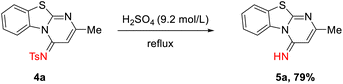Open Access Article
Open Access ArticleMild and efficient synthesis of benzothiazolopyrimidine derivatives via CuAAC/ring cleavage/cyclization reaction†
Weiguang Yang *ab,
Danyang Luoa,
Guanrong Lia,
Weigao Hua,
Jia Zheng*a and
Lanmei Chen*a
*ab,
Danyang Luoa,
Guanrong Lia,
Weigao Hua,
Jia Zheng*a and
Lanmei Chen*a
aKey Laboratory of Big Data Mining and Precision Drug Design of Guangdong Medical University, The Marine Biomedical Research Institute, Guangdong Medical University, Zhanjiang, 524023, China. E-mail: 09ywg@163.com; jiatiger@163.com; lanmeichen@126.com
bGuangDong Engineering Technology Research Center for the Development and Utilization of Mangrove Wetland Medicinal Resources, The Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, Guangdong 524023, China
First published on 28th July 2023
Abstract
An operationally mild and efficient synthesis of benzothiazolopyrimidine is achieved by a three-component reaction of 2-aminebenzo[d]thiazoles, sulfonyl azides and terminal ynones. This cascade process involved a CuAAC/ring cleavage/cyclization reaction. Particularly, most of the benzothiazolopyrimidine derivatives could be isolated by filtration without further purification.
Introduction
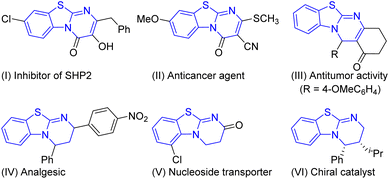
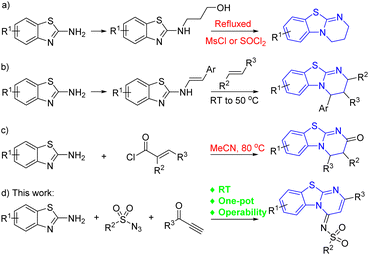
Benzothiazolopyrimidine derivatives are well established as privileged scaffolds which are commonly encountered in many pharmacologically active molecules that may be good drug candidates.1–7 Most of the benzothiazolopyrimidines possess various biological activities like inhibition of SHP2 (Fig. 1, I),2 anticancer agent (II),3 antitumor activity (III),4 analgesic (IV),5 and nucleoside transporter (V).6 Also, some benzothiazolopyrimidine derivatives are commercially available as chiral catalysts (VI).7 Therefore, the development of novel methods for the synthesis of these benzothiazolopyrimidine is important in the field of synthetic organic and pharmaceutical chemistry.Numerous synthetic methods for the preparation of benzothiazolopyrimidines reported in the literature involve three major strategies: (a) intramolecular cyclization reaction with amino alcohol substrates under reflux conditions (Scheme 1a);8 (b) [4 + 2] cycloaddition of 2-benzothiazolimines and alkenes or other unsaturated compounds (Scheme 1b);9 (c) a one-pot acylation–cyclization of 2-aminobenzothiazole with α,β-unsaturated acid chlorides (Scheme 1c).10 Each of these methods has considerable merit, including synthesis of stereoselective benzothiazolopyrimidine derivatives and polysubstituted products. However, there synthetic utility is impaired by the requirement of multistep synthesis, high temperatures and complex purification. Under this background, the development of operationally mild multicomponent one-pot synthetic strategies for the preparation of benzothiazolopyrimidines still remains highly desirable.
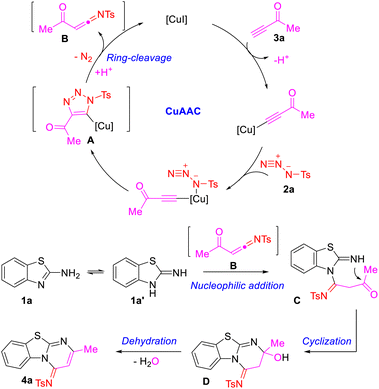
Since reported by Chang's group,11 copper-catalyzed sulfonyl azide–alkyne cycloaddition/ring cleavage (CuAAC/ring cleavage reaction) has been recognized as a mild multicomponent reaction and high efficient method for the synthesis of various N-heterocyclic compounds, which was applied to the structural modification of natural products, drugs or biological macromolecules.12 Our group has also applied the CuAAC/ring cleavage reaction to the construction of pyridines, fused heterocycles, coumarins, indoles and other N-heterocyclic compounds.13 Accordingly, we here describe a mild and efficient synthesis of benzothiazolopyrimidine derivatives via CuAAC/ring cleavage reaction (Scheme 1d). This protocol encompasses stirring a three-component reaction of 2-aminebenzo[d]thiazoles, sulfonyl azides and terminal ynones.
Results and discussion
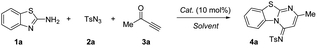
Our investigations began with an examination of the synthesis of (Z)-4-methyl-N-(2-methyl-4H-benzo[4,5]thiazolo [3,2-a]pyrimidin-4-ylidene)benzenesulfonamide 4a by using 2-aminobenzothiazole 1a, TsN3 2a and 3-butyn-2-one 3a (Table 1).| Entry | Cat. | Solvent | Yieldb (%) 4a |
|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), cat. (10 mol%) in the solvent (3 mL) was added 2a (1.5 equiv.) and 3a (1.5 equiv.) stirring at room temperature for 12 h.b Isolated yields.c n.d. = not detected the target product.d The reaction temperature was 40 °C.e The temperature was 60 °C.f The temperature was 100 °C. | |||
| 1 | CuI | CHCl3 | 75 |
| 2 | CuI | DCE | 85 |
| 3 | CuI | Toluene | 70 |
| 4 | CuI | MeCN | 93 |
| 5 | CuI | THF | 60 |
| 6 | CuI | 1,4-Dioxane | 51 |
| 7 | CuI | DMSO | 81 |
| 8 | CuI | DMA | 78 |
| 9 | CuI | EtOH | 93 |
| 10 | CuI | Acetone | 68 |
| 11 | CuCl | EtOH | 90 |
| 12 | CuBr | EtOH | 83 |
| 13 | CuBr2 | EtOH | 80 |
| 14 | Cu(OAc)2 | EtOH | 90 |
| 15 | Cu(OTf)2 | EtOH | 80 |
| 16 | Cu(acac)2 | EtOH | 85 |
| 17 | AgTFA | EtOH | n.d.c |
| 18 | CuI | EtOH | 93d |
| 19 | CuI | EtOH | 90e |
| 20 | CuI | EtOH | 87f |
The solvent screening revealed that by using CuI as the catalysts, EtOH and MeCN delivering product 4a in highest yield (93%) (Table 1, entries 1–10). Since EtOH is less toxic and cheaper than MeCN, the optimal solvent was determined to be EtOH. Encouraged by this promising result, variety of catalysts were screened. Among the copper catalysts used, both CuI or CuII catalysts exhibited high catalytic reactivity while AgTFA failed to produce the desired product (Table 1, entries 11–17). Lastly, the effect of temperature was evaluated (Table 1, entries 18–20). The results revealed room temperature is the best. Considering atomic economy, reaction rate and efficiency, the optimal reaction conditions have been defined to be Table 1, entry 9.
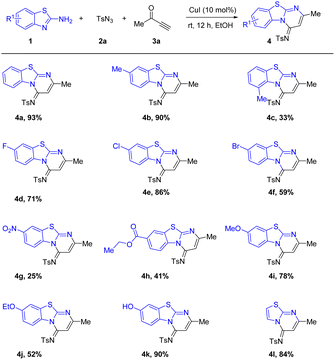
Under the optimized conditions (Table 1, entry 9), we performed a substrates screening using a series of 2-aminobenzothiazoles. Agreeably, as shown in Table 2, various 2-Aminobenzothiazoles with either electron-donating groups (–Me, –OMe, –OEt, –OH) or electron-withdrawing groups (–F, –Cl, –Br, –NO2 –COOCH2CH3) exhibited good functional-group tolerance to produced the desired products (4a−4k). It is worth noting that the yield of 4c is relatively low due to the influence of steric effect, while the yield of 4g is the lowest with the influence of the strong pull electron effect. Gratifyingly, 2-aminothiazole proceeded smoothly in this transformation, which generated 4l in decent yield. However, 2-aminobenzothiazole bearing electron-withdrawing group (–CN) gave a complex reaction system and difficult to isolate the desired product.
| a Reaction conditions: 1 (0.5 mmol), CuI (10 mol%) in EtOH (3 mL) was added 2a (1.5 equiv.) and 3a (1.5 equiv.) stirring at room temperature for 12 h. |
|---|
 |
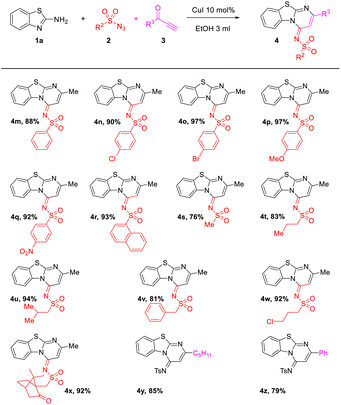
Next, the scope and limitation of substrates sulfonyl azides 2 were tested (Table 3). It is noteworthy that the substrates sulfonyl azides showed slight influence on this reaction. With R3 changed by aliphatic or aromatic substituents, such as phenyl, –(4-C6H5), –(4-ClC6H4), –(4-BrC6H4), –(4-OMeC6H4), –(4-NO2C6H4), –Me, –Pr, etc., the reaction could smoothly give the anticipated products (4m–4x) in comparable yields of 76–97%.
| a Reaction conditions: 1a (0.5 mmol), CuI (10 mol%) in the solvent (3 mL) was added 2 (1.5 equiv.) and 3 (1.5 equiv.) stirring at room temperature for 12 h. |
|---|
 |
Products 4a–4z are stable towards purification under conventional conditions. Nevertheless, N-sulfonyl benzothiazolo-pyrimidine 4a could undergo hydrolysis and converted into imine 5a under forcing conditions (Scheme 2).
None of the product imidazo[1,2-a]pyridines 4a–4z have been reported previously, which were subject to full spectroscopic characterization in the experimental section and the derived data were in complete accord with the assigned structures. The structure of 4o was confirmed by single-crystal X-ray analysis (Fig. 2).
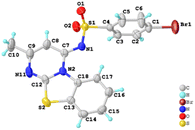 | ||
| Fig. 2 Single-crystal X-ray analysis of 4o (CCDC 2270393). | ||
A possible reaction pathway for the formation of benzothiazolopyrimidine (4a) from precursors 1a, 2a and 3a is shown in Scheme 3. As described in the literature,12,13 the substrates 2a and 3a reacted in the presence of the copper(I) catalyst to form the metallated triazole A through the CuAAC pathway. Then, the complex A underwent a ring-cleavage rearrangement, leading to a highly active intermediate N-sulfonyl α-acylketenimine B. The species B was captured by 1a via nucleophilic addition to generate the intermediate C, which delivered the intermediate D by intramolecular cyclization, and generated the final product 4a after dehydration.
Conclusions
We have developed an operationally mild and high efficient reaction for preparing benzothiazolopyrimidines by a three-component reaction of 2-aminebenzo[d]thiazoles, sulfonyl azides and terminal ynones. From a mechanistic perspective, the cascade process involved a CuAAC/ring cleavage/cyclization reaction. This methodology appears quite flexible and offers a capacity to generate forms of the title products that will be particularly useful in drug development studies.Experimental
General
1H and 13C{1H} NMR spectra were recorded at ambient temperatures on a 400 MHz Bruker spectrometer using CDCl3 or DMSO-d6 as solvent and tetramethylsilane (TMS) as the internal standard. Chemical shifts are presented as δ values relative to TMS and 1H–1H coupling constants (J values) are given in Hz. IR spectra were recorded on a BUCHI IRAffinity-1S spectrometer while HRMS measurements were carried out on a Bruker micrOTOF-Q II spectrometer. Melting points were determined on a BUCHI melting point M-565 apparatus and are uncorrected.Preparation and characterizations of compounds 4a–4z
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2
H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to obtain product 4a (171.6 mg, 93%) as a white solid, mp 252–254 °C (Rf = 0.37 in 1
1 to obtain product 4a (171.6 mg, 93%) as a white solid, mp 252–254 °C (Rf = 0.37 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.26 (d, J = 10.4 Hz, 1H), 7.94 (d, J = 5.6 Hz, 2H), 7.70 (d, J = 10.0 Hz, 1H), 7.45–7.53 (m, 2H), 7.40 (s, 1H), 7.30 (d, J = 5.6 Hz, 2H), 2.44 (d, J = 14.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.1, 161.5, 156.2, 142.8, 140.0, 136.0, 129.5 (2C), 128.0, 127.4, 126.7 (2C), 124.6, 122.7, 122.0, 104.8, 24.2, 21.7; IR ν 2920, 1609, 1493, 1450, 1402, 1283, 1140, 1088, 984, 812, 793, 756, 692 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H16N3O2S2+, [M + H]+ 370.0679; found 370.0672.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.26 (d, J = 10.4 Hz, 1H), 7.94 (d, J = 5.6 Hz, 2H), 7.70 (d, J = 10.0 Hz, 1H), 7.45–7.53 (m, 2H), 7.40 (s, 1H), 7.30 (d, J = 5.6 Hz, 2H), 2.44 (d, J = 14.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.1, 161.5, 156.2, 142.8, 140.0, 136.0, 129.5 (2C), 128.0, 127.4, 126.7 (2C), 124.6, 122.7, 122.0, 104.8, 24.2, 21.7; IR ν 2920, 1609, 1493, 1450, 1402, 1283, 1140, 1088, 984, 812, 793, 756, 692 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H16N3O2S2+, [M + H]+ 370.0679; found 370.0672.The products 4b–4z were prepared by the similar procedure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.11 (d, J = 8.8 Hz, 1H), 7.94 (d, J = 10.0 Hz, 2H), 7.49 (s, 1H), 7.39 (s, 1H), 7.29 (t, J = 11.4 Hz, 3H), 2.46 (d, J = 4.4 Hz, 6H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.9, 161.5, 156.0, 142.7, 140.1, 138.6, 133.9, 129.5 (2C), 128.5, 126.7 (2C), 124.7, 122.3, 122.0, 104.8, 24.2, 21.7, 21.5; IR ν 2924, 1599, 1493, 1277, 1142, 1086, 982, 822, 810, 789, 733, 700, 671 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C19H18O2N3S2+, [M + H]+ 384.0835; found 384.0827.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.11 (d, J = 8.8 Hz, 1H), 7.94 (d, J = 10.0 Hz, 2H), 7.49 (s, 1H), 7.39 (s, 1H), 7.29 (t, J = 11.4 Hz, 3H), 2.46 (d, J = 4.4 Hz, 6H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.9, 161.5, 156.0, 142.7, 140.1, 138.6, 133.9, 129.5 (2C), 128.5, 126.7 (2C), 124.7, 122.3, 122.0, 104.8, 24.2, 21.7, 21.5; IR ν 2924, 1599, 1493, 1277, 1142, 1086, 982, 822, 810, 789, 733, 700, 671 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C19H18O2N3S2+, [M + H]+ 384.0835; found 384.0827.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 6.8 Hz, 1H), 7.37 (t, J = 6.4 Hz, 2H), 7.26 (d, J = 4.4 Hz, 3H), 2.49 (s, 3H), 2.41 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 162.5, 162.3, 157.0, 143.0, 139.6, 134.2, 132.8, 131.3, 129.4 (2C), 127.8, 126.9 (2C), 125.5, 119.4, 104.5 25.6, 23.8, 21.7; IR ν 2926, 1597, 1487, 1398, 1298, 1283, 1146, 1086, 978, 810, 787, 739, 706, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C19H18O2N3S2+, [M + H]+ 384.0835; found 384.0828.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 6.8 Hz, 1H), 7.37 (t, J = 6.4 Hz, 2H), 7.26 (d, J = 4.4 Hz, 3H), 2.49 (s, 3H), 2.41 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 162.5, 162.3, 157.0, 143.0, 139.6, 134.2, 132.8, 131.3, 129.4 (2C), 127.8, 126.9 (2C), 125.5, 119.4, 104.5 25.6, 23.8, 21.7; IR ν 2926, 1597, 1487, 1398, 1298, 1283, 1146, 1086, 978, 810, 787, 739, 706, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C19H18O2N3S2+, [M + H]+ 384.0835; found 384.0828.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.27 (t, J = 5.0 Hz, 1H), 7.93 (d, J = 8.0 Hz, 2H), 7.41 (s, 2H), 7.31 (d, J = 6.8 Hz, 2H), 7.18 (t, J = 9.2 Hz, 1H), 2.44 (d, J = 13.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.1, 161.20 (d, J = 250.1 Hz, 1C), 161.17, 155.9, 142.9, 139.9, 132.4 (d, J = 2.4 Hz, 1C), 129.6 (2C), 126.7 (2C), 126.4 (d, J = 10.3 Hz, 1C), 124.3 (d, J = 8.4 Hz, 1C), 115.1 (d, J = 22.9 Hz, 1C), 109.1 (d, J = 27.0 Hz, 1C), 105.0, 24.2, 21.7; IR ν 2920, 2851, 1595, 1491, 1460, 1400, 1148, 1088, 984, 854, 806, 789, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H15O2N3FS2+, [M + H]+ 388.0584; found 388.0579.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.27 (t, J = 5.0 Hz, 1H), 7.93 (d, J = 8.0 Hz, 2H), 7.41 (s, 2H), 7.31 (d, J = 6.8 Hz, 2H), 7.18 (t, J = 9.2 Hz, 1H), 2.44 (d, J = 13.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.1, 161.20 (d, J = 250.1 Hz, 1C), 161.17, 155.9, 142.9, 139.9, 132.4 (d, J = 2.4 Hz, 1C), 129.6 (2C), 126.7 (2C), 126.4 (d, J = 10.3 Hz, 1C), 124.3 (d, J = 8.4 Hz, 1C), 115.1 (d, J = 22.9 Hz, 1C), 109.1 (d, J = 27.0 Hz, 1C), 105.0, 24.2, 21.7; IR ν 2920, 2851, 1595, 1491, 1460, 1400, 1148, 1088, 984, 854, 806, 789, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H15O2N3FS2+, [M + H]+ 388.0584; found 388.0579.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.17 (d, J = 8.8 Hz, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.68 (s, 1H), 7.42 (d, J = 9.2 Hz, 2H), 7.31 (d, J = 8.8 Hz, 2H), 2.44 (d, J = 13.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.3, 161.0, 156.0, 143.0, 139.8, 134.5, 134.0, 129.6 (2C), 127.8, 126.7 (2C), 126.2, 123.5, 121.7, 105.0, 24.3, 21.7; IR ν 2924, 1597, 1495, 1395, 1312, 1150, 1092, 982, 824, 808, 791, 675, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H15O2N3ClS2+, [M + H]+ 404.0289; found 404.0284.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.17 (d, J = 8.8 Hz, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.68 (s, 1H), 7.42 (d, J = 9.2 Hz, 2H), 7.31 (d, J = 8.8 Hz, 2H), 2.44 (d, J = 13.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.3, 161.0, 156.0, 143.0, 139.8, 134.5, 134.0, 129.6 (2C), 127.8, 126.7 (2C), 126.2, 123.5, 121.7, 105.0, 24.3, 21.7; IR ν 2924, 1597, 1495, 1395, 1312, 1150, 1092, 982, 824, 808, 791, 675, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H15O2N3ClS2+, [M + H]+ 404.0289; found 404.0284.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.10 (d, J = 9.6 Hz, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.82 (s, 1H), 7.56 (d, J = 6.4 Hz, 1H), 7.42 (s, 1H), 7.31 (d, J = 5.6 Hz, 2H), 2.45 (d, J = 12.4 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.3, 160.9, 156.0, 143.0, 139.9, 134.9, 130.6, 129.6 (2C), 126.7 (2C), 126.5, 124.6, 123.7, 121.6, 105.1, 24.3, 21.7; IR ν 1597, 1487, 1474, 1391, 1362, 1302, 1287, 1144, 1086, 982, 814, 791, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H15O2N3BrS2+, [M + H]+ 447.9784; found 447.9778.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.10 (d, J = 9.6 Hz, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.82 (s, 1H), 7.56 (d, J = 6.4 Hz, 1H), 7.42 (s, 1H), 7.31 (d, J = 5.6 Hz, 2H), 2.45 (d, J = 12.4 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.3, 160.9, 156.0, 143.0, 139.9, 134.9, 130.6, 129.6 (2C), 126.7 (2C), 126.5, 124.6, 123.7, 121.6, 105.1, 24.3, 21.7; IR ν 1597, 1487, 1474, 1391, 1362, 1302, 1287, 1144, 1086, 982, 814, 791, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H15O2N3BrS2+, [M + H]+ 447.9784; found 447.9778.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.39 (d, J = 9.6 Hz, 1H), 8.60 (s, 1H), 8.31 (d, J = 10.4 Hz, 1H), 7.94 (d, J = 8.8 Hz, 2H), 7.50 (s, 1H), 7.35 (d, J = 8.0 Hz, 2H), 2.48 (d, J = 17.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.5, 161.3, 156.1, 146.2, 143.4, 139.7, 139.6, 129.8 (2C), 126.7 (2C), 126.2, 122.9, 122.7, 117.7, 105.4, 24.4, 21.7; IR ν 2920, 1694, 1597, 1539, 1504, 1458, 1337, 1315, 1152, 1090, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H15O4N4S2+, [M + H]+ 415.0529; found 415.0522.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.39 (d, J = 9.6 Hz, 1H), 8.60 (s, 1H), 8.31 (d, J = 10.4 Hz, 1H), 7.94 (d, J = 8.8 Hz, 2H), 7.50 (s, 1H), 7.35 (d, J = 8.0 Hz, 2H), 2.48 (d, J = 17.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 163.5, 161.3, 156.1, 146.2, 143.4, 139.7, 139.6, 129.8 (2C), 126.7 (2C), 126.2, 122.9, 122.7, 117.7, 105.4, 24.4, 21.7; IR ν 2920, 1694, 1597, 1539, 1504, 1458, 1337, 1315, 1152, 1090, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H15O4N4S2+, [M + H]+ 415.0529; found 415.0522.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.27 (d, J = 9.2 Hz, 1H), 8.39 (s, 1H), 8.11 (d, J = 11.6 Hz, 1H), 7.94 (d, J = 10.8 Hz, 2H), 7.45 (s, 1H), 7.33 (d, J = 8.0 Hz, 2H), 4.40–4.46 (m, 2H), 2.46 (d, J = 13.6 Hz, 6H), 1.42 (t, J = 9.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 164.9, 163.3, 161.7, 156.2, 143.0, 139.9, 138.8, 130.0, 129.6 (2C), 128.6, 126.7 (2C), 125.0, 123.4, 122.3, 105.1, 62.0, 24.3, 21.7, 14.4; IR ν 2924, 1717, 1605, 1493, 1458, 1395, 1268, 1150, 1087, 984, 785, 762, 692 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C21H20O4N3S2+, [M + H]+ 442.0890; found 442.0883.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.27 (d, J = 9.2 Hz, 1H), 8.39 (s, 1H), 8.11 (d, J = 11.6 Hz, 1H), 7.94 (d, J = 10.8 Hz, 2H), 7.45 (s, 1H), 7.33 (d, J = 8.0 Hz, 2H), 4.40–4.46 (m, 2H), 2.46 (d, J = 13.6 Hz, 6H), 1.42 (t, J = 9.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 164.9, 163.3, 161.7, 156.2, 143.0, 139.9, 138.8, 130.0, 129.6 (2C), 128.6, 126.7 (2C), 125.0, 123.4, 122.3, 105.1, 62.0, 24.3, 21.7, 14.4; IR ν 2924, 1717, 1605, 1493, 1458, 1395, 1268, 1150, 1087, 984, 785, 762, 692 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C21H20O4N3S2+, [M + H]+ 442.0890; found 442.0883.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.18 (d, J = 9.6 Hz, 1H), 7.94 (d, J = 9.6 Hz, 2H), 7.37 (s, 1H), 7.31 (d, J = 8.0 Hz, 2H), 7.16 (s, 1H), 6.99 (d, J = 9.2 Hz, 1H), 3.88 (s, 3H), 2.43 (d, J = 6.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 162.7, 161.1, 159.1, 155.8, 142.7, 140.2, 129.8, 129.5 (2C), 126.7 (2C), 126.3, 123.8, 114.3, 106.1, 104.8, 56.0, 24.2, 21.7; IR ν 2926, 1612, 1503, 1493, 1271, 1140, 1090, 1022, 984, 831, 737, 702, 673 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C19H18O3N3S2+ [M + H]+ 400.0784; found 400.0778.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.18 (d, J = 9.6 Hz, 1H), 7.94 (d, J = 9.6 Hz, 2H), 7.37 (s, 1H), 7.31 (d, J = 8.0 Hz, 2H), 7.16 (s, 1H), 6.99 (d, J = 9.2 Hz, 1H), 3.88 (s, 3H), 2.43 (d, J = 6.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 162.7, 161.1, 159.1, 155.8, 142.7, 140.2, 129.8, 129.5 (2C), 126.7 (2C), 126.3, 123.8, 114.3, 106.1, 104.8, 56.0, 24.2, 21.7; IR ν 2926, 1612, 1503, 1493, 1271, 1140, 1090, 1022, 984, 831, 737, 702, 673 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C19H18O3N3S2+ [M + H]+ 400.0784; found 400.0778.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.15 (d, J = 13.2 Hz, 1H), 7.93 (d, J = 8.8 Hz, 2H), 7.36 (s, 1H), 7.30 (d, J = 8.0 Hz, 2H), 7.14 (s, 1H), 6.97 (d, J = 11.2 Hz, 1H), 4.06–4.12 (m, 2H), 2.43 (d, J = 6.8 Hz, 6H), 1.45 (t, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 162.6, 161.1, 158.5, 155.7, 142.7, 140.1, 129.6, 129.5 (2C), 126.7 (2C), 126.2, 123.7, 114.7, 106.6, 104.7, 64.4, 24.2, 21.7, 14.8; IR ν 1603, 1497, 1288, 1184, 1144, 1094, 982, 941, 829, 816, 665, cm−1; HRMS (ESI-TOF) (m/z). Calcd for C20H20O3N3S2+ [M + H]+ 414.0941; found 414.0933.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.15 (d, J = 13.2 Hz, 1H), 7.93 (d, J = 8.8 Hz, 2H), 7.36 (s, 1H), 7.30 (d, J = 8.0 Hz, 2H), 7.14 (s, 1H), 6.97 (d, J = 11.2 Hz, 1H), 4.06–4.12 (m, 2H), 2.43 (d, J = 6.8 Hz, 6H), 1.45 (t, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 162.6, 161.1, 158.5, 155.7, 142.7, 140.1, 129.6, 129.5 (2C), 126.7 (2C), 126.2, 123.7, 114.7, 106.6, 104.7, 64.4, 24.2, 21.7, 14.8; IR ν 1603, 1497, 1288, 1184, 1144, 1094, 982, 941, 829, 816, 665, cm−1; HRMS (ESI-TOF) (m/z). Calcd for C20H20O3N3S2+ [M + H]+ 414.0941; found 414.0933.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 10.50 (s, 1H), 8.98 (d, J = 9.6 Hz, 1H), 7.82 (d, J = 6.8 Hz, 2H),7.36 (d, J = 8.4 Hz, 3H), 7.11 (s, 1H), 6.90 (d, J = 8.4 Hz, 1H), 2.35 (d, J = 10.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 162.8, 161.7, 157.2, 155.4, 142.7, 139.9, 129.8 (2C), 128.4, 126.7, 126.5 (2C), 122.8, 115.0, 108.8, 103.5, 23.8, 21.2; IR ν 3277, 2926, 1618, 1503, 1452, 1261, 1130, 1090, 1063, 988, 845, 835, 704, 687 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H16O3N3S2+ [M + H]+ 386.0628; found 386.0621.
1 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 10.50 (s, 1H), 8.98 (d, J = 9.6 Hz, 1H), 7.82 (d, J = 6.8 Hz, 2H),7.36 (d, J = 8.4 Hz, 3H), 7.11 (s, 1H), 6.90 (d, J = 8.4 Hz, 1H), 2.35 (d, J = 10.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 162.8, 161.7, 157.2, 155.4, 142.7, 139.9, 129.8 (2C), 128.4, 126.7, 126.5 (2C), 122.8, 115.0, 108.8, 103.5, 23.8, 21.2; IR ν 3277, 2926, 1618, 1503, 1452, 1261, 1130, 1090, 1063, 988, 845, 835, 704, 687 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H16O3N3S2+ [M + H]+ 386.0628; found 386.0621.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.29 (t, J = 4.2 Hz, 1H), 7.96 (d, J = 7.2 Hz, 2H), 7.35 (s, 3H), 7.26 (t, J = 4.0 Hz, 1H), 2.54 (s, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.8, 162.0, 153.4, 142.7, 139.9, 129.4 (2C), 126.7 (2C), 123.3, 113.3, 102.4, 24.8, 21.6; IR ν 2920, 1587, 1456, 1412, 1279, 1140, 1083, 1045, 1003, 937, 822, 787, 706, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C14H14O2N3S2+ [M + H]+ 320.0522; found 320.0516.
1 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.29 (t, J = 4.2 Hz, 1H), 7.96 (d, J = 7.2 Hz, 2H), 7.35 (s, 3H), 7.26 (t, J = 4.0 Hz, 1H), 2.54 (s, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.8, 162.0, 153.4, 142.7, 139.9, 129.4 (2C), 126.7 (2C), 123.3, 113.3, 102.4, 24.8, 21.6; IR ν 2920, 1587, 1456, 1412, 1279, 1140, 1083, 1045, 1003, 937, 822, 787, 706, 664 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C14H14O2N3S2+ [M + H]+ 320.0522; found 320.0516.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.24 (d, J = 12.0 Hz, 1H), 8.07 (d, J = 8.4 Hz, 2H), 7.71 (d, J = 8.0 Hz, 1H), 7.42–7.54 (m, 6H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.3, 161.6, 156.3, 142.9, 136.0, 132.2, 129.0 (2C), 128.0, 127.4, 126.7 (2C), 124.7, 122.7, 122.0, 104.9, 24.3; IR ν 2924, 1605, 1503, 1495, 1452, 1287, 1144, 1088, 984, 814, 758, 694 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H14O2N3S2+ [M + H]+ 356.0522; found 356.0517.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.24 (d, J = 12.0 Hz, 1H), 8.07 (d, J = 8.4 Hz, 2H), 7.71 (d, J = 8.0 Hz, 1H), 7.42–7.54 (m, 6H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.3, 161.6, 156.3, 142.9, 136.0, 132.2, 129.0 (2C), 128.0, 127.4, 126.7 (2C), 124.7, 122.7, 122.0, 104.9, 24.3; IR ν 2924, 1605, 1503, 1495, 1452, 1287, 1144, 1088, 984, 814, 758, 694 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H14O2N3S2+ [M + H]+ 356.0522; found 356.0517.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.19 (d, J = 8.8 Hz, 1H), 7.99 (d, J = 4.8 Hz, 2H), 7.72 (d, J = 10.8 Hz, 1H), 7.48–7.55 (m, 4H), 7.40 (s, 1H), 2.49 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.6, 161.6, 156.3, 141.5, 138.5, 136.0, 129.2 (2C), 128.2 (2C), 128.1, 127.5, 124.8, 122.6, 122.1, 104.9, 24.3; IR ν 1607, 1491, 1454, 1400, 1292, 1140, 1088, 984, 812, 756, 683 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H13O2N3ClS2+ [M + H]+ 390.0132; found 390.0126.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.19 (d, J = 8.8 Hz, 1H), 7.99 (d, J = 4.8 Hz, 2H), 7.72 (d, J = 10.8 Hz, 1H), 7.48–7.55 (m, 4H), 7.40 (s, 1H), 2.49 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.6, 161.6, 156.3, 141.5, 138.5, 136.0, 129.2 (2C), 128.2 (2C), 128.1, 127.5, 124.8, 122.6, 122.1, 104.9, 24.3; IR ν 1607, 1491, 1454, 1400, 1292, 1140, 1088, 984, 812, 756, 683 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H13O2N3ClS2+ [M + H]+ 390.0132; found 390.0126.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.20 (d, J = 5.6 Hz, 1H), 7.92 (d, J = 9.2 Hz, 2H), 7.73 (d, J = 8.0 Hz, 1H), 7.65 (d, J = 9.2 Hz, 2H), 7.47–7.56 (m, 2H), 7.40 (s, 1H), 2.49 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.7, 161.7, 156.3, 142.0, 135.9, 132.2 (2C), 128.3 (2C), 128.2, 127.5, 127.0, 124.8, 122.6, 122.1, 104.9, 24.3; IR ν 3088, 1605, 1491, 1452, 1402, 1292, 1136, 1086, 982, 812, 756, 739, 677 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H13O2N3BrS2+ [M + H]+ 435.9607; found 435.9598.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.20 (d, J = 5.6 Hz, 1H), 7.92 (d, J = 9.2 Hz, 2H), 7.73 (d, J = 8.0 Hz, 1H), 7.65 (d, J = 9.2 Hz, 2H), 7.47–7.56 (m, 2H), 7.40 (s, 1H), 2.49 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.7, 161.7, 156.3, 142.0, 135.9, 132.2 (2C), 128.3 (2C), 128.2, 127.5, 127.0, 124.8, 122.6, 122.1, 104.9, 24.3; IR ν 3088, 1605, 1491, 1452, 1402, 1292, 1136, 1086, 982, 812, 756, 739, 677 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H13O2N3BrS2+ [M + H]+ 435.9607; found 435.9598.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.26 (d, J = 11.2 Hz, 1H), 7.99 (d, J = 10.0 Hz, 2H), 7.70 (d, J = 8.0 Hz, 1H), 7.46–7.54 (m, 2H), 7.41 (s, 1H), 6.98 (d, J = 10.4 Hz, 2H), 3.87 (s, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.0, 162.6, 161.6, 156.2, 136.1, 134.9, 128.7 (2C), 128.0, 127.4, 124.7, 122.7, 122.0, 114.1 (2C), 104.8, 55.7, 24.3; IR ν 1595, 1487, 1443, 1406, 1285, 1250, 1140, 1086, 1026, 984, 812, 758, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H16O3N3S2+ [M + H]+ 386.0628; found 386.0622.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.26 (d, J = 11.2 Hz, 1H), 7.99 (d, J = 10.0 Hz, 2H), 7.70 (d, J = 8.0 Hz, 1H), 7.46–7.54 (m, 2H), 7.41 (s, 1H), 6.98 (d, J = 10.4 Hz, 2H), 3.87 (s, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.0, 162.6, 161.6, 156.2, 136.1, 134.9, 128.7 (2C), 128.0, 127.4, 124.7, 122.7, 122.0, 114.1 (2C), 104.8, 55.7, 24.3; IR ν 1595, 1487, 1443, 1406, 1285, 1250, 1140, 1086, 1026, 984, 812, 758, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H16O3N3S2+ [M + H]+ 386.0628; found 386.0622.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.15 (d, J = 10.4 Hz, 1H), 8.37 (d, J = 9.6 Hz, 2H), 8.25 (d, J = 7.2 Hz, 2H), 7.75 (d, J = 5.2 Hz, 1H), 7.56 (t, J = 7.6 Hz, 1H), 7.49 (t, J = 8.6 Hz, 1H), 7.44 (s, 1H), 2.52 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.3, 161.8, 156.4, 149.9, 148.6, 135.8, 128.4, 128.0 (2C), 127.6, 124.9, 124.3 (2C), 122.5, 122.3, 104.9, 24.4; IR ν 2922, 1557, 1522, 1493, 1342, 1146, 1090, 984, 818, 764, 739, 685 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H13O4N4S2+ [M + H]+ 401.0373; found 401.0366.
3 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.15 (d, J = 10.4 Hz, 1H), 8.37 (d, J = 9.6 Hz, 2H), 8.25 (d, J = 7.2 Hz, 2H), 7.75 (d, J = 5.2 Hz, 1H), 7.56 (t, J = 7.6 Hz, 1H), 7.49 (t, J = 8.6 Hz, 1H), 7.44 (s, 1H), 2.52 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.3, 161.8, 156.4, 149.9, 148.6, 135.8, 128.4, 128.0 (2C), 127.6, 124.9, 124.3 (2C), 122.5, 122.3, 104.9, 24.4; IR ν 2922, 1557, 1522, 1493, 1342, 1146, 1090, 984, 818, 764, 739, 685 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C17H13O4N4S2+ [M + H]+ 401.0373; found 401.0366.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.27 (d, J = 11.6 Hz, 1H), 8.63 (s, 1H), 8.05 (d, J = 11.6 Hz, 1H), 7.98 (t, J = 8.2 Hz, 2H), 7.90 (d, J = 7.2 Hz, 1H), 7.70 (d, J = 11.2 Hz, 1H), 7.60 (s, 2H), 7.42–7.52 (s, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.3, 161.6, 156.3, 139.8, 136.0, 134.8, 132.3, 129.4, 129.3, 128.5, 128.0 (2C), 127.4 (2C), 127.3, 124.7, 122.74, 122.68, 122.0, 104.9, 24.3; IR ν 1607, 1495, 1452, 1404, 1285, 1144, 1124, 1074, 986, 860, 806, 748, 691 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C21H16O2N3S2+ [M + H]+ 406.0679; found 406.0671.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.27 (d, J = 11.6 Hz, 1H), 8.63 (s, 1H), 8.05 (d, J = 11.6 Hz, 1H), 7.98 (t, J = 8.2 Hz, 2H), 7.90 (d, J = 7.2 Hz, 1H), 7.70 (d, J = 11.2 Hz, 1H), 7.60 (s, 2H), 7.42–7.52 (s, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.3, 161.6, 156.3, 139.8, 136.0, 134.8, 132.3, 129.4, 129.3, 128.5, 128.0 (2C), 127.4 (2C), 127.3, 124.7, 122.74, 122.68, 122.0, 104.9, 24.3; IR ν 1607, 1495, 1452, 1404, 1285, 1144, 1124, 1074, 986, 860, 806, 748, 691 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C21H16O2N3S2+ [M + H]+ 406.0679; found 406.0671.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.29 (d, J = 5.2 Hz, 1H), 7.74 (d, J = 3.2 Hz, 1H), 7.55 (d, J = 3.6 Hz, 2H), 7.35 (s, 1H), 3.22 (s, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.9, 161.5, 156.3, 136.0, 128.0, 127.2, 124.7, 122.3, 122.2, 105.1, 42.9, 24.2; IR ν 2922, 1605, 1503, 1493, 1454, 1277, 1252, 1115, 1063, 982, 953, 824, 761, 660 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C12H12O2N3S2+ [M + H]+ 294.0366; found 294.0359.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.29 (d, J = 5.2 Hz, 1H), 7.74 (d, J = 3.2 Hz, 1H), 7.55 (d, J = 3.6 Hz, 2H), 7.35 (s, 1H), 3.22 (s, 3H), 2.47 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.9, 161.5, 156.3, 136.0, 128.0, 127.2, 124.7, 122.3, 122.2, 105.1, 42.9, 24.2; IR ν 2922, 1605, 1503, 1493, 1454, 1277, 1252, 1115, 1063, 982, 953, 824, 761, 660 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C12H12O2N3S2+ [M + H]+ 294.0366; found 294.0359.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.28 (d, J = 4.4 Hz, 1H), 7.73 (d, J = 4.0 Hz, 1H), 7.55 (d, J = 3.6 Hz, 2H), 7.40 (s, 1H), 3.25 (t, J = 8.0 Hz, 2H), 2.46 (s, 3H), 2.00–2.07 (m, 2H), 1.14 (t, J = 9.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 162.6, 161.5, 156.5, 136.1, 127.9, 127.1, 124.7, 122.2 (2C), 105.3, 56.8, 24.2, 17.6, 13.3; IR ν 2967, 1603, 1493, 1452, 1406, 1275, 1250, 1115, 1059, 984, 820, 758, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C14H16O2N3S2+ [M + H]+ 322.0679; found 322.0674.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.28 (d, J = 4.4 Hz, 1H), 7.73 (d, J = 4.0 Hz, 1H), 7.55 (d, J = 3.6 Hz, 2H), 7.40 (s, 1H), 3.25 (t, J = 8.0 Hz, 2H), 2.46 (s, 3H), 2.00–2.07 (m, 2H), 1.14 (t, J = 9.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 162.6, 161.5, 156.5, 136.1, 127.9, 127.1, 124.7, 122.2 (2C), 105.3, 56.8, 24.2, 17.6, 13.3; IR ν 2967, 1603, 1493, 1452, 1406, 1275, 1250, 1115, 1059, 984, 820, 758, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C14H16O2N3S2+ [M + H]+ 322.0679; found 322.0674.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.29 (d, J = 6.8 Hz, 1H), 7.73 (d, J = 4.0 Hz, 1H), 7.55 (d, J = 4.0 Hz, 2H), 7.40 (s, 1H), 3.20 (s, 2H), 2.46 (s, 4H), 1.19 (d, J = 5.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 162.6, 161.5, 156.4, 136.1, 127.9, 127.1, 124.7, 122.3, 122.2, 105.2, 62.7, 25.1, 24.2, 23.0 (2C); IR ν 2922, 1605, 1495, 1450, 1402, 1369, 1283, 1113, 982, 835, 814, 758, 667 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C15H18O2N3S2+ [M + H]+ 336.0835; found 336.0828.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.29 (d, J = 6.8 Hz, 1H), 7.73 (d, J = 4.0 Hz, 1H), 7.55 (d, J = 4.0 Hz, 2H), 7.40 (s, 1H), 3.20 (s, 2H), 2.46 (s, 4H), 1.19 (d, J = 5.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 162.6, 161.5, 156.4, 136.1, 127.9, 127.1, 124.7, 122.3, 122.2, 105.2, 62.7, 25.1, 24.2, 23.0 (2C); IR ν 2922, 1605, 1495, 1450, 1402, 1369, 1283, 1113, 982, 835, 814, 758, 667 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C15H18O2N3S2+ [M + H]+ 336.0835; found 336.0828.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.99 (d, J = 8.4 Hz, 1H), 7.70 (d, J = 7.2 Hz, 1H), 7.48 (d, J = 28.0 Hz, 5H), 7.30 (s, 3H), 4.46 (s, 2H), 2.42 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.0, 161.4, 156.9, 135.8, 134.5, 132.3, 131.0, 130.0, 129.1, 128.8, 128.0, 127.2, 124.6, 122.5, 122.1, 105.1, 60.6, 24.1; IR ν 2924, 1603, 1493, 1400, 1256, 1152, 1099, 982, 824, 756, 704, 677 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H16O2N3S2+ [M + H]+ 370.0679; found 370.0681.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 8.99 (d, J = 8.4 Hz, 1H), 7.70 (d, J = 7.2 Hz, 1H), 7.48 (d, J = 28.0 Hz, 5H), 7.30 (s, 3H), 4.46 (s, 2H), 2.42 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.0, 161.4, 156.9, 135.8, 134.5, 132.3, 131.0, 130.0, 129.1, 128.8, 128.0, 127.2, 124.6, 122.5, 122.1, 105.1, 60.6, 24.1; IR ν 2924, 1603, 1493, 1400, 1256, 1152, 1099, 982, 824, 756, 704, 677 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C18H16O2N3S2+ [M + H]+ 370.0679; found 370.0681.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.28 (d, J = 4.8 Hz, 1H), 7.74 (t, J = 4.4 Hz, 1H), 7.56 (d, J = 5.2 Hz, 2H), 7.37 (s, 1H), 3.78 (t, J = 7.2 Hz, 2H), 3.46 (t, J = 6.0 Hz, 2H), 2.48 (s, 5H); 13C NMR (100 MHz, CDCl3) δ 163.1, 161.6, 156.6, 136.0, 128.1, 127.4, 124.7, 122.3, 122.2, 105.3, 52.1, 43.4, 27.4, 24.2; IR ν 1601, 1491, 1452, 1445, 1406, 1281, 1250, 1111, 1061, 984, 820, 758, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C14H15O2N3ClS2+, [M + H]+ 356.0289; found 356.0283.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.28 (d, J = 4.8 Hz, 1H), 7.74 (t, J = 4.4 Hz, 1H), 7.56 (d, J = 5.2 Hz, 2H), 7.37 (s, 1H), 3.78 (t, J = 7.2 Hz, 2H), 3.46 (t, J = 6.0 Hz, 2H), 2.48 (s, 5H); 13C NMR (100 MHz, CDCl3) δ 163.1, 161.6, 156.6, 136.0, 128.1, 127.4, 124.7, 122.3, 122.2, 105.3, 52.1, 43.4, 27.4, 24.2; IR ν 1601, 1491, 1452, 1445, 1406, 1281, 1250, 1111, 1061, 984, 820, 758, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C14H15O2N3ClS2+, [M + H]+ 356.0289; found 356.0283.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.37 (d, J = 10.8 Hz, 1H), 7.72 (d, J = 5.6 Hz, 1H), 7.56 (m, 2H), 7.47 (s, 1H), 3.90 (d, J = 14.8 Hz, 1H), 3.14 (d, J = 14.8 Hz, 1H), 2.76 (t, J = 14.8 Hz, 1H), 2.48 (s, 3H), 2.39 (d, J = 18.4 Hz, 1H), 2.02–2.11 (m, 2H), 1.92 (d, J = 18.8 Hz, 1H), 1.72–1.79 (m, 1H), 1.40 (t, J = 11.0 Hz, 1H), 1.18 (s, 3H), 0.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 215.6, 162.9, 161.5, 156.7, 136.0, 128.0, 127.4, 124.6, 122.8, 122.0, 105.3, 58.6, 50.4, 48.3, 42.9, 42.7, 27.2, 24.7, 24.2, 20.2, 20.0; IR ν 2953, 1740, 1611, 1503, 1456, 1398, 1281, 1121, 1053, 986, 818, 752, 683 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C21H24O3N3S2+, [M + H]+ 430.1254; found 430.1246.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.37 (d, J = 10.8 Hz, 1H), 7.72 (d, J = 5.6 Hz, 1H), 7.56 (m, 2H), 7.47 (s, 1H), 3.90 (d, J = 14.8 Hz, 1H), 3.14 (d, J = 14.8 Hz, 1H), 2.76 (t, J = 14.8 Hz, 1H), 2.48 (s, 3H), 2.39 (d, J = 18.4 Hz, 1H), 2.02–2.11 (m, 2H), 1.92 (d, J = 18.8 Hz, 1H), 1.72–1.79 (m, 1H), 1.40 (t, J = 11.0 Hz, 1H), 1.18 (s, 3H), 0.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 215.6, 162.9, 161.5, 156.7, 136.0, 128.0, 127.4, 124.6, 122.8, 122.0, 105.3, 58.6, 50.4, 48.3, 42.9, 42.7, 27.2, 24.7, 24.2, 20.2, 20.0; IR ν 2953, 1740, 1611, 1503, 1456, 1398, 1281, 1121, 1053, 986, 818, 752, 683 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C21H24O3N3S2+, [M + H]+ 430.1254; found 430.1246.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.31 (d, J = 6.8 Hz, 1H), 7.94 (d, J = 7.2 Hz, 2H), 7.71 (d, J = 6.8 Hz, 1H), 7.50 (m, 2H), 7.37 (s, 1H), 7.30 (d, J = 7.6 Hz, 2H), 2.66 (t, J = 8.6 Hz, 2H), 2.42 (s, 3H), 1.71 (s, 2H), 1.34 (s, 4H), 0.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 167.0, 161.6, 156.5, 142.8, 140.0, 136.1, 129.5 (2C), 127.9, 127.4, 126.8 (2C), 124.7, 122.8, 122.0, 104.2, 37.8, 31.5, 28.0, 22.5, 21.7, 14.1; IR ν 2926, 1601, 1493, 1450, 1404, 1279, 1146, 1086, 1047, 810, 752, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C22H24O2N3S2+, [M + H]+ 426.1305; found 426.1297.
4 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.31 (d, J = 6.8 Hz, 1H), 7.94 (d, J = 7.2 Hz, 2H), 7.71 (d, J = 6.8 Hz, 1H), 7.50 (m, 2H), 7.37 (s, 1H), 7.30 (d, J = 7.6 Hz, 2H), 2.66 (t, J = 8.6 Hz, 2H), 2.42 (s, 3H), 1.71 (s, 2H), 1.34 (s, 4H), 0.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 167.0, 161.6, 156.5, 142.8, 140.0, 136.1, 129.5 (2C), 127.9, 127.4, 126.8 (2C), 124.7, 122.8, 122.0, 104.2, 37.8, 31.5, 28.0, 22.5, 21.7, 14.1; IR ν 2926, 1601, 1493, 1450, 1404, 1279, 1146, 1086, 1047, 810, 752, 669 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C22H24O2N3S2+, [M + H]+ 426.1305; found 426.1297.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.36 (s, J = 3.6 Hz, 1H), 8.09 (s, 2H), 7.98 (s, 3H), 7.72 (s, 1H), 7.51 (s, 5H), 7.32 (d, J = 6.4 Hz, 2H), 2.42 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.9, 159.2, 156.8, 142.9, 140.1, 136.0, 135.1, 131.9, 129.6 (2C), 129.1 (2C), 128.0 (3C), 127.5, 126.8 (2C), 125.0, 122.8, 122.0, 101.0, 21.7; IR ν 2922, 1589, 1483, 1443, 1396, 1310, 1279, 1261, 1144, 1080, 818, 750, 660 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C23H18O2N3S2+, [M + H]+ 432.0835; found 432.0830.
6 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.36 (s, J = 3.6 Hz, 1H), 8.09 (s, 2H), 7.98 (s, 3H), 7.72 (s, 1H), 7.51 (s, 5H), 7.32 (d, J = 6.4 Hz, 2H), 2.42 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.9, 159.2, 156.8, 142.9, 140.1, 136.0, 135.1, 131.9, 129.6 (2C), 129.1 (2C), 128.0 (3C), 127.5, 126.8 (2C), 125.0, 122.8, 122.0, 101.0, 21.7; IR ν 2922, 1589, 1483, 1443, 1396, 1310, 1279, 1261, 1144, 1080, 818, 750, 660 cm−1; HRMS (ESI-TOF) (m/z). Calcd for C23H18O2N3S2+, [M + H]+ 432.0835; found 432.0830.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.41 (d, J = 8.4 Hz, 1H), 7.58 (d, J = 6.4 Hz, 1H), 7.45 (t, J = 9.0 Hz, 1H), 7.38 (t, J = 8.6 Hz, 1H), 6.95 (s, 1H), 5.97 (s, 1H), 2.20 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.4, 159.3, 153.9, 137.3, 126.6, 126.0, 123.9, 121.4, 121.0, 107.7, 23.0; IR ν 2922, 2853, 2205, 1636, 1524, 1456, 1271, 1182, 976, 748, 698 cm−1.
2 v/v ethyl acetate/60–90 petroleum ether). 1H NMR (400 MHz, CDCl3) δ 9.41 (d, J = 8.4 Hz, 1H), 7.58 (d, J = 6.4 Hz, 1H), 7.45 (t, J = 9.0 Hz, 1H), 7.38 (t, J = 8.6 Hz, 1H), 6.95 (s, 1H), 5.97 (s, 1H), 2.20 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.4, 159.3, 153.9, 137.3, 126.6, 126.0, 123.9, 121.4, 121.0, 107.7, 23.0; IR ν 2922, 2853, 2205, 1636, 1524, 1456, 1271, 1182, 976, 748, 698 cm−1.All NMR spectra please see ESI Section 3.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Science and Technology Planning Program of Zhanjiang (2021A05247); Medical Scientific Research Foundation of Guangdong Province (A2021037); Key Discipline Construction Project of Guangdong Medical University (4SG23004G); Innovation and Entrepreneurship Team Leads the Pilot Program of Zhanjiang (2020LHJH005); Science and technology program of Guangdong Province (2019B090905011); Zhanjiang Ocean Young Talent Innovation Project (2022E05008) and Scientific Research Project of General Universities in Guangdong Province (2022KTSCX046) for support.Notes and references
- (a) R. A. Glennon, J. J. Gaines and M. E. Rogers, J. Med. Chem., 1981, 24, 766–769 CrossRef CAS PubMed; (b) P. Ulrich and A. Cerami, J. Med. Chem., 1982, 25, 654–657 CrossRef CAS PubMed; (c) A. K. Bhattacharjee, M. G. Hartell, D. A. Nichols, R. P. Hicks, B. Stanton, J. E. van Hamont and W. K. Milhous, Eur. J. Med. Chem., 2004, 39, 59–67 CrossRef CAS; (d) Y. Zhang, J.-H. Yang, Y.-Q. Xia, L. Dong and F.-E. Chen, Chem.–Eur. J., 2021, 27, 6183–6186 CrossRef CAS PubMed.
- J. R. LaRochelle, M. Fodor, J. M. Ellegast, X. Liu, V. Vemulapalli, M. Mohseni, T. Stams, S. J. Buhrlage, K. Stegmaier, M. J. LaMarche, M. G. Acker and S. C. Blacklow, Bioorg. Med. Chem., 2017, 25, 6479–6485 CrossRef CAS PubMed.
- S. G. Badne, D. K. Swamy, V. N. Bhosale and S. V. Kuberkar, J. Heterocycl. Chem., 2011, 48, 849–855 CrossRef CAS.
- J. N. Sangshetti, D. K. Lokwani, R. S. Chouthe, A. Ganure, B. Raval, F. A. K. Khan and D. B. Shinde, Med. Chem. Res., 2014, 23, 4893–4900 CrossRef CAS.
- M. A. Abdel-Rahman, A. El-Badieh, A. G. Ghattas, G. A. El-Saraf and A. K. Mahmoud, Rev. Roum. Chim., 1995, 40, 165–172 CAS.
- (a) M. A. El-Sherbeny, Arzneimittelforschung, 2000, 50, 848–853 CAS; (b) S. B. Mekapati, A. Kurup, R. P. Verma and C. Hansch, Bioorg. Med. Chem., 2005, 13, 3737–3762 CrossRef CAS PubMed.
- (a) L. Jarrige, D. Glavač, G. Levitre, P. Retailleau, G. Bernadat, L. Neuville and G. Masson, Chem. Sci., 2019, 10, 3765–3769 RSC; (b) A. Matviitsuk, M. D. Greenhalgh, J. E. Taylor, X. B. Nguyen, D. B. Cordes, A. M. Z. Slawin, D. W. Lupton and A. D. Smith, Org. Lett., 2020, 22, 335–339 CrossRef CAS PubMed; (c) F. Zhao, C. Shu, C. M. Young, C. Carpenter-Warren, A. M. Z. Slawin and A. D. Smith, Angew. Chem., Int. Ed., 2021, 133, 11999–12007 CrossRef.
- (a) V. B. Birman, X. Li and Z. Han, Org. Lett., 2007, 9, 37–40 CrossRef CAS PubMed; (b) V. B. Birman and X. Li, Org. Lett., 2008, 10, 1115–1118 CrossRef CAS; (c) P. A. Woods, L. C. Morrill, T. Lebl, A. M. Z. Slawin, R. A. Bragg and A. D. Smith, Org. Lett., 2010, 12, 2660–2663 CrossRef CAS PubMed; (d) D. Belmessieri, C. Joannesse, P. A. Woods, C. MacGregor, C. Jones, C. D. Campbell, C. P. Johnston, N. Duguet, C. Concellón, R. A. Braggb and A. D. Smith, Org. Biomol. Chem., 2011, 9, 559–570 RSC; (e) M. Brindisi, S. Maramai, S. Gemma, S. Brogi, A. Grillo, L. D. C. Mannelli, E. Gabellieri, S. Lamponi, S. Saponara, B. Gorelli, D. Tedesco, T. Bonfiglio, C. Landry, K.-M. Jung, A. Armirotti, L. Luongo, A. Ligresti, F. Piscitelli, C. Bertucci, M.-P. Dehouck, G. Campiani, S. Maione, C. Ghelardini, A. Pittaluga, D. Piomelli, V. D. Marzo and S. Butini, J. Med. Chem., 2016, 59, 2612–2632 CrossRef CAS PubMed; (f) N. A. Ahlemeyer, E. V. Streff, P. Muthupandi and V. B. Birman, Org. Lett., 2017, 19, 6486–6489 CrossRef CAS PubMed.
- (a) L. Jarrige, D. Glavač, G. Levitre, P. Retailleau, G. Bernadat, L. Neuville and G. Masson, Chem. Sci., 2019, 10, 3765–3769 RSC; (b) Q. Ni, X. Wang, F. Xu, X. Chen and X. Song, Chem. Commun., 2020, 56, 3155–3158 RSC; (c) D. Lu, J.-H. Wu, J. Pan, X. Chen, X. Ren and T. Wang, Chem. Commun., 2020, 56, 11231–11234 RSC; (d) J. M. Honnanayakanavar, B. Harish, J. B. Nanubolu and S. Suresh, J. Org. Chem., 2020, 85, 8780–8791 CrossRef CAS PubMed; (e) Y. Zhang, J.-H. Yang, Y.-Q. Xia, L. Dong and F.-E. Chen, Chem.–Eur. J., 2021, 27, 6183–6186 CrossRef CAS; (f) X.-P. Chen, K.-Q. Hou, F. Zhou, A. S. C. Chan and X.-F. Xiong, J. Org. Chem., 2021, 86, 1667–1675 CrossRef CAS PubMed; (g) J.-M. Wang, Y.-X. Chen, C.-S. Yao and K. Zhang, Asian J. Org. Chem., 2022, 11, e202200238 CAS.
- B. Ranieri, O. Robles and D. Romo, J. Org. Chem., 2013, 78, 6291–6296 CrossRef CAS PubMed.
- (a) I. Bae, H. Han and S. Chang, J. Am. Chem. Soc., 2005, 127, 2038–2039 CrossRef CAS PubMed; (b) S. H. Cho, E. J. Yoo, I. Bae and S. Chang, J. Am. Chem. Soc., 2005, 127, 16046–16047 CrossRef CAS PubMed.
- (a) S. H. Kim, S. H. Park, J. H. Choi and S. Chang, Chem.–Asian J., 2011, 6, 2618–2634 CrossRef CAS PubMed; (b) P. Lu and Y. Wang, Chem. Soc. Rev., 2012, 41, 5687–5705 RSC; (c) S. Bahadorikhalili, M. Divar, T. Damghani, F. Moeini, S. Ghassamipour, A. Iraji, M. A. Miller, B. Larijani and M. Mahdavi, J. Organomet. Chem., 2021, 939, 121773 CrossRef CAS.
- (a) Y. Zhao, Z. Zhou, L. Liu, M. Chen, W. Yang, Q. Chen, M. G. Gardiner and M. G. Banwell, J. Org. Chem., 2021, 86, 9155–9162 CrossRef CAS PubMed; (b) W. Yang, Y. Zhao, Q. Bu, L. Li, B. Zhou and Z. Huang, Org. Lett., 2022, 24, 457–461 CrossRef CAS PubMed; (c) X. Luo, Z. Yang, J. Zheng, G. Liang, H. Luo and W. Yang, Org. Lett., 2022, 24, 7300–7304 CrossRef CAS PubMed; (d) Z. Yang, X. Luo, Z. Zhang, X. Luo, J. Zheng, H. Luo and W. Yang, Adv. Synth. Catal., 2022, 364, 4433–4439 CrossRef CAS; (e) D. Luo, H. Zhang, W. Yi, G. Li, L. Chen and W. Yang, Eur. J. Org. Chem., 2022, 2022, e202201214 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2270393. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra04082h |
| This journal is © The Royal Society of Chemistry 2023 |