Open Access Article
Open Access ArticleIntramolecular cyclization of N-cyano sulfoximines by N–CN bond activation†
Ye Ji Seo‡
ab,
Eunsil Kim‡ac,
In Seok Oh‡§
ac,
Ji Young Hyunab,
Ji Ho Songab,
Hwan Jung Lim *ab and
Seong Jun Park
*ab and
Seong Jun Park *ab
*ab
aDepartment of Drug Discovery, Korea Research Institute of Chemical Technology (KRICT), 141 Gajeong-ro, Yuseong-gu, Daejeon 34114, Republic of Korea. E-mail: sjunpark@krict.re.kr; Fax: +82 42 860 7160; Tel: +82 42 860 7175
bPharmaceutical Chemistry, University of Science & Technology, Daejeon 34113, Republic of Korea
cDepartment of Chemistry, Sogang University, 35 Baekbeom-ro, Mapo-gu, Seoul 04107, Republic of Korea
First published on 14th August 2023
Abstract
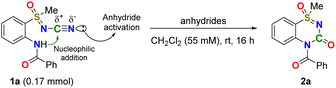
Metal-free halogenated anhydrides promote the intramolecular cyclization of N-cyano sulfoximines. Trifluoro- or trichloroacetic anhydride (TFAA or TCAA, respectively) activate the N-cyano groups of N-cyano sulfoximines, leading to the intramolecular cyclization of 2-benzamide-N-cyano sulfoximines 1. This method results in excellent yields of thiadiazinone 1-oxides 2. A full intramolecular cyclization pattern was suggested by (i) labeling experiments with 13C, (ii) isolating of N-trifluoroacetyl sulfoximine 1ac, and (iii) confirming the generation of the intermediate 1ad by LC/MS analysis.
Introduction
N-Cyano sulfoximines (CN group-substituted sulfoximidoyl moieties) are readily accessible1–6 and remarkably stable.7 Consequently, they are widely accepted as key molecules for drug development8–10 and crop protection (Fig. 1a).11–13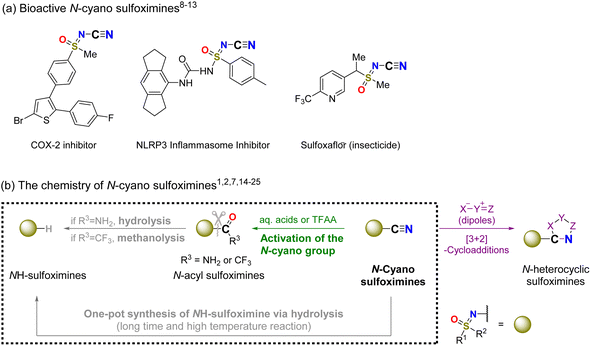 | ||
| Fig. 1 (a) Bioactive N-cyano sulfoximes,8–13 (b) cleavage of the N-cyano group under aqueous acidic conditions,1,2,7,14–16 [3 + 2]-cycloadditions of N-cyano sulfoximines,17–20 and activation of N-cyano group.21–25 | ||
In addition, owing to the existence of a well-designed method for the cleavage of the N–CN bond, N-cyano sulfoximines have been applied as useful intermediates in the synthesis of NH sulfoximines (Fig. 1b).1,2,7,14–16
While transformations of N-cyano sulfoximines, such as [3 + 2]-cycloadditions, occur at both the carbon and nitrogen atoms of the N-cyano group,17–20 acid-catalyzed hydrolysis methods have been reported for the cleaving of bonds between nitrogen and the cyano groups.1,2,7,14–16 For hydrolysis with aqueous acids, the choice of acid influences the hydrolysis of product; thus, N-urea sulfoximines, a synthetic intermediate of NH sulfoximine, can be produced.15,26 Furthermore, owing to its strong electrophilic properties, trifluoroacetic anhydride (TFAA) tends to react with relatively weak nucleophiles, such as nitrile groups; based on this strategy, interesting transformations have been applied in the synthesis of N-trifluoroacetyl sulfoximines (Fig. 1b).24,25
As a representative example of cyclic sulfoximines, benzothiadiazine-1-oxide derivatives, which exhibit improved pharmacological properties, showed enhanced water solubility compared to the 4-aminoquinazoline group of the reference compound Prazosin.27 Previously, using the strategy of cleaving the N–CN bonds of N-cyano sulfoximines, we reported the synthesis of thiadiazine 1-oxides via acid-catalyzed intramolecular cyclization (Fig. 2).26
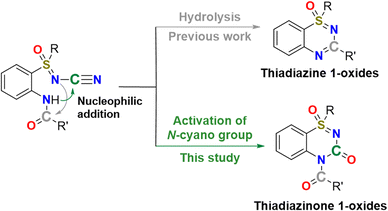 | ||
| Fig. 2 Our approaches for the synthesis of thiadiazine 1-oxides26 and thiadiazinone 1-oxides. | ||
The highlight of our method is that it is a metal-free, one-pot reaction using an aqueous acid solution. However, despite the impressive progress made in the development of synthetic routes in recent decades, the introduction of a sulfoximinoyl moiety into a heterocyclic ring system remains challenging owing to the requirement for harsh reaction conditions, expensive transition-metal catalysts, and noncommercial amination reagents.26,28 To establish highly efficient intramolecular cyclization under mild conditions, our study focused on the N–CN bond activation approach (Fig. 2). Specifically, chemical modifications were designed to maintain the carbon atom of the N-cyano groups of N-cyano sulfoximines in the molecular structures of the products.29 Because of the presence of a lone pair of electrons on the nitrogen atom, the cyano group acts as a Brønsted and Lewis base.30–34
Results and discussion
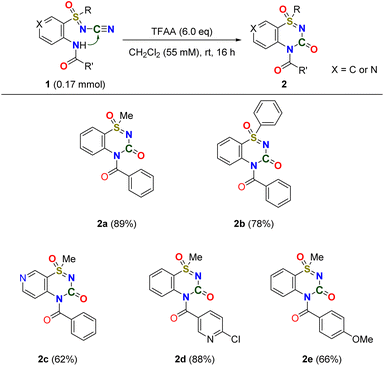
We examined metal-free nitrile activation using various anhydrides as the electrophilic reagents. Reacting N-cyano sulfoximine 1a with trichloroacetic anhydride (TCAA) and trifluoroacetic anhydride (TFAA)24,25 in CH2Cl2 for 16 h at room temperature afforded the desired thiadiazinone 1-oxide 2a in yield of 26% and 45%, respectively (entry 1, Table 1). Interestingly, compared to the trichloromethyl (–CCl3) group, the trifluoromethyl (–CF3) group showed an enhanced yield owing to its strong electron-withdrawing properties.35–37 We then screened the amount of anhydrides; the best result (89% yield) was obtained when 6 equiv. of TFAA was added (entry 3, Table 1). The use of other anhydrides, such as acetic, benzoic, and Boc anhydrides, was unsuccessful. Similarly, the use of trifluoromethanesulfonic anhydride (Tf2O)38 or methanesulfonic anhydride (Ms2O) as the electrophilic reagent did not afford the desired 2a. Thus, the influence of the choice of electrophilic reagent on this reaction was demonstrated. It is reasonable to assume that the relationship between electrophiles and nucleophiles, such as halogenated anhydride and nitrile, is crucial for obtaining the desired product.The scope of the reaction was examined under optimized reaction conditions (Scheme 1). For S-methyl and S-phenyl sulfoximines, the desired benzothiadiazinone 1-oxides 2a and 2b were obtained in excellent yields (89% and 78%, respectively). For both heterocycle-substituted N-cyano sulfoximines 1c and 1d, the cyclized products 2c and 2d were obtained in excellent yields (62% and 88%). The electron-donating methoxy group in the N-benzamide position also provided an excellent yield, affording 2e in 66% yield.
We report the X-ray crystal structure of thiadiazinone 1-oxide 2b′, as shown in Fig. 3.39,40
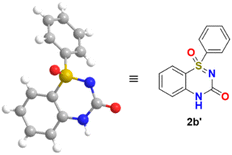 | ||
| Fig. 3 X-ray crystal structures of 2b′.39,40 | ||
We considered a mechanism involving N–CN activation, Mumm rearrangement involving O-to-N acyl group migration,41,42 and intramolecular nucleophilic addition. It is reasonable to propose the formation of intermediate 1aa by TFAA-promoted N-cyano group activation. The hypothesis involving O-to-N acyl group migration is attractive because the carbonyl group is a typical site for intramolecular nucleophilic addition.43 This hypothesis was successfully confirmed by the generation of the intermediate 1ad (Scheme 2, supported by LC/MS analysis experiments).44 The results of our study on N-acylated sulfoximine 1ac, which was unreactive, isolable, and very stable, clearly support the proposed mechanism of intramolecular cyclization.45
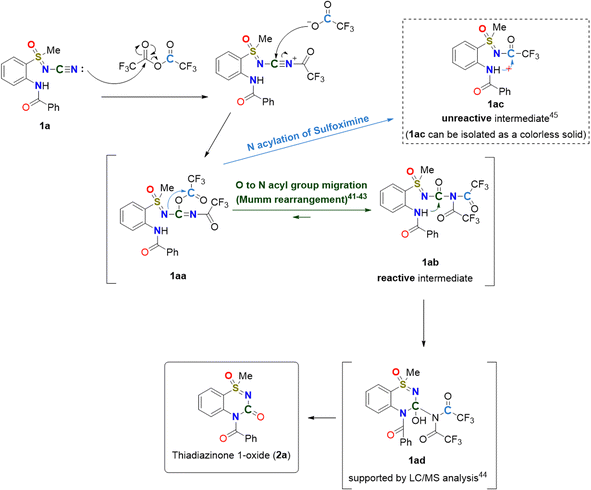 | ||
| Scheme 2 Proposed mechanism.41–45 | ||
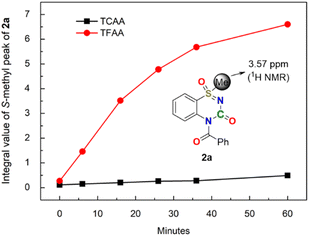
The kinetics of nitrile activation using halogenated anhydrides were monitored using time-resolved NMR spectroscopy. The spectra, recorded during the reaction of 1a with TFAA for 1 h, is illustrated in ESI.† By plotting the integral value of the S-methyl peak of the desired product 2a (3.57 ppm, when integral value of the TMS peak is 1), it can be observed that the concentration of 2a rapidly increased with time when TFAA was used, whereas it remained almost unchanged when TCAA was used (Fig. 3). This experiment proves that the strongly electrophilic TFAA readily reacts with weakly nucleophilic cyano groups (Fig. 4).
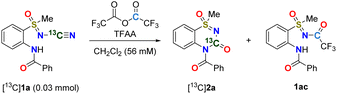
To demonstrate that the carbon atom in the resulting molecular structure was derived from the N-cyano group, N-cyano sulfoximine [13C]1a was prepared using a13C-labeled cyanamide reagent. The reaction of N-cyano sulfoximine [13C]1a with TFAA (6 equiv.) in CH2Cl2 for 16 h at room temperature readily afforded the desired thiadiazinone 1-oxide [13C]2a (entry 1, Table 2). In the case of low temperatures (−10 °C), however, N-trifluoroacetyl sulfxomine 1ac was obtained (entry 2, Table 2). Regarding the mechanism (Scheme 2), compound 1ac was formed by the N-acylation of sulfoximine with TFAA. Importantly, this hypothesis was supported by the experimental results.
Conclusions
In summary, we have developed a method for the anhydride-promoted intramolecular cyclization of N-cyano sulfoximines. Transition metal catalysts or harsh reaction conditions were not required. We believe that we have identified a clear mechanistic pathway for the activation of the N-cyano groups of N-cyano sulfoximines via the addition of commercially available halogenated anhydrides. We demonstrated the intramolecular cyclization of N-cyano sulfoximines to prepare an important class of sulfoximidoyl heterocycles, thiadiazinone 1-oxides 2, in excellent yields. It is predicted by the KRICT AI platform that thiadiazinone 1-oxides 2 will exhibit excellent drug-like properties with low toxicities (in detail, please see the ESI†).46–52 Current efforts by our group are directed toward the further application of these attractive molecules for drug discovery.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry and Energy, and Ministry of Health and Welfare (HN21C1078, Republic of Korea) and by KRICT (KK2331-30 and SI2231-30).Notes and references
- P. Stoss and G. Satzinger, Tetrahedron Lett., 1973, 14, 267–268 CrossRef.
- O. Garciá Mancheño, O. Bistri and C. Bolm, Org. Lett., 2007, 9, 3809–3811 CrossRef PubMed.
- A. Pandey and C. Bolm, Synthesis, 2010, 2922–2925 CAS.
- P. Cutler, R. Slater, A. J. F. Edmunds, P. Maienfirsch, R. G. Hall, F. G. P. Earley, T. Pitterna, S. Pal, V.-L. Paul, J. Goodchild, M. Blacker, L. Hagmann and A. J. Crossthwaite, Pest Manage. Sci., 2013, 69, 607–619 CrossRef CAS PubMed.
- F. Teng, J.-T. Yu, Y. Jiang, H. Yang and J. Cheng, Chem. Commun., 2014, 50, 8412–8415 RSC.
- C. A. Dannenberg, L. Fritze, F. Krauskopf and C. Bolm, Org. Biomol. Chem., 2017, 15, 1086–1090 RSC.
- S. Wiezorek, P. Lammers and C. Bolm, Chem. Soc. Rev., 2019, 48, 5408–5423 RSC.
- S. J. Park, H. Baars, S. Mersmann, H. Buschmann, J. M. Baron, P. M. Amann, K. Czaja, H. Hollert, K. Bluhm, R. Redelstein and C. Bolm, ChemMedChem, 2013, 8, 217–220 CrossRef CAS PubMed.
- A.-D. Steinkamp, N. Seling, S. Lee, E. Boedtkjer and C. Bolm, MedChemComm, 2015, 6, 2163–2169 RSC.
- S. Agarwal, S. Sasane, H. A. Shah, J. P. Pethani, P. Deshmukh, V. Vyas, P. Iyer, H. Bhavsar, K. Viswanathan, D. Bandyopadhyay, P. Giri, J. Mahapatra, A. Chatterjee, M. R. Jain and R. Sharma, ACS Med. Chem. Lett., 2020, 11, 414–418 CrossRef CAS PubMed.
- Y. Zhu, M. R. Loso, G. B. Watson, T. C. Sparks, R. B. Rogers, J. X. Huang, B. C. Gerwick, J. M. Babcock, D. Kelly, V. B. Hedge, B. M. Nugent, J. M. Renga, I. Denholm, K. Gorman, G. J. DeBoer, J. Hasler, T. Meade and J. D. Thomas, J. Agric. Food Chem., 2011, 59, 2950–2957 CrossRef CAS PubMed.
- J. M. Babcock, C. B. Gerwick, J. X. Huang, M. R. Loso, G. Nakamura, S. P. Nolting, R. B. Rogers, T. C. Sparks, J. Thomas, G. B. Watson and Y. Zhu, Pest Manage. Sci., 2011, 67, 328–334 CrossRef CAS PubMed.
- G. B. Watson, M. R. Loso, J. M. Babcock, J. M. Hasler, T. J. Letherer, C. D. Young, Y. Zhu, J. E. Casida and T. C. Sparks, Insect Biochem. Mol. Biol., 2011, 41, 432–439 CrossRef CAS PubMed.
- V. B. Pandya and P. R. Patel, Cadila Healthcare Limited. WO2009053999A2, 2009.
- O. G. Mancheño, J. Dallimore, A. Plant and C. Bolm, Adv. Synth. Catal., 2010, 352, 309 CrossRef.
- S. J. Park, PhD thesis, RWTH Aachen University, Aachen, Germany, 2013.
- O. G. Mancheño and C. Bolm, Org. Lett., 2007, 9, 2951–2954 CrossRef PubMed.
- S. Kim, J. E. Kim, J. Lee and P. H. Lee, Adv. Synth. Catal., 2015, 357, 3707–3717 CrossRef CAS.
- M. L. C. Reddy, F. R. N. Kahn and V. Saravanan, Org. Biomol. Chem., 2019, 17, 9187–9199 RSC.
- F. Krauskopf, K.-N. Truong, K. Rissanen and C. Bolm, Eur. J. Org. Chem., 2020, 2761–2765 CrossRef CAS.
- T. Mukaiyama, S. Ohishi and H. Takamura, Bull. Chem. Soc. Jpn., 1954, 27, 416–421 CrossRef CAS.
- P. Stoss and G. Satzinger, Tetrahedron Lett., 1973, 14, 267–268 CrossRef.
- O. G. Mancheño, J. Dallimore, A. Plant and C. Bolm, Adv. Synth. Catal., 2010, 352, 309–316 CrossRef.
- O. G. Mancheño, O. Bistri and C. Bolm, Org. Lett., 2007, 9, 3809–3811 CrossRef PubMed.
- T. A. Khan, J. D. Scott and J. N. Cumming, WO2014150331A1, 2014.
- I. S. Oh, Y. J. Seo, J. Y. Hyun, H. J. Lim, D. –H. Lee and S. J. Park, ACS Omega, 2022, 7, 2160–2169 CrossRef CAS PubMed.
- R. D. Dillard, T. T. Yen, P. Stark and D. E. Pavey, J. Med. Chem., 1980, 23, 717–722 CrossRef CAS PubMed.
- C. Wu, R. Huang, M. Zhang and Z. Chen, J. Org. Chem., 2020, 85, 841–850 CrossRef CAS PubMed.
- Y. Xia, H. Jiang and W. Wu, Eur. J. Org. Chem., 2021, 6658–6669 CrossRef CAS.
- S. P. Larissa, I. F. S. Marra and G. W. Amarante, Quim. Nova, 2022, 45, 712–727 Search PubMed.
- Note that a Lewis acid promoted intramolecular cyclization of N-cyano sulfoximine was unsuccessful. The combination of N-cyanosulfoximine 1a with various Lewis acid additives, such as ZnBr2, SnCl4, and B(C6F5)3,32,33 (each 3 equiv.) in toluene for 16 h at 90 °C did not produce the desired thiadiazinone 1-oxide 2a. These results may be related to the weak nucleophilicity of nitrile group due to the strong electron-withdrawing ability of N-cyano sulfoximine group.34.
- U. P. Saikia, G. Borah and P. Pahari, Eur. J. Org. Chem., 2018, 1211–1217 CrossRef CAS.
- V. D. Cerón, L. A. Illicachi and B. Insuasty, Molecules, 2023, 28, 257 CrossRef PubMed.
- U. Lücking, Angew. Chem., Int. Ed., 2023, 52, 9399–9408 CrossRef PubMed.
- G. A. Olah, R. D. Chambers and G. K. S. Prakash, Synthetic Fluorine Chemistry, Wiley, New York, 1992 Search PubMed.
- M. Schlosser, Angew. Chem., Int. Ed., 1998, 37, 1496 CrossRef CAS.
- G. A. Olah, G. K. S. Prakash, A. Molnar and J. Sommer, Superacid Chemistry, Wiley, New York, 2nd edn, 2009 Search PubMed.
- Q. Qin, Z. Cheng and N. Jiao, Angew. Chem., Int. Ed., 2023, 62, e202215008 CAS.
- Crystallization of 2b from different solvents, such as MeOH, toluene, and n-hexane, has been performed with evaporative crystallization, and it produced the dibenzoyl crystalized product 2b′.
- CCDC 2271515 (2b′) contain the supplementary crystallographic data for this paper. These data are provided free of by The Cambridge Crystallographic Centre.
- O. Mumm, Ber. Dtsch. Chem. Ges., 1910, 43, 886–893 CrossRef CAS.
- O. Mumm, H. Hesse and H. Volquartz, Ber. Dtsch. Chem. Ges., 1915, 48, 379–391 CrossRef CAS.
- W. P. Norris, L. H. Merwin and G. S. Ostrom, J. Org. Chem., 1997, 62, 9070–9075 CrossRef CAS.
- For the identification of reaction intermediate, we have used LC/MS analysis. Under the finetuned reaction condition, the reaction mixture was aliquoted in small portion. And then the aliquoted reaction mixture was analyzed by LC/MS. According to the LC/MS results, we could find the exact mass of fragments of intermediate 1ad. In detail, please see the ESI.†.
- The intramolecular cyclization reactions of 1ac using various reaction conditions were unsuccessful. In detail, please see the ESI.†.
- For cardiotoxicity: J. Y. Ryu, M. Y. Lee, J. H. Lee, B. H. Lee and K.-S. Oh, Bioinformatics, 2020, 36, 3049–3055 CrossRef CAS PubMed.
- For BBB permeability: B. Shaker, M.-S. Yu, J. S. Song, S. Ahn, J. Y. Ryu, K.-S. Oh and D. Na, Bioinformatics, 2021, 37, 1135–1139 CrossRef CAS PubMed.
- For cardiotoxicity v2.0: H.-M. Lee, M.-S. Yu, S. R. Kazmi, S. Y. Oh, K.-H. Rhee, M.-A. Bae, B. H. Lee, D.-S. Shin, K.-S. Oh, H. Cheong, D. Lee and D. Na, BMC Bioinf., 2019, 20, 68–80 CrossRef PubMed.
- For metabolic stability: J. Y. Ryu, J. H. Lee, B. H. Lee, J. S. Song, S. Ahn and K.-S. Oh, Bioinformatics, 2022, 38, 364–368 CrossRef CAS PubMed.
- For hepatotoxicity: J. Lee, M.-S. Yu and D. Na, Curr. Bioinf., 2022, 17, 296–303 CrossRef CAS.
- For reproductive toxicity: M.-S. Yu, J. Lee, Y. Lee and D. Na, BMC Bioinf., 2020, 245, 2–8 Search PubMed.
- For PredAOT: J. Y. Ryu, W. D. Jang, J. Jang and K.-S. Oh, BMC Bioinf., 2023, 24, 2–10 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2271515. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra04208a |
| ‡ These authors contributed equally to this work. |
| § Current address: New Drug Development Center, Daegu-Gyengbuk Medical Innovation Foundation, 80 Cheombok-ro, Dong-gu, Daegu 41061, Republic of Korea. |
| This journal is © The Royal Society of Chemistry 2023 |