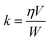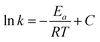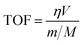Open Access Article
Open Access ArticleCoupling effect of reaction conditions on the catalytic activity of Cu–Mn composite oxide catalysts for toluene
Yungang Wang a,
Xu Lianga,
Yanjun Dai*a,
Li Zoua,
Dou Suna and
Feixiang Li*b
a,
Xu Lianga,
Yanjun Dai*a,
Li Zoua,
Dou Suna and
Feixiang Li*b
aKey Laboratory of Thermo-Fluid Science and Engineering (Ministry of Education), Xi'an Jiaotong University, Xi'an 710049, Shaanxi, China. E-mail: daiyanjun2015@xjtu.edu.cn
bHubei Special Equipment Inspection and Testing Institute, Wuhan, 430077, China. E-mail: 26005838@qq.com
First published on 1st September 2023
Abstract
Volatile organic compounds (VOCs) are one of the major components of air pollution. Catalytic combustion is a promising technology for the treatment of VOCs and at its center is the preparation of efficient and cheap catalysts. In this study, by loading copper (Cu) and manganese (Mn) on Santa Barbara Amorphous-15 (SBA-15) molecular sieve, the Cux–Mny/SBA-15 (x = 1, 2; y = 1, 2) composite metal oxide catalyst was prepared using the equal volume impregnation method. Their performance in the toluene catalytic combustion reaction was investigated by adjusting the molar ratio (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
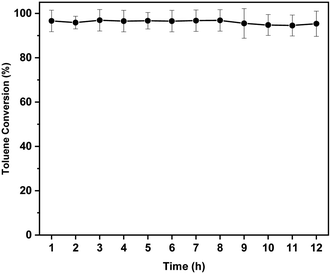
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y), and the loading of Cu and Mn. The results of the Brunner–Emmett–Teller (BET), X-ray diffraction (XRD), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS) analyses show that the CuMnO spinel phase can be detected in the Cu–Mn composite metal oxide catalyst doped with a low concentration of Cu. The overall rod-like structure of the fibrous network structure provides a large specific surface area, and the particle crystallinity is low and the dispersion is good. Due to the synergistic effect of Cu and Mn, the greater the amount of Mn3+ and adsorbed oxygen species (Oads) that are available, and the higher the turnover frequency (TOF) value, the better and more superior catalytic performance and excellent stability is obtained, when compared with the single-component oxides used in toluene catalytic combustion. After a continuous catalytic reaction for 12 h, the toluene conversion rate remained above 95%. The coupling effect of the catalytic reaction temperature and concentration of oxygen on the catalytic combustion of toluene was also studied. At a low reaction temperature (<250 °C), the increase of the concentration of oxygen played a superior role in promoting the conversion of toluene. The kinetic analysis of the toluene catalytic combustion process showed that the catalytic combustion of toluene by Cu–Mn/SBA-15 followed both the Mars–Van Krevelen (MVK) and Langmuir–Hinshelwood (L–H) reaction mechanisms. With the increase of the Oads amount caused by the decrease of the Cu ratio, the proportion of the L–H reaction mechanism increases.
y), and the loading of Cu and Mn. The results of the Brunner–Emmett–Teller (BET), X-ray diffraction (XRD), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS) analyses show that the CuMnO spinel phase can be detected in the Cu–Mn composite metal oxide catalyst doped with a low concentration of Cu. The overall rod-like structure of the fibrous network structure provides a large specific surface area, and the particle crystallinity is low and the dispersion is good. Due to the synergistic effect of Cu and Mn, the greater the amount of Mn3+ and adsorbed oxygen species (Oads) that are available, and the higher the turnover frequency (TOF) value, the better and more superior catalytic performance and excellent stability is obtained, when compared with the single-component oxides used in toluene catalytic combustion. After a continuous catalytic reaction for 12 h, the toluene conversion rate remained above 95%. The coupling effect of the catalytic reaction temperature and concentration of oxygen on the catalytic combustion of toluene was also studied. At a low reaction temperature (<250 °C), the increase of the concentration of oxygen played a superior role in promoting the conversion of toluene. The kinetic analysis of the toluene catalytic combustion process showed that the catalytic combustion of toluene by Cu–Mn/SBA-15 followed both the Mars–Van Krevelen (MVK) and Langmuir–Hinshelwood (L–H) reaction mechanisms. With the increase of the Oads amount caused by the decrease of the Cu ratio, the proportion of the L–H reaction mechanism increases.
