Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design, synthesis and antiproliferative screening of newly synthesized acrylate derivatives as potential anticancer agents†
Dalal Sulaiman Alshayaa,
Rana M. O. Tawakulb,
Islam Zaki *c,
Ali H. Abu Almaatyb,
Eman Fayadd and
Yasmin M. Abd El-Azizb
*c,
Ali H. Abu Almaatyb,
Eman Fayadd and
Yasmin M. Abd El-Azizb
aDepartment of Biology, College of Science, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh 11671, Saudi Arabia
bZoology Department, Faculty of Science, Port Said University, Port Said 42526, Egypt
cPharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Port Said University, Port Said 42526, Egypt. E-mail: Eslam.Zaki@pharm.psu.edu.eg
dDepartment of Biotechnology, Faculty of Sciences, Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia
First published on 4th August 2023
Abstract
A new series of acrylic acid and acrylate ester derivatives as modified analogs of tubulin polymerization inhibitors were designed and synthesized. The antiproliferative activity of the constructed molecules was investigated against MCF-7 breast carcinoma cells using CA-4 as positive molecule. Methyl acrylate ester 6e emerged as the most potent cytotoxic agent against MCF-7 cells, with an IC50 value of 2.57 ± 0.16 μM. Also, methyl acrylate ester molecule 6e showed good β-tubulin polymerization inhibition activity. Cellular cycle analysis showed that compound 6e can arrest MCF-7 cells at the G2/M phase. In addition, this compound produced a significant increase in apoptotic power as compared to control untreated MCF-7 cells. Furthermore, the effect of acrylate ester 6e on the gene expression levels of p53, Bax and Bcl-2 was investigated. This molecule increased the expression levels of both p53 and Bax, and decreased the gene expression level of Bcl-2 as compared to control untreated MCF-7 carcinoma cells.
1. Introduction
The importance of microtubule polymerization in cancer control has been emphasized.1,2 Microtubule formation, comprising α/β tubulin heterodimers, has a crucial role in cellular processes such as maintaining cellular shape and cellular division of eukaryotic cells and is therefore regarded as an outstanding molecular target for chemotherapy.3–5 Tubulin polymerization is required for microtubule formation.6 Tubulin assembly inhibitors interfere with the tubulin-microtubule polymerization–depolymerization process and are becoming an attractive strategy for the development of highly efficient anticancer drugs.7–9 Several natural products, such as colchicine, paclitaxel, and the vinca alkaloids, inhibit tubulin polymerization by binding to tubulin at their respective binding sites.10,11 In the case of tubulin polymerization inhibition at the colchicine binding site, combretastatin A-4 (CA-4) is a cis-stilbenoid molecule that elicited remarkable β-tubulin polymerization suppression activity acting at the colchicine site.12–14 CA-4 is the lead antimitotic molecule within the combretastatin family which exerts outstanding antitumor activity on various cancer cells due to β-tubulin polymerization suppression activity and anti-vascular effect.15,16 This is in addition to its potency against multidrug resistant cancer cell line.17 A property that makes analogs of CA-4 attractive for further development of more appropriate anticancer molecules which could be utilized in clinical use to overcome the drawbacks of conventional anticancer regimens, especially development of drug resistance.18–20Acrylate moiety is a common structural scaffold in the structure of numerous natural and synthetic small compounds displaying versatile biological interests.21,22 It has been reported to exhibit diverse biomedical activities, including anticancer activity.23 Their development has introduced new molecules acting as anticancer agents through different mechanisms such as β-tubulin inhibition and protein kinase inhibition.24,25 These findings suggested that acrylate pharmacophore is a promising molecular scaffold for further modification to develop more effective anticancer drug candidates.26
The two most frequently utilized methods in medicinal chemistry in the design of novel molecules are bioisosterism and molecular hybridization.27 The isosteric modification method is an efficient and often used technique that many drug candidates employ to enhance their pharmacodynamic behavior.28 It was of interest to further exploit the lead antimitotic agent, CA-4, with the possibility of producing effective drugs active against breast carcinoma cells.29 Encouraged by the above findings, we desired to design and synthesize a new series of acrylic acids and acrylate esters-containing scaffolds in the hope of getting more potent congeners (Fig. 1). The synthesized molecules were evaluated in vitro against MCF-7 breast cancer cells to assess their cytotoxic and β-tubulin polymerization inhibition activities (Fig. 2).
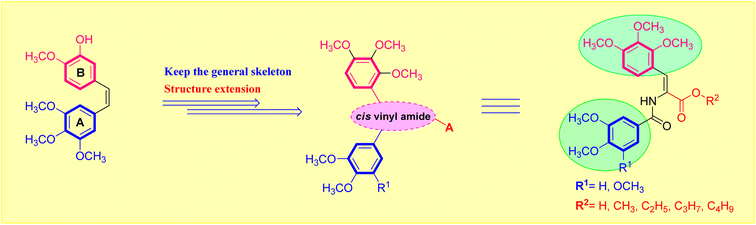 | ||
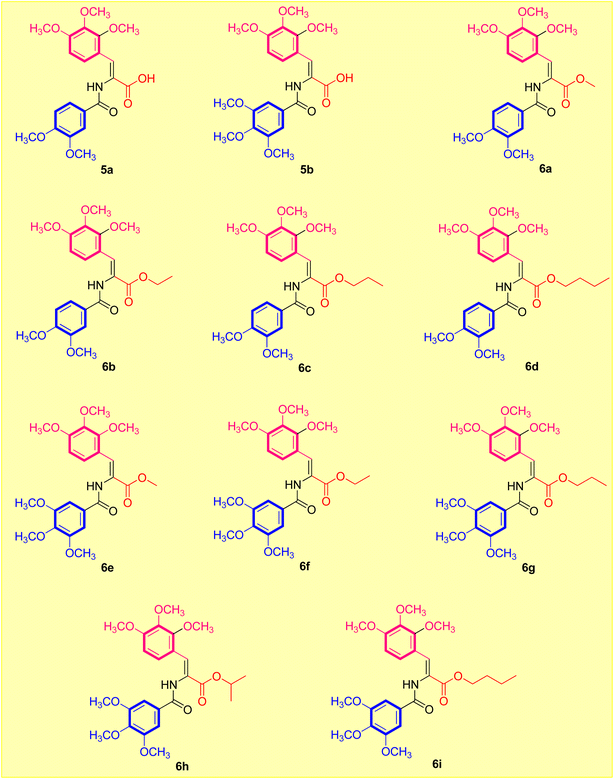
| Fig. 1 Designed strategy of the target acrylate derivatives 5a,b and 6a–i. A = carboxylic acid or ester group. | ||
2. Results and discussion
2.1. Chemistry
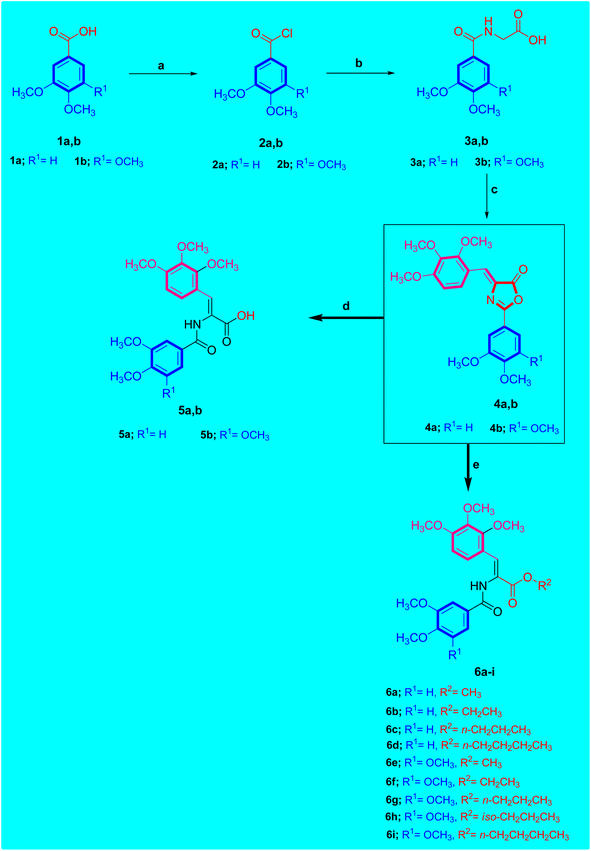
The targeted novel derivatives 5a,b and 6a–i were synthesized as depicted in Scheme 1. To prepare the key intermediates 4a,b, the Knoevenagel reaction was utilized. 2,3,4-Trimethoxybenzaldehyde was condensed with Respective hippuric acid 3a,b by refluxing in a solution of sodium acetate and acetic anhydride to give the desired (Z)-2-aryl-4-(2,3,4-trimethoxybenzylidene)oxazol-5(4H)-ones 4a,b.Consequently, treatment of compound 4a,b with aqueous K2CO3 for 3–4 h followed by neutralization in acidic solution afforded the corresponding carboxylic acid derivative 5a,b. The 1H-NMR spectrum of acrylic acid compound 5b showed two singlet signals at δ 12.65 and 9.75 ppm corresponding to OH proton of carboxylic acid moiety and NH proton of amide function, respectively. The aromatic protons of the 2,3,4-trimethoxybenzylidene ring of compound 5b resonated as two doublet signals at δ 7.44 and 6.85 ppm integrating for one proton for each signal, respectively. In addition, the aromatic protons of 3,4,5-trimethoxyphenyl ring of compound 5b resonated as singlet signal at δ 7.32 ppm integrating for two protons. The olefinic proton resonated as singlet peak at δ 7.57 ppm integrating for one proton. Furthermore, 13C-NMR spectrum of acrylic acid compound 5b showed five peaks at δ 61.92, 60.90, 60.57, 56.52 and 56.42 ppm corresponding to six methoxy groups of 2,3,4-trimethoxybenzylidene in addition to 3,4,5-trimethoxyphenyl moieties and two peaks at δ 162.66 and 163.77 ppm related to two carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of carboxylic acid and amide moieties, respectively.
O) of carboxylic acid and amide moieties, respectively.
Treating compound 4a,b with respective alcohol; namely methyl alcohol, ethyl alcohol, propyl alcohol, iso-propyl alcohol or n-butyl alcohol in the presence of triethyl amine (Et3N) yielded the desired ester derivative 6a–i. Concerning ester derivatives 6a–i, single peak corresponding to amide (NH) group was found in the 1H-NMR spectrum between δ 9.91 and 9.79 ppm. For example, consider compound 6g which was designated as (Z)-propyl 2-(3,4,5-trimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate C25H33NO9 showed the presence of two singlet peaks at 9.88 and 7.53 ppm corresponding to amidic NH and olefinic protons, respectively. In this respect, the 1H-NMR spectrum of compound 6g revealed three sets of protons resonated as triplet at δ 4.11, sextet at δ 1.65 and as triplet at δ 0.91 ppm integrating for 2H, 2H, and 3H, respectively which was attributed to propyl function (OCH2CH2CH3). 13C-NMR spectrum of compound 6g confirmed the carbon skeleton due to the presence of three signals at δ 66.64, 22.08 and 10.75 ppm corresponding to propyl group (OCH2CH2CH3), in addition to five peaks at δ 61.94, 60.94, 60.56, 56.52 and 56.44 ppm corresponding to six methoxy (OCH3) functions. Furthermore, 13C-NMR spectrum of compound 6g revealed the presence of two singlet peaks at δ 165.91 and 165.58 ppm due to carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of ester and amide moieties respectively.
O) of ester and amide moieties respectively.
2.2. Biology
| Comp. no. | IC50 value (μM) | |
|---|---|---|
| MCF-7 | MCF-10A | |
| a NT; not tested. | ||
| 5a | 9.31 ± 0.35 | NT |
| 5b | 5.12 ± 0.32 | NT |
| 6a | 6.74 ± 0.78 | NT |
| 6b | 17.08 ± 0.49 | NT |
| 6c | 20.26 ± 0.44 | NT |
| 6d | 42.08 ± 0.96 | NT |
| 6e | 2.57 ± 0.16 | 19.06 ± 0.31 |
| 6f | 3.26 ± 0.21 | NT |
| 6g | 11.34 ± 0.75 | NT |
| 6h | 7.08 ± 0.29 | NT |
| 6i | 33.02 ± 1.03 | NT |
| CA-4 | 1.25 ± 0.08 | 13.18 ± 0.19 |
3. Conclusions
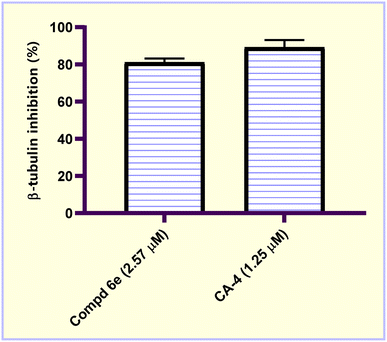
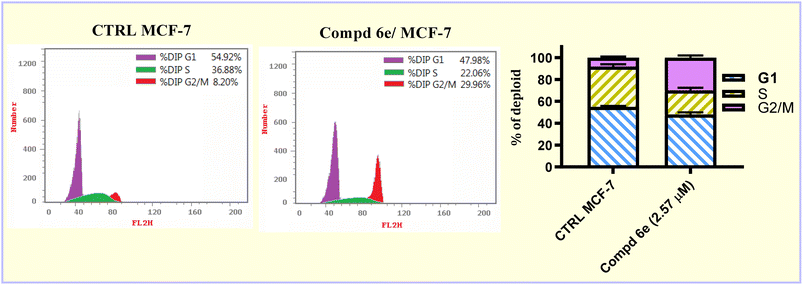
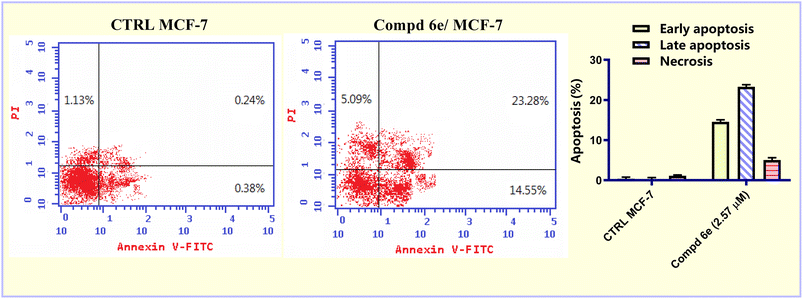
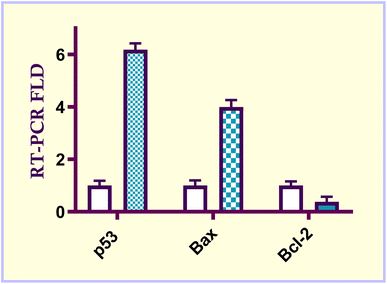
In the current study, a new series of acrylic acid and acrylate ester derivatives structurally related to CA-4 were designed and synthesized. The chemical structures of the constructed acrylate derivatives were substantiated on the basis of 1H-NMR, 13C-NMR spectroscopic studies and elemental analyses. The results revealed that methyl acrylate compound 6e (IC50 = 2.57 ± 0.16 μM) was the most potent against the MCF-7 breast carcinoma cell line. Methyl acrylate molecule 6e showed good β-tubulin polymerization inhibition activity (81.16% polymerization inhibition) relative to CA-4 as a positive control (82.82% polymerization inhibition). Compound 6e exerted an increase in the percentage of MCF-7 cells at the G2/M phase from 8.20% to 29.96% compared to the untreated control. In addition, methyl acrylate molecule 6e elicited a significant increase in the apoptotic power of MCF-7 cells. In the early stage from 0.38% to 14.55% and in the late stage from 0.24% to 23.28% compared to the untreated control. Moreover, compound 6e boosted the gene expression levels of both p53, Bax by 6.18- and 3.99-fold, respectively, relative to the untreated control. On the other hand, it caused a significant reduction in the Bcl-2 gene expression level by 0.38-fold relative to untreated MCF-7 breast carcinoma cells.4. Experimental
4.1. Synthesis
4.1.1.1 (Z)-2-(3,4-Dimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylic acid (5a). White powder (0.30 g, 48%), mp 201–203 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 12.60 (s, 1H, OH), 9.65 (s, 1H, NH), 7.65–7.59 (m, 1H, arom. CH), 7.55 (s, 2H, arom. CH and olefinic CH), 7.44 (d, J = 8.9 Hz, 1H, arom. CH), 7.07 (d, J = 8.5 Hz, 1H, arom. CH), 6.83 (d, J = 9.0 Hz, 1H, arom. CH), 3.85 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.76 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 167.07, 165.80, 154.93, 152.79, 152.15, 148.77, 142.00, 127.26, 126.82, 126.29, 124.44, 121.55, 120.64, 111.43, 111.42, 108.57, 61.92, 60.91, 56.40, 56.14, 56.04. Anal. calcd for C21H23NO8 (417.41): C, 60.43; H, 5.55; N, 3.36. Found: C, 60.32; H, 5.42; N, 3.44.
4.1.1.2 (Z)-2-(3,4,5-Trimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylic acid (5b). White powder (0.29 g, 43%), mp 217–219 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 12.65 (s, 1H, OH), 9.75 (s, 1H, NH), 7.57 (s, 1H, olefinic CH), 7.44 (d, J = 8.9 Hz, 1H, arom. CH), 7.32 (s, 2H, arom. CH), 6.85 (d, J = 9.0 Hz, 1H, arom. CH), 3.86 (s, 3H, OCH3), 3.85 (s, 6H, 2OCH3), 3.79 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.73 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 166.95, 165.64, 155.00, 153.12, 152.81, 142.01, 140.86, 129.10, 127.36, 126.60, 124.45, 120.53, 108.63, 105.73, 61.92, 60.90, 60.57, 56.52, 56.42. Anal. calcd for C22H25NO9 (447.44): C, 59.06; H, 5.63; N, 3.13. Found: C, 58.85; H, 5.87; N, 3.19.
4.1.2.1 (Z)-Methyl 2-(3,4-dimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate (6a). White powder (0.45 g, 69%), mp 177–179 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.81 (s, 1H, NH), 7.63 (d, J = 8.4 Hz, 1H, arom. CH), 7.55 (s, 1H, olefinic CH), 7.51 (s, 1H, arom. CH), 7.44 (d, J = 8.9 Hz, 1H, arom. CH), 7.08 (d, J = 8.4 Hz, 1H, arom. CH), 6.85 (d, J = 9.0 Hz, 1H), 3.86 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.73 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 166.22, 165.91, 155.15, 152.88, 152.29, 148.83, 142.02, 127.37, 126.06, 125.95, 124.53, 121.61, 120.31, 111.48, 111.41, 108.62, 61.96, 60.91, 56.44, 56.16, 56.05, 52.65. Anal. calcd for C22H25NO8 (431.44): C, 61.25; H, 5.84; N, 3.25. Found: C, 61.33; H, 6.04; N, 3.13.
4.1.2.2 (Z)-Ethyl 2-(3,4-dimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate (6b). White powder (0.41 g, 61%), mp 154–156 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.78 (s, 1H, NH), 7.68–7.60 (m, 1H, arom. CH), 7.55 (s, 1H, olefinic CH), 7.50 (s, 1H, arom. CH), 7.45 (d, J = 8.5 Hz, 1H, arom. CH), 7.08 (d, J = 7.7 Hz, 1H, arom. CH), 6.85 (d, J = 8.4 Hz, 1H, arom. CH), 4.19 (q, J = 1.6 Hz, 2H, OCH2CH3), 3.86 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 1.23 (t, J = 1.3 Hz, 3H, OCH2CH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 165.95, 165.66, 155.08, 152.86, 152.25, 148.82, 142.02, 127.06, 126.38, 126.09, 124.53, 121.57, 120.39, 111.48, 111.39, 108.60, 61.95 (OCH2CH3), 61.19, 60.91, 56.42, 56.15, 56.05, 14.62 (OCH2CH3). Anal. calcd for C23H27NO8 (445.46): C, 62.01; H, 6.11; N, 3.14. Found: C, 61.88; H, 6.16; N, 3.02.
4.1.2.3 (Z)-Propyl 2-(3,4-dimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate (6c). White powder (0.44 g, 64%), mp 119–121 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.79 (s, 1H, NH), 7.62 (d, J = 7.0 Hz, 1H, arom. CH), 7.54 (s, 1H, olefinic CH), 7.51 (s, 1H, arom. CH), 7.46 (d, J = 8.9 Hz, 1H, arom. CH), 7.08 (d, J = 8.5 Hz, 1H, arom. CH), 6.85 (d, J = 9.0 Hz, 1H, arom. CH), 4.10 (t, J = 6.4 Hz, 2H, OCH2CH2CH3), 3.85 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 1.62 (h, J = 7.0 Hz, 2H, OCH2CH2CH3), 0.90 (t, J = 7.4 Hz, 3H, OCH2CH2CH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 166.03, 165.73, 155.10, 152.87, 152.23, 148.80, 142.04, 127.09, 126.37, 126.12, 124.55, 121.56, 120.38, 111.48, 111.38, 108.64, 66.59 (OCH2CH2CH3), 61.94, 60.92, 56.43, 56.15, 56.05, 22.08 (OCH2CH2CH3), 10.76 (OCH2CH2CH3). Anal. calcd for C24H29NO8 (459.49): C, 62.73; H, 6.36; N, 3.05. Found: C, 62.86; H, 6.29; N, 2.93.
4.1.2.4 (Z)-Butyl 2-(3,4-dimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate (6d). White powder (0.37 g, 52%), mp 132–134 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.81 (s, 1H, NH), 7.66–7.60 (m, 1H, arom. CH), 7.57–7.53 (m, 1H, arom. CH), 7.51 (s, 1H, olefinic CH), 7.44 (d, J = 9.0 Hz, 1H, arom. CH), 7.08 (d, J = 8.5 Hz, 1H, arom. CH), 6.85 (d, J = 9.0 Hz, 1H, arom. CH), 4.31–3.91 (m, 2H, OCH2CH2CH2CH3), 3.86 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.73 (s, 2H, OCH2CH2CH2CH3), 1.62 (q, J = 6.9 Hz, 2H, OCH2CH2CH2CH3), 0.90 (t, J = 7.4 Hz, 3H, OCH2CH2CH2CH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 166.22, 165.91, 155.15, 152.88, 152.29, 148.83, 142.02, 127.36, 126.06, 125.96, 124.53, 121.61, 120.31, 111.48, 111.41, 108.62, 66.59 (OCH2CH2CH2CH3), 61.96, 60.91, 56.44, 56.16, 56.05, 52.65 (OCH2CH2CH2CH3), 22.09 (OCH2CH2CH2CH3), 10.76 (OCH2CH2CH2CH3). Anal. calcd for C25H31NO8 (473.52): C, 63.41; H, 6.60; N, 2.96. Found: C, 63.26; H, 6.68; N, 3.08.
4.1.2.5 (Z)-Methyl 2-(3,4,5-trimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate (6e). White powder (0.50 g, 72%), mp 133–135 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.91 (s, 1H, NH), 7.53 (s, 1H, olefinic CH), 7.44 (d, J = 8.9 Hz, 1H, arom. CH), 7.33 (s, 2H, arom. CH), 6.87 (d, J = 9.0 Hz, 1H, arom. CH), 3.86 (s, 3H, OCH3), 3.85 (s, 6H, 2OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.74 (s, 6H, 2OCH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 166.10, 165.76, 155.23, 153.17, 152.91, 142.02, 140.99, 128.75, 127.48, 125.82, 124.55, 120.18, 108.68, 105.77, 61.97, 60.91, 60.57, 56.53, 56.45, 52.70. Anal. calcd for C23H27NO9 (461.46): C, 59.86; H, 5.90; N, 3.04. Found: C, 59.98; H, 5.81; N, 2.93.
4.1.2.6 (Z)-Ethyl 2-(3,4,5-trimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate (6f). White powder (0.51 g, 71%), mp 127–129 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.87 (s, 1H, NH), 7.50 (s, 1H, olefinic CH), 7.44 (d, J = 8.9 Hz, 1H, arom. CH), 7.31 (s, 2H, arom. CH), 6.86 (d, J = 9.0 Hz, 1H, arom. CH), 4.19 (q, J = 7.1 Hz, 2H, OCH2CH3), 3.85 (s, 3H, OCH3), 3.84 (s, 6H, 2OCH3), 3.79 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 1.23 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 165.80, 165.53, 155.17, 153.16, 152.88, 142.02, 140.95, 128.91, 127.22, 126.13, 124.54, 120.25, 108.67, 105.73, 61.95 (OCH2CH3), 61.25, 60.91, 60.56, 56.53, 56.44, 14.64 (OCH2CH3). Anal. calcd for C24H29NO9 (475.49): C, 60.62; H, 6.15; N, 2.95. Found: C, 60.53; H, 6.22; N, 3.03.
4.1.2.7 (Z)-Propyl 2-(3,4,5-trimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate (6g). White powder (0.46 g, 63%), mp 111–113 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.88 (s, 1H, NH), 7.53 (s, 1H, olefinic CH), 7.46 (d, J = 8.9 Hz, 1H, arom. CH), 7.31 (s, 2H, arom. CH), 6.88 (d, J = 9.0 Hz, 1H, arom. CH), 4.11 (t, J = 6.4 Hz, 2H, OCH2CH2CH3), 3.86 (s, 3H, OCH3), 3.85 (s, 6H, 2OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 1.63 (h, J = 7.2 Hz, 2H, OCH2CH2CH3), 0.91 (t, J = 7.4 Hz, 3H, OCH2CH2CH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 165.91, 165.58, 155.18, 153.14, 152.89, 142.03, 140.91, 128.95, 127.26, 126.09, 124.56, 120.23, 108.69, 105.71, 66.64 (OCH2CH2CH3), 61.94, 60.94, 60.56, 56.52, 56.44, 22.08 (OCH2CH2CH3), 10.75 (OCH2CH2CH3). Anal. calcd for C25H31NO9 (489.51): C, 61.34; H, 6.38; N, 2.86. Found: C, 61.42; H, 6.29; N, 3.02.
4.1.2.8 (Z)-Isopropyl 2-(3,4,5-trimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate (6h). Pale yellow powder (0.44 g, 60%), mp 105–107 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.84 (s, 1H, NH), 7.47 (s, 1H, olefinic CH), 7.44 (d, J = 8.9 Hz, 1H, arom. CH), 7.30 (s, 2H, arom. CH), 6.87 (d, J = 9.0 Hz, 1H, arom. CH), 4.99 (dt, J = 12.5, 6.2 Hz, 1H, OCH(CH3)2), 3.85 (s, 3H, OCH3), 3.85 (s, 6H, 2OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 1.24 (d, J = 6.2 Hz, 6H, OCH(CH3)2). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 165.83, 165.05, 155.10, 153.15, 152.85, 142.02, 140.90, 129.04, 126.86, 126.48, 124.54, 120.31, 108.66, 105.69, 68.70 (OCH(CH3)2), 61.94, 60.91, 60.56, 56.53, 56.43, 22.11 (OCH(CH3)2). Anal. calcd for C25H31NO9 (489.51): C, 61.34; H, 6.38; N, 2.86. Found: C, 61.39; H, 6.44; N, 2.77.
4.1.2.9 Butyl 2-(3,4,5-trimethoxybenzamido)-3-(2,3,4-trimethoxyphenyl)acrylate (6i). White powder (0.39 g, 52%), mp 118–120 °C. 1H-NMR (400 MHz, DMSO-d6, δ ppm): 9.90 (d, J = 11.2 Hz, 1H, NH), 7.53 (d, J = 4.4 Hz, 1H, arom. CH), 7.44 (d, J = 8.8 Hz, 1H, arom. CH), 7.32 (d, J = 6.7 Hz, 2H, arom. CH), 6.87 (dd, J = 9.0, 1.8 Hz, 1H, arom. CH), 4.22–3.96 (m, 2H, OCH2CH2CH2CH3), 3.86 (s, 3H, OCH3), 3.85 (s, 6H, 2OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 3.74 (s, 2H, OCH2CH2CH2CH3), 1.64 (dt, J = 13.9, 7.0 Hz, 2H, OCH2CH2CH2CH3), 0.91 (t, J = 7.4 Hz, 3H, OCH2CH2CH2CH3). 13C-NMR (100 MHz, DMSO-d6, δ ppm): 166.10, 165.77, 155.23, 153.15, 152.91, 142.04, 141.00, 128.75, 127.48, 125.83, 124.55, 120.18, 108.68, 105.77, 66.65 (OCH2CH2CH2CH3), 61.96, 60.91, 60.57, 56.53, 56.45, 52.69 (OCH2CH2CH2CH3), 22.09 (OCH2CH2CH2CH3), 10.75 (OCH2CH2CH2CH3). Anal. calcd for C26H33NO9 (503.54): C, 62.02; H, 6.61; N, 2.78. Found: C, 61.89; H, 6.67; N, 2.91.
4.2. Biological study
Conflicts of interest
The authors declare no conflict of interest.References
- P. Dhyani, C. Quispe, E. Sharma, A. Bahukhandi, P. Sati, D. C. Attri, A. Szopa, J. Sharifi-Rad, A. O. Docea, I. Mardare, D. Calina and W. C. Cho, Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine, Cancer Cell Int., 2022, 22, 206–213 CrossRef CAS PubMed.
- O. Ebenezer, M. Shapi and J. A. Tuszynski, A Review of the Recent Developments of Molecular Hybrids Targeting Tubulin Polymerization, Int. J. Mol. Sci., 2022, 23, 4001–4013 CrossRef CAS PubMed.
- L. Huang, Y. Peng, X. Tao, X. Ding, R. Li, Y. Jiang and W. Zuo, Microtubule Organization Is Essential for Maintaining Cellular Morphology and Function, Oxid. Med. Cell. Longevity, 2022, 2022, 1623181–1623194 Search PubMed.
- A. K. E. Kiermaier, Dynamic Microtubule Arrays in Leukocytes and Their Role in Cell Migration and Immune Synapse Formation, Front. Cell Dev. Biol., 2021, 9, 1–18 Search PubMed.
- R. Vona, A. M. Mileo and P. Matarrese, Microtubule-Based Mitochondrial Dynamics as a Valuable Therapeutic Target in Cancer, Cancers, 2021, 13, 5812 CrossRef CAS PubMed.
- H. A. J. Saunders, D. M. Johnson-Schlitz, B. V. Jenkins, P. J. Volkert, S. Z. Yang and J. Wildonger, Acetylated α-tubulin K394 regulates microtubule stability to shape the growth of axon terminals, Curr. Biol., 2022, 32, 614–630.e5 CrossRef CAS PubMed.
- B. Karahalil, S. Yardım-Akaydin and S. Nacak Baytas, An overview of microtubule targeting agents for cancer therapy, Arh. Hig. Rada Toksikol., 2019, 70, 160–172 CrossRef CAS PubMed.
- W. Liu, M. He, Y. Li, Z. Peng and G. Wang, A review on synthetic chalcone derivatives as tubulin polymerisation inhibitors, J. Enzyme Inhib. Med. Chem., 2022, 37, 9–38 CrossRef CAS PubMed.
- V. Čermák, V. Dostál, M. Jelínek, L. Libusová, J. Kovář, D. Rösel and J. Brábek, Microtubule-targeting agents and their impact on cancer treatment, Eur. J. Cell Biol., 2020, 99, 151075 CrossRef PubMed.
- J. Yang, D. Song, B. Li, X. Gao, Y. Wang, X. Li, C. Bao, C. Wu, Y. Bao, S. Waxman, G. Chen and Y. Jing, Replacing the tropolonic methoxyl group of colchicine with methylamino increases tubulin binding affinity with improved therapeutic index and overcomes paclitaxel cross-resistance, Drug Resistance Updates, 2023, 68, 100951–100962 CrossRef CAS PubMed.
- X.-L. Sun, Y.-R. Xie, N. Zhang, C.-T. Zi, X.-J. Wang and J. Sheng, Recent advances on small-molecule tubulin inhibitors, Med. Res., 2021, 5, 200024–200028 Search PubMed.
- T. Al-Warhi, L. S. Alqahtani, G. Alsharif, M. Abualnaja, O. A. Abu Ali, S. H. Qahl, H. A. E. Althagafi, F. Alharthi, I. Jafri, F. G. Elsaid, A. A. Shati, S. Aloufi, E. Fayad, I. Zaki and M. M. Morcoss, Design, Synthesis, and Investigation of Cytotoxic Activity of cis-Vinylamide-Linked Combretastatin Analogues as Potential Anticancer Agents, Symmetry, 2022, 14, 2088 CrossRef CAS.
- Y. Lu, J. Chen, M. Xiao, W. Li and D. D. Miller, An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site, Pharm. Res., 2012, 29, 2943–2971 CrossRef CAS PubMed.
- S. N. Baytas, Recent Advances in Combretastatin A-4 Inspired Inhibitors of Tubulin Polymerization: An Update, Curr. Med. Chem., 2022, 29, 3557–3585 CrossRef CAS PubMed.
- X. Yang, B. Cheng, Y. Xiao, M. Xue, T. Liu, H. Cao and J. Chen, Discovery of novel CA-4 analogs as dual inhibitors of tubulin polymerization and PD-1/PD-L1 interaction for cancer treatment, Eur. J. Med. Chem., 2021, 213, 113058–113070 CrossRef CAS PubMed.
- X.-E. Jian, F. Yang, C.-S. Jiang, W.-W. You and P.-L. Zhao, Synthesis and biological evaluation of novel pyrazolo[3,4-b]pyridines as cis-restricted combretastatin A-4 analogues, Bioorg. Med. Chem. Lett., 2020, 30, 127025–127033 CrossRef CAS PubMed.
- M. A. Rahman, A. S. M. Saikat, M. S. Rahman, M. Islam, M. A. K. Parvez and B. Kim, Recent Update and Drug Target in Molecular and Pharmacological Insights into Autophagy Modulation in Cancer Treatment and Future Progress, Cells, 2023, 12, 458–479 CrossRef CAS PubMed.
- R. G. D. Khodyuk, R. Bai, E. Hamel, E. M. G. Lourenço, E. G. Barbosa, A. Beatriz, E. D. A. dos Santos and D. P. de Lima, Diaryl disulfides and thiosulfonates as combretastatin A-4 analogues: Synthesis, cytotoxicity and antitubulin activity, Bioorg. Chem., 2020, 101, 104017–104024 CrossRef CAS PubMed.
- Z. Huo, K. Liu, X. Zhang, Y. Liang and X. Sun, Discovery of pyrimidine-bridged CA-4 CBSIs for the treatment of cervical cancer in combination with cisplatin with significantly reduced nephrotoxicity, Eur. J. Med. Chem., 2022, 235, 114271 CrossRef CAS PubMed.
- Z.-H. Chen, R.-M. Xu, G.-H. Zheng, Y.-Z. Jin, Y. Li, X.-Y. Chen and Y.-S. Tian, Development of Combretastatin A-4 Analogues as Potential Anticancer Agents with Improved Aqueous Solubility, Molecules, 2023, 28, 1717 CrossRef CAS PubMed.
- E. Veignie, C. Ceballos, C. Len and C. Rafin, Design of New Antifungal Dithiocarbamic Esters Having Bio-Based Acrylate Moiety, ACS Omega, 2019, 4, 4779–4784 CrossRef CAS.
- G. Verma, G. Chashoo, A. Ali, M. F. Khan, W. Akhtar, I. Ali, M. Akhtar, M. M. Alam and M. Shaquiquzzaman, Synthesis of pyrazole acrylic acid based oxadiazole and amide derivatives as antimalarial and anticancer agents, Bioorg. Chem., 2018, 77, 106–124 CrossRef CAS PubMed.
- I. Zaki, S. A. Eid, M. S. Elghareb, A.-S. M. Abas, G. Mersal and F. Z. Mohammed, In Vitro Antitumor Evaluation of Acrylic Acid Derivatives Bearing Quinolinone Moiety as Novel Anticancer Agents, Anti-Cancer Agents Med. Chem., 2022, 22, 1634–1642 CrossRef CAS PubMed.
- K. O. Mohamed, I. Zaki, I. M. El-Deen and M. K. Abdelhameid, A new class of diamide scaffold: Design, synthesis and biological evaluation as potent antimitotic agents, tubulin polymerization inhibition and apoptosis inducing activity studies, Bioorg. Chem., 2019, 84, 399–409 CrossRef CAS PubMed.
- N. E. A. A. El-Sattar, S. E. S. A. El-Hddad, M. M. Ghobashy, A. A. Zaher and K. El-Adl, Nanogel-mediated drug delivery system for anticancer agent: pH stimuli responsive poly(ethylene glycol/acrylic acid) nanogel prepared by gamma irradiation, Bioorg. Chem., 2022, 127, 105972–105981 CrossRef CAS PubMed.
- M. Hao, Y. Guo, Z. Zhang, H. Zhou, Q. Gu and J. Xu, 6-acrylic phenethyl ester-2-pyranone derivative induces apoptosis and G2/M arrest by targeting GRP94 in colorectal cancer, Bioorg. Chem., 2022, 123, 105802–105809 CrossRef CAS PubMed.
- F. A. R. Barbosa, M. P. Rode, R. F. Santos Canto, A. H. Silva, T. B. Creczynski-Pasa and A. L. Braga, Antiproliferative Effect and Autophagy Inhibition of Dihydropyrimidinone-Cinnamic Acid Hybrids, ChemistrySelect, 2022, 7, e202200274 CrossRef CAS.
- B. Jayashree, P. S. Nikhil and S. Paul, Bioisosterism in Drug Discovery and Development-An Overview, Med. Chem., 2022, 18, 915–925 CrossRef CAS PubMed.
- I. Zaki, A. M. Y. Moustafa, B. Y. Beshay, R. E. Masoud, M. A. I. Elbastawesy, M. A. S. Abourehab and M. Y. Zakaria, Design and synthesis of new trimethoxylphenyl-linked combretastatin analogues loaded on diamond nanoparticles as a panel for ameliorated solubility and antiproliferative activity, J. Enzyme Inhib. Med. Chem., 2022, 37, 2679–2701 CrossRef CAS PubMed.
- O. W. V. Pongrakhananon, Post-translational modifications of tubulin: their role in cancers and the regulation of signaling molecules, Cancer Gene Ther., 2023, 30, 521–528 CrossRef PubMed.
- T. Solomon, M. Rajendran, T. Rostovtseva and L. Hool, How cytoskeletal proteins regulate mitochondrial energetics in cell physiology and diseases, Philos. Trans. R. Soc., B, 2022, 377, 20210324–20210332 CrossRef CAS PubMed.
- M. Hawash, Recent Advances of Tubulin Inhibitors Targeting the Colchicine Binding Site for Cancer Therapy, Biomolecules, 2022, 12, 1843 CrossRef CAS PubMed.
- M. Mustafa, Y. A Mostafa, A. E. Abd Elbaky, M. Mohamed, D. Abdelhamid, E. MN Abdelhafez and O. M. Aly, Combretastatin A-4 analogs: Past, present, and future directions, Octahedron Drug Res, 2022, 1, 55–64 Search PubMed.
- Q. Zhu, Y. A. An and P. E. Scherer, Mitochondrial regulation and white adipose tissue homeostasis, Trends Cell Biol., 2022, 32, 351–364 CrossRef CAS PubMed.
- H.-M. Zhou, J.-G. Zhang, X. Zhang and Q. Li, Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents, Signal Transduction Targeted Ther., 2021, 6, 62–74 CrossRef PubMed.
- F. J. Bock and S. W. G. Tait, Mitochondria as multifaceted regulators of cell death, Nat. Rev. Mol. Cell Biol., 2020, 21, 85–100 CrossRef CAS PubMed.
- K. Cosentino, V. Hertlein, A. Jenner, T. Dellmann, M. Gojkovic, A. Peña-Blanco, S. Dadsena, N. Wajngarten, J. S. H. Danial, J. V. Thevathasan, M. Mund, J. Ries and A. J. Garcia-Saez, The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation, Mol. Cell, 2022, 82, 933–949.e9 CrossRef CAS PubMed.
- I. Zaki, M. K. Abdelhameid, I. M. El-Deen, A. H. A. Abdel Wahab, A. M. Ashmawy and K. O. Mohamed, Design, synthesis and screening of 1,2,4-triazinone derivatives as potential antitumor agents with apoptosis inducing activity on MCF-7 breast cancer cell line, Eur. J. Med. Chem., 2018, 156, 563–579 CrossRef CAS PubMed.
- M. K. Abdelhameid, I. Zaki, M. R. Mohammed and K. O. Mohamed, Design, synthesis, and cytotoxic screening of novel azole derivatives on hepatocellular carcinoma (HepG2 Cells), Bioorg. Chem., 2020, 101, 103995 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03849a |
| This journal is © The Royal Society of Chemistry 2023 |