Open Access Article
Open Access ArticleSimultaneous saccharification of hemicellulose and cellulose of corncob in a one-pot system using catalysis of carbon based solid acid from lignosulfonate
Qiong Wang†
ab,
Longjun Chang†b,
Wen Wang a,
Yunzi Hua,
Jun Yue
a,
Yunzi Hua,
Jun Yue c,
Zhongming Wanga,
Cuiyi Liang*a and
Wei Qi
c,
Zhongming Wanga,
Cuiyi Liang*a and
Wei Qi *a
*a
aGuangzhou Institute of Energy Conversion, Chinese Academy of Sciences, CAS Key Laboratory of Renewable Energy, Guangdong Key Laboratory of New and Renewable Energy Research and Development, Guangzhou, Guangdong Province 510640, China. E-mail: qiwei@ms.giec.ac.cn; liangcy@ms.giec.ac.cn
bInstitute of Zhejiang University-Quzhou, 99 Zheda Road, Quzhou, Zhejiang Province 324000, China
cDepartment of Chemical Engineering, Engineering and Technology Institute of Groningen, University of Groningen, 9747 AG Groningen, The Netherland
First published on 28th September 2023
Abstract
The drive towards sustainable chemistry has inspired the development of active solid acids as catalysts and ionic liquids as solvents for an efficient release of sugars from lignocellulosic biomass for future biorefinery practices. Carbon-based solid acid (SI–C–S–H2O2) prepared from sodium lignosulfonate, a waste of the paper industry, was used with water or ionic liquid to hydrolyze corncob in this study. The effects of various reaction parameters were investigated in different solvent systems. The highest xylose yield of 83.4% and hemicellulose removal rate of 90.6% were obtained in an aqueous system at 130 °C for 14 h. After the pretreatment, cellulase was used for the hydrolysis of residue and the enzymatic digestibility of 92.6% was obtained. Following these two hydrolysis steps in the aqueous systems, the highest yield of total reducing sugar (TRS) was obtained at 88.1%. Further, one-step depolymerization and saccharification of corncob hemicellulose and cellulose to reducing sugars in an IL-water system catalyzed by SI–C–S–H2O2 was conducted at 130 °C for 10 h, with a high TRS yield of 75.1% obtained directly. After recycling five times, the solid acid catalyst still showed a high catalytic activity for sugar yield in different systems, providing a green and effective method for lignocellulose degradation.
Introduction
Lignocellulosic biomass is a non-edible, renewable and abundant natural resource consisting of cellulose, hemicellulose, and lignin, which has significant potential for the sustainable production of biobased chemicals and fuels by innovative biorefinery approaches.1–3 However, this potential remains largely unexploited and is still not economical for the biofuel industry due to the lack of economically feasible and eco-friendly strategies that can achieve a significant release of high-quality sugar platform from lignocellulose.4,5From the aspect of the reaction pathway, the transformation of lignocellulose into biofuels or chemicals mainly involves two stages, releasing of cellulose and hemicellulose into reducing sugars, and the subsequent transformation of these sugars into chemicals and biofuels.6 Due to the tightly and recalcitrantly connected matrix of cellulose, hemicellulose and lignin, necessary pretreatment procedures have been recognized as a critical step before the enzymatic hydrolysis.7,8 Various approaches including diluted liquid acid, ionic liquid, alkaline or base, hot water or steam, ammonia explosion, etc. have been developed to accomplish the pretreatment step.9,10 Among them, diluted liquid acidic pretreatment is one of the most common methods, which can decrease the biomass recalcitrance effectively and release a partial amount of sugars from raw biomass simultaneously.11 Even though diluted liquid acidic pretreatment is an easy and low-cost approach, it has various drawbacks such as low selectivity of sugar, wastewater discharge and equipment corrosion. Solid acid pretreatment can address some of these problems by the employment of mild operating conditions with various advantages of a high selectivity, an easy separating process of products, and the option of catalyst reusability.12–14
The hydrolysis of wheat straw with solid acid catalyst SO42−/Fe2O3 was researched by Zhong et al.,15 although the 63.5% of hemicellulose hydrolysis yield was obtained at 140 °C for 4 h, cellulose and lignin were kept inactive, thus leading to large amounts of sugar unexploited from the perspective of biorefinery. Xu et al.16 used glucose and p-toluenesulfonic acid as raw materials for catalyst preparation, and the 78.0% of xylose yield was achieved when hydrolysis of corncob was carried out at 140 °C for 14 h, however, the hydrolysis activity to cellulose was low. Besides, a carbon-based solid acid was synthesized by a simple one-step hydrothermal carbonization method using microcrystalline cellulose and sulfuric acid for corncob hydrolysis, the yield of xylose was 78.1% with an enzymatic digestibility of the pretreated residue of 91.6%.17 The above studies indicate that the solid acid catalyst has high catalytic activity for the hemicellulose, but poor activity for cellulose.
To achieve the saccharification of hemicellulose and cellulose in one step, many kinds of solvents were tested. Deep eutectic solvents (DES), frequently considered to be in the same category as Ionic liquids (ILs) as solvents, have received significant attention as solvents for the conversion of lignocellulosic biomass.18 However, they are mostly applied in the fields of lignocellulosic pretreatment,8,19 and the studies aiming at the one-step saccharification of hemicellulose and cellulose via DES have rarely been reported. Thanks to the properties of low vapor pressure, low flammability, thermal stability, superior solvation effect, tunable physicochemical properties, etc., ILs have always been a research hotspot in recent years.20–22 It's revealed that the enzyme toxicity of imidazolium-based ILs have a close relationship with their structure, such as the type of anions and the length of alkyl chain.23 For example, the inhibitory effect of anions of N-methylimidazolium-based ILs on lactic dehydrogenase enzyme follows the order: CF3SO3− > BF4− > Br− > Cl−, and the longer the alkylated side chain, the severer the inhibition.24,25 Ben Hmad et al. reported that a novel endoglucanase, a halophilic enzyme, retained its 80% activity in 10% 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]).26 Therefore, engineering IL-tolerant enzymes is a feasible strategy to make ILs biocompatible for high-efficiency biomass deconstruction and bioconversion.27
Imidazolium-based ILs are known as the most well-investigated ILs, Rinaldi et al.28 investigated the hydrolysis of spruce wood in 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]) catalysed by a sulfonated resin (Amberlyst 15DRY) and obtained a total reducing sugar (TRS) yield of ∼22% at 100 °C for 5 h. Guo et al.29 prepared a superparamagnetic cellulose-derived solid acid catalyst by incomplete hydrothermal carbonization of cellulose followed by Fe3O4 grafting and –SO3H group functionalization for the hydrolysis of rice straw, and a TRS yield of 35.5% was obtained at 150 °C for 2 h in [BMIM][Cl]. Besides, Babool wood (Acacia nilotica) pretreated by 1 M H2SO4 was hydrolyzed in [BMIM][Cl] catalyzed by H2SO4-modified activated carbon, ultimately realizing the maximum TRS yield of 83.7% in 60 min at 120 °C. However, the employment of homogeneous mineral acid and the tedious two-step reaction procedures are expected to be improved.30 In another study, Bai et al.31 studied the hydrolysis of rice straw in [BMIM][Cl] catalysed by a black liquor-derived carbonaceous solid acid and achieved a TRS yield of 36.6% at 140 °C for 150 min. Cheng et al.32 synthesized a chitosan-based solid acid catalyst immobilized with Fe3+ to hydrolyze bamboo powder in [BMIM][Cl], and a 73.4% of TRS yield was obtained at 120 °C for 24 h. Furthermore, Si et al.33 used Tween 80 to improve the bamboo hydrolysis catalyzed by a sulfonated chitosan based solid acid in [BMIM][Cl] and a TRS yield of 68.0% was achieved at 120 °C after 24 h. Hu et al.34 used a chlorine-doped magnetic carbon solid acid with chlorine groups as cellulose-binding domains and sulfonic groups as cellulose-hydrolyzing domains for the hydrolysis of rice straw-derived cellulose in [BMIM][Cl], and a TRS yield of 73.2% was obtained at 130 °C after 4 h.
In this study, corncob hydrolyses respective in an aqueous solution and [BMIM][Cl] with carbon-based solid acid prepared from sodium lignosulfonate were investigated, where hemicellulose and cellulose were hydrolysed to reducing sugars directly and simultaneously by the catalysis of a carbon-based solid acid. In the previous study, we studied the preparation of this catalyst (SI–C–S–H2O2) for the hydrolysis of hemicellulose in corncob, which clarified that the phenolic hydroxyl and carboxyl sites acted as binding regions, and the sulfonic groups acted as hydrolysis regions, and achieved a relatively high xylose yield of 84.2% at 130 °C for 12 h.35 In this paper, we first used SI–C–S–H2O2 to catalyze corncob hydrolysis in a water system, followed by an enzymatic hydrolysis. Further, we investigated the case of SI–C–S–H2O catalyzing one-step depolymerization of corncob to reducing sugars in an IL–water system. As shown in Fig. 1, neither the employment of surfactants nor the excessive solid acid consumption was required. Herein, this study provides a high-efficiency way for lignocellulosic conversion, which allows the one-step saccharification of hemicellulose and cellulose without a time-consuming enzymatic hydrolysis.
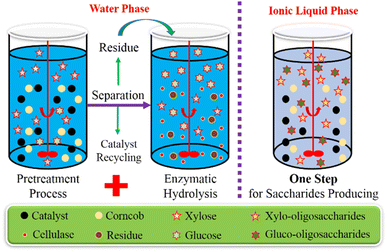 | ||
| Fig. 1 Simplified block flow diagram of the two saccharification processes; two-step process (left) and one-step process (right). | ||
Experimental
Materials
[BMIM][Cl] (purity 99%) was purchased from Lanzhou Greenchem ILs, China. Sodium lignosulfonate (96%) was purchased from Shanghai Macklin Biochemical Ltd, China. Corncob was obtained from a farm in Shangdong province, China and oven-dried to a constant weight at 80 °C for 12 h containing 35.1% cellulose, 34.5% hemicellulose and 14.1% lignin. It was ground into particles with a size of 40–60 mesh. Cellulase (190 FPU g−1) was obtained from Imperial Jade Bio-Technology Co., Ltd, China. All other chemicals were obtained from Guangzhou Chemical Regent Factory, China.Preparation of solid acid catalyst
Under nitrogen (N2) protection, sodium lignosulfonate was carbonized at 250 °C for 6 h in a tube furnace and then washed with a large amount of deionized water until the filtrate was colorless and then dried at 105 °C overnight. One gram (1.0 g) of carbonized solid and 20 mL concentrated sulfuric acid (H2SO4) were added to the thick wall pressure bottle (Beijing Synthware Glass Co. Ltd. China) and reacted at 130 °C for 10 h and the resulting powder was washed with deionized water until the pH became neutral. It was dried in an oven at 105 °C for 6 h, and denoted as Sl–C–S. One gram (1.0 g) of Sl–C–S sample and 75 mL 10.0% H2O2 were reacted at 50 °C for 90 min and the same treatment method as above and denoted as Sl–C–S–H2O2. There are three main functional groups (–OH, –COOH and –SO3H) in Sl–C–S–H2O2, and its total acid amount is 4.8 mmol g−1.35Hydrolysis of corncob
For the two-step process (Fig. 1), 0.2 g catalyst, 0.2 g corncob, and 20 mL deionized water were loaded in a 75 mL thick wall pressure bottle and kept at 130 °C in an oil bath for 14 h. After completing the reaction, the reactant was centrifuged at 8000 rpm for 3 min and the supernatant for analysis was sampled and analyzed. The solid was dried overnight at 50 °C and 200 mesh sieves were used for screening and separating the catalyst and the residue. The corncob residue was then added into 100 mL conical flasks containing 40 mL 0.05 M sodium citrate buffer (pH = 4.8). The solid concentration was 5.0% (w/v), and the cellulase loading was 40 FPU per g cellulose. The enzymatic hydrolysis was performed at 50 °C, 150 rpm for 72 h. The concentration of 0.5 g enzymatic hydrolysate was diluted 10 times with deionized water at each interval of 12 h and then analyzed by HPLC.For the one-step process (Fig. 1), 0.05 g catalyst, 0.0125–0.1 g corncob and 0–0.4 g water and 2 g ionic liquid ([BMIM][Cl]) were loaded in a 35 mL thick wall pressure bottle and reacted at 115–160 °C in an oil bath for 4–14 h. After the reaction was complete, the mixture was cooled and 10 mL water was added into the tube which was then shaken for 1 min to obtain an even mixing. The solution obtained was centrifuged at 8000 rpm for 3 min and the supernatant was sampled and analyzed. The solid was dried overnight at 50 °C and screened by a 200-mesh sieve for the catalyst recovery assessment.
To analyze the amount of oligosaccharide, the supernatant was treated by 4.0 wt% H2SO4 to depolymerize oligosaccharide into monosaccharide in an autoclave at 121 °C for 60 min. After cooling, the solution was neutralized with CaCO3, followed by centrifugal treatment to separate the supernatant.36 The concentration of glucose and xylose in the treated and untreated hydrolysate was analyzed by high-performance liquid chromatograph (HPLC).
Hydrolysis of cellobiose
A total of 0.05 g catalyst, 0.05 g cellobiose and 0–2.0 g water or ionic liquid were added into a 35 mL thick wall pressure bottle and reacted at 130 °C in an oil bath for 8 h. After completing the reaction, the mixture was cooled and 10 mL water was added into the tube which was then vigorously shaken for 1 min to achieve an even mixing. The solution was then centrifuged at 8000 rpm for 3 min. A part of the supernatant was withdrawn and analysed by HPLC.Analytical methods
Glucose and xylose were identified by HPLC (Waters 2695) with a Shodex SH-1011 column. Total 5 mM H2SO4 was employed as the mobile phase with the flow rate of 0.5 mL min−1 at 50 °C. Calibration curves were established for quantitative calculations.The yields of xylose and glucose were calculated by the following eqn (1):
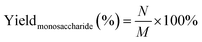 | (1) |
The enzymatic digestibility of the pretreated corncob was calculated according to the following eqn (2):
 | (2) |
The yield of TRS was calculated according to the following eqn (3):
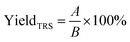 | (3) |
Results and discussion
Two-step saccharification in an aqueous system
A two-step method to sequentially obtain the C5 and C6 sugars was adopted at first, as shown in Fig. 2. In the 20 mL aqueous system with 0.2 g catalyst and 0.2 g corncob, the maximum xylose yield (83.4%) in the hydrolysate was achieved at 130 °C after 14 h. Meanwhile, a little glucose was released from the corncob with a yield lower than 10.0%. Then the residue was sent to the enzymatic hydrolysis step, resulting in a 92.6% of enzymatic digestibility. Thus, a total reducing sugar (TRS) was obtained as 88.1%.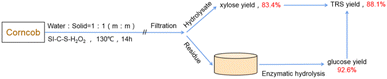 | ||
| Fig. 2 Schematic representation of two-step saccharification of corncob at the optimal reaction conditions. | ||
One-step saccharification: effect of ionic liquid on the decomposition of corncob
Based on the research above, just a small amount of cellulose has been decomposed in the aqueous phase. In order to get xylose and glucose in high yields in one hydrolysis step (i.e., without the subsequent enzymatic hydrolysis step), the ionic liquid was applied, given its solvability of cellulose. At first, the effect of water content on the yield of TRS catalyzed by Sl–C–S–H2O2 in BMIM][Cl] was studied, as shown in Fig. 3. It was indicated that water content had a significant effect on the decomposition of corncob, thus the amount of added water visibly affected the TRS yield. The results showed that the TRS yield increased with increasing water content and a maximum TRS yield of 68.7% was obtained when 3.0% (w/w%) water was added. Further addition of water over 3% into the reaction system led to a decrease in the glucose yield. The reason was due to the presence of water not only can decrease the viscosity of the ionic liquid which could accelerate the mass transfer between the reactants, but also could be conducive to the dynamic equilibrium of hydrolysis processes at a low water content.28 With a further increase in the water content, the viscosity of the solvent and the dissolving ability of ionic liquid for cellulose were of decreasing and the catalyst activity was reduced by hydration of the acid sites.37,38 The hemicellulose could be hydrolyzed easily in the aqueous system, thus the increase in water content resulted in a higher yield of xylose.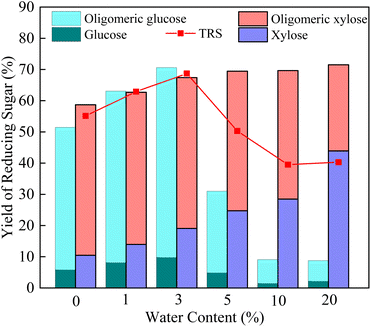 | ||
| Fig. 3 Effect of water content on TRS yield obtained by hydrolysis of corncob with Sl–C–S–H2O2 (reaction conditions: 130 °C for 8 h, 0.05 g corncob, 0.05 g catalyst, 2 g [BMIM][Cl]). | ||
Compared with the hydrolysis results in the aqueous phase, the hydrolysis of corncob in ionic liquid produced a large amount of gluco-oligosaccharide in ionic liquid. The reason was that the ionic liquid had a good solubility of cellulose, the hydrogen bonds between the cellulose molecules were interrupted,39 making cellulose to be more easily hydrolyzed and thus the yield of glucose was higher.
One-step saccharification: effect of reaction condition on the sugar yield in ionic liquid
The effects of temperature and time on the corncob hydrolysis in ionic liquid were found to be more pronounced than that in the aqueous solution. As can be seen from Fig. 4, when the hydrolysis was conducted at 115 °C, the yield of TRS was continuously increased with the reaction time and reached a maximum of 35.5% at 14 h. As the reaction temperature increased to 130 °C and 145 °C, the yields of TRS started to increase within a certain period of time, but gradually decreased after reaching a peak value. The maximum TRS yields of 75.1% (at 10 h) and 41.7% (at 6 h) were achieved, respectively. However, the yield of TRS was decreased continuously as the reaction time increased at temperatures higher than 160 °C. It is speculated that the maximum yield had been reached before 4 h under this temperature. To conclude, the hydrolysis rate was enhanced with the increase of reaction temperature, consequently, the time to achieve the maximum TRS yield was shortened.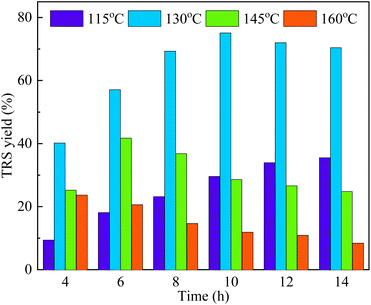 | ||
| Fig. 4 Effects of reaction temperature and time on TRS obtained by corncob hydrolysis catalyzed by Sl–C–S–H2O2 (reaction conditions: 0.05 g corncob, 0.05 g catalyst, 0.06 g H2O and 2 g [BMIM][Cl]). | ||
In general, the variation of TRS yield was contributed by two competitive reactions: a positive increase derived from the decomposition of polysaccharides, and a negative decrease resulted from a further degradation of intermediates into formic acid, furfural, 5-hydroxymethylfurfural, and other byproducts.31,35,37,38 Under the investigated reaction temperature levels, the TRS yield increased at the initial reaction period, due to the hydrolysis rates of cellulose and hemicellulose being higher than the sugar degradation rates. However, with the build-up of sugar concertation in the course of the reaction, the sugar degradation became more significant at a longer reaction time, leading to a decline in the TRS yield afterward.
Fig. 5 showed the influence of catalyst dosage on the TRS yield. Only a very low yield of 4.0% of TRS was observed at 14 h without the adding of the Sl–C–S–H2O2 catalyst. The reason for the formation of a small amount of reducing sugars is due to the formation of H+ from water in the reaction system worked as catalysts for the hydrolysis of corncob.40 The results showed that the catalyst dosage was positively correlated with the hydrolysis rate in a certain range. TRS yield gradually increased from 17.3% at 4 h to 47.7% at 14 h with 0.0125 g of catalyst. TRS yields reached up to 69.5% (12 h) and 75.1% (10 h) after the addition of 0.025 g and 0.05 g catalysts to the reaction mixture, respectively. However, TRS yield declined when the catalyst amount was further raised to 0.1 g. An excessive amount of catalyst used in this reaction will lead to the degradation of part of TRS into other by-products.40,41
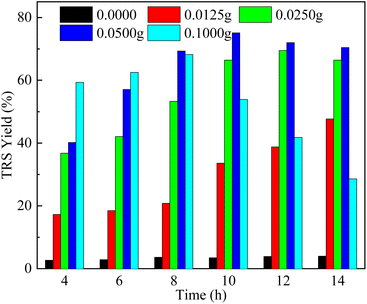 | ||
| Fig. 5 Influence of catalyst amount on TRS yield obtained by corncob hydrolysis catalyzed by Sl–C–S–H2O2 (reaction conditions: 130 °C, 0.05 g corncob, 0.06 g H2O and 2 g [BMIM][Cl]). | ||
As is shown in Fig. 6, the loading of corncob had a significant effect on TRS yield when Sl–C–S–H2O2 loading was fixed at 0.05 g. When 0.0125 g and 0.025 g corncob were used, the TRS yields were up to 63.4% and 74.3%, respectively. After the loading amount of the corncob was increased to 0.05 g, an optimum TRS yield of 75.1% was obtained at 130 °C for 10 h. A further increase of the loading amount of corncob led to a decrease in TRS yields (70.6%) and the time required to reach the maximum value was also increased, which is probably related to the resulting decrease in the mass transfer efficiency. Therefore, an appropriate substrate loading was conducive to the formation of TRS in this reaction system.
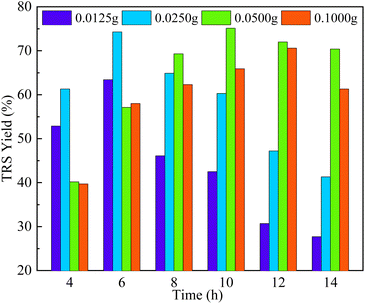 | ||
| Fig. 6 Effects of corncob concentration on the TRS yields during the hydrolysis of the corncob catalyzed by Sl–C–S–H2O2 (reaction conditions: 130 °C, 0.05 g catalyst, 0.06 g H2O, 2 g [BMIM][Cl]). | ||
Hydrolysis of cellobiose
In order to verify whether the ionic liquid also functioned as a co-catalyst on the hydrolysis of cellulose, hydrolysis experiments of cellobiose has been performed, which was soluble in both water and ionic liquid. It can be seen from Fig. 7 that, the yield of glucose obtained by hydrolysis of cellobiose was gradually increased with time, reaching a maximum at 3 h in the aqueous system. The yield of glucose was gradually decreased when the reaction time was further prolonged. For ionic liquid, the glucose concentration was much higher than that obtained in the aqueous solution, and there was no sign of decline after 6 h of reaction time. Moreover, the generating rate of glucose in ionic liquid was 0.0111 g L−1 min−1 which is about 3 times of that in water phase (0.0031 g L−1 min−1). Based on the above results, it is speculated that the ionic liquid has a catalytic effect during cellulose hydrolysis. The specific reaction mechanism might be that the cation in the ionic liquid displaced the H+ of –SO3H on the catalysts,42 more H+ was present to favour the hydrolysis reaction and thus increased the glucose concentration.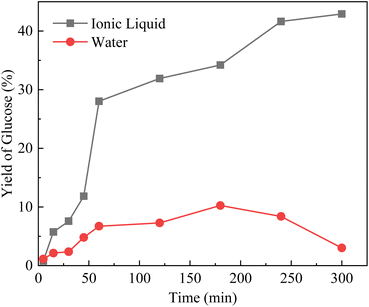 | ||
