Open Access Article
Open Access ArticleNew insights into nanosystems for non-small-cell lung cancer: diagnosis and treatment
Piao Jiang†
ab,
Bin Liang†a,
Zhen Zhang†c,
Bing Fand,
Lin Zenga,
Zhiyong Zhoua,
Zhifang Maoa,
Quan Xu*e,
Weirong Yao*a and
Qinglin Shen *ac
*ac
aDepartment of Oncology, Jiangxi Provincial People's Hospital, The First Affiliated Hospital of Nanchang Medical College, No. 152 Aiguo Road, Donghu District, Nanchang 330006, China. E-mail: qinglinshen@whu.edu.cn
bThe First Clinical Medical College, Nanchang University, Nanchang, China
cInstitute of Clinical Medicine, Jiangxi Provincial People's Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
dDepartment of Radiology, Jiangxi Provincial People's Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
eDepartment of Thoracic Surgery, Jiangxi Provincial People's Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
First published on 28th June 2023
Abstract
Lung cancer is caused by a malignant tumor that shows the fastest growth in both incidence and mortality and is also the greatest threat to human health and life. At present, both in terms of incidence and mortality, lung cancer is the first in male malignant tumors, and the second in female malignant tumors. In the past two decades, research and development of antitumor drugs worldwide have been booming, and a large number of innovative drugs have entered clinical trials and practice. In the era of precision medicine, the concept and strategy of cancer from diagnosis to treatment are experiencing unprecedented changes. The ability of tumor diagnosis and treatment has rapidly improved, the discovery rate and cure rate of early tumors have greatly improved, and the overall survival of patients has benefited significantly, with a tendency to transform to a chronic disease with tumor. The emergence of nanotechnology brings new horizons for tumor diagnosis and treatment. Nanomaterials with good biocompatibility have played an important role in tumor imaging, diagnosis, drug delivery, controlled drug release, etc. This article mainly reviews the advancements in lipid-based nanosystems, polymer-based nanosystems, and inorganic nanosystems in the diagnosis and treatment of non-small-cell lung cancer (NSCLC).
1. Introduction
Lung cancer, a major cause of cancer-related mortality worldwide, can be categorized into small cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC), with the latter accounting for approximately 80–85% of all cases.1,2 Traditionally, NSCLC is diagnosed based on patients' medical history, signs, and symptoms, using imaging techniques such as X-ray, CT, MRI, fine needle aspiration biopsy of lung tissue, and thoracocentesis.3,4 However, patients developing NSCLC are often detected as late as at stage III or IV, attributed to the absence of clinical symptoms in incipient stages, thus setting requirements for the early diagnosis of NSCLC.The treatments of NSCLC are primarily composed of chemotherapy, radiotherapy, surgery, and emerging technologies such as targeted therapy, immunotherapy, photothermal therapy, and photodynamic therapy (PDT).5–7 Despite some progress in the treatment of NSCLC, the five-year survival rate has only increased by 5% in recent years partially due to the lack of tumor targeting.8 Accordingly, novel therapy with increased anti-tumor effect and reduced systemic cytotoxicity against NSCLC is urgently demanded.
Notably, natural or acquired resistance is one of the major challenges related to NSCLC treatment. Generally, resistance to chemotherapy is categorized into three types: kinetic, biochemical, and pharmacologic. Cell kinetics-related resistance is a special problem with many tumors because certain cells are in a plateau growth phase with a small growth fraction. Methods to overcome cell kinetics-related resistance involve: diminishing the volume of the tumors via surgery or radiotherapy; impacting G0 cells via combination drugs; and scheduling drugs to avoid phase escape or to synchronize cell groups and enhance cancer cell removal. The mechanism of biochemical resistance is not fully understood. Cancer cells undergoing this kind of resistance may show declined drug uptake, enhanced efflux, altered levels or structure of the intracellular target, downregulated intracellular activation, augmented drug inactivation, or accelerated repair of damaged DNA. Another example is multidrug resistance (MDR), a complex phenotype whose predominant feature is resistance to a wide range of structurally unrelated cytotoxic compounds. MDR is associated with a variety of mechanisms, including enhanced efflux of drugs, genetic factors (gene mutations, amplifications, and epigenetic alterations), growth factors, increased DNA repair capacity, and elevated metabolism of xenobiotics. As for pharmaceutical resistance, it can be caused by poor tumor blood supply, poor or erratic absorption, enhanced excretion or catabolism, and drug interactions, all of which result in insufficient drug content in the blood.9,10
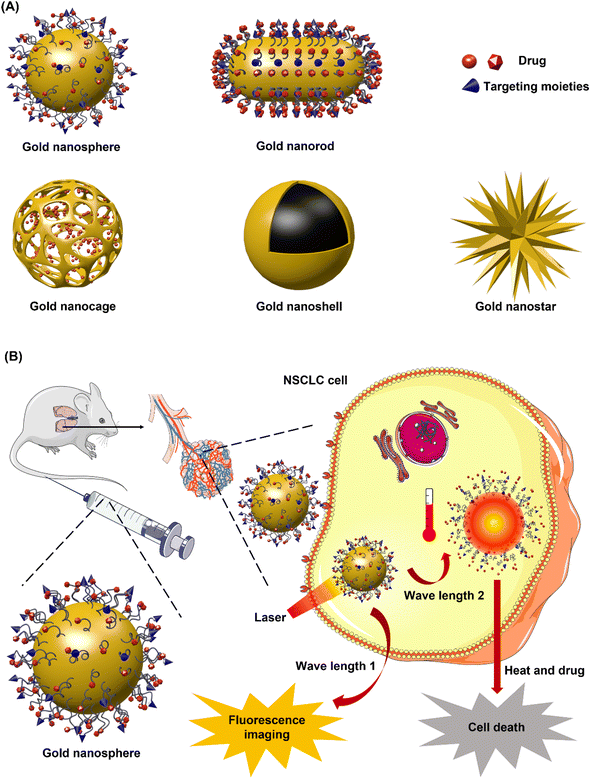
To overcome these limitations, it is paramount to develop innovative diagnosis and treatment technology targeted NSCLC. Nanomedicine, emerging as a propitious paradigm in cancer detection and therapy, has attracted more and more attention in the field of anti-NSCLC, because nanosystems offer flexible and fast drug design and production on the basis of tumor genetic profiles, leading to much more rational and effective drug selection for personalized patient treatment. The diagnosis or therapeutic efficacy of nanosystems can be manipulated by regulating their structure, geometry, materials, and surface chemistry.11–13 Aside from utilizing biosensor devices-based nanoparticle-like gold nanorods that possess the property of imaging triggered by near-infrared light (NIR) irradiation,14–16 another approach for theranostic nanomedicine is to conjugate or encapsulate both therapeutic and imaging agents to the designed nanoparticles.
Given the critical role of nanomedicine in the theranostics of NSCLC, this article aims to review the recent advance and progress of nanosystems in the early detection and improved anti-tumor effect against NSCLC. This review elucidates a wide variety of nanosystems including lipid-based nanoparticles, polymer-based nanoparticles, and inorganic nanosystems. We focus on the application of these nanosystems in the diagnosis and therapy of NSCLC and summarize recent advances in all kinds of nanosystems, which are illustrated by examples. Finally, we provide our perspective on the challenges and potential opportunities of nanosystems applied in the theranostics of NSCLC in the future.
2. Common characteristics of nanosystems
2.1. Advantages of nanosystems
The pH in the tumor tissues (pH of 5.4) and late endosomes and lysosomes (pH of 4.5–5.5) is lower than that in the healthy tissues and blood circulation (pH of 7.4). The acidic microenvironment in the tumor may lead to partial protonation of the amino, carboxylate, and phosphate groups of the nanosystems, and/or the cleavage of certain chemical bonds, which are unstable under acidic conditions such as hydrazide bonds. This impairs the interaction between nanosystems and guest molecules, thereby resulting in the selective release of cargo into tumors.22
Light-triggered theranostics has gained increasing attention owing to its high spatiotemporal precision, real-time dose control, wide clinical application in tumor imaging, photosensitizers (PSs)-based cancer killing through local hyperthermia, ROS or 1O2, and the option for on-demand switching on and off modes. However, the major issue of limited tissue penetration heavily restricts the application against tumors located deep in the tissues. The NIR region (650 to 900 nm) is considered the ‘biological window’ because most of the body components such as blood and soft tissues do not absorb or scatter light in this wavelength region, providing access to non-invasive and deep tissue penetration for imaging and therapeutic goals. Heat and 1O2 production are the most crucial effectors in light-triggered treatment. Hyperthermia (HT) caused by PS-induced heat generation leads to cell death, which is referred to as photothermal therapy (PTT). Increasing the temperature of the tumor microenvironment to 42 °C can cause cell damage and make cancer cells vulnerable to combined treatments such as irradiation and chemotherapy. When the temperature increases to 45 °C or above, it can have immediate lethal effects on the cells. On the other hand, 1O2 is responsible for causing irreversible damage to intracellular organelles, also known as photodynamic therapy (PDT). Notably, the extremely short lifespan (<3.5 μs) and highly restricted diffusion (∼10 to 20 nm) of 1O2 can be used for localized apoptosis, necrosis, or autophagy-induced cell death.23–25 Besides, light-based imaging techniques such as fluorescence, photoacoustic (PA) signal, and surface-enhanced Raman scattering (SERS) are useful in live bioimaging, namely optical imaging.26
Magnetic field (MF) is a widely applied physical trigger of magnetic nanosystems that can produce heat under an oscillating MF, or be magnetically directed to lesion sites. Also, MF is widely utilized for magnetic resonance imaging (MRI) of tumor tissue. Unlike optical imaging, MF at frequencies below 400 Hz, is not readily absorbed by tissues, allowing remote monitoring without physical contact.27
Ultrasound (US) is another non-invasive stimulus utilized to trigger site-specific drug release and allows for spatiotemporal control with millimeter precision. A focused US beam can lead to drug release through localized heating produced by the accumulation of acoustic energy at the focused region.28 The US imaging contrast agents (UCAs) are widely used in clinical examinations in order to improve the image resolution of the US. UCAs are acoustically active to external US energy, thus amplifying echo signals and improving image resolution based on differential echogenicity. The larger acoustic impedance difference between the solid phase and soft tissues indicates that compared with softer UCAs, inorganic NPs with rigid structures can yield better US image resolution.29
Recently, Liang et al. reported the development of novel stealth and matrix metalloproteinase 2 (MMP2)-activated biomimetic nanosystems against NSCLC, which are constructed using MMP2-responsive peptides to bind miR-126-3p (known as MAIN), and further camouflaged with RBCM (thus named REMAIN). Liang et al. demonstrated that REMAIN could effectively transduce miRNA into NSCLC cells and release the cargo through MMP2 responsiveness. The advantages of the natural RBCM mentioned above, such as prolonged circulation, cell-specific targeting, and immune escape, were observed in REMAIN. Furthermore, in vitro and in vivo results showed that REMAIN effectively induced apoptosis of NSCLC cells and inhibited NSCLC progression by targeting ADAM9.31 Interestingly, Zhang et al.32 reported an integrated hybrid nanosystem named Clip-PC@CO-LC NPs to specifically target NSCLC, which fused cancer cell membranes (Cm) and matrix metallopeptidase 9 (MMP-9)-switchable peptide-based charge-reversal liposome membranes (Lipm) to coat lipoic acid-modified polypeptides (LC) co-loaded with phosphoglycerate mutase 1 (PGAM1) siRNA (siPGAM1) and DTX. This nanosystem exhibited a negatively charged coating (citraconic anhydride-grafted poly-L-lysine, PC) in the middle layer for pH-triggered charge conversion functionalization. The use of Cm endowed the final nanosystems with homologous targeting ability, elevated the efficiency of drug delivery to target cells, and boosted the therapeutic effect. Furthermore, in contrast to mono-membrane coating, liposome membranes are more easily modified and can be integrated into a single biomimetic platform to attain various functions for the precise treatment of NSCLC.
2.2. Routes of administration
Given the pivotal role of administration routes in the site-specific accumulation of drugs delivered by nanoparticles, which determines the therapeutic efficacy of NSCLC, great efforts have been devoted to the research to seek the best approach and routes to achieve the anticipated outcomes.33–35 In general, NSCLC-targeted transportation can be implemented through four methods containing inhalation, intravenous (IV), oral, and parenteral (subcutaneous, intraperitoneal, etc.) administration, with the first two being preferred.36 Here, we list recent applications of these administration routes employed in NSCLC using nanoparticles, as shown in Table 1.| Administration routes | Nanoparticles | Results | Year and ref. |
|---|---|---|---|
| Intravenous administration | Radioactive 153Sm encapsulated multi-walled carbon nanotubes (153SmCl3@MWCNTs-NH2) | 153SmCl3@MWCNTs-NH2 showed improved water dispersibility for intravenous administration, high accumulation in site-specific NSCLC tissues, and no leakage of the encapsulated radioactive material for targeted radiotherapy | 2021 (ref. 37) |
| Mesenchymal stem cell membranes (MSCs)-engineered Fe(III) and cypate-loaded PMAA nanomedicines (Cyp-PMAA-Fe@MSCs) | Cyp-PMAA-Fe@MSCs after intravenous administration were identified 21% higher fluorescence signal and 30% lower T1-weighted MRI signal than Cyp-PMAA-Fe@RBCs, indicating promising application for bioimaging-guided photothermal-enhanced radiotherapy against NSCLC | 2021 (ref. 38) | |
| Inhalation | Anti-tumor siRNA-entrapped nanoparticles | Compared with intravenous administration, inhalation administration of the siRNA-loaded nanoparticles showed stronger fluorescent signals in the lung tumor tissues with little systemic toxicity | 2019 (ref. 39) |
| Silibinin-loaded poly caprolactone/pluronic F68 inhalable nanoparticles (SB-loaded PCL/Pluronic F68 NPs) | Inhalable SB-loaded PCL/Pluronic F68 NPs demonstrated prolonged circulation time, sustained drug release, and excellent NSCLC (A549 cells)-targeting capacity | 2022 (ref. 40) | |
| Oral administration | Topotecan-loaded PLGA nanoparticles | Increased bioavailability and prolonged retention of topotecan were found in target organs such as the lung | 2021 (ref. 41) |
| Intraperitoneal administration | Hyaluronic acid-based microRNA-125b encapsulated nanoparticles | Successful repolarization of tumor-associated macrophages was identified in KRAS/p53 double mutant genetically engineered (KP-GEM) NSCLC mice, remarkably improving the efficacy of anticancer immunotherapy | 2018 (ref. 42) |
2.3. Mechanism of targeting
The targeting mechanism of nanoparticles can be classified as passive and active targeting. Passive targeting of nanoparticles is principally dependent on the enhanced permeability and retention (EPR) effect supported by the permeable blood vessels in tumors in contrast to the normal capillaries. The enhanced permeability allows macromolecules to escape circulation due to the inherent leakiness of the underdeveloped tumor vasculature. Besides, the lack of an efficient lymphatic system results in the retention of those macromolecules in the tumor bed. Generally, to capitalize on the EPR effect a drug carrier must be in a narrow size range from about 10 nm to 100 nm. Entities smaller than 10 nm are rapidly cleared by the kidneys or through extravasation and larger entities (∼100–200 nm) are cleared by the reticuloendothelial system.20,43 Therefore, nanosystems with suitable size (∼10–100 nm) are allowed to extravasate in the tumor tissues, thus achieving NSCLC-targeted drug delivery.Active targeting is realized through the functionalization of the surface of nanoparticles. Peculiar ligands-conjugated nanoparticles permit a selective recognition of distinguished antigens overexpressed in the NSCLC cell surfaces, improving the therapeutic efficacy of nanoparticles. Based on the category of ligands, active targeting can be divided into various groups including albumin, hyaluronic acid, folate, transferrin, aptamer, monoclonal antibodies, and peptide fragment-based targeting, as shown in Table 2.44
| Conjugated ligands | Targeted receptors | Nanoparticles | Results | Year and ref. |
|---|---|---|---|---|
| Albumin | Albumin-binding protein BM-40 | Albumin liposomes loaded with PD-L1 and siRNA | Albumin liposomes showed efficient accumulation in lung tumor cells | 2022 (ref. 45) |
| Hyaluronic acid | Glycoprotein CD44 | CD44 targeting hyaluronic acid-conjugated nanoparticles entrapping microRNA-125 | Hyaluronic acid-conjugated nanoparticles exert their strength in site-specific accumulation in mouse models mimicking NSCLC compared to non-targeted nanoparticles or the free drug | 2018 (ref. 42) |
| Folate | Folate receptors | Paclitaxel-loaded folic acid-modified PLGA nanoparticle with glutathione | Due to the high affinity of folate to the receptors, enhanced intracellular uptake of the particles by NSCLC cells was observed, boosting the cytotoxicity of the free paclitaxel or the paclitaxel-entrapped particles without the targeting ligand | 2021 (ref. 46) |
| Transferrin | Transferrin receptors | Transferrin-conjugated protein-lipid hybrid nanoparticles delivering cisplatin and docetaxel (Tf-CIS/DTX-PLHN) | Tf-CIS/DTX-PLHN showed prolonged circulation time, increased drug accumulation, and improved antitumor activity in A549 cells-injected mice compared to non-targeted CIS/DTX-PLHN or free CIS/DTX | 2021 (ref. 47) |
| Aptamer | Complementary DNA or RNA | Aptamers-modified fluorescent superparamagnetic microparticles were paired with the complementary DNA conjugated Au nanoparticles, generating fluorescence resonance energy transfer (FRET) magnetic aptamer-based targeting sensors named Fe3O4@Au | Fe3O4@Au, using aptamers as targeting ligands specifically binding to CD63, exhibited efficient capture of NSCLC exosomes, which reached as high as 91.5% with a dosage of 200 μL | 2021 (ref. 48) |
| Monoclonal antibodies | Protein biomarkers (EGFR, HER2, etc.) | EGFR antibody conjugated gold nanorods stimulated by pulsed lasers | In vitro experiments with NSCLC cells (A549), EGFR antibody modified nanorods exhibited enhanced attenuation of 93% ± 13% in the cell viability compared with untargeted nanorods | 2020 (ref. 49) |
| Peptide fragments (e.g. arginine-glycine-aspartic acid (RGD)) | Integrin αv β3 as receptors of RGD | Integrin αvβ3-targeted RGD-modified liposomes delivering doxorubicin (DOX) | RGD-modified liposomes exhibited increased cellular uptake and resultant targeted delivery of DOX, leading to enhanced suppression of tumor growth in mice and less toxicity compared with non-RGD functionalized liposomes | 2021 (ref. 50) |
On the other hand, according to the targeted goal of NSCLC cells, active targeting can also be classified as tumor microenvironment-targeted, NSCLC biomarkers-targeted, organelles-targeted, and genes or epigenetics-targeted strategies. Extensive applications of these four kinds of targeting strategies are shown in Table 3.
| Targeting strategies | Nanoparticles | Results | Year and ref. |
|---|---|---|---|
| Tumor microenvironment-targeted | Hypoxia-responsive nitro-benzyl conjugated chitosan nanoparticles (HRCNs) | HRCNs rapidly determine the hypoxic status of lung cancer cells (A549) within 30 min compared to the conventional method needing several hours, indicating a potential role in the diagnosis and treatment of NSCLC. | 2016 (ref. 51) |
| GSH-responsive and pH-responsive cisplatin prodrug and paclitaxel co-loaded nanoparticles (DDP-P/PTX NPs) | High tumor accumulation, pH-triggered drug release, low systemic toxicity, and remarkable antitumor effects were observed by DDP-P/PTX NPs in NSCLC-bearing mice | 2021 (ref. 52) | |
| Biomarkers -targeted | CD44-targeted, indocyanine green-paclitaxel-loaded albumin nanoparticles | CD44-targeted NPs achieved image-guided drug delivery, exhibiting selective accumulation in CD44-positive NSCLC compared to the normal CD44-negative lung fibroblast cell line (MRC-5) | 2022 (ref. 53) |
| EGFR-targeted nanoparticles encapsulated erlotinib and quercetin (EQNPs) | In vivo experiments showed EQNPs promoted the uptake of nanoparticles in the cancer cells of resistant NSCLC-bearing mice compared to non-targeted NPs. Furthermore, downregulation of nuclear EGFR, improved uptake of Ertb and Quer and induced apoptosis were demonstrated in resistant A549 cells | 2022 (ref. 54) | |
| Organelles-targeted | Mitochondria-targeted nonyl acridine orange (NAO)-loaded gold nanorods (NAO-AuNRs) | NAO-AuNRs conjugated with monoclonal antibody Cetuximab have demonstrated their ability in increasing the uptake of the membrane mitochondria-targeted nanomedicine compared to non-targeted ones in NSCLC cells, accompanied by reduced progression of EGFR-positive NSCLC | 2022 (ref. 55) |
| Genes or epigenetics-targeted | βIII-tubulin and polo-like kinase 1 (PLK1)-targeted star polymer-siRNA nanoparticles | The star-siRNA nanoparticles were found accumulated in mouse lung tumors mimicking NSCLC, leading to the silencing of the expression of βIII-tubulin and PLK1 as well as inhibited tumor growth | 2022 (ref. 56) |
| PEG-PLA hybrid nanocarriers (HNCs) entrapped with cisplatin caprylate and ABCC3-siRNA | ABCC3-siRNA was utilized to specifically target and silence ABCC3 mRNA. Compared with cisplatin solution and cisplatin-loaded HNCs without ABCC3-siRNA, in vitro cellular uptake level was increased and cell viability of A549 cells was significantly reduced in the co-loaded nanocarriers, along with a higher rate of cell arrest in G2-M phase | 2021 (ref. 57) |
3. Overview of nanosystems
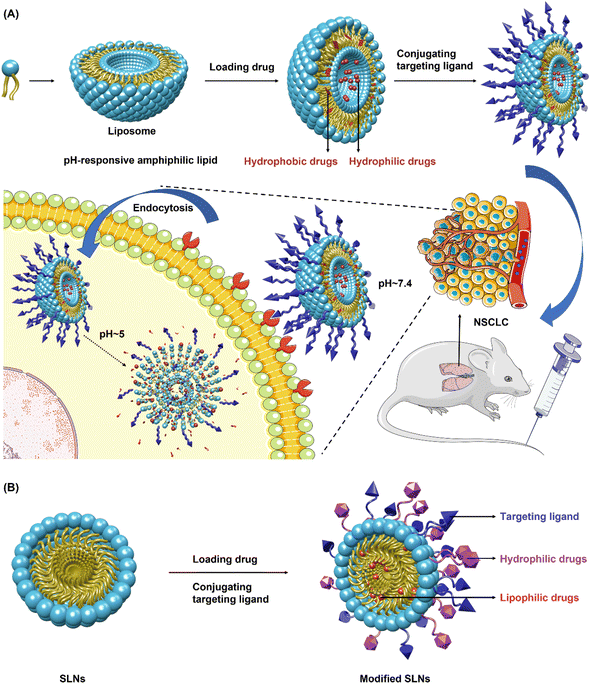
3.1. Lipid-based nanosystems
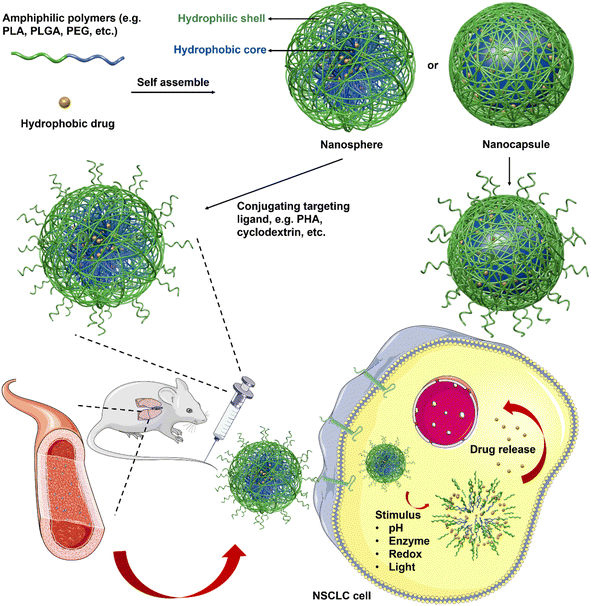
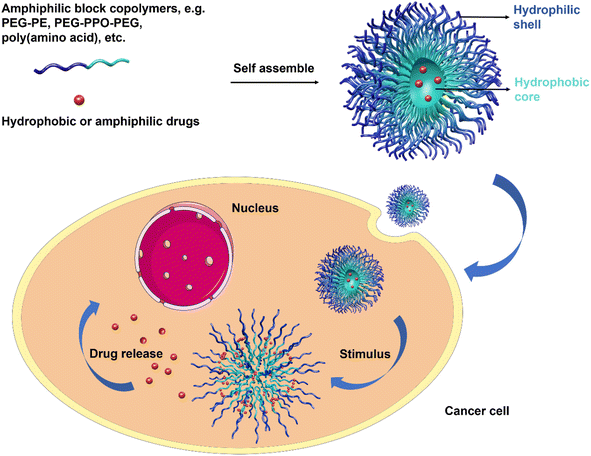
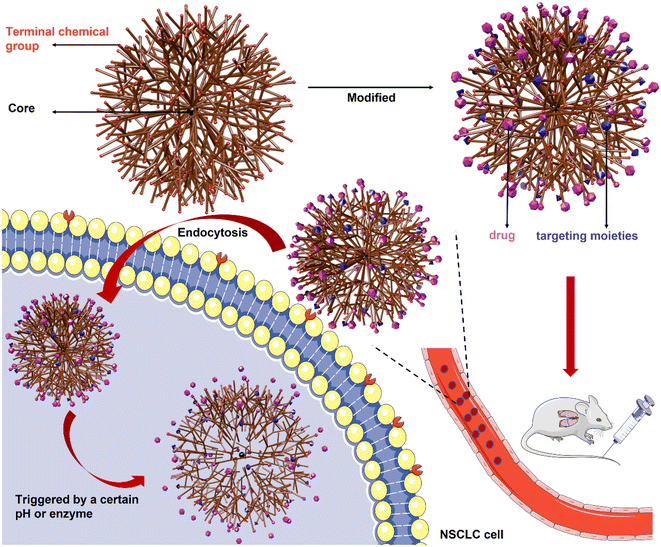
3.2. Polymer-based nanosystems
3.3. Inorganic nanosystems
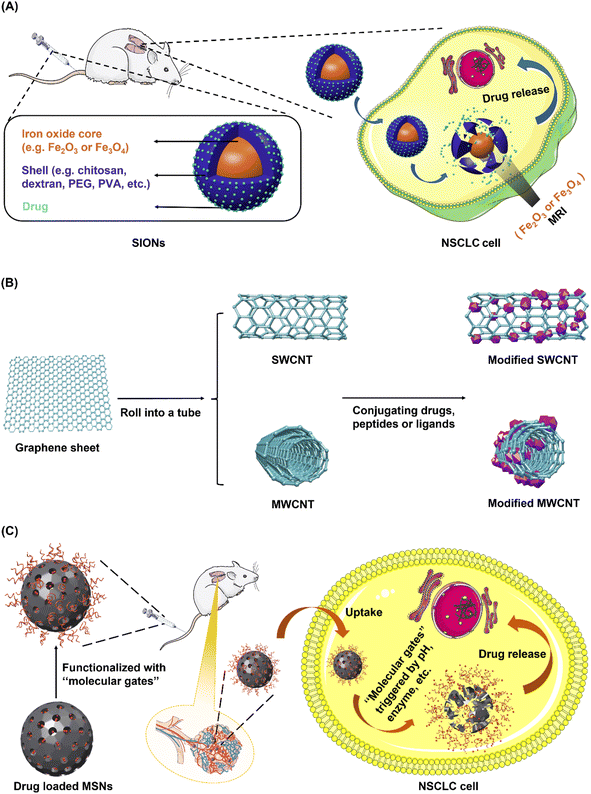
Generally, inorganic nanosystems can be divided into two groups consisting of metallic nanosystems including gold and silver, and non-metallic nanosystems including iron oxides, carbon nanotubes, silica, selenium (Se), metallic chalcogenide and metallic oxides.82,83CNTs have a cylinder-structure third allotrophic form of carbon fullerene consisting of graphene sheets that roll up into an open or capped cylinder.92–94 Generally, CNTs can be divided into two forms: single-walled carbon nanotube (SWCNT) and multi-walled carbon nanotube (MWCNT) (Fig. 6B). SWCNT, which gains more flexibility attributed to van der Waals forces, is constituted by a single sheet of graphene rolled up to form a tube, while MWCNT comprised of several graphene sheets surrounding one layer of graphene rolled into a tube.95 Owing to their unique characteristics such as nano-needle shape, hollow monolithic structure, high surface area, extremely light weight, and surface functionalization, CNTs are growing in popularity as one of the most promising nanocarriers.96 Moreover, CNTs enter cells using “needle-like penetration” and deliver molecules into the cytoplasm, thus further improving the efficiency of drug accumulation.9
4. Nanosystems for NSCLC diagnosis
4.1. Lipid-based nanosysytems
Lipid-based nanosystems have been successfully used for NSCLC diagnosis. Badea et al.102 investigated the utility of a liposomal-iodinated nanoparticle contrast agent and CT imaging for the characterization of primary nodules in genetically engineered mouse models of NSCLC. The resultant nanosystem enabled visualization of blood supply to the nodules during the early-phase imaging. Delayed-phase imaging enabled the characterization of slow-growing and rapidly-growing nodules based on signal enhancement. This agent could facilitate the early detection and diagnosis of pulmonary lesions as well as have implications on the treatment response and monitoring.Exon 2 deletion in aminoacyl tRNA synthetase complex-interacting multifunctional protein 2 (named AIMP2-DX2) has been suggested to be associated with the progression of various cancers such as lung cancer. Jo et al.103 demonstrated the rapid and simple detection of the AIMP2-DX2 mutation by molecular beacons and its relation to lung cancer. Real-time PCR with molecular beacons allowed sensitive detection of the AIMP2-DX2 mutation as low as 0.3 pg initial template. Dual-conjugated liposomes with folate and molecular beacons enabled fluorescence imaging of cancer cells harboring the AIMP2-DX2 mutation with high resolution. The association of the AIMP2-DX2 mutation with lung cancer was shown by analyzing tissue samples from lung cancer patients using real-time PCR. Approximately, 60% of lung cancer patients harbored the AIMP2-DX2 mutation, which implies a potential of the AIMP2-DX2 mutation as a prognostic biomarker for lung cancer. This study indicated that molecular beacon-based liposomes had great potential in the simple and rapid detection of mutations on nucleotides for diagnosing and monitoring the progression of relevant cancers.
4.2. Polymer-based nanosystems
Macromolecular contrast agents were first investigated for MR angiography (MRA) considering that the conventional low molecular weight gadolinium (Gd)(III) agents fail to offer vessel opacification times sufficient to allow MR imaging. Compared with conventional agents that exhibit rapid equilibration with the extravascular extracellular space (EES) and are rapidly cleared by renal filtration, macromolecular agents exhibit extended vascular retention times, a critical property for MR imaging.104Among these macromolecular agents, dendrimers are promising nanosystems used for NSCLC imaging. Two types of dendrimers have been widely explored as imaging agents, one type within the poly(propyleneime) (PPI) dendrimer series, composed of a 1,4-diaminobutane (DAB) core, the other of the poly(amidoamine) (PAMAM) dendrimer series composed of a 1,2-diaminoethane core. The defined structures and availability of the surface amino groups of dendrimers enable the development of dendrimer-conjugates with various chelates for the application as MR contrast agents.105,106 Zhu et al.107 built Lox-Stop-lox K-ras G12D transgenic mice imitating lung cancer. It was found that miR-155 and somatostatin receptor 2 (SSTR2) were expressed in all the disease stages of transgenic mice. The authors synthesized octreotide-conjugated chitosan-molecular beacon nanoparticles (CS-MB-OCT) that can specifically bind to SSTR2 expressed by the lung cancer cells to achieve the goal of identification of lung cancer cells and imaging miR-155 in vivo and in vitro. Fluorescence imaging at different disease stages of lung cancer in the transgenic mice was performed, and could dynamically monitor the occurrence and development of lung cancer by different fluorescence intensity ranges. This research provided new ideas, new methods, and new technology for the early screening of lung cancer.
4.3. Inorganic nanosystems
SWNTs are also emerging as a potent type of nanosystems for molecular imaging. Given their relative ease of synthesis compared with metallofullerenes and the significantly huger relaxivity realized by gadolinium-based SWNT contrast agents compared with conventional contrast agents, SWNT-based contrast agents may show prominent progress for MRI. Also, their intrinsic near-infrared fluorescence enables SWNTs to be prospective optical agents.115 Aasi et al. introduced SWCNTs decorated by platinum–group transition metals (Pt, Pd, Rh, or Ru) as potential nanosensors for the detection of toluene, an important biomarker in the exhaled breath of the lung cancer patients. It was observed that toluene is intensely chemisorbed on Rh- and Ru-SWCNT systems with the ascendant response (−96.98% and −99.98%, respectively), and moderately chemisorbed on Pt-SWCNTs (−27.3%) and Pd-SWCNTs (61.60%), testifying metal decorated SWCNTs sensors as attractive candidates for the early detection of NSCLC.120
Herein, we summarize all the above-mentioned studies of nanosystems for NSCLC diagnosis, as shown in Table 4.
| Nanocarrier | Imaging agent | Targeting group | Advantages | Drawbacks | Ref. |
|---|---|---|---|---|---|
| Liposome | Iodinated-liposome | — | This nanosystem enabled the visualization of blood supply to the nodules during the early-phase imaging. Delayed-phase imaging enabled the characterization of slow-growing and rapidly-growing nodules based on signal enhancement | The routine analysis is challenging in rodent studies due to small feature size and high noise levels on micro-CT scanners | 102 |
| Liposome | Molecular beacon detecting AIMP2-DX2 mutation | Folate | Simple and rapid detection of mutations on nucleotides for diagnosing and monitoring the progression of relevant cancers | Lack of in vivo studies and toxicity assessment | 103 |
| Chitosan PNPs | miR-155 molecular beacon | Octreotide (OCT) | Fluorescence imaging at different disease stages of lung cancer in transgenic mice could dynamically monitor the occurrence and development of lung cancer | Lack of toxicity assessment | 107 |
| AuNPs | Organic ligands provided broadly cross-selective adsorption sites for the breath VOCs | — | These nanosensors are suitable for detecting lung cancer (LC) specific patterns of volatile organic compounds (VOCs) profiles, allowing early differential diagnosis of LC subtypes | Lack of in vivo studies and toxicity assessment | 111 |
| AuNPs | Ti3C2Tx and MoS2 | — | The detection boundary can reach as low as 0.03 pg ml−1 within the concentration of CYFRA21-1 ranging from 0.5 pg ml−1 to 50 ng ml−1 | Lack of toxicity assessment | 112 |
| SIONs | 64Cu and Fe3O4 | Folate | This nanosystem yielded good radiochemical purity of 82.17% without the presence of a free 64Cu mobile phase and exhibited ∼90% stability in both buffer solution and human serum for 24 h, making it a potential nanoprobe for PET/MRI-based diagnosis of NSCLC. | Lack of in vivo studies and toxicity assessment | 114 |
| Metallofullerene | Gd | — | Avoided RES accumulation and spontaneous aggregation in vivo as a promising MRI contrast agent | Lack of toxicity assessment | 119 |
| SWCNTs | SWCNTs and platinum-group transition metals (Pt, Pd, Rh, or Ru) | — | Attractive candidates for the detection of toluene, a lung cancer biomarker in the exhaled breath of lung cancer patients | The lack of reversibility originated from the strong chemical reaction between the toluene and the metal atom | 120 |
| C-dot-induced hollow mesoporous organosilica nanocarriers | Carbon dot and silica | — | Enabled multi-color visualization in vitro, and exhibited strong optical signals in vivo along with high optical stability (over a week) | Can be further explored with modifications such as targeting moiety and PEGylation | 122 |
Notably, in standard practice, the US Food and Drug Administration demands that agents administered for diagnostic purposes must be cleared completely from the body within a reasonable time period. Longmire et al. suggested that renal filtration was the ideal route for nanomedicine removal from the body, and nanosystems with size <6 nm and zwitterionic or cationic surface charge may be optimized to boost renal clearance. Clearance of metal-containing nanosystems is particularly significant owing to agent toxicity and the likelihood of interference with other diagnostic imaging modalities. For instance, metal nanosystems may interfere with X-ray imaging owing to changes in linear attenuation coefficient, MRI owing to proton-free voids, ultrasound owing to enhanced echogenicity, and probably even single photon emission computed tomography and PET owing to photon attenuation.17,115
5. Nanosystems for NSCLC treatment with/without image guidance
As mentioned above, nanotechnology has revolutionized the use of carriers for treating NSCLC, which are described in detail in the following sections. Apart from summarizing the recent application of lipid-based nanosystems, polymer-based nanosystems, and inorganic nanosystems against NSCLC, we additionally introduce vaccine and gene delivery nanosystems given their increasingly important role as novel approaches for NSCLC treatment.5.1. Lipid-based nanosysytems
Though liposomes are widely utilized for NSCLC theranostics, challenges still lie in the variation between batch to batch, expensive mass production cost, induced pro-inflammatory cytokines, possible drug leakage, etc.124 Nevertheless, some functionalized liposomes have entered the clinical stage, herein we summarize main examples of liposomal formulations in clinical trials (Table 5).
| Product name | Composition | Clinical phase | Clinical use | Clinical trials (https://clinicaltrials.gov) identifier or ref. |
|---|---|---|---|---|
| L-BLP25 (Stimuvax®) | MUC1-targeted liposome vaccine | Phase III | Unresectable stage III NSCLC | NCT00157196 (ref. 125) |
| Lipoplatin | Liposome encapsulated formulation of cisplatin | Phase II | Metastatic NSCLC | NCT00006036 (ref. 126) |
| OTAP: Chol-fus1 | DOTAP:cholesterol liposomal nanoparticles complexed with a plasmid expression cassette encoding human FUS1 protein | Phase I | Advanced NSCLC | NCT00059605 |
| ATRC-101 | Pegylated liposomal doxorubicin (PLD) | Phase IB | Advanced solid malignancies containing NSCLC, breast cancer, colorectal cancer, ovarian cancer, etc. | NCT04244552 |
| ATI-1123 | Liposomal docetaxel formulation | Phase I | Solid tumors including NSCLC and breast cancer | NCT01041235 |
| STM 434 | Liposomal doxorubicin | Phase I | Solid tumors such as NSCLC, ovarian cancer, fallopian tube cancer, endometrial cancer | NCT02262455 |
| MM-310 | EphA2 receptor-targeted liposomal formulation of a docetaxel prodrug | Phase I | NSCLC, solid tumors, urothelial carcinoma, gastric carcinoma, squamous cell carcinoma of the head and neck, etc. | NCT03076372 |
| MnSOD | Manganese superoxide dismutase (MnSOD) plasmid liposome (PL) | Phase I | Advanced stage III NSCLC | NCT00618917 (ref. 127) |
| Aroplatin (L-NDPP) | Liposomal formulation of cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexane platinum(II) | Phase II | NSCLC, advanced colorectal cancer | 128 |
| SPI-77 | Sterically stabilized liposomal cisplatin | Phase II | Advanced NSCLC | 129 |
However, similar to other colloidal nanocarriers, SLNs suffer rapid elimination from circulation for the sake of the reticular endothelial system (RES), thus resulting in limited drug delivery. In addition, a significant challenge occurring in the drug-loading process is the dissolution of drug molecules into lipids.132
5.2. Polymer-based nanosystems
However, disadvantages including leakage of the encapsulated drug during the delivery, weak solubility of small-sized micelles, and limited loading competence are still persecuting reseachers.75
Extensive applications of polymer-based nanoparticles containing PNPs, PMs, and dendrimers that have entered clinical trials are shown in Table 6.
| Product name | Composition | Clinical phase | Clinical use | Clinical trials (https://clinicaltrials.gov) identifier or ref. |
|---|---|---|---|---|
| CRLX101 (NLG207) | Cyclodextrin and camptothecin loaded polymer nanoparticle | Phase II | Advanced NSCLC, advanced solid tumor malignancies | NCT01380769 (ref. 138) |
| Genexol-PM | Cremorphor EL (CrEL)-free polymeric micelle formulation of paclitaxel | Phase II | Advanced NSCLC | NCT01770795 (ref. 139) |
| NC-6004 | Polymeric micelle formulation of cisplatin | Phase I/II | Advanced unresectable NSCLC, biliary tract, or bladder cancer | NCT02240238 (ref. 140) |
| NC-4016 | 1,2-Diaminocyclohexane platinum(II) (DACHPt)-incorporating micelles | Phase I | Various solid tumors | NCT03168035 (ref. 61) |
| NC-6300 | Epirubicin-incorporating micelle | Phase IB | Advanced solid tumors including NSCLC, soft tissue sarcoma, metastatic sarcoma, sarcoma | NCT03168061 (ref. 141) |
| BIND-014 | PSMA-targeted docetaxel-containing polymeric micelle | Phase I | Advanced solid tumors including NSCLC | NCT02283320 (ref. 142) |
| XMT-1001 | Camptothecin (CPT) conjugated Fleximer® | Phase I | Non-small cell lung cancer, small cell lung cancer | NCT00455052 |
5.3. Inorganic nanosystems
5.4. Vaccine delivery nanosystems
Cancer vaccines that elicit a specific cytotoxic immune response to tumor antigens are a promising strategy for NSCLC immunotherapy. A major obstacle to developing a novel NSCLC vaccine is the successful and effective delivery of NSCLC antigens to specific cell populations, NK cells, and antigen-presenting cells (APCs). Antigens are relatively fragile in the blood microenvironment and readily susceptible to degradation. Employing nanosystems as delivery systems is a prospective way to address ineffective antigen delivery.Liposome-based NSCLC vaccines have been advanced into clinical studies. Tecemotide (L-BLP25) is a MUC1 glycoprotein immunotherapy liposomal vaccine combined with MPLA, which is capable of inducing antigen-specific T-cell responses.151 This vaccine formulation was demonstrated to induce a dominant Th1 response and CTL specific to MUC1. Interestingly, Butts et al.125 carried out a phase III trial and found no significant difference in overall survival with the administration of L-BLP25 after chemoradiotherapy compared with a placebo for all patients with unresectable stage III NSCLC. Therefore, more liposomal vaccine studies are worth conducting depending on the stage of NSCLC relative to the treatment regimen employed. Parayath et al. utilized hyaluronic acid nanoparticles loaded with microRNA-125b to reprogram M2 phenotype MØs in an NSCLC murine model. These nanoparticles were observed to target CD44+ MØs and modified to enable negatively charged nucleotide encapsulation.152 The repolarization of tumor-associated MØs to M1 phenotype was testified by altered surface biomarker expression.
These studies suggested that nanosystems are promising vectors for antigen delivery in the NSCLC vaccine. Nanovaccines can reduce the dose and number of immunizations for many vaccines, provide rapid and long-lived immunity in a single dose, activate humoral and cell-mediated immunity, allow room temperature storage for long periods of time, and enhance patient compliance. However, challenges that still need to be overcome include translation from lab to clinic, scale-up costs, and regulatory approvals.
5.5. Gene delivery nanosystems
The efficient delivery of the nucleic acid gained attention to its target tissue and nanocarriers. Generally, the exogenous genetic material must be delivered to the nucleus of the targeted cells, where they manufacture the protein products of the introduced gene. The ideal vector transfers a precise amount of genetic material into a specific cell type that achieves the level and duration of transgene expression sufficient to correct the defect and be non-immunogenic and harmless, allowing expression of the gene product without causing toxicity.153 In the following sections, we discuss the advancements of nanocarriers used to deliver miRNA, siRNA, antisense oligonucleotides, and plasmid DNA for NSCLC treatment.Herein, we summarize all the above-mentioned studies of nanosystems for NSCLC treatment, as shown in Table 7.
| Nanocarrier | Cargo | Targeting group | Advantages | Drawbacks | Ref. |
|---|---|---|---|---|---|
| Liposome | Erastin and MT1DP | Folate | Sensitized erastin-induced ferroptosis with decreased cellular GSH levels and elevated lipid ROS, and showed a favorable therapeutic effect on lung cancer xenografts | — | 123 |
| NSCLC cell membrane-hybrid liposome | Lipoic acid-modified polypeptides (LC) loaded with phosphoglycerate mutase 1 (PGAM1) siRNA (siPGAM1) and DTX | NSCLC cell membrane | pH-controlled membrane disruption and redox-responsive DTX and siRNA release, thus resulting in highly robust glycolysis-related gene silencing and enhanced antiproliferation ability of chemotherapy | — | 32 |
| SLNs | Quantum dots, paclitaxel and Bcl-2 targeted siRNA | — | Imaging-guided synergistic chemotherapy: this nanosystem showed powerful fluorescence derived from quantum dots and efficient transportation of paclitaxel and siRNA in specific sites of lung tumor tissues | Lack of in vivo study and toxicity evaluation | 130 |
| SLNs | Afatinib and paclitaxel | — | Showed a superior treatment effect in EGFR TKIs-resistant NSCLC cells | In vivo study showed slight liver toxicity, which can recover over time | 131 |
| PNPs (PMAA) | Fe(III) and cypate | Mesenchymal stem cell membranes | Realized fluorescence/MRI bimodal imaging and imaging-guided photothermal-therapy-enhanced radiotherapy against NSCLC | Lack of toxicity evaluation | 38 |
| PNPs | Gefitinib (Gef) and Yes-associated protein (YAP)-siRNA | — | Achieved a targeted drug/gene/photodynamic therapy against EGFR-TKI-resistant NSCLC. | — | 133 |
| PMs | DTX | — | Considerably enhanced DTX accumulation, thus achieving better therapeutic efficacy | — | 134 |
| PMs | DOX | Folate | Resolved the dilemma between systemic stability and rapid intracellular drug release | — | 135 |
| Fluorinated dendrimers | Gef and hematoporphyrin (Hp) | Aptamer | Greatly promoted the production of the intracellular ROS and amplified the therapeutic effect against EGFR-TKI resistant NSCLC | Lack of in vivo study and toxicity evaluation | 136 |
| PAMAM dendrimers (G5) | microRNA Mimic let-7b and chloroquine | Hyaluronic acid | Expression study of three genes linked with cancer initiation and development in NSCLC, including KRAS, p-21, and BCL-2, indicated a decrease in KRAS and BCL-2 (oncogenic and anti-apoptotic genes) and an increase in p-21 (apoptotic gene) | Lack of in vivo study | 137 |
| Gold nanorod | miR-320a | RGD peptide | Achieved integrin αvβ3-targeted therapy, photosensitive therapy by laser irradiation, and gene-targeted therapy by miR-320a | — | 143 |
| Gold nanoparticles-dextran nanoparticles | Paclitaxel dimeric prodrug and photosensitizer Ce6 | — | Enhanced the radiosensitivity of NSCLC. | — | 144 |
| trans-10, cis-12 conjugated linoleic acid (CLA)-coated SIONs | Paclitaxel | — | CLA (with potential anticancer activity) could yield a more efficacious nanomedicine for NSCLC chemotherapy with enhanced anti-proliferative activity and biocompatibility | Lack of in vivo study and toxicity evaluation | 145 |
| Hybrid nanosystem (SIONs + RPPs) | Immune adjuvant resiquimod (R848) and the phase transition agent perfluoropentane (PFP) | — | A novel strategy for enhanced mild magnetic hyperthermia immunotherapy and ultrasound imaging with great clinical translation potential | Lack of toxicity evaluation | 146 |
| Oxidized graphene nanoribbons | Cisplatin | — | Showed an average inhibition of 22.72% at a lower dose of cisplatin (>25%) by passive targeting on cell line A549 by DNA alkylation | Lack of in vivo study and toxicity evaluation | 147 |
| SWCNTs | Cadmium | — | Unavoidable side effects of Cd, such as induced cell viability reduction, reactive oxygen species, and cell cycle arrest were remarkably abrogated by joint effects of SWCNTs in A549 cells | Lack of in vivo study and toxicity evaluation | 148 |
| MSNs | Ag and Geb | Folic acid | Combined photothermal therapy and molecular targeted therapy, and achieved pH-responsive drug release by the degradation of residual MnO2 | — | 149 |
| MSNs | PDLIM5 siRNA | — | Very effective for siRNA delivery and ultrasound imaging, and the sensitivity of drug-resistant cells to gefitinib was restored | — | 150 |
| Liposomal vaccine (L-BLP25) | A synthetic 25-amino acid lipopeptide derived from the tandem repeat region of MUC1, and nonspecific adjuvant monophosphoryl lipid A | — | Induced a dominant Th1 response and CTL specific to MUC1 in the study by Mehta et al. | A phase III trial found no significant difference in overall survival with the administration of L-BLP25 after chemoradiotherapy compared with a placebo | 125,151 |
| Hyaluronic acid-based nanoparticles | miRNA-125b | Hyaluronic acid | Reprogrammed tumor-associated macrophages to overcome immunosuppression | — | 152 |
| SLNs | miR-34a | — | Provided a new strategy to deliver miRNA | Liver toxicity | 157 |
| Liposome | miR-let-7a | Ephrin-A1 | Obviously inhibit the RAS signaling pathway, hence impeding the proliferation and growth of NSCLC cells | — | 82 |
| Dendrimers | iNOP-7-PLK1 siRNA | — | A novel strategy for the treatment of NSCLC which aberrantly express PLK1 | Lack of toxicity evaluation | 160 |
| PNPs (PLGA) | Antisense oligonucleotide 2′-O-methyl-RNA | — | Remarkably inhibit the telomerase activity in the lung cancer cells | — | 164 |
| PEI/PEG NPs | Wild-type p53 and miR-125b expressing plasmid DNA | Hyaluronic acid (HA) | Showed tremendous promise of wt-p53 and miR-125b gene therapy using dual CD44/EGFR-targeting HA NP vector for effective treatment of lung cancer | Lack of toxicity evaluation | 165 |
| Liposome | TUSC2-expressing plasmid DNA | — | Reduced the number of metastatic nodules and increased survival rates in metastasis mouse models | — | 166 |
6. Discussion and perspectives
Huynh and Zheng167 introduced two design concepts for engineering multifunctional nanosystems: a conventional approach called ‘all-in-one’, and a novel approach called ‘one-for-all’. The all-in-one approach means components that own a specific singular function such as a drug or imaging contrast agent are combined, leading to multiple single components packaged in a single nanosystem. This approach is generally achieved by either encapsulating agents within the core, conjugating or adsorbing agents to the surface of the nanosystem, or a combination of these ways, thus making the resultant nanosystem become increasingly complex with the addition of multiple imaging agents and drugs. On the other hand, one-for-all means a multifunctional nanosystem can be made from a single building block that owns multiple intrinsic functionalities such as being a carrier, drug, and imaging agent, simultaneously. This approach aims to simplify the composition of the multifunctional nanosystem while retaining the required properties. Many inorganic nanosystems have both imaging and treatment feasibility, such as SIONs used for MRI and thermal therapy,168 AuNPs for CT and radiation therapy,169 gold nanorods for CT contrast enhancement, and PTT.170Both, all-in-one and one-for-all design approaches, possess merits and demerits. The aspects most remarkably discriminating the two approaches are clinical translation—manufacturing methodologies and presumable toxicity.
The major superiority of the all-in-one approach is the readily available components, many of which are based on materials already utilized in the clinic, thereby reducing challenges related to regulatory approval. However, the inferiority of the all-in-one approach is still troublesome, featuring relatively higher clinical translation hurdles and more complicated toxicity studies. In this approach, the synthesis of multifunctional nanosystems entails the addition of singular functional components in a step-by-step manner, hence demanding multiple purification courses. Accordingly, this synthesis approach is highly time-consuming, and multistep purification usually reduces the final yield, thereby causing expensive scale-up costs and impeding their clinical use. Moreover, presumable heterogeneous formulations may occur due to multiple cargos packaged in one single nanosystem. Although a step-by-step procedure may attenuate the heterogeneity and elevate reproducibility, it will result in increased synthesis time and costs. On the other hand, combining multiple steps is capable of reducing the synthesis steps, but resulting in declined reproducibility and enhanced heterogeneity amongst nanosystems in the same production batch. Moreover, biodistribution, biocompatibility, toxicity, and biodegradation also limit progress towards clinical use, entailing to ponder on each component of the formulation, because many multifunctional nanosystems consist of both organic and inorganic components, which are not cleared from the body in the same manner. Besides, the size of nanosystems also affects the routes of clearance via either liver or renal filtration. For this reason, a multifunctional nanosystem with smaller nanoparticles packaged within it may have several routes of clearance, thus demanding complex toxicity studies for multiple components.
As for the one-for-all approach, the major superiority lies in relatively lower clinical translation hurdles and simpler toxicity studies. In this approach, the synthesis procedure of multifunctional nanosystems is simplified because a single building block does not demand complicated fabrication steps, while ensuring a homogenous formulation and only demands biodistribution, biocompatibility, toxicity, and biodegradation studies for the single component. However, most of the multifunctional nanomaterials are inorganic, which poses threats to long-term toxicity and in vivo clearance considering that they are not biodegradable. Moreover, inorganic nanomaterials are usually confined to be specific for an individual imaging modality, resulting in finite flexibility for multiple desired therapeutic and imaging functionalities. Besides, the one-for-all approach challenges researchers to synthesize inherently multifunctional building blocks, which will demand their own regulatory approval.
7. Conclusions
Non-small-cell lung cancer (NSCLC) is a devastating disease of high incidence and mortality all over the world. Sensitive diagnosis and effective therapy methods are crucial for alleviating the conditions of patients whose lives are threatened by the disease. Nanoparticles have been established to be tools with enormous benefits and widely applied in the clinic against NSCLC. The structure of nanoparticles can be manipulated and regulated, which permits limitations and problems existing in conventional theranostic treatments to be overcome such as solubility and stability issues through surface chemistry.Multi-drug resistance (MDR), which has a close relationship with tumor cell heterogeneity, gene mutations, efflux pump, tumor microenvironment, etc., has been a huge obstacle to the long-term therapeutic outcomes of NSCL.9 Fortunately, nanoparticles have displayed prospective possibilities for overcoming MDR. It is well demonstrated that diverse chemotherapeutic or immunotherapeutic drugs delivered as nanoparticle cargoes are endowed with the ability to bypass MDR partially because drugs delivered by nanocarriers cannot be recognized as substrates by the ABC drug efflux systems.
Although there has been a great deal of research concerning nanoparticles delivering NSCLC drugs, only a handful of such nanomedicine have entered the market. This situation is mainly attributed to the fact that the in vivo performance of nanomedicine is of great possibility to be very distinguished from its performance in vitro, which has something to do with aspects of cell interactions, tissue transportation, diffusion, and biocompatibility in different settings. Nevertheless, nanoparticles have made great progress over the last decades, and considering the market prospect of nanoparticles against NSCLC, opportunities primarily exist in combination with conventional treatments such as chemotherapeutics, NSCLC-oriented, and patient-featured therapy guidelines.
Author contributions
Piao Jiang and Qinglin Shen: conceptualization, writing—original draft preparation. Piao Jiang, Bin Liang, Zhen Zhang, Quan Xu, Weirong Yao, Qinglin Shen: writing—review and editing. Piao Jiang, Qinglin Shen: Visualization. Bing Fan, Lin Zeng, Zhiyong Zhou, Zhifang Mao: supervision, project administration. Qinglin Shen, Weirong Yao, Quan Xu: funding acquisition. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
P. J., B. L., and Z. Z contributed equally to this work. This work was financially supported by the Scientific research projects of the Jiangxi Provincial Health Commission (202130003), the Scientific research projects of the Jiangxi Administration of Traditional Chinese Medicine (2021A370), and the Scientific research projects of the Department of Education of Jiangxi Provincial (GJJ218909).References
- J. R. Molina, P. Yang, S. D. Cassivi, S. E. Schild and A. A. Adjei, Mayo Clin. Proc., 2008, 83, 584–594 CrossRef PubMed.
- Y. Wang, Y. Zhang, Z. Du, M. Wu and G. Zhang, Int. J. Nanomed., 2012, 7, 2315–2324 CAS.
- C. García-Fernández, C. Fornaguera and S. Borrós, Cancers, 2020, 12, 1609 CrossRef PubMed.
- L. Chang, J. Li and R. Zhang, Biochim. Biophys. Acta, Rev. Cancer, 2022, 1877, 188729 CrossRef CAS PubMed.
- C. Gridelli, A. Rossi, D. P. Carbone, J. Guarize, N. Karachaliou, T. Mok, F. Petrella, L. Spaggiari and R. Rosell, Nat. Rev. Dis. Primers, 2015, 1, 15009 CrossRef PubMed.
- M. E. Daly, N. Singh, N. Ismaila, M. B. Antonoff, D. A. Arenberg, J. Bradley, E. David, F. Detterbeck, M. Früh, M. A. Gubens, A. C. Moore, S. K. Padda, J. D. Patel, T. Phillips, A. Qin, C. Robinson and C. B. Simone, J. Clin. Oncol., 2022, 40, 1356–1384 CrossRef PubMed.
- P. Chen, Y. Liu, Y. Wen and C. Zhou, Cancer Commun., 2022, 42, 937–970 CrossRef PubMed.
- M. S. Goldberg, Nat. Rev. Cancer, 2019, 19, 587–602 CrossRef CAS PubMed.
- J. L. Markman, A. Rekechenetskiy, E. Holler and J. Y. Ljubimova, Adv. Drug Delivery Rev., 2013, 65, 1866–1879 CrossRef CAS PubMed.
- K. Bukowski, M. Kciuk and R. Kontek, Int. J. Mol. Sci., 2020, 21, 3233 CrossRef CAS PubMed.
- H. K. Sajja, M. P. East, H. Mao, Y. A. Wang, S. Nie and L. Yang, Curr. Drug Discovery Technol., 2009, 6, 43–51 CrossRef CAS PubMed.
- A. Selmani, D. Kovačević and K. Bohinc, Adv. Colloid Interface Sci., 2022, 303, 102640 CrossRef CAS PubMed.
- Y. Xu, T. Fourniols, Y. Labrak, V. Préat, A. Beloqui and A. des Rieux, ACS Nano, 2022, 16, 7168–7196 CrossRef CAS PubMed.
- Y. Yang, X. Zheng, L. Chen, X. Gong, H. Yang, X. Duan and Y. Zhu, Int. J. Nanomed., 2022, 17, 2041–2067 CrossRef PubMed.
- W. He, G. Ma, Q. Shen and Z. Tang, Nanomaterials, 2022, 12, 1738 CrossRef CAS PubMed.
- B. B. Oliveira, D. Ferreira, A. R. Fernandes and P. V. Baptista, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2023, 15, e1836 CAS.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef CAS PubMed.
- S. M. Moghimi, H. Hedeman, I. S. Muir, L. Illum and S. S. Davis, Biochim. Biophys. Acta, 1993, 1157, 233–240 CrossRef CAS PubMed.
- C. J. Porter, S. M. Moghimi, L. Illum and S. S. Davis, FEBS Lett., 1992, 305, 62–66 CrossRef CAS PubMed.
- R. A. Petros and J. M. DeSimone, Nat. Rev. Drug Discovery, 2010, 9, 615–627 CrossRef CAS PubMed.
- J. D. Byrne, T. Betancourt and L. Brannon-Peppas, Adv. Drug Delivery Rev., 2008, 60, 1615–1626 CrossRef CAS PubMed.
- J. Chen, Y. Zhang, Z. Meng, L. Guo, X. Yuan, Y. Zhang, Y. Chai, J. L. Sessler, Q. Meng and C. Li, Chem. Sci., 2020, 11, 6275–6282 RSC.
- H. Kim, K. Chung, S. Lee, D. H. Kim and H. Lee, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016, 8, 23–45 CAS.
- M. P. Melancon, M. Zhou and C. Li, Acc. Chem. Res., 2011, 44, 947–956 CrossRef CAS PubMed.
- S. M. Mohamed, S. Veeranarayanan, T. Maekawa and S. K. Dasappan Nair, Adv. Drug Delivery Rev., 2019, 138, 18–40 CrossRef PubMed.
- P. Zhang, C. Hu, W. Ran, J. Meng, Q. Yin and Y. Li, Theranostics, 2016, 6, 948–968 CrossRef CAS PubMed.
- X. Li, W. Li, M. Wang and Z. Liao, J. Controlled Release, 2021, 335, 437–448 CrossRef CAS PubMed.
- Y. Z. Zhao, L. N. Du, C. T. Lu, Y. G. Jin and S. P. Ge, Int. J. Nanomed., 2013, 8, 1621–1633 Search PubMed.
- L. Fu and H. T. Ke, Cancer Biol. Med., 2016, 13, 313–324 CAS.
- Q. Lin, Y. Peng, Y. Wen, X. Li, D. Du, W. Dai, W. Tian and Y. Meng, Beilstein J. Nanotechnol., 2023, 14, 262–279 CrossRef PubMed.
- L. Liang, H. Cen, J. Huang, A. Qin, W. Xu, S. Wang, Z. Chen, L. Tan, Q. Zhang, X. Yu, X. Yang and L. Zhang, Mol. Cancer, 2022, 21, 186 CrossRef CAS PubMed.
- W. Zhang, C. Gong, Z. Chen, M. Li, Y. Li and J. Gao, J. Nanobiotechnol., 2021, 19, 339 CrossRef CAS PubMed.
- L. Wang, Y. Rao, X. Liu, L. Sun, J. Gong, H. Zhang, L. Shen, A. Bao and H. Yang, J. Nanobiotechnol., 2021, 19, 56 CrossRef CAS PubMed.
- P. Graván, A. Aguilera-Garrido, J. A. Marchal, S. A. Navarro-Marchal and F. Galisteo-González, Adv. Colloid Interface Sci., 2023, 314, 102871 CrossRef PubMed.
- M. B. McGuckin, J. Wang, R. Ghanma, N. Qin, S. D. Palma, R. F. Donnelly and A. J. Paredes, J. Controlled Release, 2022, 345, 334–353 CrossRef CAS PubMed.
- A. K. Thakur, D. K. Chellappan, K. Dua, M. Mehta, S. Satija and I. Singh, Expert Opin. Ther. Pat., 2020, 30, 375–387 CrossRef CAS PubMed.
- A. Gajewska, J. T. Wang, R. Klippstein, M. Martincic, E. Pach, R. Feldman, J. C. Saccavini, G. Tobias, B. Ballesteros, K. T. Al-Jamal and T. Da Ros, J. Mater. Chem. B, 2021, 10, 47–56 RSC.
- Y. Yin, Y. Li, S. Wang, Z. Dong, C. Liang, J. Sun, C. Wang, R. Chai, W. Fei, J. Zhang, M. Qi, L. Feng and Q. Zhang, J. Nanobiotechnol., 2021, 19, 80 CrossRef CAS PubMed.
- Y. Kanehira, K. Togami, K. Ishizawa, S. Sato, H. Tada and S. Chono, Pharm. Dev. Technol., 2019, 24, 1095–1103 CrossRef CAS PubMed.
- P. Patel, M. Raval, A. Manvar, V. Airao, V. Bhatt and P. Shah, PLoS One, 2022, 17, e0267257 CrossRef CAS PubMed.
- S. H. Jeong, J. H. Jang and Y. B. Lee, J. Controlled Release, 2021, 335, 86–102 CrossRef CAS PubMed.
- N. N. Parayath, A. Parikh and M. M. Amiji, Nano Lett., 2018, 18, 3571–3579 CrossRef CAS PubMed.
- S. V. Vinogradov, T. K. Bronich and A. V. Kabanov, Adv. Drug Delivery Rev., 2002, 54, 135–147 CrossRef CAS PubMed.
- E. Pérez-Herrero and A. Fernández-Medarde, Eur. J. Pharm. Biopharm., 2015, 93, 52–79 CrossRef PubMed.
- L. Zhang, G. Xie, X. Xiao and C. Cheng, J. Cancer Res. Clin. Oncol., 2022 DOI:10.1007/s00432-022-04298-2.
- W. Yao, J. Yao, F. Qian, Z. Que, P. Yu, T. Luo, D. Zheng, Z. Zhang and J. Tian, Acta Biochim. Biophys. Sin., 2021, 53, 1027–1036 CrossRef CAS PubMed.
- K. Mao, W. Zhang, L. Yu, Y. Yu, H. Liu and X. Zhang, Drug Des., Dev. Ther., 2021, 15, 3475–3486 CrossRef PubMed.
- N. Zhu, G. Li, J. Zhou, Y. Zhang, K. Kang, B. Ying, Q. Yi and Y. Wu, J. Mater. Chem. B, 2021, 9, 2483–2493 RSC.
- O. Knights, S. Freear and J. R. McLaughlan, Nanomaterials, 2020, 10, 1307 CrossRef CAS PubMed.
- S. Fu, Y. Zhao, J. Sun, T. Yang, D. Zhi, E. Zhang, F. Zhong, Y. Zhen, S. Zhang and S. Zhang, Colloids Surf., B, 2021, 201, 111623 CrossRef CAS PubMed.
- L. Ren, Y. J. Kim, S. Y. Park, S. Lee, J. Y. Lee, C. P. Park and Y. T. Lim, J. Mater. Chem. B, 2016, 4, 4832–4838 RSC.
- B. Wang, W. Hu, H. Yan, G. Chen, Y. Zhang, J. Mao and L. Wang, Biomed. Pharmacother., 2021, 136, 111249 CrossRef CAS PubMed.
- K. Thangavel, A. Lakshmikuttyamma, C. Thangavel and S. A. Shoyele, Colloids Surf., B, 2022, 209, 112162 CrossRef CAS PubMed.
- P. D. Ganthala, S. Alavala, N. Chella, S. B. Andugulapati, N. B. Bathini and R. Sistla, Colloids Surf., B, 2022, 211, 112305 CrossRef CAS PubMed.
- S. González-Rubio, C. Salgado, V. Manzaneda-González, M. Muñoz-Úbeda, R. Ahijado-Guzmán, P. Natale, V. G. Almendro-Vedia, E. Junquera, J. O. Barcina, I. Ferrer, A. Guerrero-Martínez, L. Paz-Ares and I. López-Montero, Nanoscale, 2022, 14, 8028–8040 RSC.
- Z. Ma, S. W. Wong, H. Forgham, L. Esser, M. Lai, M. N. Leiske, K. Kempe, G. Sharbeen, J. Youkhana, F. Mansfeld, J. F. Quinn, P. A. Phillips, T. P. Davis, M. Kavallaris and J. A. McCarroll, Biomaterials, 2022, 285, 121539 CrossRef CAS PubMed.
- V. Patel, R. Lalani, I. Vhora, D. Bardoliwala, A. Patel, S. Ghosh and A. Misra, Drug Delivery Transl. Res., 2021, 11, 2052–2071 CrossRef CAS PubMed.
- S. Wang, Y. Chen, J. Guo and Q. Huang, Int. J. Mol. Sci., 2023, 24, 2643 CrossRef CAS PubMed.
- H. Abbasi, N. Rahbar, M. Kouchak, P. Khalil Dezfuli and S. Handali, J. Liposome Res., 2022, 32, 195–210 CrossRef CAS PubMed.
- M. Dymek and E. Sikora, Adv. Colloid Interface Sci., 2022, 309, 102757 CrossRef CAS PubMed.
- H. S. Oberoi, N. V. Nukolova, A. V. Kabanov and T. K. Bronich, Adv. Drug Delivery Rev., 2013, 65, 1667–1685 CrossRef CAS PubMed.
- A. Akbarzadeh, R. Rezaei-Sadabady, S. Davaran, S. W. Joo, N. Zarghami, Y. Hanifehpour, M. Samiei, M. Kouhi and K. Nejati-Koshki, Nanoscale Res. Lett., 2013, 8, 102 CrossRef PubMed.
- W. Men, P. Zhu, S. Dong, W. Liu, K. Zhou, Y. Bai, X. Liu, S. Gong and S. Zhang, Drug Delivery, 2020, 27, 180–190 CrossRef CAS PubMed.
- J. German-Cortés, M. Vilar-Hernández, D. Rafael, I. Abasolo and F. Andrade, Pharmaceutics, 2023, 15, 831 CrossRef PubMed.
- S. V. Khairnar, P. Pagare, A. Thakre, A. R. Nambiar, V. Junnuthula, M. C. Abraham, P. Kolimi, D. Nyavanandi and S. Dyawanapelly, Pharmaceutics, 2022, 14, 1886 CrossRef CAS PubMed.
- V. Gugleva and V. Andonova, Pharmaceuticals, 2023, 16, 474 CrossRef CAS PubMed.
- E. Salah, M. M. Abouelfetouh, Y. Pan, D. Chen and S. Xie, Colloids Surf., B, 2020, 196, 111305 CrossRef CAS PubMed.
- A. R. SA, A. Mohd Gazzali, F. A. Fisol, M. A. Ibrahim, T. Parumasivam, N. Mohtar and A. W. Habibah, Cancers, 2021, 13, 400 CrossRef PubMed.
- V. Mishra, K. K. Bansal, A. Verma, N. Yadav, S. Thakur, K. Sudhakar and J. M. Rosenholm, Pharmaceutics, 2018, 10, 191 CrossRef CAS PubMed.
- M. Najlah, Z. Ahmed, M. Iqbal, Z. Wang, P. Tawari, W. Wang and C. McConville, Eur. J. Pharm. Biopharm., 2017, 112, 224–233 CrossRef CAS PubMed.
- M. Haim Zada, Y. Rottenberg and A. J. Domb, J. Colloid Interface Sci., 2022, 622, 904–913 CrossRef CAS PubMed.
- S. R. Schaffazick, A. R. Pohlmann, T. Dalla-Costa and S. S. Guterres, Eur. J. Pharm. Biopharm., 2003, 56, 501–505 CrossRef CAS PubMed.
- N. A. N. Hanafy, M. El-Kemary and S. Leporatti, Cancers, 2018, 10, 238 CrossRef PubMed.
- M. Zhang, Z. Zhang, X. Song, J. Zhu, J. A. Sng, J. Li and Y. Wen, Biomacromolecules, 2022, 23, 4586–4596 CrossRef CAS PubMed.
- S. Perumal, R. Atchudan and W. Lee, Polymers, 2022, 14, 2510 CrossRef CAS PubMed.
- C. Oerlemans, W. Bult, M. Bos, G. Storm, J. F. Nijsen and W. E. Hennink, Pharm. Res., 2010, 27, 2569–2589 CrossRef CAS PubMed.
- A. P. Sherje, M. Jadhav, B. R. Dravyakar and D. Kadam, Int. J. Pharm., 2018, 548, 707–720 CrossRef CAS PubMed.
- A. D. Dey, A. Bigham, Y. Esmaeili, M. Ashrafizadeh, F. D. Moghaddam, S. C. Tan, S. Yousefiasl, S. Sharma, A. Maleki, N. Rabiee, A. P. Kumar, V. K. Thakur, G. Orive, E. Sharifi, A. Kumar and P. Makvandi, Semin. Cancer Biol., 2022, 86, 396–419 CrossRef PubMed.
- X. Li, A. Naeem, S. Xiao, L. Hu, J. Zhang and Q. Zheng, Pharmaceutics, 2022, 14, 1292 CrossRef CAS PubMed.
- C. Sandoval-Yañez and C. Castro Rodriguez, Materials, 2020, 13, 570 CrossRef PubMed.
- A. S. Chauhan, Molecules, 2018, 23, 938 CrossRef PubMed.
- H. Y. Lee, K. A. Mohammed and N. Nasreen, Am. J. Cancer Res., 2016, 6, 1118–1134 CAS.
- M. Sun, T. Wang, L. Li, X. Li, Y. Zhai, J. Zhang and W. Li, Front. Pharmacol., 2021, 12, 702445 CrossRef CAS PubMed.
- Y. Liu, W. Ma and J. Wang, Curr. Pharm. Des., 2018, 24, 2719–2728 CrossRef CAS PubMed.
- P. Singh, S. Pandit, V. Mokkapati, A. Garg, V. Ravikumar and I. Mijakovic, Int. J. Mol. Sci., 2018, 19, 1979 CrossRef PubMed.
- A. Bardestani, S. Ebrahimpour, A. Esmaeili and A. Esmaeili, J. Nanobiotechnol., 2021, 19, 327 CrossRef CAS PubMed.
- V. Frantellizzi, M. Conte, M. Pontico, A. Pani, R. Pani and G. De Vincentis, Nucl. Med. Mol. Imaging, 2020, 54, 65–80 CrossRef CAS PubMed.
- Z. Wang, R. Qiao, N. Tang, Z. Lu, H. Wang, Z. Zhang, X. Xue, Z. Huang, S. Zhang, G. Zhang and Y. Li, Biomaterials, 2017, 127, 25–35 CrossRef CAS PubMed.
- L. M. Ngema, S. A. Adeyemi, T. Marimuthu and Y. E. Choonara, Int. J. Pharm., 2021, 606, 120870 CrossRef CAS PubMed.
- M. Nejabat, F. Charbgoo and M. Ramezani, J. Biomed. Mater. Res., 2017, 105, 2355–2367 CrossRef CAS PubMed.
- V. Mishra, A. Patil, S. Thakur and P. Kesharwani, Drug Discovery Today, 2018, 23, 1219–1232 CrossRef CAS PubMed.
- C. Woodman, G. Vundu, A. George and C. M. Wilson, Semin. Cancer Biol., 2021, 69, 349–364 CrossRef CAS PubMed.
- J. Ackermann, J. T. Metternich, S. Herbertz and S. Kruss, Angew. Chem., Int. Ed. Engl., 2022, 61, e202112372 CrossRef CAS PubMed.
- Z. Pu, Y. Wei, Y. Sun, Y. Wang and S. Zhu, Int. J. Nanomed., 2022, 17, 6157–6180 CrossRef PubMed.
- D. Cai, D. Blair, F. J. Dufort, M. R. Gumina, Z. Huang, G. Hong, D. Wagner, D. Canahan, K. Kempa, Z. F. Ren and T. C. Chiles, Nanotechnology, 2008, 19, 1–10 Search PubMed.
- A. M. Elhissi, W. Ahmed, I. U. Hassan, V. R. Dhanak and A. D'Emanuele, J. Drug Delivery, 2012, 2012, 837327 Search PubMed.
- J. Y. Oh, G. Yang, E. Choi and J. H. Ryu, Biomater. Sci., 2022, 10, 1448–1455 RSC.
- Y. Feng, Z. Liao, M. Li, H. Zhang, T. Li, X. Qin, S. Li, C. Wu, F. You, X. Liao, L. Cai, H. Yang and Y. Liu, Adv. Healthcare Mater., 2022, e2201884, DOI:10.1002/adhm.202201884.
- S. Shah, P. Famta, D. Bagasariya, K. Charankumar, A. Sikder, R. Kashikar, A. K. Kotha, M. B. Chougule, D. K. Khatri, A. Asthana, R. S. Raghuvanshi, S. B. Singh and S. Srivastava, Mol. Pharm., 2022, 19, 4428–4452 CrossRef CAS PubMed.
- P. Yang, S. Gai and J. Lin, Chem. Soc. Rev., 2012, 41, 3679–3698 RSC.
- I. I. Slowing, J. L. Vivero-Escoto, C. W. Wu and V. S. Lin, Adv. Drug Delivery Rev., 2008, 60, 1278–1288 CrossRef CAS PubMed.
- C. T. Badea, K. K. Athreya, G. Espinosa, D. Clark, A. P. Ghafoori, Y. Li, D. G. Kirsch, G. A. Johnson, A. Annapragada and K. B. Ghaghada, PLoS One, 2012, 7, e34496 CrossRef CAS PubMed.
- S. M. Jo, Y. Kim, Y. S. Jeong, Y. Hee Oh, K. Park and H. S. Kim, Biosens. Bioelectron., 2013, 46, 142–149 CrossRef CAS PubMed.
- D. A. Tomalia, L. A. Reyna and S. Svenson, Biochem. Soc. Trans., 2007, 35, 61–67 CrossRef CAS PubMed.
- H. Kobayashi, C. Wu, M. K. Kim, C. H. Paik, J. A. Carrasquillo and M. W. Brechbiel, Bioconjugate Chem., 1999, 10, 103–111 CrossRef CAS PubMed.
- E. C. Wiener, M. W. Brechbiel, H. Brothers, R. L. Magin, O. A. Gansow, D. A. Tomalia and P. C. Lauterbur, Magn. Reson. Med., 1994, 31, 1–8 CrossRef CAS PubMed.
- H. Z. Zhu, J. Hou, Y. Guo, X. Liu, F. L. Jiang, G. P. Chen, X. F. Pang, J. G. Sun and Z. T. Chen, Drug Delivery, 2018, 25, 1974–1983 CrossRef CAS PubMed.
- P. Iranpour, M. Ajamian, A. Safavi, N. Iranpoor, A. Abbaspour and S. Javanmardi, J. Mater. Sci.: Mater. Med., 2018, 29, 48 CrossRef PubMed.
- J. F. Hainfeld, D. N. Slatkin, T. M. Focella and H. M. Smilowitz, Br. J. Radiol., 2006, 79, 248–253 CrossRef CAS PubMed.
- H. S. Zhou, I. I. Honma, H. Komiyama and J. W. Haus, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 12052–12056 CrossRef CAS PubMed.
- O. Barash, N. Peled, U. Tisch, P. A. Bunn, Jr., F. R. Hirsch and H. Haick, Nanomed, 2012, 8, 580–589 CrossRef CAS PubMed.
- K. Hu, J. Cheng, K. Wang, Y. Zhao, Y. Liu, H. Yang and Z. Zhang, Talanta, 2022, 238, 122987 CrossRef CAS PubMed.
- S. Benderbous, C. Corot, P. Jacobs and B. Bonnemain, Acad. Radiol., 1996, 3(Suppl 2), S292–S294 CrossRef PubMed.
- S. Park, B. B. Cho, J. R. Anusha, S. Jung, C. Justin Raj, B. C. Kim and K. H. Yu, J. Nanosci. Nanotechnol., 2020, 20, 2040–2044 CrossRef CAS PubMed.
- M. Longmire, P. L. Choyke and H. Kobayashi, Nanomedicine, 2008, 3, 703–717 CrossRef CAS PubMed.
- Z. Zhao, M. Zhen, C. Zhou, L. Li, W. Jia, S. Liu, X. Li, X. Liao and C. Wang, J. Mater. Chem. B, 2021, 9, 5722–5728 RSC.
- J. Grebowski and G. Litwinienko, Eur. J. Med. Chem., 2022, 238, 114481 CrossRef CAS PubMed.
- N. B. Fernandes, R. U. K. Shenoy, M. K. Kajampady, D. C. CEM, R. K. Shirodkar, L. Kumar and R. Verma, Environ. Sci. Pollut. Res. Int., 2022, 29, 58607–58627 CrossRef CAS PubMed.
- R. D. Bolskar, A. F. Benedetto, L. O. Husebo, R. E. Price, E. F. Jackson, S. Wallace, L. J. Wilson and J. M. Alford, J. Am. Chem. Soc., 2003, 125, 5471–5478 CrossRef CAS PubMed.
- A. Aasi, S. M. Aghaei and B. Panchapakesan, Nanotechnology, 2020, 31, 415707 CrossRef CAS PubMed.
- J. L. Vivero-Escoto, K. M. Taylor-Pashow, R. C. Huxford, J. Della Rocca, C. Okoruwa, H. An, W. Lin and W. Lin, Small, 2011, 7, 3519–3528 CrossRef CAS PubMed.
- M. S. Kang, R. K. Singh, T. H. Kim, J. H. Kim, K. D. Patel and H. W. Kim, Acta Biomater., 2017, 55, 466–480 CrossRef CAS PubMed.
- C. Gai, C. Liu, X. Wu, M. Yu, J. Zheng, W. Zhang, S. Lv and W. Li, Cell Death Dis., 2020, 11, 751 CrossRef CAS PubMed.
- R. Kedmi, N. Ben-Arie and D. Peer, Biomaterials, 2010, 31, 6867–6875 CrossRef CAS PubMed.
- C. Butts, M. A. Socinski, P. L. Mitchell, N. Thatcher, L. Havel, M. Krzakowski, S. Nawrocki, T. E. Ciuleanu, L. Bosquée, J. M. Trigo, A. Spira, L. Tremblay, J. Nyman, R. Ramlau, G. Wickart-Johansson, P. Ellis, O. Gladkov, J. R. Pereira, W. E. Eberhardt, C. Helwig, A. Schröder and F. A. Shepherd, Lancet Oncol., 2014, 15, 59–68 CrossRef CAS PubMed.
- A. Ravaioli, M. Papi, E. Pasquini, M. Marangolo, B. Rudnas, M. Fantini, S. V. Nicoletti, F. Drudi, I. Panzini, E. Tamburini, L. Gianni and G. Pasini, J. Chemother., 2009, 21, 86–90 CrossRef CAS PubMed.
- A. A. Tarhini, C. P. Belani, J. D. Luketich, A. Argiris, S. S. Ramalingam, W. Gooding, A. Pennathur, D. Petro, K. Kane, D. Liggitt, T. Championsmith, X. Zhang, M. W. Epperly and J. S. Greenberger, Hum. Gene Ther., 2011, 22, 336–342 CrossRef CAS PubMed.
- L. Kelland, Expert Opin. Invest. Drugs, 2007, 16, 1009–1021 CrossRef CAS PubMed.
- S. C. White, P. Lorigan, G. P. Margison, J. M. Margison, F. Martin, N. Thatcher, H. Anderson and M. Ranson, Br. J. Cancer, 2006, 95, 822–828 CrossRef CAS PubMed.
- K. H. Bae, J. Y. Lee, S. H. Lee, T. G. Park and Y. S. Nam, Adv. Healthcare Mater., 2013, 2, 576–584 CrossRef CAS PubMed.
- Y. Yang, Z. Huang, J. Li, Z. Mo, Y. Huang, C. Ma, W. Wang, X. Pan and C. Wu, Adv. Healthcare Mater., 2019, 8, e1900965 CrossRef PubMed.
- C. Tapeinos, M. Battaglini and G. Ciofani, J. Controlled Release, 2017, 264, 306–332 CrossRef CAS PubMed.
- J. Huang, C. Zhuang, J. Chen, X. Chen, X. Li, T. Zhang, B. Wang, Q. Feng, X. Zheng, M. Gong, Q. Gong, K. Xiao, K. Luo and W. Li, Adv. Mater., 2022, 34, e2201516 CrossRef PubMed.
- F. Gong, R. Wang, Z. Zhu, J. Duan, X. Teng and Z. K. Cui, Drug Delivery, 2020, 27, 238–247 CrossRef CAS PubMed.
- Z. Wang, J. Wen, H. Liu, L. Zheng, H. Luo, Z. Liu, X. Chen, F. Wang, D. Li, H. Pei, W. Li and L. Chen, J. Biomed. Nanotechnol., 2018, 14, 1225–1238 CrossRef CAS PubMed.
- F. Zhu, L. Xu, X. Li, Z. Li, J. Wang, H. Chen, X. Li and Y. Gao, Eur. J. Pharm. Sci., 2021, 167, 106004 CrossRef CAS PubMed.
- N. Maghsoudnia, R. B. Eftekhari, A. N. Sohi and F. A. Dorkoosh, Curr. Drug Delivery, 2021, 18, 31–43 CrossRef CAS PubMed.
- G. J. Weiss, J. Chao, J. D. Neidhart, R. K. Ramanathan, D. Bassett, J. A. Neidhart, C. H. J. Choi, W. Chow, V. Chung, S. J. Forman, E. Garmey, J. Hwang, D. L. Kalinoski, M. Koczywas, J. Longmate, R. J. Melton, R. Morgan, J. Oliver, J. J. Peterkin, J. L. Ryan, T. Schluep, T. W. Synold, P. Twardowski, M. E. Davis and Y. Yen, Invest. New Drugs, 2013, 31, 986–1000 CrossRef CAS PubMed.
- H. K. Ahn, M. Jung, S. J. Sym, D. B. Shin, S. M. Kang, S. Y. Kyung, J. W. Park, S. H. Jeong and E. K. Cho, Cancer Chemother. Pharmacol., 2014, 74, 277–282 CrossRef CAS PubMed.
- S. R. Volovat, T. E. Ciuleanu, P. Koralewski, J. E. G. Olson, A. Croitoru, K. Koynov, S. Stabile, G. Cerea, A. Osada, I. Bobe and C. Volovat, Oncotarget, 2020, 11, 3105–3117 CrossRef PubMed.
- S. P. Chawla, S. Goel, W. Chow, F. Braiteh, A. S. Singh, J. E. G. Olson, A. Osada, I. Bobe and R. F. Riedel, Clin. Cancer Res., 2020, 26, 4225–4232 CrossRef CAS PubMed.
- D. D. Von Hoff, M. M. Mita, R. K. Ramanathan, G. J. Weiss, A. C. Mita, P. M. LoRusso, H. A. Burris, 3rd, L. L. Hart, S. C. Low, D. M. Parsons, S. E. Zale, J. M. Summa, H. Youssoufian and J. C. Sachdev, Clin. Cancer Res., 2016, 22, 3157–3163 CrossRef CAS PubMed.
- J. Peng, R. Wang, W. Sun, M. Huang, R. Wang, Y. Li, P. Wang, G. Sun and S. Xie, Biomater. Sci., 2021, 9, 6528–6541 RSC.
- J. Ma, C. Wen, M. Chen, W. Zhang, L. Wang and H. Yin, ACS Biomater. Sci. Eng., 2023, 9, 2793–2805 CrossRef CAS PubMed.
- L. M. Ngema, S. A. Adeyemi, T. Marimuthu, P. Ubanako, D. Wamwangi and Y. E. Choonara, Pharmaceutics, 2022, 14, 829 CrossRef CAS PubMed.
- Q. Qin, Y. Zhou, P. Li, Y. Liu, R. Deng, R. Tang, N. Wu, L. Wan, M. Ye, H. Zhou and Z. Wang, J. Nanobiotechnol., 2023, 21, 131 CrossRef CAS PubMed.
- S. Augustine, B. Prabhakar and P. Shende, Curr. Drug Delivery, 2022, 19, 697–705 CrossRef CAS PubMed.
- M. Ahamed, M. J. Akhtar and H. A. Alhadlaq, Environ. Sci. Pollut. Res. Int., 2022, 29, 87844–87857 CrossRef CAS PubMed.
- J. Lin, R. Zheng, L. Huang, Y. Tu, X. Li and J. Chen, Colloids Surf., B, 2022, 217, 112639 CrossRef CAS PubMed.
- H. Wu, W. H. Lv, Y. Y. Zhu, Y. Y. Jia and F. Nie, Eur. J. Pharm. Sci., 2023, 182, 106372 CrossRef CAS PubMed.
- N. R. Mehta, G. T. Wurz, R. A. Burich, B. E. Greenberg, S. Griffey, A. Gutierrez, K. E. Bell, J. L. McCall, M. Wolf and M. DeGregorio, Clin. Cancer Res., 2012, 18, 2861–2871 CrossRef CAS PubMed.
- N. N. Parayath, S. K. Gandham and M. M. Amiji, Nanomedicine, 2022, 17, 1355–1373 CrossRef CAS PubMed.
- E. J. Shillitoe, Head Neck Oncol., 2009, 1, 7 CrossRef PubMed.
- N. Yanaihara, N. Caplen, E. Bowman, M. Seike, K. Kumamoto, M. Yi, R. M. Stephens, A. Okamoto, J. Yokota, T. Tanaka, G. A. Calin, C. G. Liu, C. M. Croce and C. C. Harris, Cancer Cell, 2006, 9, 189–198 CrossRef CAS PubMed.
- A. L. Kasinski and F. J. Slack, Cancer Res., 2012, 72, 5576–5587 CrossRef CAS PubMed.
- M. S. Kumar, S. J. Erkeland, R. E. Pester, C. Y. Chen, M. S. Ebert, P. A. Sharp and T. Jacks, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3903–3908 CrossRef CAS PubMed.
- J. F. Wiggins, L. Ruffino, K. Kelnar, M. Omotola, L. Patrawala, D. Brown and A. G. Bader, Cancer Res., 2010, 70, 5923–5930 CrossRef CAS PubMed.
- Z. Bai, J. Wei, C. Yu, X. Han, X. Qin, C. Zhang, W. Liao, L. Li and W. Huang, J. Mater. Chem. B, 2019, 7, 1209–1225 RSC.
- J. McCarroll, J. Teo, C. Boyer, D. Goldstein, M. Kavallaris and P. A. Phillips, Front. Physiol., 2014, 5, 2 Search PubMed.
- J. A. McCarroll, T. Dwarte, H. Baigude, J. Dang, L. Yang, R. B. Erlich, K. Kimpton, J. Teo, S. M. Sagnella, M. C. Akerfeldt, J. Liu, P. A. Phillips, T. M. Rana and M. Kavallaris, Oncotarget, 2015, 6, 12020–12034 CrossRef PubMed.
- X. Zhu, Y. Xu, L. M. Solis, W. Tao, L. Wang, C. Behrens, X. Xu, L. Zhao, D. Liu, J. Wu, N. Zhang, I. I. Wistuba, O. C. Farokhzad, B. R. Zetter and J. Shi, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 7779–7784 CrossRef CAS PubMed.
- A. M. Davies, D. R. Gandara, P. N. Lara, Jr., P. C. Mack, D. H. Lau and P. H. Gumerlock, Clin. Lung Cancer, 2003, 4(Suppl 2), S68–S73 CrossRef PubMed.
- S. Chaudhary, A. Singh, P. Kumar and M. Kaushik, J. Biochem. Mol. Toxicol., 2021, 35, e22784 CrossRef CAS PubMed.
- N. Amreddy, A. Babu, R. Muralidharan, A. Munshi and R. Ramesh, Top. Curr. Chem., 2017, 375, 35 CrossRef PubMed.
- M. Talekar, M. Trivedi, P. Shah, Q. Ouyang, A. Oka, S. Gandham and M. M. Amiji, Mol. Ther., 2016, 24, 759–769 CrossRef CAS PubMed.
- I. Ito, L. Ji, F. Tanaka, Y. Saito, B. Gopalan, C. D. Branch, K. Xu, E. N. Atkinson, B. N. Bekele, L. C. Stephens, J. D. Minna, J. A. Roth and R. Ramesh, Cancer Gene Ther., 2004, 11, 733–739 CrossRef CAS PubMed.
- E. Huynh and G. Zheng, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2013, 5, 250–265 CAS.
- X. H. Do, T. D. Nguyen, T. T. H. Le, T. T. To, T. V. K. Bui, N. H. Pham, K. Lam, T. M. N. Hoang and P. T. Ha, Pharmaceutics, 2023, 15, 1523 CrossRef CAS PubMed.
- S. Wang, Q. You, J. Wang, Y. Song, Y. Cheng, Y. Wang, S. Yang, L. Yang, P. Li, Q. Lu, M. Yu and N. Li, Nanoscale, 2019, 11, 6270–6284 RSC.
- Q. Xiao, Y. Lu, M. Chen, B. Chen, Y. Yang, D. Cui, B. Pan and N. Xu, Nanoscale Res. Lett., 2018, 13, 77 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |