Open Access Article
Open Access ArticleMXene/graphene oxide heterojunction as a high performance anode material for lithium ion batteries
Li Wanga,
Kun Yuanb,
Hongyu Baic,
Ping Xuand,
Na Xud,
Chaofan Yina,
Kechen Lib,
Pengju Haob,
Yang Zhou *b and
Binbin Dong
*b and
Binbin Dong *a
*a
aSchool of Materials Science and Engineering, Henan Province International Joint Laboratory of Materials for Solar Energy Conversion and Lithium Sodium Based Battery, Luoyang Institute of Science and Technology, Luoyang 471023, PR China. E-mail: dongbb@lit.edu.cn
bFaculty of Materials Metallurgy and Chemistry Engineering Research Institute, Jiangxi University of Science and Technology, Ganzhou 341000, PR China
cYanshi Zhongyue Refractory Co. LTD, Luoyang 471900, PR China
dAnhui Product Quality Supervision & Inspection Research Institute, PR China
First published on 4th September 2023
Abstract
MXene/graphene oxide composites with strong interfacial interactions were constructed by ball milling in vacuum. Graphene oxide (GO) acted as a bridge between Ti3C2Tx nanosheets in the composite material, which could buffer the mechanical shear force during the ball milling process, avoid the structural damage of nanosheets and improve the structural stability of the composite material during the lithium process. Partial oxidation of Ti3C2Tx nanosheets is caused by high temperatures during ball milling, which is beneficial to improve the intercalation of lithium ions in the material, reduce the stress and electrostatic repulsion between adjacent layers, and cause the composite to have better lithium storage performance. Under the high current density of 2.5 A g−1, the reversible capacity of the Ti3C2Tx/GO composite material after 2000 cycles was 116.5 mA h g−1, and the capacity retention was as high as 116.6%.
1. Introduction
With the development of portable electronic devices and electric vehicles, lithium-ion batteries became a research hotspot because of their high energy density, lack of memory effect and wide operating voltage window. Ti3C2Tx has the advantages of adjustable layer spacing, abundant surface functional groups and multiple active sites, which had unique advantages in the field of lithium-ion battery electrode materials. However, the layer spacing decreased due to the van der Waals attraction between the layers, which seriously hindered the penetration of electrolyte, reducing the rapid migration of lithium ions, and affecting the electrochemical performance.1,2In order to further improve the lithium storage performance of Ti3C2Tx, one of the most promising strategies was to combine Ti3C2Tx nanosheets with other nanomaterials to form heterogeneous structures.3,4 At present, many materials such as transition metal oxides (TMOs),5–7 transition metal sulphides (TMDCs),8,9 phosphorus,10,11 silicon,12,13 polymers,14,15 and carbon materials16–19 are loaded between Ti3C2Tx nanosheets, which could increase the ion transport rate and buffer the volume expansion of the nanoparticles, and ensure the fast charge transport in the heterojunction materials. The existence of a Ti3C2Tx nano-sheet layer could avoid the agglomeration of nano-particles, which is beneficial to the effective utilization of surface active sites, thus improving the electrochemical properties of heterojunction composites.
In this paper, the Ti3C2Tx/GO heterojunction composites were prepared by high-speed ball milling in vacuum. Graphene oxide acted as a spacer between Ti3C2Tx nano-layers in order to enlarge the space between them, which increased the charge storage capacity of the composite and promoted the diffusion of electrolyte in the electrode reaction. Moreover, graphene oxide exhibited high conductivity, which contributed to interlayer charge transfer and improved the multiples properties of the composites.
