 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stabilization of propene molybdenum and tungsten half–sandwich complexes by intramolecular coordination of a thioether function†
Lukáš Hanzl a,
Jaromír Vinklárek
a,
Jaromír Vinklárek a,
Libor Dostál
a,
Libor Dostál a,
Ivana Císařováb,
Miroslava Litecká
a,
Ivana Císařováb,
Miroslava Litecká c and
Jan Honzíček
c and
Jan Honzíček *d
*d
aDepartment of General and Inorganic Chemistry, Faculty of Chemical Technology, University of Pardubice, Studentská 573, 532 10 Pardubice, Czech Republic
bDepartment of Inorganic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 2030/8, 128 43, Prague 2, Czech Republic
cDepartment of Materials Chemistry, Institute of Inorganic Chemistry of the CAS, Husinec-Řež 1001, 25068 Řež, Czech Republic
dInstitute of Chemistry and Technology of Macromolecular Materials, Faculty of Chemical Technology, University of Pardubice, Studentská 573, 532 10 Pardubice, Czech Republic. E-mail: jan.honzicek@upce.cz
First published on 30th June 2023
Abstract
This study reports the stabilizing effect of an intramolecularly coordinated thioether function in propene complexes of the general formula [{η5:κS-C5H4(CH2)2SR}M(CO)2(η2-C2H3Me)][BF4] (M = Mo, W; R = Et, Ph). They are formed by protonation of allyl analogues [{η5-C5H4(CH2)2SR}M(CO)2(η3-C3H5)] by tetrafluoroboric acid in non-coordinating solvents. In contrast to analogues with unsubstituted Cp ligands, these propene complexes are isolable in a pure form and characterized by NMR spectroscopy. The molybdenum compounds are stable at low temperature and the propene ligand can easily be exchanged by thioethers or acetonitrile. Several representatives of the reaction products were characterized by X-ray structure analysis. The stabilization effect in tungsten complexes [{η5:κS-C5H4(CH2)2SR}W(CO)2(η2-C2H3Me)][BF4] (R = Et, Ph) was unusually high. The compounds are long-term stable at room temperature and do not undergo ligand exchange reactions even with strong chelators such as 1,10-phenanthroline. The molecular structure of the tungsten propene complex was confirmed by X-ray diffraction analysis on a single crystal.
Introduction
The ability of transition metals to form coordination compounds with alkenes was discovered almost two hundred years ago when W. C. Zeise1 synthesized the first compound bearing π-bonded ethylene, K[PtCl3(η2-C2H4)]·H2O, currently known as Zeise's salt.1,2 After elucidation of the alkene coordination mode in the 1950s,1,3 a variety of complexes with π-coordinated alkenes have been described, which was motivated by their key role in catalytic olefin oligomerization and polymerization reactions.4–6 For example, the specific design of π-alkene intermediates permits the living polymerization catalysis of ethylene at unusually high temperatures to afford ultra-high molecular weight polyethylene with low dispersity.7 The effects of supporting ligands on the stability of intermediate π-alkene species are further exemplified in recent studies dealing with ethylene oligomerization.8,9Alkene intermediates are involved in metathesis reactions (e.g., ring-closing metathesis, cross-metathesis, and metathesis polymerization).10 These processes are commonly catalyzed by high-valent molybdenum and tungsten complexes11 and by ruthenium compounds.10,12 The potential of iron catalysts is currently under comprehensive investigation.13–15 Gold(I)-ethylene complexes, stabilized with bulky phosphine ligands were investigated as catalysts for the hydroamination of ethylene.16,17 Stereoregularity of transition metal catalyzed alkene hydroformylation is controlled upon transfer of hydride ligand to the coordinated alkene. Formed branched aldehydes serve as attractive synthetic precursors for pharmaceuticals.18
Alkene intermediates are formed upon C–H activation of hydrocarbons by cyclopentadienyl tungsten complexes [(η5-Cp*)W(NO)(R)(η3-allyl)] (Cp* = C5Me5; R = H, neopentyl), as documented on adducts bearing trapped carbon monoxide [(η5-Cp*)W(NO)(CO)(η2-alkene)] (Scheme 1, reaction A).19,20
 | ||
| Scheme 1 Stabilization of η2-alkene tungsten complexes by (a) CO coordination.19 (b) Intramolecular coordination.21 | ||
Recently, we have proven formation of η2-propene intermediates upon protonation of η3-allyl molybdenum and tungsten compounds [(η5-Ind′)M(CO)2(η3-allyl)] (Ind’ = substituted indenyl; M = Mo, W) by strong acid in non-coordinating solvents. The η2-propene intermediates were stabilized by intramolecular coordination of 1-(quinol-8-yl)indenyl ligand. A tungsten complex, presented in Scheme 1 (reaction B), was found to be stable up to 0 °C.21 This study further documented that the η2-propene ligand, in this type of complex, can be easily exchanged by labile ligands (e.g., dimethyl sulfide) while stronger ligands (e.g., acetonitrile) induce η3-to-η5-indenyl ring slippage.21
The aim of this work is to describe stabilizing effects of the intramolecular coordination on cyclopentadienyl molybdenum and tungsten complexes without an annulated benzene ring. For this purpose, the thioether moiety in the side chain was chosen due to expected hemilabile coordination to the metal. Our secondary aim involves reactivity of η2-propene complexes, prepared in situ, with thioethers and acetonitrile, as both ligands traditionally form labile complexes with early transition metals.
Results and discussion
The allyl(cyclopentadienyl) complexes 1–4 were prepared by the reaction of [(η3-C3H5)(MeCN)2M(CO)2Cl] (M = Mo, W) with lithium cyclopentadienides bearing a thioether group in the side chain LiC5H4(CH2)2SR (R = Et, Ph), see Scheme 2. These precursors of propene complexes were isolated and characterized using 1H NMR and IR spectroscopy. | ||
| Scheme 2 Synthesis of the precursors 1–4 and their protonation in a non-coordinating solvent: (I) [(η3-C3H5)(MeCN)2M(CO)2Cl] (M = Mo, W); (II) HBF4·Et2O, CH2Cl2, −40 °C. | ||
1H spectra of the complexes 1–4 contained two sets of signals for the allyl ligand, expected for complexes of the [(η5-Cp′)M(CO)2(η3-C3H5)],22 due to the presence of the exo/endo isomerism of the allyl ligand (Scheme 2). At room temperature, exo- and endo-conformers appear in molar ratio 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for 1 and 2, 5
2 for 1 and 2, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for 3 and 2
2 for 3 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 4. The assignment allyl resonances in 1–4 was aided by data from previously prepared analogues with unsubstituted cyclopentadienyl ligands.23 The infrared spectra of 1–4 exhibit two absorption bands in the region of 2000–1800 cm−1, assigned to the asymmetric and symmetric stretching modes of the terminal carbonyl ligands (Table 1).
1 for 4. The assignment allyl resonances in 1–4 was aided by data from previously prepared analogues with unsubstituted cyclopentadienyl ligands.23 The infrared spectra of 1–4 exhibit two absorption bands in the region of 2000–1800 cm−1, assigned to the asymmetric and symmetric stretching modes of the terminal carbonyl ligands (Table 1).
| Compound | νa(CO) | νs(CO) | ν(BF) |
|---|---|---|---|
| 1 | 1935 | 1850 | — |
| 2 | 1936 | 1850 | — |
| 3 | 1929 | 1840 | — |
| 4 | 1929 | 1839 | — |
| 7 | 2025 | 1946 | 1034 |
| 8 | 2025 | 1915 | 1026 |
| 9 | 1975 | 1883 | 1019 |
| 10 | 1974 | 1882 | 1048 |
| 11 | 1986 | 1885 | 1026 |
| 12 | 1978 | 1890 | 1022 |
| 13 | 1975 | 1890 | 1022 |
| 14 | 1984 | 1901 | 1030 |
| 15 | 1982 | 1888 | 1029 |
| 16 | 1960 | 1898 | 1027 |
| 17 | 1981 | 1903 | 1049 |
| 18 | 1994 | 1886 | 1054 |
| 19 | 1989 | 1896 | 1054 |
| 20 | 1990 | 1882 | — |
Reaction of the complexes 1–4 with HBF4·Et2O in dichloromethane at −40 °C afforded the complexes bearing η2-coordinated propene ligand [{η5:κS-C5H4(CH2)2SR}M(CO)2(η2-CH2CHCH3)][BF4] (Scheme 2).
The absence of coordinating solvents enabled isolation of molybdenum complexes 5 and 6 at low temperature. They were stored for days at −40 °C without signs of decomposition. We note that they were characterized by 1H NMR spectroscopy only owing to their thermal instability.
The 1H NMR spectra of complexes 5 and 6, measured at room temperature, contained two sets of signals attributed to two isomeric species of [{η5:κS-C5H4(CH2)2SR}Mo(CO)2(η2-C2H3Me)][BF4] with different propene ligand orientations (Scheme 2). The origin of these two isomers is assumed to be correlated to whether the allyl ligand being protonated in 1 and 2 is in the exo- or endo-confirmation. Relative abundances of a and b isomers were determined from integration of well resolved doublets attributed to propene methyl groups (δ = 1.8–2.3 ppm). The resonance at higher field was assigned to a isomer due to the effect of proximity of carbonyl ligand.
In both cases (5 and 6), isomer b is formed as a major protonation product since the spectra measured immediately upon dissolution contain isomers 5a/5b and 6a/6b in molar ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 3
5 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively. The lower thermodynamic stability of the b isomers become evident from repeated measurements. After two hours at room temperature, the composition of the mixtures has changed considerably. The isomers 5a/5b and 6a/6b were observed in molar ratios 6
5, respectively. The lower thermodynamic stability of the b isomers become evident from repeated measurements. After two hours at room temperature, the composition of the mixtures has changed considerably. The isomers 5a/5b and 6a/6b were observed in molar ratios 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. We note that the rearrangement of a to b is accompanied with slow decomposition of the propene complexes. After prolonged storage at room temperature, full decomposition was evidenced by disappearance of signals attributed to a and b and detection of free propene giving characteristic signal at 1.70 ppm (dt, 3J(1H,1H) = 6.5 Hz, 4J(1H,1H) = 1.5 Hz).
1, respectively. We note that the rearrangement of a to b is accompanied with slow decomposition of the propene complexes. After prolonged storage at room temperature, full decomposition was evidenced by disappearance of signals attributed to a and b and detection of free propene giving characteristic signal at 1.70 ppm (dt, 3J(1H,1H) = 6.5 Hz, 4J(1H,1H) = 1.5 Hz).
Conversion of a to b, was verified by NMR measurement with internal standard. Such experiments have shown the increase of a isomer concentration up to 130% and 160% of original concentration for 5a and 6a, respectively.
The tungsten complexes 7 and 8 are thermally stable. They were isolated at room temperature and can be long-term stored without signs of decomposition. The thermodynamic stability of tungsten-propene bond was further evidenced by their inertness toward coordinating solvents (e.g., MeCN) and aromatic amines (e.g., pyridine) including strong N,N-chelators (e.g., 1,10-phenanthroline).
The complexes 7 and 8 were characterized by mass spectrometry, 1H NMR, 13C NMR and IR spectroscopy. In both cases, only one set of signals was observed in 1H NMR spectra attributed to single isomer (presumably isomer a). The coordinated propene ligand gives three signals assigned to methyl group (doublet at ∼2.1 ppm), methylidene group (7: 3.05 ppm; 8: 2.64 ppm) and alkene CH group (7: 3.52 ppm; 8: 3.22 ppm), with the use 1H–1H COSY technique. The 1H NMR spectra also contained four signals of the cyclopentadienyl CH groups and four multiplets of ethylene chain, which implies intramolecular coordination of the thioether sulfur atom of the side chain. Low symmetry of the compounds 7 and 8 is also apparent from the 13C NMR spectra. For instance, each of the two carbonyl ligands gives an independent signal (7: 213.3 and 214.3 ppm; 8: 214.0 and 214.7 ppm). Detailed assignment of 13C NMR spectra was done with use of 1H–13C HSQC technique.
The successful protonation of the allyl ligand is also evidenced in the IR spectra. The carbonyl stretching bands are shifted by ∼90 cm−1 to higher wavenumbers compared to the precursor complexes 3 and 4 (Table 1).
Formation of the isomer 8a was confirmed by X-ray diffraction analysis on a single crystal (Fig. 1). The coordination sphere of the tungsten atom adopts a distorted square pyramidal geometry. The apical position of the pyramid is occupied by the η5-coordinated cyclopentadienyl ligand. The square base contains the two carbonyl ligands in cis-configuration, the intramolecularly coordinated sulfur atom of the thioether moiety and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the propene ligand. Methyl group of propene ligand appears at cis-position with respect to neighboring carbonyl ligand C1O1 (isomer a in Scheme 2). Selected bond lengths and angles are presented in Table 2.
C double bond of the propene ligand. Methyl group of propene ligand appears at cis-position with respect to neighboring carbonyl ligand C1O1 (isomer a in Scheme 2). Selected bond lengths and angles are presented in Table 2.
 | ||
| Fig. 1 X-ray structure of 8a (left) and 9-cis (right). Thermal ellipsoids set to 30% probability. Only one of the three crystallographically independent cations of 9-cis is shown for clarity. | ||
| 8a | 9-cisa | 9-cisa | 9-cisa | 11-cis | 12-cis | 15-trans | 16-cis | 18 | 19a | |
|---|---|---|---|---|---|---|---|---|---|---|
a Three crystallographically independent molecules in the unit cell.b Cg(C5) = center of the cyclopentadienyl ring.c L1 = sulfur atom of the pendant arm (8a, 9-cis, 11-cis, 12-cis and 18), sulfur atom of the monodentate thioether (15-trans, 16-cis) or the nitrogen atom of the acetonitrile (19a).d L2 = center of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of propene (8a), sulfur atom of the monodentate thioether (9-cis, 11-cis, 12-cis, 15-trans, 16-cis) or the nitrogen atom of the acetonitrile (18, 19a). C double bond of propene (8a), sulfur atom of the monodentate thioether (9-cis, 11-cis, 12-cis, 15-trans, 16-cis) or the nitrogen atom of the acetonitrile (18, 19a). |
||||||||||
| M–Cg(C5)b | 1.9877(15) | 1.974(4) | 1.984(4) | 1.984(5) | 1.9797(15) | 1.9820(9) | 2.0023(18) | 1.9880(11) | 1.9820(11) | 1.9871(8) |
| M–C1 | 2.027(3) | 1.99(1) | 2.00(1) | 2.01(1) | 2.006(4) | 2.000(2) | 2.017(4) | 1.975(2) | 1.998(3) | 1.984(2) |
| M–C2 | 1.983(3) | 1.94(1) | 1.95(1) | 1.96(1) | 1.964(3) | 1.963(2) | 1.959(4) | 1.965(2) | 1.951(2) | 1.981(2) |
| M–L1c | 2.5362(8) | 2.509(2) | 2.519(2) | 2.516(2) | 2.516(1) | 2.5232(5) | 2.505(1) | 2.5416(5) | 2.5120(7) | 2.166(1) |
| M–L2d | 2.210(2) | 2.546(3) | 2.530(2) | 2.489(3) | 2.5147(9) | 2.5314(5) | 2.490(1) | 2.5395(6) | 2.163(2) | 2.168(1) |
| C1–M–Cg(C5) | 105.53(11) | 117.9(3) | 110.9(3) | 113.5(4) | 117.67(11) | 113.30(6) | 134.23(13) | 114.69(8) | 118.54(10) | 116.30(5) |
| C2–M–Cg(C5) | 123.04(10) | 122.4(4) | 123.7(3) | 126.6(3) | 122.82(11) | 125.97(6) | 123.48(11) | 115.15(7) | 121.28(7) | 117.91(5) |
| L1–M–Cg(C5)b,c | 104.41(5) | 107.75(16) | 106.60(14) | 106.07(16) | 107.42(5) | 107.93(3) | 105.68(7) | 120.36(4) | 107.20(4) | 113.91(4) |
| L2–M–Cg(C5)b,d | 133.96(8) | 118.14(17) | 124.13(14) | 123.37(16) | 118.92(5) | 120.98(3) | 106.40(7) | 121.60(4) | 120.21(6) | 116.86(4) |
| C1–M–L1c | 149.8(1) | 134.4(3) | 142.0(3) | 140.3(3) | 134.7(1) | 138.43(6) | 147.85(3) | 124.84(7) | 134.17(9) | 129.78(6) |
| C2–M–L2d | 102.9(1) | 119.4(3) | 112.2(3) | 109.8(3) | 118.1(1) | 113.03(6) | 102.3(2) | 123.23(6) | 118.41(8) | 125.23(6) |
| C1–M–C2 | 79.1(1) | 74.3(5) | 78.5(4) | 77.7(4) | 75.4(1) | 76.74(8) | 102.3(2) | 75.66(9) | 75.2(1) | 74.85(7) |
| L1–M–L2c,d | 77.53(7) | 76.38(9) | 75.53(8) | 75.09(9) | 74.61(3) | 75.17(2) | 147.85(3) | 74.98(2) | 75.15(5) | 77.47(5) |
It is well established that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the alkene ligand elongates upon coordination and that the substituents on the C
C double bond of the alkene ligand elongates upon coordination and that the substituents on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C carbon atoms bend away from the metal atom. This appears because of the sharing of electron density between the π-orbitals of the alkene and the orbitals of the metal center.21 The dihedral angle defined by the metal atom, the C
C carbon atoms bend away from the metal atom. This appears because of the sharing of electron density between the π-orbitals of the alkene and the orbitals of the metal center.21 The dihedral angle defined by the metal atom, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C carbon atoms and carbon atom of the propene methyl group (α) can be used for quantifying distortion of the propene ligand. Its deviation from 90° express degree of its bending (α′ = α − 90). Elongation of the C
C carbon atoms and carbon atom of the propene methyl group (α) can be used for quantifying distortion of the propene ligand. Its deviation from 90° express degree of its bending (α′ = α − 90). Elongation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in η2-propene complexes usually vary between 0.03–0.13 Å (ref. 24–27) compared to value determined for free propene molecule (1.341(2) Å) by the gas electron diffraction.28 In the case of compound 8a, the C
C double bond in η2-propene complexes usually vary between 0.03–0.13 Å (ref. 24–27) compared to value determined for free propene molecule (1.341(2) Å) by the gas electron diffraction.28 In the case of compound 8a, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond elongates by 0.057 Å, which is comparable to data previously reported for indenyl tungsten(II) complex [{η5:κN-1-(C9H6N)C9H6}(η2-C2H3Me)W(CO)2][BF4]21 (0.068 Å) and calixarene tungsten(IV) complex [{p-But-calix[4]-(O)4}W(η2-C2H3Me)], (0.058 Å).25
C bond elongates by 0.057 Å, which is comparable to data previously reported for indenyl tungsten(II) complex [{η5:κN-1-(C9H6N)C9H6}(η2-C2H3Me)W(CO)2][BF4]21 (0.068 Å) and calixarene tungsten(IV) complex [{p-But-calix[4]-(O)4}W(η2-C2H3Me)], (0.058 Å).25
Complexes of electron rich metals such as Pt(II)29,30 and Cu(I)24 show low values of bending angle α′ not exceeding 15°. It implies a low effect of the coordination distortion on geometry of the propene ligand. The complexes of less electron rich metals, such as Ta(III),27 Mo(II),26 W(II)21,31 and W(IV),25 show higher α′ values. They vary between 21° and 30°, which documents a stronger effect of the coordination.
In our case (8a), the bending angle (21.9(3)°) fits into this range being close to value recently reported to structurally related indenyl tungsten(II) compound [{η5:κN-1-(C9H6N)C9H6}(η2-C2H3Me)W(CO)2][BF4]21 (24.5(9)°).
To assess the stability of molybdenum(II)-to-alkene bond in 5 and 6, protonation of their precursors (1 and 2) was done in presence of weak coordinating thioether ligands (Scheme 3). Infrared spectra of the isolated products 9–14 revealed shift of the carbonyl stretching bands by ∼40 cm−1 to higher wavenumbers compared to the precursors 1 and 2 (Table 1), which documents lower covalency of the Mo–CO bond in the cationic products due to reduced π-backbonding of the carbonyl ligands.
 | ||
Scheme 3 Protonation of 1 and 2 in the presence of thioethers: (I) HBF4·Et2O, CH2Cl2, 1.1 equiv.  , 0 °C. , 0 °C. | ||
1H NMR spectra of the compounds 9–14 contain four sharp signals at 5.0–6.5 ppm assigned to hydrogen atoms of the cyclopentadienyl ring. It indicates that the sulfur atom of the thioether side chain is intramolecularly coordinated. Additionally, the spectra of compounds 9, 10 and 13 contained a second set of four signals in this region implying a presence of two isomeric species. They appear in molar ratios 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 13
1, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in solutions of 9, 10 and 13, respectively. The presence of two isomers is also evidenced in the 13C NMR spectra of 9, 10 and 13. They contain two sets of signals for the carbon atoms of the carbonyl ligands. The 1H and 13C NMR spectra indicate low molecular symmetry of both isomers due to coordination of thioether side arm. The existence of two discrete isomeric species is also confirmed by the presence of two separate sets of signals for the ethylene spacers, observed in the 1H NMR spectrum of 13, which were assigned using the 1H–1H COSY technique. We note that signals of the ethylene spacer were not unambiguously assigned in the 1H NMR spectra of 9 and 10 owing to the presence of other aliphatic functions.
2 in solutions of 9, 10 and 13, respectively. The presence of two isomers is also evidenced in the 13C NMR spectra of 9, 10 and 13. They contain two sets of signals for the carbon atoms of the carbonyl ligands. The 1H and 13C NMR spectra indicate low molecular symmetry of both isomers due to coordination of thioether side arm. The existence of two discrete isomeric species is also confirmed by the presence of two separate sets of signals for the ethylene spacers, observed in the 1H NMR spectrum of 13, which were assigned using the 1H–1H COSY technique. We note that signals of the ethylene spacer were not unambiguously assigned in the 1H NMR spectra of 9 and 10 owing to the presence of other aliphatic functions.
Molecular structure of the compound 9-cis was revealed by X-ray diffraction analysis on a single crystal (Fig. 1). The compound has the expected square-pyramidal structure with carbonyl ligands in expected cis-configuration. Such evidence together with similarities observed in NMR spectra led us to attribute the main product in spectra of 9–14 to the same isomer (see, cis in Scheme 3). The minor species, in the spectra of 9, 10 and 13, is ascribed to the isomer with the carbonyl ligands in the trans-configuration. Although this configuration is less common for compounds of general formula [Cp′M(CO)2L2], it was previously reported for derivatives bearing thioether ligands without intramolecular coordination.32
The formation of cis-isomers was evidenced by X-ray analysis also for compounds 11-cis and 12-cis (Fig. 2). They are isostructural with compound 9-cis mentioned afore. Presence of simple thioether ligand in these structures enables to quantify effects of geometric constrain, caused by intramolecularly coordinated side arm. As evident from data given in Table 2, the intramolecular coordination causes only minor shortening of the Mo–S bond but the reduction of the bond angle Cg(C5)–Mo–S is substantial (from ∼120 to ∼107°).
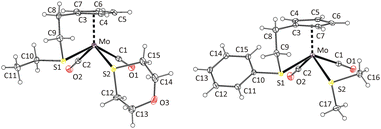 | ||
| Fig. 2 X-ray structure of 11-cis (left) and 12-cis (right). Thermal ellipsoids set to 30% probability. | ||
Observation of the rather unusual trans-isomers in solutions of 10, 12 and 13 let us to investigate protonation of parent allyl complex [(η5-C5H5)Mo(CO)2(η3-C3H5)] in the presence of simple thioethers (Scheme 4). Infrared spectra of reaction products 15–17 show carbonyl stretching bands at similar wavenumbers as the compounds with intramolecular coordination (Table 1). 1H NMR spectra contain one singlet at 5.7 ppm assigned to cyclopentadienyl hydrogens, and one set of signals of given coordinated thioether. The integral intensities of the signals prove that two thioether ligands are coordinated. 13C NMR spectra of 15–17 contain a sole signal in the region of carbonyl ligands at ∼244 ppm, which is in line with expected Cs molecular symmetry. Unfortunately, our NMR experiments are not conclusive about configuration of carbonyl ligands. They imply formation of a single isomer or a fast equilibrium between the cis- and trans-isomers. The later interpretation in more convenient, as representatives of both isomers (15-trans and 16-cis) were evidenced by X-ray crystallography (Fig. 3).
 | ||
| Fig. 3 X-ray structure of 15-trans (left) and 16-cis (right). Thermal ellipsoids set to 30% probability. | ||
Complex cations in 15-trans and 16-cis adopt expected distorted square pyramidal structures with cyclopentadienyl ligand in the apical position. Two carbonyl ligands and sulfur atoms of thioethers occupy the basal plane. The trans-configuration of the carbonyl ligands in 15-trans causes a greater distortion of the square pyramidal geometry than is observable for the cationic complexes in cis-configuration. This is evident from the values of the C1–Mo–C2 (102.3(2)°) and S1–Mo–S2 (147.85(3)°) angles. They differ by ∼45°, which contrasts with the structures of the other two complexes with unsubstituted cyclopentadienyl ligands (Fig. 3 and 4). The difference between C1–M–L1 and C2–M–L2 is only 1.61° and 4.55° in structures 16-cis and 19a, respectively (Table 2). Despite this difference in bond angles, there is only a negligible difference in corresponding Mo–S and Mo–C bond lengths in 15-trans and 16-cis.
The protonation of the allyl molybdenum complex 1 was further studied in the presence of acetonitrile. Such ligand is usually coordinated to molybdenum(II) stronger than thioethers21 but the coordination is weak enough to undergo of ligand exchange reactions. Hence, acetonitrile complexes are known as stable precursors for the assembly variety of structural motifs including bis(cyclopentadienyl) compounds33 or complexes bearing N,N-bidentate ligands.34
Compounds 1 reacts with HBF4·Et2O in acetonitrile to give cationic complex 18 (Scheme 5). The carbonyl stretching bands in the IR spectrum of 18 are shifted by about 40 cm−1 to higher energy compared to the precursor complex 1, which resembles behavior of the thioether complexes (Table 1).
The 1H NMR spectrum of compound 18 contains four sharp signals in the region 5.0–6.5 ppm, those were assigned to the hydrogen atoms of the cyclopentadienyl ring. It further contains four multiplets (2.62, 2.96, 3.68 and 4.08 ppm) attributed to the hydrogen atoms of the methylene spacers and signals for the ethyl group (1.39 and 2.88 ppm). Such pattern is very similar to analogues 9–11 mentioned afore, which implies appearance of the same structural motif. Signal of acetonitrile ligand appears at 2.54 ppm. Its integral intensity proves that only one molecule of the ligand is coordinated.
The structure of 18 was confirmed by X-ray diffraction analysis (Fig. 4). The coordination sphere adopts a distorted square pyramidal geometry resembling other cationic complexes under the study. The carbonyl ligands are in expected cis-configuration. The geometrical constrain induced by the intramolecular coordination of thioether function Cg(C5)–Mo–S angle (107.20(4)°) is comparable to that in crystal structures of 8, 9-cis, 11-cis and 12-cis (Table 2).
As protonation of [(η5-C5H5)Mo(CO)2(η3-C3H5)] by HBF4·Et2O in presence of acetonitrile is well described in literature,35,36 we decided to study the protonation with another strong acid CF3SO3H. The reaction was done in acetonitrile at 0 °C. 1H NMR spectrum in CD2Cl2 revealed a formation of two molybdenum complexes 19a and 19b in solution (Scheme 6). They give two sets of signals assigned to cyclopentadienyl (5.74 ppm for b and 5.75 ppm for a) and the coordinated acetonitrile ligands (2.50 ppm for b and 2.54 ppm for a). The spectrum further contains a singlet at 1.97 ppm which originates from free acetonitrile. It implies that recrystallization from acetonitrile produces [(η5-C5H5)Mo(CO)2(MeCN)2](CF3SO3) (19a) in a pure form and dissolution in CD2Cl2 leads to partial exchange of acetonitrile ligand with triflate. Such interpretation well correlates with formation equimolar amounts of 19b and free acetonitrile, evidenced by the 1H NMR technique.
Single crystal of 19a, prepared by a slow diffusion of diethyl ether into an acetonitrile solution, enabled to verify its solid-state structure (Fig. 4). We note that geometric parameters describing coordination sphere of molybdenum (Table 2) are in line with those reported previously for [(η5-C5H5)Mo(CO)2(NCMe)2][BF4].35
Conclusions
This study describes protonation of allyl ligand in molybdenum and tungsten complexes [(η5-Cp′)(η3-allyl)M(CO)2] (M = Mo, W) bearing cyclopentadienyl ligand decorated with a thioether function in the side chain. After allyl ligand protonation, the thioether moiety coordinates intramolecularly to the central metal and stabilizes the η2-bond of appeared propene ligand.Molybdenum complexes [{η5:κS-C5H4(CH2)2SR}Mo(CO)2(η2-C2H3Me)][BF4] (R = Et, Ph), formed by this pathway, are isolable at low temperature while tungsten analogues are long-term stable at room temperature. Unusually high thermodynamic stability of tungsten complexes is documented by inertness toward coordinating solvents (e.g., acetonitrile) and strong chelators (e.g., 1,10-phenanthroline). In the case of less stable molybdenum compounds, η2-propene ligand can be easily exchanged even by labile ligands (e.g., thioethers). The products of ligand exchange [{η5:κS-C5H4(CH2)2SR}Mo(CO)2 ][BF4] form both cis- and trans-isomers in solution, though structure of cis-isomers were resolved by X-ray crystallography. Rather unusual trans-isomer was structurally characterized in the case of analoque without intramolecular coordination [(η5-C5H5)Mo(CO)2(SMe2)2][BF4] formed by protonation of cyclopentadienyl complex [(η5-C5H5)Mo(CO)2(η3-C3H5)] in the presence of dimethyl sulfide.
][BF4] form both cis- and trans-isomers in solution, though structure of cis-isomers were resolved by X-ray crystallography. Rather unusual trans-isomer was structurally characterized in the case of analoque without intramolecular coordination [(η5-C5H5)Mo(CO)2(SMe2)2][BF4] formed by protonation of cyclopentadienyl complex [(η5-C5H5)Mo(CO)2(η3-C3H5)] in the presence of dimethyl sulfide.
Experimental
Materials
Synthesis of the organometallic compounds was done under an argon atmosphere using conventional Schlenk-line techniques. The products were stored under argon atmosphere at −20 °C. The solvents were dried using standard methods.37 The reagents were purchased from comercial sources (Sigma-Aldrich, Acros Organics and Penta) or prepared according to literature procedures: [(η5-C5H5)Mo(CO)2(η3-C3H5)];38 [(η3-C3H5)(MeCN)2Mo(CO)2Cl];39 [(η3-C3H5)(EtCN)2W(CO)2Cl].40Methods
1H, 13C{1H}, 13C-APT, 1H–1H COSY and 1H–13C HSQC NMR spectra were measured on the Bruker Avance 500 MHz and Bruker Avance 400 MHz spectrometers. The spectra were calibrated to the residual signal of the solvent relative to Me4Si. Poly(dimethylsiloxane) was used as internal standard in stability studies. Infrared spectra were recorded on a Nicolet iS50 FTIR spectrometer using a diamond Smart Orbit ATR in the region 4000–400 cm−1. Mass spectra were collected on a quadruple mass spectrometer LCMS 2010 (Shimadzu, Japan). The samples were dissolved in acetone and injected into the mass spectrometer with an infusion mode at a constant flow rate of 10 μL min−1. Electrospray ionization-mass spectrometry (ESI-MS) was used for the identification of the analyzed samples.X-ray crystallography
Data for 9-cis, 11-cis, 12-cis, 16-cis and 19a was collected on the Rigaku XtaLAB Synergy S diffractometer equipped with micro-focus CuKα/MoKα radiation and a Hybrid Pixel Array Detector (HyPix-6000HE). An Oxford Cryosystems (Cryostream 800) cooling device was used for data collection and the crystals were kept at 100 K during data collection. CrysAlisPro software41 was used for data collection, cell refinement and data reduction. Data were corrected for absorption effects using empirical absorption correction (spherical harmonics), implemented in SCALE3 ABSPACK scaling algorithm and numerical absorption correction based on gaussian integration over a multifaceted crystal model. Using Olex2,42 the structures were solved with the SHELXT43 and SHELXS44 structure solution program using intrinsic phasing (9-cis, 12-cis, 16-cis and 19a) and direct methods (11-cis) and refined with the SHELXL45 refinement package using least squares minimization. Hydrogen atoms of all molecules were placed in calculated positions. The diffraction experiments for 8a, 15-trans and 18 were performed on Bruker D8 VENTURE Kappa Duo PHOTONIII by IμS micro-focus sealed tube CuKα (λ = 1.54178) radiation at low temperature. The structures were solved by direct methods (SHELXS)42 and refined by full matrix least squares based on F2 (SHELXL2018).44 The hydrogen atoms on carbon were fixed into idealized positions (riding model) and assigned atomic displacement parameters either Uiso(H) = 1.2 Ueq (pivot atom) or Uiso(H) = 1.5 Ueq (pivot atom) for methyl moiety.Synthesis of C5H5(CH2)2SEt
The substituted cyclopentadiene was prepare using modification of the previously described procedures.46,47Freshly monomerized cyclopentadiene (5.2 mL, 62 mmol) was added dropwise to a suspension of sodium sand (1.6 g, 70 mmol) in 30 mL of THF. The mixture was stirred at room temperature for 16 h and then it was filtered from excess of sodium using a Schlenk frit. The sodium cyclopentadienide solution was precooled to −40 °C, treated with Cl(CH2)2SEt (7.0 g, 56 mmol) dropwise and the reaction mixture was stirred at room temperature for 16 h. The reaction was quenched with a ice/water mixture and the crude product was extracted with diethyl ether (2 × 10 mL). The volatiles were removed on a rotavapor and the product purified by vacuum distillation (bp = 110 °C at 67 Pa). Yield: 3.9 g (19 mmol, 34%). Yellow liquid. 1H NMR [400 MHz, CDCl3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1- and 2-isomers (a
1 mixture of 1- and 2-isomers (a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b)]: δ = 1.27 (t, 3J(1H,1H) = 7.4 Hz, 3H, SCH2CH3); 2.57 (q, 3J(1H,1H) = 7.4 Hz, 2H, SCH2CH3); 2.63–2.76 (m, 4H of a and 4H b, C5H5CH2CH2S); 2.93 (q, 3J(1H,1H) = 1.4 Hz, 2H, H5,5 of a, C5H5); 2.96–2.98 (m, 2H, H5,5 of b, C5H5); 6.06–6.09 (m, H4 of a, C5H5); 6.22 (s, H4 of b, C5H5); 6.28 (dq, 3J(1H,1H) = 5.4 Hz, 4J(1H,1H) = 1.5 Hz, H3 of b, C5H5); 6.41–6.47 (m, 3H, H3,2 of a, H1 of b, C5H5).
b)]: δ = 1.27 (t, 3J(1H,1H) = 7.4 Hz, 3H, SCH2CH3); 2.57 (q, 3J(1H,1H) = 7.4 Hz, 2H, SCH2CH3); 2.63–2.76 (m, 4H of a and 4H b, C5H5CH2CH2S); 2.93 (q, 3J(1H,1H) = 1.4 Hz, 2H, H5,5 of a, C5H5); 2.96–2.98 (m, 2H, H5,5 of b, C5H5); 6.06–6.09 (m, H4 of a, C5H5); 6.22 (s, H4 of b, C5H5); 6.28 (dq, 3J(1H,1H) = 5.4 Hz, 4J(1H,1H) = 1.5 Hz, H3 of b, C5H5); 6.41–6.47 (m, 3H, H3,2 of a, H1 of b, C5H5).
Synthesis of C5H5(CH2)2SPh
The steps of synthesis followed the procedure for C5H5(CH2)2SEt, but with 4.88 mL (58.0 mmol) of freshly monomerized cyclopentadiene, 1.47 g (63.9 mmol) of elemental sodium and 9.56 g (55.4 mmol) of Cl(CH2)2SPh. The desired product came over as the second fraction (100 °C, 13 mm Hg). Yield: 2.59 g (12.8 mmol, 23%). Yellow liquid. 1H NMR [400 MHz, CDCl3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1- and 2-isomers (a
1 mixture of 1- and 2-isomers (a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b)] 2.80–2.89 (m, 2H of a and 2H of b, C5H5CH2CH2S); 3.03 (s, 2H, H5,5 of a, C5H5); 3.09 (s, 2H, H5,5 of b, C5H5); 3.20–3.28 (m, 2H of a and 2H of b, C5H5CH2CH2S); 6.22 (s, 1H, H4 of a, C5H5); 6.37 (s, 1H, H4 of b, C5H5); 6.41 (d, 3J(1H,1H) = 5.3 Hz, 1H, H3 of b, C5H5); 6.57 (s, 3H, H2,3 of a, H1 of b, C5H5); 7.29 (t, 3J(1H,1H) = 7.2 Hz, 2H of a and 2H of b, H2,6 C6H5); 7.40 (t, 3J(1H,1H) = 7.5 Hz, 2H of a and 2H of b, H3,5 C6H5); 7.47 (d, 3J(1H,1H) = 7.5 Hz, 1H of a and 1H of b, H4 C6H5).
b)] 2.80–2.89 (m, 2H of a and 2H of b, C5H5CH2CH2S); 3.03 (s, 2H, H5,5 of a, C5H5); 3.09 (s, 2H, H5,5 of b, C5H5); 3.20–3.28 (m, 2H of a and 2H of b, C5H5CH2CH2S); 6.22 (s, 1H, H4 of a, C5H5); 6.37 (s, 1H, H4 of b, C5H5); 6.41 (d, 3J(1H,1H) = 5.3 Hz, 1H, H3 of b, C5H5); 6.57 (s, 3H, H2,3 of a, H1 of b, C5H5); 7.29 (t, 3J(1H,1H) = 7.2 Hz, 2H of a and 2H of b, H2,6 C6H5); 7.40 (t, 3J(1H,1H) = 7.5 Hz, 2H of a and 2H of b, H3,5 C6H5); 7.47 (d, 3J(1H,1H) = 7.5 Hz, 1H of a and 1H of b, H4 C6H5).
Synthesis of [{η5-C5H4(CH2)2SEt}Mo(CO)2(η3-C3H5)] (1)
C5H5(CH2)2SEt (469 mg, 3.04 mmol) was dissolved in 15 mL of THF, cooled to −60 °C and treated dropwise with a solution of butyllithium (1.6 M in hexanes, 1.9 mL, 3.04 mmol). The reaction mixture was stirred for 1 h and then added dropwise to a solution of [(η3-C3H5)(MeCN)2Mo(CO)2Cl] (922 mg, 2.97 mmol) in 10 mL of THF. The reaction mixture was stirred 16 h at room temperature. The volatiles were vacuum evaporated, the crude product extracted with hexane (3 × 25 mL, 60 °C). The volume of the extract was halved by vacuum evaporation and the product was precipitated as a yellow powder by cooling the mixture to −80 °C. The leftover solvent was decanted off and the product was dried in vacuum. Yield: 766 mg (2.21 mmol, 75%). Yellow powder. Anal. calc. for C14H18SO2Mo: C: 48.56; H: 5.24; S: 9.26. Found: C: 48.44; H: 5.10; S: 9.05. 1H NMR [500 MHz, CD2Cl2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of isomers exo
2 mixture of isomers exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo]: δ = 0.91 (d, 3J(1H,1H) = 10,8 Hz, 2H, Hanti of exo, C3H5); 1.22 (t, 3J(1H,1H) = 7.3 Hz, 3H, SCH2CH3); 1.65 (d, 3J(1H,1H) = 10.5 Hz, 2H, Hanti of endo, C3H5); 2.47 (t, 3J(1H,1H) = 7.4 Hz, 2H, C5H4CH2CH2S); 2.52 (q, 3J(1H,1H) = 7.3 Hz, 2H, SCH2CH3); 2.64 (t, 3J(1H,1H) = 7.4 Hz, 2H, C5H4CH2CH2S); 2.74 (d, 3J(1H,1H) = 7.0 Hz, 2H, Hsyn of exo, C3H5); 2.78–2.83 (m, 2H, Hsyn of endo, C3H5); 3.59–3.69 (m, 1H, Hmeso of endo, C3H5); 3.92 (tt, 3J(1H,1H) = 10.8 Hz, 4J(1H,1H) = 7.0 Hz, 1H, Hmeso of exo, C3H5); 5.17 (s, 2H, C5H4); 5.22 (s, 2H, C5H4). IR (ATR, cm−1): 1935 vs. [νa(CO)], 1850 vs. [νs(CO)].
endo]: δ = 0.91 (d, 3J(1H,1H) = 10,8 Hz, 2H, Hanti of exo, C3H5); 1.22 (t, 3J(1H,1H) = 7.3 Hz, 3H, SCH2CH3); 1.65 (d, 3J(1H,1H) = 10.5 Hz, 2H, Hanti of endo, C3H5); 2.47 (t, 3J(1H,1H) = 7.4 Hz, 2H, C5H4CH2CH2S); 2.52 (q, 3J(1H,1H) = 7.3 Hz, 2H, SCH2CH3); 2.64 (t, 3J(1H,1H) = 7.4 Hz, 2H, C5H4CH2CH2S); 2.74 (d, 3J(1H,1H) = 7.0 Hz, 2H, Hsyn of exo, C3H5); 2.78–2.83 (m, 2H, Hsyn of endo, C3H5); 3.59–3.69 (m, 1H, Hmeso of endo, C3H5); 3.92 (tt, 3J(1H,1H) = 10.8 Hz, 4J(1H,1H) = 7.0 Hz, 1H, Hmeso of exo, C3H5); 5.17 (s, 2H, C5H4); 5.22 (s, 2H, C5H4). IR (ATR, cm−1): 1935 vs. [νa(CO)], 1850 vs. [νs(CO)].
Synthesis of [(η5-C5H4(CH2)2SPh)Mo(CO)2(η3-C3H5)] (2)
The steps of the synthesis followed the procedure for 1, but with C5H5(CH2)2SPh (616 mg, 3.05 mmol), butyllithium (1.6 M in hexanes, 1.99 mL, 3.18 mmol) and [(η3-C3H5)(MeCN)2Mo(CO)2Cl] (925 mg, 2.98 mmol). Yield: 805 mg (2.10 mmol, 68%). Pale yellow powder. Anal. calc. for C18H18SO2Mo: C: 54.82; H: 4.60; S: 8.13. Found: C, 54.63; H, 4.48; S: 8.03. 1H NMR [500 MHz, CD2Cl2; 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of isomers exo
2 mixture of isomers exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo]: δ = 0.91 (d, 3J(1H,1H) = 10.8 Hz, 2H, Hanti of exo, C3H5); 1.63 (d, 3J(1H,1H) = 10.4 Hz, 2H, Hanti of endo, C3H5); 2.54 (t, 3J(1H,1H) = 7.5 Hz, 2H, C5H5CH2CH2SPh); 2.73 (d, 3J(1H,1H) = 6.9 Hz, 2H, Hsyn of exo, C3H5); 2.80 (d, 3J(1H,1H) = 5.3 Hz, 2H, Hsyn of endo, C3H5); 3.01 (t, 3J(1H,1H) = 7.5 Hz, 2H, C5H5CH2CH2SPh); 3.59–3.67 (m, 1H, Hmeso of endo, C3H5); 3.90 (tt, 3J(1H,1H) = 10.7 Hz, 3J(1H,1H) = 7.1 Hz, 1H, Hmeso of exo, C3H5); 5.18 (s, 2H, C5H4); 5.22 (s, 2H, C5H4); 7.20 (t, 3J(1H,1H) = 7.1 Hz, 1H, C6H5); 7.28–7.34 (m, 4H, C6H5). IR (ATR; cm−1): 1936 vs. [νa(CO)], 1850 vs. [νs(CO)].
endo]: δ = 0.91 (d, 3J(1H,1H) = 10.8 Hz, 2H, Hanti of exo, C3H5); 1.63 (d, 3J(1H,1H) = 10.4 Hz, 2H, Hanti of endo, C3H5); 2.54 (t, 3J(1H,1H) = 7.5 Hz, 2H, C5H5CH2CH2SPh); 2.73 (d, 3J(1H,1H) = 6.9 Hz, 2H, Hsyn of exo, C3H5); 2.80 (d, 3J(1H,1H) = 5.3 Hz, 2H, Hsyn of endo, C3H5); 3.01 (t, 3J(1H,1H) = 7.5 Hz, 2H, C5H5CH2CH2SPh); 3.59–3.67 (m, 1H, Hmeso of endo, C3H5); 3.90 (tt, 3J(1H,1H) = 10.7 Hz, 3J(1H,1H) = 7.1 Hz, 1H, Hmeso of exo, C3H5); 5.18 (s, 2H, C5H4); 5.22 (s, 2H, C5H4); 7.20 (t, 3J(1H,1H) = 7.1 Hz, 1H, C6H5); 7.28–7.34 (m, 4H, C6H5). IR (ATR; cm−1): 1936 vs. [νa(CO)], 1850 vs. [νs(CO)].
Synthesis of [{η5-C5H4(CH2)2SEt}W(CO)2(η3-C3H5)] (3)
The steps of synthesis followed the procedure for compound 1, but with C5H5(CH2)2SEt (936 mg, 6.07 mmol), butyllithium (1.6 M in hexanes, 3.8 mL, 6.08 mmol) and [(η3-C3H5)(EtCN)2W(CO)2Cl] (2.25 g, 5.65 mmol). Yield: 2.35 g (5.41 mmol, 96%). Orange liquid. Anal. calc. for C14H18SO2W: C:38.73; H: 4.18; S: 7.38. Found: C: 38.57; H: 3.94; S: 7.11. 1H NMR [400 MHz, C6D6, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of isomers exo
2 mixture of isomers exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo]: δ = 1.03 (t, 3J(1H,1H) = 7.4 Hz, 3H, SCH2CH3); 1.08–1.15 (m, 2H, Hanti of exo and endo, C3H5); 2.12 (t, 3J(1H,1H) = 7.1 Hz, 2H, C5H4CH2CH2S); 2.19 (q, 3J(1H,1H) = 7.3 Hz, 2H, SCH2CH3); 2.26 (t, 3J(1H,1H) = 6.8 Hz, 2H, C5H4CH2CH2S); 2.38 (d, 3J(1H,1H) = 6.1 Hz, 2H, Hsyn of exo, C3H5); 2.61–2.67 (m, 2H, Hsyn of endo, C3H5); 2.84–2.95 (m, 1Hmeso of exo, C3H5); 3.51–3.62 (m, 1Hmeso of endo, C3H5); 4.50 (s, 2H, C5H4); 4.57 (s, 2H, C5H4). IR (ATR, cm−1): 1929 vs. [νa(CO)], 1840 vs. [νs(CO)].
endo]: δ = 1.03 (t, 3J(1H,1H) = 7.4 Hz, 3H, SCH2CH3); 1.08–1.15 (m, 2H, Hanti of exo and endo, C3H5); 2.12 (t, 3J(1H,1H) = 7.1 Hz, 2H, C5H4CH2CH2S); 2.19 (q, 3J(1H,1H) = 7.3 Hz, 2H, SCH2CH3); 2.26 (t, 3J(1H,1H) = 6.8 Hz, 2H, C5H4CH2CH2S); 2.38 (d, 3J(1H,1H) = 6.1 Hz, 2H, Hsyn of exo, C3H5); 2.61–2.67 (m, 2H, Hsyn of endo, C3H5); 2.84–2.95 (m, 1Hmeso of exo, C3H5); 3.51–3.62 (m, 1Hmeso of endo, C3H5); 4.50 (s, 2H, C5H4); 4.57 (s, 2H, C5H4). IR (ATR, cm−1): 1929 vs. [νa(CO)], 1840 vs. [νs(CO)].
Synthesis of [{η5-C5H4(CH2)2SPh}W(CO)2(η3-C3H5)] (4)
The steps of synthesis followed the procedure for compound 1, but with C5H5(CH2)2SPh (297 mg, 1.47 mmol), butyllithium (1.6 M in hexanes, 1.0 mL, 1.6 mmol) and [(η3-C3H5)(EtCN)2W(CO)2Cl] (545 mg, 1.37 mmol). Yield: 498 mg (1.15 mmol, 82%). Orange liquid. Anal. calc. for C18H18SO2W: C: 44.83; H: 3.76; S: 6.65. Found: C: 44.55; H: 3.59; S: 6.37. 1H NMR [400 MHz, CDCl3, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of isomers exo
1 mixture of isomers exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo]: δ = 1.19 (d, 3J(1H,1H) = 9.9 Hz, 2H, Hanti of exo, C3H5); 1.32–1.39 (m, 2H, Hanti of endo, C3H5); 2.66 (t, 3J(1H,1H) = 7.5 Hz, 2H, C5H4CH2CH2S); 2.72 (d, 3J(1H,1H) = 5.9 Hz, 2H, Hsyn of exo, C3H5); 2.79–2.85 (m, 2H, Hsyn of endo, C3H5); 3.13 (t, 3J(1H,1H) = 7.4 Hz, 2H, C5H4CH2CH2S); 3.46–3.56 (m, 1H, Hmeso of exo, C3H5); 3.74–3.82 (m, 1H, Hmeso of endo, C3H5); 5.25–5.38 (m, 4H, C5H4); 7.32–7.43 (m, 5H, C6H5). IR (ATR, cm−1): 1929 vs. [νa(CO)], 1839 vs. [νs(CO)].
endo]: δ = 1.19 (d, 3J(1H,1H) = 9.9 Hz, 2H, Hanti of exo, C3H5); 1.32–1.39 (m, 2H, Hanti of endo, C3H5); 2.66 (t, 3J(1H,1H) = 7.5 Hz, 2H, C5H4CH2CH2S); 2.72 (d, 3J(1H,1H) = 5.9 Hz, 2H, Hsyn of exo, C3H5); 2.79–2.85 (m, 2H, Hsyn of endo, C3H5); 3.13 (t, 3J(1H,1H) = 7.4 Hz, 2H, C5H4CH2CH2S); 3.46–3.56 (m, 1H, Hmeso of exo, C3H5); 3.74–3.82 (m, 1H, Hmeso of endo, C3H5); 5.25–5.38 (m, 4H, C5H4); 7.32–7.43 (m, 5H, C6H5). IR (ATR, cm−1): 1929 vs. [νa(CO)], 1839 vs. [νs(CO)].
Synthesis of [{η5:κS-C5H4(CH2)2SEt}Mo(CO)2(η2-C2H3Me)][BF4] (5)
Compound 1 (252 mg, 0.73 mmol) was dissolved in 10 mL of dichloromethane, the solution was cooled to −40 °C and treated with HBF4·Et2O (98 μL, 0.73 mmol) dropwise. The solution was stirred at −40 °C for 16 h, the solvent was vacuum evaporated, and the crude product was purified by dissolution in a small amount of dichloromethane (∼0.5 mL) and subsequent precipitation by the addition of diethyl ether (10 mL), the solvents were decanted and the solid was vacuum dried. This process was repeated up to three times. Care was taken to keep the reaction mixture at or below −40 °C and to measure the 1H NMR spectra directly after isolation. Yield: 316 mg (0.60 mmol, 82%). Orange solid. 1H NMR [500 MHz, CD2Cl2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixture of isomers a
5 mixture of isomers a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b]: δ = 1.27 (t, 3J(1H,1H) = 7.4 Hz, 3H of b, SCH2CH3); 1.31 (t, 3J(1H,1H) = 7.4 Hz, 3H of a, SCH2CH3); 1.88 (d, 3J(1H,1H) = 5.5 Hz, 3H of a, CH2CHCH3); 2.21 (d, 3J(1H,1H) = 6.0 Hz, 3H of b, CH2CHCH3); 4.90 (s, 1H of b, C5H4); 4.95 (s, 1H of a, C5H4); 5.35 (s, 1H of b, C5H4); 6.08 (s, 1H of a, C5H4); 6.10 (s, 1H of b, C5H4); 6.25 (s, 1H of a, C5H4); 6.35 (s, 1H of b, C5H4).
b]: δ = 1.27 (t, 3J(1H,1H) = 7.4 Hz, 3H of b, SCH2CH3); 1.31 (t, 3J(1H,1H) = 7.4 Hz, 3H of a, SCH2CH3); 1.88 (d, 3J(1H,1H) = 5.5 Hz, 3H of a, CH2CHCH3); 2.21 (d, 3J(1H,1H) = 6.0 Hz, 3H of b, CH2CHCH3); 4.90 (s, 1H of b, C5H4); 4.95 (s, 1H of a, C5H4); 5.35 (s, 1H of b, C5H4); 6.08 (s, 1H of a, C5H4); 6.10 (s, 1H of b, C5H4); 6.25 (s, 1H of a, C5H4); 6.35 (s, 1H of b, C5H4).
Synthesis of [{η5:κS-C5H4(CH2)2SPh}Mo(CO)2(η2-C2H3Me)][BF4] (6)
The steps of synthesis followed the procedure for compound 5, but with 2 (105 mg, 0.27 mmol), HBF4·Et2O (38 μL, 0.28 mmol). Yield: 101 mg (0.21 mmol, 79%). Orange solid. 1H NMR [500 MHz, CD2Cl2, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixture of isomers a
5 mixture of isomers a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b]: δ = 1.98 (d, 3J(1H,1H) = 5.7 Hz, 3H of a, CH2CHCH3); 2.24 (d, 3J(1H,1H) = 6.0 Hz, 3H of b, CH2CHCH3); 4.96 (s, 1H of b, C5H4); 5.10 (s, 2H of a, C5H4); 5.36 (s, 1H of b, C5H4); 5.37 (s, 1H of b, C5H4); 6.33 (s, 1H of a, C5H4); 6.35 (s, 1H of a, C5H4); 6.43 (s, 1H of b, C5H4).
b]: δ = 1.98 (d, 3J(1H,1H) = 5.7 Hz, 3H of a, CH2CHCH3); 2.24 (d, 3J(1H,1H) = 6.0 Hz, 3H of b, CH2CHCH3); 4.96 (s, 1H of b, C5H4); 5.10 (s, 2H of a, C5H4); 5.36 (s, 1H of b, C5H4); 5.37 (s, 1H of b, C5H4); 6.33 (s, 1H of a, C5H4); 6.35 (s, 1H of a, C5H4); 6.43 (s, 1H of b, C5H4).
Synthesis of [{η5:κS-C5H4(CH2)2SEt}W(CO)2(η2-C2H3Me)][BF4] (7)
Compound 3 (731 mg, 1.68 mmol) was dissolved in 10 mL of acetonitrile, the solution was cooled to 0 °C and treated with HBF4·Et2O (250 μL, 1.85 mmol) dropwise. The solution was stirred at room temperature for 17 h, the solvent was vacuum evaporated, and the crude product was purified by dissolution in a small amount of dichloromethane (∼0.5 mL), the solution was cooled to −40 °C and subsequently the product was precipitated by the addition of diethyl ether (10 mL), the solvents were decanted and the solid was dried in vacuo. This process was repeated up to three times. Yield: 537 mg (1.03 mmol, 61%). Yellow solid. Anal. calc. for C14H19SO2WBF4: C: 32.21; H: 3.67; S: 6.14. Found: C: 32.01; H: 3.43; S: 5.90. Positive-ion MS (acetone): m/z (%) = 393 (100) [M − C3H6]+. 1H NMR [500 MHz, CD2Cl2]: δ = 1.22 (t, 3J(1H,1H) = 7.4 Hz, 3H, SCH2CH3); 2.09 (d, 3J(1H,1H) = 5.2 Hz, 3H, CH2CHCH3); 2.64 (d, 3J(1H,1H) = 10.0 Hz, 2H, CH2CHCH3); 2.75–2.80 (m, 1H, SCH2CH3); 2.82–2.89 (m, 1H, C5H4CH2CH2S); 2.92–3.00 (m, 1H, SCH2CH3); 3,12 (ddd, 3J(1H,1H) = 14.6 Hz, 4J(1H,1H) = 5.4 Hz, 5J(1H,1H) = 2.2 Hz, 1H, C5H4CH2CH2S); 3.18–3.26 (m, 1H, CH2CHCH3); 3.77 (td, 3J(1H,1H) = 13.7 Hz, 4J(1H,1H) = 5.5 Hz, 1H, C5H4CH2CH2S); 3.89 (ddd, 3J(1H,1H) = 10.9 Hz, 4J(1H,1H) = 4.7 Hz, 5J(1H,1H) = 2.8 Hz, 1H, C5H4CH2CH2S); 5.01 (m, 1H, C5H4); 5.08 (s, 1H, C5H4); 6.24 (m, 1H, C5H4); 6.37 (s, 1H, C5H4). 13C NMR [126 MHz, CD2Cl2]: δ = 13.6 (SCH2CH3); 25.9 (CH2CHCH3); 26.5 (C5H4CH2CH2S); 34.8 (SCH2CH3); 45.0 (CH2CHCH3); 51.4 (C5H4CH2CH2S); 51.8 (CH2CHCH3); 76.1 (C5H4); 85.1 (C5H4); 88.0 (C5H4); 94.0 (C5H4); 139.8 (C5H4); 214.0 (CO); 214.7 (CO). IR (ATR, cm−1): 2025 vs. [νa(CO)], 1946 vs. [νs(CO)], 1034 vs. [ν(BF)].Synthesis of [{η5:κS-C5H4(CH2)2SPh}W(CO)2(η2-C2H3Me)][BF4] (8)
The steps of synthesis followed the procedure for compound 7, but with 4 (135 mg, 0.28 mmol) and HBF4·Et2O (40 μL, 0.30 mmol). Yield: 102 mg (0.18 mmol, 64%). Yellow solid. Anal. calc. for C18H19SO2WBF4: C: 37.93; H: 3.36; S: 5.62. Found: C: 37.60; H: 3.14; S: 5.35. Positive-ion MS (acetone): m/z (%) = 441 (100) [M – C3H6]+. 1H NMR [500 MHz, CD2Cl2]: δ = 2.14 (d, 3J(1H,1H) = 4.7 Hz, 3H, CH2CHCH3); 2.45–2.57 (m, 1H, C5H4CH2CH2S); 2.66 (dd, 2J(1H,1H) = 11.8 Hz, 3J(1H,1H) = 5.3 Hz, 1H, C5H4CH2CH2S); 2.99–3.11 (m, 2H, CH2CHCH3); 3.52 (s, 1H, CH2CHCH3); 3.97–4.04 (m, 1H, C5H4CH2CH2S); 4.12–4.19 (m, 1H, C5H4CH2CH2S); 5.07 (s, 1H, C5H4); 5.16 (s, 1H, C5H4); 6.45 (s, 1H, C5H4); 6.53 (s, 1H, C5H4); 7.51 (5H, C6H5). 13C NMR [126 MHz, CD2Cl2]: δ = 25.9 (CH2CHCH3); 27.5 (CH2CHCH3); 45.5 (CH2CHCH3); 51.6 (C5H4CH2CH2S); 57.9 (C5H4CH2CH2S); 76.9 (C5H4); 84.4 (C5H4); 86.9 (C5H4); 95.1 (C5H4); 129.4 (C6H5); 130.7 (C6H5); 132.1 (C6H5); 139.0 (C5H4); 213.3 (CO); 214.3 (CO). IR (ATR, cm−1): 2025 vs. [νa(CO)], 1915 vs. [νs(CO)], 1026 vs. [ν(BF)]. Single crystals of 8 suitable for X-ray diffraction analysis were prepared by overlayering of the acetonitrile solution of 8 with diethyl ether.Synthesis of [{η5:κS-C5H4(CH2)2SEt}Mo(CO)2(SMe2)][BF4] (9)
Compound 1 (119 mg, 0.34 mmol) was dissolved in 10 mL of dichloromethane, the solution was treated with Me2S (50 μL, 0.68 mmol) cooled to 0 °C and treated with HBF4·Et2O (46 μL, 0.34 mmol) dropwise. The color of the solution changed from orange to red immediately upon the addition of acid. The reaction mixture was stirred at room temperature for 16 h, the solvent was vacuum evaporated, and the crude product was purified by dissolution in a small amount of dichloromethane (∼0.5 mL), the solution was cooled to −40 °C and subsequently the product was precipitated by the addition of diethyl ether (10 mL), the solvents were decanted and the solid was dried in vacuo. This process was repeated up to three times. Yield: 122 mg (0.27 mmol, 79%). Red solid. Anal. calc. for C13H19S2O2MoBF4: C: 34.38; H: 4.22; S: 14.12. Found: C: 34.20; H: 4.07; S: 13.88. Positive-ion MS (acetone): m/z (%) = 369 (100) [M]+. 1H NMR [500 MHz, CD2Cl2, mixture 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of isomers cis
1 of isomers cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans]: δ = 1.38 (t, 3J(1H,1H) = 7.3 Hz, 3H, SCH2CH3); 2.52 (s, 6H, (CH3)2S); 2.65–2.75 (m, 1H, C5H4CH2CH2S); 2.89–2.99 (m, 3H, 1H of C5H4CH2CH2S; 2H of SCH2CH3); 3.71–3.78 (m, 1H, C5H4CH2CH2S); 3.81–3.88 (m, 1H, C5H4CH2CH2S); 4.88–4.90 (m, 1H, C5H4 of cis); 5,03 (m, 1H, C5H4 of trans); 5.16–5.19 (m, 1H, C5H4 of trans); 5.35–5.36 (m, 1H, C5H4 of trans); 5.56 (s, 1H, C5H4 of cis); 5.64 (s,1H, C5H4 of cis); 6.19–6.10 (m, 1H, C5H4 of trans); 6.29 (s, 1H, C5H4 of cis). 13C NMR [126 MHz, CD2Cl2]: δ = 13.7 (SCH2CH3 of cis); 13.9 (SCH2CH3 of trans); 25.9 (SCH2CH3); 28.2 ((CH3)2S of cis); 28.5 ((CH3)2S of trans); 34.1 (C5H4CH2CH2S of cis); 36.3 (C5H4CH2CH2S of trans); 54.0 (C5H4CH2CH2S of cis); 66.2 (C5H4CH2CH2S of trans); 82.8 (C5H4 of cis); 87.5 (C5H4 of cis); 89.2 (C5H4 of cis); 89.8 (C5H4 of trans); 92.7 (C5H4 of trans); 93.9 (C5H4 of trans); 95.4 (C5H4 of trans); 100.4 (C5H4 of cis); 130.9 (C5H4 of trans); 142.3 (C5H4 of cis); 230.6 (CO of trans); 235.8 (CO of trans); 241.8 (CO of cis); 244.2 (CO of cis). IR (ATR, cm−1): 1975 vs. [νa(CO)], 1883 vs. [νs(CO)], 1019 vs. [ν(BF)]. Single crystals of 9-cis suitable for X-ray diffraction analysis were prepared by the overlayering of dichloromethane solution of 9 with diethyl ether.
trans]: δ = 1.38 (t, 3J(1H,1H) = 7.3 Hz, 3H, SCH2CH3); 2.52 (s, 6H, (CH3)2S); 2.65–2.75 (m, 1H, C5H4CH2CH2S); 2.89–2.99 (m, 3H, 1H of C5H4CH2CH2S; 2H of SCH2CH3); 3.71–3.78 (m, 1H, C5H4CH2CH2S); 3.81–3.88 (m, 1H, C5H4CH2CH2S); 4.88–4.90 (m, 1H, C5H4 of cis); 5,03 (m, 1H, C5H4 of trans); 5.16–5.19 (m, 1H, C5H4 of trans); 5.35–5.36 (m, 1H, C5H4 of trans); 5.56 (s, 1H, C5H4 of cis); 5.64 (s,1H, C5H4 of cis); 6.19–6.10 (m, 1H, C5H4 of trans); 6.29 (s, 1H, C5H4 of cis). 13C NMR [126 MHz, CD2Cl2]: δ = 13.7 (SCH2CH3 of cis); 13.9 (SCH2CH3 of trans); 25.9 (SCH2CH3); 28.2 ((CH3)2S of cis); 28.5 ((CH3)2S of trans); 34.1 (C5H4CH2CH2S of cis); 36.3 (C5H4CH2CH2S of trans); 54.0 (C5H4CH2CH2S of cis); 66.2 (C5H4CH2CH2S of trans); 82.8 (C5H4 of cis); 87.5 (C5H4 of cis); 89.2 (C5H4 of cis); 89.8 (C5H4 of trans); 92.7 (C5H4 of trans); 93.9 (C5H4 of trans); 95.4 (C5H4 of trans); 100.4 (C5H4 of cis); 130.9 (C5H4 of trans); 142.3 (C5H4 of cis); 230.6 (CO of trans); 235.8 (CO of trans); 241.8 (CO of cis); 244.2 (CO of cis). IR (ATR, cm−1): 1975 vs. [νa(CO)], 1883 vs. [νs(CO)], 1019 vs. [ν(BF)]. Single crystals of 9-cis suitable for X-ray diffraction analysis were prepared by the overlayering of dichloromethane solution of 9 with diethyl ether.
Synthesis of [{η5:κS-C5H4(CH2)2SEt}Mo(CO)2{S(CH2)4}][BF4] (10)
The steps of synthesis followed the procedure for compound 9, but with 1 (176 mg, 0.51 mmol), tetrahydrothiophene (67 μL, 0.76 mmol) and HBF4·Et2O (70 μL, 0.52 mmol). Yield: 186 mg (0.39 mmol, 76%). Red solid. Anal. calc. for C15H21S2O2MoBF4: C: 37.52; H: 4.41; S: 13.35. Found: C: 37.27; H: 4.19; S: 13.09. 1H NMR [500 MHz, CD2Cl2, mixture 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of isomers cis
1 of isomers cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans]: δ = 1.37 (t, 3J(1H,1H) = 7.3 Hz, 3H, SCH2CH3); 2.10–2.19 (m, 4H, H3,3,4,4, C4H8S); 2.70–2.78 (m, 1H, C5H4CH2CH2S); 2.87–3.02 (m, 3H, 1H of C5H4CH2CH2S, 2H of SCH2CH3); 3.03–3.20 (m, 4H, H2,2,5,5, C4H8S); 3.70–3.85 (m, 2H, C5H4CH2CH2S); 4.87–4.89 (m, 1H, C5H4 of cis); 5.06 (m, 1H, C5H4 of trans); 5.11 (m, 1H, C5H4 of trans); 5.54 (s, 1H, C5H4 of cis); 5.72 (s, 1H, C5H4 of cis); 6.09 (m, 1H, C5H4 of trans); 6.12 (m, 1H, C5H4 of trans); 6.23 (s, 1H, C5H4 of cis). 13C NMR [126 MHz, CD2Cl2]: δ = 13.8 (SCH2CH3 of cis); 15.5 (SCH2CH3 of trans); 25.3 (SCH2CH3 of trans); 25.8 (SCH2CH3 of cis); 31.0 (C3,4, C4H8S of trans); 31.2 (C3,4, C4H8S of cis); 34.0 (C5H4CH2CH2S); 44.4 (C2,5, C4H8S of trans); 45.0 (C2,5, C4H8S of cis); 54.0 (C5H4CH2CH2S); 83.2 (C5H4 of cis); 87.4 (C5H4 of cis); 89.0 (C5H4 of cis); 89.9 (C5H4 of trans); 92.6 (C5H4 of trans); 93.9 (C5H4 of trans); 95.0 (C5H4 of trans); 100.6 (C5H4 of cis); 131.1 (C5H4 of trans); 142.1 (C5H4 of cis); 231.4 (CO of trans); 235.3 (CO of trans); 242.7 (CO of cis); 245.1 (CO of cis). IR (ATR, cm−1): 1974 vs. [νa(CO)], 1882 vs. [νs(CO)], 1048 vs. [ν(BF)].
trans]: δ = 1.37 (t, 3J(1H,1H) = 7.3 Hz, 3H, SCH2CH3); 2.10–2.19 (m, 4H, H3,3,4,4, C4H8S); 2.70–2.78 (m, 1H, C5H4CH2CH2S); 2.87–3.02 (m, 3H, 1H of C5H4CH2CH2S, 2H of SCH2CH3); 3.03–3.20 (m, 4H, H2,2,5,5, C4H8S); 3.70–3.85 (m, 2H, C5H4CH2CH2S); 4.87–4.89 (m, 1H, C5H4 of cis); 5.06 (m, 1H, C5H4 of trans); 5.11 (m, 1H, C5H4 of trans); 5.54 (s, 1H, C5H4 of cis); 5.72 (s, 1H, C5H4 of cis); 6.09 (m, 1H, C5H4 of trans); 6.12 (m, 1H, C5H4 of trans); 6.23 (s, 1H, C5H4 of cis). 13C NMR [126 MHz, CD2Cl2]: δ = 13.8 (SCH2CH3 of cis); 15.5 (SCH2CH3 of trans); 25.3 (SCH2CH3 of trans); 25.8 (SCH2CH3 of cis); 31.0 (C3,4, C4H8S of trans); 31.2 (C3,4, C4H8S of cis); 34.0 (C5H4CH2CH2S); 44.4 (C2,5, C4H8S of trans); 45.0 (C2,5, C4H8S of cis); 54.0 (C5H4CH2CH2S); 83.2 (C5H4 of cis); 87.4 (C5H4 of cis); 89.0 (C5H4 of cis); 89.9 (C5H4 of trans); 92.6 (C5H4 of trans); 93.9 (C5H4 of trans); 95.0 (C5H4 of trans); 100.6 (C5H4 of cis); 131.1 (C5H4 of trans); 142.1 (C5H4 of cis); 231.4 (CO of trans); 235.3 (CO of trans); 242.7 (CO of cis); 245.1 (CO of cis). IR (ATR, cm−1): 1974 vs. [νa(CO)], 1882 vs. [νs(CO)], 1048 vs. [ν(BF)].
Synthesis of [{η5:κS-C5H4(CH2)2SEt}Mo(CO)2{κS-S(CH2)4O}][BF4] (11)
The steps of synthesis followed the procedure for compound 9, but with 1 (139 mg, 0.40 mmol), 1,4-oxathiane (56 μL, 0.60 mmol) and HBF4·Et2O (55 μL, 0.40 mmol). Yield: 147 mg (0.30 mmol, 74%). Red solid. Anal. calc. for C15H21S2O3MoBF4: C: 36.31; H: 4.27; S: 12.92. Found: C: 36.10; H: 4.09; S: 12.69. Positive-ion MS (acetone): m/z (%) = 411 (100) [M]+. 1H NMR [500 MHz, CD2Cl2]: δ = 1.38 (t, 3J(1H,1H) = 7.4 Hz, 3H, SCH2CH3); 2.66–2.73 (m, 1H, C5H4CH2CH2S); 2.75–2.81 (m, 2H, CH2, SCH2CH3); 2.90–3.00 (m, 4H of OC4H8S, 1H of C5H4CH2CH2S); 3.70–3.76 (m, 1H, C5H4CH2CH2S); 3.84–3.92 (m, 1H, C5H4CH2CH2S); 3.96–4.04 (m, 4H, OC4H8S); 4.86–4.87 (m, 1H, C5H4); 5.58 (s, 1H, C5H4); 5.69 (s, 1H, C5H4); 6.38 (s, 1H, C5H4). 13C NMR [126 MHz, CD2Cl2]: δ = 13.7 (SCH2CH3); 26.0 (C5H4CH2CH2S); 34.1 (OC4H8S); 38.4 (SCH2CH3); 54.0 (C5H4CH2CH2S); 68.9 (OC4H8S); 82.6 (C5H4); 87.2 (C5H4); 89.2 (C5H4); 100.0 (C5H4); 142.5 (C5H4); 241.5 (CO); 243.5 (CO). IR (ATR, cm−1): 1986 vs. [νa(CO)], 1885 vs. [νs(CO)], 1026 vs. [ν(BF)]. Single crystals of 11-cis suitable for X-ray diffraction analysis were prepared by overlayering of the dichloromethane solution of 11 with diethyl ether.Synthesis of [{η5:κS-C5H5(CH2)2SPh}Mo(CO)2(SMe2)][BF4] (12)
The steps of synthesis followed the procedure for compound 9, but with 2 (98 mg, 0.25 mmol), Me2S (37 μL, 0.50 mmol) and HBF4·Et2O (33 μL, 0.25 mmol). Yield: 98 mg (0.20 mmol, 78%). Red solid. Anal. calc. for C17H19S2O2MoBF4: C: 40.66; H: 3.81; S: 12.77. Found: C: 40.38; H: 3.57; S: 12.52. Positive-ion MS (acetone): m/z (%) = 355 (100) [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SC2H6]+. 1H NMR [400 MHz, CD2Cl2]: δ = 2.53 (s, 6H, (CH3)2S); 2.72–2.81 (m, 1H, C5H4CH2CH2S); 2.93–3.01 (m, 1H, C5H4CH2CH2S); 3.87–3.95 (m, 1H, C5H4CH2CH2S); 4.19–4.28 (m, 1H, C5H4CH2CH2S); 4.96 (s, 1H, C5H4); 5.56 (s, 1H, C5H4); 5.85 (s,1H, C5H4); 6.39 (s, 1H, C5H4); 7.50–7.58 (m, 5H, C6H5). 13C NMR [126 MHz, CD2Cl2]: δ = 27.0 (C5H4CH2CH2S); 28.3 ((CH3)2S); 61.3 (C5H4CH2CH2S); 82.8 (C5H4); 87.2 (C5H4); 89.5 (C5H4); 100.8 (C5H4); 130.6 (C6H5); 131.6 (C6H5); 131.8 (C6H5); 142.1 (C5H4); 240.2 (CO); 243.9 (CO). IR (ATR, cm−1): 1978 vs. [νa(CO)], 1890 vs. [νs(CO)], 1022 vs. [ν(BF)]. Single crystals of 12-cis suitable for X-ray diffraction analysis were prepared by the overlayering of dichloromethane solution of 12 with hexane.
SC2H6]+. 1H NMR [400 MHz, CD2Cl2]: δ = 2.53 (s, 6H, (CH3)2S); 2.72–2.81 (m, 1H, C5H4CH2CH2S); 2.93–3.01 (m, 1H, C5H4CH2CH2S); 3.87–3.95 (m, 1H, C5H4CH2CH2S); 4.19–4.28 (m, 1H, C5H4CH2CH2S); 4.96 (s, 1H, C5H4); 5.56 (s, 1H, C5H4); 5.85 (s,1H, C5H4); 6.39 (s, 1H, C5H4); 7.50–7.58 (m, 5H, C6H5). 13C NMR [126 MHz, CD2Cl2]: δ = 27.0 (C5H4CH2CH2S); 28.3 ((CH3)2S); 61.3 (C5H4CH2CH2S); 82.8 (C5H4); 87.2 (C5H4); 89.5 (C5H4); 100.8 (C5H4); 130.6 (C6H5); 131.6 (C6H5); 131.8 (C6H5); 142.1 (C5H4); 240.2 (CO); 243.9 (CO). IR (ATR, cm−1): 1978 vs. [νa(CO)], 1890 vs. [νs(CO)], 1022 vs. [ν(BF)]. Single crystals of 12-cis suitable for X-ray diffraction analysis were prepared by the overlayering of dichloromethane solution of 12 with hexane.
Synthesis of [{η5:κS-C5H4(CH2)2SPh}Mo(CO)2{S(CH2)4}][BF4] (13)
The steps of synthesis followed the procedure for compound 9, but with 2 (68 mg, 0.17 mmol), tetrahydrothiophene (22 μL, 0.25 mmol) and HBF4·Et2O (23 μL, 0.17 mmol). Yield: 80 mg (0.15 mmol, 89%). Red solid. Anal. calc. for C19H21S2O2MoBF4: C: 43.20; H: 4.01; S: 12.14. Found: C: 42.98; H: 3.83; S: 11.96. Positive-ion MS (acetone): m/z (%) = 443 (100) [M]+. 1H NMR [500 MHz, CD2Cl2, mixture 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of isomers cis
2 of isomers cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans]: δ = 2.07–2.21 (m, 4H, H3,3,4,4, C4H8S); 2.37–2.48 (m, 1H, C5H4CH2CH2S of trans); 2.62–2.71 (m, 1H, C5H4CH2CH2S of trans); 2.74–2.85 (m,1H, C5H4CH2CH2S of cis); 2.90–3.00 (m, 1H, C5H4CH2CH2S of cis); 3.17 (t, 3J(1H,1H) = 5.8 Hz, 4H, H2,2,5,5, C4H8S); 3.52–3.61 (m, 1H, C5H4CH2CH2S of trans); 3.73–3.80 (m, 1H, C5H4CH2CH2S of trans); 3.94 (dt, 2J(1H,1H) = 12.7 Hz, 3J(1H,1H) = 5.3 Hz, 1H, C5H4CH2CH2S of cis); 4.15–4.22 (m, 1H, C5H4CH2CH2S of cis); 4.97 (d, 3J(1H,1H) = 1.4 Hz, 1H, C5H4 of cis); 5.15 (s, 1H, C5H4 of trans); 5.24 (s, 1H, C5H4 of trans); 5.54 (s, 1H, C5H4 of cis); 5.87 (s, 1H, C5H4 of cis); 6.17 (s, 1H, C5H4 of trans); 6.35 (s, 1H, C5H4 of cis); 6.42 (s, 1H, C5H4 of trans); 7.49–7.54 (m, 5H, C6H5 of cis); 7.55–7.57 (m, 3H, C6H5 of trans); 7.63–7.66 (m, 2H, C6H5 of trans). 13C NMR [126 MHz, CD2Cl2]: δ = 26.7 (C5H4CH2CH2S); 31.0 (SC4H8); 31.2 (SC4H8); 44.4 (SC4H8); 45.0 (SC4H8); 61.5 (C5H4CH2CH2S); 83.3 (C5H4 of cis); 87.2 (C5H4 of cis); 89.1 (C5H4 of cis); 90.4 (C5H4 of trans); 92.7 (C5H4 of trans); 94.6 (C5H4 of trans); 95.2 (C5H4 of trans); 101.1 (C5H4 of cis); 130.5 (C6H5); 130.6 (C6H5); 131.9 (C6H5); 141.5 (C5H4); 231.3 (CO of trans); 232.3 (CO of trans); 241.3 (CO of cis); 244.8 (CO of cis). IR (ATR, cm−1): 1978 vs. [νa(CO)], 1890 vs. [νs(CO)], 1022 vs. [ν(BF)].
trans]: δ = 2.07–2.21 (m, 4H, H3,3,4,4, C4H8S); 2.37–2.48 (m, 1H, C5H4CH2CH2S of trans); 2.62–2.71 (m, 1H, C5H4CH2CH2S of trans); 2.74–2.85 (m,1H, C5H4CH2CH2S of cis); 2.90–3.00 (m, 1H, C5H4CH2CH2S of cis); 3.17 (t, 3J(1H,1H) = 5.8 Hz, 4H, H2,2,5,5, C4H8S); 3.52–3.61 (m, 1H, C5H4CH2CH2S of trans); 3.73–3.80 (m, 1H, C5H4CH2CH2S of trans); 3.94 (dt, 2J(1H,1H) = 12.7 Hz, 3J(1H,1H) = 5.3 Hz, 1H, C5H4CH2CH2S of cis); 4.15–4.22 (m, 1H, C5H4CH2CH2S of cis); 4.97 (d, 3J(1H,1H) = 1.4 Hz, 1H, C5H4 of cis); 5.15 (s, 1H, C5H4 of trans); 5.24 (s, 1H, C5H4 of trans); 5.54 (s, 1H, C5H4 of cis); 5.87 (s, 1H, C5H4 of cis); 6.17 (s, 1H, C5H4 of trans); 6.35 (s, 1H, C5H4 of cis); 6.42 (s, 1H, C5H4 of trans); 7.49–7.54 (m, 5H, C6H5 of cis); 7.55–7.57 (m, 3H, C6H5 of trans); 7.63–7.66 (m, 2H, C6H5 of trans). 13C NMR [126 MHz, CD2Cl2]: δ = 26.7 (C5H4CH2CH2S); 31.0 (SC4H8); 31.2 (SC4H8); 44.4 (SC4H8); 45.0 (SC4H8); 61.5 (C5H4CH2CH2S); 83.3 (C5H4 of cis); 87.2 (C5H4 of cis); 89.1 (C5H4 of cis); 90.4 (C5H4 of trans); 92.7 (C5H4 of trans); 94.6 (C5H4 of trans); 95.2 (C5H4 of trans); 101.1 (C5H4 of cis); 130.5 (C6H5); 130.6 (C6H5); 131.9 (C6H5); 141.5 (C5H4); 231.3 (CO of trans); 232.3 (CO of trans); 241.3 (CO of cis); 244.8 (CO of cis). IR (ATR, cm−1): 1978 vs. [νa(CO)], 1890 vs. [νs(CO)], 1022 vs. [ν(BF)].
Synthesis of [{η5:κS-C5H4(CH2)2SPh}Mo(CO)2{κS-S(CH2)4O}][BF4] (14)
The steps of synthesis followed the procedure for compound 9, but with 2 (198 mg, 0.50 mmol), 1,4-oxathiane (70 μL, 0.75 mmol) and HBF4·Et2O (67 μL, 0.50 mmol). Yield: 221 mg (0.41 mmol, 81%). Red solid. Anal. calc. for C19H21S2O3MoBF4: C: 41.93; H: 3.89; S: 11.78. Found: C: 41.67; H: 3.66; S: 11.57. Positive-ion MS (acetone): m/z (%) = 355 (100) [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OC4H8S]+. 1H NMR [500 MHz, CD2Cl2]: δ = 2.63–2.83 (m, 2H, C5H4CH2CH2S); 2.93–3.06 (m, 4H, OC4H8S); 3.86–3.96 (1H, C5H4CH2CH2S); 3.99 (s, 4H, OC4H8S); 4.22–4.30 (m, 1H, C5H4CH2CH2S); 4.94 (s, 1H, C5H4); 5.58 (s, 1H, C5H4); 5.86 (s, 1H, C5H4); 6.47 (s, 1H, C5H4); 7.49–7.58 (m, 5H, C6H5). 13C NMR [126 MHz, CD2Cl2]: δ = 27.0 (C5H4CH2CH2S); 38.6 (OC4H8S); 54.4 (C5H4CH2CH2S); 69.0 (OC4H8S); 82.8 (C5H4); 87.0 (C5H4); 89.4 (C5H4); 100.6 (C5H4); 130.6 (C6H5); 131.5 (C6H5); 131.6 (C6H5); 142.0 (C5H4); 240.1 (CO); 243.4 (CO). IR (ATR, cm−1): 1984 vs. [νa(CO)], 1901 vs. [νs(CO)], 1030 vs. [ν(BF)].
OC4H8S]+. 1H NMR [500 MHz, CD2Cl2]: δ = 2.63–2.83 (m, 2H, C5H4CH2CH2S); 2.93–3.06 (m, 4H, OC4H8S); 3.86–3.96 (1H, C5H4CH2CH2S); 3.99 (s, 4H, OC4H8S); 4.22–4.30 (m, 1H, C5H4CH2CH2S); 4.94 (s, 1H, C5H4); 5.58 (s, 1H, C5H4); 5.86 (s, 1H, C5H4); 6.47 (s, 1H, C5H4); 7.49–7.58 (m, 5H, C6H5). 13C NMR [126 MHz, CD2Cl2]: δ = 27.0 (C5H4CH2CH2S); 38.6 (OC4H8S); 54.4 (C5H4CH2CH2S); 69.0 (OC4H8S); 82.8 (C5H4); 87.0 (C5H4); 89.4 (C5H4); 100.6 (C5H4); 130.6 (C6H5); 131.5 (C6H5); 131.6 (C6H5); 142.0 (C5H4); 240.1 (CO); 243.4 (CO). IR (ATR, cm−1): 1984 vs. [νa(CO)], 1901 vs. [νs(CO)], 1030 vs. [ν(BF)].
Synthesis of [(η5-C5H5)Mo(CO)2(SMe2)2][BF4] (15)
The steps of synthesis followed the procedure for compound 9, but with [(η5-C5H5)Mo(CO)2(η3-C3H5)] (120 mg, 0.47 mmol), Me2S (104 μL, 1.41 mmol) and HBF4·Et2O (63 μL, 0.47 mmol). Yield: 157 mg (0.37 mmol, 78%). Red solid. Anal. calc. for C11H17S2O2MoBF4: C: 30.86; H: 4.00; S: 14.98. Found: C: 30.62; H: 3.77; S: 14.74. Positive-ion MS (acetone): m/z (%) = 335 (100) [M − 2 SC2H6 + 2 C3H6O]+. 1H NMR [500 MHz, CD2Cl2]: δ = 2.52 (s, 12H, (CH3)2S); 5.76 (s, 5H, C5H5). 13C NMR [126 MHz, CD2Cl2]: δ = 26.9 ((CH3)2S); 95.7 (C5H5); 244.9 (CO). IR (ATR, cm−1): 1982 vs. [νa(CO)], 1888 vs. [νs(CO)], 1029 vs. [ν(BF)]. Single crystals of 15-trans suitable for X-ray diffraction analysis were prepared by overlayering of the dichloromethane solution of 15 with diethyl ether.Synthesis of [(η5-C5H5)Mo(CO)2{(CH2)4S}2][BF4] (16)
The steps of synthesis followed the procedure for compound 9, but with [(η5-C5H5)Mo(CO)2(η3-C3H5)] (109 mg, 0.42 mmol), tetrahydrothiophene (93 μL, 1.05 mmol) and HBF4·Et2O (57 μL, 0.42 mmol)). Yield: 169 mg (0.35 mmol, 84%). Red solid. Anal. calc. for C15H21S2O2MoBF4: C: 37.52; H: 4.41; S: 13.35. Found: C: 37.25; H: 4.14; S: 13.11. Positive-ion MS (acetone): m/z (%) = 307 (100) [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SC4H8]+. 1H NMR [500 MHz, CD2Cl2]: δ = 2.15–2.18 (m, 4H, H3,3,4,4, C4H8S); 3.08–3.20 (m, 4H, H2,2,5,5, C4H8S); 5.72 (s, 5H, C5H5). 13C NMR [126 MHz, CD2Cl2]: δ = 31.2 (C3,4, C4H8S); 44.2 (C2,5, C4H8S); 95.8 (C5H5); 246.1 (CO). IR (ATR, cm−1): 1960 vs. [νa(CO)], 1898 vs. [νs(CO)], 1027 vs. [ν(BF)]. Single crystals of 16-cis suitable for X-ray diffraction analysis were prepared by overlayering of the dichloromethane solution of 16 with diethyl ether.
SC4H8]+. 1H NMR [500 MHz, CD2Cl2]: δ = 2.15–2.18 (m, 4H, H3,3,4,4, C4H8S); 3.08–3.20 (m, 4H, H2,2,5,5, C4H8S); 5.72 (s, 5H, C5H5). 13C NMR [126 MHz, CD2Cl2]: δ = 31.2 (C3,4, C4H8S); 44.2 (C2,5, C4H8S); 95.8 (C5H5); 246.1 (CO). IR (ATR, cm−1): 1960 vs. [νa(CO)], 1898 vs. [νs(CO)], 1027 vs. [ν(BF)]. Single crystals of 16-cis suitable for X-ray diffraction analysis were prepared by overlayering of the dichloromethane solution of 16 with diethyl ether.
Synthesis of [(η5-C5H5)Mo(CO)2{κS-S(CH2)4O}2][BF4] (17)
The steps of synthesis followed the procedure for compound 9, but with [(η5-C5H5)Mo(CO)2(η3-C3H5)] (180 mg, 0.70 mmol), 1,4-oxathiane (164 μL, 1.75 mmol) and HBF4·Et2O (95 μL, 0.70 mmol). Yield: 295 mg (0.58 mmol, 82%). Red solid. Anal. calc. for C15H21S2O4MoBF4: C: 35.17; H: 4.13; S: 12.52. Found: C: 34.93; H: 3.89; S: 12.30. Positive-ion MS (acetone): m/z (%) = 295 (100) [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OC4H8S − CO]+. 1H NMR [500 MHz, CD2Cl2]: δ = 2.79–2.83 (m, 4H, OC4H8S); 3.00–3.04 (m, 4H, OC4H8S); 4.01–4.04 (m, 8H, OC4H8S); 5.79 (s, 5H, C5H5). 13C NMR [126 MHz, CD2Cl2]: δ = 37.6 (OC4H8S); 69.0 (OC4H8S); 95.7 (C5H5); 243.9 (CO). IR (ATR, cm−1): 1981 vs. [νa(CO)], 1903 vs. [νs(CO)], 1049 vs. [ν(BF)].
OC4H8S − CO]+. 1H NMR [500 MHz, CD2Cl2]: δ = 2.79–2.83 (m, 4H, OC4H8S); 3.00–3.04 (m, 4H, OC4H8S); 4.01–4.04 (m, 8H, OC4H8S); 5.79 (s, 5H, C5H5). 13C NMR [126 MHz, CD2Cl2]: δ = 37.6 (OC4H8S); 69.0 (OC4H8S); 95.7 (C5H5); 243.9 (CO). IR (ATR, cm−1): 1981 vs. [νa(CO)], 1903 vs. [νs(CO)], 1049 vs. [ν(BF)].
Synthesis of [{η5:κS-C5H4(CH2)2SEt}Mo(CO)2(NCMe)][BF4] (18)
The steps of synthesis followed the procedure for compound 7, but with 1 (391 mg, 1.13 mmol) and HBF4·Et2O (152 μL, 1.13 mmol). Yield: 454 mg (1.05 mmol, 93%). Red solid. Anal. calc. for C13H16SO2NMoBF4: C: 36.05; H: 3.72; N: 3.23; S: 7.40. Found: C: 35.88; H: 3.47; N: 2.98; S: 7.17. Positive-ion MS (acetone): m/z (%) = 365 (100) [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN + C3H6O]+. 1H NMR [500 MHz, CD2Cl2]: δ = 1.39 (t, 3J(1H,1H) = 7.2 Hz, 3H, SCH2CH3); 2.54 (s, 3H, CH3CN); 2.59–2.66 (m, 1H, C5H4CH2CH2S); 2.81–2.95 (m, 2H, SCH2CH3); 2.93–2.99 (m, 1H, C5H4CH2CH2S); 3.65–3.71 (m, 1H, C5H4CH2CH2S); 4.04–4.12 (m, 1H, C5H4CH2CH2S); 4.82 (s, 1H, C5H4); 5.44 (s, 1H, C5H4); 5.76 (s, 1H, C5H4); 6.52 (s, 1H, C5H4). 13C NMR [126 MHz, CD2Cl2]: δ = 5.5 (CH3, CH3CN); 13.7 (SCH2CH3); 25.9 (C5H4CH2CH2S); 33.4 (SCH2CH3); 53.9 (C5H4CH2CH2S); 83.6 (C5H4); 87.8 (C5H4); 88.0 (C5H4); 104.9 (C5H4); 142.2 (C5H4); 144.7 (CN, CH3CN); 244.2 (CO); 247.8 (CO). IR (ATR, cm−1): 1994 vs. [νa(CO)], 1886 vs. [νs(CO)], 1054 vs. [ν(BF)]. Single crystals of 18 suitable for X-ray diffraction analysis were prepared by overlayering of the dichloromethane solution of 18 with hexane.
MeCN + C3H6O]+. 1H NMR [500 MHz, CD2Cl2]: δ = 1.39 (t, 3J(1H,1H) = 7.2 Hz, 3H, SCH2CH3); 2.54 (s, 3H, CH3CN); 2.59–2.66 (m, 1H, C5H4CH2CH2S); 2.81–2.95 (m, 2H, SCH2CH3); 2.93–2.99 (m, 1H, C5H4CH2CH2S); 3.65–3.71 (m, 1H, C5H4CH2CH2S); 4.04–4.12 (m, 1H, C5H4CH2CH2S); 4.82 (s, 1H, C5H4); 5.44 (s, 1H, C5H4); 5.76 (s, 1H, C5H4); 6.52 (s, 1H, C5H4). 13C NMR [126 MHz, CD2Cl2]: δ = 5.5 (CH3, CH3CN); 13.7 (SCH2CH3); 25.9 (C5H4CH2CH2S); 33.4 (SCH2CH3); 53.9 (C5H4CH2CH2S); 83.6 (C5H4); 87.8 (C5H4); 88.0 (C5H4); 104.9 (C5H4); 142.2 (C5H4); 144.7 (CN, CH3CN); 244.2 (CO); 247.8 (CO). IR (ATR, cm−1): 1994 vs. [νa(CO)], 1886 vs. [νs(CO)], 1054 vs. [ν(BF)]. Single crystals of 18 suitable for X-ray diffraction analysis were prepared by overlayering of the dichloromethane solution of 18 with hexane.
Synthesis of [(η5-C5H5)Mo(CO)2(MeCN)2](CF3SO3) (19)
The steps of synthesis followed the procedure for compound 7, but with [(η5-C5H5)Mo(CO)2(η3-C3H5)] (109 mg, 0.42 mmol) and CF3SO3H (37 μL, 0.42 mmol). Yield: 150 mg (0.33 mmol, 80%). Red solid. Anal. calc. for C12H11N2SO5MoF3: C: 32.16; H: 2.47; N: 6.25; S: 7.15. Found: C: 31.93; H: 2.22; N: 6.02; S: 6.87. 1H NMR [400 MHz, CD2Cl2, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixture of complexes [(η5-C5H5)Mo(CO)2(MeCN)2](CF3SO3) and [(η5-C5H5)Mo(CO)2(MeCN)(CF3SO3)] (a
5 mixture of complexes [(η5-C5H5)Mo(CO)2(MeCN)2](CF3SO3) and [(η5-C5H5)Mo(CO)2(MeCN)(CF3SO3)] (a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b)]: δ = 1.97 (s, 3H, free CH3CN); 2.50 (s, 3H, CH3CN of b); 2.55 (s, 6H, CH3CN of a); 5.74 (s, 5H, C5H5 of b); 5.75 (s, 5H, C5H5 of a). IR (ATR, cm−1): 1990 vs. [νa(CO)], 1882 vs. [νs(CO)], 1261 vs. [νa(SO3)], 1224 m [νs(CF3)], 1151 s [νa(CO)], 1032 s [νs(SO3)]. Single crystals of 19 suitable for X-ray diffraction analysis were prepared by overlayering of the acetonitrile solution of 19 with diethyl ether.
b)]: δ = 1.97 (s, 3H, free CH3CN); 2.50 (s, 3H, CH3CN of b); 2.55 (s, 6H, CH3CN of a); 5.74 (s, 5H, C5H5 of b); 5.75 (s, 5H, C5H5 of a). IR (ATR, cm−1): 1990 vs. [νa(CO)], 1882 vs. [νs(CO)], 1261 vs. [νa(SO3)], 1224 m [νs(CF3)], 1151 s [νa(CO)], 1032 s [νs(SO3)]. Single crystals of 19 suitable for X-ray diffraction analysis were prepared by overlayering of the acetonitrile solution of 19 with diethyl ether.
Author contributions
The authors contributed equally.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Ministry of Education, Youth and Sports of the Czech Republic for support through project No. UPA/SGS_2023_009 and to the Charles University Cooperation Program, research area DIAG.References
- D. Seyferth, Organometallics, 2001, 20, 2–6 CrossRef CAS.
- K. Meyer and H. Braunschweig, Organometallics, 2018, 37, 625–627 CrossRef CAS.
- R. A. Love, T. F. Koetzle, G. J. B. Williams, L. C. Andrews and R. Bau, Inorg. Chem., 1975, 14, 2653–2657 CrossRef CAS.
- J. Petit, L. l Magna and N. Mézailles, Coord. Chem. Rev., 2022, 450, 214227 CrossRef CAS.
- H. Olivier-Bourbigou, P. A. R. Breuil, L. Magna, T. Michel, M. Fernandez Espada Pastor and D. Delcroix, Chem. Rev., 2020, 120, 7919–7983 CrossRef CAS PubMed.
- A. Nakamura, S. Ito and K. Nozaki, Chem. Rev., 2009, 109, 5215–5244 CrossRef CAS PubMed.
- X. Hu, X. Kang and Z. Jian, Angew. Chem., Int. Ed., 2022, 61, e202207363 CAS.
- O. S. Soficheva, G. E. Bekmukhamedov, A. B. Dobrynin, J. W. Heinicke, O. G. Sinyashin and D. G. Yakhvarov, Mendeleev Commun., 2019, 29, 575–577 CrossRef CAS.
- A. A. Kagileva, A. A. Kagilev, A. O. Kantyukov, Z. N. Gafurov, I. F. Sakhapov, G. E. Bekmukhamedov, K. R. Khayarov, E. M. Zueva, O. S. Soficheva and D. G. Yakhvarov, New J. Chem., 2022, 46, 17303–17312 RSC.
- O. M. Ogba, N. C. Warner, D. J. O'Leary and R. H. Grubbs, Chem. Soc. Rev., 2018, 47, 4510–4544 RSC.
- R. R. Schrock, in Handbook of Metathesis, ed. R. H. Grubbs, A. G. Wenzel, D.l J. O'Leary and E. Khosravi, Wiley-VCH, Weinheim, 2nd edition, 2015, ch. 1, pp. 1–32 Search PubMed.
- K. M. Dawood and K. Nomura, Adv. Synth. Catal., 2021, 363, 1970–2019 CrossRef CAS.
- B. W. Grau, A. Neuhauser, S. Aghazada, K. Meyer and S. B. Tsogoeva, Chem. – Eur. J., 2022, 28, e2022014 Search PubMed.
- S. Takebayashi, M. A. Iron, M. Feller, O. Rivada-Wheelaghan, G. Leitus, Y. Diskin-Posner, L. J. W. Shimon, L. Avram, R. Carmieli, S. G. Wolf, I. Cohen-Ofri, R. A. Sanguramath, R. Shenhar, M. Eisen and D. Milstein, Nat. Catal., 2022, 5, 494–502 CrossRef CAS.
- D. S. Belov, L. Mathivathanan, M. J. Beazley, W. B. Martin and K. V. Bukhryakov, Angew. Chem., Int. Ed., 2021, 60, 2934–2938 CrossRef CAS PubMed.
- M. Navarro, M. G. Alférez, M. de Sousa, J. Miranda-Pizarro and J. Campos, ACS Catal., 2022, 12, 4227–4241 CrossRef CAS PubMed.
- M. Navarro, J. Miranda-Pizarro, J. J. Moreno, C. Navarro-Gilabert, I. Fernández and J. Campos, Chem. Commun., 2021, 57, 9280–9283 RSC.
- Y. Ning, T. Ohwada and F. Chen, Green Synth. Catal., 2021, 2, 247–266 CrossRef.
- R. A. Baillie, G. P. Lefèvre, R. J. Wakeham, A. S. Holmes and P. Legzdins, Organometallics, 2015, 34, 4085–4092 CrossRef CAS.
- R. J. Wakeham, R. A. Baillie, B. O. Patrick, P. Legzdins and D. C. Rosenfeld, Organometallics, 2017, 36, 39–52 CrossRef CAS.
- O. Mrózek, L. Dostál, I. Císařová, J. Honzíček and J. Vinklárek, Dalton Trans., 2019, 48, 12210–12218 RSC.
- D. E. Ryan, D. J. Cardin and F. Hartl, Coord. Chem. Rev., 2017, 335, 103–149 CrossRef CAS.
- J. W. Faller, C.-C. Chen, M. J. Mattina and A. Jakubowski, J. Organomet. Chem., 1973, 52, 361–386 CrossRef CAS.
- D. Parasar, A. H. Elashkar, A. A. Yakovenko, N. B. Jayaratna, B. L. Edwards, S. G. Telfer, H. V. Rasika Dias and M. G. Cowan, Angew. Chem., 2020, 132, 21187–21192 CrossRef.
- L. Giannini, G. Guillemot, E. Solari, C. Floriani, N. Re, A. Chiesi-Villa and C. Rizzoli, J. Am. Chem. Soc., 1999, 121, 2797–2807 CrossRef CAS.
- P. W. Dyer, V. C. Gibson, J. A. K. Howard, B. Whittle and C. Wilson, Polyhedron, 1995, 14, 103–111 CrossRef CAS.
- E. Otten, A. Meetsma and B. Hessen, J. Am. Chem. Soc., 2007, 129, 10100–10101 CrossRef CAS PubMed.
- I. Tokue, T. Fukuyama and K. Kuchitsu, J. Mol. Struct., 1973, 17, 207–223 CrossRef CAS.
- C. R. Barone, R. Cini, S. de Pinto, N. G. Di Masi, L. Maresca, G. Natile and G. Tamasi, Inorg. Chim. Acta, 2010, 363, 205–212 CrossRef CAS.
- R. Pryadun, D. Sukumaran, R. Bogadi and J. D. Atwood, J. Am. Chem. Soc., 2004, 126, 12414–12420 CrossRef CAS PubMed.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., 2016, B72, 171–179 Search PubMed.
- N. Kuhn, H. Schumann and E. Zauder, J. Organomet. Chem., 1988, 354, 161–167 CrossRef CAS.
- J. R. Ascenso, C. G. de Azevedo, I. S. Goncalves, E. Herdtweck, D. S. Moreno, C. C. Romao and J. Zuehlke, Organometallics, 1994, 13, 429–431 CrossRef CAS.
- O. Mrózek, L. Šebestová, J. Vinklárek, M. Řezáčová, A. Eisner, Z. Růžičková and J. Honzíček, Eur. J. Inorg. Chem., 2016, 2016, 519–529 CrossRef.
- C. C. L. Pereira, S. S. Braga, F. A. Almeida Paz, M. Pillinger, J. Klinowski and I. S. Gonçalves, Eur. J. Inorg. Chem., 2006, 2006, 4278–4288 CrossRef.
- J. Honzíček, P. Kratochvíl, J. Vinklárek, A. Eisner and Z. Padělková, Organometallics, 2012, 31, 2193–2202 CrossRef.
- W. L. F. Armarego and D. D. Perin, Purification of Laboratory Chemicals, Butterworth Heinemann, Oxford, Boston, 4rd edn, 1996 Search PubMed.
- J. R. Ascenso, C. G. de Azevedo, I. l S. Goncalves, E. Herdtweck, D. S. Moreno, M. Pessanha and C. C. Romao, Organometallics, 1995, 14, 3901–3919 CrossRef CAS.
- D. A. Clark, D. L. Jones and R. J. Mawby, J. Chem. Soc., Dalton Trans., 1980, 4, 565–569 RSC.
- I. S. Gonçalves and C. C. Romão, J. Organomet. Chem., 1995, 48, 155–161 CrossRef.
- Rigaku, CrysAlisPRO, Version 1.0.43, Rigaku Oxford Diffraction, Yarnton, UK, 2022 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., 2015, A71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., 2008, A64, 112–122 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., 2015, C71, 3–8 CrossRef PubMed.
- X.-F. Hou, X.-C. Wang, J.-Q. Wang and G.-X. Jin, J. Organomet. Chem., 2004, 689, 2228–2235 CrossRef CAS.
- H. Schumann, K. Herrmann, S. H. Mühle and S. Dechert, Z. Anorg. Allg. Chem., 2003, 629, 1184–1194 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra and CCDC 2244384–2244391. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra03383j |
| This journal is © The Royal Society of Chemistry 2023 |





