Open Access Article
Open Access ArticleSynthesis of an eco-inspired anticorrosive composite for mild steel applications
Anoja Kawsihanab,
D. M. S. N. Dissanayake b,
N. P. W. Rathuwaduc,
H. C. S. Perera
b,
N. P. W. Rathuwaduc,
H. C. S. Perera g,
K. E. D. Y. T. Dayanandad,
K. R. Koswattageef,
Rajesh Mahadevag,
Arnab Gangulyg,
G. Das
g,
K. E. D. Y. T. Dayanandad,
K. R. Koswattageef,
Rajesh Mahadevag,
Arnab Gangulyg,
G. Das *g and
M. M. M. G. P. G. Mantilaka
*g and
M. M. M. G. P. G. Mantilaka *df
*df
aAcademy of Sri Lanka Institute of Nanotechnology, Nanotechnology and Science Park, Mahenwatte, Pitipana, Homagama, Sri Lanka
bSri Lanka Institute of Nanotechnology, Nanotechnology and Science Park, Mahenwatte, Pitipana, Homagama, Sri Lanka
cInstitute for Combinatorial Advanced Research and Education, General Sir John Kotelawala Defence University, Ratmalana, Sri Lanka
dInstitute of Materials Engineering and Technopreneurships (IMETECHNO), Kandy, Sri Lanka. E-mail: mantilaka.publications@gmail.com; mantilaka@gmail.com
eDepartment of Engineering Technology, Faculty of Technology, Sabaragamuwa University of Sri Lanka, 70140 Belihuloya, Sri Lanka
fCentre for Nanodevices Fabrication and Characterization (C. N. F. C.), Faculty of Technology, Sabaragamuwa University of Sri Lanka, 70140 Belihuloya, Sri Lanka
gDepartment of Physics, Khalifa University, United Arab Emirates. E-mail: gobind.das@ku.ac.ae
First published on 2nd October 2023
Abstract
We synthesised a polyaniline/mica (Mica–PANI) nanocomposite using naturally occurring muscovite mica by a top-down approach. The developed coating materials were characterised using a different technique to investigate their chemical and structural properties using Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), and thermogravimetric analysis (TGA). Furthermore, the electrochemical properties of the coating materials were investigated by linear sweep voltammetry (LSV). SEM images elucidate the composite's average particle diameter of the prepared nano-mica, approximately 80 nm. The existence of relevant functional groups and bonding in the prepared Mica–PANI composite material was confirmed by means of XPS and FTIR techniques. Moreover, the synthesised composite with 5% w/w shows high anticorrosion protection, i.e. 84 μm per year, compared to competing materials, including commercial paint and individual raw materials (0.35 mm per year). The anti-corrosive effect occurs mainly due to two opposing effects: the formation of an Fe(OH)3 passive layer on the steel surface by oxidation of surface iron atoms by the PANI and the barrier effect of mica NPs through inhibition of corrosive agents. Therefore, the eco-inspired composite could be an ideal cost-effective coating material to prevent the corrosion of mild steel surfaces.
Introduction
Degrading metals through the corrosion process has become a significant challenge to ensure the durability of metal products and surfaces. Accordingly, corrosion protection has been a vital topic among scientists, engineers, manufacturers, and product consumers in recent years and for many decades.1 The currently available techniques to protect metal structures from corrosion include applying special protective organic or metallic coatings, galvanising, corrosion inhibitors, etc.2–4 Nonetheless, the development of anticorrosive coatings using organic and inorganic materials as composites has attained great interest in the field of research and industries due to their cost effectiveness, the availability of raw materials, effectiveness, superior performance in corrosion protection, and the synergetic properties on the finished product or surface.5,6 Moreover, the anticorrosive coating, fabricated by grafting corrosion inhibitors directly into nanocarriers and forming nanocomposite coatings, is a promising strategy for protecting metals and metal alloys against a corrosive environment.PANI is highly utilised due to its fascinating and unique properties such as redox tunability, environmental stability, electrochromic properties, cost-effective/flexible method of production, and forming passivation of oxide layer between metal and coating.7–9 It is also more attractive as it shows a simple acid–base doping–developing process and three distinct oxidation states with different colours.10 Alkyd or epoxy resins are generally used to synthesise PANI-based anticorrosive paints, which can protect metals and metal alloys from corrosion.11 Nevertheless, there are also limitations to using PANI in anticorrosive paint due to its poor adhesion, thermal stability, and low abrasion resistance.9,12,13
Muscovite is a phyllosilicate mica mineral with a chemical composition KAl2(Al, Si3O10)(F, OH) widely found in the natural deposits abundantly.14 The previous study confirms that muscovite is a more convenient compound that can be utilised for coatings.15 It also exhibits excellent properties for producing anticorrosive paint, including structure with chemical stability, low reactivity, compatibility in producing polymer matrices, high electrical and thermal insulation, and protection from U.V. radiation.16
This paper describes a novel method to synthesise Mica–PANI nanocomposite using naturally occurring muscovite mica as an anticorrosive coating material on mild steel surfaces. Although, there have been plenty of research based on PANI/montmorillonite nanocomposites, PANI doped with p-toluenesulfonate for the anticorrosive performance, this study contributes significantly to the surface protective coating industry as it uses Muscovite mica nanoparticles made with a convenient top-down approach, which is readily available, cost-effective, uncomplicated, nontoxic, insulative, and flexible material. This effort is also significant in value-addition to natural mica deposits in a sustainable manner. Preparing new composite material as an anticorrosive coating for mild steel surfaces is the critical element contributing novelty to the study. Earlier studies have shown that PANI inhibits corrosion through the barrier effect and electrochemical process of forming a passive layer of Fe(OH)3 on the steel surface due to the oxidation of surface iron atoms.17,18 In this study, the anticorrosion ability of PANI is further increased by the addition of mica nanoparticles due to the barrier effect provided by mica NPs towards corrosive agents.
Experimental
Synthesis
Analytical grades of aniline (∼99.5%), stearic acid (octadecanoic acid ∼95%), hydrochloric acid (∼37%), sodium persulfate (∼99.9%), and ethanol (∼99.8%) were purchased from Sigma-Aldrich. Commercial grade 2K clear, 2K hardener (Alkyd resin), xylene (thinner), and mild steel were purchased from Sri Lankan open market. Mica samples were collected from Badulla, situated in the lower central hills of Sri Lanka.Preparation of mica nanoparticles (NPs)
Mica nanoparticles (NPs) were synthesised using a top-down approach. Bulk mica sheets were ground to micron size in the synthesis using a grinder. Then, the ground mica was sieved using a 300 μm mesh strainer to obtain lesser size particles. After that, sieved mica particles were ground using 1 mm silica balls of nano-grinder for 2 h. Finally, the gravity filtration technique separated mica nanoparticles from silica balls.Preparation of Mica–PANI nanocomposites
To prepare Mica–PANI nanocomposites, 2 g of mica NPs was added to 0.1 M, 25 mL stearic acid in ethanol solution. The solution was heated to 80 °C, and then, 1.5 M, 25 mL of HCl was added and stirred well using a magnetic stirrer until the solution became homogenous. Then, 2 mL of aniline was added to the same solution and stirred further for 30 min. Later, 0.4 M, 25 mL of Na2S2O8 was added dropwise into the mixture at the rate of 1 drop per sec. The prepared Mica–PANI composite material was thoroughly washed with 200 mL of distilled water and collected by centrifugation at 6000 rpm. Finally, the collected Mica–PANI composite was dried in a vacuum oven at 40 °C for 8 h.Chemical characterisation
Physical characterisation
Study of anticorrosive property
Mica–PANI composites of 0.34 g (5% w/w), 0.7 g (10% w/w), and 1.25 g (16% w/w) were dispersed separately in a mixture of 2K clear (2.5 g), 2K hardener (0.625 g), and xylene (3.3 g) to prepare the composite mixtures for the coating “a”, “b” and “c” respectively to compare the activity of Mica–PANI composite percentages. The ratios of each material of 2K clear, 2K hardener, and xylene were selected according to the procedure mentioned on the label of the products. To compare the activity of individual components of PANI and mica NPs, mixtures for the coatings “d” and “e” were prepared by of 5% w/w PANI and 5% w/w of mica NPs separately with the mixture of 2K clear (2.5 g), 2K hardener (0.625 g) and xylene (3.3 g). The coating “f,” which is the “blank” sample, is prepared by mixing 2K clear (2.5 g), 2K hardener (0.625 g), and xylene (3.3 g). The coating “g” with purchased commercial paint was prepared to compare the corrosion inhibition efficiency of the Mica–PANI composite. Brush coating technique was found to be effective for preparation of each coating among other techniques including spray coating, dip coating and flow coating after series of SEM image investigation. Though, these seven coatings were applied on mild steel immediately using the brush coating technique after polishing the surface with 220 and 400 emery papers and cleaning with acetone and ethanol. The coated area is 1 cm × 1 cm. The thicknesses of the coatings were measured using a digital micrometre screw gauge.Corrosion studies were carried out on each coated and uncoated (bare) mild steel surface initially (soon after dipping the coating inside the NaCl solution) and after 24 h in the same condition to observe the changes after attaining the equilibrium position inside the solution to confirm the stability of the coatings. The coated and uncoated mild steel was used as the working electrode with a platinum sheet counter electrode and Ag/AgCl reference electrode in 0.1 M NaCl (aq) solution. Linear sweep voltammetry (LSV) studies were carried out for each coating cast on mild steel surfaces. LSV was performed in the potential range from −0.5 V to +0.5 V with respect to the open circuit potential of each sample at the scan rate of 5 mV s−1. The corrosion studies were performed using a potentiostat (Metrohm Autolab PGSTAT 302N). Electrochemical Impedance Spectroscopic (EIS) study was carried out in the frequency range 105 Hz to 0.1 Hz with an amplitude of 10 mV under no applied DC bias.
The corrosion efficiency of the coatings (in percentages) on mild steel surfaces were calculated by taking the corrosion rate of bare mild steel surface as a reference as 100%.
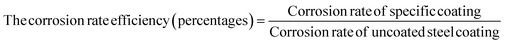 | (1) |
Results and discussion
Natural muscovite sample and synthesised intermediate and final products
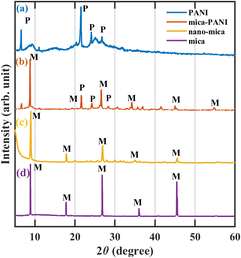
The formation of short chains of polymers or oligomers of PANI was confirmed in the experimental procedure by the appearance of thick and brown colour colloidal solution at the initial stage of polymerisation of the PANI. The continual process of polymerisation leads to long PANI polymer chains in which the colloidal turns blue-green at the final stage.10 A schematic representation of the anticorrosive coatings is shown in Fig. 1 below.The XRD spectrum of the raw mica material (Fig. 2d) reveals that it consists of peaks at 2θ values of 8.82°, 17.98°, 26.74°, 36.2°, 45.36°, and 54.92° are attributed to the corresponding basal planes of (002), (004), (006), (112), (0010), and (139) that can be assigned to muscovite (JCPDS card no. 01-089-5401). The muscovite consists of the chemical composition of KAl2.20(Si3Al)0.975O10((OH)1.72O0.28) with a monoclinic crystallographic structure. The intermediate product nano ground mica (nano-mica) shows similar XRD peak positions in the pattern with the slight broadening of the peak that relates to the width of the peak due to increment in the high surface area and breakage along weaker cleavage planes (Fig. 2c). The pure PANI exhibit a sharp peak at the 2θ value of 21.13° and two other peaks around 24.29° and 26.99° with the corresponding planes (011), (020), and (200) respectively (Fig. 2a). The peaks at the 2θ values of 21.77° and 24.55° representing PANI are also observed in Fig. 2b, which indicates the PANI-coated nano-mica (mice-PANI). The XRD pattern of Mica–PANI consists of the peaks of both nano-mica and PANI, confirming the process of coating PANI on mica. The spectra reveal the certainty of the lattice structure of the formation of the nano-mica and polymer of PANI-coated nano-mica.
FE-SEM images of the raw, intermediate product materials “nano-mica and PANI” and the final product “Mica–PANI” are revealed in Fig. 3a–c, respectively, with different magnifications. Images of FE-SEM (Fig. 3a) describe the changes in the layered silicate structure of muscovite during the top-down approach of mechanical milling to produce nano-mica. It shows that muscovite's distinct layered silicate structure with sharp edges has changed to an agglomerated ball-like structure with an average particle diameter of 80 nm. Fig. 3b confirms the worm-like agglomerated heterogeneous polyaniline formation with the average particle length and width of 500 nm and 75 nm, respectively. A similar morphology of the PANI is observed as the coating of the nanomica in the image Fig. 3c. It has an uneven surface with the blunted edges of muscovite. It is completely covered with PANI, which explains that nano-mica has offered the active sites to initiate the nucleation process of PANI during polymerisation.9,10
The chemical structure of the PANI, Mica–PANI, nano-mica and mica are illustrated in Fig. 4a–d, respectively. FT-IR spectrum confirms the presence of the synthesised intermediate nano-mica and product PANI-coated muscovite (Mica–PANI) by the appearance of the corresponding peaks. The characteristic band at 830–900 cm−1 in Fig. 4d is referred to the octahedral sheets occupied by a trivalent central atom O–H bending bands to the silicate sheets in the structure of muscovite.19 The weak band at around 3624 cm−1 represents the stretching of the free hydroxyl group between tetrahedral and octahedral sheets in the structure of muscovite. The Al–OH group in the mica lattice does not show any peak due to no accessible reaction for steric hindrance. The bands in the range 600–750 cm−1 exhibit the bending vibrations of the Si–O bond.20 The Al–O out-of-plane vibrations absorb at 810 cm−1, and Al–O–Si in-plane vibrations absorb at 750 cm−1. Liberational vibrations involving OH lie between 950–800 cm−1.19,21–23 The FT-IR spectra of nano-mica (Fig. 4c) show similar trend spectra like bulk mica with precise change. The peak around 3406 cm−1 displays a broad peak attributed to the hydroxyl (OH) group between tetrahedral and octahedral sheets. The difference between bulk and nano-mica is due to the rise in the surface area that leads to the effective vibration mode of the hydroxyl group.24
 | ||
| Fig. 4 FT-IR focused images in the ranges of wavenumber 4000–600 cm−1 of (a) PANI, (b) Mica–PANI composite, (c) nano-mica, and (d) mica. | ||
The characteristic bands (Fig. 4b) of Mica–PANI confirm the presence of PANI and Muscovite by the characteristic peaks of each compound. The presence of PANI is confirmed chemically in Fig. 4a. The characteristic bands at 2800 cm−1 to 3200 cm−1 are referred to as the stretching mode of N–H.25 The bands at the ranges from 1697 cm−1 and 1470 cm−1 corresponded to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–C stretching modes for the quinoid and benzenoid rings.9,10,26 The bands at about 1090 cm−1 have been referred to as the C–N stretching mode for the benzenoid ring. The band at 780 cm−1 is specified to an in-plane bending vibration of C–H (mode of N
N and C–C stretching modes for the quinoid and benzenoid rings.9,10,26 The bands at about 1090 cm−1 have been referred to as the C–N stretching mode for the benzenoid ring. The band at 780 cm−1 is specified to an in-plane bending vibration of C–H (mode of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, Q
N, Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ H
N+ H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B, and B–N+ H–B; where Q is a quinonoid unit. B is a benzenoid unit) formed during protonation.12,13 Similar bands detected at 3406 cm−1, and 938 cm−1 represent the presence of characteristic muscovite in the final product Mica–PANI illustrated in Fig. 4b.
B, and B–N+ H–B; where Q is a quinonoid unit. B is a benzenoid unit) formed during protonation.12,13 Similar bands detected at 3406 cm−1, and 938 cm−1 represent the presence of characteristic muscovite in the final product Mica–PANI illustrated in Fig. 4b.
The thermal properties of the PANI, prepared composite Mica–PANI, nano-mica, and bulk mica, are interpreted using thermograms which are shown in Fig. 5a–d, respectively. The TGA graphs of the nano-mica and Muscovite (Fig. 5c and d) interpret that muscovite exhibit higher thermal stability comparatively than the nano-mica due to lower surface area and lower concentration of free water molecule and a hydroxyl group. The mass-loss (0.37%) at the temperature range between 150 °C and 400 °C at the TGA curve of muscovite is due to adsorbed water that is different from simple moisture. In the case of nano-mica, the TGA curve shows two stages of weight loss at the temperature ranges between 100 –700 °C and 700–890 °C that show weight loss as 10.03% and 0.98%, respectively. The weight loss results from adsorbed water content and the removal of hydroxyl ions below the temperature of 890 °C. The total mass loss of muscovite and nano-mica is 4.7% and 11.01%, respectively.
The TGA curve of PANI (Fig. 5a) shows three stages of weight loss. The first weight loss of 11.37% at 130 °C is probably due to the evaporation of physisorbed water molecules. The second weight loss of 24.25% at the temperature of 460 °C can be attributed to the decomposition and evaporation of unreacted solvents. The third weight loss of 30.98% at the temperature of 907 °C is due to the degradation of the unsaturated group present in the polymer. The total weight loss of PANI is 66.6%. The TGA curve of composite Mica–PANI also exhibits a similar curve to PANI as it has the coating of the PANI on the surface of muscovite. However, the composite show comparatively higher thermal stability than the PANI as it consists of thermally stable nano-mica. The Mica–PANI composite exposes three stages of weight loss, as clearly indicated in Fig. 5b. The first weight loss of 8.97% in the composite's TGA curve can be associated with removing physisorbed water molecules via evaporation. The second weight loss at the temperature of 320 °C shows 19% due to the decomposition and evaporation of the unreacted solvents. 15% of weight loss in the third stage of the TGA curve can be related to the removal of hydroxyl ions of the muscovite nano-mica and the decomposition of the unsaturated group present in the polymer matrix. The TGA curve of the composite can be concluded that the composite is stable enough up to the temperature of 800 °C that the structure can be preserved due to the polymer coating.
Mechanism of composite of Mica–PANI formation
Muscovite composition, which is (K, Na)(Al, Mg, Fe)2(Si3Al2.90H2KO12Si3.10), has plenty of hydroxyl groups due to water adsorbed molecules on the surface of mica NPs. The electrochemical interaction between the carbonyl group of the stearic acid and the negatively surface-charged mica NPs forms the highly reactive COO− group to the surface. The hydrogen bonding interaction between the surface hydroxyl groups, or the hydroxyl groups in ethanol, makes it possible to weaken the strong particle agglomeration of mica NPs and form a dispersion. Then, adding aniline to the mixed homogeneous suspension of HCl and mica NPs produces the adsorbed aniline on the surface of muscovite NPs due to the electrostatic interaction. When the initiative oxidant sodium persulfate is added to the solution, polymerisation is initiated just as at adsorbing sites producing a large number of PANI nuclei on the surface of the muscovite NPs by heterogeneous nucleation. The initial lower molecular weight positively charged PANI molecular chains would spontaneously be surrounded by free negative ions (Cl−), forming an excellent protective layer to restrain the growth of PANI. The excess ionic liquids and ethanol can be washed out at the end of the polymerisation process.12,27Anticorrosive property of prepared product Mica–PANI nanocomposites
 | ||
| Fig. 6 Tafel plots of composition weight percentage (a1) initial (a2) 24 h and individual components at (b1) initial and (b2) 24 h after dipping the coating inside the 0.1 M, 30 mL NaCl solution. | ||
As shown in Fig. 6a2, even after 24 h, the composites showed better anticorrosion activity with less negative Ecorr and smaller Jcorr compared to that of bare steel and blank samples and hence lower corrosion rates. This 24 h result also indicates the appreciable composite coating stability. The weight percentage increase of Mica–PANI composites has not significantly improved the anticorrosion performance of the coating. This could be due to several reasons. At higher weight percentages it is possible that composite material aggregates28 and the film integrity,29 homogeneity,30 diffusion properties,31 and the electrochemical performance32 are altered. Moreover, 5% composite showed the highest Rp at 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 Ω compared to all other samples indicating the greater resistance to corrosion (Table 1).
620 Ω compared to all other samples indicating the greater resistance to corrosion (Table 1).
| Coating | Content | Electrochemical corrosion studies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | 2K clear (g) | 2K hardener (g) | Xylene (g) | Ecorr|Ecorr 24 h (V) | Jcorr|Jcorr 24 h (A cm−2) | Rp|Rp 24 h (Ω) | RM|RM 24 h (mm per year) | RM|RM 24 h (%) | |
| a | 5% compo | 2.5 | 0.625 | 3.3 | −0.68822 | 2.23 × 10−6 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620 |
0.025958 | 2.7 |
| −0.66259 | 7.27 × 10−6 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380 380 |
0.08444 | 0.23 | |||||
| b | 10% compo | 2.5 | 0.625 | 3.3 | −0.70924 | 2.66 × 10−6 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 403 403 |
0.030895 | 3.9 |
| −0.57674 | 1.07 × 10−5 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 158 158 |
0.12472 | 0.3 | |||||
| c | 16% compo | 2.5 | 0.625 | 3.3 | −0.67273 | 2.57 × 10−6 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 568 568 |
0.029855 | 3.1 |
| −0.69614 | 7.16 × 10−6 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 534 534 |
0.08315 | 0.22 | |||||
| d | 5% PANI | 2.5 | 0.625 | 3.3 | −0.66647 | 2.67 × 10−5 | 3108.1 | 0.30973 | 31.9 |
| −0.69416 | 4.44 × 10−5 | 2525.6 | 0.51567 | 1.4 | |||||
| e | 5% mica NPs | 2.5 | 0.625 | 3.3 | −0.78363 | 3.54 × 10−6 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 803 803 |
0.041167 | 4.2 |
| −0.76444 | 1.63 × 10−5 | 9953.1 | 0.18946 | 0.5 | |||||
| f | Blank | 2.5 | 0.625 | 3.3 | −0.84339 | 7.25 × 10−5 | 9355.2 | 0.84257 | 86.7 |
| — | −0.80752 | 1.28 × 10−4 | 4345 | 1.4908 | 3.96 | ||||
| g | Commercial | — | — | — | −0.66857 | 5.76 × 10−6 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.06688 | 6.9 |
| −0.72038 | 3.02 × 10−5 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 503 503 |
0.35107 | 0.9 | |||||
| h | Uncoated mild steel | — | — | — | −0.8088 | 8.36 × 10−5 | 404.72 | 0.97151 | 100 |
| −0.93131 | 0.0032355 | 192.26 | 37.596 | 100 | |||||
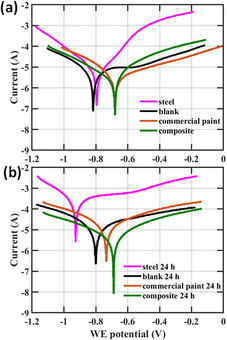
Fig. 6b1 and b2 show Tafel plots for coatings with individual components of the composite, mica and PANI, after reaching the initial equilibrium and after continuing to be in the corrosive conditions for 24 h respectively. According to Fig. 6b1, Ecorr are most negative for the bare mild steel at −0.8088 V and the blank at −0.84339 V. The lowest Ecorr was obtained for PANI is −0.66647 V, which is comparable for that of the composite at −0.67273 V indicating the corrosion resistant nature. Similarly, Jcorr are highest for the bare mild steel at 8.36 × 10−5 A cm−2 and the blank at 7.25 × 10−5 A cm−2. Jcorr are the lowest for composite material at 2.57 × 10−6 A cm−2 and then for mica at 3.54 × 10−6 A cm−2 which indicated the anticorrosion behaviour. Hence the composite has the lowest corrosion rate compared to that of bare mild steel, blank, and the individual components, mica (4.2%) and PANI (31.9%). A similar trend could be observed in Fig. 6b2 after 24 h as well. The variation of Jcorr suggests that incorporation of mica NPs into the composite has enhanced the anticorrosive performance of coating as it acts as a barrier for the penetration of atmospheric oxygen and forming a passive layer on steel surface. In addition, mica has the highest Rp among all the samples, further confirming its great barrier effect for corrosion. The variation of Ecorr shows that incorporation of PANI into the composite has enhanced the anticorrosive performance of coating via its electrochemical processes. This confirms that the composite of Mica–PANI significantly improved the anticorrosion performance of the coating compared to individual components due to combined barrier effect and electrochemical process of mica and PANI.
The Tafel plots in Fig. 7 show the initial and after 24 h comparison of the composite Mica–PANI with the commercially available anticorrosive paint. According to Fig. 7a, initially both the composite and the commercially available anticorrosion paint performs in a similar manner comparing Ecorr and RP. Jcorr is better for the composite and hence smaller Rm and corrosion efficiency compared to the commercial paint. Interestingly, after 24 h, the composite had better values for Ecorr, Jcorr, RP, Rm, and corrosion efficiency indicating a better anticorrosion activity. This confirms that the anticorrosion activity and the coating stability is superior in the composite.
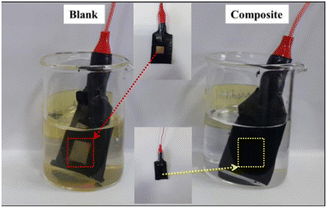
Visual observations on the comparison of corrosion efficiency of coated mild steel with coatings blank paint and composite Mica–PANI paint against the bare mild steel and, which were dipped for 120 h into the solution of 0.1 M, 30 mL of NaCl solution were depicted in Fig. 11 below. The high corrosion protection is observed for the composite than that the blank paint and bare mild steel even after 120 h.
Fig. 12 below represents the graphical representation of percentage of corrosion rate efficiency RM% for different paint coatings w.r.t. bare mild steel (100%). The synthesised Mica–PANI nanocomposite material exhibits the highest corrosion resistance of around 84 μm per year (RM ∼ 0.23%) compared to blank sample of alkyd resin (1490 μm per year, 3.96%), existing commercial paint (351 μm per year, 0.9%), and other individual components such as PANI coatings (∼516 μm per year, 1.4%) on mild steel surfaces. The results clarify that Mica–PANI nanocomposite is a challenging material for high-tech applications for surface coating.
Conclusions
Mica–PANI nanocomposite material was fabricated using natural muscovite and showed high potential as an anticorrosive coating on mild steel surfaces. The anticorrosive property study confirmed that the high anticorrosion protection of the product is mainly due to the electrochemical process and barrier effect through inhibition of corrosive agents like atmospheric O2 and acidic environment. The muscovite mica in the composite product is attributed to high corrosion resistance due to its attractive properties, including high electrical and thermal insulation and chemically inertness. The synthesised eco-inspired Mica–PANI nanocomposite material exhibits the highest corrosion resistance of around 84 μm per year compared to blank sample of alkyd resin (1490 μm per year), existing commercial paint (351 μm per year), and other individual components such as PANI coatings (∼516 μm per year) coated on mild steel surfaces. The results clarify that Mica–PANI nanocomposite is a challenging material for high-tech applications for surface coating. The study generally reveals prosperous novel techniques for fabricating Mica–PANI nanocomposite utilising naturally available muscovite mica and PANI. Furthermore, it indicates the capability of Mica–PANI nanocomposite as a highly anticorrosive and low-cost surface coating material for mild steel surfaces.Author contributions
Anoja Senthilnathan: investigation, formal analysis, writing-original draft; D. M. S. N. Dissanayake: investigation, data curation; N. P. W. Rathuwadu: methodology, formal analysis, writing-review and editing, supervision; H. C. S. Perera: writing-review and editing, funding acquisition, project administration, validation; K. E. D. Y. T. Dayananda: writing-review and editing; K. R. Koswattage: supervision, resources, writing-review and editing; Rajesh Mahadeva: writing-review and editing, software, visualization; Arnab Ganguly: writing-review and editing, software, visualization; G. Das: writing-review and editing, resources, funding acquisition; M. M. M. G. P. G. Mantilaka: conceptualization, supervision, resources, writing-review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. M. M. G. P. G. M. and K. R. K. acknowledges the Science and Technology Human Resource Development Project, Ministry of Higher Education, Sri Lanka, funded by the Asian Development Bank (Grant no. CRG-R2-SB-1) and the National Research Council, Sri Lanka (Grant no. 19-064). G. D., H. C. S. P. and A. G. acknowledge the support of the Khalifa University internal funding FSU-2020-08 (8474000233) and CIRA-2021-071 (8474000416).References
- B. Kulyk, M. A. Freitas, N. F. Santos, F. Mohseni, A. F. Carvalho, K. Yasakau, A. J. S. Fernandes, A. Bernardes, B. Figueiredo, R. Silva, J. Tedim and F. M. Costa, Crit. Rev. Solid State Mater. Sci., 2022, 47, 309–355 CrossRef CAS.
- A. S. H. Makhlouf, Handb. Smart Coatings Mater. Prot., 2014, pp. 121–131 Search PubMed.
- F. Olivieri, R. Castaldo, M. Cocca, G. Gentile and M. Lavorgna, Nanoscale, 2021, 13, 9091–9111 RSC.
- L. Liu, K. Shi, Y. Lu and C. Yu, Micro Nano Lett., 2020, 15, 728–731 CrossRef CAS.
- M. Faccini, L. Bautista, L. Soldi, A. M. Escobar, M. Altavilla, M. Calvet, A. Domènech and E. Domínguez, Appl. Sci., 2021, 11(8), 3446 CrossRef CAS.
- L. Nurdiwijayanto, H. Nishijima, Y. Miyake, N. Sakai, M. Osada, T. Sasaki and T. Taniguchi, Nano Lett., 2021, 21, 7044–7049 CrossRef CAS PubMed.
- D. Sazou and P. P. Deshpande, Chem. Pap., 2017, 71, 459–487 CrossRef CAS.
- Z. Tian, H. Yu, L. Wang, M. Saleem, F. Ren, P. Ren, Y. Chen, R. Sun, Y. Sun and L. Huang, RSC Adv., 2014, 4, 28195–28208 RSC.
- K. G. C. Senarathna, M. M. M. G. P. G. Mantilaka, T. A. N. Peiris, H. M. T. G. A. Pitawala, D. G. G. P. Karunaratne and R. M. G. Rajapakse, Electrochim. Acta, 2014, 117, 460–469 CrossRef CAS.
- R. M. N. M. Rathnayake, M. M. M. G. P. G. Mantilaka, M. Hara, H. H. Huang, H. W. M. A. C. Wijayasinghe, M. Yoshimura and H. M. T. G. A. Pitawala, Appl. Surf. Sci., 2017, 410, 445–453 CrossRef CAS.
- E. Armelin, R. Pla, F. Liesa, X. Ramis, J. I. Iribarren and C. Alemán, Corros. Sci., 2008, 50, 721–728 CrossRef CAS.
- Y. Guo, D. He, S. Xia, X. Xie, X. Gao and Q. Zhang, J. Nanomater., 2012, 2012, 202794 Search PubMed.
- E. T. Kang, K. G. Neoh and K. L. Tan, Prog. Polym. Sci., 1998, 23, 277–324 CrossRef CAS.
- S. Chen, F. Chen, Y. Di, S. Han and X. Zhu, Micro Nano Lett., 2020, 15, 509–513 CrossRef CAS.
- A. Kalendová, Pigm. Resin Technol., 2002, 31, 216–225 CrossRef.
- P. Kalenda, A. Kalendová, V. A. Štengl, P. Antoš, J. Šubrt, Z. Kváča and S. Bakardjieva, Prog. Org. Coat., 2004, 49, 137–145 CrossRef CAS.
- N. Maruthi, M. Faisal, N. Raghavendra, B. P. Prasanna, S. R. Manohara and M. Revanasiddappa, Colloids Surf., A, 2021, 621, 126611 CrossRef CAS.
- D. K. Gupta, S. Neupane, S. Singh, N. Karki and A. P. Yadav, Prog. Org. Coat., 2021, 152, 106127 CrossRef CAS.
- W. Vedder and R. S. McDonald, J. Chem. Phys., 1963, 38, 1583–1590 CrossRef CAS.
- G. Mariotto, G. Das, A. Quaranta, G. Della Mea, F. Corni and R. Tonini, J. Appl. Phys., 2005, 97, 113502 CrossRef.
- L. Cheng, D. Lin and K. Yang, J. Membr. Sci., 2000, 172, 157–166 CrossRef CAS.
- A. M. El-toni, S. Yin and T. Sato, Appl. Surf. Sci., 2006, 252, 5063–5070 CrossRef CAS.
- G. Das, G. Mariotto and A. Quaranta, Mater. Sci. Semicond. Process., 2004, 7, 295–300 CrossRef CAS.
- M. V Maslova, L. G. Gerasimova and W. Forsling, Colloid J., 2004, 66, 322–328 CrossRef.
- N. Daldosso, G. Das, S. Larcheri, G. Mariotto, G. Dalba, L. Pavesi, A. Irrera, F. Priolo, F. Iacona and F. Rocca, J. Appl. Phys., 2007, 101, 113510 CrossRef.
- Z. A. Boeva and V. G. Sergeyev, Polym. Sci., Ser. C, 2014, 56, 144–153 CrossRef CAS.
- B. A. Bhanvase and S. H. Sonawane, Chem. Eng. J., 2010, 156, 177–183 CrossRef CAS.
- Y. Ge, X. Guo, D. Zhou and J. Liu, Nanoscale, 2022, 14, 12358–12376 RSC.
- W. Taleb, F. Pessu, C. Wang, T. Charpentier, R. Barker and A. Neville, npj Mater. Degrad., 2017, 1, 13 CrossRef.
- S. Pourhashem, A. Rashidi, M. R. Vaezi and M. R. Bagherzadeh, Surf. Coat. Technol., 2017, 317, 1–9 CrossRef CAS.
- S. Pourhashem, M. R. Vaezi, A. Rashidi and M. R. Bagherzadeh, Corros. Sci., 2017, 115, 78–92 CrossRef CAS.
- Y. Zhao, S. Tian, D. Lin, Z. Zhang and G. Li, Mater. Des., 2022, 216, 110589 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |