Open Access Article
Open Access ArticleUltrasound-assisted degradation of organophosphorus pesticide methidathion using CuFe2O4@SiO2-GOCOOH as a magnetic separable sonocatalyst
Houda Maati*ab,
Othmane Amadine *ab,
Younes Essamlaliab,
Soumia Aboulhrouz
*ab,
Younes Essamlaliab,
Soumia Aboulhrouz ab,
Ilham jiouiab,
Karim Dânounab and
Mohamed Zahouily*abc
ab,
Ilham jiouiab,
Karim Dânounab and
Mohamed Zahouily*abc
aMoroccan Foundation for Advanced Science, Innovation and Research (MAScIR), Benguerir, Morocco. E-mail: m.zahouily@mascir.com; o.amadine@mascir.com
bMohammed VI Polytechnic University, Lot 660 – Hay Moulay Rachid, Ben Guerir, 43150, Morocco
cLaboratory of Materials, Catalysis & Valorization of Natural Resources, Hassan II University, FST-Mohammedia, Morocco
First published on 28th June 2023
Abstract
Water contamination by pesticides is a critical environmental issue, necessitating the development of sustainable and efficient degradation methods. This study focuses on synthesizing and evaluating a novel heterogeneous sonocatalyst for degrading pesticide methidathion. The catalyst consists of graphene oxide (GO) decorated CuFe2O4@SiO2 nanocomposites. Comprehensive characterization using various techniques confirmed the superior sonocatalytic activity of the CuFe2O4@SiO2-GOCOOH nanocomposite compared to CuFe2O4@SiO2 alone. The enhanced performance is attributed to the combined effects of GO and CuFe2O4@SiO2, including increased surface area, enhanced adsorption capabilities, and efficient electron transfer pathways. Reaction parameters such as time, temperature, concentration, and pH significantly influenced the degradation efficiency of methidathion. Longer reaction times, higher temperatures, and lower initial pesticide concentrations favored faster degradation and higher efficiency. Optimal pH conditions were identified to ensure effective degradation. Remarkably, the catalyst demonstrated excellent recyclability, indicating its potential for practical implementation in pesticide-contaminated wastewater treatment. This research contributes to the development of sustainable methods for environmental remediation, highlighting the promising potential of the graphene oxide decorated CuFe2O4@SiO2 nanocomposite as an effective heterogeneous sonocatalyst for pesticide degradation.
1. Introduction
For more than two decades, pesticides have been extensively employed in various sectors, including agriculture, road maintenance, rail infrastructure, wood treatment, and even for private use such as gardening and premises treatment.1 While they aid in controlling pests, they also pose a significant threat to water pollution. Pesticide use has been steadily increasing over the past six decades, with tonnages reaching alarming levels.2 Pesticides are not only a concern for applicators who are most exposed to them, but also for the general population due to the real public health problem they pose.3,4 The body absorbs pesticides, particularly by ingestion, through the skin, and by the respiratory tract.5 Furthermore, epidemiological studies have demonstrated that individuals who frequently use pesticides without protection are more likely to develop certain illnesses such as cancer, birth defects, sterility, neurological disorders, or weakened immune systems.6 Pesticide residues are ubiquitous, found not only in water but also in air, mist, soil, food, and even in the blood.7,8Pesticides are a major cause of water contamination.9,10 When they are spread over soil, they can infiltrate and contaminate groundwater.11 This contamination can affect water quality and lead to the need for additional treatments.12 The majority of pesticides found in rivers and groundwater are herbicides,13,14 which are used to kill weeds in agricultural and non-agricultural areas.
To protect the environment, it is essential to treat urban, industrial, and agricultural wastewater. Various methods are currently available and well-controlled on a laboratory scale, which can be applied on a large scale. These methods include membrane technologies, adsorption techniques, ion exchange, and solid–liquid separation processes.15–18 However, these processes are only separative, not degradative, and have several disadvantages, such as producing pollutant concentrates, sludge, and requiring significant consumption of chemical reagents. Biological purification processes are commonly used for treating polluted waters,19 but this process generates large quantities of biological sludge that must be treated. Additionally, these methods may not be applicable to effluents with a high concentration of pollutants. Therefore, it is necessary to use more reactive systems than those adopted in conventional purification processes.
To address this problem, advanced oxidation processes (AOPs) have been developed and are increasingly being used.20,21 These methods involve the formation of hydroxyl radicals (HO˙), which are highly reactive chemicals that break down pollutants into biologically degradable molecules or mineral compounds, such as CO2 and H2O.22 Because hydroxyl radicals are very reactive and unstable,23 they must be continuously produced through various means, including chemical, photochemical, biological, or electrochemical methods.24 Among these processes, we are particularly interested in sonolysis for the degradation of organic pollutants.25 Ultrasound waves are propagated in the solution to be treated, but using ultrasound alone for pollutant degradation requires high energy and long reaction times.26,27 To overcome these problems, low-power ultrasonic irradiation has been combined with heterogeneous catalysis to create a sonocatalytic system,28–30 which can increase the rate of pollutant degradation and reduce reaction times. Various materials have been used as sonocatalysts for organic pollutant degradation, including Rutile TiO2,31 ZnO/biochar,32 TiO2/ZnO,33 Zr/TiO2,34 SiO2,35 CuO.36 Among the various heterogeneous catalysts, magnetic materials have caught the attention of the industrial community because they are less expensive, readily available, recyclable, and exhibit significant improvement due to their high surface area, small particle size, and high active sites.37 Additionally, these magnetic nanoparticles can be efficiently separated after completion of the reaction by a simple external magnet, which is advantageous compared to classical techniques such as centrifugation and filtration. In particular, the spinel CuFe2O4, a magnetic material, has gained intensive attention due to its high catalytic activity and ease of separation from the reaction system.38 However, the traditional methods used for the preparation of CuFe2O4 produce agglomerated particles that influence their catalytic properties.39 To overcome this problem, different approaches have been developed. Among them, coating CuFe2O4 with silica has been found to be an effective approach to reduce the tendency of particle agglomeration.40 Furthermore, silanol groups presented on the surface of silica offer the possibility for the immobilization of a variety of functionalized materials that can improve the catalytic activity of CuFe2O4.41,42 Graphene oxide (GO) is a promising candidate for the immobilization of CuFe2O4 materials, as it can improve the degradation rate of organic pollutants through an increase in the production of hydroxyl radicals (HO˙) from the functional groups on the surface of GO.43,44
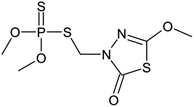
In this study, a CuFe2O4@SiO2 material decorated with graphene oxide (GO) was synthesized using a simple and efficient method. The physical and chemical properties of the samples were characterized through various techniques including nitrogen adsorption–desorption, scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR). The performance of the magnetic material CuFe2O4@SiO2-GOCOOH as a sonocatalytic system for degrading organic pollutants was evaluated, with methidathion pesticide (Scheme 1) selected as the target pollutant. Optimization of reaction parameters such as reaction kinetics, temperature, methidathion concentration, and pH was conducted. Furthermore, the reusability of the CuFe2O4@SiO2-GOCOOH sonocatalytic system was investigated.
2. Experimental
2.1 Chemical reagents
Methidathion pesticide (C6H11N2O4PS3), hydrogen peroxide H2O2 (30% w/w), iron chloride hexahydrate (FeCl3·6H2O), copper nitrate (Cu(NO3)·3H2O), polyvinylpyrrolidone (PVP), sodium hydroxide (NaOH), graphite, sodium nitrate (NaNO3), sulfuric acid (H2SO4) (98% w/w), tetra-ethyl-ortho-silicate (TEOS), sodium acetate anhydrous (NaAc) 3-(trimethoxysilyl)propylamine (APTMS), ethylene glycol (EG) and potassium permanganate (KMnO4) were purchased from Aldrich chemical company. All the reagents were used without further purification. Water used in all experiments was deionized.2.2 Materials preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The mixture was stirred vigorously for 60 min and then transferred to a 50 mL Teflon-lined stainless-steel autoclave, which was sealed and heated at 200 °C for 8 h. The resulting brown product was collected using an external magnetic field, washed several times with ethanol and deionized water, and then dried at 80 °C for 1 h in a hot vacuum desiccator.
2. The mixture was stirred vigorously for 60 min and then transferred to a 50 mL Teflon-lined stainless-steel autoclave, which was sealed and heated at 200 °C for 8 h. The resulting brown product was collected using an external magnetic field, washed several times with ethanol and deionized water, and then dried at 80 °C for 1 h in a hot vacuum desiccator.2.3 Characterization
X-ray diffraction (XRD) measurements were conducted using Cu-Kα radiation in Bragg–Brentano geometry (2θ) on a Bruker AXS D-8 diffractometer. Fourier transform infrared spectroscopy (FTIR) was performed on all samples in the range of 4000–400 cm−1, using an ABB Bomem FTLA 2000 spectrometer with a 16 cm−1 resolution. Scanning electron microscopy (SEM) and scanning transmission electron microscopy (STEM) micrographs were obtained using a Tecnai G2 microscope at 120 kV. The elemental composition of the CuFe2O4@SiO2-GOCOOH nanocomposite was confirmed by energy dispersive X-ray analysis (EDX). The magnetic properties of CuFe2O4 nanoparticles and the CuFe2O4@SiO2-GOCOOH nanocomposite were investigated using a MPMS-XL-7AC superconducting quantum interference device (SQUID) magnetometer. Magnetic measurements were performed at room temperature from −15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 15
000 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. Zeta potential measurements were carried out using a Zetasizer Nano Series (ZS90).
000 Oe. Zeta potential measurements were carried out using a Zetasizer Nano Series (ZS90).
2.4 Catalytic test procedure
The sonocatalytic degradation of methidathion was carried out using the CuFe2O4@SiO2-GOCOOH catalyst in the presence of H2O2. The catalytic test was carried out in triplicate to obtain the mean value. The reaction was performed in a 50 mL beaker for 60 minutes. Sonication was carried out in an ultrasonic cleaning bath (ELMAS60H) operating at 37 kHz and a power of 600 W. A water-circulating unit was used to control the water bath temperature. In a typical procedure, 25 mL of H2O2 was added to the methidathion solution under ultrasound irradiation. Subsequently, 10 mg of the catalyst was added to initiate the reaction. At specific time intervals, a predetermined volume (2.0 mL) of the reaction mixture was withdrawn and filtered using a micro-filter (45 mm) to remove the solid catalyst. The concentration of methidathion was determined using high-performance liquid chromatography (HPLC Shimadzu Kyoto, Japan) equipped with a reversed-phase C18 column (150 mm × 4.6 mm × 5 μm) using methanol/water ((40/60) volume ratio) as the mobile phase at a flow rate of 0.5 mL min−1 with UV detection.3. Results and discussion
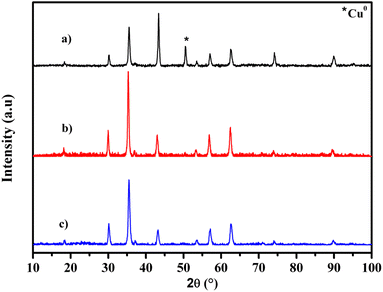
The crystalline structure of CuFe2O4, CuFe2O4@SiO2 and CuFe2O4@SiO2-GOCOO was investigated by X-ray diffraction (XRD). As shown in Fig. 1, the diffraction peaks located at 30.1°, 35.5°, 43.1°, 57.0°, and 62.9°, which correspond to the crystal planes of (202), (311), (004), (333), and (440), respectively, can be readily attributed to the tetragonal-type CuFe2O4 (JCPDS 34-0425).48 However, we observed the presence of an XRD peak of metallic copper in the CuFe2O4 sample, which can be explained by the reduction of copper in the presence of ethylene glycol.49 Similar peaks characteristic of CuFe2O4 were also detected in the case of CuFe2O4@SiO2 and CuFe2O4@SiO2-GOCOOH, indicating the stability of our materials after silica coating. Furthermore, we observed the disappearance of metallic copper diffraction peaks in CuFe2O4@SiO2, CuFe2O4@SiO2-GOCOOH materials, which can be attributed to the oxidation of copper during the preparation of both materials. In addition, the narrow and strong peaks of all samples indicate good crystallinity. The crystal sizes of CuFe2O4, CuFe2O4@SiO2 and CuFe2O4@SiO2-GOCOOH were calculated based on the Scherrer equation and are 21.8, 22.4, and 19.8 nm, respectively (as shown in Table 1).| Samples | Lattice parameter a (Å) | Cell volume (Å3) | Crystallite size (nm) |
|---|---|---|---|
| CuFe2O4 | 8.3783 | 588.1182 | 21.8 |
| CuFe2O4@SiO2 | 8.4140 | 595.6643 | 22.4 |
| CuFe2O4@SiO2-GOCOOH | 8.3799 | 588.4558 | 19.8 |
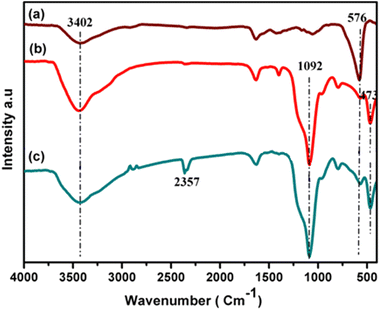
The structures and chemical species present in CuFe2O4, CuFe2O4@SiO2 and CuFe2O4@SiO2-GOCOOH were identified by FT-IR analysis. As depicted in Fig. 2, the broad peaks at 3402 and 1620 cm−1 correspond to the hydroxyl (OH) stretching vibrations caused by the presence of adsorbed water. The metal oxide stretching vibration peaks were observed at 576 cm−1 for all samples. The characteristic peaks of silica were identified at 1092 and 473 cm−1 for CuFe2O4@SiO2 and CuFe2O4@SiO2-GOCOOH, confirming the presence of SiO2 coating on the surface of CuFe2O4 materials. Furthermore, minor peaks in the range of 2000–2800 cm−1 were observed for CuFe2O4@SiO2 and CuFe2O4@SiO2-GOCOOH, which can be attributed to the C–H stretching vibration caused by the grafting of APTMS and GO.
The morphology of CuFe2O4, CuFe2O4@SiO2 and CuFe2O4@SiO2-GOCOOH samples was investigated by SEM analysis. As shown in Fig. 3a, the CuFe2O4 nanoparticles are spherical, narrowly distributed, and uniform in shape and size. The SEM image of CuFe2O4@SiO2 materials shown in Fig. 3b indicates that the CuFe2O4 nanoparticles retain their morphological properties, except for a slight change in their shape and particle size. This result confirms that the silica groups were successfully coated on the surface of CuFe2O4 nanoparticles, preventing the interaction and agglomeration of CuFe2O4 magnetic nanoparticles. Furthermore, Fig. 3c and d show the presence of GO sheets in the CuFe2O4@SiO2-GOCOOH nanoparticles, and we observed that the surface of CuFe2O4 materials becomes smoother. Additionally, the element composition and distribution of CuFe2O4@SiO2-GOCOOH were determined using energy-dispersive X-ray (EDX) analysis. As shown in Fig. 4, the EDX spectrum confirms the presence of copper, iron, silica, and oxygen.
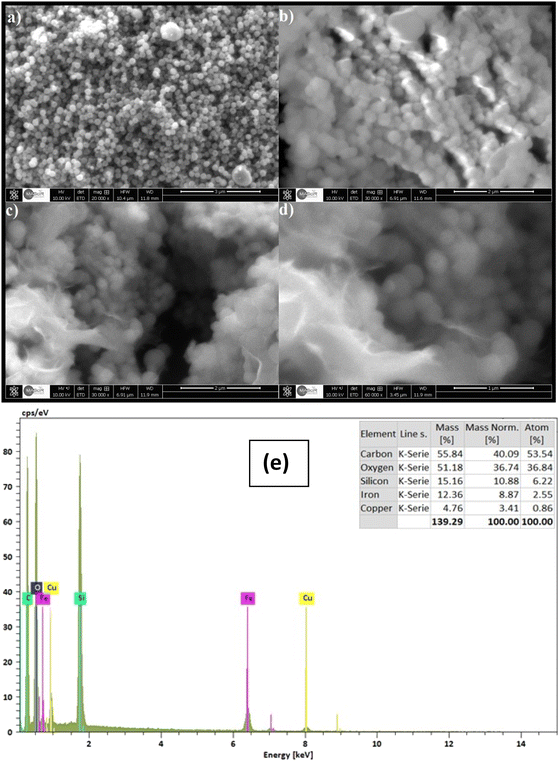 | ||
| Fig. 3 SEM images of CuFe2O4 (a), CuFe2O4@SiO2 (b) CuFe2O4@SiO2-GOCOOH (c and d) and EDX spectrum of CuFe2O4@SiO2-GOCOOH (e). | ||
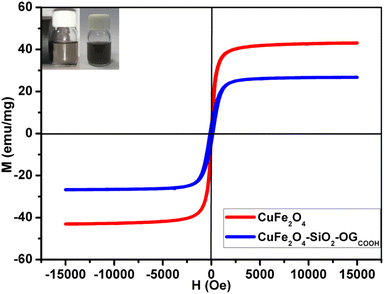 | ||
| Fig. 4 Magnetization curves of CuFe2O4 and CuFe2O4@SiO2-GOCOOH. The inset shows the photographs of the separation processes of CuFe2O4@SiO2-GOCOOH with external magnetic field. | ||
The magnetic properties of CuFe2O4, CuFe2O4@SiO2-GOCOOH samples were determined using a SQUID magnetometer from ±15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe at room temperature. Fig. 4 shows the magnetic hysteresis loops of CuFe2O4 and CuFe2O4@SiO2-GOCOOH. The saturation magnetization (Ms) value for CuFe2O4 was 43.24 emu g−1. Compared to the Ms value of CuFe2O4, CuFe2O4@SiO2-GOCOOH had a lower value of 26.35 emu g−1, which can be explained by the surface coating of CuFe2O4 magnetic particles with SiO2. The magnetization curves of CuFe2O4 and CuFe2O4@SiO2-GOCOOH show negligible remanence (Mr) and coercivity (Hc), indicating superparamagnetic behavior of both materials. Additionally, the CuFe2O4@SiO2-GOCOOH materials can be dispersed in deionized water to form a black solution before magnetic separation (as shown in the inserted Fig. 4). By applying an external magnetic field, the CuFe2O4@SiO2-GOCOOH sample can be easily separated, and the solution becomes colorless.
000 Oe at room temperature. Fig. 4 shows the magnetic hysteresis loops of CuFe2O4 and CuFe2O4@SiO2-GOCOOH. The saturation magnetization (Ms) value for CuFe2O4 was 43.24 emu g−1. Compared to the Ms value of CuFe2O4, CuFe2O4@SiO2-GOCOOH had a lower value of 26.35 emu g−1, which can be explained by the surface coating of CuFe2O4 magnetic particles with SiO2. The magnetization curves of CuFe2O4 and CuFe2O4@SiO2-GOCOOH show negligible remanence (Mr) and coercivity (Hc), indicating superparamagnetic behavior of both materials. Additionally, the CuFe2O4@SiO2-GOCOOH materials can be dispersed in deionized water to form a black solution before magnetic separation (as shown in the inserted Fig. 4). By applying an external magnetic field, the CuFe2O4@SiO2-GOCOOH sample can be easily separated, and the solution becomes colorless.
The surface charge of all samples was investigated using zeta potential measurements. To prepare the samples, all materials were dispersed in deionized water to form a homogeneous solution. As shown in Fig. 5, the zeta potentials of CuFe2O4 and CuFe2O4@SiO2 were −13.3 and −41.3 mV, respectively, indicating that the surface of both materials was negatively charged. After amine functionalization of CuFe2O4@SiO2, the surface charge became positive due to the presence of NH2 groups on the material's surface.50 Subsequently, CuFe2O4@SiO2-NH2 nanoparticles immobilized on GO showed a zeta potential value of −40.8 mV, indicating that the surface of CuFe2O4@SiO2-GOCOOH was negatively charged due to the formation of covalent bonding between the carboxylic groups of GO and amine groups. These results suggest that the CuFe2O4@SiO2-GOCOOH material could be used as a potential adsorbent for the removal of negatively charged pollutants.
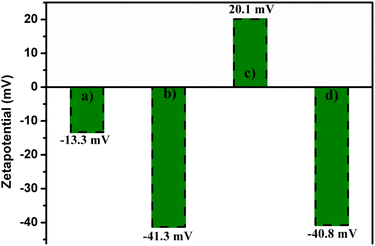 | ||
| Fig. 5 Zeta potential of CuFe2O4 (a), CuFe2O4@SiO2 (b), CuFe2O4@SiO2-NH2 (c) and CuFe2O4@SiO2-GOCOOH (d). | ||
The performance of CuFe2O4@SiO2-GOCOOH as a sonocatalyst for methidathion degradation was evaluated. Fig. 6 shows the concentration of methidathion degradation (Ct/C0) as a function of reaction time using various sonocatalytic systems. Firstly, it can be observed that ultrasonic irradiations alone had no significant effect on the degradation of methidathion . Slight increase in the degradation of methidathion was observed when using both H2O2 as an oxidizing agent and ultrasonic irradiations. However, when combined with CuFe2O4@SiO2-GOCOOH as a sonocatalyst, ultrasonic irradiations had a significant effect on the degradation of methidathion. The highest degradation of methidathion was achieved when ultrasound irradiations, H2O2, and CuFe2O4@SiO2-GOCOOH were combined as sonocatalyst. This result can be explained by the high concentration of hydroxyl radicals formed, leading to great degradation efficiency.51,52
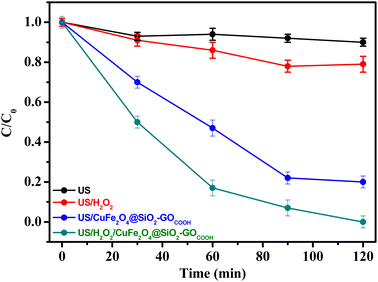 | ||
| Fig. 6 Degradation efficiency (C/C0) of methidathion by various sonocatalytic systems. Reaction conditions: [methidathion] = 5 ppm, H2O2 5%, T = 25 °C. | ||
The influence of catalyst concentration on the degradation rate of methidathion was investigated by using different concentrations of CuFe2O4@SiO2-GOCOOH sonocatalyst. The results of this study are presented in Fig. 7. Firstly, it should be noted that the reaction in the absence of the catalyst gave a low degradation rate of methidathion. Increasing the amount of the catalyst from 5 to 10 mg led to an increase in the degradation rate of methidathion. However, increasing the amount of the catalyst beyond 10 mg did not result in an increase in the degradation rate of methidathion. Therefore, we have chosen 10 mg as the optimum amount of the catalyst for methidathion degradation.
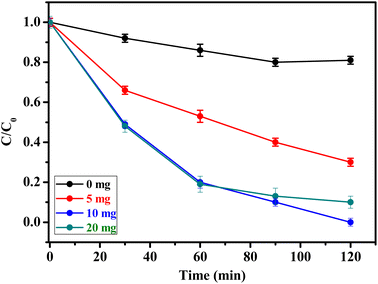 | ||
| Fig. 7 Effect of amount of catalyst on methidathion degradation over CuFe2O4@SiO2-GOCOOH. Reaction conditions: [methidathion] = 5 ppm, H2O2 5%, T = 25 °C. | ||
The concentration of H2O2 is important factors influencing the rat degradation of pesticide. In order, different concentrations of H2O2 were evaluated in the rate degradation of methidathion. As can be shown in the Fig. 8, the rate degradation of methidathion increase with increasing the concentration of H2O2 from 1.25 to 5%, respectively. This result can be explain by the formation of high concentration of ˙OH radicals responsible for the degradation of methidathion.53 However, increasing the H2O2 concentration to 10% leads to decrease the rate degradation of methidathion due to the hydroxyl radical (˙OH) scavenging effect.54 Finally, the concentration of 5% was chosen as the optimum for the degradation of methidathion.
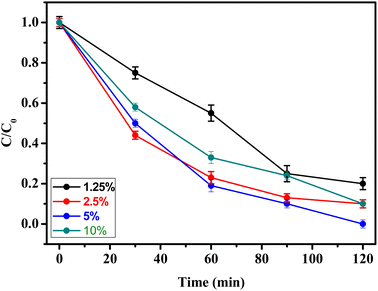 | ||
| Fig. 8 Effect of H2O2 on pesticide degradation over CuFe2O4@SiO2-GOCOOH. Reaction conditions: [methidathion] = 5 ppm, amount of catalyst = 10 mg, T = 25 °C. | ||
The influence of temperature on the degradation of methidathion over CuFe2O4@SiO2-GOCOOH sonocatalyst was investigated at various temperatures. The results of this study are presented in Fig. 9. The kinetic constants were calculated from the plot of ln(C0/Ct) versus time, and it was observed that the degradation of methidathion over CuFe2O4@SiO2-GOCOOH sonocatalyst at all temperatures followed the pseudo-first-order kinetics:
| ln(C0/Ct) = kappt |
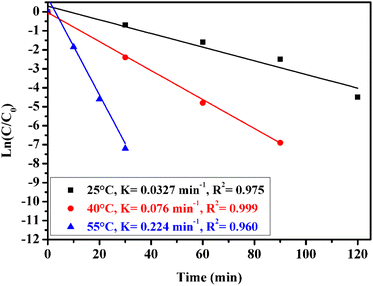 | ||
| Fig. 9 Effect of reaction temperature on methidathion degradation over the CuFe2O4@SiO2-GOCOOH sono-catalyst. Reaction conditions: [methidathion] = 5 ppm, catalyst amount = 10 mg and H2O2 5%. | ||
As shown in Fig. 9, when the temperature of the reaction was increased from 25 to 55 °C, the rate constant increased from 0.032 to 0.224 min−1, respectively. This increase can be attributed to the high concentration of ˙OH radicals formed at higher temperatures, which are responsible for the degradation of methidathion.
Thereafter, the activation energy was determined by using an Arrhenius plot. Fig. 10 shows the natural logarithm of the rate constant, ln(k), as a function of 1000/T according to the Arrhenius equation:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = ln k = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − Ea/RT A − Ea/RT |
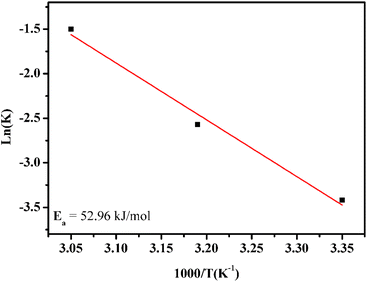 | ||
| Fig. 10 Arrhenius plot with linear regression. Reaction conditions: [methidathion] = 5 ppm, catalyst amount = 10 mg and H2O2 5%. | ||
The activation energy for methidathion degradation using CuFe2O4@SiO2-GOCOOH as a sonocatalyst was found to be 52.96 kJ mol−1.
The initial concentration of methidathion plays an important role in the rate of degradation. To investigate this, different methidathion concentrations (2.5, 5, and 10 ppm) were used, and the rate of degradation was determined. As shown in Fig. 11, the rate of degradation remains constant when increasing the methidathion concentration from 2.5 to 5 ppm, but it decreases for 10 ppm methidathion concentration. This result can be explained by the decreasing number of active sites at higher methidathion concentrations due to their adsorption on the surface of the CuFe2O4@SiO2-GOCOOH sonocatalyst.
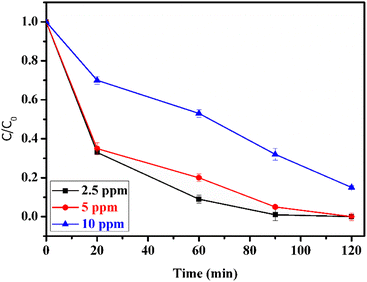 | ||
| Fig. 11 Effect of methidathion concentration. Reaction conditions: H2O2 5%, catalyst amount of = 10 mg. | ||
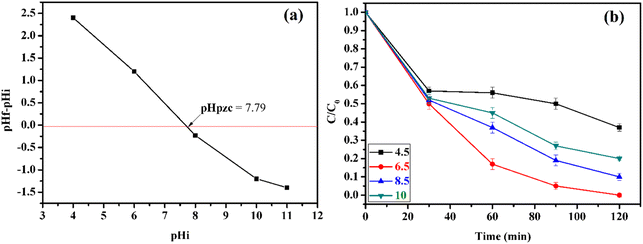
The pH is a critical factor that influences the rate of degradation of methidathion, as it affects the adsorption/desorption of methidathion on the surface of the CuFe2O4@SiO2-GOCOOH sonocatalyst. Firstly, the surface charge of CuFe2O4@SiO2-GOCOOH sonocatalyst as a function of pH value was investigated. As shown in Fig. 12a, the surface charge of CuFe2O4@SiO2-GOCOOH is negatively charged for a pH value lower than 7.79. However, the surface charge of CuFe2O4@SiO2-GOCOOH becomes positive for a pH value higher than 7.79. On the other hand, to study the effect of pH on methidathion degradation, the pH of the solution was adjusted to the desired value by adding NaOH (0.1 M) and HCl (0.1 M). Different pH values were obtained and their effect on methidathion degradation was evaluated. According to Fig. 12b, it can be observed that the rate of methidathion degradation increases with increasing pH value from 4.5 to 6.5. However, increasing the pH value greater than 6.5 leads to a decrease in the rate of methidathion degradation. These results can be explained by the electrostatic interaction between the positive charge of CuFe2O4@SiO2-GOCOOH sonocatalyst and the negative charge of methidathion at a pH lower than 7.79.
To further investigate methidathion degradation, we measured the total organic carbon (TOC) using the US/CuFe2O4@SiO2-GOCOOH/H2O2 system, which is widely used to evaluate the degree of mineralization of organic species. As shown in Fig. 13, the TOC removal efficiency of methidathion reached 90% after 120 minutes in the presence of the US/CuFe2O4@SiO2-GOCOOH/H2O2 system. This finding confirms that the as-prepared samples can mineralize methidathion to residual organic molecules.
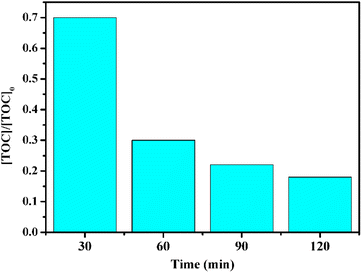 | ||
| Fig. 13 TOC removal efficiency of methidathion using CuFe2O4@SiO2-GOCOOH as a catalyst. Reaction conditions: [pesticide] = 5 ppm, catalyst mount = 10 mg and H2O2 5%. | ||
The reusability of the catalyst is a crucial factor to consider for large-scale industrial usage. Therefore, we investigated the reusability of our sonocatalyst, CuFe2O4@SiO2-GOCOOH. The sonocatalyst was easily separated using an external magnet and washed several times with water and dichloroethane to remove any organic traces. The recuperated sonocatalyst was then used as a recyclable sonocatalyst for the degradation of Methiadation in multiple runs. As shown in Fig. 14, the CuFe2O4@SiO2-GOCOOH sonocatalyst could be used consecutively for the degradation of Methiadation with a slight decrease in performance after three runs. This decrease could be attributed to the deposition of organic matter on the surface of CuFe2O4@SiO2-GOCOOH sonocatalyst, which blocks active sites and reduces the effectiveness of the catalyst in a catalyzed reaction. In addition, the CuFe2O4@SiO2-GOCOOH exhibits superior or comparable catalytic activity compared to most state-of-the-art highly active catalysts reported for pesticide degradation using H2O2 as an oxidizing agent and ultrasonic irradiation (Table 2).
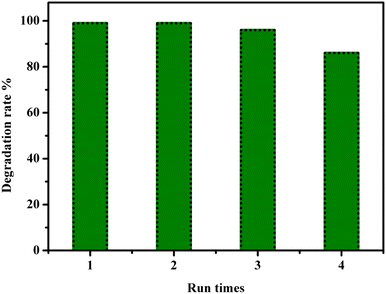 | ||
| Fig. 14 Reuse performance of the CuFe2O4@SiO2-GOCOOH catalyst in pesticide degradation. Reaction conditions: [pesticide] = 5 ppm, catalyst amount = 10 mg and H2O2 5%. | ||
| Sample | Pesticides | Catalyst dosage | Aiders | Oxidant | Time (min) | Removal efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a US: ultrasonic; PC: photocatalysis. | |||||||
| Sepiolite/Fe3O4 | Diuron (30 mg L−1) | 1 g L−1 | US | H2O2 (40 mM) | 80 | 100 | 55 |
| Fe3O4@MOF-2 | Diazinon (30 mg L−1) | 0.7 g L−1 | US | Persulfate (10 mmol L−1) | 120 | 98 | 56 |
| TiO2-Fe3+ | Terbuthylazine (5 mg L−1) | TiO2 (1 g L−1), Fe3+ (34 mg L−1) | PC | H2O2 (1.32 mmol L−1) | 20 | 100 | 57 |
| Ag-TiO2 | p-Chlorophenol (100 mg L−1) | 1 g L−1 | US | H2O2 (450 mg L−1) | 75 | 58 | |
| CuFe2O4@SiO2-GOCOOH | Methidathion (5 mg L−1) | 0.4 g L−1 | US | H2O2 (5%) | 120 | 100 | This work |
4. Conclusion
In this study, we synthesized and characterized CuFe2O4@SiO2-GOCOOH using various techniques, including XRD, FTIR, SEM, TEM, and SQUID magnetometry. We utilized the synthesized CuFe2O4@SiO2-GOCOOH as a sonocatalyst for the degradation of Methdation pesticide under ultrasound irradiation. Our results indicated that the rate of Methdation degradation was affected by several reaction parameters, including pH, Methdation concentration, and the amount of CuFe2O4@SiO2-GOCOOH sonocatalyst. The optimal conditions for Methdation degradation were found to be at pH 6.5, with 10 mg of CuFe2O4@SiO2-GOCOOH sonocatalyst, and 5 ppm Methdation concentration. Notably, CuFe2O4@SiO2-GOCOOH sonocatalyst could be easily separated using an external magnet, without the need for traditional separation and purification methods, aligning with green and sustainable chemistry principles. Furthermore, the sonocatalyst demonstrated reusability for up to four cycles, with only a slight decrease in its sonocatalytic activity for the degradation of Methdation pesticides. Our findings suggest that CuFe2O4@SiO2-GOCOOH sonocatalyst can be a promising candidate for industrial application in the removal of Methdation pesticide from contaminated environments.Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.Acknowledgements
We are thankful Moroccan Foundation for Advanced Science, Innovation and Research (MAScIR) for the financial assistance towards this research.References
- C. Wilson and C. Tisdell, Ecol. Econ., 2001, 39, 449–462 CrossRef.
- J. Popp, K. Pető and J. Nagy, Agron. Sustainable Dev., 2013, 33, 243–255 CrossRef.
- K.-H. Kim, E. Kabir and S. A. Jahan, Sci. Total Environ., 2017, 575, 525–535 CrossRef CAS PubMed.
- C. A. Damalas, SRE, 2009, 4, 945–949 Search PubMed.
- C. A. Damalas and I. G. Eleftherohorinos, Int. J. Environ. Res. Public Health, 2011, 8, 1402–1419 CrossRef CAS PubMed.
- A. Amaral, Front. Public Health, 2014, 2, 6 Search PubMed.
- P. D. Stivaktakis, M. P. Kavvalakis, M. N. Tzatzarakis, A. K. Alegakis, M. N. Panagiotakis, P. Fragkiadaki, E. Vakonaki, E. Ozcagli, W. A. Hayes, V. N. Rakitskii and A. M. Tsatsakis, Chemosphere, 2016, 149, 108–113 CrossRef CAS PubMed.
- S. E. Anderson and B. J. Meade, Environ. Health Insights, 2014, 8, 51–62 Search PubMed.
- W. Aktar, D. Sengupta and A. Chowdhury, Interdiscip. Toxicol., 2009, 2, 1–12 Search PubMed.
- S. Bulut, S. F. Erdogmuş, M. Konuk and M. Cemek, Ekoloji, 2010, 19, 24–31 CAS.
- S. Piel, E. Baurès and O. Thomas, Int. J. Environ. Res. Public Health, 2012, 9, 4433–4451 CrossRef PubMed.
- K. Narita, Y. Matsui, K. Iwao, M. Kamata, T. Matsushita and N. Shirasaki, Environ. Int., 2014, 63, 114–120 CrossRef CAS PubMed.
- J. E. Barbash, G. P. Thelin, D. W. Kolpin and R. J. Gilliom, J. Environ. Qual., 2001, 30, 831–845 CrossRef CAS PubMed.
- K. F. Mendes, A. P. J. Régo, V. Takeshita and V. L. Tornisielo, in Organic Pollutants, IntechOpen, 2019 Search PubMed.
- P. A. Shivajirao, Int. J. Adv. Eng. Res. Stud., 2012, 275–283 Search PubMed.
- N. Matsumoto, H. Uemoto and H. Saiki, Water Res., 2007, 41, 2541–2550 CrossRef CAS PubMed.
- J. Wang and C. Chen, Biotechnol. Adv., 2009, 27, 195–226 CrossRef CAS PubMed.
- L. Chai, Q. Li, Q. Wang and X. Yan, Environ. Sci. Pollut. Res. Int., 2018, 25, 17250–17267 CrossRef PubMed.
- S. Mohamed, Wastewater Treatment Engineering, 2015 Search PubMed.
- J. Gomes, E. Domingues, M. Gmurek, R. M. Quinta-Ferreira and R. C. Martins, Energy Rep., 2020, 6, 666–671 CrossRef.
- C. Amor, L. Marchão, M. S. Lucas and J. A. Peres, Water, 2019, 11, 205 CrossRef CAS.
- P. R. Gogate and A. B. Pandit, Adv. Environ. Res., 2004, 8, 501–551 CrossRef CAS.
- S. Gligorovski, R. Strekowski, S. Barbati and D. Vione, Chem. Rev., 2015, 115, 13051–13092 CrossRef CAS PubMed.
- M. A. Oturan and J.-J. Aaron, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2577–2641 CrossRef CAS.
- Y. Guo, X. Mi, G. Li and X. Chen, J. Chem., 2017, 2017, 2830138 Search PubMed.
- A. A. Pradhan and P. R. Gogate, J. Hazard. Mater., 2010, 173, 517–522 CrossRef CAS PubMed.
- M. T. Taghizadeh and A. Mehrdad, Ultrason. Sonochem., 2003, 10, 309–313 CrossRef CAS PubMed.
- H. Liu, M. Y. Liang, C. S. Liu, Y. X. Gao and J. M. Zhou, Chem. Eng. J., 2009, 153, 131–137 CrossRef CAS.
- C. Minero, M. Lucchiari, D. Vione and V. Maurino, Environ. Sci. Technol., 2005, 39, 8936–8942 CrossRef CAS PubMed.
- H. A. Oualid, O. Amadine, Y. Essamlali, I. M. Kadmiri, H. E. Arroussi and M. Zahouily, Nanoscale Adv., 2019, 1, 3151–3163 RSC.
- M. Pirsaheb and N. Moradi, RSC Adv., 2020, 10, 7396–7423 RSC.
- P. Gholami, L. Dinpazhoh, A. Khataee and Y. Orooji, Ultrason. Sonochem., 2019, 55, 44–56 CrossRef CAS PubMed.
- J. Wang, Z. Jiang, L. Zhang, P. Kang, Y. Xie, Y. Lv, R. Xu and X. Zhang, Ultrason. Sonochem., 2009, 16, 225–231 CrossRef CAS PubMed.
- J. Wang, Y. Lv, L. Zhang, B. Liu, R. Jiang, G. Han, R. Xu and X. Zhang, Ultrason. Sonochem., 2010, 17, 642–648 CrossRef CAS PubMed.
- J. Hartmann, P. Bartels, U. Mau, M. Witter, W. V. Tümpling, J. Hofmann and E. Nietzschmann, Chemosphere, 2008, 70, 453–461 CrossRef CAS PubMed.
- N. B. Bokhale, S. D. Bomble, R. R. Dalbhanjan, D. D. Mahale, S. P. Hinge, B. S. Banerjee, A. V. Mohod and P. R. Gogate, Ultrason. Sonochem., 2014, 21, 1797–1804 CrossRef CAS PubMed.
- A. M. Abu-Dief and S. M. Abdel-Fatah, Beni-Suef University J. Basic Appl. Sci., 2018, 7, 55–67 CrossRef.
- S. Kameoka, T. Tanabe and A. P. Tsai, Catal. Lett., 2005, 100, 89–93 CrossRef CAS.
- L. S. Mdletshe, P. R. Makgwane and S. S. Ray, Nanomaterials, 2019, 9, 1140 CrossRef CAS PubMed.
- J. Plocek, A. Hutlová, D. Niž and J. Buršík, Mater. Sci., 2005, 23, 697–705 CAS.
- F. Liu, F. Niu, N. Peng, Y. Su and Y. Yang, RSC Adv., 2015, 5, 18128–18136 RSC.
- E. Aslani, A. Abri and M. Pazhang, Colloids Surf., B, 2018, 170, 553–562 CrossRef CAS PubMed.
- R. Dhanda and M. Kidwai, RSC Adv., 2016, 6, 53430–53437 RSC.
- R. Tabit, O. Amadine, Y. Essamlali, K. Dânoun, A. Rhihil and M. Zahouily, RSC Adv., 2018, 8, 1351–1360 RSC.
- Y. Zhang, Q. Zhu, Y. Zhao, X. Yang and L. Jiang, Chin. J. Chem. Phys., 2023 DOI:10.1063/1674-0068/cjcp2210150.
- F. Liu, F. Niu, N. Peng, Y. Su and Y. Yang, RSC Adv., 2015, 5, 18128–18136 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- K. Ali, A. Bahadur, A. Jabbar, S. Iqbal, I. Ahmad and M. I. Bashir, J. Magn. Magn. Mater., 2017, 434, 30–36 CrossRef CAS.
- J. Zheng, Z. Lin, W. Liu, L. Wang, S. Zhao, H. Yang and L. Zhang, J. Mater. Chem. B, 2014, 2, 6207–6214 RSC.
- Y. Li, L. Yang, X. Liu, N. Li, L. Zhang, Q. Li, Y. Yang, Y. Duan and F. Zhang, J. Mater. Sci., 2015, 50, 5960–5969 CrossRef CAS.
- M. Sayed, L. A. Shah, J. A. Khan, N. S. Shah, H. M. Khan, R. A. Khan, A. R. Khan and A. M. Khan, J. Chil. Chem. Soc., 2016, 61, 2949–2953 CrossRef CAS.
- G. Lyngsie, L. Krumina, A. Tunlid and P. Persson, Sci. Rep., 2018, 8, 1–9 CAS.
- M. Xing, W. Xu, C. Dong, Y. Bai, J. Zeng, Y. Zhou, J. Zhang and Y. Yin, Chem, 2018, 4, 1359–1372 CAS.
- C. D. Fernando and P. Soysa, MethodsX, 2015, 2, 283–291 CrossRef.
- K. Hou, G. Wang, Y. Zhu, N. Ezzatahmadi, L. Fu, A. Tang, H. Yang and Y. Xi, Appl. Clay Sci., 2019, 181, 105243 CrossRef CAS.
- S. Sajjadi, A. Khataee, N. Bagheri, M. Kobya, A. Şenocak, E. Demirbas and A. G. Karaoğlu, J. Ind. Eng. Chem., 2019, 77, 280–290 CrossRef CAS.
- J. Tang, Y. Chen and Z. Dong, J. Environ. Sci., 2019, 79, 153–160 CrossRef CAS PubMed.
- S. K. Nandwani, A. K. Mungray and M. Chakraborty, Indian J. Chem. Technol., 2015, 22, 73–77 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |