Open Access Article
Open Access ArticleNew supramolecules of bis(acylhydrazones)-linked bisphenol sulfide for Alzheimer's: targeting cholinesterases by in vitro and in silico approaches†
Muhammad Ibrahima,
Mumtaz Ali *a,
Sobia Ahsan Halimb,
Abdul Latif
*a,
Sobia Ahsan Halimb,
Abdul Latif *a,
Manzoor Ahmada,
Sajid Alia,
SameeUllah
*a,
Manzoor Ahmada,
Sajid Alia,
SameeUllah a,
Ajmal Khanb,
Alany Ingrido Rebierioc,
Jalal Uddind and
Ahmed Al-Harrasi*b
a,
Ajmal Khanb,
Alany Ingrido Rebierioc,
Jalal Uddind and
Ahmed Al-Harrasi*b
aDepartment of Chemistry, University of Malakand, Dir Lower, Chakdara 18800, Khyber Pakhtunkhwa, Pakistan. E-mail: mumtazphd@gmail.com
bNatural and Medical Sciences Research Centre, University of Nizwa, PO Box 33, 616 Birkat Al Mauz, Nizwa, Oman. E-mail: ajmalkhan@unizwa.edu.com; aharrasi@unizwa.edu.com
cDepartment of Chemistry, Federal University of São Carlos, Rod. Washington Luís, Km 265, São Carlos 13565-905, Brazil
dDepartment of Pharmaceutical Chemistry, College of Pharmacy, King Khalid University, Abha, 62529, Kingdom of Saudi Arabia
First published on 24th August 2023
Abstract
In current research, two functional components, i.e., hydrazone and bisphenol sulfide were combined to get useful supramolecules in medicinal chemistry. Herein 25 new 4,4′-thiodiphenol bis-acylhydrazones were synthesized in good to excellent yields. Initially ethyl-2-chloroacetate was reacted with 4,4′-thiodiphenol, which was further reacted with excess hydrazine hydrate to produce 2,2′-((thiobis(4,1-phenylene))bis(oxy))di(acetohydrazide), which was then combined with various aromatic and aliphatic aldehydes to get the desired products (hydrazones, 4a–4y). The synthesized supramolecules were characterized by contemporary spectroscopic techniques such as 1H NMR, 13C NMR, and mass spectroscopy. The synthetic compound's cholinesterase blocking activity was tested against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes where compounds 4n, and 4h showed excellent inhibitory potential for AChE, while 4b, and 4h, demonstrated most potent inhibition of BChE. The starting compound (SM3) and compounds 4h and SM3 depicted excellent dual inhibitory capabilities for both enzymes. The chemical basis of anticholinesterase activity was investigated using a structure-based molecular docking approach. The biological significance and the ease of synthesis of this class of compounds should be considered in therapeutic development for Alzheimer's disease treatments.
1 Introduction
Alzheimer's disease is a degenerative neurological condition that affects cognition and behavior and makes everyday tasks difficult. The loss of cholinergic neurons is the primary reason for these alterations. In the end, it causes intellectual changes, memory loss, and social impairment.1 The two main enzymes involved in the beginning of Alzheimer's disease are acetyl (AChE) and butyrylcholinesterase (BChE). Acetylcholinesterase (AChE) hydrolyzes acetylcholine, a neurotransmitter present in cholinergic synapses, while BChE co-regulates the activity of AChE. Cholinesterase inhibitors work against the actions of these enzyme to increase the amount of acetylcholine required for the neurotransmission process.2 Cholinesterase inhibitors are among the most effective Alzheimer's disease treatments available right now. By suppressing AChE, certain medications treat Alzheimer's effectively. The current medications only produce symptomatic effects, therefore finding new cholinesterase inhibitors with more potency and less side effects is urgently needed to combat the effects of Alzheimer's.The sulfide group and the Schiff's bases (hydrazide-hydrazones) are two important pharmacophores that have drawn medicinal chemists to create novel medications.3 The hydrazones of aromatic carbonyl (aldehydes and ketones) compounds, have a strong conjugated system, excellent stability,4 and are significant intermediates in the field of organic chemistry.5 The unusual biological potential of Schiff's bases, including antibacterial7 anti-inflammatory,10 anti-tumor,9 anticancer,6 analgesic11 and antifungal8 properties, has received a lot of attention recently. Researchers are attracted to the synthesis of hydrazide-hydrazone derivatives, because of intriguing biological activities of this pharmacophore like anti-proliferative,12 potential inhibitor to different enzymes,13 anti-convulsant,14 anti-microbial,18 anti-malarial,16 anti-leishmanial,15 anti-mycobacterial,17 anti-viral,21 anti-HIV,20 anti-pyretic23 and anti-protozoal,24 anti-trypanosomal,22 anti-HIV,25 anti-depressant,26 anti-tubercular,19 and anti-oxidant27 activities which are crucial to organic and medicinal chemistry. Several commercially marketed hydrazide-hydrazone derivatives are available, for example nifurtimox (treats Chagas disease),28 nifuroxazide (antibiotic to treat colitis and diarrhea), and nitrofurazone (antimicrobial agent), furazolidone (antibacterial agent and monoamine oxidase inhibitor), and nitrofurantoin (antibacterial medicine to treat urinary tract infections)1 which shows the importance of hydrazide-hydrazone scaffold.29
In an effort to enhance the bio-chemical properties of compounds, the sulfide group and hydrazide-hydrazones were incorporated into a single molecule. This study sought to find new compounds with improved anticholinesterase activities30–32 by synthesizing a series of bis(acylhydrazones) from bisphenol sulfide. Additionally, a well-known structure-based drug design approach i.e., molecular docking was used to investigate the binding behavior of the highest, moderate, and least active molecules against both studied enzymes.
2 Results and discussion
2.1. Chemistry of compounds
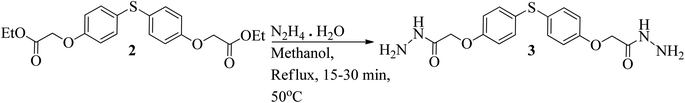
The esterification process was followed by hydrazination reaction to synthesize the aforementioned compounds (Schemes 1–3).33 Diethyl 2,2′-((thiobis(4,1-phenylene))bis(oxy)) diacetate (2) was obtained by subjecting 4,4′-thiodiphenol (1) to reflux in dimethyl formamide (DMF) and reacting it with ethyl-2-chloroacetate (Scheme 1).34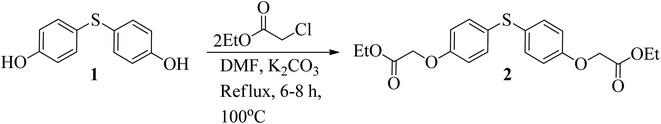 | ||
| Scheme 1 Thiodiphenol (1) is esterified to produce diethyl 2,2′-((thiobis(4,1-phenylene))bis(oxy))diacetate (2). | ||
In the process of being synthesized, 2,2′-((thiobis(4,1-phenylene))bis(oxy))di (acetohydrazide) (3) involved the reaction of 2,2′-((thiobis(4,2-phenylene))bis(oxy))diacetate (2) with an excess of hydrazine hydrate. This reaction was carried out in anhydrous methanol as the solvent (Scheme 2).35
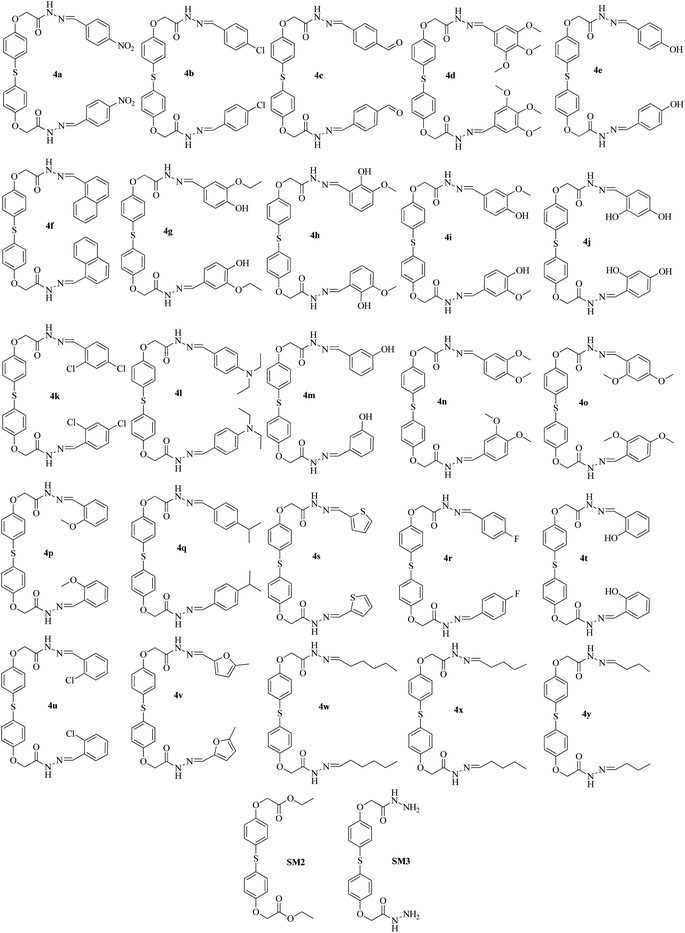
The hydrazide (3) was utilized in reactions with several aldehydes, both aromatic and aliphatic, in either DMF or ethyl alcohol which led to the synthesis of the matching bis(acylhydrazones) (4a–4y) with high yields ranging from 80% to 90% (Fig. 1). While considering the overall yields of the products obtained using different solvents, it can be inferred that DMF surpasses ethanol as a solvent for this reaction. This is likely due to the complete solubility of the hydrazide (3) in DMF. The enhanced solubility of the hydrazides in DMF makes it straightforward to separate the hydrazones by dissolving the reaction mixture in ice-cold water (Scheme 3). A collection of twenty-five (25) different bis(acylhydrazones) were prepared (Fig. 1) and their medicinal properties were evaluated in vitro by targeting acetyl and butyrylcholinesterase enzymes (Table 1).
| Compounds | AChE | BChE |
|---|---|---|
| IC50 (μM) ± SD | IC50 (μM) ± SD | |
| a NA = not active, SD = standard deviation. | ||
| 4a | NA | NA |
| 4b | NA | 14.7 ± 0.8297 |
| 4c | 148.1 ± 0.7700 | 93.3 ± 0.8208 |
| 4d | 67.0 ± 0.8623 | 44.7 ± 0.8461 |
| 4e | 74.2 ± 0.7970 | 142.5 ± 0.7698 |
| 4f | 131.4 ± 0.8634 | 117.1 ± 0.8046 |
| 4g | 36.3 ± 0.7874 | 239.2 ± 0.8209 |
| 4h | 27.8 ± 0.7232 | 19.0 ± 0.8625 |
| 4i | 45.4 ± 0.6736 | 65.7 ± 0.7662 |
| 4j | 39.1 ± 0.7811 | 84.6 ± 0.7276 |
| 4k | 47.3 ± 0.7912 | 75.1 ± 0.8628 |
| 4l | 33.5 ± 0.9318 | 109.4 ± 0.8708 |
| 4m | 51.1 ± 0.7283 | 60.3 ± 0.6995 |
| 4n | 25.7 ± 0.8137 | 48.6 ± 0.6727 |
| 4o | 40.2 ± 0.8223 | 55.4 ± 0.7870 |
| 4p | 49.1 ± 0.6934 | 110.2 ± 0.8135 |
| 4q | 192.8 ± 0.6493 | 119.6 ± 0.6420 |
| 4r | NA | NA |
| 4s | 91.5 ± 0.6153 | 36.4 ± 0.7022 |
| 4t | 37.7 ± 0.6853 | 78.9 ± 0.6850 |
| 4u | 235.1 ± 0.7335 | 113.5 ± 0.6950 |
| 4v | NA | NA |
| 4w | NA | NA |
| 4x | 186.3 ± 0.8761 | 103.1 ± 0.8765 |
| 4y | 122.6 ± 0.9429 | 101.1 ± 0.9378 |
| SM2 | NA | NA |
| SM3 | 23.1 ± 0.6540 | 21.8 ± 0.8761 |
| Galantamine | 29.5 ± 0.9036 | 27.8 ± 0.8740 |
2.2. Anticholinesterase activity
The cooperative function of AChE and BChE enzymes in preserving cholinomimetic activity has been shown by their inhibition studies. The rise in BChE activity in response to decrease in AChE activity suggests a different mechanism for the cholinergic breakdown. Additionally, clinical trials have shown that dual cholinesterase inhibitors outperform selective AChE inhibitors in terms of effectiveness. The inhibitory capability of the 25 synthetic supramolecules (4a–4y) were evaluated against both AChE and BChE to search dual cholinesterase inhibitors that may be considered as Alzheimer's disease therapy.Among all the compounds, SM3, 4n, 4h, 4l, 4g, 4t, 4j and 4o showed good inhibitory activity for AChE with IC50 value in range of 23.1 to 40.2 μM, while 4b, 4h, SM3, 4s, 4d, and 4n effectively inhibited the activity of BChE with IC50 ranging from 14.7 to 48.6 μM. We observed that compound 4h and the starting molecule SM3 have dual inhibitory properties for both AChE and BChE (Table 1). A well-known cholinesterase inhibitor, galantamine was used as a positive control which bears IC50 values of 29.5 μM and 27.8 μM for AChE and BChE, respectively. These findings suggests that bis(acylhydrazones), particularly SM3 and 4h holds promise as alternative therapeutic agents for the treatment of Alzheimer's disease.
2.3. Molecular docking analysis
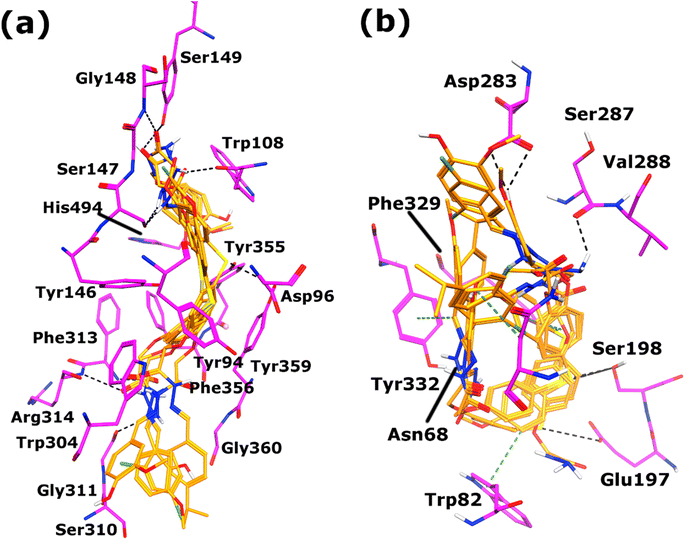
The binding modes of most active, moderate, and least active inhibitors of both AChE and BChE were deduced by in silico structure-based drug design approach, i.e., molecular docking. Compounds SM3 and 4h are among the most potent inhibitors of both the enzymes, similarly, 4i and 4k serves as moderate active inhibitors and 4q is among the least active inhibitors for both AChE and BChE enzymes, therefore, only these molecules were selected for docking studies.Before docking our synthesized compounds, the standard inhibitor, galantamine was manually docked into the active site of Electric eel AChE (https://alphafold.com/entry/O42275) and Equine BChE (https://alphafold.com/entry/P81908) to deduce its binding mode in these enzymes. The electric eel AChE and equine BChE structures was selected for docking studies because these sources were used in the in vitro experiments. Several residues including Asp96, Trp108, Gly142-Gly144, Tyr146, Ser147, Tyr155, Glu224, Ser225, Trp258, Phe313, Phe315, Tyr355, Phe356, Tyr359, Glu352, and His494, Gly495 constitutes the active site of electric eel AChE, where galantamine binds with the side chain of Glu224 through hydrogen bond, while Trp108 provides hydrophobic interactions to galantamine. The active site of equine BChE is constituted by Asp70, Ser79, Trp82, Asn83, Tyr114, Gly115-Gly117, Phe118, Gly121, Tyr128, Glu197, Ser198, Ala199, Trp231, Phe329, Tyr332, His438, Gly439, and Ile442. In the active site of equine BChE, Glu197, and Gly116 mediates hydrogen bonds with the –OH group of galantamine. When our selected inhibitors were docked into the active site of AChE, these molecules showed excellent binding interactions and the bisphenol sulfide moiety of these molecules resides in the middle of AChE active site, while one of the acylhydrazone moieties is located at the gorge of the active site, and the other acylhydrazone moiety is located near the entrance of AChE active site. The acylhydrazone moieties of most active inhibitor of AChE, SM3 mediates strong hydrogen bonds with multiple residues including Gly311, Trp108, and Arg314, these multiple hydrogen bonding within the active site is responsible for the higher AChE inhibitory activity of SM3. Similarly, one of the acyl hydrazone moiety of 4h and 4i formed H-bond with side chain of Ser147 and Tyr155, respectively, while their substituted methoxy-phenol ring forms hydrophobic interaction with the side chain of Trp108 in the active site of AChE. The less hydrogen bond interactions of 4h and 4i with the active site residues of AChE as compared to SM3 leads to the moderate activity of 4h and 4i. Likewise, the compound 4k mediates H-bond and hydrophobic interaction with Ser147 and Trp108 through its acylhydrazone and its linked dicholorphenyl ring, respectively. In addition, the other acylhydrazone also forms H-bond with backbone of Phe313 at the entrance of the active site. While the least active molecule, 4q mediates only bidentate interaction with Ser149 and Tyr155. The compounds possess docking scores in range of −7.73 to −3.04 kcal mol−1, where both highly active compounds, SM3 (−7.73kcal mol−1) and 4h (−7.18 kcal mol−1) exhibited higher docking score than galantamine (−5.69 kcal mol−1) which is in good agreement with the IC50 values of these compounds. The binding interactions of the selected compounds are shown in Fig. 2A.
The compound 4h shows excellent inhibitory potency for BChE, when docked into the active site of BChE, this compound reflects multiple interactions with Asp283, Tyr332, and Trp82. We observed that one of the substituted methoxyphenol group formed H-bond with the side chain of Asp283 instead of the acylhydrazone moiety of the compound. While the diphenylsulfane ring forms hydrophobic interaction (π–π) with Trp82, similarly the other substituted acylhydrazone moiety formed hydrophobic interaction (π–H) with Tyr332. In compound SM3, both the acetohydrazide groups formed strong H-bonds with the side chains of Ser287 and Gly115. Interestingly, the diphenylsulfane of compounds 4i, 4k and 4q accepts H-bonds with the side chain of Ser198, while the diphenylsulfane of 4i and 4k and the substituted cumene of 4q formed hydrophobic interactions with trp82, Phe329, and Asn68, respectively. It is clear that due to the higher number of binding interactions with the residues of active site, these molecules exhibits high inhibitory activity, while the activity of compounds are reduced when their binding interactions are reduced. The compounds' docking scores vary from −9.52 to −3.36 kcal mol−1 (Table 2). Again, we obtained higher docking scores of 4h (−9.52 kcal mol−1), SM3 (−7.15 kcal mol−1) and 4i (−6.98 kcal mol−1) as compared to galantamine (−6.66 kcal mol−1) while 4k and 4q bears lesser docking scores than the standard inhibitor. The molecular interactions of compounds 4h, SM3, 4i, 4k, and 4q are summarized in Table 2 and presented in Fig. 2B.
| Compounds | Docking score (kcal mol−1) | AChE-ligands interactions | |||
|---|---|---|---|---|---|
| LA | RA | Interaction | Distance (Å) | ||
| a LA = ligand atom, RA = receptor Atom, HBA = hydrogen bond acceptor, HBD = hydrogen bond donor. | |||||
| SM3 | −7.73 | N31 | O-GLY311 | HBD | 2.09 |
| N38 | O-TRP108 | HBD | 2.54 | ||
| O30 | N-ARG314 | HBA | 2.19 | ||
| 4h | −7.18 | N28 | OG-SER147 | HBD | 2.52 |
| 6-Ring | 6-Ring-TRP108 | π–π | 2.81 | ||
| Galantamine | −5.69 | O18 | OE2-GLU224 | HBD | 2.70 |
| 4k | −5.41 | N36 | OG-SER147 | HBD | 2.87 |
| O30 | N-PHE313 | HBA | 2.18 | ||
| 6-Ring | 6-Ring-TRP108 | π–π | 2.61 | ||
| 4i | −5.39 | O38 | OH-TYR155 | HBA | 2.44 |
| 6-Ring | 5-Ring-TRP108 | π–π | 2.90 | ||
| 6-Ring | 6-Ring-TRP108 | π–π | 2.65 | ||
| 4q | −3.04 | O38 | N-SER149 | HBA | 2.13 |
| O38 | OH-TYR155 | HBA | 2.10 | ||
| Compounds | BChE IC50 (μM) | BChE-ligands interactions | |||
|---|---|---|---|---|---|
| 4h | −9.52 | O72 | OD1-ASP283 | HBD | 2.38 |
| O72 | OD2-ASP283 | HBD | 2.30 | ||
| C42 | 6-Ring-TYR332 | H–π | 2.70 | ||
| 6-Ring | 5-Ring-TRP82 | π–π | 2.61 | ||
| SM3 | −7.15 | N41 | O-SER287 | HBD | 2.14 |
| O30 | N-GLY115 | HBA | 2.39 | ||
| 4i | −6.98 | S11 | OG-SER198 | HBA | 2.12 |
| C8 | 5-Ring-TRP82 | H–π | 2.81 | ||
| Galantamine | −6.66 | O18 | OE2-GLU197 | HBD | 2.76 |
| O18 | N-GLY116 | HBA | 2.04 | ||
| 4k | −4.12 | S11 | OG-SER198 | HBA | 2.51 |
| 6-Ring | CE1-PHE329 | H–π | 2.94 | ||
| 4q | −3.36 | S11 | OG-SER198 | HBA | 2.52 |
| 6-Ring | CB-ASN68 | H–π | 2.56 | ||
3 Methodology
3.1. General procedure
In addition to freshly distilled solvents, dry solvents were sometimes utilized as well. The progress of the reactions was monitored through Thin Layered Chromatography performed on pre-coated Kieselgel-60 HF 254 cards. Standard protocols were followed, and all compounds were synthesized using clean glassware. Chemical reactions were carried out using reagents purchased from Sigma Aldrich. A number of contemporary spectroscopic methods were used to characterize the newly synthesized molecules, including 13C NMR, 1H NMR, and HRMS-EIMS.3.2. Synthesis of diethyl 2,2′-((thiobis(4,1-phenylene))bis(oxy))diacetate (2)
8 mmol of 4,4′-thiodiphenol and 16 mmol of K2CO3 were dissolved in DMF, and the mixture was then refluxed for 30 minutes. The reaction mixture was then given 12 mmol of ethyl-2-chloroacetate for esterification. An additional 6 to 8 hours were spent refluxing the reaction mixture. TLC was used to continually track the reaction's progress using the n-hexane/ethyl acetate solvent combination (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). White precipitates were created when the hot reaction mixture was poured into ice-cold water at the end of the reaction. The precipitates were filtered and then let to air dry.
4). White precipitates were created when the hot reaction mixture was poured into ice-cold water at the end of the reaction. The precipitates were filtered and then let to air dry.
Yield: 8 g (97%); white solid; mp 107–109 °C.
HR-MS (EIMS): m/z [M + H]+ calcd for C20H22O6S: 390.4500, found: 390.1031.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 1.230 (t, J = 7.0 Hz, 6H, 2 –CH3), 4.902 (q, J = 7.5 Hz, 4H, 2 –CH2–), 4.6 s (4H, 2 –CH2–), 7.16 (d, J = 8.2 Hz, 4H, Ar–H), 7.38 (d, J = 8.2 Hz, 4H, Ar–H).
13C NMR (600.153 MHz, DMSO-d6): (δ ppm): 168.49, 161.16, 134.04, 129.30, 115.45, 64.68, 60.85, 51.96, 13.99.
3.3. 2,2′-((Thiobis(4,1-phenylene)bis(oxy))di(acetohydrazide)) syntheses (3)
8000 mg (8 g, 8 mmol) of diethyl 2,2′-((thiobis(4,1-phenylene))bi(oxy))diacetate (2) and an excess quantity of hydrazine hydrate were combined in 50 mL of methanol to create the hydrazide of the ester. A white precipitate of compound (3) was created after a 30 minute reflux time. The precipitate was separated, filtered, and then dried.Yield: 90% (5581 mg); white solid; mp 227–229 °C. HR-MS (EIMS): m/z [M + H]+ calcd for C16H18N4O4S: 362.4020, found: 362.1020.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 9.08 (s, 4H, 2-NH2), 4.22 (s, 2H, 2-NH–), 4.590 (s, 4H, 2-CH2–), 7.160 (d, J = 8.2 Hz, 4H, Ar–H), 7.381 (d, J = 8.2 Hz, 4H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.11, 163.66, 134.54, 129.80, 115.95, 66.36.
3.4. Basic steps for producing 2,2′-((thiobis(4,1-phenylene))bis(oxy))di(acetohydrazide) bis(acylhydrazones) (3)
Using compound (3) as the starting material, bis(acylhydrazones) were synthesized by reacting it with various aromatic and aliphatic aldehydes in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The mixture was refluxed at 120 °C for 8–16 hours while the reactions took place using DMF as the solvent. The resulting mixture was quickly cooled by submerging it in ice-cold water after refluxing. The resultant bi(acylhydrazone) precipitates were filtered, separated, and dried for further use.
1 ratio. The mixture was refluxed at 120 °C for 8–16 hours while the reactions took place using DMF as the solvent. The resulting mixture was quickly cooled by submerging it in ice-cold water after refluxing. The resultant bi(acylhydrazone) precipitates were filtered, separated, and dried for further use.
HR-MS (EIMS): m/z [M + H]+ calcd for C30H24N6O8S: 628.1400, found: 628.2525.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.686 (s, 2H, 2-NH–), 4.733 (s, 4H, 2-CH2–), 8.428, 8.408 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.999, 7.053 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.510, 7.558 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.929, 7.981 (d, d, J = 8.4 Hz, 4H, Ar–H), 8.252, 8.283 (d, d, J = 8.4 Hz, 4H, Ar–H).
CH–), 6.999, 7.053 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.510, 7.558 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.929, 7.981 (d, d, J = 8.4 Hz, 4H, Ar–H), 8.252, 8.283 (d, d, J = 8.4 Hz, 4H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.52, 164.85, 162.33, 157.37, 147.98, 147.82, 145.52, 141.54, 140.47, 140.32, 132.71, 128.11, 127.95, 127.36, 127.26, 124.08, 124.02, 115.16, 114.99, 66.60, 64.85, 35.81, 30.80.
HR-MS (EIMS): m/z [M + H]+ calcd for C30H24Cl2N4O4S: 606.0900, found: 606.1993.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.687 (s, 2H, 2-NH–), 4.689 (s, 4H, 2-CH2–), 8.311, 7.997 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.980, 7.046 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.484, 7.506 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.542, 7.566 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.705, 7.740 (d, d, J = 8.4 Hz, 4H, Ar–H).
CH–), 6.980, 7.046 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.484, 7.506 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.542, 7.566 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.705, 7.740 (d, d, J = 8.4 Hz, 4H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.15, 164.42, 162.33, 157.47, 157.30, 156.93, 146.67, 142.59, 134.65, 134.41, 133.11, 132.98, 132.67, 128.96, 128.90, 128.80, 127.34, 127.28, 127.24, 115.15, 114.96, 66.62, 64.80, 35.80, 30.80.
HR-MS (EIMS): m/z [M + H]+ calcd for C32H26N4O6S: 594.1600, found: 594.2686.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.686 (s, 2H, 2-NH–), 10.015 (s, 2H, 2CHO), 4.723 (s, 4H, 2-CH2–), 8.403, 8.085 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.997, 7.054 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.523, 7.558 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.896, 7.910 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.918, 7.945 (d, d, J = 8.4 Hz, 4H, Ar–H).
CH–), 6.997, 7.054 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.523, 7.558 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.896, 7.910 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.918, 7.945 (d, d, J = 8.4 Hz, 4H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 192.72, 169.39, 164.67, 162.33, 146.57, 142.55, 139.66, 139.51, 136.74, 129.96, 129.93, 127.68, 127.52, 127.36, 127.30, 127.25, 115.17, 114.99, 66.64, 64.85, 35.80, 30.81.
HR-MS (EIMS): m/z [M + H]+ calcd for C36H38N4O10S: 718.2300, found: 718.3526.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.599 (s, 2H, 2-NH–), 4.691 (s, 4H, 2-CH2–), 3.804 (s, 6H, 2-CH3), 3.688 (s, 12H, 4-CH3), 8.256, 7.925 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.977, 7.051 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.521, 7.559 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.012 (s, 4H, Ar–H).
CH–), 6.977, 7.051 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.521, 7.559 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.012 (s, 4H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.14, 164.22, 153.19, 148.02, 143.66, 139.38, 139.17, 129.62, 129.54, 127.38, 127.31, 127.27, 127.20, 115.17, 114.91, 104.43, 104.29, 66.64, 64.89, 60.15, 55.99.
HR-MS (EIMS): m/z [M + H]+ calcd for C30H26N4O6S: 570.1600, found: 570.2664.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.368 (s, 2H, 2-NH–), 9.934 (s, 2H, 2-OH), 4.723 (s, 4H, 2-CH2–), 8.214, 7.903 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.972, 7.046 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.505, 7.518 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.798, 6.812 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.541, 7.556 (d, d, J = 8.4 Hz, 4H, Ar–H).
CH–), 6.972, 7.046 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.505, 7.518 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.798, 6.812 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.541, 7.556 (d, d, J = 8.4 Hz, 4H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.68, 163.88, 162.33, 159.53, 159.32, 157.51, 157.43, 157.04, 156.97, 148.30, 144.18, 133.14, 132.98, 132.63, 132.45, 128.93, 128.69, 127.37, 127.32, 127.28, 127.22, 125.09, 125.03, 115.72, 115.70, 115.16, 114.92, 66.67, 64.78, 35.81, 30.81.
HR-MS (EIMS): m/z [M + H]+ calcd for C38H30N4O4S: 638.2000, found.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.666, 11.648 (s, 2H, 2-NH–), 4.755 (s, 4H, 2-CH2–), 9.010, 8.687 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.023, 7.110 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.535, 7.550 (d, d, J = 8.2 Hz, 4H, Ar–H), 8.799, 8.818 (dd, J = 8.4 Hz, 2H, Ar–H), 8.012, 8.022 (ddd, J = 8.4 Hz, 2H, Ar–H), 8.614, 8.628 (dd, J = 8.4 Hz, 2H, Ar–H), 7.910, 7.999 (dd, J = 8.4 Hz, 2H, Ar–H), 7.565, 7.591 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.601, 7.659 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.635, 7.659 (dd, J = 8.4 Hz, 2H, Ar–H).
CH–), 7.023, 7.110 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.535, 7.550 (d, d, J = 8.2 Hz, 4H, Ar–H), 8.799, 8.818 (dd, J = 8.4 Hz, 2H, Ar–H), 8.012, 8.022 (ddd, J = 8.4 Hz, 2H, Ar–H), 8.614, 8.628 (dd, J = 8.4 Hz, 2H, Ar–H), 7.910, 7.999 (dd, J = 8.4 Hz, 2H, Ar–H), 7.565, 7.591 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.601, 7.659 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.635, 7.659 (dd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.68, 163.88, 162.33, 159.53, 159.32, 157.51, 157.43, 157.04, 156.97, 148.30, 144.18, 133.14, 132.98, 132.63, 132.45, 128.93, 128.69, 127.37, 127.32, 127.28, 127.22, 125.09, 125.03, 115.74, 115.70, 115.16, 114.92, 66.67, 64.78, 35.81, 30.81.
HR-MS (EIMS): m/z [M + H]+ calcd for C34H34N4O8S: 658.2100, found: 658.3287.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.418, 11.378 (s, 2H, 2-NH–), 9.448 (s, 2H, 2-OH), 4.653 (s, 4H, 2-CH2–), 1.307, 1.350 (t, J = 7.0 Hz, 6H, 2-CH3), 4.029, 4.040 (q, J = 7.5 Hz, 4H, 2-CH2–) 8.188, 7.783 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.046, 7.079 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.532, 7.569 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.506, 7.519 (d, J = 8.4 Hz, 2H, Ar–H), 7.240, 7.250 (dd, J = 8.4 Hz, 2H, Ar–H), 6.953, 6.957 (dd, J = 8.4 Hz, 2H, Ar–H).
CH–), 7.046, 7.079 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.532, 7.569 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.506, 7.519 (d, J = 8.4 Hz, 2H, Ar–H), 7.240, 7.250 (dd, J = 8.4 Hz, 2H, Ar–H), 6.953, 6.957 (dd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.77, 163.89, 162.33, 157.54, 149.36, 149.08, 148.55, 147.19, 147.12, 144.27, 127.37, 127.30, 127.26, 127.19, 125.46, 122.10, 121.33, 115.62, 115.57, 115.16, 114.90, 110.96, 110.51, 66.68, 6486, 63.91, 35.80, 30.80, 14.74, 14.71.
HR-MS (EIMS): m/z [M + H]+ calcd for C32H30N4O8S: 630.1800, found: 630.2939.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.525 (s, 2H, 2-NH–), 10.730 (s, 2H, 2-OH), 4.795 (s, 4H, 2-CH2–), 3.811 (s, 6H, 2-OCH3) 8.569, 8.334 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.124, 7.309 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.509, 7.851 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.965, 6.992 (d, J = 8.4 Hz, 2H, Ar–H), 6.795, 6.853 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.024, 7.066 (dd, J = 8.4 Hz, 2H, Ar–H).
CH–), 7.124, 7.309 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.509, 7.851 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.965, 6.992 (d, J = 8.4 Hz, 2H, Ar–H), 6.795, 6.853 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.024, 7.066 (dd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.72, 164.21, 157.48, 156.97, 156.89, 148.22, 148.02, 147.98, 147.13, 146.01, 141.36, 133.23, 133.05, 127.41, 127.35, 127.30, 127.24, 120.63, 120.46, 119.21, 119.09, 118.93, 117.91, 115.19, 114.94, 113.96, 66.57, 64.86, 55.89.
HR-MS (EIMS): m/z [M + H]+ calcd for C32H30N4O8S: 630.1800, found: 630.2958.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.429, 11.392 (s, 2H, 2-NH–), 9.570 (s, 2H, 2-OH), 4.658 (s, 4H, 2-CH2–), 3.800 (s, 6H, 2-OCH3) 8.203, 7.940 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.957, 6.970 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.519, 7.570 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.258, 7.269 (d, J = 8.4 Hz, 2H, Ar–H), 7.048, 7.088 (dd, J = 8.4 Hz, 2H, Ar–H), 6.808, 6.820 (dd, J = 8.4 Hz, 2H, Ar–H).
CH–), 6.957, 6.970 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.519, 7.570 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.258, 7.269 (d, J = 8.4 Hz, 2H, Ar–H), 7.048, 7.088 (dd, J = 8.4 Hz, 2H, Ar–H), 6.808, 6.820 (dd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.78, 163.89, 162.34, 159.46, 157.55, 157.46, 157.05, 156.96, 149.12, 148.84, 148.54, 148.06, 147.98, 144.27, 132.98, 132.43, 127.37, 127.31, 127.27, 127.20, 125.50, 125.46, 122.20, 121.36, 115.55, 115.47, 115.17, 114.91, 109.69, 109.12, 66.68, 64.85, 55.63, 55.60, 35.81, 20.21.
HR-MS (EIMS): m/z [M + H]+ calcd for C30H26N4O8S: 602.1500, found: 602.2595.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.257 (s, 2H, 2-NH–), 10.038 (s, 2H, 2-OH), 4.684 (s, 4H, 2-CH2–), 8.413, 8.172 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.967, 7.058 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.532, 7.575 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.474, 7.488 (d, J = 8.4 Hz, 2H, Ar–H), 6.303, 6.337 (dd, J = 8.4 Hz, 2H, Ar–H), 7.267, 7.281 (d, J = 8.4 Hz, 2H, Ar–H).
CH–), 6.967, 7.058 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.532, 7.575 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.474, 7.488 (d, J = 8.4 Hz, 2H, Ar–H), 6.303, 6.337 (dd, J = 8.4 Hz, 2H, Ar–H), 7.267, 7.281 (d, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm: 172.69, 168.26, 163.83, 162.29, 160.67, 159.67, 158.14, 157.14, 149.55, 142.67, 133.03, 131.21, 127.39, 127.33, 127.28, 115.19, 114.92, 112.07, 107.86, 102.68, 102.44, 66.85, 65.42, 35.81, 30.72.
HR-MS (EIMS): m/z [M + H]+ calcd for C30H22Cl4N4O4S: 674.0100, found: 674.1286.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.866, (s, 2H, 2-NH–), 4.715 (s, 4H, 2-CH2–), 8.703, 8.342 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.992, 7.061 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.500, 7.564 (d, d, J = 8.2 Hz, 4H, Ar–H), 8.028, 8.043 (dd, J = 8.4 Hz, 2H, Ar–H), 7.952, 7.967 (dd, J = 8.4 Hz, 2H, Ar–H), 7.12 (d, J = 8.4 Hz, 2H, Ar–H).
CH–), 6.992, 7.061 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.500, 7.564 (d, d, J = 8.2 Hz, 4H, Ar–H), 8.028, 8.043 (dd, J = 8.4 Hz, 2H, Ar–H), 7.952, 7.967 (dd, J = 8.4 Hz, 2H, Ar–H), 7.12 (d, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.30, 164.66, 157.37, 142.86, 138.86, 135.24, 135.00, 133.97, 133.68, 132.70, 130.58, 130,41, 129.41, 129.38, 128.33, 128.17, 128.07, 127.97, 127.40, 127.35, 127.28, 127.24, 115.19, 114.99, 66.69, 64.84, 35.86, 14.30.
HR-MS (EIMS): m/z [M + H]+ calcd for C38H44N6O4S: 680.3100, found: 680.4315.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.272, 11.216 (s, 2H, 2-NH–), 4.646 (s, 4H, 2-CH2–), 8.143, 7.843 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 1.081, 1.100 (t, J = 7.0 Hz, 12H, 4-CH3), 3.297, 3.373 (q, J = 7.5 Hz, 8H, 4-CH2–), 6.966, 7.048 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.405, 7.519 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.660, 6.688 (dd, J = 8.4 Hz, 4H, Ar–H), 7.544, 7.570 (dd, J = 8.4 Hz, 4H, Ar–H).
CH–), 1.081, 1.100 (t, J = 7.0 Hz, 12H, 4-CH3), 3.297, 3.373 (q, J = 7.5 Hz, 8H, 4-CH2–), 6.966, 7.048 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.405, 7.519 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.660, 6.688 (dd, J = 8.4 Hz, 4H, Ar–H), 7.544, 7.570 (dd, J = 8.4 Hz, 4H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 189.48, 168.36, 163.50, 148.97, 148.86, 148.78, 144.74, 128.85, 128.56, 127.36, 127.30, 127.22, 120.44, 115.15, 114.90, 111.11, 110.63, 66.71, 64.80, 43.75, 12.45.
HR-MS (EIMS): m/z [M + H]+ calcd for C30H26N4O6S: 570.1600, found: 570.2672.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.542, 11.523 (s, 2H, 2-NH–), 4.679 (s, 4H, 2-CH2–), 8.239, 8.229 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 9.611 (s, 2H, 2-OH), 7.094, 7.148 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.920, 7.940 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.966, 6.980 (d, J = 8.4 Hz, 2H, Ar–H), 6.806, 6.819 (dd, J = 8.4 Hz, 2H, Ar–H), 7.212, 7.238 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.512, 7.576 (ddd, J = 8.4 Hz, 2H, Ar–H).
CH–), 9.611 (s, 2H, 2-OH), 7.094, 7.148 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.920, 7.940 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.966, 6.980 (d, J = 8.4 Hz, 2H, Ar–H), 6.806, 6.819 (dd, J = 8.4 Hz, 2H, Ar–H), 7.212, 7.238 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.512, 7.576 (ddd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.95, 164.26, 162.33, 157.70, 157.66, 157.47, 157.40, 156.95, 148.06, 144.08, 135.40, 135.24, 133.17, 133.00, 132.68, 132.52, 129.89, 127.39, 127.34, 127.31, 127.26, 118.86, 118.45, 117.56, 117.31, 115.16, 114.92, 112.77, 112.71, 66.65, 64.74, 35.80, 30.80.
HR-MS (EIMS): m/z [M + H]+ calcd for C34H34N4O8S: 658.2100, found: 658.5057.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.466 (s, 2H, 2-NH–), 4.672 (s, 4H, 2-CH2–), 8.249, 7.940 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.783 (s, 6H, 2-OCH3), 3.789 (s, 6, 2-OCH3), 7.050, 7.193 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.570, 7.940 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.974, 7.000 (dd, J = 8.4 Hz, 2H, Ar–H), 7.289, 7.313 (dd, J = 8.4 Hz, 2H, Ar–H), 7.520, 7.545 (d, J = 8.4 Hz, 2H, Ar–H).
CH–), 3.783 (s, 6H, 2-OCH3), 3.789 (s, 6, 2-OCH3), 7.050, 7.193 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.570, 7.940 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.974, 7.000 (dd, J = 8.4 Hz, 2H, Ar–H), 7.289, 7.313 (dd, J = 8.4 Hz, 2H, Ar–H), 7.520, 7.545 (d, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.90, 164.02, 162.33, 157.55, 157.47, 157.04, 156.96, 150.88, 150.64, 149.10, 149.07, 148.21, 143.92, 133.16, 132.99, 132.44, 127.38, 127.31, 127.26, 127.21, 126.83, 126.79, 121.95, 121.27, 115.17, 114.92, 111.55, 108.77, 108.40, 66.68, 64.88, 5561, 55.51, 38.30, 30.80.
HR-MS (EIMS): m/z [M + H]+ calcd for C34H34N4O8S: 658.2100, found: 658.3267.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.458, 11.409 (s, 2H, 2-NH–), 4.636 (s, 4H, 2-CH2–), 8.574, 8.249 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.783 (s, 6H, 2-OCH3), 3.789 (s, 6H, 2-OCH3), 7.032, 7.046 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.509, 7.569 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.581, 6.616 (d, J = 8.4 Hz, 2H, Ar–H), 6.954, 6.968 (dd, J = 8.4 Hz, 2H, Ar–H), 7.744, 7.776 (d, J = 8.4 Hz, 2H, Ar–H).
CH–), 3.783 (s, 6H, 2-OCH3), 3.789 (s, 6H, 2-OCH3), 7.032, 7.046 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.509, 7.569 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.581, 6.616 (d, J = 8.4 Hz, 2H, Ar–H), 6.954, 6.968 (dd, J = 8.4 Hz, 2H, Ar–H), 7.744, 7.776 (d, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.66, 163.83, 162.56, 162.35, 159.24, 159.06, 157.52, 157.44, 143.55, 139.65, 132.62, 127.35, 127.30, 127.21, 126.76, 115.14, 114.93, 106.52, 106.44, 98.34, 98.17, 66.63, 64.83, 55.82, 55.48, 35.81, 30.80.
HR-MS (EIMS): m/z [M + H]+ calcd for C32H30N4O6S: 598.1900, found: 598.3018.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.615, 11.567 (s, 2H, 2-NH–), 4.680 (s, 4H, 2-CH2–), 8.698, 8.364 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.861 (s, 6H, 2-OCH3), 7.058, 7.117 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.826, 7.813 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.983, 7.025 (dd, J = 8.4 Hz, 2H, Ar–H), 7.023, 7.428 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.523, 7.579 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.875, 7.863 (dd, J = 8.4 Hz, 2H, Ar–H).
CH–), 3.861 (s, 6H, 2-OCH3), 7.058, 7.117 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.826, 7.813 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.983, 7.025 (dd, J = 8.4 Hz, 2H, Ar–H), 7.023, 7.428 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.523, 7.579 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.875, 7.863 (dd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.13, 164.34, 159.57, 147.92, 143.70, 135.57, 135.43, 132.65, 129.98, 129.94, 127.39, 127.33, 127.28, 127.23, 120.09, 119.63, 116.37, 115.92, 115.17, 114.94, 111,64, 111.34, 66.65, 64.83, 55.21.
HR-MS (EIMS): m/z [M + H]+ calcd for C36H38N4O4S: 622.2600, found: 622.3745.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.544 (s, 2H, 2-NH–), 4.679 (s, 4H, 2-CH2–), 8.303, 7.982 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 1.194–1.205 (d, J = 7.4 Hz, 12H, 4-CH3), 2.980 (heptet, J = 7.5 Hz, 2H, 2-CH–), 6.966, 7.055 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.512, 7.572 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.602, 7.623 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.295, 7.320 (d, d, J = 8.4 Hz, 4H, Ar–H).
CH–), 1.194–1.205 (d, J = 7.4 Hz, 12H, 4-CH3), 2.980 (heptet, J = 7.5 Hz, 2H, 2-CH–), 6.966, 7.055 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.512, 7.572 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.602, 7.623 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.295, 7.320 (d, d, J = 8.4 Hz, 4H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.95, 164.18, 157.50, 157.43, 156.96, 150.83, 150.60, 148.03, 142.93, 133.16, 132.66, 132.50, 131.84, 131.72, 127.38, 127.33, 127.26, 127.05, 126.83, 126.78, 115.16, 114.93, 66.66, 64.81, 33.40, 33.38, 23.68.
HR-MS (EIMS): m/z [M + H]+ calcd for C26H22N4O4S3: 550.0800, found: 550.1864.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.550 (s, 2H, 2-NH–), 4.668 (s, 4H, 2-CH2–), 8.549, 8.190 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.943, 7.047 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.439, 7.521 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.116, 7.130 (dd, J = 8.4 Hz, 2H, Ar–H), 7.631, 7.663 (dd, J = 8.4 Hz, 2H, Ar–H), 7.535, 7.544 (dd, J = 8.4 Hz, 2H, Ar–H).
CH–), 6.943, 7.047 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.439, 7.521 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.116, 7.130 (dd, J = 8.4 Hz, 2H, Ar–H), 7.631, 7.663 (dd, J = 8.4 Hz, 2H, Ar–H), 7.535, 7.544 (dd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.65, 164.15, 157.44, 156.99, 156.92, 143.17, 139.03, 138.86, 138.67, 133.02, 132.15, 130.56, 139.12, 128.62, 127.94, 127.89, 127.39, 127.32, 127.27, 115.18, 114.98, 66.68, 64.57, 38.90, 35.80.
HR-MS (EIMS): m/z [M + H]+ calcd for C30H24F2N4O4S: 574.1500, found: 574.2565.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.600 (s, 2H, 2-NH–), 4.687 (s, 4H, 2-CH2–), 8.335, 8.007 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.993, 7.052 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.510, 7.560 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.258, 7.279 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.750, 7.787 (d, d, J = 8.4 Hz, 4H, Ar–H).
CH–), 6.993, 7.052 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.510, 7.560 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.258, 7.279 (d, d, J = 8.4 Hz, 4H, Ar–H), 7.750, 7.787 (d, d, J = 8.4 Hz, 4H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.08, 164.33, 164.03, 162.38, 162.24, 157.49, 157.41, 156.94, 146.88, 142.73, 133.00, 132.67, 132.49, 130.77, 130.66, 129.38, 129.32, 129.20, 129.14, 127.34, 127.28, 127.24, 127.01, 116.01, 115.94, 115.86, 115.80, 115.16, 114.95, 66.65, 64.81, 38.30, 35.80, 30.40.
HR-MS (EIMS): m/z [M + H]+ calcd for C30H26N4O6S: 570.6200, found: 570.2663.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.819, 11.535 (s, 2H, 2-NH–), 4.720 (s, 4H, 2-CH2–), 8.560, 8.306 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 11.044 and 10.049 (s, 2H, 2-OH), 6.969, 7.055 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.511, 7.583 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.839, 6.851 (dd, J = 8.4 Hz, 2H, Ar–H), 6.864, 6.914 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.223, 7.297 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.699, 7.712 (dd, J = 8.4 Hz, 2H, Ar–H).
CH–), 11.044 and 10.049 (s, 2H, 2-OH), 6.969, 7.055 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.511, 7.583 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.839, 6.851 (dd, J = 8.4 Hz, 2H, Ar–H), 6.864, 6.914 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.223, 7.297 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.699, 7.712 (dd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.72, 164.25, 157.41, 156.98, 156.90, 156.44, 148.37, 141.52, 133.24, 133.06, 131.54, 131.23, 129.34, 127.41, 127.35, 127.30, 127.24, 126.58, 120.05, 119.44, 119.40, 118.65, 116.42, 116.17, 112.20, 114.95, 66.56,64.89, 38.40, 35.90, 30.80.
HR-MS (EIMS): m/z [M + H]+ calcd for C30H24Cl2N4O4S: 606.5100, found: 606.2000.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.851, 11.778 (s, 2H, 2-NH–), 4.710 (s, 4H, 2-CH2–), 8.749, 8.397 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.982, 7.067 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.512, 7.556 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.570, 7.585 (dd, J = 8.4 Hz, 4H, Ar–H), 7.409, 7.448 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.956, 8.030 (dd, J = 8.4 Hz, 2H, Ar–H).
CH–), 6.982, 7.067 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.512, 7.556 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.570, 7.585 (dd, J = 8.4 Hz, 4H, Ar–H), 7.409, 7.448 (ddd, J = 8.4 Hz, 2H, Ar–H), 7.956, 8.030 (dd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.25, 164.59, 157.39, 143.91, 139.90, 133.28, 132.98, 132.69, 131.65, 131.41, 131.29, 129.96, 129.91, 127.67, 127.63, 127.41, 127.35, 127.30, 127.24, 127.11, 126.98, 115.20, 114.98, 66.69, 64.85, 35.80, 30.70.
HR-MS (EIMS): m/z [M + H]+ calcd for C28H26N4O6S: 546.6000, found: 546.0000.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.453, 11.436 (s, 2H, 2-NH–), 4.657 (s, 4H, 2-CH2–), 8.123, 7.810 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.324 (s, 6H, 2-CH3), 6.936, 7.028 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.499, 7.571 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.244 (dd, J = 8.4 Hz, 2H, Ar–H), 6.783, 6.795 (dd, J = 8.4 Hz, 2H, Ar–H).
CH–), 2.324 (s, 6H, 2-CH3), 6.936, 7.028 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.499, 7.571 (d, d, J = 8.2 Hz, 4H, Ar–H), 6.244 (dd, J = 8.4 Hz, 2H, Ar–H), 6.783, 6.795 (dd, J = 8.4 Hz, 2H, Ar–H).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.67, 164.08, 157.46, 154.71, 154.55, 147.71, 147.53, 137.67, 134.17, 127.38, 127.32, 127.28, 127.23, 115.64, 115.39, 115.18, 114.92, 108.59, 66.72, 64.62, 13.56, 13.49.
HR-MS (EIMS): m/z [M + H]+ calcd for C28H38N4O4S: 526.7000, found: 526.0000.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.605 (s, 2H, 2-NH–), 4.811 (s, 4H, 2-CH2–), 7.940, 7.810 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.324 (s, 6H, 2-CH3), 6.921, 6.935 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.489, 7.500 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.381 (t, J = 7.5 Hz, 1H, –N
CH–), 2.324 (s, 6H, 2-CH3), 6.921, 6.935 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.489, 7.500 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.381 (t, J = 7.5 Hz, 1H, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.974 (t, J = 7.5 Hz, 1H, –N
CH–), 7.974 (t, J = 7.5 Hz, 1H, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.180 (q, J = 7.2 Hz, 4H, 2-CH2–), 1.460–1.500 (quintet, J = 7.3 Hz, 4H, 2-CH2–), 1.236–1.276 (sextet, J = 7.3 Hz, 4H. 2-CH2–), 0.839–0.903 (t, J = 7.5 Hz, 6H. 2-CH3).
CH–), 2.180 (q, J = 7.2 Hz, 4H, 2-CH2–), 1.460–1.500 (quintet, J = 7.3 Hz, 4H, 2-CH2–), 1.236–1.276 (sextet, J = 7.3 Hz, 4H. 2-CH2–), 0.839–0.903 (t, J = 7.5 Hz, 6H. 2-CH3).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 192.72, 169.39, 164.67, 162.33, 146.57, 142.55, 139.66, 139.51, 136.74, 129.96, 129.93, 127.68, 127.52, 127.36, 127.30, 127.25, 115.17, 114.99, 66.64, 64.85, 35.80, 30.81,24.80, 22.30, 18.40, 14.30.
HR-MS (EIMS): m/z [M + H]+ calcd for C26H34N4O4S: 498.6400, found: 498.0000.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.605 (s, 2H, 2-NH–), 4.811 (s, 4H, 2-CH2–), 7.940, 7.810 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.324 (s, 6H, 2-CH3), 6.921, 6.935 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.489, 7.500 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.381 (t, J = 7.5 Hz, 1H, –N
CH–), 2.324 (s, 6H, 2-CH3), 6.921, 6.935 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.489, 7.500 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.381 (t, J = 7.5 Hz, 1H, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.974 (t, J = 7.5 Hz, 1H, –N
CH–), 7.974 (t, J = 7.5 Hz, 1H, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.180 (q, J = 7.2 Hz, 4H, 2-CH2–), 1.460–1.500 (quintet, J = 7.3 Hz, 4H, 2-CH2–), 1.236–1.276 (sextet, J = 7.3 Hz, 4H. 2-CH2–), 0.839–0.903 (t, J = 7.5 Hz, 6H. 2-CH3).
CH–), 2.180 (q, J = 7.2 Hz, 4H, 2-CH2–), 1.460–1.500 (quintet, J = 7.3 Hz, 4H, 2-CH2–), 1.236–1.276 (sextet, J = 7.3 Hz, 4H. 2-CH2–), 0.839–0.903 (t, J = 7.5 Hz, 6H. 2-CH3).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 169.52, 164.85, 162.33, 157.37, 147.98, 147.82, 145.52, 141.54, 140.47, 140.32, 132.71, 128.11, 127.95, 127.36, 127.26, 124.08, 124.02, 115.16, 114.99, 66.60.64.85, 35.81, 30.80, 28.70, 26.80, 14.30.
HR-MS (EIMS): m/z [M + H]+ calcd for C24H30N4O4S: 470.5900, found: 470.0000.
1H NMR (DMSO-d6, 600.150 MHz, δ ppm): 11.632 (s, 2H, 2-NH–), 4.812 (s, 4H, 2-CH2–), 7.940, 7.810 (s, 2H, 2 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.324 (s, 6H, 2-CH3), 6.896, 6.990 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.386, 7.459 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.648 (t, J = 7.5 Hz, 1H, –N
CH–), 2.324 (s, 6H, 2-CH3), 6.896, 6.990 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.386, 7.459 (d, d, J = 8.2 Hz, 4H, Ar–H), 7.648 (t, J = 7.5 Hz, 1H, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.928 (t, J = 7.5 Hz, 1H, –N
CH–), 6.928 (t, J = 7.5 Hz, 1H, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.232–2.243 (q, J = 7.2 Hz, 4H, 2-CH2–), 1.223–1.531 (heptet, J = 7.3 Hz, 4H. 2-CH2–), 0.863–0.915 (t, J = 7.5 Hz, 6H. 2-CH3).
CH–), 2.232–2.243 (q, J = 7.2 Hz, 4H, 2-CH2–), 1.223–1.531 (heptet, J = 7.3 Hz, 4H. 2-CH2–), 0.863–0.915 (t, J = 7.5 Hz, 6H. 2-CH3).
13C NMR (600.150 MHz, DMSO-d6): (δ ppm): 168.95, 164.18, 157.50, 157.43, 156.96, 150.83, 150.60, 148.03, 143.94, 133.16, 132.66, 132.50, 131.84, 131.72, 127.38, 127.33, 127.29, 127.26, 127.05, 126.83, 126.78, 115.16, 114.93, 66.66, 64.81, 33.40, 33.38, 23.68, 20.20, 18.80.
3.5. Anticholinesterase activity
DTNB, butyrylcholine iodide, potassium phosphate buffer (pH 8.0), acetylcholine iodide, galantamine, and two enzymes—equine butyrylcholinesterase and electric eel acetylcholinesterase (type-IV-S) were utilized in this study. The spectrophotometric approach developed by Elman was used to perform the inhibition investigations on AChE and BChE. The substrates used in this approach were butyrylcholine iodide and acetylcholine iodide.Finally, two mixes were made with 5 mL each of AChE (0.03 g mL−1) and BChE (0.01 g mL−1). The sample chemical was added to each combination in amounts ranging from 125 to 1000 g mL−1 along with DNTB (5 μL), and each mixture was then incubated for 15 minutes at 30 °C. 5 μL of substrates were then added to each mixture after the incubation period, and the reaction was then observed using a UV-Visible spectrophotometer for 4 minutes at 412 nm. The mixture took on a yellow hue, which was an indication that the 5-thio-2-nitrbenzoate anion was formed as a consequence of the interaction between the thiocholines and the DNTB. A control reaction mixture without enzymes and samples was also looked at to investigate the non-enzymatic hydrolysis of the substrate. Using the supplied formulae, enzyme activity and percent inhibition were computed.
| % Age enzyme activity = 100 − % enzyme activity |
3.6. Molecular docking simulations
Molecular docking was performed on Molecular Operating Environment (MOE version 2020.0901)36,37 by using the three-dimensional structures of electric eel AChE (https://alphafold.com/entry/O42275) and equine BChE (https://alphafold.com/entry/P81908) from AlphaFold protein structure database (https://alphafold.com/). Both the structures were treated by MOE's QuickPrep module to add hydrogen atoms and to calculate partial charges (according to AMBER10:EHT force field). Tetronarce californica acetylcholinesterase (PDB code: 1DX6) in complex with galantamine was taken from Protein data bank and superimposed with both the models to deduce the binding configuration of galantamine in electric eel AChE and equine BChE. Active site was defined as 3.5 Å vicinity of galantamine binding region to dock the compounds. The ligands were drawn on MOE and partial charges (with MMFF-94x forcefield) were added on ligands, and each structure was minimized with gradient of 0.1 RMS kcal mol−1 Å−1. After preparing ligands and protein files, the selected compounds were docked at the active site (around 3 Å of galantamine binding region) by the Triangle Matcher docking algorithm and London dG scoring function, and 30 conformations of each ligand were saved. After docking, the protein ligand interactions were analyzed on MOE's interface and diagrams are illustrated by MOE. The 2D-interactions of docked molecules within the active site of acetyl and butyryl cholinesterase enzymes are shown in ESI (Fig. S82 and S83†).4 Conclusion
Using straightforward, simple, varied, and sequential synthetic approach, 25 (4a–y) novel supramolecules comprising of bisphenol sulfide and hydrazone functionalities were synthesized. Herein, DMF, methanol, and acetic acid are found as the most efficient solvents for these alterations. Phenolic hydroxy was often used for synthetic modifications (CH3COOH). The synthesized functionalities were tested in vitro for their potential cholinergic activities by blocking acetylcholinesterase and butyrylcholinesterase enzymes. Among the synthesized hits, 4n/4h and 4b/4h exhibited excellent inhibitory potential for AChE and BChE, respectively. While the starting compound, SM3 and compound 4h have dual inhibitory capabilities for AChE and BChE. In addition, the molecular docking results of most active (4h and SM3), intermediate (4i and 4k), and least active (4q) inhibitors of both AChE and BChE shows strong correlation with our in vitro findings. The results indicate a strong potential for bis(acylhydrazones) as promising drug candidates for the treatment of Alzheimer's disease.Author contributions
All authors declare that they have all participated in the design, execution, and analysis of the paper and approved the final version.Conflicts of interest
There is no conflict of interest.Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the Large Groups Project under grant number (RGP 2/100/44). The authors would like to thank Higher Education Commission of Pakistan (HEC) to support this project under NRPU #14622.References
- X. Zheng, Z. Zhang, G. Chou, T. Wu, X. Cheng, C. Wang and Z. Wang, Acetylcholinesterase inhibitive activity-guided isolation of two new alkaloids from seeds of Peganum nigellastrum Bunge by an in vitro TLC-bioautographic assay, Arch. Pharm. Res., 2009, 32, 1245–1251 CrossRef CAS PubMed.
- Y. K. Yoon, M. A. Ali, A. C. Wei, T. S. Choon, K. Y. Khaw, V. Murugaiyah, H. Osman and V. H. Masand, Synthesis, characterization, and molecular docking analysis of novel benzimidazole derivatives as cholinesterase inhibitors, Bioorg. Chem., 2013, 49, 33–39 CrossRef CAS PubMed.
- D. C. Meadows and J. Gervay-Hague, Vinyl sulfones: synthetic preparations and medicinal chemistry applications, Med. Res. Rev., 2006, 26, 793–814 CrossRef CAS PubMed.
- N. S. El-Gohary, Arylidene derivatives as synthons in heterocyclic synthesis, Open Access Libr. J., 2014, 1, 1–47 Search PubMed.
- K. Rana, A. Pandurangan, N. Singh and A. K. Tiwari, A systemic review of Schiff bases as an analgesic, anti-inflammatory, Int. J. Curr. Pharm. Res., 2012, 4, 5–11 CAS.
- J. D. Modi, S. S. Sabnis and C. V. Deliwala, Potential anticancer agents. III. Schiff bases from benzaldehyde nitrogen mustards and aminophenylthiazoles, J. Med. Chem., 1970, 13, 935–941 CrossRef CAS PubMed.
- P. G. More, R. B. Bhalvankar and S. C. Pattar, Schiff bases derived from substituted-2-aminothiazole and substituted salicylaldehyde and 2-hydroxy-1-napthaldehyde exhibits antibacterial and antifungal activity, J. Indian Chem. Soc., 2001, 78, 474–475 CAS.
- M. S. Chaitanya, G. Nagendrappa and V. P. Vaidya, Synthesis, biological and pharmacological activities of 2-methyl-4H-pyrimido [2, 1-b][1, 3] benzothiazoles, J. Chem. Pharm. Res., 2010, 2, 206–213 CAS.
- E. M. Hodnett and W. J. Dunn, Structure-antitumor activity correlation of some Schiff bases, J. Med. Chem., 1970, 13, 768–770 CrossRef CAS PubMed.
- B. S. Sathe, E. Jaychandran, V. A. Jagtap and G. M. Sreenivasa, Synthesis characterization and anti-inflammatory evaluation of new fluorobenzothiazole schiff's bases, Int. J. Pharm. Res. Dev., 2011, 3, 164–169 Search PubMed.
- R. P. Chinnasamy, R. Sundararajan and S. Govindaraj, Synthesis, characterization, and analgesic activity of novel schiff base of isatin derivatives, J. Adv. Pharm. Technol. Res., 2010, 1, 342 CrossRef CAS PubMed.
- R. Sreenivasulu, K. T. Reddy, P. Sujitha, C. G. Kumar and R. R. Raju, Synthesis, antiproliferative and apoptosis induction potential activities of novel bis(indolyl)hydrazide–hydrazone derivatives, Bioorg. Med. Chem., 2019, 27, 1043 CrossRef CAS PubMed.
- J. Xu, D. C. Cole, C. P. Chang, R. Ayyad, M. Asselin, W. Hao, J. Gibbons, S. A. Jelinsky, K. A. Saraf and K. Park, Inhibition of the signal transducer and activator of transcription-3 (STAT3) signaling pathway by 4-oxo-1-phenyl-1,4-dihydroquinoline-3-carboxylic acid esters, J Med. Chem., 2008, 51, 4115–4121 CrossRef CAS PubMed.
- P. Kumar, B. Shrivastava, S. N. Pandeya, L. Tripathi and J. P. Stables, Design, synthesis, and anticonvulsant evaluation of some novel 1,3 benzothiazol-2-yl hydrazones/acetohydrazones, Med. Chem. Res., 2012, 21, 2428 CrossRef CAS.
- R. M. Ohareb and J. Schatz, Anti-tumor and anti-leishmanial evaluations of 1,3,4-oxadiazine, pyran derivatives derived from cross-coupling reactions of b-bromo-6H-1,3,4-oxadiazine derivatives, Bioorg. Med. Chem., 2011, 19, 2707–2713 CrossRef PubMed.
- P. Melnyk, v. Leroux, C. Sergheraert and P. Grellier, Design, synthesis and in vitro antimalarial activity of an acylhydrazone library, Bioorg. Med. Chem. Lett., 2006, 16, 31–35 CrossRef CAS PubMed.
- S. Küçükgüzel, g. S. Rollas, I. Küçükgüzel and M. Kiraz, Synthesis and anti-mycobacterial activity of some coupling products from 4-aminobenzoic acid hydra zones, Eur. J. Med. Chem., 1999, 34, 1093–1100 CrossRef.
- L. Popiołek, Hydrazide–hydrazones as potential antimicrobial agents: overview of the literature since 2010, Med. Chem. Res., 2017, 26, 287 CrossRef PubMed.
- Y. Q. Hu, S. Zhang, F. Zhao, C. Gao, L. S. Feng, Z. S. Lv, Z. Xu and X. Wu, Isoniazid derivatives and their antitubercular activity, Eur. J. Med. Chem., 2017, 133, 255 CrossRef CAS PubMed.
- P. Vicini, M. I. Incerti, P. L. Colla and R. Loddo, Anti-HIV evaluation of benzo[d]isothiazole hydrazones, Eur. J. Med. Chem., 2009, 44, 1801–1807 CrossRef CAS PubMed.
- S. Şenkardes, N. Kaushik-Basu, I. Durmaz, D. Manvar, A. Basu, R. Atalay and S. G. Küçükgüzel, Synthesis of novel diflunisal hydrazideehydrazones as anti-hepatitis C virus agents and hepatocellular carcinoma inhibitors, Eur. J. Med. Chem., 2016, 108, 301 CrossRef PubMed.
- L. Troeberg, X. Chen, T. M. Flaherty, R. E. Morty, M. Cheng, H. Hua, C. Springer, J. H. McKerrow, G. L. Kenyon, J. D. Lonsdale-Eccles, T. H. Coetzer and F. E. Cohen, Chalcone, acyl hydrazide and related amides kill cultured Trypanosoma brucei brucei, Mol. Med., 2000, 6(8), 660–669 CAS.
- M. T. Cocco, C. Congiu, V. Lilliu and V. Onnis, Syn-thesis and in vitro Antitumoral Activity of New Hy-drazinopyrimidine-5-carbonitrile Derivatives, Bioorg. Med. Chem., 2006, 14(2), 366–372, DOI:10.1016/j.bmc.2005.08.012.
- A. Inam, S. M. Siddiqui, T. S. Macedo, D. R. M. Moreira, A. C. L. Leite, M. B. P. Soares and A. Azam, Design, synthesis and biological evaluation of 3-[4-(7-chloroquinolin-4-yl)-piperazin-1-yl]-propionic acid hydrazones as antiprotozoal agents, Eur. J. Med. Chem., 2014, 75, 67 CrossRef CAS PubMed.
- P. Vicini, M. I. Incerti, P. L. Colla and R. Loddo, Anti-HIV evaluation of benzo[d]isothiazole hydrazones, Eur. J. Med. Chem., 2009, 44, 1801–1807 CrossRef CAS PubMed.
- N. Ergenç, N. S. Günay and R. Demirdamar, Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives, Eur. J. Med. Chem., 1998, 33(2), 143–148 CrossRef.
- R. Narang, B. Narasimhan and S. Sharma, A Review on Biological Activities and Chemical Synthesis of Hydrazide Derivatives, Curr. Med. Chem., 2012, 19, 569–612 CrossRef CAS PubMed.
- P. Melnyk, V. Leroux, C. Sergheraert and P. Grellier, Design, Synthesis and in vitro Antimalarial Activity of an Acylhydrazone Library, Bioorg. Med. Chem. Lett., 2006, 16(1), 31–35, DOI:10.1016/j.bmcl.2005.09.058.
- S. G. Küçükgüzel, A. Mazi, F. Sahin, S. Öztürk and J. P. Stables, Synthesis and Biological Activities of Diflunisal Hydrazide-Hydrazones, Eur. J. Med. Chem., 2003, 38(11), 1005–1013, DOI:10.1016/j.ejmech.2003.08.004.
- G. L. Ellman, K. D. Courtney, V. Andres and R. M. Featherstone, A new and rapid colorimetric determination of acetylcholinesterase activity, Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS PubMed.
- V. P. Chen, Y. Gao, L. Geng, R. J. Parks, Y. P. Pang and S. Brimijoin, Plasma butyrylcholinesterase regulates ghrelin to control aggression, Proc. Natl. Acad. Sci. USA, 2015, 112, 2251–2256 CrossRef CAS PubMed.
- M. Ayaz, M. Junaid, F. Subhan, F. Ullah, A. Sadiq, S. Ahmad, M. Imran, Z. Kamal, S. Hussain and S. M. Shah, Heavy metals analysis, phytochemical, phytotoxic and anthelmintic investigations of crude methanolic extract, subsequent fractions and crude saponins from polygonum hydropiper L, BMC Complement, Altern. Med., 2014, 14, 1–9 Search PubMed.
- M. E. Azab, S. A. Rizk and N. F. Mahmoud, Facile synthesis, characterization, and antimicrobial evaluation of novel heterocycles, schiff bases, and N-nucleosides bearing phthalazine moiety, Chem. Pharm. Bull., 2016, 64, 439–450 CrossRef CAS PubMed.
- M. Rabnawaz, B. Khan, M. R. Shah, I. Anis and S. W. Ng, Methyl (2-hydroxybiphenyl-2-yloxy) acetate, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, 0931 CrossRef PubMed.
- A. Boguszewska-Czubara, A. Lapczuk-Krygier, K. Rykala, A. Biernasiuk, A. Wnorowski, L. Popiolek, A. Maziarka, A. Hordyjewska and R. Jasinski, Novel synthesis scheme and in vitro antimicrobial evaluation of a panel of (E)-2-aryl-1-cyano-1-nitroethenes, J. Enzym. Inhib. Med. Chem., 2016, 31, 900–907 CrossRef CAS PubMed.
- G. Jones, P. Willett, R. C. Glen, A. R. Leach and R. Taylor, Development and validation of a genetic algorithm for flexible docking, J. Mol. Biol., 1997, 267, 727–748 CrossRef CAS PubMed.
- C. C. G. U. Molecular Operating Environment (MOE), 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2022.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03908k |
| This journal is © The Royal Society of Chemistry 2023 |