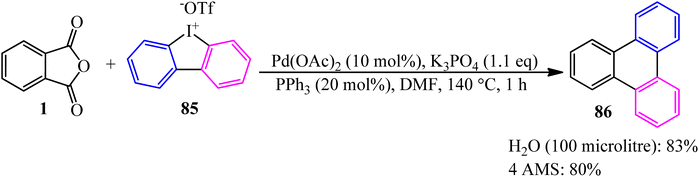Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Phthalic anhydride (PA): a valuable substrate in organic transformations
Kobra Nikoofar * and
Mansoorehsadat Sadathosainy
* and
Mansoorehsadat Sadathosainy
Department of Organic Chemistry, Faculty of Chemistry, Alzahra University, P.O. Box 1993891176, Tehran, Iran. E-mail: k.nikoofar@alzahra.ac.ir; kobranikoofar@yahoo.com; Fax: +98 2188041344; Tel: +98 2188041344
First published on 15th August 2023
Abstract
This review has been centralized on applications of phthalic anhydride (PA) as a valuable and significant heterocyclic substrate in two- and multicomponent organic reactions. The article has been subdivided into the following parts: (i) PA introduction by focusing on its characterization, synthesizing procedure, and multiple-aspect applications. In addition, the previous review articles based on PA have also been indicated; (ii) the applications of PA as a substrate have been subdivided into parts with a glance on the reaction components numbers; (iii) the applications of PA in esterification reactions; and (iv) some examples of PA in multistep synthesis. The review covers the corresponding literature up to the end of 2022. According to the abovementioned classifications, PA is a potent substrate to design a wide range of heterocyclic compounds that possess various kinds of properties and applications in chemistry, industry, and pharmaceuticals.
1. Introduction
2-Benzofuran-1,3-dione (isobenzofuran-1,3-dione, 1,3-isobenzofuranidone, 1,3-dioxophthalan, phthalandione, 1,3-phthalandione, 1,2-benzenedicarboxylic acid anhydride, phthalic acid anhydride, 1,2-benzenedicarboxylic anhydride, 1,3-dihydro-1,3-dioxoisobenzofuran) with the common/popular name of phthalic anhydride (PA) is a versatile organic compound with the formula C6H4(CO)2O. It is a white (or somehow colorless) powder (or flakes and sometimes needles) with a melting point of 131 °C, molar mass of 148.1 g mol−1, and some acidic odor. Actually, this fused bicycle with the LD50 = 1.530 mg kg−1 (oral rat) is the anhydride form of phthalic acid. It is soluble in ethanol, acetone, water, and benzene but sparingly in ether and chloroform.1 Contact with skin and eye may cause an allergic skin reaction and serious eye damage. Basketter and Kimber published a review article in 2016, in which the absence of both skin- and respiratory-sensitizing capacity of PA was confirmed based on interpreting predictive toxicology tests for skin sensitization.2This white solid was first synthesized by Auguste Laurent in 1836. The first procedure involved liquid-phase mercury-catalyzed oxidation of naphthalene.3 In the modern process, vanadium pentoxide was used as the catalyst in a gas-phase reaction with naphthalene using molecular oxygen.4 Rindone group in 2010 prepared PA via the ozonation of naphthalene.5
An alternative synthetic procedure is also based on the oxidation of o-xylene by vanadium-based catalysts such as V2O5/TiO2 and anatase-supported vanadium oxide.6–8 In 2018, Co–Mn/H3PW12O40@TiO2 was also utilized as the catalyst for the selective vapor-phase oxidation of o-xylene to PA in the presence of green oxidant.9 Boger and Menegola also used extruded monoliths with high thermal conductivity as improved operated and economic catalysts to prepare PA from the oxidation of o-xylene.10 This technology was also studied in detail by Tronconi group in 2012.11 The air oxidation of o-xylene over mesoporous V-Mo-MCM-41 molecular sieves was also applied for PA synthesis by Selvaraj and Lee in 2005.12 PA can be also produced from phthalic acid.13 Nikolov's research group published a review article on the synthesis of PA from o-xylene in detail.14 Dias and Portela in 1997 issued a review article that discussed about various catalysts to gain PA from two main routes for its synthesis, which concerns the oxidation of o-xylene and naphthalene.15
Recently some green and novel protocols have been presented to obtain PA such as renewable production from biomass-derived furan and maleic anhydride.16 Deng group in 2021 also reported green production of PA from biobased furan and maleic anhydride by an acid resin catalyst.17 PA obtained renewably form 5-hydroxymethfurfural (HMF) using MoO3/Cu(NO3)2 as a catalyst.18 Sha group in 2016 designed a conceptual process to prepare PA from corn stover, an agricultural residue. Their techno-economic assessment contends energy integration alternatives as well as water consumption and life cycle greenhouse emissions.19 Ierapetritou and coworkers in 2015 accounted a novel route for PA production from hemicellulose solutions, in which they focused on synthetic process, technoeconomic analysis, and life cycle assessment (LCA) of the protocol.20
Kinney and Pincus in 1951 reported PA production from catalytic air oxidation of some substrates such as certain higher aromatics and coal tar fractions.21 The PA synthesis by the oxidation of tar oils was also represented by Shelmerdine's group in 1953.22
Prichard in 1956 obtained PA from the reaction of bromobenzene and carbon monoxide in the presence of sodium carbonate and nickel carbonyl.23
Zazhigalov and Kiziun in 2017 reported PA production by Diels–Alder reaction during n-pentane oxidation on vanadium phosphorus oxide (VPO) catalyst.24 Kim and Yang in 2000 studied the synthesis of maleic and phthalic anhydrides from the mixture of cyclopentene and 1-pentene via their selective oxidations under different reaction parameters.25
In 1919, Gibbs published the title “phthalic anhydride. I-introduction”, which discussed the different synthetic procedures of PA.26 Gibbs in 1920 also interpreted the title “history of the preparation and properties of pure phthalic anhydride”.27
The chemical engineering design of the PA preparation reactor is considered based on the computer model that simulates the operation of the reactor over a range of conditions that lead to both satisfactory mass transfer and heat transfer requirements.28 Wainwright and Foster in 1979, reviewed the “catalysts, kinetics, and reactor design in phthalic anhydride synthesis”.29
PA has been utilized in different fields of science and technology. In polymerization technologies, some examples such as preparation of ternary polymeric system including polysulfide (PS), diglycidylether of bisphenol A resin, and PA have been reported. PA plays a role in the curing reaction of this ternary polymer.30 The copolymerization of PA with epoxides catalyzed by amine-bis(phenolate)chromium(III) complexes also performed in 2021.31 Amin's group in 2011 prepared two hyperbranched polyesteramides (HB1 and HB2) by the bulk reaction between PA and diisopropanolamine (DiPA) or diethanolamine (DEA), respectively. The effects of various solutions of HB1 and HB2 on the properties (such as measurements of water of consistency, setting times, bulk density, apparent porosity, and compressive strength for the cement pastes) of ordinary Portland cement (OPC) and Portland limestone cement (PLC) were studied.32 Duchateau's group constructed a partially renewable polyester via the catalytic ring-opening copolymerization of limonene oxide and PA.33
Pooley and coworkers in 2005 copolymerized PA as an electrophilic monomer with aziridine or 2-methylaziridine as nucleophilic monomers in the absence of initiator under various experimental conditions.34
PA was utilized as a bridge for the grafting of chitosan biopolymer onto wool fabric to prepare antibacterial agents.35 It also played a role in the chemical modification of chitosan to obtain bacteria inhibitors.36 In 2020, a novel biodegradable diblock/triblock poly(ester-bicarbonate)s was prepared from cyclohexene oxide (CHO), propylene oxide (PO), PA, and CO2 in a one-pot/one-step protocol.37 The surface modification of silk fiber using PA to graft the polysaccharide chitosan has been reported in 2009, which lead to the dyeing ability of the grafted silk. The grafted samples possess antibacterial potential.38
PA is also a critical substrate to prepare a series of new optically active and thermally stable polyamides (PAs).39 It plays the role of a chain end group in the fluorescent PMMAs polymers. These polymers can detect the intermacromolecular reaction in reactive polymer blends at a low concentration using fluorescence-gel permeation chromatography.40
It has also utilized as a key substrate for the multistep preparation of novel optically active polyamides derived from 5-(3-methyl-2-phthalimidylpentanoylamino)isophthalic acid.41 PA was also utilized for the preparation of chiral polyesters through enantioselective terpolymerization with racemic and meso-epoxides and also sequence-controlled block copolymers.42
PA was also applied as a modifier in poly(butylene succinate-co-butylene adipate) (PBSA)/cellulose nanocrystal (CNC) composites. The modified PBSA/CNC composites demonstrated elevated mechanical properties, fast crystallization, and improved hydrophobicity.43 PA has been added in the preparation procedure of poly(butylenes succinate)/cellulose nanocrystals (PBS/CNC) composite via melt blending, which yielded higher crystallinity and smaller crystals.44
To obtain phthalylated cellulosic compounds with higher DS (degree of substitution), the chemical modification of sugarcane bagasse cellulose has been reported with PA in the 1-butyl-3-methyl imidazolium chloride solvent and 4-dimethylaminopyridine (DAMP) catalyst.45
PA is also significant in different fields of biological and pharmaceutical applications. It is a key substrate in the synthesis of symmetrical novel organoselenocyanates and diselenides dye stuffs, which was evaluated for the antitumor properties.46 In 2012, N-benzoyl 3-nitro-phthalimide, which possesses anxiolytic activity in mice model, was prepared from PA as the substrate.47
Bis[aqua-1,8-(1,2-dicarboxamido benzene)3,6-diazaoctane copper(II)/nickel(II)] tetrachloride, as two binuclear complex, was synthesized by a two-component one-pot metal template condensation between PA and 1,8-diamino-3,6-diazaoctane. The complexes are able to bind to calf thymus (CT)-DNA under physiological pH.48 Lepoittevin et al. in 2021 investigated the reactivity of PA as a chemical respiratory sensitizer toward reconstructed human epidermis (RHE).49
Spanedd and Bourel-Bonnet published a review article about the potential of cyclic anhydrides such as PA in bioconjugation to functionalize the biomolecules and carriers. The pH-dependent stability and reactivity, as well as the physical properties, can be tuned by the structure of the cyclic anhydride used. Thus, their application in smart delivery systems has become very important.50
Alheety in 2021 prepared new complexes of PA (as ligand) with some cations (such as Co(II), Ni(II), Cu(II), Mn(II), and Zn(II)) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The microbicide activity studies of the synthesized complexes against four types of bacteria (E. coli, S. epidermidis, K. pneumoniae, and S. aureus) was also reported. The complexes also demonstrated stability on laser beams for 10–30 s.51
1 molar ratio. The microbicide activity studies of the synthesized complexes against four types of bacteria (E. coli, S. epidermidis, K. pneumoniae, and S. aureus) was also reported. The complexes also demonstrated stability on laser beams for 10–30 s.51
Marzouk's group in 2016 designed and manufactured some new phthalazinones containing benzyl moiety via a multistep reaction started from PA. Some of the heterocycles demonstrated antitumor activity.52 Yamaguchi and coworkers in 1998 constructed some 2-[2-(L-imidazolyl)alkyl]-1(2H)-phthalazinones as novel antiasthmatic agents with dual activities of thromboxane A2 synthetase inhibition and bronchodilation.53 Costa Silva group in 2021 modified Chicha gum with PA to obtain a new biologically active material that demonstrated excellent inhibitory effect against P. aeruginosa and K. pneumoniae species (rating 100% inhibition) and could also inhibit Escherichia coli growth.54 Bold et al. in 2000 synthesized new anilinophthalazines as potent and orally well absorbed inhibitors of the VEGF receptor tyrosine kinases useful as antagonists of tumor-driven angiogenesis. The key substrate for the preparation procedure starts from PA.55
PA also has a special role in sensors. In 2016, phthaloylchitosan (PHCS) was synthesized simply and cost-effectively using chitosan and PA by microwave irradiation, which was applied to determine tyrosine with high sensitivity and good selectivity through carbon nanotube film-coated glassy carbon electrode.56 Zhang et al. in 2022 reported a novel phthalic anhydride-based room-temperature phosphorescence (RTP) emitter with the lifetime longer than one second.57
A novel fluorescent probe IMPD, based on imidazo[1,2-a]pyridine that contained PA moiety in its structure, was designed and synthesized by Huang's research group in 2021. The probe could detect hydrazine via its maleimide as the recognition group. In addition, the probe IMPD could dye the HepG2 cell with blue color in the presence of hydrazine.58
Another notable application of PA is in the dye industry. It utilized to prepare the nanosized copper phthalocyanin blue (CuPc) pigments.59 The phenol precipitation and dye bleaching capabilities of phthalic anhydride-modified horseradish peroxidase C (PA-HRP) were also investigated in 2000.60
Polymeric surfactants have been synthesized by the reaction of maleic anhydride (MA), polyethylene glycol (PEG), and PA, which exhibit excellent surface-active properties (including surface tension, low-foaming, solubilization, and dispersant properties) in disperse dye systems.61
PA also plays a role in separation and waste removal. In 2016, the Gurgel group prepared chemically-modified sugarcane bagasse, named as carboxylate-functionalized sugarcane bagasse (SPA), via the reaction of PA with sugarcane bagasse. The SPA adsorbent was used to remove Co2+, Cu2+, and Ni2+ from aqueous solution in mono- and multicomponent systems in the batch mode.62
Another aspect of the PA usage is in the field of catalyst and protecting group. It was utilized as a part of the co-catalytic system (in combination with Zn(OTf)2) to promote Beckmann rearrangement.63 The efficient and clean oxidation of sulfides to sulfones (not the probable sulfoxide) with urea–hydrogen peroxide in the presence of PA in ethyl acetate was also performed in 2018.64 PA also worked as a remarkable protective group in the synthesis of a dipeptide (β-alanine-L-histidine) via two procedures, which are solution phase peptide synthesis (SPS) and solid phase peptide synthesis (SPPS).65
Liu et al. in 2017 utilized PA as a low-temperature activator in the H2O2 bleaching system for cotton fabric. The performance of the H2O2/PA bleaching system was investigated by measuring the CIE whiteness index (WI) of the bleached cotton fabric, H2O2 decomposition rate, and bursting strength, respectively.66
Maldas and Kokta in 1990 considered the performance of PA as a coupling agent in wood fiber-filled polystyrene composites. Its presence evaluated the mechanical properties of the composite materials.67
Duan in 2019 interpreted PA-promoted ring-opening cationic polymerization of cyclohexene oxide catalyzed by dinuclear chromium complex supported by piperazine-bridged [ONSO] ligand to obtain atactic poly(cyclohexeneoxide) polymer. The formation of carbocation species by interaction between PA and bimetallic chromium complexes is the real initiator center.68
The hydrogenation of PA is another important reaction that has been evaluated extensively. Liquid phase selective hydrogenation of PA to phthalide (an important industrial intermediate for pharmaceuticals, fine chemicals, and organic synthesis) was reported in 2015 in the presence of Au/FeOx–TiO2 catalyst.69,70 Liquid phase hydrogenation of PA to phthalide over Au/TiO2 catalysts (with different gold loadings) was reported in 2009.71 Different catalytic systems were reported for PA hydrogenation to phthalide, such as acid-tolerant intermetallic cobalt–nickel silicides as noble metal like catalysts,72 CoSix/CNTs,73 hydrophobic activated carbon supported Ni-based acid-resistant catalyst,74 and Al2O3-supported NiCu alloy.75
PA is applicable in some other fields as well. Al-Sawaad and Alwaaly research group in 2021 reported bisthioureaphthalatonickel(II) complex (PTUNi) via the reaction of NiCl2·6H2O with thiourea (2 mol) and PA (1 mol). The complex was evaluated as a corrosion inhibitor for carbon steel alloy (C1010) against a corrosive medium of 0.1 M hydrochloric acid at 298 K.76 Fouda group in 2013 also reported anhydride derivatives (such as PA) as corrosion inhibitors for carbon steel in hydrochloric acid solutions.77 In 2022, glycerol/PA novel nanocomposite was introduced for microwave absorbing applications.78
Velayutham and coworkers in 2009 demonstrated the synthesis and characterization of polyurethane (PUR) coatings derived from polyols synthesized with glycerol, PA, and oleic acid. The utilized polyols were designated as Alk28, Alk40, and Alk65, in which 28%, 40%, and 65% of oleic acid was present, respectively. The coatings obtained from polyol Alk28, with the lowest percentage of oleic acid content, exhibited the best overall physicochemical properties, followed by Alk40. PUR from polyol with the highest percentage of oleic acid content, Pualk65 coatings, were softer and their anticorrosive properties were less satisfactory.79 Son in 1975 studied the role of PA in cure retardation of rubbers.80
Based on the importance of PA in diverse fields of science and technology, many review articles have been written. Dubey and coworkers in 1996 published a review article about the importance of phthalic anhydride as a petrochemical agent.81 In 2002, a review article entitled “Some new applications of phthalic anhydride in organic synthesis” was published by the Iordache research group.82 In 2021, Elgharbawy reported a mini-article entitled “A review on phthalic anhydride industry and uses”.83 In 2016, Basketter and Kimber discussed about PA as a chemical allergen in detail. They claimed that it displays a differentiated behavior, whereas most respiratory sensitizers are known also to give rise to delayed skin reactions; evidence for PA suggests that it only causes immediate type allergy.84 Siddiqui and Javed research group study the computational, spectroscopic, Hirshfeld surface, electronic state, and molecular docking properties (with 21 different protein receptors) of PA.85 Hong et al. in 2017 investigated the antiinflammatory effect of titrated extract of Centella asiatica in PA.86 In 1977, a patient with occupational asthma caused by PA was reported.87 Yanagimoto in 1956 discussed about the reaction between urea and PA under pressure.88 The Martin group in 2015 published a book-chapter entitled “Anhydride-based multicomponent reactions”, which contains some reactions of PA.89
2. Applications of PA in two-component reactions
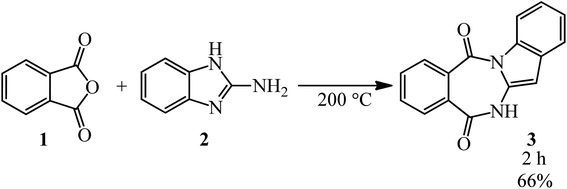
Katritzky and Yates in 1976 prepared 6H-benzimidazo[1,2-b][2,4]benzodiazepine-7,12-dione (3) via the catalyst-free reaction of PA (1) and 2-aminobenzimidazole (2) (Scheme 1).90Gitis group in 2000 reported the reaction of PA with 2-methylimidazole that formed amide.91 The authors also examined the reaction of maleic anhydride that leads to the formation of molecular complexes.
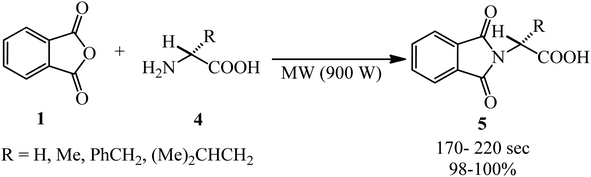
Hajipour et al. in 2000 interpreted the phthloyation microwave-assisted reaction of PA (1) with amino acids (4) under solvent-free conditions to obtain phthalimide derivatives without racemization (5) (Scheme 2).92 The substrates were mixed with silica gel and irradiated with microwave (900 W) for an appropriate time.
Billman and Harting in 1948 obtained the phthalyl derivatives of different kinds of amino acids (5) via the reaction of PA (1) with (4) at 180–185 °C within 15 min with 30–92% yield.93 Zeng research group in 2004 also reported the N-phthaloylation of amino acids (Gly, Ala, Phe, Val, 10 mmol) with PA or phthalic acid (11 mmol) with amino acids at 130–135 °C under pressure (about 40 mmHg) for 15–30 min with 79.4–90.5% yield.94 Kidd and King in 1948 also reported the preparation of phthalyl-L-glutamic acid.95 Leite et al. in 2014 demonstrated the microwave-assisted synthesis of some phthaloyl amino acids from the reaction of PA (1) and amino acids (4, Gly, Ala, Val, Glu, Phe, Ile, and Trp) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in TEA or 4-DMAP (0.5 mL) and DMF (three drops) within 2 min by 31.9–96%. The products presented antioral inflammatory activity comparable to thalidomide. Most of the compounds effectively suppressed nitric oxide production in murine cells stimulated with lipopolysaccharide.96 Ökten and coworkers in 2022 reported the facile, expeditious, and cost-effective preparation of N-phthaloyl (S)-amino acids and evaluated their in silico activities against Staphylococcus aureus.97
1 molar ratio in TEA or 4-DMAP (0.5 mL) and DMF (three drops) within 2 min by 31.9–96%. The products presented antioral inflammatory activity comparable to thalidomide. Most of the compounds effectively suppressed nitric oxide production in murine cells stimulated with lipopolysaccharide.96 Ökten and coworkers in 2022 reported the facile, expeditious, and cost-effective preparation of N-phthaloyl (S)-amino acids and evaluated their in silico activities against Staphylococcus aureus.97
In 1958, the reaction of PA with different amino acids by refluxing in nonpolar solvents (such as benzene and toluene) in the presence of triethylamine was performed. By separating the water formed in the reaction, the phthalimide derivatives were prepared in good yields and without racemization. Phthaloylation without racemization may also be carried out in N,N-dimethylformamide medium.98
Homsi and Kasideh in 2015 obtained N-phthalimide amino acids from PA and amino acids (Gly, Ala, Phe, Val, Leu, and Asp) in refluxing glacial AcOH for 2 h with 66.8–95.8% yield. The synthesized compounds, which were purified through recrystallization from ethanol, were screened for their antimicrobial activity against four microorganisms, namely, Streptococcus epidermidis, Escherichia coli, Mycobacterium tuberculosis, and Candida albicans.99
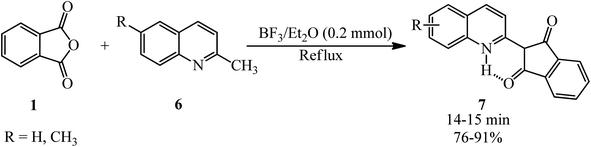
Safari et al. in 2009 achieved quinophthalone pigments (7) through the reaction of PA (1) and 2-methylquinolines (6) in the presence of BF3/Et2O as the catalyst under solvent-free and reflux conditions (Scheme 3).100
Loghmani-Khouzani in 2004 also gained some quinophthalones (7) via the microwave-assisted (700 W) reaction of PA (1) and 2-methylquinolines (6) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in the presence of silica gel (silica gel 60, 230–240 mesh Merck, 300 mg) within 2 min by 85–97%.101
1 molar ratio in the presence of silica gel (silica gel 60, 230–240 mesh Merck, 300 mg) within 2 min by 85–97%.101
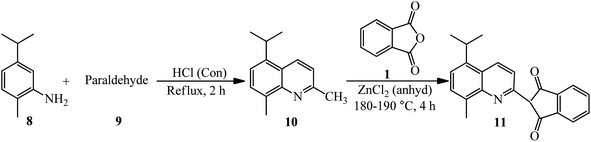
Phillips and Goss in 1926 obtained methyl-isopropyl-quinoline yellow (11) through the reaction of PA with methylisopropyl-quinaldine (10). The product (10) was also achieved from 2-amino-p-cymene (8) and paraldehyde (9) (Scheme 4).102
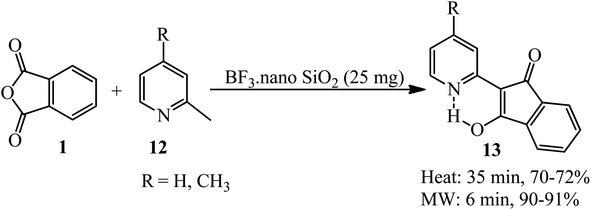
Safari et al. in 2012 performed simple regioselective one-pot solvent-free reaction of 2-methylpyridines (12) and PA (1) in the presence of BF3·nano SiO2 (as a solid supported catalyst) at conventional heating and also under microwave irradiation to prepare the corresponding pyrophthalones (13) (Scheme 5).103
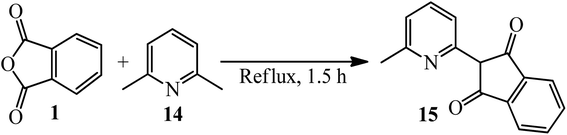
Cook and Martin in 1954 acquired orange crystals of 2-(6-methyl-2-pyridyl)-l,3-indanedione (15) via the reaction of PA (1) and 2,6-dimethylpyridine (14) in equimolar ratio under reflux conditions within 1.5 h. Their attempts to perform the condensation of both methyl groups through using 2 equimolar of PA were not successful, and only the mono-condensation product (15) as orange crystals was isolated (Scheme 6).104 The authors discussed about the chelating tendency of the obtained product with some bivalent-metal ions at 30 °C in a 75% (v/v) dioxane–water solution.
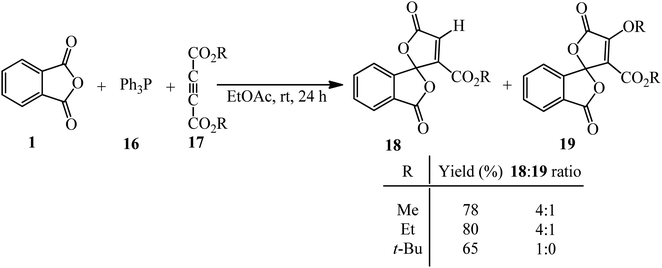
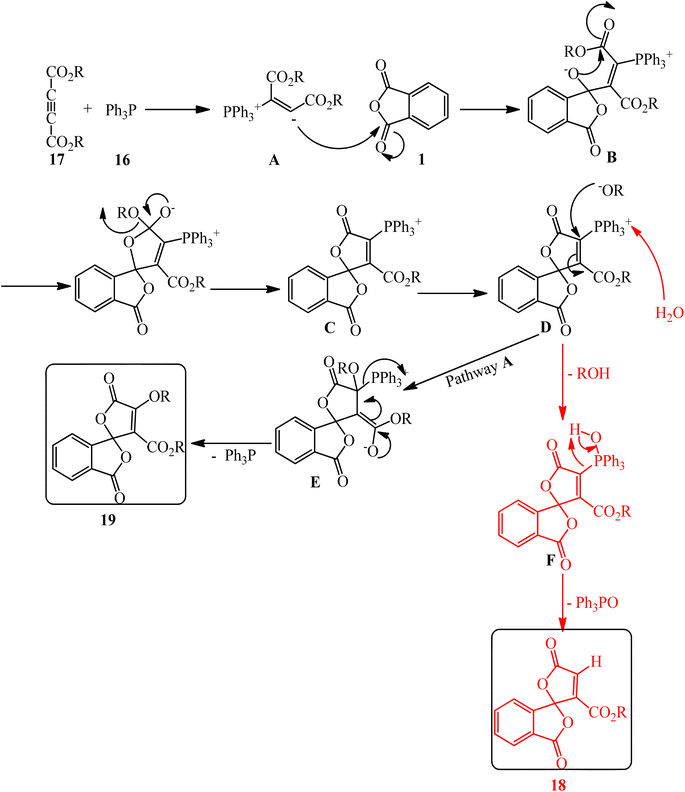
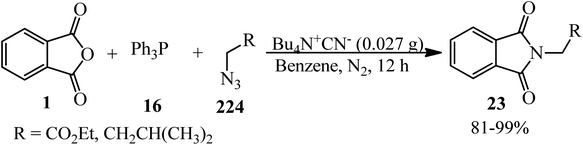
Bayat et al. in 2010 reacted PA (1) and dialkyl acetylenedicarboxylates (17) to afford the novel spirocyclic compounds (18, 19) at room temperature via the promoting effect of triphenylphosphine (16) (Scheme 7).105 Although Ph3P has been utilized in 1 mmol amount, similar to the other two substrates, but according to the proposed mechanism (Scheme 8) and also the products structure, its role is just that of a promoting agent. According to the mechanism, the first step is the formation of 1,3-dipolar intermediate (A), which attacked PA (1) to obtain zwitterionic intermediate (B), which cyclized to the spiro intermediate (D). Water attack to the positively charged phosphorus ion of (D) formed (F), followed by a proton transfer and loss of Ph3PO, which led to product (18). In pathway A, intermediate (D) was attacked by the alkoxy anion, and the subsequent loss of Ph3P gave compound (19).
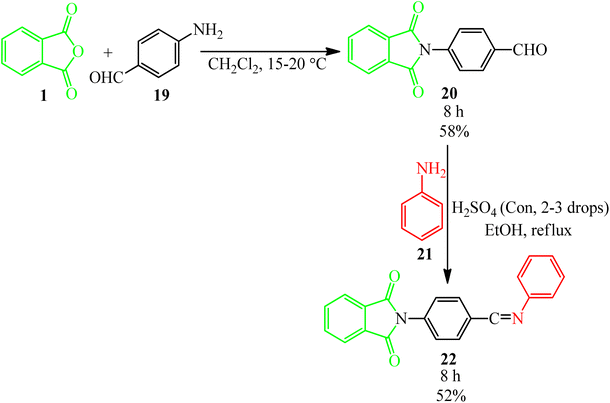
Sharma's research group in 2012 synthesized some Schiff bases possessing imide moieties through multistep reactions initiated from the reaction of PA (1) and 4-aminobenzaldehyde (19) in dichloromethane to obtain 4-(1,3-dioxoisoindolin-2-yl)benzaldehyde (20). In the second step (20), reacted with anilines (21) in the presence of glacial acetic acid to obtain the Schiff bases (22), which demonstrated analgesic and antiinflammatory activities (Scheme 9).106
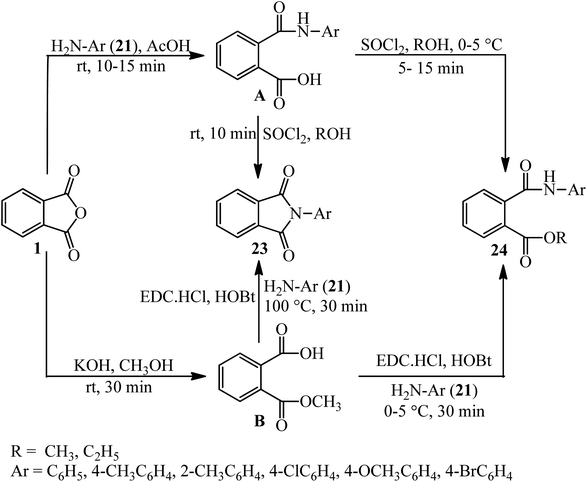
Kumar et al. in 2014 described the reaction of PA (1) with amines (21) to obtain monoacid monoamides (A), which get the corresponding cyclic imide derivatives (23) in the presence of SOCl2. The PA also reacted with KOH to obtain monomethyl ester (B), which reacted with amines in the presence of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimid hydrochlorid (DEC·HCl) and N-hydroxy benzotriazole (HOBt) to get the cyclic imide derivatives (24) (Scheme 10).107
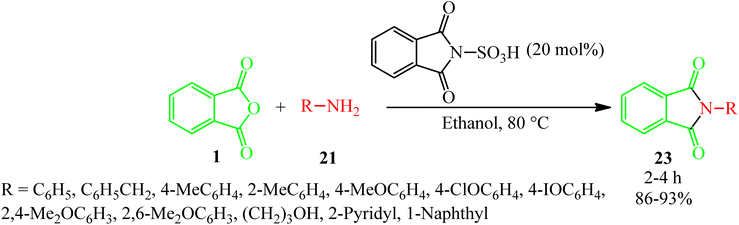
Habibi and Mohammadkhani Pordanjani in 2017 developed an efficient and easy catalytic protocol for the preparation of various isoindoline-1,3-diones (23) via the reaction of PA (1) with aromatic, aliphatic, and benzylic amines (21), using a catalytic amount of phthalimide N-sulfonic acid in ethanol at 80 °C (Scheme 11).108
Chia group in 2019 prepared the isoindoline-1,3-diones via the reaction of anilines and PA in the presence of “water extract of onion peel ash” (WEOPA) (2 mL) as a green catalytic-solvent system at 80 °C within 8 min by 64–92%.109 The WEOPA was recycled and reused for 5 runs without significant activity loss.
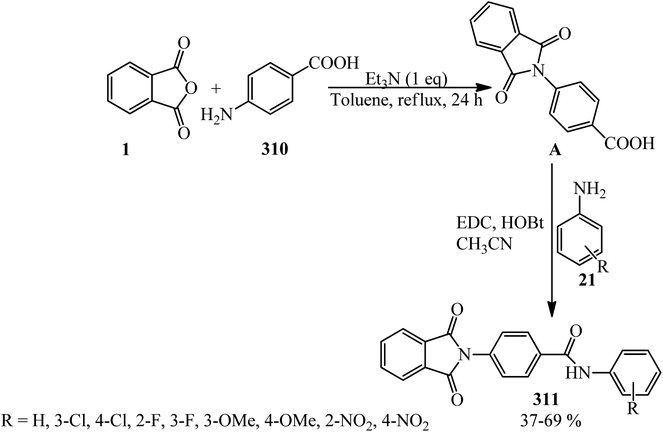
Aliabadi's group in 2014 obtained a series of phthalimides (23) through the reaction of PA (1) and anilines (21) in toluene solvent (reflux, 24 h) in the presence of Et3N with 21–80% yield. The antiepileptic activity of the products was investigated using two experimental models, namely, maximal electroshock (MES) and pentylenetetrazole (PTZ), and the obtained results were compared with diazepam as the reference drug. The neurotoxicity of the compounds was also evaluated using the rotarod model. The presence of para-methoxy substituent in the product showed anticonvulsant activity in the MES (maximal electroshock) model. None of the tested compounds demonstrated acceptable protection in subcutaneous PTZ (pentylenetetrazole) model.110
Patel's group in 2022 achieved some new classes of isoindoline-1,3-diones (23) via the reaction of PA (1) and the primary amino-containing compounds (21) in refluxing glacial acetic acid as the solvent-catalyst within 3 h with 62–78% yield. The quantum chemistry-based investigations of the products as antimycobacterial agents (toward the H37Rv strain by a dual read-out assay method) was also performed. Computational studies such as density functional theory (DFT) study, molecular docking, and dynamic simulation studies illustrated the reactivity and stability of the synthesized compounds as InhA inhibitors.111
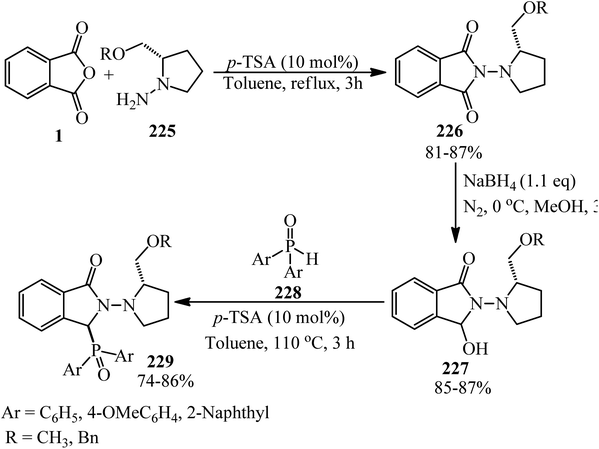
Hamdi et al. investigated some phthalimides via the reaction of PA (1) and amines (21) (aromatic, aliphatic, and benzylic) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 molar ratio in the presence of p-TSA (50 mg) in refluxing toluene within 3 h with 29–88% yield. The synthesized compounds were screened for their antimicrobial activities against Gram-positive bacterial strains (Micrococcus luteus, Listeria monocytogenes, Staphylococcus aureus, and Bacillus cereus), a Gram-negative bacterial strain (Salmonella typhimurium), and a fungus (Candida albicans). The cytotoxicity studies of the phthalimides were conducted in two human cancer cell lines, namely, MDA-MB-231 and MCF-7.112
1.1 molar ratio in the presence of p-TSA (50 mg) in refluxing toluene within 3 h with 29–88% yield. The synthesized compounds were screened for their antimicrobial activities against Gram-positive bacterial strains (Micrococcus luteus, Listeria monocytogenes, Staphylococcus aureus, and Bacillus cereus), a Gram-negative bacterial strain (Salmonella typhimurium), and a fungus (Candida albicans). The cytotoxicity studies of the phthalimides were conducted in two human cancer cell lines, namely, MDA-MB-231 and MCF-7.112
Al-Mousawi in 2010 achieved 2-phenylisoindole-1,3-dione (23) via the reaction of PA (1) and aniline (21) under solvent-free conditions for 30 min in a focused microwave oven at 160 °C with 96% yield.113
Singh Bisht and Rajat Bisht in 2021 reported the reaction of PA with some amines (such as urea, glycine, aniline, and sulphanilic acid) to yield various phthalimide derivatives (23) using domestic microwave with 70.7–80.21% yield. All synthesized derivatives were subjected to DDPH scavenging activity, which showed good to high antioxidant potential (69.56%) in the presence of ascorbic acid as the standard.114
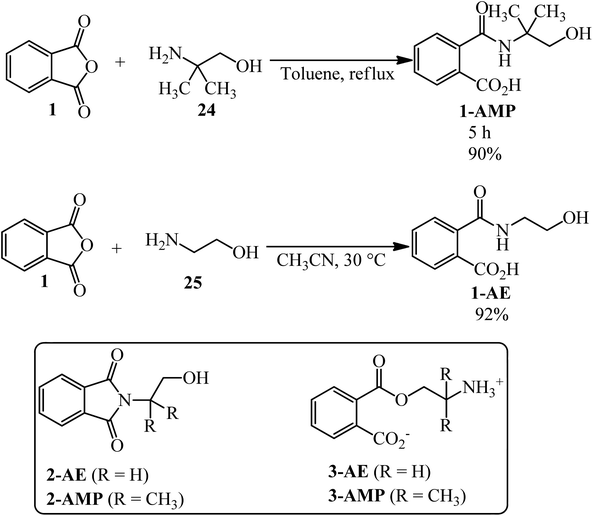
Wicks and Chen in 1979 mixed equimolar amounts of PA with two kinds of alcohols. First, the reaction of PA (1) and 2-amino-2-methyl-1-propanol (AMP) (24) yielded amide 1-AMP, imide 2-AMP, and/or ester 3-AMP. Amide 1-AMP undergoes an acyl shift to ester 3-AMP, apparently representing the first example of an N- to O-acyl shift in the absence of strong acid. Second, the reaction of PA (1) and 2-aminoethanol (AE) (25) yielded only amide 1-AE or imide 2-AE. No acyl shift of amide 1-AE to ester 3-AE was detected in this case (Scheme 12).115
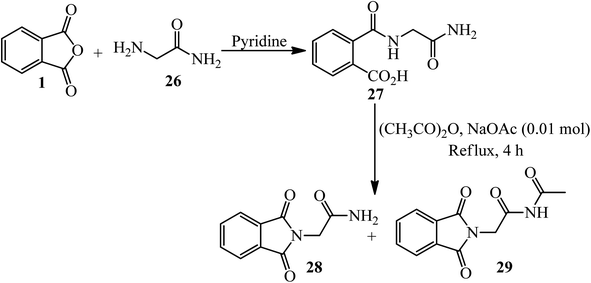
Hassanzadeh's group in 2017 supplied cyclic imides. In the first step, PA (1) reacted with glycinamide (26) in freshly distilled and dried pyridine under reflux conditions to yield the corresponding amic acid (27) within 5 h. The amic acid underwent ring closure with acetic anhydride and anhydrous sodium acetate to form imides (28, 29), which were isolated via column chromatography (Scheme 13).116
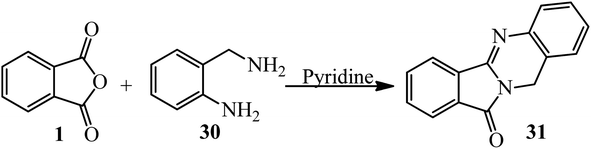
In another procedure, the reaction of PA and 2-amino-benzylamine (30) in pyridine within 5 h under reflux conditions gave the corresponding cyclic imide (31) (Scheme 14).116 The imides were screened for their antimicrobial activities against three types of bacteria and one type of fungi. Phthalimide derived from benzylamine exhibited remarkable antimicrobial activity against E. coli.109
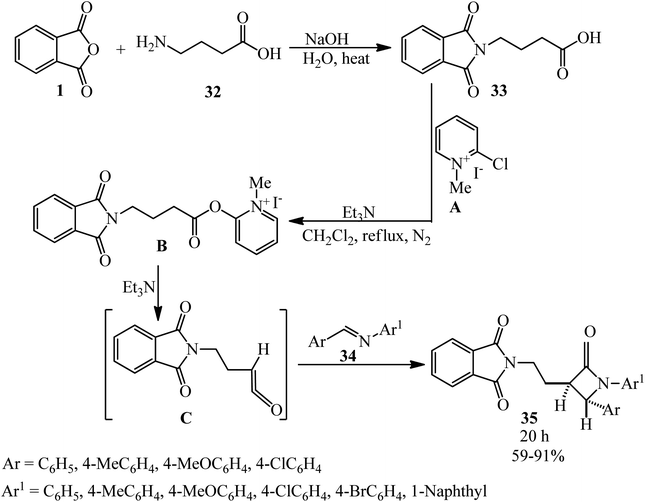
Islami et al. in 2015 obtained 4-(2H-isoindol-2-yl)butanoic acid (33) by the reaction of PA (1) with 4-aminobutanoic acid (32), which was followed by the reaction with Mukaiyama's reagent (2-chloro-1-methylpyridinium iodide, A) in the presence of triethylamine in dichloromethane. In the second step, novel diaryl-3-[2-(2H-isoindol-2-yl)ethyl]azetidin-2-ones (35) was synthesized via the reaction of 4-(2H-isoindol-2-yl)butanoic acid (33) with aromatic imines (34). The reaction proceeded through the in situ generation of a novel ketene containing an isoindole ring (C) and the subsequent electrocyclic reaction of a zwitterionic intermediate. The reaction was found to be highly stereoselective, and the trans-isomers of (E) were obtained as the only products (Scheme 15).117
Chandrasekhar and coworkers in 2009 identified (33) as an N-alkyl imide of PA from a microwave-assisted (450 W) procedure of polymer bound γ-amino butyric acid (36) (1 mmol) and PA (1.25 mmol) in the presence of activated silica gel and TaCl5–SiO2 (Scheme 16).118 The organic product was isolated from the resin–silica mixture using TFA.
