Open Access Article
Open Access ArticleRecent advances in bio-based polybenzoxazines as an interesting adhesive coating
Hafsah A. Klfout
 a,
Abdullah M. Asiri
a,
Abdullah M. Asiri
 ab,
Khalid A. Alamry
ab,
Khalid A. Alamry
 a and
Mahmoud A. Hussein
a and
Mahmoud A. Hussein
 *ac
*ac
aChemistry Department, Faculty of Science, King Abdulaziz University, P.O. Box 80203, Jeddah, 21589, Saudi Arabia. E-mail: Arabiamaabdo@kau.edu.sa; mahussein74@yahoo.com
bCenter of Excellence for Advanced Materials Research (CEAMR), King Abdulaziz University, Jeddah 21589, Saudi Arabia
cChemistry Department, Faculty of Science, Assiut University, Assiut 71516, Egypt
First published on 3rd July 2023
Abstract
Polybenzoxazine (PBz) is an excellent and highly intriguing resin for various sophisticated uses. Benzoxazines have piqued the curiosity of academics worldwide because of their peculiar properties. Nonetheless, most benzoxazine resin manufacturing and processing methods, notably bisphenol A-based benzoxazine, rely on petroleum resources. Because of the environmental consequences, bio-based benzoxazines are being researched as alternatives to petroleum-based benzoxazines. As a result of the environmental implications, bio-based benzoxazines are being developed to replace petroleum-based benzoxazines, and they are gaining traction. Bio-based polybenzoxazine, epoxy, and polysiloxane-based resins have piqued the interest of researchers in coatings, adhesives, and flame-retardant thermosets in recent years due to their anticorrosion, ecologically friendly, affordable, and low water absorption properties. As a result, numerous scientific studies and patents on polybenzoxazine continues to rise in polymer research. Based on its mechanical, thermal, and chemical characteristics, bio-based polybenzoxazine has several applications, including coatings (anticorrosion and antifouling), adhesives (highly crosslinked network, outstanding mechanical and thermal capabilities), and flame retardants (with the high charring capability). This review reports an overview of polybenzoxazine, highlighting the current advances and progress in synthesizing bio-based polybenzoxazine, their properties, and their use in coating applications.
Hafsah A. Klfout is a PhD candidate in the Chemistry Department at King Abdulaziz University (KAU), Jeddah, Saudi Arabia. She is working as a collaborating lecturer at KAU. She obtained her master's degree in organic synthesis from Eastern Illinois University (EIU) in the USA in 2015. She received the IGCD Student Travel Award from EIU for achieving excellence in research. She received Optional Practical Training (OPT) to work for one year at EIU, USA, Charleston, Illinois. She published several papers and conference (poster) for the Materials Research Society Spring Meeting and Exhibit in San Francisco, CA, and Grand Rapids, MI. Her PhD research focuses on the synthesis and development of bio-based benzoxazine-containing polymers for variable applications. |
1 Introduction
Metals are prevalent materials as they are used in many fields, including medicine, transportation, and architecture. However, the metal layer must be protected to stay long, which is often covered by paints. On the other perspectives, coatings can be applied for anticorrosion, anti-fogging, anti-icing, anti-biofilm attachment, or for other different applications to improve protection and decrease possible financial losses.1Corrosion is one of the enormous problems that causes numerous economic and environmental losses annually. It is defined as the destruction and deterioration of materials generated by the reaction between the materials and their surroundings environments.2 It harms the chemical, shipping, manufacturing, and architectural industries.2 Many methods have been widely applied and investigated to solve corrosion problems. Organic coatings are considered one of the most used methods to protect against corrosion, significantly prolonging metallic substrates' service life and reducing financial losses. Nevertheless, the anticorrosion efficiency of standard organic coatings is generally insufficient for use in highly aggressive environments.
Polymer coatings are an effective way to provide promising solutions to these problems. Polymer coatings give a physical barrier against corrosion. These physical barriers prevent the metal substrate from getting into contact with oxygen, water, and corrosive ions to protect it from decay.2 Coatings can be endowed with active corrosion protection (known as self-healing) to increase anti-corrosion effectiveness by introducing blocker-loaded containers (such as multilayer double hydroxides, halloysite nanotubes, and mesoporous SiO2 nanocontainers).3–6 Furthermore, anticorrosion can be inhibited and reduced using some green nanofiller to promote anticorrosion or by adopting some rules for anticorrosion inhibition.7
Among all these polymer coatings, polybenzoxazine (PBZ) is a promising thermosetting resin used in various domains, including many advanced coating applications.8 Researchers may be able to produce polymers with remarkable molecular structural flexibility due to the intricate molecular structure of benzoxazines and the available varieties of phenolic and amino derivatives.9 As an example, coating mild steel (MS) with cured polybenzoxazine (PBA-ddm) resulted in vital corrosion prevention and a reduction in the corrosion rate by two orders of magnitude compared to that with the untreated MS.7 At increased temperatures, various initiatives employed polybenzoxazine coatings for electronic, fire resistance, and ultra hydrophobic surfaces. In addition, silane-functionalized polybenzoxazine was applied to steel surfaces as an anticorrosion coating to broaden the uses of polybenzoxazine. Regarding the efficiency of lowering the corrosion current five times than that of a pure MS surface, this protective coating was earmarked significantly to reduce the rate of corrosion on steel.10,11
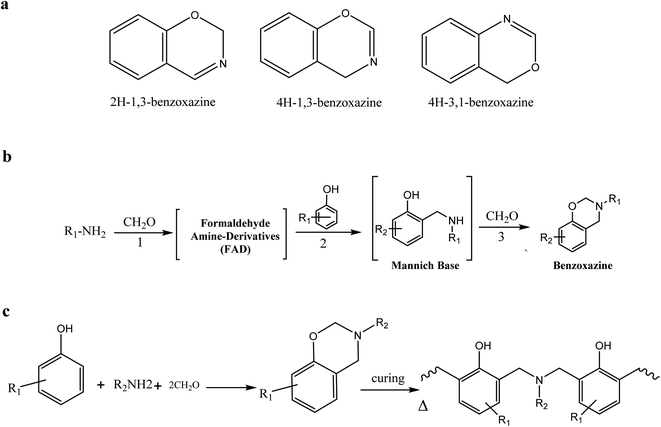
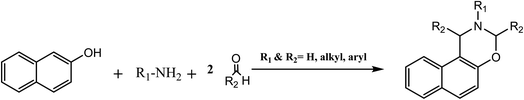
Benzoxazine (Bz) is a monomer in the phenolic resin class. This resin becomes a new thermosetting resin that contains heterocyclic oxygen and nitrogen chains. As the name implies, benzoxazine is a chemical compound consisting of a benzene ring connected to an oxazine ring. The oxazine ring is a six-membered heterocyclic molecule that contains an oxygen atom and one nitrogen atom as heteroatoms. The arrangements of benzoxazine structures are determined by the positions of these heteroatoms within the oxazine ring. According to the oxygen and nitrogen chain position, benzoxazine has six isomers; however, only the isomers that contain oxygen and nitrogen in 1,3 positions are active in forming poly-benzoxazine, as shown in Fig. 1a. This claim was made because of the simplicity of the way that it can be polymerized using ring-opening polymerization (ROP). Bz is typically produced in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio starting with a phenolic molecule, paraformaldehyde (PFA), and amine.12 The unique characteristic of benzoxazine monomer is that it has a simple preparation method from primary or secondary amines, phenol, or phenol derivatives, and paraformaldehyde (PFA) in one pot reaction called the Mannich condensation reaction which proceeds in three stages, as depicted in Fig. 1b. The first stage is the Mannich condensation reaction, in this step, PFA-amine compound derivatives (FAD) are formed by a reaction between amine and PFA. The reaction between PFA-amine compounds and phenol is then followed by the formation of the Mannich base (MB) in the second stage, which reacts in the final stage with the PFA to form the benzoxazine monomer.13 After the Bz monomer is prepared, it is cured under carefully designed conditions by cationic ring-opening polymerization (ROP), which gives polybenzoxazine PBz, as shown in Fig. 1c.
1 molar ratio starting with a phenolic molecule, paraformaldehyde (PFA), and amine.12 The unique characteristic of benzoxazine monomer is that it has a simple preparation method from primary or secondary amines, phenol, or phenol derivatives, and paraformaldehyde (PFA) in one pot reaction called the Mannich condensation reaction which proceeds in three stages, as depicted in Fig. 1b. The first stage is the Mannich condensation reaction, in this step, PFA-amine compound derivatives (FAD) are formed by a reaction between amine and PFA. The reaction between PFA-amine compounds and phenol is then followed by the formation of the Mannich base (MB) in the second stage, which reacts in the final stage with the PFA to form the benzoxazine monomer.13 After the Bz monomer is prepared, it is cured under carefully designed conditions by cationic ring-opening polymerization (ROP), which gives polybenzoxazine PBz, as shown in Fig. 1c.
The curing process does not need a strong acid catalyst. From these perspectives, Bz has attracted wide attention from academics worldwide because of its special characteristics. Nevertheless, because of the environmental implications, the invention of renewable resources benzoxazines, such as bio-based in place of petroleum-based benzoxazines, is gaining attention.14 Producing monomers and polymers using bio-based precursors is a current direction in polymer science research. This enthusiasm fueled a surge in demand for environmentally friendly materials and the superior chemical functionality of biologically produced compounds over traditional petroleum-derived building blocks.
Over the past few decades, most chemicals utilized for synthesizing benzoxazine monomers have been derived from non-biobased resources, specifically petroleum resources, which are not readily renewable. The need to explore alternative raw materials has arisen due to concerns regarding the depletion of fossil fuels, environmental and economic considerations. The extensive consumption of fossil fuels and consequent emission of carbon oxides has led to a crucial energy shortfall that hinders the attainment of sustainable human development. The traditional utilization of benzoxazine, which originates from petroleum-based origins, is linked to significant obstacles, such as the utilization of non-renewable raw materials, vulnerability to combustion, and insufficient toughness. Polybenzoxazine has been widely utilized in diverse fields. Notably, a significant percentage of polybenzoxazine that originates from bisphenol A is manufactured using non-renewable resources derived from petroleum. Additionally, Bisphenol A (BPA) has been found to cause permanent damage to the reproductive and immune systems of human beings. Various polymerizable petroleum-based groups, namely acetylene, allyl, nitrile, and maleimide, are incorporated into the benzoxazine structure to enhance its thermal and mechanical properties. The widespread usage of fossil fuels during this procedure has led to environmental concerns that require mitigation. Hence, substituting chemical materials derived from fossils with bio-based materials of natural origin is a crucial area of investigation.14,15
Natural and bioengineered metabolic pathways can give access to various monomers and synthetic precursors with different structures and features that are difficult to obtain using synthetic approaches alone. Furthermore, the invention of high-throughput genomic engineering technologies that merge automation, machine learning, and molecular biology may provide cost-effective and industrial-scale access to genetic diversity in biological systems. With worldwide environmental pollution on the rise, researchers have paid close attention by actively investigating more sustainable and high-performance materials, such as bio-phenols, as renewable raw materials in synthesizing benzoxazines to generate ecologically benign, sustainable, and renewable bio-based benzoxazines.15–17 It has been reported that the bio-based benzoxazine, which was prepared using a range of renewable phenolic and amine compounds for benzoxazines, such as stearyl amine, furfuryl amine, cardanol, eugenol, guaiacol, rosin, sesamol, catechol, and other compounds to generate ecologically benign material, which has attracted great interest.18 This distinctive source of prospective starting materials comprises compounds with phenolic groups currently being investigated as structural components of 1,3-benzoxazine monomers.19
Polybenzoxazine (PBz) is a polymer derived from the benzoxazine monomer. It is considered a type of high-performance thermoset phenolic resin with various properties that overcome some of the drawbacks of resole and novolac-type phenolics. These resins are considered promising thermoset resins due to their remarkable thermal stability and outstanding mechanical strength. It is considered a new type of low-surface-energy material with outstanding properties and effective UV shielding.20 It offers unique characteristics, for instance, lower water absorption along with outstanding stability in dimension owing to near-zero shrinkage upon curing, which overcomes the drawbacks of existing phenolic resins and offers extensive application possibilities in the sector of anti-corrosion.21 The absence of shrinkage while curing is regarded as a distinct characteristic of polymers based on benzoxazine. These additionally remain thermally resistant, as indicated by their significant glass transition, deterioration temperature, and char yield (28–66%). Although polybenzoxazine polymers are extremely strong and have several advantages, their use is still limited due to their brittle nature. Using pure polybenzoxazine-based polymers have a few disadvantages, including a high curing temperature, difficulties in processing, low crosslinking density, poor toughness, and brittleness; several solutions could be utilized to address these challenges and eliminate the associated drawbacks, such as modified monomers with enhanced functionality, new polymeric precursors, and combining with a high-performance polymer or fillers and fiber. Two ways were recently discovered to increase the material toughness of PBz to use their potential benefits and help increase the performance of polybenzoxazine. First, novel benzoxazines were synthesized by modifying their structure, secondly, generate polymer-inorganic filler composites to increase polybenzoxazine effectiveness.22–24 Due to PBZs' outstanding qualities, current research has focused on investigating several unconventional uses for polybenzoxazine, including electrochromic components, shape-memory substances, superhydrophobic substrates, intelligent protective coatings, self-healing infrastructure, porous Bz polymers, batteries and porous Bz polymer compounds.20,25
Lu et al. synthesized polybenzoxazine coating-based bisphenol A (BA) for specimens made of mild steel (MS). The PBz coating was discovered to lower the corrosion rate by two orders of magnitude of the bare steel substrate. Several studies have also focused on using nanofillers alloy PBz coatings with long-term anticorrosive performance in severe circumstances.1,26
J. Huang et al. have investigated and characterized amino-modified SiO2 nanoparticles (SiO2–NH2) and a cardanol-based poly-benzoxazine coating that combined to form a superhydrophobic surface (PC-a/SiO2) on raw MS by simply applying and heating to cure. The cardanol-based poly-benzoxazine superhydrophobic layers demonstrated remarkable heat resistance, chemical endurance, and self-cleaning properties. Furthermore, the long-term corrosion resistance of PC-a/SiO2 coatings was tested by immersing it in a 3.5 wt% NaCl aqueous solution. Furthermore, the anti-corrosion mechanism of the PC-a/SiO2 coating on mild steel was studied.27
Y. Deng et al. developed novel multi-functional double-layer coatings to limit microbe settling, adhesion, growth, and corrosion attack while taking environmental sustainability, low expenses, and high productivity into consideration. These high-performance poly-benzoxazine coatings were created using curcumin, 3-aminopropyltriethoxysilane, and PFA. Curcumin-based polybenzoxazine could be applied as a sub-layer preserving barrier that effectively prevented the attack of corrosion on the substrate metal in environments with high corrosion rates.28
A. Mahdy et al. studied a unique approach for creating water-repellent/UV-protective cotton textiles using in situ/thermal polymerization of BZs within a cotton matrix. Their study produced two Schiff bases of functionalized benzoxazines, indicated as Ph-para-BZ and Ph-ortho-BZ, as shown in Fig. 2. The functionalized benzoxazines were synthesized in one pot reaction using the Mannich condensation procedure of the produced Schiff base functionalized diphenol, CH2O, and para-toluidine in a toluene/ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent. The structure of poly-(Ph-ortho-BZ) and poly-(Ph-para-BZ) is highly crystalline/cross-linked, resulting in high Tg values of 210 and 195 °C. The endothermic peak for (Ph-p-Benzoxazine) was at 65 °C. This work could benefit the military textile sector by producing water-repellent/UV-protective fabrics by modifying cotton using PBs.20
1) mixed solvent. The structure of poly-(Ph-ortho-BZ) and poly-(Ph-para-BZ) is highly crystalline/cross-linked, resulting in high Tg values of 210 and 195 °C. The endothermic peak for (Ph-p-Benzoxazine) was at 65 °C. This work could benefit the military textile sector by producing water-repellent/UV-protective fabrics by modifying cotton using PBs.20
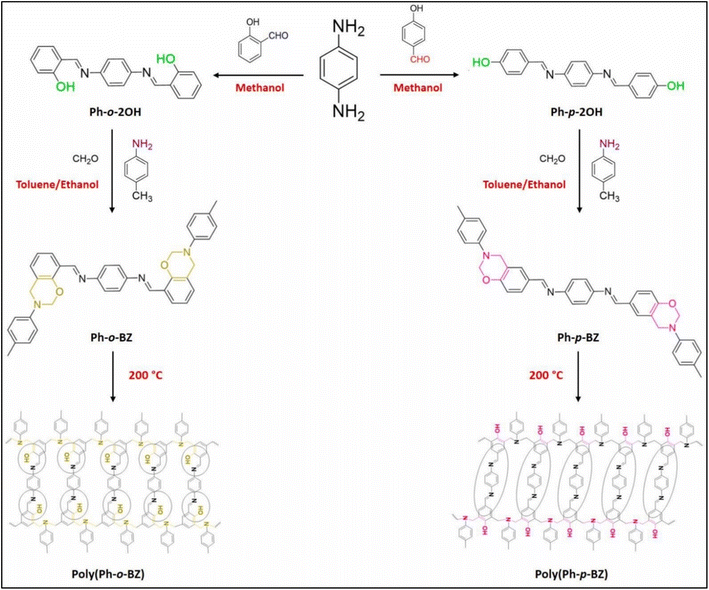 | ||
| Fig. 2 Synthesis of benzoxazine derivatives and their polymers. Reprinted with permission from ref. 20. Copyright 2023 Polymer Testing. | ||
Bio-based polybenzoxazine polymer composites (PCs) generated from natural sources have also resentful the scientific society's attention, particularly for antimicrobial applications.29–31 Because of their strong penetration power inside biofilms, PCs offer a viable alternative solution to this problem.32
2 Different synthetic methodologies for benzoxazine production
This section described several methods for synthesizing benzoxazine (and related naphthoxazine). Since numerous phenol and amine compounds are available for purchase as synthetic chemicals or as naturally occurring and sustainable resources, each synthesis method produces a significant number of benzoxazines. More sophisticated development approaches significantly increase the capacity to synthesize additional benzoxazines. Numerous appropriate chemistries can be used to produce polybenzoxazine prepolymers. Hydrosilylation, Diels–Alder, Mannich, specific coupling processes, poly-etherification, and poly-esterification, for example, have all been employed effectively.33 Still, Mannich condensation is the more commonly employed procedure because of its ease of preparation. Because of their considerable molecular structure flexibility, benzoxazines and polymers derived from them are the optimum choices for materials for customizing desired properties.34 Mannich-condensation synthesis is the most often used method for synthesizing benzoxazine, as described in the introductory section. This section will shed light on the different ways of synthesizing benzoxazine, which will be reviewed briefly.2.1 Synthesis of benzoxazine via ortho-hydroxybenzyl amine structure
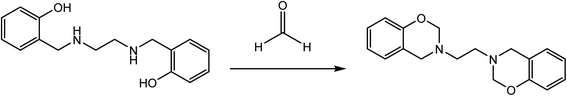
In 1963, Billman and Dorman synthesized difunctional benzoxazine from bis(ortho-hydroxy benzyl amino) ethane and formaldehyde, as illustrated in Scheme 1.35 Despite requiring several reaction steps, this N-substituted ortho-hydroxybenzyl amine has been widely used as a precursor in synthesizing benzoxazine. By reducing a Schiff base consisting of ortho-hydroxybenzaldehyde (salicylaldehyde) and a primary amine, this ortho-hydroxybenzyl amine was generated in high yield. This synthesis benefits from the functional group substitution on the oxazine ring. Ortho-hydroxybenzyl amine is a building block for flame retardant benzoxazines. It can be prepared when DOPO (9,10-dihydro-9-oxa-10-phosphenanthrene-10-oxide) attacks amino carbon in a nucleophilic way. The Betti reaction with phenol, primary amine, and benzaldehyde produces ortho-hydroxybenzyl amine, a precursor for phenyl group-substituted benzoxazine.36 Ring-closing ortho-hydroxybenzaldehyde with different aldehydes can substitute formaldehyde on the oxazine ring.37 Moreover, ortho-hydroxybenzaldehyde can close the oxazine ring with aldehydes and methylene bromide, similar to the one-pot Mannich condensation synthesis by adding substituents to ortho-hydroxybenzaldehyde and amine produces many benzoxazine compounds. Furthermore, this method enhances the production of benzoxazine due to its intramolecular nature. Since intramolecular cyclization permits the reaction conditions to vary, this technique improves the production of benzoxazine by minimizing side reactions induced by high temperatures. Using the one-pot Mannich condensation method frequently necessitates a high temperature for sealing the oxazine ring.342.2 Synthesis of polycyclic benzoxazine via N,O-acetal forming reaction
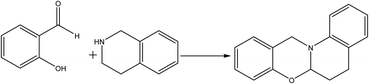
The reaction of cyclic secondary amines with salicylaldehyde or its derivatives led to a fused-ring benzoxazine through the N,O-acetal intermediate (Scheme 2), which differs from the typical Mannich condensation production. The unique structure and synthesis method allow for a wider variety of benzoxazine compounds, which are expected to exhibit exceptional properties as monomers and polymers.382.3 Synthesis of benzoxazine via cycloaddition
The alternative structure for benzoxazine is cycloaddition. In the presence of Brønsted acid at ambient temperature, for instance, the [3 + 3] cycloaddition of quinone monoimine and azomethine ylide produced a high yield of the benzoxazine structure (Scheme 3a).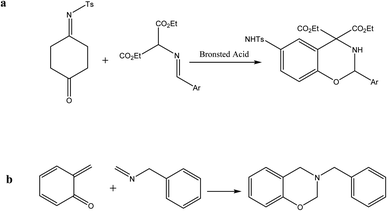 | ||
| Scheme 3 (a) [3 + 3] Cycloaddition of azomethine ylide with quinone monoamine. (b) The Diels–Alder reaction of ortho-quinone methide with benzyl methylene amine. | ||
Another cycloaddition benzoxazine synthesis technique is the [4 + 2] Diels–Alder reaction of a short-lived intermediate, ortho-quinone methide, with benzylmethyle-enamine (Scheme 3b). Due to the reactants' compatibility complexity, this cyclic addition approach is less common than others; however, it contributes to the diversity of the benzoxazine structure.34,39,40
2.4 Alternative synthesis reactions for benzoxazine
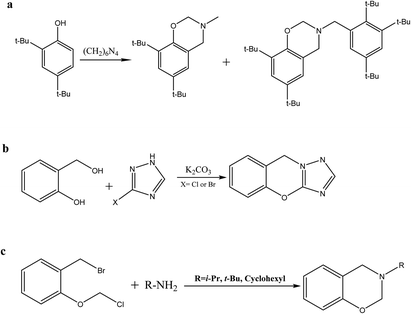
Recently, alternative synthetic benzoxazine techniques have emerged at the forefront of benzoxazine chemistry due to their widespread use in the pharmaceutical, biological, and polymer science industries. Benzoxazine can be synthesized using a variety of methods, such as the Duff reaction (Scheme 4a) with phenol and hexamethylenetetramine, the reaction of a halogenated triazole with salicyl alcohol (Scheme 4b), and the cyclization procedure of methyl-ortho-tolyl ether with a primary amine (Scheme 4c).34,41–432.5 Synthesis of benzoxazine with alternative energy sources
Different heating techniques were used in synthesizing benzoxazine, which was more efficient than the standard heating process. Ultrasound, microwave irradiation, and mechanical grinding are examples of alternate energy application methods. The most critical issue with the classic benzoxazine manufacturing process is the undesirable reaction that occurs during high-temperature heat. Nevertheless, these other methods may avoid the side reaction by reacting at ambient conditions or by shortening the duration of the reaction. As shown in Scheme 5a, linear aliphatic ether-linked benzoxazine can be produced at room temperature using ultrasonic irradiation. In contrast, the typical traditional heating setting was 65 °C for 5 hours.44 Response time can be substantially reduced by microwave irradiation. The provided reaction depicted in (Scheme 5b) demonstrated that the reaction could be abbreviated from several hours to a couple of days by heating for just a few minutes using a microwave method.34,44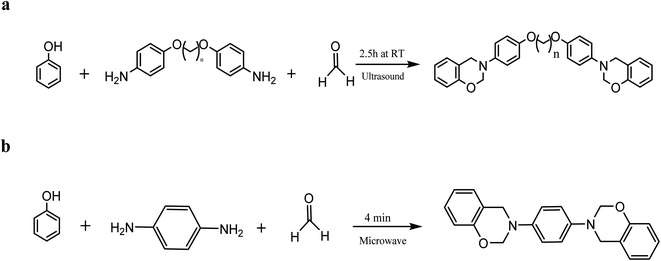 | ||
| Scheme 5 (a) Synthesis of benzoxazine via the ultrasound-assisted method. (b) Synthesis of benzoxazine using the microwave-assisted method. | ||
2.6 Synthesis of naphthoxazine and benzoxazine analogues
In this method, we focus on the synthesis of naphthoxazine. Comparable substances, such as naphthoxazine, have been investigated, whereas benzoxazines have demonstrated promising pharmacological and polymeric implications. The polymer naphthoxazine is predicted to have exceptional heat stability and a condensed polynuclear aromatic structure. Since the first naphthoxazine synthesis from 2-naphthol, methyl amine, and formaldehyde was reported in 1952 by Burke et al., as shown in Scheme 6, several naphthoxazines have been prepared in the same manner as benzoxazines. However, because of naphthol's strong reactivity, the reaction conditions for the naphthoxazine production are mild. Heat oligomerization byproducts are small throughout the process; therefore, naphthoxazine may be lowered. Economically advantageous synthesis methods have been introduced, including room-temperature and aqueous solvent reactions. As a result, naphthoxazine is more than just a benzoxazine analog with identical properties. It can also be synthesized under more reaction conditions compared to benzoxazines, resulting in various aromatic ring-fused oxazines with specific characteristics.34,45–483 Preparation and synthesis of bio-based benzoxazine monomers
Although there are many benzoxazine isomers (based on the location of the nitrogen and oxygen in the oxazine ring), as stated in the introduction, this section focuses mainly on synthesizing 1,3-benzoxazines because these compounds are typically employed for polymerization. There have been numerous published methods for synthesizing benzoxazine monomer structures. The Mannich-condensation approach, a one-pot reaction comprising an amine, an aldehyde, and PFA, is the most widely employed synthetic method.343.1 Synthesis of cardanol-based benzoxazine monomer
As is widely recognized, cardanol is a byproduct of cashew nutshell liquid. It constitutes a mono-hydroxy phenol with an extensive unsaturated fatty chain that processes well and is hydrophobic. In the present study, a cardanol-based polybenzoxazine coating and amino-modified SiO2 nanoparticles (SiO2–NH2) were coupled to produce a superhydrophobic surface (PC-a/SiO2) on bare MS by straightforward spraying and heat curing, as illustrated in Scheme 7.28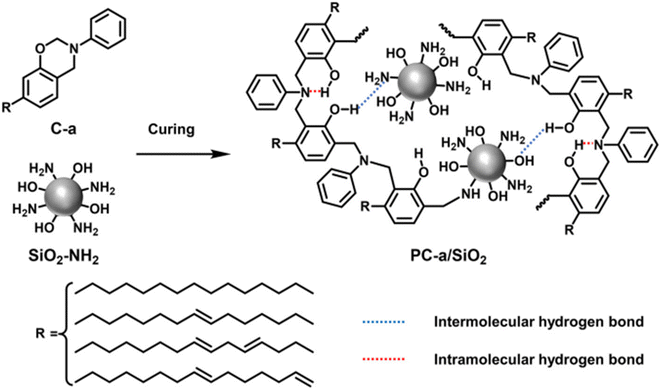 | ||
| Scheme 7 The fabrication of the PC-a/SiO2 coating. Reprinted with permission from ref. 28. Copyright 2019 Progress in Organic Coatings. | ||
A cardanol-based benzoxazine was created using the technique outlined in the literature.28,49,50 Using a three-necked procedure, chloroform was used in a three-necked flask for dissolving paraformaldehyde and aniline using the Mannich procedure. After dissolving cardanol in chloroform, it was introduced to the flask while stirring. The reaction mixture was then refluxed, and the crude products were extracted with aqueous NaOH and cleansed with deionized water until the pH reached neutral. The solvent was then extracted to form the final product.
Another study focused on synthesizing a bio-based benzoxazine monomer using cardanol and stearyl amine instead of amine. A polybenzoxazine double-layer coating with extremely high hydrophobic and active protection was applied to carbon steel. The monomer was synthesized following the scientific literature.18 In a three-necked flask, cardanol, paraformaldehyde, and octadecyl amine were heated at 80 °C for 0.5 hours, then at 125 °C for 2 hours. The crude products were dissolved in chloroform and rinsed with deionized water multiple times after the reaction. Scheme 8 depicts the production of a liquid bio-based benzoxazine monomer (C-s), following overnight lyophilization.
3.2 Synthesis of eugenol-based benzoxazine monomer
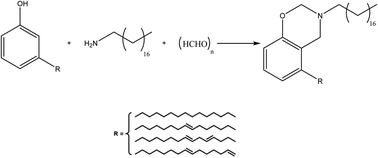
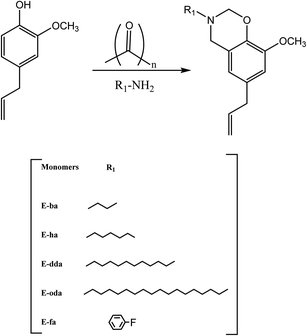
Eugenol is a bio-phenolic compound derived mainly from clove oil, nutmeg, cinnamon, basil, and bay leaf. The ortho and para locations in the molecular structure of eugenol are occupied by methoxy and allyl groups, respectively. Eugenol has lately been employed as a non-toxic feedstock chemical in the production of benzoxazines.51–54The monofunctional benzoxazine monomers are prepared, as shown in Scheme 9, from long-chain aliphatic amines and fluorine-substituted aromatic amines. Briefly, eugenol was dissolved in 1,4-dioxane, and amines such as butylamine [(ba)] or heptylamine [(ha)] or dodecyl amine [(dda)] or octadecyl amine [(oda)] or fluoroaniline [(fa)] were added, along with paraformaldehyde. The reaction temperature was then gradually increased to 110 °C, allowed to reflux, and continually stirred. Meanwhile, TLC was used to monitor the reaction's development.
3.3 Synthesis of curcumin-based benzoxazine monomers
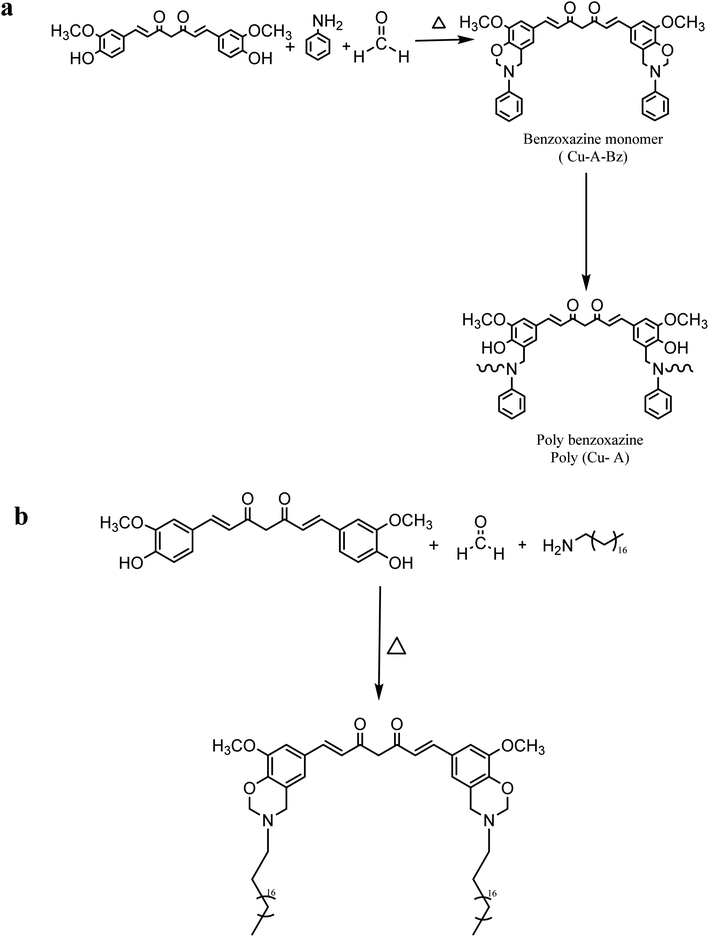
Curcumin is a yellow polyphenolic compound extracted from turmeric's rhizomes. 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione is another name for this compound. Curcumin has the potential for pharmacological activity and significant beneficial effects on patients due to its biochemical activities, which include antioxidant, antibacterial, antiviral, anti-inflammatory, and antiproliferative characteristics. In addition, the functional phenolic component of curcumin facilitates the production of high-performance polymers. To our knowledge, however, no previous research has utilized curcumin as a phenolic resource to synthesize benzoxazine. Curcumin is anticipated to increase resin durability, antibacterial and antiviral activity, physical and chemical resistance, and anticorrosion properties when used as a benzoxazine chemical feedstock.1,54–59As depicted in (Scheme 10a), the benzoxazine-based curcumin monomer CB was synthesized by Mannich condensation using renewable curcumin, 3-aminopropyltriethoxysilane, and paraformaldehyde to form the oxazine ring.1,60 As shown in (Scheme 10b), the monomer was then polymerized via ring-opening polymerization (ROP). The cationic constituent of zwitterionic intermediates was produced through the cleavage of O–CH2–N in the oxazine ring. The electrophilic substitution was used by adjacent benzoxazine monomers to attack the cationic amine moiety.
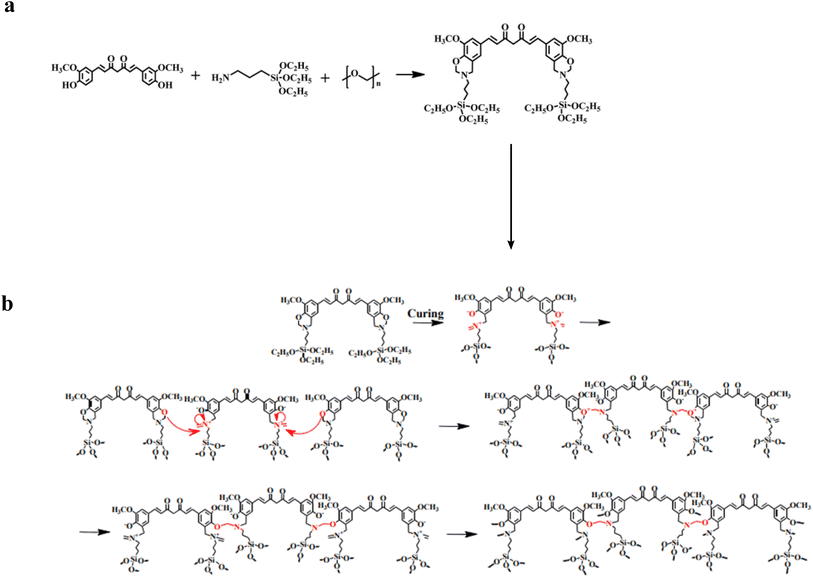 | ||
| Scheme 10 (a) Synthesis reaction of the curcumin-based benzoxazine monomer (CB). (b) Thermally-induced ring-opening polymerization of functional curcumin-based benzoxazine. | ||
Consequently, the structure was reconfigured to build a new type of Mannich bridge. In addition, hydrochloric acid promoted the formation of Si–OH groups from triethoxysilane groups of benzoxazine monomers. Along with the Mannich bridge section, the Si–OH groups would transform into Si–O–Si linkages, thereby strengthening the polybenzoxazine.
Curcumin, 3-aminopropyltriethoxysilane, and paraformaldehyde were used as basic materials in this recent study to create a bio-based, high-performance benzoxazine. Numerous loadings of hydrophilic poly(ethylene glycol) PEG molecules in the monomer were added to the produced benzoxazine to acquire the resin's intrinsic properties and enhance its anti-biofouling performance by modifying the resin's framework. In designing coatings from these chemical materials, the following characteristics are taken into consideration: curcumin functions intrinsically as a natural and renewable phenol, and two phenolic hydroxyl groups on a curcumin molecule can increase the crosslinking density of bioactive bis-benzoxazine resin. Further, 3-aminopropyltriethoxysilane (APTES) was used as a common and inexpensive amine source, and the Si–O–Si framework can enhance resin crosslinking after polymerization. As prepared, the coatings had minimal water absorption, antifouling, and corrosion resistance. Due to the advantageous effect of a particular PEG concentration, the resins exhibit extraordinary repellent properties even in the absence of biocides.
Utilizing the Mannich condensation method, a novel curcumin-based polybenzoxazine Cu-A-Bzo polymer composite (poly(Cu-A)PC) was created and evaluated as an antibiofilm and anticorrosive candidate against Candida albicans. Furthermore, their anticorrosive properties were investigated. PC exhibited potent antibiofilm activity against C. albicans DAY185, as evidenced by the morphological transformation of yeast through hyphae, and a greater dosage of PC demonstrated >90% anticorrosive effectiveness.32,61
As depicted in (Scheme 11a), PFA and DMSO were added to a round-bottomed flask equipped with a reflux condenser and stirred for a few minutes at 70 °C. After the PFA started dissolving, aniline and curcumin were introduced separately to the solution under stirring. Next, the mixture was heated to 120 °C and agitated for five hours. After cooling the mixture to room temperature, a 1 N NaOH solution was added to precipitate the benzoxazine monomer. Finally, the residue was washed with DI water before being filtered and desiccated at 70 °C.
Another type of benzoxazine-based curcumin with cellulose-grafted films was synthesized by Kiskan and Yagci as follows. Anhydrous chloroform was added to a round-bottomed flask containing curcumin, PFA, stearyl amine, and CaH2, as shown in (Scheme 11b). The reactants were stirred and refluxed at 120 °C for several hours. After the reaction was completed, the liquid was extracted from the solution after it was cooled and purified and then evaporated at low pressure. The remaining solution was saturated with an excess of methanol. After being dried in a vacuum for 24 hours, the residue was cleaned many times with deionized water.22
3.4 Synthesis of lignin-based benzoxazine
Lignin is the main source of phenolic chemicals utilized in developing bio-derived products.62 Lignin is a low-value fuel generally created during pulp and paper refining. Nevertheless, the development of biorefineries offers a new source of lignin manufacturing, along with its growing abundance and significant attempts to valorize, it pave the way for more cost-effective innovations.63,64Lignin-based benzoxazine was prepared and synthesized according to the methods described in the literature.65 As shown in Fig. 3, freshly prepared eLig, the amine precursor, and PFA were dissolved in a round-bottom flask containing a minimal amount of solvent THF. The mixture was stirred magnetically at room temperature in a nitrogen atmosphere. The temperature was raised to 80 °C and held constant for 24 hours. After cooling to room temperature, the crude product was concentrated under decreased pressure using a rotary evaporator. The extract was subsequently redissolved in diethyl ether and washed thoroughly with excess water and diethyl ether. eLig-Bz was recovered after a final dehydrating process at 50 °C for 24 hours at decreased pressure.
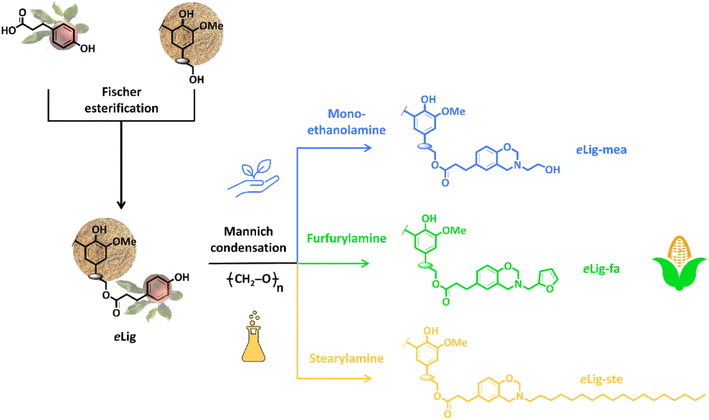 | ||
| Fig. 3 Illustration of synthesizing lignin-based benzoxazine precursors. Reprinted with permission from ref. 65. Copyright 2023, Chemical Engineering Journal. | ||
3.5 Synthesis of daidzein based benzoxazine monomer
In this study, as illustrated in Scheme 12, several kinds of bio-based main chain benzoxazine (MCBZ) polymers have been developed, starting with daidzein as the phenolic source and long-chain aliphatic primary amines, furfuryl amine, and poly(propylene glycol)bis(2-aminopropyl ether) (D400) as the amine source of information. Daidzein is a biobased MCBZ polymer with bacteriostatic properties against microorganisms. Liposoluble amines with long alkyl chains increase cell membrane permeability and synergize with anti-fouling groups to provide a more significant antifouling coating impact. Further, by composition optimization, the bio-based furfuryl amine and main chain type structure will produce excellent toughness and substrate adherence.66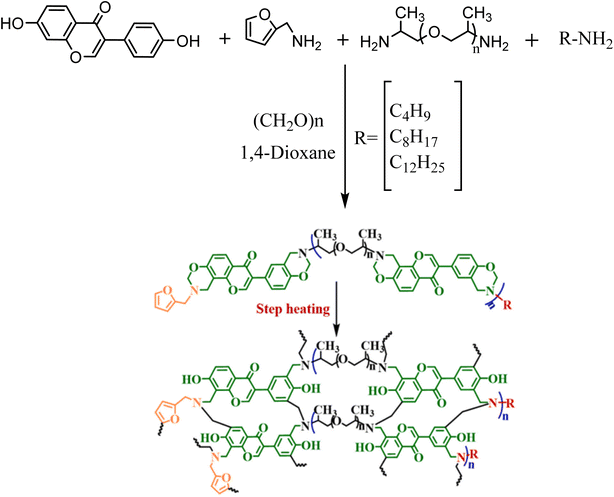 | ||
| Scheme 12 Synthesis of poly(4/8/12)-functionalized. Reproduced with permission from ref. 66. Copyright 2023, Progress in Organic Coatings. | ||
In a round-bottom flask, butylamine, furfuryl amine, and D400 were added and stirred at room temperature in a one-pot reaction via the Mannich condensation. During this time, PFA and 1,4-dioxane were introduced to the flask directly. Daidzein and 1,4-dioxane were added to the mixture while it was refluxed. The mixture was subsequently heated and maintained at that temperature before being washed using petroleum ether several times. Butylamine- and furfuryl amine-capped benzoxazine (4-fu-MCBZ) was synthesized after removing the remaining solvent in a vacuum oven. Using similar processes, octylamine- and furfuryl amine-capped benzoxazine (8-fu-MCBZ) and dodecyl amine- and furfuryl amine-capped benzoxazine (12-fu-MCBZ) were produced, but butylamine was substituted by octylamine and dodecyl amine.66
The properties of the three synthesized PBz products, poly(4/8/12)-functionalized-MCBZs, were investigated using dynamic mechanical analysis (DMA). The results exhibited that the three cured polymers had a comparable storage capacity of approximately 1900 MPa at 25 °C, which was lower than that of polybenzoxazine that was prepared from bisphenol A. However, they showed a high Tg which was above 200 °C. A comparable benzoxazine monomer was prepared from daidzein, and furfuryl amine previously reported a high Tg after curing, which was above 300 °C. In this investigation, the existence of many flexible chains was responsible for the decrease in Tg of the three cured resins. Furthermore, the furfuryl amine furan ring offered more crosslinking sites for benzoxazine's hydrogen bonding. The long alkyl chains boosted the density of the crosslinking in the system, resulting in a higher storage modulus and Tg.
The weight loss temperature (Td,10%) was higher than 300 °C, and the char yield was approximately 40% at 800 °C, as shown in Table 1, demonstrating that the three cured resins had high char yield and thermal stability, with the shorter alkyl chain giving the most heat stability. The resin's exceptional heat stability is due to the unique benzopyrone geometry of daidzein and the furan ring in the end group.66
| Sample | Td,10% (°C) | Char yield (%) | Tg (°C) | Flexural strain (%) | Hardness | Adhesion |
|---|---|---|---|---|---|---|
| Poly(4-functionalized-MCBZ) | 346.9 | 41.66 | 263.0 | 3.3 ± 1.2 | 2H | 4.79 MPa |
| Poly(8-functionalized-MCBZ) | 338.0 | 39.73 | 263.6 | 3.5 ± 0.8 | 3H | 4.97 MPa |
| Poly(12-functionalized-MCBZ) | 331.3 | 39.43 | 268.5 | 5.5 ± 1.4 | 4H | 5.02 MPa |
Polybenzoxazine derived from benzoxazine monomer is often brittle, making it unsuitable for durable uses.67 When the end-group chain length was extended from butyl to dodecyl, the cured resins' tensile stress and strain were improved. As a result, it was demonstrated that stretched alkyl chain length did not reduce resin strength. Consequently, poly(12-functionalized-MCBZ) gave the best mechanical and thermal performance, as shown in Table 1.
3.6 Synthesis of BA/PA-based benzoxazine monomer
This study used tannic acid (TA) to modify the synthesized polybenzoxazine, as illustrated in (Fig. 4a). As such, the choice of TA was based on its advantages. TA has been reported to have the ability to adhere to a wide range of substrates, including organic and inorganic materials, hydrophilic and hydrophobic materials, particles, and planar materials. TA obtained from biological sources has many catechol groups as shown in Fig. 4b and a lot of surface energy because of its hydrophilicity. It is worth mentioning that adherence is often high. TA and substrate interactions cause adhesive. In addition, it possesses hydrophobic regions; therefore, a hydrophobic interaction is expected. It has typically been used to modify the surface of polymeric membranes. Since polymeric membrane materials contain hydrophobic patches, hydrophobic interaction is expected to play an essential role in the successful surface modification. It has abundant phenolic hydroxyl groups, which can interact with materials via hydrogen bonding. Due to its many hydroxyl groups, it could form hydrogen bonds with proteins and other biomolecules. The usage of TA to increase the binding strength of PBz coatings is highly possible. In this study, as inspired by mussels, applying TA to increase the binding strength of PBZ coatings is extremely practical. It may be utilized to improve the mechanical characteristics of natural and synthetic hydrogels and polymers by acting as a natural crosslinking agent. In addition, it has been utilized to create thin film coatings and drug-delivery nanoparticles. TA has applications in medicines, biomaterials, and drug delivery techniques. To the best of our knowledge, just a minimal amount of study on TA-modified PBZ coating has been documented in the context of the bionic approach.68,69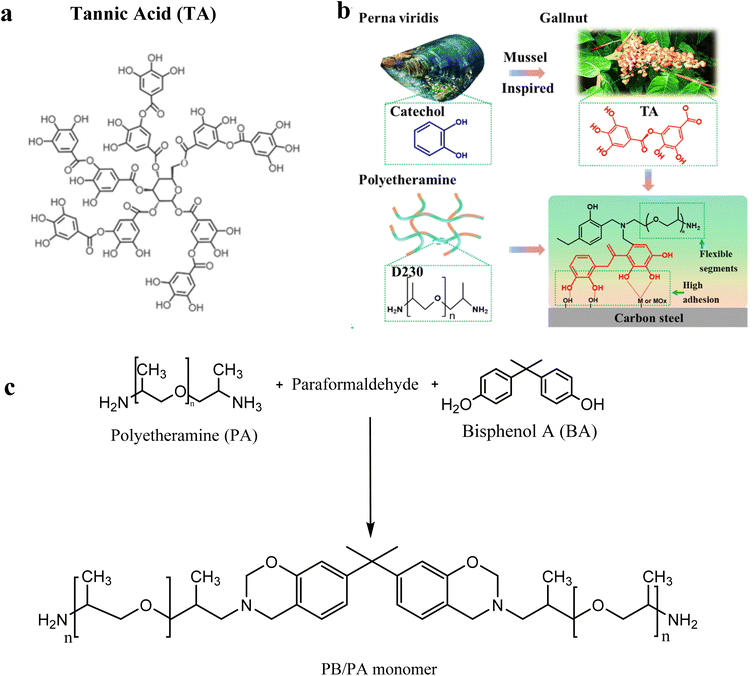 | ||
| Fig. 4 (a) The chemical structure of tannic acid (TA). (b) Bioinspired architecture with excellent adhesion and flexible PBz-TA coatings. Reprinted with permission from ref. 70. Copyright 2023, Progress in Organic Coatings. (c) Synthesis of the PB/PA monomer. | ||
The approach for synthesizing BA/PA-based benzoxazine monomers described in the literature was followed,70 as shown in (Fig. 4c). In a three-necked flask with nitrogen flow, paraformaldehyde, and 1 M NaOH were added to chloroform with minimal stirring. The fluid was then progressively heated to 65 °C, and PA was dissolved in it while vigorously stirring. Following that, at 85 °C, bisphenol A (BA) was added to the mixture and refluxed to form a homogenous solution. Following that, the mixture was vacuum purified using column chromatography to obtain the resulting viscous liquid as BA/PA monomers (Table 2).
| Phenol | Amine | Aldehyde | Modification | Advantages | Ref. |
|---|---|---|---|---|---|
| Cardanol | Aniline | PFA | SiO2–NH2 nanoparticles | The PC-a/SiO2 coating blocks entry of corrosive fluids | 28 |
| Eugenol | ba, ha, dda, oda, fa | PFA | Coated cotton fabrics | It provides the best oil–water separation efficiency (98%), improved cycle repeatability and anti-icing properties | 15 |
| Curcumin | 3-Aminopropyltriethoxysilane | PFA | Poly(ethylene glycol) (PEG) | Bio-adhesive and antifouling agent are used to improve surface resistance | 1 |
| Poly-dopamine (PDA) | |||||
| BA | D230 (PA) | PFA | Tannic acid (TA) | Improves PBz coatings' mechanical characteristics and adhesion strength | 70 |
| Daidzein | Furfurylamine | PFA | (D400) n-butylamine, n-octylamine, dodecylamine | Excellent curing activity, processability, and reduced curing temperature | 66 |
| Curcumin | Aniline | PFA | PC | Inhibited the growth of C. albicans biofilms and served as a suitable corrosion inhibitor | 32 |
| Lignin | Stearylamine | PFA | Stearylamine | Improves thermoset thermal properties and Tg, and fire parcels, due to increased hydrophobicity and coating capability | 65 |
| Furfuryl amine | Furfurylamine | ||||
| Mono-éthanolamine | Mono- éthanolamine | ||||
| Curcumin | Stearyl-amine | PFA | Amino Cellulose (AC) | Reduces initial curing temperature, improving mechanical properties | 22 |
4 Application of bio-based benzoxazine as coatings
Recently, researchers in flame retardant thermosets, adhesives, and coatings have shown interest in bio-based polybenzoxazine due to its anti-corrosion, low-water absorption properties, and environmentally friendly cost-effectiveness.8,9 Benzoxazine-based materials, as previously indicated, can respond to stimuli and behave unpredictably. Furthermore, polybenzoxazine can adhere to metal surfaces, making them suitable metal coating materials for resolving corrosion issues, as most metals corrode, causing problems in terms of cost and safety. Furthermore, polybenzoxazine contains phenolic –OH groups that can react with metals, making its surface adhesion stronger than that of other resins.33 As a result, several benzoxazine resins were utilized to protect steel items against corrosion. Using silane-functional polybenzoxazine as a corrosion protection coating on mild steel (MS) surfaces, for instance, the reduced corrosive current by more than four times when compared to bare MS. Further research found that BA-a-derived polybenzoxazine coatings achieved a corrosion efficiency of 99.9% with a corrosion rate of 5.07 × 10−5 mm per year.10,71Y. Deng et al. reported in their work that curcumin-based polybenzoxazine resin was shown to be an efficient barrier layer capable of reducing substrate metal corrosion and greatly resisting fouling attachment. Furthermore, it was discovered that increasing the resin's PEG content could enhance the coating's antifouling efficacy. Molecular dynamics simulations revealed that the density of PEG molecules in the polybenzoxazine framework could substantially affect the hydration layer and energy interactions.1
In this study, various innovative multifunctional double-layer coatings were developed that could inhibit the settling, adhesion, and development of microorganisms and corrosion assaults and fulfill sustainability, cost-effectiveness, and outstanding performance criteria. Curcumin, 3-aminopropyltriethoxysilane, and paraformaldehyde were employed to create excellent-performance polybenzoxazine coatings, and the curcumin-based polybenzoxazine was capable of being applied as a sub-layer protecting barrier that effectively prevented corrosion attack on the substrate metal in environments with high corrosion rates. Even after months of exposure to various corrosive conditions, the coating demonstrated exceptional corrosion resistance over a prolonged period. Due to its robust hydration layer and high interaction energy at the molecular level, this coating could potentially be used as an up-layer through cooperative interaction between curcumin and the PEG chain in the polybenzoxazine resin framework, resulting in an exceptional anti-fouling capability against the adsorption of proteins or the attachment of bacteria and microalgae. This double-layer coating system is anticipated to enable numerous uses in the marine sector and other relevant disciplines.1 Overall, polyethylene glycol (PEG) is a functional polymer and modifying agent used to increase surface resistance to antimicrobial adhesion and settling. By forming a hydration barrier between PEG chains and water molecules or through the “steric repulsion” generated by the chains, PEG and PEG-based copolymers have been widely recognized as having anti-biofouling effects against proteins, bacteria, cells, and various other organisms. PDA-PEG coatings were developed using PDA and PEG as bio-adhesive and antifouling agents to drastically reduce biofouling attachment on diverse substrates.1
The cardanol-based polybenzoxazine superhydrophobic coating (PC-a/SiO2) with a static WCA of 162.8 2.9° was effectively created by spraying amino-modified silica nanoparticles into the cardanol-based polybenzoxazine and then thermally curing the coating. Due to the incorporation of amino-modified silica nanoparticles into cardanol-based polybenzoxazine, the superhydrophobic PC-a/SiO2 coating exhibited excellent self-cleaning properties and retained its superhydrophobic feature. The superhydrophobic PC-a/SiO2 coating is self-cleaning and retained its superhydrophobic characteristic after 1 hour of heat treatment at 250 °C or 10 days of immersion in a 3.5 wt% NaCl aqueous solution. In addition, electrochemical experiments demonstrated that the PC-a/SiO2 coating had superior corrosion resistance compared to the PC-a coating. In comparison to the PC-a coating, the superior superhydrophobicity of the PC-a/SiO2 coating and the incorporation of SiO2–NH2 nanoparticles could effectively prevent the penetration of corrosive fluids. The amino groups of SiO2–NH2 nanoparticles can generate intermolecular hydrogen interactions with polybenzoxazine's phenol groups. In addition, during the curing procedure, the amino groups of SiO2–NH2 nanoparticles may react with benzoxazine. As a result, it is anticipated that the PC-a/SiO2 coating will have promising growth prospects in uses such as waterproofing and corrosion resistance. Therefore, the superhydrophobic properties of the PC-a/SiO2 coating and the incorporation of SiO2–NH2 nanoparticles offer exceptional corrosion resistance.27,49
The creation of lignin coatings is a burgeoning subject because lignin is naturally hydrophobic. As stated in recent research by S. Ohashi and H. Ishida such coatings have no less than 15 applications, including antibacterial and anticorrosive coatings, to name a few.35 However, the limited solubility of lignin in solvents frequently impedes the production of such substances. The solubility of lignin is essential when endeavoring to produce coatings by dipping, dropping, spraying, or spinning. Even though the isolation process modifies lignin's ability to dissolve, chemical modifications can also improve this parameter for producing homogeneous and productive coatings.51 Therefore, the solubility (S) of each eLig-Bz was studied in 19 organic solvents of varying polarity. eLig-ste is readily soluble in a variety of solvents, such as chlorinated, non-polar, and polar aprotic solvents (S > 80 wt%). Despite the polarity of the solvent, the long alkyl alternatives (C18) limit the expanded structure of lignin chains.36 To demonstrate the utility of this property, eLig-ste was cast from THF onto a glass substrate, resulting in a homogenous coating with an optimal weight and surface concentration of 75 μL cm.67
In their study, W. Yan et al. demonstrated that increasing the reactivity of soda lignin with a bio-based phenolic molecule enables the synthesis of a diverse range of benzoxazine precursors. The lignin-based benzoxazines thermosets were produced through a chemical process that adheres to most green chemistry standards. The only waste product of simple synthesis is water. The process yields materials with a lignin mass fraction between 46 and 66 wt%. Due to lignin's structure and benzoxazine's characteristics, these materials possess desirable mechanical and thermal properties.72 Temperatures of glass transition fluctuate between 136 and 197 °C, and storage moduli range from 0.5 to 3.1 GPa. The most intriguing aspect of this work is the demonstration of the fact that the properties of these precursors can be altered based on the type of amine applied to seal the oxazine ring. Stearyl amine benzoxazine precursors derived from this amine could profit from increased hydrophilic properties and coating capabilities. Furfuryl amine, the furan ring plays a role in the polymerization of benzoxazines and enhances thermosets' Tg and combustion properties. Mono-ethanolamine, the ester and monoethanolamine constituents of benzoxazine, conduct dynamic exchanges, resulting in poly-benzoxazine with trimeric properties. Stearylamine had been used to produce hydrophobic coatings that were durable and simple to apply by casting. Experimentation with heat and flame demonstrated that a lignin-based benzoxazine containing furan has emerged as fire-resistant and self-extinguishing. Eventually, an OH-terminated amine was utilized to generate a catalyst-free lignin-based trimer. The aliphatic groups containing OH and ester bonds underwent topological rearrangements via internally catalyzed transesterification procedures, rendering the lignin-based thermoset re-processable and degradable. By altering the amine employed, additional lignin derivatives could be produced, and their potential as antioxidant additives or anti-corrosion coatings could be investigated. Moreover, the combination of various amines can be tailored to produce bio-based trimers with a high Tg and minimal fire smoke and toxicity, paving the way for entirely circular composites.72
X. Cao et al. used molecular design to incorporate long-chain polyether amine with high flexibility into PBZ monomers, which were then copolymerized using TA to create PB-TA composite coatings employing a bioinspired technique. The results of their investigation indicate that TA additives may enhance the mechanical properties and adhesion strength of PB-TA coatings by increasing both the crosslinking density of PBZ coatings and their binding strength to the steel matrix. Moreover, because of the abundance of phenolic hydroxyl groups in TA, it can form strong hydrogen bonds with benzoxazine molecules. PB-6TA (with a 6% TA concentration) had the most favorable overall performance among all PB-TA coatings, including intact morphology, enhanced durability, adhesive force, and high tensile strength.70 The salt spray test (SST) indicates that the PB-6TA coating has greater resistance to corrosion compared to the prevalent epoxy coatings (>1 × 109 cm2 in the 600 h Salt Spray Test (SST) and nearly 1 × 108 cm2 even after 1200 h). The frictional coefficient of PBZ-based coatings demonstrates a two-stage rise, including abrasion of the surface and bulk abrasion, suggesting that increasing the amount of TA may enhance their anti-friction performance. Furthermore, the cyclic wear-corrosion experiments degraded the PBZ coating more notably than the PB-6TA coating. Overdosing TA could result in an excessive increase in the cross-linking density of PBZ coatings, leading to increased surface brittleness and hardness, which reduces the bond strength of PBZ-based coatings. Nonetheless, this research could contribute to the development of further polybenzoxazine-based coatings for infrastructure serving in severe conditions.73
Qing Chen's group successfully developed a range of bio-based main chain type benzoxazine (MCBZs) resins to solve the problem of marine fouling by proving coatings that are antifouling.66 Strong intramolecular hydrogen bonding interactions provide polybenzoxazine with a surface with low energy and weak binding to substrates, based on the findings of Q. Chen et al. In this study, the MCBZs solved the problem of poor adherence by modifying their structure. The inclusion of longer alkyl chains and furan groups offered additional reactive locations for the formation of hydrogen bonds with the substrate, which improved adhesion. Consistent with the results of the preceding flexural property test, the hardness, adhesion, and impact strength of poly(4, 8, and 12 fu-MCBZ) were progressively enhanced. Notable was the fact that the poly(12-fu-MCBZ) adhesion met the hull coatings adhesion standards.
Compared to standard benzoxazines, the advantages of using the developed MCBZs demonstrated outstanding processability, superior curing activity, and a reduced curing temperature. In addition, the resins demonstrated high thermal stability and mechanical qualities after curing, along with high adherence to the substrate. Most notably, the resins demonstrated remarkable antifouling performance and dual defense-bactericidal action. In addition, the anti-adhesion, and antibacterial properties of long alkyl chains, daidzein, and the furan moiety were attributable to the relatively small synergistic influence on surface energy.66
Metal coatings using benzoxazines may be regarded as a traditional use. However, developing appropriate benzoxazine monomers may create stimuli-sensitive polybenzoxazine coatings. Furthermore, the adherence characteristic of polybenzoxazines can be altered by varying the number of polyphenol derivatives that contain OH groups in their network, which can be considered an intelligent approach. Of all the benzoxazines-coated cotton textiles, poly(E-dda) has the maximum oil–water separation efficiency (98%), anti-icing, and enhanced cyclic repeatability properties. Therefore, the poly(E-dda) developed in this study can be utilized as an effective hydrophobic/oleophilic substance for oil–water separation and anti-icing purposes. Moreover, E-dda had a minor surface free energy and exhibited the most significant contact angle with water (151°).15
5 Conclusion and future perspectives
To sum up, benzoxazine chemistry is exceptionally versatile because of its direct synthesis and wide range of phenols and primary amines. Moreover, in comparison to many other high-performance polymers, the production of high-performance thermosets for a variety of applications is relatively straightforward. Another fascinating aspect of this polymer science is the ring-opening polymerization of the corresponding benzoxazine monomers, which is triggered only through heat and does not require a catalyst or addition. In addition to their simplicity of production and curing, PBz and their derivatives have unique structural properties such as intramolecular and intermolecular hydrogen bonding, good thermal stability, and mechanical endurance. Despite substantial academic research studies, most other polymers have yet to be commercialized in the past two decades. However, in the case of PBz, several commercial uses have been developed and are still expanding, resulting in the patenting of numerous benzoxazines. Furthermore, researchers have discovered that this adaptable chemistry may be used to create unique polymers, which might be referred to as inventive materials. Some examples of uses are porous benzoxazine polymers, intelligent coatings, self-healing systems, electrochromic materials, batteries, superhydrophobic surfaces, and shape memory PBz. Although these aspects of benzoxazine chemistry are still in their early stages, the number of papers published suggests that they will progress quickly because the adaptability of bio-based benzoxazine chemistry goes far beyond expectations. There may be no doubt that environmentally friendly growth and preservation of the environment have grown significantly in recent decades and gained more attention. To replace petroleum-based benzoxazines, benzoxazines produced from natural renewable raw materials such as catechol, vanillin, eugenol, cardanol, guaiacol, and daidzein are being extensively studied and produced. Bio-based PBz was successfully synthesized using bio-inspired strategies and showed high performance for coating applications. From a future perspective, combining rigid and flexible segments in the main chain provides new inspiration for high-performance bio-based polymers. Also, using renewable polyphenols such as lignin, matcha, and chitosan as phenol sources to synthesize benzoxazine monomers or as reinforcement would enhance these synthesized polymers' adhesive properties, making them suitable for coating applications.Conflicts of interest
There are no conflicts to declare.References
- Y. Deng, L. Xia, G.-L. Song, Y. Zhao, Y. Zhang, Y. Xu and D. Zheng, Development of a curcumin-based antifouling and anticorrosion sustainable polybenzoxazine resin composite coating, Composites, Part B, 2021, 225, 109263, DOI:10.1016/j.compositesb.2021.109263.
- Study on the synergistic anticorrosion property of a fully bio-based polybenzoxazine copolymer resin.
- E. Abdullayev, R. Price, D. Shchukin and Y. Lvov, Halloysite tubes as nanocontainers for anticorrosion coating with benzotriazole, ACS Appl. Mater. Interfaces, 2009, 1, 1437–1443, DOI:10.1021/am9002028.
- K. A. Zahidah, S. Kakooei, M. C. Ismail and P. B. Raja, Halloysite nanotubes as nanocontainer for smart coating application: a review, Prog. Org. Coat., 2017, 111, 175–185, DOI:10.1016/j.porgcoat.2017.05.018.
- E. Shchukina, D. Grigoriev, T. Sviridova and D. Shchukin, Comparative study of the effect of halloysite nanocontainers on autonomic corrosion protection of polyepoxy coatings on steel by salt-spray tests, Prog. Org. Coat., 2017, 108, 84–89, DOI:10.1016/j.porgcoat.2017.03.018.
- N. Y. Abu-Thabit and A. S. Hamdy, Stimuli-responsive polyelectrolyte multilayers for fabrication of self-healing coatings – a review, Surf. Coat. Technol., 2016, 303, 406–424, DOI:10.1016/j.surfcoat.2015.11.020.
- A. M. Soliman, K. I. Aly, M. G. Mohamed, A. A. Amer, M. R. Belal and M. Abdel-Hakim, Synthesis, characterization and protective efficiency of novel polybenzoxazine precursor as an anticorrosive coating for mild steel, Sci. Rep., 2023, 13, 5581, DOI:10.1038/s41598-023-30364-x.
- P. Thirukumaran, A. Shakila and S. Muthusamy, Synthesis and characterization of novel bio-based benzoxazines from eugenol, RSC Adv., 2014, 4(16), 7959, 10.1039/c3ra46582a.
- K. Zhang, Mengchao Han, Yuqi Liu, and Pablo Froimowicz, ACS Sustainable Chem. Eng., 2019, 7(10), 9399–9407, DOI:10.1021/acssuschemeng.9b00603.
- C. Zhou, X. Lu, Z. Xin and J. Liu, Corrosion resistance of novel silane-functional polybenzoxazine coating on steel, Corros. Sci., 2013, 70, 145–151, DOI:10.1016/j.corsci.2013.01.023.
- L. Wang, M. Yuan, Y. Zhao, Z. Guo, X. Lu and Z. Xin, Fabrication of superhydrophobic bio-based polybenzoxazine/hexagonal boron nitride composite coating for corrosion protection, Prog. Org. Coat., 2022, 167, 106863, DOI:10.1016/j.porgcoat.2022.106863.
- A. Forchetti Casarino, S. A. Bortolato, N. Casis, D. A. Estenoz and M. E. Spontón, Novel polybenzoxazine and polybenzoxazine/epoxy thermosetting copolymers containing polysilsesquioxane nanostructures for high-performance thermal protection systems, Eur. Polym. J., 2023, 182, 111722, DOI:10.1016/j.eurpolymj.2022.111722.
- Q. Zhang, P. Yang, Y. Deng, C. Zhang, R. Zhu and Y. Gu, Effect of phenol on the synthesis of benzoxazine, RSC Adv., 2015, 5(125), 103203–103209, 10.1039/c5ra17395g.
- A. Adjaoud, L. Puchot, C. E. Federico, R. Das and P. Verge, Lignin-based benzoxazines: A tunable key-precursor for the design of hydrophobic coatings, fire resistant materials and catalyst-free vitrimers, Chem. Eng. J., 2023, 453, 139895, DOI:10.1016/j.cej.2022.139895.
- H. Ding, X. Wang, L. Song and Y. Hu, Recent advances in flame retardant bio-based benzoxazine resins, J. Renewable Mater., 2022, 10(4), 871–895, DOI:10.32604/jrm.2022.018150.
- G. Dinesh Kumar, P. Prabunathan, M. Manoj, A. Hariharan and M. Alagar, Fluorine free bio-based Polybenzoxazine coated substrates for oil–water separation and anti-icing applications, J. Polym. Environ., 2020, 28(9), 2444–2456, DOI:10.1007/s10924-020-01782-z.
- X. Liu, Z. Li and G. Zhan, et al) Bio-based benzoxazines based on sesamol: synthesis and properties, J. Appl. Polym. Sci., 2019, 136, 48255, DOI:10.1002/app.48255.
- X. Liu, R. Zhang and T. Li, et al) Novel fully biobased benzoxazines from rosin: synthesis and properties, ACS Sustainable Chem. Eng., 2017, 5, 10682–10692, DOI:10.1021/acssuschemeng.7b02650.
- Y. Cao, C. Chen, X. Lu, D. Xu, J. Huang and Z. Xin, Bio-based polybenzoxazine superhydrophobic coating with active corrosion resistance for carbon steel protection, Surf. Coat. Technol., 2021, 405, 126569, DOI:10.1016/j.surfcoat.2020.126569.
- C. J. Higginson, K. G. Malollari, Y. Xu, A. V. Kelleghan, N. G. Ricapito and P. B. Messersmith, Bioinspired design provides high-strength Benzoxazine structural adhesives, Angew. Chem., 2019, 131(35), 12399–12407, DOI:10.1002/ange.201906008.
- A. Mahdy, M. G. Mohamed, K. I. Aly, H. B Ahmed and H. E. Emam, Liquid crystalline polybenzoxazines for manufacturing of technical textiles: Water repellency and ultraviolet shielding, Polym. Test., 2023, 119, 107933, DOI:10.1016/j.polymertesting.2023.107933.
- B. Kiskan and Y. Yagci, Thermally curable benzoxazine monomer with a photodimerizable coumarin group, J. Polym. Chem. A, 2007, 45(9), 1670–1676, DOI:10.1002/pola.21934.
- T. Periyasamy, S. P. Asrafali and S. Kim, Bio-based polybenzoxazine–cellulose grafted films: Material fabrication and properties, Polymers, 2023, 15(4), 849, DOI:10.3390/polym15040849.
- A. Singh, S. S. Narvi, P. K. Dutta and N. D. Pandey, External stimuli response on a novel chitosan hydrogel crosslinked with formaldehyde, Bull. Mater. Sci., 2006, 29, 233–238 CrossRef CAS.
- H. shida, Handbook of Benzoxazine Resins, ed. Ishida H. and Agag T., Elsevier, Amsterdam, The Netherlands, 2011, pp. 3–81 Search PubMed.
- X. Lu, Y. Liu, W. Zhang, X. Zhang, C. Zhou and Z. Xin, Crosslinked main-chain-type polybenzoxazine coatings for corrosion protection of mild steel, J. Coat. Technol. Res., 2017, 14, 937–944 CrossRef CAS.
- D. Xu, C. Lou, J. Huang, X. Lu, Z. Xin and C. Zhou, Effect of inhibitor-loaded halloysite nanotubes on active corrosion protection of polybenzoxazine coatings on mild steel, Prog. Org. Coat., 2019, 134, 126–133 CrossRef CAS.
- J. Huang, C. Lou, D. Xu, X. Lu, Z. Xin and C. Zhou, Cardanol-based polybenzoxazine superhydrophobic coating with improved corrosion resistance on mild steel, Prog. Org. Coat., 2019, 136, 105191, DOI:10.1016/j.porgcoat.2019.06.037.
- Y. Deng, L. Xia, G.-L. Song, Y. Zhao, Y. Zhang, Y. Xu and D. Zheng, Development of a curcumin-based antifouling and anticorrosion sustainable polybenzoxazine resin composite coating, Composites, Part B, 2021, 225, 109263, DOI:10.1016/j.compositesb.2021.109263.
- W. Shi, X. Zhang, Y. Ji, Z. Zhao, W. Li and X. Jia, Sustainable preparation of bio-based polybenzoxazine resins from amino acid and their application in CO2 adsorption, ACS Sustainable Chem. Eng., 2019, 7, 17313–17324 CrossRef CAS.
- J. R. Oliveira, L. R. V. Kotzebue, D. B. Freitas, A. L. A. Mattos, A. E. da Costa Júnior, S. E. Mazzetto and D. Lomonaco, Towards novel high-performance bio-composites: Polybenzoxazine-based matrix reinforced with Manicaria saccifera fabrics, Composites, Part B, 2020, 194, 108060 CrossRef CAS.
- C. Peng, Z. Wu and D. Zhou, Synthesis of a benzoxazine-type dispersant and its application on epoxy/benzoxazine/ZrO2 composite: Dispersion performance and tensile behavior, Composites, Part B, 2019, 167, 507–516 CrossRef CAS.
- C. J. Raorane, T. Periyasamy, R. Haldhar, S. P. Asrafali, V. Raj and S. Kim, Synthesis of bio-based Polybenzoxazine and its Antibiofilm and anticorrosive activities, Materials, 2023, 16(6), 2249, DOI:10.3390/ma16062249.
- C. J. Raorane, T. Periyasamy, R. Haldhar, S. P. Asrafali, V. Raj and S. Kim, Synthesis of bio-based Polybenzoxazine and its Antibiofilm and anticorrosive activities, Materials, 2023, 16(6), 2249, DOI:10.3390/ma16062249.
- S. Ohashi and H. Ishida, Various synthetic methods of Benzoxazine monomers, Advanced and Emerging Polybenzoxazine Science and Technology, 2017, vol. 3–8. DOI:10.1016/b978-0-12-804170-3.00001-9.
- J. H. Billman and L. C. Dorman, Imidazolidines. 111. 2-Substituted 1,3-bis-(o-hydroxybenzyl)-imidazolidines, J. Med. Chem., 1963, 6, 701–705 CrossRef CAS PubMed.
- M. R. Shushizadeh and S. A. Jundishapur, Solvent-free preparation ofnovel 2-[phenyl (pyridine-2-ylamino) methyl] phenols as pseudo- Betti processor for natural products, J. Nat. Pharm. Prod., 2014, 9(4), e17808, DOI:10.17795/jjnpp-17808.
- Z. Tang, Z. Zhu, Z. Xia, H. Liu, J. Chen, W. Xiao and X. Ou, Synthesis and fungicidal activity of novel 2,3-disubstituted-1,3-benzoxazines, Molecules, 2012, 17, 8174–8185 CrossRef CAS PubMed.
- M. T. Richers, M. Breugst, A. Y. Platonova, A. Ullrich, A. Dieckmann, K. N. Houk and D. Seidel, Redox-neutral a-oxygenation of amines: reaction development and elucidation of the mechanism, J. Am. Chem. Soc., 2014, 136, 6123–6135 CrossRef CAS PubMed.
- Y. Wu, G. Qiao, H. Liu, L. Zhang, Z. Sun, Y. Xiao and H. Guo, Brønsted acid-promoted [3 + 3] cycloaddition of azomethine ylides with quinone monoimine: a practical method towards dihydrobenzoxazine, RSC Adv., 2015,(5), 84290–84294 RSC.
- H. Sugimoto, S. Nakamura and T. Ohwada, Generation and application of o-quinone methides bearing various substituents on the benzene ring, Adv. Synth. Catal., 2007, 349, 669–679 CrossRef CAS.
- I. S. Belostotskaya, N. L. Komissarova, T. I. Prokof'eva, L. N. Kurkovskaya and V. B. Vol'eva, New opportunities for Duff reaction, Russ. J. Org. Chem., 2005, 41, 703–706 CrossRef CAS.
- V. A. Osyanin, D. V. Osipov and Y. N. Klimochkin, Synthesis of 1, 2, 4-triazolo [5,1-b][1,3] benzoxazines, Chem. Heterocycl. Compd., 2010, 46, 377–378 CrossRef CAS.
- J. L. Colin and B. Loubinoux, Nouvelle voie d’acces aux dihydro-3, 4-2H-benzoxazines-1, 3, Tetrahedron Lett., 1982, 23, 4245–4246 CrossRef CAS.
- M. R. Vengatesan, S. Devaraju, D. Kannaiyan, J. K. Song and M. Alagar, Ultrasound-assisted synthesis of benzoxazine monomers: thermal and mechanical properties of polybenzoxazines, Polym. Int., 2013, 62, 127–133 CrossRef.
- M. Ramaiyan and S. Muthusubramanian, Synthesis and biological activity of 6-alkyl/chloro-3-{4-(6-alkyl/chloro-2H-benzo [e][1, 3] oxazin-3 (4H)-yl) phenyl}-3, 4-dihydro-2H-benzo [e][1, 3] oxazines, Indian J. Chem., 2010, 49, 1083–1087 Search PubMed.
- W. J. Burke, M. J. Kolbezen and C. W. Stephens, Condensation of naph-thols with formaldehyde and primary amines, J. Am. Chem. Soc., 1952, 74, 3601–3605 CrossRef CAS.
- I. Szatmari and F. F€ul€op, Syntheses, transformations and applications of aminonaphthol derivatives prepared via modified Mannich reactions, Tetrahedron, 2013, 69, 1255–1278 CrossRef CAS.
- V. D. Dhakane, S. S. Gholap, U. P. Deshmukh, H. V. Chavan and B. P. Bandgar, An efficient and green method for the synthesis of [1, 3] oxazine derivatives catalyzed by thiamine hydrochloride (VB1) in water, C. R. Chim., 2014, 17, 431–436 CrossRef CAS.
- P. Thirukumaran, R. Sathiyamoorthi, A. Shakila Parveen and M. Sarojadevi, New benzoxazines from renewable resources for green composite applications, Polym. Compos., 2016, 37, 573–582, DOI:10.1002/pc.23214.
- Y. Cao, C. Chen, X. Lu, D. Xu, J. Huang and Z. Xin, Bio-based polybenzoxazine superhydrophobic coating with active corrosion resistance for carbon steel protection, Surf. Coat. Technol., 2020, 126569, DOI:10.1016/j.surfcoat.2020.126569.
- R. Ambrožič, U. Šebenik and M. Krajnc, Synthesis, curing kinetics, thermal and mechanical behavior of novel cardanol-based benzoxazines, Polymer, 2015, 76, 203–212, DOI:10.1016/j.polymer.2015.08.065.
- A. A. Khalil, U. U. Rahman and M. R. Khan, et al) Essential oil euge-nol: sources, extraction techniques and nutraceutical perspectives, RSC Adv., 2017, 7, 32669–32681, 10.1039/c7ra04803c.
- P. Thirukumaran, A. Shakila and S. Muthusamy, Synthesis and characterization of novel bio-based benzoxazines from eugenol, RSC Adv., 2014, 4, 7959–7966, 10.1039/c3ra46582a.
- L. Dumas, L. Bonnaud and M. Olivier, et al) Eugenol-based ben-zoxazine: from straight synthesis to taming of the network properties, J. Mater. Chem. A, 2015, 3, 6012–6018, 10.1039/c4ta06636g.
- P. Thirukumaran, A. S. Parveen and M. Sarojadevi, Synthesis and copolymerization of fully biobased benzoxazines from renewable resources, ACS Sustainable Chem. Eng., 2014, 2, 2790–2801, DOI:10.1021/sc500548c.
- J. R. Oliveira, L. R. V. Kotzebue, D. B. Freitas, A. L. A. Mattos, A. E. da Costa Júnior and S. E. Mazzetto, et al., Towards novel high-performance bio-composites: polybenzoxazine-based matrix reinforced with Manicaria saccifera fabrics, Composites, Part B, 2020, 194, 108060 CrossRef CAS.
- C. Peng, Z. Wu and D. Zhou, Synthesis of a benzoxazine-type dispersant and its application on epoxy/benzoxazine/ZrO2 composite: dispersion performance and tensile behavior, Composites, Part B, 2019, 167, 507–516 CrossRef CAS.
- D. N. Heo, W.-K. Ko, H.-J. Moon, H.-J. Kim, S. J. Lee and J. B. Lee, et al., Inhibition of osteoclast differentiation by gold nanoparticles functionalized with cyclodextrin curcumin complexes, ACS Nano, 2014, 8(12), 12049–12062 CrossRef CAS PubMed.
- J. Barry, M. Fritz, J. R. Brender, P. E. S. Smith, D.-K. Lee and A. Ramamoorthy, Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin, J. Am. Chem. Soc., 2009, 131(12), 4490–4498 CrossRef CAS PubMed.
- G. A. Phalak, D. M. Patil and S. T. Mhaske, Synthesis and characterization of thermally curable guaiacol based poly(benzoxazine-urethane) coating for corrosion protection on mild steel, Eur. Polym. J., 2017, 88, 93–108 CrossRef CAS.
- J. Xie, Y. Yong, X. Dong, J. Du, Z. Guo and L. Gong, et al., Therapeutic nanoparticles based on curcumin and bamboo charcoal nanoparticles for chemo-photothermal synergistic treatment of cancer and radioprotection of normal cells, ACS Appl. Mater. Interfaces, 2017, 9(16), 14281–14291 CrossRef CAS PubMed.
- N. Mahato, T. V. Sreekanth, K. Yoo and J. Kim, Semi-polycrystalline polyaniline-activated carbon composite for supercapacitor application, Molecules, 2023, 28(4), 1520, DOI:10.3390/molecules28041520.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, A concise review of current lignin production, applications, products and their environmental impact, Ind. Crops Prod., 2019, 139, 111526, DOI:10.1016/j.indcrop.2019.111526.
- A. Adjaoud, L. Puchot, C. E. Federico, R. Das and P. Verge, Lignin-based benzoxazines: A tunable key-precursor for the design of hydrophobic coatings, fire resistant materials and catalyst-free vitrimers, Chem. Eng. J., 2023, 453, 139895, DOI:10.1016/j.cej.2022.139895.
- Q. Chen, L. Zhang, J. Zhang, S. Habib, G. Lu, J. Dai and X. Liu, Bio-based polybenzoxazines coatings for efficient marine antifouling, Prog. Org. Coat., 2023, 174, 107298 CrossRef CAS.
- E. Paone, T. Tabanelli and F. Mauriello, The rise of lignin biorefinery, Current Opinion in Green and Sustainable, Chemistry, 2020, 24, 1–6, DOI:10.1016/j.cogsc.2019.11.004.
- Y. Cao, S. S. Chen, S. Zhang, Y. S. Ok, B. M. Matsagar, K. C. W. Wu and D. C. W. Tsang, Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery, Bioresour. Technol., 2019, 291, 121878, DOI:10.1016/j.biortech.2019.121878.
- Z. Deliballi, B. Kiskan and Y. Yagci, Main-chain benzoxazine precursor block copolymers, Polym. Chem., 2018, 9, 178–183, 10.1039/C7PY01873H.
- X. Cao, G. Cai, Y. Wang, J. Pan, X. Zhang and Z. Dong, Bio-inspired polybenzoxazine coating: Anti-corrosion and anti-abrasion performance enhancement through monomer design, Prog. Org. Coat., 2023, 174, 107283 CrossRef CAS.
- M. C. Etter, Encoding and decoding hydrogen-bond patterns of organic compounds, Acc. Chem. Res., 1990, 23, 120–126 CrossRef CAS.
- W. Yan, M. Shi, C. Dong, L. Liu and C. Gao, Applications of tannic acid in membrane technologies: A review, Adv. Colloid Interface Sci., 2020, 284, 102267, DOI:10.1016/j.cis.2020.102267.
- X. Lu, Y. Liu, C. Zhou, W. Zhang and Z. Xin, Corrosion protection of hydrophobic bisphenol A-based polybenzoxazine coatings on mild steel, RSC Adv., 2016, 6(7), 5805–5811 RSC.
| This journal is © The Royal Society of Chemistry 2023 |