Open Access Article
Open Access ArticleLipase-mediated flow synthesis of nature-inspired phenolic carbonates†
Sara Vicinanza‡
 a,
Francesca Annunziata‡
a,
Francesca Annunziata‡ a,
Desirèe Pecora
a,
Desirèe Pecora a,
Andrea Pinto
a,
Andrea Pinto b and
Lucia Tamborini
b and
Lucia Tamborini *a
*a
aDepartment of Pharmaceutical Sciences (DISFARM), University of Milan, Via Mangiagalli 25, Milan 20133, Italy. E-mail: lucia.tamborini@unimi.it
bDepartment of Food, Environmental and Nutritional Sciences (DeFENS), University of Milan, Via Celoria 2, Milan 20133, Italy
First published on 28th July 2023
Abstract
A facile and convenient lipase-catalyzed flow approach for the chemoselective synthesis of tyrosol and hydroxytyrosol methyl carbonates has been developed in neat dimethylcarbonate. The products were obtained in quantitative yield with high catalyst productivity. The biocatalytic approach was then exploited for the preparation of value-added symmetrical tyrosol and hydroxytyrosol carbonates.
Introduction
Organic carbonates are important classes of compounds and chemical intermediates, which are renowned for their great versatility, and high biodegradability and, consequently, are widely used for a variety of industrial and synthetic applications.1,2 A plethora of methods have been reported for synthesizing non-symmetrical carbonates on a variety of substrates such as alcohols,3 olefins4,5 and epoxides,6–8 either relying on metal-based catalysts9–12 or organic metal-free catalysts.13 However, carbonating agents such as phosgene and its derivatives, e.g., chloroformates and 1,1-carbonyldiimidazole, are now not recommended because of their inherent toxicity,14,15 as well as CO2, whose thermodynamic stability and kinetic inertness limit its use,16 despite its huge potential as carbonation reagent.Therefore, the attention for the obtainment of alkyl carbonates is currently focused on the development of eco-compatible methodologies employing non-toxic and biodegradable chemicals.
In the last decades, food-derived phenolic compounds have raised great attention in the food, cosmetic and pharmaceutical sectors for their health-promoting effects such as antioxidant, metal chelator, free radical scavenger, antimicrobial and anti-inflammatory properties.17,18 However, due to their solubility, metabolic/chemical stability and bioavailability issues, their applicability as active ingredients is still limited. With the aim to overcome this limitation, a growing interest has been devoted to the obtainment of more lipophilic derivatives.19,20 For example, one of the most common strategies to increase their oil solubility and miscibility in emulsion-based systems is the esterification of phenolic compounds with medium or long aliphatic chains, preserving the –OH phenolic moieties, which are responsible of the desired bioactivity.21 Other strategies include the incorporation of one or more halogen atoms22 or the introduction of different aliphatic chains into the molecular skeleton.23
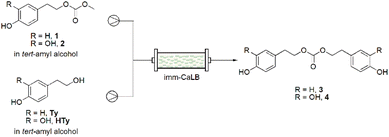
In this context, we explored the potential of flow biocatalysis for the sustainable synthesis of tyrosol (Ty) and hydroxytyrosol (HTy) carbonates (Fig. 1) characterized by increased lipophilicity compared to the parent phenolic compounds, which are natural antioxidants present in olive oil and wine. Besides the production of methyl carbonates 1 and 2, we became particularly intrigued in the obtainment of symmetric carbonated 3 and 4. Notably, compound 3 has been recently described as monomer of a new class of biodegradable, aromatic polycarbonates deriving from Ty.24 The synthesis of compound 3 reported until now is far away from being environmentally friendly and sustainable, requiring the use of tyrosol chloroformate and triphosgene under inert atmosphere.24,25
Results and discussion
First, the chemoselective carbomethoxylation of the alcoholic function of Ty and HTy was performed under flow conditions using a cheap, non-toxic and green carboxymethylating agent as dimethyl carbonate (DMC),26,27 exploited also as reaction medium, and Novozyme 435® as biocatalyst (imm-CaLB).28,29 The carbomethoxylation reaction usually relies on the use of organocatalyst under heating30,31 and, until now, the synthesis of compounds 1 and 2 has been achieved mainly using an acidic catalyst under prolonged heating.32–34 The immobilized biocatalyst was packed into a glass reactor column and a solution of Ty in DMC was flowed through it (Table 1); temperature (T, range: 40–80 °C), Ty concentration (CTy, range: 100–250 mM), and residence time (Rt, range: 5–15 min) were varied and conversion (c) was evaluated by HPLC (Table 1).| Entrya | CTyb (mM) | Rt (min) | T (°C) | cc (%) |
|---|---|---|---|---|
| a Optimization of reaction parameters to achieve the highest molar conversion was performed using Ty as substrate.b Solution in DMC.c Molar conversion determined by HPLC analysis. | ||||
| 1 | 100 | 15 | 50 | 100 |
| 2 | 100 | 15 | 40 | 100 |
| 3 | 100 | 10 | 40 | 74 |
| 4 | 100 | 10 | 50 | 85 |
| 5 | 100 | 10 | 60 | 100 |
| 6 | 100 | 5 | 60 | 58 |
| 7 | 250 | 10 | 60 | 43 |
| 8 | 150 | 10 | 60 | 81 |
| 9 | 250 | 10 | 70 | 57 |
| 10 | 250 | 10 | 80 | 70 |
| 11 | 250 | 15 | 80 | 94 |
Using a 100 mM solution of Ty in DMC, the flow biotransformation was complete after only 15 min both at 50 °C and lowering the temperature at 40 °C (Table 1, entries 1 and 2). Keeping constant the temperature at 50 °C and decreasing the residence time at 10 min resulted in only a partial conversion of Ty into product 1 (Table 1, entry 4), but raising the temperature at 60 °C, in only 10 min, full conversion was achieved (Table 1, entry 5). Under these conditions, to improve the productivity of the system, higher concentration of Ty was tested, but the biotransformation was not complete, even increasing temperature up to 80 °C and residence time to 15 min (Table 1, entry 11).
The selected conditions (CTy: 100 mM in DMC, T = 60 °C, Rt: 10 min) were then successfully applied for the synthesis of compound 2. Considering the HTy low availability in nature and its price, it was obtained from Ty through a continuous biocatalyzed oxidation using a free tyrosinase.35 Then, to obtain compounds 3 and 4, carbonates 1 and 2 were used as substrates and Ty or HTy as nucleophiles. The biotransformation was performed in tert-amyl alcohol which was chosen as unconventional medium for its safety profile, low freezing point in comparison to t-BuOH, and ability to solubilize polar compounds.36,37 A solution of compound 1 in tert-amyl alcohol was mixed with a solution of Ty and the resulting mixture was flowed through a reactor packed with imm-CaLB (Scheme 1).
Different experiments were performed changing residence time, stoichiometry and temperature and the outcome was monitored by HPLC (Table 2). Unsatisfactory conversions were achieved using a stoichiometric ratio between compound 1 and Ty, even after 120 min of residence time (Table 2, entries 1–3). So, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio was tested (Table 2, entries 4–7). The reaction did not reach completion even after 180 min of residence time at 80 °C and a significant hydrolysis of carbonate 2 was observed, leading to the formation of Ty that was then recovered by column chromatography obtaining the desired product in 25% yield. It was not possible to avoid or reduce this side reaction even reducing the temperature or the residence time, changing the solvent, or adding molecular sieves to produced a mixed bed reactor.38 Since the differences in the reaction outcome between 120 min and 180 min were not significant, the final set-up was settled for a residence time of 120 min which allowed to obtain a conversion of 91% and an isolated yield of 23%. It is worth noting that the same results were reached in batch after 4 days.
2 ratio was tested (Table 2, entries 4–7). The reaction did not reach completion even after 180 min of residence time at 80 °C and a significant hydrolysis of carbonate 2 was observed, leading to the formation of Ty that was then recovered by column chromatography obtaining the desired product in 25% yield. It was not possible to avoid or reduce this side reaction even reducing the temperature or the residence time, changing the solvent, or adding molecular sieves to produced a mixed bed reactor.38 Since the differences in the reaction outcome between 120 min and 180 min were not significant, the final set-up was settled for a residence time of 120 min which allowed to obtain a conversion of 91% and an isolated yield of 23%. It is worth noting that the same results were reached in batch after 4 days.
Conclusions
Alkyl carbonates are an important class of compounds widely used for a variety of industrial and synthetic applications, including as protecting groups for the alcoholic function. In this work, the high-yield biocatalyzed chemoselective carbomethoxylation of the alcoholic chain of natural phenolic compounds was performed in flow conditions, without affecting the phenolic moiety. The obtained methyl carbonates 1 and 2 were then reacted to obtain the symmetric carbonates 3 and 4. The biotransformations were carried out in DMC or tert-amyl alcohol as the solvents and the biocatalyst (Novozyme 435®) showed very good stability in these unconventional media. Concerning the synthesis of methyl carbonates derivatives 1 and 2, the switch from a batch traditional set up to a continuous allowed a reduction of the reaction time, an easier work-up and a higher yield: in fact, the batch reaction needed 6 h to bring the reaction to completeness followed by a filtration and washing under pressure and by a flash chromatography purification to isolate the pure product 1 in 93% yield; the flow biotransformation required only 10 min of residence time to reach the completeness, did not require downstream processes giving quantitative yield. Using a packed bed reactor with a volume of 1.6 mL (545 mg of imm-CaLB), a multi-gram amount of compounds 1 (4.5 g day−1) and 2 (4.8 g day−1) were produced in 24 h, that means a biocatalyst productivity of more that 8 gproduct gimm-CaLB−1 day−1. Moreover, DMC was recovered by evaporation and reused. Carbonates 3 and 4 were obtained in moderate yield but the present protocol represents the first biocatalyzed approach towards these symmetric scaffolds, that were synthesised without the need of protecting the phenolic group(s).Experimental
Materials and methods
Reagents and solvents were obtained from commercial suppliers and were used without further purification. NMR spectra were recorded on a Varian Gemini 300 MHz spectrometer using the residual signal of the deuterated solvent as internal standard. 1H chemical shifts (δ) are expressed in ppm, and coupling constants (J) in hertz (Hz). Continuous flow biotransformations were performed using a R4 Vapourtec flow reactor and Asia Flow Chemistry Syringe pumps (Syrris) equipped with an Omnifit® glass column (i.d. 6.6 mm × 100 mm length). The temperature sensor sits on the wall of the reactors. Immobilized lipase from Candida antarctica B (CaLB, Novozyme®) was purchased from Merk (Milan, Italy). HTy was synthetized from Ty as previously reported.35 Analytical thin layer chromatography (TLC) was carried out on TLC plates precoated with silica gel 60 F254. Spots were further evidenced using a dilute alkaline solution of KMnO4 or an acidic solution of vanillin in ethanol. HPLC analyses were performed using a Waters 1525 Binary HPLC Pump, equipped with a Waters 2489 UV-vis detector (Waters, Milford, MA).HPLC method
For the analysis of tyrosol, hydroxytyrosol and compounds 1–4 an isocratic method H2O/acetonitrile (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was used. Waters C18 column μBondapack (10 μm, 125 Å), 210 nm (λ). Injection volume: 10 μL. Flow rate: 1 mL min−1. Retention times: Ty = 3.4 min, HTy = 3.1 min, 1 = 5.1 min, 2 = 4.2 min, 3 = 6.8 min, 4 = 4.5 min.
4) was used. Waters C18 column μBondapack (10 μm, 125 Å), 210 nm (λ). Injection volume: 10 μL. Flow rate: 1 mL min−1. Retention times: Ty = 3.4 min, HTy = 3.1 min, 1 = 5.1 min, 2 = 4.2 min, 3 = 6.8 min, 4 = 4.5 min.
4-Hydroxyphenethyl methyl carbonate (1). Yield: quantitative; white solid; Rf = 0.66 (cyclohexane/ethyl acetate 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (300 MHz, CDCl3) δ 7.09 (d, J = 8.5 Hz, 2H), 6.77 (d, J = 8.5 Hz, 2H), 4.98 (s, 1H, OH), 4.30 (t, J = 7.2 Hz, 2H), 3.77 (s, 3H), 2.90 (t, J = 7.2 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 155.8, 154.3, 130.1, 129.3, 115.4, 68.7, 54.6, 34.2.39
4); 1H NMR (300 MHz, CDCl3) δ 7.09 (d, J = 8.5 Hz, 2H), 6.77 (d, J = 8.5 Hz, 2H), 4.98 (s, 1H, OH), 4.30 (t, J = 7.2 Hz, 2H), 3.77 (s, 3H), 2.90 (t, J = 7.2 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 155.8, 154.3, 130.1, 129.3, 115.4, 68.7, 54.6, 34.2.39
3,4-Dihydroxyphenetyl methyl carbonate (2). Yield: quantitative; colourless oil; Rf = 0.52 (cyclohexane/ethyl acetate 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (300 MHz, CD3OD) δ 6.69–6.66 (m, 2H), 6.55–6.51 (m, 1H), 4.22 (t, J = 7.1 Hz, 2H), 3.71 (s, 3H), 2.78 (t, J = 7.1 Hz, 2H). 13C NMR (75 MHz, CD3OD) δ 155.9, 144.9, 143.8, 128.8, 119.7, 115.5, 114.9, 68.5, 53.7, 34.0.40
4); 1H NMR (300 MHz, CD3OD) δ 6.69–6.66 (m, 2H), 6.55–6.51 (m, 1H), 4.22 (t, J = 7.1 Hz, 2H), 3.71 (s, 3H), 2.78 (t, J = 7.1 Hz, 2H). 13C NMR (75 MHz, CD3OD) δ 155.9, 144.9, 143.8, 128.8, 119.7, 115.5, 114.9, 68.5, 53.7, 34.0.40
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for compound 3, dichloromethane/methanol 98
2 for compound 3, dichloromethane/methanol 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–95
2–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for compound 4).
5 for compound 4).
Bis(4-hydroxyphenethyl) carbonate (3). Yield: 23%; white solid; Rf = 0.45 (cyclohexane/ethyl acetate 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (300 MHz, CD3OD) δ 7.02 (d, J = 8.5 Hz, 4H), 6.70 (d, J = 8.5 Hz, 4H), 4.21 (t, J = 7.1 Hz, 4H), 2.82 (t, J = 7.1 Hz, 4H). 13C NMR (75 MHz, CD3OD) δ 155.7, 155.2, 129.5, 128.1, 114.8, 68.3, 33.8.
4); 1H NMR (300 MHz, CD3OD) δ 7.02 (d, J = 8.5 Hz, 4H), 6.70 (d, J = 8.5 Hz, 4H), 4.21 (t, J = 7.1 Hz, 4H), 2.82 (t, J = 7.1 Hz, 4H). 13C NMR (75 MHz, CD3OD) δ 155.7, 155.2, 129.5, 128.1, 114.8, 68.3, 33.8.
Bis(3,4-dihydroxyphenethyl) carbonate (4). Yield: 25%; white solid; Rf = 0.58 (dichloromethane/methanol 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CD3OD) δ 6.70–6.66 (m, 4H), 6.54–6.51 (m, 2H), 4.20 (t, J = 7.1 Hz, 4H), 2.77 (t, J = 7.1 Hz, 4H). 13C NMR (75 MHz, CD3OD) δ 155.7, 144.8, 143.5, 128.9, 119.8, 115.6, 114.9, 68.4, 29.1.
1); 1H NMR (300 MHz, CD3OD) δ 6.70–6.66 (m, 4H), 6.54–6.51 (m, 2H), 4.20 (t, J = 7.1 Hz, 4H), 2.77 (t, J = 7.1 Hz, 4H). 13C NMR (75 MHz, CD3OD) δ 155.7, 144.8, 143.5, 128.9, 119.8, 115.6, 114.9, 68.4, 29.1.
Author contributions
Conceptualization, L. T. and A. P.; methodology, S. V., F. A. and D. P.; investigation, S. V., F. A. and D. P.; resources, L. T. and A. P.; data curation, S. V. and F. A.; writing—original draft preparation, L. T., S. V. and F. A.; writing—review and editing, all authors; project administration, L. T. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was realised within the MUSA – Multilayered Urban Sustainability Action – project, funded by the European Union – NextGenerationEU, under the National Recovery and Resilience Plan (NRRP) Mission 4 Component 2 Investment Line 1.5: Strengthening of research structures and creation of R&D “innovation ecosystems”, set up of “territorial leaders in R&D”. The authors acknowledge the support of the APC central fund of the University of Milan.Notes and references
- M. Azam Rasool, P. P. Pescarmona and I. F. J. Vankelecom, ACS Sustainable Chem. Eng., 2019, 7, 13774–13785 CrossRef.
- S. Huang, B. Yan, S. Wang and X. Ma, Chem. Soc. Rev., 2015, 44, 3079–3116 RSC.
- S. Dabral, U. Licht, P. Rudolf, G. Bollmann, A. S. K. Hashmi and T. Schaub, Green Chem., 2020, 22, 1553–1558 RSC.
- N. Eghbali and C.-J. Li, Green Chem., 2007, 9, 213–215 RSC.
- J. Wu, J. A. Kozak, F. Simeon, T. A. Hatton and T. F. Jamison, Chem. Sci., 2014, 5, 1227–1231 RSC.
- M. North, R. Pasquale and C. Young, Green Chem., 2010, 12, 1514–1539 RSC.
- X. Wu, C. Chen, Z. Guo, M. North and A. C. Whitwood, ACS Catal., 2019, 9, 1895–1906 CrossRef CAS.
- K. R. Roshan, G. Mathai, J. Kim, J. Tharun, G.-A. Park and D.-W. Park, Green Chem., 2012, 14, 2933–2940 RSC.
- F. Chen, N. Liu and B. Dai, ACS Sustainable Chem. Eng., 2017, 5, 9065–9075 CrossRef CAS.
- S. Ramesh, K. Indukuri, O. Riant and D. P. Debecker, Org. Process Res. Dev., 2018, 22, 1846–1851 CrossRef CAS.
- H. Büttner, J. Steinbauer and T. Werner, ChemSusChem, 2015, 8, 2655–2669 CrossRef PubMed.
- D. Chevella, A. K. Macharla, R. Banothu, K. S. Gajula, V. Amrutham, M. Boosa and N. Nama, Green Chem., 2019, 21, 2938–2945 RSC.
- M. Cokoja, M. E. Wilhelm, M. H. Anthofer, W. A. Herrmann and F. E. Kühn, ChemSusChem, 2015, 8, 2436–2454 CrossRef CAS.
- F. Liang, M. Yanai, Y. Suzuki and A. Tsuda, Org. Lett., 2020, 22, 3566–3569 CrossRef CAS PubMed.
- J. Pauluhn, Toxicology, 2021, 450, 152682 CrossRef CAS PubMed.
- J. Schneider, H. Jia, J. T. Muckerman and E. Fujita, Chem. Soc. Rev., 2012, 41, 2036–2051 RSC.
- N. Kumar and N. Goel, Biotechnol. Rep., 2019, 24, e00370 CrossRef PubMed.
- A. Crozier, I. B. Jaganath and M. N. Clifford, Nat. Prod. Rep., 2009, 26, 1001–1043 RSC.
- S. J. Marathe, N. Dedhia and R. S. Singhal, Curr. Opin. Food Sci., 2022, 43, 163–173 CrossRef CAS.
- B. Liu and W. Yan, Food Chem., 2019, 272, 663–669 CrossRef CAS PubMed.
- S. Farooq, Abdullah, H. Zhang and J. Weiss, Compr. Rev. Food Sci. Food Saf., 2021, 20, 4250–4277 CrossRef CAS.
- R. Bernini, M. Barontini, V. Cis, I. Carastro, D. Tofani, R. A. Chiodo, P. Lupattelli and S. Incerpi, Molecules, 2018, 23, 208 CrossRef PubMed.
- Y. Sun, D. Zhou and F. Shahidi, Food Chem., 2018, 245, 1262–1268 CrossRef CAS PubMed.
- S. D. Sommerfeld, N. S. Murthy, J. Cohen, Z. Zhang, J. A. Kaduk and J. Kohn, Macromolecules, 2020, 53, 9878–9889 CrossRef CAS.
- D. Bolikal and P. J. Kohn, US Pat., WO/2013/116804, 2013.
- P. Tundo, M. Musolino and F. Aricò, Green Chem., 2018, 20, 28 RSC.
- S.-H. Pyo, J. Hoon Park, T.-S. Chang and R. Hatti-Kaul, Curr. Opin. Green Sustainable Chem., 2017, 5, 61–66 CrossRef.
- V. Gotor-Fernández, E. Busto and V. Gotor, Adv. Synth. Catal., 2006, 348, 797–812 CrossRef.
- Y. Zhou, Q. Jin, Z. Gao, H. Guo, H. Zhang and X. Zhou, RSC Adv., 2014, 4, 7013–7018 RSC.
- M. Barontini, R. Bernini, I. Carastro, P. Gentili and A. Romani, New J. Chem., 2014, 38, 809–816 RSC.
- T. Toupy, L. Bovy and J.-C. M. Monbaliu, J. Flow Chem., 2022, 12, 207–217 CrossRef CAS.
- R. Bernini, F. Crisante, N. Merendino, R. Molinari, M. C. Soldatelli and F. Velotti, Eur. J. Med. Chem., 2010, 46, 439–446 CrossRef PubMed.
- E. Fortunati, F. Luzi, L. Dugo, C. Fanali, G. Tripodo, L. Santi, J. M. Kenny, L. Torre and R. Bernini, Polym. Int., 2016, 65, 872–882 CrossRef CAS.
- M. Barontini, I. Proietti Silvestri, V. Nardi, P. Bovicellu, L. Pari, F. Gallucci, R. Spezia and G. Righi, Tetrahedron Lett., 2013, 54, 5004–5006 CrossRef CAS.
- F. Annunziata, M. L. Contente, C. Pinna, L. Tamborini and A. Pinto, Antioxidants, 2021, 10, 1142 CrossRef CAS PubMed.
- E. Catoni, E. Cernia and C. Palocci, J. Mol. Catal. A: Chem., 1996, 105, 79–86 CrossRef CAS.
- S. D. Ramgren, L. Hie, Y. Ye and N. K. Garg, Org. Lett., 2013, 15, 3950–3953 CrossRef CAS PubMed.
- B. Zieniuk, A. Fabiszewska and E. Białecka-Florjańczyk, Bioprocess Biosyst. Eng., 2020, 43, 605–613 CrossRef CAS PubMed.
- K. M. Jones, T. Hillringhaus and M. Klussmann, Tetrahedron Lett., 2103, 54, 3294–3297 CrossRef.
- R. Bernini, E. Mincione, F. Crisante, M. Barontini, G. Fabrizi and P. Gentili, Tetrahedron Lett., 2007, 48, 7000–7003 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra04735k |
| ‡ These Authors equally contributed to the work. |
| This journal is © The Royal Society of Chemistry 2023 |