 Open Access Article
Open Access ArticlePreparation of Gd-doped AuNBP@mSiO2 nanocomposites for the MR imaging, drug delivery and chemo-photothermal synergistic killing of breast cancer cells†
Shiyi Tanga,
Ruohan Liab,
Tao Luoa,
Tianhao Huanga,
Xiaotong Lua,
Xinyao Wua,
Yulin Donga,
Changyu Wu a,
Kai Xu*ab and
Yong Wang
a,
Kai Xu*ab and
Yong Wang *ab
*ab
aSchool of Medical Imaging, Xuzhou Medical University, Xuzhou, Jiangsu 221004, China. E-mail: wangyong@xzhmu.edu.cn; xkpaper@163.com
bDepartment of Radiology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu 221002, China
First published on 10th August 2023
Abstract
Under near-infrared (NIR) light, gold nanobipyramids (AuNBPs) exhibit a high photothermal conversion rate and photothermal stability, making them ideal mediators for photothermal therapy (PTT). In this study, highly purified AuNBPs are prepared, followed by coating their surfaces with mesoporous silica (mSiO2). The obtained AuNBP@mSiO2 nanocomplex exhibits an ellipsoidal shape with a relatively large specific surface, pore diameter and pore volume. To achieve MRI guided chemo-photothermal therapy of breast cancer cells, the nanocomplex is further coupled with the MRI contrast agent Gd-DTTA and the chemotherapeutic drug doxorubicin (DOX). The results indicated that under NIR light irradiation, AuNBPs exhibited promising PTT effects, while the cumulative release rate of DOX was significantly enhanced to 81.40%. Moreover, the chemo-photothermal therapy approach effectively eradicated 4T1 breast cancer cells. This work successfully confirms that chemo-photothermal synergistic therapy is an effective tumor treatment strategy and demonstrates the potential application of AuNBP@mSiO2 as a nano-drug delivery platform. Additionally, it introduces new ideas for the integrated study of breast cancer diagnosis and treatment.
Introduction
In the past decades, cancers have been considered as a primary threat to human health.1 Currently, surgery, radiotherapy, chemotherapy and endocrine therapy are major clinical approaches used in the treatment of various cancers.2,3 However, the actual treatment efficacy is still limited due to the low specificity and severe side effects of these methods. For example, most drugs are poor in water solubility, high in metabolic turnover, and insufficient in targeting tumor cells in the postoperative adjuvant chemotherapy, resulting in systemic adverse reactions, damage to healthy normal tissues and the development of drug resistance in tumors.4,5 Fortunately, nanotechnology has proposed a novel methodology to address the problems faced by the traditional treatment approaches. At this point, versatile multifunctional materials have been developed for precise imaging, surgical navigation, radiosensitivity enhancement, drug delivery and phototherapy (PTT), and etc.6–8As an emerging therapeutic modality, PTT kills tumor cells by using a photothermal reagent, that converts light into heat when exposed to NIR. It shows great value in cancer treatment and controlled drug release due to its non-invasive nature, minimal damage to normal tissues and other advantages compared to traditional therapeutic methods.8,9 Currently, various NIR laser absorbing nanomaterials were proposed to improve the photothermal conversion efficiency of PTT, including carbon nanomaterials, semiconductor nanoparticles and gold nanostructures.10–12 Among these materials, gold-based nanomaterials have been widely investigated, such as nanorods, nanostars, nanocages and nanoshells, and etc.10,13–15 Compared with other shapes of Au nanomaterials, AuNBPs, a novel type of Au nanostructures with two sharp tips, have attracted great attentions owing to their stronger and narrower localized surface plasmon resonance (LSPR) and photostability.16 The strong NIR absorption capability makes the Au NBPs a promising mediator for PTT.17–19
Mesoporous silica nanoparticles (mSiO2) are considered ideal drug nanocarriers and have been approved by the FDA for clinical trials in cancer treatment and imaging applications. Due to their stable skeleton structure, easy-to-modify inner and outer surfaces, large surface area, adjustable pore volume and low physiological toxicity, mSiO2 can load drugs into its pores and achieve a controlled-release effect to improve drug durability.20,21 Moreover, the surface of mSiO2 is easily to be modified with functional groups, providing it with multifunctionality. Furthermore, combining chemotherapy and PTT can further improve the therapeutic effect, including overcoming the resistance of tumor cells and reducing the adverse effects of chemotherapy drugs. To achieve this, mSiO2 nanolayers can be coated on the surfaces of AuNBPs to obtain AuNBP@mSiO2 nanocomposites, which offer high PTT efficiency and drug loading rate.
Magnetic resonance imaging (MRI) plays a crucial role in the precise diagnosis of cancers among other diagnostic methods such as fluorescence imaging, computer tomography (CT), ultrasounds, positron emission tomography (PET), and etc. This is due to its high resolution of soft tissue, lack of radiation damage, and the ability to conduct multi-sequence, multi-parameter, and multi-azimuth imaging.22,23 However, currently used MRI contrast agents are mainly small molecule gadolinium chelates that lack sensitivity and often do not provide satisfactory image contrast enhancement in early disease stages. Previous studies have indicated that Gd3+-chelates loaded silica nanoparticles exhibit highly enhanced T1 MR relaxivity.24,25 Hence, it is desirable to modify mSiO2 with gadolinium chelates to enhance the MRI signal intensities.
In this study, we prepared mSiO2 coated AuNBP (AuNBP@mSiO2) with a core–shell structure. Then, we modified the surface with the Gd3+ chelated 3-aminopropyl(trimethoxysilyl)diethylenetriamine tetraacetic acid (Si-DTTA), a type of MRI contrast agent. Subsequently, we loaded the nanoprobes with DOX, resulting in the development of AuNBP@mSiO2-Gd-DTTA@DOX. This integration allowed for MRI and chemo-photothermal killing of breast cancer cells. The findings of work provide a relevant research basis for the diagnosis, evaluation, and treatment of cancers.
Experimental
Reagents and materials
Gold chloride trihydrate (HAuCl4·3H2O, 99.9%), sodium borohydride (NaBH4, 98%), trisodium citrate (99.5%), gadolinium chloride (GdCl3, 99%), hydrochloric acid (HCl, 36.0–38.0%), nitric acid (HNO3, 65.0–68.0%), silver nitrate (AgNO3, 99.8%), L-ascorbic acid (99.7%), sodium hydroxide (NaOH, 96.0%), cyclohexane (99.7%), tetraethyl orthosilicate and ethanol absolute (C2H6O, 99.7%) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Triethanolamine (TEA, AR), bromoacetic acid (AR), hexadecyltrimethylammonium bromide (CTAB, 99%) and cetyltrimethylammonium (CTAC, 97%) were bought from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). 3-(Trimethoxysilylpropyl)diethylene triamine was purchased from Gelest (Morrisville, US). Fetal bovine serum (FBS, Gibco), phosphate buffered saline (PBS), trypsin–EDTA and Dulbecco's modified Eagle medium (DMEM) were obtained from Beyotime Biotechnology (Nantong, China).Preparation of AuNBP@mSiO2-Gd-DTTA
The AuNBP growth solution was then prepared as follows: 200 mL of 0.1 M CTAB was added to a clean and dry flask, followed by sequentially adding 10 mL of 0.01 M HAuCl4, 2 mL of 0.01 M AgNO3, 4 mL of 1 M HCl and 1.6 mL of 0.1 M ascorbic acid with continuously stirring. Then 1.46 mL of Au nanocrystal seeds solution was added under gentle stirring for 10 s, and left in a water bath at 25 °C for 12 h. The purification of AuNBP is a crucial step in the process. To initiate the growth of silver nanorods from AuNBP, the AuNBP growth solution underwent centrifugation at 8000 rpm for 15 min. The resulting precipitate was then dispersed in 160 mL of 0.08 M CTAC solution, using sonication. Subsequently, 16 mL of 0.01 M AgNO3 solution and 8 mL of 0.1 mol L−1 ascorbic acid solution were added to the solution with stirring. The Ag nanorods were grown on the AuNBP surface by letting the solution stand at 65 °C for 4 h, after which the solution was naturally cooled to room temperature and centrifuged to remove the supernatant. The precipitate was further dispersed in 160 mL of 0.05 M CTAB solution and left to stand at room temperature for several days. The precipitates obtained in the reaction solution were AuNBP with Ag nanorods.
Next, the Ag nanorods on the AuNBP surface were etched away. The precipitated AuNBP with Ag nanorods was dispersed in 100 mL of deionized water, followed by the gentle addition of 2.5 mL of ammonia and 2 mL of hydrogen peroxide with stirring. The solution was allowed to stand while monitoring the etching process of Ag nanorods from the surface of AuNBP by UV-vis absorption. After complete etching, the solution was centrifuged and the precipitate was dispersed in 50 mL of CTAB solution with a concentration of 2 mM and stored at 4 °C for further use.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min and the precipitate was washed in a 1% NaCl methanol solution, followed by repeated washing with ethanol. This step was taken to remove the template molecules, i.e., CTAC, which act as pore-making agents in the encapsulation process of AuNBP with mesoporous mSiO2.
000 rpm for 15 min and the precipitate was washed in a 1% NaCl methanol solution, followed by repeated washing with ethanol. This step was taken to remove the template molecules, i.e., CTAC, which act as pore-making agents in the encapsulation process of AuNBP with mesoporous mSiO2.Characterization of the nanocomplexes
Transmission electron microscopy (TEM) images were obtained using the JEM-200 EX transmission electron microscope (JEOL, Japan). For investigating the elements distribution, STEM equipped with EDS (Talos F200X, FEI, USA) was employed. The UV-Vis absorbance measurements were recorded using a UH-4150 spectrophotometer (Hitachi, Japan). Surface charges were analyzed using a Zetasizer Nano ZS90 equipment (Malvern Instruments Ltd, Malvern, UK). The specific surface area and pore size distribution of AuNBP@mSiO2 were determined by performing BET tests using a TriStar II 3020 instrument (Micromeritics, USA). Furthermore, the gadolinium ion concentration in the AuNBP@mSiO2-Gd-DTTA solution was measured using ICP-MS (Agilent ICPOES730, USA).DOX loading and release
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm min−1 for 15 min, and this centrifugation step was repeated 3 times to effectively remove any free DOX from the solution.
000 rpm min−1 for 15 min, and this centrifugation step was repeated 3 times to effectively remove any free DOX from the solution.In vitro MRI performance
In vitro cytotoxicity of AuNBP@mSiO2-Gd-DTTA
The in vitro cytotoxicity of AuNBP@mSiO2-Gd-DTTA was assessed using the MTT assay. Both 4T1 breast cancer cells and 3T3 fibroblasts at logarithmic growth stage were seeded in a 96-well plate at a concentration of approximately 105 cells per mL. The cells were allowed grow until they covered the culture wells, as observed under an inverted microscope (IX51, Olympus, Japan). Subsequently, AuNBP@mSiO2-Gd-DTTA solutions at various concentrations, ranging from 0 to 0.8 mg mL−1, were added to the wells of the 96-well plate, resulting in a total volume in each well. After an additional 24 h of incubation, 100 μL of MTT solution was dispensed into each well, that had been rinsed twice with PBS, followed by an 4 h incubation. Next, the culture medium was removed, and 100 μL of DMSO was added to each well to dissolve the formazan crystals formed by viable cells. The optical density (OD) value of each well was measured at 490 nm using a microplate reader (BioTek Epoch, Service Card) to determine cell viability.Photothermal efficiency
Results and discussion
Morphology and structure of AuNBP, AuNBP@mSiO2 and AuNBP@mSiO2-Gd-DTTA
Products during the fabrication process of AuNBP were characterized using TEM and UV-vis spectroscope. As shown in Fig. 1A, the AuNBP growth solution consisted of the mixture of Au nanoparticles and AuNBP, which exhibited two main absorption peaks at 550 nm and 815 nm (Fig. 1D). The size of Au nanoparticles was similar to that of AuNBP, making it challenging to separate them using conventional centrifugation methods. To address this, silver was grown onto the surface of both Au nanoparticles and AuNBP, resulting in the formation of core–shell Au/Ag nanoparticles and Au/Ag nanorods, respectively, as evident from the TEM images (Fig. 1B) and the wide UV-Vis absorption band observed between 330–550 nm (Fig. 1D). The core–shell Au/Ag nanoparticles remained in the dispersive solution while the Au/Ag nanorods gradually precipitated when the mixture of core–shell Au/Ag nanoparticles and Au/Ag nanorods was left to stand for several days with dispersion in CTAB solution. After centrifugation, the Au/Ag nanorods were successfully separated, exhibiting a strong UV-Vis absorption peak at 380 nm, which corresponds to the transverse plasmon resonance absorption peak of Au/Ag nanorods, and a length of about 212 nm. As shown in Fig. 1C, it is evident that each AuNBP is enveloped in the middle of the Ag/Au nanorods.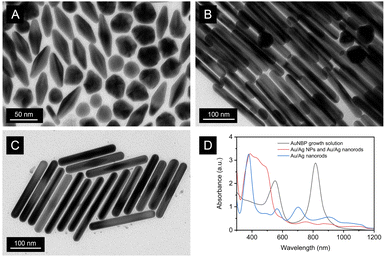 | ||
| Fig. 1 TEM images of AuNBP growth solution (A), mixture of core–shell Au/Ag nanoparticles and Au/Ag nanorods (B), and Au/Ag nanorod dispersion (C), and UV-Vis absorption spectra of them (D). | ||
Subsequently, pure AuNBP was obtained by etching the Au/Ag nanorods with ammonia and hydrogen peroxide to remove the silver from the two poles of AuNBP. As shown in Fig. 2A, the resulting AuNBP exhibited a bipyramid structure with a length of approximately 66.6 nm and a width of 24.9 nm. These findings provided evidence that well-dispersed and uniformly sized AuNBP were successfully fabricated. Furthermore, HRTEM (Fig. 2B) revealed the lattice fringes with a spacing of 0.22 nm, which corresponds to the {111} facets of face-centered-cubic (FCC) Au crystalline.29,30
To facilitate the loading of MR contrasts and chemotherapeutic agents, AuNBP was encapsulated with mesoporous silica. As shown in Fig. 2C, the resulting ellipsoid-like AuNBP@mSiO2 exhibited excellent dispersion and uniform size, with a mesoporous silica shell approximately 30 nm in thickness. After the loading of Gd-Si-DTTA, AuNBP@mSiO2-Gd-DTTA maintained its bipyramidal core and elliptical encapsulation structure, as observed in the TEM results (Fig. 2D). EDS mapping was utilized to analyze the elemental content and spatial distribution within AuNBP@mSiO2-Gd-DTTA (Fig. 2E–J). The element Au was predominantly concentrated at the center of AuNBP@mSiO2-Gd-DTTA, while Si, O, and Gd were distributed throughout the nanocomplex. As shown in Fig. 2E–J and Table S1,† the mass fraction of O, Si, Gd, and Au were 32.02, 15.42, 23.27, and 29.30 wt%, respectively.
The obtained results suggested that Gd-Si-DTTA has been successfully loaded onto the mesoporous AuNBP@mSiO2, and the encapsulated AuNBP@mSiO2-Gd-DTTA still retained sufficient pores, indicating promising potential for loading DOX, the chemotherapeutic agents.
Surface analysis of AuNBP@mSiO2-Gd-DTTA@DOX
Fig. 3A and B depict the N2 adsorption–desorption BET isotherm of AuNBP@mSiO2. The pore size distribution for AuNBP@mSiO2-Gd-DTTA ranged from 4 to 12 nm, with an average pore size of 9.5 nm. Additionally, the pore volume and specific surface area of AuNBP@mSiO2-Gd-DTTA were measured to be 2.4 cm3 g−1 and 961 m2 g−1, respectively. It is noteworthy that the average pore size, pore volume, and specific surface area achieved in this study are higher compared to most previous reports.31,32The surface charges of the nanocomplexes were assessed as shown in the zeta potential diagram (Fig. 3C). The zeta potentials of AuNBP, AuNBP@mSiO2 and AuNBP@mSiO2-Gd-DTTA were about 26.7, −24.6 and −8.88 mV, respectively. Notably, DOX possesses a positively charged protonatable amino group. Therefore, the interaction between DOX and AuNBP@mSiO2-Gd-DTTA resulted in the formation of AuNBP@mSiO2-Gd-DTTA@DOX, as evidenced by the change in Zeta potential to 11.43 mV, confirming the successful loading of DOX within the mesoporous silicon pores.
The difference observed in the UV-Vis spectra lies in the absorption characteristics of AuNBP@mSiO2-Gd-DTTA compared with AuNBP and AuNBP@mSiO2 (Fig. 3D). AuNBP@mSiO2-Gd-DTTA exhibited a prominent absorption peak at 817 nm, which was redshifted in comparison to AuNBP and AuNBP@mSiO2, attributed to the successful loading of Gd-DTTA onto the nanocomplex. The adsorption peak at 500 nm of AuNBP@mSiO2-Gd-DTTA@DOX was attributed to DOX. The redshift of adsorption peak at 834 nm for AuNBP@mSiO2-Gd-DTTA@DOX can be attributed to the specific chromophore and auxochrome structures of DOX. Thus, the UV-Vis serve as the evidence supporting the successful loading of DOX onto AuNBP@mSiO2-Gd-DTTA.
DOX loading and release with AuNBP@mSiO2-Gd-DTTA
The optimization of DOX loading with AuNBP@mSiO2-Gd-DTTA was depicted in Fig. 4A. The encapsulation efficiency and loading rate were found to reach their peak values of 74.03% and 56.30%, respectively, when the DOX concentration was set as 200 μg mL−1 and the pH level was maintained at 7.4. Based on these results, the condition of 200 μg mL−1 DOX concentration and pH 7.4 was selected as the optimal condition for DOX loading with AuNBP@mSiO2-Gd-DTTA for the subsequent experiments. | ||
| Fig. 4 (A) Encapsulation efficiency and loading rate of DOX for AuNBP@mSiO2-Gd-DTTA. (B) DOX release from AuNBP@mSiO2-Gd-DTTA@DOX under different pH values and NIR laser triggering conditions. | ||
Fig. 4B illustrates the release profile of DOX from AuNBP@mSiO2-Gd-DTTA@DOX under neutral and acidic conditions, both in the presence and absence of NIR laser irradiation. Notably, AuNBP@mSiO2-Gd-DTTA@DOX exhibited a slow release rate of 27.95% at pH 7.4 after 24 h. In contrast, a relatively higher release rate of 45.46% was observed at pH 5.3 during the same period. Moreover, the release rate was significantly increased when NIR laser irradiation was applied. Specifically, the total release of DOX from AuNBP@mSiO2-Gd-DTTA@DOX reached 81.40% at pH 5.3 after undergoing two NIR irradiation cycles at 4 and 12 h. These findings demonstrate that NIR irradiation under acidic conditions can expedite the release of DOX from AuNBP@mSiO2-Gd-DTTA@DOX, showcasing its potential for controlled drug release at specific sites, such as tumors.
T1 MR performance of AuNBP@mSiO2-Gd-DTTA
The concentration of gadolinium ions within AuNBP@mSiO2-Gd-DTTA measured by ICP-MS was 0.123 mM (corresponding to 19.34 wt%, the results was shown in Table S2†). The T1 MR performance of AuNBP@mSiO2-Gd-DTTA was showed in Fig. 5. With the increasing concentration of gadolinium ions, the T1 signal gradually increased, and the pseudo-colored T1 map changed from dark blue to red-yellow in sync (Fig. 6A). The T1 relaxation rate (r1) value of AuNBP@mSiO2-Gd-DTTA, calculated from the slope of linear fit of T1 relaxation time and concentration of gadolinium ions, was approximately 24.90 s−1 mM−1 (Fig. 6A), which was much higher than values reported in previously ref. 33 and 34. | ||
| Fig. 5 (A) T1WI and T1 map of AuNBP@mSiO2-Gd-DTTA in vitro. (B) Linear fit of T1 relaxation time with the concentration of gadolinium ions. (C) T1WI and T1 map of 4T1 cells incubated with AuNBP@mSiO2-Gd-DTTA of various concentrations. The bright-field image of cells in EP tube was shown in Fig. S1.† | ||
As shown in Fig. 5, MR imaging of 4T1 breast cancer cells was successfully achieved. The T1 imaging signals of 4T1 cells exhibited an increase with the rising concentration of AuNBP@mSiO2-Gd-DTTA, as evident from the pseudo-colored T1 map images. These findings suggest that AuNBP@mSiO2-Gd-DTTA can serve as a contrast agent, enhancing the MR imaging of cells.
Photothermal effect of AuNBP@mSiO2-Gd-DTTA
The photothermal effect of AuNBP@mSiO2-Gd-DTTA at various concentrations was investigated under irradiation with an 808 nm NIR laser at different power intensities and irradiation times. As depicted in Fig. 6A and B, the PBS solution without AuNBP@mSiO2-Gd-DTTA nanocomposites exhibited a minimal temperature increase of only 3.7 °C after 10 min of laser irradiation, indicating a negligible photothermal effect of PBS alone. However, upon NIR laser irradiation for 10 min, the temperature of AuNBP@mSiO2-Gd-DTTA nanocomposites increased significantly by 14.8, 21.8, 32.4, 38.1 and 41.8 °C at concentrations of 0.1, 0.2, 0.3, 0.6 and 1.0 mg L−1, respectively. This result indicated that AuNBP@mSiO2-Gd-DTTA nanocomposites exhibit excellent photothermal conversion efficiency. According to previous reports,35 cancer cells can be effectively killed through photothermal treatment when exposed to temperature above 45 °C for 5 min. Here, the temperature of AuNBP@mSiO2-Gd-DTTA solution rapidly increased above 45 °C at low concentrations and power intensities. Moreover, the temperature elevation was directly proportional to the power intensities of the NIR laser (Fig. 6C).Furthermore, the photothermal stability of AuNBP@mSiO2-Gd-DTTA was investigated through several irradiation cycles of NIR laser on/off switches (Fig. 6D). After four repeated heating/cooling steps, the peak temperatures, reached by AuNBP with the irradiation of the NIR laser remained consistent, with a relative standard deviation (RSD) of 0.2%. These results demonstrated the excellent stability of AuNBP as a photothermal therapeutic application.
In vitro chemo-photothermal synergistic therapy of AuNBP@mSiO2-Gd-DTTA@DOX
The cytotoxicity of AuNBP@mSiO2-Gd-DTTA was evaluated using the MTT assay on 4T1 breast cancer cells and 3T3, as shown in Fig. 7A. Both cell types displayed robust growth, with cell viabilities of 96.1% and 95.8%, respectively, after 24 h of treatment with a high concentration of the nanocomplex (0.3 mg mL−1). The cytotoxicity test revealed that AuNBP@mSiO2-Gd-DTTA exhibited excellent biocompatibility with both tumor and normal cells in vitro, and there were no statistically significant differences (P > 0.05).The in vitro chemo-photothermal synergistic therapeutic effects of AuNBP@mSiO2-Gd-DTTA@DOX (250 μg mL−1) on 4T1 cells were assessed using the MTT method. As shown in Fig. 7B, the cell survival rates for the control group and AuNBP@mSiO2-Gd-DTTA without NIR laser irradiation were both above 90%. Treatment with DOX (11.4 μg mL−1) with or without NIR laser irradiation, resulted in an about 40% cell killing efficiency. In contrast, AuNBP@mSiO2-Gd-DTTA and AuNBP@mSiO2-Gd-DTTA@DOX with NIR laser irradiation induced an additional 43% and 47% cell mortality rate in tumor cells, respectively, which was statistically significant (P < 0.05).
Meanwhile, for further investigate the chemo-photothermal synergistic therapeutic efficiency, various concentrations of AuNBP@mSiO2-Gd-DTTA and AuNBP@mSiO2-Gd-DTTA@DOX along with NIR laser irradiation were applied to treat 4T1 cells, as represented in Fig. 7C. The cell viabilities were highly concentration-dependent and could decrease to 17.1% for 0.3 mg mL−1 of AuNBP@mSiO2-Gd-DTTA@DOX with NIR laser irradiation. When the concentrations of the nanocomplex were higher than 0.3 mg mL−1, there were statistically significant differences (P < 0.05) between AuNBP@mSiO2-Gd-DTTA and AuNBP@mSiO2-Gd-DTTA@DOX.
Furthermore, the synergistic effect was also investigated using the live/dead staining method (Fig. 8). Cells in the control group (with/without NIR laser irradiation) and the AuNBP@mSiO2-Gd-DTTA group (without NIR laser irradiation) were mostly labeled with green, indicating that the cells remained in good condition with high viability. For the DOX only group, nearly half of the cells were labeled with green, suggesting the cell viability was above 60%, which is consistent with the above results. In contrast, for the AuNBP@mSiO2-Gd-DTTA with NIR laser irradiation group, only a fraction of cells were labeled with green, while all cells were labeled with red for the AuNBP@mSiO2-Gd-DTTA@DOX with NIR laser irradiation group. These results collectively demonstrated that AuNBP@mSiO2-Gd-DTTA@DOX exhibited a significant chemo-photothermal synergistic effect for tumor treatment.
 | ||
| Fig. 8 Live/dead cell viability images of 4T1 cells treated with different nanocomplex (250 mg mL−1) with/without NIR laser irradiation (scale bar 100 μm). | ||
Indeed, the results suggest that AuNBP@mSiO2-Gd-DTTA, loaded with DOX, combined with NIR laser irradiation, exerted a pronounced synergistic therapeutic effect on 4T1 cells, leading to enhanced therapeutic efficacy and potential applications in cancer therapy.
Conclusions
In this study, we designed a core–shell structured nanocomplex by using AuNBP with excellent photothermal effect as the core and mSiO2 as the shell. Further modification with Gd-Si-DTTA allowed us to obtain AuNBP@mSiO2-Gd-DTTA, which demonstrated good biocompatibility, excellent photothermal conversion efficiency, and a high T1 relaxation rate for MR imaging. The loading of DOX onto AuNBP@mSiO2-Gd-DTTA resulted in AuNBP@mSiO2-Gd-DTTA@DOX, which exhibited a high DOX loading rate, and demonstrated pH-sensitive and NIR laser-triggered DOX release. Remarkably, this nanocomplex showed a significant improvement in synergistic therapeutic effect by combining DOX-induced chemotherapy and AuNBP-induced photothermal therapy. This approach holds great promise for MR imaging guided cancer treatment with synergistic chemo-photothermal therapy in vivo.Author contributions
Shiyi Tang: investigation, data curation, writing – original draft preparation, funding acquisition. Ruohan Li: investigation, data curation. Tao Luo: investigation, data curation, validation. Tianhao Huang: investigation, data curation, funding acquisition. Xiaotong Lu: investigation, data curation, funding acquisition. Xinyao Wu: data curation, funding acquisition. Yulin Dong: data curation, funding acquisition. Changyu Wu: visualization, writing – original draft preparation, validation. Kai Xu: funding acquisition, project administration. Yong Wang: conceptualization, methodology, writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National College Student Innovation and Entrepreneurship Training Program (202110313035), Jiangsu Provincial College Student Innovation and Entrepreneurship Training Program (202110313035Z) and National Natural Science Foundation of China (82172005, 81901835).Notes and references
- R. L. Siegel, K. D. Miller, N. S. Wagle and A. Jemal, Ca-Cancer J. Clin., 2023, 73, 17–48 CrossRef PubMed.
- J. Zugazagoitia, C. Guedes, S. Ponce, I. Ferrer, S. Molina-Pinelo and L. Paz-Ares, Clin. Ther., 2016, 38, 1551–1566 CrossRef PubMed.
- A. G. Waks and E. P. Winer, JAMA, J. Am. Med. Assoc., 2019, 321, 288–300 CrossRef CAS.
- W. Zhou, T. Pan, H. Cui, Z. Zhao, P. K. Chu and X.-F. Yu, Angew. Chem., Int. Ed., 2019, 58, 769–774 CrossRef CAS.
- E. F. Blackley and S. Loi, Breast, 2019, 48(Suppl 1), S44–S48 CrossRef PubMed.
- V. Jain, H. Kumar, H. V. Anod, P. Chand, N. V. Gupta, S. Dey and S. S. Kesharwani, J. Controlled Release, 2020, 326, 628–647 CrossRef CAS.
- L. N. Borgheti-Cardoso, J. S. R. Viegas, A. V. P. Silvestrini, A. L. Caron, F. G. Praça, M. Kravicz and M. V. L. B. Bentley, Adv. Drug Delivery Rev., 2020, 153, 109–136 CrossRef CAS PubMed.
- B. Nasseri, E. Alizadeh, F. Bani, S. Davaran, A. Akbarzadeh, N. Rabiee, A. Bahadori, M. Ziaei, M. Bagherzadeh, M. R. Saeb, M. Mozafari and M. R. Hamblin, Appl. Phys. Rev., 2022, 9, 011317 CAS.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- X. Luo, B. Zhang, Y. Zhang, Z. Meng, P. Li, X. Jiang, J. Xiao, C. Lin and W. Su, Photodiagn. Photodyn. Ther., 2022, 39, 102988 CrossRef CAS PubMed.
- H. Shi, R. Yan, L. Wu, Y. Sun, S. Liu, Z. Zhou, J. He and D. Ye, Acta Biomater., 2018, 72, 256–265 CrossRef CAS PubMed.
- S. Feng, J. Lu, K. Wang, D. Di, Z. Shi, Q. Zhao and S. Wang, Chem. Eng. J., 2022, 435, 134886 CrossRef CAS.
- D. Kalinowska, I. Grabowska-Jadach, M. Liwinska, M. Drozd, M. Pietrzak, A. Dybko and Z. Brzozka, Biosens. Bioelectron., 2019, 126, 214–221 CrossRef CAS PubMed.
- S. Wang, Y. Song, K. Cao, L. Zhang, X. Fang, F. Chen, S. Feng and F. Yan, Acta Biomater., 2021, 134, 621–632 CrossRef CAS PubMed.
- R.-T. Li, M. Chen, Z.-C. Yang, Y.-J. Chen, N.-H. Huang, W.-H. Chen, J. Chen and J.-X. Chen, Nanoscale, 2022, 14, 9818–9831 RSC.
- C. Fang, G. Zhao, Y. Xiao, J. Zhao, Z. Zhang and B. Geng, Sci. Rep., 2016, 6, 36706 CrossRef CAS PubMed.
- T. H. Chow, N. Li, X. Bai, X. Zhuo, L. Shao and J. Wang, Acc. Chem. Res., 2019, 52, 2136–2146 CrossRef CAS PubMed.
- W. Rao, Q. Li, Y. Wang, T. Li and L. Wu, ACS Nano, 2015, 9, 2783–2791 CrossRef CAS PubMed.
- C. Li, E. Mei, C. Chen, Y. Li, B. Nugasur, L. Hou, X. Ding, M. Hu, Y. Zhang, Z. Su, J. Lin, Y. Yang, P. Huang and Z. Li, ACS Appl. Mater. Interfaces, 2020, 12, 12541–12548 CrossRef CAS PubMed.
- C. Mo, L. Lu, D. Liu and K. Wei, J. Nanobiotechnol., 2020, 18, 55 CrossRef CAS PubMed.
- X. Li, X. Zhang, Y. Zhao and L. Sun, J. Inorg. Biochem., 2020, 202, 110887 CrossRef CAS.
- A. R. Padhani, J. Barentsz, G. Villeirs, A. B. Rosenkrantz, D. J. Margolis, B. Turkbey, H. C. Thoeny, F. Cornud, M. A. Haider, K. J. Macura, C. M. Tempany, S. Verma and J. C. Weinreb, Radiology, 2019, 292, 464–474 CrossRef PubMed.
- T. Anani, S. Rahmati, N. Sultana and A. E. David, Theranostics, 2021, 11, 579–601 CrossRef CAS PubMed.
- W. J. Rieter, J. S. Kim, K. M. L. Taylor, H. An, W. Lin, T. Tarrant and W. Lin, Angew. Chem., Int. Ed., 2007, 46, 3680–3682 CrossRef CAS PubMed.
- K. M. L. Taylor, J. S. Kim, W. J. Rieter, H. An, W. Lin and W. Lin, J. Am. Chem. Soc., 2008, 130, 2154–2155 CrossRef CAS PubMed.
- Q. Li, X. Zhuo, S. Li, Q. Ruan, Q.-H. Xu and J. Wang, Adv. Opt. Mater., 2015, 3, 801–812 CrossRef CAS.
- J. Feng, L. Chen, Y. Xia, J. Xing, Z. Li, Q. Qian, Y. Wang, A. Wu, L. Zeng and Y. Zhou, ACS Biomater. Sci. Eng., 2017, 3, 608–618 CrossRef CAS PubMed.
- C. Xu, F. Chen, H. F. Valdovinos, D. Jiang, S. Goel, B. Yu, H. Sun, T. E. Barnhart, J. J. Moon and W. Cai, Biomaterials, 2018, 165, 56–65 CrossRef CAS PubMed.
- X. Kou, W. Ni, C.-K. Tsung, K. Chan, H.-Q. Lin, G. D. Stucky and J. Wang, Small, 2007, 3, 2103–2113 CrossRef CAS PubMed.
- A. Mehere and N. B. Chaure, Appl. Phys. A, 2020, 126, 662 CrossRef CAS.
- S. Shen, H. Tang, X. Zhang, J. Ren, Z. Pang, D. Wang, H. Gao, Y. Qian, X. Jiang and W. Yang, Biomaterials, 2013, 34, 3150–3158 CrossRef CAS PubMed.
- H. Tang, S. Shen, J. Guo, B. Chang, X. Jiang and W. Yang, J. Mater. Chem., 2012, 22, 16095–16103 RSC.
- Y. Wang, M. Li, T. Luo, M. Jiao, S. Jin, P. Dou, F. Zuo, C. Wu, C. Han, J. Li, K. Xu and S. Zheng, Mater. Sci. Eng., C, 2021, 127, 112190 CrossRef CAS.
- J. Li, J. You, Y. Dai, M. Shi, C. Han and K. Xu, Anal. Chem., 2014, 86, 11306–11311 CrossRef CAS.
- Z. Chen, Q. Wang, H. Wang, L. Zhang, G. Song, L. Song, J. Hu, H. Wang, J. Liu, M. Zhu and D. Zhao, Adv. Mater., 2013, 25, 2095–2100 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03753c |
| This journal is © The Royal Society of Chemistry 2023 |




