 Open Access Article
Open Access ArticleCombating drug-resistant bacteria with sulfonium cationic poly(methionine)†
Lizhong Zhu‡
,
Jie Li‡ and
Weiwei Huan
and
Weiwei Huan *
*
Zhejiang Provincial Key Laboratory of Chemical Utilization of Forestry Biomass, College of Chemistry and Materials Engineering, Zhejiang A&F University, Hangzhou, Zhejiang 311300, China
First published on 15th September 2023
Abstract
Abstract antibiotic resistance and drug-resistant bacterial infections pose significant threats to public health. Antimicrobial peptides (AMPs) are a promising candidate for related-infection therapy, but their clinical application is limited by their high synthesis cost and susceptibility to protease degradation. To address these issues, cationic poly(α-amino acid)s based on lysine have been developed as synthetic mimics of AMPs. In this study, we introduce a new class of cationic AMP synthetic mimics based on functional poly(methionine)s. We synthesized a series of sulfonium cationic poly(D,L-methionine)s with varying chain lengths via a convenient polymerization on α-amino acid thiocarboxyanhydride (α-NTA) using tert-butyl-benzylamine as the initiator, followed by alkylation with iodomethane. Our optimal methionine polymer demonstrated potent and broad-spectrum antibacterial activity against antibiotic-resistant bacteria, as well as excellent biocompatibility with mammalian cells and rapid bactericidal performance. Our findings suggest that sulfonium poly(methionine)s have the potential to address the challenge of drug-resistant bacterial infections.
The emergence of antibiotic resistance and drug-resistant bacteria-related infections is one of the most significant threats to public health.1 It is estimated that drug-resistant microorganisms cause 700
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths annually, and this figure is projected to increase to 10 million by 2050 if there are no effective measures and strategies.2 Furthermore, the challenges posed by antibiotic resistance have been exacerbated by the extensive use of antibiotics and prolonged hospitalization of patients during the COVID-19 pandemic outbreak.3 Therefore, there is an urgent need to develop effective antibacterial agents against drug-resistant bacteria.4–6
000 deaths annually, and this figure is projected to increase to 10 million by 2050 if there are no effective measures and strategies.2 Furthermore, the challenges posed by antibiotic resistance have been exacerbated by the extensive use of antibiotics and prolonged hospitalization of patients during the COVID-19 pandemic outbreak.3 Therefore, there is an urgent need to develop effective antibacterial agents against drug-resistant bacteria.4–6
Antimicrobial peptides (AMPs) are natural peptides that play a crucial role in the immune system and have been identified as promising therapeutic candidates against various drug-resistant bacteria.7–9 Typically consisting of 10–50 amino acids, AMPs are characterized by highly positive charges.10 However, the clinical application of AMPs is greatly limited by their low stability upon proteolysis, difficulty in large-scale synthesis and high cost.11 To overcome these limitations, synthetic mimics of AMPs have received increasing attention due to their high antibacterial activity against drug-resistant bacteria and strong proteolytic stability.12,13 Among them, cationic amino acid polymers based on natural skeletons have been extensively developed as synthetic mimics of AMPs.14 Additionally, various synthetic mimics of AMPs based on unnatural skeletons have been widely studied in recent years.15–20
However, the structure and function of antibacterial amino acid polymers were limited by the class of available amino acids with positive charge, such as lysine or arginine.21–24 Herein, we designed the sulfonium cationic poly(methionine)s as a class of synthetic AMP synthetic mimics because of many advantages of poly(methionine)s, such as excellent biocompatibility, low-cost and nontoxicity (Fig. 1). A series of sulfonium cationic poly(D,L-methionine)s with different chain length were synthesized via facile polymerization on D,L-methionine N-thiocarboxyanhydride (D,L-met NTA) and subsequently alkylation. The optimal methionine polymer demonstrated potent and broad-spectrum antibacterial activity against drug-resistant bacteria and negligible cytotoxicity against mammalian cell, and the rapid bactericidal performance.
The synthesis of sulfonium cationic poly(D,L-methionine)s was illustrated in Scheme 1. Firstly, the monomer of D,L-met NTA was prepared using D,L-methionine as the starting material according to the literature reported by Zhang et al.25 Briefly, the nucleophilic addition reaction of D,L-methionine and ethylxanthogenacetic acid produced the intermediate of N-alkoxythiocarbonyl methionine, subsequently used for cyclization to afford the D,L-met NTA in the presence of PBr3 (Fig. S1–S3 in ESI†). Secondly, a ring-opening polymerization (ROP) of D,L-met NTA was performed in the open vessel using tert-butyl-benzylamine (tBuBnNH2) as the initiator to synthesize poly(D,L-methionine)s with different chain lengths by following the reported methods by Ling et al.26 which were characterized by gel permeation chromatography (GPC) (Fig. S4 and Table S1 in ESI†). This was followed by the modification with iodomethane in order to yield the sulfonium cationic poly(D,L-methionine)s, which were characterized by 1H NMR spectrum (Fig. 1). The chemical structures and functionalization degrees of the samples were confirmed by 1H NMR spectroscopy, as shown in Fig. 2a and b. It was mentioned that such a synthetic route selection reduced the cost of polypeptides synthesis and simplified the operation. The sulfonium polymers were directly used for further biological activity study.
 | ||
| Fig. 2 (a) The chemical structures and (b) 1H NMR characterization of sulfonium poly(D,L-methionine)s. | ||
The antibacterial activities against drug-resistant bacteria of these sulfonium pendant poly(D,L-methionine)s were evaluated through the determination of minimum inhibitory concentration (MIC) values. The representative methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus epidermidis (S. e) ATCC12228 and methicillin-resistant Gram-negative bacteria of Escherichia coli (E. coli) ATCC25922 and Pseudomonas aeruginosa O1 (PA O1) were employed for antibacterial activities testing, respectively. It was found that all those sulfonium cationic poly(D,L-methionine)s displayed the potent and broad-spectrum activities against drug-resistant Gram-positive bacteria and Gram-negative bacteria with the MIC values at a concentration of 7.8–62.5 μg mL−1, whereas, methicillin as a commonly used antibiotic in clinic showed very low activity against those bacterial with the MIC values at 125 to 250 μg mL−1 (Table 1). We also found that the MIC values against drug-resistant bacteria decreased gradually as the polymer chain length increased from 5 mer to 40 mer, which indicated the characteristic of polymer length dependent antibacterial activity. It may be caused by the stronger electrostatic interaction between the long-chain cationic poly(D,L-methionine)s and the anionic plasma membrane of bacteria, resulting in higher antimicrobial activities.
| Bacterial strains | MICs (μg mL−1) | |||||
|---|---|---|---|---|---|---|
| P5 | P10 | P20 | P40 | Met | PLL20 | |
| a Met means methicillin. MRSA means methicillin-resistant S. aureus. | ||||||
| MRSA | 31.3 | 31.3 | 7.8 | 7.8 | 250 | 15.6 |
| S. epidermidis ATCC12228 | 31.3 | 15.6 | 7.8 | 15.6 | 250 | 7.8 |
| E. coli ATCC25922 | 62.5 | 15.6 | 15.6 | 15.6 | 125 | 15.6 |
| P. aeruginosa PA O1 | 62.5 | 31.2 | 15.6 | 15.6 | 250 | 15.6 |
In addition, we also synthesized poly(L-lysine) with 20 mer chain length via tBuBnNH2-initiated L-lysine NCA polymerization as a comparison (Fig. S5 and S6 in ESI†). It was found that for Gram negative bacteria, sulfonium poly(D,L-met)20 exhibited the identical activity to poly(L-lysine)20 with MIC values at 15.6 μg mL−1. For Gram positive bacteria of MRSA, sulfonium poly(D,L-met)20 exhibited the superior activity with MIC values at 7.8 μg mL−1. Moreover, these results indicate that both poly(L-lysine)20 and poly(D,L-met)20 were more susceptible Gram positive bacteria in comparison to Gram-negative. It may be due to the presence of an outer membrane composed of LPS on the membrane of negative bacteria compared to positive bacteria.27
We also determined the minimum bactericidal concentration (MBC) values against drug-resistant Gram-positive and Gram-negative bacteria that was defined as the lowest polymer concentration to kill over 99.9% of bacteria. It was found that sulfonium poly(D,L-methionine)s showed the strong bactericidal activity and the MBCs of those poly(D,L-methionine)s was ranged from 7.8 to 125 μg mL−1, whereas, methicillin as the comparison showed no bactericidal activity with the MBC values at ≥250 μg mL−1 (Table 2). It is noteworthy that the MBC values of sulfonium pendant poly(D,L-methionine)s was comparable to the MIC values and sulfonium poly(D,L-met)20 exhibited comparable or superior bactericidal activity compared to poly(L-lysine)20, which showing that sulfonium poly(D,L-methionine)s can act as the promising an ultra-high effect and wide-spectrum bactericide.
| Bacterial strains | MBCs (μg mL−1) | |||||
|---|---|---|---|---|---|---|
| P5 | P10 | P20 | P40 | Met | PLL20 | |
| a Met means methicillin. MRSA means methicillin-resistant S. aureus. | ||||||
| MRSA | 62.5 | 31.3 | 7.8 | 15.6 | >250 | 15.6 |
| S. epidermidis ATCC12228 | 62.5 | 313 | 15.6 | 15.6 | >250 | 15.6 |
| E. coli ATCC25922 | 125 | 31.3 | 15.6 | 15.6 | 250 | 31.2 |
| P. aeruginosa PA O1 | 125 | 31.3 | 15.6 | 15.6 | 250 | 15.6 |
Biocompatibility testing of polymers and related biomaterials is the most fundamental evaluation to describe appropriate biological requirements used in medical field. Herein, the hemolytic toxicity and cell cytotoxicity of all those polymers were evaluated by using human red blood cells (hRBCs) and NIH/3T3 fibroblast cells, respectively. For hemolytic toxicity, all polymers exerted the low hemolysis against hRBCs with the HC10 values (the minimum polymer concentration to cause 10% hemolysis) of ≥400 μg mL−1 (Fig. 3a). For cytotoxicity against mammalian, the polymers with DP = 5, 10 and 20 showed very low cytotoxicity against NIH/3T3 fibroblast cells with the IC50 values (the minimum polymer concentration to cause 50% reduction in cell viability) upon 200 μg mL−1. IC50 values tends to decrease as the chain length of polymers gradually increase, when the average chain length is 40, polymer have significantly high cytotoxicity with the IC50 values of 50 μg mL−1 (Fig. 3b). HC10 and IC50 values altogether demonstrated that sulfonium pendant poly(D,L-methionine)s presented very low hemolysis and toxicity, it may be due to the fact that the surface of mammalian cell membranes is mainly composed of neutral charged phospholipids, resulting in weaker interactions with positively charged polymers than bacteria membranes.28 Moreover, the cell cytotoxicity depended on the polymer chain length compared to hemolytic toxicity. P20 and P40 exhibited the obvious toxicity on 3T3 fibroblast cell, these results may be attributed to the fact that large amounts of positive charge enhance the interaction between polymers and mammalian membranes, potentially leading to the penetration of mammalian cells and interaction with intracellular substances.29,30
The higher selectivity index (SI) values imply the more effectivity and safety of antibacterial agent. We calculated the SI of antibacterial poly(D,L-methionine)s from the ratio of HC10/MIC and IC50/MIC, respectively. Based on the SI calculated from HC10/MIC, the sulfonium poly(D,L-methionine)s of P10 and P20 exhibited very higher selectivity for both drug-resistant Gram-positive and Gram-negative bacteria with HC10/MIC at 13–51 (Fig. 4a–d). For Gram-positive bacteria, P20 showed better selectivity than other polymers calculated form HC10/MIC or HC50/MIC (Fig. 4a and c). Considering better antibacterial activity with MIC values at 7.8–15.6 μg mL−1 and higher selectivity index against all tested bacterial strains at 26–51 of P20 (Fig. 4a–d), we choose P20 as the optimal candidate of those antibacterial agent because of the potent, broad-spectrum activities and high selectivity.
 | ||
| Fig. 4 SI values of polymers on drug-resistant Gram-positive bacteria and Gram-negative bacteria calculated from (a and b) HC10/MIC and (c and d) IC50/MIC, respectively. | ||
The bactericidal kinetics of the optimal poly(D,L-methionine) P20 candidate polymers was further investigated by using the representative strains of MRSA and E. coli, in comparison with antibiotics vancomycin and imipenem as the control for the Gram-positive bacteria and Gram-negative bacteria, respectively. For MRSA, P20 achieved more than 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction for 0.5 h at 2 × MBC concentration, whereas, vancomycin achieved about 2
log reduction for 0.5 h at 2 × MBC concentration, whereas, vancomycin achieved about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction for 6 h at 2 × MBC (Fig. 5a). For E. coli, P20 achieved more than 3
log reduction for 6 h at 2 × MBC (Fig. 5a). For E. coli, P20 achieved more than 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction (99.9% sterilization efficiency) for 2 h at 2 × MBC concentration, whereas, imipenem achieved about 1.2
log reduction (99.9% sterilization efficiency) for 2 h at 2 × MBC concentration, whereas, imipenem achieved about 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction until 6 hours at 2 × MBC (Fig. 5b). These results clearly reflected a rapid killing efficiency of more than 99.9% within 2 h against both Gram-positive and Gram-negative MDR bacteria.
log reduction until 6 hours at 2 × MBC (Fig. 5b). These results clearly reflected a rapid killing efficiency of more than 99.9% within 2 h against both Gram-positive and Gram-negative MDR bacteria.
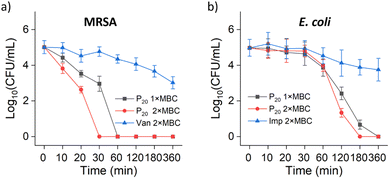 | ||
| Fig. 5 Time-kill kinetics assay of P20 against MRSA (a) and E. coli (b) at 1 × MBC and 2 × MBC concentration, respectively. | ||
Conclusions
As an important component of AMP and synthetic mimics, lysine amino acid maintain the positive charge and contribute to antibacterial function of cationic poly(amino acid)s. Here, a general strategy was reported to design AMP synthetic mimics by using a type of sulfonium methionine as positive charge. We design and synthesize a series of sulfonium ion-based poly(D,L-methionine)s with different chain length via one-pot and open-vessel α-NTA polymerization. Those sulfonium poly(D,L-methionine)s exert potent and broad-spectrum antibacterial activity against drug resistant Gram-positive and Gram-negative bacteria, as well as the low hemolytic toxicity and cell cytotoxicity. The preferred poly(D,L-methionine) P20 present the high antibacterial selectivity and rapid sterilization performance within 1–2 hours. This study shows that sulfonium methionine can act as effectively positive charge of poly(peptide)s. Sulfonium poly(D,L-methionine)s can serve as the promising antibacterial agent against drug-resistant bacteria and have the potential application of addressing the challenge of drug-resistant bacteria.Methionine-S-methyl sulfonium chloride, when used as a dietary supplement, has been found to inhibit prostaglandin synthesis, demonstrating the medicinal potential and excellent biocompatibility of sulfonium poly(methionine)s. It is important to note that the catabolic pathway involved in converting it into S-adenosylmethionine through methionine adenosyl-S-transferase in animals may impact the antibacterial function of polymers in vivo due to the shared sidechain structure between methionine-S-methyl sulfonium and sulfonium poly(D,L-methionine)s. Furthermore, to further explore the potential of sulfonium poly(D,L-methionine)s, it is crucial to investigate their stability in various biological media, considering the differences in components such as pH, inorganic salts, and enzymes present in different buffer solutions.
Methionine-S-methyl sulfonium chloride has been used as a dietary supplement and found to inhibit the synthesis of prostaglandins, demonstrating the medicinal potential and excellent biocompatibility of sulfonium poly(methionine)s.31 It is noteworthy that the catabolism path involved in converting into S-adenosylmethionine via methionine adenosyl-S-transferase in animal may impact the antibacterial function of polymers in vivo due to the shared sidechain structure of methionine-S-methyl sulfonium and sulfonium poly(D,L-methionine)s.32 In addition, in order to further explore the potential of sulfonium poly(D,L-methionine)s, it is crucial to investigate their stability in various biological media, considering the differences such as pH, inorganic salts and enzymes in different buffer solutions.
Author contributions
W. H. directed the whole project. W. H. and J. L. conceived the idea, designed the experiments and wrote the manuscript together. L. Z. performed a majority of the experiments. All authors proofread the manuscript.Conflicts of interest
L. Z. have a pending patent application that covering the synthesis and application of sulfonium poly(methionine).Notes and references
- M. C. Fisher, N. J. Hawkins, D. Sanglard and S. J. Gurr, Science, 2018, 360, 739–742 CrossRef CAS PubMed.
- M. A. Farha and E. D. Brown, Nat. Microbiol., 2019, 4, 565–577 CrossRef CAS PubMed.
- A. R. Collaborators, Lancet, 2022, 399, 629–655 CrossRef PubMed.
- C. Zhou, Y.-H. Li, Z.-H. Jiang, K.-D. Ahn, T.-J. Hu, Q.-H. Wang and C.-H. Wang, Chin. Chem. Lett., 2016, 27, 681–684 CrossRef CAS.
- J. Shi, N. Luo, M. Ding and X. Bao, Chin. Chem. Lett., 2020, 31, 434–438 CrossRef CAS.
- W. Xu, Z. Ma, G. Dhanda, J. Haldar and H. Xie, Chin. Chem. Lett., 2023, 34 CAS.
- R. E. Hancock and H. G. Sahl, Nat. Biotechnol., 2006, 24, 1551–1557 CrossRef CAS PubMed.
- Z. Z. Wang, X. Q. Ye, M. Shi, F. Li, Z. H. Wang, Y. N. Zhou, Q. J. Gu, X. T. Wu, C. L. Yin, D. H. Guo, R. M. Hu, N. N. Hu, T. Chen, B. Y. Zheng, J. N. Zou, L. Q. Zhan, S. J. Wei, Y. P. Wang, J. H. Huang, X. D. Fang, M. R. Strand and X. X. Chen, Nat. Commun., 2018, 9, 2205 CrossRef PubMed.
- Z. Z. Wang, X. L. Bing, S. S. Liu and X. X. Chen, Pest Manage. Sci., 2017, 73, 1421–1427 CrossRef CAS PubMed.
- N. Mookherjee, M. A. Anderson, H. P. Haagsman and D. J. Davidson, Nat. Rev. Drug Discov., 2020, 19, 311–332 CrossRef CAS PubMed.
- S. Mukhopadhyay, A. S. Bharath Prasad and C. H. Mehta, World J. Microbiol. Biotechnol., 2020, 36, 131 CrossRef CAS PubMed.
- K. Kuroda and G. A. Caputo, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2013, 5, 49–66 CAS.
- B. P. Mowery, S. E. Lee, D. A. Kissounko, R. F. Epand, R. M. Epand, B. Weisblum, S. S. Stahl and S. H. Gellman, J. Am. Chem. Soc., 2007, 129, 15474–15476 CrossRef CAS PubMed.
- W. Shen, P. He, C. Xiao and X. Chen, Adv. Healthcare Mater., 2018, 7, e1800354 CrossRef PubMed.
- J. Sun, M. Li, M. Lin, B. Zhang and X. Chen, Adv. Mater., 2021, 33, e2104402 CrossRef PubMed.
- M. Zhou, Y. Qian, J. Xie, W. Zhang, W. Jiang, X. Xiao, S. Chen, C. Dai, Z. Cong, Z. Ji, N. Shao, L. Liu, Y. Wu and R. Liu, Angew. Chem., Int. Ed., 2020, 59, 6412–6419 CrossRef CAS PubMed.
- M. Zhou, X. Xiao, Z. Cong, Y. Wu, W. Zhang, P. Ma, S. Chen, H. Zhang, D. Zhang, D. Zhang, X. Luan, Y. Mai and R. Liu, Angew. Chem., Int. Ed., 2020, 59, 7240–7244 CrossRef CAS PubMed.
- M. Zhou, J. Zou, L. Liu, X. Xiao, S. Deng, Y. Wu, J. Xie, Z. Cong, Z. Ji and R. Liu, iScience, 2021, 24, 103124 CrossRef CAS PubMed.
- X. Li, H. Bai, Y. Yang, J. Yoon, S. Wang and X. Zhang, Adv. Mater., 2019, 31, e1805092 CrossRef PubMed.
- X. D. Zhao, D. N. Pei, Y. X. Yang, K. Xu, J. Yu, Y. C. Zhang, Q. Zhang, G. He, Y. F. Zhang, A. Li, Y. L. Cheng and X. S. Chen, Adv. Funct. Mater., 2021, 31 Search PubMed.
- R. Durrani, Y. Meiyun, B. Yang, E. Durand, A. Delavault, H. Bowen, H. Weiwei, L. Yiyang, S. Lili and G. Fei, Food Chem., 2023, 405, 134843 CrossRef CAS PubMed.
- J. Chen, H. Fang, Y. Hu, J. Wu, S. Zhang, Y. Feng, L. Lin, H. Tian and X. Chen, Bioact. Mater., 2022, 7, 167–180 CrossRef CAS PubMed.
- V. Gribova, L. Petit, L. Kocgozlu, C. Seguin, S. Fournel, A. Kichler, N. E. Vrana and P. Lavalle, Macromol. Biosci., 2022, 22, e2200043 CrossRef PubMed.
- H. D. Lu, C. X. Tu, T. Zhou, W. Y. Zhang, Y. B. Zhan, J. Ding, X. Y. Wu, Z. J. Yang, W. B. Cao, L. W. Deng, C. Y. Gao and F. Xu, Chem. Eng. J., 2022, 436, 135130 CrossRef CAS.
- J. B. Cao, D. Siefker, B. A. Chan, T. Y. Yu, L. Lu, M. A. Saputra, F. R. Fronczek, W. W. Xie and D. H. Zhang, ACS Macro Lett., 2017, 6, 836–840 CrossRef CAS.
- X. Tao, J. Du, Y. Wang and J. Ling, Polym. Chem., 2015, 6, 3164–3174 RSC.
- J. Sun, S. T. Rutherford, T. J. Silhavy and K. C. Huang, Nat. Rev. Microbiol., 2022, 20, 236–248 CrossRef CAS PubMed.
- P. Kumar, J. Kizhakkedathu and S. Straus, Biomolecules, 2018, 8, 4 CrossRef PubMed.
- J. R. Kramer, N. W. Schmidt, K. M. Mayle, D. T. Kamei, G. C. Wong and T. J. Deming, ACS Cent. Sci., 2015, 1, 83–88 CrossRef CAS PubMed.
- S. T. Hemp, M. H. Allen Jr, A. E. Smith and T. E. Long, ACS Macro Lett., 2013, 2, 731–735 CrossRef CAS PubMed.
- Y.-H. Lee, D. Ren, B. Jeon and H.-w. Liu, Nat. Prod. Rep., 2023 10.1039/D2NP00086E.
- Y. Ouyang, Q. Wu, J. Li, S. Sun and S. Sun, Cell Proliferation, 2020, 53(11), e12891 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03925k |
| ‡ These authors contributed equally to this manuscript. |
| This journal is © The Royal Society of Chemistry 2023 |



