Open Access Article
Open Access ArticleEfficient Buchwald–Hartwig and nitrene-mediated five-membered ring closure approaches to the total synthesis of quindoline. Unexpected direct conversion of a nitro group into a phosphazene†
Elida N. Thobokholt ,
Sebastián O. Simonetti
,
Sebastián O. Simonetti ,
Teodoro S. Kaufman
,
Teodoro S. Kaufman ,
Enrique L. Larghi
,
Enrique L. Larghi * and
Andrea B. J. Bracca
* and
Andrea B. J. Bracca *
*
Instituto de Química Rosario (IQUIR, CONICET-UNR), Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Suipacha 531, 2000, Rosario, Argentina. E-mail: bracca@iquir-conicet.gov.ar; larghi@iquir-conicet.gov.ar
First published on 5th May 2023
Abstract
Two total syntheses of quindoline, which take place through the intermediacy of 3-nitroquinoline derivatives, are reported. The general synthetic sequence involves construction of the latter by mechanochemical condensation of benzaldehydes with 2-amino-nitrostyrene, followed either by reduction of the nitro group of the heterocycle and Buchwald–Hartwig cyclization or by a nitrene-mediated cyclization under solventless conditions. Use of PPh3 to generate the nitrene resulted in the unprecedented formation of a phosphazene in place of quindoline. This unexpected transformation was explained by means of DFT computations.
Introduction
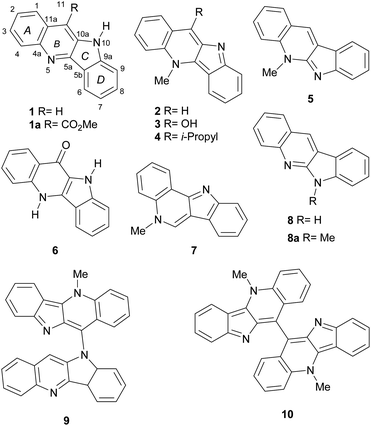
The indoloquinolines are a small family of heterocyclic natural products, which display fused indole and quinoline rings. They have been isolated from various plants around the world belonging to three different genera. These include the African thin-stemmed twining and scrambling shrub Cryptolepis sanguinolenta (Periplocaceae), the Chinese Isatis indigotica and I. tinctoria (Brassicaceae), the indian Justicia betonica L. (Acanthaceae),1 the American evergreen climbing shrub J. secunda Vahl and the Brazilian Sida acuta,2 S. rhombifolia and S. cordifolia3 (Malvaceae).The natural indoloquinolines share two main characteristics: their vegetal sources enjoy wide use in different traditional medicine systems, being employed to treat a variety of illnesses and conditions;4 in addition, the natural products are scarcely decorated by alkyl or oxygen bearing functionalities.5
Most of the natural indoloquinolines are monomeric (Fig. 1) and display their heterocyclic components fused to yield different skeletal arrangements. These comprise, among others, quindoline (1),6 methylquindoline-11-carboxylate (1a),7 cryptolepine (2),8 11-hydroxycryptolepine (3),9 11-isopropyl cryptolepine (4),10a neocryptolepine (5),11 quindolinone (6),10b isocryptolepine (7)9b,10c and quinindoline (8).1
Enlarging the family, a handful of dimeric indoloquinolines have also been reported as minor alkaloids from Cryptolepis sanguinolenta. Among them are cryptoquindoline (9)9 and biscryptolepine (10),9b as well as quindolinocryptotackieine12a cryptolepicarboline,12b cryptospirolepine,12c and cryptomisrine.12d
We have recently focused on the synthesis of indoloquinolines,13 contributing with the total syntheses of quindoline (1)14 and neocryptolepine (5).15 Access to the unnatural 6-methylquinindoline (8a) was also disclosed.15
The synthesis of indoloquinolines remains an active field. Quindoline (1) has been isolated from different plants worldwide;3,5a,6,16,17 this simple and bioactive monomeric indoloquinoline is a synthetic precursor of the most widely known cryptolepine (2).18
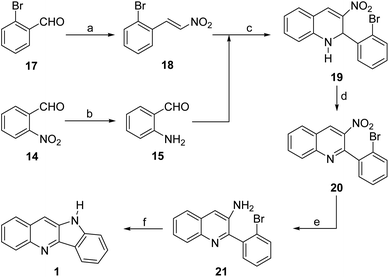
During our previous synthesis of quindoline, we observed that attempts to perform a nitrene-based cyclization of the five-membered ring by PPh3-mediated reduction of the nitrobenzene derivative 11 resulted exclusively in the formation of the indazolo[2,3a]quinoline 13,14 whereas an alternative Buchwald–Hartwig approach from the bromo-aniline 12 gave the product (1) in good yield (Scheme 1). In an attempt to avoid these diverging paths, we considered exchanging the places of the nitrogen functionality and the cyclization facilitating group, when required, as a viable alternative.
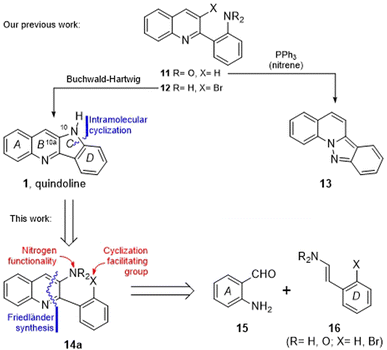 | ||
| Scheme 1 Synthetic approaches to quindoline. Previous strategy and a proposed new retrosynthetic analysis of quindoline (1). | ||
Retrosynthetically, this concept is shown in 14a, where the nitrogen functionality is supported by the quinoline moiety. In turn, appropriate C–C and C–N disconnections of the heterocyclic ring in the latter unveil the aminobenzaldehyde 15 and the nitrostyrene derivative 16 as its starting materials, conjecturing that a Friedländer condensation would serve as a constructive solution.
Therefore, herein we wish to report a new strategic approach toward the total synthesis of quindoline, employing a ring building route of the type A + D → ABD → ABCD, in compliance with the retrosynthetic analysis of Scheme 1.
Results and discussion
According to the retrosynthetic analysis, the synthetic sequence commenced with the preparation of the proposed 2,3-disubstituted quinoline intermediate exemplified by 14. To that end, 2-bromobenzaldehyde (17) was reacted with nitromethane and subjected to a Henry nitroaldol condensation under NH4AcO promotion, in refluxing AcOH, to afford 18 in 62% isolated yield,19 whereas the slightly unstable 2-aminobenzaldehyde (15) was conveniently obtained in almost quantitative yield, by an iron-mediated reduction of 2-nitrobenzaldehyde (14) in an EtOH-0.1 N HCl medium (Scheme 2).14Next, the projected Friedländer-type condensation was undertaken, employing a solvent-free strategy where 15 and 18 were thoroughly mixed with neutral alumina. Under these conditions, a hardly separable mixture of 1,2-dihydroquinoline 19 and the corresponding quinoline 20 were obtained in 98% combined yield.20
Considering that formation of the quinoline could not be driven to completion, it was assumed that its presence among the products could be the result of an air-mediated oxidation of the reaction mixture. Further, in view of the difficulties posed by the separation of both products, the dehydrogenation stage was optimized on the mixture, as shown in Table 1.
| Entry no. | Reagents | Solvent | Temp. (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a Performed under microwave irradiation (maximum power: 150 W); complex mixture of unidentifiable compounds. | |||||
| 1 | MnO2 | CH2Cl2 | 100 | 0.5 | 0a |
| 2 | I2 | EtOH | RT | 3 | 4 |
| 3 | KMnO4 | Me2CO | RT | 10 | 6 |
| 4 | FeCl3, DDQ (cat.) | CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (10 H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
RT | 22 | 29 |
| 5 | p-Benzoquinone | Dioxane | Reflux | 72 | 35 |
| 6 | KHSO4, K2CrO4, SiO2 | CH2Cl2 | RT | 20 | 38 |
| 7 | Mn(OAc)3.2H2O DDQ (cat.) | CH2Cl2 | RT | 12 | 51 |
| 8 | CuI | DMSO | 120 | 1 | 78 |
| 9 | DDQ | CH2Cl2 | RT | 0.5 | 98 |
Unexpectedly, the microwaves-assisted reaction with activated MnO2 in CH2Cl2 gave a complex mixture of unidentified products (entry 1), whereas exposure to iodine in EtOH for 3 h at room temperature (entry 2) and to KMnO4 in acetone (entry 3) gave unsatisfactory yields (4 and 6%, respectively).
A slightly better performance was observed with FeCl3 in a CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (10
H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solvent mixture (29% yield) under catalytic amounts of DDQ after 22 h at room temperature (entry 4). However, still unsatisfactory results were obtained with p-benzoquinone (entry 5) in refluxing dioxane (72 h, 35% yield) and with the K2CrO4/KHSO4 reagent supported on SiO2 (entry 6) under solventless conditions, when it was left to react for 20 h at room temperature (38% yield).
1, v/v) solvent mixture (29% yield) under catalytic amounts of DDQ after 22 h at room temperature (entry 4). However, still unsatisfactory results were obtained with p-benzoquinone (entry 5) in refluxing dioxane (72 h, 35% yield) and with the K2CrO4/KHSO4 reagent supported on SiO2 (entry 6) under solventless conditions, when it was left to react for 20 h at room temperature (38% yield).
In view of these meagre results, an additional series of conditions was tested. The use of Mn(OAc)3.2H2O as oxidant, with the addition of catalytic amounts of DDQ, for 12 h at room temperature gave 20 in a moderate 51% yield (entry 7). However, an improved 78% yield was achieved after a 1 h reaction with CuI in DMSO at 120 °C (entry 8). Finally, it was found that exposure of 19 to DDQ at room temperature was capable of affording the required quinoline 20 in 98% yield after 30 min (entry 9).
With an efficient protocol to access the 3-nitroquinoline in hand, the next step in the sequence involved its modification toward a cyclizable substrate.
Therefore, 20 was reduced to the 3-aminoquinoline 21 in 99% yield with iron turnings in EtOH to which 0.1 N HCl was added.14
Then, aiming to optimize the yields, the final cyclization stage was performed under different conditions, as shown in Table 2.
The first attempt, an intramolecular amination of the bromide under tBuOK promotion in DMSO at 130 °C for 24 h afforded the expected product in 43% yield (entry 1),21a whereas an analogous photostimulated cyclization at room temperature using violet LED light proceeded in 57% yield (entry 2). On the other hand, the reaction with CuI and K2CO3 in DMF at 110 °C, under modified Ullman conditions and proline as ligand, gave 1 in 56% yield after 24 h (entry 3).
Gratefully, the moderate performance of these reactions was surpassed by the Buchwald–Hartwig cyclization of 21 in THF under Pd(MeCN)2Cl2 catalysis, which proceeded uneventfully to afford 1 in 78% yield, when DPPF was employed as ligand and tBuOK as base (entry 4). The spectroscopic data of the tetracycle were in full agreement with those of the literature.14
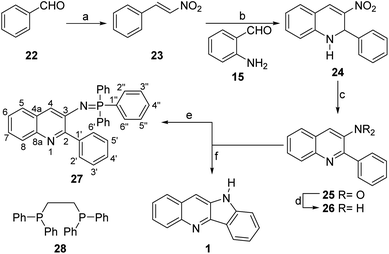
After demonstrating that the new strategy was capable to successfully afford quindoline through a Buchwald–Hartwig reaction, our attention was turned toward the use of a nitrene as cyclization intermediate. To that end, benzaldehyde (22) was submitted to a Henry reaction under microwaves irradiation, affording nitrostyrene (23) in 70% yield after 1.5 h (Scheme 3).
Next, the reaction of 23 and freshly prepared 15 under promotion by basic alumina, gave a mixture of the heterocycles 24 and 25 in 99% combined yield, after 24 h at 50 °C. Conversion of the mixture to 3-nitroquinoline 25 took place in 82% yield by stirring for 30 min at room temperature, with DDQ as oxidant.
This set the stage for the final cyclization step. Unexpectedly, however, reaction of 25 with PPh3 in refluxing toluene under MoO2Cl2(DMF)2 catalysis gave aminoquinoline 26 in 52% yield, whereas reaction with PPh3 at 150 °C under solventless conditions afforded phosphazene 27 in 87% yield, in place of the sought tetracycle 1. Interestingly, however, under the same conditions, the use of DPPE (28), a chelating phosphine, afforded quindoline in 82% yield.21b The NMR data of the heterocycle fully agreed with those recorded for the Buchwald–Hartwig product and for natural quindoline.14
The unexpected isolation of 27 prompted for the need of studying the reasons underlying its generation. Phosphazenes (iminophosphoranes) have been prepared by reaction of phosphines with different nitrogen functionalities, including mainly amines22 and azides (Staudinger reaction)23 which, in turn, were accessed from the corresponding nitro-aromatic precursors.
While phosphazenes are directly formed from azides and phosphines, their access from amines requires an activation agent (PPh3.Br2 PPh3/CCl4, PPh3/I2 or the like).24 Interestingly, however, to the best of our knowledge, this is the first time a phosphazene is obtained by direct reaction between a nitro-aromatic moiety and PPh3. Furthermore, the surprisingly high yield of the transformation suggests that it may be synthetically useful.
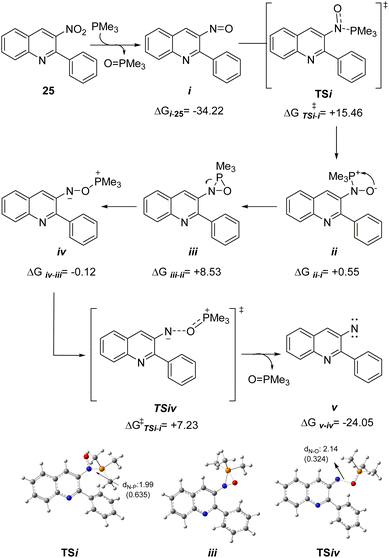
Despite the most intimate details of the reaction mechanism may still remain unknown, based on previous reports, a proposal was devised to explain our observations regarding the generation of phosphazene 27 from the nitroquinoline 25, which involves the sequential participation of three molecules of PPh3 for each one of the starting nitroquinoline. In order to gain more insight about the mechanism that affords the phosphazene intermediate, a DFT study was performed at the m062x/6-31g*//6-311+g** theoretical level, using the less computationally demanding Me3P instead of Ph3P for the calculations, with the results displayed in Scheme 4.
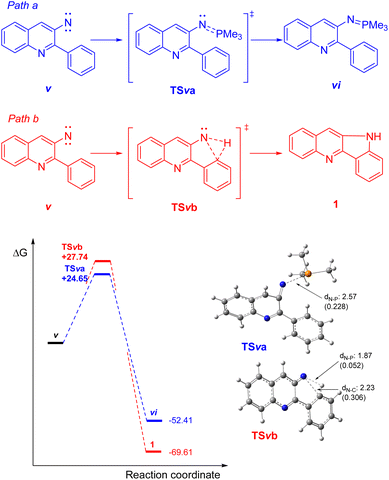
It has been shown that nitroso derivatives result from nucleophilic attack to the oxygen atom of the nitro moiety by a lone electron pair from the phosphine, with further release of phosphine oxide.25 This is in line with our previous proposal;14 hence, compound i was considered as a logical intermediate in the sequence and our calculations show that i is 34.22 kcal mol−1 more stable than 25 (Scheme 4).
In turn, i could experience a nucleophilic attack by a second molecule of the phosphine at the nitrogen atom and subsequently lead to formation of a betaine (ii), which could undergo ring closure to the oxazaphosphiridine iii. This kind of strained heterocycles has been earlier proposed in the context of the mechanism of the Cadogan or Cadogan–Sundberg type synthesis of indoles and analogous pyrrole derivatives from nitroaromatics.26 Further, their formation has been recently demonstrated by low temperature 31P NMR spectroscopy,27 and they were also proposed as key intermediates during the Mitsunobu esterification of alcohols mediated by nitrosobenzene.28
The formation of the betaine intermediate from i through the TSi involves an activation energy of 15.46 kcal mol−1, with an endothermic balance for ii of 0.55 kcal mol−1. Afterwards, the ring closure of ii to obtain iii shows that the balance of these two intermediates has a thermodynamic penalty of 8.53 kcal mol−1 through iii. The intermediate iii could afford the nitrene intermediate by two paths: (1) a stepwise path that involves the break of the N–P bond in iii and the formation of a “N–O–P” species as iv. Subsequently, iv could release Me3PO with the concomitant formation of the nitrene intermediate. (2) Another alternative is the formation of the nitrene intermediate v through the concerted elimination of Me3PO as previously was described for bisphospines.27
Whereas it was not possible to find out the transition state of the concerted mechanism (even a dissociate transition state), the intermediate iv was observed in an optimization of iii. Therefore, this intermediate was re-optimized and furnished an energy which resulted −0.12 kcal mol−1 lower than iii. The transition state (TSiv) for the formation of the nitrene intermediate (v) afforded an activation energy of 7.23 kcal mol−1 with and exergonic energy of −24.05 kcal mol−1 in favour of v.
This nitrene intermediate could continue the reaction along two paths (Scheme 5).29 One of them is the formation of the cyclized product through the insertion of the nitrene species at C–H bond of the aromatic ring (Path b). The other possibility (observed experimentally) is the formation of the phosphazene through the coupling between the nitrene and the phosphine (Path a). The calculations of these paths show that the formation of the phosphazene product presents an activation energy of 24.65 kcal mol−1 (TSva), whereas the formation of the cyclized product (TSvb) is 3 kcal mol−1 higher than the phosphazene product. While the formation of the cyclized product presents a lower energy balance regards to the nitrene than the phosphazene product (−69.61 kcal mol−1 vs. −52.41 kcal mol−1, respectively) the energy gap in the activation energy is enough to explain the results obtained experimentally.
Therefore, based on our theoretical calculations, it can be assumed that the phosphazene product could be accessed from nitrene v, which, in turn, is obtained through the gradual cleavage of the oxaazaphosphane iii intermediate (Scheme 4). Exchange of oxygen atoms in the case of bisphosphines (such as DPPF) probably also provides the intermediate nitrene, but cyclization might be favored instead of a phosphazene product due to steric hindrance. However, a deeper mechanistic investigation may be required to confirm this hypothesis and rule out an electronic factor.
Conclusions
In conclusion, we have developed simple and efficient total syntheses of quindoline, a naturally-occurring bioactive indoloquinoline, based on Buchwald–Hartwig and nitrene-mediated five-membered ring closure approaches, employing 3-nitroquinoline derivatives as key intermediates. The Buchwald–Hartwig alternative afforded quindoline in 5 linear steps and 46% overall yield from 2-bromobenzaldehyde (73% from 2-nitrobenzaldehyde), whereas the nitrene-based synthesis afforded the natural product in only 4 steps and 47% overall yield (66% from 2-nitrobenzaldehyde).Interestingly, phosphazene 27 was obtained in an unprecedented way by reaction with 3-nitroquinoline 25 and PPh3. DFT calculations were carried out to shed light on its formation and confirmed a nitrene as key intermediate in the mechanistic pathway. Further investigation on the scope and limitations of the formation of phosphazenes with other nitroarenes is underway and the results will be reported in due time.
Both strategies, which were devoid of protecting groups, use 2-nitrobenzaldehyde as starting material. Simplicity of these routes suggests that they can inspire the synthesis of more complex heterocycles as analogs of the natural product.
Experimental
General information
The reactions were executed with oven-dried glassware under dry nitrogen or argon atmospheres. Anhydrous DMF was obtained by heating the PA grade product over BaO for 4 h, followed by distillation under reduced pressure. Anhydrous CH2Cl2 was obtained by refluxing 4 h the analytical grade product over CaH2, followed by distillation. Anhydrous toluene was obtained by reflux of the solvent over sodium, to which benzophenone was added, followed by distillation from the deep-blue sodium benzophenone ketyl. Anhydrous EtOH was obtained by refluxing the analytical grade solvent for 4 h over magnesium ethoxide, prepared in situ from magnesium turnings activated with iodine, and distilling the solvent from the thus formed magnesium ethoxide. Anhydrous solvents were stored in dry Young ampoules. All other reagents were used as received.The flash column chromatographies were carried out employing Merck's silica gel 60H. The elutions were performed under positive pressure with mixtures of hexane and EtOAc, and employing gradient of solvent polarity techniques. The new compounds exhibited single spots on TLC plates (silica gel 60 GF254) developed in different hexane/EtOAc solvent systems. The spots were detected by exposure of the plates to 254 nm UV light, followed by spraying with ethanolic p-anisaldehyde/sulfuric acid reagent or with the Dragendorff reagent (Munier and Macheboeuf modification).30 The plates were carefully heated, for improving selectivity.
Apparatus
The melting points were determined on a model 350 Ernst Leitz Wetzlar hot-stage microscope and are reported uncorrected. The IR spectra were acquired in a Prestige 21 spectrophotometer (Shimadzu Corp., Tokyo, Japan), as thin films between NaCl plates or as solid dispersions in KBr disks.The NMR spectra were acquired in CDCl3 at 300.13 (1H), 75.48 (13C) or 188.31 (19P) MHz on a Bruker Avance spectrometer. The peak of residual CHCl3 (δH = 7.26 ppm), and the signal of CDCl3 (δC = 77.0 ppm) were used as internal standards, respectively. The chemical shifts are informed in parts per million (δ scale) and the J-values are given in Hertz. When required, selective TOCSY and 2D-NMR experiments (COSY, HMBC and HMQC) were also obtained. The high-resolution mass spectra were obtained from UMYMFOR (Buenos Aires, Argentina) with a Bruker MicroTOF-Q II instrument. Detection of the ions was performed in electrospray ionization, positive ion mode.
(E)-1-Bromo-2-(2-nitrovinyl)benzene (18)
A solution of 2-bromobenzaldehyde (17, 113 mg, 0.61 mmol) in glacial acetic acid (1.5 mL) was treated portion-wise with NH4AcO (94 mg, 1.22 mmol) and the mixture was stirred for 15 minutes at room temperature. Then, MeNO2 (149.3 mg, 2.44 mmol) was slowly transferred to the reaction medium and the system was heated under reflux for 24 h. Brine was added (30 mL) and the product was extracted with EtOAc (3 × 30 mL). The combined organic phases were dried (MgSO4), filtered and concentrated under reduced pressure. The crude material was purified by chromatography to give 18 (86 mg, 62%), as a yellow solid. Mp 88–90 °C (Lit.: 87–88 °C);19a IR (KBr, υ): 3395, 3750, 3422, 1636, 1458, 1340, 1111, 945 and 758 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.40 (1H, d, J = 13.7, H-2′), 7.69 (1H, dd, J = 7.4 and 1.9, H-3), 7.58 (1H, dd, J = 7.4 and 2.1, H-6), 7.54 (1H, d, J = 13.7, H-1′), 7.38 (1H, td, J = 7.4 and 1.9, H-5) and 7.33 (1H, td, J = 7.4 and 2.1, H-1′); 13C NMR (75 MHz, CDCl3): δ = 139.0 (C-1′), 137.5 (C-2′), 134.0 (C-3), 133.0 (C-4), 130.0 (C-2), 128.5 (C-6), 128.1 (C-5) and 126.3 (C-1).2-Aminobenzaldehyde (15)
A stirred solution of 2-nitrobenzaldehyde (14, 206 mg, 1.36 mmol) in EtOH (8 mL) to which a 0.1 N HCl solution (680 μL, 68 μmol) was added, was treated with Fe0 turnings (299 mg, 5.34 mmol) and the reaction was heated under reflux for 4 h. After cooling to room temperature, the reaction mixture was diluted with EtOAc (30 mL), filtered through Celite®, and the filtrate was neutralized with saturated NaHCO3 solution and washed with brine (10 mL). The product was extracted with EtOAc (3 × 30 mL), and the combined organic phases were dried (MgSO4), filtered and concentrated under reduced pressure. After filtration through a shorth pad of Celite, the desired product 15 was obtained (160 mg, 99%), as a yellow solid.18 Mp 32–34 °C; IR (KBr, υ): 3458, 3352, 3063, 2746, 1651, 1556, 1396, 1157, 1028 and 752 cm−1; 1H NMR (300 MHz, CDCl3): δ = 9.87 (1H, s, CHO), 7.48 (1H, dd, J = 7.8 and 1.5, H-6), 7.34–7.28 (1H, m, H-4), 6.75 (1H, dt, J = 7.4 and 0.7, H-5), 6.64 (1H, d, J = 8.3, H-3) and 6.11 (2H, bs, NH2); 13C NMR (75 MHz, CDCl3): δ = 194.1 (CHO), 149.9 (C-2), 135.7 (C-4), 135.2 (C-6), 118.9 (C-5), 116.4 (C-1) and 116.0 (C-3).2-(2-Bromophenyl)-3-nitroquinoline (20)
2-Bromonitrostyrene (21.6 mg, 0.095 mmol) was added to a mixture containing neutral Al2O3 (95 mg, 1.07 mmol) and 2-aminobenzaldehyde (11.5 mg, 0.095 mmol) and the reaction was heated at 50 °C for 24 h. After cooling to room temperature, the crude material was chromatographed to give a barely separable mixture of 2-(2-bromophenyl)-3-nitro-1,2-dihydroquinoline (19) and quinoline 20 (30.9 mg, 98%), as a reddish solid.202-(2-Bromophenyl)-3-nitro-1,2-dihydroquinoline (19)
Mp 104–106 °C; IR (KBr, υ): 3995, 3977, 3400, 3381, 1634, 1606, 748, 603 and 588 cm−1. 1H NMR (300 MHz, CDCl3): δ = 8.19 (1H, s, H-4), 7.61 (1H, dd, J = 7.7 and 1.0, H-5), 7.24–7.21 (3H, m, H-7, H-4′ and H-5′), 7.18–7.12 (2H, m, H-3′ and H-6′), 6.70 (1H, td, J = 7.5 and 0.8, H-6), 6.45 (1H, d, J = 6.4, H-8), 6.43 (s, 1H, H-1′) and 5.09 (s, 1H, H-1); 13C NMR (75 MHz, CDCl3): δ = 143.8 (C-8a), 139.2 (C-4a), 139.1 (C-3), 134.2 (C-6′), 133.5 (C-5), 133.0 (C-4), 131.3 (C-5′), 130.1 (C-3′), 128.3 (C-7), 127.9 (C-4′), 121.4 (C-2′), 118.9 (C-6), 115.2 (C-1′), 114.0 (C-8) and 53.8 (C-2).Without further purification, a stirred solution of dihydroquinoline 19 (15.7 mg, 0.05 mmol) in anhydrous CH2Cl2 (1 mL) was treated with DDQ (12.8 mg, 0.05 mmol) and the reaction was stirred at room temperature for 30 minutes. Next, saturated NaHCO3 solution (7 mL) was added and the products were extracted with EtOAc (3 × 30 mL). The combined organic phases were dried (MgSO4), filtered and concentrated under reduced pressure. The residue was purified by chromatography to give the desired nitroquinoline 20 (15.3 mg, 98%), as a yellow solid. Mp 95–97 °C (Lit.: 95–97 °C);31 IR (KBr, υ): 1603, 1553, 1520, 1348, 1335, 831, 789 and 760 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.99 (1H, s, H-4), 8.26 (1H, d, J = 8.7, H-5), 8.07 (1H, d, J = 8.2, H-8), 7.96 (1H, dt, J = 7.7 and 1.4, H-7), 7.76 (1H, dt, J = 7.6 and 1.0, H-6), 7.66 (1H, d, J = 7.8, H-3′), 7.51 (ddd, 2H, J = 9.9, 7.6 and 2.3) and 7.36 (ddd, 1H, J = 8.8, 6.7 and 2.3, H-4′); 13C NMR (75 MHz, CDCl3): δ = 152.0 (C-2), 148.3 (C-8a), 142.8 (C-1′), 139.2 (C-3), 133.2 (C-4), 133.1 (C-7), 132.3 (C-3′), 130.1 (C-4′), 129.9 (C-5′), 129.6 (C-5), 128.8 (C-8), 128.7 (C-6), 127.5 (C-6′), 125.8 (C-4a) and 121.6 (C-2′).
2-(2′-Bromophenyl)-3-aminoquinoline (21)21,31
Fe0 turnings (67.7 mg, 1.20 mmol) were added to a stirred solution of the nitroquinoline 18 (66.4 mg, 0.2 mmol) in EtOH (2.7 mL). The mixture was then treated with a 0.1 N solution of HCl (100 μL, 100 μmol) and heated under reflux for 4 h. Then, the reaction system was diluted with EtAcO and filtered through Celite®. After diluting with EtOAc (30 mL) and washing with saturated NaHCO3 (10 mL), the product was extracted with EtOAc (3 × 30 mL); the combined organic layers were dried (MgSO4), filtered and concentrated under reduced pressure, affording 21 (60 mg, 99%), as a light yellow oil. IR (film, υ): 3997, 3462, 3129, 2851, 1556, 1417, 1250, 1188, 881 and 665 cm−1; 1H NMR (300 MHz, CDCl3): δ = 7.90 (1H, t, J = 4.7, H-8), 7.60 (1H, d, J = 7.9, H-5), 7.55–7.52 (1H, m, H-6), 7.38–7.33 (4H, m, H-7, H-3′, H-5′ and H-6′), 7.25–7.19 (1H, m, H-4), 7.14 (1H, s, H-4) and 3.65 (2H, bs, NH2); 13C NMR (75 MHz, CDCl3): δ = 149.4 (C-2), 141.3 (C-3), 138.0 (C-1′), 137.1 (C-8a), 132.2 (C-3′), 130.0 (C-6′), 129.4 (C-4′), 128.4 (C-4a), 128.2 (C-8), 127.2 (C-5′), 126.0 (C-6), 124.9 (C-5), 124.5 (C-7), 121.6 (C-2′) and 115.2 (C-4).10H-Indolo[3,2-b]quinoline (quindoline, 1)
A mixture of Pd(MeCN)2Cl2 (1.6 mg, 0.006 mmol) in THF (1.2 mL) was treated with DPPF (16.0 mg, 0.03 mmol) and stirred at room temperature under inert atmosphere for 12 h. Then, the aminoquinoline 21 (18.4 mg, 0.06 mmol) was added and the reaction was stirred at room temperature for 1 h. After the addition of tBuOK (19.3 mg, 0.14 mmol), the mixture was stirred under reflux for 72 h. The reaction mixture was diluted with EtOAc and filtered through Celite®. After evaporation of the solvent under reduced pressure, the residue was purified by chromatography to give the desired quindoline (1, 10.2 mg, 78%), as a yellow solid.32 Mp 201–203 °C; IR (KBr, υ): 3163, 3086, 2922, 2851, 1608, 1481, 1338, 1223, 737 and 716 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.54 (1H, d, J = 7.9, H-6), 8.33 (1H, d, J = 8.6, H-4), 8.27 (1H, b, H-10), 8.02 (1H, s, H-11), 7.92 (1H, dd, J = 8.3 and 1.2, H-1), 7.65 (1H, ddd, J = 7.6, 6.9 and 1.5, H-3), 7.55 (2H, ddd, J = 8.5, 8.4 and 1.2, H-8 and H-2), 7.45 (1H, d, J = 8.2, H-9) and 7.33 (1H, td, J = 7.5 and 0.8, H-7); 13C NMR (75 MHz, CDCl3): δ = 146.5 (C-5a), 144.4 (C-4a), 143.6 (C-9a), 132.4 (C10-a), 129.9 (C-8), 129.1 (C-4), 127.2 (C-1), 127.0 (C-11a), 126.6 (C-3), 125.3 (C-2), 122.2 (C-6), 122.1 (C-5b), 120.4 (C-7), 113.2 (C-11) and 111.0 (C-9).(E)-(2-Nitrovinyl)benzene (23)
A mixture of benzaldehyde (22, 106.1 mg, 1 mmol), MeNO2 (2 mL) and NH4Ac (39.7 mg, 0.51 mmol) was transferred to a microwave tube and irradiated in a microwave oven at 90 °C for 60 min. Once the reaction was finished, the volatiles were removed under reduced pressure and the crude material was purified by chromatography to give the nitrostyrene 23 (105 mg, 70.4%), as a yellow solid. Mp 58–60 °C (Lit.: 57–58 °C);19b IR (KBr, υ): 1634, 1514, 1495, 1449, 1346 and 594 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.0 (1H, d, J = 14.1, H-2′), 7.59 (2H, d, J = 13.7, H-2 and H-6), 7.54 (1H, d, J = 1.9, H-1′) and 7.51–7.42 (3H, m, H-3, H-4 and H-5); 13C NMR (75 MHz, CDCl3): δ = 139.1 (C-2′), 137.1 (C-2 and C-6), 132.1 (C-3 and C-5), 130.1 (C-1), 129.4 (C-1′) and 129.1 (C-4).2-Phenyl-3-nitroquinoline (25)
A mixture of basic Al2O3 (172 mg, 1.67 mmol), 2-amino benzaldehyde (15, 20.9 mg, 0.17 mmol) and nitrostyrene (23, 36.1 mg, 0.24 mmol) was heated at 50 °C for 24 h. Then, the system was cooled at room temperature and the crude material was purified by chromatography to give a mixture of 2-phenyl-3-nitrodihydro-quinoline (24) and 2-phenyl-3-nitroquinoline (25) as a red solid (43 mg, 99%).Compound 24: HRMS (ESI+) calcd. for C15H12N2NaO2 ([M + Na]+) m/z = 275.0791; found: m/z = 275.0794.
Without further treatment, the mixture of dihydroquinoline 24 and nitroquinoline 25 (28.2 mg, 0.11 mmol) was dissolved in anhydrous CH2Cl2 (1 mL) and treated with DDQ (28.1 mg, 0.12 mmol). The reaction was stirred at room temperature for 30 min, when saturated NaHCO3 solution (5 mL) was added to the reaction mixture and the product was extracted with EtOAc (3 × 30 mL). The combined organic layers were dried (MgSO4), filtered and concentrated under reduced pressure, and the residue was chromatographed, giving the desired nitroquinoline 25 (23.3 mg, 82%), as a light yellow solid. Mp 146–148 °C. IR (KBr, υ): 3447, 3422, 1636, 1607, 1524, 831, 762 and 692 cm−1. 1H NMR (300 MHz, CDCl3): δ = 8.70 (1H, s, H-4), 8.24 (1H, d, J = 8.5, H-5), 7.99 (1H, d, J = 7.9, H-8), 7.91 (1H, td, J = 7.7 and 1.4, H-6), 7.72 (1H, dd, J = 6.9 and 1.0, H-7), 7.68–7.65 (2H, m, H-2′ and H-6′) and 7.50 (t, 3H, J = 3.3, H-3′, H-4′ and H-5′). 13C NMR (75 MHz, CDCl3): δ = 152.1 (C-2), 148.4 (C-8a), 144.0 (C-3), 137.1 (C-1′), 132.9 (C-6), 132.7 (C-4), 129.8 (C-5), 129.6 (C-3′, C-4′ and C-5′), 128.8 (C-8), 128.6 (C-7), 128.5 (C-6′), 128.1 (C-2′) and 125.6 (C-4a). HRMS (ESI+) calcd. for C15H11N2O2 ([M + 1]+) m/z = 251.0815; found: m/z = 251.0819.
2-Phenyl-3-aminoquinoline (26)
A mixture of PPh3 (47.0 mg, 0.18 mmol) and 3-nitroquinoline 25 (18.9 mg, 0.07 mmol) in PhMe (1 mL) was treated with MoO2Cl2(DMF)2 (2.8 mg, 0.008 mmol). The reaction mixture is heated under reflux for 72 h. Then, the solvent was removed under reduced pressure and the crude material was purified by chromatography to give 2-phenyl-3-aminoquinoline 26 (8.5 mg, 52%) as a yellow solid, mp 121–123 °C (Lit.: 123 °C).33 IR (KBr, υ): 3321, 2924, 1614, 1435, 1354, 1190, 881, 766 and 700 cm−1. 1H NMR (300 MHz, CDCl3): δ = 8.03–8.00 (1H, m, H-8), 7.75 (2H, dt, J = 6.6 and 1.7, H-2′ and H-6′), 7.64–7.60 (1H, m, H-4), 7.56–7.51 (2H, m, H-6 and H-7), 7.49–7.40 (3H, m, H-3′, H-4′ and H-5′), 7.34 (1H, s, H-4) and 3.98 (2H, bs, NH2). 13C NMR (75 MHz, CDCl3): δ = 150.7 (C-2), 142.8 (C-3), 138.4 (C-8a), 138.1 (C-1′), 129.3 (C-8), 129.1 (C-4a), 129.0 (C- 2′ y C-6′) 128.9 (C-6), 128.7 (C-7),126.7 (C-4′), 125.7 (C-5), 125.4 (C-3′) and 116.1 (C-4).Quindoline (1)
A solution of 2-phenyl-3-nitroquinoline 25 (15.61 mg, 0.06 mmol) in CH2Cl2 (0.5 mL) was treated with DPPE (27.1 mg, 0.07 mmol); the solvent was evaporated under a N2 atmosphere and the remaining solid mixture was heated at 150 °C for 5 h. Then, the crude material was purified by chromatography, affording quindoline (1, 11 mg, 82%), as a yellow solid, mp 201–203 °C.34,5d The 1H and 13C NMR spectroscopic data of this synthetic compound were in agreement with those of synthetic and natural quindoline.Phosphazene 27
In order to ensure an intimate mixture of reagents, PPh3 (70 mg, 0.27 mmol) was added to a solution of 2-phenyl-3-nitroquinoline (25, 19.4 mg, 0.08 mmol) in CH2Cl2 (0.5 mL). The solvent was evaporated under high vacuum in a N2 atmosphere, and the resulting solventless solid mixture was heated at 150 °C for 4 h. The crude material was purified by chromatography to give phosphazene 27 (33.4 mg, 87%) as a yellow solid, mp 117–119 °C. IR (KBr, υ): 3053, 2959, 2849, 2779, 1416, 1360, 1271, 1107, 747 and 694 cm−1. 1H NMR (300 MHz, CDCl3): δ = 8.05 (2H, dd, H-2′ and H-6′), 7.97 (1H, d, J = 8, H-8), 7.74–7.66 (6H, m, 3 × H-2′′ and 3 × H-6′′), 7.57–7.48 (3H, m, 3 × H-4′′), 7.47–7.38 (9H, m, 3 × H-3′′, 3 × H-5′′, H-3′, H-4′ and H-5′), 7.32 (1H, dd, J = 7.5 and 8, H-7), 7.24 (2H, m, H-5 and H-6), and 6.97 (1H, bs, H-4). 13C NMR (75 MHz, CDCl3): δ = 121.4 (d, 3JP-C = 10.4, C-4), 124.5 (C-7), 125.0 (C-5), 125.5 (C-6), 127.3 (H-3′ and H-5′), 127.5 (H-4′), 128.7 (d, 3JP-C = 12.0, 3 × C-3′′ and 3 × C-5′′), 129.0 (2C, C-4a and C-8), 129.4 (C-8a), 130.3 (C-2′ and C-6′), 130.5 (d, 1JP-C = 100.3, 3 × C-1′′), 131.9 (d, 4JP-C = 2.6, 3 × H-4′′), 132.6 (d, 2JP-C = 9.8, 3 × C-2′′ and 3 × C-6′′), 141.6 (d, 2JP-C = 33.8, C-3), 143.7 (C-1′) and 159.0 (d, 3JP-C = 22.8, C-2). 31P NMR (188.31 MHz, CDCl3): δ = 3.97. HRMS (ESI+) calcd. for C33H26N2P ([M + 1]+) m/z = 481.1828; found: m/z = 481.1830.Computational methods
Conformational searches for the reactants, transition structures (TS) and products were run using the conformational search module of Hyperchem with the MM+ method.35 Selected structures were then successively optimized at the B3LYP/6-31G* and M062X/6-311+G** level single point was performed.36 This level has been shown as a suitable level of theory for this type of structures.27Frequency calculations were made to confirm the nature of the stationary points and to evaluate their thermochemical properties. The molecular orbitals of the reactants were calculated to analyze the frontier orbital interactions at the M062X/6-311+G** level of theory. Intrinsic reaction coordinate (IRCs) calculations were run to verify the connectivity between reactants, TSs and products. To examine the most important interactions in the TSs, natural bond orbital calculations were performed and Wiberg bond indexes (WBIs) analyzed.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PUE IQUIR 2016) and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICT 2018-1933 and PICT 2019-3969) for financial support. E. N. T. also thanks ANPCyT for her doctoral fellowship. SOS acknowledges to AvH Foundation for support.Notes and references
- G. V. Subbaraju, J. Kavitha, D. Rajasekhar and J. I. Jimenez, J. Nat. Prod., 2004, 67, 461–462 CrossRef CAS PubMed.
- D. S. Jang, E. J. Park, Y.-H. Kang, B.-N. Su, M. E. Hawthorne, J. Schunke Vigo, J. G. Graham, F. Cabieses, H. H. S. Fong, R. G. Mehta, J. M. Pezzuto and A. D. Kinghorn, Arch. Pharmacal Res., 2003, 26, 585–590 CrossRef CAS PubMed.
- O. Souza Chaves, Y. C. Ferreira Teles, M. Morais de Oliveira Monteiro, L. G. Mendes Junior, M. F. Agra, V. de Andrade Braga, T. M. Sarmento Silva and M. F. Vanderlei de Souza, Molecules, 2017, 22, 94 CrossRef PubMed.
- J. F. Liu, Z. Y. Jiang, R. R. Wang, Y. T. Zheng, J. J. Chen, X. M. Zhang and Y. B. Ma, Org. Lett., 2007, 9, 4127–4129 CrossRef CAS PubMed.
- (a) P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, Curr. Org. Chem., 2011, 15, 1036–1057 CrossRef CAS; (b) J. Lavrado, R. Moreira and A. Paul, Curr. Med. Chem., 2010, 17, 2348–2370 CrossRef CAS PubMed; (c) L. R. Whittell, K. T. Batty, R. P. M. Wong, E. M. Bolitho, S. A. Fox, T. M. E. Davis and P. E. Murray, Bioorg. Med. Chem., 2011, 19, 7519–7525 CrossRef CAS PubMed; (d) J. Lavrado, G. G. Cabal, M. Prudêncio, M. M. Mota, J. Gut, P. J. Rosenthal, C. Díaz, R. C. Guedes, D. J. V. A. dos Santos, E. Bichenkova, K. T. Douglas, R. Moreira and A. Paulo, J. Med. Chem., 2011, 54, 734–750 CrossRef CAS PubMed.
- D. Dwuma−Badu, J. S. K. Ayim, N. Y. Y. Fiagbe, J. E. Knapp, P. L. Schiff Jr and D. J. Slatkin, J. Pharm. Sci., 1978, 67, 433–434 CrossRef PubMed.
- (a) L. Yang, C. He, Sudunabuqi, X. Bao, X. Wang and W. Ao, Biochem. Syst. Ecol., 2020, 93, 104163 CrossRef CAS; (b) S. W. Yang, M. Abdel-Kader, S. Malone, M. C. M. Werkhoven, J. H. Wisse, I. Bursuker, K. Neddermann, C. Fairchild, C. Raventos-Suarez, A. T. Menendez, K. Lane and D. G. I. Kingston, J. Nat. Prod., 1999, 62, 976–983 CrossRef CAS PubMed; (c) Z. Y. Xu, Y. F. Xi, W. Y. Zhou, L. L. Lou, X. B. Wang, X. X. Huang and S. J. Song, Biochem. Syst. Ecol., 2020, 92, 104089 CrossRef CAS.
- (a) S. D. Ablordeppey, C. D. Hufford, R. F. Borne and D. Dwama-Badu, Planta Med., 1990, 56, 416–417 CrossRef CAS PubMed; (b) A. N. Tackie, M. H. M. Sharaf, P. L. Schiff Jr, G. L. Boye, R. C. Crouch and G. E. Martin, J. Heterocycl. Chem., 1991, 28, 1429–1435 CrossRef CAS.
- (a) A. Paulo, E. T. Gomes and P. J. Houghton, J. Nat. Prod., 1995, 58, 1485–1491 CrossRef CAS; (b) K. Cimanga, T. De Bruyne, L. Pieters, M. Claeys and A. Vlietnick, Tetrahedron Lett., 1996, 37, 1703–1706 CrossRef CAS; (c) D. M. Fort, J. Litvak, J. L. Chen, Q. Lu, P. W. Phuan, R. Cooper and D. E. Bierer, J. Nat. Prod., 1998, 61, 1528–1530 CrossRef CAS PubMed.
- (a) C. E. Hadden, M. H. M. Sharaf, J. E. Guido, R. H. Robins, A. N. Tackie, C. H. Phoebe Jr, P. L. Schiff Jr and G. E. Martin, J. Nat. Prod., 1999, 62, 238–240 CrossRef CAS PubMed; (b) R. C. Crouch, A. O. Davis, T. D. Spitzer, G. E. Martin, M. H. M. Sharaf, P. L. Schiff Jr, C. H. Phoebe and A. N. Tackie, J. Heterocycl. Chem., 1995, 32, 1077–1080 CrossRef CAS; (c) J.-L. Pousset, M.-T. Martin, A. Jossang and B. Bodo, Phytochemistry, 1995, 39, 735–736 CrossRef CAS.
- M. H. M. Sharaf, P. L. Schiff Jr, A. N. Tackie, C. H. Phoebe Jr and G. E. Martin, J. Heterocycl. Chem., 1996, 33, 239–243 CrossRef CAS.
- (a) K. Blinov, M. Elyashberg, E. R. Martirosian, S. G. Molodtsov, A. J. Williams, A. N. Tackie, M. H. M. Sharaf, P. L. Schiff Jr, R. C. Crouch, G. E. Martin, C. E. Hadden, J. E. Guido and K. A. Mills, Magn. Reson. Chem., 2003, 41, 577–584 CrossRef CAS; (b) M. H. M. Sharaf, P. L. Schiff Jr, A. N. Tackie, C. H. Phoebe Jr, L. Howard, C. Myers, C. E. Hadden, S. K. Wrenn, A. O. Davis, C. W. Andrews, D. Minick, R. L. Johnson, J. P. Shockcor, R. C. Crouch and G. E. Martin, Magn. Reson. Chem., 1995, 33, 767–778 CrossRef CAS; (c) A. N. Tackie, G. L. Boye, M. H. M. Sharaf, P. L. Schiff Jr, R. C. Crouch, T. D. Spitzer, R. L. Johnson, J. Dunn, D. Minick and G. E. Martin, J. Nat. Prod., 1993, 56, 653–670 CrossRef CAS; (d) M. H. M. Sharaf, P. L. Schiff Jr, A. N. Tackie, C. H. Phoebe Jr, R. L. Johnson, D. Minick, C. W. Andrews, R. C. Crouch and G. E. Martin, J. Heterocycl. Chem., 1996, 33, 789–797 CrossRef CAS.
- (a) E. L. Larghi, A. B. J. Bracca, A. A. Arroyo Aguilar, D. A. Heredia, J. L. Pergomet, S. O. Simonetti and T. S. Kaufman, Curr. Top. Med. Chem., 2015, 17, 1683–1707 CrossRef PubMed; (b) A. B. J. Bracca, D. A. Heredia, E. L. Larghi and T. S. Kaufman, Eur. J. Org. Chem., 2014, 7979–8003 CrossRef CAS; (c) M. V. Méndez, A. B. J. Bracca and T. S. Kaufman, Synthesis, 2018, 50, 1417–1429 CrossRef.
- M. V. Méndez, S. O. Simonetti, T. S. Kaufman and A. B. J. Bracca, New J. Chem., 2019, 43, 10803–10813 RSC.
- M. V. Méndez, D. A. Heredia, E. L. Larghi, A. B. J. Bracca and T. S. Kaufman, RSC Adv., 2017, 7, 28298–28307 RSC.
- T. D. Spitzer, R. C. Crouch, G. E. Martin, M. H. M. Sharaf, P. L. Schiff Jr., A. N. Tackie and L. B. Gilbert, J. Heterocycl. Chem., 1991, 28, 2065–2070 CrossRef CAS.
- A. I. Calderon, A. Hodel, J.-L. Wolfender, M. P. Gupta, M. Correa and K. Hostettmann, Nat. Prod. Res., 2013, 27, 1335–1342 CrossRef CAS PubMed.
- (a) H. C. Pal and S. K. Katiyar, Molecules, 2016, 21, 1758 CrossRef PubMed; (b) O. A. Olajide, H. S. Bhatia, A. C. de Oliveira, C. W. Wright and B. L. Fiebich, Eur. J. Med. Chem., 2013, 63, 333–339 CrossRef CAS PubMed; (c) C. Ansah and K. B. Mensah, Ghana Med. J., 2013, 47, 137–147 CAS; (d) H. C. Pal, R. Prasad and S. K. Katiyar, Sci. Rep., 2017, 7, 1498 CrossRef PubMed.
- (a) C.-F. Chang, C.-Y. Huang, Y.-C. Huang, K.-Y. Lin, Y.-J. Lee and C.-J. Wang, Synth. Commun., 2010, 40, 3452–3466 CrossRef CAS; (b) T. Naveen, S. Maity, U. Sharma and D. Maiti, J. Org. Chem., 2013, 78, 5949–5954 CrossRef CAS PubMed.
- R. Ballini, G. Bosica, D. Fiorini and A. Palmieri, Green Chem., 2005, 7, 825–827 RSC.
- (a) A. Awasthi, P. Yadav, S. Yadav and D. K. Tiwari, Adv. Synth. Catal., 2022, 364, 41–46 CrossRef CAS; (b) V. Y. Shuvalov, A. S. Rupp, A. S. Fisyuk, A. K. Kuratova, A. A. Nefedov and G. P. Sagitullina, ChemistrySelect, 2019, 4, 1696–1699 CrossRef CAS.
- (a) P. Molina, M. Alajarin, A. Vidal and M. C. Foces-Foces, Tetrahedron, 1995, 51, 12127–12142 CrossRef CAS; (b) Y. Matsuno, M. Mitsunaga, J. Oda, M. Furuki, S. Shoji, T. Kitamura and Y. Shibata, US Pat., US9650350, 2017; (c) P. Molina, M. Alajarin, P. Sanchez-Andrada, J. Elguero and M. L. Jimeno, J. Org. Chem., 1994, 59, 7306–7315 CrossRef CAS.
- (a) M. Pattarawarapan, D. Yamano, N. Wiriya, S. Yimklan and W. Phakhodee, J. Org. Chem., 2020, 85, 13330–13338 CrossRef CAS PubMed; (b) M. Alajarin, A. Vidal and M.-M. Ortin, Tetrahedron Lett., 2003, 44, 3027–3030 CrossRef CAS; (c) T. Alaime, M. Daniel, M.-A. Hiebel, E. Pasquinet, F. Suzenet and G. Guillaumet, Chem. Commun., 2018, 54, 8411–8414 RSC; (d) R. S. Foster, J. P. A. Harrity and H. Jakobi, Org. Lett., 2012, 14, 4858–4861 CrossRef CAS PubMed; (e) P. Molina, M. Alajarín, P. Sánchez-Andrada, J. S. Carrió, M. Martínez-Ripoll, J. E. Anderson, M. L. Jimeno and J. Elguero, J. Org. Chem., 1996, 61, 4289–4299 CrossRef CAS PubMed.
- (a) S. Ramírez-Rave, M. T. Ramírez-Apan, H. Tlahuext, D. Morales-Morales, R. A. Toscano and J.-M. Grevy, J. Organomet. Chem., 2016, 814, 16–24 CrossRef; (b) F. Schömberg, I. Vilotijevic, K. Wagner and Y. Zi, Org. Lett., 2020, 22, 3407–3411 CrossRef PubMed.
- (a) H. Majgier-Baranowska, J. D. Williams, B. Li and N. P. Peet, Tetrahedron Lett., 2012, 53, 4785–4788 CrossRef CAS; (b) G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045–1075 RSC.
- (a) R. J. Sundberg, J. Org. Chem., 1965, 30, 3604–3610 CrossRef CAS; (b) J. I. G. Cadogan and M. J. Todd, J. Chem. Soc. C, 1969, 2808–2813 RSC; (c) K. Zhang, A. El Bouakher, H. Lévaique, J. Bignon, P. Retailleau, M. Alami and A. Hamzé, J. Org. Chem., 2019, 84, 13807–13823 CrossRef CAS PubMed; (d) M. Castiñeira Reis, M. Marín-Luna, C. Silva López and O. N. Faza, ACS Omega, 2018, 3, 7019–7026 CrossRef PubMed.
- T. V. Nykaza, A. Ramirez, T. S. Harrison, M. R. Luzung and A. T. Radosevich, J. Am. Chem. Soc., 2018, 140, 3103–3113 CrossRef CAS PubMed.
- A. Pokluda, M. Kohout, J. Chudoba, M. Krupička and R. Cibulka, ACS Omega, 2019, 4, 5012–5018 CrossRef CAS PubMed.
- The calculation of the Yamaguchi’s spin contamination at the um062x/6-31g* level affords a contribution of the triplet state to the free energy value of less than 2%; hence it was not considered for the calculations.
- H. Wagner, S. Bladt and V. Rickl, Drug Analysis: A Thin Layer Chromatography Atlas, Springer, Heidelberg, Germany, 2nd edn, 2009, p. 360 Search PubMed.
- L. Zheng, Z. Zeng, Q. Yan, F. Jia, L. Jia and Y. Chen, Adv. Synth. Catal., 2018, 360, 4037–4042 CrossRef CAS.
- (a) R. Sanz, J. Escribano, M. R. Pedrosa, R. Aguado and F. J. Arnáiz, Adv. Synth. Catal., 2007, 349, 713–718 CrossRef CAS; (b) A. W. Freeman, U. Urvoy and M. E. Criswel, J. Org. Chem., 2005, 70, 5014–5019 CrossRef CAS PubMed.
- J. Letessier and H. Detert, Synthesis, 2012, 290–296 CAS.
- H. Peng, X. Chen, Y. Chen, Q. He, Y. Xie and C. Yang, Tetrahedron, 2011, 67, 5725–5731 CrossRef CAS.
- Hyperchem Professional Release 7.52, Hypercube, Inc., 2005 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman,G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra of the compounds and details of DFT calculations. See DOI: https://doi.org/10.1039/d3ra02468g |
| This journal is © The Royal Society of Chemistry 2023 |