Open Access Article
Open Access ArticleTheoretical study of Ponceau S oxidation using the electro-Fenton process under optimal operational conditions
Yasmine Laftani *,
Baylassane Chatib
*,
Baylassane Chatib ,
Abdelghani Boussaoud and
Mohsine Hachkar
,
Abdelghani Boussaoud and
Mohsine Hachkar
Laboratory of Process, Signals, Industrial Systems and Computer Science, Graduate School of Technology, Cadi Ayyad University, Morocco. E-mail: laftani90yasmine@gmail.com; Tel: +21-2639941253
First published on 2nd November 2023
Abstract
Electrochemical methods as one of the Advanced Oxidation Processes (AOPs) have been applied effectively to the degradation of recalcitrant organic molecules in aqueous solutions. In the present study, the performance of the electro-Fenton (EF) process on the oxidation of Ponceau S(PS) dye was studied. The experimental study performed at the optimal factors like the solution pH, the PS concentration and the ferrous ions dose provided 74.35% of PS degradation. The results, however, showed a decreased removal efficiency of PS when using sodium sulphate as the supporting electrolyte. From a theoretical point of view, the hydroxyl radical being an electron acceptor and the PS dye an electron donor, from a theoretical point of view, the hydroxyl radical being an electron acceptor and the PS dye an electron donor, furthermore, the nitrogen atom 2N being the most nucleophilic site of the PS dye with the most electrophilic site of the hydroxyl radical being the oxygen atom, the first stage of the reaction between PS and the hydroxyl radical was suggested.
1. Introduction
The textile industries are one of the most water-consuming sectors, estimated at several thousand cubic meters (m3) per day.1 Their effluents can be very coloured and resistant to biodegradation.2,3 Consequently, they can cause a wide variety of environmental and health problems. Regarding synthetic dyes released in effluents from textile industries, azo dyes represent almost 50% of about 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of dyes produced in the world.4,5 As a di-azo dye, Ponceau S (PS), frequently detected in various water environments, is a kind of refractory organic matter that can put humans and other organisms in danger.6,7
000 tons of dyes produced in the world.4,5 As a di-azo dye, Ponceau S (PS), frequently detected in various water environments, is a kind of refractory organic matter that can put humans and other organisms in danger.6,7
Several technologies such as biodegradation, adsorption and oxidation methods have not yet shown success in degrading such refractory organics.8 Depending on the nature and extent of the pollution, different processes can be employed for the purification of such effluents. Advanced oxidation processes (AOPs) are considered as high-efficiency physical–chemical processes due to their thermodynamic viability and capability to degrade or even mineralize a wide range of contaminants via the participation of free radicals,9,10 mainly HO˙ hydroxyl radicals.11,12 The electro-Fenton (EF) process is a potentially effective AOP for treating several kinds of effluents.1 There are notable advantages of electrochemistry, including energy efficiency, versatility and environmental suitability as the electrons and main-stream reagents are clean. Hence, by coupling electrochemistry with the Fenton process, the oxidation efficiency can be significantly improved.13
The electro-Fenton oxidation process involves either oxidizing Fe2+ or reducing Fe3+ electrochemically along with the simultaneous production of H2O2. The H2O2 is electro-generated by the reduction of O2 on the cathode, and then H2O2 reacts with Fe2+ to produce ferric ions, OH− and hydroxyl radicals for the degradation of organics.14,15 The molecular oxygen necessary for the production of hydrogen peroxide is regenerated at the anode by the oxidation of water.16
The electro-Fenton process can overcome the drawbacks of the traditional Fenton process, including the risk derived from storage and transportation of the concentrated H2O2, and the formation of large quantities of iron sludge. Meanwhile, due to the main performed reaction, it can avoid mass transfer limitations encountered during electrochemical oxidation. Moreover, the electro-Fenton process does not use any harmful and toxic materials; it is an environmentally friendly and low-cost method for treating wastewater.17 However, the electro-Fenton process has been found to suffer from the low efficiency of Fe3+/Fe2+ cycle and is limited to operating at conditions of low pH.18
Researchers have been using computational methods for achieving descriptions of molecular structures, spectroscopic properties and molecular reactivity.19 Depending on the overall electronic character of the bond formation and/or bond breaking during the reaction, organic reactions can be categorised as non-polar or polar. Most organic molecules with polarised functional groups present a polar reactivity, which is characterised by a nucleophilic/electrophilic interaction. While electrophiles are molecules able to accept electron density during the reaction, nucleophiles are molecules able to donate electron density during the reaction. The electrophilic or nucleophilic power of a molecule is associated with its ability to exchange electron density during a reaction.20
In the current work, we applied electrochemical Fenton processes to the degradation of an aromatic pollutant, namely the PS dye, and investigated the reaction mechanism of the PS dye degradation using theoretical studies, namely density functional theory (DFT) with the DFT B3LYP calculation method and the 6-31G(d,p) basis set. The experimental studies were performed under optimal operating conditions.
2. Materials and methods
2.1. Materials
The PS dye, a tetrasodium salt of 3-hydroxy-4-({2-sulfo-4-[(4-sulfophenyl)-diazenyl]-phenyl}-diazenyl)-naphthalene-2,7-disulfonic acid, was purchased from Reactifs Ral. Hydrogen peroxide (H2O2, 50%) was obtained from Prochilabo. FeSO4·7H2O (99%) and H2SO4 (98%) were obtained from Sigma-Aldrich. NaOH was purchased from Scharlau.All chemical reagents were of analytical grade. Synthetic solutions of PS dye were prepared with distilled water obtained using a GFL 2004 apparatus.
2.2. Experimental
The electrochemical treatment was carried out in an electrochemical cell, which consisted of a stirred reactor with two electrodes: vitreous carbon as the working electrode and platinum (Pt) as the anode. The current applied between the electrodes was produced using a current generator under 2.5 V of voltage.The treated solutions were each kept stirred to avoid mass transport to/from the electrodes. The cell was filled with 25 mL of a solution containing 0.06 mM of PS at 25 °C. During each experiment, oxygen saturation was provided by using an air pump for 30 minutes. The solutions were each acidified with sulfuric acid (H2SO4) to avoid precipitation of ferric ions as hydroxides, and a pH meter (Hach Sension+) was used to measure the pH of the solutions. Samples with volumes of 2 mL were taken at regular intervals and analysed to evaluate the variation of the residual PS dye dose.
2.3. Analytical methods
UV-visible spectra of PS diazo dye were recorded from 200 to 800 nm using a UV-visible spectrophotometer (Rayleigh UV-1800) with a spectrometric quartz cell (1 cm path length). The maximum absorbance wavelength (λmax) of the PS dye could be found at 520 nm. The obtained data were analysed using Excel software.The PS disappearance efficiency was calculated using the equation
 | (1) |
2.4. Theoretical
All calculations were optimized using the B3LYP (Becke – 3 parameters – Lee Yang Parr) functional with the standard 6-31G(d) basis set. All computations were carried out with Gaussian 09 software. Combined with the 6-31G(d) basis set, B3LYP has become the method of choice for efficient and accurate computation of most chemical properties. It is known for its relatively high accuracy in predicting molecular structures, energies, and properties of a wide range of chemical systems including organic, inorganic, and biological molecules. B3LYP has played a crucial role in predicting reaction mechanisms, electronic structures, and spectroscopic properties. Its accuracy and versatility make it an appreciated tool in these areas.21Furthermore, 6-31G is known for being a reasonably accurate and computationally efficient basis set for a wide range of molecular systems, making it a popular choice for many applications.20
3. Results and discussion
3.1. Degradation of PS dye using the EF process
| PS degradation efficiency (%) | |
|---|---|
| Graphite carbon electrode | Vitreous carbon electrode |
| 17.93% | 74.35% |
When using the vitreous carbon cathode, a PS dye degradation efficiency of 74.35% was achieved. This cathode having also been shown to display technologically important characteristics such as high hydrogen overpotential, good conductivity, strong chemical resistance, and a smooth surface to which gas and dirt do not easily adhere,28 it was used for the rest of this work.
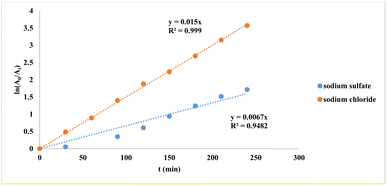
Na2SO4 was tested instead as the supporting electrolyte to avoid the effects of Cl2 and ClO− generated at the anode when using NaCl. However, the use of Na2SO4 might have led to a passivation of the platinum electrode in the current work. NaCl showed the apparent advantage of increasing the solution conductivity during electrolysis and hence increasing the PS degradation efficiency.
3.2. Theoretical study
Many investigations have confirmed hydroxyl radicals to be the primary active substance for oxidizing PS dye. In order to elucidate the first step of the mechanism of the reaction between the PS dye and the hydroxyl radicals, a pathway based on DFT concepts was modelled. This approach in general allows access to global and local parameters to justify and predict the chemoselectivity and regioselectivity of the attack of reactive species on a substrate.22The global electrophilicity descriptor (ω) is given using the expression
| ω = (μ2/2η) | (2) |
| S = (1/η). | (3) |
Using the theorem developed by Koopmans and Kohn–Sham, both quantities may be approached in terms of frontier molecular orbitals, namely the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), using the equations
| μ = (EHOMO + ELUMO)/2 | (4) |
| η = (ELUMO − EHOMO)/2. | (5) |
In 2008, an empirical (relative) nucleophilicity N index for closed-shell organic molecules based on the HOMO energies, obtained within the Kohn–Sham theorem, was proposed24—with this index defined using the equation
| N = EHOMO(nucleophile) − EHOMO(TCE), | (6) |
| EHOMO (eV) | −5.864048 |
| ELUMO (eV) | −3.05184 |
| μ | −4.457944 |
| η (eV) | 2.812208 |
| ω (eV) | 3.533391 |
| N | 3.130448 |
| Dipole moment (debye) | 7.4105 |
| S | 0.355592 |
| Eα,0HOMO | −9.74 |
| Eβ,0HOMO | −9.00 |
| Eα,0LOMO | −1.21 |
| Eβ,0LOMO | −5.07 |
| μ° | −7.40 |
| η° | 4.67 |
| S° | 0.21 |
| ω° | 5.87 |
| N0 | — |
| Dipole moment | 2.07 |
(a). Global reactivity descriptors. No electron density exchange occurs during non-polar organic reactions involving neutral radical species. However, certain substitutions in the radical species may favour the occurrence of electron density exchange, thereby determining the chemical reactivity of these radical species.6 Consequently, a set of DFT reactivity indices have recently been proposed for the study of the reactivity of free radicals. The global nucleophilicity N0 of free radicals is presented by the equation using the equation
| N0 = Eα,0HOMO(nucleophile) − Eα,0HOMO(DCM), | (7) |
A higher value of ω indicates a more electrophilic agent. The PS molecule was determined to have a global electrophilicity index (ω) of 3.533391 eV, compared to the value of the hydroxyl radical (ω° = 5.87 eV), indicating a role as a nucleophile agent played by the PS dye and an electrophile role played by the hydroxyl radical.
A low chemical potential (high electronegativity) is associated with a good electrophile. The values of the calculated chemical potentials and those of the hardness were calculated to be μ = −4.457944 eV and η = 2.812208 eV for PS and μ° = −7.40 eV and η° = 4.67 eV for the hydroxyl radical, in agreement with the above results.
The reaction between the most electrophilic centre of an electrophilic reactant and the most nucleophilic centre of a nucleophilic reactant is the most important step of the reaction pathway during a reaction involving asymmetric reactants. Note that in the Fukui (HOMO and LUMO) frontier orbital approximation, a smaller HOMO–LUMO separation indicates a stronger interaction and a more favoured reaction.
(b). Local reactivity descriptors. In general in a local reactivity study, the Parr functions Pk+ and Pk− are essential tools for characterizing the most electrophilic and nucleophilic centres of the reactants involved in a polar reaction process, and for subsequently explaining any regioselectivity.25
Recent studies of polar cycloaddition reactions have shown that the most favourable regioisomeric channel is that involving the bond formation between the most electrophilic and most nucleophilic centres of the reagents. Consequently, it is desirable to have local reactivity indices able to characterise these relevant centres in organic molecules.
Remarkably, analysis of the atomic spin density (ASD) at the radical cation and the radical anion gives a picture of the distribution of the electron density in the electrophile and the nucleophile when they approach each other during the progress of the reaction.26
In 2014, Domingo proposed the Parr functions P(r), which are given by the equations
| Pk− = ρrcs for electrophilic attacks | (8) |
| Pk+ = ρras for nucleophilic attacks, | (9) |
| Atom | Pk+ | Pk+ | ωk | Nk |
|---|---|---|---|---|
| 1 N | 0.050112 | 0.190216 | 0.672107632 | 0.15687301 |
| 2 N | 0.169089 | 0.047884 | 0.169192927 | 0.529324322 |
| 3 C | 0.00261 | 0.092006 | 0.325093235 | 0.008170469 |
| 4 C | 0.068599 | 0.019359 | 0.06840293 | 0.214745602 |
| 5 C | −0.040773 | 0.009958 | 0.035185514 | −0.127637756 |
| 6 C | −0.033596 | 0.054368 | 0.192103439 | −0.105170531 |
| 7 C | 0.112171 | 0.039881 | 0.140915194 | 0.351145483 |
| 8 H | 0.001281 | −0.000759 | −0.002681844 | 0.004010104 |
| 9 H | 0.00097 | −0.002899 | −0.010243302 | 0.003036535 |
| 10 N | −0.040637 | 0.083247 | 0.294144257 | −0.127212015 |
| 11 N | 0.061533 | 0.206282 | 0.728875103 | 0.192625857 |
| 12 C | −0.007344 | −0.037652 | −0.133039264 | −0.02299001 |
| 13 C | 0.028599 | 0.082266 | 0.290678 | 0.089527682 |
| 14 C | 0.030137 | 0.063158 | 0.223161952 | 0.094342311 |
| 15 C | −0.016321 | −0.041681 | −0.147275299 | −0.051092042 |
| 16 H | −0.001152 | −0.003941 | −0.013925097 | −0.003606276 |
| 17 C | −0.017562 | −0.031342 | −0.110743562 | −0.054976928 |
| 18 H | −0.001304 | −0.003053 | −0.010787445 | −0.004082104 |
| 19 C | 0.040118 | 0.084638 | 0.299059205 | 0.125587313 |
| 20 H | 0.00054 | 0.001476 | 0.005215286 | 0.001690442 |
| 21 H | 0.000586 | 0.001061 | 0.003748929 | 0.001834443 |
| 22 S | −0.002229 | −0.000179 | −0.000632477 | −0.006977769 |
| 23 C | 0.181536 | −0.063465 | −0.224246703 | 0.568289008 |
| 24 C | −0.054631 | 0.055936 | 0.197643797 | −0.171019505 |
| 25 C | 0.087416 | 0.091947 | 0.324884765 | 0.273651242 |
| 26 C | −0.005565 | −0.039358 | −0.13906723 | −0.017420943 |
| 27 C | 0.096312 | −0.028065 | −0.099164638 | 0.301499708 |
| 28 C | −0.046193 | −0.041896 | −0.148034978 | −0.144604784 |
| 29 C | 0.108007 | 0.126196 | 0.445899897 | 0.338110297 |
| 30 C | 0.036175 | 0.030762 | 0.108694195 | 0.113243956 |
| 31 C | −0.01274 | 0.036523 | 0.129050064 | −0.039881908 |
| 32 H | −0.003546 | 0.0005 | 0.001766696 | −0.011100569 |
| 33 H | −0.004312 | −0.005815 | −0.020546673 | −0.013498492 |
| 34 C | 0.050838 | −0.024772 | −0.087529179 | 0.159145715 |
| 35 H | −0.001405 | −0.001271 | −0.004490941 | −0.004398279 |
| 36 H | 0.000092 | −0.001662 | −0.005872497 | 0.000288001 |
| 37 S | 0.001359 | 0.000393 | 0.001388623 | 0.004254279 |
| 38 S | −0.003368 | 0.000384 | 0.001356822 | −0.010543349 |
| 39 O | 0.00536 | −0.000789 | −0.002787846 | 0.016779201 |
| 40 O | −0.000489 | −0.001128 | −0.003985666 | −0.001530789 |
| 41 O | 0.001448 | 0.001393 | 0.004922015 | 0.004532889 |
| 42 O | 0.003904 | 0.003645 | 0.012879213 | 0.012221269 |
| 43 O | 0.002754 | −0.000219 | −0.000773813 | 0.008621254 |
| 44 O | −0.000154 | −0.000621 | −0.002194236 | −0.000482089 |
| 45 O | 0.088401 | 0.020142 | 0.071169575 | 0.276734734 |
| 46 H | −0.002092 | −0.000542 | −0.001915098 | −0.006548897 |
| 47 S | −0.004888 | 0.000398 | 0.00140629 | −0.01530163 |
| 48 O | 0.007007 | 0.001361 | 0.004808946 | 0.021935049 |
| 49 O | −0.000279 | 0.000608 | 0.002148302 | −0.000873395 |
| 50 C | 0.058706 | −0.01584 | −0.055968924 | 0.18377608 |
| 51 H | −0.002461 | 0.000227 | 0.00080208 | −0.007704033 |
| 52 O | 0.001584 | 0.00131 | 0.004628743 | 0.00495863 |
| 53 O | 0.003464 | 0.000422 | 0.001491091 | 0.010843872 |
| 54 O | 0.003123 | −0.000476 | −0.001681894 | 0.009776389 |
| 55 O | −0.000418 | −0.000569 | −0.0020105 | −0.001308527 |
| 56 Na | 0.000051 | 0.000267 | 0.000943416 | 0.000159653 |
| 57 Na | −0.000159 | 0.00007 | 0.000247337 | −0.000497741 |
| 58 Na | −0.000195 | 0.00004 | 0.000141336 | −0.000610437 |
| 59 Na | −0.000072 | −0.000333 | −0.001176619 | −0.000225392 |
The local radical Parr function P0k is defined as
| P0k = ρsk, | (10) |
| ω0k = ω0P0k | (11) |
| N0k = N0P0k. | (12) |
The analysis of the Parr functions of the PS dye indicated the 2N atom (N2N = 0.529 eV) to be the most nucleophilic centre of this molecule. On the other hand, the Parr function analysis of the hydroxyl radical indicated the oxygen atom (6.02 eV) to be the centre concentrating the local electrophilicity of this species.
Due to the hydroxyl radicals being electrophilic species, the dye hydroxylation would only take place at electron-rich sites, such as amino groups or those of the azo bond.
According to the literature, 60% of electrophilic attack reactions involving azo-containing compounds start with an addition onto the double bond of the azo group. The addition of hydroxyl radicals to the azo double bond could be considered as the first step in the degradation process. Once the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond has been broken, the aromatic compounds can in turn be attacked by hydroxyl radicals.
N– bond has been broken, the aromatic compounds can in turn be attacked by hydroxyl radicals.
The diazinyl group was confirmed, based on analysis of the regioselectivity indicators, to be the most nucleophilic centre of PS dye, with this centre first attacked by the hydroxyl radicals.
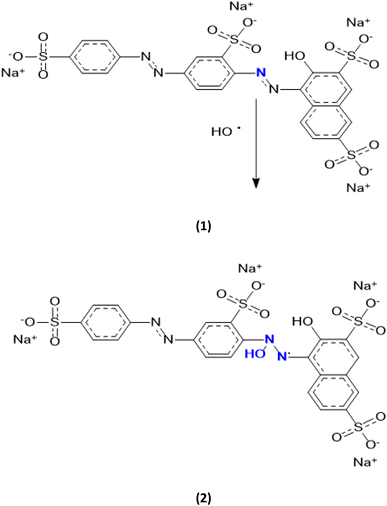
Thus, the most favourable electrophilic/nucleophilic interaction would be between the 2N nucleophilic centre of PS and the O electrophilic centre of the hydroxyl radicals and which would give rise to the compound (2) presented in Fig. 3.
4. Conclusion
The combination of chemical and electrochemical procedures is considered to be a good alternative to water treatment. At the optimized conditions, PS dye was removed at an efficiency of 74.35%. Under the optimized conditions, the maximum degradation of PS dye was obtained by using sodium chloride as a supporting electrolyte.Theoretical DFT calculation estimating the global and local reactivity indices demonstrated an electron donor behaviour displayed by the PS dye and an electron acceptor behaviour displayed by the hydroxyl radical. An analysis involving Parr functions indicated the local attack to be between nitrogen atom 2N of the PS dye and the oxygen atom of hydroxyl radical.
Conflicts of interest
There are no conflicts to declare.References
- B. Louhichi, F. Gaied, K. Mansouri and M. R. Jeday, Chem. Eng. J., 2021, 427, 131735 CrossRef.
- Y. Laftani, A. Boussaoud, B. Chatib, M. El Makhfouk, M. Hachkar and M. Khayar, Maced. J. Chem. Chem. Eng., 2019, 38, 197 CrossRef CAS.
- B. Chatib, Y. Laftani, M. Khayar, M. El Makhfouk, M. Hachkar and A. Boussaoud, Iran. J. Chem. Chem. Eng., 2021, 40, 111–121 Search PubMed.
- N. Bougdour, A. Sennaoui, I. Bakas and A. Assabbane, Sci. Technol. Adv. Mater., 2018, 1–9 Search PubMed.
- N. P. Tantak and S. Chaudhari, J. Hazard. Mater., 2006, 136, 698–705 CrossRef CAS PubMed.
- F. Z. Ankouri, H. Lamkhanter, A. Jaafar, Z. Lakbaibi and H. Mountacer, Chem. Afr., 2023, 6, 1495–1513, DOI:10.1007/s42250-022-00579-y.
- M. Behrouzeh, et al. , Arabian J. Chem., 2022, 15, 104229, DOI:10.1016/j.arabjc.2022.104229.
- Z. He, C. Gao, M. Qian, Y. Shi, J. Chen and S. Song, Ind. Eng. Chem. Res., 2014, 53, 3435–3447 CrossRef CAS.
- Y. Laftani, B. Chatib, A. Boussaoud, M. El Makhfouk, M. Hachkar and M. Khayar, Water Sci. Technol., 2019, 80, 1731–1739, DOI:10.2166/wst.2019.424.
- Y. Laftani, B. Chatib, A. Boussaoud, M. Hachkar and M. El Makhfouk, Water Qual. Res. J., 2022, 1–16 Search PubMed.
- Y. Laftani, A. Boussaoud, B. Chatib, M. Hachkar, M. El Makhfouk and M. Khayar, Environ. Eng. Res., 2021, 27, 210002 CrossRef.
- M. A. Quiroz, E. R. Bandala and C. A. Martínez-huitle, Pesticides, in Advanced Oxidation Processes (AOPs) for Removal of Pesticides from Aqueous Media, 2007 Search PubMed.
- R. Javaid and U. Y. Qazi, Int. J. Environ. Res. Public Health, 2019, 16, 1–27 Search PubMed.
- E. Brillas, M. Á. Baños, M. Skoumal, P. L. Cabot, J. A. Garrido and R. M. Rodríguez, Chemosphere, 2007, 68, 199–209 CrossRef CAS PubMed.
- Y. Laftani, Procédés de dépollution par oxydation avancée: cinetique & planification experimentale-cas du colorant ponceau S, Cadi Ayyad University, 2021 Search PubMed.
- J. Li, Y. Li, Z. Xiong, G. Yao and B. Lai, Chin. Chem. Lett., 2019, 30, 2139–2146 CrossRef CAS.
- A. R. Rahmani, A. Shabanloo, J. Mehralipou, M. Fazlzadeh and Y. Poureshgh, Res. J. Environ. Sci., 2015, 9, 332–341 CrossRef CAS.
- D. Li, T. Zheng, J. Yu, H. He, W. Shi and J. Ma, J. Ind. Eng. Chem., 2022, 105, 405–413 CrossRef CAS.
- A. Jaafar, et al., Res. J. Chem. Environ., 2021, 25, 74–78 CAS.
- L. R. Domingo, P. Pe and J. A. Saez, RCS Adv., 2013, 1486–1494 CAS.
- K. Raghavachari, J. Chem. Phys., 2000, 361–363 CAS.
- H. Chermette, J. Comput. Chem., 1999, 20, 129–154 CrossRef CAS.
- L. R. Domingo and P. Pérez, Org. Biomol. Chem., 2011, 9, 7168–7175 RSC.
- L. R. Domingo, M. Ríos-gutiérrez and P. Pérez, Molecules, 2016, 21(6), 7482016 CrossRef.
- A. Jaafar, et al., J. Chem. Res., 2019, 2, 53–61 Search PubMed.
- P. Caregnato, et al., J. Phys. Chem. A, 2008, 112, 1188–1194 CrossRef CAS PubMed.
- S. Singireddy, et al., Origins Life Evol. Biospheres, 2012, 42, 275–294 CrossRef CAS PubMed.
- J. M. Friedrich, C. Ponce-de-León, G. W. Reade and F. C. Walsh, J. Electroanal. Chem, 2004, 561, 203–217 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |