Open Access Article
Open Access ArticleDegradation of three β-O-4 lignin model compounds via organic electrolysis and elucidation of the degradation mechanisms†
Yoshiyuki Uruma *a,
Tomohiro Yamadab,
Tsubasa Kojimaa,
Tianyuan Zhang
*a,
Tomohiro Yamadab,
Tsubasa Kojimaa,
Tianyuan Zhang b,
Chen Quc,
Moe Ishiharaa,
Takashi Watanabe
b,
Chen Quc,
Moe Ishiharaa,
Takashi Watanabe c,
Kan Wakamatsu
c,
Kan Wakamatsu d and
Hirofumi Maekawa
d and
Hirofumi Maekawa b
b
aDepartment of Integrated Engineering, Chemistry and Biochemistry Division, National Institute of Technology, Yonago College, 4448, Hikona-cho, Yonago City, Tottori 683-8502, Japan. E-mail: uruma@yonago-k.ac.jp
bDepartment of Materials Science and Technology, Nagaoka University of Technology, 1603-1, Kamitomioka-cho, Nagaoka, Niigata 940-2188, Japan
cResearch Institute for Sustainable Humanosphere, Kyoto University, Gokasho, Uji, Kyoto 611-0011, Japan
dDepartment of Chemistry, Faculty of Science, Okayama University of Science, 1-1 Ridaicho, Kita-ku, Okayama 700-0005, Japan
First published on 14th June 2023
Abstract
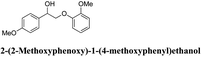
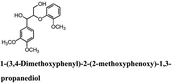
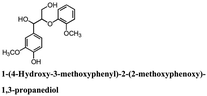
Woody biomass comprising cellulose, hemicellulose, and lignin has been the focus of considerable attention as an alternative energy source to fossil fuel for various applications. However, lignin has a complex structure, which is difficult to degrade. Typically, lignin degradation is studied using β-O-4 lignin model compounds as lignin contains a large number of β-O-4 bonds. In this study, we investigated the degradation of the following lignin model compounds via organic electrolysis: 2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)ethanol 1a, 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 2a, and 1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 3a. The electrolysis was conducted for 2.5 h at a constant current of 0.2 A using a carbon electrode. Various degradation products such as 1-phenylethane-1,2-diol, vanillin, and guaiacol were identified upon separation via silica-gel column chromatography. The degradation reaction mechanisms were elucidated using electrochemical results as well as density functional theory calculations. The results suggest that the organic electrolytic reaction can be used for the degradation reaction of a lignin model with β-O-4 bonds.
1. Introduction
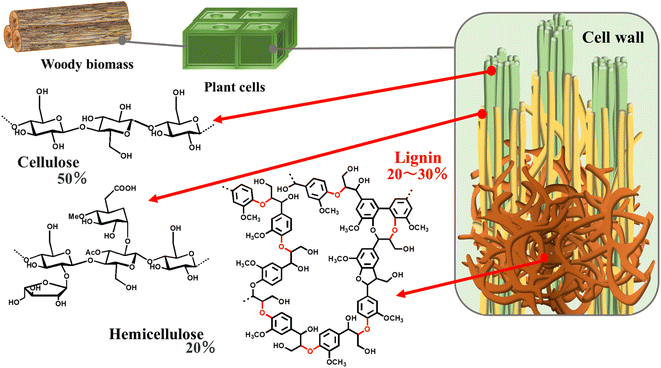
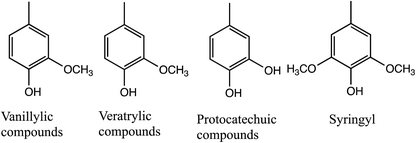
Petroleum resources have been widely used for various applications ever since the Industrial Revolution. However, owing to the depletion of oil reserves and climate change, the demand for sustainable energy resources to replace oil is increasing.1 Woody biomass, a general term for cellulose, hemicellulose, and lignin, which make up the cell walls of plants, is a naturally abundant chemical resource and common alternative to petroleum (Fig. 1). If explored via nanotechnology, lignocellulosic biomass (LCB) can be refined to yield high-performance fuel sources. The toxicity and cost of conventional methods can be reduced by applying nanoparticles in the refining of LCB.2 It can be used for manufacturing value-added chemicals and fuels and facilitates industrial production from renewable biomass, known as “biorefinery,” named after “oil refinery” (industrial production from petroleum resources).3However, the complex structure of woody biomass makes its applications challenging. Efficient lignin degradation is required for effectively using woody biomass. Lignin has a complex structure of C–C bonds or C–O bonds in phenylpropane units such as syringyl, guaiacyl, and hydroxyphenyl (Table 1).4
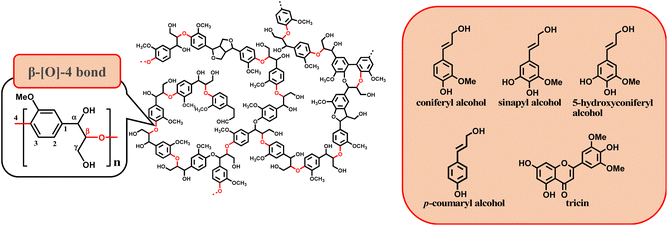
The β-O-4 bond is characteristic of lignin, and many studies have been conducted on the oxidative lignin degradation of the β-O-4 bonds in lignin.5 For example, lignin peroxidase, manganese peroxidase, and laccase isolated from white-rot fungi are lignin-degrading enzymes. Lignin degradation by lignin peroxidase reduces methylated lignin and cleaves the Cα–Cβ bond in the side chain.6,7
Nonaka et al. used a different method for the β-O-4 bond cleavage. They subjected lignin to organic electrolytic reactions under mild conditions without special reagents or catalysts and reported four types of oxidative degradation products (Fig. 2). They also concluded that the generation of a single product is difficult and that a robust method for the separation of the degradation products is essential.8 For example, thermochemical decomposition of lignin using a catalyst and internal heating, which are energy-saving and mild conditions, resulted in low yield and lack of reaction selectivity (Fig. 3).9
Gao et al. investigated β-O-4 lignin model compounds via electrochemical oxidation with an iodide ion mediator. They used 2,2-dimethoxy-2-arylacetaldehyde as a β-O-4 lignin model to investigate the electrochemical selective C–O bond cleavage.10 In this study, we analyzed the lignin degradation products of the following β-O-4-type lignin model compounds via constant current electrolysis: 2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)ethanol 1a, 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 2a, and 1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 3a. The experimental conditions (solvent, supporting salt, and electrodes) without additives were optimized. The degradation products were isolated using silica-gel column chromatography and the degradation mechanisms were elucidated via GC-MS analysis and density functional theory (DFT) calculations.
2. Results and discussion
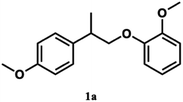
2.1. β-O-4 model compounds
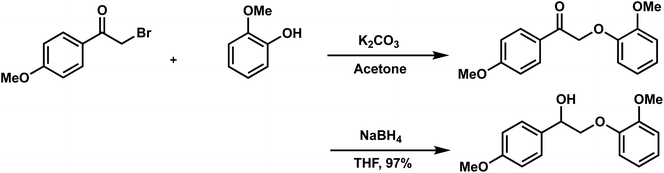
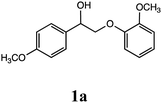
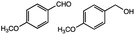
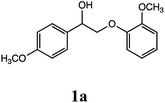
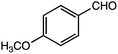
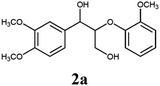
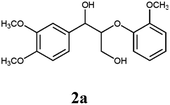
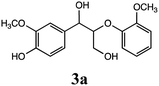
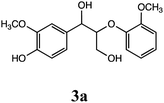
Lignin has a complex structure with a large number of β-O-4 bonds and, therefore, its degradation mechanism is typically studied using model compounds. 2-Aryloxyl-aryl ethanol is commonly used as a lignin model compound.11 Several studies on the degradation of lignin models via electrolysis have been conducted. Pardini et al. reported the electrolytic oxidation of a dimeric lignin model.12 In this study, 2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)ethanol 1a was used as the primary lignin model compound. In addition, 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 2a and 1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 3a, which has similar structures to that of lignin, were used for the electrolysis experiments. Compound 1a was synthesized in two steps using a previously reported method (Scheme 1).102.2. Organic electrolysis
Organic electrolytic reactions are pollution-free, energy-saving, and resource-saving and, thus, have attracted attention as clean chemical reactions occurring at the electrodes.13 Therefore, we chose this method for lignin degradation to establish a method that promotes the use of lignocellulosic biomass as a clean energy source. Several studies on lignin electrolysis have been previously reported. Li et al. reported the catalytic electrochemical degradation of aspen lignin in a three-dimensional electrode reactor using Pb/PbO2 anodes.14 Zang et al. demonstrated selective and efficient oxidation of the Cα–Cβ bond in 2-phenoxy-1-phenyl ethanol, a typical β-O-4 lignin model compound, utilizing multiphase active interfaces and smooth pore channels; a degradation rate of 93.6% and benzoic acid yield of 83.8% were reported.15Organic electrolysis reactions entail electron transfer (E process) and chemical reactions (C process). The product is obtained via an intermediate E process. Therefore, the selection of solvent and electrode is important.16 When a carbon (C) electrode was used, precipitates adhered to the electrode after 2 hours of electrolysis, and subsequent processes, such as an increase in voltage and electrolytic reaction temperature, were observed (Table 2).
| Time (h) | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
|---|---|---|---|---|---|---|---|
| Voltage (V) | 17 | 17 | 17 | 19 | 20 | 27 | 45 |
| Temperature (°C) | 22 | 25 | 26 | 28 | 28 | 30 | 33 |
The endpoint of the reaction was set at 2.5 hours when the lignin model compound was used as much as possible in the reaction and disappeared, and the electrolysis reaction could be performed stably.
The reason why a little methanol was added to the electrolysis reaction was to suppress the increase in voltage (Fig. 4).
Therefore, 2.5 hours was considered optimal for electrolysis. The reaction was monitored by thin layer chromatography (TLC), which confirmed the complete degradation of the lignin model compound detected at Rf 0.22 (EtOAc/hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), with a new spot appearing at Rf 0.00 that appeared to be a degradation product (Fig. 5).
3), with a new spot appearing at Rf 0.00 that appeared to be a degradation product (Fig. 5).
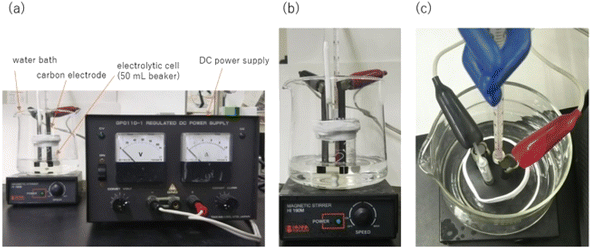 | ||
| Fig. 5 (a) Setup of the electrolysis equipment: DC power supply, carbon electrode, and electrolytic cell; (b) side-view and (c) top-view of the electrolysis cell. | ||
After each electrolysis, the electrode was checked, deposits were removed, and the electrode was polished. The thickness of the electrode was also measured after each electrolysis, and it was found that the electrode was corroded by about 0.1 mm after one reaction.
2.3. Organic electrolysis of 1a, 2a, and 3a
Electrolysis of the lignin models 1a, 2a, and 3a required the use of tetraethylammonium p-toluenesulfonate (Et4NOTs) as the supporting electrolyte.Initially, complete degradation of the raw materials was attempted. However, the reaction voltage increased because of the residues on the electrodes and, consequently, the temperature of the reaction solution increased. A stable electrolytic reaction was observed for only 2.5 h. The depth of immersion of the electrodes was varied to suppress the increase in the reaction temperature under mild reaction conditions. In the initial phase of the reaction, the solution in the electrolytic cell, which was initially colorless and transparent, turned brown. After extraction with ethyl acetate, the solvent was distilled under reduced pressure to afford a brown oily electrolyte mixture. The electrolyte was a mixture containing multiple components, and the degradation products were confirmed by TLC using a developing solvent of ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
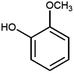
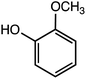
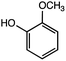
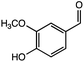
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with 1% acetic acid. Furthermore, electrolytic reactions of 2a and 3a, which have comparable structures to that of lignin, were performed. Guaiacol was isolated from 2a and its degradation reaction rate was 75% using CH3OH/CH3CN. In contrast, guaiacol and vanillin were isolated from 3a at a degradation rate of 65% (Table 3).
2 with 1% acetic acid. Furthermore, electrolytic reactions of 2a and 3a, which have comparable structures to that of lignin, were performed. Guaiacol was isolated from 2a and its degradation reaction rate was 75% using CH3OH/CH3CN. In contrast, guaiacol and vanillin were isolated from 3a at a degradation rate of 65% (Table 3).
The assignment of degradation products were evaluated using 1H NMR spectroscopy and GC-MS. The cylindrical C electrode had a diameters of 0.8 cm. The plate Pt electrode has a size dimension of 20 mm × 10 mm × 0.5 mm. The C electrode (SEG-R, Nippon Carbon Co. Ltd) was immersed in the reaction solution at a depth of 1.2 cm from the surface. The reaction was conducted under constant current at a current density of 0.13 A cm−2. The reaction progression was monitored using TLC, and the products were isolated by silica-gel column chromatography. The degradation rate was calculated using eqn (1):
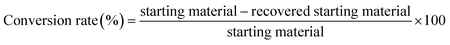 | (1) |
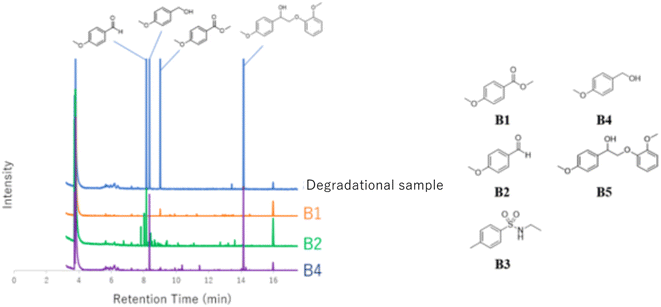
In the case of using a Pt electrolyte, even though lignin model compounds degradation occurred, the reaction rate was low when a Pt electrode was used. Moreover, the reaction rate decreased in the absence of acetonitrile (Run 3). For 1a, the reaction rate was 82% in a mixed methanol/acetonitrile solvent, whereas it was 77% when only methanol was used. This is attributed to the excellent electrolytic oxidation observed in organic solvents with dielectric constants of ≥30.17 Notably, when the Pt electrode was used, even though the reaction rate was low, the degradation products were clean, and complex degradation products were not observed. This suggests that a large number of degradation products were generated in the electrolytic oxidation under harsh conditions in Runs 1 and 2. However, the structure elucidation of these products was difficult. Furthermore, the electrolytic oxidation of 2a, which has a similar β-O-4 bond to that in lignin model compound, proceeded at a reaction rate of 87%, and guaiacol was confirmed as a degradation product (Run 4). Similarly, guaiacol and vanillin were detected as the degradation products of 3a at reaction rates of ≥65% (Runs 6 and 7). The degradation products and samples were purified by silica-gel column chromatography and evaluated by GC-MS. The degradation products of 1a were determined to be 4-methoxy benzoic acid (B1), 4-methoxy benzaldehyde (B2), and 4-methoxybenzyl alcohol (B4) (Fig. 6).
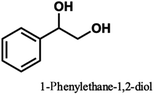
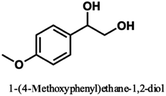
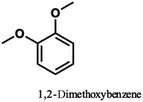
Pardini et al. reported that the Cα–Cβ bond was cleaved during electrolysis using a mediator, yielding an aldehyde.12 As the guaiacyl-type benzene ring is electron-rich, cation radical species could be easily generated by a one-electron oxidation reaction. To prove that the guaiacol aromatic rings are more easily oxidized, we conducted cyclic voltammetry tests on 1a using 1-phenylethane-1,2-diol, 1-(4-methoxy)-ethane-1,2-diol, and 1,2-dimethoxybenzene as the standard materials (Table 4).
| Substrate | Oxidation potentials |
|---|---|
| a Working electrode: Pt; counter electrode: Pt; reference electrode: Ag/AgCl; solvent: MeCN; supporting electrolyte: 0.1 M n-Bu4NClO4; scan rate: 0.2 V s−1. | |
 |
1.47 V |
| 1.85V | |
| 2.12 V | |
 |
2.25 V |
| 2.81 V | |
| 2.81 V | |
 |
1.69 V |
| 2.08 V | |
 |
1.05 V |
| 1.70 V | |
| 2.12 V | |
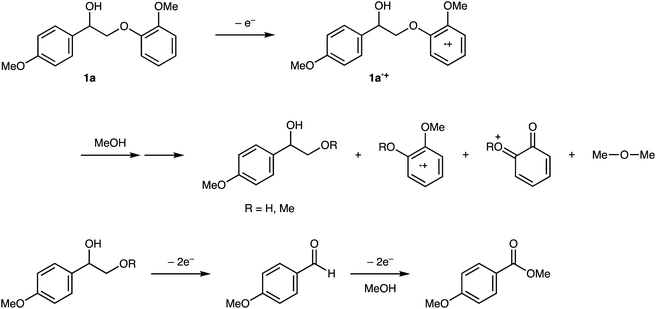
The oxidation potentials of 1a were determined to be 1.47, 1.85, and 2.12 V, indicating that the guaiacyl-type aromatic rings were easily oxidized. This may be attributed to the presence of a methoxy group at the 4-position of the benzene ring. This suggests that a nucleophilic attack on the cationic species by MeOH affords an ortho-quinone acetal, and the subsequent electrogenerated acid-catalyzed transesterification of the acetal generates 1-(4-methoxyphenyl)ethane-1,2-diol.
We hypothesized that we could confirm the feasibility of using electrolysis for lignin degradation by investigating the degradation of a structure comparable to that of lignin (2a and 3a). The reactions were evaluated by GC-MS without separation to confirm the degradation products.
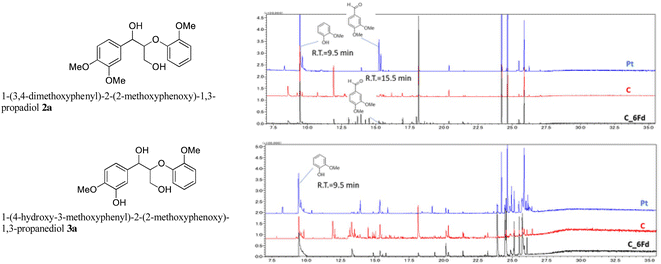
The GC-MS analysis of 2a and 3a is shown in Fig. 7. The blue and red spectra indicate the reaction electrolysis using Pt and C electrodes, respectively. The product mixture was evaluated by GC-MS without product isolation. The column temperature was programmed as follows: the temperature was initially maintained at 50 °C for 3 min, then increased at 3 °C min−1 to 300 °C and maintained for 7.5 min. In the case of 2a, guaiacol and methoxyvanillin were detected on both the Pt and C electrodes. When the reaction was performed at 6 F using a C electrode, a small amount of methoxyvanillin was detected, along with the main product guaiacol. Similarly, the decomposition of 3a yielded guaiacol as the major product, along with a small amount of methoxyvanillin, irrespective of the electrode type.
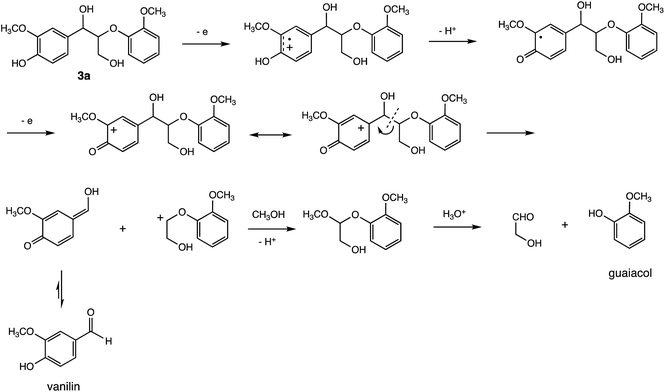
The degradation mechanism of 3a is shown in Scheme 3. Oxidation of the phenolic hydroxyl group to the quinone form leads to C–C bond cleavage owing to the electron transfer from the benzyl position. However, the intermediates have not yet been identified. Moreover, the easily oxidizable portion in 2a was difficult to identify, thus limiting our ability to elucidate its degradation mechanism. Therefore, computational studies were conducted.
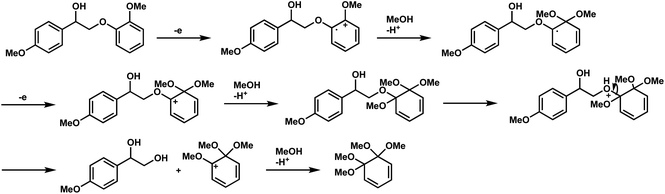
The proposed degradation mechanism for 1a is outlined in Scheme 2. To gain more insight into the mechanism, DFT calculations were performed at the B3LYP/6-31G(d) level using the Gaussian 16 program.18 The natural population analysis19 of the radical cation of 1a (1a˙+) revealed that both charge and spin were nearly localized in the guaiacyl-type aromatic moiety (+0.95 for charge and 1.00 for spin in conformer 1, see Fig. S1 and S2†). Because the nucleophilic attack of MeOH may occur at several positions on the guaiacyl moiety, the elucidation of the degradation mechanism is challenging (see Scheme S1 and Table S1†). However, 1-(4-methoxyphenyl)-1,2-ethanediol or its 2-methyl ether derivative is produced as a common intermediate at a sufficiently low oxidation potential. The 1,2-diol derivative was immediately oxidized to 4-methoxybenzaldehyde and methyl 4-methoxybenzoate. 4-Methoxybenzyl alcohol was likely formed via hydrogen transfer from the MeOH adduct of the aldehyde radical cation to the neutral form of the aldehyde; however, further investigation is required (Scheme 4).
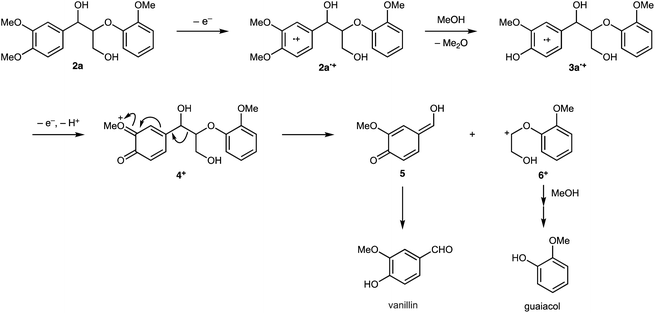
The proposed degradation mechanisms of 2a and 3a are outlined in Schemes 5 and 3. Both charge and spin of the radical cations of 2a (2a˙+) and 3a (3a˙+) are mainly distributed in the 3,4-dimethoxybenzylic or 3-methoxy-4-hydroxybenzylic moiety (+0.81 (2a˙+) and +0.66 (3a˙+) for charge; 0.84 (2a˙+) and 0.68 (3a˙+) for spin in conformer 2, see Fig. S1 and S2†). The 2a˙+ moiety can react with MeOH to afford 3a˙+ and Me2O exergonically (ΔG = −6.11 kcal mol−1). Proton abstraction from the hydroxy group of 3a˙+ yields the corresponding neutral radical, which undergoes oxidation to produce the quinoid cation 4+. The oxonium cationic center would facilitate the cleavage of the central C–C bond to produce 5, which isomerizes to vanillin and 6+ (ΔG = −7.32 kcal mol−1). Fragment 6+ reacts with MeOH to form an acetal intermediate, which is then hydrolyzed to produce guaiacol.
3. Conclusion
In this study, we investigated the degradation of lignin model compounds 2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)ethanol 1a, 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 2a and 1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 3a via electrolysis. The optimal degradation duration was 2.5 h. Methoxyvanillin, benzaldehyde, and 1-phenylethane-1,2-diol were identified as the degradation products of 1a, whereas guaiacol and vanillin were the degradation products of 2a and 3a. Duan et al. reported reactions using a Pt electrode and tBuOOH as an oxidant to obtain lignin model dimers, polymeric compounds, and even natural lignin as the desired aromatic aldehyde.20 We also proposed degradation mechanisms for 1a, 2a, and 3a based on GC-MS analysis and DFT calculations. This study used carbon and platinum electrodes to degrade lignin model compounds. It was found that useful products were obtained at high degradation rates without oxidants.This new finding differs from previous reports of electrolytic reactions with oxidants. Investigation into the applications of these degradation products is currently underway.
4. Experimental
4.1. Instruments and reagents
1H and 13C NMR spectra were recorded on a BRUKER 400 instrument at 400 and 100 MHz, respectively. The chemical shifts are reported as δ values in relation to the internal standards tetramethylsilane (0 ppm) and CHCl3 (77.0 ppm) for 1H and 13C NMR spectra, respectively. High-resolution mass spectra (HRMS) were obtained using JMS-AX 500 and JMS-700T spectrometers at the Analytical Center of Osaka City University. Silica gel (silica gel 60, 230–400 mesh) was used for flash chromatography. Pre-coated silica gel plates (Merck 5715 and 60F254) were used for TLC. 2-(2-Methoxyphenyl)oxy-1-phenetanol, which was used as the starting material for OEM, was synthesized using a previously reported method. Carbon electrode (SEG-R; Nippon Carbon Co., Ltd) was purchased from TCI. 1-(3,4-Dimethoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 2a was purchased from Wako. 1-(4-Hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 3a was purchased from TCI.4.2. Preparation of β-O-4 lignin model
We prepared the β-O-4 lignin model 2-(2-methoxyphenyl)oxy-1-phenethanol using a method previously reported by Nichols at al.84.3. Organic electrolysis
A 50 mL beaker cell was equipped with a carbon electrode, thermometer, and DC-regulated power supply. Lignin model substrate (0.2 g; 0.82 mmol), tetraethylammonium p-toluenesulfonate (1.0 g; 3.32 mmol), 1.5 mL of methanol, and 28.5 mL of acetonitrile were added to the cell. The mixture was electrolyzed under constant current at 0.2 A for 2.5 h with continuous stirring and cooling in a water bath. After electrolysis, the mixture was extracted with ethyl acetate (50 mL × 3). The organic phase was washed with brine and dried over Na2SO4. The organic extracts were then concentrated under reduced pressure. The residue was subjected to column chromatography to separate the products.4.4. Degradation products
The degradation mixture obtained from organic electrolysis was separated and purified using silica-gel column chromatography (ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1% acetic acid). Six degradation products were obtained by distilling the solvent at reduced pressure for each fraction. These products were further purified by preparative TLC, and their structures were determined by NMR and mass spectrometry. GC-MS measurements were used to evaluate lignin models 2a and 3a. A GCMS-QP2010SE instrument (Shimadzu, Kyoto, Japan) was connected to a DB-5MS column (30 m × 0.25 mm id, 0.25 μm film thickness; Agilent Technologies). MS measurements were performed in the electron ionization mode at a voltage of 0.7 eV. The MS scanning range was m/z 30–700. The column temperature was initially maintained at 50 °C for 3 min and then raised to 300 °C at 3 °C min−1, where it was maintained for 7.5 min. The compounds were identified by a GCMS-QP2010SE similarity search.
2, 1% acetic acid). Six degradation products were obtained by distilling the solvent at reduced pressure for each fraction. These products were further purified by preparative TLC, and their structures were determined by NMR and mass spectrometry. GC-MS measurements were used to evaluate lignin models 2a and 3a. A GCMS-QP2010SE instrument (Shimadzu, Kyoto, Japan) was connected to a DB-5MS column (30 m × 0.25 mm id, 0.25 μm film thickness; Agilent Technologies). MS measurements were performed in the electron ionization mode at a voltage of 0.7 eV. The MS scanning range was m/z 30–700. The column temperature was initially maintained at 50 °C for 3 min and then raised to 300 °C at 3 °C min−1, where it was maintained for 7.5 min. The compounds were identified by a GCMS-QP2010SE similarity search.
4.5. DFT calculations
Calculations were performed using the Gaussian 16 program package.18 The structures of the closed-shell species were optimized at the restricted B3LYP/6-31G(d) level, whereas the structures of open-shell species (1a˙+, 2a˙+, and 3a˙+) were optimized using the unrestricted theory at the same level. Frequency analysis was performed for each optimized structure to confirm that no imaginary frequencies were obtained for the energy-minimum structures. These calculations were performed in an MeOH solution using a polarizable continuum model. A built-in Gaussian natural population analysis was used to estimate the charge and spin distributions.19Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We acknowledge Prof. Yuzo Fujii for his valuable suggestions and discussions. This study was funded by the Nagaoka University of Technology (NUT) grant for collaborative research with the National Institute of Technology (NIT) and the Research Institute for Sustainable Humanosphere (RISH), Kyoto University grant for the System for Development. In addition, this study was partly funded by the Assessment of Sustainable Humanosphere, NIT and Yonago College financial support.References
- V. A. Rebecca, V. Ruben, S. Véronique, C. M. Jennife, D. Paul and B. Wout, Biotechnol. Bioeng., 2013, 6, 64 Search PubMed.
- N. Dey, G. Kumar, A. S. Vickram, M. Mohan, R. R. Singhania, A. K. Patel, C. D. Dong, K. Anbarasu, S. Thanigaivel and V. K. Ponnusamy, Bioresour. Technol., 2022, 344(Pt A), 126171 CrossRef CAS PubMed.
- G. Chatel and R. D. Rogers, ACS Sustainable Chem. Eng., 2014, 2, 322–339 CrossRef CAS.
- G. Zhu, X. Qiu, Y. Zjao, Y. Qian, Y. Pang and X. Ouyang, Bioresour. Technol., 2016, 218, 718–722 CrossRef CAS PubMed.
- (a) Y. Liu, Q. Luo, Q. Qiang, H. Wang, Y. Ding, C. Wang, J. Xiao, C. Li and T. Zhang, ChemSusChem, 2022, 15(21), e202201401 CrossRef CAS PubMed; (b) C. Scimmi, L. Sancineto, J. Drabowicz and C. Santi, Int. J. Mol. Sci., 2022, 23(8), 4378 CrossRef CAS PubMed.
- M. Tien and T. K. Kirk, Science, 1983, 221, 661–663 CrossRef CAS PubMed.
- J. K. Glenn, M. A. Morgan, M. B. Mayfield, M. Kuwahara and M. H. Gold, Biochem. Biophys. Res. Commun., 1983, 114, 1077–1083 CrossRef CAS PubMed.
- A. A. Yoshiyama, T. Fuchigami, T. Nonaka, T. C. Chou and H. J. Tien, Chem. Express, 1986, 1, 212 CAS.
- A. Ashutosh, R. Masud and P. Jeong-Hun, Fuel Process. Technol., 2018, 181, 115–132 CrossRef.
- (a) W.-J. Gao, C. M. Lam, B.-G. Sun, R. D. Little and C.-C. Zeng, Tetrahedron, 2017, 73, 2447–2454 CrossRef CAS; (b) A. Yoshiyama, T. Fuchigami, T. Nonaka, T.-C. Chou and H.-J. Tien, Chem. Express, 1986, 1, 212 CAS.
- J. M. Nichols, L. M. Bishop, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2010, 132, 12554–12555 CrossRef CAS PubMed.
- V. L. Pardini, C. Z. Smith, J. H. P. Utley, R. R. Vargas and H. Viertler, J. Org. Chem., 1991, 56, 7305–7313 CrossRef CAS.
- S. Rodrigo, D. Gunasekera, J. P. Mahajan and L. Luo, Curr. Opin. Electrochem., 2021, 28, 100712 CrossRef CAS.
- Y. S. Wang, F. Yang, Z. H. Liu, L. Yuan and G. Li, Catal. Commun., 2015, 67, 49–53 CrossRef CAS.
- N. Wang, R. Xue, N. Yang, H. Sun, B. Zhang, Z. Ma, Y. Ma and L. Zang, J. Alloys Compd., 2022, 929, 167324 CrossRef CAS.
- Y. Liu, C. Li, W. Miao, W. Tang, D. Xue, C. Li, B. Zhang, J. Xiao, A. Wang, T. Zhang and C. Wang, ACS Catal., 2019, 9, 4441–4447 CrossRef CAS.
- N. L. Weinberg and H. R. Weinberg, Chem. Rev., 1968, 68, 449 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- E. D. Glendening, A. E. Reed, J. E. Carpenter and F. Weinhold, The Gaussian Package Includes the Program of Natural Bond Orbital Analysis: NBO program version 3.1 Search PubMed.
- L. Ma, H. Zhou, X. Kong, Z. Li and H. Duan, ACS Sustainable Chem. Eng., 2021, 9, 1932–1940 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02486e |
| This journal is © The Royal Society of Chemistry 2023 |