Open Access Article
Open Access ArticleDCID-mediated esterification of carboxylic acids with alcohols under mild conditions†
Farzaneh Nasiria,
Javad Mokhtari *a,
Salman Taheri
*a,
Salman Taheri b and
Zohreh Mirjafarya
b and
Zohreh Mirjafarya
aDepartment of Chemistry, Science and Research Branch, Islamic Azad University, PO Box 14515/775, Tehran, Iran. E-mail: j.mokhtari@srbiau.ac.ir
bChemistry & Chemical Engineering Research Center of Iran, PO Box 14335-186, Tehran, Iran
First published on 13th September 2023
Abstract
DCID (Dichloroimidazolidinedione) 2 is used as a novel coupling reagent for the esterification of carboxylic acids with alcohols at room temperature. The reaction represents the first DCID-promoted esterification under mild conditions with good to excellent yields. Reactions can proceed smoothly with those bearing electron-withdrawing and donating group(s) on the carboxylic acids and benzyl alcohols at ambient temperature. Furthermore, we proposed a plausible mechanism and confirmed it by isolating and characterizing intermediates 3a and 7. The structures of the synthesized compounds were confirmed by comparison of melting points and NMR spectra.
Introduction
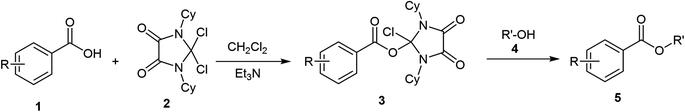
The esterification of carboxylic acids is an important reaction in organic synthesis.1,2 Esters play a noteworthy role in living systems and the chemical industry.3–9 There are many methods for the synthesis of esters available in the literature. The common method is the Fischer esterification, which is a reversible condensation reaction between acids and alcohols and is catalyzed by the acid. On the other hand, there are many irreversible reactions such as the Mitsunobu, and Steglich (activation by carbodiimides) esterification which is catalyzed by one or more catalysts.10 The reaction of carboxylic acids with alcohols for the preparation of esters is among the efficient and mildest of organic transformations, which is mainly the result of high accessibility and stability of reactants. Although the literature is supplied with different methods for esterification, novel approaches such as the use of oxalyl chloride and triphenylphosphine oxide,11 Dowex H+/NaI,12 lipase-catalyzed alkyl valerates,13 etc.14 have been reported recently. Generally, maybe one of the sampling methods to prepare esters is the use of reactive carboxylic anhydride or acid halides with alcohol. These reactions are simple, with high yield and can be done mostly without special catalyst. In view of the above insights and in continuation of previous works for the developing of the role of DCID (Dichloroimidazolidinedione) in organic synthesis,15 we wish to report a general esterification of carboxylic acids using DCID as a new coupling reagent (Scheme 1).Experimental section
Materials and methods
All materials including ethanol (C2H5OH), dry dichloromethane (CH2Cl2), oxalyl chloride (COCl)2, N,N′-dicyclohexylcarbodiimide (DCC), trithylamine (Et3N), pyridine, DIEA, benzoic acids and alcohols derivatives were obtained from commercially available sources such as Merck and Sigma-Aldrich without further purification. NMR spectra were recorded on Bruker NMR spectrometers (400 MHz for 1H-NMR and 100 MHz for 13C-NMR). 1H-NMR chemical shifts (δH) are quoted in ppm downfield from tetramethylsilane (TMS) in 400 MHz spectrometer. Melting points were obtained on an Electrothermal 9100 apparatus.Synthesis of DCID (dichloroimidazolidinedione)
DCID was synthesized with minor modifications.15a To the 4.0 g (19.40 mmol) of N,N′-Dicyclohexylcarbodiimine (DCC) in 50 mL dry dichloromethane (CH2Cl2) was added 1.8 mL (21.0 mmol) of oxalyl chloride dropwise at 0 °C and the reaction mixture stirred for 1 hours at room temperature. The resulting precipitate was filtered and washed with cold dichloromethane. The recrystallization of white solid in ethanol gives 5.5 gr of DCID (yield: 85%); m.p. = 174–176 °C. 1H-NMR (400 MHz, CDCl3): δ (ppm) = 3.97–4.00 (m, 2H), 2.02–2.10 (m, 4H, Cy), 1.73–1.87 (m, 4H, Cy), 1.71–1.75 (m, 4H, Cy), 1.66–1.69 (m, 2H, Cy), 1.17–1.36 (m, 6H, Cy). 13C-NMR (100 MHz, DMSO-d6): 157.3, 115.2, 51.5, 29.5, 25.6, 25.2 ppm.General procedure for the synthesis of esters
Benzoic acid (1.0 mmol), benzyl alcohol (1.0 mmol), DCID (1.20 mmol) and triethyl amine (1.0 mmol) were added to CH2Cl2 (5 mL) and stirred at 25 °C for 24 h and completion of the reaction monitored by TLC. Products were purified by chromatography on silica gel (eluents: EtOAc–hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3).
3).
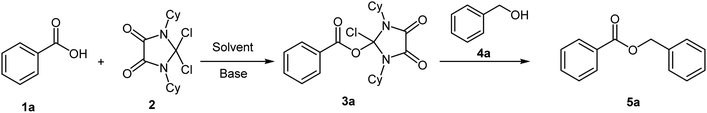
Synthesis and isolation of 2-chloro-1,3-dicyclohexyl-4,5-dioxoimidazolidin-2-yl benzoate intermediate (3a)
Typically, to and 25 mL round-bottom flask containing dry CH2Cl2 (5 mL) was added benzoic acid (1.0 mmol), DCID (1.2 mmol) and triethyl amine (1.0 mmol). The reaction mixture was stirred for 24 h at 25 °C. The completion of the reaction monitored by TLC. The salt was filtered, and the filtrate was evaporated under reduced pressure to yield an oily solid. 1HNMR (400 MHz, DMSO-d6): = 8.17 (t, J = 7.0 Hz, 2H), 7.83 (t, J = 7.0 Hz, 1H), 7.79 (d, J = 6.9 Hz, 2H), 3.79–3.83 (m, 2H), 1.83–1.90 (m, 4H), 1.71–1.78 (m, 9H), 1.59–1.62 (m, 2H), 1.09–1.29 (m, 6H) ppm. 13C-NMR (100 MHz, DMSO-d6): 165.6, 158.8, 133.6, 131.6, 131.2, 130.0, 129.4, 51.5, 29.5, 25.6, 25.2 ppm.Results and discussion
Initial investigations for suitable reaction conditions for the catalyst-free one-pot procedure for ester formation using benzoic acid and benzyl alcohol as the model substrates were performed. The model reaction was performed in the presence of different bases such as Et3N, pyridine and DIEA; among them Et3N was found to be the most effective base. Then, the reaction was done in different solvents including CH2Cl2, CH3CN, THF and DMF. Among the solvents examined, CH2Cl2 was proved to be the best reaction medium. For the optimization of time, completion of the reaction monitored by TLC at different times, the best time was between 18 and 24 hours. In this reaction, the molar ratio of DCID was also effective on the reaction yield; by increasing the amount of DCID to 1.2 equivalent the yield of 5a increased to 86% (Table 1, entry 2). On the other hand, when the reaction was done without DCID, no product was observed, and this shows its effective role in this reaction (Table 1, entry 8). Therefore, it was concluded that the optimum reaction conditions involved benzoic acid (1 mmol), benzyl alcohol DCID (1.2 mmol), and Et3N (1.0 mmol) in CH2Cl2 (5 mL) at 25 °C (Table 2, entry 2).| Entry | Base | Solvent | DCID 2 (eq.) | Yield (%) |
|---|---|---|---|---|
| a Reaction condition: benzoic acid (1 mmol), base (1 mmol), benzyl alcohol (1.0 mmol), solvent (5 mL), time: 24 h at room temperature. | ||||
| 1 | Et3N | CH2Cl2 | 1 | 75 |
| 2 | Et3N | CH2Cl2 | 1.20 | 86 |
| 3 | Pyridine | CH2Cl2 | 1.20 | 70 |
| 4 | DIEA | CH2Cl2 | 1.20 | 82 |
| 5 | Et3N | DMF | 1.20 | 45 |
| 6 | Et3N | THF | 1.20 | 52 |
| 7 | Et3N | CH3CN | 1.20 | 60 |
| 8 | Et3N | CH2Cl2 | — | — |
| Entry | Benzoic acids | Alcohols | Products | Yield (%) | Mp. (°C) |
|---|---|---|---|---|---|
| 1 | 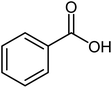 |
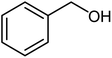 |
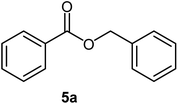 |
86 | Oil |
| 2 | 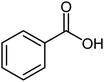 |
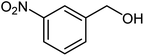 |
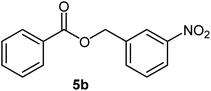 |
74 | 70–72 (ref. 16) |
| 3 | 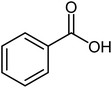 |
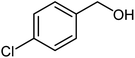 |
 |
84 | 58–60 (ref. 17) |
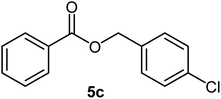
| 4 | 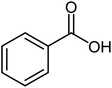 |
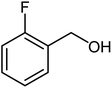 |
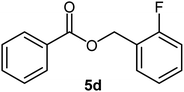 |
85 | Oil |
| 5 | 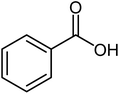 |
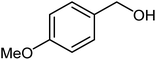 |
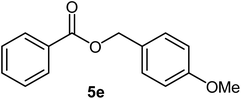 |
83 | 29–30 (ref. 18) |
| 6 | 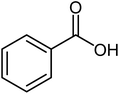 |
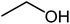 |
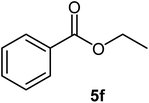 |
79 | Oil |
| 7 | 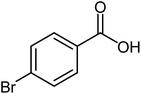 |
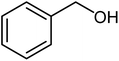 |
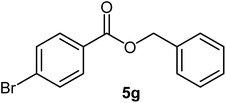 |
89 | 51–53 (ref. 19) |
| 8 | 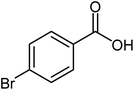 |
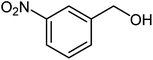 |
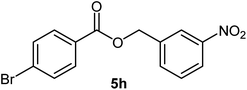 |
76 | 114–116 (ref. 20) |
| 9 | 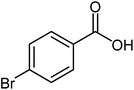 |
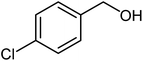 |
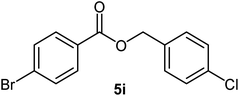 |
84 | Oil |
| 10 | 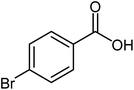 |
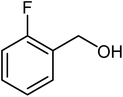 |
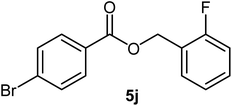 |
86 | Oil |
| 11 | 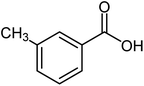 |
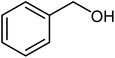 |
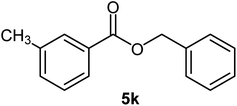 |
91 | Oil |
| 12 | 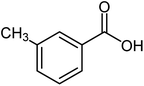 |
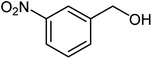 |
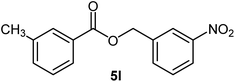 |
75 | 89–91 (ref. 21) |
| 13 | 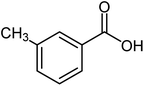 |
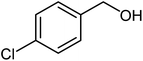 |
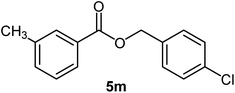 |
84 | Oil |
| 14 | 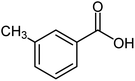 |
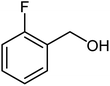 |
 |
82 | Oil |
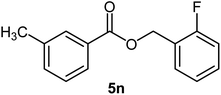
| 15 | 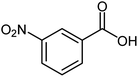 |
 |
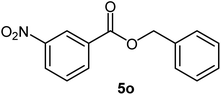 |
68 | 48–50 (ref. 22) |
| 16 | 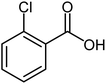 |
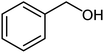 |
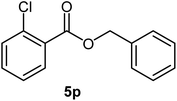 |
78 | 18–20 (ref. 22) |
| 17 | 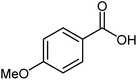 |
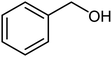 |
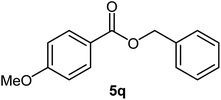 |
82 | 24–26 (ref. 22) |
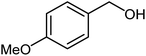
| 18 | 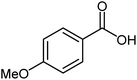 |
 |
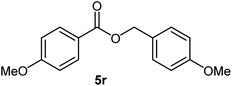 |
87 | 28–30 (ref. 23) |
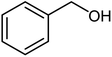
| 19 | 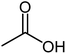 |
 |
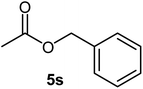 |
58 | Oil |
| 20 | 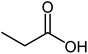 |
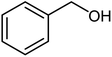 |
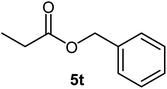 |
54 | Oil |
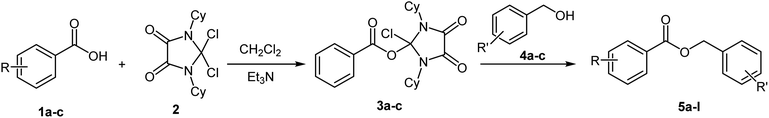
Encouraged by our initial studies, we then investigated the generality and versatility of DCID for the esterification of different carboxylic acids with alcohols (Table 2). As shown in Table 2, most carboxylic acids underwent esterification to afford the corresponding esters in good yields (74% to 86%). The introduction of substituents often changes the activity of the aromatic ring and in this reaction changing the aromatic substitution from an electron-donating group (Table 2, entries 17 and 18) to an electron-withdrawing group (Table 2, entry 15) significantly influence yields to products as clearly showed in Table 2, because the rate of nucleophilic addition of the carboxylate ion to DCID decreases (Scheme 2). This fact also applies to benzyl alcohols and the yields decreases with the change of electron donating groups with electron withdrawing groups (Table 2, entries 2, 8 and 12). The reaction of aliphatic carboxylic acids with alcohols in this work show obvious differences and the corresponding esters were obtained in low yields (Table 2, entries 19 and 20) and the reason for that is the less activity of carbonyl aliphatic group in acyl nucleophilic substitution reaction. The solvent-free reaction was also performed. As example, the reaction between benzoic acid and benzyl alcohol under optimized conditions for 24 h could provide only an ester yield of 25%. So, present of a solvent seem to have an effective role in completion of the reaction.
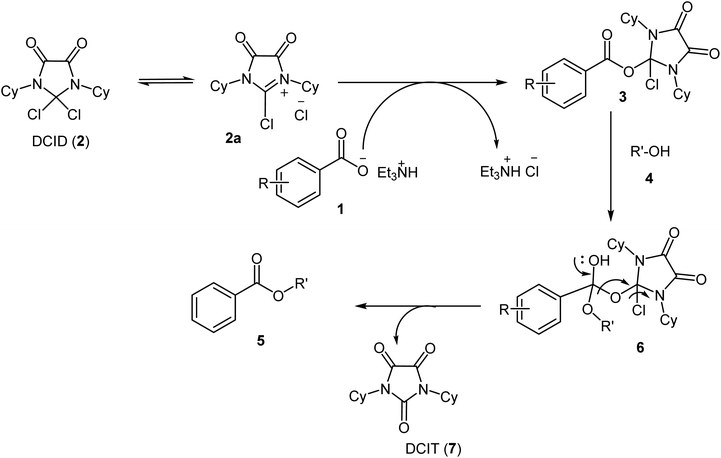
Based on experimental observations and isolation and characterization of intermediate 3 a plausible mechanism for a DCID-mediated esterification of carboxylic acids is proposed in Scheme 2. At the first, the heterolytic cleavage of the C–Cl bond in DCID, given imidazolinium chloride 2a. Subsequently, the nucleophilic addition of benzoate anion on C2 of compound 2 led to the intermediate 3. Nucleophilic acyl substitution of intermediate 3 with benzyl alcohols generate a tetrahedral intermediate 6. Finally, removing of 1,3-dicyclohexylimidazolidine-2,4,5-trione (DCIT) 7 provides the esterification products 5. Compound 7 was isolated and characterized by 13C-NMR and mass spectra (ESI, Fig. S16 and S17†).
Conclusion
In summary, we have developed a one-pot and catalyst-free procedure for the synthesis of a range of esters using DCID as mild and readily available material with good to excellent yields. In comparison with the previous methods for the esterification, this system offered several advantages such as mild reaction conditions, no formation of side products, simple experimental operation, high yields of product and etc. Also, a wide range of benzoic acids and benzyl alcohols gave the corresponding esterification products in good yields. A proposed mechanism for this procedure is also provided. Further study on the DICD-promoted organic transformation is ongoing in our laboratory and would be presented in the future.Conflicts of interest
There are no conflicts to declare.References
- (a) M. A. Ogliaruso and J. F. Wolfe, The Chemistry of Functional Groups, ed. S. Patai, Wiley, Chichester, 1985, Suppl. B. Part, vol. 1, p. 411 Search PubMed; (b) H. Pielatzik, B. Irmisch-Pielatzik and T. Eicher, Methods of Organic Chemistry, Houben Weyl, 2004, vol. 5, p. 659 Search PubMed; (c) R. Caputo, E. Corrado, C. Ferreri and G. Palumbo, Synth. Commun., 1986, 16, 1081 CrossRef CAS.
- J. Mulzer, Comprehensive Organic Synthesis, ed. B. M. Trost and L. Fleming, Pergamon Press, Oxford, 1991, vol. 6, p. 323 Search PubMed.
- D. L. Nelson and M. M. Cox, Lehninger Principles of Biochemistry, Worth Publishing, New York, 3rd edn, 2000 Search PubMed.
- V. Fernández, P. Guzman-Delgado, J. Graca, S. Santos and L. Gil, Front. Plant Sci., 2016, 7, 427 Search PubMed.
- https://www.plasticscolor.com/en-US/Media/News/article/, from-resin-to-product: -plastics-production, -uses-and-properties, (accessed February 11, 2019).
- M. Espino-Díaz, D. R. Sepúlveda, G. González-Aguilar and G. I. Olivas, Food Technol. Biotechnol., 2016, 54, 375 Search PubMed.
- http://www.newworldencyclopedia.org/entry/Ester, (accessed February 11, 2019).
- https://www.prweb.com/releases/2014/02/prweb11619424.htm, (accessed February 11, 2019).
- M. Krieger, M. P. Scott, P. T. Matsudaira, H. F. Lodish, J. E. Darnell, Z. Lawrence, C. Kaiser and A. Berk, Section 4.1: Structure of Nucleic Acids, Molecular Cell Biology, W. H. Freeman and CO, New York, 2004 Search PubMed.
- J. Otera and J. Nishikido, Esterification: Methods, Reactions, and Applications, Wiley-VCH, Weinheim, Germany, 2nd edn, 2009 Search PubMed.
- M. Jia, L. Jiang, F. Niu, Y. Zhang and X. Sun, R. Soc. Open Sci., 2018, 5, 171988 CrossRef PubMed.
- P. A. Turhanen, J. Leppänen and J. J. Vepsäläinen, ACS Omega, 2019, 4, 8974 CrossRef CAS PubMed.
- S. Cebrián-García, A. M. Balu and R. Luque, Front. Chem., 2018, 6, 197 CrossRef PubMed.
- (a) F. U. Nigiz and N. D. Hilmioglu, Chem. Eng. Technol., 2018, 41, 836 CrossRef CAS; (b) X.-Y. Zhou and X. Chen, Synlett, 2018, 29, 2321 CrossRef CAS; (c) M. Finnveden, S. Brännström, M. Johansson, E. Malmström and M. Martinelle, RSC Adv., 2018, 8, 24716 RSC.
- (a) K. Madankar, J. Mokhtari and Z. Mirjafary, Appl. Organomet. Chem., 2020, e5383 CrossRef CAS; (b) K. Madankar, J. Mokhtari and Z. Mirjafary, Synlett, 2020, 31, 1725 CrossRef CAS; (c) N. Hosseini, J. Mokhtari and I. Yavari, New J. Chem., 2022, 46, 5588 RSC; (d) N. Hosseini, J. Mokhtari and I. Yavari, ChemistrySelect, 2021, 6, 5198 CrossRef CAS; (e) F. Nasiri, J. Mokhtari, S. Taheri and Z. Mirjafary, Tetrahedron Lett., 2023, 118, 154392 CrossRef CAS; (f) J. P. Moerdyk and C. W. Bielawski, Chem.–Eur. J., 2014, 20, 13487 CrossRef CAS PubMed; (g) Y. Gao, J. Liu, Z. Li, T. Guo, S. Xu, H. Zhu, F. Wei, S. Chen, H. Gebru and K. Guo, J. Org. Chem., 2018, 83, 2040 CrossRef CAS PubMed; (h) Y. Gao, Z. Zhang, Z. Li, T. Guo, Y. Zhu, Z. Yao, B. Liu, Y. Li and K. Guo, J. Org. Chem., 2020, 85, 1087 CrossRef CAS PubMed; (i) J. A. Tiwari, Z. Azeem and P. K. Mandal, J. Org. Chem., 2022, 87, 3718 CrossRef PubMed.
- Y. Yasuhide, T. Yuho and S. Masami, Bull. Chem. Soc. Jpn., 1972, 45, 1198 CrossRef.
- S. A. Runikhina, D. L. Usanov, A. O. Chizhov and D. Chusov, Org. Lett., 2018, 20, 7856 CrossRef CAS PubMed.
- A. M. Harned, H. S. He, P. H. Toy, D. L. Flynn and P. R. Hanson, J. Am. Chem. Soc., 2005, 127, 52 CrossRef CAS PubMed.
- R. A. Green, D. Pletcher, S. G. Leach and R. C. D. Brown, Org. Lett., 2015, 17, 3290 CrossRef CAS PubMed.
- B. A. Fiekers and E. M. Di Geronimo, J. Am. Chem. Soc., 1948, 70, 1654 CrossRef CAS.
- J. H. Bowie and B. Nussey, Org. Mass Spectrom., 1974, 9, 310 CrossRef CAS.
- H. Rouhi-Saidabad and B. Akhlaghinia, J. Braz. Chem. Soc., 2014, 25, 253 Search PubMed.
- M. A. Pasha and B. Ravindranath, Indian J. Chem., Sect. B: Org. Med. Chem., 1985, 24, 1068 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra04048h |
| This journal is © The Royal Society of Chemistry 2023 |