| Fig. 7 Hydrolysis of cellobiose to glucose catalyzed by Sl–C–S–H2O2 in water and ionic liquid (reaction conditions: 130 °C, 0.05 g catalyst, 0.05 g cellobiose, 2 g water or [BMIM][Cl]). | ||
Comparison with other methods
To evaluate the advantages of the method we developed, the comparison of our method with other methods was investigated, and the results were showed in Table 1. Since the solvent played a key role in the hydrolysis of biomass materials, hydrolysis of hemicellulose was dominant when the hydrolysis of biomass was carried out in water.43 Ulteriorly, in [BMIM][Cl], the cellulose in the biomass can be efficiently dissolved besides hemicellulose.39| Solvent | Catalyst | Biomass | Yieldb | Temperature | Time | Reference |
|---|---|---|---|---|---|---|
| a The yield indicates xylose, TRS, and hemicellulose removal, with solvent corresponding to water, IL, and DES, respectively.b H2SO4 aqueous solution was used for the pretreatment of Babool wood.c KOH aqueous solution was used for the pretreatment of rice straw.d Surfactant was added.e ChCl: choline chloride, LA: lactic acid, FA: formic acid, OA: oxalic acid, EG: ethylene glycol. | ||||||
| H2O | SO42−/Fe2O3 | Wheat straw | 63.5% | 140 °C | 4 h | 15 |
| H2O | Gp–SO3H–H2O2 | Corncob | 78.0% | 140 °C | 14 h | 16 |
| H2O | C–SO3H | Corncob | 78.1% | 140 °C | 6 h | 17 |
| H2O | Sl–C–S–H2O2 | Corncob | 83.4% | 130 °C | 14 h | This work |
| [BMIM][Cl] | Sl–C–S–H2O2 | Corncob | 75.1% | 130 °C | 10 h | This work |
| [BMIM][Cl] | PCM–SO3H | Rice straw | 35.5% | 150 °C | 2 h | 29 |
| [BMIM][Cl] | ACS | Babool woodb | 83.7% | 120 °C | 1 h | 30 |
| [BMIM][Cl] | BLSCM | Rice strawc | 63.4% | 140 °C | 2.5 h | 31 |
| [BMIM][Cl] | Fe3+-SCCR | Bamboo | 73.4% | 120 °C | 24 h | 32 |
| [BMIM][Cl] | SCCAC | Bambood | 68.0% | 120 °C | 24 h | 33 |
| ChCl/LAe | — | Wheat straw | 73.4% | 150 °C | 6 h | 44 |
| ChCl/LA:ChCl/FAe | — | Napier grass | 76.5% | 120 °C | 1.5 h | 45 |
| ChCl/OA/EGe | — | Eucommia ulmoides seed shells | 79.7% | 100 °C | 3 h | 46 |
As can be seen from the Table 1, under the moderate temperature of 130 °C in water, the catalyst prepared in this work had the highest activity and the yield of xylose was 83.4%. In [BMIM][Cl], compared with the hydrolysis of rice straw,29,31 although the hydrolysis reaction time was longer in our work, the hydrolysis temperature was lower and the TRS yield was much higher. In contrast, the hydrolysis of bamboo at a lower temperature,32,33 but the corresponding reaction time was much longer and the yield of TRS was slight lower than ours (75.1%). Even if the maximum TRS yield of 83.7% in [BMIM][Cl]was achieved in 60 min at 120 °C by Tyagi et al.,30 the employment of homogeneous mineral acid and the tedious two-step reaction procedures are expected to be improved. Besides, DES system has been frequently investigated to remove hemicellulose, extract lignin, and retain cellulose, primarily contributed by the cleavages of lignin-carbohydrate linkages and β-O-4 bond.44–46 However, few works reported its application in one-step sugar conversion.
Through the comparison with other reports, our work not only provided suitable temperature and time for biomass hydrolysis, but also had a relative high yield of TRS, which provides an efficient method for releasing sugars from lignocellulosic materials.
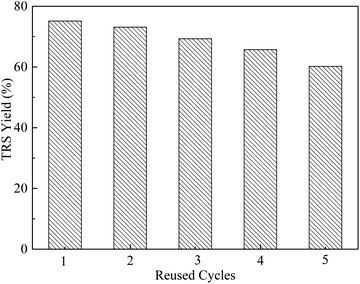
Catalyst reusability
In order to further study the recyclability and stability of the as-prepared catalyst. The catalyst was subjected to 5 cycles of use under the optimized reaction conditions (130 °C for 10 h, 0.05 g catalyst, 0.05 g corncob, 0.06 g H2O, and 2 g ionic liquid). After each reaction cycle, 10 g of water was added to the reaction solution, and the catalyst was separated by centrifugation. The catalyst was alternately washed with acetone and deionized water and then dried in an oven at 105 °C for 6 h before its use in the next cycle. It can be seen from Fig. 8 that the yield of TRS decreased from 75.1% to 60.2% after 5 times of cycling, which is probably ascribed to the mass loss of –SO3H group during the washing process.34 After repeated cycles of reaction, the catalyst still has good activity. The reusability of the catalyst in the aqueous also exhibited excellent stability and catalytic activity.35Conclusions
In this work, a carbon-based solid acid derived from sodium lignosulfonate was used to hydrolyze corncob and the hydrolyzing behaviors in different phases were studied. In the aqueous phase, the carbon-based solid acid has a high catalytic activity and selectivity for the hydrolysis of hemicellulose into xylose (the highest xylose yield is 83.4%) with a high retention rate of cellulose (86.3%). After the enzymatic hydrolysis, a TRS yield of 88.1% was obtained. In an ionic liquid system, cellulose and hemicellulose were hydrolysed at the same time with high efficiency and the yield of TRS was 75.1%. In short, both of the two strategies were demonstrated to be efficient and environmental to convert biomass into sugars, which shed light on the sustainable production of biobased chemicals and fuels by innovative biorefinery approaches.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (51976224 and 52006227), the Key Fundamental Research Project of Guangdong-Guangxi Joint Funding (2020B1515420005), the Youth Innovation Promotion Association, CAS (2021351), and Science and Technology Program of Guangzhou (202201010563).References
- Z. Sun, G. Bottari, A. Afanasenko, M. C. A. Stuart, P. J. Deuss, B. Fridrich and K. Barta, Nat. Catal., 2018, 1, 82–92 CrossRef CAS.
- Y. Su, W. Zhang, A. Zhang and W. Shao, Appl. Sci., 2020, 10, 8222 CrossRef CAS.
- M. Garedew, F. Lin, B. Song, T. M. DeWinter, J. E. Jackson, C. M. Saffron, C. H. Lam and P. T. Anastas, ChemSusChem, 2020, 13, 4214–4237 CrossRef CAS PubMed.
- Z. Zhou, D. Liu and X. Zhao, Renew. Sust. Energ. Rev., 2021, 146, 111169 CrossRef CAS.
- Q. Qing, Z. Ma, P. Chen, Q. Zhang, D. Chen, L. Wang and Y. Zhang, Biomass Convers. Biorefin., 2022 DOI:10.1007/s13399-021-02202-5.
- A. Adewuyi, Front. Energy Res., 2022, 10, 741570 CrossRef.
- S. S. Hassan, G. A. Williams and A. K. Jaiswal, Bioresour. Technol., 2018, 262, 310–318 CrossRef CAS PubMed.
- V. Sharma, M. L. Tsai, C. W. Chen, P. P. Sun, A. K. Patel, R. R. Singhania, P. Nargotra and C. D. Dong, Bioresour. Technol., 2022, 360, 127631 CrossRef CAS PubMed.
- A. K. Kumar and S. Sharma, Bioresour. Bioprocess., 2017, 4, 7 CrossRef PubMed.
- S. Soltanian, M. Aghbashlo, F. Almasi, H. Hosseinzadeh-Bandbafha, A.-S. Nizami, Y. S. Ok, S. S. Lam and M. Tabatabaei, Energy Convers. Manage., 2020, 212, 112792 CrossRef CAS.
- V. Oriez, J. Peydecastaing and P.-Y. Pontalier, Molecules, 2019, 24, 4273 CrossRef CAS PubMed.
- Q. Xu, W. Yang, G. Liu, C. Liang, S. Lu, Z. Qi, J. Hu, Q. Wang and W. Qi, ACS Omega, 2019, 4, 17864–17873 CrossRef CAS PubMed.
- B. Zhang, M. Gao, W. Tang, X. Wang, C. Wu, Q. Wang, S. M. Cheung and X. Chen, Energy, 2023, 263, 125606 CrossRef CAS.
- S. Lu, Q. Wang, Z. Liang, W. Wang, C. Liang, Z. Wang, Z. Yuan, P. Lan and W. Qi, Ind. Crops Prod., 2021, 160, 113159 CrossRef CAS.
- C. Zhong, C. Wang, F. Huang, F. Wang, H. Jia, H. Zhou and P. Wei, Carbohydr. Polym., 2015, 131, 384–391 CrossRef CAS PubMed.
- Y. Xu, X. Li, X. Zhang, W. Wang, S. Liu, W. Qi, X. Zhuang, Y. Luo and Z. Yuan, Bioresources, 2016, 11, 10469–10482 CAS.
- W. Qi, C. He, Q. Wang, S. Liu, Q. Yu, W. Wang, N. Leksawasdi, C. Wang and Z. Yuan, ACS Sustain. Chem. Eng., 2018, 6, 3640–3648 CrossRef CAS.
- J. Cheng, C. Huang, Y. Zhan, X. Liu, J. Wang, C. Huang, G. Fang, A. J. Ragauskas, Z. Xie and X. Meng, Bioresour. Technol., 2023, 387 Search PubMed.
- A. A. M. Elgharbawy, M. Hayyan, A. Hayyan, W. J. Basirun, H. M. Salleh and M. E. S. Mirghani, Biomass Bioenergy, 2020, 137, 105550 CrossRef CAS.
- Z. Zhang, J. Song and B. Han, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS PubMed.
- S. Suzuki and K. Takahashi, Chem. Rec., 2023 DOI:10.1002/tcr.202200264.
- K. Kuroda, New J. Chem., 2022, 46, 20047–20052 RSC.
- C.-W. Cho, T. P. T. Pham, Y. Zhao, S. Stolte and Y.-S. Yun, Sci. Total Environ., 2021, 786 Search PubMed.
- X. Dong, Y. Fan, H. Zhang, Y. Zhong, Y. Yang, J. Miao and S. Hua, Int. J. Biol. Macromol., 2016, 86, 155–161 CrossRef CAS PubMed.
- F. A. R. Mota, S. A. P. Pereira, A. R. T. S. Araujo and M. L. M. F. S. Saraiva, Molecules, 2021, 26 Search PubMed.
- I. Ben Hmad, M. Boudabbous, H. Belghith and A. Gargouri, Process Biochem., 2017, 54, 59–66 CrossRef CAS.
- P. Wolski, B. W. Blankenship, A. Umar, M. Cabrera, B. A. Simmons, K. L. Sale and E. C. Achinivu, Front. Energy Res., 2023, 11 Search PubMed.
- R. Rinaldi, R. Palkovits and F. Schuth, Angew Chem. Int. Ed. Engl., 2008, 47, 8047–8050 CrossRef CAS PubMed.
- H. Guo, Y. Lian, L. Yan, X. Qi and R. L. Smith, Green Chem., 2013, 15, 2167–2174 RSC.
- U. Tyagi, N. Anand and D. Kumar, Renewable Energy, 2020, 146, 56–65 CrossRef CAS.
- C. Bai, L. Zhu, F. Shen and X. Qi, Bioresour. Technol., 2016, 220, 656–660 CrossRef CAS PubMed.
- J. Cheng, N. Wang, D. Zhao, D. Qin, W. Si, Y. Tan, S. Wei and D. Wang, Bioresour. Technol., 2016, 220, 457–463 CrossRef CAS PubMed.
- W. Si, Y. Li, J. Zheng, S. Wei and D. Wang, Carbohydr. Polym., 2017, 174, 154–159 CrossRef CAS PubMed.
- L. Hu, Z. Li, Z. Wu, L. Lin and S. Zhou, Ind. Crops Prod., 2016, 84, 408–417 CrossRef CAS.
- X. Li, F. Shu, C. He, S. Liu, N. Leksawasdi, Q. Wang, W. Qi, M. A. Alam, Z. Yuan and Y. Gao, RSC Adv., 2018, 8, 10922–10929 RSC.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton, D. Crocker, Determination of Structural Carbohydrates and Lignin in Biomass, National Renewable Energy Laboratory (NREL), 2012, pp. 1−15 Search PubMed.
- Y. Lian, L. Yan, Y. Wang and X. Qi, Acta Chim. Sin., 2014, 72, 502–507 CrossRef CAS.
- H. Guo, X. Qi, L. Li and R. L. Smith, Bioresour. Technol., 2012, 116, 355–359 CrossRef CAS PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- X. Liu, Q. Xu, J. Liu, D. Yin, S. Su and H. Ding, Fuel, 2016, 164, 46–50 CrossRef CAS.
- Y. Xiong, Z. Zhang, X. Wang, B. Liu and J. Lin, Chem. Eng. J., 2014, 235, 349–355 CrossRef CAS.
- R. Rinaldi, N. Meine, J. vom Stein, R. Palkovits and F. Schuth, ChemSusChem, 2010, 3, 266–276 CrossRef CAS PubMed.
- C. Huang, G. Fang, Y. Zhou, X. Du, L. Yu, X. Meng, M. Li, C. G. Yoo, B. Chen, S. Zhai, Q. Guan, Q. Yong and A. J. Ragauskas, ACS Sustain. Chem. Eng., 2020, 8, 7380–7393 CrossRef CAS.
- H. Ma, P. Fu, J. Zhao, X. Lin, W. Wu, Z. Yu, C. Xia, Q. Wang, M. Gao and J. Zhou, Molecules, 2022, 27, 7955 CrossRef CAS PubMed.
- M. P. Gundupalli, K. Cheenkachorn, S. Chuetor, S. Kirdponpattara, S. P. Gundupalli, P. L. Show and M. Sriariyanun, Carbohydr. Polym., 2023, 306, 120599 CrossRef CAS PubMed.
- C.-J. Duan, X. Han, Y.-H. Chang, J. Xu, G.-L. Yue, Y. Zhang and Y.-J. Fu, Bioresour. Technol., 2023, 370, 128570 CrossRef CAS PubMed.
Footnote |
| † These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |