DOI:
10.1039/D3RA05235D
(Paper)
RSC Adv., 2023,
13, 28362-28370
Microwave assisted one-pot access to pyrazolo quinolinone and tetrahydroisoxazolo quinolinone derivatives via T3P®-DMSO catalysed tandem oxidative–condensation reaction†
Received
2nd August 2023
, Accepted 18th September 2023
First published on 3rd October 2023
Abstract
A new approach for the synthesis of two important annulated pyrazolo quinolinone and tetrahydroisoxazolo quinolinone derivatives from multicomponent reactions was achieved by using T3P®-DMSO-catalysed reactions of stable alcohols, cyclic 1,3-dicarbonyl compounds and amino derivatives of phenyl pyrazoles and isoxazole and has been reported for the first time. This reaction occurred via a tandem oxidative–condensation reaction under microwave irradiation and notable characteristics of this protocol are MCR reactions, shorter reaction time, less waste creation, ease of workup, stable precursors, broad substrate scope and functional group tolerance.
Introduction
The construction of annulated bioactive heterocyclic small molecules using MW irradiation from MCR is of great importance in the field of synthetic organic chemistry. These MCRs are frequently employed in synthetic organic chemistry as instruments for producing simple C–C bonds1a because they provide a simple workup technique, increased selectivity, and improved yields by simultaneously producing C–C and C-heteroatom bonds.1b Due to its capacity to produce higher yields in shorter reaction times, microwave (MW) irradiation has emerged as a crucial tool in the generation of heterocyclic compounds and drug discovery processes in organic synthesis.2a–d In particular, two important biological structures with a wide range of applications in the pharmaceutical and medical industries are pyrazoloquinolines and tetrahydroisoxazolo quinolinone derivatives. Out of these two, fused pyrazole derivatives exhibit a wide range of biological applications, such as anticancer,3a antimicrobial,3b antimalarial,3c antiviral,3d anti-inflammatory,3e antiplatelet,3f pulmonary hypertension,3g cytotoxicity,3h and translocator protein ligands.3i Furthermore, utilisation of these compounds as therapeutic agents for the treatment of Alzheimer's disease4a and influenza virus4b has been reported. Pyrazolo quinolines, which are also used in advanced dye chemistry, second-order nonlinear optical materials,5 electroluminescent materials,6a,b and fluorescence sensors,7a,b occasionally reach unity and are extremely fluorescent in the blue or greenish-blue portion of the spectrum.8a,b Numerous synthetic techniques have been devised for the synthesis of different substituted pyrazoloquinolines due to their broad variety of biological applications and technical interest. One of the main synthetic techniques for creating bioactive pyrazoloquinoline nuclei from commonly available precursors like o-amino benzaldehydes, o-amino acetophenones, and o-aminobenzophenones via condensation reactions9a–f has been reported, and the same annulated derivatives are synthesised from commonly available precursors such as 5-aminopyrazoles, less stable aldehydes, and suitable cyclic ketones.10a–d Similarly, isoxazolo quinolinones are important heterocyclic compounds with a wide range of pharmacological properties. These properties include hypoglycemic, analgesic, anti-inflammatory, antibacterial, anti-HIV, and anticancer activity.11a–c They also have positive effects on conditions like schizophrenia, hypertension, and Alzheimer's disease.12a–c Isoxazolopyridines are isoxazole derivatives that have raised attention and concern due to their CNS depressive, anticonvulsant, and muscle relaxant properties.12d Because of this wide range of biological applications, a lot of synthetic strategies have been documented for the synthesis of fused isoxazole derivatives from aminoisoxazole, aldehydes, and ketones.13a–d The significant disadvantages of the reported protocols for the synthesis of both the heterocycles are the use of less stable aldehydes as precursors. In addition to that, reported methods exhibit a few more disadvantages, such as poor substrate scope, longer reaction time, less functional group tolerance, costly synthesis, tedious work-up procedures, and undesirable side products. In continuation of our research interest from the past few years, the synthetic utility of T3P® in the presence of DMSO towards the development of various synthetic strategies for the synthesis of very important bioactive heterocycles via the oxidation–condensation reaction has been reported.14a–e We report herein a new strategic approach for the synthesis of both pyrazolo quinolinones and tetrahydroisoxazolo quinolinone from stable electronically diversified alcohols, cyclic 1,3 diketones and amino derivative of both phenyl pyrazole and isoxazole using T3P®-DMSO as a catalyst under microwave irradiation.
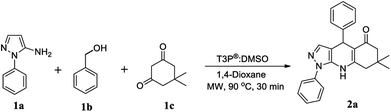
To optimise the reaction conditions, initially we conducted the experiments by choosing amino phenyl pyrazole 1a and dimedone 1c with benzyl alcohol 1b in a mixed solvent of 1,4-dioxane:DMSO in the ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
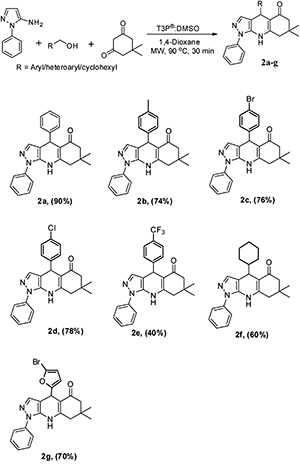
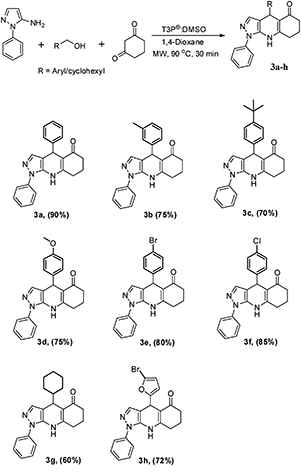
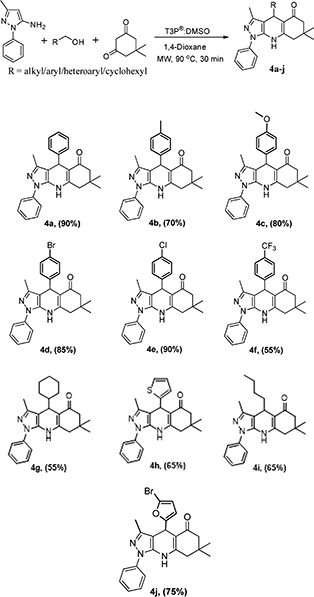
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, followed by the addition of 1 equivalent T3P® (50% in EtOAc) at RT to afford the desired pyrazoloquinoline in a 4% yield (Scheme 1). The same reaction was transferred from RT to MW irradiation at 90 °C for 30 min, yielding the desired pyrazoloquinoline in 24% yield. Further, varying different equivalents of T3P® could affect the course of the reaction; the best result was obtained by choosing 2.5 equivalents of T3P® and keeping the temperature at 90 °C for 30 min, which afforded the desired compound at around 90% yield. To determine the precise experimental parameter, the same reaction was carried out by varying the time and temperature while keeping the equivalent of T3P® (2.5 eq.) constant. Notably, the product yield was not adversely affected. And also, by changing the solvent conditions, we could not find any further improvement in the desired product yield. The overall optimisation results are summarised in Table 1. Thus, a clear optimisation reaction condition to obtain desired pyrazolo quinolinone from dimedone and benzyl alcohol was revealed from our studies, which entailed employing 2.5 eq. of T3P® at 90 °C and a duration of 30 min in the presence of 1,4-dioxane as a solvent under MW irradiation. The product was isolated by initially washing the reaction mixture with water followed by brine, then purified by column chromatography using 60–120 silica gel. With the clear optimisation condition in our hands, we carried out a series of reactions with amino phenyl pyrazole with 1,3-dicarbonyl compounds and various electronically diversified alcohols in the presence of an optimised reaction condition to afford the desired pyrazolo quinolinone derivatives. 2a–g are summarised in Scheme 2. To increase the substrate scope, the titled reactions are carried out by changing substituents in the 1,3-dicarbonyl compound to afford the desired compounds. 3a–g are summarised under Scheme 3. Similarly, by choosing the methyl group on pyrazolo quinolinone, it reacts with methyl substituted 1,3-dicarbonyl compounds and various alcohols to afford the desired pyrazolo quinolinone derivatives (4a–i), and the results are summarised under Scheme 4. Further, to broaden the substrate scope even more, the same methyl substituted phenyl pyrazole amine reacts with various alcohols and 1,3-dicarbonyl compounds containing without substitution the desired pyrazolo quinolinone derivatives (5a–f), and the results are summarised in Scheme 5. The overall product yields of the pyrazolo quinolinone derivatives were moderate to good. In all circumstances, the substitution that is present in the alcohols has a significant impact on the overall product yield. In Schemes 2 and 3, alcohols without substitution and alcohols containing halogens are the substituents, which comparatively give higher yields than alcohols containing electron-donating groups, followed by heterocyclic and cyclohexyl alcohols. Similarly, in Schemes 4 and 5, alcohols containing without substitution and alcohols containing halogens, particularly chloro substitution, comparatively gives equal and higher yield than the other categories of alcohols. But electron withdrawing groups other than hallogens like –CF3 affords poor yield compared to other substituents present on the alcohols as mentioned in the case of 2e and 4f.
1, followed by the addition of 1 equivalent T3P® (50% in EtOAc) at RT to afford the desired pyrazoloquinoline in a 4% yield (Scheme 1). The same reaction was transferred from RT to MW irradiation at 90 °C for 30 min, yielding the desired pyrazoloquinoline in 24% yield. Further, varying different equivalents of T3P® could affect the course of the reaction; the best result was obtained by choosing 2.5 equivalents of T3P® and keeping the temperature at 90 °C for 30 min, which afforded the desired compound at around 90% yield. To determine the precise experimental parameter, the same reaction was carried out by varying the time and temperature while keeping the equivalent of T3P® (2.5 eq.) constant. Notably, the product yield was not adversely affected. And also, by changing the solvent conditions, we could not find any further improvement in the desired product yield. The overall optimisation results are summarised in Table 1. Thus, a clear optimisation reaction condition to obtain desired pyrazolo quinolinone from dimedone and benzyl alcohol was revealed from our studies, which entailed employing 2.5 eq. of T3P® at 90 °C and a duration of 30 min in the presence of 1,4-dioxane as a solvent under MW irradiation. The product was isolated by initially washing the reaction mixture with water followed by brine, then purified by column chromatography using 60–120 silica gel. With the clear optimisation condition in our hands, we carried out a series of reactions with amino phenyl pyrazole with 1,3-dicarbonyl compounds and various electronically diversified alcohols in the presence of an optimised reaction condition to afford the desired pyrazolo quinolinone derivatives. 2a–g are summarised in Scheme 2. To increase the substrate scope, the titled reactions are carried out by changing substituents in the 1,3-dicarbonyl compound to afford the desired compounds. 3a–g are summarised under Scheme 3. Similarly, by choosing the methyl group on pyrazolo quinolinone, it reacts with methyl substituted 1,3-dicarbonyl compounds and various alcohols to afford the desired pyrazolo quinolinone derivatives (4a–i), and the results are summarised under Scheme 4. Further, to broaden the substrate scope even more, the same methyl substituted phenyl pyrazole amine reacts with various alcohols and 1,3-dicarbonyl compounds containing without substitution the desired pyrazolo quinolinone derivatives (5a–f), and the results are summarised in Scheme 5. The overall product yields of the pyrazolo quinolinone derivatives were moderate to good. In all circumstances, the substitution that is present in the alcohols has a significant impact on the overall product yield. In Schemes 2 and 3, alcohols without substitution and alcohols containing halogens are the substituents, which comparatively give higher yields than alcohols containing electron-donating groups, followed by heterocyclic and cyclohexyl alcohols. Similarly, in Schemes 4 and 5, alcohols containing without substitution and alcohols containing halogens, particularly chloro substitution, comparatively gives equal and higher yield than the other categories of alcohols. But electron withdrawing groups other than hallogens like –CF3 affords poor yield compared to other substituents present on the alcohols as mentioned in the case of 2e and 4f.
 |
| | Scheme 1 The T3P®-DMSO mediated synthesis of 2a under different reaction conditions and solvents. | |
Table 1 Optimization of reaction conditions
| Entry |
Solventa |
T3P®b |
Time (min) |
T [°C] |
Yieldc [%] of 2a |
Reactions were performed with solvent and DMSO were taken in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1,4-dioxane 1 (1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO) volume ratio, 1mmol of 1a, 1mmol of 1c and 1.2 mmol of alcohol in case of 2a.
T3P® (50% solution in EtOAc) was used to carry out the reactions.
Isolated yield after purified by column chromatography. DMSO) volume ratio, 1mmol of 1a, 1mmol of 1c and 1.2 mmol of alcohol in case of 2a.
T3P® (50% solution in EtOAc) was used to carry out the reactions.
Isolated yield after purified by column chromatography.
|
| 1 |
Dioxane |
1.0 |
30 |
RT |
4 |
| 1 |
Dioxane |
1.0 |
30 |
90 |
24 |
| 2 |
Dioxane |
1.5 |
30 |
90 |
41 |
| 3 |
Dioxane |
2.0 |
30 |
90 |
76 |
|
4
|
Dioxane
|
2.5
|
30
|
90 |
90
|
| 5 |
Dioxane |
3.0 |
30 |
100 |
88 |
| 7 |
Dioxane |
2.5 |
35 |
90 |
90 |
| 8 |
Dioxane |
2.5 |
40 |
90 |
86 |
| 9 |
Dioxane |
2.5 |
45 |
90 |
80 |
| 10 |
Toluene |
2.5 |
30 |
100 |
60 |
| 11 |
EtOAc |
2.5 |
30 |
75 |
40 |
| 12 |
MeCN |
2.5 |
30 |
80 |
18 |
| 13 |
Benzene |
2.5 |
30 |
80 |
21 |
| 14 |
DMF |
2.5 |
30 |
140 |
27 |
 |
| | Scheme 2 Synthesis of pyrazolo quinolinone derivatives from 1-phenyl-1H-pyrazol-5-amine and dimedone. | |
 |
| | Scheme 3 Synthesis of pyrazolo quinolinone derivatives from 1-phenyl-1H-pyrazol-5-amine and 1,3-cyclohexanedione. | |
 |
| | Scheme 4 Synthesis of pyrazolo quinolinone derivatives from 3-methyl-1-phenyl-1H-pyrazol-5-amine and dimedone. | |
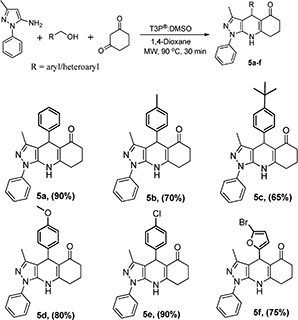 |
| | Scheme 5 Synthesis of pyrazolo quinolinone derivatives from 3-methyl-1-phenyl-1H-pyrazol-5-amine and 1,3-cyclohexanedione. | |
We succeeded in extending the scope of this synthetic methodology for the synthesis of biologically most important tetrahydroisoxazolo quinolinone derivatives by using methyl substituted amino isoxazole with same optimised reaction parameters, which afforded the desired tetrahydroisoxazolo quinolinones (6a–g) in moderate to good yield. The results are summarized in Scheme 6. In this instance also, alcohol without substitution gives a higher yield, and in the remaining cases, yields are mostly influenced by the substitutions that are present in the alcohols. Alcohols with halogens have a higher yield than alcohols without substitution, followed by alcohols with electron-donating groups.
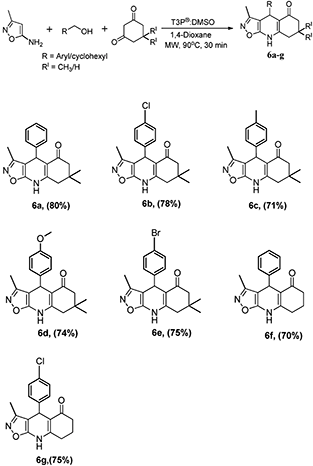 |
| | Scheme 6 Synthesis of tetrahydroisoxazolo quinolinone derivative from 3-methylisoxazol-5-amine, 1,3-cyclohexanedione with alcohols. | |
The possible mechanism involves the reaction of DMSO with T3P® followed by the substitution reaction of alcohol, which results in the cleavage of the phosphoester bond and the subsequent elimination of dimethyl sulphide, affords respective carbonyl compound (a). The formed carbonyl compound undergoes condensation reaction with the active methylene group of the 1,3-dicarbony compound to form intermediate (b); this intermediate undergoes 1,4 addition reaction with amino phenyl pyrazole in the presence of another equivalence of T3P® to form the desired pyrazolo quinolinone derivative with the elimination of propylphosphonic acid (PPA) (Scheme 7).
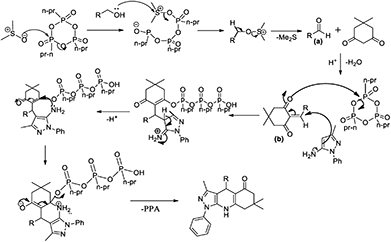 |
| | Scheme 7 Possible mechanism for the synthesis of 1-phenyl-1H-pyrazol-5-amine 2a from the Scheme 2. | |
X-ray crystallography analysis
The compound 3b crystallizes in the monoclinic space group P21/n. The unit cell parameters are a = 10.7713(16) Å, b = 13.2410(18) Å, c = 13.3621(18) Å, β = 108.828(5)° and Z = 4. The ORTEP of compound 3b with 50% probability ellipsoids is shown in Fig. 1. The various hydrogen bond interactions, along with the geometry, are listed in Table 2. The packing of the molecules viewed down the b axis is shown in Fig. 2.
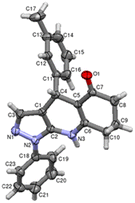 |
| | Fig. 1 The ORTEP of 3b with 50% probability (CCDC: 2267726). | |
Table 2 The calaculated hydrogen bond geometry details of the compound 3ba
| Sl. no |
Atoms |
D–H (Å) |
H⋯A (Å) |
D⋯A (Å) |
D–H⋯A (Å) |
|
a: 1/2 + x, 3/2 − y,1/2 + z.
|
| 1 |
N3–H3⋯O1a |
0.86 |
2.19 |
2.9418(16) |
146 |
| 2 |
C10–H10B⋯O1a |
0.97 |
2.54 |
3.3484(19) |
140 |
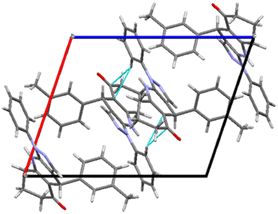 |
| | Fig. 2 Packing diagram of compound 3b along b axis. | |
The plane of the quinolin ring is oriented at −169.8(1)° from the pyrazole ring. The ring C18–C23 has trigonal geometry as it is indicated by the bond angle values of C18–C19–C20 = 119.2(2)°, C19–C20–C21 = 120.5(2)°, C20–C21–C22 = 119.7(2)°, C21–C22–C23 = 119.8(2)°, C22–C23–C18 = 119.8(2)°, C23–C18–C19 = 120.3(5)°.
Conclusion
In summary, we have described three component annulation reaction for the synthesis of two crucial heterocycles exhibiting broad biological spectrum, such as pyrazolo quinolinone and tetrahydroisoxazolo quinolinone derivatives, from stable alcohols using T3P®-DMSO as a catalyst under microwave irradiation. This method requires less reaction time and energy usage, less waste creation, and an atom economy under MW irradiation. And also, this gives insight into the synthesis of libraries of bioactive pyrazolo quinolinone and tetrahydroisoxazolo quinolinone derivatives. Further studies on this and related reactions are ongoing.
Experimental section
Materials and instruments
Using Sorbent Technologies standard-grade silica gel, reaction products were purified using standard column chromatography (60–120 mesh). On Merck silica gel 60 F254 plates, analytical thin-layer chromatography was carried out. Visualization was accomplished with UV light. Melting points were recorded on a Thomas Hoover capillary melting point apparatus and are uncorrected. Infrared spectra were recorded on an ATI Mattson Genesis Series FT-Infrared spectrophotometer. Proton nuclear magnetic resonance spectra (1H NMR) were recorded on Agilent-400 MHz and are reported in ppm using CDCl3 (7.24 ppm) and DMSO-d6 (2.47 ppm) are the internal standards. Proton-decoupled carbon nuclear magnetic resonance spectra (13C-NMR) were recorded on an Agilent-100 MHz and reported in ppm using CDCl3 as the internal standard (77.0 ppm). Mass spectra were recorded on Waters' mass spectrum.
General procedure for the synthesis of pyrazolo quinolinone and tetrahydroisoxazolo quinolinone derivative
The stirred suspension of alcohol (1.2 mmol) was added to the mixture of amino phenyl pyrazole or amino isoxazole (1 mmol) and cyclic 1,3 cyclic diketone in 1,4-dioxane as a solvent. It was followed by the addition of T3P® (2.0 mmol), and then the reaction mixture was kept under microwave irradiation at 90 °C for 30 minutes. The progress of the reaction was monitored by TLC. The reaction mixture was quenched by NaHCO3 solution under an ice bath, and the reaction mixture was extracted with EtOAc (2). The collected organic layer was washed with brine solution and dried over anhydrous sodium sulphate. The organic layer was removed under reduced pressure using a rotoevaporator to afford the desired compounds, which were purified by column chromatography using hexane and ethyl acetate as an eluent.
7,7-Dimethyl-1,4-diphenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (2a).
White solid; 90% yield (Rf = 0.45 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 138–142 °C; IR (KBr): 3236, 3055, 2956, 1578, 1549, 1247, 767 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (d, J = 4.4 Hz, 4H), 7.39–7.36 (m, 1H), 7.27–7.21 (m, 5H), 7.13–7.11 (m, 1H), 6.53 (s, 1H), 5.22 (s, 1H), 2.36 (s, 2H), 2.19 (d, J = 1.6 Hz, 2H), 1.06 (s, 3H), 1.03 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.0, 147.2, 138.7, 138.0, 135.0, 129.9, 128.2, 127.3, 127.3, 126.0, 122.9, 111.5, 106.5, 50.8, 42.5, 36.2, 32.5, 28.9, 27.4; HRMS (ESI) [M + H]+ calculated C24H23N3O 370.1919 found 370.1923.
40 v/v); MP: 138–142 °C; IR (KBr): 3236, 3055, 2956, 1578, 1549, 1247, 767 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (d, J = 4.4 Hz, 4H), 7.39–7.36 (m, 1H), 7.27–7.21 (m, 5H), 7.13–7.11 (m, 1H), 6.53 (s, 1H), 5.22 (s, 1H), 2.36 (s, 2H), 2.19 (d, J = 1.6 Hz, 2H), 1.06 (s, 3H), 1.03 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.0, 147.2, 138.7, 138.0, 135.0, 129.9, 128.2, 127.3, 127.3, 126.0, 122.9, 111.5, 106.5, 50.8, 42.5, 36.2, 32.5, 28.9, 27.4; HRMS (ESI) [M + H]+ calculated C24H23N3O 370.1919 found 370.1923.
7,7-Dimethyl-1-phenyl-4-(p-tolyl)-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (2b).
White solid; 75% yield (Rf = 0.40 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 142–146 °C; IR (KBr): 3225, 3028, 2963, 1569, 1541, 1253, 775 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.45 (m, 4H), 7.37–7.34 (m, 1H), 7.27 (d, J = 6.0 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 7.04 (t, J = 7.4 Hz, 2H), 6.93 (d, J = 7.6 Hz, 1H), 6.70 (s, 1H), 5.14 (s, 1H), 2.34 (d, J = 6.8 Hz, 2H), 2.28 (s, 3H), 2.18 (s, 2H), 1.048 (s, 6H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.3, 147.2, 138.7, 138.0, 137.6, 135.1, 129.9, 128.1, 128.0, 127.6, 126.8, 124.3, 123.0, 111.4, 106.6, 50.8, 42.4, 36.1, 32.5, 29.0, 27.5, 21.5; HRMS (ESI) [M + H]+ calculated C25H25N3O 384.2076 found 384.2079.
40 v/v); MP: 142–146 °C; IR (KBr): 3225, 3028, 2963, 1569, 1541, 1253, 775 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.45 (m, 4H), 7.37–7.34 (m, 1H), 7.27 (d, J = 6.0 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 7.04 (t, J = 7.4 Hz, 2H), 6.93 (d, J = 7.6 Hz, 1H), 6.70 (s, 1H), 5.14 (s, 1H), 2.34 (d, J = 6.8 Hz, 2H), 2.28 (s, 3H), 2.18 (s, 2H), 1.048 (s, 6H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.3, 147.2, 138.7, 138.0, 137.6, 135.1, 129.9, 128.1, 128.0, 127.6, 126.8, 124.3, 123.0, 111.4, 106.6, 50.8, 42.4, 36.1, 32.5, 29.0, 27.5, 21.5; HRMS (ESI) [M + H]+ calculated C25H25N3O 384.2076 found 384.2079.
4-(4-Bromophenyl)-7,7-dimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (2c).
Brown solid; 80% yield (Rf = 0.36 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 197–201 °C; IR (KBr): 3231, 3120, 2958, 1575, 1541, 1256, 791 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (s, 4H), 7.35 (d, J = 7.6 Hz, 3H), 7.25 (d, J = 4.4 Hz, 1H), 7.14 (d, J = 7.6 Hz, 2H), 6.63 (s, 1H), 5.18 (s, 1H), 2.36 (s, 2H), 2.19 (d, J = 2.0 Hz, 2H), 1.05 (s, 3H), 1.01 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.2, 146.2, 138.6, 137.9, 135.1, 131.2, 129.9, 129.2, 127.8, 123.0, 119.8, 111.1, 105.9, 50.8, 42.5, 35.8, 32.5, 28.9, 27.4; HRMS (ESI) [M + H]+ calculated C24H22BrN3O 448.1024 found 448.1021.
40 v/v); MP: 197–201 °C; IR (KBr): 3231, 3120, 2958, 1575, 1541, 1256, 791 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (s, 4H), 7.35 (d, J = 7.6 Hz, 3H), 7.25 (d, J = 4.4 Hz, 1H), 7.14 (d, J = 7.6 Hz, 2H), 6.63 (s, 1H), 5.18 (s, 1H), 2.36 (s, 2H), 2.19 (d, J = 2.0 Hz, 2H), 1.05 (s, 3H), 1.01 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.2, 146.2, 138.6, 137.9, 135.1, 131.2, 129.9, 129.2, 127.8, 123.0, 119.8, 111.1, 105.9, 50.8, 42.5, 35.8, 32.5, 28.9, 27.4; HRMS (ESI) [M + H]+ calculated C24H22BrN3O 448.1024 found 448.1021.
4-(4-Chlorophenyl)-7,7-dimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (2d).
White solid; 85% yield (Rf = 0.38 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 194–198 °C; IR (KBr): 3254, 3070, 2954, 1572, 1545, 1251, 771 cm−1; 1H NMR (CDCl3, 400 MHz): 7.57–7.53 (m, 6H), 7.44–7.37 (m, 2H), 7.17 (d, J = 8.0 Hz, 2H), 6.53 (s, 1H), 5.22 (s, 1H), 2.41 (s, 2H), 2.24 (d, J = 3.2 Hz, 2H), 1.09 (s, 3H), 1.05 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.8, 150.8, 139.6, 137.9, 136.7, 129.9, 129.6, 123.0, 122.6, 121.2, 110.6, 102.9, 50.9, 42.3, 35.5, 32.3, 29.2, 27.3; HRMS (ESI) [M + H]+ calculated C24H22ClN3O 404.1530 found 404.1525.
40 v/v); MP: 194–198 °C; IR (KBr): 3254, 3070, 2954, 1572, 1545, 1251, 771 cm−1; 1H NMR (CDCl3, 400 MHz): 7.57–7.53 (m, 6H), 7.44–7.37 (m, 2H), 7.17 (d, J = 8.0 Hz, 2H), 6.53 (s, 1H), 5.22 (s, 1H), 2.41 (s, 2H), 2.24 (d, J = 3.2 Hz, 2H), 1.09 (s, 3H), 1.05 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.8, 150.8, 139.6, 137.9, 136.7, 129.9, 129.6, 123.0, 122.6, 121.2, 110.6, 102.9, 50.9, 42.3, 35.5, 32.3, 29.2, 27.3; HRMS (ESI) [M + H]+ calculated C24H22ClN3O 404.1530 found 404.1525.
4-Cyclohexyl-7,7-dimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (2e).
Light brown solid; 60% yield (Rf = 0.42 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 136–140 °C; IR (KBr): 3436, 2868, 1653, 1589, 1375, 832, 695 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.35 (m, 6H), 6.41 (s, 1H), 4.01 (s, 1H), 2.30–2.14 (m, 2H), 1.74–1.44 (m, 5H), 1.24–0.87 (m, 14H); 13C NMR (CDCl3, 100 MHz): 195.8, 150.6, 139.3, 138.0, 136.7, 129.9, 127.6, 122.9, 110.8, 103.0, 51.0, 43.1, 42.4, 35.7, 32.4, 30.9, 29.3, 27.4, 27.3, 26.5, 26.4, 26.2; HRMS (ESI) [M + H]+ calculated C24H29N3O 376.2389 found 376.2394.
40 v/v); MP: 136–140 °C; IR (KBr): 3436, 2868, 1653, 1589, 1375, 832, 695 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.35 (m, 6H), 6.41 (s, 1H), 4.01 (s, 1H), 2.30–2.14 (m, 2H), 1.74–1.44 (m, 5H), 1.24–0.87 (m, 14H); 13C NMR (CDCl3, 100 MHz): 195.8, 150.6, 139.3, 138.0, 136.7, 129.9, 127.6, 122.9, 110.8, 103.0, 51.0, 43.1, 42.4, 35.7, 32.4, 30.9, 29.3, 27.4, 27.3, 26.5, 26.4, 26.2; HRMS (ESI) [M + H]+ calculated C24H29N3O 376.2389 found 376.2394.
4-(5-Bromofuran-2-yl)-7,7-dimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (2f).
Pale yellowsolid; 70% yield (Rf = 0.34 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 162–166 °C; IR (KBr): 3430, 2935, 1663, 1557, 1392, 1327, 695 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49–7.43 (m, 6H), 7.24 (s, 1H), 6.12 (d, J = 3.6 Hz, 1H), 5.98 (d, J = 3.2 Hz, 1H), 5.32 (s, 1H), 2.37 (s, 2H), 2.25 (d, J = 3.2 Hz, 2H), 1.09 (s, 3H), 1.06 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 159.6, 150.2, 138.7, 137.8, 135.2, 129.9, 123.1, 119.2, 111.9, 107.5, 103.1, 50.7, 45.4, 42.5, 32.5, 30.2, 30.1, 27.2; HRMS (ESI) [M + H]+ calculated C22H20BrN3O2 438.0817 found 438.0820.
40 v/v); MP: 162–166 °C; IR (KBr): 3430, 2935, 1663, 1557, 1392, 1327, 695 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49–7.43 (m, 6H), 7.24 (s, 1H), 6.12 (d, J = 3.6 Hz, 1H), 5.98 (d, J = 3.2 Hz, 1H), 5.32 (s, 1H), 2.37 (s, 2H), 2.25 (d, J = 3.2 Hz, 2H), 1.09 (s, 3H), 1.06 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 159.6, 150.2, 138.7, 137.8, 135.2, 129.9, 123.1, 119.2, 111.9, 107.5, 103.1, 50.7, 45.4, 42.5, 32.5, 30.2, 30.1, 27.2; HRMS (ESI) [M + H]+ calculated C22H20BrN3O2 438.0817 found 438.0820.
1,4-Diphenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (3a).
White solid; 90% yield (Rf = 0.36 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 128–132 °C; IR (KBr): 3243, 3058, 2963, 1563, 1549, 1248, 768 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.42 (m, 5H), 7.37–7.33 (m, 1H), 7.28–7.24 (m, 4H), 7.16–7.12 (m, 1H), 7.11 (s, 1H), 5.24 (s, 1H), 2.49–2.43 (m, 2H), 2.29–2.22 (m, 2H), 1.99–1.92 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 151.5, 147.3, 138.6, 137.9, 135.1, 129.8, 128.2, 127.6, 127.3, 126.0, 123.1, 112.3, 106.5, 37.1, 36.1, 28.7, 21.2; HRMS (ESI) [M + H]+ calculated C22H19N3O 342.1606 found 342.1612.
40 v/v); MP: 128–132 °C; IR (KBr): 3243, 3058, 2963, 1563, 1549, 1248, 768 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.42 (m, 5H), 7.37–7.33 (m, 1H), 7.28–7.24 (m, 4H), 7.16–7.12 (m, 1H), 7.11 (s, 1H), 5.24 (s, 1H), 2.49–2.43 (m, 2H), 2.29–2.22 (m, 2H), 1.99–1.92 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 151.5, 147.3, 138.6, 137.9, 135.1, 129.8, 128.2, 127.6, 127.3, 126.0, 123.1, 112.3, 106.5, 37.1, 36.1, 28.7, 21.2; HRMS (ESI) [M + H]+ calculated C22H19N3O 342.1606 found 342.1612.
1-Phenyl-4-(m-tolyl)-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (3b).
White solid; 75% yield (Rf = 0.37 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 158–162 °C; IR (KBr): 3238, 3016, 2964, 1568, 1556, 1239, 772 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.47 (m, 5H), 7.37–7.25 (m, 2H), 7.13–7.07 (m, 3H), 6.71 (s, 1H), 5.22 (s, 1H), 2.34–2.29 (m, 6H), 1.99–1.98 (m, 3H); 13C NMR (CDCl3, 100 MHz): 195.6, 147.2, 138.7, 138.1, 138.0, 137.6, 135.0, 129.9, 128.9, 128.5, 128.3, 128.1, 128.0, 127.7, 126.9, 124.8, 124.4, 123.0, 121.9, 112.6, 106.6, 87.4, 37.1, 35.9, 28.9, 21.5; HRMS (ESI) [M + H]+ calculated C23H21N3O 356.1763 found 356.1758.
40 v/v); MP: 158–162 °C; IR (KBr): 3238, 3016, 2964, 1568, 1556, 1239, 772 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.47 (m, 5H), 7.37–7.25 (m, 2H), 7.13–7.07 (m, 3H), 6.71 (s, 1H), 5.22 (s, 1H), 2.34–2.29 (m, 6H), 1.99–1.98 (m, 3H); 13C NMR (CDCl3, 100 MHz): 195.6, 147.2, 138.7, 138.1, 138.0, 137.6, 135.0, 129.9, 128.9, 128.5, 128.3, 128.1, 128.0, 127.7, 126.9, 124.8, 124.4, 123.0, 121.9, 112.6, 106.6, 87.4, 37.1, 35.9, 28.9, 21.5; HRMS (ESI) [M + H]+ calculated C23H21N3O 356.1763 found 356.1758.
4-(4-(tert-Butyl)phenyl)-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (3c).
White solid; 70% yield (Rf = 0.38 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 144–148 °C; IR (KBr): 3228, 3051, 2965, 1569, 1548, 1245, 771 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.24 (m, 9H), 7.20–7.16 (m, 2H), 5.21 (S, 1H), 2.53–2.2.41 (m, 2H), 2.25–2.19 (m, 2H), 1.94–1.87 (m, 2H), 1.25 (s, 9H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.7, 148.5, 144.2, 138.6, 138.0, 135.2, 129.7, 127.6, 127.3, 126.8, 125.5, 125.1, 123.1, 121.9, 112.3, 106.6, 37.1, 35.3, 34.2, 31.3, 28.6, 21.1; HRMS (ESI) [M + H]+ calculated C26H27N3O 398.2232 found 398.2238.
40 v/v); MP: 144–148 °C; IR (KBr): 3228, 3051, 2965, 1569, 1548, 1245, 771 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.24 (m, 9H), 7.20–7.16 (m, 2H), 5.21 (S, 1H), 2.53–2.2.41 (m, 2H), 2.25–2.19 (m, 2H), 1.94–1.87 (m, 2H), 1.25 (s, 9H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.7, 148.5, 144.2, 138.6, 138.0, 135.2, 129.7, 127.6, 127.3, 126.8, 125.5, 125.1, 123.1, 121.9, 112.3, 106.6, 37.1, 35.3, 34.2, 31.3, 28.6, 21.1; HRMS (ESI) [M + H]+ calculated C26H27N3O 398.2232 found 398.2238.
4-(4-Methoxyphenyl)-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (3d).
Light brown solid; 75% yield (Rf = 0.40 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 163–167 °C; IR (KBr): 3248, 3076, 2973, 1580, 1556, 1254, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.44–7.38 (m, 4H), 7.33–7.24 (m, 3H), 7.17 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 5.16 (s, 1H), 3.72 (s, 3H), 2.53–2.40 (m, 2H), 2.25–2.18 (m, 2H), 1.90 (t, J = 6.0 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.8, 157.7, 151.5, 139.8, 138.6, 138.0, 135.2, 129.7, 128.3, 127.6, 123.1, 113.5, 112.5, 106.6, 55.1, 37.1, 35.1, 28.6, 21.1; HRMS (ESI) [M + H]+ calculated C23H21N3O2 372.1712 found 372.1715.
40 v/v); MP: 163–167 °C; IR (KBr): 3248, 3076, 2973, 1580, 1556, 1254, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.44–7.38 (m, 4H), 7.33–7.24 (m, 3H), 7.17 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 5.16 (s, 1H), 3.72 (s, 3H), 2.53–2.40 (m, 2H), 2.25–2.18 (m, 2H), 1.90 (t, J = 6.0 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.8, 157.7, 151.5, 139.8, 138.6, 138.0, 135.2, 129.7, 128.3, 127.6, 123.1, 113.5, 112.5, 106.6, 55.1, 37.1, 35.1, 28.6, 21.1; HRMS (ESI) [M + H]+ calculated C23H21N3O2 372.1712 found 372.1715.
4-(4-Bromophenyl)-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (3e).
White solid; 80% yield (Rf = 0.33 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 212–216 °C; IR (KBr): 3239, 3115, 2948, 1568, 1575, 1256, 776 cm−1; 1H NMR (CDCl3, 400 MHz): 7.53–7.43 (m, 5H), 7.37 (d, J = 8 Hz, 3H), 7.16 (d, J = 8.0 Hz, 2H), 6.76 (s, 1H), 5.23 (s, 1H), 2.55–2.50 (m, 2H), 2.40–2.29 (m, 2H), 2.02–1.98 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.5, 151.1, 146.2, 138.6, 137.9, 135.0, 131.2, 129.5, 128.3, 123.0, 119.8, 116.0, 112.2, 105.8, 105.8, 37.1, 35.7, 28.8, 21.1; HRMS (ESI) [M + H]+ calculated C22H18BrN3O 421.3098 found 421.3091.
40 v/v); MP: 212–216 °C; IR (KBr): 3239, 3115, 2948, 1568, 1575, 1256, 776 cm−1; 1H NMR (CDCl3, 400 MHz): 7.53–7.43 (m, 5H), 7.37 (d, J = 8 Hz, 3H), 7.16 (d, J = 8.0 Hz, 2H), 6.76 (s, 1H), 5.23 (s, 1H), 2.55–2.50 (m, 2H), 2.40–2.29 (m, 2H), 2.02–1.98 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.5, 151.1, 146.2, 138.6, 137.9, 135.0, 131.2, 129.5, 128.3, 123.0, 119.8, 116.0, 112.2, 105.8, 105.8, 37.1, 35.7, 28.8, 21.1; HRMS (ESI) [M + H]+ calculated C22H18BrN3O 421.3098 found 421.3091.
4-(4-Chlorophenyl)-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (3f).
White solid; 85% yield (Rf = 0.35 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 200–204 °C; IR (KBr): 3254, 3071, 2953, 1572, 1546, 1256, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.54–7.48 (m, 5H), 7.42–7.25 (m, 6H), 6.58 (s, 1H), 5.25 (s, 1H), 2.54–2.52 (m, 2H), 2.38–2.31 (m, 2H), 2.03–1.98 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.5, 150.8, 145.6, 138.6, 137.9, 130.0, 128.8, 128.3, 127.9, 123.0, 112.4, 105.9, 37.1, 35.6, 28.9, 21.183; HRMS (ESI) [M + H]+ calculated C22H18ClN3O 376.1217 found 376.1224.
40 v/v); MP: 200–204 °C; IR (KBr): 3254, 3071, 2953, 1572, 1546, 1256, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.54–7.48 (m, 5H), 7.42–7.25 (m, 6H), 6.58 (s, 1H), 5.25 (s, 1H), 2.54–2.52 (m, 2H), 2.38–2.31 (m, 2H), 2.03–1.98 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.5, 150.8, 145.6, 138.6, 137.9, 130.0, 128.8, 128.3, 127.9, 123.0, 112.4, 105.9, 37.1, 35.6, 28.9, 21.183; HRMS (ESI) [M + H]+ calculated C22H18ClN3O 376.1217 found 376.1224.
4-Cyclohexyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (3g).
Pale yellow solid; 60% yield (Rf = 0.41 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 132–136 °C; IR (KBr): 3238, 3048, 2964, 1575, 1543, 1249, 773 cm−1; 1H NMR (CDCl3, 400 MHz): 7.40–7.27 (m, 6H), 6.64 (s, 1H), 3.95 (s, 1H), 2.39–2.38 (m, 4H), 1.92 (s, 2H), 1.66–1.1.49 (m, 7H), 1.14–1.12 (m, 4H); 13C NMR (CDCl3, 100 MHz): 196.2, 152.6, 139.2, 137.9, 136.6, 129.8, 127.9, 122.9, 111.7, 103.0, 42.9, 37.3, 35.6, 35.4, 30.7, 28.7, 27.0, 26.4, 26.4, 26.2, 21.2; HRMS (ESI) [M + H]+ calculated C22H25N3O 348.2076 found 348.2069.
40 v/v); MP: 132–136 °C; IR (KBr): 3238, 3048, 2964, 1575, 1543, 1249, 773 cm−1; 1H NMR (CDCl3, 400 MHz): 7.40–7.27 (m, 6H), 6.64 (s, 1H), 3.95 (s, 1H), 2.39–2.38 (m, 4H), 1.92 (s, 2H), 1.66–1.1.49 (m, 7H), 1.14–1.12 (m, 4H); 13C NMR (CDCl3, 100 MHz): 196.2, 152.6, 139.2, 137.9, 136.6, 129.8, 127.9, 122.9, 111.7, 103.0, 42.9, 37.3, 35.6, 35.4, 30.7, 28.7, 27.0, 26.4, 26.4, 26.2, 21.2; HRMS (ESI) [M + H]+ calculated C22H25N3O 348.2076 found 348.2069.
3,7,7-Trimethyl-1,4-diphenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (4a).
White solid; 90% yield(Rf = 0.40 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 126–130 °C(lit. 128–132 °C);15 IR (KBr): 3231, 3062, 2958, 1585, 1543, 1256, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (d, J = 4.0 Hz, 4H), 7.36 (d, J = 4.4 Hz, 1H), 7.31–7.22 (m, 4H), 7.14 (d, J = 7.2 Hz, 1H), 6.58 (s, 1H), 5.15 (s, 1H), 2.37 (s, 2H), 2.192 (d, J = 6.8 Hz, 2H), 2.03 (s, 3H), 1.07 (s, 3H), 1.02 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 148.6, 147.6, 146.4, 138.0, 135.5, 129.8, 128.0, 127.9, 127.3, 125.9, 122.7, 112.1, 104.7, 65.8, 50.8, 42.5, 36.1, 32.5, 29.0, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C25H25N3O 384.2076 found 384.2079.
40 v/v); MP: 126–130 °C(lit. 128–132 °C);15 IR (KBr): 3231, 3062, 2958, 1585, 1543, 1256, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (d, J = 4.0 Hz, 4H), 7.36 (d, J = 4.4 Hz, 1H), 7.31–7.22 (m, 4H), 7.14 (d, J = 7.2 Hz, 1H), 6.58 (s, 1H), 5.15 (s, 1H), 2.37 (s, 2H), 2.192 (d, J = 6.8 Hz, 2H), 2.03 (s, 3H), 1.07 (s, 3H), 1.02 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 148.6, 147.6, 146.4, 138.0, 135.5, 129.8, 128.0, 127.9, 127.3, 125.9, 122.7, 112.1, 104.7, 65.8, 50.8, 42.5, 36.1, 32.5, 29.0, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C25H25N3O 384.2076 found 384.2079.
3,7,7-Trimethyl-1-phenyl-4-(p-tolyl)-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (4b).
White solid; 70% yield (Rf = 0.42 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 196–200 °C (lit. 200–201 °C);15 IR (KBr): 3236, 3025, 2957, 1578, 1545, 1249, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.41 (m, 4H), 7.32 (d, J = 6.4 Hz, 1H), 7.10–7.04 (m, 3H), 6.92 (d, J = 7.2 Hz, 1H), 6.82 (s, 1H), 5.05 (s, 1H), 2.32 (d, J = 3.2 Hz, 2H), 2.28 (s, 3H), 2.15 (d, J = 5.6 Hz, 2H), 2.02 (s, 3H), 1.03 (S, 3H), 1.00 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 149.1, 147.5, 146.4, 138.0, 137.4, 135.5, 129.8, 128.5, 127.9, 127.2, 126.8, 124.9, 122.8, 111.9, 104.8, 50.8, 42.3, 36.1, 32.5, 29.0, 27.3, 21.5, 12.2; HRMS (ESI) [M + H]+ calculated C26H27N3O 398.2232 found 398.2228.
40 v/v); MP: 196–200 °C (lit. 200–201 °C);15 IR (KBr): 3236, 3025, 2957, 1578, 1545, 1249, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.41 (m, 4H), 7.32 (d, J = 6.4 Hz, 1H), 7.10–7.04 (m, 3H), 6.92 (d, J = 7.2 Hz, 1H), 6.82 (s, 1H), 5.05 (s, 1H), 2.32 (d, J = 3.2 Hz, 2H), 2.28 (s, 3H), 2.15 (d, J = 5.6 Hz, 2H), 2.02 (s, 3H), 1.03 (S, 3H), 1.00 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 149.1, 147.5, 146.4, 138.0, 137.4, 135.5, 129.8, 128.5, 127.9, 127.2, 126.8, 124.9, 122.8, 111.9, 104.8, 50.8, 42.3, 36.1, 32.5, 29.0, 27.3, 21.5, 12.2; HRMS (ESI) [M + H]+ calculated C26H27N3O 398.2232 found 398.2228.
4-(4-Methoxyphenyl)-3,7,7-trimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (4c).
White solid; 80% yield (Rf = 0.43 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 170–174 °C (lit. 166–170 °C);15 IR (KBr): 3237, 3060, 2958, 1596, 1541, 1248, 761 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47 (d, J = 4.4 Hz, 4H), 7.34 (t, J = 4.4 Hz, 1H), 7.19 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 6.53 (s, 1H), 5.08 (s, 1H), 3.74 (s, 3H), 2.34 (s, 2H), 2.17 (d, J = 5.2 Hz, 2H), 2.01 (s, 3H), 1.04 (s, 3H), 0.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 157.7, 148.4, 147.6, 138.9, 138.0, 135.5, 129.8, 128.8, 127.3, 122.7, 113.4, 112.3, 104.8, 55.1, 50.8, 42.5, 35.2, 32.5, 28.9, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C26H27N3O2414.2182 found 414.2185.
40 v/v); MP: 170–174 °C (lit. 166–170 °C);15 IR (KBr): 3237, 3060, 2958, 1596, 1541, 1248, 761 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47 (d, J = 4.4 Hz, 4H), 7.34 (t, J = 4.4 Hz, 1H), 7.19 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 6.53 (s, 1H), 5.08 (s, 1H), 3.74 (s, 3H), 2.34 (s, 2H), 2.17 (d, J = 5.2 Hz, 2H), 2.01 (s, 3H), 1.04 (s, 3H), 0.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 157.7, 148.4, 147.6, 138.9, 138.0, 135.5, 129.8, 128.8, 127.3, 122.7, 113.4, 112.3, 104.8, 55.1, 50.8, 42.5, 35.2, 32.5, 28.9, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C26H27N3O2414.2182 found 414.2185.
4-(4-Bromophenyl)-3,7,7-trimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (4d).
White solid; 85% yield (Rf = 0.36 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 208–212 °C (lit. 206 – 210 °C);15 IR (KBr): 3228, 3121, 2950, 1571, 1543, 1251, 786 cm−1; 1H NMR (CDCl3, 400 MHz): 7.51–7.46 (m, 5H), 7.35 (t, J = 7.0 Hz, 2H), 7.16–7.10 (m, 2H), 6.65 (s, 1H), 5.09 (s, 1H), 2.34 (s, 2H), 2.22–2.11 (m, 2H), 1.98 (s, 3H), 1.04 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 148.9, 147.5, 145.5, 137.9, 135.5, 131.1, 129.9, 129.7, 129.0, 128.9, 127.7, 126.0, 121.1, 119.7, 50.7, 42.4, 35.7, 32.5, 29.0, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C25H24BrN3O462.1181 found 462.1175.
40 v/v); MP: 208–212 °C (lit. 206 – 210 °C);15 IR (KBr): 3228, 3121, 2950, 1571, 1543, 1251, 786 cm−1; 1H NMR (CDCl3, 400 MHz): 7.51–7.46 (m, 5H), 7.35 (t, J = 7.0 Hz, 2H), 7.16–7.10 (m, 2H), 6.65 (s, 1H), 5.09 (s, 1H), 2.34 (s, 2H), 2.22–2.11 (m, 2H), 1.98 (s, 3H), 1.04 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 148.9, 147.5, 145.5, 137.9, 135.5, 131.1, 129.9, 129.7, 129.0, 128.9, 127.7, 126.0, 121.1, 119.7, 50.7, 42.4, 35.7, 32.5, 29.0, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C25H24BrN3O462.1181 found 462.1175.
4-(4-Chlorophenyl)-3,7,7-trimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (4e).
White solid; 90% yield (Rf = 0.37 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 210–214 °C (lit. 212–216 °C);15 IR (KBr): 3258, 3073, 2952, 1573, 1547, 1255, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.51–7.45 (m, 4H), 7.36 (t, J = 3.2 Hz, 1H), 7.25–7.17 (m, 4H), 6.48 (s, 1H), 5.11 (s, 1H), 2.35 (d, J = 2.4 Hz, 2H), 2.17 (d, J = 8.4 Hz, 2H), 1.98 (s, 3H), 1.05 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 149.3, 147.4, 145.0, 137.9, 135.6, 131.5, 129.8, 129.3, 128.2, 127.4, 122.9, 111.4, 104.2, 50.7, 42.2, 35.7, 35.6, 32.4, 29.0, 27.2, 12.1; HRMS (ESI) [M + H]+ calculated C25H24ClN3O 418.1686 found 418.1682.
40 v/v); MP: 210–214 °C (lit. 212–216 °C);15 IR (KBr): 3258, 3073, 2952, 1573, 1547, 1255, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.51–7.45 (m, 4H), 7.36 (t, J = 3.2 Hz, 1H), 7.25–7.17 (m, 4H), 6.48 (s, 1H), 5.11 (s, 1H), 2.35 (d, J = 2.4 Hz, 2H), 2.17 (d, J = 8.4 Hz, 2H), 1.98 (s, 3H), 1.05 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 149.3, 147.4, 145.0, 137.9, 135.6, 131.5, 129.8, 129.3, 128.2, 127.4, 122.9, 111.4, 104.2, 50.7, 42.2, 35.7, 35.6, 32.4, 29.0, 27.2, 12.1; HRMS (ESI) [M + H]+ calculated C25H24ClN3O 418.1686 found 418.1682.
4-Cyclohexyl-3,7,7-trimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (4f).
White solid; 55% yield (Rf = 0.44 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 168–172 °C; IR (KBr): 3435, 2928, 1643, 1504, 1385, 1065, 694 cm−1; 1H NMR (CDCl3, 400 MHz): 9.17 (broad s, 1H), 7.53–7.35 (m, 5H), 3.87 (s, 1H), 2.44 (s, 2H), 2.20–2.09 (m, 5H), 1.67–1.53 (m, 5H), 1.34–1.11 (m, 1H), 1.10–0.96 (m, 10H), 0.84–0.74 (m, 1H); 13C NMR (CDCl3, 100 MHz): 197.5, 162.6, 147.3, 137.4, 130.0, 129.1, 198.9, 122.0, 120.9, 116.5, 102.5, 52.2, 47.3, 32.9, 32.4, 28.2, 28.1, 27.4, 27.3, 26.5, 26.4, 12.2; HRMS (ESI) [M + H]+ calculated C25H31N3O 390.2545 found 390.2543.
40 v/v); MP: 168–172 °C; IR (KBr): 3435, 2928, 1643, 1504, 1385, 1065, 694 cm−1; 1H NMR (CDCl3, 400 MHz): 9.17 (broad s, 1H), 7.53–7.35 (m, 5H), 3.87 (s, 1H), 2.44 (s, 2H), 2.20–2.09 (m, 5H), 1.67–1.53 (m, 5H), 1.34–1.11 (m, 1H), 1.10–0.96 (m, 10H), 0.84–0.74 (m, 1H); 13C NMR (CDCl3, 100 MHz): 197.5, 162.6, 147.3, 137.4, 130.0, 129.1, 198.9, 122.0, 120.9, 116.5, 102.5, 52.2, 47.3, 32.9, 32.4, 28.2, 28.1, 27.4, 27.3, 26.5, 26.4, 12.2; HRMS (ESI) [M + H]+ calculated C25H31N3O 390.2545 found 390.2543.
3,7,7-Trimethyl-1-phenyl-4-(thiophen-2-yl)-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (4g).
Off white solid; 65% yield (Rf = 0.40 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 223–227 °C (lit. 226–229 °C);16 IR (KBr): 3439, 2846, 1648, 1514, 1373, 971, 695 cm−1; 1H NMR (DMSO-d6, 400 MHz): 9.54 (broad s, 1H), 7.55–7.41 (m, 4H), 7.39–7.38 (m, 1H), 7.21 (d, J = 4.4 Hz, 1H), 6.87–6.81 (m, 2H), 5.37 (s, 1H), 2.54–2.50 (m, 2H), 2.23–2.19 (m, 1H), 2.10–1.97 (m, 4H), 1.01 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 199.9, 155.5, 153.7, 151.8, 142.2, 140.0, 134.1, 130.8, 130.7, 127.8, 127.3, 108.4, 55.0, 46.5, 36.8, 34.9, 29.7, 27.8, 12.2; HRMS (ESI) [M + H]+ calculated C23H23N3OS 390.1640 found 390.1649.
40 v/v); MP: 223–227 °C (lit. 226–229 °C);16 IR (KBr): 3439, 2846, 1648, 1514, 1373, 971, 695 cm−1; 1H NMR (DMSO-d6, 400 MHz): 9.54 (broad s, 1H), 7.55–7.41 (m, 4H), 7.39–7.38 (m, 1H), 7.21 (d, J = 4.4 Hz, 1H), 6.87–6.81 (m, 2H), 5.37 (s, 1H), 2.54–2.50 (m, 2H), 2.23–2.19 (m, 1H), 2.10–1.97 (m, 4H), 1.01 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 199.9, 155.5, 153.7, 151.8, 142.2, 140.0, 134.1, 130.8, 130.7, 127.8, 127.3, 108.4, 55.0, 46.5, 36.8, 34.9, 29.7, 27.8, 12.2; HRMS (ESI) [M + H]+ calculated C23H23N3OS 390.1640 found 390.1649.
4-Butyl-3,7,7-trimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (4h).
White solid; 65% yield (Rf = 0.44 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 154–158 °C; IR (KBr): 3463, 2945, 2868, 1645, 1524, 1375, 698 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42 (d, J = 4.0 Hz, 5H), 7.30–7.25 (m, 1H), 6.81 (s, 1H), 4.12–4.10 (m, 1H), 2.28 (s, 2H), 2.21 (s, 3H), 2.16 (d, J −5.6 Hz, 2H), 2.15–1.62 (m, 1H), 1.48 (t, J = 6.8 Hz, 1H), 1.35–1.12 (m, 2H), 1.11–1.08 (m, 2H), 1.06 (s, 3H), 1.01 (s, 3H), 0.80 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 195.9, 150.8, 147.0, 138.0, 136.6, 129.7, 128.9, 127.1, 122.7, 111.1, 103.9, 50.9, 42.2, 35.6, 32.3, 29.4, 29.3, 27.4, 27.1, 22.8, 14.1, 12.4; HRMS (ESI) [M + H]+ calculated C23H29N3O 364.2389 found364.2387.
40 v/v); MP: 154–158 °C; IR (KBr): 3463, 2945, 2868, 1645, 1524, 1375, 698 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42 (d, J = 4.0 Hz, 5H), 7.30–7.25 (m, 1H), 6.81 (s, 1H), 4.12–4.10 (m, 1H), 2.28 (s, 2H), 2.21 (s, 3H), 2.16 (d, J −5.6 Hz, 2H), 2.15–1.62 (m, 1H), 1.48 (t, J = 6.8 Hz, 1H), 1.35–1.12 (m, 2H), 1.11–1.08 (m, 2H), 1.06 (s, 3H), 1.01 (s, 3H), 0.80 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 195.9, 150.8, 147.0, 138.0, 136.6, 129.7, 128.9, 127.1, 122.7, 111.1, 103.9, 50.9, 42.2, 35.6, 32.3, 29.4, 29.3, 27.4, 27.1, 22.8, 14.1, 12.4; HRMS (ESI) [M + H]+ calculated C23H29N3O 364.2389 found364.2387.
4-(5-Bromofuran-2-yl)-3,7,7-trimethyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (4i).
Light yellow solid; 75% yield (Rf = 0.38 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 172–176 °C; IR (KBr): 3433, 2861, 1651, 1575, 1378, 1056, 699 cm−1; 1H NMR (CDCl3, 400 MHz): 7.43–7.23 (m, 5H), 6.89 (s, 1H), 6.12 (d, J = 3.2 Hz, 1H), 6.01 (d, J = 2.8 Hz, 1H), 5.24 (s, 1H), 2.35 (s, 2H), 2.27–2.18 (m, 4H), 1.22 (s, 1H), 1.076 (s, 3H), 1.05 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.4, 163.3, 159.1, 150.6, 147.4, 138.8, 137.8, 135.8, 121.2, 120.9, 111.9, 107.6, 101.3, 53.8, 50.6, 48.3, 32.5, 28.2, 28.1, 12.3; HRMS (ESI) [M + H]+ calculated C23H22BrN3O2 452.0974 found 452.0979.
40 v/v); MP: 172–176 °C; IR (KBr): 3433, 2861, 1651, 1575, 1378, 1056, 699 cm−1; 1H NMR (CDCl3, 400 MHz): 7.43–7.23 (m, 5H), 6.89 (s, 1H), 6.12 (d, J = 3.2 Hz, 1H), 6.01 (d, J = 2.8 Hz, 1H), 5.24 (s, 1H), 2.35 (s, 2H), 2.27–2.18 (m, 4H), 1.22 (s, 1H), 1.076 (s, 3H), 1.05 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.4, 163.3, 159.1, 150.6, 147.4, 138.8, 137.8, 135.8, 121.2, 120.9, 111.9, 107.6, 101.3, 53.8, 50.6, 48.3, 32.5, 28.2, 28.1, 12.3; HRMS (ESI) [M + H]+ calculated C23H22BrN3O2 452.0974 found 452.0979.
3-Methyl-1,4-diphenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (5a).
White solid; 90% yield (Rf = 0.38in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 125–129 °C(lit.122–126 °C);15 IR (KBr): 3239, 3053, 2946, 1575, 1543, 1248, 762 cm−1; 1H NMR (CDCl3, 400 MHz): 7.43 (t, J = 5.2 Hz, 4H), 7.33–7.31 (m, 3H), 7.29–7.23 (m, 2H), 7.12 (t, 1H, 7 Hz), 6.90 (s, 1H), 5.14 (s, 1H), 2.50–2.44 (m, 2H), 2.28–2.22 (m, 2H), 2.00 (s, 3H), 1.93 (t, J = 4.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 150.8, 147.5, 146.6, 138.0, 135.5, 129.8, 128.1, 128.0, 127.2, 126.0, 122.8, 113.2, 104.6, 37.1, 36.0, 28.7, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C23H21N3O 356.1763 found 356.1759.
40 v/v); MP: 125–129 °C(lit.122–126 °C);15 IR (KBr): 3239, 3053, 2946, 1575, 1543, 1248, 762 cm−1; 1H NMR (CDCl3, 400 MHz): 7.43 (t, J = 5.2 Hz, 4H), 7.33–7.31 (m, 3H), 7.29–7.23 (m, 2H), 7.12 (t, 1H, 7 Hz), 6.90 (s, 1H), 5.14 (s, 1H), 2.50–2.44 (m, 2H), 2.28–2.22 (m, 2H), 2.00 (s, 3H), 1.93 (t, J = 4.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 150.8, 147.5, 146.6, 138.0, 135.5, 129.8, 128.1, 128.0, 127.2, 126.0, 122.8, 113.2, 104.6, 37.1, 36.0, 28.7, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C23H21N3O 356.1763 found 356.1759.
3-Methyl-1-phenyl-4-(p-tolyl)-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (5b).
White solid; 70% yield (Rf = 0.39 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 188–192 °C; IR (KBr): 3234, 3018, 2963, 1565, 1529, 1236, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.34 (m, 5H), 7.27 (d, J = 7.2 Hz, 1H), 7.11–7.04 (m, 3H), 6.92 (d, J = 6.8 Hz, 1H), 5.06 (s, 1H), 2.47 (s, 1H), 2.39 (d, J = 6.8 Hz, 1H), 2.28 (s, 3H), 2.22–2.16 (m, 2H), 2.00 (s, 3H), 1.89 (d, J = 5.6 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.6, 147.4, 146.6, 138.0, 137.4, 135.6, 129.6, 128.6, 127.1, 126.8, 125.0, 123.0, 112.9, 104.7, 37.1, 36.0, 28.5, 21.5, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C24H23N3O 370.1919 found 370.1922.
40 v/v); MP: 188–192 °C; IR (KBr): 3234, 3018, 2963, 1565, 1529, 1236, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.34 (m, 5H), 7.27 (d, J = 7.2 Hz, 1H), 7.11–7.04 (m, 3H), 6.92 (d, J = 6.8 Hz, 1H), 5.06 (s, 1H), 2.47 (s, 1H), 2.39 (d, J = 6.8 Hz, 1H), 2.28 (s, 3H), 2.22–2.16 (m, 2H), 2.00 (s, 3H), 1.89 (d, J = 5.6 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.6, 147.4, 146.6, 138.0, 137.4, 135.6, 129.6, 128.6, 127.1, 126.8, 125.0, 123.0, 112.9, 104.7, 37.1, 36.0, 28.5, 21.5, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C24H23N3O 370.1919 found 370.1922.
4-(4-(tert-Butyl)phenyl)-3-methyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (5c).
Pale yellow solid; 65% yield (Rf = 0.40 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 155–159 °C; IR (KBr): 3239, 3056, 2943, 1565, 1573, 1242, 769 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.35 (m, 4H), 7.29–7.23 (m, 4H), 7.197 (d, J = 8.8 Hz, 2H), 5.11 (s, 1H), 2.55–2.51 (m, 2H), 2.24–2.19 (m, 2H), 2.03 (s, 3H), 1.92 (t, J = 5.6 Hz, 2H), 1.27 (s, 9H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.3, 148.4, 147.4, 143.6, 138.0, 135.6, 129.6, 127.3, 127.1, 124.9, 122.9, 113.1, 104.9, 37.1, 35.4, 34.2, 31.3, 28.6, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C27H29N3O 412.2389 found 412.2381.
40 v/v); MP: 155–159 °C; IR (KBr): 3239, 3056, 2943, 1565, 1573, 1242, 769 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.35 (m, 4H), 7.29–7.23 (m, 4H), 7.197 (d, J = 8.8 Hz, 2H), 5.11 (s, 1H), 2.55–2.51 (m, 2H), 2.24–2.19 (m, 2H), 2.03 (s, 3H), 1.92 (t, J = 5.6 Hz, 2H), 1.27 (s, 9H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.3, 148.4, 147.4, 143.6, 138.0, 135.6, 129.6, 127.3, 127.1, 124.9, 122.9, 113.1, 104.9, 37.1, 35.4, 34.2, 31.3, 28.6, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C27H29N3O 412.2389 found 412.2381.
4-(4-Methoxyphenyl)-3-methyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (5d).
White solid; 80% yield (Rf = 0.41 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 122–126 °C (lit. 124–128 °C);15 IR (KBr): 3234, 3055, 2952, 1580, 1541, 1253, 755 cm−1; 1H NMR (CDCl3, 400 MHz): 7.53–7.48 (m, 4H), 7.39–7.35 (m, 1H), 7.22 (t, J = 5.8 Hz, 2H), 6.78 (d, J = 8.8 Hz, 2H), 6.47 (s, 1H), 5.16 (s, 1H), 3.76 (s, 3H), 2.51 (t, J = 5.6 Hz, 2H), 2.40–2.31 (m, 2H), 2.02 (s, 3H), 1.98 (s, 2H); 13C NMR (CDCl3, 100 MHz): 190.9, 145.3, 142.9, 134.2, 133.3, 130.6, 125.1, 123.9, 123.7, 122.5, 118.0, 109.3, 109.3, 109.0, 108.6, 50.4, 32.4, 30.4, 24.1, 16.4, 7.4; HRMS (ESI) [M + H]+ calculated C24H23N3O2 386.1869 found 386.1864.
40 v/v); MP: 122–126 °C (lit. 124–128 °C);15 IR (KBr): 3234, 3055, 2952, 1580, 1541, 1253, 755 cm−1; 1H NMR (CDCl3, 400 MHz): 7.53–7.48 (m, 4H), 7.39–7.35 (m, 1H), 7.22 (t, J = 5.8 Hz, 2H), 6.78 (d, J = 8.8 Hz, 2H), 6.47 (s, 1H), 5.16 (s, 1H), 3.76 (s, 3H), 2.51 (t, J = 5.6 Hz, 2H), 2.40–2.31 (m, 2H), 2.02 (s, 3H), 1.98 (s, 2H); 13C NMR (CDCl3, 100 MHz): 190.9, 145.3, 142.9, 134.2, 133.3, 130.6, 125.1, 123.9, 123.7, 122.5, 118.0, 109.3, 109.3, 109.0, 108.6, 50.4, 32.4, 30.4, 24.1, 16.4, 7.4; HRMS (ESI) [M + H]+ calculated C24H23N3O2 386.1869 found 386.1864.
4-(4-Chlorophenyl)-3-methyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (5e).
White solid; 90% yield (Rf = 0.35 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 202–206 °C (lit. 202–204 °C);15 IR (KBr): 3257, 3066, 2958, 1577, 1546, 1255, 745 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.44 (m, 4H), 7.37–7.33 (m, 1H), 7.25–7.18 (m, 4H), 6.71 (s, 1H), 5.13 (s, 1H), 2.50–2.46 (m, 2H), 2.31–2.26 (m, 2H), 1.98–1.91 (m, 5H); 13C NMR (CDCl3, 100 MHz): 195.5, 150.6, 147.4, 145.0, 137.8, 135.4, 131.6, 129.4, 128.1, 127.4, 122.8, 113.0, 104.1, 37.1, 35.6, 28.8, 21.1, 12.1; HRMS (ESI) [M + H]+ calculated C23H20ClN3O 390.1373 found 390.1378.
40 v/v); MP: 202–206 °C (lit. 202–204 °C);15 IR (KBr): 3257, 3066, 2958, 1577, 1546, 1255, 745 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.44 (m, 4H), 7.37–7.33 (m, 1H), 7.25–7.18 (m, 4H), 6.71 (s, 1H), 5.13 (s, 1H), 2.50–2.46 (m, 2H), 2.31–2.26 (m, 2H), 1.98–1.91 (m, 5H); 13C NMR (CDCl3, 100 MHz): 195.5, 150.6, 147.4, 145.0, 137.8, 135.4, 131.6, 129.4, 128.1, 127.4, 122.8, 113.0, 104.1, 37.1, 35.6, 28.8, 21.1, 12.1; HRMS (ESI) [M + H]+ calculated C23H20ClN3O 390.1373 found 390.1378.
4-(5-Bromofuran-2-yl)-3-methyl-1-phenyl-6,7,8,9-tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-one (5f).
Pale yellow solid; 75% yield (Rf = 0.35 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 166–170 °C; IR (KBr): 3228, 3045, 2952, 1573, 1541, 1239, 772 cm−1; 1H NMR (CDCl3, 400 MHz): 7.41–7.38 (m, 5H), 7.31–7.30 (m, 1H), 6.12 (t, J = 3.2 Hz, 1H), 5.97 (d, J = 2.8 Hz, 1H), 5.25 (s, 1H), 2.48–2.28 (m, 3H), 2.17–2.15 (m, 4H), 1.97–1.95 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 164.5, 159.1, 152.1, 150.3, 135.7, 134.5, 122.0, 121.3, 121.2, 113.8, 112.7, 108.9, 101.3, 40.0, 34.6, 28.8, 21.6, 12.2; HRMS (ESI) [M + H]+ calculated C21H18BrN3O2 424.0661 found 424.0669.
40 v/v); MP: 166–170 °C; IR (KBr): 3228, 3045, 2952, 1573, 1541, 1239, 772 cm−1; 1H NMR (CDCl3, 400 MHz): 7.41–7.38 (m, 5H), 7.31–7.30 (m, 1H), 6.12 (t, J = 3.2 Hz, 1H), 5.97 (d, J = 2.8 Hz, 1H), 5.25 (s, 1H), 2.48–2.28 (m, 3H), 2.17–2.15 (m, 4H), 1.97–1.95 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 164.5, 159.1, 152.1, 150.3, 135.7, 134.5, 122.0, 121.3, 121.2, 113.8, 112.7, 108.9, 101.3, 40.0, 34.6, 28.8, 21.6, 12.2; HRMS (ESI) [M + H]+ calculated C21H18BrN3O2 424.0661 found 424.0669.
3,7,7-Trimethyl-4-phenyl-6,7,8,9-tetrahydroisoxazolo[5,4-b]quinolin-5(4H)-one (6a).
White solid; 75% yield (Rf = 0.40 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 182–186 °C (lit. 183–184 °C);17 IR (KBr): 3436, 2927, 1645, 1563, 1504, 1452, 1257; 1H NMR (CDCl3, 400 MHz): 9.40 (broad s, 1H), 7.49–7.27 (m, 5H), 5.28–5.27 (m, 1H), 2.63 (s, 2H), 2.45–2.42 (m, 2H), 2.15 (s, 3H), 1.36–1.22 (m, 6H); 13C NMR (CDCl3, 100 MHz): 196.1, 165.4, 161.1, 149.6, 138.8, 129.4, 128.7, 128.5, 128.1, 126.5, 117.1, 113.0, 96.9, 51.1, 43.2, 37.2, 33.2, 28.2, 28.0, 11.1 HRMS (ESI) [M + H]+ calculated C19H20N2O2 309.1603 found 309.1606.
40 v/v); MP: 182–186 °C (lit. 183–184 °C);17 IR (KBr): 3436, 2927, 1645, 1563, 1504, 1452, 1257; 1H NMR (CDCl3, 400 MHz): 9.40 (broad s, 1H), 7.49–7.27 (m, 5H), 5.28–5.27 (m, 1H), 2.63 (s, 2H), 2.45–2.42 (m, 2H), 2.15 (s, 3H), 1.36–1.22 (m, 6H); 13C NMR (CDCl3, 100 MHz): 196.1, 165.4, 161.1, 149.6, 138.8, 129.4, 128.7, 128.5, 128.1, 126.5, 117.1, 113.0, 96.9, 51.1, 43.2, 37.2, 33.2, 28.2, 28.0, 11.1 HRMS (ESI) [M + H]+ calculated C19H20N2O2 309.1603 found 309.1606.
4-(4-Chlorophenyl)-3,7,7-trimethyl-6,7,8,9-tetrahydroisoxazolo[5,4-b]quinolin-5(4H)-one (6b).
White solid; 78% yield (Rf = 0.37 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 269–273 °C (lit. 270–271 °C);17 IR (KBr): 3430, 2925, 1645, 1562, 1505, 1454, 1251; 1H NMR (DMSO-d6, 400 MHz): 10.77 (broad s, 1H), 7.30 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.4 Hz, 2H), 4.98 (s, 1H), 3.34–2.53 (m, 1H), 2.51–2.40 (m, 1H), 2.19–1.91 (m, 2H), 1.86 (s, 3H), 1.05 (s, 3H), 0.85 (s, 3H); 13C NMR (CDCl3, 100 MHz): 191.2, 154.6, 153.7, 146.0, 139.2, 127.4, 124.6, 123.5, 108.2, 91.5, 53.3, 48.7, 32.3, 31.0, 26.8, 26.4, 10.4; HRMS (ESI) [M + H]+ calculated C19H19ClN2O2343.1213 found 343.1209.
40 v/v); MP: 269–273 °C (lit. 270–271 °C);17 IR (KBr): 3430, 2925, 1645, 1562, 1505, 1454, 1251; 1H NMR (DMSO-d6, 400 MHz): 10.77 (broad s, 1H), 7.30 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.4 Hz, 2H), 4.98 (s, 1H), 3.34–2.53 (m, 1H), 2.51–2.40 (m, 1H), 2.19–1.91 (m, 2H), 1.86 (s, 3H), 1.05 (s, 3H), 0.85 (s, 3H); 13C NMR (CDCl3, 100 MHz): 191.2, 154.6, 153.7, 146.0, 139.2, 127.4, 124.6, 123.5, 108.2, 91.5, 53.3, 48.7, 32.3, 31.0, 26.8, 26.4, 10.4; HRMS (ESI) [M + H]+ calculated C19H19ClN2O2343.1213 found 343.1209.
3,7,7-Trimethyl-4-(p-tolyl)-6,7,8,9-tetrahydroisoxazolo[5,4-b]quinolin-5(4H)-one (6c).
White solid; 71% yield (Rf = 0.38 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 288–232 °C (lit. 289–230 °C);17 IR (KBr): 3437, 2920, 1645, 1570, 1501, 1459, 1249; 1H NMR (DMSO-d6, 400 MHz): 10.71 (broad s, 1H), 7.24–7.10 (m, 4H), 4.95 (s, 1H), 2.54–2.41 (m, 2H), 2.18 (d, J = 16 Hz, 1H), 2.01 (t, J = 3.6 Hz, 1H), 1.86 (s, 3H), 1.34 (s, 3H), 1.02 (s, 3H), 0.96 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.9, 160.1, 159.5, 151.1, 145.5, 132.1, 129.6, 128.9, 128.4, 111.9, 96.3, 51.4, 41.7, 40.1, 33.0, 29.5, 29.1, 21.7, 11.1; HRMS (ESI) [M + H]+ calculated 323.1760 found 323.1767.
40 v/v); MP: 288–232 °C (lit. 289–230 °C);17 IR (KBr): 3437, 2920, 1645, 1570, 1501, 1459, 1249; 1H NMR (DMSO-d6, 400 MHz): 10.71 (broad s, 1H), 7.24–7.10 (m, 4H), 4.95 (s, 1H), 2.54–2.41 (m, 2H), 2.18 (d, J = 16 Hz, 1H), 2.01 (t, J = 3.6 Hz, 1H), 1.86 (s, 3H), 1.34 (s, 3H), 1.02 (s, 3H), 0.96 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.9, 160.1, 159.5, 151.1, 145.5, 132.1, 129.6, 128.9, 128.4, 111.9, 96.3, 51.4, 41.7, 40.1, 33.0, 29.5, 29.1, 21.7, 11.1; HRMS (ESI) [M + H]+ calculated 323.1760 found 323.1767.
4-(4-Methoxyphenyl)-3,7,7-trimethyl-6,7,8,9-tetrahydroisoxazolo[5,4-b]quinolin-5(4H)-one (6d).
Pale yellow solid; 76% yield (Rf = 0.36 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 226–230 °C (lit. 226–227 °C);17 IR (KBr): 3435, 2922, 1641, 1567, 1509, 1452, 1251; 1H NMR (DMSO-d6, 400 MHz): 8.02–8.01 (m, 1H), 7.87–7.80 (m, 2H), 7.57–7.46 (m, 2H), 3.97 (s, 1H), 3.32–3.30 (m, 3H), 2.28 (s, 3H), 1.86–1.69 (m, 10H); 13C NMR (CDCl3, 100 MHz): 196.1, 166.3, 162.1, 149.2, 139.1, 135.2, 131.1, 130.2, 118.1, 113.0, 100.0, 55.4, 51.1, 43.2, 38.2, 33.2, 28.2, 11.2; HRMS (ESI) [M + H]+ calculated 339.1709 found 339.1701.
40 v/v); MP: 226–230 °C (lit. 226–227 °C);17 IR (KBr): 3435, 2922, 1641, 1567, 1509, 1452, 1251; 1H NMR (DMSO-d6, 400 MHz): 8.02–8.01 (m, 1H), 7.87–7.80 (m, 2H), 7.57–7.46 (m, 2H), 3.97 (s, 1H), 3.32–3.30 (m, 3H), 2.28 (s, 3H), 1.86–1.69 (m, 10H); 13C NMR (CDCl3, 100 MHz): 196.1, 166.3, 162.1, 149.2, 139.1, 135.2, 131.1, 130.2, 118.1, 113.0, 100.0, 55.4, 51.1, 43.2, 38.2, 33.2, 28.2, 11.2; HRMS (ESI) [M + H]+ calculated 339.1709 found 339.1701.
4-(4-Bromophenyl)-3,7,7-trimethyl-6,7,8,9-tetrahydroisoxazolo[5,4-b]quinolin-5(4H)-one (6e).
White solid; 75% yield (Rf = 0.33 in hexane/EtoAc 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 224–226 °C (lit. 224–225 °C);17 IR (KBr): 3430, 2926, 1642, 1568, 1503, 1453, 1254; 1H NMR (DMSO-d6, 400 MHz): 10.61 (broad s, 1H), 7.08 (d, J = 8.0 Hz, 2H), 6.79 (d, J = 8.0 Hz, 2H), 4.90 (s, 1H), 2.51–2.39 (m, 2H), 2.19–2.15 (m, 1H), 2.03–1.99 (m, 1H), 1.861 (s, 3H), 1.18 (m, 6H), 1.09 (m, 3H); 13C NMR (DMSO-d6, 100 MHz): 194.6, 159.5, 158.8, 157.8, 150.3, 139.3, 128.8, 113.8, 110.5, 96.4, 55.3, 50.8, 35.2, 32.5, 29.0, 27.2, 10.2; HRMS (ESI) [M + H]+ calculated 345.1415 found 345.1418.
40 v/v); MP: 224–226 °C (lit. 224–225 °C);17 IR (KBr): 3430, 2926, 1642, 1568, 1503, 1453, 1254; 1H NMR (DMSO-d6, 400 MHz): 10.61 (broad s, 1H), 7.08 (d, J = 8.0 Hz, 2H), 6.79 (d, J = 8.0 Hz, 2H), 4.90 (s, 1H), 2.51–2.39 (m, 2H), 2.19–2.15 (m, 1H), 2.03–1.99 (m, 1H), 1.861 (s, 3H), 1.18 (m, 6H), 1.09 (m, 3H); 13C NMR (DMSO-d6, 100 MHz): 194.6, 159.5, 158.8, 157.8, 150.3, 139.3, 128.8, 113.8, 110.5, 96.4, 55.3, 50.8, 35.2, 32.5, 29.0, 27.2, 10.2; HRMS (ESI) [M + H]+ calculated 345.1415 found 345.1418.
3-Methyl-4-phenyl-6,7,8,9-tetrahydroisoxazolo[5,4-b]quinolin-5(4H)-one (6f).
White solid; 70% yield (Rf = 0.35 in hexane/EtoAc60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 172–176 °C (lit. 174–175 °C);17 IR (KBr): 3433, 2926, 1642, 1568, 1503, 1453, 1254; 1H NMR (CDCl3, 400 MHz): 8.94 (broad s, 1H), 7.55–7.40 (m, 5H), 4.17 (s, 1H), 2.81–74 (m, 2H), 2.53–2.39 (m, 2H), 2.22–2.16 (m, 3H), 2.09 (s, 1H), 1.48–1.42 (m, 1H); 13C NMR (CDCl3, 100 MHz): 195.9, 168.8, 165.7, 155.8, 150.4, 148.1, 147.0, 132.1, 123.0, 121.7, 111.9, 52.9, 47.3, 31.7, 29.0, 27.4, 11.4; HRMS (ESI) [M + H]+ calculated C17H16N2O2281.1290 found 281.1295.
40 v/v); MP: 172–176 °C (lit. 174–175 °C);17 IR (KBr): 3433, 2926, 1642, 1568, 1503, 1453, 1254; 1H NMR (CDCl3, 400 MHz): 8.94 (broad s, 1H), 7.55–7.40 (m, 5H), 4.17 (s, 1H), 2.81–74 (m, 2H), 2.53–2.39 (m, 2H), 2.22–2.16 (m, 3H), 2.09 (s, 1H), 1.48–1.42 (m, 1H); 13C NMR (CDCl3, 100 MHz): 195.9, 168.8, 165.7, 155.8, 150.4, 148.1, 147.0, 132.1, 123.0, 121.7, 111.9, 52.9, 47.3, 31.7, 29.0, 27.4, 11.4; HRMS (ESI) [M + H]+ calculated C17H16N2O2281.1290 found 281.1295.
4-(4-Chlorophenyl)-3-methyl-6,7,8,9-tetrahydroisoxazolo[5,4-b]quinolin-5(4H)-one (6g).
White solid; 73% yield (Rf = 0.32 in hexane/EtoAc60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 278–282 °C (lit. 278–279 °C);17 IR (KBr): 3435, 2924, 1640, 1569, 1501, 1452, 1256; 1H NMR (CDCl3, 400 MHz): 8.08 (broad s, 1H), 7.27–7.18 (m, 4H), 5.08 (s, 1H), 2.55 (t, J = 5.6 Hz, 2H), 2.38 (t, J = 5.6 Hz, 2H), 2.09–1.96 (m, 2H), 1.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): 196.5, 162.8, 157.1, 145.0, 137.8, 131.6, 129.9, 129.4, 122.8, 113.0, 104.2, 37.1, 35.6, 28.8, 22.1, 11.2; HRMS (ESI) [M + H]+ calculated C17H15ClN2O 315.0900 found 315.0908.
40 v/v); MP: 278–282 °C (lit. 278–279 °C);17 IR (KBr): 3435, 2924, 1640, 1569, 1501, 1452, 1256; 1H NMR (CDCl3, 400 MHz): 8.08 (broad s, 1H), 7.27–7.18 (m, 4H), 5.08 (s, 1H), 2.55 (t, J = 5.6 Hz, 2H), 2.38 (t, J = 5.6 Hz, 2H), 2.09–1.96 (m, 2H), 1.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): 196.5, 162.8, 157.1, 145.0, 137.8, 131.6, 129.9, 129.4, 122.8, 113.0, 104.2, 37.1, 35.6, 28.8, 22.1, 11.2; HRMS (ESI) [M + H]+ calculated C17H15ClN2O 315.0900 found 315.0908.
Conflicts of interest
The authors declare that they have no known conflict or competing financial interests that could have appeared to influence the work which is reported.
Note added after first publication
This article replaces the version published on the 3rd of October 2023, which contained errors in the scheme layout.
Acknowledgements
DG thanks to CSIR for providing JRF fellowship (Grant No. 09/119(0217)2019EMR-I), KSR thanks to Indian Science congress for providing Asutosh Mookerjee fellowship (Grant No. 595/73/2020-21) and CSIR for Emeritus fellowship (Grant No. 21(1117)/20/EMR-II). We are also thankful to the Institution of Excellence (IOE), University of Mysore, Manasagangotri, Mysuru-570006, India, for the spectral data and Indian Institute of chemical technology (IICT), Hyderabad-500007, India for X-ray crystallographic data.
References
-
(a) S. Maddila, S. B. Jonnalagadda, K. K. Gangu and S. N. Maddila, Curr. Org. Synth., 2017, 14, 634–653 CrossRef CAS;
(b) S. Maddila, K. K. Gangu, S. N. Maddila and S. B. Jonnalagadda, Mol. Diversity, 2017, 21, 247–255 CrossRef CAS PubMed.
-
(a) H. Cho, F. Török and B. Török, Green Chem., 2014, 16, 3623–3634 RSC;
(b) E. Petruzzella, C. V. Chirosca, C. S. Heidenga and J. D. Hoeschele, Dalton Trans., 2015, 44, 3384–3392 RSC;
(c) A. Kokel, C. Schäfer and B. Török, Green Chem., 2017, 19, 3729–3751 RSC;
(d) A. Kulkarni and B. Török, Green Chem., 2010, 12, 875–878 RSC.
-
(a) C. Karthikeyan, R. Malla, C. R. Ashby Jr, H. Amawi, K. L. Abbott, J. Moore, J. Chen, C. Balch, C. Lee and P. C. Flannery, Cancer Lett., 2016, 376, 118–126 CrossRef CAS PubMed;
(b) S. T. Selvi, V. Nadaraj, S. Mohan, R. Sasi and M. Hema, Bioorg. Med. Chem., 2006, 14, 3896–3903 CrossRef CAS PubMed;
(c) A. M. Hussein, F. A. Abu-Shanab and E. A. Ishak, Phosphorus, Sulfur Silicon Relat. Elem., 2000, 159, 55–68 CrossRef CAS;
(d) A. R. Azevedo, V. F. Ferreira, H. de Mello, L. R. Leão-Ferreirab, A. V. Jabor, I. C. Frugulhetti, H. S. Pereira, N. Moussatche and A. M. R. Bernardino, Heterocycl. Commun., 2002, 8, 427–432 CAS;
(e) L. W. Mohamed, M. A. Shaaban, A. F. Zaher, S. M. Alhamaky and A. M. Elsahar, Bioorg. Chem., 2019, 83, 47–54 CrossRef CAS PubMed;
(f) A. L. Lourenço, R. R. Salvador, L. A. Silva, M. S. Saito, J. F. Mello, L. M. Cabral, C. R. Rodrigues, M. A. Vera, E. M. Muri and A. M. de Souza, Eur. J. Med. Chem., 2017, 135, 213–229 CrossRef PubMed;
(g) L. Li, W. Zhang, F. Lin, X. Lu, W. Chen, X. Li, X. Zhou, R. Su, L. Wang and Z. Zheng, Eur. J. Med. Chem., 2019, 173, 107–116 CrossRef CAS PubMed;
(h) C. Kurumurthy, B. Veeraswamy, P. S. Rao, G. S. Kumar, P. S. Rao, V. L. Reddy, J. V. Rao and B. Narsaiah, Bioorg. Med. Chem. Lett., 2014, 24, 746–749 CrossRef CAS PubMed;
(i) A. Cappelli, G. Bini, S. Valenti, G. Giuliani, M. Paolino, M. Anzini, S. Vomero, G. Giorgi, A. Giordani and L. P. Stasi, J. Med. Chem., 2011, 54, 7165–7175 CrossRef CAS PubMed.
-
(a) T. Umar, S. Shalini, M. K. Raza, S. Gusain, J. Kumar, P. Seth, M. Tiwari and N. Hoda, Eur. J. Med. Chem., 2019, 175, 2–19 CrossRef CAS PubMed;
(b) L.-Y. Zeng, T. Liu, J. Yang, Y. Yang, C. Cai and S. Liu, ACS Comb. Sci., 2017, 19, 437–446 CrossRef CAS PubMed.
- I. Kanwal, N. Rasool, S. H. M. Zaidi, Z. A. Zakaria, M. Bilal, M. A. Hashmi, A. Mubarik, G. Ahmad and S. A. A. Shah, Molecules, 2022, 27, 360 CrossRef CAS PubMed.
-
(a) I. V. Taydakov, A. A. Akkuzina, R. I. Avetisov, A. V. Khomyakov, R. R. Saifutyarov and I. C. Avetissov, J. Lumin., 2016, 177, 31–39 CrossRef CAS;
(b) G. Lewińska, K. Khachatryan, K. S. Danel, Z. Danel, J. Sanetra and K. W. Marszałek, Polymers, 2020, 12, 2707 CrossRef PubMed.
-
(a) M. García, I. Romero and J. Portilla, ACS Omega, 2019, 4, 6757–6768 CrossRef PubMed;
(b) J. Orrego-Hernández, C. Lizarazo, J. Cobo and J. Portilla, RSC Adv., 2019, 9, 27318–27323 RSC.
-
(a) J. Niziol, A. Danel, G. Boiteux, J. Davenas, B. Jarosz, A. Wisla and G. Seytre, Synth. Met., 2002, 127, 175–180 CrossRef CAS;
(b) E. Gondek, A. Danel, J. Nizio, P. Armatys, I. V. Kityk, P. Szlachcic, M. Karelus, T. Uchacz, J. Chwast and G. Lakshminarayana, J. Lumin., 2010, 130, 2093–2099 CrossRef CAS.
-
(a) P. Friedlaender, BerichteDtsch. Chem. Ges., 1882, 15, 2572–2575 CrossRef;
(b) J. Marco-Contelles, E. Pérez-Mayoral and A. Samadi, Chem. Rev., 2009, 109, 2652–2671 CrossRef CAS PubMed;
(c) C.-C. Cheng and S.-J. Yan, Org. React., 2004, 28, 37–201 Search PubMed;
(d) M. Fallah-Mehrjardi, Mini-Rev. Org. Chem., 2017, 14, 187–196 CAS;
(e) G. A. Ramann and B. J. Cowen, Molecules, 2016, 21, 986 CrossRef PubMed;
(f) L. M. Nainwal, S. Tasneem, W. Akhtar, G. Verma, M. F. Khan, S. Parvez, M. Shaquiquzzaman, M. Akhter and M. M. Alam, Eur. J. Med. Chem., 2019, 164, 121–170 CrossRef CAS PubMed.
-
(a) S. L. Wang, Y. P. Liu, B. H. Xu, X. H. Wang, B. Jiang and S. J. Tu, Tetrahedron, 2011, 67, 9417–9425 CrossRef CAS;
(b) J. Quiroga, A. Hormaza, B. Insuasty and M. Marquez, J. Heterocycl. Chem., 1998, 35, 409–412 CrossRef CAS;
(c) I. Drizin, R. J. Altenbach, S. A. Buckner, K. L. Whiteaker, V. E. Scott, J. F. Darbyshire, V. Jayanti, R. F. Henry, M. J. Coghlan and M. Gopalakrishnan, Bioorg. Med. Chem., 2004, 12, 1895–1904 CrossRef CAS PubMed;
(d) J. Quiroga, J. Portilla, H. Serrano, R. Abonia, B. Insuasty, M. Nogueras and J. Cobo, Tetrahedron Lett., 2007, 48, 1987–1990 CrossRef CAS.
-
(a) K. D. Shin, M.-Y. Lee, D.-S. Shin, S. Lee, K.-H. Son, S. Koh, Y.-K. Paik, B.-M. Kwon and D. C. Han, J. Biol. Chem., 2005, 280, 41439–41448 CrossRef CAS PubMed;
(b) J. P. Demers, W. E. Hageman, S. G. Johnson, D. H. Klaubert, R. A. Look and J. B. Moore, Bioorg. Med. Chem. Lett., 1994, 4, 2451–2456 CrossRef CAS;
(c) D. Simoni, M. Roberti, F. P. Invidiata, R. Rondanin, R. Baruchello, C. Malagutti, A. Mazzali, M. Rossi, S. Grimaudo and F. Capone, J. Med. Chem., 2001, 44, 2308–2318 CrossRef CAS PubMed.
-
(a) M. Rowley, H. B. Broughton, I. Collins, R. Baker, F. Emms, R. Marwood, S. Patel, S. Patel, C. I. Ragan and S. B. Freedman, J. Med. Chem., 1996, 39, 1943–1945 CrossRef CAS PubMed;
(b) S. J. Wittenberger, J. Org. Chem., 1996, 61, 356–358 CrossRef CAS;
(c) G. Dannhardt, P. Dominiak and S. Laufer, Arzneimittelforschung, 1993, 43, 441–444 CAS;
(d) T. Tatee, K. Narita, S. Kurashige, S. I. H. Miyazaki, H. Yamanaka, M. Mizugaki and T. S. H. Fukuda, Chem. Pharm. Bull., 1987, 35, 3676–3690 CrossRef CAS PubMed.
-
(a) M. Suzuki, H. Iwasaki, Y. Fujikawa, M. Sakashita, M. Kitahara and R. Sakoda, Bioorg. Med. Chem. Lett., 2001, 11, 1285–1288 CrossRef CAS PubMed;
(b) P. Pevarello, R. Amici, M. G. Brasca, M. Villa and M. Varasi, Syst., 1999, 3, 301–339 CAS;
(c) M. S. Chande, R. S. Verma, P. A. Barve, R. R. Khanwelkar, R. B. Vaidya and K. B. Ajaikumar, Eur. J. Med. Chem., 2005, 40, 1143–1148 CrossRef CAS PubMed;
(d) V. K. DC, B. S. Chethan, D. Gowda, K. S. Rangappa and N. K. Lokanath, J. Mol. Struct., 2023, 135770 Search PubMed.
-
(a) K. S. S. Kumar, T. R. Swaroop, K. B. Harsha, K. H. Narasimhamurthy and K. S. Rangappa, Tetrahedron Lett., 2012, 53, 5619–5623 CrossRef;
(b) K. B. Harsha and K. S. Rangappa, J. Chem. Sci., 2017, 129, 141 CrossRef;
(c) A. B. Ramesha, G. M. Raghavendra, K. N. Nandeesh, K. S. Rangappa and K. Mantelingu, Tetrahedron Lett., 2013, 54, 95–100 CrossRef CAS;
(d) G. M. Raghavendra, A. B. Ramesha, C. N. Revanna, K. N. Nandeesh, K. Mantelingu and K. S. Rangappa, Tetrahedron Lett., 2011, 52, 5571–5574 CrossRef CAS;
(e) K. Vinaya, P. D. Shivaramu, G. K. Chandrashekara, S. Chandrappa and K. S. Rangappa, Lett. Org. Chem., 2018, 15, 241–245 CrossRef CAS.
- M. G. Sharma, R. M. Vala and H. M. Patel, RSC Adv., 2020, 10, 35499–35504 RSC.
- D. M. Patel, M. G. Sharma, R. M. Vala, I. Lagunes, A. Puerta, J. M. Padrón, D. P. Rajani and H. M. Patel, Bioorg. Chem., 2019, 86, 137–150 CrossRef CAS PubMed.
- E. A. Muravyova, V. V. Tkachenko, S. M. Desenko, Y. V. Sen’ko, T. J. Müller, E. V. Vashchenko and V. A. Chebanov, Arkivoc, 2013, 2013, 338–371 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2267726. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra05235d |
| ‡ Authors contributed equally. |
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *d
*d
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, followed by the addition of 1 equivalent T3P® (50% in EtOAc) at RT to afford the desired pyrazoloquinoline in a 4% yield (Scheme 1). The same reaction was transferred from RT to MW irradiation at 90 °C for 30 min, yielding the desired pyrazoloquinoline in 24% yield. Further, varying different equivalents of T3P® could affect the course of the reaction; the best result was obtained by choosing 2.5 equivalents of T3P® and keeping the temperature at 90 °C for 30 min, which afforded the desired compound at around 90% yield. To determine the precise experimental parameter, the same reaction was carried out by varying the time and temperature while keeping the equivalent of T3P® (2.5 eq.) constant. Notably, the product yield was not adversely affected. And also, by changing the solvent conditions, we could not find any further improvement in the desired product yield. The overall optimisation results are summarised in Table 1. Thus, a clear optimisation reaction condition to obtain desired pyrazolo quinolinone from dimedone and benzyl alcohol was revealed from our studies, which entailed employing 2.5 eq. of T3P® at 90 °C and a duration of 30 min in the presence of 1,4-dioxane as a solvent under MW irradiation. The product was isolated by initially washing the reaction mixture with water followed by brine, then purified by column chromatography using 60–120 silica gel. With the clear optimisation condition in our hands, we carried out a series of reactions with amino phenyl pyrazole with 1,3-dicarbonyl compounds and various electronically diversified alcohols in the presence of an optimised reaction condition to afford the desired pyrazolo quinolinone derivatives. 2a–g are summarised in Scheme 2. To increase the substrate scope, the titled reactions are carried out by changing substituents in the 1,3-dicarbonyl compound to afford the desired compounds. 3a–g are summarised under Scheme 3. Similarly, by choosing the methyl group on pyrazolo quinolinone, it reacts with methyl substituted 1,3-dicarbonyl compounds and various alcohols to afford the desired pyrazolo quinolinone derivatives (4a–i), and the results are summarised under Scheme 4. Further, to broaden the substrate scope even more, the same methyl substituted phenyl pyrazole amine reacts with various alcohols and 1,3-dicarbonyl compounds containing without substitution the desired pyrazolo quinolinone derivatives (5a–f), and the results are summarised in Scheme 5. The overall product yields of the pyrazolo quinolinone derivatives were moderate to good. In all circumstances, the substitution that is present in the alcohols has a significant impact on the overall product yield. In Schemes 2 and 3, alcohols without substitution and alcohols containing halogens are the substituents, which comparatively give higher yields than alcohols containing electron-donating groups, followed by heterocyclic and cyclohexyl alcohols. Similarly, in Schemes 4 and 5, alcohols containing without substitution and alcohols containing halogens, particularly chloro substitution, comparatively gives equal and higher yield than the other categories of alcohols. But electron withdrawing groups other than hallogens like –CF3 affords poor yield compared to other substituents present on the alcohols as mentioned in the case of 2e and 4f.
1, followed by the addition of 1 equivalent T3P® (50% in EtOAc) at RT to afford the desired pyrazoloquinoline in a 4% yield (Scheme 1). The same reaction was transferred from RT to MW irradiation at 90 °C for 30 min, yielding the desired pyrazoloquinoline in 24% yield. Further, varying different equivalents of T3P® could affect the course of the reaction; the best result was obtained by choosing 2.5 equivalents of T3P® and keeping the temperature at 90 °C for 30 min, which afforded the desired compound at around 90% yield. To determine the precise experimental parameter, the same reaction was carried out by varying the time and temperature while keeping the equivalent of T3P® (2.5 eq.) constant. Notably, the product yield was not adversely affected. And also, by changing the solvent conditions, we could not find any further improvement in the desired product yield. The overall optimisation results are summarised in Table 1. Thus, a clear optimisation reaction condition to obtain desired pyrazolo quinolinone from dimedone and benzyl alcohol was revealed from our studies, which entailed employing 2.5 eq. of T3P® at 90 °C and a duration of 30 min in the presence of 1,4-dioxane as a solvent under MW irradiation. The product was isolated by initially washing the reaction mixture with water followed by brine, then purified by column chromatography using 60–120 silica gel. With the clear optimisation condition in our hands, we carried out a series of reactions with amino phenyl pyrazole with 1,3-dicarbonyl compounds and various electronically diversified alcohols in the presence of an optimised reaction condition to afford the desired pyrazolo quinolinone derivatives. 2a–g are summarised in Scheme 2. To increase the substrate scope, the titled reactions are carried out by changing substituents in the 1,3-dicarbonyl compound to afford the desired compounds. 3a–g are summarised under Scheme 3. Similarly, by choosing the methyl group on pyrazolo quinolinone, it reacts with methyl substituted 1,3-dicarbonyl compounds and various alcohols to afford the desired pyrazolo quinolinone derivatives (4a–i), and the results are summarised under Scheme 4. Further, to broaden the substrate scope even more, the same methyl substituted phenyl pyrazole amine reacts with various alcohols and 1,3-dicarbonyl compounds containing without substitution the desired pyrazolo quinolinone derivatives (5a–f), and the results are summarised in Scheme 5. The overall product yields of the pyrazolo quinolinone derivatives were moderate to good. In all circumstances, the substitution that is present in the alcohols has a significant impact on the overall product yield. In Schemes 2 and 3, alcohols without substitution and alcohols containing halogens are the substituents, which comparatively give higher yields than alcohols containing electron-donating groups, followed by heterocyclic and cyclohexyl alcohols. Similarly, in Schemes 4 and 5, alcohols containing without substitution and alcohols containing halogens, particularly chloro substitution, comparatively gives equal and higher yield than the other categories of alcohols. But electron withdrawing groups other than hallogens like –CF3 affords poor yield compared to other substituents present on the alcohols as mentioned in the case of 2e and 4f.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1,4-dioxane
1 (1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO) volume ratio, 1mmol of 1a, 1mmol of 1c and 1.2 mmol of alcohol in case of 2a.
b T3P® (50% solution in EtOAc) was used to carry out the reactions.
c Isolated yield after purified by column chromatography.
DMSO) volume ratio, 1mmol of 1a, 1mmol of 1c and 1.2 mmol of alcohol in case of 2a.
b T3P® (50% solution in EtOAc) was used to carry out the reactions.
c Isolated yield after purified by column chromatography.







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 138–142 °C; IR (KBr): 3236, 3055, 2956, 1578, 1549, 1247, 767 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (d, J = 4.4 Hz, 4H), 7.39–7.36 (m, 1H), 7.27–7.21 (m, 5H), 7.13–7.11 (m, 1H), 6.53 (s, 1H), 5.22 (s, 1H), 2.36 (s, 2H), 2.19 (d, J = 1.6 Hz, 2H), 1.06 (s, 3H), 1.03 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.0, 147.2, 138.7, 138.0, 135.0, 129.9, 128.2, 127.3, 127.3, 126.0, 122.9, 111.5, 106.5, 50.8, 42.5, 36.2, 32.5, 28.9, 27.4; HRMS (ESI) [M + H]+ calculated C24H23N3O 370.1919 found 370.1923.
40 v/v); MP: 138–142 °C; IR (KBr): 3236, 3055, 2956, 1578, 1549, 1247, 767 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (d, J = 4.4 Hz, 4H), 7.39–7.36 (m, 1H), 7.27–7.21 (m, 5H), 7.13–7.11 (m, 1H), 6.53 (s, 1H), 5.22 (s, 1H), 2.36 (s, 2H), 2.19 (d, J = 1.6 Hz, 2H), 1.06 (s, 3H), 1.03 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.0, 147.2, 138.7, 138.0, 135.0, 129.9, 128.2, 127.3, 127.3, 126.0, 122.9, 111.5, 106.5, 50.8, 42.5, 36.2, 32.5, 28.9, 27.4; HRMS (ESI) [M + H]+ calculated C24H23N3O 370.1919 found 370.1923.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 142–146 °C; IR (KBr): 3225, 3028, 2963, 1569, 1541, 1253, 775 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.45 (m, 4H), 7.37–7.34 (m, 1H), 7.27 (d, J = 6.0 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 7.04 (t, J = 7.4 Hz, 2H), 6.93 (d, J = 7.6 Hz, 1H), 6.70 (s, 1H), 5.14 (s, 1H), 2.34 (d, J = 6.8 Hz, 2H), 2.28 (s, 3H), 2.18 (s, 2H), 1.048 (s, 6H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.3, 147.2, 138.7, 138.0, 137.6, 135.1, 129.9, 128.1, 128.0, 127.6, 126.8, 124.3, 123.0, 111.4, 106.6, 50.8, 42.4, 36.1, 32.5, 29.0, 27.5, 21.5; HRMS (ESI) [M + H]+ calculated C25H25N3O 384.2076 found 384.2079.
40 v/v); MP: 142–146 °C; IR (KBr): 3225, 3028, 2963, 1569, 1541, 1253, 775 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.45 (m, 4H), 7.37–7.34 (m, 1H), 7.27 (d, J = 6.0 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 7.04 (t, J = 7.4 Hz, 2H), 6.93 (d, J = 7.6 Hz, 1H), 6.70 (s, 1H), 5.14 (s, 1H), 2.34 (d, J = 6.8 Hz, 2H), 2.28 (s, 3H), 2.18 (s, 2H), 1.048 (s, 6H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.3, 147.2, 138.7, 138.0, 137.6, 135.1, 129.9, 128.1, 128.0, 127.6, 126.8, 124.3, 123.0, 111.4, 106.6, 50.8, 42.4, 36.1, 32.5, 29.0, 27.5, 21.5; HRMS (ESI) [M + H]+ calculated C25H25N3O 384.2076 found 384.2079.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 197–201 °C; IR (KBr): 3231, 3120, 2958, 1575, 1541, 1256, 791 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (s, 4H), 7.35 (d, J = 7.6 Hz, 3H), 7.25 (d, J = 4.4 Hz, 1H), 7.14 (d, J = 7.6 Hz, 2H), 6.63 (s, 1H), 5.18 (s, 1H), 2.36 (s, 2H), 2.19 (d, J = 2.0 Hz, 2H), 1.05 (s, 3H), 1.01 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.2, 146.2, 138.6, 137.9, 135.1, 131.2, 129.9, 129.2, 127.8, 123.0, 119.8, 111.1, 105.9, 50.8, 42.5, 35.8, 32.5, 28.9, 27.4; HRMS (ESI) [M + H]+ calculated C24H22BrN3O 448.1024 found 448.1021.
40 v/v); MP: 197–201 °C; IR (KBr): 3231, 3120, 2958, 1575, 1541, 1256, 791 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (s, 4H), 7.35 (d, J = 7.6 Hz, 3H), 7.25 (d, J = 4.4 Hz, 1H), 7.14 (d, J = 7.6 Hz, 2H), 6.63 (s, 1H), 5.18 (s, 1H), 2.36 (s, 2H), 2.19 (d, J = 2.0 Hz, 2H), 1.05 (s, 3H), 1.01 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 149.2, 146.2, 138.6, 137.9, 135.1, 131.2, 129.9, 129.2, 127.8, 123.0, 119.8, 111.1, 105.9, 50.8, 42.5, 35.8, 32.5, 28.9, 27.4; HRMS (ESI) [M + H]+ calculated C24H22BrN3O 448.1024 found 448.1021.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 194–198 °C; IR (KBr): 3254, 3070, 2954, 1572, 1545, 1251, 771 cm−1; 1H NMR (CDCl3, 400 MHz): 7.57–7.53 (m, 6H), 7.44–7.37 (m, 2H), 7.17 (d, J = 8.0 Hz, 2H), 6.53 (s, 1H), 5.22 (s, 1H), 2.41 (s, 2H), 2.24 (d, J = 3.2 Hz, 2H), 1.09 (s, 3H), 1.05 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.8, 150.8, 139.6, 137.9, 136.7, 129.9, 129.6, 123.0, 122.6, 121.2, 110.6, 102.9, 50.9, 42.3, 35.5, 32.3, 29.2, 27.3; HRMS (ESI) [M + H]+ calculated C24H22ClN3O 404.1530 found 404.1525.
40 v/v); MP: 194–198 °C; IR (KBr): 3254, 3070, 2954, 1572, 1545, 1251, 771 cm−1; 1H NMR (CDCl3, 400 MHz): 7.57–7.53 (m, 6H), 7.44–7.37 (m, 2H), 7.17 (d, J = 8.0 Hz, 2H), 6.53 (s, 1H), 5.22 (s, 1H), 2.41 (s, 2H), 2.24 (d, J = 3.2 Hz, 2H), 1.09 (s, 3H), 1.05 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.8, 150.8, 139.6, 137.9, 136.7, 129.9, 129.6, 123.0, 122.6, 121.2, 110.6, 102.9, 50.9, 42.3, 35.5, 32.3, 29.2, 27.3; HRMS (ESI) [M + H]+ calculated C24H22ClN3O 404.1530 found 404.1525.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 136–140 °C; IR (KBr): 3436, 2868, 1653, 1589, 1375, 832, 695 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.35 (m, 6H), 6.41 (s, 1H), 4.01 (s, 1H), 2.30–2.14 (m, 2H), 1.74–1.44 (m, 5H), 1.24–0.87 (m, 14H); 13C NMR (CDCl3, 100 MHz): 195.8, 150.6, 139.3, 138.0, 136.7, 129.9, 127.6, 122.9, 110.8, 103.0, 51.0, 43.1, 42.4, 35.7, 32.4, 30.9, 29.3, 27.4, 27.3, 26.5, 26.4, 26.2; HRMS (ESI) [M + H]+ calculated C24H29N3O 376.2389 found 376.2394.
40 v/v); MP: 136–140 °C; IR (KBr): 3436, 2868, 1653, 1589, 1375, 832, 695 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.35 (m, 6H), 6.41 (s, 1H), 4.01 (s, 1H), 2.30–2.14 (m, 2H), 1.74–1.44 (m, 5H), 1.24–0.87 (m, 14H); 13C NMR (CDCl3, 100 MHz): 195.8, 150.6, 139.3, 138.0, 136.7, 129.9, 127.6, 122.9, 110.8, 103.0, 51.0, 43.1, 42.4, 35.7, 32.4, 30.9, 29.3, 27.4, 27.3, 26.5, 26.4, 26.2; HRMS (ESI) [M + H]+ calculated C24H29N3O 376.2389 found 376.2394.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 162–166 °C; IR (KBr): 3430, 2935, 1663, 1557, 1392, 1327, 695 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49–7.43 (m, 6H), 7.24 (s, 1H), 6.12 (d, J = 3.6 Hz, 1H), 5.98 (d, J = 3.2 Hz, 1H), 5.32 (s, 1H), 2.37 (s, 2H), 2.25 (d, J = 3.2 Hz, 2H), 1.09 (s, 3H), 1.06 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 159.6, 150.2, 138.7, 137.8, 135.2, 129.9, 123.1, 119.2, 111.9, 107.5, 103.1, 50.7, 45.4, 42.5, 32.5, 30.2, 30.1, 27.2; HRMS (ESI) [M + H]+ calculated C22H20BrN3O2 438.0817 found 438.0820.
40 v/v); MP: 162–166 °C; IR (KBr): 3430, 2935, 1663, 1557, 1392, 1327, 695 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49–7.43 (m, 6H), 7.24 (s, 1H), 6.12 (d, J = 3.6 Hz, 1H), 5.98 (d, J = 3.2 Hz, 1H), 5.32 (s, 1H), 2.37 (s, 2H), 2.25 (d, J = 3.2 Hz, 2H), 1.09 (s, 3H), 1.06 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 159.6, 150.2, 138.7, 137.8, 135.2, 129.9, 123.1, 119.2, 111.9, 107.5, 103.1, 50.7, 45.4, 42.5, 32.5, 30.2, 30.1, 27.2; HRMS (ESI) [M + H]+ calculated C22H20BrN3O2 438.0817 found 438.0820.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 128–132 °C; IR (KBr): 3243, 3058, 2963, 1563, 1549, 1248, 768 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.42 (m, 5H), 7.37–7.33 (m, 1H), 7.28–7.24 (m, 4H), 7.16–7.12 (m, 1H), 7.11 (s, 1H), 5.24 (s, 1H), 2.49–2.43 (m, 2H), 2.29–2.22 (m, 2H), 1.99–1.92 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 151.5, 147.3, 138.6, 137.9, 135.1, 129.8, 128.2, 127.6, 127.3, 126.0, 123.1, 112.3, 106.5, 37.1, 36.1, 28.7, 21.2; HRMS (ESI) [M + H]+ calculated C22H19N3O 342.1606 found 342.1612.
40 v/v); MP: 128–132 °C; IR (KBr): 3243, 3058, 2963, 1563, 1549, 1248, 768 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.42 (m, 5H), 7.37–7.33 (m, 1H), 7.28–7.24 (m, 4H), 7.16–7.12 (m, 1H), 7.11 (s, 1H), 5.24 (s, 1H), 2.49–2.43 (m, 2H), 2.29–2.22 (m, 2H), 1.99–1.92 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 151.5, 147.3, 138.6, 137.9, 135.1, 129.8, 128.2, 127.6, 127.3, 126.0, 123.1, 112.3, 106.5, 37.1, 36.1, 28.7, 21.2; HRMS (ESI) [M + H]+ calculated C22H19N3O 342.1606 found 342.1612.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 158–162 °C; IR (KBr): 3238, 3016, 2964, 1568, 1556, 1239, 772 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.47 (m, 5H), 7.37–7.25 (m, 2H), 7.13–7.07 (m, 3H), 6.71 (s, 1H), 5.22 (s, 1H), 2.34–2.29 (m, 6H), 1.99–1.98 (m, 3H); 13C NMR (CDCl3, 100 MHz): 195.6, 147.2, 138.7, 138.1, 138.0, 137.6, 135.0, 129.9, 128.9, 128.5, 128.3, 128.1, 128.0, 127.7, 126.9, 124.8, 124.4, 123.0, 121.9, 112.6, 106.6, 87.4, 37.1, 35.9, 28.9, 21.5; HRMS (ESI) [M + H]+ calculated C23H21N3O 356.1763 found 356.1758.
40 v/v); MP: 158–162 °C; IR (KBr): 3238, 3016, 2964, 1568, 1556, 1239, 772 cm−1; 1H NMR (CDCl3, 400 MHz): 7.48–7.47 (m, 5H), 7.37–7.25 (m, 2H), 7.13–7.07 (m, 3H), 6.71 (s, 1H), 5.22 (s, 1H), 2.34–2.29 (m, 6H), 1.99–1.98 (m, 3H); 13C NMR (CDCl3, 100 MHz): 195.6, 147.2, 138.7, 138.1, 138.0, 137.6, 135.0, 129.9, 128.9, 128.5, 128.3, 128.1, 128.0, 127.7, 126.9, 124.8, 124.4, 123.0, 121.9, 112.6, 106.6, 87.4, 37.1, 35.9, 28.9, 21.5; HRMS (ESI) [M + H]+ calculated C23H21N3O 356.1763 found 356.1758.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 144–148 °C; IR (KBr): 3228, 3051, 2965, 1569, 1548, 1245, 771 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.24 (m, 9H), 7.20–7.16 (m, 2H), 5.21 (S, 1H), 2.53–2.2.41 (m, 2H), 2.25–2.19 (m, 2H), 1.94–1.87 (m, 2H), 1.25 (s, 9H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.7, 148.5, 144.2, 138.6, 138.0, 135.2, 129.7, 127.6, 127.3, 126.8, 125.5, 125.1, 123.1, 121.9, 112.3, 106.6, 37.1, 35.3, 34.2, 31.3, 28.6, 21.1; HRMS (ESI) [M + H]+ calculated C26H27N3O 398.2232 found 398.2238.
40 v/v); MP: 144–148 °C; IR (KBr): 3228, 3051, 2965, 1569, 1548, 1245, 771 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.24 (m, 9H), 7.20–7.16 (m, 2H), 5.21 (S, 1H), 2.53–2.2.41 (m, 2H), 2.25–2.19 (m, 2H), 1.94–1.87 (m, 2H), 1.25 (s, 9H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.7, 148.5, 144.2, 138.6, 138.0, 135.2, 129.7, 127.6, 127.3, 126.8, 125.5, 125.1, 123.1, 121.9, 112.3, 106.6, 37.1, 35.3, 34.2, 31.3, 28.6, 21.1; HRMS (ESI) [M + H]+ calculated C26H27N3O 398.2232 found 398.2238.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 163–167 °C; IR (KBr): 3248, 3076, 2973, 1580, 1556, 1254, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.44–7.38 (m, 4H), 7.33–7.24 (m, 3H), 7.17 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 5.16 (s, 1H), 3.72 (s, 3H), 2.53–2.40 (m, 2H), 2.25–2.18 (m, 2H), 1.90 (t, J = 6.0 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.8, 157.7, 151.5, 139.8, 138.6, 138.0, 135.2, 129.7, 128.3, 127.6, 123.1, 113.5, 112.5, 106.6, 55.1, 37.1, 35.1, 28.6, 21.1; HRMS (ESI) [M + H]+ calculated C23H21N3O2 372.1712 found 372.1715.
40 v/v); MP: 163–167 °C; IR (KBr): 3248, 3076, 2973, 1580, 1556, 1254, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.44–7.38 (m, 4H), 7.33–7.24 (m, 3H), 7.17 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 5.16 (s, 1H), 3.72 (s, 3H), 2.53–2.40 (m, 2H), 2.25–2.18 (m, 2H), 1.90 (t, J = 6.0 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.8, 157.7, 151.5, 139.8, 138.6, 138.0, 135.2, 129.7, 128.3, 127.6, 123.1, 113.5, 112.5, 106.6, 55.1, 37.1, 35.1, 28.6, 21.1; HRMS (ESI) [M + H]+ calculated C23H21N3O2 372.1712 found 372.1715.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 212–216 °C; IR (KBr): 3239, 3115, 2948, 1568, 1575, 1256, 776 cm−1; 1H NMR (CDCl3, 400 MHz): 7.53–7.43 (m, 5H), 7.37 (d, J = 8 Hz, 3H), 7.16 (d, J = 8.0 Hz, 2H), 6.76 (s, 1H), 5.23 (s, 1H), 2.55–2.50 (m, 2H), 2.40–2.29 (m, 2H), 2.02–1.98 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.5, 151.1, 146.2, 138.6, 137.9, 135.0, 131.2, 129.5, 128.3, 123.0, 119.8, 116.0, 112.2, 105.8, 105.8, 37.1, 35.7, 28.8, 21.1; HRMS (ESI) [M + H]+ calculated C22H18BrN3O 421.3098 found 421.3091.
40 v/v); MP: 212–216 °C; IR (KBr): 3239, 3115, 2948, 1568, 1575, 1256, 776 cm−1; 1H NMR (CDCl3, 400 MHz): 7.53–7.43 (m, 5H), 7.37 (d, J = 8 Hz, 3H), 7.16 (d, J = 8.0 Hz, 2H), 6.76 (s, 1H), 5.23 (s, 1H), 2.55–2.50 (m, 2H), 2.40–2.29 (m, 2H), 2.02–1.98 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.5, 151.1, 146.2, 138.6, 137.9, 135.0, 131.2, 129.5, 128.3, 123.0, 119.8, 116.0, 112.2, 105.8, 105.8, 37.1, 35.7, 28.8, 21.1; HRMS (ESI) [M + H]+ calculated C22H18BrN3O 421.3098 found 421.3091.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 200–204 °C; IR (KBr): 3254, 3071, 2953, 1572, 1546, 1256, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.54–7.48 (m, 5H), 7.42–7.25 (m, 6H), 6.58 (s, 1H), 5.25 (s, 1H), 2.54–2.52 (m, 2H), 2.38–2.31 (m, 2H), 2.03–1.98 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.5, 150.8, 145.6, 138.6, 137.9, 130.0, 128.8, 128.3, 127.9, 123.0, 112.4, 105.9, 37.1, 35.6, 28.9, 21.183; HRMS (ESI) [M + H]+ calculated C22H18ClN3O 376.1217 found 376.1224.
40 v/v); MP: 200–204 °C; IR (KBr): 3254, 3071, 2953, 1572, 1546, 1256, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.54–7.48 (m, 5H), 7.42–7.25 (m, 6H), 6.58 (s, 1H), 5.25 (s, 1H), 2.54–2.52 (m, 2H), 2.38–2.31 (m, 2H), 2.03–1.98 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.5, 150.8, 145.6, 138.6, 137.9, 130.0, 128.8, 128.3, 127.9, 123.0, 112.4, 105.9, 37.1, 35.6, 28.9, 21.183; HRMS (ESI) [M + H]+ calculated C22H18ClN3O 376.1217 found 376.1224.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 132–136 °C; IR (KBr): 3238, 3048, 2964, 1575, 1543, 1249, 773 cm−1; 1H NMR (CDCl3, 400 MHz): 7.40–7.27 (m, 6H), 6.64 (s, 1H), 3.95 (s, 1H), 2.39–2.38 (m, 4H), 1.92 (s, 2H), 1.66–1.1.49 (m, 7H), 1.14–1.12 (m, 4H); 13C NMR (CDCl3, 100 MHz): 196.2, 152.6, 139.2, 137.9, 136.6, 129.8, 127.9, 122.9, 111.7, 103.0, 42.9, 37.3, 35.6, 35.4, 30.7, 28.7, 27.0, 26.4, 26.4, 26.2, 21.2; HRMS (ESI) [M + H]+ calculated C22H25N3O 348.2076 found 348.2069.
40 v/v); MP: 132–136 °C; IR (KBr): 3238, 3048, 2964, 1575, 1543, 1249, 773 cm−1; 1H NMR (CDCl3, 400 MHz): 7.40–7.27 (m, 6H), 6.64 (s, 1H), 3.95 (s, 1H), 2.39–2.38 (m, 4H), 1.92 (s, 2H), 1.66–1.1.49 (m, 7H), 1.14–1.12 (m, 4H); 13C NMR (CDCl3, 100 MHz): 196.2, 152.6, 139.2, 137.9, 136.6, 129.8, 127.9, 122.9, 111.7, 103.0, 42.9, 37.3, 35.6, 35.4, 30.7, 28.7, 27.0, 26.4, 26.4, 26.2, 21.2; HRMS (ESI) [M + H]+ calculated C22H25N3O 348.2076 found 348.2069.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 126–130 °C(lit. 128–132 °C);15 IR (KBr): 3231, 3062, 2958, 1585, 1543, 1256, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (d, J = 4.0 Hz, 4H), 7.36 (d, J = 4.4 Hz, 1H), 7.31–7.22 (m, 4H), 7.14 (d, J = 7.2 Hz, 1H), 6.58 (s, 1H), 5.15 (s, 1H), 2.37 (s, 2H), 2.192 (d, J = 6.8 Hz, 2H), 2.03 (s, 3H), 1.07 (s, 3H), 1.02 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 148.6, 147.6, 146.4, 138.0, 135.5, 129.8, 128.0, 127.9, 127.3, 125.9, 122.7, 112.1, 104.7, 65.8, 50.8, 42.5, 36.1, 32.5, 29.0, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C25H25N3O 384.2076 found 384.2079.
40 v/v); MP: 126–130 °C(lit. 128–132 °C);15 IR (KBr): 3231, 3062, 2958, 1585, 1543, 1256, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.49 (d, J = 4.0 Hz, 4H), 7.36 (d, J = 4.4 Hz, 1H), 7.31–7.22 (m, 4H), 7.14 (d, J = 7.2 Hz, 1H), 6.58 (s, 1H), 5.15 (s, 1H), 2.37 (s, 2H), 2.192 (d, J = 6.8 Hz, 2H), 2.03 (s, 3H), 1.07 (s, 3H), 1.02 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.2, 148.6, 147.6, 146.4, 138.0, 135.5, 129.8, 128.0, 127.9, 127.3, 125.9, 122.7, 112.1, 104.7, 65.8, 50.8, 42.5, 36.1, 32.5, 29.0, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C25H25N3O 384.2076 found 384.2079.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 196–200 °C (lit. 200–201 °C);15 IR (KBr): 3236, 3025, 2957, 1578, 1545, 1249, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.41 (m, 4H), 7.32 (d, J = 6.4 Hz, 1H), 7.10–7.04 (m, 3H), 6.92 (d, J = 7.2 Hz, 1H), 6.82 (s, 1H), 5.05 (s, 1H), 2.32 (d, J = 3.2 Hz, 2H), 2.28 (s, 3H), 2.15 (d, J = 5.6 Hz, 2H), 2.02 (s, 3H), 1.03 (S, 3H), 1.00 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 149.1, 147.5, 146.4, 138.0, 137.4, 135.5, 129.8, 128.5, 127.9, 127.2, 126.8, 124.9, 122.8, 111.9, 104.8, 50.8, 42.3, 36.1, 32.5, 29.0, 27.3, 21.5, 12.2; HRMS (ESI) [M + H]+ calculated C26H27N3O 398.2232 found 398.2228.
40 v/v); MP: 196–200 °C (lit. 200–201 °C);15 IR (KBr): 3236, 3025, 2957, 1578, 1545, 1249, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.41 (m, 4H), 7.32 (d, J = 6.4 Hz, 1H), 7.10–7.04 (m, 3H), 6.92 (d, J = 7.2 Hz, 1H), 6.82 (s, 1H), 5.05 (s, 1H), 2.32 (d, J = 3.2 Hz, 2H), 2.28 (s, 3H), 2.15 (d, J = 5.6 Hz, 2H), 2.02 (s, 3H), 1.03 (S, 3H), 1.00 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 149.1, 147.5, 146.4, 138.0, 137.4, 135.5, 129.8, 128.5, 127.9, 127.2, 126.8, 124.9, 122.8, 111.9, 104.8, 50.8, 42.3, 36.1, 32.5, 29.0, 27.3, 21.5, 12.2; HRMS (ESI) [M + H]+ calculated C26H27N3O 398.2232 found 398.2228.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 170–174 °C (lit. 166–170 °C);15 IR (KBr): 3237, 3060, 2958, 1596, 1541, 1248, 761 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47 (d, J = 4.4 Hz, 4H), 7.34 (t, J = 4.4 Hz, 1H), 7.19 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 6.53 (s, 1H), 5.08 (s, 1H), 3.74 (s, 3H), 2.34 (s, 2H), 2.17 (d, J = 5.2 Hz, 2H), 2.01 (s, 3H), 1.04 (s, 3H), 0.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 157.7, 148.4, 147.6, 138.9, 138.0, 135.5, 129.8, 128.8, 127.3, 122.7, 113.4, 112.3, 104.8, 55.1, 50.8, 42.5, 35.2, 32.5, 28.9, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C26H27N3O2414.2182 found 414.2185.
40 v/v); MP: 170–174 °C (lit. 166–170 °C);15 IR (KBr): 3237, 3060, 2958, 1596, 1541, 1248, 761 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47 (d, J = 4.4 Hz, 4H), 7.34 (t, J = 4.4 Hz, 1H), 7.19 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 6.53 (s, 1H), 5.08 (s, 1H), 3.74 (s, 3H), 2.34 (s, 2H), 2.17 (d, J = 5.2 Hz, 2H), 2.01 (s, 3H), 1.04 (s, 3H), 0.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 157.7, 148.4, 147.6, 138.9, 138.0, 135.5, 129.8, 128.8, 127.3, 122.7, 113.4, 112.3, 104.8, 55.1, 50.8, 42.5, 35.2, 32.5, 28.9, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C26H27N3O2414.2182 found 414.2185.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 208–212 °C (lit. 206 – 210 °C);15 IR (KBr): 3228, 3121, 2950, 1571, 1543, 1251, 786 cm−1; 1H NMR (CDCl3, 400 MHz): 7.51–7.46 (m, 5H), 7.35 (t, J = 7.0 Hz, 2H), 7.16–7.10 (m, 2H), 6.65 (s, 1H), 5.09 (s, 1H), 2.34 (s, 2H), 2.22–2.11 (m, 2H), 1.98 (s, 3H), 1.04 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 148.9, 147.5, 145.5, 137.9, 135.5, 131.1, 129.9, 129.7, 129.0, 128.9, 127.7, 126.0, 121.1, 119.7, 50.7, 42.4, 35.7, 32.5, 29.0, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C25H24BrN3O462.1181 found 462.1175.
40 v/v); MP: 208–212 °C (lit. 206 – 210 °C);15 IR (KBr): 3228, 3121, 2950, 1571, 1543, 1251, 786 cm−1; 1H NMR (CDCl3, 400 MHz): 7.51–7.46 (m, 5H), 7.35 (t, J = 7.0 Hz, 2H), 7.16–7.10 (m, 2H), 6.65 (s, 1H), 5.09 (s, 1H), 2.34 (s, 2H), 2.22–2.11 (m, 2H), 1.98 (s, 3H), 1.04 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 148.9, 147.5, 145.5, 137.9, 135.5, 131.1, 129.9, 129.7, 129.0, 128.9, 127.7, 126.0, 121.1, 119.7, 50.7, 42.4, 35.7, 32.5, 29.0, 27.3, 12.1; HRMS (ESI) [M + H]+ calculated C25H24BrN3O462.1181 found 462.1175.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 210–214 °C (lit. 212–216 °C);15 IR (KBr): 3258, 3073, 2952, 1573, 1547, 1255, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.51–7.45 (m, 4H), 7.36 (t, J = 3.2 Hz, 1H), 7.25–7.17 (m, 4H), 6.48 (s, 1H), 5.11 (s, 1H), 2.35 (d, J = 2.4 Hz, 2H), 2.17 (d, J = 8.4 Hz, 2H), 1.98 (s, 3H), 1.05 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 149.3, 147.4, 145.0, 137.9, 135.6, 131.5, 129.8, 129.3, 128.2, 127.4, 122.9, 111.4, 104.2, 50.7, 42.2, 35.7, 35.6, 32.4, 29.0, 27.2, 12.1; HRMS (ESI) [M + H]+ calculated C25H24ClN3O 418.1686 found 418.1682.
40 v/v); MP: 210–214 °C (lit. 212–216 °C);15 IR (KBr): 3258, 3073, 2952, 1573, 1547, 1255, 770 cm−1; 1H NMR (CDCl3, 400 MHz): 7.51–7.45 (m, 4H), 7.36 (t, J = 3.2 Hz, 1H), 7.25–7.17 (m, 4H), 6.48 (s, 1H), 5.11 (s, 1H), 2.35 (d, J = 2.4 Hz, 2H), 2.17 (d, J = 8.4 Hz, 2H), 1.98 (s, 3H), 1.05 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.3, 149.3, 147.4, 145.0, 137.9, 135.6, 131.5, 129.8, 129.3, 128.2, 127.4, 122.9, 111.4, 104.2, 50.7, 42.2, 35.7, 35.6, 32.4, 29.0, 27.2, 12.1; HRMS (ESI) [M + H]+ calculated C25H24ClN3O 418.1686 found 418.1682.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 168–172 °C; IR (KBr): 3435, 2928, 1643, 1504, 1385, 1065, 694 cm−1; 1H NMR (CDCl3, 400 MHz): 9.17 (broad s, 1H), 7.53–7.35 (m, 5H), 3.87 (s, 1H), 2.44 (s, 2H), 2.20–2.09 (m, 5H), 1.67–1.53 (m, 5H), 1.34–1.11 (m, 1H), 1.10–0.96 (m, 10H), 0.84–0.74 (m, 1H); 13C NMR (CDCl3, 100 MHz): 197.5, 162.6, 147.3, 137.4, 130.0, 129.1, 198.9, 122.0, 120.9, 116.5, 102.5, 52.2, 47.3, 32.9, 32.4, 28.2, 28.1, 27.4, 27.3, 26.5, 26.4, 12.2; HRMS (ESI) [M + H]+ calculated C25H31N3O 390.2545 found 390.2543.
40 v/v); MP: 168–172 °C; IR (KBr): 3435, 2928, 1643, 1504, 1385, 1065, 694 cm−1; 1H NMR (CDCl3, 400 MHz): 9.17 (broad s, 1H), 7.53–7.35 (m, 5H), 3.87 (s, 1H), 2.44 (s, 2H), 2.20–2.09 (m, 5H), 1.67–1.53 (m, 5H), 1.34–1.11 (m, 1H), 1.10–0.96 (m, 10H), 0.84–0.74 (m, 1H); 13C NMR (CDCl3, 100 MHz): 197.5, 162.6, 147.3, 137.4, 130.0, 129.1, 198.9, 122.0, 120.9, 116.5, 102.5, 52.2, 47.3, 32.9, 32.4, 28.2, 28.1, 27.4, 27.3, 26.5, 26.4, 12.2; HRMS (ESI) [M + H]+ calculated C25H31N3O 390.2545 found 390.2543.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 223–227 °C (lit. 226–229 °C);16 IR (KBr): 3439, 2846, 1648, 1514, 1373, 971, 695 cm−1; 1H NMR (DMSO-d6, 400 MHz): 9.54 (broad s, 1H), 7.55–7.41 (m, 4H), 7.39–7.38 (m, 1H), 7.21 (d, J = 4.4 Hz, 1H), 6.87–6.81 (m, 2H), 5.37 (s, 1H), 2.54–2.50 (m, 2H), 2.23–2.19 (m, 1H), 2.10–1.97 (m, 4H), 1.01 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 199.9, 155.5, 153.7, 151.8, 142.2, 140.0, 134.1, 130.8, 130.7, 127.8, 127.3, 108.4, 55.0, 46.5, 36.8, 34.9, 29.7, 27.8, 12.2; HRMS (ESI) [M + H]+ calculated C23H23N3OS 390.1640 found 390.1649.
40 v/v); MP: 223–227 °C (lit. 226–229 °C);16 IR (KBr): 3439, 2846, 1648, 1514, 1373, 971, 695 cm−1; 1H NMR (DMSO-d6, 400 MHz): 9.54 (broad s, 1H), 7.55–7.41 (m, 4H), 7.39–7.38 (m, 1H), 7.21 (d, J = 4.4 Hz, 1H), 6.87–6.81 (m, 2H), 5.37 (s, 1H), 2.54–2.50 (m, 2H), 2.23–2.19 (m, 1H), 2.10–1.97 (m, 4H), 1.01 (s, 3H), 0.98 (s, 3H); 13C NMR (CDCl3, 100 MHz): 199.9, 155.5, 153.7, 151.8, 142.2, 140.0, 134.1, 130.8, 130.7, 127.8, 127.3, 108.4, 55.0, 46.5, 36.8, 34.9, 29.7, 27.8, 12.2; HRMS (ESI) [M + H]+ calculated C23H23N3OS 390.1640 found 390.1649.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 154–158 °C; IR (KBr): 3463, 2945, 2868, 1645, 1524, 1375, 698 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42 (d, J = 4.0 Hz, 5H), 7.30–7.25 (m, 1H), 6.81 (s, 1H), 4.12–4.10 (m, 1H), 2.28 (s, 2H), 2.21 (s, 3H), 2.16 (d, J −5.6 Hz, 2H), 2.15–1.62 (m, 1H), 1.48 (t, J = 6.8 Hz, 1H), 1.35–1.12 (m, 2H), 1.11–1.08 (m, 2H), 1.06 (s, 3H), 1.01 (s, 3H), 0.80 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 195.9, 150.8, 147.0, 138.0, 136.6, 129.7, 128.9, 127.1, 122.7, 111.1, 103.9, 50.9, 42.2, 35.6, 32.3, 29.4, 29.3, 27.4, 27.1, 22.8, 14.1, 12.4; HRMS (ESI) [M + H]+ calculated C23H29N3O 364.2389 found364.2387.
40 v/v); MP: 154–158 °C; IR (KBr): 3463, 2945, 2868, 1645, 1524, 1375, 698 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42 (d, J = 4.0 Hz, 5H), 7.30–7.25 (m, 1H), 6.81 (s, 1H), 4.12–4.10 (m, 1H), 2.28 (s, 2H), 2.21 (s, 3H), 2.16 (d, J −5.6 Hz, 2H), 2.15–1.62 (m, 1H), 1.48 (t, J = 6.8 Hz, 1H), 1.35–1.12 (m, 2H), 1.11–1.08 (m, 2H), 1.06 (s, 3H), 1.01 (s, 3H), 0.80 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): 195.9, 150.8, 147.0, 138.0, 136.6, 129.7, 128.9, 127.1, 122.7, 111.1, 103.9, 50.9, 42.2, 35.6, 32.3, 29.4, 29.3, 27.4, 27.1, 22.8, 14.1, 12.4; HRMS (ESI) [M + H]+ calculated C23H29N3O 364.2389 found364.2387.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 172–176 °C; IR (KBr): 3433, 2861, 1651, 1575, 1378, 1056, 699 cm−1; 1H NMR (CDCl3, 400 MHz): 7.43–7.23 (m, 5H), 6.89 (s, 1H), 6.12 (d, J = 3.2 Hz, 1H), 6.01 (d, J = 2.8 Hz, 1H), 5.24 (s, 1H), 2.35 (s, 2H), 2.27–2.18 (m, 4H), 1.22 (s, 1H), 1.076 (s, 3H), 1.05 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.4, 163.3, 159.1, 150.6, 147.4, 138.8, 137.8, 135.8, 121.2, 120.9, 111.9, 107.6, 101.3, 53.8, 50.6, 48.3, 32.5, 28.2, 28.1, 12.3; HRMS (ESI) [M + H]+ calculated C23H22BrN3O2 452.0974 found 452.0979.
40 v/v); MP: 172–176 °C; IR (KBr): 3433, 2861, 1651, 1575, 1378, 1056, 699 cm−1; 1H NMR (CDCl3, 400 MHz): 7.43–7.23 (m, 5H), 6.89 (s, 1H), 6.12 (d, J = 3.2 Hz, 1H), 6.01 (d, J = 2.8 Hz, 1H), 5.24 (s, 1H), 2.35 (s, 2H), 2.27–2.18 (m, 4H), 1.22 (s, 1H), 1.076 (s, 3H), 1.05 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.4, 163.3, 159.1, 150.6, 147.4, 138.8, 137.8, 135.8, 121.2, 120.9, 111.9, 107.6, 101.3, 53.8, 50.6, 48.3, 32.5, 28.2, 28.1, 12.3; HRMS (ESI) [M + H]+ calculated C23H22BrN3O2 452.0974 found 452.0979.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 125–129 °C(lit.122–126 °C);15 IR (KBr): 3239, 3053, 2946, 1575, 1543, 1248, 762 cm−1; 1H NMR (CDCl3, 400 MHz): 7.43 (t, J = 5.2 Hz, 4H), 7.33–7.31 (m, 3H), 7.29–7.23 (m, 2H), 7.12 (t, 1H, 7 Hz), 6.90 (s, 1H), 5.14 (s, 1H), 2.50–2.44 (m, 2H), 2.28–2.22 (m, 2H), 2.00 (s, 3H), 1.93 (t, J = 4.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 150.8, 147.5, 146.6, 138.0, 135.5, 129.8, 128.1, 128.0, 127.2, 126.0, 122.8, 113.2, 104.6, 37.1, 36.0, 28.7, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C23H21N3O 356.1763 found 356.1759.
40 v/v); MP: 125–129 °C(lit.122–126 °C);15 IR (KBr): 3239, 3053, 2946, 1575, 1543, 1248, 762 cm−1; 1H NMR (CDCl3, 400 MHz): 7.43 (t, J = 5.2 Hz, 4H), 7.33–7.31 (m, 3H), 7.29–7.23 (m, 2H), 7.12 (t, 1H, 7 Hz), 6.90 (s, 1H), 5.14 (s, 1H), 2.50–2.44 (m, 2H), 2.28–2.22 (m, 2H), 2.00 (s, 3H), 1.93 (t, J = 4.8 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 150.8, 147.5, 146.6, 138.0, 135.5, 129.8, 128.1, 128.0, 127.2, 126.0, 122.8, 113.2, 104.6, 37.1, 36.0, 28.7, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C23H21N3O 356.1763 found 356.1759.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 188–192 °C; IR (KBr): 3234, 3018, 2963, 1565, 1529, 1236, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.34 (m, 5H), 7.27 (d, J = 7.2 Hz, 1H), 7.11–7.04 (m, 3H), 6.92 (d, J = 6.8 Hz, 1H), 5.06 (s, 1H), 2.47 (s, 1H), 2.39 (d, J = 6.8 Hz, 1H), 2.28 (s, 3H), 2.22–2.16 (m, 2H), 2.00 (s, 3H), 1.89 (d, J = 5.6 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.6, 147.4, 146.6, 138.0, 137.4, 135.6, 129.6, 128.6, 127.1, 126.8, 125.0, 123.0, 112.9, 104.7, 37.1, 36.0, 28.5, 21.5, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C24H23N3O 370.1919 found 370.1922.
40 v/v); MP: 188–192 °C; IR (KBr): 3234, 3018, 2963, 1565, 1529, 1236, 765 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.34 (m, 5H), 7.27 (d, J = 7.2 Hz, 1H), 7.11–7.04 (m, 3H), 6.92 (d, J = 6.8 Hz, 1H), 5.06 (s, 1H), 2.47 (s, 1H), 2.39 (d, J = 6.8 Hz, 1H), 2.28 (s, 3H), 2.22–2.16 (m, 2H), 2.00 (s, 3H), 1.89 (d, J = 5.6 Hz, 2H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.6, 147.4, 146.6, 138.0, 137.4, 135.6, 129.6, 128.6, 127.1, 126.8, 125.0, 123.0, 112.9, 104.7, 37.1, 36.0, 28.5, 21.5, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C24H23N3O 370.1919 found 370.1922.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 155–159 °C; IR (KBr): 3239, 3056, 2943, 1565, 1573, 1242, 769 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.35 (m, 4H), 7.29–7.23 (m, 4H), 7.197 (d, J = 8.8 Hz, 2H), 5.11 (s, 1H), 2.55–2.51 (m, 2H), 2.24–2.19 (m, 2H), 2.03 (s, 3H), 1.92 (t, J = 5.6 Hz, 2H), 1.27 (s, 9H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.3, 148.4, 147.4, 143.6, 138.0, 135.6, 129.6, 127.3, 127.1, 124.9, 122.9, 113.1, 104.9, 37.1, 35.4, 34.2, 31.3, 28.6, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C27H29N3O 412.2389 found 412.2381.
40 v/v); MP: 155–159 °C; IR (KBr): 3239, 3056, 2943, 1565, 1573, 1242, 769 cm−1; 1H NMR (CDCl3, 400 MHz): 7.42–7.35 (m, 4H), 7.29–7.23 (m, 4H), 7.197 (d, J = 8.8 Hz, 2H), 5.11 (s, 1H), 2.55–2.51 (m, 2H), 2.24–2.19 (m, 2H), 2.03 (s, 3H), 1.92 (t, J = 5.6 Hz, 2H), 1.27 (s, 9H); 13C NMR (CDCl3, 100 MHz): 195.8, 151.3, 148.4, 147.4, 143.6, 138.0, 135.6, 129.6, 127.3, 127.1, 124.9, 122.9, 113.1, 104.9, 37.1, 35.4, 34.2, 31.3, 28.6, 21.1, 12.2; HRMS (ESI) [M + H]+ calculated C27H29N3O 412.2389 found 412.2381.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 122–126 °C (lit. 124–128 °C);15 IR (KBr): 3234, 3055, 2952, 1580, 1541, 1253, 755 cm−1; 1H NMR (CDCl3, 400 MHz): 7.53–7.48 (m, 4H), 7.39–7.35 (m, 1H), 7.22 (t, J = 5.8 Hz, 2H), 6.78 (d, J = 8.8 Hz, 2H), 6.47 (s, 1H), 5.16 (s, 1H), 3.76 (s, 3H), 2.51 (t, J = 5.6 Hz, 2H), 2.40–2.31 (m, 2H), 2.02 (s, 3H), 1.98 (s, 2H); 13C NMR (CDCl3, 100 MHz): 190.9, 145.3, 142.9, 134.2, 133.3, 130.6, 125.1, 123.9, 123.7, 122.5, 118.0, 109.3, 109.3, 109.0, 108.6, 50.4, 32.4, 30.4, 24.1, 16.4, 7.4; HRMS (ESI) [M + H]+ calculated C24H23N3O2 386.1869 found 386.1864.
40 v/v); MP: 122–126 °C (lit. 124–128 °C);15 IR (KBr): 3234, 3055, 2952, 1580, 1541, 1253, 755 cm−1; 1H NMR (CDCl3, 400 MHz): 7.53–7.48 (m, 4H), 7.39–7.35 (m, 1H), 7.22 (t, J = 5.8 Hz, 2H), 6.78 (d, J = 8.8 Hz, 2H), 6.47 (s, 1H), 5.16 (s, 1H), 3.76 (s, 3H), 2.51 (t, J = 5.6 Hz, 2H), 2.40–2.31 (m, 2H), 2.02 (s, 3H), 1.98 (s, 2H); 13C NMR (CDCl3, 100 MHz): 190.9, 145.3, 142.9, 134.2, 133.3, 130.6, 125.1, 123.9, 123.7, 122.5, 118.0, 109.3, 109.3, 109.0, 108.6, 50.4, 32.4, 30.4, 24.1, 16.4, 7.4; HRMS (ESI) [M + H]+ calculated C24H23N3O2 386.1869 found 386.1864.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 202–206 °C (lit. 202–204 °C);15 IR (KBr): 3257, 3066, 2958, 1577, 1546, 1255, 745 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.44 (m, 4H), 7.37–7.33 (m, 1H), 7.25–7.18 (m, 4H), 6.71 (s, 1H), 5.13 (s, 1H), 2.50–2.46 (m, 2H), 2.31–2.26 (m, 2H), 1.98–1.91 (m, 5H); 13C NMR (CDCl3, 100 MHz): 195.5, 150.6, 147.4, 145.0, 137.8, 135.4, 131.6, 129.4, 128.1, 127.4, 122.8, 113.0, 104.1, 37.1, 35.6, 28.8, 21.1, 12.1; HRMS (ESI) [M + H]+ calculated C23H20ClN3O 390.1373 found 390.1378.
40 v/v); MP: 202–206 °C (lit. 202–204 °C);15 IR (KBr): 3257, 3066, 2958, 1577, 1546, 1255, 745 cm−1; 1H NMR (CDCl3, 400 MHz): 7.47–7.44 (m, 4H), 7.37–7.33 (m, 1H), 7.25–7.18 (m, 4H), 6.71 (s, 1H), 5.13 (s, 1H), 2.50–2.46 (m, 2H), 2.31–2.26 (m, 2H), 1.98–1.91 (m, 5H); 13C NMR (CDCl3, 100 MHz): 195.5, 150.6, 147.4, 145.0, 137.8, 135.4, 131.6, 129.4, 128.1, 127.4, 122.8, 113.0, 104.1, 37.1, 35.6, 28.8, 21.1, 12.1; HRMS (ESI) [M + H]+ calculated C23H20ClN3O 390.1373 found 390.1378.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 166–170 °C; IR (KBr): 3228, 3045, 2952, 1573, 1541, 1239, 772 cm−1; 1H NMR (CDCl3, 400 MHz): 7.41–7.38 (m, 5H), 7.31–7.30 (m, 1H), 6.12 (t, J = 3.2 Hz, 1H), 5.97 (d, J = 2.8 Hz, 1H), 5.25 (s, 1H), 2.48–2.28 (m, 3H), 2.17–2.15 (m, 4H), 1.97–1.95 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 164.5, 159.1, 152.1, 150.3, 135.7, 134.5, 122.0, 121.3, 121.2, 113.8, 112.7, 108.9, 101.3, 40.0, 34.6, 28.8, 21.6, 12.2; HRMS (ESI) [M + H]+ calculated C21H18BrN3O2 424.0661 found 424.0669.
40 v/v); MP: 166–170 °C; IR (KBr): 3228, 3045, 2952, 1573, 1541, 1239, 772 cm−1; 1H NMR (CDCl3, 400 MHz): 7.41–7.38 (m, 5H), 7.31–7.30 (m, 1H), 6.12 (t, J = 3.2 Hz, 1H), 5.97 (d, J = 2.8 Hz, 1H), 5.25 (s, 1H), 2.48–2.28 (m, 3H), 2.17–2.15 (m, 4H), 1.97–1.95 (m, 2H); 13C NMR (CDCl3, 100 MHz): 195.6, 164.5, 159.1, 152.1, 150.3, 135.7, 134.5, 122.0, 121.3, 121.2, 113.8, 112.7, 108.9, 101.3, 40.0, 34.6, 28.8, 21.6, 12.2; HRMS (ESI) [M + H]+ calculated C21H18BrN3O2 424.0661 found 424.0669.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 182–186 °C (lit. 183–184 °C);17 IR (KBr): 3436, 2927, 1645, 1563, 1504, 1452, 1257; 1H NMR (CDCl3, 400 MHz): 9.40 (broad s, 1H), 7.49–7.27 (m, 5H), 5.28–5.27 (m, 1H), 2.63 (s, 2H), 2.45–2.42 (m, 2H), 2.15 (s, 3H), 1.36–1.22 (m, 6H); 13C NMR (CDCl3, 100 MHz): 196.1, 165.4, 161.1, 149.6, 138.8, 129.4, 128.7, 128.5, 128.1, 126.5, 117.1, 113.0, 96.9, 51.1, 43.2, 37.2, 33.2, 28.2, 28.0, 11.1 HRMS (ESI) [M + H]+ calculated C19H20N2O2 309.1603 found 309.1606.
40 v/v); MP: 182–186 °C (lit. 183–184 °C);17 IR (KBr): 3436, 2927, 1645, 1563, 1504, 1452, 1257; 1H NMR (CDCl3, 400 MHz): 9.40 (broad s, 1H), 7.49–7.27 (m, 5H), 5.28–5.27 (m, 1H), 2.63 (s, 2H), 2.45–2.42 (m, 2H), 2.15 (s, 3H), 1.36–1.22 (m, 6H); 13C NMR (CDCl3, 100 MHz): 196.1, 165.4, 161.1, 149.6, 138.8, 129.4, 128.7, 128.5, 128.1, 126.5, 117.1, 113.0, 96.9, 51.1, 43.2, 37.2, 33.2, 28.2, 28.0, 11.1 HRMS (ESI) [M + H]+ calculated C19H20N2O2 309.1603 found 309.1606.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 269–273 °C (lit. 270–271 °C);17 IR (KBr): 3430, 2925, 1645, 1562, 1505, 1454, 1251; 1H NMR (DMSO-d6, 400 MHz): 10.77 (broad s, 1H), 7.30 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.4 Hz, 2H), 4.98 (s, 1H), 3.34–2.53 (m, 1H), 2.51–2.40 (m, 1H), 2.19–1.91 (m, 2H), 1.86 (s, 3H), 1.05 (s, 3H), 0.85 (s, 3H); 13C NMR (CDCl3, 100 MHz): 191.2, 154.6, 153.7, 146.0, 139.2, 127.4, 124.6, 123.5, 108.2, 91.5, 53.3, 48.7, 32.3, 31.0, 26.8, 26.4, 10.4; HRMS (ESI) [M + H]+ calculated C19H19ClN2O2343.1213 found 343.1209.
40 v/v); MP: 269–273 °C (lit. 270–271 °C);17 IR (KBr): 3430, 2925, 1645, 1562, 1505, 1454, 1251; 1H NMR (DMSO-d6, 400 MHz): 10.77 (broad s, 1H), 7.30 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.4 Hz, 2H), 4.98 (s, 1H), 3.34–2.53 (m, 1H), 2.51–2.40 (m, 1H), 2.19–1.91 (m, 2H), 1.86 (s, 3H), 1.05 (s, 3H), 0.85 (s, 3H); 13C NMR (CDCl3, 100 MHz): 191.2, 154.6, 153.7, 146.0, 139.2, 127.4, 124.6, 123.5, 108.2, 91.5, 53.3, 48.7, 32.3, 31.0, 26.8, 26.4, 10.4; HRMS (ESI) [M + H]+ calculated C19H19ClN2O2343.1213 found 343.1209.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 288–232 °C (lit. 289–230 °C);17 IR (KBr): 3437, 2920, 1645, 1570, 1501, 1459, 1249; 1H NMR (DMSO-d6, 400 MHz): 10.71 (broad s, 1H), 7.24–7.10 (m, 4H), 4.95 (s, 1H), 2.54–2.41 (m, 2H), 2.18 (d, J = 16 Hz, 1H), 2.01 (t, J = 3.6 Hz, 1H), 1.86 (s, 3H), 1.34 (s, 3H), 1.02 (s, 3H), 0.96 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.9, 160.1, 159.5, 151.1, 145.5, 132.1, 129.6, 128.9, 128.4, 111.9, 96.3, 51.4, 41.7, 40.1, 33.0, 29.5, 29.1, 21.7, 11.1; HRMS (ESI) [M + H]+ calculated 323.1760 found 323.1767.
40 v/v); MP: 288–232 °C (lit. 289–230 °C);17 IR (KBr): 3437, 2920, 1645, 1570, 1501, 1459, 1249; 1H NMR (DMSO-d6, 400 MHz): 10.71 (broad s, 1H), 7.24–7.10 (m, 4H), 4.95 (s, 1H), 2.54–2.41 (m, 2H), 2.18 (d, J = 16 Hz, 1H), 2.01 (t, J = 3.6 Hz, 1H), 1.86 (s, 3H), 1.34 (s, 3H), 1.02 (s, 3H), 0.96 (s, 3H); 13C NMR (CDCl3, 100 MHz): 195.9, 160.1, 159.5, 151.1, 145.5, 132.1, 129.6, 128.9, 128.4, 111.9, 96.3, 51.4, 41.7, 40.1, 33.0, 29.5, 29.1, 21.7, 11.1; HRMS (ESI) [M + H]+ calculated 323.1760 found 323.1767.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 226–230 °C (lit. 226–227 °C);17 IR (KBr): 3435, 2922, 1641, 1567, 1509, 1452, 1251; 1H NMR (DMSO-d6, 400 MHz): 8.02–8.01 (m, 1H), 7.87–7.80 (m, 2H), 7.57–7.46 (m, 2H), 3.97 (s, 1H), 3.32–3.30 (m, 3H), 2.28 (s, 3H), 1.86–1.69 (m, 10H); 13C NMR (CDCl3, 100 MHz): 196.1, 166.3, 162.1, 149.2, 139.1, 135.2, 131.1, 130.2, 118.1, 113.0, 100.0, 55.4, 51.1, 43.2, 38.2, 33.2, 28.2, 11.2; HRMS (ESI) [M + H]+ calculated 339.1709 found 339.1701.
40 v/v); MP: 226–230 °C (lit. 226–227 °C);17 IR (KBr): 3435, 2922, 1641, 1567, 1509, 1452, 1251; 1H NMR (DMSO-d6, 400 MHz): 8.02–8.01 (m, 1H), 7.87–7.80 (m, 2H), 7.57–7.46 (m, 2H), 3.97 (s, 1H), 3.32–3.30 (m, 3H), 2.28 (s, 3H), 1.86–1.69 (m, 10H); 13C NMR (CDCl3, 100 MHz): 196.1, 166.3, 162.1, 149.2, 139.1, 135.2, 131.1, 130.2, 118.1, 113.0, 100.0, 55.4, 51.1, 43.2, 38.2, 33.2, 28.2, 11.2; HRMS (ESI) [M + H]+ calculated 339.1709 found 339.1701.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 224–226 °C (lit. 224–225 °C);17 IR (KBr): 3430, 2926, 1642, 1568, 1503, 1453, 1254; 1H NMR (DMSO-d6, 400 MHz): 10.61 (broad s, 1H), 7.08 (d, J = 8.0 Hz, 2H), 6.79 (d, J = 8.0 Hz, 2H), 4.90 (s, 1H), 2.51–2.39 (m, 2H), 2.19–2.15 (m, 1H), 2.03–1.99 (m, 1H), 1.861 (s, 3H), 1.18 (m, 6H), 1.09 (m, 3H); 13C NMR (DMSO-d6, 100 MHz): 194.6, 159.5, 158.8, 157.8, 150.3, 139.3, 128.8, 113.8, 110.5, 96.4, 55.3, 50.8, 35.2, 32.5, 29.0, 27.2, 10.2; HRMS (ESI) [M + H]+ calculated 345.1415 found 345.1418.
40 v/v); MP: 224–226 °C (lit. 224–225 °C);17 IR (KBr): 3430, 2926, 1642, 1568, 1503, 1453, 1254; 1H NMR (DMSO-d6, 400 MHz): 10.61 (broad s, 1H), 7.08 (d, J = 8.0 Hz, 2H), 6.79 (d, J = 8.0 Hz, 2H), 4.90 (s, 1H), 2.51–2.39 (m, 2H), 2.19–2.15 (m, 1H), 2.03–1.99 (m, 1H), 1.861 (s, 3H), 1.18 (m, 6H), 1.09 (m, 3H); 13C NMR (DMSO-d6, 100 MHz): 194.6, 159.5, 158.8, 157.8, 150.3, 139.3, 128.8, 113.8, 110.5, 96.4, 55.3, 50.8, 35.2, 32.5, 29.0, 27.2, 10.2; HRMS (ESI) [M + H]+ calculated 345.1415 found 345.1418.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 172–176 °C (lit. 174–175 °C);17 IR (KBr): 3433, 2926, 1642, 1568, 1503, 1453, 1254; 1H NMR (CDCl3, 400 MHz): 8.94 (broad s, 1H), 7.55–7.40 (m, 5H), 4.17 (s, 1H), 2.81–74 (m, 2H), 2.53–2.39 (m, 2H), 2.22–2.16 (m, 3H), 2.09 (s, 1H), 1.48–1.42 (m, 1H); 13C NMR (CDCl3, 100 MHz): 195.9, 168.8, 165.7, 155.8, 150.4, 148.1, 147.0, 132.1, 123.0, 121.7, 111.9, 52.9, 47.3, 31.7, 29.0, 27.4, 11.4; HRMS (ESI) [M + H]+ calculated C17H16N2O2281.1290 found 281.1295.
40 v/v); MP: 172–176 °C (lit. 174–175 °C);17 IR (KBr): 3433, 2926, 1642, 1568, 1503, 1453, 1254; 1H NMR (CDCl3, 400 MHz): 8.94 (broad s, 1H), 7.55–7.40 (m, 5H), 4.17 (s, 1H), 2.81–74 (m, 2H), 2.53–2.39 (m, 2H), 2.22–2.16 (m, 3H), 2.09 (s, 1H), 1.48–1.42 (m, 1H); 13C NMR (CDCl3, 100 MHz): 195.9, 168.8, 165.7, 155.8, 150.4, 148.1, 147.0, 132.1, 123.0, 121.7, 111.9, 52.9, 47.3, 31.7, 29.0, 27.4, 11.4; HRMS (ESI) [M + H]+ calculated C17H16N2O2281.1290 found 281.1295.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v); MP: 278–282 °C (lit. 278–279 °C);17 IR (KBr): 3435, 2924, 1640, 1569, 1501, 1452, 1256; 1H NMR (CDCl3, 400 MHz): 8.08 (broad s, 1H), 7.27–7.18 (m, 4H), 5.08 (s, 1H), 2.55 (t, J = 5.6 Hz, 2H), 2.38 (t, J = 5.6 Hz, 2H), 2.09–1.96 (m, 2H), 1.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): 196.5, 162.8, 157.1, 145.0, 137.8, 131.6, 129.9, 129.4, 122.8, 113.0, 104.2, 37.1, 35.6, 28.8, 22.1, 11.2; HRMS (ESI) [M + H]+ calculated C17H15ClN2O 315.0900 found 315.0908.
40 v/v); MP: 278–282 °C (lit. 278–279 °C);17 IR (KBr): 3435, 2924, 1640, 1569, 1501, 1452, 1256; 1H NMR (CDCl3, 400 MHz): 8.08 (broad s, 1H), 7.27–7.18 (m, 4H), 5.08 (s, 1H), 2.55 (t, J = 5.6 Hz, 2H), 2.38 (t, J = 5.6 Hz, 2H), 2.09–1.96 (m, 2H), 1.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): 196.5, 162.8, 157.1, 145.0, 137.8, 131.6, 129.9, 129.4, 122.8, 113.0, 104.2, 37.1, 35.6, 28.8, 22.1, 11.2; HRMS (ESI) [M + H]+ calculated C17H15ClN2O 315.0900 found 315.0908.


