Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design of electron-donating group substituted 2-PAM analogs as antidotes for organophosphate insecticide poisoning†
Nalinee Kongkaew a,
Kowit Hengphasatpornb,
Yuwanda Injongkola,
Pitchayathida Mee-udornc,
Liyi Shide,
Panupong Mahalapbutrf,
Phornphimon Maitarad
a,
Kowit Hengphasatpornb,
Yuwanda Injongkola,
Pitchayathida Mee-udornc,
Liyi Shide,
Panupong Mahalapbutrf,
Phornphimon Maitarad *de,
Ryuhei Harada
*de,
Ryuhei Harada b,
Yasuteru Shigeta
b,
Yasuteru Shigeta b,
Thanyada Rungrotmongkol
b,
Thanyada Rungrotmongkol *ag and
Alisa S. Vangnai*g
*ag and
Alisa S. Vangnai*g
aProgram in Bioinformatics and Computational Biology, Graduate School, Chulalongkorn University, Bangkok 10330, Thailand. E-mail: t.rungrotmongkol@gmail.com
bCenter for Computational Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8577, Japan
cNational Center for Genetic Engineering and Biotechnology, 113 Thailand Science Park, Pathumthani 12120, Thailand
dResearch Center of Nano Science and Technology, Department of Chemistry, College of Science, Shanghai University, Shanghai 200444, China. E-mail: pmaitarad@shu.edu.cn
eDepartment of Chemistry, College of Sciences, State Key Laboratory of Advanced Special Steel, Research Center of Nano Science and Technology, School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China
fDepartment of Biochemistry, Center for Translational Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
gCenter of Excellence in Biocatalyst and Sustainable Biotechnology, Department of Biochemistry, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand. E-mail: Alisa.V@chula.ac.th
First published on 2nd November 2023
Abstract
The use of organophosphate (OPs) pesticides is widespread in agriculture and horticulture, but these chemicals can be lethal to humans, causing fatalities and deaths each year. The inhibition of acetylcholinesterase (AChE) by OPs leads to the overstimulation of cholinergic receptors, ultimately resulting in respiratory arrest, seizures, and death. Although 2-pralidoxime (2-PAM) is the FDA-approved drug for treating OP poisoning, there is difficulty in blood–brain barrier permeation. To address this issue, we designed and evaluated a series of 2-PAM analogs by substituting electron-donating groups on the para and/or ortho positions of the pyridinium core using in silico techniques. Our PCM-ONIOM2 (MP2/6-31G*:PM7//B3LYP/6-31G*:UFF) binding energy results demonstrated that 13 compounds exhibited higher binding energy than 2-PAM. The analog with phenyl and methyl groups substituted on the para and ortho positions, respectively, showed the most favorable binding characteristics, with aromatic residues in the active site (Y124, W286, F297, W338, and Y341) and the catalytic residue S203 covalently bonding with paraoxon. The results of DS-MD simulation revealed a highly favorable apical conformation of the potent analog, which has the potential to enhance reactivation of AChE. Importantly, newly designed compound demonstrated appropriate drug-likeness properties and blood–brain barrier penetration. These results provide a rational guide for developing new antidotes to treat organophosphate insecticide toxicity.
1 Introduction
Due to the increasing demand for food, higher crop yields have been produced, leading to the prevalent use of organophosphate (OPs) pesticides in agriculture for insect pest management. The Asia-Pacific region countries that utilize the most pesticides include China, India, Vietnam, and Thailand.1 The World Health Organization reports that OPs are accountable for more than 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fatalities and 3 million poisonings annually.2 OPs can infiltrate the body through ingestion, skin absorption, and inhalation and quickly move to specific toxicity target organs within the nervous system.3 Acetylcholinesterase (AChE) is an enzyme responsible for terminating the activity of acetylcholine (ACh).
000 fatalities and 3 million poisonings annually.2 OPs can infiltrate the body through ingestion, skin absorption, and inhalation and quickly move to specific toxicity target organs within the nervous system.3 Acetylcholinesterase (AChE) is an enzyme responsible for terminating the activity of acetylcholine (ACh).
The primary toxic effect of OPs is the inhibition of AChE by phosphorylation of the serine hydroxyl group, resulting in decreased AChE activity caused by acetylcholine discharge. The resulting pathophysiology comprises respiratory arrest, seizures, and often death due to the paralysis of the diaphragm and intercostal muscles.4,5
Two procedures for treating OPs poisoning exist, one before and one after exposure to OPs, which involve the use of an acetylcholine receptor antagonist, anticonvulsant, and acetylcholinesterase reactivator.6 However, these treatments have several limitations, and recent research suggests that they require further study.7 Pralidoxime, obidoxime, HI-6, trimedoxime, and methoxime are common oxime-based reactivators or nerve antidotes. Only pralidoxime also known as 2-pyridinium aldoxime or 2-PAM received FDA8 and became the first commercial antidote for treating organophosphorus nerve agents (OPNAs) in 1956.9 However, in vivo studies have shown that approximately 90% of the administered dose of 2-PAM reactivators remains in the blood and peripheral tissues due to their low efficiency in crossing the blood–brain barrier (BBB).4,10 Consequently, only 24–34% reactivation of AChE occurs in the brain. Therefore, there is a need to enhance the efficiency of antidotes in crossing the BBB and reactivating AChE.11 To overcome this limitation, the researchers endeavored to develop novel antidotes by methyl scanning on the pyridinium core of the 2-PAM template.12
Theoretical research has been utilized as an alternative to study the interactions between inhibited enzymes and reactivators to gain additional structural insights into these interactions. Our own N-layered Integrated molecular Orbital and Molecular mechanics (ONIOM) method is a hybrid approach that distinguishes itself from the “generic” two-layer QM/MM method (i.e., the IMOMM and IMOMO methods), which is a “subtractive” or “extrapolative” scheme. ONIOM was developed by Morokuma et al.13,14 and has been applied in numerous studies to investigate binding energy, including the binding of HUP A and GAL to the binding pocket of AChE,15 analysis of the hybrid transfer mechanism for NiAc2,16 binding of ellagic acid to the CK2 protein.17 Similar to ONIOM methods, fragment molecular orbital (FMO) calculations provide insights into the electronic structure and energetics of complex molecular interactions in a large biomolecular system, reducing time-consuming computational costs, as shown in previous studies, the binding interaction of potent compounds to SARS-CoV-2 main protease.18,19 Previous studies have also shown that electron-donating groups (EDGs) can improve effectively cross the blood–brain barrier (BBB) in new series of activatable photoacoustic (PA) probes for Alzheimer's disease20 and the substitution of EDGs can increase electron density in a molecule through the adjacent carbon atoms' effect, making nucleophiles stronger. For example, installing a strong EDG on the ortho or para position of some polyhalogenated pyridines produced more negative changes in the system.21 Moreover, boosting the electron density within the structure of 2-PAM, oriented in an apical (in-line) conformation towards paraoxon, enhances interactions with several residues in the catalytic active site (CAS) residues of AChE. These residues, namely W86, Y119, Y124, Y133, E202, S203, W439, H447, Y449, and also glycines.22 The successful drug design has been reported, such as the substitution of benzothiadiazide for interaction with phospholipid,23 phenolic dendritic for antioxidant activity,24 and tyramine oxidase inhibition.25
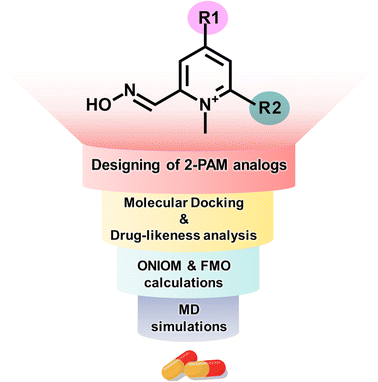
In this study, we employed computational methods to design novel analogs of 2-PAM by incorporating EDGs onto the pyridinium core to enhance binding affinity and BBB permeability (Fig. 1). We utilized four computational techniques: molecular docking, drug-likeness analysis, ONIOM calculations, and FMO method. We evaluated the ligand-binding stability using distance-based selection molecular dynamics (DS-MD) simulations. These approaches can yield insights into the development of a more potent AChE reactivator.
2 Methodology
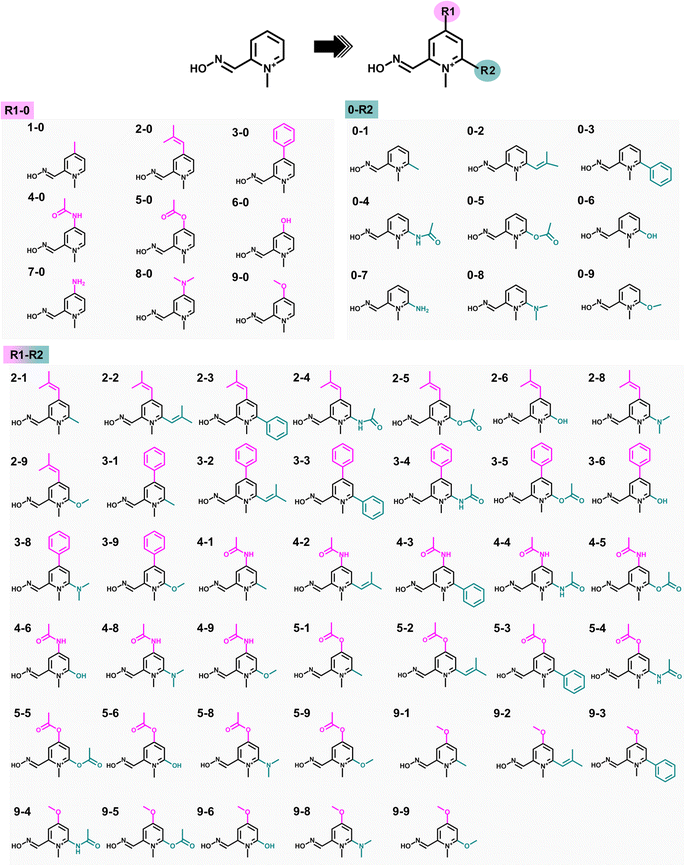
2.1 Designing a series of 2-PAM analogs
Substituting the electron-donating groups (EDGs) at the para (R1) and/or ortho (R2) positions of the pyridinium ring of 2-PAM (Fig. 2) was a strategic choice, as these positions can tolerate methylation,12 and the oxime binding pocket consists of high aromatic content residues.26 The addition of EDGs can increase electron density to the aromatic system, owing to their strong impact on the electronic structure, making it more nucleophilic, hydrophobic, and, thus, better able to cross the blood–brain barrier (BBB).12,20 This study included three weak, two moderate, and four strong EDGs (ESI Table S1†). The nine distinct EDGs were individually introduced at R1 or R2 positions to generate the 2-PAM analogs. Furthermore, the combination of potential substituents at both positions was considered to produce R1-R2 analogs.2.2 Molecular docking and drug-likeness prediction
The study utilized the three-dimensional structure of human acetylcholinesterase in complex with paraoxon and 2-PAM in an unproductive binding mode, obtained from the Protein Data Bank with PDB entry code 5HFA.27 The missing amino acids at 257–264 position were added by homology modeling method using SWISS-MODEL webtool.28 Then, the covalent bond between paraoxon and S203 was created following the Amber Basic Tutorials – Tutorial A26.29 The protonation states of all ionizable residues and ligands were assigned using the PROPKA version 3.1 in PDB2PQR web server30 and MarvinSketch software31 at pH 7.0, respectively. The 3D structure of analogs was constructed using GaussView6 and subsequently optimized using density functional theory (DFT) with the B3LYP/6-31G* basis set implemented in Gaussian16.32 The structure of AChE with paraoxon covalently bound was prepared for refinement by undergoing relaxation through a 100 ns molecular dynamics (MD) simulation in an aqueous solution using AMBER20,33 following the previous study.34 The last snapshot, excluding waters and neutralized ions, was used for docking study.The Autodock Vina 1.2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19,35 was employed for molecular docking of 2-PAM and designed compounds in an “apical” orientation that fulfilled two criteria. Firstly, the distance between the oxime oxygen atom of compound and the phosphorus atom of paraoxon (dOP) was restricted to less than 8 Å. Secondly, the approaching oxime oxygen was oriented at approximately 180° to the paraoxon phosphorus and S203 hydroxyl oxygen (θOPO).36 The analogs with a single substitution at the para or ortho position, denoted as R1-0 or 0-R2, were subjected to docking with 20 poses for each compound. A 15 × 15 × 15 Å cubical grid box centered on the paraoxon phosphorus was created to cover the AChE active site. The resulting pose was visualized using Accelrys Discovery Studio37 and UCSF Chimera,38 taking into account the near attack conformation (NAC) approach, where dOP was considered.39
19,35 was employed for molecular docking of 2-PAM and designed compounds in an “apical” orientation that fulfilled two criteria. Firstly, the distance between the oxime oxygen atom of compound and the phosphorus atom of paraoxon (dOP) was restricted to less than 8 Å. Secondly, the approaching oxime oxygen was oriented at approximately 180° to the paraoxon phosphorus and S203 hydroxyl oxygen (θOPO).36 The analogs with a single substitution at the para or ortho position, denoted as R1-0 or 0-R2, were subjected to docking with 20 poses for each compound. A 15 × 15 × 15 Å cubical grid box centered on the paraoxon phosphorus was created to cover the AChE active site. The resulting pose was visualized using Accelrys Discovery Studio37 and UCSF Chimera,38 taking into account the near attack conformation (NAC) approach, where dOP was considered.39
The analogs R1-0 and 0-R2, which showed better binding affinity than 2-PAM, were chosen to construct the combined analogs (R1-R2, Fig. 2). All focused analogs were then evaluated for their drug-likeness, taking into consideration various pharmacological characteristics such as molecular weight (M.W.), number of hydrogen bond donors (HBD) and acceptors (HBA), rotatable bonds (R. B.), polar surface area (PSA), and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, as per Lipinski's rule of five (Ro5), as well as their ability to penetrate the blood–brain barrier (BBB)40,41 using freely available software such as DataWarrior42 and SwissADME.43 These characteristics are essential in the rational design of orally active drugs and central nervous system (CNS) active candidates.44 After that, the screened R1-R2 analogs were subjected to perform molecular docking, following the previously described protocol.
P, as per Lipinski's rule of five (Ro5), as well as their ability to penetrate the blood–brain barrier (BBB)40,41 using freely available software such as DataWarrior42 and SwissADME.43 These characteristics are essential in the rational design of orally active drugs and central nervous system (CNS) active candidates.44 After that, the screened R1-R2 analogs were subjected to perform molecular docking, following the previously described protocol.
2.3 ONIOM and FMO calculations
Theoretical investigation of the binding energy of 2-PAM analogs bound to inhibited AChE was carried out using two-layer ONIOM calculations (ONIOM2)13 implemented in Gaussian16.32 The high-level energy of the actual system (Ehighreal), which is the target energy, was approximated by eqn (1).13| Ehighreal ≈ Ehigh:lowONIOM2 = Ehighmodel + Elowreal − Elowmodel | (1) |
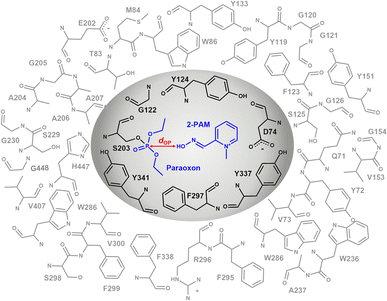
The inner layer, represented by the grey region in Fig. 3, comprising 2-PAM or its analogs, paraoxon covalently bonded with S203 and key interacting residues (D74, G122, Y124, F297, Y337, and Y341) suggested by iGEMDOCK,45 was treated at the B3LYP/6-31G* level of theory. In the outer layer, the side chains of the residues within 10 Å of the oxime oxygen, as shown in the white region of Fig. 3, were permitted to move freely, while the remaining atoms were held fixed. The outer layer, was treated with the computationally less expensive universal force field (UFF).46 Single-point calculations were performed using B3LYP/6-31G*:PM7 and MP2/6-31G*:PM7, taking into account the polarizable continuum model (PCM) solvation effect to obtain accurate calculations of large molecules in solution.47
For the computation of pair interaction energy (PIE) and its decomposition (PIEDA) between the focused compound and individual amino acids exhibiting significant binding energies, fragment molecular orbital (FMO) calculations were employed at the PCM-RIMP2/6-31G* level of theory. The PIE calculation for each compound bound to inhibited AChE was conducted utilizing the subsequent equation:
| PIE = EESij + ECT+mixij + EDIij + EEXij + GPCMSol | (2) |
The molecular electrostatic potential (MEP) map of the potent analog and 2-PAM was created from the result of ligand optimization based on PCM-MP2/6-31G* level of theory, acquired through natural bond orbital (NBO) charges analysis.49 Additionally, the frontier molecular orbital theory of MO theory was used to determine and describe HOMO/LUMO interactions using Multiwfn.50
2.4 Molecular dynamics simulation
To assess the stability of ligand binding, we performed distance-based selection molecular dynamics (DS-MD) simulation using the concept of parallel cascade selection molecular dynamics (PaCS-MD).51–55 Briefly, the original PaCS-MD independently repeats multiple MD simulations from a set of initial structures, where they are selected by referring to several measures. In contrast, DS-MD repeats a single MD simulation starting from an initial structure selected by referring to the distance between a given ligand and the target protein. More specifically, DS-MD repeatedly conducts a MD simulation from the snapshot with the shortest distance for cycles, enabling one to assess the ligand-binding stability.The optimized configuration of either 2-PAM or the most potent designed analog binding to inhibited AChE derived from ONIOM calculations was prepared using the tLeap module in AmberTools21.56 Gaussian16 was used to calculate the electrostatic potential charges (ESP) for the considered ligand using the B3LYP/6-31+G(d,p) method.32,57 The ligand charges were then fitted using restrained ESP and topology parameters with the antechamber module in AMBER20.33 The generalized Amber force field (GAFF)19,58,59 was applied to both ligands according to the standard protocol.60,61 The topology and coordinate files were converted to Gromacs format to conduct the DS-MD simulation.62 According to this technique, the distance between the ith and jth atoms (dij) needs to be defined. In this case, the dij was set between the oxime oxygen of the ligand and paraoxon phosphorus (dOP), as mentioned previously. For this study, three-independent DS-MD simulations were performed. Each simulation encompassed 100 cycles, and each cycle extended for 100 ps, leading to a total simulation time of 1 ns. During the DS-MD simulations, we conducted each cycle for 100 ps, selecting the structure with the shortest value of dij as the initial structure for the subsequent cycle. The reactive trajectories of each replication were analyzed in terms of dOP and θOPO using the CPPTRAJ program63 to assess the ligand-binding stability and mechanism.
We calculated 2D-free energy landscapes (2D FELs) of both compounds to quantitively characterize their binding processes. To calculate the 2D FELs, Markov state models (MSMs) were built using all the trajectories of each system. More specifically, 3 trials of the DS-MD simulations for each compound (100 ps × 100 cycles per the DS-MD simulation). Indeed, we used the trajectories generated after the 30th cycle (Fig. S1†) to build each MSM by specifying a lag time (25 ps) using the EMMA program.64 Finally, the 2D PMFs were calculated as a function of X and Y, which are the coordinates of dOP and θOPO using matplotlib65 and UCSF Chimera 1.16 software.66
3 Results and discussion
3.1 Docking study and drug-likeness analysis of 2-PAM analogs
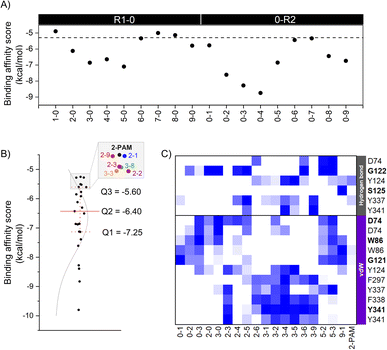
The eighteen designed analogs with EDGs substitutions at the para (R1) or ortho (R2) position on the 2-PAM pyridinium ring in Fig. 2 were individually docked into the inhibited AChE active site using the Autodock Vina. By considering the apical conformation, the binding affinity scores for the R1-0 and 0-R2 analogs in complex with the inhibited AChE are plotted in Fig. 4A. There were five analogs from R1-0 group (2-0, 3-0, 4-0, 5-0, and 9-0) and eight analogs from 0-R2 group (0-1, 0-2, 0-3, 0-4, 0-5, 0-6, 0-8, and 0-9) that exhibited superior binding affinity scores than 2-PAM (−5.3 kcal mol−1, dashed line in Fig. 4A). Consequently, these potent analogs from R1-0 and 0-R2 groups were combined to form the R1-R2 analogs, consisting of 40 compounds.A total of 53 analogs and 2-PAM were investigated for the drug-likeness and pharmacological properties using DataWarrior and SwissADME.42,43 The results were presented in ESI Table S2,† which included molecular weight (M.W.), number of hydrogen bond donors (HBD) and acceptors (HBA), calculated lipophilicity (c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P), polar surface area (PSA), number of heavy atoms, and BBB permeability. Only 24 analogs, including 2 R1-0, 3 0-R2, and 19 R1-R2, passed Lipinski's rule of five criteria, which is crucial for drug discovery. These criteria included M.W. ≤ 450 Da, HBD ≤ 7, HAD ≤ 3, c
P), polar surface area (PSA), number of heavy atoms, and BBB permeability. Only 24 analogs, including 2 R1-0, 3 0-R2, and 19 R1-R2, passed Lipinski's rule of five criteria, which is crucial for drug discovery. These criteria included M.W. ≤ 450 Da, HBD ≤ 7, HAD ≤ 3, c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ≤ 5, PSA ≤ 60–70 Å, #heavy atoms 12 ≤ X ≤ 30, and BBB permeability.
P ≤ 5, PSA ≤ 60–70 Å, #heavy atoms 12 ≤ X ≤ 30, and BBB permeability.
Moreover, BBB permeability plays an important role in the treatment of 2-PAM poisoning in both the peripheral nervous system (PNS) and CNS,36 making it a focal point of this study. Computational calculations in Table S2† predicted a slight increase in lipophilicity (c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) and a slight decrease in solubility for these analogs, which is an indicator of lipophilicity.67 Additionally, when compared with isatin derivatives,68 which are novel reactivator candidates, our 2-PAM analogs demonstrated significantly higher values in terms of c
P) and a slight decrease in solubility for these analogs, which is an indicator of lipophilicity.67 Additionally, when compared with isatin derivatives,68 which are novel reactivator candidates, our 2-PAM analogs demonstrated significantly higher values in terms of c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, especially 2-3 and 3-3. Such compounds with higher c
P, especially 2-3 and 3-3. Such compounds with higher c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, indicating increased lipophilicity, are more likely to cross the BBB and enter the CNS. However, this relationship is not straightforward and may be influenced by other factors such as M.W., charge, and polarity. While lipophilic compounds can cross the BBB more efficiently, they may also be more likely to accumulate in adipose tissues and have a longer duration of action. Very high c
P values, indicating increased lipophilicity, are more likely to cross the BBB and enter the CNS. However, this relationship is not straightforward and may be influenced by other factors such as M.W., charge, and polarity. While lipophilic compounds can cross the BBB more efficiently, they may also be more likely to accumulate in adipose tissues and have a longer duration of action. Very high c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values may also result in poor solubility and formulation challenges. Hence, striking the right balance between BBB permeability and physicochemical properties emerges as vital in drug discovery and development. Thus, only these 24 analogs could be promising and effective AChE reactivators by considering the permeable ability.
P values may also result in poor solubility and formulation challenges. Hence, striking the right balance between BBB permeability and physicochemical properties emerges as vital in drug discovery and development. Thus, only these 24 analogs could be promising and effective AChE reactivators by considering the permeable ability.
To further identify potent combined (R1-R2) analogs, we conducted molecular docking of 19 R1-R2 analogs towards AChE using criteria employed in the first-round screening. Analyzing the docking results statistically (Fig. 4B), we observed that both 2-PAM and 6 analogs fell within the lowest quartile (Q4) range, highlighted in the grey box. Therefore, only 18 analogs in within the Q1–Q3 range were chosen for subsequent ONIOM calculations, in comparison with 2-PAM.
In the ONIOM calculation, the important residues (D74, G122, Y124, F297, Y337, and Y341) identified through a consensus score of interacting residues ≥50% (Fig. 4C) analyzed from iGEMDOCK were set in the inner layer of the ONIOM calculations. Remarkably, we observed that G122 played a pivotal role in forming a hydrogen bond with these screened analogs.
By introducing EDGs at the para and/or ortho positions on the 2-PAM structure, the interaction profile of these analogs was confirmed to possess the capability to establish the vdW, π–π, and hydrogen bond interactions with several AChE residues in the CAS and peripheral anionic site (PAS). By considering the strength of the EDGs, the weakly donating groups (methyl (1), 2-methyl-2-butene (2), and phenyl (3) groups) showed a high contribution found in all 18 potent analogs. Particularly, the phenyl group-containing analogs served as noteworthy functional groups, facilitating interactions and improving the binding affinity score, similar to the 4-pyridine aldoxime (4-PA) in earlier research.69 Among the four key interacting residues identified (Y124, F297, Y337, and Y341), most analogs exhibited vdW and π–π interactions, further emphasizing the significance of these interactions in enhancing their binding capabilities due to the aromatic residues in the AChE binding pocket. There are crucial for forming a π–π interaction with the antidote and new analog, such as LLNL-02, in an apical conformation.36 On the other hand, single phenyl group substitution analogs (0-3 and 3-0) form the vdW to another set of residues in the binding pocket, which are D74, W86, and G121 (Fig. 4C).
3.2 Extrapolated QM-based binding energy and interaction
We investigated the binding energies and geometric parameters of 2-PAM analogs using B3LYP/6-31G*:UFF optimization and single point calculations with B3LYP/6-31G*:PM7//B3LYP/6-31G*:UFF and MP2/6-31G*:PM7//B3LYP/6-31G*:UFF levels of theory, with the inclusion of PCM. As discussed earlier, the reaction distance dOP is an essential factor in determining the effectiveness of an analog as an AChE reactivator. The energetic and dOP results obtained from the ONIOM calculations are presented in Tables 1 and S3.† The ONIOM2 (B3LYP/6-31G*:UFF) calculation reported energies ranging from −4331.56 to −4752.88 kcal mol−1 and dOP ranging from 2.90 to 3.76 Å for considered analogs, while those of 2-PAM were −4292.53 kcal mol−1 and 4.42 Å. The PCM-ONIOM2 single-point calculations of 18 analogs compared to 2-PAM (ΔPCM-ONIOM2) revealed that the incorporation of various EDGs resulted in a substantial enhancement in binding affinity (≤−15 kcal mol−1) for 3 and 8 analogs in B3LYP and MP2 calculations, respectively. B3LYP includes its computational efficiency, reliability for ground state energies and geometry optimization, applicability to various systems, and ability to predict electronic excitation energies. In comparison, the MP2 method provides a more accurate description of the electrostatic, steric, polarization, and dispersion interactions between analogs and residues within the pocket.70 Only a 3-1 analog shows outstanding ONIOM2 results in both B3LYP and MP2 (Table 1 and ESI Fig. S2†). Hence, we selected a 3-1 analog to further insight evaluation regarding pair interaction energy (PIE) and decomposition analysis (PIEDA). The incorporation of a phenyl group onto the para position and a methyl group onto the ortho position of the pyridinium core led to a reduction in the dOP in the 3-1 complex, with a value of 3.54 Å. The shortened dOP could facilitate faster and more efficient reactivation via a nucleophilic attack on the phosphorus atom of the conjugated S203-paraoxon.71| ONIOM2 B3LYP/6-31G*:UFF | ΔPCM-ONIOM2a | dOP (Å) | ||
|---|---|---|---|---|
| B3LYP/6-31G*:PM7 | MP2/6-31G*:PM7 | |||
| *ONIOM2: optimization; ΔPCM-ONIOM2a: single point calculations with PCM (polarizable continuum model) of each analog compared to reference ligand (2-PAM). | ||||
| 0-1 | −4331.56 | −33.41 | −1.77 | 3.39 |
| 0-2 | −4448.27 | 22.14 | 12.40 | 3.32 |
| 0-3 | −4523.29 | −6.92 | −19.01 | 3.41 |
| 2-0 | −4448.28 | 15.54 | −9.27 | 3.47 |
| 3-0 | −4523.29 | 9.21 | −2.66 | 3.76 |
| 2-3 | −4681.05 | −10.76 | −31.65 | 2.90 |
| 2-4 | −4658.01 | 2.55 | −14.25 | 3.39 |
| 2-5 | −4676.14 | 23.00 | 5.71 | 3.28 |
| 2-6 | −4523.56 | 21.98 | 10.76 | 3.30 |
| 3-1 | −4562.62 | −32.77 | −43.43 | 3.54 |
| 3-2 | −4679.34 | 15.17 | −19.38 | 3.07 |
| 3-4 | −4733.04 | −5.67 | −24.61 | 2.90 |
| 3-5 | −4751.17 | −11.79 | −28.89 | 3.22 |
| 3-6 | −4600.48 | 4.33 | −6.89 | 3.31 |
| 3-9 | −4637.82 | 2.26 | −15.87 | 2.95 |
| 5-2 | −4676.15 | 25.09 | 11.93 | 3.14 |
| 5-3 | −4752.88 | −0.74 | −19.16 | 3.23 |
| 9-1 | −4446.09 | −26.59 | 2.55 | 3.30 |
| 2-PAM | −4292.53 | 0 | 0 | 4.42 |
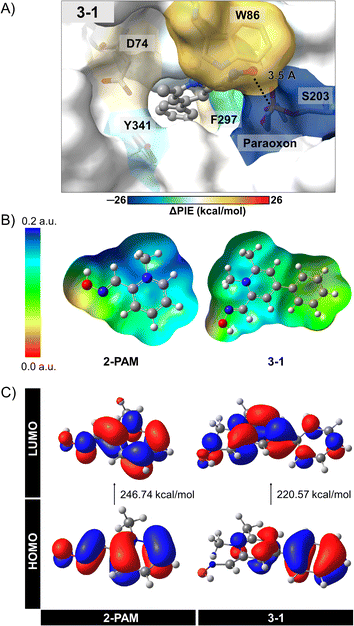
The individual interactions from PCM-RIMP2/6-31G* FMO calculations can provide insights into the specific interactions between 3-1 analog and the active site residues of inhibited AChE compared to those of 2-PAM. By analyzing these individual interactions, we can identify key interacting residues involved in binding and reactivation, thereby guiding the design of more effective AChE reactivators. We observed significant interactions of certain amino acids in the 3–1 complex (Fig. 5A, ESI Table S4, and Fig. S3†).
According to the FMO calculation, the positively charged quaternary nitrogen atom of the pyridinium ion in both compounds exhibits a robust electrostatic interaction (EESij) with D74 (Fig. S3A†). The pyridinium ring can form a π-donor hydrogen bond and vdW interactions with Y124, considered by dispersion (EDIij). In the 3-1 analog, an additional phenyl ring interacts with W286 and F297 near the back door of AChE, mainly through EDIij and EESij, respectively. This interaction potentially stabilizes and maintains ligand binding after approaching the phosphorus atom of S203-paraoxon, facilitating the mechanism. Although an extra methyl group in the 3-1 analog does not frequently interact with the bound residues, it likely contributes to optimizing the ligand arrangement within the binding pocket. As a result, the oxygen atom in the 3-1 analog demonstrates a relatively strong interaction with S203-paraoxon (−33.09 kcal mol−1), involving electrostatic, charge exchange, dispersion, and solvation energies (Fig. S3†). These interactions could stabilize the more flexible oxime portion of the molecule.36
To better understand the interactions of 3-1 analog, we conducted further investigations using electronic properties, including MEP maps, NBO, and Egap with the PCM-MP2/6-31G* computational level. The designed 3-1 involved adding more EDGs, making the –OH group more reactive and possibly resulting in more free enzymes. Fig. 5B and C illustrate the MEP maps and the molecular orbitals (HOMO and LUMO) of 3-1 compared to 2-PAM. Charge distribution in Fig. 5B showed a significant difference among these ligands, i.e., adding the phenyl and methyl groups at the para and ortho positions in 3-1 led to an increased electron density distribution within the molecule. This enlargement can elevate the nucleophilic attack potential of the 3-1 analog compared to 2-PAM.
The HOMO–LUMO gap provides insights nucleophilic attack capability of these compounds (Fig. 5C). A narrower HOMO–LUMO gap indicates greater electron availability for nucleophilic interactions. In this study, 3–1 analog exhibited a HOMO–LUMO gap of 220.57 kcal mol−1, while 2-PAM showed 246.74 kcal mol−1. The Egap of 3-1 analog showed smaller than 2-PAM, about 26 kcal mol−1, indicating that a small HOMO–LUMO gap can enhance charge transfer,72 leading to the occurrence of nucleophilic attacks and increased ability to cross the BBB. These insights could be helpful in the development of more effective AChE reactivators.
3.3 Molecular dynamics simulation
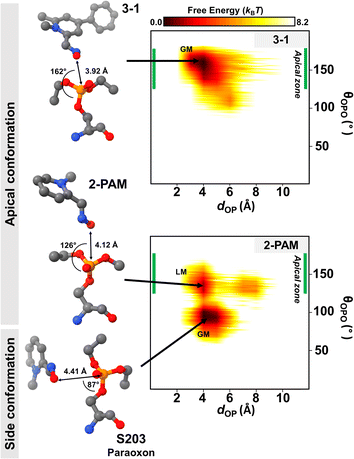
Generally, the antidote should not bind tightly to AChE because it acts as a nucleophilic agent, forming a transient covalent bond with the phosphorus of paraoxon in the active site of AChE, which is essential for the reactivation process.73,74 Thus, we conducted DS-MD simulations to thoroughly examine the conformational behavior and stability of the 3-1 analog binding to inhibited AChE, comparing it with 2-PAM. The conformational sampling from the DS-MD simulation was analyzed regarding free energy landscapes (FELs) as a function of dOP and θOPO. Notably, the optimal θOPO for an apical conformation is 120° < θOPO < 180°, as shown in the green line in Fig. 6. The 2D FELs (Fig. 6) provide valuable insights into significant ligand stability observed in 2-PAM and 3-1 analog. The initial structures of these ligands derived from the ONIOM calculation were in the apical conformation. According to the 2D FELs, only the 3-1 analog in the apical conformation (global minimum, GM) was sustained. In contrast, 2-PAM exhibited multiple states, including side (GM) and apical conformations (local minimum, LM) (Fig. 6). Once 2-PAM moves to the GM, it becomes difficult to escape from this location and return to its original orientation at LM due to a high free-energy barrier (∼5.0 kBT), which means 2-PAM tends to approach the paraoxon at the side conformation based on our free-energy analyses. This confirmed that the novel-designed analog, 3-1, shows a higher chance to reactivate by forming a covalent bond with the phosphorus of paraoxon, which is superior to 2-PAM. This finding firmly establishes the superiority of the novel potent analog in achieving an optimal binding orientation, especially concerning the reactivation reaction, facilitating highly efficient enzyme re-inhibition.75 Our comprehensive 2D FEL analyses shed light on the superior binding orientation and stability of the 3-1 analog compared to 2-PAM, offering valuable insights for designing and developing more effective AChE reactivators with enhanced enzyme re-inhibition properties.4 Conclusions
This study has demonstrated the potential of electron substitution with donating groups at the para and ortho positions of the pyridinium core of 2-PAM for producing effective reactivators to treat organophosphate pesticide poisoning. Our computational methods identified the most promising functional group for these novel compounds to increase binding affinity score, AChE reactivation, and BBB permeability was the phenyl and methyl groups added to para and ortho. The key residues in the active site of inhibited AChE showed significant interactions with the 3-1 complexes were G121, Y124, W286, F297, W338, Y341, and the catalytic residue S203 covalently bonding with paraoxon calculated by the FMO method. The increase in charge distribution and nucleophilic property of 3-1 was confirmed by MEP map and Egap analyses. The DS-MD simulations of 3-1 analog displayed high stability in the apical conformation, enhancing the reactivation mechanism. In conclusion, the superiority of the novel 3-1 analog over 2-PAM in achieving an optimal binding orientation and ligand-binding stability offers valuable insights for designing more effective AChE reactivators with enhanced enzyme re-inhibition properties, benefiting organophosphate poisoning treatments.Conflicts of interest
The authors report no conflict of interest, financial or otherwise.Acknowledgements
This study is funded by Thailand Science Research, Innovation Fund, Chulalongkorn University (CU_FRB65_hea(63)_126_23_56). N. K. gratefully acknowledges the Development and Promotion of Science and Technology Talents Project (DPST) for the scholarship, the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund: GCUGR1125661074M), and the Overseas Presentations of Graduate Level Academic Thesis from Graduate School, Chulalongkorn University. Y. I. thanks the Second Century Fund (C2F), Chulalongkorn University. National Research Council of Thailand (NRCT) N42A640329 support is appreciated. Y. S. acknowledges the support received from various funding sources, including the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan through the Grant-in-aid for innovative research “Biometal Sciences” (grant no. 22H04800), as well as the Japan Agency for Science and Technology (JST) through the CREST program “Precise arrangement toward functionality” (grant no. JPMJCR20B3). The research was also funded by the Mishima Kaiun Memorial Foundation, the Sumitomo Foundation in Japan, and the Multidisciplinary Cooperative Research Program in CCS at the University of Tsukuba.References
- R. Sapbamrer and S. Hongsibsong, Environ. Sci. Pollut. Res., 2019, 26, 18267–18290 CrossRef CAS PubMed.
- O. World Health and P. United Nations Environment, Public health impact of pesticides used in agriculture, https://apps.who.int/iris/handle/10665/39772, accessed January, 2022 Search PubMed.
- F. Sánchez-Santed, M. T. Colomina and E. H. Hernández, Cortex, 2016, 74, 417–426 CrossRef PubMed.
- M. Jokanović, M. P. Stojiljković, B. Kovač and D. Ristić, in Handbook of Toxicology of Chemical Warfare Agents, Elsevier, 2020, pp. 1145–1159 Search PubMed.
- A. S. Iyengar and A. H. Pande, Protein J., 2016, 35, 424–439 CrossRef CAS PubMed.
- A. Blumenberg, R. Benabbas, I. S. deSouza, A. Conigliaro, L. Paladino, E. Warman, R. Sinert and S. W. Wiener, J. Med. Toxicol., 2018, 14, 91–98 CrossRef CAS PubMed.
- P. Masson, Toxicol. Lett., 2011, 206, 5–13 CrossRef CAS PubMed.
- J. Dhuguru, E. Zviagin and R. Skouta, Pharmaceuticals, 2022, 15(6), 121 Search PubMed.
- S. Mukherjee and R. D. Gupta, J. Toxicol., 2020, 2020, 3007984 Search PubMed.
- M. N. F. Norrrahim, M. A. I. A. Razak, N. A. A. Shah, H. Kasim, W. Y. W. Yusoff, N. A. Halim, S. A. M. Nor, S. H. Jamal, K. K. Ong and W. M. Z. W. Yunus, RSC Adv., 2020, 10, 4465–4489 RSC.
- J. Acharya, D. K. Dubey, A. K. Srivastava and S. K. Raza, Toxicol. In Vitro, 2011, 25, 251–256 CrossRef CAS.
- A. Gambino, J. C. Burnett and K. Koide, ACS Med. Chem. Lett., 2020, 11, 1893–1898 CrossRef CAS.
- L. W. Chung, W. Sameera, R. Ramozzi, A. J. Page, M. Hatanaka, G. P. Petrova, T. V. Harris, X. Li, Z. Ke and F. Liu, Chem. Rev., 2015, 115, 5678–5796 CrossRef CAS PubMed.
- T. Vreven and K. Morokuma, Annu. Rep. Comput. Chem., 2006, 2, 35–51 CAS.
- N. Kitisripanya, P. Saparpakorn, P. Wolschann and S. Hannongbua, Nanomed. Nanotechnol. Biol. Med., 2011, 7, 60–68 CrossRef CAS PubMed.
- I. Yildiz, ACS Omega, 2021, 6, 22422–22428 CrossRef CAS PubMed.
- A. Shahraki and A. Ebrahimi, New J. Chem., 2019, 43, 15983–15998 RSC.
- K. Hengphasatporn, P. Wilasluck, P. Deetanya, K. Wangkanont, W. Chavasiri, P. Visitchanakun, A. Leelahavanichkul, W. Paunrat, S. Boonyasuppayakorn and T. Rungrotmongkol, J. Chem. Inf. Model., 2022, 62, 1498–1509 CrossRef CAS.
- N. Darai, K. Hengphasatporn, P. Wolschann, M. Wolfinger, Y. Shigeta, T. Rungrotmongkol and R. Harada, Bull. Chem. Soc. Jpn., 2023, 96, 677–685 CrossRef CAS.
- S. Wang, Z. Sheng, Z. Yang, D. Hu, X. Long, G. Feng, Y. Liu, Z. Yuan, J. Zhang, H. Zheng and X. Zhang, Angew. Chem., Int. Ed., 2019, 58, 12415–12419 CrossRef CAS PubMed.
- E. Benassi, T. Vaganova, E. Malykhin and H. Fan, Phys. Chem. Chem. Phys., 2022, 24, 4002–4021 RSC.
- Y. Pourshojaei, A. Abiri, K. Eskandari, Z. Haghighijoo, N. Edraki and A. Asadipour, Sci. Rep., 2019, 9, 19855 CrossRef CAS PubMed.
- Z. Hu and J. Marti, Membranes, 2022, 12, 331 CrossRef CAS PubMed.
- C. Y. Lee, A. Sharma, J. Semenya, C. Anamoah, K. N. Chapman and V. Barone, Antioxidants, 2020, 9, 189 CrossRef CAS PubMed.
- S. Hruschka, S. Yoshida, K. L. Kirk and G. Haufe, J. Fluorine Chem., 2008, 129, 875–880 CrossRef CAS PubMed.
- Y. Xu, J.-P. Colletier, M. Weik, H. Jiang, J. Moult, I. Silman and J. L. Sussman, Biophys. J., 2008, 95, 2500–2511 CrossRef CAS PubMed.
- M. C. Franklin, M. J. Rudolph, C. Ginter, M. S. Cassidy and J. Cheung, Proteins: Struct., Funct., Bioinf., 2016, 84, 1246–1256 CrossRef CAS PubMed.
- A. Waterhouse, M. Bertoni, S. Bienert, G. Studer, G. Tauriello, R. Gumienny, F. T. Heer, T. A. P. de Beer, C. Rempfer, L. Bordoli, R. Lepore and T. Schwede, Nucleic Acids Res., 2018, 46, W296–W303 CrossRef CAS PubMed.
- S. Ubonprasert, J. Jaroensuk, W. Pornthanakasem, N. Kamonsutthipaijit, P. Wongpituk, P. Mee-Udorn, T. Rungrotmongkol, O. Ketchart, P. Chitnumsub, U. Leartsakulpanich, P. Chaiyen and S. Maenpuen, J. Biol. Chem., 2019, 294, 10490–10502 CrossRef CAS PubMed.
- T. J. Dolinsky, P. Czodrowski, H. Li, J. E. Nielsen, J. H. Jensen, G. Klebe and N. A. Baker, Nucleic Acids Res., 2007, 35, W522–W525 CrossRef PubMed.
- T. Ten Brink and T. E. Exner, J. Comput.-Aided Mol. Des., 2010, 24, 935–942 CrossRef PubMed.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, G. Petersson and H. Nakatsuji, Program computer, Wallingford, CT, 2016 Search PubMed.
- D. Case, K. Belfon, I. Ben-Shalom, S. R. Brozell, D. Cerutti, T. Cheatham, Cruzeiro III, T. A. Darden, R. Duke, G. Giambasu, M. Gilson, H. Gohlke, A. W. Goetz, R. Harris, S. Izadi, S. A. Izmailov, K. Kasavajahala, A. Kovalenko, R. Krasny and P. Kollman, Amber20, 2020 Search PubMed.
- X.-Z. He and R. A. Dixon, Arch. Biochem. Biophys., 1996, 336, 121–129 CrossRef CAS PubMed.
- J. Eberhardt, D. Santos-Martins, A. F. Tillack and S. Forli, J. Chem. Inf. Model., 2021, 61, 3891–3898 CrossRef CAS PubMed.
- B. J. Bennion, M. A. Malfatti, N. A. Be, H. A. Enright, S. Hok, C. L. Cadieux, T. S. Carpenter, V. Lao, E. A. Kuhn and M. W. McNerney, Sci. Rep., 2021, 11, 15567 CrossRef CAS PubMed.
- BIOVIA, Program computer, Dassault Systèmes, San Diego, CA, USA, 2020 Search PubMed.
- E. F. Pettersen, T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng and T. E. Ferrin, J. Comput. Chem., 2004, 25, 1605–1612 CrossRef CAS PubMed.
- S. Hur and T. C. Bruice, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 12015–12020 CrossRef CAS PubMed.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 2012, 64, 4–17 CrossRef.
- T. Zorbaz and Z. Kovarik, Period. Biol., 2020, 35–54 CrossRef.
- T. Sander, J. Freyss, M. von Korff and C. Rufener, J. Chem. Inf. Model., 2015, 55, 460–473 CrossRef CAS PubMed.
- A. Daina, O. Michielin and V. Zoete, Sci. Rep., 2017, 7, 42717 CrossRef PubMed.
- R. Daneman and A. Prat, Cold Spring Harbor Perspect. Biol., 2015, 7, a020412 CrossRef PubMed.
- K.-C. Hsu, Y.-F. Chen, S.-R. Lin and J.-M. Yang, BMC Bioinf., 2011, 12, S33 CrossRef PubMed.
- A. K. Rappe, C. J. Casewit, K. S. Colwell, W. A. Goddard III and W. M. Skiff, J. Am. Chem. Soc., 1992, 114, 10024–10035 CrossRef CAS.
- T. Vreven, B. Mennucci, C. O. da Silva, K. Morokuma and J. Tomasi, J. Chem. Phys., 2001, 115, 62–72 CrossRef CAS.
- D. Fedorov, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2017, 7, e1322 Search PubMed.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- R. Harada and A. Kitao, J. Chem. Phys., 2013, 139, 035103 CrossRef PubMed.
- R. Harada, Y. Takano, T. Baba and Y. Shigeta, Phys. Chem. Chem. Phys., 2015, 17, 6155–6173 RSC.
- A. Kitao, R. Harada, Y. Nishihara and D. P. Tran, AIP Conf. Proc., 2016, 1790, 020013 CrossRef.
- T. Baba, R. Harada, M. Nakano and Y. Shigeta, J. Comput. Chem., 2014, 35, 1240–1247 CrossRef CAS PubMed.
- R. Harada and Y. Shigeta, J. Comput. Chem., 2017, 38, 2671–2674 CrossRef CAS PubMed.
- D. A. Case, T. E. Cheatham III, T. Darden, H. Gohlke, R. Luo, K. M. Merz Jr, A. Onufriev, C. Simmerling, B. Wang and R. J. Woods, J. Comput. Chem., 2005, 26, 1668–1688 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, Acc. Chem. Res., 2008, 41, 157–167 CrossRef CAS PubMed.
- K. Hengphasatporn, P. Wilasluck, P. Deetanya, K. Wangkanont, W. Chavasiri, P. Visitchanakun, A. Leelahavanichkul, W. Paunrat, S. Boonyasuppayakorn, T. Rungrotmongkol, S. Hannongbua and Y. Shigeta, J. Chem. Inf. Model., 2022, 62, 1498–1509 CrossRef CAS PubMed.
- K. Hengphasatporn, R. Harada, P. Wilasluck, P. Deetanya, E. R. Sukandar, W. Chavasiri, A. Suroengrit, S. Boonyasuppayakorn, T. Rungrotmongkol, K. Wangkanont and Y. Shigeta, Sci. Rep., 2022, 12, 17984 CrossRef CAS PubMed.
- J. Wang, R. M. Wolf, J. W. Caldwell, P. A. Kollman and D. A. Case, J. Comput. Chem., 2004, 25, 1157–1174 CrossRef CAS PubMed.
- K. Hengphasatporn, T. Aiebchun, P. Mahalapbutr, A. Auepattanapong, O. Khaikate, K. Choowongkomon, C. Kuhakarn, J. Meesin, Y. Shigeta and T. Rungrotmongkol, ACS Omega, 2023, 8, 19645–19655 CrossRef CAS PubMed.
- M. J. Abraham, T. Murtola, R. Schulz, S. Páll, J. C. Smith, B. Hess and E. Lindahl, SoftwareX, 2015, 1–2, 19–25 CrossRef.
- D. R. Roe and T. E. Cheatham III, J. Chem. Theory Comput., 2013, 9, 3084–3095 CrossRef CAS PubMed.
- M. K. Scherer, B. Trendelkamp-Schroer, F. Paul, G. Pérez-Hernández, M. Hoffmann, N. Plattner, C. Wehmeyer, J.-H. Prinz and F. Noé, J. Chem. Theory Comput., 2015, 11, 5525–5542 CrossRef CAS PubMed.
- J. D. Hunter, Comput. Sci. Eng., 2007, 9, 90–95 Search PubMed.
- E. F. Pettersen, T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng and T. E. Ferrin, J. Comput. Chem., 2004, 25, 1605–1612 CrossRef CAS PubMed.
- W. A. Banks, BMC Neurol., 2009, 9, S3 CrossRef PubMed.
- D. A. Kitagawa, R. B. Rodrigues, T. N. Silva, W. V. Dos Santos, V. C. da Rocha, J. S. de Almeida, L. B. Bernardo, T. Carvalho-Silva, C. N. Ferreira and A. A. da Silva, J. Enzyme Inhib. Med. Chem., 2021, 36, 1369–1376 CrossRef PubMed.
- J. A. V. da Silva, E. Nepovimova, T. C. Ramalho, K. Kuca and T. Celmar Costa França, J. Enzyme Inhib. Med. Chem., 2019, 34, 1018–1029 CrossRef CAS PubMed.
- S. Tsuzuki and H. P. Lüthi, J. Chem. Phys., 2001, 114, 3949–3957 CrossRef CAS.
- A. da Silva Gonçalves, T. C. C. França, M. S. Caetano and T. C. Ramalho, J. Biomol. Struct. Dyn., 2014, 32, 301–307 CrossRef PubMed.
- Y. Ruiz-Morales, J. Phys. Chem. A, 2002, 106, 11283–11308 CrossRef CAS.
- A. Gonçalves, T. França, J. Figueroa-Villar and P. Pascutti, J. Braz. Chem. Soc., 2011, 22, 155–165 CrossRef.
- A. Kovalevsky, D. K. Blumenthal, X. Cheng, P. Taylor and Z. Radić, Ann. N. Y. Acad. Sci., 2016, 1378, 41–49 CrossRef CAS PubMed.
- V. E. Semenov, I. V. Zueva, S. V. Lushchekina, O. A. Lenina, L. M. Gubaidullina, L. F. Saifina, M. M. Shulaeva, R. M. Kayumova, A. F. Saifina, A. T. Gubaidullin, S. A. Kondrashova, S. K. Latypov, P. Masson and K. A. Petrov, Eur. J. Med. Chem., 2020, 185, 111787 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03087c |
| This journal is © The Royal Society of Chemistry 2023 |