2. Experimental
2.1 Preparation of Ti3C2Tx and GO
Graphene oxide (GO) was prepared by the oxidation reaction of graphite powder (99.9% purity, Alfa Aesar, USA) using a modified Hummers' method [18]. H2SO4 (12 mL), K2S2O8 (2.5 g) and P2O5 (2.5 g) were mixed and heated in a flask, and graphite powder (3 g, 325 mesh) was added at 80 °C and kept at this temperature for 4.5 h. After heating, dilute it with 500 mL deionized water and cool it to room temperature naturally. Let it stand for 10 h before removing it. The seed water is drained and filtered to remove residual acid and dried. Subsequently, the product was placed in concentrated sulfuric acid (120 mL) under ice bath conditions and KMnO4 (15 g) was slowly added. After stirring for 2 h, dilute with 250 mL deionized water and continue stirring for 2 h. After that, 700 mL of deionized water was added to dilute the solution, and 20 mL of 30%H2O2 was used to neutralize the solution until the color of the mixture turned bright yellow with bubbling. The mixture is then filtered and washed with 10% HCl solution to remove metal ions, and then washed repeatedly with deionized water until the pH value of the filtrate is ∼6. Finally, the precipitate is dried in a freezer to obtain graphene oxide (GO). It was then ball milled at 500 rpm for 12 h to be used as backup for reserve.The commercial Ti3AlC2 powder was purchased from 11 Technology Co., Ltd (Jilin, China). The layered Ti3AlC2 was chemically etched to remove the Al layer by wet etching as follows: after 1 g LiF and 20 mL 9 M HCl solution were magnetically stirred in a Teflon beaker for 30 min, 1 g Ti3AlC2 powder was slowly added into the above solution, and the etching reaction was carried out for 36 h at 35 °C under magnetic stirring. Subsequently, the precipitation was obtained by centrifugation at 6000 rpm and washed with deionized water several times until the pH value reached ∼6. Then, the sediment was placed in 200 mL 0.25 M LiOH solution, ultrasonic treatment and stirred for 24 h. The alkalized mixture is repeatedly filtered and washed with deionized water until the pH of the solution reaches ∼8. Finally, the precipitate was dried in a freeze-dryer to obtain multilayer Ti3C2Tx MXene (multi-Ti3C2Tx). Subsequently, Ti3C2Tx was obtained by ball milling at 500 rpm for 12 h in a vacuum environment for backup.
2.2 Preparation of Ti3C2Tx/GO composite
The multi-Ti3C2Tx and GO nanosheets were taken in a mass ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and placed in a vacuum environment with mixed ball milling at a speed of 500 rpm for 12 h. After that, by calcination at 300 °C for 3 h under inert atmosphere with a heating rate of 2 °C min−1. The as-prepared product was designated as Ti3C2Tx/GO. Control the GO content but different composite materials was prepared.
1 and placed in a vacuum environment with mixed ball milling at a speed of 500 rpm for 12 h. After that, by calcination at 300 °C for 3 h under inert atmosphere with a heating rate of 2 °C min−1. The as-prepared product was designated as Ti3C2Tx/GO. Control the GO content but different composite materials was prepared.
2.3 Electrochemical measurements
As for the lithium-ion battery tests, all of the CR2032-type coin cells were assembled in an Ar-filled glove box. The working electrode was prepared by mixing Ti3C2Tx or S–Ti3C2Tx, acetylene black, and polyvinylidene fluoride (PVDF) in N-methyl-2-pyrrolidinone (NMP) with a weight ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Lithium foil was used as counter electrode, Celgard polypropylene as separators and 1 M LiPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) a volumetric ratio of 1
2. Lithium foil was used as counter electrode, Celgard polypropylene as separators and 1 M LiPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) a volumetric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the electrolyte. All electrochemical performance tests were conducted under 25 °C. Cyclic voltammetry experiments were performed by LAND CT2001A battery testing system at a scan rate of 0.1 mV s−1 between 0.05 and 3 V. Electrochemical impedance spectroscopy (EIS) measurements were performed using an AC amplitude of 10 mV and a frequency range of 200 kHz to 0.01 Hz.
1 as the electrolyte. All electrochemical performance tests were conducted under 25 °C. Cyclic voltammetry experiments were performed by LAND CT2001A battery testing system at a scan rate of 0.1 mV s−1 between 0.05 and 3 V. Electrochemical impedance spectroscopy (EIS) measurements were performed using an AC amplitude of 10 mV and a frequency range of 200 kHz to 0.01 Hz.
2.4 Characterization
The morphology and structural information were conducted by SEM (SU-8020, Hitachi) and TEM (JEOL JEM-2100F). The XRD patterns were collected on a Bruker D8 diffractometer (Cu Kα radiation, λ = 1.5406 Å). The element surface analysis was carried out by XPS (Axis Ultra DLD, Kratos Analytical) with Al Kα radiation. The specific surface area of the sample was determined by N2 absorption at 77 K with a BeiShiDe 3H-2000PM1 instrument. The Raman spectra were collected on a Raman spectrometer (Labram-010) using 532 nm laser.3. Results and discussion
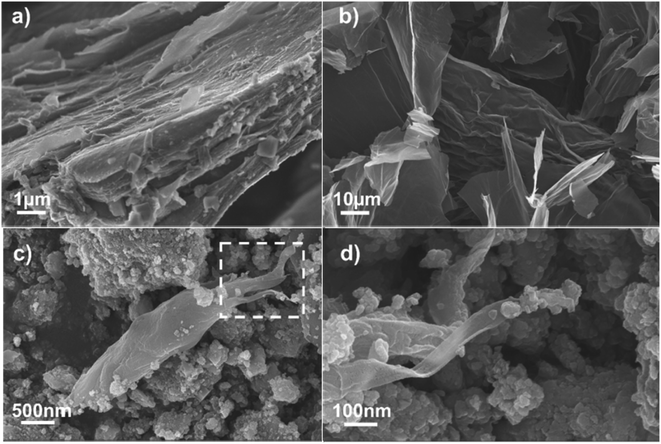
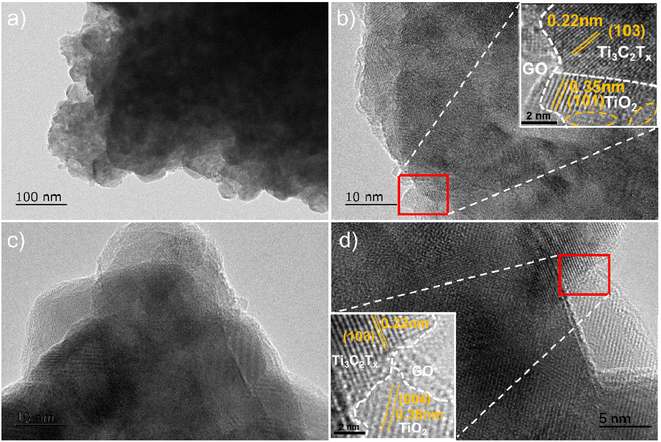
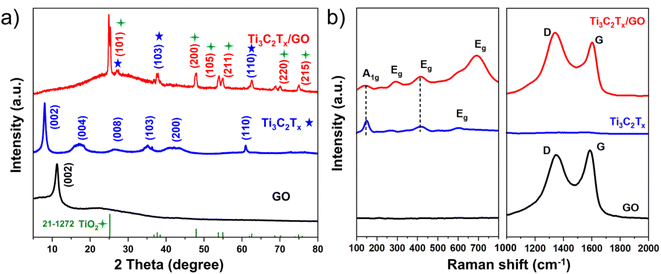
As shown in Fig. 1a, Ti3C2Tx presented a typical layered structure after chemical etching, but there was still a serious self-stacking phenomenon. GO had very large horizontal and vertical dimensions, with internal three-dimensional holes connected to each other (Fig. 1b).20 When the two mixtures were milled and treated at high temperature, the size of the particles was significantly reduced, but the flake graphene was like a ribbon floating between the Ti3C2Tx particles (Fig. 1c and d).In order to deeply analyze the morphological structure of Ti3C2Tx/GO composites, transmission electron microscopy (TEM) was used for further characterization. According to Fig. 2a and b, it can be clearly seen that GO is coated in the outermost layer of the material, where the lattice spacing of 0.22 nm and 0.35 nm corresponds to Ti3C2Tx (103) and TiO2 (110) crystal planes, respectively. In addition, the interface structure of TiO2/Ti3C2Tx appeared in Fig. 2c and d,21–23 and there is an obvious lattice fringe interruption phenomenon, which indicated that Ti3C2Tx is partially oxidized due to mechanical ball milling and large transverse shear force. The XRD results (Fig. 3a) further confirm the presence of a small amount of TiO2,22,24 which was consistent with TEM results. Raman spectra (Fig. 3b) further confirmed the successful recombination of Ti3C2Tx and GO. The vibrational peak at 141.9 cm−1 in the Ti3C2Tx/GO composite represented the A1g symmetric out-of-plane vibrations of the titanium atoms in the material, while the vibrational peaks at 417.2 and 692.6 cm−1 indicated the in-plane shear vibrations (Eg) of Ti, C and surface oxygen-containing functional groups in the Ti3C2Tx material, respectively, where the vibrational peak at 292.3 cm−1 peak then verified the production of TiO2 in the material.25,26 Meanwhile, the typical peaks of the carbon material were located at 1346.5 and 1599.9 cm−1, corresponding to the D and G peaks of graphene oxide, respectively, indicating the generation of amorphous carbon during the reaction.25,27 After graphene oxide composite Ti3C2Tx, the intensity ratio (ID/IG) of the two peaks increased from 0.92 to 1.08, indicating that most of the oxygen functional groups on its surface were removed and TiO2 was directly embedded into the graphene surface, increasing the large number of defects generated and exposing more active sites on the composite surface, which is conducive to increasing the contact area of the electrolyte and promoting the ion and charge diffusion.28
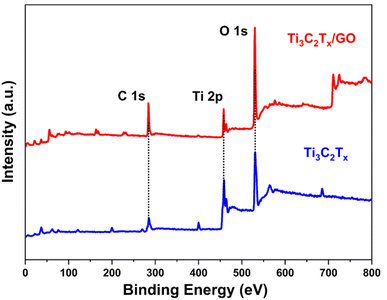
In order to analyze the chemical composition and surface electronic states of Ti3C2Tx/GO composites, XPS spectroscopy was performed, as shown in Fig. 4. In the XPS full spectrum, the addition of GO material resulted in a significant increase in the intensity of the C 1s characteristic peak of the composites, and the increase in the intensity of the O 1s characteristic peak was attributed to the oxidation of Ti3C2Tx nanosheets by high temperature during the ball milling composite. In the XPS high-resolution spectra of Ti 2p orbitals (Fig. 5a and b), there are four distinct characteristic peaks in Ti3C2Tx. The characteristic peaks at 455.1 and 460.6 eV correspond to Ti(II) (2p3/2 and 2p1/2), 456.6 eV corresponds to Ti(III) (2p3/2), while the Ti–C peak at 461.3 eV corresponds to the Ti 2p1/2 orbital, and the characteristic peaks at 458.5 and 464.3 eV correspond to Ti(2p3/2 and 2p1/2).29,30 After the composite graphene oxide, the characteristic peaks on the Ti 2p orbitals show a significant change and the Ti–C intensity in the structure is weak, which is still mainly due to the generation of TiO2 in the material and is wrapped by the graphene oxide on its surface along with Ti3C2Tx. As shown in Fig. 5c and d), the main characteristic peaks present in the C 1s are C–C (284.8 eV), C–O (286.2 eV), C–Ti–O (282.2 eV) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.8 eV), and the intensity of the C–C peak in the composite was significantly higher than the other characteristic peaks, which then indicated the presence of a large number of C–C bonds in it, which could be attributed to the graphene oxide. Fig. 5e and f) showed the O 1s high-resolution spectra of Ti3C2Tx, Ti3C2Tx/GO composites with characteristic peaks corresponding to Ti–O (530.1 eV), C–Ti-(OH)x (531.2 eV) and O
O (288.8 eV), and the intensity of the C–C peak in the composite was significantly higher than the other characteristic peaks, which then indicated the presence of a large number of C–C bonds in it, which could be attributed to the graphene oxide. Fig. 5e and f) showed the O 1s high-resolution spectra of Ti3C2Tx, Ti3C2Tx/GO composites with characteristic peaks corresponding to Ti–O (530.1 eV), C–Ti-(OH)x (531.2 eV) and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH (533.2 eV), respectively, where O was mainly present in Ti3C2Tx materials in the form of Ti–O bonds, followed in the form of oxygen-containing functional groups on the surface of Ti3C2Tx, while in the composites, the residual oxygen functional groups of GO caused a slight increase in the peak intensity.
C–OH (533.2 eV), respectively, where O was mainly present in Ti3C2Tx materials in the form of Ti–O bonds, followed in the form of oxygen-containing functional groups on the surface of Ti3C2Tx, while in the composites, the residual oxygen functional groups of GO caused a slight increase in the peak intensity.
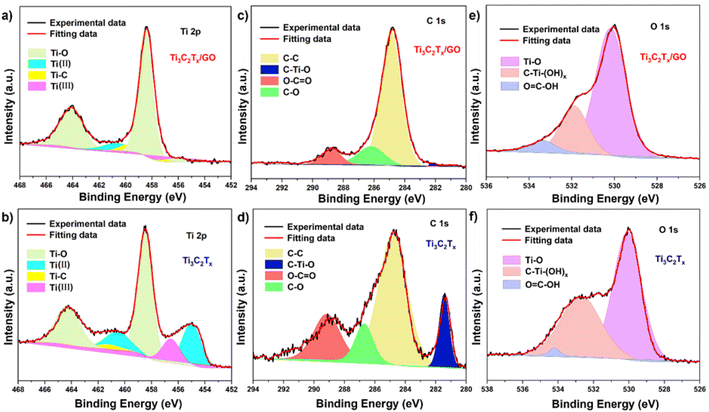 | ||
| Fig. 5 High resolution XPS spectra of Ti3C2Tx/GO and Ti3C2Tx: (a and b) Ti 2p; (c and d) C 1s; (e and f) O 1s. | ||
Ti3C2Tx/GO composite was used as working electrode and lithium sheet as paired electrode to investigate its electrochemical properties in lithium-ion battery. In the voltage range of 0.05–3.0 V, the CV curve of the Ti3C2Tx/GO composite at sweep velocity of 0.1 mV s−1 is shown in Fig. 6a). At 0.99 V, the irreversible peak appeared on the first discharge curve, which was mainly due to the formation of SEI film on the electrode surface and the irreversible reaction between lithium ion and the functional groups on the material surface. In the subsequent cycle, redox peaks at 0.68 V and 1.7 V correspond to lithium ion intercalation/desorption by the following mechanisms:26,31
| Ti3C2Tx + yLi + ye− ↔ Ti3C2TxLiy | (1-1) |
 | ||
| Fig. 6 (a and b) CV curve and charge–discharge curve of Ti3C2Tx/GO composites; (c and d) Ti3C2Tx and Ti3C2Tx/GO composites multiplier properties and cycle properties. | ||
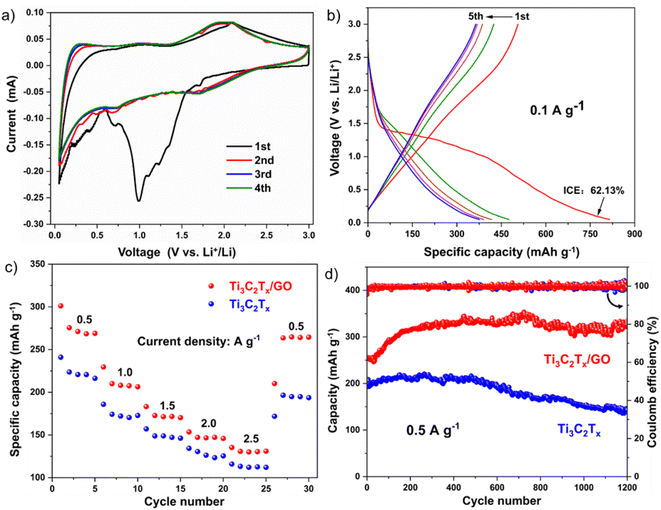
The good overlap of the CV curves in the 3rd and 4th circles then indicated that the Ti3C2Tx/GO composite has excellent cycling stability in the electrochemical lithium storage process. Fig. 6b shows the charge/discharge curves of the Ti3C2Tx/GO composite electrode at a current density of 0.1 A g−1, which could correspond well with the CV curves with initial charge/discharge specific capacities of 506.1 and 814.6 mA h g−1, respectively, and a first Coulomb efficiency of 62.13%, due to the presence of abundant functional groups on the surfaces of GO and Ti3C2Tx nanosheets that the hybrid ball milling of GO and Ti3C2Tx nanosheets exposed a large number of active sites at the same time as reducing their corresponding sizes, increasing their lithium storage capacity. The subsequent overlap of charge/discharge curves also confirmed the more excellent cycling stability of Ti3C2Tx/GO composites.32
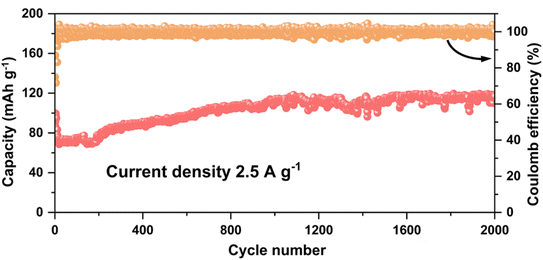
After ball milling, the reduction of material particle size shortened the diffusion path of ions and charges and promoted the penetration of electrolyte, which made Ti3C2Tx/GO exhibit a more excellent multiplicative performance than Ti3C2Tx, as shown in Fig. 6c. The reversible specific capacities of Ti3C2Tx/GO reached 275.6, 210.1, 172.8, 147.2, and 130.8 mA h g−1 at current densities of 0.5, 1.0, 1.5, 2.0, and 2.5 A g−1, respectively, and the specific capacities at each current density were much larger than those of the Ti3C2Tx material after ball milling alone. When the current density was reduced to 0.5 A g−1, Ti3C2Tx/GO could recover to a reversible specific capacity of 263.6 mA h g−1, showing excellent reversibility. Subsequently, Ti3C2Tx/GO as well as Ti3C2Tx materials were tested for 1200 cycles of performance, as shown in Fig. 6d. At a current density of 0.5 A g−1, the specific capacity of Ti3C2Tx/GO was much higher than that of Ti3C2Tx. During the first 500 cycles, the electrolyte continuously penetrated between the active particles and the lithium ions were de-embedded several times, which enabled the exposure of more surface active sites, resulting in a gradual increase in the lithium storage capacity of both. However, the capacity of Ti3C2Tx materials decayed afterwards, mainly due to the small size of the particles after ball milling, which prompted the agglomeration of small particles under the continuous embedding of lithium ions and impeded the wetting of the electrolyte, and the irreversible SEI film on the surface of the particles, which seriously affected the rapid ion and charge transport, and the internal lithium storage sites were not fully utilized, resulting in the decay of their capacity.28 However, graphene oxide in Ti3C2Tx/GO composites, due to its unique ribbon structure, played a bridging role in it, prompting the activation process of the material to continuously expose the active sites and utilize them effectively, showing extremely excellent cycling stability. Then, the Ti3C2Tx/GO composite was further tested for long cycles at a high current density of 2.5 A g−1, as shown in Fig. 7. The specific capacity of Ti3C2Tx/GO composite after 2000 cycles was 116.5 mA h g−1, with a capacity retention rate of 116.6%, which fully indicated that the Ti3C2Tx/GO composite retained good structural stability and had excellent cycling performance after graphene oxide composite. It has excellent cycling performance.
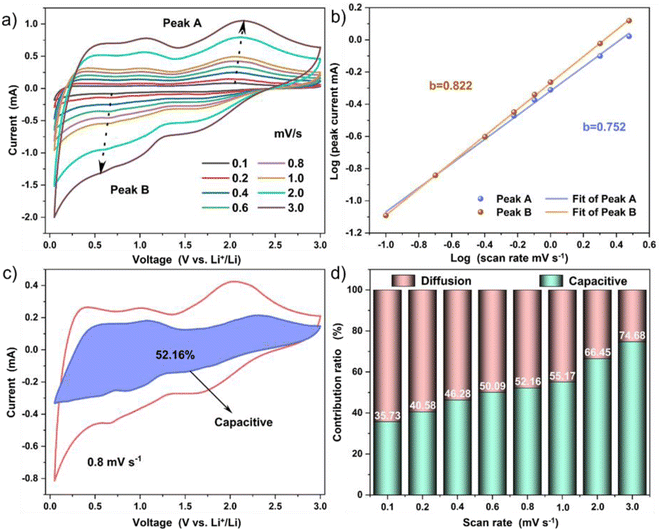
In order to further investigate the electrochemical behavior of Ti3C2Tx/GO composites, the CV curves of different sweep rates were analyzed accordingly (Fig. 8a). Based on the formula i = avb, where a is a constant and the value of b can be determined by the slope of the log(v) − log(i) curve. From the fitting curve of Fig. 8b, the b values of reduction peak and oxidation peak are 0.752 and 0.822, respectively. In contrast, diffusion-controlled embedding mechanism and surface pseudocapacitance mechanism coexist in Ti3C2Tx/GO electrodes, but the surface-induced pseudo-capacitance process still dominated. In addition, the capacitance contribution ratio of the two reaction mechanisms could be calculated by the following formula:14,33 i(v) = k1ν + k2ν1/2, where i(v), k1v, k2v1/2 and v are the current, capacitive current, diffusion control current and scanning rate at a given voltage, respectively. According to the CV curve, when the scanning rate is 0.8 mV s−1, the pseudo-capacitance contribution of Ti3C2Tx/GO electrode is 52.16%. With the increase in scanning rate, the corresponding pseudo-capacitance contribution also increased (Fig. 8c and d). This showed that in the process of charge and discharge, the mechanical ball milling led to a sharp decrease in the particle size of the material, shortening the ion and charge diffusion path, coupled with the good electrical conductivity of graphene oxide and Ti3C2Tx nanosheets, which made the charge transfer process in the surface of Ti3C2Tx/GO composites in a dominant position.34
4. Conclusion
Ti3C2Tx nanosheets and GO were ball milled in vacuum by high energy ball mill self-assembly method. Ti3C2Tx/GO composites with strong interfacial interaction could be constructed by forming chemical bonds between the abundant functional groups on the surface of GO and Ti3C2Tx, which could effectively promote the transport of ions and electrons at the heterogeneous interface and improve its electrochemical behavior. The specific capacity of Ti3C2Tx/GO composite electrode could reach 506.1 mA h g−1 at the current density of 0.1 A g−1, and the reversible capacity of Ti3C2Tx/GO composite electrode after 2000 cycles is 116.5 mA h g−1 at the high current density of 2.5 A g−1. The capacity retention rate is as high as 116.6%.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was financially supported by National Natural Science Foundation of China (21865012, 52202064), Education Department Project Fund of Jiangxi Province (GJJ190427), Ganzhou innovative talent project, the Program for Excellent Young Talents, JXUST, Science and Technology Department of Henan Province (222102230054), New Energy Electric Vehicles High-Voltage Components Inspection and Testing Public Service Platform, Henan Province Education Department of Key Scientific Research Project in Colleges and Universities (21B430012, 23B430012).References
- W. Nie, H. Cheng and Q. Sun, et al., Design strategies toward high-performance zn metal anode, Small Methods, 2023, 2201572 CrossRef.
- Q. Sun, H. Cheng and Y. Yuan, et al., Uncovering the fundamental role of interlayer water in charge storage for bilayered V2O5·nH2O xerogel cathode materials, Adv. Energy Mater., 2023, 13(3), 2202515 CrossRef CAS.
- D. Xiong, X. Li and Z. Bai, et al., Recent advances in layered Ti3C2Tx MXene for electrochemical energy storage, Small, 2018, 14(17), e1703419 CrossRef PubMed.
- Y. Zhang, L. Tao and C. Xie, et al., Defect engineering on electrode materials for rechargeable batteries, Adv. Mater., 2020, 32(7), e1905923 CrossRef PubMed.
- Q. Chen, Z. Wen and J. Zhang, et al., Fe3O4 nanorods in N-doped carbon matrix with pseudo-capacitive behaviors as an excellent anode for subzero lithium-ion batteries, J. Alloys Compd., 2018, 772, 557–564 CrossRef.
- X. Li, J. Zhu and Y. Fang, et al., Hydrothermal preparation of CoO/Ti3C2 composite material for lithium-ion batteries with enhanced electrochemical performance, J. Electroanal. Chem., 2018, 817, 1–8 CrossRef CAS.
- B. Ahmed, D. Anjum and Y. Gogotsi, et al., Atomic layer deposition of SnO2 on MXene for Li-ion battery anodes, Nano Energy, 2017, 34, 249–256 CrossRef CAS.
- S. Zhang, H. Ying and P. Huang, et al., Rational design of pillared SnS/Ti3C2Tx MXene for superior lithium-ion storage, ACS Nano, 2020, 14(12), 17665–17674 CrossRef CAS PubMed.
- H. Liu, Y. He and H. Zhang, et al., Heterostructure engineering of ultrathin SnS2/Ti3C2Tx nanosheets for high-performance potassium-ion batteries, J. Colloid Interface Sci., 2021, 606(1), 167–176 CrossRef PubMed.
- H. Liu, S. Zhang and Q. Zhu, et al., Fluffy carbon-coated redhorus as a highly stable and high-rate anode for lithium-ion batteries, J. Mater. Chem. A, 2019, 7(18), 11205–11213 RSC.
- S. Zhang, H. Liu and B. Cao, et al., An MXene/CNTs@P nanohybrid with stable Ti-O-P bonds for enhanced lithium ion storage, J. Mater. Chem. A, 2019, 7(38), 21766–21773 RSC.
- G. Tritsaris, K. Zhao and O. Okeke, et al., Diffusion of lithium in bulk amorphous silicon: a theoretical study, J. Phys. Chem. C, 2012, 116(42), 22212–22216 CrossRef CAS.
- M. Xia, B. Chen and F. Gu, et al., Ti3C2Tx MXene nanosheets as a robust and conductive tight on Si anodes significantly enhance electrochemical lithium storage performance, ACS Nano, 2020, 14(4), 5111–5120 CrossRef CAS PubMed.
- X. Xie, M. Zhao and B. Anasori, et al., Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices, Nano Energy, 2016, 26, 513–523 CrossRef CAS.
- C. Chen, M. Boota and X. Xie, et al., Charge transfer induced polymerization of EDOT confined between 2D titanium carbide layers, J. Mater. Chem. A, 2017, 5(11), 5260–5265 RSC.
- C. Zhang, X. Wang and W. Wei, et al., Recent advances in the synthesis and energy applications of 2D MXenes, ChemElectroChem, 2021, 8(20), 3804–3826 CrossRef CAS.
- M. Lu, W. Han and H. Li, et al., Tent-pitching-inspired high-valence period 3-cation pre-intercalation excels for anode of 2D titanium carbide (MXene) with high Li storage capacity, Energy Storage Mater., 2019, 16, 163–168 CrossRef.
- K. Yuan, P. Hao, X. Hu, J. Zhang and Y. Zhou, Experimental and computational studies on S-decorated Ti3C2 MXene as anode material in Li-ion batteries, J. Mater. Sci., 2022, 57(13), 7001–7011 CrossRef CAS.
- K. Yuan, P. Hao, Y. Zhou, X. Hu, J. Zhang and S. Zhong, A two-dimensional MXene/BN van der Waals heterostructure as an anode material for lithium-ion batteries, Phys. Chem. Chem. Phys., 2022, 24(22), 13713–13719 RSC.
- M. Yoo and H. Park, Effect of hydrogen peroxide on properties of graphene oxide in Hummers method, Carbon, 2019, 141, 515–522 CrossRef CAS.
- Y. Zhang, Y. Tang and W. Li, et al., Nanostructured TiO2-based anode materials for high-performance rechargeable lithium-ion batteries, ChemNanoMat, 2016, 2(8), 764–775 CrossRef CAS.
- C. Guo, Q. Tian and L. Yang, The size-controllable spindle TiO2 nanograins and their lithium storage properties, J. Alloys Compd., 2018, 776, 740–745 CrossRef.
- Z. Liu, R. Guo and F. Li, et al., Reduced graphene oxide bridged, TiO2 modified and MnO4 intercalated Ti3C2Tx sandwich-like nanocomposite as a high performance anode for enhanced lithium storage applications, J. Alloys Compd., 2018, 762, 643–652 CrossRef CAS.
- H. Ren, J. Sun and R. Yu, et al., Controllable synthesis of mesostructures from TiO2 hollow to porous nanospheres with superior rate performance for lithium ion batteries, Chem. Sci., 2015, 7(1), 793–798 RSC.
- Z. Li, G. Chen and J. Deng, et al., Creating sandwich-like Ti3C2/TiO2/rGO as anode materials with high energy and power density for Li-ion hybrid capacitors, ACS Sustain. Chem. Eng., 2019, 7(18), 15394–15403 CrossRef CAS.
- J. Huang, R. Meng and L. Zu, et al., Sandwich-like Na0.23TiO2 nanobelt/Ti3C2 MXene composites from a scalable in situ transformation reaction for long-life high-rate lithium/sodium-ion batteries, Nano Energy, 2018, 46, 20–28 CrossRef CAS.
- C. Shen, L. Wang and A. Zhou, et al., Synthesis and electrochemical properties of two-dimensional RGO/Ti3C2Tx nanocomposites, Nanomaterials, 2018, 8(2), 80 CrossRef PubMed.
- Y. Zhang, L. Tao and C. Xie, et al., Defect engineering on electrode materials for rechargeable batteries, Adv. Mater., 2020, 32(7), e1905923 CrossRef PubMed.
- C. Zhang, S. Kim and M. Ghidiu, et al., Layered orthorhombic Nb2O5@Nb4C3Tx and TiO2@Ti3C2Tx hierarchical composites for high performance Li-ion batteries, Adv. Funct. Mater., 2016, 26(23), 4143–4151 CrossRef CAS.
- L. Jia, Y. Li and L. Su, et al., TiO2 nanoparticles in situ formed on Ti3C2 nanosheets by a one-step ethanol-thermal method for enhanced reversible lithium-ion storage, Chemistryselect, 2020, 5(10), 3124–3129 CrossRef CAS.
- C. Yang, Y. Liu and X. Sun, et al., In-situ construction of hierarchical accordion-like TiO2/Ti3C2 nanohybrid as anode material for lithium and sodium ion batteries, Electrochim. Acta, 2018, 271, 165–172 CrossRef CAS.
- Z. Ma, X. Zhou and W. Deng, et al., 3D porous MXene (Ti3C2)/reduced graphene oxide hybrid films for advanced lithium storage, ACS Appl. Mater. Interfaces, 2018, 10(4), 3634–3643 CrossRef CAS PubMed.
- H. Huang, J. Cui and G. Liu, et al., Carbon-coated MoSe2/MXene Hybrid nanosheets for superior potassium storage, ACS Nano, 2019, 13(3), 3448–3456 CrossRef CAS PubMed.
- Q. Zhao, Q. Zhu and J. Miao, et al., Flexible 3D porous MXene foam for high performance lithium-ion batteries, Small, 2019, 15(51), e1904293 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |