Open Access Article
Open Access ArticlePd-catalyzed intermolecular consecutive double Heck reaction “on water” under air: facile synthesis of substituted indenes†
Dong Wei abc,
Han-Yu Lu
abc,
Han-Yu Lu c,
Han-Zhe Miaoc,
Chen-Guo Feng
c,
Han-Zhe Miaoc,
Chen-Guo Feng *d,
Guo-Qiang Lin*cd and
Yingbin Liu
*d,
Guo-Qiang Lin*cd and
Yingbin Liu *ae
*ae
aState Key Laboratory of Systems Medicine for Cancer, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China. E-mail: laoniulyb@shsmu.edu.cn
bShanghai Key Laboratory of Biliary Tract Disease Research, Department of General Surgery, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200092, China
cKey Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
dThe Research Center of Chiral Drugs, Innovation Research Institute of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
eDepartment of Biliary-Pancreatic Surgery, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200127, China
First published on 26th June 2023
Abstract
An efficient and environmentally benign method for the preparation of substituted indene derivatives has been developed by using water as the sole solvent. This reaction proceeded under air, tolerated a wide range of functional-groups and was easily scaled up. Bioactive natural products like indriline were synthesized via the developed protocol. Preliminary results demonstrate that the enantioselective variant can also be achieved.
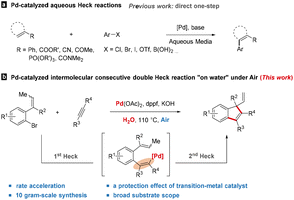
Solvent selection is perhaps the major consideration in green chemical design, and usually accounts for the vast majority of mass and energy wasted in syntheses and processes.1 Thus, water has gained wide attention within the last several decades for many potential advantages in organic chemistry such as abundance, low cost, simple operation and environmental benefits.2 Despite the great achievements in green and sustainable chemistry, less attention has been paid to the potential of water to promote organic reactions, especially in transition-metal catalysis. Indeed, “on-water” catalysis3 which showed significant rate acceleration in water compared to those performed in organic solvents, has recently sparked research interest and an impressive variety of modifications have been reported.4 However, the relevant studies on multi-step transition-metal catalyzed reactions remain largely unexplored.
Pd-catalyzed Heck-type reactions currently stand as highly reliable methods for the construction of carbo- and heterocyclic complex molecules.5 In view of numerous benefits of using water as the reaction medium, extensive efforts has been devoted to the development of Pd-catalyzed aqueous Heck reactions.6 These strategies were mainly focusing on the coupling of aryl (pseudo)halides or boronic acids with conjugated alkenes, involving a direct intermolecular one-step Heck-type transformation, and organic solvents were generally employed as the co-solvents to improve the poor solubility of substrates (Scheme 1a). With regard to multi-step reactions, although the sequential intermolecular Heck-type reactions have been well-studied in normal organic solvents,7 the aqueous version is a continuing challenge. A possible reason is the increased difficulty in reconciling the added reaction steps with the aqueous reaction condition.
Indenes are important structural skeletons which are integral parts of a wide variety of biologically active natural products8a and medicines,8b as well as functional materials8c and chiral ligands.8d Although a great number of synthetic methods have been explored to afford indene derivatives,9 the development of green methods for the synthesis of indene derivatives with simple operation is still highly desirable. Herein, we report a Pd-catalyzed aqueous domino process involving the “on-water” mechanism under air to construct functionalized indenes featuring all-carbon quaternary stereocenters via an intermolecular consecutive double Heck reaction of alkenes and alkynes (Scheme 1b).
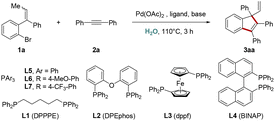
This domino process was initially investigated by using tri-substituted alkene 1a and diphenylacetylene 2a as model substrates in the presence of Pd(OAc)2, ligand and base. With DPPPE as a ligand, KOH as a base and dioxane as a solvent, the desired product 3aa was obtained in 47% yield (entry 1). However, there was no improvement in the yield of 3aa by the screening of several kinds of bases (entries 2–5) and reaction media (entries 6–8). Based on the effect of water in Heck reactions that the aqueous media might exert an influence on the rate of cyclization,6 we then turn our attention to examine solvents, using water as a co-solvent. To our delight, the yields were sharply increased to 74–77% (entries 9 and 10) and further attempt by using pure H2O as a solvent gave an increased reaction yield of 87% (entry 11). Next, the outcome was further improved by introducing several well-known mono- or bis-phosphorus ligands (entries 12–17), and dppf was identified as the optimal choice (entry 13). The reaction would be totally suppressed in the absence of a ligand (entry 18). Gratifyingly, the control experiments indicated that the reaction could also perform efficiently without insert gas protection (entry 19), and the same excellent reaction yield was maintained under air atmosphere. Interestingly, good reaction yield was also observed under O2 atmosphere (entry 20), which hints a protection effect of H2O to the active Pd intermediate from oxidation.10 Finally, efforts to lower the reaction temperature or decrease the catalyst/ligand loading were unsuccessful, and markedly lower yields of 3aa were obtained (entries 21 and 22) (Table 1).
| Entry | Ligand | Base | Solvent | Atmosphere | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.20 mmol, 1.0 equiv.), 2a (1.2 equiv.), Pd(OAc)2 (0.05 equiv.), L1–L4 (0.1 equiv.), L5–L7 (0.2 equiv.), base (0.40 mmol, 2.0 equiv.), solvent (1 mL). Yields determined by NMR analysis using CH2Br2 as an internal standard.b 90 °C.c Pd(OAc)2 (0.025 equiv.), L3 (0.05 equiv.).d PdCl2(dppf) (0.05 equiv.). | |||||
| 1 | L1 | KOH | Dioxane | Ar | 47 |
| 2 | L1 | KOAc | Dioxane | Ar | 23 |
| 3 | L1 | K2CO3 | Dioxane | Ar | 37 |
| 4 | L1 | NaHCO3 | Dioxane | Ar | 43 |
| 5 | L1 | TEA | Dioxane | Ar | 14 |
| 6 | L1 | KOH | DMF | Ar | 27 |
| 7 | L1 | KOH | DMSO | Ar | 14 |
| 8 | L1 | KOH | CH3CN | Ar | n.r. |
| 9 | L1 | KOH | Dioxane/H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Ar | 74 |
| 10 | L1 | KOH | Dioxane/H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Ar | 77 |
| 11 | L1 | KOH | H2O | Ar | 87 |
| 12 | L2 | KOH | H2O | Ar | 75 |
| 13 | L3 | KOH | H2O | Ar | 99 |
| 14 | L4 | KOH | H2O | Ar | 73 |
| 15 | L5 | KOH | H2O | Ar | 83 |
| 16 | L6 | KOH | H2O | Ar | 59 |
| 17 | L7 | KOH | H2O | Ar | 88 |
| 18 | — | KOH | H2O | Ar | n.r. |
| 19 | L3 | KOH | H2O | Air | 99 |
| 20 | L3 | KOH | H2O | O2 | 65 |
| 21b | L3 | KOH | H2O | Air | 41 |
| 22c | L3 | KOH | H2O | Air | 54 |
| 23d | — | KOH | H2O | Air | 67 |
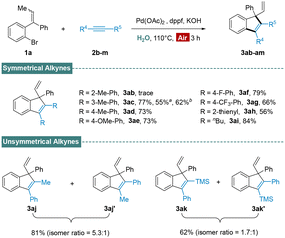
With the optimal catalytic system established, we examined the generality of this protocol (Scheme 2). Various tri-substituted alkenes were found to be compatible with the present procedure to afford the corresponding indenyl products in good to excellent yields. Substrates with electron withdrawing and donating mono/di substituents on phenyl ring A exhibited excellent reactivity, producing substituted indenes in the yield of 77–92% (3aa–ha). Furthermore, we undertook X-ray crystal structure analysis of 3ha to determine the existence of quaternary center. Alkenes bearing substituents on the phenyl ring B were suitable as well, and expected products were generated in comfortable yields (3ia–na). Moreover, the transformations were also effective when employing naphthyl (3oa), thienyl (3pa) or alkyl (3qa and 3ra) group instead of the phenyl ring B.
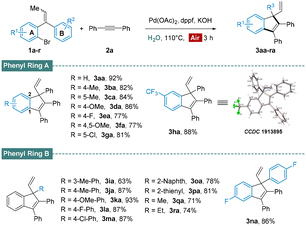 | ||
| Scheme 2 Substrate scope of alkenes. Reaction conditions: 1a–s (0.30 mmol, 1.0 equiv.), 2a (1.2 equiv.), Pd(OAc)2 (0.05 equiv.), dppf (0.1 equiv.), KOH (0.60 mmol, 2.0 equiv.), H2O (1.5 mL). | ||
Subsequently, we turned our attention to the scope of the alkyne coupling partner. As summarized in Scheme 3, most diphenylacetylenes were well-tolerated and delivered desired indenes in good yields (3ac–ag). As a comparison, When surfactants (sodium dodecyl sulfate, SDS) and TPGS-750 M were used to the reaction under standard conditions (3ac), the yields of 3ac were sharply reduced to 55% and 62%. A possible reason is the oxidation of metal catalyst. However, while increasing the steric hindrance of the phenyl group, it disfavoured the process and led to trace yield (3ab). Additionally, the reactions employed heteroaryl alkyne (3ah) or dialkyl alkyne (3ai) also proceeded smoothly and afforded indenes in acceptable yield. Encouraged by the success of the scope with symmetrical alkynes, we then submitted two unsymmetrical substrates (2j, 2k) to this aqueous process. Reaction of 1a with 1-phenyl-1-propyne delivered the expected product in good yield with 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivity (3aj + 3aj′). Nevertheless, the outcome was significant reduced when trimethyl(phenyl-ethynyl)silane was examined (3ak + 3ak′). The observed effect is likely due to the steric effects of palladium species involved in the alkyne insertion step.
1 regioselectivity (3aj + 3aj′). Nevertheless, the outcome was significant reduced when trimethyl(phenyl-ethynyl)silane was examined (3ak + 3ak′). The observed effect is likely due to the steric effects of palladium species involved in the alkyne insertion step.
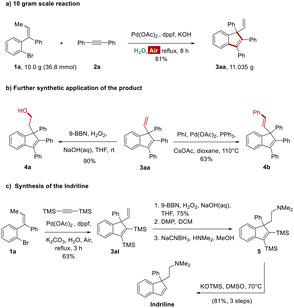
The practicability of this protocol was also demonstrated by the synthesis on the 10 gram level and versatile transformations of terminal alkenes. The presented methodology could be scaled up easily and afford compound 3aa in 81% yield, similar to that run on small scale (Scheme 4a). The terminal alkene moiety of 3aa could be elaborated through hydroboration–oxidation, which would be further transformed into other indenes possessing all-carbon quaternary centers contained different functional groups. Furthermore, the length of conjugated chain could be extended via a Heck coupling (Scheme 4b). The scope of the present methodology was also successfully extended to the efficient synthesis of biologically important indene derivatives like indriline.11 First, bis(trimethylsilyl)acetylene was subjected to our developed Pd-catalyzed protocol with alkene 1a, which furnished indene 3al. This intermediate was then converted to the imine 5 through a three-step sequence: (i) borohydride oxidation using 9-BBN, (ii) Dess–Martin oxidation, (iii) reductive amination by treatment with NaBH3CN and HNMe2. Finally, treatment of imine 5 with KOTMS at 70 °C for 4 hours afforded indriline in good yield (Scheme 4c).
We have begun to develop an enantioselective variant of this process. We anticipated that this might be challenging, due to issues such as the effect of E/Z isomers of the starting material 1 on the enantioselectivity of the reaction and the difficulty in controlling facial selectivity of the second Heck process in aqueous media under air. Yet we were pleased to measure a promising enantiomeric excess of 12% in the presence of (S)-BINAP at 60 °C (see the ESI† for details), which provides an example of using water-insoluble ligand in transition-metal catalyzed asymmetric aqueous transformations.
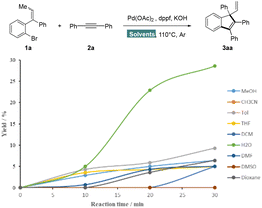
In order to understand “on water” reactivity, an array of kinetic experiments were performed under the same catalytic system (Scheme 5). Different organic solvents were introduced to assess the rate enhancement as compared to water. As a remarkable improvement of reaction rate was observed in water, this process could be defined to follow an “on-water” mechanism. In this regard, the properties of Pd catalysts at the macroscopic phase boundary between water and oil12 seem to play a role in reaction acceleration. In addition, the redistribution of surface species driven by surface-tension energetics,13 high concentration of starting materials and good solubility of bases may also be relevant.
Thereafter, we conducted several control experiments (see the ESI† for details) to gain the support of our hypothesis. Treatment of 1a with 2a under Ar in dioxane furnished the desired product in 47% yield (entry 1), while the outcome was sharply reduced to 4% under air (entry 2). Moreover, the transformation does not proceed in dioxane under O2 (entry 3). In contrast, indene 3aa was also successfully obtained under air without a decrease in the yield by using water as a solvent (entry 4 and 5). Surprisingly, replacement of air with O2 still maintained the reactivity (entry 6). Next, the stability of PdCl2(dppf) was tested under O2, which exhibited good efficiency to afford the desired product 3aa in 67% yield (Scheme 1, entry 23). After stirring at 110 °C under O2 for 3 hours, there was a small decline of catalyst, only about 8% in the presence of water (see the ESI† for details). On the contrary, the use of dioxane led to deactivation. The above experiments demonstrate convincingly that aqueous medium has shown to protect catalysts. This effect likely arises from the low solubility of catalysts and dissolved oxygen in water, which significantly prevents the unexpected behavior of O2 at the air–water interface.
In conclusion, we have developed a Pd-catalyzed consecutive double Heck coupling of substituted alkenes with internal alkynes in water under open air involving the “on-water” mechanism and a catalyst protection effect, which affords substituted indene derivatives bearing all-carbon quaternary centers with broad substrate scope and functional group tolerance. In addition, the synthetic utility of the aqueous process was demonstrated by a 10 gram scale reaction, several transformations and the synthesis of indriline. Preliminary studies have also shown that an enantioselective variant can be achieved.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge support from the National Natural Science Foundation of China (22271195, 2019ZX09301158, 81874181, 21871284), Emerging Frontier Program of Hospital Development Centre (SHDC12018107), the STCSM (18401933500), and the State Key Laboratory of Systems Medicine for Cancer (SB23-09). We would like to thank Prof. Qingyang Zhao from Shenzhen Campus of Sun Yat-sen University and Prof. Zhilong Chen from Huazhong University of Science and Technology for their kind help during manuscript preparation.Notes and references
- For selected reviews: (a) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC; (b) V. Polshettiwar and R. S. Varma, Chem. Soc. Rev., 2008, 37, 1546–1557 RSC.
- For selected reviews: (a) E. Breynaert, M. Houlleberghs, S. Radhakrishnan, G. Grübel, F. Taulelle and J. A. Martens, Chem. Soc. Rev., 2020, 49, 2557–2569 RSC; (b) T. Sahoo, J. Panda, J. Sahu, D. Sarangi, S. K. Sahoo, B. B. Nanda and R. Sahu, Curr. Org. Synth., 2020, 17, 426–439 CrossRef CAS PubMed; (c) M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC.
- (a) U. M. Lindström and F. Andersson, Angew. Chem., Int. Ed., 2006, 45, 548–551 CrossRef PubMed; (b) S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS PubMed; (c) D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816–7817 CrossRef CAS.
- For selected reviews: (a) T. Kitanosono and S. Kobayashi, Chem.–Eur. J., 2020, 26, 9408–9429 CrossRef CAS PubMed; (b) M. B. Gawande, V. D. B. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522–5551 RSCfor recent examples: (c) R. C. Cioc, T. J. Smak, M. Crockatt, J. C. van der Waal and P. C. A. Bruijnincx, Green Chem., 2021, 23, 5503–5510 RSC; (d) T. Kitanosono and S. Kobayashi, ACS Cent. Sci., 2021, 7, 739–747 CrossRef CAS PubMed; (e) H. C. Lam, H. P. Pepper, C. J. Sumby and J. H. George, Angew. Chem., Int. Ed., 2017, 56, 8532–8535 CrossRef CAS PubMed.
- For selected reviews: (a) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS PubMed; (b) G. T. Crisp, Chem. Soc. Rev., 1998, 27, 427–436 RSCfor recent examples: (c) W. Yao, G. Zhao, Y. Wu, L. Zhou, U. Mukherjee, P. Liu and M. Ngai, J. Am. Chem. Soc., 2022, 144, 3353–3359 CrossRef CAS PubMed; (d) B. Zhu, Z. Li, F. Chen, W. Xiong, X. Tan, M. Lei, W. Wu and H. Jiang, Chem. Commun., 2022, 58, 12688–12691 RSC; (e) W.-X. Wei, Y. Li, Y.-T. Wen, M. Li, X.-S. Li, C.-T. Wang, H.-C. Liu, Y. Xia, B.-S. Zhang, R.-Q. Jiao and Y.-M. Liang, J. Am. Chem. Soc., 2021, 143, 7868–7875 CrossRef CAS PubMed; (f) F. Garnes-Portolés, R. Greco, J. Oliver-Meseguer, J. Castellanos-Soriano, M. C. Jiménez, M. López-Haro, J. C. Hernández-Garrido, M. Boronat, R. Pérez-Ruiz and A. Leyva-Pérez, Nat. Catal., 2021, 4, 293–303 CrossRef.
- For recent review: F. Christoffel and T. R. Ward, Catal. Lett., 2018, 148, 489–511 CrossRef CAS ; for recent examples: H. Pang, Y. Hu, J. Yu, F. Gallou and B. H. Lipshutz, J. Am. Chem. Soc., 2021, 143, 3373–3382 CrossRef PubMed; X. Wang, H. Sun, J. Liu, W. Zhong, M. Zhang, H. Zhou, D. Dai and X. Lu, Org. Lett., 2019, 21, 719–723 CrossRef PubMed; N. Kaur, G. Kaur, A. Bhalla, J. S. Dhau and G. R. Chaudhary, Green Chem., 2018, 20, 1506–1514 RSC; S. N. Jadhav and C. V. Rode, Green Chem., 2017, 19, 5958–5970 RSC.
- For recent examples: (a) P. Yang, R.-Q. Xu, C. Zheng and S.-L. You, Chin. J. Chem., 2020, 38, 235–241 CrossRef CAS; (b) T. Yao, F. Zhang, J. Zhang and L. Liu, Org. Lett., 2020, 22, 5063–5067 CrossRef CAS PubMed; (c) T. R. Huffman, Y. Wu, A. Emmerich and R. A. Shenvi, Angew. Chem., Int. Ed., 2019, 58, 2371–2376 CrossRef CAS PubMed; (d) S. Teng, M. E. Tessensohn, R. D. Webster and J. S. Zhou, ACS Catal., 2018, 8, 7439–7444 CrossRef CAS.
- (a) J. Banothu, S. Basavoju and R. Bavantula, J. Heterocycl. Chem., 2015, 52, 853–860 CrossRef CAS; (b) G. Majetich and J. M. Shimkus, J. Nat. Prod., 2010, 73, 284–298 CrossRef CAS PubMed; (c) H. Minegishi, Y. Futamura, S. Fukashiro, M. Muroi, M. Kawatani, H. Osada and H. Nakamura, J. Med. Chem., 2015, 58, 4230–4241 CrossRef CAS PubMed; (d) M. Voets, I. Antes, C. Scherer, U. Müller-Vieira, K. Biemel, S. Marchais-Oberwinkler and R. W. Hartmann, J. Med. Chem., 2006, 49, 2222–2231 CrossRef CAS PubMed; (e) C. E. Diesendruck, B. D. Steinberg, N. Sugai, M. N. Silberstein, N. R. Sottos, S. R. White, P. V. Braun and J. S. Moore, J. Am. Chem. Soc., 2012, 134, 12446–12449 CrossRef CAS PubMed; (f) Y. He, G. Zhao, B. Peng and Y. Li, Adv. Funct. Mater., 2010, 20, 3383 CrossRef CAS; (g) Z. Zheng, Y. Cao, Q. Chong, Z. Han, J. Ding, C. Luo, Z. Wang, D. Zhu, Q.-L. Zhou and K. Ding, J. Am. Chem. Soc., 2018, 140, 10374–10381 CrossRef CAS PubMed; (h) S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45, 1365–1377 CrossRef CAS PubMed.
- (a) H. Chu, J. Cheng, J. Yang, Y.-L. Guo and J. Zhang, Angew. Chem., Int. Ed., 2020, 59, 21991–21996 CrossRef CAS PubMed; (b) L. Yang, H. Zheng, L. Luo, J. Nan, J. Liu, Y. Wang and X. Luan, J. Am. Chem. Soc., 2015, 137, 4876–4879 CrossRef CAS PubMed; (c) W. Fang, Y. Wei and M. Shi, Adv. Synth. Catal., 2019, 361, 3446–3450 CrossRef CAS; (d) Z. Wu, D. Ma, B. Zhou, X. Ji, X. Ma, X. Wang and Y. Zhang, Angew. Chem., Int. Ed., 2017, 56, 12288–12291 CrossRef CAS PubMed; (e) B. Gabriele, R. Mancuso and L. Veltri, Chem.–Eur. J., 2016, 22, 5056–5094 CrossRef CAS PubMed.
- (a) W. C. Ho, K. Chung, A. J. Ingram and R. M. Waymouth, J. Am. Chem. Soc., 2018, 140, 748–757 CrossRef CAS PubMed; (b) M. M. Konnick and S. S. Stahl, J. Am. Chem. Soc., 2008, 130, 5753–5762 CrossRef CAS PubMed; (c) S. S. Stahl, Science, 2005, 309, 1824–1826 CrossRef CAS PubMed.
- X. Abel-Snape, G. Wycich and M. Lautens, ACS Catal., 2022, 12, 3291–3301 CrossRef CAS.
- For selected reviews: (a) E. G. Percástegui, T. K. Ronson and J. R. Nitschke, Chem. Rev., 2020, 120, 13480–13544 CrossRef PubMed; (b) T. Jarisz, S. Roy and D. K. Hore, Acc. Chem. Res., 2018, 51, 2287–2295 CrossRef CAS PubMed.
- J. T. Koberstein, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 2942–2956 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1913895. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra03510g |
| This journal is © The Royal Society of Chemistry 2023 |