1. Introduction
Organic compounds involved in atmospheric photochemical reactions are known as volatile organic compounds (VOCs).1 As important precursors of photochemical smog and fine particulate matter (PM2.5),2 VOCs not only induce haze and pollute the atmosphere, but also damage human health and biological growth.3 The VOCs treatment technologies include adsorption, condensation, catalytic combustion, photocatalysis, non-thermal plasma methods, and so on.4–8 For catalytic combustion technology, the final major products are harmless carbon dioxide (CO2) and water. It has become one of the most promising treatment technologies due to its low energy consumption and high efficiency.9,10Catalysts consist of an active component and a carrier, and depending on the active component, they can be classified as noble metal catalysts (Au, Pd, Pt, and so on)11–13 and transition metal catalysts (Co, Cu, Mn, and so on).7,14 Although the noble metal catalysts have a high catalytic activity at low temperatures, the properties of unsustainability, high cost, and susceptibility to sintering and carbon deposition, restrict their wide application in industry.15 The development of non-noble metal catalysts with a high catalytic activity has become the focus of research. Among them, the Cu and Mn oxides became the focus of current research because of their high catalytic activity and high stability.16,17 The Mn-based catalysts have an excellent catalytic performance due to the double exchange behaviour between the Mn3+ and Mn4+ that can promote electron transfer.18 Adding other metals to the Mn oxide catalyst to form a composite catalyst can improve its performance, but it does not mean the higher the ratio, the better the performance. The main drawback of metal oxide catalysts is their tendency to deactivate due to aggregation. Therefore, they can be supported on a carrier to improve their dispersion. The carriers primarily include Al2O3, SiO2, activated carbon, and molecular sieves.19–22 Among them, mesoporous molecular sieves have the potential to generate well-dispersed and stable metal particles, thereby offering further enhancement of the catalytic activity of the metal oxide catalysts. The molecular sieve, SBA-15 has gained extensive attention due to its regular hexagonal pore arrangement, uniform pore size, extremely high surface area, and large pore volume. It also exhibits inertness and stability at high temperatures, as well as good mechanical stability.9,10 Appropriate loading is also an important factor affecting catalyst activity.
Various reaction conditions, such as reaction temperature, space velocity, toluene concentration, and so on, remarkably effect the efficiency of the VOCs catalytic combustion.23–25 However, there are few studies on the impacts of concentration of oxygen in the reaction gas and the coupling with other reaction conditions, on the VOCs catalytic combustion. Hu et al.24 examined the toluene catalytic combustion over a Cu/MnO catalyst with toluene gas switched with oxygen and nitrogen (O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1
N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). In the same method that other researchers used to explore the catalytic combustion of toluene, the reaction gas was also a mixture of nitrogen and oxygen with a fixed proportion.26,27 In addition, the reaction of toluene gas in catalytic combustion with nitrogen was investigated by Wang et al.25 In related studies, the effect of the concentration of oxygen on the catalytic combustion of toluene was not studied. Oxygen plays a vital role in the catalytic combustion of toluene, and a low concentration of oxygen will lead to insufficient combustion of toluene gas and reduce the removal rate. An excessive content will increase the treatment cost. In actual industrial production, the appropriate concentration of oxygen can be selected, according to different reaction conditions, to achieve the highest toluene removal rate at the minimum energy consumption. Based on the previous analysis, the impacts of the concentration of oxygen in the reaction gas and coupling with other reaction conditions on VOC catalytic oxidation is investigated extensively in the research discussed in this paper, especially when non-precious metal catalysts are selected to catalyze oxidation. The results can provide a reference for the design and optimization of catalysts, reaction systems, and reaction parameters in industrial processes. This is significant for the development of more efficient and sustainable processes for the combustion of VOCs.
4). In the same method that other researchers used to explore the catalytic combustion of toluene, the reaction gas was also a mixture of nitrogen and oxygen with a fixed proportion.26,27 In addition, the reaction of toluene gas in catalytic combustion with nitrogen was investigated by Wang et al.25 In related studies, the effect of the concentration of oxygen on the catalytic combustion of toluene was not studied. Oxygen plays a vital role in the catalytic combustion of toluene, and a low concentration of oxygen will lead to insufficient combustion of toluene gas and reduce the removal rate. An excessive content will increase the treatment cost. In actual industrial production, the appropriate concentration of oxygen can be selected, according to different reaction conditions, to achieve the highest toluene removal rate at the minimum energy consumption. Based on the previous analysis, the impacts of the concentration of oxygen in the reaction gas and coupling with other reaction conditions on VOC catalytic oxidation is investigated extensively in the research discussed in this paper, especially when non-precious metal catalysts are selected to catalyze oxidation. The results can provide a reference for the design and optimization of catalysts, reaction systems, and reaction parameters in industrial processes. This is significant for the development of more efficient and sustainable processes for the combustion of VOCs.
As a consequence, the present paper is focused on exploring the catalytic combustion characteristics and reaction mechanism of VOCs under different reaction temperatures and concentrations of oxygen. The Cu–Mn/SBA-15 catalysts with various loadings and different relative contents of Cu/Mn were synthesized using the equal volume impregnation method. The effects of the catalyst active components and reaction conditions on the catalytic combustion of the VOCs were explored in a fixed bed reactor with toluene as a molecular probe, and a stability test was carried out. The catalysts were characterized using BET, XRD, SEM and XPS. Combined with kinetic analysis, the catalytic combustion mechanism of toluene on the Cu–Mn/SBA-15 was proposed. This study aimed to provide a low-cost and efficient non-precious metal composite oxide catalyst, and to explore its coupling effect under different reaction conditions, to promote the development of the VOC's removal technology.
2. Experimental
2.1 Materials
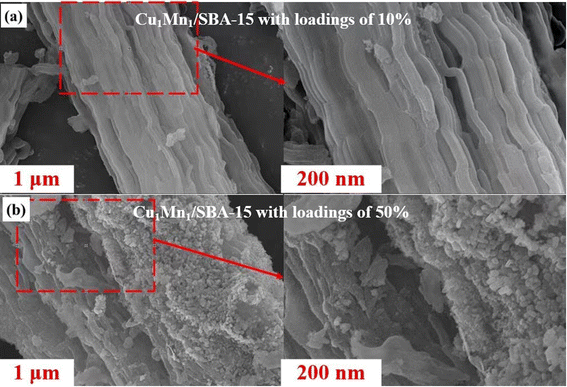
Manganese nitrate solution (50% Mn(NO3)2), copper nitrate trihydrate (Cu(NO3)2·3H2O), quartz sand, toluene and ethanol were purchased as AR from Sinopharm Chemical Reagent Company. Nitrogen (99.99%) and oxygen (99.99%) were procured from the Xi'an Tenglong Chemical Company.The Cu–Mn/SBA-15 samples were prepared using the impregnation method. Firstly, a specific amount of SBA-15 was soaked in an aqueous mixture of Cu(NO3)2 and Mn(NO3)2 with constant stirring. After keeping the mixture at room temperature (ca. 25 °C) for 12 h, the products were then dried at 110 °C for 12 h. Then these products were calcined in air for 4 h at 400 °C. Using the previous steps, the Cu and Mn were loaded onto the SBA-15 carrier Cu1Mn1/SBA-15 with loadings of 10%, 20%, 30%, 40% and 50%. For Cu1Mn2/SBA-15 and Cu2Mn1/SBA-15, only samples with a 40% loading were prepared. Abbreviation and detailed experimental conditions of the Cu–Mn/SBA-15 samples are shown in Table 1. In Table 1, ‘Cu1Mn2’ indicates that the molar ratio of Cu to Mn is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and so on.
2, and so on.
| Abbreviation | Loading of active components (wt%) | Metal content (wt%) | |
|---|---|---|---|
| Cu | Mn | ||
| Cu/SBA-15 | 40 | 40 | 0 |
| Mn/SBA-15 | 40 | 0 | 40 |
| Cu1Mn2/SBA-15 | 40 | 14.7 | 25.3 |
| Cu1Mn1/SBA-15 | 10 | 5.4 | 4.6 |
| Cu1Mn1/SBA-15 | 20 | 10.7 | 9.3 |
| Cu1Mn1/SBA-15 | 30 | 16.1 | 13.9 |
| Cu1Mn1/SBA-15 | 40 | 21.5 | 18.5 |
| Cu1Mn1/SBA-15 | 50 | 26.8 | 23.2 |
| Cu2Mn1/SBA-15 | 40 | 27.9 | 12.1 |
2.2 Instrumental measurements
The textural characteristics of the samples were determined from the adsorption isotherm of N2 at −196 °C which was obtained using an ASAP 2020 Plus HD88 (Micromeritics) automatic physical adsorption instrument. All the samples were heat-treated at 150 °C under vacuum overnight before the analysis.The XRD patterns were obtained by using an XRD-6100 (Shimadzu, Japan) instrument, with Cu-Kα radiation (λ = 1.5418 Å), focusing geometry θ–2θ in the scanning mode within a range of angles of 2θ from 10° to 80° with a step of 0.02°.
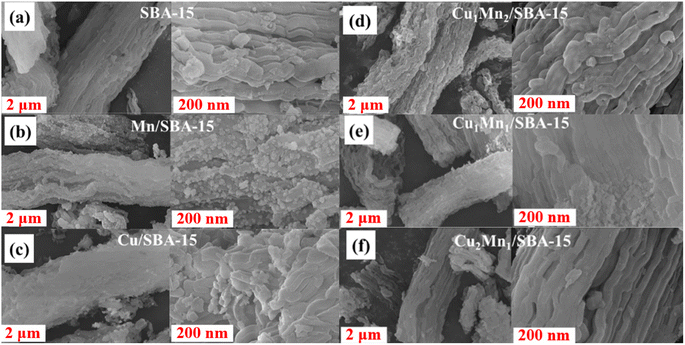
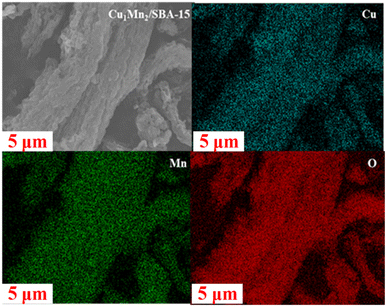
The surface morphology was observed using field-emission scanning electron microscopy (FE-SEM) with a Gemini 500 instrument (Zeiss, Germany).
The XPS using an Axis Ultra DLD (Kratos, UK) was adopted for determining the elemental composition and chemical states.
2.3 Catalytic combustion experiments
A fixed-bed reactor (inner diameter = 20 mm) was utilized to conduct the toluene catalytic combustion experiments. For each experiment, 0.1 g of catalyst and 0.9 g of quartz sand were weighed and evenly mixed before being placed into the reactor. With a toluene concentration of 1500 mg m−3, the total rate of the gas mixture (N2 and O2) was 200 mL min−1 with a weight hourly space velocity (WHSV) of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. The volume concentration of the oxygen was changed by changing the oxygen flow rate. Each set of experiments was performed for 30 min under various reaction conditions. The toluene concentration was monitored using a toluene gas detector. Each set of experiments was performed for 30 min under various reaction conditions and repeated three times to determine the average toluene concentration. The toluene conversion was calculated using eqn (1):
000 mL g−1 h−1. The volume concentration of the oxygen was changed by changing the oxygen flow rate. Each set of experiments was performed for 30 min under various reaction conditions. The toluene concentration was monitored using a toluene gas detector. Each set of experiments was performed for 30 min under various reaction conditions and repeated three times to determine the average toluene concentration. The toluene conversion was calculated using eqn (1):
 | (1) |
3. Results and discussion
3.1 Catalyst characterization
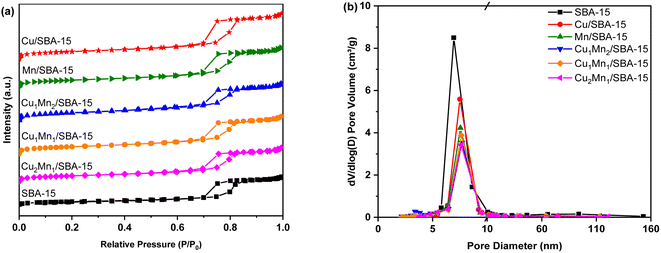
| Sample | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) |
|---|---|---|---|
| SBA-15 | 309 | 1.13 | 8.03 |
| Cu/SBA-15 | 260 | 0.64 | 8.12 |
| Mn/SBA-15 | 240 | 0.55 | 8.36 |
| Cu1Mn2/SBA-15 | 268 | 0.52 | 7.64 |
| Cu1Mn1/SBA-15 | 250 | 0.53 | 8.26 |
| Cu2Mn1/SBA-15 | 209 | 0.48 | 8.39 |
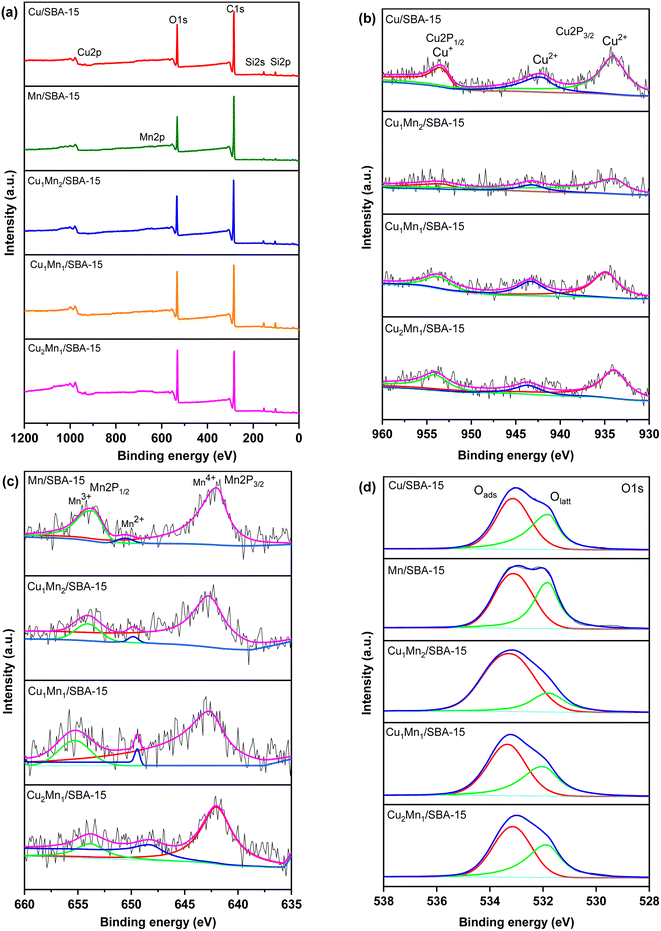 | ||
| Fig. 5 (a) The XPS spectra, (b) Cu 2p, (c) Mn 2p, and (d) the O 1s XPS spectra of different catalysts. | ||
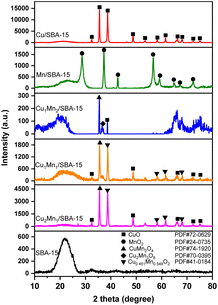
Table 3 shows the XPS results of the different catalysts. The Cu1Mn2/SBA-15 sample had more Mn3+ and a larger Oads than Cu1Mn1/SBA-15 and Cu2Mn1/SBA-15. The greater the Oads in the catalysts, the more oxygen vacancies and active species on the surface of catalysts there were, which made it more favorable for toluene catalytic combustion.26,34 A higher Mn3+ concentration was favorable for generating more oxygen vacancies, which could improve the catalytic activity and redox performance.35 The crystallinity of the catalysts continuously increased with the increase in Olatt concentration. The variation in the Olatt concentration of different catalysts indicated that the Cu2Mn1/SBA-15 had the highest crystallinity amongst the samples, which was compatible with the XRD characterization results.
| Sample | Cu+ (%) | Cu2+ (%) | Mn3+ (%) | Mn4+ (%) | Mn2+ (%) | Olatt (%) | Oads (%) |
|---|---|---|---|---|---|---|---|
| Cu1Mn2/SBA-15 | 20.88 | 79.12 | 25.99 | 56.50 | 17.51 | 24.81 | 75.19 |
| Cu1Mn1/SBA-15 | 24.04 | 75.96 | 22.76 | 68.97 | 8.28 | 36.31 | 63.69 |
| Cu2Mn1/SBA-15 | 29.63 | 70.37 | 16.34 | 65.36 | 18.30 | 39.02 | 60.98 |
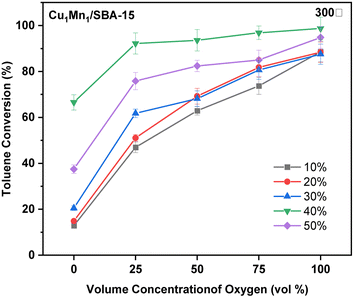
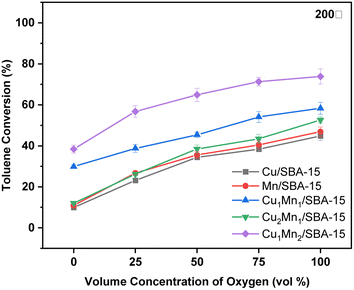
3.2 Catalytic activity
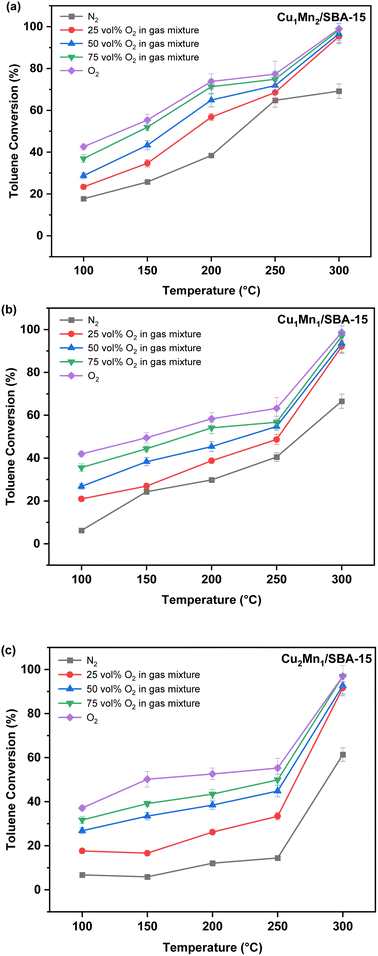 | ||
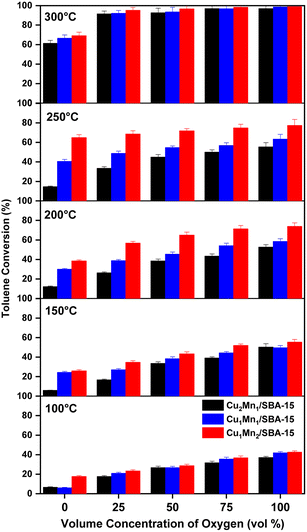
| Fig. 9 The effects of temperature and concentration of oxygen on toluene removal efficiency: (a) Cu1Mn2/SBA-15, (b) Cu1Mn1/SBA-15, (c) Cu2Mn1/SBA-15. | ||
When the temperature was lower than 250 °C, the toluene conversion of the Cu2Mn1/SBA-15 increased slightly with the increasing reaction temperature unlike those of the Cu1Mn1/SBA-15 and Cu1Mn2/SBA-15. When the reaction temperature was raised from 100 to 250 °C, the toluene removal efficiency only increased by 7.7% at 100 vol% N2. However, the toluene removal efficiency increased by 46.9% when the reaction temperature was increased from 250 to 300 °C, which was attributed to the high content of Olatt in the Cu2Mn1/SBA-15. The reaction temperature of Olatt was higher in the absence of oxygen.36
The Mn3+ and Oads concentrations decreased as the Cu/Mn mole ratio increased, and the toluene conversion increased with the increasing Mn3+ and Oads concentration. Amongst the catalysts studied, the Cu1Mn2/SBA-15 had the largest specific surface area and the active components were evenly distributed, which was conducive to improving the catalytic performance.38 The Cu1Mn2/SBA-15 catalyst in this study had a toluene conversion of 68.5% at 250 °C, and this catalytic performance was comparable to that of precious metals (such as Ag),39 while saving costs.
3.3 Catalytic combustion mechanism of toluene
The reaction rate k (mol gcat−1 s−1) can be calculated as follows:
The apparent Ea (kJ mol−1) is calculated according to the Arrhenius formula:
The turnover frequency (TOFCu or TOFMn) (s−1) is given by:40
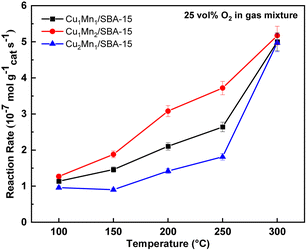
Fig. 12 displays the reaction rates of the catalysts at different temperatures. It can be seen that the reaction rates of Cu–Mn/SBA-15 with different Cu/Mn molar ratios show a trend of continuous increase as the temperature increased. Among them, the reaction rate of toluene combustion catalyzed by Cu1Mn2/SBA-15 showed a positive correlation with the reaction temperature. In addition, for the toluene catalytic combustion by other Cu/Mn molar ratio catalysts, the relationship between the reaction rate and reaction temperature can be analyzed in two bands according to temperature. In the temperature range of 100–250 °C, the reaction rate increased slowly with increasing temperature. However, the reaction rate increased significantly as the temperature increased from 250 to 300 °C, which was consistent with the experimental results on the effect of temperature on toluene conversion.
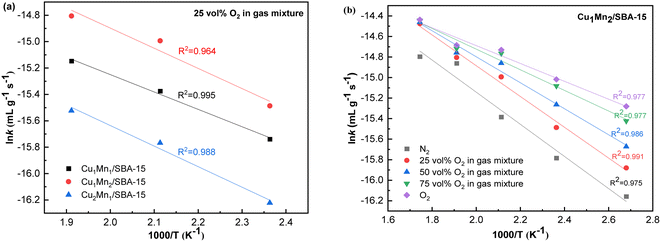
Fig. 13 displays the Arrhenius plots of toluene combustion, and Table 4 lists the results of apparent Ea, reaction rate and TOF for different Cu/Mn molar ratio catalysts. The coefficients of determination (R2) were all greater than 0.95, indicating that the model had a good fit. The apparent Ea of Cu1Mn2/SBA-15 (10.2 kJ mol−1) was the minimum, which suggested that the toluene was more prone to combustion by Cu1Mn2/SBA-15. Meanwhile, the reaction rate of Cu1Mn2/SBA-15 (0.96 × 10−7 mol gcat−1 s−1) was more than twice that of those of Cu2Mn1/SBA-15 and Cu1Mn1/SBA-15 under the same reaction conditions. The Cu1Mn2/SBA-15 also had the highest values of TOFCu and TOFMn, indicating that it had an outstanding catalytic performance.
| Sample | Eaa (kJ mol−1) | R2b | kc (10−7 mol gcat−1 s−1) | TOFCub (10−5 s−1) | TOFMnb (10−5 s−1) |
|---|---|---|---|---|---|
| a The apparent activation energy (Ea) was calculated at 25 vol% O2.b R2 is an indicator to evaluate the quality of the regression model, i.e., the degree of fit between the regression line and the experimental data points.c The reaction rates (k) and TOF calculations were at the experimental conditions of 100 °C and 100 vol% N2 for catalytic combustion. | |||||
| Cu2Mn1/SBA-15 | 13.0 | 0.988 | 0.37 | 0.83 | 1.68 |
| Cu1Mn1/SBA-15 | 11.7 | 0.964 | 0.34 | 1.00 | 1.00 |
| Cu1Mn2/SBA-15 | 10.2 | 0.995 | 0.96 | 4.15 | 2.08 |
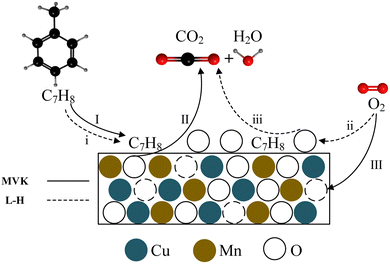
Fig. 14 shows the toluene reaction mechanism of Cu–Mn/SBA-15. Firstly, the toluene molecules are adsorbed on the surface of the catalyst, and then these adsorbed toluene molecules react with the Olatt in the catalyst to generate CO2 and H2O. Finally, the oxygen vacancy formed by the reaction is supplemented by gas phase oxygen. The reaction process described previously is the MVK mechanism. While the MVK mechanism reaction is taking place, the L–H mechanism reaction also takes place. This demonstrates that the gas phase oxygen can be adsorbed by the catalyst surface to form adsorbed oxygen, and then reacts with the toluene molecules adsorbed on the catalyst surface to generate CO2 and H2O.
4. Conclusions
The Cu–Mn/SBA-15 catalysts with various loadings and the Cu/Mn mole ratios were produced using an impregnation method for exploring the effects of different reaction conditions on toluene conversion. The Cu1Mn2/SBA-15 showed the maximum catalytic performance for the toluene combustion at 300 °C because of the synergy between both the Cu and Mn and higher content of the Mn3+ and Oads. Furthermore, the Cu1Mn2/SBA-15 also has the lowest apparent Ea, the highest reaction rate and the highest TOF content, all of which were conducive for the improvement of the toluene catalytic combustion. The toluene removal efficiency increased with the increase in reaction temperature, and the concentration of oxygen, and the increase of the concentration of oxygen showed a remarkable effect on the improvement of toluene removal efficiency, especially under a low reaction temperature (<250 °C). The toluene conversion could be improved by increasing the concentration of oxygen when the reaction temperature was relatively low. The toluene catalytic combustion for the Cu–Mn/SBA-15 followed both the MVK and L–H mechanism, but the proportion of both reaction mechanisms varied according to the content of Oads and Olatt in the catalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Shaanxi Provincial Innovation Capability Support Program (Grant No. 2019KJXX-042), the Hubei Market Supervision Bureau Technical Support Project (Grant No. Hbscjg-JS2022004) and the Wang Kuan-cheng Education Fund.References
- A. He, J. Cheng, X. Zhang, M. Douthwaite, S. Pattisson and Z. Hao, Chem. Rev., 2019, 119, 4471–4568 CrossRef PubMed.
- Y. Xu, Z. Qu, Y. Ren and C. Dong, Appl. Surf. Sci., 2021, 560, 149983 CrossRef CAS.
- C. Yang, G. Miao, Y. Pi, Q. Xia, J. Wu, Z. Li and J. Xiao, Chem. Eng. J., 2019, 370, 1128–1153 CrossRef CAS.
- A. Hamad and M. Fayed, Chem. Eng. Res. Des., 2004, 82, 895–906 CrossRef CAS.
- Y. Huang, S. S. H. Ho, Y. Lu, R. Niu, L. Xu, J. Cao and S. Lee, Molecules, 2016, 21, 56 CrossRef PubMed.
- K. M. R. S. H. MM, Atmos. Environ., 2016, 140, 117–134 CrossRef.
- R. Li, L. Zhang, S. Zhu, S. Fu, X. Dong, S. Ida, L. Zhang and L. Guo, Appl. Catal., A, 2020, 602, 117715 CrossRef CAS.
- A. M. Vandenbroucke, R. Morent, N. De Geyter and C. Leys, J. Hazard. Mater., 2011, 195, 30–54 CrossRef CAS PubMed.
- J.-L. Blin, L. Michelin, B. Lebeau, A. Naydenov, R. Velinova, H. Kolev, P. Gaudin, L. Vidal, A. Dotzeva, K. Tenchev and S. Todorova, Catalysts, 2021, 11, 366 CrossRef CAS.
- S. Todorova, J. L. Blin, A. Naydenov, B. Lebeau, H. Kolev, P. Gaudin, A. Dotzeva, R. Velinova, D. Filkova, I. Ivanova, L. Vidal, L. Michelin, L. Josien and K. Tenchev, Catal. Today, 2020, 357, 602–612 CrossRef CAS.
- X. Chen, Z. Zhao, Y. Zhou, Q. Zhu, Z. Pan and H. Lu, Appl. Catal., A, 2018, 566, 190–199 CrossRef CAS.
- Y. Feng, L. Wei, Z. Wang, Y. Liu, H. Dai, C. Wang, H.-C. Hsi, E. Duan, Y. Peng and J. Deng, J. Hazard. Mater., 2022, 439, 129612 CrossRef CAS PubMed.
- J. Hu, X. Gao, Q. Fan and X. Gao, RSC Adv., 2021, 11, 16547–16556 RSC.
- R. Fang, Z. Yang, X. Liu, Y. Yan, J. Ran and L. Zhang, Fuel, 2021, 286, 119311 CrossRef CAS.
- J. Chen, X. Chen, W. Xu, Z. Xu, H.-p. Jia and J. Chen, Appl. Catal., B, 2018, 224, 825–835 CrossRef CAS.
- P. N. H. Thi, N. C. Chien and N. D. M. Tuan, RSC Adv., 2023, 13, 13354–13364 RSC.
- M. Li, W. Zhang, X. Zhang, Y. Lian, X. Niu and Y. Zhu, J. Colloid Interface Sci., 2022, 630, 301–316 CrossRef PubMed.
- C. Xu, S. Dong, T. Chen, H. Liu, X. Zou, M. Ji, Z. Han, D. Shu, C. Wang and D. Chen, Fuel, 2023, 347, 128401 CrossRef CAS.
- T. F. Garetto, E. Rincón and C. Apesteguıa, Appl. Catal., B, 2004, 48, 167–174 CrossRef CAS.
- Q. Guo, Y. Liu, G. Qi and W. Jiao, Energy, 2019, 179, 431–441 CrossRef CAS.
- J. Meng, F. Fang, N. Feng, H. Wan and G. Guan, RSC Adv., 2020, 10, 2472–2482 RSC.
- L. Schick, R. Sanchis, V. González-Alfaro, S. Agouram, J. M. López, L. Torrente-Murciano, T. García and B. Solsona, Chem. Eng. J., 2019, 366, 100–111 CrossRef CAS.
- J.-L. Blin, L. Michelin, B. Lebeau, A. Naydenov, R. Velinova, H. Kolev, P. Gaudin, L. Vidal, A. Dotzeva and K. Tenchev, Catalysts, 2021, 11, 366 CrossRef CAS.
- J. Hu, W. Li and R. Liu, Catal. Today, 2018, 314, 147–153 CrossRef CAS.
- Y. Wang, D. Yang, S. Li, L. Zhang, G. Zheng and L. Guo, Chem. Eng. J., 2019, 357, 258–268 CrossRef CAS.
- J.-R. Li, W.-P. Zhang, C. Li, H. Xiao and C. He, Appl. Surf. Sci., 2021, 550, 149179 CrossRef CAS.
- Y. Zhang, Z. Zeng, Y. Li, Y. Hou, J. Hu and Z. Huang, Fuel, 2021, 288, 119700 CrossRef CAS.
- P. Rayo, M. S. Rana, J. Ramírez, J. Ancheyta and A. Aguilar-Elguézabal, Catal. Today, 2008, 130, 283–291 CrossRef CAS.
- S. Mo, Q. Zhang, J. Li, Y. Sun, Q. Ren, S. Zou, Q. Zhang, J. Lu, M. Fu and D. Mo, Appl. Catal., B, 2020, 264, 118464 CrossRef CAS.
- Y. Ding, Q. Xian, E. Wang, X. He, Z. Jiang, H. Dan and W. Zhu, New J. Chem., 2020, 44, 13707–13715 RSC.
- S. Hosseini, A. Niaei, D. Salari, M. C. Alvarez-Galvan and J. Fierro, Ceram. Int., 2014, 40, 6157–6163 CrossRef CAS.
- J.-R. Li, W.-P. Zhang, C. Li and C. He, J. Colloid Interface Sci., 2021, 591, 396–408 CrossRef CAS.
- H. Zhao, K. Fang, F. Dong, M. Lin, Y. Sun and Z. Tang, J. Ind. Eng. Chem., 2017, 54, 117–125 CrossRef CAS.
- Y. Luo, Y. Zheng, J. Zuo, X. Feng, X. Wang, T. Zhang, K. Zhang and L. Jiang, J. Hazard. Mater., 2018, 349, 119–127 CrossRef CAS PubMed.
- X. Zhang, J. Zhao, Z. Song, W. Liu, H. Zhao, M. Zhao, Y. Xing and H. Du, J. Colloid Interface Sci., 2020, 562, 170–181 CrossRef CAS PubMed.
- P. Wang, Y. He, Z. Yang, X. Liu, J. Ran and M. Guo, ChemistrySelect, 2020, 5, 1122–1129 CrossRef CAS.
- S. Behar, P. Gonzalez, P. Agulhon, F. Quignard and D. Świerczyński, Catal. Today, 2012, 189, 35–41 CrossRef CAS.
- Y. Qin, H. Wang, C. Dong and Z. Qu, J. Catal., 2019, 380, 21–31 CrossRef CAS.
- Y. Qin, Z. Qu, C. Dong and N. Huang, Chin. J. Catal., 2017, 38, 1603–1612 CrossRef CAS.
- Y. Luo, D. Lin, Y. Zheng, X. Feng, Q. Chen, K. Zhang, X. Wang and L. Jiang, Appl. Surf. Sci., 2020, 504, 144481 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |