 Open Access Article
Open Access ArticleCarbon dots with tissue engineering and regenerative medicine applications
Nima Farshidfar a,
Saba Fooladib,
Mohammad Hadi Nematollahi
a,
Saba Fooladib,
Mohammad Hadi Nematollahi *cd and
Siavash Iravani
*cd and
Siavash Iravani *e
*e
aOrthodontic Research Center, School of Dentistry, Shiraz University of Medical Sciences, Shiraz, Iran
bStudent Research Committee, Kerman University of Medical Sciences, Kerman, Iran
cApplied Cellular and Molecular Research Center, Kerman University of Medical Sciences, Kerman, Iran. E-mail: mh.nematollahi@yahoo.com
dDepartment of Biochemistry, Faculty of Medicine, Kerman University of Medical Sciences, Kerman, Iran
eFaculty of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, 81746-73461, Isfahan, Iran. E-mail: siavashira@gmail.com
First published on 15th May 2023
Abstract
Carbon dots (CDs) with unique physicochemical features such as exceptional biocompatibility, low cost, eco-friendliness, abundant functional groups (e.g., amino, hydroxyl, and carboxyl), high stability, and electron mobility have been broadly investigated in nano- and biomedicine. In addition, the controlled architecture, tunable fluorescence emission/excitation, light-emitting potential, high photostability, high water solubility, low cytotoxicity, and biodegradability make these carbon-based nanomaterials suitable for tissue engineering and regenerative medicine (TE-RM) purposes. However, there are still limited pre- and clinical assessments, because of some important challenges such as the scaffold inconsistency and non-biodegradability in addition to the lack of non-invasive methods to monitor tissue regeneration after implantation. In addition, the eco-friendly synthesis of CDs exhibited some important advantages such as environmentally friendly properties, low cost, and simplicity compared to the conventional synthesis techniques. Several CD-based nanosystems have been designed with stable photoluminescence, high-resolution imaging of live cells, excellent biocompatibility, fluorescence properties, and low cytotoxicity, which make them promising candidates for TE-RM purposes. Combining attractive fluorescence properties, CDs have shown great potential for cell culture and other biomedical applications. Herein, recent advancements and new discoveries of CDs in TE-RM are considered, focusing on challenges and future perspectives.
1. Introduction
The main goal of tissue engineering and regenerative medicine (TE-RM) is to fabricate substitutes to maintain, promote, or restore damaged tissues/organs.1,2 It is well known that biomaterials play a pivotal role in TE-RM by producing functional and biological tissues/organs.3,4 In this context, three-dimensional (3D) frameworks can provide the necessary conditions for the proliferation and differentiation of cells using various interactions such as biochemical, bioelectrical, or biomechanical signals in order to promote tissue/organ regeneration.5,6 However, the use of a single biomaterial cannot entirely mimic the natural composition of the extracellular matrix (ECM); thus, it will not be able to provide all the requirements for cellular interactions. Nanotechnology has the potential to address the aforementioned issue through the synthesis and incorporation of various nanomaterials.7–9 To this purpose, carbon-based nanomaterials are a unique class of materials with fascinating physicochemical properties for gene/drug delivery, cancer theranostics, antimicrobials/antivirals, photodynamic therapy, imaging/biosensing, and TE-RM applications.10–13As a zero-dimensional class of carbon-based nanomaterials, carbon dots (CDs) are promising candidates for environmental and biomedical applications due to their ultrafine sizes of <10 nm, quasi-spherical morphology, good biocompatibility, high quantum yield, strong absorption, among others (Table 1).14 CDs have fascinating optical features such as ultraviolet absorption, photoluminescence, up-conversion fluorescence, and long-wavelength and multicolor emission.15–17 CDs were discovered by Xu et al. in 2004, due to their high fluorescence characteristics during the electrophoretic purification of arc-synthesized single-walled carbon nanotubes (SWCNTs).18 These carbon-based nanomaterials are generally classified into three different types, including graphene quantum dots (GQDs), carbon quantum dots (CQDs), and carbonized polymeric dots (CPDs).19 These nanomaterials are majorly made up of a sp2/sp3 hybridized carbon core with surface functional groups, but each type has its own unique characteristics, as well.20 For instance, GQDs consist of mono- or multi-layer nanoscale graphite sheets and surface/edge functional groups or interlayer defects, which are anisotropic with lateral dimensions wider than their height. Their optical characteristics are also affected by the size of π-conjugated domains and the surface/edge structures.19,20 Contrary to GQDs, both CQDs and CPDs have spherical cores connected to their surface functional groups.21 CQDs are comprised of multi-layer graphite structures within their spherical core, and their optical properties are primarily dominated by the intrinsic state luminescence and quantum size effect. On the other hand, CPDs are hybrid nanocomposites which composed of aggregated/crosslinked carbon cores and polymeric chains shells, and their optical characteristics are mainly affected by their molecular state and crosslink structures.19,20
| Size (nm) | Structure | Shape | Solubility | Toxicity | Excitation range | Excitation FWHM (nm) | Emission range | Emission FWHM (nm) | Photoluminescence QY | τ (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| a FWHM: full-width at half-maximum, QY: quantum yield, τ: lifetime. | ||||||||||
| 1–10 | Amorphous-crystalline | Discoidal/quasi-spherical | High | Low | Ultraviolet-visible | 50–200 | Visible | 30–200 | 0.05–0.9 | 10−9–10−8 |
There are numerous fabrication methods for synthesizing CDs, to be used for various biomedical applications, which can be generally classified into “top-down” and “bottom-up” strategies.22 The “top-down” approach includes the breaking of bulk carbon structures which is generally used for synthesizing GQDs, while the “bottom-up” approach consists of the formation of nanostructures from molecular precursors through chemical reactions which is typically used to synthesize both CQDs and CPDs.22 Fig. 1A demonstrates different types of these synthetic approaches for the preparation of CDs along with their advantages and disadvantages.23 Although various approaches have been applied for synthesizing CDs, CDs synthesized from green resources have caught the attention of researchers worldwide.24 These CDs are synthesized from renewable green sources such as animals or plants (Fig. 1B).23 Green resources are favorable sources for synthesizing CDs due to their high contents of carbon, high affordability and availability, excellent stability, simple extraction protocol, safeness, eco-friendliness, etc.11,25,26 The comparison between “top-down” and “bottom-up” approaches also showed that CDs obtained from these two methods had different photoluminescence characteristics and chemical states.22 In this context, the synthesized CDs may have a different distribution of functional groups (e.g., hydroxyl, carbonyl, carboxyl, and amine) and significant variations in sp3-carbon content depending on the specific synthetic conditions.27
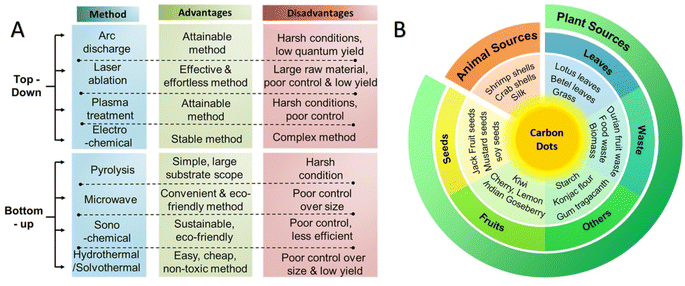 | ||
| Fig. 1 (A) Synthetic approaches for the preparation of CDs along with their advantages and disadvantages, (B) overview of sustainable resources used for the synthesis of CDs. Reproduced with permission from ref. 23 Copyright 2021 American Chemical Society. | ||
Over the past decades, CDs also received extensive and enormous attention in TE-RM due to their numerous physicochemical and biological properties. In this context, various in vitro studies on a series of cell lines showed low cytotoxicity and excellent biocompatibility of CDs even at a high concentration level.19 These nanomaterials have abundant functional groups (e.g., hydroxyl, carboxyl, and amino) that can be easily incorporated with other biomaterials to achieve rapid soft and hard tissue regeneration.28 In addition, CDs have unique optical characteristics such as tunable fluorescence emission/excitation, light-emitting potential, and high photostability which can be used to track the action and biodegradation of scaffold biomaterials after tissue regeneration.28 They also have favorable photothermal effects that can be used to kill tumor and cancer cells.29,30 These nanomaterials are highly biodegradable, and no remarkable symptoms of local or systemic inflammation could be detected after their usage in animal models.19,28 In vivo experiments demonstrated that CDs could be rapidly excreted through the kidney and/or hepatobiliary system due to their high-water solubility.31,32 These pieces of information prove the safety of these carbon-based nanomaterials for various biomedical applications, particularly TE-RM. CDs also have other advantages such as controlled architecture, low cost, eco-friendliness, high stability, electron mobility, etc. which justifies their broad application in various fields of bio- or nanomedicine.33 In light of the aforementioned merits, the application of CDs, a newly developed carbonaceous nanomaterial, has become one of the most promising tools for various biomedical engineering fields, especially TE-RM. This review aimed to elaborately discuss the current applications of CDs in TE-RM, with a focus on its recent advancements, important challenges, and future perspectives.
2. CDs and TE-RM applications
Because of unique physicochemical, optical, electrical, and biological properties of CDs, they are exploited for versatile applications of energy storage, (photo)catalysis, solar cells, biosensing/imaging, pollutant removal/detection, photodynamic therapy, cancer theranostics, drug/gene delivery, TE-RM, among others.11,19,34,35 Several novel CD-based nanosystems have been designed for TE-RM applications (Table 2). For instance, CDs synthesized by hydrothermal technique exhibited low cytotoxicity, high-resolution imaging of live cells, and stable photoluminescence. These multifunctional CDs were incorporated into electrospun nanofiber mats for cell proliferation and TE purposes, showing high biocompatibility and fluorescence features.36 CDs could improve the hydrophilicity and biocompatibility of scaffolds obtained from polycaprolactone, showing enhanced cell proliferation/attachment, surface roughness, and pore structure.37 In addition, fluorescent metal-doped CDs with low toxicity, good biocompatibility and cellular uptake exhibited superb interaction with cells during differentiation and outgrowth; these CDs with photostability and suitable selective affinity ought to be further explored for multifunctional TE-RM applications.38 On the other hand, to overcome the monitoring challenge of biodistribution and delivery of growth factors to injured tissues for tissue regeneration, photoluminescent CDs can be employed.39 For instance, CDs were conjugated with human vascular endothelial growth factor for in vitro imaging of human umbilical vein endothelial cells; they can be applied for tracking growth factor protein in angiogenic therapy.39| CDs | Applications | Advantages/properties | Ref. |
|---|---|---|---|
| Hydroxyl functionalized CDs | Stimulation of cell proliferation | •Great photostability and low cytotoxicity | 40 |
| •Strong fluorescence | |||
| •The ability to promote cell proliferation depends on the hydroxyl groups content | |||
| CD/tungsten disulfide (WS2) heterojunctions | Photothermal therapy of osteosarcoma and bone TE-RM | •Efficient osteosarcoma ablation | 41 |
| •Enhanced osteogenic differentiation and photothermal effects in the second near-infrared (NIR) window (1000–1350 nm) | |||
| •Good biocompatibility and significant up-regulation of bone-related gene expression | |||
| 3D-printed bioactive scaffolds bearing CDs | Bone regeneration; bioimaging | •Efficient monitoring of healing progress | 42 |
| •Bioglass-assisted bone regeneration using hydroxyapatite | |||
| •Good biocompatibility and robust luminescence | |||
| CQDs loaded electroactive silk fibroin/polylactic acid nanofibrous bioactive scaffolds (electrospun nanofibers) | Cardiac TE | •Highly porous with fully consistent pores; good biocompatibility and mechanical properties | 43 |
| •Improved swelling ability and young modulus | |||
| •Enhanced metabolic activity and viability of the cardiomyocytes in scaffolds | |||
| Electrospun captopril-loaded polycaprolactone-CQDs nanocomposite scaffolds | Bone tissue regeneration | •Decreased diameter of fiber | 44 |
| •Increase of the surface hydrophilicity in scaffolds | |||
| •Enhancement in the cells' proliferation and alkaline phosphatase activity | |||
| CD-peptide functionalized water dispersible hyperbranched polyurethane gel | Bone TE | •Suitable osteoblast adhesion, proliferation, and differentiation | 45 |
| •Good biocompatibility | |||
| •Moderate biodegradation | |||
| Fluorescent CDs loaded nanocomposites chitosan film | Wound healing; skin tissue regeneration | •Reduction in water absorption and moisture permeation | 46 |
| •Improved flexibility | |||
| •Excellent antioxidant properties | |||
| Collagen derived CQDs | Cell imaging and TE | •Excellent biocompatibility | 47 |
| •Long-term photostability and pH flexible fluorescence | |||
| •Deep cellular and scaffold imaging | |||
| Sodium alginate-hemoglobin-CQDs injectable hydrogels | Skin tissue regeneration | •Enhanced antibacterial effects | 48 |
| •Improved biocompatibility | |||
| •Efficient monitoring of wound pH | |||
| Polyurethane/GQDs electrospun nanofibers | TE | •Improved mechanical strength | 49 |
| •Enhanced electrical conductivity | |||
| •The scaffold had suitable interaction with 3T3 fibroblast cells (in vitro) | |||
| Metformin-CDs | Periodontal tissue regeneration | •Excellent biocompatibility | 50 |
| •Improved osteogenic activity (in vitro) | |||
| •Favorable bone regeneration ability (in vivo) | |||
| Mg2+-doped CDs | Cell imaging and TE | •High biocompatibility | 51 |
| •Improved fluorescence properties | |||
| •Enhancement in MC3T3 cells' proliferation and differentiation (in vitro) | |||
| Functionalized CDs | Dentin-pulp complex regeneration | •Enhanced ECM secretion of dental pulp stem cells and increased cell adhesion | 52 |
| •Strong osteogenic/odontogenic activity (in vitro) | |||
| •Efficient regeneration of dentin-pulp complex and blood vessels (in vivo) | |||
| Alendronate-polyethyleneimine-CDs | Bone tissue regeneration | •Excellent biocompatibility | 53 |
| •Enhanced osteoblast differentiation and bone regeneration (in vitro) | |||
| •Inhibition of formation and function of osteoclasts (in vitro) | |||
| Linezoid- bovine serum albumin CDs | Wound healing; skin tissue regeneration | •Enhanced antibacterial effects | 54 |
| •Excellent biocompatibility | |||
| •Low toxicity against human skin fibroblasts (in vitro) | |||
| •Intense fluorescence characteristics |
2.1. Bone and cartilage TE-RM
Because of good biocompatibility, water dispersibility, simplicity of fabrication, and bright multicolor luminescence, CDs have received significant interest. Different composite scaffolds have been designed using CDs by altering or mixing them with other therapeutic/functional materials for bone and cartilage TE-RM purposes, especially for bone defect healing. In this context, one of the main challenges in bone regeneration is the elevated levels of reactive oxygen species (ROS) that can lead to aggravated inflammation and disrupted tissue repair. Thus, several studies have focused on designing novel multifunctional systems for inhibiting the possible inflammatory reactions while promoting the bone tissue regeneration.55 In one study, dexamethasone CDs were designed through a simple hydrothermal technique utilizing citric acid, ammonium fluoride, and a trace amount of dexamethasone.55 These CDs with good biocompatibility promoted the differentiation of rat bone marrow mesenchymal stem cells under inflammatory and normal circumstances. They could also be deployed for scavenging ROS (˙OH) and keeping the pharmacological performance of dexamethasone owing to their abundant-reducing groups, leading to the reduction of inflammatory responses. Besides, dexamethasone CDs exhibited suitable osteoimmunomodulatory performance to stimulate a bone immune microenvironment, promoting the differentiation of rat bone marrow mesenchymal stem cells and accelerating the bone regeneration. They promoted phenotype switching of macrophages from M1-type to M2-type under inflammatory circumstances, providing beneficial effects for anti-inflammatory responses.55 In addition, CD-decorated carboxymethyl cellulose-hydroxyapatite nanocomposites (<100 nm) were constructed using a simple thermal treatment strategy, with fluorescence emission band ∼440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm upon excitation with 340 nm wavelength.56 These nanocomposites with suitable osteogenic features could be deployed for bone TE and exhibited high selectivity/sensitivity toward Fe3+ ions, showing capability of cell differentiation with excellent alkaline phosphatase performance of MG63 cells followed by extracellular mineralization.56
nm upon excitation with 340 nm wavelength.56 These nanocomposites with suitable osteogenic features could be deployed for bone TE and exhibited high selectivity/sensitivity toward Fe3+ ions, showing capability of cell differentiation with excellent alkaline phosphatase performance of MG63 cells followed by extracellular mineralization.56
Egg shell-derived calcium phosphate/CD nanofibrous scaffolds were constructed by simultaneous electrospinning of poly (ε-caprolactone) with calcium phosphate, polyvinyl alcohol, and CDs for bone TE. These composite scaffolds exhibited improved alkaline phosphatase performance and cell proliferation rate, showing excellent osteogenic differentiation and proliferation rate.57 The osteogenic capability of nitrogen (N)-doped CDs conjugated with hydroxyapatite nanoparticles (NPs) was evaluated (Fig. 2).58 As a result, these CDs with suitable luminescent emission (in vitro) could efficiently induce the MC3T3-E1 osteoblast proliferation, differentiation, and mineralization. Notably, they had suitable effects on bone regeneration of zebrafish jawbone model, showing excellent potential for cell imaging along with improved alkaline phosphatase function, mineralization, and expression of the osteogenic genes in osteoblast cells. These NPs can be deployed as promising theranostic platforms with multifunctional TE-RM capabilities, especially in bone regeneration and fracture healing.58
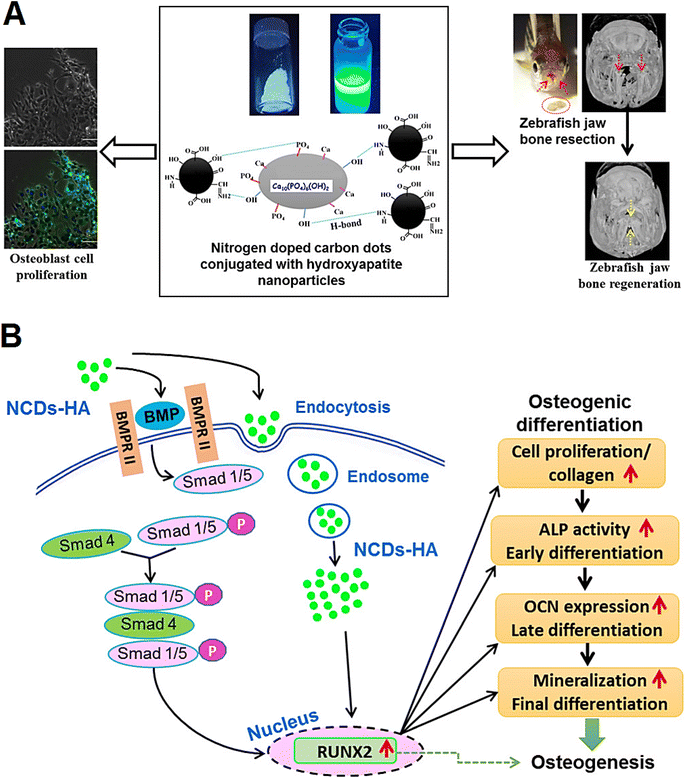 | ||
| Fig. 2 (A) N-doped CDs functionalized with hydroxyapatite NPs (NCDs-HA) with enhanced bone regeneration activity. (B) Possible molecular mechanisms for accelerated osteogenesis by functionalized CDs through the activation of bone morphogenetic protein (BMP) signaling pathway along with the internalization of CDs into the osteoblast cells. RUNX2: runt-related transcription factor 2; ALP: alkaline phosphatase; OCN: osteocalcin. Reproduced with permission from ref. 58 Copyright 2018 American Chemical Society. | ||
Wang et al.59 developed a nanocomposite composed of graphene quantum dots loaded with immunomodulatory layered double hydroxide NPs to enhance the osteogenic differentiation of rat bone marrow mesenchymal stem cells, providing immense potential for bone defects regeneration. Remarkably, the anti-inflammatory regulation of lactate dehydrogenase could facilitate the phenotypic transition of macrophage in this nanocomposite.59 Bone morphogenetic protein-2 (BMP-2)-CDs loaded pectin microparticles were incorporated into the scaffolds with sustained release behavior (up to 21 days) comprised from gelatin, elastin, and hyaluronic acid for bone tissue regeneration. In vitro analyses on MG-63 cells revealed the intercellular uptake of BMP-2-CDs, showing improved biological features and pro-osteogenic effects.60 Shao et al.61 constructed citric acid-based CDs for labeling and tracking of rat bone marrow mesenchymal stem cells. The highly fluorescent probes provided labeling of these mesenchymal stem cells through the internalization without influencing the cell viability or stimulating apoptosis once the concentration was <50 µg mL−1. These CDs facilitated osteogenic differentiation of rat bone marrow mesenchymal stem cells with high efficiency by stimulating the osteogenic transcription and enhancing the matrix mineralization. They provided long-term tracking and promoted the differentiation of the mesenchymal stem cells towards osteoblasts via the ROS-mediated mitogen-activated protein kinase (MAPK) pathway.61
The CD-doped chitosan/nanohydroxyapatite scaffold was introduced for promoting the bone regeneration.62 In rat bone mesenchymal stem cells, the scaffold could enhance the cell adhesion and osteoinductivity through the up-regulation of genes involved in focal adhesion and osteogenesis (in vitro). Compared to pure chitosan/nanohydroxyapatite scaffold, the as-obtained bone repair scaffold could highly enhance the generation of vascularized new bone tissue at four weeks (in vivo). The CDs applied in scaffolds exhibited suitable photothermal effects for tumor photothermal therapy under NIR irradiation. The scaffold could highly inhibit the osteosarcoma cell proliferation (in vitro) and successfully suppress the growth of tumors (in vivo).62 Geng et al.63 studied the effect of surface charge on osteogenic differentiation by applying graphene quantum dots (GQDs). Accordingly, the negatively charged GQDs− highly enhanced the osteogenic differentiation of human mesenchymal stem cells through the activation of bone morphogenetic protein (BMP)/Smad signaling pathway (Fig. 3). 3D injectable hydrogel scaffolds could be obtained after the encapsulation of GQD− in gelatin methacrylamide (GelMA) hydrogels through a UV light-activated photopolymerization in the existence of photo-initiator. Notably, this hydrogel scaffold could successfully accelerated bone regeneration in the mouse calvarial defect model.63
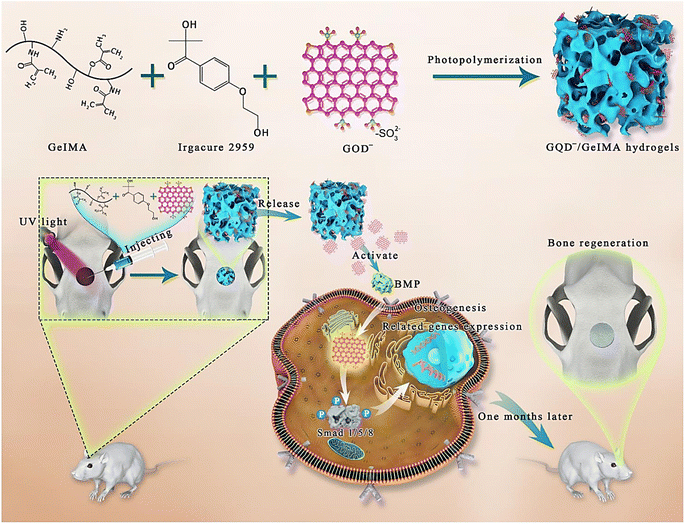 | ||
| Fig. 3 The preparative process of hydrogel scaffolds constructed from GQDs− and GelMA using a UV light-activated photopolymerization; GQDs− incorporated in the porous hydrogel composites could gradually release at the bone defect sites to stimulate in situ bone regeneration. Reproduced with permission from ref. 63 Copyright 2020 Elsevier. | ||
Nanohybrids of CD decorated hydroxyapatite were prepared from the aqueous extract of corms of Colocasia esculenta (as the CD precursor) and egg shell (to obtain CaO as the precursor of hydroxyapatite) using a simple one-pot hydrothermal technique.64 The nanohybrids with suitable cytocompatibility, cell proliferation and alkaline phosphatase performance exhibited osteogenic activities for bone tissue regeneration.64 On the other hand, for cartilage tissue regeneration, thermosensitive alginate-gelatin-N-doped CDs scaffolds were developed as injectable hydrogels with small pore sizes and improved mechanical features.65 Remarkably, changing the amount of N-doped CDs could affect the mechanical features, water uptake, biodegradation, and pore sizes. It was revealed that hydrogels comprising 0.06% N-doped CDs had smaller pore sizes, lower water uptake, improved mechanical features, and less weight loss because of the robust interplay between these CDs and hydrogels. The composite hydrogels with excellent mechanical and stability properties could be injected for cartilage TE purposes, showing great cell adhesion to MG63 cells and good cell viability (>97%).65
2.2. Cardiovascular TE-RM
CDs with unique mechanical, electrical, and optical properties have been employed in cardiovascular TE-RM studies. However, there have been very limited studies in this field and there is a need for comprehensive studies on the potential of CDs in cardiovascular TE-RM. For instance, multifunctional nanocomposite scaffolds were designed with suitable electrical conductivity using poly glycerol sebacate, polycaprolactone, and CQDs through a simple electrospinning technique for cardiac muscle regeneration.66 The addition of polyglycerol sebacate and CQDs led to decrease in the mean fiber diameter of the scaffold; the electrical conductivity of scaffold was also enhanced after the addition of CQDs.66 In addition, nanocomposites were fabricated utilizing bioresorbable polylactic acid and CQDs, showing attractive potential for noninvasive imaging and monitoring of composite conditions and cell growth.67 The as-prepared 3D-printed nanocomposites with cell proliferation, tensile strength, outstanding processability, radial stability, hydrophilicity, and compressive strength could be deployed for in situ monitoring of cardiovascular stents. CQDs could significantly enhance stent features, including composite hydrophilicity by ∼25%, tensile strength by ∼24%, compressive strength by ∼66%, and cell proliferation by ∼50% compared to polylactic acid alone.67Yan et al.43 constructed nanofibrous scaffolds for cardiac TE by incorporating p-phenylenediamine surface functionalized CQDs into silk fibroin–polylactic acid (CQDs–SF–PLA) scaffolds. Accordingly, the scaffolds with improved mechanical properties could be obtained. The presence of CQDs in the SF–PLA scaffolds also led to an enhancement in cell adhesion, proliferation, and mRNA expression of cardiac genes such as Tnnc1, Tnnt2, Cx43, and Atp2a2, even without external electrical stimulation.43 Tabish et al.68 developed GQDs-based electrochemical biosensing platforms to detect biomarkers for acute myocardial infarction detection include cardiac troponin I, cardiac troponin T, myoglobin, lactate dehydrogenase, C-reactive protein, creatine kinase, and myoglobin. Such GQDs-integrated electrochemical biosensors exhibited excellent electrochemical responses owing to the fascinating properties of GQDs such as high specific surface area, π–π interactions with the analyte, interplay between bandgap and photoluminescence, simplistic electron-transfer mechanisms, simple functionalization, size-dependent optical properties, electrochemical luminescence emission potential, and good biocompatibility. Remarkably, the existence of functional groups such as hydroxyl, carboxyl, carbonyl, and epoxide groups could increase the solubility and dispersibility of GQDs in different solvents and biological media.68
2.3. Skin TE-RM and wound healing
CQDs were prepared with low-drug resistance using gentamicin sulfate and diammonium citrate as precursors.69 Accordingly, after the reaction of carboxymethyl chitosan-mixed CQDs with oxidized dextran via the Schiff base linkage, the hydrogel network could be obtained. The obtained hydrogels decorated with antibacterial CQDs (2 mg mL−1) exhibited suitable multifunctionality with self-healing, injectability, stretchability, compressive properties, and pH-responsive release behavior, providing efficient obstruction of biofilm formation and bacterial infections treatment (Fig. 4). These hydrogels also displayed in vivo wound healing with anti-inflammatory and wound repair stimulatory effects, which make them promising candidates for skin tissue regeneration purposes.69 In another study, polyvinyl alcohol (PVA)-based nanofibers containing CQD, silica nanoparticle, and silk fibroin were fabricated with enhanced antibacterial effects.70 The results of cell toxicity, viability, and proliferation studies on NIH 3T3 fibroblast cells demonstrated that these nanocomposites not only had no toxicity but also accelerated cell viability and proliferation, promoting wound healing (in vivo); they could effectively accelerated the skin and hair follicle regeneration, offering attractive candidates for obstructing the bacterial growth and stimulating skin wound repair and hair regeneration.70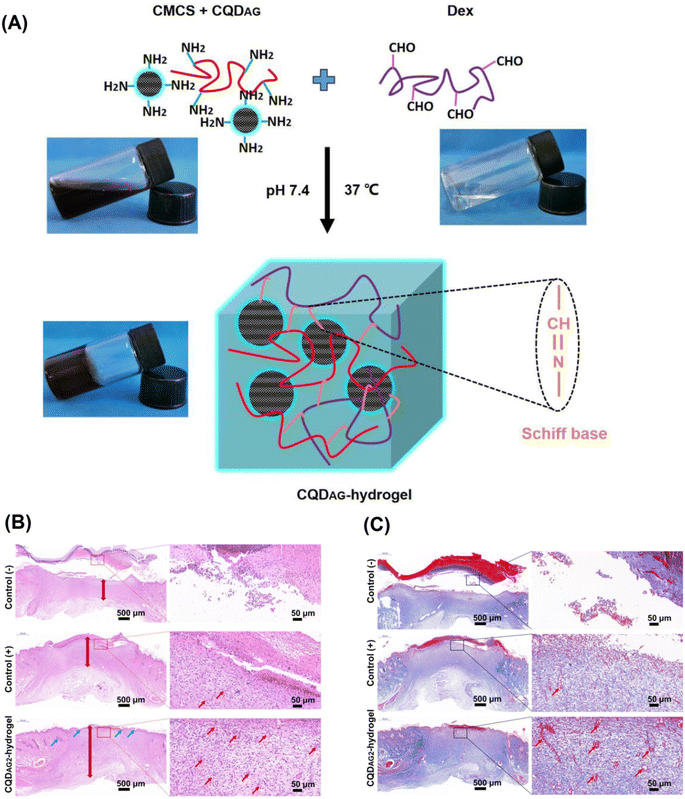 | ||
| Fig. 4 (A) The preparative process of antibacterial CQDs (CQDAG)-hydrogel system using carboxymethyl chitosan (CMCS), CQDAG, and dextran (Dex). (B) Hematoxylin and eosin (H&E) staining of tissues at wound sites to evaluate the healing of wounds for each group after 14 days treatment (blood vessels: red arrows, hair follicles: blue arrows). (C) Masson's trichrome staining of tissues at wound sites to evaluate collagen generation for each group after 14 days treatment (blood vessels: red arrows). Reproduced with permission from ref. 69 Copyright 2020 Elsevier. | ||
A CQDs-loaded chitosan film was fabricated by simultaneous functionalization using amino acids under microwave-assisted conditions and solvent casting method for skin regeneration applications.46 The results demonstrated that the impregnation of CQDs into chitosan film decreased the water absorption, bovine serum albumin absorption, and moisture permeation capacities of the film. This would be beneficial for wounds with low exudates since the presence of sufficient crosslinks in the film containing CQDs will prevent it from absorbing excessive amounts of wound fluid, which consequently helps to maintain the appropriate moisture level in the wound. They also showed that the incorporation of CQDs in chitosan films could result in better antioxidant properties than pure chitosan film, as CQDs have favorable properties in scavenging free radicals.46 Zhang et al.48 also synthesized a multifunctional sodium alginate (SA) hydrogel loaded with hemoglobin (Hb) and pH-sensitive fluorescent changing CQDs for monitoring wound healing as well as preventing bacterial infections and tumor recurrence for postoperative cancer treatment. Their findings showed that SA-Hb-CQDs had potential in rapidly arresting bleeding, promoting wound healing, and providing real-time updates on wound conditions. They demonstrated that Fe2+ in Hb, in the presence of tumor endogenous H2O2, was able to generate toxic ˙OH molecules, which proved effective elimination of residual recurrent cancer cells and infected bacteria.48
Dong et al.71 prepared an electrospun zein (ZE)-based wound dressing membrane containing tea CDs (TCDs) and CaO2 for diabetic wound disinfection and healing (Fig. 5). TCDs and CaO2 were doped into this dressing membrane to synergistically enhance the antibacterial and healing efficacy of the membrane. It was revealed that TCDs-CaO2-ZE had excellent biocompatibility and anti-inflammatory effects as well as enhanced wound healing properties in diabetic rats without any cytotoxicity.71 In another study, Qu et al.72 designed sprayable positively charged CDs for promoting infected wound healing. These CDs were synthesized from p-phenylenediamine and polyethyleneimine by a facile one-pot solvothermal method. The positively charged CDs displayed favorable biocompatibility after in vitro cytotoxicity, hemolysis assay, and in vivo toxicity studies. These NPs also exhibited superior antibacterial activity against Staphylococcus aureus at very low concentrations as well as strong antioxidant activity by scavenging excess free radicals. Its sprayable forms were also used to treat a skin infection in mouse model, and the results were promising in promoting would healing without causing any noticeable side effects.72
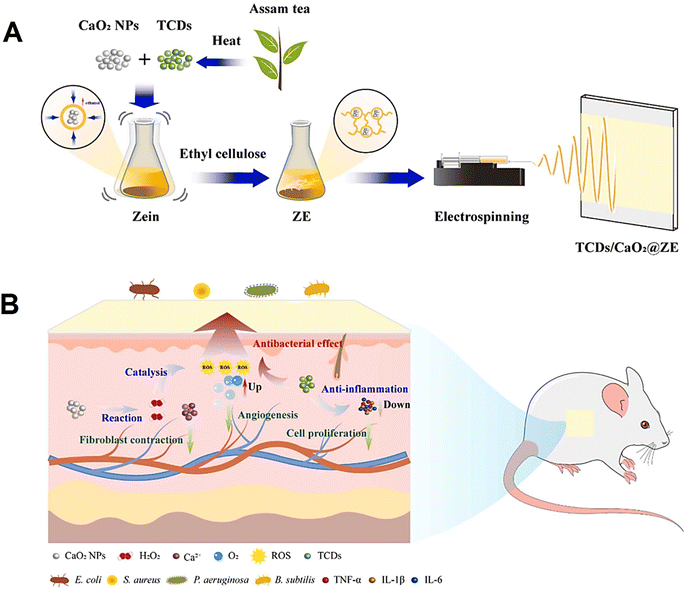 | ||
| Fig. 5 (A) The preparative process of TCDs/CaO2@ZE film dressing. (B) The mechanism of TCDs/CaO2@ZE for stimulating chronic wound healing on the full-thickness wound in the streptozotocin-induced diabetic rat model. Reproduced with permission from ref. 71 Copyright 2023 Elsevier. | ||
CDs impregnated fluorescent nanofibers were introduced in vivo monitoring and enhancing wound healing.73 The application of CDs in scaffolds could promote fibroblast cell adhesion, migration, and proliferation, accelerating the healing progression. Remarkably, the regenerated skin displayed a stratified epithelial layer with mature dermal tissue. These scaffolds with suitable antioxidant activities, excellent photoluminescence, good biocompatibility, and photostability could be able to scavenge free radicals and reduce the expression of antioxidative enzymes; they could also be deployed for noninvasive in vivo evaluation of wound healing kinetics using two-photon microscopy.73 Omidi et al.74 developed chitosan-CDs nanocomposites to monitor the pH of wounds as an important diagnosis factor during the healing process, offering multifunctional nanosystems with antibacterial and pH-sensitive behavior to enhance the wound healing process. These nanocomposites with good biocompatibility and nontoxicity as well as improved mechanical properties exhibited efficient antibacterial features. They could be deployed as wound healing bandage, showing suitable antibacterial properties due to the ROS formation.74
3. Challenges and perspectives
CD-based nanosystems have gained significant attentions in recent years due to their distinctive structures and properties, which make them potential materials for biomedical applications.75,76 CDs offer several advantages that make them ideal candidates for TE-RM applications, including low toxicity, exceptional biodegradability, biocompatibility, mechanical strength, antimicrobial characteristic, and biomineralization as well as their application in real-time tissue monitoring and visualization.77,78 It has been indicated that the hydrogels incorporating CDs show enhanced cell proliferation and migration within scaffolds, higher osteogenic differentiation, increased tissue granulation, and collagen deposition during the tissue regeneration process.79 This is possibly because of their large number of surface functional groups (such as hydroxyl, carboxyl and amino) that have the potential to be readily conjugated with other components to enhance tissue regeneration.55 The incorporation of CDs into scaffolds can be served as a cell culture substrate, directing cell expansion along the scaffold's nanofibers and increasing cellular activity.28,80 This suggests that the huge number of functional groups on the surface of CDs serve an important role in supporting cellular activities. Current research mostly focuses on the design and implementation of CDs and scaffold materials, with little attention paid to the mechanism of action underlying their biological effects.28 Hence, comprehensive research on the relationship between the characterizations of CDs and their biological mechanisms of activities should be performed, offering a theoretical foundation for the rational design of scaffolds incorporating CDs for TE purposes.28 Given that CDs may cause bacterial cells to lyse by producing intracellular ROS, their chemical cross-link with hydrogels exhibited increased antibacterial efficacy.81–83 Thus, it is suggested to investigate the effects of CDs incorporation within scaffolds in prospective studies due to the excellent antibacterial properties of CDs, which can be employed to prevent or treat frequent infections following the tissue repair.69Developing scaffolds with appropriate mechanical features is also an important requirement, since the cell adhesion, proliferation, and differentiation are all highly dependent on the scaffold's mechanical properties.84 Currently, carbon nanomaterials have been employed as secondary structural reinforcing agents to enhance the mechanical properties of 2- and 3D cell culture scaffolds; however, tissue scaffolds made using CDs have outstanding mechanical properties due to CDs' cross-link formation and effective cell interactions.85,86 Meanwhile, several studies suggested that CDs might significantly improve the mechanical strength of different kinds of scaffolds used for TE applications such as bone TE.87 Thus, future explorations should be directed toward the evaluation of mechanical properties of scaffolds incorporated with CDs as well as the improvement of their mechanical strength.88 With regard to CD's toxicity, CDs have great biocompatibility and low cytotoxicity.89–91 A number of in vitro and in vivo cytotoxicity experiments revealed that CDs display toxic effects at high doses but no discernible toxic effect was detected at lower concentrations. The important point is that although there have been few findings on controlling scaffold cytotoxicity, CD's cytotoxic or disruptive effects on different pathways remain unclear, and need further investigation.88 Although CDs offer promising features for the usage in nanomedical applications because of their low toxicity both in vitro and in vivo, it is urgently necessary to ascertain the proper CD concentration in animal and cell culture models as well as their long-term effects on the organisms before their therapeutic use.31 There is also a need to pay greater attention to examining the biocompatibility and cytotoxicity of CDs on various tissues to assess the practical impacts and biosafety of CDs thoroughly and precisely.28
The optical characteristics of CDs have also generated a lot of interest and excitement in a variety of biological applications because of their wide excitation and emission wavelength range.92 Several investigations have demonstrated the fluorescence characteristic of CDs through a variety of conceivable explanations, including size dependency, surface defects, surface states, degree of oxidation, surface functional groups, etc., and there is no question that the superb fluorescent characteristic of CDs has a great deal of promise in TE applications such as deep-tissue imaging.92–96 The highly stable fluorescent of CDs present in the scaffold made it possible to study the kinetics of tissue regeneration over a long period of time without invasion.97,98 The long-term non-invasive imaging of cell–scaffold interaction during in vivo tissue regeneration would therefore be an intriguing real-time imaging technique that may be exploited in future investigations.73 The biodegradation of scaffold materials following tissue regeneration may be also monitored using CDs' distinctive optical characteristics.42 However, it is noteworthy to consider that although several studies have shown the capacity of CDs to be employed for cellular tracking, the majority of these CDs generate blue-green fluorescence when exposed to UV radiation, which can cause spontaneous fluorescence induction of tissues.99 This may significantly interfere with CDs signaling, and cause cell and tissue damage. Thus, studying CDs in the long-wavelength and NIR regions for cellular tracing will be an increasingly important field of research in the future.99,100
Considering current challenges, future efforts should be conducted on developing bacterial-resistant scaffolds that reduce the risk of infection after transplantation. These scaffolds can be used particularly for skin wound-healing, in which there is a high risk of infection due to direct contact with the surrounding area. CDs can be also used for the engineering of hard tissues such as bone, because their incorporation within scaffolds increases the scaffold's mechanical strength remarkably. Future investigations should also investigate the applicability of using red/NIR light for CD excitation. The shift from UV to red/NIR irradiation will improve deep tissue imaging, cell tracking within scaffolds, and monitoring scaffolds biodegradation by decreasing not only the risk of tissue damage but also tissue-interfering signaling while imaging. And last but not least, prospective in vitro and in vivo studies should focus on investigating the biocompatibility and biodegradation as well as the accumulation of CDs and their toxic effects on body organs. The longer emissive wavelength, increased photostability, improved physicochemical and mechanical properties, broad pH sensitivity, deep tissue penetration, enhanced antibacterial activity, and good biocompatibility of CDs are the primary factors influencing their success in TE-RM.92 Taking advantage of CDs in TE is the subject of numerous potential research projects, yet there are still a few challenges that need to be rectified.
The application of CDs is still limited by its synthesis process that may require special equipment, laborious methods, and extreme synthetic conditions. In order to increase CDs application, it is crucial to manufacture CDs with precise control over their lateral dimensions, appropriate surface chemistry, and high quantum yields by a straightforward one-step procedure.36 In addition, cytotoxicity is an important concept that should be considered when working in TE field of study. Thus, further research on CDs' cytotoxicity and their potential environmental adverse effects should be performed before any clinical utilization of CDs. These materials are the subject of studies that are significantly growing, and the next several years will reveal if their promise will be fulfilled.88
Evaluating the potential risks of CDs on human health requires the comprehension of their biocompatibility and interactions with biomolecules through in-depth in vitro and in vivo studies. In this context, several in vitro studies have focused on the biodegradation of CDs using digestive enzymes such as horseradish peroxidase, lipase, etc. These studies suggest that CDs can be broken down into smaller, less toxic compounds that can be more easily eliminated by the body.101 Since the biodegradability of CDs is an important consideration for their use in TE-RM, comprehensive and long-term in vivo studies should investigate the biodegradation, bio-clearance, and accumulation of different CDs in the main body organs such as brain, kidney, intestines, and liver in the future.102,103 On the other hand, the absorption and emission wavelengths of CDs have primarily been focused in the ultraviolet/visible area, whereas the production of CDs using red/NIR light is still uncommon, and this makes deep tissue imaging using CDs limited. To address this limitation, future research should focus on developing CDs with deep red/NIR absorption and emission capabilities in order to broaden their applicability in TE field of study.104
4. Conclusions
In conclusion, we anticipate that the ongoing study of fluorescent CD-mediated scaffolds during the coming years will expand the field of non-invasive TE by addressing the current constraints, and the advancement of manufacturing technology and characterization will allow for a deeper investigation of CD preparation, structure, characteristics, applications, and mechanisms, which lead to scaled-up production of scaffolds incorporating CDs for TE-RM in the future. To improve the properties of CD-based nanosystems, the in-depth characterization of physicochemical features of scaffolds-morphology, chemical bonds, mechanical properties, wettability, and electrical conductivity are essential. Remarkably, biological properties such as cytotoxicity, degradation rate, and cell attachment/proliferation ought to be systematically analyzed (both in vitro and in vivo). Several studies have focused on designing CD-based nanosystems for bone, cardiovascular, and skin TE as well as wound healing and antimicrobial applications; however, more explorations are still warranted to develop next-generation nanosystems and scaffolds with cost-effectiveness and multifunctionality for TE-RM purposes. In this context, clinical translation studies and long-term biosafety assessments along with the optimization of synthesis/reaction conditions and suitable functionalization are crucial aspects.Conflicts of interest
The author(s) declare no competing interest.References
- N. Farshidfar, N. Tanideh, Z. Emami, F. Sari Aslani, N. Sarafraz, Z. Khodabandeh, S. Zare, G. Farshidfar, S. Nikoofal-Sahlabadi, L. Tayebi and M. Zarei, J. Mater. Res. Technol., 2022, 21, 4558–4576 CrossRef CAS.
- F. Han, J. Wang, L. Ding, Y. Hu, W. Li, Z. Yuan, Q. Guo, C. Zhu, L. Yu and H. Wang, Front. Bioeng. Biotechnol., 2020, 8, 83 CrossRef PubMed.
- F. Habibzadeh, S. M. Sadraei, R. Mansoori, N. P. S. Chauhan and G. Sargazi, Heliyon, 2022, 8, e12193 CrossRef CAS.
- N. Farshidfar, D. Jafarpour, P. Firoozi, S. Sahmeddini, S. Hamedani, R. F. de Souza and L. Tayebi, Jpn. Dent. Sci. Rev., 2022, 58, 89–123 CrossRef PubMed.
- H. Mehdizadeh, S. I. Somo, E. S. Bayrak, E. M. Brey and A. Cinar, Ind. Eng. Chem. Res., 2015, 54, 2317–2328 CrossRef CAS.
- B. Guo and P. X. Ma, Biomacromolecules, 2018, 19, 1764–1782 CrossRef CAS PubMed.
- Z. Khodabandeh, N. Tanideh, F. S. Aslani, I. Jamhiri, S. Zare, N. Alizadeh, A. Safari, N. Farshidfar, M. Dara and M. Zarei, Mater. Today Commun., 2022, 31, 103339 CrossRef CAS.
- A. Hasan, M. Morshed, A. Memic, S. Hassan, T. J. Webster and H. El-Sayed Marei, Int. J. Nanomed., 2018, 13, 5637–5655 CrossRef CAS PubMed.
- X. Zheng, P. Zhang, Z. Fu, S. Meng, L. Dai and H. Yang, RSC Adv., 2021, 11, 19041–19058 RSC.
- S. Zheng, Y. Tian, Y. Shen, J. Ouyang, X. Wang and J. Luan, Front. Chem, 2022, 10, 1106 Search PubMed.
- S. Iravani and R. S. Varma, Environ. Chem. Lett., 2020, 18, 703–727 CrossRef CAS PubMed.
- M. Sajjadi, M. Nasrollahzadeh, B. Jaleh, G. Jamalipour Soufi and S. Iravani, J. Drug Targeting, 2021, 29, 716–741 CrossRef CAS PubMed.
- N. Tanideh, N. Azarpira, N. Sarafraz, S. Zare, A. Rowshanghiyas, N. Farshidfar, A. Iraji, M. Zarei and M. El Fray, Polymers, 2020, 12, 2588 CrossRef CAS PubMed.
- L. Đorđević, F. Arcudi, M. Cacioppo and M. Prato, Nat. Nanotechnol., 2022, 17, 112–130 CrossRef PubMed.
- Z. L. Wu, Z. X. Liu and Y. H. Yuan, J. Mater. Chem. B, 2017, 5, 3794–3809 RSC.
- G. Ge, L. Li, D. Wang, M. Chen, Z. Zeng, W. Xiong, X. Wu and C. Guo, J. Mater. Chem. B, 2021, 9, 6553–6575 RSC.
- Y. Wang and L. Chen, Nanomed.: Nanotechnol. Biol. Med., 2011, 7, 385–402 CrossRef CAS PubMed.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- J. Liu, R. Li and B. Yang, ACS Cent. Sci., 2020, 6, 2179–2195 CrossRef CAS PubMed.
- L. Cui, X. Ren, M. Sun, H. Liu and L. Xia, Nanomaterials, 2021, 11, 3419 CrossRef CAS PubMed.
- B. Wang, G. I. N. Waterhouse and S. Lu, Trends Chem., 2023, 5, 76–87 CrossRef CAS.
- B. Domingo-Tafalla, E. Martínez-Ferrero, F. Franco and E. Palomares-Gil, Molecules, 2022, 27, 1081 CrossRef CAS PubMed.
- S. A. Shaik, S. Sengupta, R. S. Varma, M. B. Gawande and A. Goswami, ACS Sustainable Chem. Eng., 2021, 9, 3–49 CrossRef CAS.
- N. Rabiee, S. Iravani and R. S. Varma, Molecules, 2022, 27, 6186 CrossRef CAS PubMed.
- H. H. Jing, F. Bardakci, S. Akgöl, K. Kusat, M. Adnan, M. J. Alam, R. Gupta, S. Sahreen, Y. Chen and S. C. B. Gopinath, J. Funct. Biomater., 2023, 14, 27 CrossRef CAS PubMed.
- M. Kurian and A. Paul, Carbon Trends, 2021, 3, 100032 CrossRef CAS.
- T. J. Pillar-Little, N. Wanninayake, L. Nease, D. K. Heidary, E. C. Glazer and D. Y. Kim, Carbon, 2018, 140, 616–623 CrossRef CAS.
- R. Zhang, Y. Hou, L. Sun, X. Liu, Y. Zhao, Q. Zhang, Y. Zhang, L. Wang, R. Li, C. Wang, X. Wu and B. Li, Nanoscale, 2023, 15, 3106–3119 RSC.
- B. Geng, D. Yang, D. Pan, L. Wang, F. Zheng, W. Shen, C. Zhang and X. Li, Carbon, 2018, 134, 153–162 CrossRef CAS.
- B. Geng, J. Hu, Y. Li, S. Feng, D. Pan, L. Feng and L. Shen, Nat. Commun., 2022, 13, 5735 CrossRef CAS PubMed.
- J. Liu, Y. Geng, D. Li, H. Yao, Z. Huo, Y. Li, K. Zhang, S. Zhu, H. Wei, W. Xu, J. Jiang and B. Yang, Adv. Mater., 2020, 32, 1906641 CrossRef CAS PubMed.
- J. Liu, D. Li, K. Zhang, M. Yang, H. Sun and B. Yang, Small, 2018, 14, 1703919 CrossRef PubMed.
- S. Ross, R.-S. Wu, S.-C. Wei, G. M. Ross and H.-T. Chang, J. Food Drug Anal., 2020, 28, 677–695 CAS.
- R. Kumar, V. B. Kumar and A. Gedanken, Ultrason. Sonochem., 2020, 64, 105009 CrossRef CAS PubMed.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS PubMed.
- L. Jin, K. Ren, Q. Xu, T. Hong, S. Wu, Y. Zhang and Z. Wang, Polym. Compos., 2018, 39, 73–80 CrossRef CAS.
- H. Ehtesabi and F. Massah, Mater. Today Sustain., 2021, 13, 100075 CrossRef.
- V. B. Kumar, R. Kumar, A. Gedanken and O. Shefi, Ultrason. Sonochem., 2019, 52, 205–213 CrossRef CAS.
- H. Basiri, A. A. Mehrizi, A. Ghaee, M. Farokhi, M. Chekini and E. Kumacheva, Langmuir, 2020, 36, 2893–2900 CrossRef CAS PubMed.
- F. Lu, S. Yang, Y. Song, C. Zhai, Q. Wang, G. Ding and Z. Kang, Mater. Res. Express, 2019, 6, 065030 CrossRef CAS.
- B. Geng, H. Qin, W. Shen, P. Li, F. Fang, X. Li, D. Pan and L. Shen, Chem. Eng. J., 2020, 383, 123102 CrossRef CAS.
- A. Saranti, A. Tiron-Stathopoulos, L. Papaioannou, C. Gioti, A. Ioannou, M. A. Karakassides, K. Avgoustakis, I. Koutselas and K. Dimos, Smart Mater. Med., 2022, 3, 12–19 CrossRef.
- C. Yan, Y. Ren, X. Sun, L. Jin, X. Liu, H. Chen, K. Wang, M. Yu and Y. Zhao, J. Photochem. Photobiol., B, 2020, 202, 111680 CrossRef CAS PubMed.
- M. Ghorghi, M. Rafienia, V. Nasirian, F. S. Bitaraf, A. M. Gharravi and A. Zarrabi, Polym. Adv. Technol., 2020, 31, 3302–3315 CrossRef CAS.
- S. Gogoi, S. Maji, D. Mishra, K. S. P. Devi, T. K. Maiti and N. Karak, Macromol. Biosci., 2017, 17, 1600271 CrossRef PubMed.
- R. Kandra and S. Bajpai, Arabian J. Chem., 2020, 13, 4882–4894 CrossRef CAS.
- A. Dehghani, S. M. Ardekani, M. Hassan and V. G. Gomes, Carbon, 2018, 131, 238–245 CrossRef CAS.
- Q. Zhang, Z. Li, M. Zhang, W. Wang, J. Shen, Z. Ye and N. Zhou, Langmuir, 2020, 36, 13263–13273 CrossRef CAS PubMed.
- S. Sarabiyan Nejad, D. Razzaghi, M. Rezaei, M. Bagheri, A. Babaie and F. Abbasi, Int. J. Polym. Mater. Polym. Biomater., 2022, 71, 1069–1077 CrossRef CAS.
- C. Ren, X. Hao, L. Wang, Y. Hu, L. Meng, S. Zheng, F. Ren, W. Bu, H. Wang and D. Li, Adv. Healthcare Mater., 2021, 10, 2100196 CrossRef CAS PubMed.
- Y. Hou, R. Zhang, H. Cheng, Y. Wang, Q. Zhang, L. Zhang, L. Wang, R. Li, X. Wu and B. Li, Colloids Surf., A, 2023, 656, 130264 CrossRef CAS.
- L. Liu, X. Li, W. Bu, N. Jin, Y. Meng, Y. Wang, D. Wang, X. Xu, D. Zhou and H. Sun, Mater. Today Bio, 2022, 16, 100344 CrossRef CAS PubMed.
- K. Zhang, J. Da, J. Wang, L. Liu, X. Liu, H. Yuan, L. Jiang, Y. Geng, X. Liu and Z. Jiang, Adv. Ther., 2023, 6, 2200149 CrossRef CAS.
- D. S. Ghataty, R. I. Amer, M. A. Amer, M. F. Abdel Rahman and R. N. Shamma, Pharmaceutics, 2023, 15, 234 CrossRef CAS PubMed.
- C. Wan, M. Hu, X. Peng, N. Lei, H. Ding, Y. Luo and X. Yu, Biomater. Sci., 2022, 10, 6291–6306 RSC.
- C. Sarkar, A. R. Chowdhuri, A. Kumar, D. Laha, S. Garai, J. Chakraborty and S. K. Sahu, Carbohydr. Polym., 2018, 181, 710–718 CrossRef CAS PubMed.
- S. Shafiei, M. Omidi, F. Nasehi, H. Golzar, D. Mohammadrezaei, M. Rezai Rad and A. Khojasteh, Mater. Sci. Eng. C, 2019, 100, 564–575 CrossRef CAS PubMed.
- D. K. Khajuria, V. B. Kumar, D. Gigi, A. Gedanken and D. Karasik, ACS Appl. Mater. Interfaces, 2018, 10, 19373–19385 CrossRef CAS PubMed.
- Z. Wang, H. Yang, Y. Bai, L. Cheng and R. Zhu, Biomed. Mater., 2022, 17, 024101 CrossRef PubMed.
- A. R. Keleshteri, F. Moztarzadeh, M. Farokhi, A. A. Mehrizi, H. Basiri and S. S. Mohseni, Int. J. Biol. Macromol., 2021, 184, 29–41 CrossRef PubMed.
- D. Shao, M. Lu, D. Xu, X. Zheng, Y. Pan, Y. Song, J. Xu, M. Li, M. Zhang, J. Li, G. Chi, L. Chen and B. Yang, Biomater. Sci., 2017, 5, 1820–1827 RSC.
- Y. Lu, L. Li, M. Li, Z. Lin, L. Wang, Y. Zhang, Q. Yin, H. Xia and G. Han, Bioconjugate Chem., 2018, 29, 2982–2993 CrossRef CAS PubMed.
- B. Geng, F. Fang, P. Li, S. Xu, D. Pan, Y. Zhang and L. Shen, Chem. Eng. J., 2021, 417, 128125 CrossRef CAS.
- S. Gogoi, M. Kumar, B. B. Mandal and N. Karak, RSC Adv., 2016, 6, 26066–26076 RSC.
- M. Ghanbari, M. Salavati-Niasari and F. Mohandes, RSC Adv., 2021, 11, 18423–18431 RSC.
- S. Rastegar, M. Mehdikhani, A. Bigham, E. Poorazizi and M. Rafienia, Mater. Chem. Phys., 2021, 266, 124543 CrossRef CAS.
- Z. Mahmud, A. Nasrin, M. Hassan and V. G. Gomes, Polym. Adv. Technol., 2022, 33, 980–990 CrossRef CAS.
- T. A. Tabish, H. Hayat, A. Abbas and R. J. Narayan, Biosensors, 2022, 12, 77, DOI:10.3390/bios12020077.
- P. Li, S. Liu, X. Yang, S. Du, W. Tang, W. Cao, J. Zhou, X. Gong and X. Xing, Chem. Eng. J., 2021, 403, 126387 CrossRef CAS.
- S. Abolghasemzade, M. Pourmadadi, H. Rashedi, F. Yazdian, S. Kianbakht and M. Navaei-Nigjeh, J. Mater. Chem. B, 2021, 9, 658–676 RSC.
- Z. Dong, J. Yin, X. Zhou, S. Li, Z. Fu, P. Liu, L. Shen and W. Shi, Colloids Surf., B, 2023, 113325 CrossRef CAS PubMed.
- X. Qu, C. Gao, L. Fu, Y. Chu, J.-H. Wang, H. Qiu and J. Chen, ACS Appl. Mater. Interfaces, 2023, 15, 18608–18619 CrossRef CAS PubMed.
- P. Pal, B. Das, P. Dadhich, A. Achar and S. Dhara, J. Mater. Chem. B, 2017, 5, 6645–6656 RSC.
- M. Omidi, A. Yadegari and L. Tayebi, RSC Adv., 2017, 7, 10638–10649 RSC.
- X. Yu, X. Tang, S. V. Gohil and C. T. Laurencin, Adv. Healthcare Mater., 2015, 4, 1268–1285 CrossRef CAS PubMed.
- Z. Nazari, M. H. Nematollahi, F. Zareh, B. Pouramiri and M. Mehrabani, ChemistrySelect, 2023, 8, e202203630 CrossRef CAS.
- S. Samadian, A. Karbalaei, M. Pourmadadi, F. Yazdian, H. Rashedi, M. Omidi and S. Malmir, Int. J. Polym. Mater. Polym. Biomater., 2022, 71, 395–402 CrossRef CAS.
- J. L. Parimi, H. Bora, B. Saha, K. Dixit, S. Dhara, B. Guntupalli and P. Manchikanti, in Carbon Dots: Next-generation materials for biomedical applications, IOP Publishing, 2022 Search PubMed.
- W. Bu, X. Xu, Z. Wang, N. Jin, L. Liu, J. Liu, S. Zhu, K. Zhang, R. Jelinek and D. Zhou, ACS Appl. Mater. Interfaces, 2020, 12, 50287–50302 CrossRef CAS PubMed.
- M. K. Kumawat, M. Thakur, R. B. Gurung and R. Srivastava, Sci. Rep., 2017, 7, 15858 CrossRef PubMed.
- J. Liang, W. Li, J. Chen, X. Huang, Y. Liu, X. Zhang, W. Shu, B. Lei and H. Zhang, ACS Appl. Bio Mater., 2021, 4, 6937–6945 CrossRef CAS PubMed.
- X. Dong, W. Liang, M. J. Meziani, Y.-P. Sun and L. Yang, Theranostics, 2020, 10, 671 CrossRef CAS PubMed.
- M. Ghirardello, J. Ramos-Soriano and M. C. Galan, Nanomaterials, 2021, 11, 1877 CrossRef CAS PubMed.
- S. Fooladi, S. Faramarz, S. Dabiri, A. Kajbafzadeh, M. H. Nematollahi and M. Mehrabani, Biomater. Res., 2022, 26, 46, DOI:10.1186/s40824-40022-00295-40821.
- Z. Peng, T. Zhao, Y. Zhou, S. Li, J. Li and R. M. Leblanc, Adv. Healthcare Mater., 2020, 9, 1901495 CrossRef CAS PubMed.
- L. Zhu, W. Kong, J. Ma, R. Zhang, C. Qin, H. Liu and S. Pan, J. Biol. Eng., 2022, 16, 1–17 CrossRef PubMed.
- Y. Chen and X. Li, Med. Novel Tech. Dev., 2022, 100168 CrossRef.
- R. Eivazzadeh-Keihan, A. Maleki, M. De La Guardia, M. S. Bani, K. K. Chenab, P. Pashazadeh-Panahi, B. Baradaran, A. Mokhtarzadeh and M. R. Hamblin, J. Adv. Res., 2019, 18, 185–201 CrossRef CAS PubMed.
- J. Liao, Y. Yao, C.-H. Lee, Y. Wu and P. Li, Pharmaceutics, 2021, 13, 1872 CrossRef CAS PubMed.
- N. Azam, M. Najabat Ali and T. Javaid Khan, Front. Mater., 2021, 8, 700403 CrossRef.
- Y. Park, Y. Kim, H. Chang, S. Won, H. Kim and W. Kwon, J. Mater. Chem. B, 2020, 8, 8935–8951 RSC.
- M. Vedhanayagam, I. S. Raja, A. Molkenova, T. S. Atabaev, K. J. Sreeram and D.-W. Han, Int. J. Mol. Sci., 2021, 22, 5378 CrossRef CAS PubMed.
- M. J. Molaei, RSC Adv., 2019, 9, 6460–6481 RSC.
- Y. Y. Wei, L. Chen, X. Zhang, J. L. Du, Q. Li, J. Luo, X. G. Liu, Y. Z. Yang, S. P. Yu and Y. D. Gao, Biomater. Sci., 2022, 10, 4345–4355 RSC.
- Y. Zhao, Y. Xie, Y. Liu, X. Tang and S. Cui, J. Mater. Chem. B, 2022, 10, 3512–3523 RSC.
- K. K. Lee, N. Raja, H.-s. Yun, S. C. Lee and C.-S. Lee, Acta Biomater., 2023, 159, 382–393 CrossRef CAS PubMed.
- L. M. T. Phan and S. Cho, Bioinorg. Chem. Appl., 2022, 2022, 1–32 CrossRef PubMed.
- B. Unnikrishnan, R.-S. Wu, S.-C. Wei, C.-C. Huang and H.-T. Chang, ACS Omega, 2020, 5, 11248–11261 CrossRef CAS PubMed.
- H. Ding, J.-S. Wei, N. Zhong, Q.-Y. Gao and H.-M. Xiong, Langmuir, 2017, 33, 12635–12642 CrossRef CAS PubMed.
- K. B. Juybari, K. Rizwan, S. Faramarz, A. Sadeghi, A. Amirkhosravi, M. H. Nematollahi and M. Mehrabani, Chem. Biodiversity, 2023, e202200721 CrossRef PubMed.
- I. Srivastava, D. Sar, P. Mukherjee, A. S. Schwartz-Duval, Z. Huang, C. Jaramillo, A. Civantos, I. Tripathi, J. P. Allain and R. Bhargava, Nanoscale, 2019, 11, 8226–8236 RSC.
- X. Tian, A. Zeng, Z. Liu, C. Zheng, Y. Wei, P. Yang, M. Zhang, F. Yang and F. Xie, Int. J. Nanomed., 2020, 6519–6529 CrossRef CAS PubMed.
- C. Fu, X. Qin, J. Zhang, T. Zhang, Y. Song, J. Yang, G. Wu, D. Luo, N. Jiang and F. J. Bikker, Heliyon, 2023, 9, e13422 CrossRef CAS PubMed.
- F. Wu, H. Su, X. Zhu, K. Wang, Z. Zhang and W.-K. Wong, J. Mater. Chem. B, 2016, 4, 6366–6372 RSC.
| This journal is © The Royal Society of Chemistry 2023 |
