j.jsamd.2022.100454
.
- M. Chang, Z. Jia, G. Wu and P. Yin, Multiple dimension-component designed Co/Co9S8/Ti3C2Tx MXene composite for enhanced microwave absorption, Appl. Phys. Lett., 2023, 122, 131901, DOI:10.1063/5.0142497
.
- A. Taufiq, et al., Studies on Nanostructure and Magnetic Behaviors of Mn-Doped Black Iron Oxide Magnetic Fluids Synthesized from Iron Sand, Nano, 2017, 12(09), 09, DOI:10.1142/S1793292017501107
.
- A. Taufiq, et al., Nanoscale Clustering and Magnetic Properties of MnxFe3−xO4 Particles Prepared from Natural Magnetite, J. Supercond. Novel Magn., 2015, 28(9), 2855–2863, DOI:10.1007/s10948-015-3111-9
.
- R. Rahmawati, et al., Preparation of MWCNT-Fe3O4 Nanocomposites from Iron Sand Using Sonochemical Route, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 202, 012013, DOI:10.1088/1757-899X/202/1/012013
.
- A. Taufiq, et al., Fabrication of Mn1-xZnxFe2O4 ferrofluids from natural sand for magnetic sensors and radar absorbing materials, Heliyon, 2020, 6(7), e04577 CrossRef PubMed
.
- M. Li, et al., Solvothermal synthesis of MnxFe3−xO4 nanoparticles with interesting physicochemical characteristics and good catalytic degradation activity, Mater. Des., 2016, 97, 341–348, DOI:10.1016/j.matdes.2016.02.103
.
- J. S. Anandhi, G. A. Jacob and R. J. Joseyphus, Factors affecting the heating efficiency of Mn-doped Fe3O4 nanoparticles, J. Magn. Magn. Mater., 2020, 512, 166992, DOI:10.1016/j.jmmm.2020.166992
.
- A. Doaga, et al., Synthesis and characterizations of manganese ferrites for hyperthermia applications, Mater. Chem. Phys., 2013, 143(1), 305–310, DOI:10.1016/j.matchemphys.2013.08.066
.
- S. T. Yazdi, P. Iranmanesh, S. Saeednia and M. Mehran, Structural, optical and magnetic properties of MnxFe3−xO4 nanoferrites synthesized by a simple capping agent-free coprecipitation route, Mater. Sci. Eng., B, 2019, 245, 55–62, DOI:10.1016/j.mseb.2019.05.009
.
- S.-D. Seo, G.-H. Lee and D.-W. Kim, Enhanced electroactivity with Li in Fe3O4/MWCNT nanocomposite electrodes, J. Alloys Compd., 2014, 615, S397–S400, DOI:10.1016/j.jallcom.2014.01.077
.
- Y. Huang, K. Yang and J.-Y. Dong, Copolymerization of Ethylene and 10-Undecen-1-ol using a Montmorillonite-Intercalated Metallocene Catalyst: Synthesis of Polyethylene/Montmorillonite Nanocomposites with Enhanced Structural Stability, Macromol. Rapid Commun., 2006, 27, 1278–1283, DOI:10.1002/marc.200600131
.
- J. Tang and J. Wang, Fe3O4-MWCNT Magnetic Nanocomposites as Efficient Fenton-Like Catalysts for Degradation of Sulfamethazine in Aqueous Solution, ChemistrySelect, 2017, 2, 10727–10735, DOI:10.1002/slct.201702249
.
- P. Yin, G. Wu, Y. Tang, S. Liu, Y. Zhang, G. Bu, J. Dai, Y. Zhao and Y. Liu, Structure regulation in N-doping biconical carbon frame decorated with CoFe2O4 and (Fe,Ni) for broadband microwave absorption, Chem. Eng. J., 2022, 446, 136975, DOI:10.1016/j.cej.2022.136975
.
- V. A. Silva and M. C. Rezende, S-parameters, electrical permittivity, and absorbing energy measurements of carbon nanotubes-based composites in X-band, J. Appl. Polym. Sci., 2021, 138, 49843, DOI:10.1002/app.49843
.
- T. Zheng, Y. Zhang, Z. Jia, J. Zhu, G. Wu and P. Yin, Customized dielectric-magnetic balance enhanced electromagnetic wave absorption performance in CuxS/CoFe2O4 composites, Chem. Eng. J., 2023, 457, 140876, DOI:10.1016/j.cej.2022.140876
.
- R. Rameshbabu, R. Ramesh, S. Kanagesan, A. Karthigeyan and S. Ponnusamy, One pot facile hydrothermal synthesis of superparamagnetic ZnFe2O4 nanoparticles and their properties, J. Sol-Gel Sci. Technol., 2014, 71(1), 147–151, DOI:10.1007/s10971-014-3347-z
.
- Z. Chen, et al., Magnetic Mn-Doped Fe3O4 hollow Microsphere/RGO heterogeneous Photo-Fenton Catalyst for high efficiency degradation of organic pollutant at neutral pH, Mater. Chem. Phys., 2019, 238, 121893, DOI:10.1016/j.matchemphys.2019.121893
.
- K. Saputra, et al., Effect of Polyethylene Glycol (PEG) on Particle Distribution of Mn0. 25Fe2.75O4-PEG 6000 Nanoparticles, J. Phys.: Conf. Ser., 2018, 012005 Search PubMed
.
- A. Taufiq, S. N. Qoidah, S. U. I. Subadra, N. Mufti, A. Hidayat and Sunaryono, Preparation of Fe3O4/MWCNT nanocomposite combined with titanium dioxide using sonochemical and precipitation methods, AIP Conf. Proc., 2020, 020007 CrossRef CAS
.
- G. F. Goya, T. S. Berquo, F. C. Fonseca and M. P. Morales, Static and dynamic magnetic properties of spherical magnetite nanoparticles, J. Appl. Phys., 2003, 94(5), 3520–3528 CrossRef CAS
.
- N. Helaïli, Y. Bessekhouad, K. Bachari and M. Trari, Synthesis and physical properties of the CuFe2−xMnxO4 (0 ≤ x ≤ 2) solid solution, Mater. Chem. Phys., 2014, 148(3), 734–743, DOI:10.1016/j.matchemphys.2014.08.042
.
- M. M. Ismail, S. N. Rafeeq, J. M. Sulaiman and A. Mandal, Electromagnetic interference shielding and microwave absorption properties of cobalt ferrite CoFe2O4/polyaniline composite, Appl. Phys. A: Mater. Sci. Process., 2018, 124, 1–12 CrossRef CAS
.
- Y. Luo, P. Yin, G. Wu, L. Zhang, G. Ma, J. Wang, X. Sun and G. Bu, Porous carbon sphere decorated with Co/Ni nanoparticles for strong and broadband electromagnetic dissipation, Carbon, 2022, 197, 389–399, DOI:10.1016/j.carbon.2022.06.084
.
- F. Jiang, X. Wei and J. Zheng, Synthesis and electromagnetic characteristics of MnFe2O4/TiO2 composite material, Mater. Res. Express, 2022, 9(10), 106101, DOI:10.1088/2053-1591/ac97de
.
- L. Ahmadian-Alam, F. Jahangiri and H. Mahdavi, Fabrication and assessment of an electrochromic and radar-absorbent dual device based on the new smart polythiophene-based/RGO/Fe3O4 ternary nanocomposite, Chem. Eng. J., 2021, 422, 130159, DOI:10.1016/j.cej.2021.130159
.
| This journal is © The Royal Society of Chemistry 2023 |