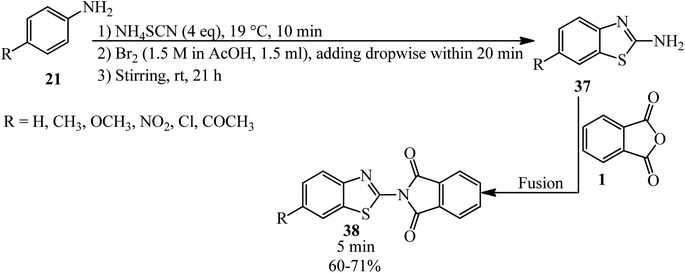
Pawar's group in 2014 synthesized 2-(6-substituted benzo[d]thiazol-2-yl)isoindoline-1,3-diones (38) from the heat-assisted reaction of PA and 6-substituted 2-amino benzothiazoles (37) within 5 min (Scheme 17).119 The 2-amino benzothiazoles (37) were prepared as using a previously reported method.120 The antiinflammatory activity of the synthesized compounds was determined using carrageenan-induced rat paw edema method (a mechanistic model for in vivo COX-2 inhibition). Compounds containing the Cl and COCH3 substituents showed antiinflammatory activity comparable to the reference drug diclofenac at 100 mg kg−1 doses.
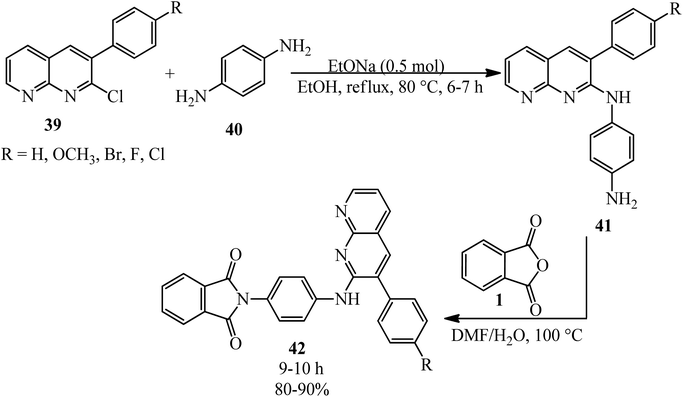
2-(4-((3-Aryl-1,8-naphthyridin-2-yl)amino)phenyl)isoindoline-1,3-diones (42) was obtained by the treatment of substituted 3-aryl-1,8-naphthyridines (41) with PA in refluxing N,N-dimethylformamide containing 5% (v/v) water by Sakram group in 2018 (Scheme 18).121 Some of the products demonstrated moderate to good antimicrobial activity compared with the streptomycin reference.
The target compounds 2-(7-fluoro-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl)isoindoline-1,3-diones (44) were constructed by Huang's group in 2005 via the reaction of PA (1) with 6-amino-7-fluoro-4-substituted-2H-3(4H)-benzo[b][1,4]oxazinones (43) in refluxing acetic acid within 4 h (Scheme 19).122 The products demonstrated protoporphyrinogen oxidase (protox) inhibitory properties. The preliminary bioassay data displayed that some of them possessed promising herbicidal activities comparable to that of the lead compound B2055 (flumioxazin and its iodo analogue).
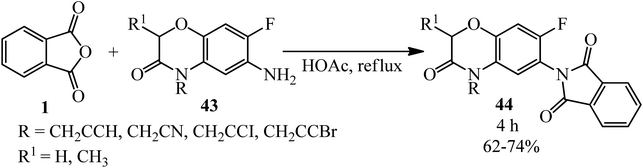 | ||
| Scheme 19 Synthesis of 2-(7-fluoro-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl)isoindoline-1,3-diones. | ||
Anwer et al. in 2019 manufactured nicotinonitriles attached to 1,3-dioxoisoindoline ring (46) via the reaction of PA (1) and 2-amino-6-(2,4-dimethoxyphenyl)-4-(4-methoxyphenyl)nicotinonitrile (45) in the presence of acetic acid that acted as the catalyst and solvent (Scheme 20).123
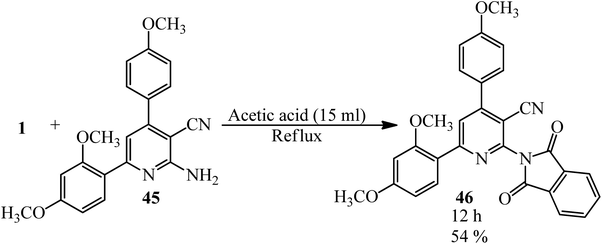 | ||
| Scheme 20 Synthesis of 6-(2,4-dimethoxyphenyl)-2-(1,3-dioxoisoindolin-2-yl)-4-(4-methoxyphenyl)nicotinonitrile. | ||
Shaban et al. in 2020 also synthesized a new derivative of (46) named 2-(1,3-dioxoisoindolin-2-yl)-4-(4-methoxyphenyl)-6-phenylnicotinonitrile via the rection of PA and 2-amino-4-(4-methoxyphenyl)-6-phenylnicotinonitrile in acetic acid under reflux conditions (22 h, 88%) and also microwave irradiation (3 min, 90.4%).124
Anwer and Sayed in 2020 reported the reaction of PA (1) and 5-amino-3-(4-(dimethylamino)phenyl)-1-phenyl-1H-pyrazole-4-carbonitrile (50) to obtain 3-(4-(dimethylamino)phenyl)-5-(1,3-dioxoisoindolin-2-yl)-1-phenyl-1H-pyrazole-4-carbonitrile (51). The adduct demonstrated high activities against Gram-negative and Gram-positive bacteria and high cytotoxic activity against MCF7 and HCT-116 (Scheme 21).125 The pyrazole-containing substrate (50) was achieved via the one-pot coupling reaction of N,N-dimethylamino benzaldehyde (47), malononitrile (48), and phenyl hydrazine (49).
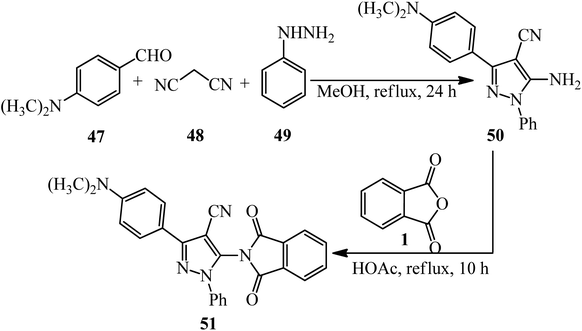 | ||
| Scheme 21 Synthesis of 3-(4-(dimethylamino)phenyl)-5-(1,3-dioxoisoindolin-2-yl)-1-phenyl-1H-pyrazole-4-carbonitrile. | ||
El-Shahat and Hasanin group in 2021 fabricated novel imidazole isoindoline-1,3-dione (58) from the reaction of PA with 1-(1-(4-bromophenyl)-2-hydrazinyl-4-methyl-1H-imidazol-5-yl)ethenone (57) (Scheme 22).126 According to Scheme 21, 1-(3-(4-bromophenyl)-5-methyl-2-thioxo-2,3-dihydro-1H-imidazol-4-yl)ethan-1-one (53) was obtained through the dropwise addition of 3-chloro-2,4-pentanedione (52) to a solution of 4-bromoaniline (21) in ethanol, followed by the addition of equimolar amount of KSCN. Subsequent alkylation of (53) with ethyl bromoacetate (54) led to the corresponding ethyl ester (55), which fused with hydrazine hydrate (56) to yield (57).
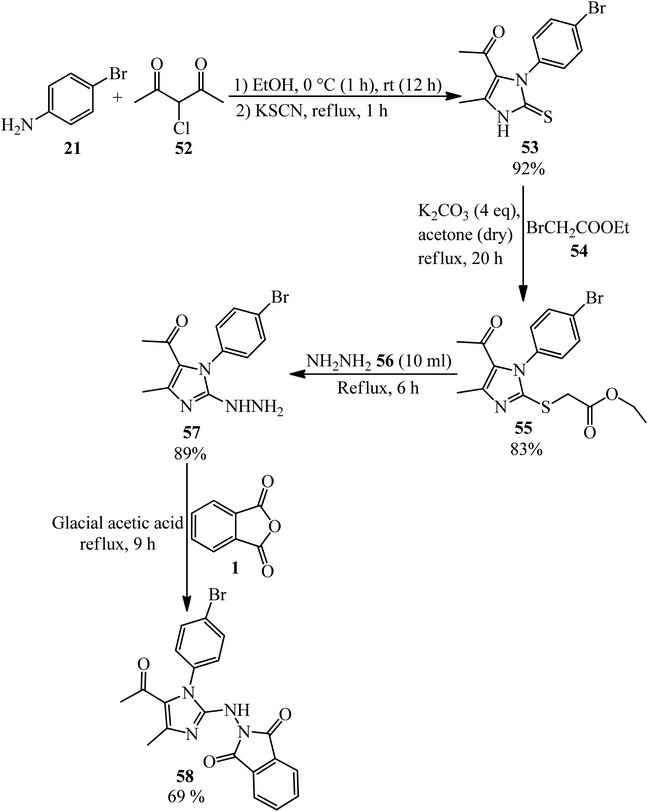 | ||
| Scheme 22 Synthesis of 2-((5-acetyl-1-(4-bromophenyl)-4-methyl-1H-midazole-2-yl) amino)isoindoline-1,3-dione. | ||
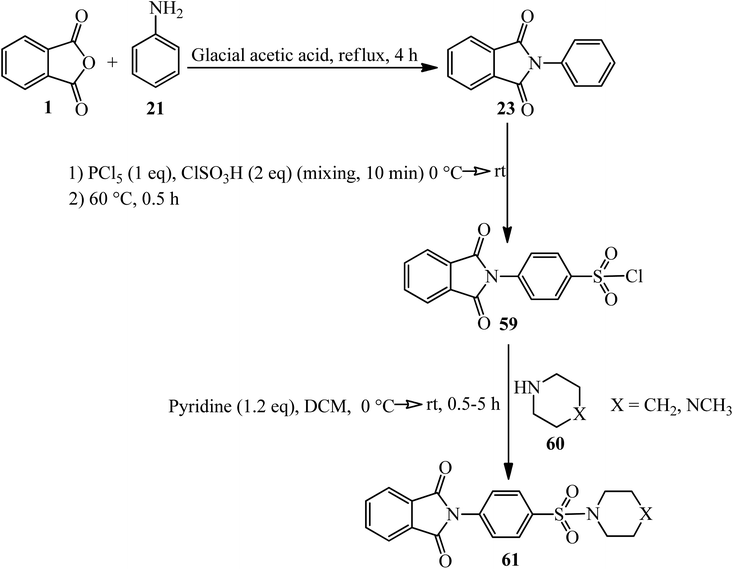
The Jayaprakash group in 2015 constructed some novel 4-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)benzene-1-sulphonamides (61). As shown in Scheme 22, in the first step, the 2-phenyl-1,3-isoindolinedione (23) was obtained by the reaction of PA (4 mmol) and aniline (3.4 mmol) in glacial acetic under nitrogen atmosphere at 120 °C within 2 h. In the next step, the product reacted with a mixture of chlorosulfonic acid (2 eq.) and phosphorus pentachloride (1 eq.) at 60 °C for 30 min. These two steps were performed based on previously-reported procedures.127 The final products were obtained via the reaction of 4-(1,3-dioxoisoindolin-2-yl)benzene-1-sulfonyl chloride (59) and piperidine/1-methylpiperazine (60) in the presence of pyridine with 68–95% yield (Scheme 23).128 The authors carried out molecular modelling studies on the adducts as Dengue virus 2 (DENV2) protease inhibitors.
Nofal group in 2014 reacted the starting compound [4-(1H-benzo[d]imidazol-2-yl)thiazol-2-amine] (62) with PA in refluxing glacial acetic acid for 6 h to obtain 2-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)isoindoline-1,3-dione (63) with 55% yield (Scheme 24).129
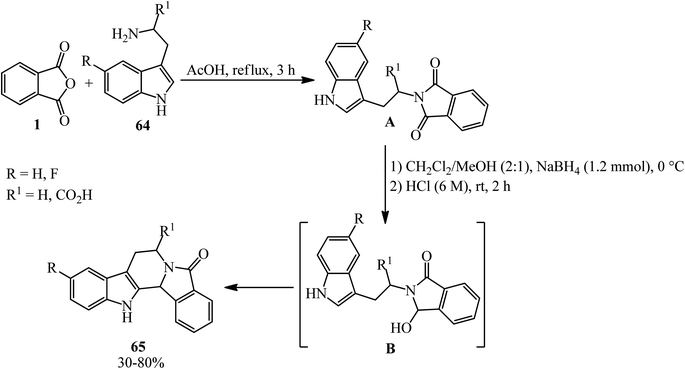
Tetrahydro-β-carbolines (65), with strictosamide skeleton, were obtained by Liu in 2014 via intermolecular condensation, selective reduction, and intramolecular cyclization starting from PA (1) and tryptamines (64) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 molar ratio (Scheme 25).130 The reaction proceeds via the intermediate (B), which has not been isolated.
1.2 molar ratio (Scheme 25).130 The reaction proceeds via the intermediate (B), which has not been isolated.
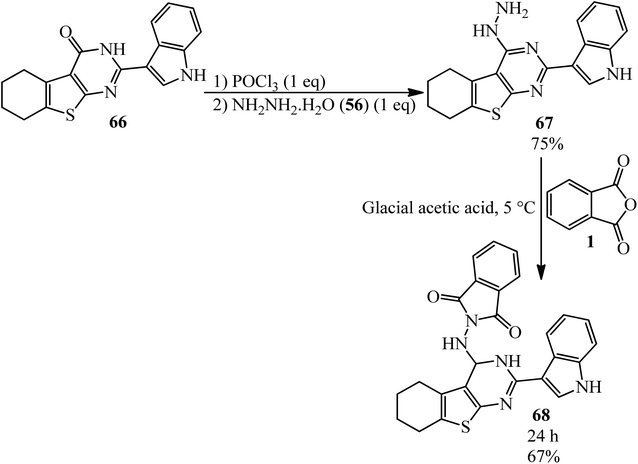
Abd El-All's research group in 2016 constructed 2-((2-(1H-indol-3-yl)-3,4,5,6,7,8-hexahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)amino)isoindoline-1,3-dione (68) via the reaction of pyrimidinohydrazine (67) and PA. The target compound was tested for its activity against Influenza A Neuraminidase virus (H3N2), which was very potent (Scheme 26).131
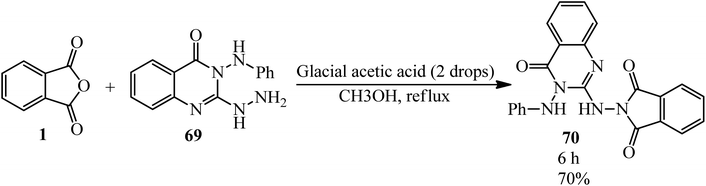
In 2003, Saleh's group constructed 2-(4-oxo-3-phenylamino-3,4-dihydroquinazolin-2-ylamino)-isoindole-1,3-dione (70) by the reaction of PA with 2-hydrazinyl-3-(phenylamino)quinazolin-4(3H)-one (69) in refluxing methanol (Scheme 27).132
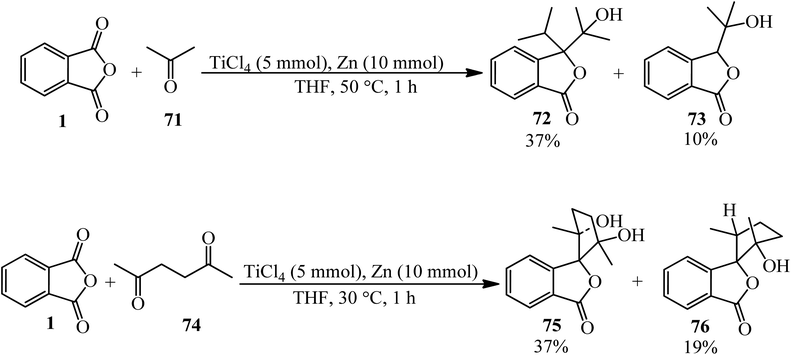
Kise et al. in 2020 presented the reductive coupling of PA (1) with acetone (71) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio by Zn–TiCl4 (in 2
10 molar ratio by Zn–TiCl4 (in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in THF) which, gave two-to-one (72) as the major and one-to-one (73) as the minor coupled products. The coupled products were transformed to 3,3-diisopropyl-, 3-isopropylidene-, and 3-isopropylphthalides. In addition, the reductive coupling of PA (1) with acetonylacetone (74) in 1
1 molar ratio in THF) which, gave two-to-one (72) as the major and one-to-one (73) as the minor coupled products. The coupled products were transformed to 3,3-diisopropyl-, 3-isopropylidene-, and 3-isopropylphthalides. In addition, the reductive coupling of PA (1) with acetonylacetone (74) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio by Zn–TiCl4 in THF gave 3-spirocyclopentanylphtalides as the selective exo-cis isomers (75, 76) (Scheme 28).133 Zn–TiCl4 was utilized as the source of low-valent titanium.
5 molar ratio by Zn–TiCl4 in THF gave 3-spirocyclopentanylphtalides as the selective exo-cis isomers (75, 76) (Scheme 28).133 Zn–TiCl4 was utilized as the source of low-valent titanium.
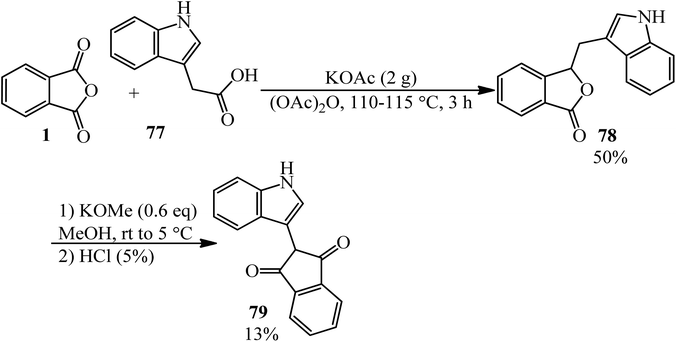
Lácová in 1969 reacted indole-3-acetic acid (77) with PA, in a 0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, under Perkin synthesis conditions (in the presence of potassium acetate and acetic anhydride) to form 3-(3-indolylmethine)phthalide (78), as yellow crystals, which underwent rearrangement in methanol in the presence of potassium methoxide to yield 2-(3-indolyl)indane-1,3-dione (79) (Scheme 29).134
1 molar ratio, under Perkin synthesis conditions (in the presence of potassium acetate and acetic anhydride) to form 3-(3-indolylmethine)phthalide (78), as yellow crystals, which underwent rearrangement in methanol in the presence of potassium methoxide to yield 2-(3-indolyl)indane-1,3-dione (79) (Scheme 29).134
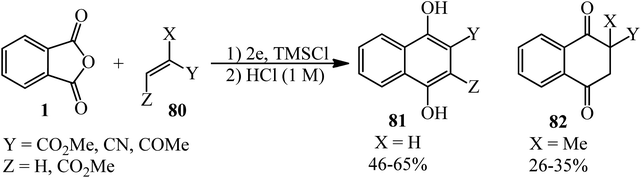
Kise and coworkers in 2020 demonstrated the electroreduction coupling of PA (1) with α,β-unsaturated carbonyl compounds (80) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio in the presence of TMSCl (5 mmol) and subsequent treatment with HCl (1 M), which gave 1,4-dihydroxynaphthalenes (81) and 2-methyl 2,3-dihydronaphthalene-1,4-diones (82) (Scheme 30).135
3 molar ratio in the presence of TMSCl (5 mmol) and subsequent treatment with HCl (1 M), which gave 1,4-dihydroxynaphthalenes (81) and 2-methyl 2,3-dihydronaphthalene-1,4-diones (82) (Scheme 30).135
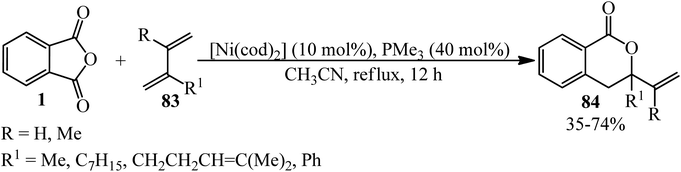
An intermolecular nickel-catalyzed decarbonylative [4 + 2] cycloaddition between PA (1) with 1,3-dienes (83) in a 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio in the presence of [Ni(cod)2] as a Ni(0) precursor to afford substituted 3-vinyldihydroisocoumarins (84) was studied by the Kurahashi group in 2011 (Scheme 31).136
3 molar ratio in the presence of [Ni(cod)2] as a Ni(0) precursor to afford substituted 3-vinyldihydroisocoumarins (84) was studied by the Kurahashi group in 2011 (Scheme 31).136
According to the proposed mechanism, the oxidative addition of (1) to Ni(0)-bearing electron-rich phosphine ligands gave the nickelacycle (A) (Scheme 32). 7,8 subsequent decarbonylation provides oxanickelacycle (B). The insertion of (83) through the more electron-rich C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond to C–Ni bond leads to the more stable acyclic3-allylnickel intermediate (D), which undergoes nucleophilic addition of oxygen to π-allylnickel at the more substituted carbon to give (84) and regenerates the starting Ni(0) complex.136,137
C bond to C–Ni bond leads to the more stable acyclic3-allylnickel intermediate (D), which undergoes nucleophilic addition of oxygen to π-allylnickel at the more substituted carbon to give (84) and regenerates the starting Ni(0) complex.136,137
In 2022, the palladium-catalyzed decarbonylative/decarboxylative [4 + 2] annulation of PA (1) with cyclic diaryliodonium salt (85) to synthesize triphenylene (86) developed by Li and coworkers (Scheme 33).138
Satchel and Stacey in 1971 investigated some products [2-(4,8-dihydroxy-1-naphthoyl)benzoic acid and its methylated form] (88, 89) from a Friedel–Crafts reaction of PA (1) with 1,5-dihydroxynaphthalene (87). The reaction of 1,5-dimethoxynaphthalene (90) with PA and in the presence of excess aluminum chloride gave o-(8-hydroxy-4-methoxy-1-naphthoyl)benzoic acid (91) and o-(4,8-dihydroxy-1-naphthoyl) benzoic acid (92), while with an excess of phthalic anhydride, the main products are dimethoxy-acid and 4,8-bis-(2-carboxybenzoyl)-l,5-dimethoxynaphthalene (Scheme 34).139
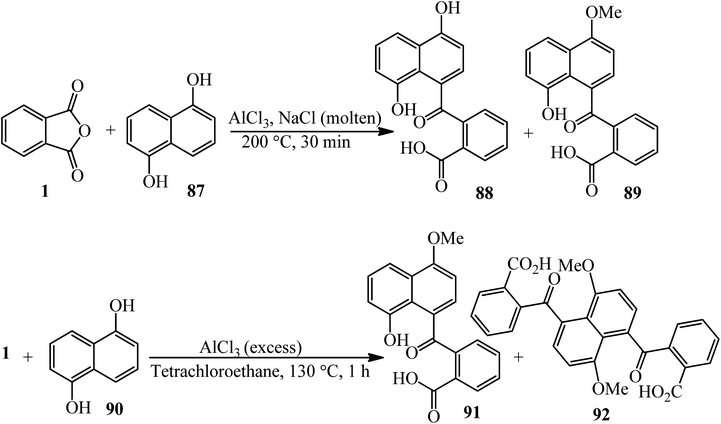 | ||
| Scheme 34 The Friedel–Crafts reaction of 1,5-dihydroxynaphthalene and 1,5-dimethoxynaphthalene with PA. | ||
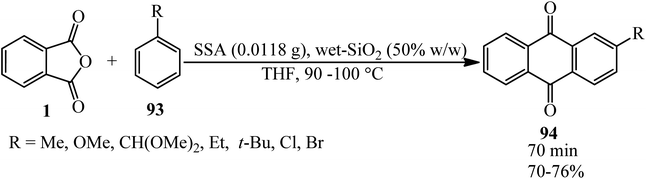
Naeimi and Shokrollah Brojerdi in 2014 gained anthraquinones (94) via the reaction of PA (1) and wide variety of substituted aromatic compounds such as (phenols, naphthols, alkyl benzenes, halo benzenes, and biphenyl) (93) using silica sulfuric acid (SSA) as a green, recyclable, and heterogeneous catalyst (Scheme 35).140 The catalyst could be reused seven times without significant activity loss.
Nie and Liu research group in 2022 provided 2-ethylanthraquinone (2-EAQ) (94) using PA (1) and ethylbenzene (93) as a feedstock by the combination of acylation and dehydration over a Sc-modified Hβ catalyst. Sc modification was used to create new strong Lewis acid and increased acid amount of Hβ.141
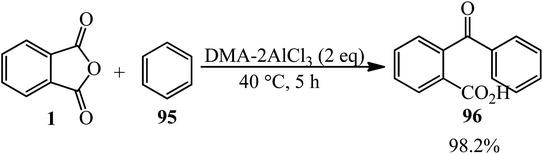
The catalytic performance of amide-AlCl3 (DMA-2AlCl3) ionic liquid analogs in synthesizing o-benzoylbenzoic acid (BBA) (96) from PA and benzene (95) was demonstrated. The catalyst was obtained by mixing AlCl3 and DMA in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at 100 °C for 3 h. Then, PA and benzene, in a 1
1 molar ratio at 100 °C for 3 h. Then, PA and benzene, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio were mixed at 40 °C within 5 h to obtain the product by 98.2% (Scheme 36).142 The authors proposed that Al2Cl7−, gained from DMA–2AlCl3, attacks the anhydride and formed (96) via an electrophilic substitution reaction.
10 molar ratio were mixed at 40 °C within 5 h to obtain the product by 98.2% (Scheme 36).142 The authors proposed that Al2Cl7−, gained from DMA–2AlCl3, attacks the anhydride and formed (96) via an electrophilic substitution reaction.
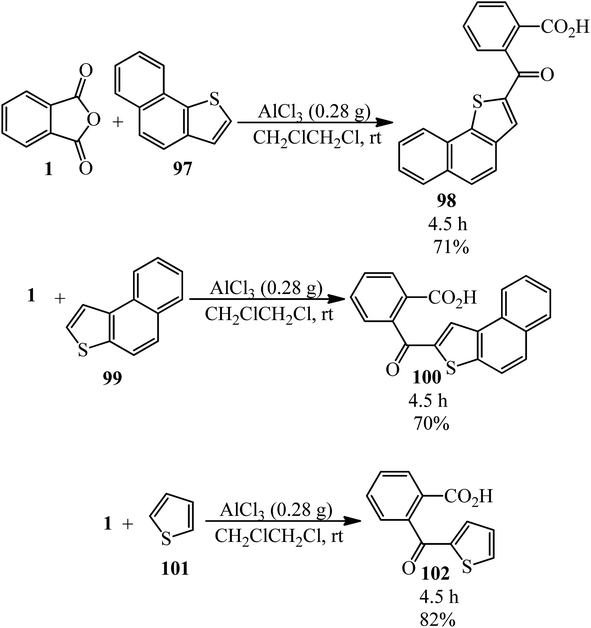
Naphto[1,2-b]thiophene (97) reacted with PA (1) to prepare ketocarboxilic acid (98). Naphto[2,1-b]thiophene (99) also reacted with PA to obtain 2-naphto[2,1-b]thienyl o-carboxyphenyl ketone (100) with 70% yield. The Friedel–Crafts reaction between PA and thiophene (101) yielded ketoacid (102) (Scheme 37).143
Naeimi and Namdari in 2009 described the direct preparation of anthraquinones (94) via the reaction of PA (1) and various benzene derivatives (93) in the presence of anhydrous AlCl3 (0.11 mmol)/methanesulfonic acid (0.01 mmol) (LAMA) at 95–100 °C within 15–65 min with 6–93% yield. Benzenes that contain electron withdrawing substituents (such as nitrobenzene and 1,3-dichlorobenzene) had the lowest reactivity in this reaction (8% and 6% yields, respectively). The compound 1,3-dinitrobenzene did not get the corresponding product even performing the reaction overnight.144
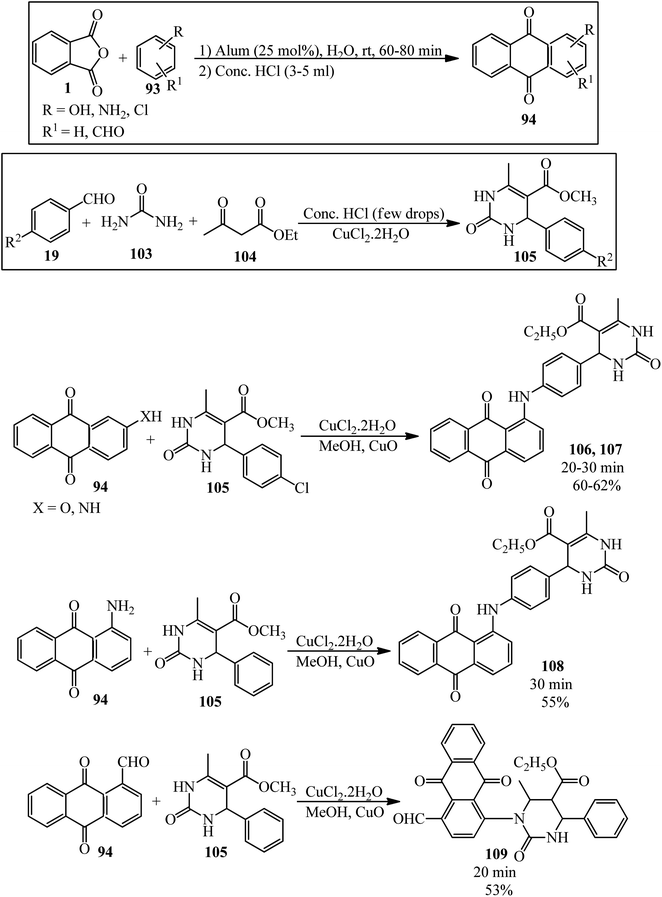
Shafiq group in 2021 reported newly synthesized anthraquinone-based pyrimidine derivatives. In the first step, the authors prepared the anthraquinone derivatives (94) via the modified procedure of Madje et al.145 in which the PA and benzene derivatives in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 molar ratio were mixed in aqueous media at room temperature in the presence of alum (KAl(SO4)2·12H2O), which was followed by the addition of concentrated HCl, washing with EtOAc, drying, and recrystallization from methanol. In the next step, the pyrimidine derivatives (105) (obtained by a procedure of Shafiq et al.146) were obtained via the three-component reaction of benzaldehydes (19), urea (103), and ethyl acetoacetate (104). In the third step, different derivatives of the precursors (94) and (105) were mixed in the presence of catalytic amount of copper chloride and cupric oxide in methanol for 20–30 minutes at ambient temperature to obtain the anthraquinone-based pyrimidines adducts (106–109) (Scheme 38).147 The antioxidant, antidiabetic, molecular docking, and QSAR studies of the products were also examined.
1.1 molar ratio were mixed in aqueous media at room temperature in the presence of alum (KAl(SO4)2·12H2O), which was followed by the addition of concentrated HCl, washing with EtOAc, drying, and recrystallization from methanol. In the next step, the pyrimidine derivatives (105) (obtained by a procedure of Shafiq et al.146) were obtained via the three-component reaction of benzaldehydes (19), urea (103), and ethyl acetoacetate (104). In the third step, different derivatives of the precursors (94) and (105) were mixed in the presence of catalytic amount of copper chloride and cupric oxide in methanol for 20–30 minutes at ambient temperature to obtain the anthraquinone-based pyrimidines adducts (106–109) (Scheme 38).147 The antioxidant, antidiabetic, molecular docking, and QSAR studies of the products were also examined.
Phillips in 1927 reported the synthesis of alizarin through a multistep reaction. First, the condensation of PA, o-dichlorobenzene, and anhydrous aluminum chloride into 3′,4′-dichloro-2-benzoylbenzoic acid, and second, the conversion of this acid by means of sulfuric acid into 2,3-dichloro-anthraquinone. Upon fusion with alkali, the dichloro-anthraquinone (94) converted into alizarin.148 The authors confirmed the claim of Sprent and Dodd149 that 2-chloro-anthraquinone is obtained by the condensation of PA and o-dichlorobenzene could not be confirmed.
Kumar et al. in 2014 reported novel 2-amino-6-(1,4-dioxo-3,4-dihydrophthalazin)-2(1H)-yl-4-phenyl-4H-pyran-3,5-dicarbonitriles (113) through a two-step reaction. First, 3-(1,4-dioxo-3,4-dihydrophthalazin-(1H)-yl)-3-oxopropanenitrile (111) was obtained from rection of PA (1) with ethyl cyanohydrazide (110) in the presence of acetic acid. The adduct reacted with benzaldehydes (19) and active methylene compounds such as malononitrile (48) and ethyl cyanoacetate (112) using L-proline as the catalyst in EtOH at ambient temperature to afford (113) (Scheme 39).150
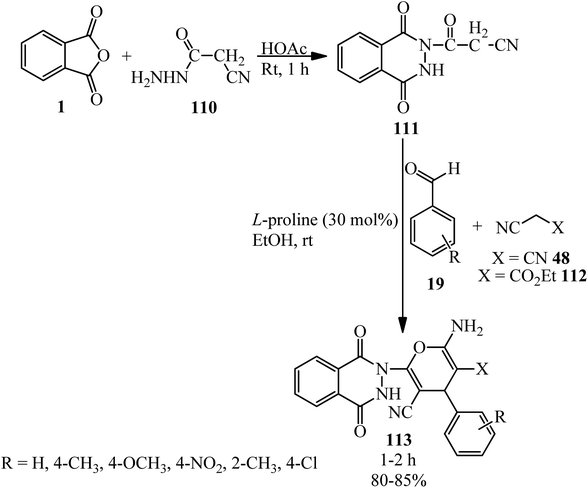 | ||
| Scheme 39 Synthesis of 2-amino-6-(1,4-dioxo-3,4-dihydrophthalazin)-2(1H)-yl-4-phenyl-4H-pyran-3,5-dicarbonitriles. | ||
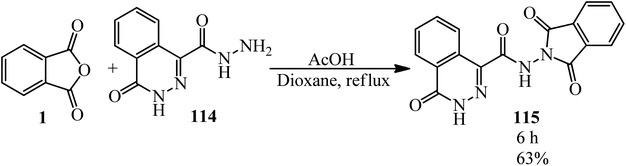
Abou-Elmagd group in 2012 achieved N-(1,3-dioxoisoindolin-2-yl)-4-oxo-3,4-dihydrophthalazine-1-carboxamide (115) via the reaction of PA and 4-oxo-3,4-dihydrophthalazine-1-carbohydrazide (114) in the presence of glacial acetic acid in refluxing dioxane (Scheme 40).151
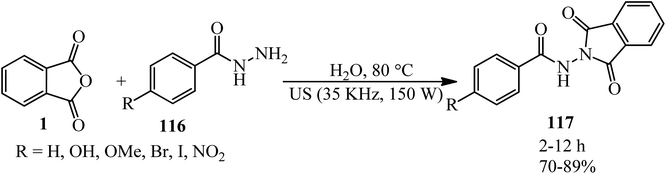
Simijonovic in 2020 described green aqua-mediated synthesis of benzamide–dioxoisoindoline derivatives (117) via the reaction of PA and benzoyl hydrazides (116) at 80 °C under ultrasonic irradiation. All compounds were subjected to experimental determination of their antioxidative potential. The DPPH test revealed that newly synthesized phenolic compounds are the best antioxidants (Scheme 41).152
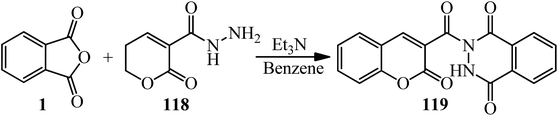
Aly's group in 2022 interpreted that the coumarin hydrazide (118) treated with PA (1) to furnish 2-(2-oxo-2H-chromene-3-carbonyl)-2,3-dihydrophthalazine-1,4-dione (119) by 76% yield (Scheme 42).153
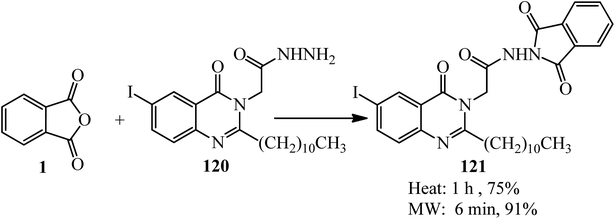
In 2019, 2-(6-iodo-4-oxo-2-undecylquinazolin-3(4H)-yl)acetohydrazide (120) reacted with PA (1) to produce 2-undecyl-4(3H)-quinazolinone (121), which was obtained by either conventional method or by the microwave-assisted technique. The adduct was tested in vitro against a panel of three human tumor cell lines, namely, Hepatocellular Carcinoma (liver) HepG2, colon cancer HCT-116, and mammary gland breast MCF-7, which demonstrated satisfactory activity (Scheme 43).154
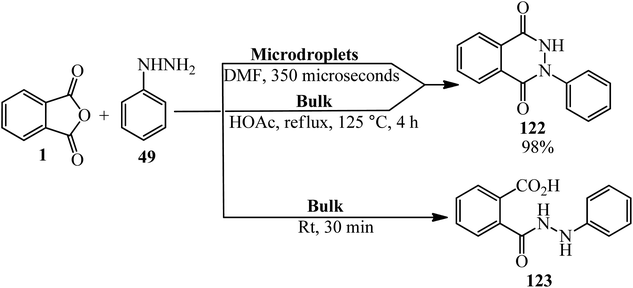
Zare's group in 2019 performed the reaction PA (1) and phenyl hydrazine (49) in (A): microdroplets (the reaction condition: N2 atmosphere, DMF was demonstrated to be the best solvent for this microdroplet reaction, with a yield of product (122) up to 98% when the distance between the spray tip to the glass vial extended to 7 cm with reaction time of about 350 μs), and (B): bulk-phase without catalyst and without refluxing (the concentration of the both substrates is 10 mM). The microdroplet reaction led to significant formation of (122) with a small amount of product (123), whereas the bulk-phase reaction of PA and (49) in acetic acid catalyst under high-temperature refluxing led to product (122), but without catalyst at room temperature only (123) was formed. Moreover, this reaction in microdroplets showed excellent selectivity, yielding only the important six-membered heterocyclic product (122). This behavior indicated an extremely different reaction pathway in the confined microdroplets. The authors claimed that the reaction rate and selectivity enhancement was attributed to a surface reaction in the confined microdroplets having low pH values, which is enhanced by the positive charging of the microdroplets (Scheme 44).155
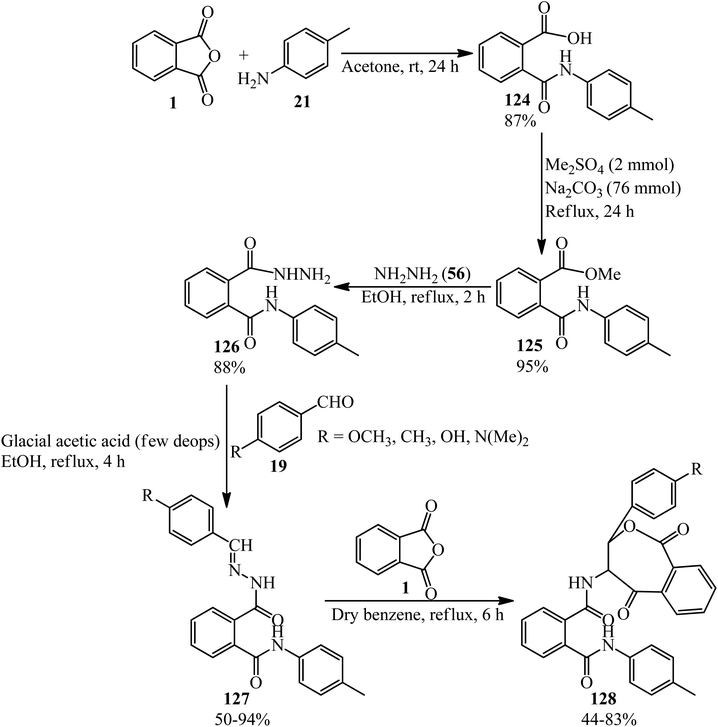
The Abbas group in 2021 achieved new Schiff bases and also their 1,3-oxazepines, derived from PA via a multistep reaction. In the first step, 2-[N-(4-methyl phenyl)]phthalamic acid (124) was prepared though the reaction of PA (1) and 4-methyl aniline (21) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. In the next step, the methylation of (124) gave 2-[N-(4-methyl phenyl)]phthalamide acetate (125), which reacted with hydrazine (56) to get 2-[N-(4-methyl phenyl)]phthalamic acid hydrazide (126). The Schiff bases (127) was obtained in refluxing ethanol in the presence of glacial acetic acid as catalyst. Finally, the 1,3-oxazepine derivatives of the Schiff bases (128) gained from the consequent reaction of (127) with PA in refluxing benzene (Scheme 45).156
1 molar ratio. In the next step, the methylation of (124) gave 2-[N-(4-methyl phenyl)]phthalamide acetate (125), which reacted with hydrazine (56) to get 2-[N-(4-methyl phenyl)]phthalamic acid hydrazide (126). The Schiff bases (127) was obtained in refluxing ethanol in the presence of glacial acetic acid as catalyst. Finally, the 1,3-oxazepine derivatives of the Schiff bases (128) gained from the consequent reaction of (127) with PA in refluxing benzene (Scheme 45).156
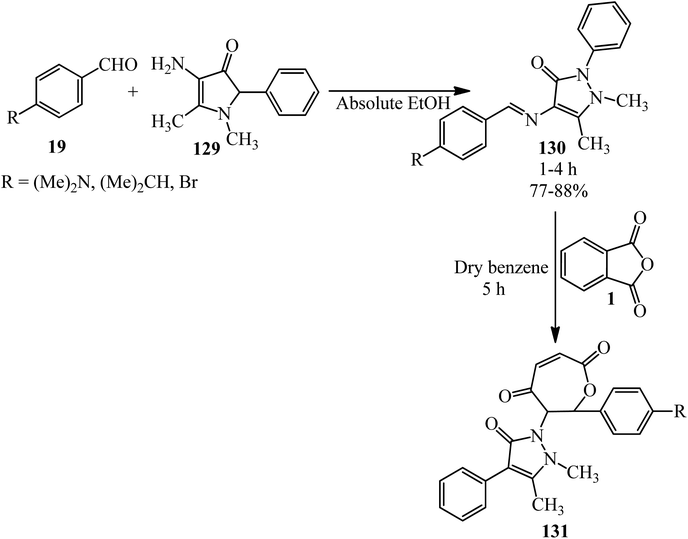
Hanoon in 2011 reported new 6-(2,3-dimethyl-5-oxo-4-phenyl-2,5-dihydro-1H-pyrazol-1-yl)-7-aryl-6,7-dihydrooxepine-2,5-diones (131) via the reaction of PA (1) and Schiff bases (130) in dry benzene. The Schiff bases was obtained via the condensation of various aromatic aldehydes (19) with 4-aminophenazone (129) in the presence of glacial acetic acid as the catalyst (Scheme 46).157
 | ||
| Scheme 46 Synthesis of new 6-(2,3-dimethyl-5-oxo-4-phenyl-2,5-dihydro-1H-pyrazol-1-yl)-7-aryl-6,7-dihydrooxepine-2,5-diones. | ||
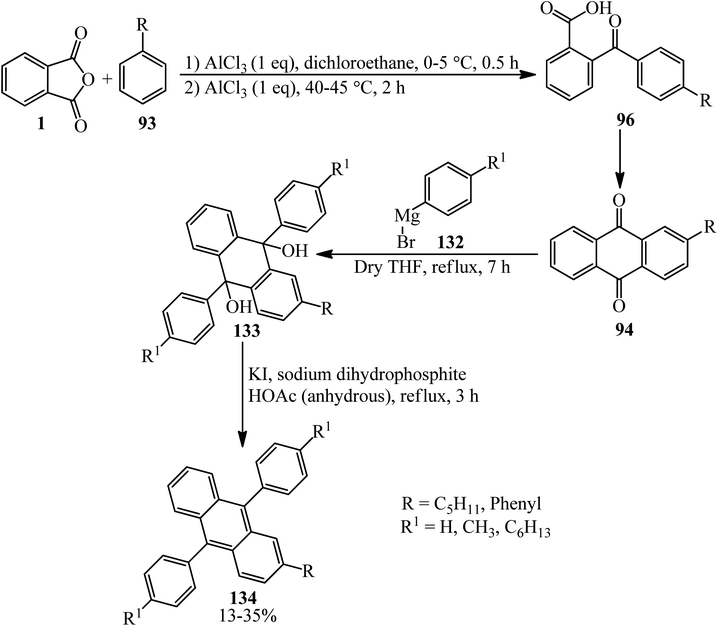
In 2014, Serevičius group constructed a series of nonsymmetric 9,10-diphenylanthracenes (DPA) through a multistep reaction, which started from the acylation reaction of PA (1) and substituted benzenes (93) (including pentyl and phenyl benzenes) to obtain substituted benzoylbenzoic acid (96) that underwent an intramolecular cyclization in heated acidic media (such as poly phosphoric acid or oleum 5%), which led to the formation of 2-substituted anthraquinones (94), which reacted with arylmagnesium bromide (132), resulting in 9,10-diaryl-9,10-dihydroxydihydroanthracene (133), which was reduced to form 2,9,10-trisubstituted anthracenes (134) (Scheme 47).158 The authors investigations affirmed that DPA compounds are deep-blue emitters with enhanced charge transport properties.
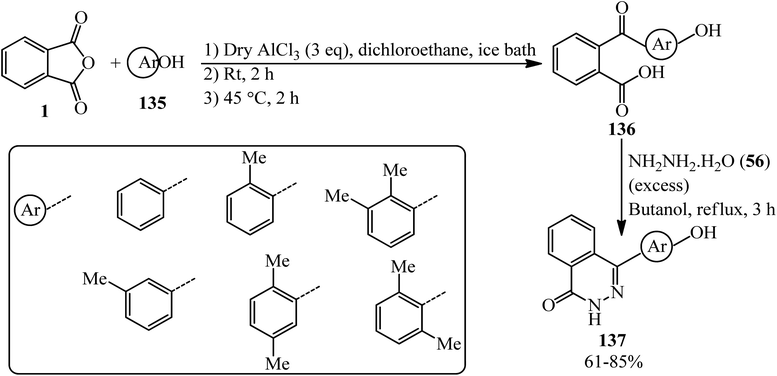
The synthesis of 4-(4′-hydroxyaryl)(2H)phthalazin-1-ones (137) was done by Cheng's group in 2007. The procedure consists of a two-step reaction, starting from the Friedel–Crafts acylation reaction of six phenols (135) with PA (1) to obtain α-keto acids (136), followed by cyclization with excess amount of hydrazine hydrate (56) in good to excellent yields with high regioselectivity to get products (137), which are methyl-substituted phthalazinone-containing bisphenol-like monomers; the adducts were utilized as monomers to obtain a number of novel heterocyclic poly(arylene ether ketone)s through the reaction with an activated difluoro monomer based on a novel N–C coupling reaction. The obtained polymers demonstrated polymers with high Tg's, good solubility, and excellent thermal stability (Scheme 48).159
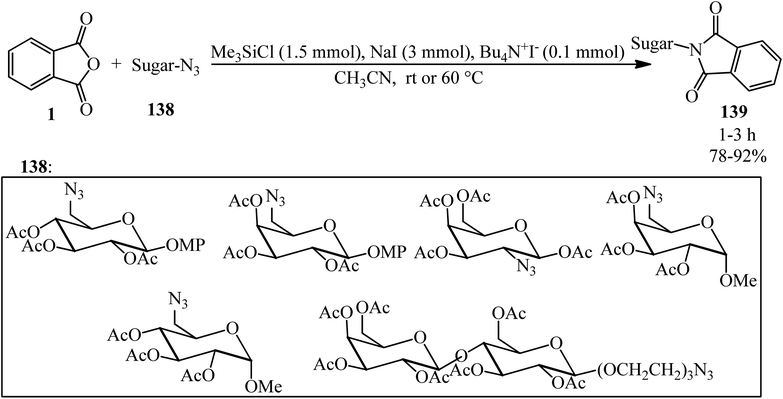
N-Sugar-substituted phthalimides (139) constructed in 2004 by Li's group via the reaction of PA (1) sugar azide (138) in a 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in the presence of tetrabutyl ammonium iodide (0.1 mmol, as catalyst) under essentially neutral conditions [which was performed by NaI (3 mmol) and Me3SiCl (1.5 mmol)] in dry acetonitrile at ambient temperature or 60 °C within 1–3 h with 78–92% yield (Scheme 49).160
1 molar ratio in the presence of tetrabutyl ammonium iodide (0.1 mmol, as catalyst) under essentially neutral conditions [which was performed by NaI (3 mmol) and Me3SiCl (1.5 mmol)] in dry acetonitrile at ambient temperature or 60 °C within 1–3 h with 78–92% yield (Scheme 49).160
Kamal in 1994 also reported N-substituted phthalimides (139) from the reaction of the corresponding azides (138) and PA (1) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, employing chlorotrimethylsilane and sodium iodide (in situ generation of iodotrimethylsilane) in acetonitrile at ambient temperature within 15 min with 95% yield.161
1 molar ratio, employing chlorotrimethylsilane and sodium iodide (in situ generation of iodotrimethylsilane) in acetonitrile at ambient temperature within 15 min with 95% yield.161
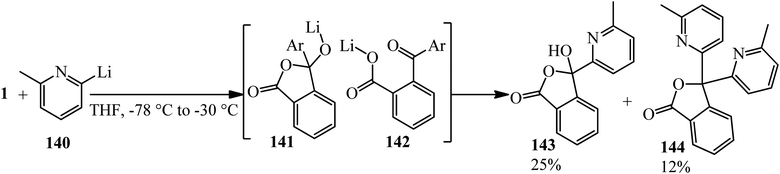
Nguyen and coworkers in 2012 performed the bisaddition of pyridinyl lithium (140) to PA, which yielded the monoaddition product 3-hydroxyisobenzofuranone (143) and 3,3-bis(6-methylpyridin-2-yl)isobenzofuran-1(3H)-one (144) as the byproduct. According to the mechanism, presumably, bisaddition arises from the ring opening of the initially formed alkoxy-isobenzofuranone (141), leading to diaryl ketone (142), which reacted with the organometallic reagent to give (144) (Scheme 50).162
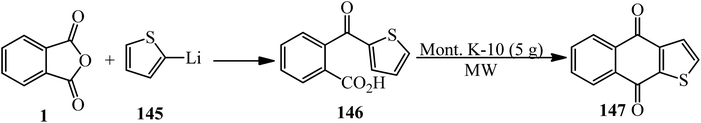
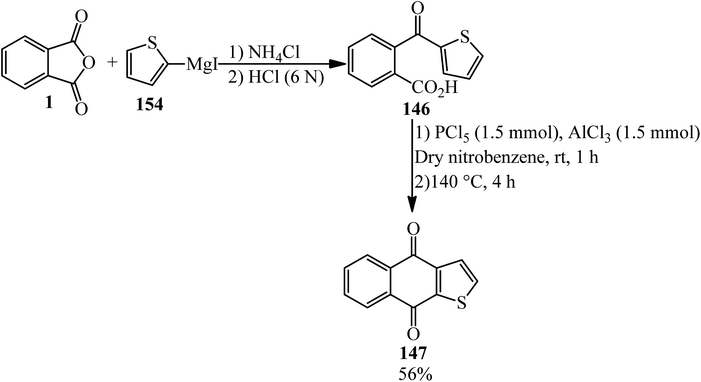
Acosta in 1995 synthesized 2-thenoylbenzoic acid (146) via the reaction of the PA (1) and 2-thienyllithium (145) by 80% yield. The product (146) cyclized in the presence of purified Mont K-10 (under microwave irradiation) to obtain thieno[2,3-b]-l,4-naphthoquinone (147) (Scheme 51).163
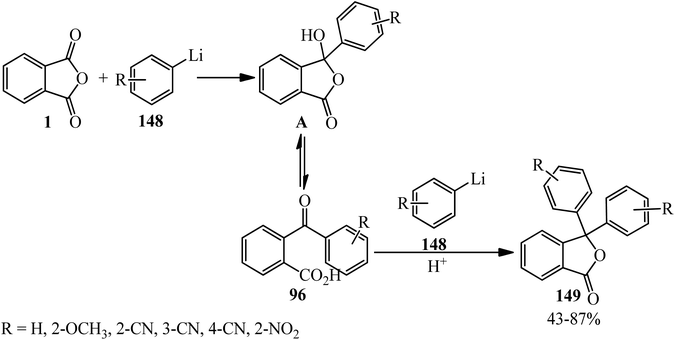
Parham and Piccirilli in 1976 claimed that when equimolar amounts of PA (1) and phenyllithium (148) were added at −78 °C, the yield of phthalide (149) was 78% based on phenyllithium. When the same ratios were maintained but the order of addition reversed, the yield of isolated (149) was 9%, while the yield of o-benzoylbenzoic acid (96) was 35%. Furthermore, the yield of o-benzoylbenzoic acid was further increased (55%) when excess (2 eq.) of PA was employed. In subsequent experiments, the aryllithium reagent was added rapidly to PA (2 eq.) in tetrahydrofuran at −100 °C, in which good yields of substituted benzoylbenzoic acids (96) were obtained (Scheme 52).164
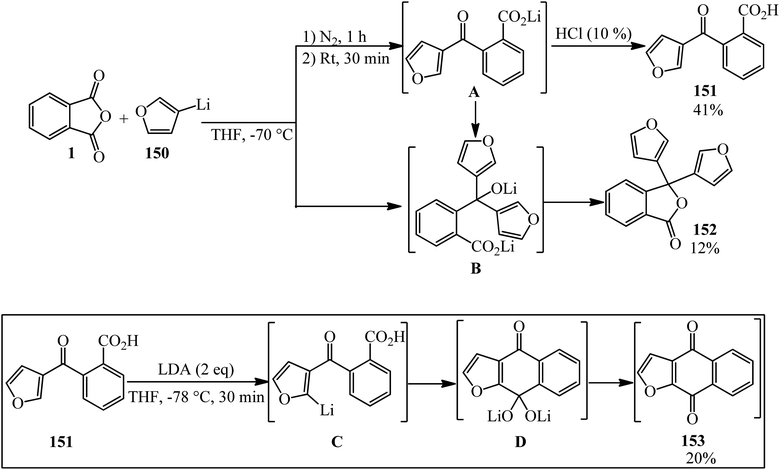
Tanaka's group in 1994 identified the reaction of PA (1) and furan-3-yllithium (150) to obtain 2-(3-furanoyl)benzoic acid (151) though the inverse addition method. On the other hand, the lactonization of (B) gave 3,3-di-(3-furyl)-1,3-dihydroisobenzofuran-1-one (152). The reaction of (151) with LDA gave naphtho[2,3-b]furan-4,9-dione (153) as yellow needles (Scheme 53).165 The authors attempted to obtain (153) via the chlorination of (151) with thionyl chloride or PCl5, which was not successful.
Martin and Seoane in 1992 claimed that 2-thenoylbenzoic acid (146), obtained from PA (1) and 2-thenoylmagnesium iodide (154), yielded thieno[2,3-b]-l,4-naphthoquinone (147) (Scheme 54).166
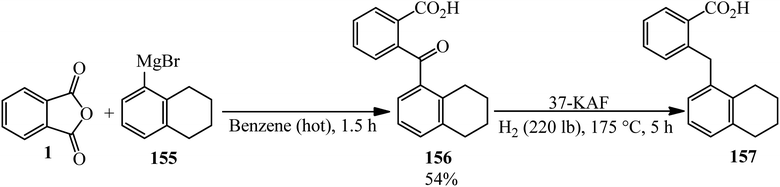
Fieser and Hershbe in 1937 reported the reaction of PA and α-tetralylmagnesium bromide (155) yielded the 2-(α-tetraloy1)-benzoic acid (156) as colorless prisms, which was reduced to 2-((5,6,7,8-tetrahydronaphthalen-1-yl)methyl)benzoic acid (157) by high pressure hydrogenation (Scheme 55).167
Baba in 1968 mixed PA (1) with triethylaluminum (158), which surprisingly got the reduced isobenzofuran-1(3H)-one (159) instead of the common ketoacid corresponding to the Grignard reaction (Scheme 56).168
Benneville in 1941 demonstrated the reaction of PA (1) and organocadmium compounds (160) (including dialkyl- and diaryl-cadmium) to obtain keto acids (96). The method consists of more satisfactory yields than the ones achieved from the reaction of PA with the Grignard reagent and is more generally applicable than the Friedel–Crafts synthesis. At first, the organocadmium was obtained from mixing the Grignard reagent and anhydrous cadmium chloride by the method of Gilman and Nelson.169 Then, a solution of PA in dry ether was added to organocadmium within fifteen to thirty minutes in an ice-bath, followed by refluxing (Scheme 57).170
In addition, McMullen in 1922 studied the Friedel–Crafts reaction of PA with benzene in the presence of AlCl3 in detail.171
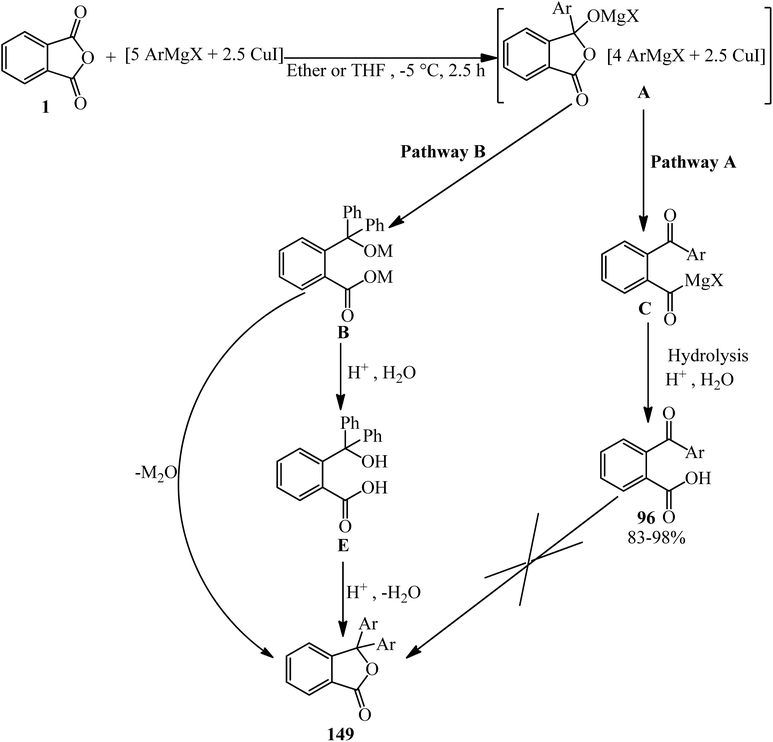
Rahman and Nahar in 1992 studied the reaction of arylcoppermagnesium reagents (prepared from ArMgX and CuI) with one equivalent of PA. The phenylcoppermagnesium reagent (Ar = C6H5) gave 2-benzoylbenzoic acid (96) and 3,3-diphenylphthalide (149) in 40% and 42% yield, respectively. The yield of 2-benzoylbenzoic acid (96) increased to 93% in the presence of dimethyl sulphide. Under these conditions, no phthalide was formed. On the other hand, lithium diphenylcuprate reacted with PA in ether–hexane to give 2-benzoylbenzoic acid (96) and 3,3-diphenylphthalide (149) in 92% and 7% yields, respectively. The reaction of phenylcopper reagent (prepared from PhMgBr and CuI) and PA under similar conditions proceed slowly to get 2-benzoylbenzoic acid (96) in 15% yield. The reaction of phenylmagnesium bromide in the presence or absence of catalytic amounts of copper(I) iodide led to unsatisfactory results. The use of two equivalents of phenyllithium with one equivalent of PA, on the other hand, afforded 3,3-diphenylphthalide (149) in 77% yield. The authors believed that the species (A) is formed as the first step. If it is stable, it would be present in the system long enough to undergo an intramolecular ring opening (pathway A) to form 2-aroylbenzoate (C), which hydrolyzed to generate 2-aroylbenzoic acid (96). On the other hand, if (A) is very unstable, it may decompose intramolecularly by the transfer of an organic group and concomitant ring opening (pathway B) to produce (B), which converted into the lactone form, which is 3,3-diphenylphthalide (149) either during hydrolysis or by the elimination of metal oxide prior to hydrolysis. Compared with diethyl ether, THF and dimethyl sulphide are expected to have greater stabilizing effects on species (A), presumably by forming more stable complexes; thus, in their presence, the reaction of (A) directed to pathway A. Since dimethyl sulphide is the most efficient of the ligands examined, it is not surprising that the yields of 2-benzoylbenzoic acid are the highest in its presence (Scheme 58).172
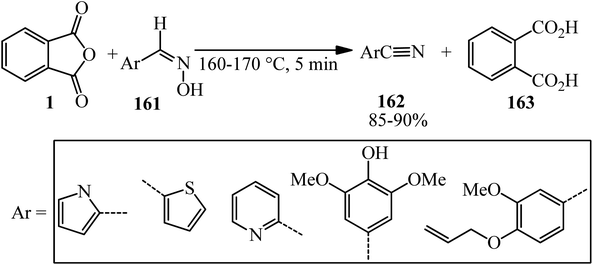
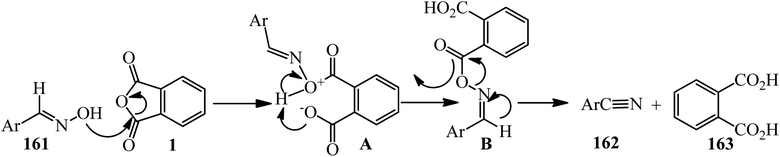
Wang in 2004 accounted an efficient method for the conversion of aldoximes (161) to nitriles (162) via the reaction of PA and aldoximes, in 1.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, which gained phthalic acid (163) as byproduct (Scheme 59).173
1 molar ratio, which gained phthalic acid (163) as byproduct (Scheme 59).173
According to the proposed mechanism, using PA (1) led to formation of the acylated aldoxime intermediate (A), which efficiently underwent a feasible [3,3]-sigmatropic rearrangement as the reaction pathway via this proposed six-membered transition state (B), which gave nitriles (162) without utilizing an external base (Scheme 60).173
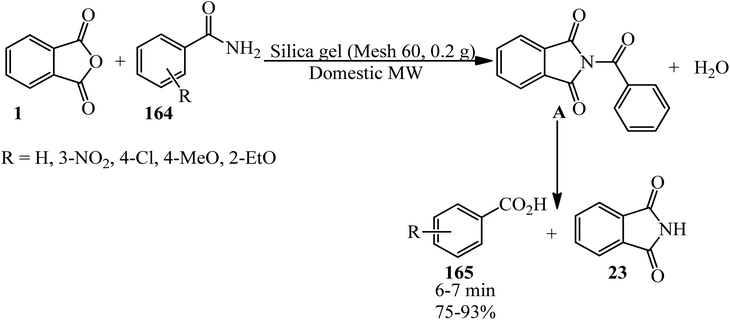
Heravi group in 2005 transformed amides (164) to carboxylic acids (165) upon reaction with PA under microwave irradiation in the absence of solvent (Scheme 61).174 They utilized different amides (such as acetamide, acrylamide, and lactamide) successfully.
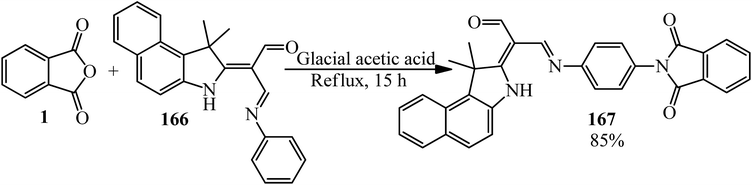
Kadhum group in 2022 supplied 2-(1,1-dimethyl-1H-benzo[e]indol-2(3H)-ylidene)-3-((4-(1,3-dioxoisoindolin-2-yl)phenyl)imino)propanal (167) via the reaction of PA (1) and Schiff base ((2E,3E)-2-(1,1-dimethyl-1H-benzo[e]indol-2(3H)-ylidene)-3-(phenylimino)propanal) (166) (Scheme 62).175
 | ||
| Scheme 62 Synthesis of 2-(1,1-dimethyl-1H-benzo[e]indol-2(3H)-ylidene)-3-((4-(1,3-dioxoisoindolin-2-yl)phenyl)imino)propanal. | ||
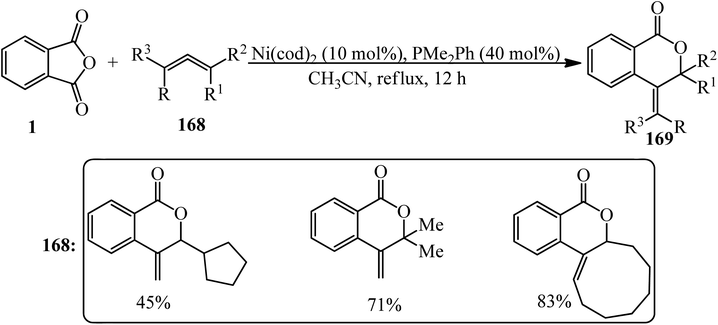
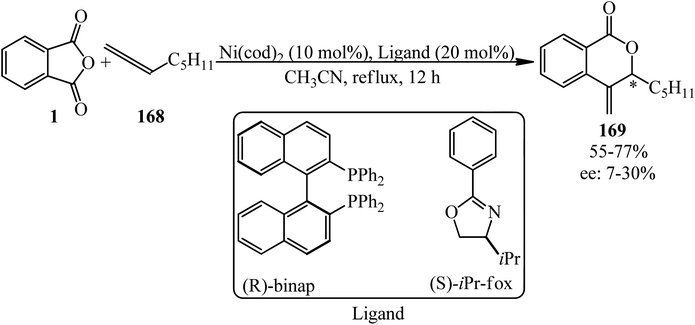
Matsubara group in 2011 dedicated the decarbonylative cycloadditions of PA (1) with allenes (168) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 molar ratio using Ni(cod)2 as the Ni(0) precursor to give δ-lactones (169) in a single step. The reaction represented an unprecedented insertion reaction of a carbon–carbon double bond into a carbon–oxygen bond (Scheme 63).176 The asymmetric variant of the cycloaddition was also achieved using chiral phosphine ligands to provide δ-lactones enantioselectively (Scheme 64).176
1.5 molar ratio using Ni(cod)2 as the Ni(0) precursor to give δ-lactones (169) in a single step. The reaction represented an unprecedented insertion reaction of a carbon–carbon double bond into a carbon–oxygen bond (Scheme 63).176 The asymmetric variant of the cycloaddition was also achieved using chiral phosphine ligands to provide δ-lactones enantioselectively (Scheme 64).176
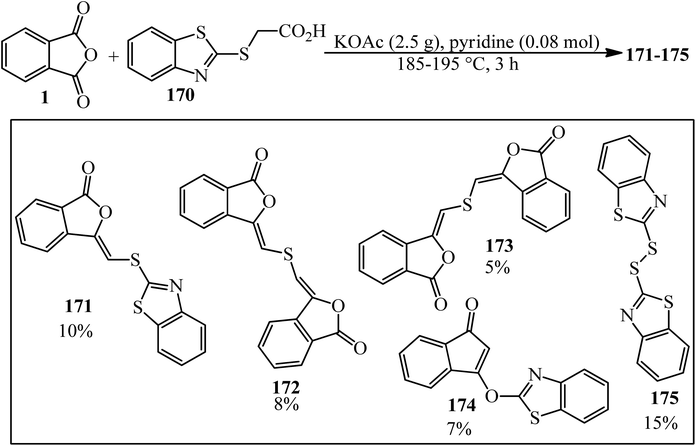
Lácová in 1986 considered the reaction of 2-benzothiazolylthioethanoic acid (170) (0.1 mol) with PA (0.2 mol) under conditions of Gabriel modification of Perkin synthesis. They claimed that in addition to the anticipated 3-(2-benzothiazolylthiomethylene)phthalide (171), four more compounds were identified as (Z,Z)-3,3′-thio-bis(methylenephthalide) (172), (E,Z)-3,3′-thio-bis(methylenephthalide) (173), l-(2-benzothiazolyloxy)-l-inden-3-one (174), and dibenzothiazolyl disulfide (175). The starting compounds did not react in the presence of acetic anhydride since 2-benzothiazolylthioethanoic acid (170) was preferentially acetylated to yield 4-methylthiazo[2,3-b]benzothiazolium 5-carboxylate by an intramolecular condensation. Only (Z)-3-(2-benzothiazolylthiomethylene)phthalide (171) corresponded to a normal course of aldol synthesis (Scheme 65).177
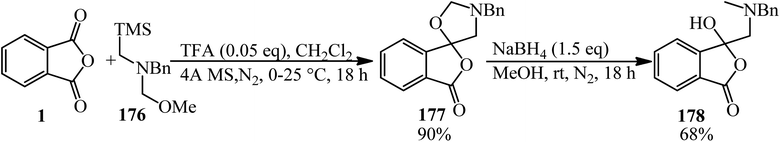
Meyer and Ryan group in 2015 reported that PA (1 eq) underwent a 1,3-dipolar cycloaddition reaction with N-benzylazomethine ylide (that formed in situ from N-(methoxymethyl)-N-(trimethylsilylmethyl)benzylamine (176) (1.1 eq.) and a catalytic amount of trifluoroacetic acid) to produce unstable spiro(isobenzofuran-1,5′-oxazolidin)-3-ones (177), which was reduced with sodium borohydride to afford 1(3H)-isobenzofuranones (178) (Scheme 66).178
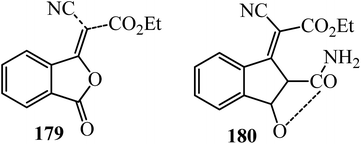
Renfrew and Bostock in 1977 performed the Knoevenagel condensation of PA (1) with ethyl cyanoacetate (112) in the presence of sodium as the catalyst in benzene for 4 h to furnish ethyl cyano(phthalidy1idene)acetate (179) in 95% yield. Utilizing triethylamine as a base in toluene at 90 °C for 24 h, a yellow color was produced immediately, and upon refluxing, an intense orange color developed. On cooling, an orange oil separated, which on acidification gave a white solid in 64% yield, identified as (2)-ethyl 2-carbamoyl-8-cyano-3-hydroxybenzofulvene-8-carboxylate (180) (Scheme 67).179
 | ||
| Scheme 67 Ethyl cyano(phthalidy1idene)acetate and (2)-ethyl 2-carbamoyl-8-cyano-3-hydroxybenzofulvene-8-carboxylate. | ||
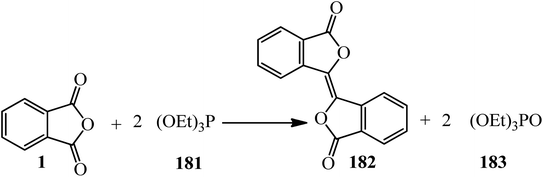
The Ramirez group in 1961 found that triethyl phosphite (181) effected the conversion of PA (1) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio into biphthalyl (182) in satisfactory yield (70%). The reaction was carried out in an excess of the phosphite as the solvent and the biphthalyl separated from the solution in nearly pure state. It was observed that most of the excess triethyl phosphite was isomerized to diethyl ethylphosphonate (183) during the reaction (Scheme 68).180
1 molar ratio into biphthalyl (182) in satisfactory yield (70%). The reaction was carried out in an excess of the phosphite as the solvent and the biphthalyl separated from the solution in nearly pure state. It was observed that most of the excess triethyl phosphite was isomerized to diethyl ethylphosphonate (183) during the reaction (Scheme 68).180
3. Applications of PA in three-component and pseudo three-component reactions
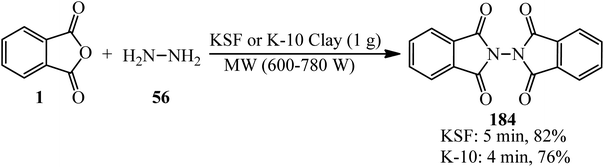
In 2006, Habibi and Marvi obtained N-phthalimidophthalimide (184) from the reaction of PA (1) and hydrazine hydrate (56) in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in the presence of montmorillonite KSF and montmorillonite K-10 clays as natural heterogeneous catalysts with the help of microwave irradiation (600–780 W) under solvent-free conditions (Scheme 69).181 The pure product achieved through recrystallization by glacial acetic acid.
1 molar ratio in the presence of montmorillonite KSF and montmorillonite K-10 clays as natural heterogeneous catalysts with the help of microwave irradiation (600–780 W) under solvent-free conditions (Scheme 69).181 The pure product achieved through recrystallization by glacial acetic acid.
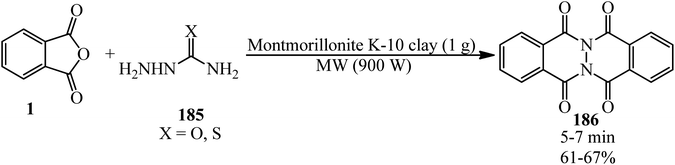
Habibi et al. in 2007 described a solvent-free and microwave-assisted procedure for the synthesis of phthalazino[2,3-b]phthalazine-5,7,12,14-tetraones (186) via the pseudo three-component reaction of PA (1) and (thio)semicarbazides (185) using montmorillonite K-10 clay. The catalyst demonstrated significant activity after recovering in another cycle (Scheme 70).182
Maccioni's group in 2003 also gained the commands (186) via the catalytic role of acetic acid in refluxing isopropyl alcohol media within 1 h with 61–75% yield.183 The antimicrobial properties of the products was also examined by the authors that was not satisfactory.
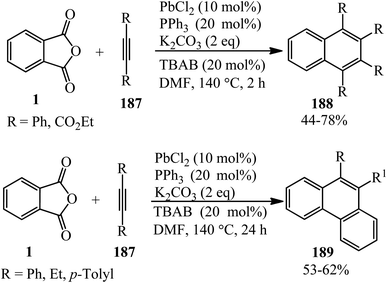
Jafarpour and coworkers in 2013 developed a new method for the decarboxylative and decarbonylative addition of cyclic anhydrides to alkynes. They performed the palladium-catalytic benzannulation of PA (1) with alkynes (187) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio to obtain the corresponding polyfunctionalized sterically condensed naphthalenes (188) and phenanthrenes (189), respectively. The sequential liberation of CO2 and CO occurred via the oxidative decomposition of anhydride (Scheme 71).184
2 molar ratio to obtain the corresponding polyfunctionalized sterically condensed naphthalenes (188) and phenanthrenes (189), respectively. The sequential liberation of CO2 and CO occurred via the oxidative decomposition of anhydride (Scheme 71).184
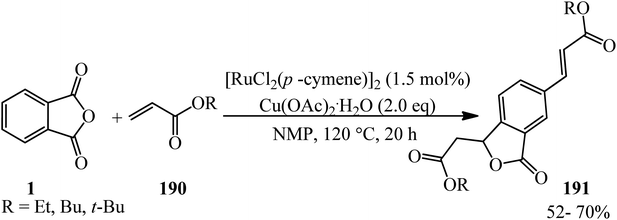
Fardpour et al. in 2019 constructed substituted vinylated phthalides (191) through a ruthenium-catalyzed cross-dehydrogenative coupling reaction of PA (1) with acrylates (190) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in the presence of Cu(OAc)2·H2O as the oxidant in N-methyl-2-pyrrolidone (NMP) solvent (Scheme 72).185 The reaction proceeded via C–H bond activation through a successive double vinylation accompanied by decarboxylation and annulation reaction.
2 molar ratio in the presence of Cu(OAc)2·H2O as the oxidant in N-methyl-2-pyrrolidone (NMP) solvent (Scheme 72).185 The reaction proceeded via C–H bond activation through a successive double vinylation accompanied by decarboxylation and annulation reaction.
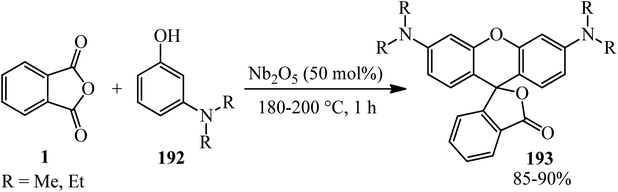
Silva's research group in 2020 described the solvent-free synthesis of rhodamine dyes (193) via the reaction of PA and m-aminophenols (192) using Nb2O5 as the catalyst. The solvatochromic study of rhodamines was also performed (Scheme 73).186 Rhodamine dyes possessed various applications due to their properties, such as high molar absorptivity, high fluorescence quantum yield, photostability, and absorption and emission wavelengths in the visible region, which make them good candidates in electronic devices (such as lasers and OLEDs).
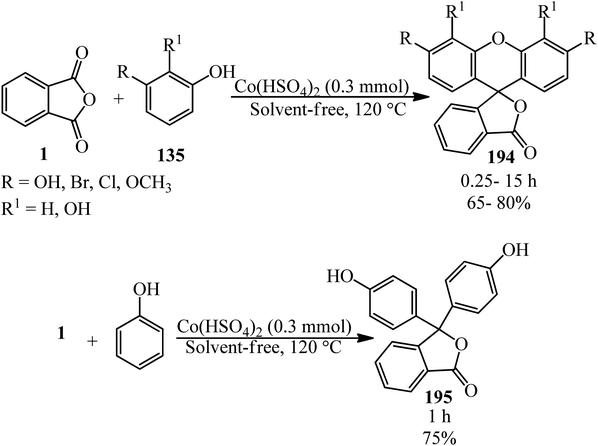
Eshghi et al. in 2015 explained the condensation of PA (1) with substituted phenols (135) in the presence of cobalt hydrogen sulfate under melt conditions that gave 3H-spiro[isobenzofuran-1,9′-xanthen]-3-one (194). In the case of phenol, the product was 3,3-bis(4-hydroxyphenyl)isobenzofuran-1(3H)-one (195) (Scheme 74).187
 | ||
| Scheme 74 Synthesis of 3H-spiro[isobenzofuran-1,9′-xanthen]-3-ones and 3-bis(4-hydroxyphenyl)isobenzofuran-1(3H)-one. | ||
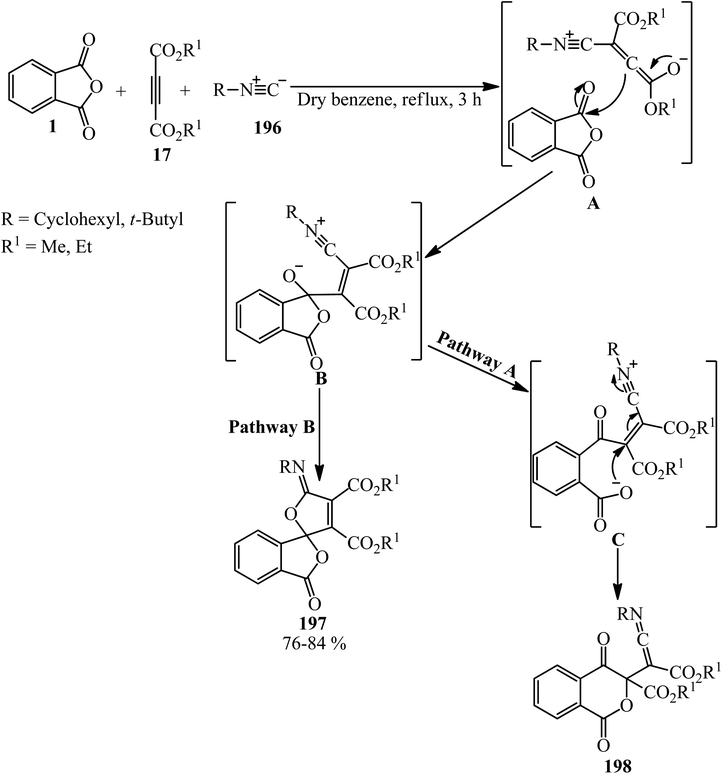
Shaabani's research group in 2002 reported the addition of PA (1), dialkyl acetylenedicarboxylates (17) and alkyl isocyanides (196), leading to highly functionalized γ-spiroiminolactones (197). The authors purposed preparing highly functionalized ketenimines (198) (pathway A), but the unusual γ-spiroiminolactones (197) were obtained in high yields (pathway B) (Scheme 75).188
Shaabani et al. in 2009 also applied catalyst-free three-component method for the synthesis of a benzo-fused spirolacton (197) from the reaction of PA (1), dimethyl acetylenedicarboxylate (17), and cyclohexyl isocyanide (196) in dichloromethane at ambient temperature within 2 h with 82% yield. In fact, the zwitterion formed from isocyanide and dialkyl acetylenedicarboxylate reacted with PA to form the benzo-fused spirolactone (197).189
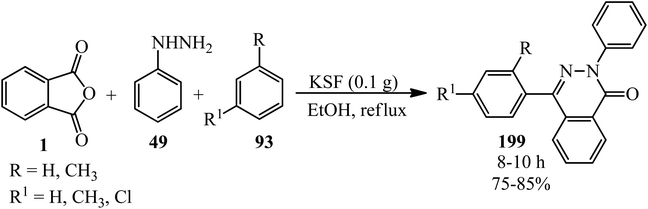
Mahmoodi's research group in 2010 demonstrated the regioselective synthesis of phthalazinones (199) from PA (1), phenyl hydrazine (49), and arenes (93) in the presence of efficient recyclable heterogeneous catalyst, montmorillonite-KSF, in high yields (Scheme 76).190
The same research group in 2012 also reported ultrasound-assisted (45 kHz) preparation of phthalazinones (199) in the presence of the recyclable catalyst [bmim]Br/AlCl3 (2 eq.) at 60 °C within 4–5 h by 65–75% yield.191
Thirupaiah and Vedula in 2013 developed a facile and efficient one-pot, three-component protocol for the synthesis of novel 2,3-dihydro-2-(6-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazin-3-yl)phthalazine-1,4-dione (202) from the reaction of PA (1), 3-(2-bromoacetyl)-4-hydroxy-6-methyl-2H-pyran-2-one (200), and 4-amino-5-hydrazino-4H-[1,2,4]triazole-3-thiol (201) in acetic acid medium (Scheme 77).192 The product was purified by simple recrystallization from ethanol.
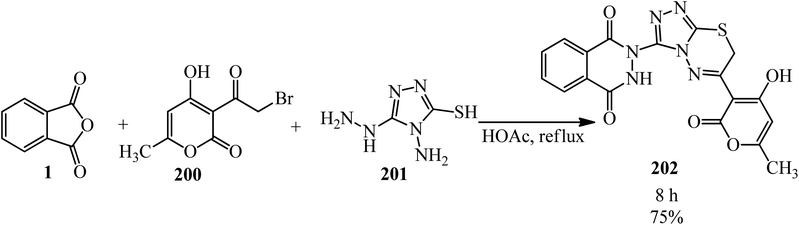 | ||
| Scheme 77 Synthesis of 2,3-dihydro-2-(6-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-7H-[1,2,4]-triazolo[3,4-b][1,3,4]thiadiazin-3-yl)phthalazine-1,4-dione. | ||
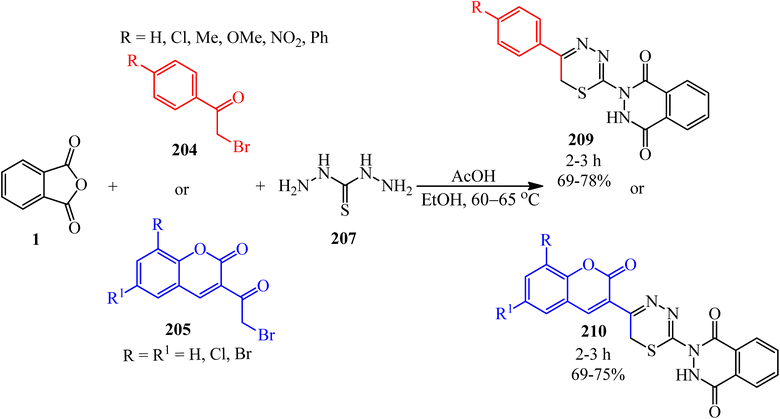
Chunduru and Vedula in 2013 reported the synthesis of aryl(hetaryl)-substituted thiazolylphthalazine-1,4-diones (206, 207) via the reaction of PA (1), thiosemicarbazide (203), and phenacyl bromides (204)/3-(2-bromoacetyl)coumarins (205), respectively (Scheme 78).193,194
Sujatha and Vedula in 2019 presented a novel one-pot multicomponent method for the synthesis of (E)-2-((benzylideneamino)-5-mercapto-4H-1,2,4-triazol-3-yl)-2,3-dihydrophthalazine-1,4-diones (211) via the reaction of PA (1), aromatic aldehyde (19), and 4-amino-5-hydrazino-4H-1,2,4-triazole-3-thiol (201) (Scheme 79).195
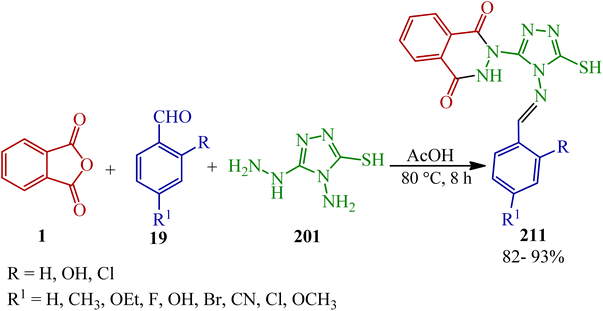 | ||
| Scheme 79 Synthesis of (E)-2-(benzylideneamino)-5-mercapto-4H-1,2,4-triazol-3-yl)-2,3 dihydrophthalazine-1,4-diones. | ||
Vedula et al. in 2020 developed the synthesis of a series of 2-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)-2,3-dihydrophthalazine-1,4-diones (212) via a one-pot multicomponent reaction of PA (1), 4-amino-5-hydrazineyl-4H-1,2,4-triazole-3-thiol (201), and substituted 2-bromo-1-phenylethanones (204) in the presence of acetic acid under reflux conditions (Scheme 80).196
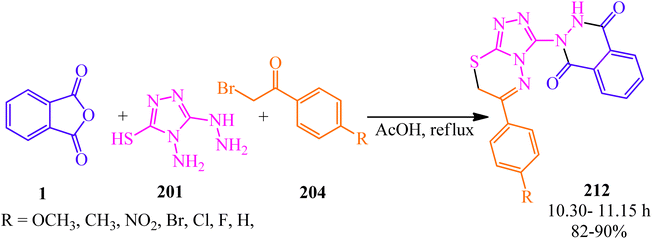 | ||
| Scheme 80 Synthesis of 2-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)-2,3-dihydrophthalazine-1,4-diones. | ||
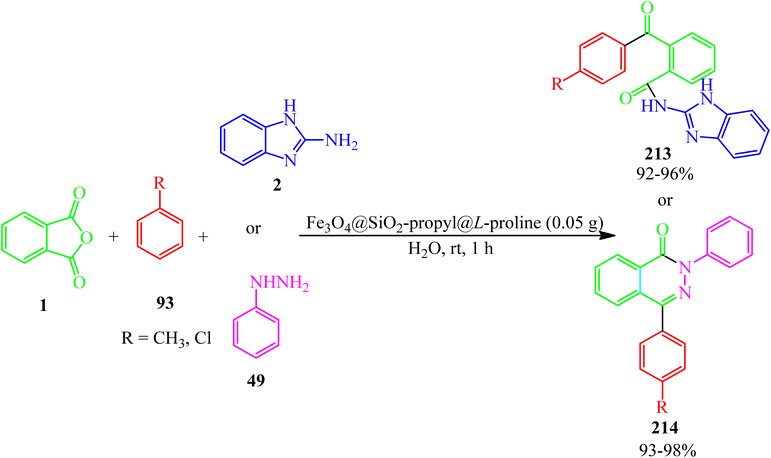
Zare Fekri and Farjood Feshalami in 2020 investigated the multicomponent reaction of PA (1) and 2-aminobenzimidazole (2), and arenes (93) to synthesize N-(1H-benzo[d]imidazol-2-yl)-2-benzoylbenzamides (213), and also, the multicomponent synthesis of pyridazinones (214) via the reaction of PA (1), phenyl hydrazine (49), and arenes (93) using L-proline-functionalized silicapropyl-modified nanomagnetic catalyst (Fe3O4@SiO2-propyl@L-proline) (Scheme 81).197
In 2021, they also obtained the amides (213) via the water-mediated the-component condensation of PA (1), 2-aminobenzimidazole (2), and (93) in the presence of nickel-ferrite silica-propyl supported glucosamine crystalline nanoparticles (NiFe2O4@SiO2-propyl@glucosamine) (0.05 g) as an efficient, reusable, and heterogeneous catalyst within 1 h with 92–96% yield.198
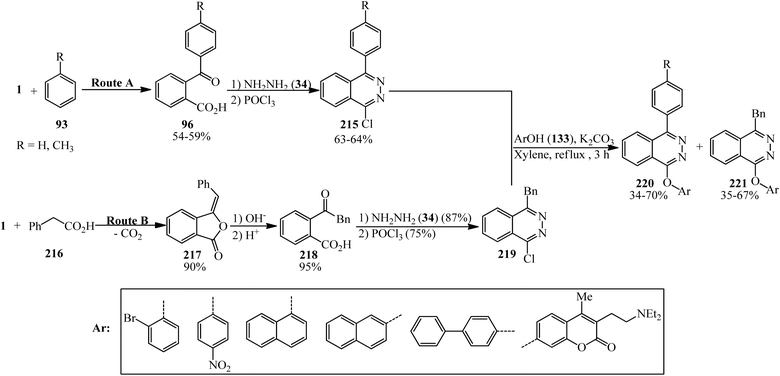
Bele and Darabantu in 2003 demonstrated the rapid synthesis of eighteen new 1,4-disubstituted phthalazines bearing an aryl or benzyl substituent at C-4 and a variety of aryloxy groups at C-l. The route A afforded the phthalazines (215) possessing a direct Ar–Ar linkage. Route B provided the L-chloro-4-benzylphthalazine (219). The neat condensation between (215, 219) and selected phenols (135) resulted in the decomposition of the reaction mixtures. Indeed, three series of new phthalazine derivatives were obtained in refluxing xylene (220, 221). All compounds were isolated simply by direct crystallization (Scheme 82).199
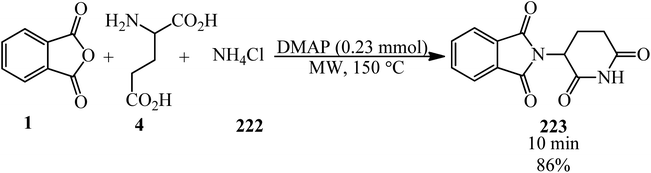
Benjamin and Hijji in 2017 developed a novel green one-pot synthetic technique for the generation of thalidomide (223) via the reaction of PA (1), glutamic acid (4), and ammonium chloride (222) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 molar ratio in the presence of catalytic amounts of 4-N,N-dimethylaminopyridine (DMAP) via microwave irradiation (Scheme 83).200
1.1 molar ratio in the presence of catalytic amounts of 4-N,N-dimethylaminopyridine (DMAP) via microwave irradiation (Scheme 83).200
Garcia and Vilarrasa in 1986 prepared N-substituted phthalimides (23) via the reaction of PA (1), triphenylphosphine (16), and azides (224) in the presence of tetrabutyl ammonium cyanide as the catalyst and benzene or toluene as solvent (Scheme 84).201
Deniau and coworkers in 2005 extended the asymmetric synthesis of diarylphosphine oxide-substituted isoindolinones (229) by a three-step reaction starting from PA (1) and (S)-1-amino-2-alkyloxymethylpyrrolidine (225) to prepare phthalhydrazides (226), which was reduced to 2-((S)-2-(alkoxymethyl)pyrrolidin-1-yl)-3-hydroxyisoindolin-1-one (227). In the second step, the final products (229) were obtained from the reaction of (227) with diarylphosphine oxides (228) (Scheme 85).202,203 The “de” of the products is more than 96% after recrystallization from hexane/toluene.
A green synthetic protocol was developed by Kalpana's group in 2021 for the synthesis of imidazo[4,5-b]pyrazine-conjugated benzamides (231) via the one-pot three-component reaction of PA (1), substituted anilines (21), and pyrazine-2,3-diamine (230) in the presence of phosphoric acid as the catalyst in heated water (Scheme 86).204 The products were evaluated for their anticancer activity against liver and ovarian cancer cell lines (HepG2 and HeLa), which demonstrated moderate to good activities. In addition, molecular modeling investigations affirmed the crucial binding interactions of the target protein and the synthesized ligands. In addition, the permeability and bioavailability properties were predicted along with molecular descriptors such as shape index, molecular complexity, and molecular flexibility.
Rasheed et al. in 2021 accomplished the reaction of PA (1) and 4,4′-(2,8-dimethyl-2,3-dihydro-1H-benzo[b][1,5]diazepine-2,4-diyl)dianiline (232) in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in the refluxing acetic acid solvent under microwave irradiation to obtain 2,2′-((2,8-dimethyl-2,3-dihydro-1H-benzo[b][1,5]diazepine-2,4-diyl)bis(4,1-phenylene))bis(isoindoline-1,3-dione) (233) as a dark brown solid (Scheme 87).205
1 molar ratio in the refluxing acetic acid solvent under microwave irradiation to obtain 2,2′-((2,8-dimethyl-2,3-dihydro-1H-benzo[b][1,5]diazepine-2,4-diyl)bis(4,1-phenylene))bis(isoindoline-1,3-dione) (233) as a dark brown solid (Scheme 87).205
 | ||
| Scheme 87 Synthesis of 2,2′-((2,8-dimethyl-2,3-dihydro-1H-benzo[b][1,5]diazepine-2,4-diyl)bis(4,1-phenylene))bis(isoindoline-1,3-dione). | ||
Eissa in 2014 obtained six N,N-substituted phthaldicarboximides (235), which was synthesized from the reaction of PA (1) with diamines (234). The synthesized compounds were screened for their antibacterial activity against four microorganisms, namely, Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Klebsiella pneumonia, and they were found to exhibit good to moderate antibacterial activity (Scheme 88).206
Chen's group in 2020 described an efficient cobalt-catalyzed intermolecular decarbonylative three-component carboamidation of alkynes (187) through the oxidative addition of cobalt into the N–C(O) bond of phthalimide and the subsequent decarbonylation. High regioselectivities achieved for unsymmetrical alkynes (including aryl-alkyl or aryl–aryl) to deliver polysubstituted isoquinolones (237–240) (Scheme 89).207
Zheng's group in 2019 described the efficient [2 + 2 + 2] benzannulation of PA (1) (or phthalic acid) with alkynes (187) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.33 molar ratio to prepare multisubstituted 1-naphthoic acids (241) via Ru-catalyzed C–H activation. The reaction proceeded well using atmospheric oxygen as the sole oxidant with high atom/step economies (Scheme 90).208
1.33 molar ratio to prepare multisubstituted 1-naphthoic acids (241) via Ru-catalyzed C–H activation. The reaction proceeded well using atmospheric oxygen as the sole oxidant with high atom/step economies (Scheme 90).208
Singh's group in 2015 demonstrated the transimidization reaction between PA (1), 2-amino-3-picoline (242), and 3-aminopropyl triethoxysilane (243) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.125 molar ratio, which transited from 2-(3-methylpyridin-2-yl)isoindoline-1,3-dione (23) though a two-step reaction to synthesize N-(triethoxysilylpropyl)phthalimide (244). New silatranes (246, 247) containing phthalimide as the exocyclic group were prepared by the transesterification reactions of (244) with triethanolamine/trisisopropanolamine (245), respectively. N-(silatranylpropyl)phthalimides (246, 247) were evaluated for the preliminary antimicrobial activity using broth microdilution method, which shows that silatranes-possessing urea group exhibited good antimicrobial activity. The investigation of the UV-vis spectra of the silatranes proved fruitful for analyzing the hydrogen bonding of trifluroacetic acid with phthalimide heterocycle possessing silatranes. Hydrogen bonding has shown major influence upon n → π* transition, resulting in blueshift phenomenon (Scheme 91).209
1.125 molar ratio, which transited from 2-(3-methylpyridin-2-yl)isoindoline-1,3-dione (23) though a two-step reaction to synthesize N-(triethoxysilylpropyl)phthalimide (244). New silatranes (246, 247) containing phthalimide as the exocyclic group were prepared by the transesterification reactions of (244) with triethanolamine/trisisopropanolamine (245), respectively. N-(silatranylpropyl)phthalimides (246, 247) were evaluated for the preliminary antimicrobial activity using broth microdilution method, which shows that silatranes-possessing urea group exhibited good antimicrobial activity. The investigation of the UV-vis spectra of the silatranes proved fruitful for analyzing the hydrogen bonding of trifluroacetic acid with phthalimide heterocycle possessing silatranes. Hydrogen bonding has shown major influence upon n → π* transition, resulting in blueshift phenomenon (Scheme 91).209
Malik's group in 2022 accessed new imidazole-based N-phenylbenzamides (249) from one-pot three-component reaction PA, substituted anilines (23), and 2,3-diaminomaleonitrile (248) in the presence of HCl in refluxing ethanol. The cytotoxic evaluation revealed that some derivatives (with para-fluorine and para-methoxy substituent) were the most active compounds, which exhibited good activity against the tested cancer cell lines (with single-digit IC50 values). Computational studies and molecular dynamic simulations were also investigated (Scheme 92).210
Xinwei's group in 2022 developed a rhodium(III)-catalyzed cascade reaction of phthalic anhydrides with cyclic 2-diazo-1,3-diketones (250) and methanol to obtain the esterified cyclohexenone-fused isocoumarins (251). The formation of C–C and two C–O bonds through C–H activation, transannulative coupling, and subsequent annulation process occurred through this strategy. Furthermore, there is no need for an additive or base. Surprisingly, the reaction proceeded well under air atmosphere with broad substrate and good functional group tolerance (Scheme 93).211
4. Applications of PA in four-component and pseudo four-component reactions
Shaterian and Mohammadnia in 2012 prepared 1H-pyrazolo[1,2-b]phthalazine-5,10-diones (252) under ambient and solvent-free conditions in the presence of mild basic ionic liquids, which are 1,8-diazabicyclo[5.4.0]-undec-7-en-8-ium acetate (DBU[CH3COO]), pyrrolidinium formate ([Pyrr][HCOO]), and pyrrolidinium acetate ([Pyrr][CH3COO]) via the domino reaction of PA (1), aromatic aldehydes (19), malononitrile (48)/ethyl cyanoacetate (112), and hydrazine monohydrate (56) (Scheme 94).212 The PA and (56) were mixed at 100 °C for 10 min to obtain solid phthalhydrazide intermediate (A). The subsequent addition of (48)/(112) and aromatic aldehydes (19) in addition with 2appropriate ionic liquid at ambient temperature yielded the products (252).In 2014, the same group also obtained 1H-pyrazolo[1,2-b]phthalazine-5,10-diones (252) via the four-component domino one-pot condensation reaction of PA (1), aromatic aldehydes (19), malononitrile(48)/ethyl cyanoacetate (112), and hydrazine monohydrate (56) in the presence of magnetic Fe3O4 nanoparticles coated with (3-aminopropyl)-triethoxysilane (APTES-MNP, 10 mol%) as the catalyst under solvent-free conditions within 4–15 min with 94–86% yield.213
Many other research groups studied the domino four-component reaction of PA (1), aromatic aldehydes (19), malononitrile(48)/ethyl cyanoacetate (112), and hydrazine monohydrate (56) to prepare the corresponding 1H-pyrazolo[1,2-b]phthalazine-5,10-diones (252) in the presence of various catalytic systems successfully. For example, Ghomi et al. in 2014 utilized CuI nanoparticles (10 mol%) within 25–30 min by 82–93% to accelerate this transformation.214
Some other researches utilized PA (1), aromatic aldehydes (19), malononitrile (48), and hydrazine monohydrate (56) to prepare 1H-pyrazolo[1,2-b]phthalazine-5,10-diones (252). They are as follows: (A) Ghorbani-Vaghei et al. in 2016 exploited piperidinium benzene-1,3-disulfonate nanomagnetic ionic liquid (NMIL, 20 mg) as a novel and reusable catalyst at 110 °C under solvent-free conditions within 40–80 min with 75–94% yield. The magnetized nanostructure was recycled and reused seven times without any loss of catalytic activity. In addition, they examined the four-component reaction in the presence of aliphatic aldehydes such as 3-phenylpropanal and fused aromatic candidates such as 2-naphthadehyde successfully.215 (B) Lashkari's group in 2018 reported zinc acetate dihydrate (Zn(OAc)·2H2O, 15 mol%) to accelerate the synthesis of 1H-pyrazolo[1,2b]phthalazine-5,10-dione derivatives (252) under solvent-free conditions at 70 °C with 93–83% yield within 2.5–4.5 h.216 (C) Mohamadpour in 2020 discussed an ecosafe green synthetic route to obtain (252) via the domino Knoevenagel–Michael cyclocondensation of PA (1), hydrazine monohydrate (56), aromatic aldehydes (19), and malononitrile (48) in the presence of carboxymethyl cellulose (CMC, 25 mol%) at 80 °C with a yield of 77–94% in 65–95 min period. Then, the catalyst was recovered after washing with ethyl acetate, filtering, and air drying, then reused for consecutive five runs with good yields and with insignificant CMC loss.217 (D) Maghsoodlou and coworkers in 2016 achieved (252) via the domino reaction of PA (1), hydrazine monohydrate (56), aromatic benzaldehyde (19), and malononitrile (48) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) with copper(II) acetate monohydrate (20 mol%) at 80 °C within 3–5 min with 73–89%.218 (E) Amini's group in 2021 prepared (251) through a one-step reaction of PA (1), aromatic aldehydes (19), malononitrile (48), and hydrazine monohydrate (56) (in a 1
1 molar ratio) with copper(II) acetate monohydrate (20 mol%) at 80 °C within 3–5 min with 73–89%.218 (E) Amini's group in 2021 prepared (251) through a one-step reaction of PA (1), aromatic aldehydes (19), malononitrile (48), and hydrazine monohydrate (56) (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 molar ratio) in the presence of NiCl2·6H2O (10 mol%) in refluxing EtOH for 2–4 h within 81–96%. They also examined the procedure with ethanal (as an aliphatic candidate) to prepare the corresponding products successfully. The authors executed the α-glucosidase inhibitory activity of the synthesized compounds using a source of the α-glucosidase enzyme (EC3.2.1.20, Saccharomyces cerevisiae at 20 U mg−1 concentration). The results revealed that some of the products are efficient inhibitors of the α-glucosidase enzyme compared to the acarbose standard.219 (F) Lingampalle's group in 2019 constructed (252) though the domino reaction, in which, firstly, equal molar ratio of PA (1) and monohydrate hydrazine (56) were mixed at 80 °C for 15 min. Then, aromatic aldehydes (19) and malononitrile (48) were added and heated at 100 °C under microwave irradiation (300 W) for 10–16 min in the presence of boric acid (10 mol%) catalyst, which led to the corresponding adducts (252) with 86–94% yield.220 (G) 3-Amino-1-aryl-5,10-dioxo-1H-pyrazolo[1,2-b]phthalazine-2-carbonitriles (252) were also obtained by Abdesheikhi and Karimi-Jaberi in 2015 though the domino reaction of PA, hydrazine hydrate, benzaldehydes, and malononitrile in the presence of K2CO3 (0.1 g) in refluxing ethanol within 50–80 min by 86–96%.221 (H) Kalhor's group in 2022 obtained novel multifunctional nanocatalyst (Mn/4-MePy-IL@ZY), namely, 4-methylpyridinium chloride ionic liquid grafted on Mn@zeolite-Y, and scanned its catalytic performance (10 mol%) in the four-component synthesis of pyrazolo[1,2-b]phthalazines (252) under mild reaction conditions (H2O/EtOH, 1
1.2 molar ratio) in the presence of NiCl2·6H2O (10 mol%) in refluxing EtOH for 2–4 h within 81–96%. They also examined the procedure with ethanal (as an aliphatic candidate) to prepare the corresponding products successfully. The authors executed the α-glucosidase inhibitory activity of the synthesized compounds using a source of the α-glucosidase enzyme (EC3.2.1.20, Saccharomyces cerevisiae at 20 U mg−1 concentration). The results revealed that some of the products are efficient inhibitors of the α-glucosidase enzyme compared to the acarbose standard.219 (F) Lingampalle's group in 2019 constructed (252) though the domino reaction, in which, firstly, equal molar ratio of PA (1) and monohydrate hydrazine (56) were mixed at 80 °C for 15 min. Then, aromatic aldehydes (19) and malononitrile (48) were added and heated at 100 °C under microwave irradiation (300 W) for 10–16 min in the presence of boric acid (10 mol%) catalyst, which led to the corresponding adducts (252) with 86–94% yield.220 (G) 3-Amino-1-aryl-5,10-dioxo-1H-pyrazolo[1,2-b]phthalazine-2-carbonitriles (252) were also obtained by Abdesheikhi and Karimi-Jaberi in 2015 though the domino reaction of PA, hydrazine hydrate, benzaldehydes, and malononitrile in the presence of K2CO3 (0.1 g) in refluxing ethanol within 50–80 min by 86–96%.221 (H) Kalhor's group in 2022 obtained novel multifunctional nanocatalyst (Mn/4-MePy-IL@ZY), namely, 4-methylpyridinium chloride ionic liquid grafted on Mn@zeolite-Y, and scanned its catalytic performance (10 mol%) in the four-component synthesis of pyrazolo[1,2-b]phthalazines (252) under mild reaction conditions (H2O/EtOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 80 °C) within 8–12 min with 88–98% yields.222
1, 80 °C) within 8–12 min with 88–98% yields.222
Jonnalagadda et al. in 2020 utilized eggshell powder (ESP, 20 mg) as a biodegradable and recyclable catalyst for the synthesis of pyrazolo-phthalazine derivatives (252) in aqueous media within 28–45 min by 93–98% at 60 °C. They performed the reaction in the presence of the substrates PA, hydrazine hydrate, aldehydes, and activated methylene groups in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in a one-pot and one-step manner. They examined the method scope with different activated methylene compounds (malononitrile, ethyl cyanoacetate, and methyl cyanoacetate) and also several benzaldehydes and indole-3-carbaldehdye successfully.223
1 molar ratio in a one-pot and one-step manner. They examined the method scope with different activated methylene compounds (malononitrile, ethyl cyanoacetate, and methyl cyanoacetate) and also several benzaldehydes and indole-3-carbaldehdye successfully.223
Roy's group in 2016 synthesized (252) through the domino reaction of PA (1), aromatic aldehyde (19), ethyl cyanoacetate (112), and hydrazine hydrate (56) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 molar ratio in the presence of L-proline (10 mol%) and LiCl (5 mol%) in ethanol/water (1
1.2 molar ratio in the presence of L-proline (10 mol%) and LiCl (5 mol%) in ethanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) media at 80 °C within 10–12 h by 87–90%. LiCl was used to elevate the yield of the reaction by elevating the electrophilicity of the aldehyde functionality.224
1) media at 80 °C within 10–12 h by 87–90%. LiCl was used to elevate the yield of the reaction by elevating the electrophilicity of the aldehyde functionality.224
The Das group in 2014 prepared a novel biodegradable SO3H-bearing carbonaceous solid catalyst (PEG-SAC, 70 mg) to achieve 1H-pyrazolo[1,2-b]phthalazine-5,10-dione carboxamides (254) through a domino aqueous media reaction of PA (1), hydrazine hydrate (56), (hetero)aromatic aldehydes (19), and malononitrile (48)/2-cyanoacetamide (253) at 60 °C. They also utilized aliphatic aldehydes such as isobutyraldehyde successfully. To improve the applicability of the Brønsted acid PEG-SAC catalyst, the authors also examined the preparation of multifunctionalized 1H-pyrazolo[1,2-b]phthalazine-5,10-dione carboxamides in a regio-controlled manner (no 1H-pyrazolo[1,2-b]phthalazine-2-carbonitriles observed) (Scheme 95).225
Raghavendra Siddaiah performed the domino synthesis of 3-amino-1-(1H-indol-2-yl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine derivatives (256) via the reaction of PA (1) with hydrazine hydrate (56), malononitrile (48)/ethyl cyanoacetate (112), and indole-3-carboxaldehydes (255) in the presence of the ionic liquid ([DBUH][OAc]) at 60–65 °C (Scheme 96).226
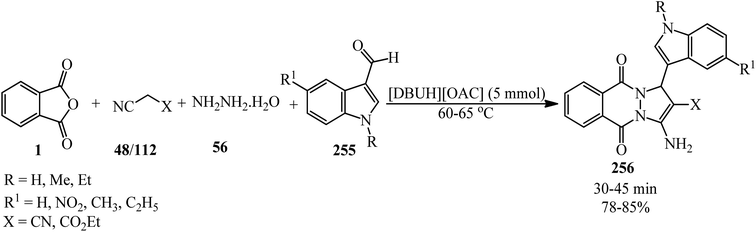 | ||
| Scheme 96 Synthesis of 3-amino-1-(1H-indol-2-yl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazines. | ||
Reddy's group in 2014 also obtained 3-amino-1-(1H-indol-2-yl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazines (256) in a four-component domino reaction between hydrazine hydrate, N-substituted-indole-3-carboxaldehydes, malononitrile/ethyl cyanoacetate, and dialkylphthalates (methyl and ethyl) in the presence of InCl3 (30 mol%) in refluxing ethanol within 1 h with 70–85% yield.227 Also, they utilized dialkylphthalates instead of PA; interestingly, the products (256) were obtained.
Shaabani's research group in 2012 developed the synthesis of structurally diverse 1H-pyrazolo[1,2-b]phthalazine-1,2-dicarboxylates (257) via a four-component reaction of PA (1), hydrazine hydrate (56), dialkyl acetylenedicarboxylates (17), and isocyanides (196) in ethanol/acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature in good to moderate yields (Scheme 97).228
1) at room temperature in good to moderate yields (Scheme 97).228
Kumar's research group in 2013 presented one-pot four-component protocol for the synthesis of structurally diverse spirooxindoles, spiroannulated with chromenopyrazolophthalazines/pyranopyrazolophthalazines/indazolophthalazine (262, 263, 264) from the reaction of PA (1), hydrazine hydrate (56), isatins (258), and cyclic ketones [4-hydroxy-2H-chromen-2-one (259), 4-hydroxy-6-methylpyran-2-one (260), dimedone (261)] in aqueous alcoholic medium (H2O/C2H5OH, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using catalytic amounts of sulphamic acid (SA) (Scheme 98).229 The reaction mechanism is considered to involve acid-catalyzed Knoevenagel condensation, Michael-type addition reaction, and consequent intramolecular dehydrative cyclization to obtain the pure products via recrystallization from ethanol.
1) using catalytic amounts of sulphamic acid (SA) (Scheme 98).229 The reaction mechanism is considered to involve acid-catalyzed Knoevenagel condensation, Michael-type addition reaction, and consequent intramolecular dehydrative cyclization to obtain the pure products via recrystallization from ethanol.
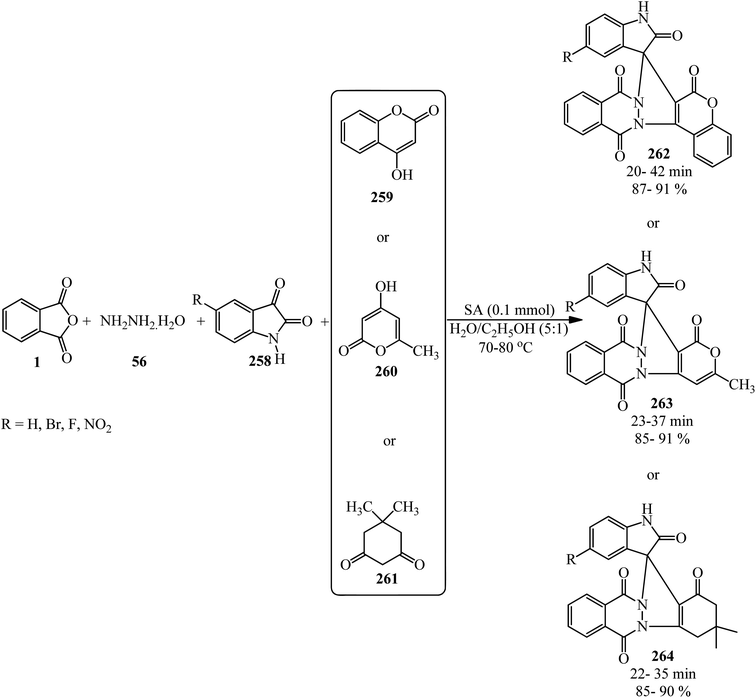 | ||
| Scheme 98 Synthesis of spirooxindoles spiroannulated with chromenopyrazolophthalazines/pyranopyrazolophthalazines/indazolophthalazine. | ||
Kumar's group in 2014 constructed indenopyrazolophthalazines (266) and pyrazolopyrimidophthalazines (267, 268) via the reaction of PA (1), hydrazine hydrate (56), isatins (258), and 1,3-indandione (265)/barbituric acids (266) in the presence of deep eutectic solvent (DES, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, 1
urea, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as a catalyst and reaction medium at 80 °C (Scheme 99).230 They also prepared chromenopyrazolophthalazines (262) and indazolophthalazine (264) in the same reaction conditions within 20–32 min with 88–90% and 86–93% yields, respectively.
2) as a catalyst and reaction medium at 80 °C (Scheme 99).230 They also prepared chromenopyrazolophthalazines (262) and indazolophthalazine (264) in the same reaction conditions within 20–32 min with 88–90% and 86–93% yields, respectively.
Biabangard and Shaterian in 2015 obtained pyrazolopyrimidophthalazines (267) via the domino four-component reaction of PA (1), aromatic aldehydes (19), hydrazine hydrate (56), and barbituric acid (266), in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, using vitamin B1 supported on alumina (VB1-Al2O3, 5 mol%) as a heterogeneous catalyst under solvent-free conditions at 70 °C within 10–14 min with 88–92% yield.231 The recovered catalyst was reutilized for at least four runs without any loss of its activity.
1 molar ratio, using vitamin B1 supported on alumina (VB1-Al2O3, 5 mol%) as a heterogeneous catalyst under solvent-free conditions at 70 °C within 10–14 min with 88–92% yield.231 The recovered catalyst was reutilized for at least four runs without any loss of its activity.
A novel catalyst obtained via the stabilization of methylene dipyridine nanoparticles on Fe3O4 (Fe3O4/SiO2/propyltriethoxysilane/methylene dipyridine nanoparticles) was prepared by Sadeghzadeh and Nasseri in 2013, which was utilized to prepare pyrazolophthalazinyl spirooxindoles (269) via a four-component solvent-free reaction at room temperature (Scheme 100).232
Maleki and Sedigh Ashrafi in 2014 reported the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones (271) and 1H-indazolo[1,2-b] phthalazine-1,6,11-triones (272) via the multicomponent and one-pot reactions of PA (1), various aldehydes (19), hydrazinium hydroxide (56), and acyclic or cyclic 1,3-diketones (261, 270) using wet 2,4,6-trichlorotriazine (TCT) as a catalyst under solvent-free conditions (Scheme 101).233 Cyanuric acid was produced as the byproduct, which was removed by washing with water.
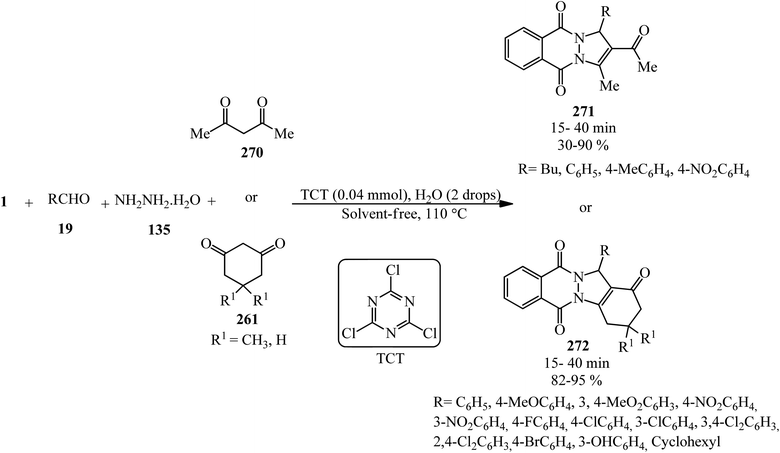 | ||
| Scheme 101 Synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones and 1H-indazolo[1,2-b] phthalazine-1,6,11-triones. | ||
Esmaeilpour and Zahmatkesh et al. in 2017 investigated the synthesis of 1H-pyrazolo[1,2-b]phthalazine-diones (271) 2H-indazolo[2,1-b]phthalazine-triones (272) using Fe3O4@SiO2-imid-PMAn nanoparticles (immobilization of H3PMo12O40 nanoparticles (PMAn) on an imidazole-functionalized Fe3O4@SiO2) as an ecofriendly magnetic catalyst from the four-component condensation, solvent-free reaction of PA (1), aldehydes (19), hydrazinium hydroxide (56), 1,3-diketones (dimedone (261), 1,3-pentandion), and in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio under conventional thermal conditions (80 °C) or ultrasound-assisted conditions at room temperature.234 Also, to enlarge the efficacy of the procedure, a vast range of aldehydes including aromatic, heteroaromatic, cyclic/acyclic aliphatic, and sterically-hindered candidates were used to perform the reaction successfully. They also examined the catalyst efficacy to prepare some new kinds of pyrazolo[1,2-b]phthalazine-5,10-diones (271) through the reaction of PA (1), aldehydes (19), hydrazine hydrate (56), and 1,3-diphenylpropanedione (273) (Scheme 102).
1 molar ratio under conventional thermal conditions (80 °C) or ultrasound-assisted conditions at room temperature.234 Also, to enlarge the efficacy of the procedure, a vast range of aldehydes including aromatic, heteroaromatic, cyclic/acyclic aliphatic, and sterically-hindered candidates were used to perform the reaction successfully. They also examined the catalyst efficacy to prepare some new kinds of pyrazolo[1,2-b]phthalazine-5,10-diones (271) through the reaction of PA (1), aldehydes (19), hydrazine hydrate (56), and 1,3-diphenylpropanedione (273) (Scheme 102).
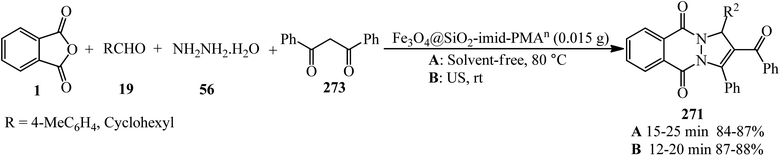 | ||
| Scheme 102 Pyrazolo[1,2-b]phthalazine-5,10-diones obtained from PA, aldehydes, hydrazine hydrate, and 1,3-diphenylpropanedione. | ||
Safaei-Ghomi et al. in 2016 described the synthesis of some 1H-pyrazolo[1,2-b]phthalazine-diones (271) and 2H-indazolo[2,1-b]phthalazine-triones (272) via the domino reaction of PA (1), aromatic aldehydes (19), hydrazine monohydrate (56), and malononitrile (48)/ethyl cyanoacetate (112)/dimedone (261), respectively. The reaction proceeded in the presence of CuFe2O4 and ZrP2O7 nanocatalysts under solvent-free conditions (Scheme 103).235
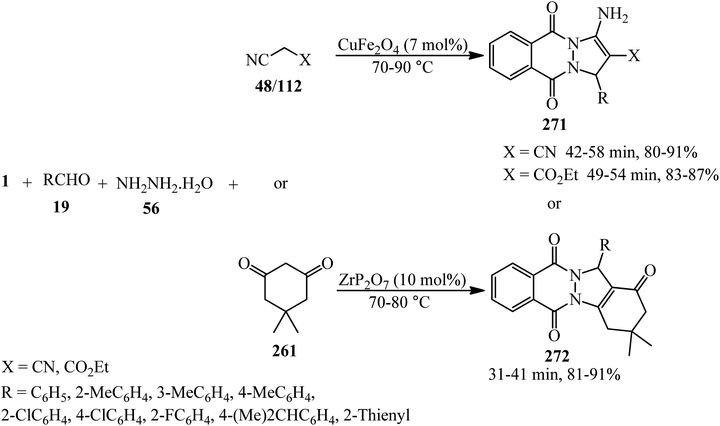 | ||
| Scheme 103 Synthesis of 1H-pyrazolo[1,2-b]phthalazine-diones and 2H-indazolo[2,1-b]phthalazine-triones. | ||
Besides the abovementioned examples, many other research groups prepared different kinds of 2H-indazolo[2,1-b]phthalazine-triones (272) via a four-component reaction of PA (1), different aldehydes (19), hydrazine hydrate (56), and dimedone (261). (A) The Javidi and Esmaeilpour group in 2016 synthesized dendrimer-encapsulated phosphotungstic acid nanoparticles immobilized on nanosilica with surface amino groups (dendrimer-PWAn) and utilized it as a reusable catalyst to accelerate the green synthesis of (272) via a one-pot, one-step, and four-component condensation reaction of PA (1), aldehydes (19), hydrazinium hydroxide (56), and dimedone (261) in a 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at both under solvent-free conditions at 80 °C and ultrasound irradiation at room temperature.236 The protocol was successful for different classes of aldehydes such as aromatic, cyclic/linear aliphatic, heteroaromatics, and sterically-hindered candidates. An example of their procedure has been demonstrated in Scheme 104 (entry 1). In addition, the catalyst could be successfully recycled and reused at least for six runs without a significant loss in activity. (B) Shaterian and Rigi in 2014 prepared (272) using cellulose-SO3H (4 mol%) as a solid-acidic reusable catalyst via the domino four-component condensation reaction under thermal (80 °C) solvent-free conditions (Scheme 104, entry 2).237 The protocol was unsuccessful in utilizing aliphatic aldehydes such as heptanal and octanal. (C) Mosaddegh and Hassankhani in 2011 described an efficient protocol for the one-pot, one-step synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-triones (272) under thermal (125 °C) solvent-free conditions using PA (1), aromatic aldehydes (19), hydrazinium hydroxide (56), and dimedone (261) in a 1
1 molar ratio at both under solvent-free conditions at 80 °C and ultrasound irradiation at room temperature.236 The protocol was successful for different classes of aldehydes such as aromatic, cyclic/linear aliphatic, heteroaromatics, and sterically-hindered candidates. An example of their procedure has been demonstrated in Scheme 104 (entry 1). In addition, the catalyst could be successfully recycled and reused at least for six runs without a significant loss in activity. (B) Shaterian and Rigi in 2014 prepared (272) using cellulose-SO3H (4 mol%) as a solid-acidic reusable catalyst via the domino four-component condensation reaction under thermal (80 °C) solvent-free conditions (Scheme 104, entry 2).237 The protocol was unsuccessful in utilizing aliphatic aldehydes such as heptanal and octanal. (C) Mosaddegh and Hassankhani in 2011 described an efficient protocol for the one-pot, one-step synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-triones (272) under thermal (125 °C) solvent-free conditions using PA (1), aromatic aldehydes (19), hydrazinium hydroxide (56), and dimedone (261) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in the presence of Ce(SO4)2·4H2O (2.5 mol%) within 5–10 min (Scheme 104, entry 3).238 (D) Shaterian and Rigi in 2011 obtained (272) via the domino four-component condensation reaction from (1), aromatic aldehydes (19), (56), and dimedone (261) in a 1
1 molar ratio in the presence of Ce(SO4)2·4H2O (2.5 mol%) within 5–10 min (Scheme 104, entry 3).238 (D) Shaterian and Rigi in 2011 obtained (272) via the domino four-component condensation reaction from (1), aromatic aldehydes (19), (56), and dimedone (261) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio using 0.8 mol% starch sulfate as a bio-supported nonhygroscopic solid-acid catalyst under thermal (80 °C) solvent-free conditions within 4–15 min period with 72–92% yield (Scheme 104, entry 4).239 The recovered catalyst was reused four times without any loss of its activities. (E) The compounds (272) were achieved by Hasaninejed et al. in 2012 through the four-component one-step condensation reaction of PA (1), aromatic aldehydes (19), hydrazinium hydroxide (56), and 1,3-cyclohexanedione/dimedone in a 1
1 molar ratio using 0.8 mol% starch sulfate as a bio-supported nonhygroscopic solid-acid catalyst under thermal (80 °C) solvent-free conditions within 4–15 min period with 72–92% yield (Scheme 104, entry 4).239 The recovered catalyst was reused four times without any loss of its activities. (E) The compounds (272) were achieved by Hasaninejed et al. in 2012 through the four-component one-step condensation reaction of PA (1), aromatic aldehydes (19), hydrazinium hydroxide (56), and 1,3-cyclohexanedione/dimedone in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12
1.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at 80 °C using sulfuric acid-modified PEG-6000 (PEG-OSO3H, 8 mol%) as a green, recyclable, and biodegradable polymeric catalyst, with the yield of 80–93% in 10–20 min (Scheme 104, entry 5).240 The authors calculated the environmental impact factor (E-factor)241 to evaluate the minimizing environmental impacts of their method. (F) Shaterian and Aghakhanizadeh in 2012 obtained (272) from the domino four-component solvent-free reaction of PA (1), arylaldehydes (19), hydrazine monohydrate (56), and dimedone/1,3-cyclohexanedion in a 1.2
1 molar ratio at 80 °C using sulfuric acid-modified PEG-6000 (PEG-OSO3H, 8 mol%) as a green, recyclable, and biodegradable polymeric catalyst, with the yield of 80–93% in 10–20 min (Scheme 104, entry 5).240 The authors calculated the environmental impact factor (E-factor)241 to evaluate the minimizing environmental impacts of their method. (F) Shaterian and Aghakhanizadeh in 2012 obtained (272) from the domino four-component solvent-free reaction of PA (1), arylaldehydes (19), hydrazine monohydrate (56), and dimedone/1,3-cyclohexanedion in a 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio using reusable ionic liquids [including A: 2-pyrrolidonium hydrogen sulfate ([Hnhp][HSO4]); B: (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogen sulfate; and C: triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate] as acidic reusable catalysts (5 mol%). The catalysts recovered within 5 runs without significant activity loss. The time and yield of utilizing these 3 ILs at 80 °C are A: 8–11 min/70–90%; B: 4–8 min/83–92%; and C: 6–15 min/80–93%, respectively (Scheme 104, entry 6).242 (G) Shekouhy and Hasaninejad in 2012 obtained (272) via the one-pot and one-step four-component reaction of PA (1), (hetero)aromatic aldehydes (19), hydrazine monohydrate (56), and dimedone (261) in a 1
1 molar ratio using reusable ionic liquids [including A: 2-pyrrolidonium hydrogen sulfate ([Hnhp][HSO4]); B: (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogen sulfate; and C: triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate] as acidic reusable catalysts (5 mol%). The catalysts recovered within 5 runs without significant activity loss. The time and yield of utilizing these 3 ILs at 80 °C are A: 8–11 min/70–90%; B: 4–8 min/83–92%; and C: 6–15 min/80–93%, respectively (Scheme 104, entry 6).242 (G) Shekouhy and Hasaninejad in 2012 obtained (272) via the one-pot and one-step four-component reaction of PA (1), (hetero)aromatic aldehydes (19), hydrazine monohydrate (56), and dimedone (261) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with a yield of 89–95% in 3–15 min using 1-butyl-3-methyl imidazolium bromide ([Bmim]Br, 0.5 g) as a neutral IL under ultrasonic irradiation and catalyst-free conditions at room temperature (Scheme 104, entry 7).243 (H) Veisi and Aminimanesh et al. in 2014 synthesized (272) in a one-pot and one-step reaction of PA (1), aliphatic/aromatic aldehydes (19), hydrazine monohydrate (56), and dimedone (261) in a 1
1 molar ratio with a yield of 89–95% in 3–15 min using 1-butyl-3-methyl imidazolium bromide ([Bmim]Br, 0.5 g) as a neutral IL under ultrasonic irradiation and catalyst-free conditions at room temperature (Scheme 104, entry 7).243 (H) Veisi and Aminimanesh et al. in 2014 synthesized (272) in a one-pot and one-step reaction of PA (1), aliphatic/aromatic aldehydes (19), hydrazine monohydrate (56), and dimedone (261) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in the presence of mildly basic ionic liquid N,N,N,N-tetramethylguanidinium acetate ([TMG][Ac], 10 mol%) in high yields (55–98%) at 80 °C within 10–30 min (Scheme 104, entry 8).244 (I) Habibi and Shamsian in 2015 utilized new reusable acid–base bifunctional ionic liquid, 1,4-dimethyl(4-sulfobutyl)piperazinium hydrogen sulfate ([DMSBP][HSO4], 3 mol%), for the synthesis of (272) via the one-step four-component reaction of reaction of PA (1), aromatic aldehydes (19), hydrazine monohydrate (56), and dimedone (261) in a 1
1 molar ratio in the presence of mildly basic ionic liquid N,N,N,N-tetramethylguanidinium acetate ([TMG][Ac], 10 mol%) in high yields (55–98%) at 80 °C within 10–30 min (Scheme 104, entry 8).244 (I) Habibi and Shamsian in 2015 utilized new reusable acid–base bifunctional ionic liquid, 1,4-dimethyl(4-sulfobutyl)piperazinium hydrogen sulfate ([DMSBP][HSO4], 3 mol%), for the synthesis of (272) via the one-step four-component reaction of reaction of PA (1), aromatic aldehydes (19), hydrazine monohydrate (56), and dimedone (261) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with 85–94% yield in 3–15 min (Scheme 104, entry 9).245 Examining the reaction with octanal as an aliphatic aldehyde, it did not form the corresponding product. The catalyst was recovered from the aqueous medium, dried under vacuum, and reused in five successive runs without a substantial loss of activity. (J) The compounds (272) were also prepared by Ebrahimipour et al. in 2015 via the one-pot and one-step, and four-component reaction in the presence of three octahedral complexes containing 2-pyrazinecarboxylate (pzca), including [Ni(pzca)2(H2O)2] (25 mol%), [Co(pzca)2(H2O)2] (20 mol%), and [Cu(pzca)2(H2O)2] (20 mol%) in acetic acid solvent at 50 °C within 10–40 min with 80–94% yields (Scheme 104, entry 10).246 (K) Bhosale et al. in 2017 developed the synthesis of (272) in the presence cesium chloride (15 mol%) in refluxing ethanol at 60 °C via the equimolar amounts of the substrates in a one-step and one-pot manner, in 3–4 h duration with a yield of 70–82% (Scheme 104, entry 11).247 (L) Gill's research group in 2017 demonstrated a synthetic route for 2H-indazolo[2,1-b]pthalazinetrione derivatives (272) through a one-pot, one-step four-component protocol in the presence of β-cyclodextrin as a supramolecular, biodegradable, and reusable catalyst in 80 °C aqueous media within 94–79%
yield during 35–25 min (Scheme 104, entry 12).248 (M) Ebrahimipour and coworkers in 2017 reported the synthesis of (272) with a yield of 84–94% in 15–25 min in the presence of imidazole 2-acetamido-N′-(3-methoxy-2-oxidobenzylidene)benzohydrazonate–nickel(II) [Ni(L)(imi)], which is a tridentate Schiff base ligand (Scheme 104, entry 13).249 The Ni(II) complex showed promising antimicrobial activities against some Gram-negative and Gram-positive bacteria such as E. coli, S. aureus, P. aeruginosa, and B. cereus. The catalyst was recovered by centrifugation and recrystallization from a mixture of ethanol and water and reused at least three times with satisfactory results. (N) Bamoniri et al. in 2020 described a method for the synthesis of 2H-Indazolo[2,1-b]phthalazinetrione derivatives (272) using nano γ-Al2O3/BFn/Fe3O4 (8 mg) via the one-step reaction of equimolar of four substrates under solvent-free conditions, which yielded the corresponding adducts with 90–97% yield in 10–17 min (Scheme 104, entry 14).250 The catalyst was recycled by washing with CH2Cl2, drying at 50 °C under vacuum for 1 h, and reused within 5 runs successfully. (O) Liu and Li et al. in 2020 prepared a novel modified core–shell magnetic nanocomposite by anchoring Ag NPs on magnetite core that was coated with chitosan-alginate dual bio-polysaccharide (Fe3O4/CS-Alg/Ag NPs). The novel nanocatalyst was utilized for the synthesis of (272) via one-step and one-pot reaction of the corresponding substrates at 80 °C within 0.2–1.5 h with 50–95% yield. The catalyst demonstrated human lung protective effects against α-Guttiferin. These events revealed that the catalyst suppressed Guttiferin-induced cell death in a dose-dependent manner in lung MRC-5, CCD-19Lu, WI-38, and BEAS-2B cell lines. The catalyst was recovered easily using an external magnet and recycled for 10 successive times with minimal reduction in activity (Scheme 104, entry 15).251 (P) In 2022, Naeimi and Zahedifar reported an immobilized copper(II) complex on microcellulose (cell-DABCO-Cu) as a novel catalyst to promote the preparation of (272) at 80 °C under solvent-free conditions in a one-step and one-pot manner (Scheme 104, entry 16).252 In addition, the CuO nanoparticles with an average size of 40 nm were obtained by the direct calcination of Cell-DABCO-Cu. (Q) Mahmoodi et al. in 2020 obtained (272) via the one-pot, one-step, four-component, and solvent-free condensation of PA (1), aromatic aldehydes (19), hydrazine hydrate (56), and dimedone (261), which was accelerated with triethanolammonium acetate ([TEAH][OAc]) at 80 °C within 10–35 min with 89–95% yield (Scheme 104, entry 17).253 The authors examined the efficacy of the protocol through utilizing succinic anhydride instead of PA. They also prepared phthalazine-diones (271) by the reaction of PA (1), aromatic aldehydes (19), hydrazine hydrate (56), and alkyl cyanoacetates (112) (methyl and ethyl) successfully in the same reaction conditions within 22–37 min with 80–95%. (R) Tigote's group in 2017 used ZnFe2O4 nanoparticles (1.5 mol%) as a catalyst for this transformation via the one-pot and two-step reaction of aromatic aldehyde (19) and the dimedone (261), which was added into the mixture of PA (1) and hydrazine hydrate (56) in a 1
1 molar ratio with 85–94% yield in 3–15 min (Scheme 104, entry 9).245 Examining the reaction with octanal as an aliphatic aldehyde, it did not form the corresponding product. The catalyst was recovered from the aqueous medium, dried under vacuum, and reused in five successive runs without a substantial loss of activity. (J) The compounds (272) were also prepared by Ebrahimipour et al. in 2015 via the one-pot and one-step, and four-component reaction in the presence of three octahedral complexes containing 2-pyrazinecarboxylate (pzca), including [Ni(pzca)2(H2O)2] (25 mol%), [Co(pzca)2(H2O)2] (20 mol%), and [Cu(pzca)2(H2O)2] (20 mol%) in acetic acid solvent at 50 °C within 10–40 min with 80–94% yields (Scheme 104, entry 10).246 (K) Bhosale et al. in 2017 developed the synthesis of (272) in the presence cesium chloride (15 mol%) in refluxing ethanol at 60 °C via the equimolar amounts of the substrates in a one-step and one-pot manner, in 3–4 h duration with a yield of 70–82% (Scheme 104, entry 11).247 (L) Gill's research group in 2017 demonstrated a synthetic route for 2H-indazolo[2,1-b]pthalazinetrione derivatives (272) through a one-pot, one-step four-component protocol in the presence of β-cyclodextrin as a supramolecular, biodegradable, and reusable catalyst in 80 °C aqueous media within 94–79%
yield during 35–25 min (Scheme 104, entry 12).248 (M) Ebrahimipour and coworkers in 2017 reported the synthesis of (272) with a yield of 84–94% in 15–25 min in the presence of imidazole 2-acetamido-N′-(3-methoxy-2-oxidobenzylidene)benzohydrazonate–nickel(II) [Ni(L)(imi)], which is a tridentate Schiff base ligand (Scheme 104, entry 13).249 The Ni(II) complex showed promising antimicrobial activities against some Gram-negative and Gram-positive bacteria such as E. coli, S. aureus, P. aeruginosa, and B. cereus. The catalyst was recovered by centrifugation and recrystallization from a mixture of ethanol and water and reused at least three times with satisfactory results. (N) Bamoniri et al. in 2020 described a method for the synthesis of 2H-Indazolo[2,1-b]phthalazinetrione derivatives (272) using nano γ-Al2O3/BFn/Fe3O4 (8 mg) via the one-step reaction of equimolar of four substrates under solvent-free conditions, which yielded the corresponding adducts with 90–97% yield in 10–17 min (Scheme 104, entry 14).250 The catalyst was recycled by washing with CH2Cl2, drying at 50 °C under vacuum for 1 h, and reused within 5 runs successfully. (O) Liu and Li et al. in 2020 prepared a novel modified core–shell magnetic nanocomposite by anchoring Ag NPs on magnetite core that was coated with chitosan-alginate dual bio-polysaccharide (Fe3O4/CS-Alg/Ag NPs). The novel nanocatalyst was utilized for the synthesis of (272) via one-step and one-pot reaction of the corresponding substrates at 80 °C within 0.2–1.5 h with 50–95% yield. The catalyst demonstrated human lung protective effects against α-Guttiferin. These events revealed that the catalyst suppressed Guttiferin-induced cell death in a dose-dependent manner in lung MRC-5, CCD-19Lu, WI-38, and BEAS-2B cell lines. The catalyst was recovered easily using an external magnet and recycled for 10 successive times with minimal reduction in activity (Scheme 104, entry 15).251 (P) In 2022, Naeimi and Zahedifar reported an immobilized copper(II) complex on microcellulose (cell-DABCO-Cu) as a novel catalyst to promote the preparation of (272) at 80 °C under solvent-free conditions in a one-step and one-pot manner (Scheme 104, entry 16).252 In addition, the CuO nanoparticles with an average size of 40 nm were obtained by the direct calcination of Cell-DABCO-Cu. (Q) Mahmoodi et al. in 2020 obtained (272) via the one-pot, one-step, four-component, and solvent-free condensation of PA (1), aromatic aldehydes (19), hydrazine hydrate (56), and dimedone (261), which was accelerated with triethanolammonium acetate ([TEAH][OAc]) at 80 °C within 10–35 min with 89–95% yield (Scheme 104, entry 17).253 The authors examined the efficacy of the protocol through utilizing succinic anhydride instead of PA. They also prepared phthalazine-diones (271) by the reaction of PA (1), aromatic aldehydes (19), hydrazine hydrate (56), and alkyl cyanoacetates (112) (methyl and ethyl) successfully in the same reaction conditions within 22–37 min with 80–95%. (R) Tigote's group in 2017 used ZnFe2O4 nanoparticles (1.5 mol%) as a catalyst for this transformation via the one-pot and two-step reaction of aromatic aldehyde (19) and the dimedone (261), which was added into the mixture of PA (1) and hydrazine hydrate (56) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 molar ratio at room temperature within 30–90 min with 76–89% yield.254 (S) Mahmoodi's group in 2020 used tetrabutyl phosphonium sulfate ([TBP]2SO4, 5 mol%) as a novel room-temperature ionic liquid (RTIL) to prepare (272) via the domino reaction of the mentioned four substrates at room temperature within 10–20 min with 89–92% yield. The authors examined the scope of the protocol utilizing succinic anhydride instead of PA. They also achieved phthalazine-diones through the reaction of phthalazine-diones (271) and the reaction of PA (1), aromatic aldehydes (19), hydrazine hydrate (56), and alkyl cyanoacetates (112) (methyl and ethyl) successfully in the same reaction conditions within 15–45 min with 65–92% yield.255
1.2 molar ratio at room temperature within 30–90 min with 76–89% yield.254 (S) Mahmoodi's group in 2020 used tetrabutyl phosphonium sulfate ([TBP]2SO4, 5 mol%) as a novel room-temperature ionic liquid (RTIL) to prepare (272) via the domino reaction of the mentioned four substrates at room temperature within 10–20 min with 89–92% yield. The authors examined the scope of the protocol utilizing succinic anhydride instead of PA. They also achieved phthalazine-diones through the reaction of phthalazine-diones (271) and the reaction of PA (1), aromatic aldehydes (19), hydrazine hydrate (56), and alkyl cyanoacetates (112) (methyl and ethyl) successfully in the same reaction conditions within 15–45 min with 65–92% yield.255
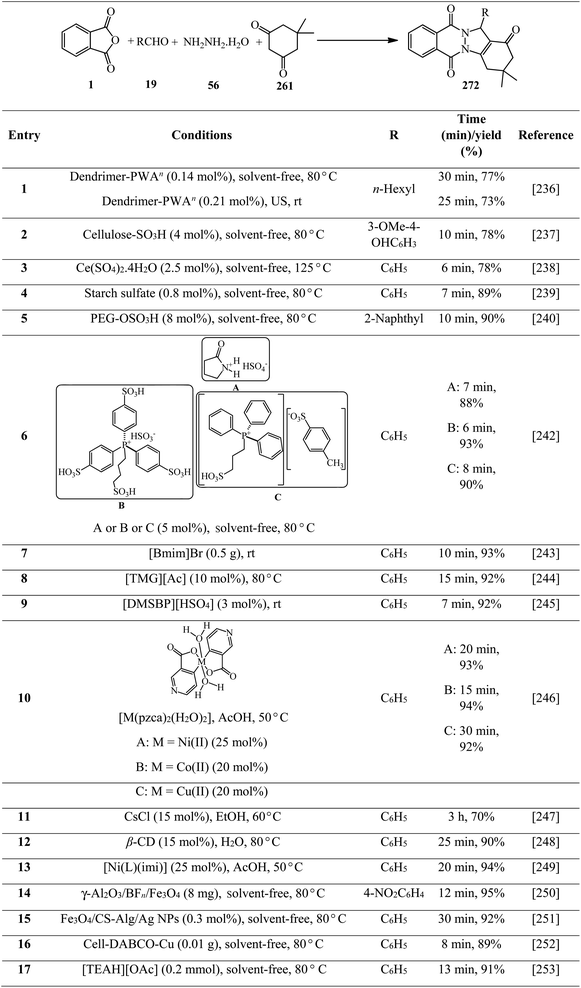 | ||
| Scheme 104 Investigation of the scope of the protocols to obtain 2H-indazolo[2,1-b]phthalazine-triones. | ||
Some research groups utilized 1,3-cyclohexanedione (273) (in addition with dimedone) to prepare (272) and 3,4-dihydro-1H-indazolo[1,2-b]phthalazine-6,11(2H,13H)-diones (274), which consist of (A). Sadek et al. in 2019 developed a highly efficient, catalyst-free, one-pot, multicomponent synthesis of various indazolophthalazines (272, 274) in glycerol as a cheap, biodegradable, and commercially available promoting solvent and catalyst under controlled microwave heating (Scheme 105).256 The reaction mixture in glycerol was heated under reflux in a Milestone microwave lab station at 60 °C. They also synthesized (277) via the same reaction conditions.
(B) Pal's group in 2016 prepared the compounds (272, 274) via the one-pot four-component reaction of PA (1), variety of aldehydes (19) (including aliphatic and (hetero)aromatic aldehydes), hydrazine hydrate (56), and active methylene compounds (dimedone (261)/1,3-cyxlohexandione (273)) in the presence of magnetic Fe3O4–glutathione core–shell (nano-FGT, 10 mg) at 80 °C under solvent-free conditions within 20–30 min with 87–97%. The magnetic catalyst could be separated easily by an external magnet and reused in five more consecutive runs without much decrease in the catalytic activities. The authors also examined the efficacy of the catalyst in the synthesis of two kinds of phthalazine-diones (271) via the reaction of PA (1), aldehydes (19) (which are 4-chloro-3-nitrobenzaldehyde and pentanal), aminonitriles/ethyl cyanoacetate (112), and hydrazine hydrate (56) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 molar ratio in the same reaction conditions within 20–25 min with 92–97%.257
1.2 molar ratio in the same reaction conditions within 20–25 min with 92–97%.257
Zaheer et al. in 2016 exploited PrxCoFe2−xO4 (x = 0.1) nanoparticles to catalyze the efficient one-pot four-component reaction of PA (1), hydrazine hydrate (56), dimedone (261), and various quinoline aldehydes (278, 279) to achieve phthalazine quinoline derivatives (280, 281) (Scheme 106).258 The synthesized adducts were evaluated for antibiofilm activity against P. aeruginosa and C. albicans.
Zahedifar's research group in 2018 constructed a series of novel pyrazolo[1,2-b]phthalazine-2-carboxylates (282) via the one-pot four-component reaction of PA (1), aromatic aldehydes (19), hydrazine hydrate (56), and alkyl acetoacetates (104) in the presence of acidic ionic liquids such as 3-methyl-1-sulfo-1H-imidazol-3-ium chloride ([Msim]Cl), 1,3-disulfo-1H-imidazol-3-ium chloride ([Dsim]Cl), and triethyl(sulfo)ammonium chloride ([Et3NSO3H]Cl) as the catalyst (Scheme 107).259
Jahanshahi and Mamaghani in 2019 described the synthesis of a wide range of novel 1H-pyrazolo[1,2-b]phthalazine-5,10-dioness (284) in the presence of acetic acid-functionalized imidazolium salt (1-carboxymethyl-2,3-dimethylimidazolium iodide, [cmdmim]I), as a newly synthesized Brønsted acid catalyst. The reaction occurred via tandem Knoevenagel cyclocondensation of PA (1), aromatic aldehydes (19), hydrazine hydrate (56), and 3-(1-methyl-1Hpyrrol-2-yl)-3-oxopropanenitrile (or 3-(1H-indol-3-yl)-3-oxopropanenitrile) (283) in the presence of [cmdmim]I in ethanol under reflux conditions (Scheme 108).260 The reusability of the catalyst was examined in 5 consecutive runs without any appreciable decrease in the activity.
In 2022, Vedula and coworkers provided alkyl/aralkyl/phenacyl thiotriazolyl isoindoline-1,3-diones (287) via the reaction of PA (1), hydrazine hydrate (56), dipotassium cyanodithioimidocarbonate salt (285), and alkyl/aralkyl/phenacyl bromides (286) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio using acetic acid and sodium acetate (0.1 mmol) via a one-pot four-component reaction at 80 °C (Scheme 109). The in vitro anticancer activity of the products revealed that some of them demonstrated cytotoxic assay against HeLa cancer cell lines. The compounds also subjected to their docking analysis and DFT calculations.261
1 molar ratio using acetic acid and sodium acetate (0.1 mmol) via a one-pot four-component reaction at 80 °C (Scheme 109). The in vitro anticancer activity of the products revealed that some of them demonstrated cytotoxic assay against HeLa cancer cell lines. The compounds also subjected to their docking analysis and DFT calculations.261
5. Applications of PA in five-component and higher-component reactions
Toru's group in 2003 reported the preparation of metal-free phthalocyanines from PA (1) with hexamethyldisilazane (HMDS) (288) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio (Scheme 110).262 The transformation is actually a pseudo eight-component reaction.
5 molar ratio (Scheme 110).262 The transformation is actually a pseudo eight-component reaction.
Park et al. in 2004 developed a green procedure for the synthesis of metal phthalocyanines (291) (MPc: M = Cu, Mn, Al, Co, and Zn) through the condensation reaction of PA (1) (0.283 mol) and urea (103) (0.816 mol) with various metal chlorides (290) (7 g) in the presence of ammonium molybdate (5 × 10−4 mol) as a catalyst under microwave-assisted solvent-free conditions (Scheme 111).263 The procedure actually is a pseudo nine-component reaction.
6. Applications of PA in esterification
Ahmad et al. in 2010 reported immobilized Candida Antarctica lipase, Novozym 435, as a biocatalyst to catalyze the esterification reaction of PA (1) and betulinic acid (292) to obtain 3-O-phthalyl betulinic acid (293) in n-hexane/chloroform. The effect of different parameters in the reaction process was predicted by the “response surface methodology” (RSM) technique. The comparison of the results of this model and experimental values revealed a good correspondence. The effect of enzyme amount was found to be less, while the reaction temperature, reaction time, and molar ratio strongly affected the ester yield. According to the “central composite rotatable design” (CCRD) optimization, in the presence of a betulinic acid to phthalic anhydride in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.11 molar ratio, the maximal yield of the ester (64.7%) was obtained using 145.6 mg enzyme at 53.9 °C in 20.3 h. This predicted that the optimum conditions are in close correlation with the experimental results (Scheme 112).264
1.11 molar ratio, the maximal yield of the ester (64.7%) was obtained using 145.6 mg enzyme at 53.9 °C in 20.3 h. This predicted that the optimum conditions are in close correlation with the experimental results (Scheme 112).264
Ahmad's research group in 2010 also considered the reaction parameters of lipase-catalyzed esterification of betulinic acid (292) using PA (1) in organic solvent media.265 The lipase from Candida antarctica immobilized on an acrylic resin (Novozym 435) was employed for esterification. The influence of different reaction parameters, such as effect of single and mixed solvents, substrate molar ratio, reaction time, temperature, amount of enzyme, effect of inorganic bases, and effect of substrate support were investigated and optimized. The optimum conditions to obtain 3-O-phthalyl-betulinic acid (293) (61.8%) are: substrate molar ratio (betulinic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phthalic anhydride, 1
phthalic anhydride, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), within 24 h at 55 °C, enzyme (176 mg), and Celite (170 mg) in 1
1), within 24 h at 55 °C, enzyme (176 mg), and Celite (170 mg) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of chloroform and n-hexane as a solvent in the presence of K2CO3 (as an inorganic base).
1 mixture of chloroform and n-hexane as a solvent in the presence of K2CO3 (as an inorganic base).
Dubey's research group in 1997 converted PA (1) to its monoesters through the reaction with simple alcohols (294) under a variety of conditions (Scheme 113).266 The monoesters (295) could be prepared via two procedures: (A) reaction of PA and alcohols in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 molar ratio, in refluxing benzene, (B) reaction of PA with alkyl alcohols in 1
2.2 molar ratio, in refluxing benzene, (B) reaction of PA with alkyl alcohols in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8 molar ratio in the presence of sodium alkoxide (0.02 g) at room temperature with 5 min. The diesters (296) were also obtained via various procedures: (I) reaction of PA and alcohols in 1
3.8 molar ratio in the presence of sodium alkoxide (0.02 g) at room temperature with 5 min. The diesters (296) were also obtained via various procedures: (I) reaction of PA and alcohols in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2 molar ratio in benzene solvent in the presence of p-TSA (5 mg) under reflux conditions in a dean-stark apparatus within 6 h; (II) the reaction of PA with thionyl chloride (297) for 1 h under reflux conditions, which was followed by separating thionyl chloride, and consequent addition of pyridine and an appropriate alcohol and refluxing for 3 h.
3.2 molar ratio in benzene solvent in the presence of p-TSA (5 mg) under reflux conditions in a dean-stark apparatus within 6 h; (II) the reaction of PA with thionyl chloride (297) for 1 h under reflux conditions, which was followed by separating thionyl chloride, and consequent addition of pyridine and an appropriate alcohol and refluxing for 3 h.
Fareghi-Alamdari and coworkers in 2017 manufactured two functionalized diacidic ionic liquids (FDAILs) including hydroxyl functionalized diacidic IL [3,3′-(2,2-bis (hydroxymethyl)propane-1,3-diyl)bis(1-methyl-1H-imidazole-3-ium)hydrogen sulfate, HFDAIL] and sulfonated diacidic IL [3,3′-(2,2-bis((sulfoxy)methyl)propane-1,3-diyl)bis(1-methyl-1H-imidazole-3-ium)bromide, SFDAIL] by a simple method in high yields, which served for the esterification reaction of anhydrides (PA, succinic anhydride, and maleic anhydride) with some alcohols in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio to give corresponding dialkyl esters (296) as plasticizers under solvent-free conditions.267 HFDAIL showed higher catalytic performance in comparison with other reported catalysts, suggesting its high acidity and hydrophilic property. Phthalates, which are a class of neutral plasticizers. Are widely used in nonmilitary industries. Recycling experiments suggested that ILs could be reused seven times without a remarkable loss in their catalytic activity. According to the proposed mechanism, HFDAIL has two hydroxyl groups, which can absorb the produced water by hydrogen bonding. Thus, the esterification reaction equilibrium will shift to the right and the product yield will increase.
5 molar ratio to give corresponding dialkyl esters (296) as plasticizers under solvent-free conditions.267 HFDAIL showed higher catalytic performance in comparison with other reported catalysts, suggesting its high acidity and hydrophilic property. Phthalates, which are a class of neutral plasticizers. Are widely used in nonmilitary industries. Recycling experiments suggested that ILs could be reused seven times without a remarkable loss in their catalytic activity. According to the proposed mechanism, HFDAIL has two hydroxyl groups, which can absorb the produced water by hydrogen bonding. Thus, the esterification reaction equilibrium will shift to the right and the product yield will increase.
Fareghi-Alamdari's group also introduced two highly acidic, imidazolium-based, functionalized dicationic ionic liquids (FDCILs), which used (in 0.2 eq.) as efficient and green catalysts in the synthesis of phthalate plasticizers through the esterification of PA with alcohols (such as ethanol, n-propanol and n-butanol) at 110 °C. Among these two FDCILs, (FDCIL 1: [(dimethyl-4-sulfobutyl-ammonium)-1,2-ethane-1H-imidazolium sulfonic acid]hydrogen sulfate and FDCIL 2: 3,3′-(1,2-ethanediyl)bis[1-(4-sulfobutyl)-1H-imidazolium sulfonic acid]hydrogen sulfate), the first one performed better. The catalytic activity of FDCIL is related to the density of acidic groups on it (higher acidity) and the length of the carbon chain (low lipophilic character) in the cationic part. The influences of the reaction temperature, catalyst dosage, and molar ratio of PA to alcohol on the esterification reaction were investigated. The reusability of the catalyst in these reactions was also studied. The yields were estimated by GC analysis.268 Phthalate plasticizers are the main plasticizers used as softening agents in various industrial applications. These compounds are mainly used as plasticizers for cellulosic resins and some vinyl ester resins, PVC, and nitrocellulose lacquers.269
Fareghi-Alamdari in 2018 also prepared supported diacidic ionic liquid on magnetic silica nanoparticles (SDAIL@magnetic nano SiO2) and investigated its catalytic activity (10 mol%) for the selective diesterification of alcohols (2-methoxy ethanol, allyl alcohol, 2-ethoxyhexanol, butanol, propanol, ethanol, and methanol) with PA in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio to afford the corresponding dialkyl plasticizers (296) under solvent-free conditions (65–180 °C) within 1–10 h. Under the optimized conditions, the conversion of PA was 100%, and the diester plasticizers were obtained with excellent yields (80–100%). The SDAIL@magnetic nano-SiO2 catalyst showed good reusability and could be easily separated from the reaction mixture using an external magnet, washed with dichloromethane, and reused for the next runs for up to 8 runs without significant activity loss.270
1 molar ratio to afford the corresponding dialkyl plasticizers (296) under solvent-free conditions (65–180 °C) within 1–10 h. Under the optimized conditions, the conversion of PA was 100%, and the diester plasticizers were obtained with excellent yields (80–100%). The SDAIL@magnetic nano-SiO2 catalyst showed good reusability and could be easily separated from the reaction mixture using an external magnet, washed with dichloromethane, and reused for the next runs for up to 8 runs without significant activity loss.270
Shahedi and Mansoori in 2018 studied the esterification reaction of PA (1) with various aliphatic and cycloaliphatic alcohols (such as propanol and butanol) in the presence of a Fe3O4@SiO2–SO3H (5 mol%) nanocatalyst. The two mentioned alcohols gave the corresponding diesters (296) in duration of 8–12 h with a yield of 71–97%.271
Kulawska's group in 2011 considered the kinetics of the syntheses of higher aliphatic alcohols (C7, C9, C11) phthalates in an isothermal, semi-batch reactor. In the first stage of the process, the formation of monoester (295) was very fast and irreversible. In the second stage, the esterification of monoester toward diester (296) in the presence sulfuric acid catalyst progressed slowly. These reactions appeared to be first order with respect to the monoester and did not depend on the concentration of the alcohol.272
Mohammadpour Amini in 2003 evaluated the esterification of PA with 2-ethylhexanol and 1-butanol and ester decomposition of dioctyl phthalate (DOP) in the presence of different kinds of heteropolyacids such as (I) Keggin H3PW12O40, H4SiW12O40, and H4SiMo12O40; (II) Wells–Dawson H6P2W18O62, H6P2W17MoO62; (III) Preyssler H14[NaP5W29MoO110], H14[NaP5W30O110]. The heteropolyacids with Preyssler and Wells–Dawson structures (due to the higher number of acidic protons) and their molybdenum substituted derivatives (attributed to the reduction of Mo(VI) to Mo(V) and enhanced acidity) demonstrated higher activity in esterification and ester decomposition reactions than Keggin type heteropolyacids. A complete conversion of PA to dioctyl phthalate and dibutyl phthalate are achieved in 2 h in the presence of molybdenum-substituted Preyssler heteropolyacid. In the decomposition of dioctyl phthalate in the presence of Preyssler heteropolyacid, 2-ethylhexene was formed in quantitative yield.273
The kinetics of esterification of PA with 2-ethylhexanol was studied by Bhutada and Pangarkar in 1986 using three kinds of catalysts such as tetrabutyl titanate (TBT), tetrabutyl zirconate (TBZ), and p-TSA. In each case, the kinetic parameters such as rate constant (order with respect to various reactants and catalyst), activation energy, and collision frequency were determined. The titanates and zirconates were found to be better than p-TSA in rate and rate/product quality, respectively. The reaction shows general first order in the monoester and alcohol. Bimolecular acyl oxygen fission satisfactorily explains the kinetics.274
Gharibe in 2020 utilized the novel ZnAl2O4/SiO2 (5 mol%) for the esterification of PA with 2-ethylhexanol to obtain dioctyl phthalate (DOP) in 99.2% yield in 45 min.275
Brennecke's group in 1994 surveyed the pressure effect on the bimolecular rate constants for the esterification of PA with methanol in supercritical carbon dioxide (SC CO2) at 40 °C and 50 °C. They observed a 25-fold decrease in the bimolecular rate constant based on bulk concentrations when increasing the pressure from 97.5 to 166.5 bar.276
Khodadadi Moghaddam and Gholami in 2006 inspected the esterification of PA with 2-octanol in the presence of sulfated titania (SO42−/TiO2 prepared by immersing titania powder in 1 N solution of sulfuric acid). The conversion reached an equilibrium composition in 20 min for dioctyl phthalate without water removal from the reactor. The linear dependence of conversion to the catalyst amount exhibited that there are no mass transfer limitations in the reaction conditions.277
Yadav's group in 1992 represented the efficacy of several solid superacidic catalysts [such as phosphate, borated, and sulfated zirconia, sulphated iron oxide, phosphotungstic acid (P2O5·24WO3·nH2O), and dodecatungstophosphoric acid (H3PO4·12WO3·nH2O)] in the preparation of the industrially important plasticizer dioctyl phthalate (DOP) from 2-ethylhexanol and PA.278
Bajracharya et al. in 2021 prepared phthalate monoesters (295) and diesters (296) via the reaction of PA (1) and alcohols (allyl, isoamyl, n-butyl, and benzyl alcohols) (294) through a one-pot two-step procedure in the presence of FeCl3 as a catalyst. Utilizing the reactants in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio at 50 °C achieved both the phthalate esters (295, 296). Addition of excess amounts of alcohol at 50 °C or 100 °C yielded the selective preparation of diesters (296). The authors claimed that the synthesis of the adducts (295) was performed through a facile addition displacement pathway, which was continued by Lewis acid catalysis providing phthalate diesters (296). The ring-closing metathesis of diallyl phthalate using the Grubb's 2nd generation catalyst led to macrolide (298) (Scheme 114).279
2 molar ratio at 50 °C achieved both the phthalate esters (295, 296). Addition of excess amounts of alcohol at 50 °C or 100 °C yielded the selective preparation of diesters (296). The authors claimed that the synthesis of the adducts (295) was performed through a facile addition displacement pathway, which was continued by Lewis acid catalysis providing phthalate diesters (296). The ring-closing metathesis of diallyl phthalate using the Grubb's 2nd generation catalyst led to macrolide (298) (Scheme 114).279
Polyifluoroalkyl mono- and diesters of PA were obtained by Rakhimova and Kudashev in 2011 through the reaction of PA (1) with polyfluorinated alcohols (299). The monesters (300) were achieved via the ultrasonic-assisted (40 kHz) reaction of equimolar amounts of PA and polyfluorinated alcohol in cyclohexanone for 2 h at 70 °C, which was followed by heating at 130 °C for 2 h to complete the transition of phthalic anhydride. The diesters (301) were also prepared as the same procedure in the presence of PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alcohols in a 1
alcohols in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio (Scheme 115).280
2 molar ratio (Scheme 115).280
Karimi Alavijeh and Amini in 2019 demonstrated that mesoporous MIL-101(Cr)-SO3H and [Cr3O(BDC-SO3H)2.4(BDC-SO3NH3Bu)0.6]n (20% BuNH2) acted as potent and robust catalysts in the esterification of PA (1) with various alcohols in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio under solvent-free conditions.281 After the completion of the reaction, the catalyst was separated by centrifugation, washing with dichloromethane, drying at 50 °C, and reusing in the next reaction for 6 runs without activity loss.
5 molar ratio under solvent-free conditions.281 After the completion of the reaction, the catalyst was separated by centrifugation, washing with dichloromethane, drying at 50 °C, and reusing in the next reaction for 6 runs without activity loss.
Fieser in 1931 discussed about the condensation of β-naphthol with phthalic anhydride in detail.282
7. Some examples of PA utilization in multistep applicable products
PA could function as a key substrate in multicomponent sequential reactions, which led to applicable products in various fields such as drugs, natural products, and industry. Some examples have been discussed below.Cavé et al. in 2008 prepared novel phosphonodipeptide conjugates of ursolic acid (3β-hydroxy-urs-12-en-28-oic acid) and their homologs via a series of reactions. First, the preparation of a series of α-phosphonodipeptides and homologs has been reported (Scheme 117). According to Scheme 116, the 1,3-dihydro-1,3-dioxo-2H-isoindole-2-acetic (or propanoic) acids (5) were obtained by mixing the grounded forms of PA (1) and amnio acids (4) (glycine and β-alanine), which was converted to acyl chloride by SOCl2 (297) to phthalimidoacetyl chloride (302). The subsequent reaction of (302) with α-aminophosphonates (303) yielded N-blocked phosphonodipeptides and homologs (304). The phosphonodipeptides and homologs (305) were obtained from hydrazinolysis with hydrazine hydrate (56). In the second step (Scheme 117), the introduction of phosphonodipeptides and their homologs (305) to naturally bioactive ursolic acid (UA) (306) at C-28 moiety afforded new classes of phosphonodipeptide conjugates of ursolic acid and their homologs (309), which was performed by (i) its acylation to 3β-acetoy-urs-12-en-28-oic acid (307); (ii) chlorination to 3β-acetoy-urs-12-en-28-oyl chloride (308), and (iii) final reaction with (305) to afford the (309) adducts.283 The phosphonodipeptide conjugates of UA and their homologs (309) are potent for anti-HT29 (human colon cancer cell line) and anti-HIV properties.
Aliabadi and coworkers in 2016 synthesized a new series of phthalimides named 4-(1,3-dioxoisoindolin-2-yl)-N-phenylbenzamides (311) via the reaction of PA (1) with 4-aminobenzoic acid (310). The first step consisted of Gabriel-like reaction in the presence of triethylamine (as a proton acceptor) under reflux conditions to obtain 4-(1,3-dioxoisoindolin-2-yl)benzoic acid (A), which was mixed with equimolar quantities of N-ethyl-N-dimethyl aminopropyl carbodiimide (EDC, as a carbodiimide coupling agent) and hydroxyl benzotriazole (HOBt, additive agent) in acetonitrile solvent and was stirred for 30 min at room temperature. Then, an equimolar quantity of appropriate anilines (21) was added to the reaction medium. On continuous stirring for 24 h, an amidation reaction through a carbodiimide coupling reaction yielded the adducts (311). The potential of the products as anti-Alzheimer agents was examined. The anti-acetylcholinesterase activity of the synthesized derivatives was assessed by Ellman’s test. The product, with ortho nitro moiety, in this series exhibited the highest inhibitory potency (IC50 = 1.1 ± 0.25 μM) compared to donepezil (IC50 = 0.41 ± 0.12 μM) as the reference drug (Scheme 118).284
Iwanaga in 2019 prepared dinaphtho[2,3-b:2′,3′-i]dihydrophenazine derivatives via a multistep reaction starting from PA. First, Friedel–Crafts reaction of PA (1) with 1,4-dibromobenzene (312), followed by dehydration, afforded 2,3-dibromoanthraquinone (313), which reduced stepwise to give 2,3-dibromoanthracene (314) with 17% overall yield. Pseudo three-component conventional Buchwald-Hartwig coupling of (314) with 4-octyloxyaniline (21)285 gave compound (315) as a yellow solid. The reaction of (315) with (313) and (314) gave 7,16-bis(4-(octyloxy)phenyl)-16,18a-dihydrodinaphtho[2,3-b:2′,3′-i]phenazine-5,18(4aH,7H)-dione (316) and 7,16-bis(4-(octyloxy)phenyl)-7,16-dihydrodinaphtho[2,3-b:2′,3′-i]phenazine (317), respectively. The X-ray crystallographic analysis of (317) indicated a planar structure. The photophysical properties were influenced by nitrogen atoms, resulting in extended π-conjugation for (317) and intramolecular donor–acceptor interactions for (316). The oxidation potentials of (316, 317) were similar due to the independence of the 2,3-diaminoanthracene unit (Scheme 119).286
4-(4-Aminophenyl)-3-(4-(dimethylamino)phenyl)-3,4-dihydrobenzo[e][1,3]oxazepine-1,5-dione (319) was constructed through the reaction of PA (1) and Schiff base (318). The Mannich reaction occurred between (319), paraformaldehyde (9), and phenytoin (320) in equimolar ratio to obtain 3-(4-(dimethylamino)phenyl)-4-(4-(((2,4-dioxo-5,5-diphenylimidazolidin-1-yl)methyl)amino)phenyl)-3,4-dihydrobenzo[e][1,3]oxazepine-1,5-dione (321). The authors investigated the preparation of some other phenytoin derivatives containing diazepines and quinazoline rings through the reaction of (318) with malic anhydride and phthalic imide. The anticancer and cytotoxicity properties of the products was tested through the effects of treating the L20B cell line (Scheme 120).287
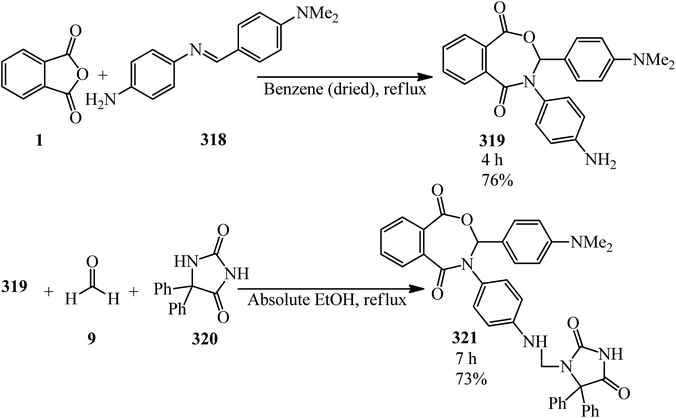 | ||
| Scheme 120 3-(4-(Dimethylamino)phenyl)-4-(4-(((2,4-dioxo-5,5-diphenylimidazolidin-1-yl)methyl)amino)phenyl)-3,4-dihydrobenzo[e][1,3]xazepane-1,5-dione preparation. | ||
Bala's group in 2014 introduced phthalic anhydride-based benzylidene-hydrazides (322) as novel, potential antiinflammatory agents. The synthetic route started from PA (1) and glycine (4) to give phthaloylglycine (5), which was subjected to chlorination by thionyl chloride to form (A). Subsequent esterification, followed by reaction with hydrazine hydrate (56), and the final reaction of intermediate (C) with benzaldehydes (19) yielded 2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-N′-(substituted benzylidene)acetohydrazides (322). The research consists of computational studies. The products were screened for in vivo antiinflammatory and analgesic activities by carrageenan-induced Rat Paw Oedema and tail immersion methods, respectively, using diclofenac sodium as a standard drug (Scheme 121).288
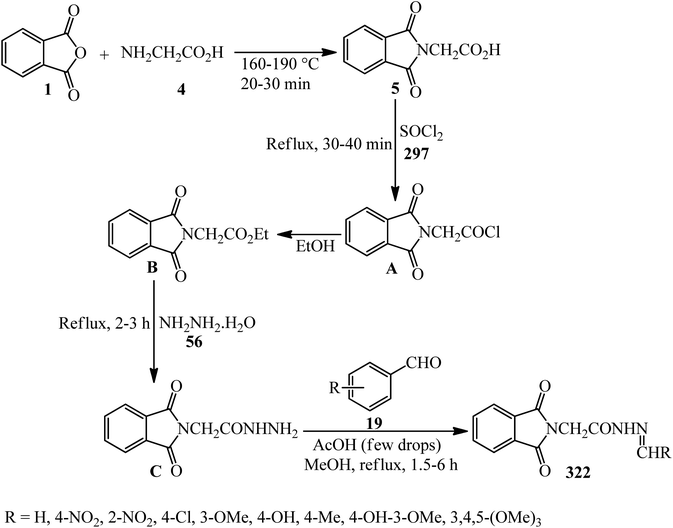 | ||
| Scheme 121 Synthesis of 2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-N′-(substituted benzylidene)acetohydrazides. | ||
8. Conclusion
In this literature survey, the authors focused on applications of phthalic anhydride (PA), a cyclic fused heterocyclic anhydride, as a versatile substrate in organic reactions. Actually, PA is an efficient substrate and/or intermediate in several organic transformations due to its bifunctional cyclic anhydride moiety, which could be opened via the attack of various nucleophilic groups. In addition to the introduction part, which identified the compound and its overall utilities in various respects, the review has been subdivided into some parts, centralizing on the organic two- and multicomponent reactions containing PA as the substrate. The importance and applicability of PA in esterification are also discussed with examples. There is also a final part that discusses the preparation of heterocycles starting from PA via multistep protocols, which possess various applications in pharmacology, treatment, natural products, industry, and some other fields of operative aspects of science and technology. In all the abovementioned parts, PA plays a crucial role as a substrate that cannot be changed with other heterocycles, which could be due to the intrinsic activity of the anhydride functional group. The authors hope that this review would be helpful and effectual for future research by chemists on PA and its analogs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Alzahra University for their support.References
- S. C. Gad, Phthalic Anhydride, in Encyclopedia of Toxicology, Elsevier, 2014, pp. 934–936 Search PubMed.
- D. A. Basketter and I. Kimber, Phthalic anhydride: illustrating a conundrum in chemical allergy, J. Immunotoxicol., 2016, 13, 767–769, DOI:10.1080/1547691X.2016.1177149.
- A. Laurent, Sur l'acide naphtalique et ses combinaisons, Ann. Chim. Phys., 1836, 61, 113–125 Search PubMed.
- P. M. Lorz, F. K. Towae, W. Enke, R. Jäckh, N. Bhargava and W. Hillesheim, Phthalic acid and derivatives, Ullmann's Encyclopedia of Industrial Chemistry, Weinheim, Wiley-VCH. 2007, DOI:10.1002/14356007.a20181.pub2.
- B. Rindone, F. Saliu and R. Suarez-Bertoa, The synthesis of phthalic anhydride via ozonation of naphthalene, Ozone: Sci. Eng., 2010, 32, 161–165, DOI:10.1080/01919511003788001.
- C. R. Dias, M. F. Portela and G. C. Bond, Oxidation of o-xylene to phthalic anhydride over V2O5/TiO2 catalysts: part 4. mathematical modelling study and analysis of the reaction network, J. Catal., 1996, 164, 276–287, DOI:10.1006/jcat.1996.0384.
- S. Luciani, N. Ballarini, F. Cavani, C. Cortelli, F. Cruzzolin, A. Frattini, R. Leanza and B. Panzacchi, Anatase-supported vanadium oxide catalysts for o-xylene oxidation: from consolidated certainties to unexpected effects, Catal. Today, 2009, 142, 132–137, DOI:10.1016/j.cattod.2008.08.044.
- J. N. Papageorgiou and G. F. Froment, Phthalic anhydride synthesis. reactor optimization aspects, Chem. Eng. Sci., 1996, 51, 2091–2098, DOI:10.1016/0009-2509(96)00066-8.
- K. Masoomi, M. Ghiaci and A. M. Botelho do Rego, Selective vapor-phase oxidation of o-xylene to phthalic anhydride over Co-Mn/H3PW12O40@TiO2 using molecular oxygen as a green oxidant, Appl. Organomet. Chem., 2018, 32, e4461, DOI:10.1002/aoc.4461.
- T. Boger and M. Menegola, Monolithic catalysts with high thermal conductivity for improved operation and economics in the production of phthalic anhydride, Ind. Eng. Chem. Res., 2005, 44, 30–40, DOI:10.1021/ie040088f.
- G. Groppi, E. Tronconi, C. Cortelli and R. Leanza, Conductive monolithic catalysts: development and industrial pilot tests for the oxidation of o-xylene to phthalic anhydride, Ind. Eng. Chem. Res., 2012, 51, 7590–7596, DOI:10.1021/ie2021653.
- M. Selvaraj and T. G. Lee, A novel route to produce phthalic anhydride by oxidation of o-xylene with air over mesoporous V-Mo-MCM-41 molecular sieves, Microporous Mesoporous Mater., 2005, 85, 39–51, DOI:10.1016/j.micromeso.2005.05.046.
- F. List and H. Alfs, Production of phthalic anhydride from phthalic acid, US Pat.US3720692A, United States, 1968 Search PubMed.
- V. Nikolov, D. Klissurski and A. Anastasov, Phthalic anhydride from o-xylene catalysis: science and engineering, Catal. Rev.: Sci. Eng., 1991, 33, 319–374, DOI:10.1080/01614949108020303.
- C. R. Dias and M. F. Portela, Synthesis of phthalic anhydride: catalysts, kinetics, and reaction modeling, Catal. Rev.: Sci. Eng., 1997, 39, 169–207, DOI:10.1080/01614949709353776.
- E. Mahmoud, D. A. Watson and F. Lobo, Renewable production of phthalic anhydride from biomass-derived furan and maleic anhydride, Green Chem., 2014, 16, 167–175, 10.1039/C3GC41655K.
- X. Shao, L. Su, J. Zhang, Z. Tian, N. Zhang, Y. Wang, H. Wang, X. Cui, X. Hou and T. Deng, Green Production of phthalic anhydride from biobased furan and maleic anhydride by an acid resin catalyst, ACS Sustainable Chem. Eng., 2021, 9, 14385–14394, DOI:10.1021/acssuschemeng.1c03974.
- (a) W. Jia, W. Li, X. Zhao, Y. Feng, M. Zuo, Y. Sun, X. Tang, X. Zeng and L. Lin, Insights into the catalytic mechanism of 5-hydroxymethfurfural to phthalic anhydride with MoO3/Cu(NO3)2 in one-pot, Catal. Sci. Technol., 2021, 11, 5656–5662, 10.1039/D1CY00940K; (b) W. Jia, Y. Sun, M. Zuo, Y. Feng, X. Tang, X. Zeng and L. Lin, One-pot synthesis of renewable phthalic anhydride from 5-hydroxymethfurfural by using MoO3/Cu(NO3)2 as catalyst, Chemsuschem, 2020, 13, 640–646, DOI:10.1002/cssc.201902590.
- S. Giarola, Ch. Romain, Ch. K. Williams, J. P. Hallett and N. Shah, Techno-economic assessment of the production of phthalic anhydride from corn stover, Chem. Eng. Res. Des., 2016, 107, 181–194, DOI:10.1016/j.cherd.2015.10.034.
- Zh. Lin, M. Ierapetritou and V. Nikolakis, Phthalic Anhydride production from hemicellulose solutions: technoeconomic analysis and life cycle assessment, AIChE J., 2015, 61, 3708–3718 CrossRef CAS.
- C. R. Kinney and I. Pincus, Catalytic air oxidation of higher aromatics to phthalic anhydride, Ind. Eng. Chem., 1951, 43, 2880–2884, DOI:10.1021/ie50504a062.
- J. Shelmerdine, F. Popper and D. McNeil, The production of phthalic anhydride by the oxidation of tar oils, J. Appl. Chem., 1953, 3, 513–521, DOI:10.1002/jctb.5010031106.
- W. W. Prichard, Reactions of carbon monoxide at high temperature. I. a new synthesis of phthalic anhydrides, J. Org. Chem., 1956, 78, 6137–6139, DOI:10.1021/ja01604a050.
- V. A. Zazhigalov and E. V. Kiziun, Naphthoquinoneation of phthalic anhydride by Diels-Alder reaction during n-pentane oxidation on VPO catalysts and control of the process selectivity, Theor. Exp. Chem., 2017, 53, 194–198, DOI:10.1007/s11237-017-9515-9.
- Y. H. Kim and H. S. Yang, Synthesis of maleic and phthalic anhydrides from the mixture of cyclopentene and 1-pentene, Korean J. Chem. Eng., 2000, 17, 357–364, DOI:10.1007/BF02699053.
- H. D. Gibbs, Phthalic anhydride. I-introduction, Ind. Eng. Chem., 1919, 11, 1031–1032, DOI:10.1021/ie50119a010.
- H. D. Gibbs, History of the preparation and properties of pure phthalic anhydride, Ind. Eng. Chem., 1920, 12(10), 1017–1018, DOI:10.1021/ie50130a031.
- M. S. Ray, Case Study-Phthalic anhydride reactor design, Chemical engineering design project, a case study approach, CRC press; London, 2nd edn, 1989, ch. 10 Search PubMed.
- M. S. Wainwright and N. R. Foster, Catalysts, kinetics and reactor design in phthalic anhydride synthesis, Catal. Rev.: Sci. Eng., 1979, 19, 211–292, DOI:10.1080/03602457908068056.
- A. Soroush, H. Rezaie Haghighat and S. H. Sajadinia, Thermal and mechanical properties of polysulfide/epoxy copolymer system: The effect of anhydride content, Polym. Adv. Technol., 2014, 25, 184–190, DOI:10.1002/pat.3221.
- W. Bukowski, A. Bukowska, A. Sobota, M. Pytel and K. Bester, Copolymerization of phthalic anhydride with epoxides catalyzed by amine-bis(phenolate) chromium(III) complexes, Polymers, 2021, 13, 1785, DOI:10.3390/polym13111785.
- A. Amin, H. H. M. Darweesh, S. M. M. Morsi and M. M. H. Ayoub, Effect of phthalic anhydride-based hyperbranched polyesteramide on cement characteristics, J. Appl. Polym. Sci., 2011, 120, 3054–3064, DOI:10.1002/app.33484.
- E. Hosseini Nejad, A. Paoniasari, C. G. W. van Melis, C. E. Koning and R. Duchateau, Catalytic ring-opening copolymerization of limonene oxide and phthalic anhydride: toward partially renewable polyesters, Macromolecules, 2013, 46, 631–637, DOI:10.1021/ma301904y.
- S. A. Pooley, G. S. Canessa and B. L. Rivas, Spontaneous copolymerization of phthalic anhydride with aziridine or 2-methylaziridine, Eur. Polym. J., 1995, 31, 547–553, DOI:10.1016/0014-3057(94)00204-5.
- M. Ranjbar-Mohammadi, M. Arami, H. Bahrami, F. Mazaheri and N. M. Mahmoodi, Grafting of chitosan as a biopolymer onto wool fabric using anhydride bridge and its antibacterial property, Colloids Surf., B, 2010, 76, 397–403, DOI:10.1016/j.colsurfb.2009.11.014.
- E. M. A. Braz, S. C. C. C. Silva, C. A. R. S. Brito, L. M. Brito, H. M. Barreto, F. A. A. Carvalho, L. S. S. Junior, A. O. Lobo, J. A. Osajima, K. S. Sousa and E. C. Silva-Filho, Spectroscopic, thermal characterizations and bacteria inhibition of chemically modified chitosan with phthalic anhydride, Mater. Chem. Phys., 2020, 240, 122053, DOI:10.1016/j.matchemphys.2019.122053.
- S. Ye, W. Wang, J. Liang, S. Wang, M. Xiao and Y. Meng, Metal-free approach for a one-pot construction of biodegradable block copolymers from epoxides, phthalic anhydride, and CO2, ACS Sustainable Chem. Eng., 2020, 8, 17860–17867, DOI:10.1021/acssuschemeng.0c07283.
- S. Davarpanah, N. M. Mahmoodi, M. Arami, H. Bahrami and F. Mazaheri, Environmentally friendly surface modification of silk fiber: chitosan grafting and dyeing, Appl. Surf. Sci., 2009, 255, 4171–4176, DOI:10.1016/j.apsusc.2008.11.001.
- S. Mallakpour and S. Sepehri, Preparation of new optically active polyamides containing a L-Phenylalanine, phthalimide side-chain via the diisocyanate route by microwave energy: comparison with conventional heating, Des. Monomers Polym., 2008, 11, 535–546, DOI:10.1163/156855508X363825.
- B. Moona, T. R. Hoyea and Ch. W. Macosko, Synthesis and application of fluorescently labeled phthalic anhydride (PA) functionalized polymers by ATRP, Polymer, 2002, 43, 5501–5509, DOI:10.1016/S0032-3861(02)00406-8.
- S. Mallakpour and M. Taghavi, A facile, microwave-assisted synthesis of novel optically activepolyamides derived from 5-(3-methyl-2-phthalimidylpentanoylamino)isophthalic acid and different diisocyanates, Eur. Polym. J., 2008, 44, 87–97, DOI:10.1016/j.eurpolymj.2007.10.027.
- (a) J. Li, Y. Liu, W.-M. Ren and X.-B. Lu, Enantioselective terpolymerization of racemic and meso-epoxides with anhydrides for preparation of chiral polyesters, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 15429–15436, DOI:10.1073/pnas.2005519117; (b) H.-Y. Ji, D.-P. Song, B. Wang, L. Pan and Y.-S. Li, Organic Lewis pairs for selective copolymerization of epoxides with anhydrides to access sequencecontrolled block copolymers, Green Chem., 2019, 21, 6123–6132, 10.1039/c9gc02429h.
- X. Zhang and Y. Zhang, Poly(butylene succinate-co-butylene adipate)/cellulose nanocrystal composites modified with phthalic anhydride, Carbohydr. Polym., 2015, 134, 52–59, DOI:10.1016/j.carbpol.2015.07.078.
- X. Zhang and X. Wang, Polybutylene succinate/cellulose nanocrystals: role of phthalic anhydride in squeeze oriented bionanocomposites, Carbohydr. Polym., 2018, 196, 254–261, DOI:10.1016/j.carbpol.2018.04.124.
- W. Li, L. Wu, C. Di, C. Liu and R. Sun, DMAP-catalyzed phthalylation of cellulose with with phthalic anhydride in [bmim]Cl, Bioresour, 2011, 6, 2375–2385 CAS.
- S. Shaaban, M. A. Arafat, E. Gaffer and W. S. Hamama, Synthesis and anti-tumor evaluation of novel organoselenocyanates and symmetrical diselenides dyestuffs, Der Pharma Chem., 2014, 6, 186–193 Search PubMed.
- F. Hassanzadeh, M. Rabbani, G. A. Khodarahmi and M. Moosavi, Synthesis and evaluation of the anxiolytic activity of some phthalimide derivatives in mice model of anxiety, Iran. J. Pharm. Res., 2012, 11, 109–115 CAS.
- S. Mathur and S. Tabassum, Template synthesis of novel carboxamide dinuclear copper (II) complex: spectral characterization and reactivity towards calf-thymus DNA, BioMetals, 2008, 21, 299–310, DOI:10.1007/s10534-007-9119-2.
- M.-Th. Khong, V. Berl, L. Kuhn, Ph. Hammann and J.-P. Lepoittevin, Chemical modifications induced by phthalic anhydride, a respiratory sensitizer, in reconstructed human epidermis: a combined HRMAS NMR and LC-MS/MS proteomic approach, Chem. Res. Toxicol., 2021, 34, 2087–2099, DOI:10.1021/acs.chemrestox.1c00172.
- M. V. Spanedd and L. Bourel-Bonnet, Cyclic anhydrides as powerful tools for bioconjugation and smart delivery, Bioconjugate Chem., 2021, 32, 482–496, DOI:10.1021/acs.bioconjchem.1c00023.
- B. D. Salih, A. H. Dalaf, M. A. Alheety, W. M. Rashed and I. Q. Abdullah, Biological activity and laser efficacy of new Co(II), Ni(II), Cu(II), Mn(II) and Zn(II) complexes with phthalic anhydride, Mater. Today, 2021, 43, 869–874, DOI:10.1016/j.matpr.2020.07.083.
- M. I. Marzouk, S. A. Shaker, A. A. Abdel Hafiz and Kh. Z. El-Baghdady, Design and synthesis of new phthalazinone derivatives containing benzyl moiety with anticipated antitumor activity, Biol. Pharm. Bull., 2016, 39, 239–251, DOI:10.1248/bpb.b15-00656.
- M. Yamaguchi, K. Kamei, T. Koga, M. Akima, T. Kuroki and N. Ohi, Novel antiasthmatic agents with dual activities of thromboxane A2 synthetase inhibition and bronchodilation. 1. 2-[2-(l-Imidazolyl)alkyl]-1(2EI)-phthalazinones, J. Med. Chem., 1993, 36, 4052–4060, DOI:10.1021/jm00077a009.
- S. C. C. Costa Silva, E. M. A. Braz, C. A. R. S. Brito, M. M. M. Alves, F. A. A. Carvalho, H. M. Barreto, A. L. Oliveira, D. A. Silva and E. C. Silva-Filho, Phthalic anhydride esterified chicha gum: characterization and antibacterial activity, Carbohydr. Polym., 2021, 251, 117077, DOI:10.1016/j.carbpol.2020.117077.
- G. Bold, K.-H. Altmann, J. Frei, M. Lang, P. W. Manley, P. Traxler, B. Wietfeld, J. Brü ggen, E. Buchdunger, R. Cozens, S. Ferrari, P. Furet, F. Hofmann, G. Martiny-Baron, J. Mestan, J. Rösel, M. Sills, D. Stover, F. Acemoglu, E. Boss, R. Emmenegger, L. Lä sser, E. Masso, R. Roth, Ch. Schlachter, W. Vetterli, D. Wyss and J. M. Wood, New anilinophthalazines as potent and orally well absorbed inhibitors of the VEGF receptor tyrosine kinases useful as antagonists of tumor-driven angiogenesis, J. Med. Chem., 2000, 43, 2310–2323, DOI:10.1021/jm9909443.
- F. Chekin and S. Bagheri, Tyrosine sensing on phthalic anhydride functionalized chitosan and carbon nanotube film coated glassy carbon electrode, Russ. J. Electrochem., 2016, 52, 174–180, DOI:10.1134/S1023193515120034.
- Z. Yin, Q. Sun, L. Chen, M. Du, Y.-Y. Huang, Q. Peng, G. Zhang and D. Zhang, New phthalic anhydride-based room-temperature phosphorescence emitter with lifetime longer than one second, Adv. Opt. Mater., 2023, 11, 2202224, DOI:10.1002/adom.202202224.
- D. Li, Q. Wang, N. Rao, Y. Zhang, Y. Le, L. Liu, L. Li, L. Huang and L. Yan, Development of Imidazo[1,2-a]pyridine-based probe for detection of hydrazine and its applications in imaging of HepG2 cell, J. Mol. Struct., 2021, 1239, 130521, DOI:10.1016/j.molstruc.2021.130521.
- M. Edrissi, B. Nasernejad and B. Sayedi, Novel method for the preparation of copper phthalocyanin blue nanoparticles in an electrochemical cell irradiated by microwave, Int. J. Eng., Trans. B, 2007, 20, 257–262 Search PubMed.
- A. M. O'Brien and C. Ó'Fágáin, Dye bleaching and phenol precipitation by phthalic anhydride-modified horseradish peroxidase, J. Chem. Technol. Biotechnol., 2000, 75, 363–368, DOI:10.1002/(SICI)1097-4660(200005).
- K. M. Chen and C. C. Tsai, Synthesis and surface activity of maleic anhydride-polyethylene glycoi-phthalic anhydride polymeric surfactants, J. Am. Oil Chem. Soc., 1988, 65, 1346–1349, DOI:10.1007/BF02542419.
- S. N. C. Ramos, A. L. P. Xavier, F. S. Teodoro, L. F. Gil and L. V. A. Gurgel, Removal of cobalt(II), copper(II), and nickel(II) ions from aqueous solutions using phthalate-functionalized sugarcane bagasse: Monoand multicomponent adsorption in batch mode, Ind. Crops Prod., 2016, 79, 116–130, DOI:10.1016/j.indcrop.2015.10.035.
- Z.-F. Xu, T. Zhang and W. Hong, O-phthalic anhydride/Zn(OTf)2 co-catalyzed Beckmann rearrangement under mild conditions, Tetrahedron, 2019, 75, 3113–3117, DOI:10.1016/j.tet.2019.04.056.
- M. Lutz, M. Wenzler and I. Likhotvorik, An efficient oxidation of sulfides to sulfones with urea–hydrogen peroxide in the presence of phthalic anhydride in ethyl acetate, Synthesis, 2018, 50, 2231–2234, DOI:10.1055/s-0037-1609446.
- M. Khosravi, R. Rahimi, M. Rabbani and E. Safavi, Microwave-assisted solid-phase(SPPS) and solution-phase (SPS) synthesis of biological dipeptide (β-alanine-L histidine), Quat. J. Appl. Chem. Res., 2017, 11, 8–18 Search PubMed.
- K. Liu, X. Zhang and K. Yan, Development of o-phthalic anhydride as a low-temperature activator in H2O2 bleaching system for cotton fabric, Cellulose, 2018, 25, 859–867, DOI:10.1007/s10570-017-1578-1.
- D. Maldas and B. V. Kokta, Influence of phthalic anhydride as a coupling agent on the mechanical behavior of wood fiber-polystyrene composites, J. Appl. Polym. Sci., 1990, 41, 185–194, DOI:10.1002/app.1990.070410116.
- M. Wang, J. Huang, Y. Xu, B. Han and Zh. Duan, Phthalic anhydride promoted ring-opening polymerization of cyclohexene oxide catalyzed by dinuclear chromium complex supported by piperazine-bridged [ONSO] ligand, Polym. Int., 2019, 68, 1704–1709, DOI:10.1002/pi.5874.
- Y. Liu, Z. Wei, T. Xing, M. Lu and X. Li, Performance of Au/FeOx-TiO2 catalyst for liquid phase selective hydrogenation of phthalic anhydride to phthalide, J. Ind. Eng. Chem., 2015, 3, 321–327, DOI:10.1016/j.jiec.2014.08.036.
- Y. X. Liu, T. F. Xing, Y. M. Luo, X. N. Li and W. Yan, Au/FeOx-TiO2 as an efficient catalyst for the selective hydrogenation of phthalic anhydride to phthalide, Chin. Chem. Lett., 2010, 21, 1322–1325, DOI:10.1016/j.cclet.2010.06.042.
- Y. Liu, T. Xing, Z. Wei, X. Li and W. Yan, Liquid phase selective hydrogenation of phthalic anhydride to phthalide over titania supported gold catalysts, Catal. Commun., 2009, 10, 2023–2026, DOI:10.1016/j.catcom.2009.07.021.
- L. Zhang, X. Chen, Y. Chen, Z. Peng and Ch. Liang, Acid-tolerant Intermetallic Cobalt-nickel silicides as noble metal like catalysts for selective hydrogenation of phthalic anhydride to phthalide, Catal. Sci. Technol., 2019, 9, 1108–1116, 10.1039/C8CY02258E.
- L. Zhang, X. Chen, S. Jin, J. Guan, Ch. T. Williams, Zh. Peng and Ch. Liang, Rapid microwaves synthesis of CoSix/CNTs as novel catalytic materials for hydrogenation of phthalic anhydride, J. Solid State Chem., 2014, 217, 105–112, DOI:10.1016/j.jssc.2014.05.021.
- Y. Liu, Y. Gu, Y. Hou, Y. Yang, S. Deng and Z. Wei, Hydrophobic activated carbon supported Ni-based acid-resistant catalyst for selective hydrogenation of phthalic anhydride to phthalide, J. Chem. Eng., 2015, 275, 271–280, DOI:10.1016/j.cej.2015.04.051.
- T. Xiao, J. Xie, J. Cheng, X. Dai, S. Lu, R. Zuo, Z. Li and Zh. Yang, Al2O3 supported NiCu alloy as a stable catalyst for selective hydrogenation of phthalic anhydride to phthalide, Appl. Catal., A, 2023, 660, 119189, DOI:10.1016/j.apcata.2023.119189.
- I. M. Al-Jubanawi, H. Z. Al-Sawaad and A. A. Alwaaly, Synthesis characterization and corrosion inhibition of thiourea and phthalic anhydride complex with Ni(II) for carbon steel alloy C1010 0.1 M hydrochloric acid, Surf. Eng. Appl., 2021, 57, 595–606, DOI:10.3103/S1068375521050057.
- A. S. Fouda, A. M. El-desoky and D. M. Ead, Anhydride derivatives as corrosion inhibitors for carbon steel in hydrochloric acid solutions, Int. J. Electrochem. Sci., 2013, 8, 8823–8847 CrossRef CAS.
- B. A. R. Zaki, F. L. Rashid and M. N. Al-Baiati, Glycerol/phthalic anhydride novel nano composite for microwave absorbing applications, Rev. Compos. Mater. Av., 2022, 32, 133–139 Search PubMed.
- T. S. Velayutham, W. H. Abd Majid, A. B. Ahmad, G. Y. Kang and S. N. Gan, Synthesis and characterization of polyurethane coatings derived from polyols synthesized with glycerol, phthalic anhydride and oleic acid, Prog. Org. Coat., 2009, 66, 367371, DOI:10.1016/j.porgcoat.2009.08.013.
- P. N. Son, The chemistry of phthalic anhydride in cure retardation of rubbers, Rubber Chem. Technol., 1976, 49, 118–125, DOI:10.5254/1.3534939.
- P. K. Dubey, S. M. G. Mohiuddin and R. Kumar, Phthalic anhydride - a valuable petrochemical, Asian J. Chem., 1996, 8, 341–345 CAS.
- F. Iordache, S. A. Iordache, I. Costea, C. Simion, A. Simion, D. Mihaescu and F. Badea, Some new applications of phthalic anhydride in organic synthesis, Rev. Roum. Chim., 2002, 46, 835–845, DOI:10.1002/CHIN.200246249.
- A. S. Elgharbawy, A Review on phthalic anhydride industry and uses, Trends Pet. Eng., 2021, 1, 1–2 Search PubMed.
- D. A. Basketter and I. Kimber, Phthalic anhydride: illustrating a conundrum in chemical allergy, J. Immunotoxicol., 2016, 13, 767–769, DOI:10.1080/1547691X.2016.1177149.
- A. Fatima, Gh. Khanum, A. Sharma, K. Garima, S. Savita, I. Verma, N. Siddiqui and S. Javed, Computational, spectroscopic, Hirschfeld surface, electronic state and molecular docking studies on phthalic anhydride, J. Mol. Struct., 2022, 1249, 131571, DOI:10.1016/j.molstruc.2021.131571.
- J. H. Park, J. Y. Choi, D. J. Son, E. K. Park, M. J. Song, M. Hellström and J. T. Hong, Anti-inflammatory effect of titrated extract of centella asiatica in phthalic anhydride-induced allergic dermatitis animal model, Int. J. Mol. Sci., 2017, 18, 738, DOI:10.3390/ijms18040738.
- E. H. Chester, H. J. Schwartz, C. B. Payne JR and S. Greenstein, Phthalic anhydride asthma, Clin. Exp. Allergy, 1977, 7, 15–20, DOI:10.1111/j.1365-2222.1977.tb01419.x.
- T. Yanagimoto, The reaction between urea and phthalic anhydride under pressure, Int. Rev. Phys. Chem., 1956, 25, 71–77 Search PubMed.
- K. S. Martin, J. T. Shaw and A. Younai, Anhydride-based multi-component reactions, Multicomponent reactions in organic synthesis ed. J. Zhu, Q. Wang and M. Wang, John Wiley & Sons, 2014, ch. 13, pp. 379–400 Search PubMed.
- A. R. Katritzky and F. Yates, Phthaloylation of amino-azoles and amino-azines, J. Chem. Soc., Perkin Trans. 1, 1976, 309–312, 10.1039/P19760000309.
- Y. M. Evtushenko, B. E. Zaitsev, V. M. Ivanov and K. M. Gitis, The reaction of maleic and phthalic anhydrides with 2-methylimidazole, Chem. Heterocycl. Compd., 2000, 36, 1054–1057, DOI:10.1023/A:1002781815067.
- A. R. Hajipour, S. E. Mallakpour and G. Imanzadeh, Microwave-assisted rapid synthesis of phthalimide derivatives, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2001, 40, 250–251 Search PubMed.
- J. H. Billman and W. F. Harting, Amino acids. V.1 phthalyl derivatives, J. Am. Chem. Soc., 1948, 70, 1473–1474, DOI:10.1021/ja01184a051.
- Q. Zeng, Z. Liu, B. Li and F. Wang, Mild and effective N-phthaloylation of amino acids, Amino Acids, 2004, 27, 183–186, DOI:10.1007/s00726-004-0109-1.
- D. A. Kidd and F. E. King, Preparation of phthalyl-L-glutamic acid, Nature, 1984, 162, 776, DOI:10.1038/162776b0.
- A. C. L. Leite, F. F. Barbosa, M. V. de Oliveira Cardoso, D. R. M. Moreira, L. C. D. Coêlho, E. B. da Silva, G. B. de Oliveira Filho, V. M. O. de Souza, V. R. A. Pereira, L. C. Reis, P. M. P. Ferreira, C. Pessoa, A. Gonc, A. Wanderley, F. V. B. Mota and T. G. da Silva, Phthaloyl amino acids as anti-inflammatory and immunomodulatory prototypes, Med. Chem. Res., 2014, 23, 1701–1708, DOI:10.1007/s00044-013-0730-1.
- A. Saddiqa, I. Shahzadi, M. Usman, O. Çakmak and S. Ökten, Facile, expeditious and cost-effective preparation of N-phthaloyl (S)-amino acids and their in silico activities against Staphylococcus Aureus, Org. Prep. Proced. Int., 2022, 54, 583–593, DOI:10.1080/00304948.2022.2113724.
- A. K. Bose, F. Greer and Ch. C. Price, A procedure for phthaloylation under mild conditions, J. Org. Chem., 1958, 23, 1335–1338, DOI:10.1021/jo01103a025.
- A. Homsi and A. Kasideh, Synthesis of some N-phthalimide amino acids derivatives and evaluation their biological activity, Int. J. ChemTech Res., 2015, 8, 1817–1825 CAS.
- S. D. Khalili, S. H. Banitabai Koupaie and J. Safari, Lewis acid catalyzed synthesis of quinophthalone pigments under solvent-free conditions, Sci. Iran., 2009, 16, 1–6 CAS.
- H. Loghmani-Khouzani, M. M. Sadeghi and J. Safari, Silica gel catalyzed synthesis of quinophthalone pigments under solvent-free conditions using microwave irradiation, Molecules, 2002, 7, 135–139, DOI:10.3390/70200135.
- M. Phillips and M. J. Goss, The preparation and properties of methylisopropyl-quinoline yellow, J. Am. Chem. Soc., 1926, 48, 823–826, DOI:10.1021/ja01414a051.
- J. Safari, S. H. Banitaba and S. D. Khalili, BF3-nano SiO2 as a catalytic system for one-pot green synthesis of pyrophthalone derivatives under microwave conditions, Arabian J. Chem., 2012, 5, 419–424, DOI:10.1016/j.arabjc.2010.09.031.
- J. R. Cook and D. F. Martin, Chelating tendencies of some substituted 2-(pyridyl)-1,3-indanediones with bivalentmetal ions, J. Inorg. Nucl. Chem., 1964, 26, 571–577, DOI:10.1016/0022-1902(64)80290-6.
- M. Bayat, H. Imanieh and M. H. Farjam, Triphenylphosphine-promoted synthesis of spiroketals from phthalic anhydride with dialkyl acetylenedicarboxylates, Synth. Commun., 2010, 40, 2475–2482, DOI:10.1080/00397910903267913.
- S. Sharma, A. D. K. Jain, A. Aggarwal and N. S. Gill, Synthesis, characterization and pharmacological evaluation of novel Schiff bases of imide moiety, J. Med. Sci., 2012, 12, 61–69, DOI:10.3923/jms.2012.61.69.
- P. P. Kumar, Y. D. Reddy, Y. B. Kumari, B. R. Devi and P. K. Dubey, n expeditious synthesis of imides from phthalic, maleic and succinic anhydrides and chemoselective C=C reduction of maleic amide esters, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2014, 53, 392–398 Search PubMed.
- D. Habibi and H. M. Pordanjani, Phthalimide-N-sulfonic acid, an efficient catalyst for the synthesis of various isoindoline-1,3-dione derivatives, Chem. Pap., 2017, 71, 2293–2299, DOI:10.1007/s11696-017-0223-7.
- P. W. Chia, P. S. Chee, M. Z. H. Aziz, S. F. M. Radzi and S.-Y. Kan, Water extract of onion peel ash: an efficient green catalytic system for the synthesis of isoindoline-1,3-dione derivatives, Malaysian J. Anal. Sci., 2019, 23, 23–30, DOI:10.17576/mjas-2019-2301-03.
- A. Aliabadi, B. Gholamine and T. Karimi, Synthesis and antiseizure evaluation of isoindoline-1,3-dione derivatives in mice, Med. Chem. Res., 2014, 23, 2736–2743, DOI:10.1007/s00044-013-0870-3.
- I. Ahmad, R. H. Pawara, R. T. Girase, A. Y. Pathan, V. R. Jagatap, N. Desai, Y. O. Ayipo, S. J. Surana and H. Patel, Synthesis, molecular modeling study, and quantum-chemical-based investigations of isoindoline-1,3-diones as antimycobacterial agents, ACS Omega, 2022, 7, 21820–21844, DOI:10.1021/acsomega.2c01981.
- H. Jelali, L. Mansour, E. Deniau, M. Sauthier and N. Hamdi, An efficient synthesis of phthalimides and their biological activities, Polycyclic Aromat. Compd., 2022, 4, 1806–1813, DOI:10.1080/10406638.2020.1809468.
- S. M. Al-Mousawi, M. A. El-Apasery and M. H. Elnagdi, Enaminones in heterocyclic synthesis: a novel route to tetrahydropyrimidines, dihydropyridines, triacylbenzenes and naphthofurans under microwave irradiation, Molecules, 2010, 15, 58–67, DOI:10.3390/molecules15010058.
- A. S. Bisht and R. Bisht, Microwave assested synthesis of phthalimide amino derivatives with their antioxidant potential, Curr. Trends Biotechnol. Pharm., 2021, 3, 23–27, DOI:10.18231/j.ctppc.2021.007.
- Z. W. Wicks Jr and G.-F. Chen, Reactions of phthalic anhydride with 2-amino alcohols, J. Org. Chem., 1979, 44, 1244–1247, DOI:10.1021/jo01322a011.
- E. Jafari, N. Taghi jarah-Najafabadi, A. Jahanian-Najafabadi, S. Poorirani, F. Hassanzadeh and S. Sadeghian-Rizi, Synthesis and evaluation of antimicrobial activity of cyclic imides derived from phthalic and succinic anhydrides, Res. Pharm. Sci., 2017, 12, 526–534, DOI:10.4103/1735-5362.217433.
- B. Hosseinkhani, M. R. Islami and S. Hosseinkhani, Highly Stereoselective synthesis of isoindole derivatives containing an azetidinone ring, Synlett, 2015, 26, 2277–2279, DOI:10.1055/s-0035-1560066.
- S. Chandrasekhar, M. B. Padmaja and A. Raza, Solid phase-solid state synthesis of N-alkyl imides from anhydrides, Synlett, 1999, 1597–1599, DOI:10.1055/s-1999-2894.
- S. Pawar, S. Sawant, A. Nerkar and A. Bhosale, In silico design, synthesis and pharmacological screening of novel 2-(6-substituted benzo[d]thiazol-2-yl)isoindoline-1,3-diones as potential COX-2inhibitors for anti-inflammatory activity, Int. J. Pharm. Pharm. Sci., 2014, 6, 353–357 Search PubMed.
- P. Jimonet, F. Audiau, M. Barreau, J.-Ch. Blanchard, A. Boireau, Y. Bour, M.-A. Coléno, A. Doble, G. Doerflinger, C. D. Huu, M.-H. Donat, J. M. Duchesne, P. Ganil, C. Guérémy, E. Honoré, B. Just, R. Kerphirique, S. Gontier, P. Hubert, P. M. Laduron, J. Le Blevec, M. Meunier, J.-M. Miquet, C. Nemecek, M. Pasquet, O. Piot, J. Pratt, J. Rataud, M. Reibaud, J.-M. Stutzmann and S. Mignani, Riluzole series. synthesis and in Vivo “antiglutamate” activity of 6-substituted-2-benzothiazolamines and 3-substituted-2-imino-benzothiazolines, J. Med. Chem., 1999, 42(15), 2828–2843, DOI:10.1021/jm980202u.
- D. Ravi, B. Sakram, K. Ashok, S. Rambabu, B. Sonyanaik, A. Kurumanna and P. Madhu, Novel synthesis of substituted N-(3-aryl-1,8-naphthyridin-2-yl)and N,N′-bis(3-aryl-1,8-naphthyridin-2-yl)benzene-1,4-diamines and 2-(4-((3-aryl-1,8-naphthyridin-2-yl)amino)phenyl)isoindoline-1,3-diones and their biological evaluation, Synth. Commun., 2018, 48, 50–58, DOI:10.1080/00397911.2017.1386790.
- M.-Z. Huang, K.-L. Huang, Y.-G. Ren, M.-X. Lei, L. Huang, Z.-K. Hou, A.-P. Liu and X.-M. Ou, Synthesis and herbicidal activity of 2-(7-fluoro-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl)isoindoline-1,3-diones, J. Agric. Food Chem., 2005, 53, 7908–7914, DOI:10.1021/jf051494s.
- K. E. Anwer, G. H. Sayed, H. H. Hassanan and M. E. Azab, Conventional and microwave synthesis of some new pyridine derivatives and evaluation their antimicrobial and cytotoxic activities, Egypt. J. Chem., 2019, 62, 707–726, DOI:10.21608/ejchem.2018.5115.1452.
- S. Y. Mansour, G. H. Sayed, M. I. Marzouk and S. S. Shaban, Synthesis and anticancer assessment of some new 2-amino-3-cyanopyridine derivatives, Synth. Commun., 2021, 51, 1160–1170, DOI:10.1080/00397911.2020.1870698.
- K. E. Anwer and G. H. Sayed, Conventional and microwave reactions of 1,3-diaryl-5,4-enaminonitrile-pyrazole derivative with expected antimicrobial and anticancer activities, J. Heterocycl. Chem., 2020, 1–15, DOI:10.1002/jhet.3946.
- M. El-Shahat, W. I. El-Sofany, A. A. Soliman and M. Hasanin, Newly synthesized imidazolotriazole, imidazolotriazine, and imidazole-pyrazole hybrid derivatives as promising antimicrobial agents, J. Mol. Struct., 2021, 1250, 131727, DOI:10.1016/j.molstruc.2021.131727.
- L. M. Lima, P. Castro, A. L. Machado, C. A. M. Fraga, C. Lugnier, V. L. G. de Moraes and E. J. Barreiro, Synthesis and anti-inflammatory activity of phthalimide derivatives, designed as new thalidomide analogues, Bioorg. Med. Chem., 2002, 10, 3067–3073, DOI:10.1016/s0968-0896(02)00152-.9.
- A. K. Timiri, S. Subasri, M. Kesherwani, V. Vishwanathan, B. N. Sinha, D. Velmurugan and V. Jayaprakash, Synthesis and molecular modelling studies of novel sulphonamide derivatives as dengue virus 2 protease inhibitors, Bioorg. Chem., 2015, 62, 74–82, DOI:10.1016/j.bioorg.2015.07.005.
- Z. M. Nofal, E. A. Soliman, S. S. Abd El-Karim, M. I. El-Zahar, A. M. Srour, S. Sethumadhavan and T. J. Maherc, Synthesis of some new benzimidazole-thiazole derivatives as anticancer agents, J. Heterocycl. Chem., 2014, 51, 1797–1806, DOI:10.1002/jhet.1886.
- Y.-N. Sun, C.-L. Wang, N. Zhang, Zh. Wang, Zh.-L. Liu and J.-L. Liu, Synthesis of tetrahydro-β-carbolines from phthalic anhydrides and tryptamine, Chin. Chem. Lett., 2014, 25, 1503–1506, DOI:10.1016/j.cclet.2014.06.012.
- A. S. Abd El-All, S. M. S. Atta, H. M. F. Roaiah, E. M. Awad and M. M. Abdalla, New potent SARS-CoV 3C-like protease inhibitors derived from thieno[2,3-d]pyrimidine derivatives, Arch. Pharm. Chem. Life Sci., 2016, 349, 202–210, DOI:10.1002/ardp.201500407.
- M. A. Saleh, Y. A. Hafez, F. E. Abdel-hay and W. I. Gad, Synthesis and Reactions of 3-amino-2-methyl-3H-[1,2,4]triazolo[5,1-b]quinazolin-9-one and 2-hydrazino-3-phenylamino-3H-quinazolin-4-one, J. Heterocycl. Chem., 2003, 40, 973–978, DOI:10.1002/jhet.5570400604.
- N. Kise, S. Yamamoto and T. Sakurai, Reductive coupling of phthalic anhydrides with aliphatic ketones by low-valent titanium: unusual two-to-one coupling for preparation of 3,3-disubstituted phthalides, Tetrahedron, 2020, 76, 130820, DOI:10.1016/j.tet.2019.130820.
- M. Lácová, Condensation of 3-indoleacetic acid with phthalic anhydride by the Gabriel and Oglialoro-modification of the Perkin synthesis, Chem. Pap., 1969, 23, 450–453 Search PubMed.
- N. Kise, S. Yamamoto and T. Sakurai, Electroreductive coupling of phthalic anhydrides with α,β-unsaturated carbonyl compounds: synthesis of 1,4-dihydroxynaphthalenes, J. Org. Chem., 2020, 85, 13973–13982, DOI:10.1021/acs.joc.0c02000.
- K. Fujiwara, T. Kurahashi and S. Matsubara, Nickel-catalyzed decarbonylative cycloaddition of phthalic anhydrides with 1,3-dienes, Chem. Lett., 2011, 40, 322–323, DOI:10.1246/cl.2011.322.
- (a) E. M. O'Brien, E. A. Bercot and T. Rovis, Decarbonylative cross-coupling of cyclic anhydrides: introducing stereochemistry at an sp3 carbon in the cross-coupling even, J. Am. Chem. Soc., 2003, 125, 10498–10499, DOI:10.1021/ja036290b; (b) J. B. Johnson, E. A. Bercot, J. M. Rowley, G. W. Coates and T. Rovis, Ligand-dependent catalytic cycle and role of styrene in nickel-catalyzed anhydride cross-coupling: evidence for turnover-limiting reductive elimination, J. Am. Chem. Soc., 2007, 129, 2718–2725, DOI:10.1021/ja067845g; (c) B. M. Trost and F. Chen, Transition metal mediated eliminations in anhydrides and thioanhydrides, Tetrahedron Lett., 1971, 12, 2603–2607, DOI:10.1016/S0040-4039(01)96930-8; (d) K. Sano, T. Yamamoto and A. Yamamoto, Kinetic and thermodynamic control of asymmetric induction by chiral diphosphine in ring contraction of (chiral diphosphine)NiCH2CH2CH2COO to (chiral diphosphine) NiCH (CH3)CH2COO, Chem. Lett., 1984, 13, 941–944, DOI:10.1246/cl.1984.941; (e) K. Sano, T. Yamamoto and A. Yamamoto, preparation of Ni-or Pt-containing cyclic esters by oxidative addition of cyclic carboxylic anhydrides and their properties, Bull. Chem. Soc. Jpn., 1984, 57, 2741–2747 CrossRef CAS; (f) T. Yamamoto, K. Sano and A. Yamamoto, Effect of ligand on ring contraction of six-membered nickel-containing cyclic esters, LnNiCH2CH2CH2COO, to their five-membered-ring isomers, LnNiCH(CH3)CH2COO. kinetic and thermodynamic control of asymmetric induction by chiral diphosphines in the ring contraction, J. Am. Chem. Soc., 1987, 109, 1092–1100, DOI:10.1021/ja00238a017; (g) R. Fischer, D. Walther, R. Kempe, J. Sieler and B. Schönecker, Metallacyclische acyl-carboxylate des nickels: Reaktivität der acylgruppe und synthesepotential bei kreuzkopplungsreaktionen mit alkylhalogeniden, J. Organomet. Chem., 1993, 447, 131–136, DOI:10.1016/0022-328X(93)80282-G; (h) K. Fujiwara, T. Kurahashi and S. Matsubara, Decarbonylative cycloaddition of phthalimides with 1,3-dienes, Org. Lett., 2010, 12, 4548–4551, DOI:10.1021/ol101842y.
- L. Huang, R. Xie, Ch. Wen, Y. Yang, Y. Wang, S. Ren, B. Huang and S. Li, Decarbonylative/decarboxylative [4+2] annulation of phthalic anhydrides and cyclic iodonium towards triphenylenes, Org. Biomol. Chem., 2022, 20, 3913–3916, 10.1039/D2OB00500J.
- M. P. Satchel and B. E. Stacey, Reactions of 1,5-disubstituted naphthalenes with phthalic anhydride, J. Chem. Soc. C, 1971, 469–474, 10.1039/J39710000469.
- H. Naeimi and S. S. Brojerdi, Facile and efficient one-pot synthesis of anthraquinones from benzene derivatives catalyzed by silica sulfuric acid, Polycyclic Aromat. Compd., 2014, 34, 504–517, DOI:10.1080/10406638.2014.910238.
- C. Ji, H. Wang, S. Sun, Q. Li, J.-J. Zou, S. Yu, H. Shi, G. Nie and S. Liu, One-pot synthesis of 2-ethylanthraquinone from phthalic anhydride and ethylbenzene over a Sc-modified Hβ catalyst, Chem. Eng. Sci., 2022, 251, 117480, DOI:10.1016/j.ces.2022.117480.
- Y. Wang, G. Wang, Y. Yan, X. Zhao and Y. Wang, Acylation reaction of benzene with phthalic anhydride catalyzed by amide-AlCl3 ionic liquid analogs, Acta Pet. Sin., 2022, 3, 1001–8719, DOI:10.3969/j.issn.1001-8719.2022.02.008.
- M. L. Tedjamulia, Y. Tominaga, R. N. Castle and M. L. Lee, The synthesis of dinaphthothiophenes, J. Heterocycl. Chem., 1983, 20, 1143–1148, DOI:10.1002/jhet.5570200502.
- H. Naeimi and R. Namdari, Rapid, efficient and one pot synthesis of anthraquinone derivatives catalyzed by Lewis acid/methanesulfonic acid under heterogeneous conditions, Dyes Pigm., 2009, 81, 259–263, DOI:10.1016/j.dyepig.2008.10.019.
- B. R. Madje, K. F. Shelke, S. B. Sapkal, G. K. Kakade and M. S. Shingare, An efficient one-pot synthesis of anthraquinone derivatives catalyzed by alum in aqueous media, Green Chem. Lett. Rev., 2010, 3, 269–273, DOI:10.1080/17518251003776877.
- N. Shafiq, S. Ashraf, S. Parveen and B. Ali, A one-pot rapid synthesis, docking study and biological evaluation of some tetrahydropyrimidine-5-carboxylates, Indian J. Heterocycl. Chem., 2019, 29, 249–253 Search PubMed.
- G. Zarren, N. Shafiq, U. Arshad, N. Rafiq, S. Parveen and Z. Ahmad, Copper-catalyzed one-pot relay synthesis of anthraquinone based pyrimidine derivative as a probe for antioxidant and antidiabetic activity, J. Mol. Struct., 2021, 1227, 129668, DOI:10.1016/j.molstruc.2020.129668.
- M. Phillips, The preparation of alizarin from phthalic anhydride and ortho-dichlorobenzene, J. Am. Chem. Soc., 1927, 49, 473–478, DOI:10.1021/ja01401a021.
- C. Sprent and W. Dodd, Brit. Pat., 1923, 204, p. 528 Search PubMed.
- P. P. Kumar, Y. D. Reddy, C. V. R. Reddy, B. R. Devi and P. K. Dubey, One pot, three-component synthesis of novel 3,4-dihydrophthalazin-2(1H)-yl-4-phenyl-4H-pyrans, Tetrahedron Lett., 2014, 55, 2177–2182, DOI:10.1016/j.tetlet.2014.02.020.
- M. R. Mahmoud, W. S. I. Abou-Elmagd, H. A. Derbala and M. H. Hekal, Synthesis and spectral characterisation of some phthalazinone derivatives, J. Chem. Res., 2012, 75–82 CrossRef CAS.
- V. M. Milovanovi, Z. D. Petrovi, S. Novakovi, G. A. Bogdanovi, V. P. Petrovi and D. Simijonovi, Green synthesis of benzamide-dioxoisoindoline derivatives and assessment of their radical scavenging activity-experimental and theoretical approach, Tetrahedron, 2020, 76, 131456, DOI:10.1016/j.tet.2020.131456.
- A. A. Aly, M. A. Abo-Riya, A. A. El-Shiekh and A. I. Elkala, Synthesis and biological evaluation of new series of phthalazinone, pyridazinone, oxadiazole, pyrazolone scaffolds based on coumarin-3-carbohydrazide, Benha J. Appl. Sci., 2022, 7, 21–29 CrossRef.
- M. H. Hekal, F. S. M. A. El-Azm and S. R. Atta-Allah, Ecofriendly and highly efficient microwaveinduced synthesis of novel quinazolinone-undecyl hybrids with in vitro antitumor activity, Synth. Commun., 2019, 49, 2630–2641, DOI:10.1080/00397911.2019.1637001.
- D. Gao, F. Jin, X. Yan and R. N. Zare, Selective synthesis in microdroplets of 2-phenyl-2,3-dihydrophthalazine-1,4-dione from phenyl hydrazine with phthalic anhydride or phthalic acid, Chem. - Eur. J., 2019, 25, 1466–1471, DOI:10.1002/chem.201805585.
- S. F. Abbas, J. H. Tomma and E. T. Ali, Synthesis and characterization of new Schiff bases and their 1,3-oxazepines derived from phthalic anhydride, Sys. Rev. Pharm., 2021, 12, 260–265 CAS.
- H. D. Hanoon, Synthesis and characterization of new seven-membered heterocyclic compounds from reaction of new Schiff-bases with maleic and phthalic anhydrides, J. Chem., 2011, 41, 77–89 Search PubMed.
- T. Serevičius, R. Komskis, P. Adome˙nas, O. Adomeniene, V. Jankauskas, A. Gruodis, K. Kazlauskas and S. Juršėnas, Non-symmetric 9,10-diphenylanthracene-based deep-blue emitters with enhanced charge transport properties, Phys. Chem. Chem. Phys., 2014, 16, 7089–7101, 10.1039/c4cp00236a.
- L. Cheng, L. Ying, J. Feng, C. H. Y. Wang, J. L. Li and Z. Xu, Novel heterocyclic poly(arylene ether ketone)s: synthesis and polymerization of 4-(40-hydroxyaryl)(2H)phthalazin-1-ones with methyl groups, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1525–1535, DOI:10.1002/pola.21880.
- S.-N. Zhang, Zh.-J. Li and M.-S. Cai, Synthesis of N-sugar-substituted phthalimides and their derivatives from sugar azides and phthalic anhydride, Carbohydr. Res., 2004, 339, 1419–1420, DOI:10.1016/j.carres.2004.03.032.
- A. Kamal, E. Laxman, N. Laxman and N. V. Rao, Efficient one-pot synthesis of n-substituted phthalimides∼aphthalimides from azides and anhydrides by iodotrimethylsilane, Tetrahedron Lett., 1998, 39, 8733–8734, DOI:10.1016/S0040-4039(98)01937-6.
- H. N. Nguyen, V. J. Cee, H. L. Deak, B. Du, K. P. Faber, H. Gunaydin, B. L. Hodous, S. L. Hollis, P. H. Krolikowski, Ph. R. Olivieri, V. F. Patel, K. Romero, L. B. Schenkel and S. D. Geuns-Meyer, Synthesis of 4-substituted chlorophthalazines, dihydrobenzoazepinediones, 2-pyrazolylbenzoic acid, and 2-pyrazolylbenzohydrazide via 3-substituted-3-hydroxyisoindolin-1-ones, J. Org. Chem., 2012, 77, 3887–3906, DOI:10.1021/jo3000628.
- A. Acosta, P. de ia Cruz, P. De Miguel, E. Diez-Barra, A. de la Hoz, F. Langa, A. Loupy, M. Majdoub, N. Martin, C. Sanchez and C. Seoane, Microwave assisted synthesis of heterocyclic fused quinones in dry media, Tetrahedron Lett., 1995, 36, 2165–2168, DOI:10.1016/0040-4039(95)00202-N.
- W. E. Parham and R. M. Piccirilli, Preparation of aroylbenzoic acid. reaction of aryllithium reagents with phthalic anhydride, J. Org. Chem., 1976, 41, 1268–1269, DOI:10.1021/jo00869a046.
- J. Koyanagi, K. Yamamoto, K. Nakayama and A. Tanaka, A short-step synthesis of naphtho[2,3-b]furan-4,9-dione, J. Heterocycl. Chem., 1994, 31, 1303–1304, DOI:10.1002/jhet.5570310534.
- P. de la Cruz, N. Martin, F. Miguel and C. Seoane, Novel pi.-extended thiophene-fused electron acceptors for organic metals, J. Org. Chem., 1992, 57, 6192–6198, DOI:10.1021/jo00049a027.
- L. Fieser and E. B. Hershberg, 1',2',3',4'-Tetrahydro-10-isopropyl-l,2-benzanthracene, J. Am. Chem. Soc., 1937, 59(11), 2331–2335, DOI:10.1021/ja01290a069.
- Y. Baba, The reaction of triethylaluminum with phthalic anhydride, Bull. Chem. Soc. Jpn., 1968, 41, 1020–1021, DOI:10.1246/bcsj.41.1020.
- H. Gilman and J. F. Nelson, Relative reactivities of organometallic compounds. IX. cadmium, Recl. Trav. Chim., 1936, 55, 518–530, DOI:10.1002/recl.19360550607.
- P. L. de Benneville, The synthesis of keto acids and ketones by the reaction of acid anhydrides with cadmium alkyls, J. Org. Chem., 1941, 6, 462–466, DOI:10.1021/jo01203a012.
- T. C. McMullen, The Friedel and Crafts reaction with phthalic anhydride, J. Am. Chem. Soc., 1922, 44(9), 2055–2060, DOI:10.1021/ja01430a027.
- M. T. Rahman and S. K. Nahar, The reaction of arylcoppermagnesium and other organometallic reagents with phthalic anhydride, J. Organomet. Chem., 1992, 425, 201–208, DOI:10.1016/0022-328X(92)80036-W.
- E.-Ch. Wang, K.-S. Huang, H.-M. Chen, Ch.-Ch. Wu and G.-J. Lin, An Efficient method for the preparation of nitriles via the dehydration of aldoximes with phthalic anhydride, J. Chin. Chem. Soc., 2004, 51, 619–627, DOI:10.1002/jccs.200400093.
- M. M. Heravi, D. Zargarani, R. Hekmat Shoar and S. Khaleghi, Hydrolysis of amides to carboxylic acids using phthalic anhydride under microwave irradiation and solvent-free conditions, J. Chem. Res., 2005, 119–120 CrossRef CAS.
- S. H. Kadhum, W. B. Ali and A. A. Jarullah, Synthesis and characterization of some compounds derived from 2-(1,1-dimethyl-1,3-dihydro-2H-benzo[e]indol-2-ylidene) malonaldehyde, HIV Nurs., 2022, 22, 3625–3631 Search PubMed.
- Y. Ochi, T. Kurahashi and S. Matsubara, Decarbonylative cycloaddition of phthalic anhydrides with allenes, Org. Lett., 2011, 13, 1374–1377, DOI:10.1021/ol200044y.
- M. Lácová, Reaction of 2-benzothiazolylthioethanoic acid with phthalic anhydride under conditions of Gabriel modification of Perkin synthesis, Chem. Pap., 1986, 40, 95–102 Search PubMed.
- H. Santos, A. Distiller, A. M. D'Souza, Q. Arnoux, J. M. White, A. G. Meyer and J. H. Ryan, 1,3-Dipolar cycloadditions with an azomethine ylide, Org. Chem. Front., 2015, 2, 705–712, 10.1039/C5QO00062A.
- A. H. Renfrew and S. B. Bostock, Reaction of phthalic anhydride with ethyl cyanoacetate: a route to the 2H-indeno[2,1-c]pyridine-1,9-dione,2H-indeno[2,1-c]pyridine-3,9-dione,2H-indeno[2,1-c]pyridazine-3,9-dione, and indeno[2,1-c]pyran-1,9-dione systems, J. Chem. Soc., Perkin Trans. 1, 1977, 84–90, 10.1039/P19770000084.
- F. Ramirez, H. Yamanaka and O. H. Basedow, New reactions of phosphite esters: the conversion of phthalic anhydride into biphthalyl by trialkyl phosphites and into phthalide-3-phosphonates by dialkyl phosphites, J. Am. Chem. Soc., 1961, 83, 173–178, DOI:10.1021/ja01462a034.
- D. Habibi and O. Marvi, Montmorillonite KSF and montmorillonite K-10 clays as efficient catalysts for the solventless synthesis of bismaleimides and bisphthalimides using microwave irradiation, Arkivoc, 2006, 8–15, DOI:10.3998/ark.5550190.0007.d02.
- D. Habibi, N. Mahmoudi and O. Marvi, Green Procedure for the synthesis of phthalazino[2,3-b]phthalazine-5,7,12,14-tetraones, Synth. Commun., 2007, 37, 3165–3171, DOI:10.1080/00397910701545247.
- M. C. Cardia, S. Distinto, E. Maccioni, L. Bonsignore and A. DeLogu, Synthesis and biological activity evaluation of differently substituted 1,4-dioxo-3,4-dihydrophthalazine-2(1H)-carboxamides and -carbothioamides, J. Heterocycl. Chem., 2003, 40, 1011–1015, DOI:10.1002/jhet.5570400609.
- F. Jafarpour, H. Hazrati and S. Nouraldinmousa, Three-bond breaking of cyclic anhydrides: easy access to polyfunctionalized naphthalenes and phenanthrenes, Org. Lett., 2013, 15, 3816–3819, DOI:10.1021/ol401318v.
- M. Fardpour, A. Darvish, E. Kianmehr and A. N. Kharat, Ru-catalyzed synthesis of substituted phthalides through C–H bond activation and functionalization, Tetrahedron Lett., 2019, 60, 699–702, DOI:10.1016/j.tetlet.2018.12.005.
- B. H. S. Torquato da Silva, L. O. Riehl, G. Carvalho dos Santos, J. C. Roldao and L. C. Silva-Filho, Solvent-free synthesis using Nb2O5 and a theoretical-experimental study of solvent effect in new rhodamine dyes, ChemistrySelect, 2020, 5, 1455–1463, DOI:10.1002/slct.201904486.
- H. Eshghi, M. Eftekhar, M. Rahimizadeh, M. Hassanpour and M. Bakavoli, Efficient Solvent free synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones and 3H-spiro[isobenzofuran-1,9’-xanthen]-3-one derivatives using cobalt hydrogensulfate as a green, heterogeneous and reusable catalyst, J. Org. Chem. Res., 2016, 2, 20–27, DOI:10.22036/ORG.CHEM.2016.13465.
- A. Shaabani, M. B. Teimouri and H. R. Bijanzadeh, The reaction of Alkyl Isocyanides and Dialkylacetylene Dicarboxylates with Phthalic Anhydride Derivatives: A Novel Synthesis of γ-spiroiminolactones, J. Chem. Res., 2002, 2002, 381–383, DOI:10.3184/030823402103172383.
- A. Shaabani, A. H. Rezayan, S. Ghasemi and A. Sarvary, A mild and efficient method for the synthesis of 2,5-dihydro-5-imino-2-methylfuran-3,4-dicarboxylates via an isocyanide-based multicomponent reaction, Tetrahedron Lett., 2009, 50, 1456–1458, DOI:10.1016/j.tetlet.2009.01.069.
- L. Zare, N. O. Mahmoodi, A. Yahyazadeh, M. Mamaghani and K. Tabatabaeian, An efficient chemo- and regioselective three-component synthesis of pyridazinones and phthalazinones using activated KSF, Chin. Chem. Lett., 2010, 21, 538–541, DOI:10.1016/j.cclet.2009.11.032.
- L. Zare, N. O. Mahmoodi, A. Yahyazadeh and M. Nikpassand, Ultrasound-promoted regio and chemoselective synthesis of pyridazinones and phthalazinones catalyzed by ionic liquid [bmim]Br/AlCl3, Ultrason. Sonochem., 2012, 19, 740–744, DOI:10.1016/j.ultsonch.2011.11.008.
- B. Thirupaiah and R. R. Vedula, Novel one-pot multicomponent synthesis of substituted 2,3-dihydro-2-(6-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)phthalazine-1,4-diones and substituted 3-[3-(N′-benzylidene-hydrazino)-7H-[1,2,4] triazolo[3,4-b][1,3,4]thiadiazin-6-yl]-4-hydroxy-6-methyl-pyran-2-ones, Synth. Commun., 2014, 44, 513–519, DOI:10.1080/00397911.2013.817584.
- V. S. R. Chunduru and R. R. Vedula, One-pot synthesis of aryl(hetaryl)-thiazolylphthalazine-1,4-diones via a multicomponent approach, Chem. Heterocycl. Compd., 2013, 49, 586–590, DOI:10.1007/s10593-013-1285-2.
- V. S. R. Chunduru and V. R. Rao, Synthesis of aryl and heteryl 1,3,4-thiadiazinyl-phthalazine-1,4-dione derivatives via a multicomponent approach, Synth. Commun., 2013, 43, 923–929, DOI:10.1080/00397911.2011.604147.
- K. Sujatha and R. R. Vedula, Novel one-pot multicomponent synthesis of (E)-2-(benzylideneamino)-5-mercapto-4H-1,2,4-triazol-3-yl-2,3-dihydrophthalazine-1,4-dione derivatives, Synth. Commun., 2019, 50, 129–135, DOI:10.1080/00397911.2019.1689571.
- P. C. Jilloju, M. Srikanth, S. V. Kumar and R. R. Vedula, One-pot, multi-component synthesis of substituted 2-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)-2,3-dihydrophthalazine-1,4-diones, Polycyclic Aromat. Compd., 2021, 41, 2043–2054, DOI:10.1080/10406638.2019.1709212.
- L. Z. Fekri and M. F. Feshalami, Green, practical and scalable multicomponent reaction for the synthesis of amides and pyridazinones from arenes using L-Proline functionalized silicapropyl modified nanomagnetic as a heterogeneous bronsted acid catalyst, Polycyclic Aromat. Compd., 2022, 42, 690–710, DOI:10.1080/10406638.2020.1749091.
- L. Z. Fekri and M. F. Feshalami, A new multicomponent reaction for the synthesis of amides, Org. Prep. Proced. Int., 2021, 53, 291–300, DOI:10.1080/00304948.2021.1880217.
- C. Bele and M. Darabantu, New 1,4-disubstituted phthalazines: synthesis, structure and herbicidal evaluation, Heterocycl. Commun., 2003, 9, 641–646, DOI:10.1515/HC.2003.9.6.641.
- E. Benjamin and Y. M. Hijji, A Novel green synthesis of thalidomide and analogs, J. Chem., 2017, 2017, 6436185, DOI:10.1155/2017/6436185.
- J. Garcia, J. Vilarrasa, X. Bordas and A. Banaszek, New synthetic “tricks” one-pot preparation of N-substituted phthalimides from azides and phthalic anhydride, Tetrahedron Lett., 1986, 27, 639–640, DOI:10.1016/S0040-4039(00)84061-7.
- E. Deniau, D. Enders, A. Couture and P. Grandclaudon, Asymmetric synthesis of 3-hetero-substituted 2,3-dihydro-1H-isoindol-1-ones, Tetrahedron: Asymmetry, 2005, 16, 875–881, DOI:10.1016/j.tetasy.2005.01.012.
- E. Deniau, D. Enders, A. Couture and P. Grandclaudon, A new synthetic route to highly enantioenriched 3-substituted-2,3-dihydro-1H-isoindol-1-ones, Tetrahedron: Asymmetry, 2003, 14, 2253–2258, DOI:10.1016/S0957-4166(03)00409-9.
- K. Kalpana, R. Syed, J. Saranya, M. Rafi and B. Ravi Kiran, Synthesis and theoretical study of novel imidazo[4,5-b]pyrazine-conjugated benzamides as potential anticancer agents, Russ. J. Org. Chem., 2021, 57, 1487–1494, DOI:10.1134/S1070428021090153.
- M. K. Rasheed, D. A. Al-rifaie and D. I. Madab, Synthesis and evaluation of antibacterial and antifungal activity of new 1,5-Benzodiazepine derivatives contain cyclic imides and Mannich bases, Int. J. Pharm. Res., 2021, 13, 2175, DOI:10.31838/ijpr/2021.13.01.348.
- H. H. Eissa, Synthesis of bisphthaldicarboximide via the reaction of phthalic anhydridewith diamines and evaluation of its biological activity, Int. J. ChemTech Res., 2014, 6, 95–101 Search PubMed.
- X.-T. Min, D.-W. Ji, H. Zheng, B.-Zh. Chen, Y.-Ch. Hu, B. Wan and Q.-A. Chen, Cobalt-catalyzed regioselective carboamidation of alkynes with imides enabled by cleavage of C−N and C−C bonds, Org. Lett., 2020, 22, 3386–3391, DOI:10.1021/acs.joc.9b00926.
- H. Chen, L. Ouyang, J. Liu, W.-J. Shi, G. Chen and L. Zheng, Synthesis of multisubstituted 1-naphthoic acids via Ru-catalyzed C−H activation and double-alkyne annulation under air, J. Org. Chem., 2019, 84, 12755–12763, DOI:10.1021/acs.joc.9b00926.
- G. Singh, A. Saroa, S. Girdhar, S. Rani, S. Sahoo and D. Choquesillo-Lazarte, Synthesis, characterization, electronic absorption and antimicrobial studies of N-(silatranylpropyl)phthalimide derived from phthalic anhydride, Inorg. Chim. Acta, 2015, 427, 232–239, DOI:10.1016/j.ica.2015.01.011.
- M. S. Malik, R. I. Alsantali, Q. M. S. Jamal, Z. S. Seddigi, M. Morad, M. A. Alsharif, E. M. Hussein, R. S. Jassas, M. M. Al-Rooqi, Z. Abduljaleel, A. O. Babalgith, H. M. Altass, Z. Moussa and S. A. Ahmed, New imidazole-based N-phenylbenzamide derivatives as potential anticancer agents: key computational insights, Front. Chem., 2022, 9, 808556, DOI:10.3389/fchem.2021.808556.
- S. Mingzhou, Zh. Lanlan, Ch. Miaomiao, H. Wangcheng, H. Xinwei and L. Hongjian, Synthesis of esterified/fused isocoumarins via Rh-catalyzed C-H activation/transannulative coupling/annulation of phthalic anhydrides with cyclic 2-diazo-1,3-diketones and methanol, Chin. J. Org. Chem., 2022, 42, 3816–3823, DOI:10.6023/cjoc202205039.
- H. R. Shaterian and M. Mohammadnia, Mild basic ionic liquids catalyzed new four-component synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones, J. Mol. Liq., 2012, 173, 55–61, DOI:10.1016/j.molliq.2012.06.007.
- H. R. Shaterian and M. Mohammadnia, Mild preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives with magnetic Fe3O4 nanoparticles coated by (3-aminopropyl)-triethoxysilane as catalyst under ambient and solvent-free conditions, Res. Chem. Intermed., 2014, 40, 371–383, DOI:10.1007/S11164-012-0969-Z/TABLES/3.
- J. Safaei-Ghomi, H. Shahbazi-Alavi, A. Ziarati, R. Teymuri and M. R. Saberi, A highly flexible green synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives with CuI nanoparticles as catalyst under solvent-free conditions, Chin. Chem. Lett., 2014, 25, 401–405, DOI:10.1016/j.cclet.2013.11.046.
- R. Ghorbani-Vaghei, J. Mahmoodi and Y. Maghbooli, Preparation and characterization of nanomagnetic piperidinium benzene-1,3-disulfonate ionic liquid as a novel, green and heterogeneous catalyst and its use in the synthesis of 1H–pyrazolo[1,2-b]phthalazine-5,10-diones and 1H–pyrazolo[1,2-a]pyridazine-5,8-diones under solvent-free conditions, Appl. Organomet. Chem., 2017, 31, e3717, DOI:10.1002/aoc.3717.
- F. Mohamadpour, M. Lashkari, R. Heydari and N. Hazeri, Four-component clean process for the eco-friendly synthesis of 1H-pyrazolo [1,2-b]phthalazine-5,10-dione derivatives using Zn(OAc)2.2H2O as an efficient catalyst under solvent-free conditions, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2018, 57, 843–851 Search PubMed.
- F. Mohamadpour, Carboxymethyl cellulose (CMC) as a recyclable green catalyst promoted eco-friendly protocol for the solvent-free synthesis of 1H-yrazolo[1,2-b]PHthalazine-5,10-dione derivatives, Polycyclic Aromat. Compd., 2022, 42, 1091–1102, DOI:10.1080/10406638.2020.1768412.
- F. Mohamadpour, M. T. Maghsoodlou, R. Heydari and M. Lashkari, Copper(II) acetate monohydrate: an efficient and ecofriendly catalyst for the one-pot multi-component synthesis of biologically active spiropyrans and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives under solvent-free conditions, Res. Chem. Intermed., 2016, 42, 7841–7853, DOI:10.1007/s11164-016-2565-0.
- M. Hosseinpoor Tehrani, S. A. Mirshokraie, M. Khoobi and M. Amini, Synthesis of pyrazolo[1,2-b]phthalazine-5,10-dione derivatives: a new class of α-glucosidase inhibitors, Pharm. Biomed. Res., 2021, 7, 121–132, DOI:10.18502/pbr.v7i2.7365.
- S. R. Kolsepatil, A. V. Sapkal, S. Chandole and D. L. Lingampalle, One pot synthesis of pyrazolo phthalazine dione derivatives under microwave irradiation, Rasayan J. Chem., 2019, 12, 415–420 CrossRef CAS.
- M. Abdesheikhi and Z. Karimi-Jaberi, Four-component synthesis of 3-amino-1-aryl-5,10-dioxo-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile derivatives promoted by potassium carbonate, J. Chem. Res., 2015, 39, 482–483, DOI:10.3184/174751915X14382558688543.
- M. Kalhor, Z. Orouji and M. Khalaj, 4-Methylpyridinium chloride ionic liquid grafted on Mn@zeolite-Y: design, fabrication and performance as a novel multi-functional nanocatalyst in the four-component synthesis of pyrazolophthalazine-diones, Microporous Mesoporous Mater., 2022, 329, 111498, DOI:10.1016/j.micromeso.2021.111498.
- N. Kerru, L. Gummidi, S. V. H. S. Bhaskaruni, S. N. Maddila and S. B. Jonnalagadda, One-pot green synthesis of novel 5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine derivatives with eco-friendly biodegradable eggshell powder as efficacious catalyst, Res. Chem. Intermed., 2020, 46, 3067–3083, DOI:10.1007/s11164-020-04135-6.
- H. N. Roy, M. Rana, A. Z. Al Munsur, K.-I. Lee and A. K. Sarker, Efficient and convenient synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives mediated by L-proline, Synth. Commun., 2016, 46, 1370–1376, DOI:10.1080/00397911.2016.1192650.
- K. Pradhan, S. Paul and A. R. Das, Synthesis of a diversified combinatorial library of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives applying sustainable carbon-based solid acid catalyst involving a domino four-component reaction, Monatsh. Chem., 2014, 145, 1343–1352, DOI:10.1007/s00706-014-1195-8.
- G. J. Raghavendra and V. Siddaiah, One-pot four component synthesis of 3-amino-1-(1H-indol-2-yl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine derivatives mediated by [DBUH][OAc], Russ. J. Gen. Chem., 2018, 88, 1892–1898, DOI:10.1134/S1070363218090219.
- Y. D. Reddy, B. S. Narayana, Ch. V. R. Reddy and P. K. Dubey, Four-component domino reaction for the synthesis of 1-(1H-indol-2-yl)-1H-pyrazolo[1,2-b]phthalazine-5,10-diones, Synth. Commun., 2014, 44, 3037–3046, DOI:10.1080/00397911.2014.928326.
- S. Keshipour, S. Shojaei and A. Shaabani, A novel one-pot isocyanide-based four-component reaction: synthesis of highly functionalized 1H-pyrazolo[1,2-b]phthalazine-1,2-dicarboxylates and 1H-pyrazolo[1,2-a]pyridazine-1,2-dicarboxylates, Tetrahedron, 2012, 68, 6141–6145, DOI:10.1016/j.tet.2012.05.078.
- S. Khandelwal, A. Rajawat and M. Kumar, An efficient and environmentally benign four-component domino protocol for synthesis of spirooxindoles spiroannulated with fused heterosystems of privileged heterocycles, Curr. Bioact. Compd., 2013, 9, 203–210, DOI:10.2174/15734072113099990014.
- A. Rajawat, S. Khandelwal and M. Kumar, Deep eutectic solvent promoted efficient and environmentally benign four-component domino protocol for synthesis of spirooxindoles, RSC Adv., 2014, 4, 5105, 10.1039/c3ra44600j.
- A. Biabangard and H. Reza Shaterian, Vitamin B1 supported on alumina as an efficient heterogeneous catalyst for synthesis of tetrahydro-2,6-dioxopyrimidin-4-yl-2,3-dihydrophthalazine-1,4-dione derivatives, J. Iran. Chem. Soc., 2015, 12, 1529–1534, DOI:10.1007/s13738-015-0623-y.
- M. Sadeghzadeh and M. A. Nasseri, Methylene dipyridine nanoparticles stabilized on Fe3O4 as catalysts for efficient, green, and one-pot synthesis of pyrazolophthalazinyl spirooxindoles, Catal. Today, 2013, 217, 80–85, DOI:10.1016/j.cattod.2013.07.018.
- B. Maleki and S. Sedigh Ashrafi, In Situ’ generated ‘HCl’: A highly efficient catalyst for one-pot synthesis of 1H-indazolo[1,2-b]phthalazine-1,6,11-triones and 1H-pyrazolo[1,2-b]phthalazine-5,10-diones under solvent-free conditions, J. Mex. Chem. Soc., 2014, 58, 159–167, DOI:10.29356/jmcs.v58i2.172.
- M. Esmaeilpour, J. Javidi, F. N. Dodeji and S. Zahmatkesh, Solvent-free, sonochemical, one-pot, four-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones and 1H-pyrazolo[1,2-b]phthalazine-diones catalyzed by Fe3O4@SiO2-imid-PMAn magnetic nanoparticles, Res. Chem. Intermed., 2021, 47, 2629–2652, DOI:10.1007/s11164-016-2462-6.
- J. Safaei-Ghomi, A. Hatami, H. Shahbazi-Alavi and A. Ziarati, CuFe2O4 and ZrP2O7 nanoparticles as highly efficient catalysts for the one-pot synthesis of phthalazine derivatives under solvent-free conditions, Sci. Iran., 2016, 23, 27052716, DOI:10.24200/sci.2016.3979.
- M. Esmaeilpour, J. Javidi and F. Dehghani, Preparation, characterization and catalytic activity of dendrimer-encapsulated phosphotungstic acid nanoparticles immobilized on nanosilica for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones under solvent-free or sonochemical conditions, J. Iran. Chem. Soc., 2016, 13, 695–714, DOI:10.1007/s13738-015-0782-x.
- H. R. Shaterian and F. Rigi, One-pot, four-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones catalyzed by cellulose-SO3H as a reusable heterogeneous and efficient catalyst, Res. Chem. Intermed., 2014, 40, 1989–1995, DOI:10.1007/s11164-013-1096-1.
- E. Mosaddegh and A. Hassankhani, A rapid, one-pot, four-component route to 2H-indazolo[2,1-b]phthalazine-triones, Tetrahedron Lett., 2011, 52, 488–490, DOI:10.1016/j.tetlet.2010.08.099.
- H. R. Shaterian and F. Rigi, Starch sulfate as an efficient and biodegradable polymer catalyst for one-pot, four-component reaction of 2H-indazolo[2,1-b]phthalazine-triones, Starch, 2011, 63, 340–346, DOI:10.1002/star.201000144.
- A. Hasaninejed, M. R. Kazerooni and A. Zare, Solvent-free, one-pot, four-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones using sulfuric acid-modified PEG-6000 as a green recyclable and biodegradable polymeric catalyst, Catal. Today, 2012, 196, 148–155, DOI:10.1016/J.CATTOD.2012.05.026.
- R. A. Sheldon, Catalysis: the key to waste minimization, J. Chem. Technol. Biotechnol., 1997, 68, 381–388, DOI:10.1002/(SICI)1097-4660(199704).
- H. R. Shaterian and M. Aghakhanizadeh, Four-component synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives, J. Org. Chem. Res., 2012, 15, 1060–1064, DOI:10.1016/j.crci.2012.09.005.
- M. Shekouhy and A. Hasaninejad, Ultrasound-promoted catalyst-free one-pot four component synthesis of 2H-indazolo[2,1-b]phthalazine-triones in neutral ionic liquid 1-butyl-3-methylimidazolium bromide, Ultrason. Sonochem., 2012, 19, 307–313, DOI:10.1016/j.ultsonch.2011.07.011.
- H. Veisi, A. Aminimanesh, N. Khankhani and R. Ghorbani-Vaghei, Protic ionic liquid [TMG][Ac] as an efficient, homogeneous and recyclable catalyst for one-pot four-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones and dihydro-1H-pyrano[2,3-c]pyrazol-6-ones, RSC Adv., 2014, 4, 25057–25062, 10.1039/C4RA03514C.
- D. Habibi and A. Shamsian, An efficient and recyclable bifunctional acid-base ionic liquid for synthesis of 1H-indazolo[1,2-b]phthalazinetriones, Res. Chem. Intermed., 2015, 41, 6245–6255, DOI:10.1007/s11164-014-1736-0.
- B. Afzalian, J. T. Mague, M. Mohamadi, S. Yousef Ebrahimipour, B. Pouramiri and E. T. Kermani, Ni(II), Co(II), and Cu(II) complexes incorporating 2-pyrazinecarboxylic acid: Synthesis, characterization, electrochemical evaluation, and catalytic activity for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones, Chin. J. Catal., 2015, 36, 1101–1108, DOI:10.1016/S1872-2067(14)60318-1.
- S. S. Choudhare, G. S. Khansole and V. N. Bhosale, Synthesis of phenyl 1H-indazolo[1,2-b]phthalazine trione derivatives with their biological evaluation, Indo Am. J. Pharm. Sci., 2017, 2017, 4272–4279, DOI:10.5281/zenodo.1095543.
- A. V. Chate, P. K. Bhadke, M. A. Khande, J. N. Sangshetti and C. H. Gill, β-Cyclodextrin as a supramolecular catalyst for the synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives in water and their antimicrobial activities, Chin. Chem. Lett., 2017, 28, 1577–1582, DOI:10.1016/j.cclet.2017.03.007.
- F. Khajoee Nejad, M. Khosravan, S. Y. Ebrahimipour and F. Bisceglie, A mixed-ligand quinazoline-based Ni(II) Schiff base complex: Synthesis, characterization, crystal structure, antimicrobial investigation and catalytic activity for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones, Appl. Organomet. Chem., 2018, 32, e3907, DOI:10.1002/aoc.3907.
- M. Mahmoodi Fard Chegeni, A. Bamoniri and B. B. F. Mirjalili, A versatile protocol for synthesis of 2H-indazolo[2,1-b]phthalazine triones using γ-Al2O3/BFn/Fe 3O4 as an efficient magnetic nano-catalyst, Polycyclic Aromat. Compd., 2022, 42, 391–399, DOI:10.1080/10406638.2020.1735457.
- Y. Han, Y. Gao, X. Cao, M. M. Zangeneh, S. Liu and J. Li, Ag NPs on chitosan-alginate coated magnetite for synthesis of indazolo[2,1-b]phthalazines and human lung protective effects against α-Guttiferin, Int. J. Biol. Macromol., 2020, 164, 2974–2986, DOI:10.1016/j.ijbiomac.2020.08.183.
- A. Naeimi and M. Zahedifar, Immobilized copper complex on biodegradable cellulose: catalytic application for the green synthesis of 2H-indazolo[1,2-b]phthalazine trione derivatives and synthesis of CuO nanoparticles, Iran. J. Chem. Chem. Eng., 2022, 40, 49–57, DOI:10.30492/IJCCE.2020.121610.3971.
- N. Amirmahani, N. O. Mahmoodi, M. Malakootian and A. Pardakhty, Introducing new and effective catalysts for the synthesis of pyridazino[1,2-a]indazole, indazolo[2,1-b]phthalazine and pyrazolo[1,2-b]phthalazine derivatives, MethodsX, 2020, 7, 100823, DOI:10.1016/j.mex.2020.100823.
- R. M. Tigote, K. P. Haval and S. K. Kazi, ZnFe2O4 nanoparticles: an efficient and reusable catalyst for 2H-indazolo[2,1-b]phthalazine-triones synthesis under solvent free condition, J. Med. Chem. Drug Discov., 2017, 2, 654–665 Search PubMed.
- N. Amirmahani, N. O. Mahmoodi, M. Malakootian and A. Pardakhty, [TBP]2SO4 ionic liquid catalyst for 4MCR of pyridazinoindazole, indazolophthalazine and pyrazolophthalazine derivatives, Mol. Diversity, 2022, 26, 15–25, DOI:10.1007/s11030-020-10153-8.
- O. S. Zaky, M. A. Selim, M. M. Ebied and K. U. Sadek, Glycerol: A promising benign solvent for catalyst-free one-pot multi-component synthesis of 1H-pyrozolo[1,2-b]phthalazine-5,10-diones and 2H-indazolo[2,1-b]phthalazine-triones under controlled microwaves irradiation, J. Heterocycl. Chem., 2019, 56, 2796–2803, DOI:10.1002/JHET.3659.
- B. Dam, M. Saha, R. Jamatia and A. K. Pal, Nano-ferrite supported glutathione as a reusable nanoorganocatalyst for the synthesis of phthalazine-trione and dione derivatives under solvent free condition, RSC Adv., 2016, 6, 54768–54776, 10.1039/C6RA06376D.
- Z. Zaheer, F. A. K. Khan, J. N. Sangshetti, R. H. Patil and K. S. Lohar, Novel amalgamation of phthalazine-quinolines as biofilm inhibitors: one-pot synthesis, biological evaluation and in silico ADME prediction with favorable metabolic fate, Bioorg. Med. Chem. Lett., 2016, 26, 1696–1703, DOI:10.1016/j.bmcl.2016.02.057.
- B. Pouramiri, R. G. Far and M. Zahedifar, Acidic ionic liquids: highly efficient catalysts for one-pot four-component synthesis of pyrazolo[1,2-b]phthalazines under solvent-free conditions, Chem. Heterocycl. Compd., 2018, 54, 1056–1060, DOI:10.1007/s10593-018-2391-y.
- P. Jahanshahi and M. Mamaghani, Chemodivergent, multicomponent-tandem facile synthesis of novel 1H-pyrazolo[1,2-b]phthalazine-5,10-dione using acetic acid functionalized imidazolium salt {[cmdmim]I} as a recyclable catalyst, New J. Chem., 2019, 43, 8266–8278, 10.1039/C9NJ00993K.
- P. Venkatesham, P. Shyam, Pooja, R. Chedupaka and R. R. Vedula, Facile one-pot multi-component synthesis, characterization, molecular docking studies, biological evaluation of 1,2,4-triazolo isoindoline-1,3-diones and Their DFT calculations, Polycyclic Aromat. Compd., 2023, 43(3), 2283–2301, DOI:10.1080/10406638.2022.2042333.
- H. Uchida, H. Yoshiyama, P. Y. Reddy, S. Nakamura and T. Toru, Novel synthesis of metal-free phthalocyanines from phthalimides and phthalic anhydrides with hexamethyldisilazan, Synlett, 2003, 2083–2085, DOI:10.1055/s-2003-41484.
- K. S. Jung, J. H. Kwon, S. M. Shon, J. P. Ko, J. S. Shin and S. S. Park, Microwave synthesis of metal phthalocyanines under solvent-free conditions, J. Mater. Sci., 2004, 39, 723–726, DOI:10.1023/B:JMSC.0000011541.91490.88.
- F. B. H. Ahmad, M. G. Moghaddam, M. Basri and M. B. Abdul Rahman, Enzymatic synthesis of betulinic acid ester as an anticancer agent: optimization study, Biocatal. Biotransform., 2010, 28, 192–200, DOI:10.3109/10242421003753795.
- M. Ghaffari M, F. B. H. Ahmad, M. Basri and M. B. Abdul Rahm, Lipase-catalyzed esterification of betulinic acid using phthalic anhydride in organic solvent media: study of reaction parameters, J. Appl. Sci., 2010, 337–342, DOI:10.3923/jas.2010.337.342.
- P. K. Dubey, S. M. Ghouse Mohiuddin and D. Ramesh, Reactions of phthalic anhydride with alcohols, Asian J. Chem., 1997, 9, 379–387 CAS.
- R. Fareghi-Alamdari, M. Nadiri Niri and H. Hazarkhani, Synthesis and characterization of a new hydroxyl functionalized diacidic ionic liquid as catalyst for the preparation of diester plasticizers, J. Mol. Liq., 2017, 227, 153–160, DOI:10.1016/j.molliq.2016.11.121.
- N. Zekri, R. Fareghi-Alamdari and Z. Khodarahmi, Functionalized dicationic ionic liquids: Green and efficient alternatives for catalysts in phthalate plasticizers preparation, J. Chem. Sci., 2016, 128, 1277–1284, DOI:10.1007/s12039-016-1127-8.
- G. Wypych, Handbook of Plasticizers, ChemTec Publishing, New York, 2004, ch. 2, pp 36–43 Search PubMed.
- R. Fareghi-Alamdari, M. Nadiri Niri, H. Hazarkhani and N. Zekri, Diacidic ionic liquid supported on magnetic-silica nanocomposite: a novel, stable, and reusable catalyst for selective diester production, J. Iran. Chem. Soc., 2018, 15, 2615–2629, DOI:10.1007/S13738-018-1450-8.
- Z. Shahedi and Y. Mansoori, Fe3O4@SiO2-SO3H nanoparticles: An efficient magnetically retrievable catalyst for esterification reactions, J. Part. Sci. Technol., 2018, 4, 67–79, DOI:10.22104/jpst.2018.2811.1117.
- M. Kulawska, H. Moroz and A. Kasprzyk, Kinetic investigations on the esterification of phthalic anhydride with N-heptyl, N-nonyl or N-undecyl alcohol over sulfuric acid catalyst, React. Kinet., Mech. Catal., 2011, 104, 9–15, DOI:10.1007/s11144-011-0337-9.
- M. Arabi, M. Mohammadpour Amini, M. Abedini, A. Nemati and M. Alizadeh, Esterification of phthalic anhydride with 1-butanol and 2-ethylhexanol catalyzed by heteropolyacids, J. Mol. Catal. A: Chem., 2003, 200, 105–110 CrossRef CAS.
- S. R. Bhutada and V. G. Pangarkar, Esterification of phthalic anhydride with 2-ethylhexanol, J. Chem. Technol. Biotechnol., 1986, 36, 61–66, DOI:10.1002/jctb.280360204.
- S. Gharibe, ZnAl2O4/SiO2 as an efficient nanocatalyst for esterification of phthalic anhydride by 2-ethylhexanol, Iran. J. Sci. Technol., Trans. A: Sci., 2020, 44, 1349–1355, DOI:10.1007/s40995-020-00899-z.
- J. B. Ellington, K. M. Park and J. F. Brennecke, Effect of local composition enhancements on the esterification of phthalic anhydride with methanol in supercritical carbon dioxide, Ind. Eng. Chem. Res., 1994, 33(4), 965–974, DOI:10.1021/ie00028a026.
- M. Khodadadi Moghaddam and M. R. Gholami, Preparation of Titania based solid strong acid and study of its catalytic activity in esterification of phthalic anhydride and sebacic acid, Mater. Lett., 2006, 60, 715–719, DOI:10.1016/j.matlet.2005.09.069.
- T. S. Thorat, V. M. Yadav and G. D. Yadav, Esterification of phthalic anhydride with 2-ethylhexanol by solid superacidic catalysts, Appl. Catal., 1992, 90, 73–96, DOI:10.1016/0926-860X(92)85050-L.
- G. B. Bajracharya, R. Koju, S. Ojha, S. Nayak, S. Subedi and H. Sasai, Plasticizers: synthesis of phthalate esters via FeCl3-catalyzed nucleophilic addition of alcohols to phthalic anhydride, Results Chem., 2021, 3, 100190, DOI:10.1016/j.rechem.2021.100190.
- N. A. Rakhimova and S. V. Kudashev, Features of reactions of phthalic anhydride with polyfluorinated alcohols, Russ. J. Gen. Chem., 2011, 81, 1529–1534 CrossRef CAS.
- M. Karimi Alavijeh and M. M. Amini, Synthesis and characterization of butylamine-functionalized Cr(III)–MOF–SO3H: synergistic effect of the hydrophobic moiety on Cr(III)–MOF–SO3H in esterification reactions, Polyhedron, 2019, 173, 114142, DOI:10.1016/j.poly.2019.114142.
- L. F. Fieser, The condensation of β-naphthol with phthalic anhydride, J. Am. Chem. Soc., 1931, 53, 3546–3560, DOI:10.1021/ja01360a051.
- S.-L. Deng, I. Baglin, M. Nour and Ch. Cavé, Synthesis of phosphonodipeptide conjugates of ursolic acid and their homologs, Heteroat. Chem., 2008, 19, 55–65, DOI:10.1002/hc.20396.
- A. Mohammadi-Farani, S. Soltani Darbandi and A. Aliabadi, Synthesis and acetylcholinesterase inhibitory evaluation of 4-(1,3-dioxoisoindolin-2-yl)-N-phenyl benzamide derivatives as potential anti-alzheimer agents, I, Iran. J. Pharm. Sci., 2016, 15, 313–320, DOI:10.22037/ijpr.2016.1868.
- D.-Y. Kim, S.-A. Lee, H. Kim, S. M. Kim, N. Kim and K.-U. Jeong, An azobenzene-based photochromic liquid crystalline amphiphile for the remote-controllable light shutter, Chem. Commun., 2015, 51, 11080–11083, 10.1039/C5CC02834E.
- T. Iwanaga, N. Asano, H. Yamada and S. Toyota, Synthesis and photophysical properties of dinaphtho[2,3-b:2′,3′-i]dihydrophenazine derivatives, Tetrahedron Lett., 2019, 60, 1113–1116, DOI:10.1016/j.tetlet.2019.03.035.
- M. A. Mahmoud, A. Y. Abed and S. M. D. Al-Nuzal, Synthesis and anticancer evaluation of new heterocyclic compounds in cell line L20B in vitro, J. Pharm. Sci. Res., 2019, 11, 901–904 CAS.
- A. Kajal, S. Bala, S. Kamboj and V. Saini, Synthesis, characterization, and computational studies on phthalic anhydride-based benzylidene-hydrazide derivatives as novel, potential anti-inflammatory agents, Med. Chem. Res., 2014, 23, 2676–2689, DOI:10.1007/s00044-013-0848-1.
| This journal is © The Royal Society of Chemistry 2023 |