Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface modifications of biomaterials in different applied fields
Xi Huab,
Teng Wangab,
Faqi Li*ab and
Xiang Mao *ab
*ab
aState Key Laboratory of Ultrasound in Medicine and Engineering College of Biomedical Engineering, Chongqing Medical University, Chongqing 400016, P. R. China. E-mail: maox@cqmu.edu.cn
bChongqing Key Laboratory of Biomedical Engineering, College of Biomedical Engineering, Chongqing Medical University, Chongqing 400016, P. R. China
First published on 10th July 2023
Abstract
Biomaterial implantation into the human body plays a key role in the medical field and biological applications. Increasing the life expectancy of biomaterial implants, reducing the rejection reaction inside the human body and reducing the risk of infection are the problems in this field that need to be solved urgently. The surface modification of biomaterials can change the original physical, chemical and biological properties and improve the function of materials. This review focuses on the application of surface modification techniques in various fields of biomaterials reported in the past few years. The surface modification techniques include film and coating synthesis, covalent grafting, self-assembled monolayers (SAMs), plasma surface modification and other strategies. First, a brief introduction to these surface modification techniques for biomaterials is given. Subsequently, the review focuses on how these techniques change the properties of biomaterials, and evaluates the effects of modification on the cytocompatibility, antibacterial, antifouling and surface hydrophobic properties of biomaterials. In addition, the implications for the design of biomaterials with different functions are discussed. Finally, based on this review, it is expected that the biomaterials have development prospects in the medical field.
Introduction
The development of modern medicine has made it possible to regenerate and rebuild damaged human tissues and organs. Biomaterials show strong vitality and broad development prospects in different medical fields. In this case, biological materials can be divided into metal materials, ceramic materials and polymer materials.1 To date, a variety of materials based on polymers, ceramics and metals have been widely used in tissue engineering, including micro-implants for cardiovascular purposes and macroscopic devices in bone tissues, among many other applications.2 Because of their excellent mechanical properties (high strength toughness and fatigue resistance) and chemical properties, metal-based biomaterials have been successfully applied as artificial implants in the biomedical engineering field.3–5 The clinical application of ceramic and polymer materials as implants has also been widely studied. Even so, the utilized biological materials cannot avoid the occurrence of biocompatibility, protein adsorption, and bacterial adhesion. In this way, biocompatibility is the primary problem to be solved in surgical implantation.6 Traditionally, biological materials implanted in the body will inevitably be rejected by the body, and the immune mechanism will show defense against them, leading to the failure of implantation of materials.7,8 The growth of microorganisms in the implant leads to an increased rate of surgical infection. Most bacteria contaminating implants come from the skin surface and mucous membrane. These bacteria adhere to the surface of implant materials and proliferate to form biofilms, leading to infection finally.9 In this case, biofilms can damage the tissue surrounding the implant, resulting in poor vascularization, loosening, detachment and even dislocation of the implant material.10 Furthermore, the biofilm formation process occurs via two stages: one is reversible interaction between bacteria and the surface of the biomaterial and the other one is an irreversible process, i.e. proteins on the surface of bacteria and the surface of the biomaterial could bind together to produce specific and non-specific interactions.11 Biological contamination triggers foreign body reactions including nonspecific adsorption of proteins and adhesion of inflammatory cells. Protein adsorption on the surface of biomaterials plays a key role in the subsequent processes of cell behaviour and extracellular matrix (ECM) formation.12 The infection and inflammation are the main causes of complications and failure of biomaterial implantation, both of which are caused by the interaction between cells and biomaterials.9 Therefore, it is of vital importance to design medical biomaterials, which are both anti-fouling and anti-infective with highly biocompatible features. This not only significantly improves the clinical outcomes, but also reduces the financial burden of implant failure in patients.The interaction of biomaterials with tissues is determined by their surface properties. Therefore, surface modification is considered as an important means to improve the biological properties. The surface modification of implanted biomaterials is an effective way to improve biocompatibility and reduce the incidence of associated infections.13 It is common to modify the surface of biomaterials without changing the properties of the substrates on a micro or nano scale.14,15 Surface modification provides controllable and programmable surface properties for biomaterials at the same time. It provides effective physical or chemical properties to make implanted biomaterials “more compatible” inside the human body.16,17 Because of their advantages and disadvantages, these techniques can be used individually or in combination.18 In this review article, the effects of surface modification on the properties of biomaterials were discussed from the perspective of chemical treatment and its combination with physics.
There are several surface modification strategies widely used in the modification of biomaterials, such as surface coatings and synthetic films, covalent grafting, self-assembled monolayers (SAMs), and plasma treatment (Fig. 1). Recently, several researchers have found that titanium (Ti), zirconia (ZrO2), and polyetheretherketone (PEEK) have been used as orthopedic implants due to their excellent biocompatibility. The surface was modified with NaOH, which significantly improved the water contact angle, protein adhesion and bioactivity of these materials.19 Sharma et al. developed a stable, multifunctional, new-generation implantable urological biomaterial grafted with polyethyleneimine and poly(2-ethyl-2-oxazoline), which showed excellent antifouling performance and biocompatibility.20 As a coating agent, SAMs have antifouling properties on the surface of biomaterials and resist the adsorption of non-specific proteins.21 Among the surface modifications, hydrophobic surface is easy to cause protein adsorption, leading to biofilm formation. Researchers introduce hydrophilic materials to the surface, such as synthetic coatings or plasma treatments that produce hydrophilic functional groups that resist non-specific protein adsorption and bacterial adhesion.22 This review article mainly introduces several common surface modification strategies, focuses on the steps required by surface modification strategies and discusses the rationality of surface modification for biomaterials in medical applications. It includes biocompatibility, anti-infection and surface functionalization of biomaterials. In general, this kind of modification of biomaterials' surface (micro–nano scale) is beneficial for us to improve the possibility of implant surgery and reduce medical costs.
 | ||
| Fig. 1 Schematic illustration of different techniques of surface modification of micro- and nano-scale biomaterials and the impact thereof. | ||
Coating technology in surface modification processes
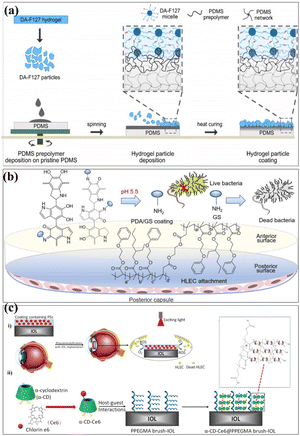
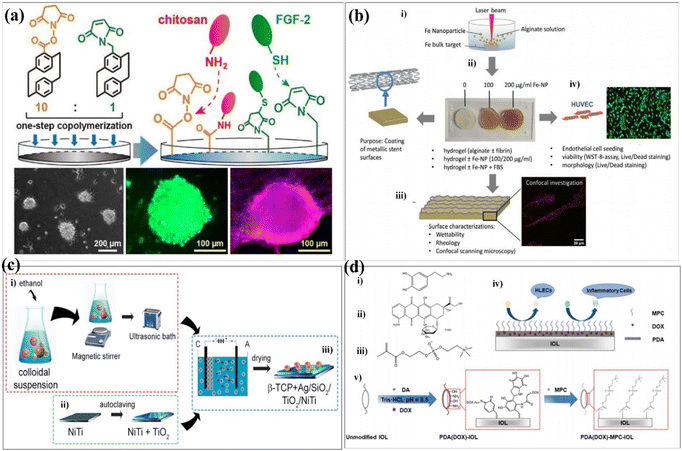
Surface modification techniques, in particular for biotechnological applications, were heavily pursued for creating extra physical or chemical properties as well as for designing and building potential interfaces.23,24 A summary of the common surface modification strategies is given in Table 1. Since the creation of 45S5 bioglass by hench, it was utilized in clinical applications as a biocompatible coating material for hard tissue replacement and regeneration.25 There have been several attempts to modify the makeup of bioactive glasses (BGs) in order to enhance their biological performance. Copper (Cu) exhibits antibacterial properties, and it has been reported that 45S5 bioactive glass can be fabricated by adding 5 wt% of CuO to the integrations.26 The investigation of the influence of Cu-containing bioactive glass on cellular behaviour has revealed that the presence of Cu induced an early differentiation of human mesenchymal stem cells (hMSCs) via the osteoblast phenotype, which promotes the expression of anti-inflammatory interleukin and reduces proinflammatory interleukin expressions. With the aim to produce coatings with antibacterial properties, the Cu-containing bioactive glass was used as the target material for the pulsed laser deposition (PLD) of bioactive thin films. Chen et al. determined a surface modification technique with excellent cohesive strength and durable coating stability, and in parallel, the modified layer exhibited a precise definition of chemical/biochemical conducts. They demonstrated a robust modification layer that was synthesized based on chemical vapor deposition (CVD) copolymerization.27 The copolymer modification layer's characteristics such as its adhesive strength and thermal stability demonstrated remarkable endurance (Fig. 2a). In 2018, in order to further improve the osteogenic performance of tantalum coatings, Ding et al. proposed a new method for developing micro/nano tantalum (MNT) with layered structures by combining plasma spraying and anodic oxidation technologies.28 During this process, it demonstrates that it can effectively enhance the proliferation and differentiation of human bone mesenchymal stem cells (hBMSCs) in vitro. Alena Richter et al. had proved that the Fe NP and fibrin can modify alginic acid salt and affect its wettability, surface roughness and elastic modulus. This, in turn, promotes the absorption of serum proteins, thereby promoting endothelial convergence by enhancing cell adhesion, proliferation, and vitality, thereby demonstrating a promising scaffold coating biomaterial.29 These synergetic effects can pave the way toward a novel strategy for the modification of various hydrogel-based biomaterials and biomaterial coatings (Fig. 2b). Dulski et al. prepared a colloidal suspension, composed of β-TCP and the Ag/SiO2 nanocomposites, which due to the electrophoretic deposition (EPD) led to the formation of structurally atypical calcium phosphosilicate coating.30 The purpose was to improve the functionality of NiTi alloys and extend their medical stability. The focus of this idea was to use biocompatible multifunctional coatings without affecting the functionality of the substrate (Fig. 2c). Taking advantage of self-polymerization of DA, a multifunctional coating of polydopamine(doxorubicin)-hydrophilic 2-methacryloyloxyethyl phosphorylcholine (PDA (DOX)-MPC) was constructed and modified on the intraocular lens (IOL) surface successfully. This coating was investigated by a series of experiments in vitro and in vivo. The measurement of water contact angle indicated that the IOL material has good hydrophilicity, which may lead to the biological adhesion between human lens epithelial cells and proteins. The drug-release behavior indicates that the PDA (DOX) mpc-modified IOL material, as an original drug delivery system, has long-term sustained drug release characteristics (Fig. 2d). Biocompatible elements (niobium (Nb) and silicon (Si) were introduced into a TiO2 matrix to change the surface chemical composition and tailor the thermophysical properties, which, in turn, leads to the generation of topographical features under specific thermal history of plasma spraying. The results indicate that the incorporation of Nb2O5 can enhance the biological performance of TiO2 coatings by changing the surface chemical composition and nanotopography, suggesting its potential use in the modification of biomedical TiO2 coatings in orthopedic applications.32 Stepulane et al. presented a polydimethylsiloxane (PDMS) surface modification strategy of antibacterial coating.33 Physical immobilization through the development of an interpenetrating polymer network allowed for the deposition of the microparticle coating made of cross-linked triblock copolymers (diacrylated Pluronic F127) on PDMS. A powerful antimicrobial peptide (AMP) was covalently immobilized on the surface of the produced coatings. With regard to Staphylococcus aureus and Staphylococcus epidermidis, it has a strong contact-killing antibacterial activity. Additionally, the coating's capacity to host polar, amphiphilic, and nonpolar medicines in a selective manner was evaluated, producing sustained release profiles (Fig. 3a). Xiang et al. synthesized a series of poly(2-phenoxyethyl methacrylate-co-2-phenoxyethyl acrylate-co-2-ethylhexyl methacrylate) (PPPE) acrylic intraocular lens (IOL) materials for “glistening-free” optimization.34 The 2-ethylhexyl methacrylate content in the chosen PPPE at 2% demonstrated good optical, foldable, and thermomechanical capabilities. Following gentamycin conjugation (PDA/GS), polydopamine was coated on the front side of PPPE. It reduced the thickness of the biofilm by 87% and prevented bacterial adherence by 74%. Bacterial growth was controlled in the inflammatory-mimicking conditions by acid-dependent GS release behavior. The PPPE surface remained hydrophobic as it approached the posterior capsule. The attachment of human lens epithelial cells, the adsorption of collagen IV and fibronectin, and the subsequent development of a “sealed sandwich structure” were all made possible by this (Fig. 3b).35 To efficiently prevent PCO, the hydrophobic surface of IOLs facilitates the inhibition of irregular migration and proliferation of human lens epithelial cells (HLECs). Therefore, an IOL material with a two-sided heterogeneous surface was needed. Photodynamic coating was introduced into the IOL surface modification. The photosensitizer chlorin e6-grafted a-cyclodextrin (a-CD-Ce6) was synthesized and self-assembled onto poly(ethylene glycol) methacrylate (PPEGMA) brushes. It established the IOL surface via supramolecular interactions between a-CD and polymer chains. The results indicated that this functional coating modification was effective in eliminating cells from the IOL surface when treated with light, while sustaining cytocompatibility in the absence of light (Fig. 3c).| Reference | Methods | Substrates | Cell experiments/microorganisms | Biocompatibility/bioactivity/antibacterial activity |
|---|---|---|---|---|
| 27 | N-hydroxysuccinimide (NHS) esterandmaleimide functionalities, chitosan, growth factor protein (FGFr2) molecules | Tissue culture polystyrene (TCPS) | Adipose-derived stem cells (ADSCs) | Cellular spheroids exhibit an enhanced growth activity in terms of size (210% larger) and number (180% less spheroids) ADSCs cell growth activity and proliferation were enhanced |
| 29 | Fe-NP, hydrogel | Metallic stent | Human umbilical cord vein endothelial cells (HUVEC) | Promoting endothelial cell adhesion, proliferation, and migration |
| 30 | Tricalcium phosphate (β-TCP), silver–silica (Ag/SiO2) nanocomposite | NiTi shape memory alloy | E. coli, Staphylococcus aureus (S. aureus), Saccharomyces cerevisiae (S. cerevisiae) | The coating with silver doped inhibits organization of biofilm created by Gram-negative bacteria, antimicrobial activity, E. coli viability decreased, non-cytotoxic |
| 31 | Doxorubicin (DOX), polydopamine (PDA), 2-methacryloyloxyethyl phosphorylcholine (MPC) | Intraocular lens (IOL) | Human lens epithelial cell line (HLE B3, CRL-11421) | The cell viability on the drug-loaded coating, surfaces decreased to around 40 or 20%, favorable biocompatibility, the excellent hydrophilicity |
| 33 | Antimicrobial peptides (AMP), diacrylate pluronic F127 (DAF127) hydrogel | Polydimethylsiloxane (PDMS) | S. Epidermidis, S. aureus | Significant reduction in the surface-adhered bacteria counts, up to 99.3% reduction of S. epidermidis, antibacterial activity of 99.1% against S. aureus |
| 34 | Polydopamine, (PDA), gentamycin sulfate (GS) | Poly(2-phenoxyethyl methacrylate-co-2-phenoxyethyl acrylate-co-2-ethylhexyl methacrylate) (PPPE) | Human lens epithelial cells (HLECs) S. aureus, P. aeruginosa | Antibacterial adhesion rates of 63.8%, 73.7% against S. aureus and P. aeruginosa respectively, biofilm formation was effectively inhibited, cell viabilities of HLECs were above 90% |
| 35 | α-Cyclodextrin (α-CD), chlorin e6, poly(poly(ethylene glycol)methacrylate) (PPEGMA) | IOL | HLE B3, CRL-11421 | Excellent biocompatibility, increased cell adhesion and proliferation |
The use of grafting, assembly and other methods to improve hydrophilicity requires special equipment or complex preparation conditions, which has limitations in practical applications. However, the preparation process of surface coating modification technology is simple, and the coating thickness and composition ratio are controllable. The surface properties of biomaterials functionalized with surface-modified layers of surface coatings have been improved, such as good blood compatibility, enhanced cell behaviour, expansibility, good hydrophilicity and extended life of biomaterials. However, it is essential to improve the stability of the surface coating, which is prone to fall off into fragments and produce toxicity to surrounding cells and tissues.
Covalent grafting technology in surface modification processes
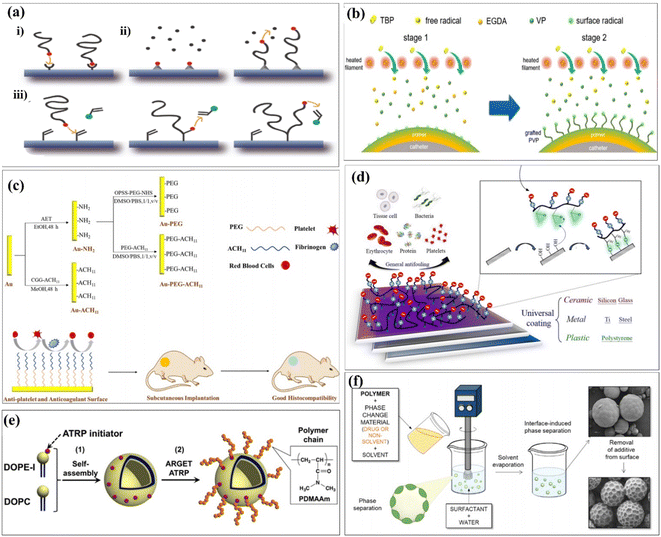
Chemical interactions are typical examples of establishing connections between different functional groups. It could be introduced as an important case of covalent grafting technology. Generally speaking, chemical grafting is more advantageous than physical methods because the covalent attachment of grafted chains onto the substrate surface can avoid the desorption and ensure long-term stability. Further, due to the strong, selective, specific, and convenient reactivity of polymer brushes, polymer brushes that can be chemically grafted to the surface have high adhesive strength.36 Chemical surface modification methods usually based on the grafting can be categorized into “grafting-to,” “grafting-from,” and “grafting-through” approaches37 (Fig. 4a). It was commonly used for ensuring covalent grafting of polymers.38,39 In the grafting-to approach, end-group-transformed polymer chains reacted with the functional groups of substrates, which finally form grafted polymer chains. In the grafting-from approaches, polymer chains propagated from surface-attached initiators and polymer chains completely propagated from surface-attached double bonds.40 The characteristic of the graft copolymer is controlled by the nature of grafted chains, including number, length and molecular structure of grafted chains. So far, extensive research has been conducted to explore the influences of these parameters on the grafting percentage and the characteristics of grafted materials.41 Covalent grafting offers the strongest link work between biomaterials and its coating candidates, and it produces a more durable interface as much as possible. A summary of the common surface modification strategies is given in Table 2.| Reference | Surface functional groups | Substrates | Cell experiments | Biocompatibility/bioactivity |
|---|---|---|---|---|
| 45 | Grafted polyvinylpyrrolidone (PVP) | Polyvinyl chloride (PVC) medical catheters | E. coli | Fouling resistance of 99.98% against E. coli |
| Good compatibility | ||||
| High surface hydrophilicity | ||||
| 58 | Poly(ethylene glycol) (PEG), antithrombotic peptide ACH11 | Au substrates | Platelet | Excellent hydrophilic property |
| Strong antiprotein and antiplatelet attachment ability | ||||
| Excellent hemocompatibility | ||||
| Inhibiting granulation formation and fibrous capsule | ||||
| 59 | Ploy(glycidyl methacrylate-co-sulfobetaine methacrylate) (poly(GMA-co-SBMA)) | Silicon, glass, titanium, stainless steel, and polymers | Human blood cells, fibroblast cells, E. coli | Reduced by 89.3%, 73.7%, 76.5%, 77.9%, and 49.6%, for platelet adhesion and by 96.1%, 96.2%, 83.7%, 88.1%, and 87% for erythrocyte adhesion with the surfaces respectively |
| A high reduction in human fibroblast (HT1080) adhesion of 96.3%, 96.5%, 91.3%, 86.3%, and 57.8% respectively | ||||
| The high reduction in bacterial attachment of 88.5%, 79.7%, 86.9%, 90.8%, and 51.4% respectively | ||||
| 60 | N,N-Dimethylacrylamide (DMAAm) | Liposomes | N/A | Increased in surface softness |
| 63 | 3-Aminopropylmethacrylamide (APMA), poly(ethylene glycol) methacrylate (PEGMA) | Poly(D,L-lactic acid) (PLA) | Human immortalized mesenchymal stem cell (hiMSCs), cardiomyocytes derived from human-induced pluripotent stem cells (hiPSC-CMs) | Non-cytotoxic to hiMSCs |
| The size and number of hiMSC | ||||
| Aggregates formed are primarily influenced by the surface chemistry of the incorporated microparticles |
Naim et al. first reported that the chemical medication of chitin by grafting with polystyrene can use ammonium persulfate (APS) as an initiator.42 Yu et al. prepared amphiphilic linoleic acid (LA) and poly(b-malic acid) (PMLA) double-grafted chitosans with various grafting degrees of linoleic acid (LA), which was modified with folate (FA) and poly(β-malic acid) PMLA. It was double-grafted by the chitosan to probe the optimized hydrophobicity and hydrophilicity in a co-delivery system. This co-delivery system showed significant enhancement in in vitro and in vivo antitumor efficiency while compared with single administration.43 Chen et al. used functionalized chitosan/hydroxyapatite (CS/HA) biomimetic composite scaffolds for the controlled delivery of BMP2-derived peptides (P24) through chemically grafting chitosan-4-thiobutylamidne (CS-TBA). Second, the resulting CS P24 was then combined with HA to prepare CS-P24/HA scaffolds. The effect of the CS-P24/HA scaffold on bone regeneration was evaluated, along with the underlying biological mechanisms. Finally, CS-P24/HA was superior to CS/HA in promotion of bone regeneration in vivo. This study highlights the enormous potential of using the CS-P24/HA scaffold for bone tissue engineering works.44 Since an ultralow fouling surface is essential for good biocompatibility, Sun et al. attempted to further enhance the surface functionality using a novel solvent-free graft-from method. The grafting was implemented by first depositing a cross-linked polyvinylpyrrolidone (PVP) prime layer via initiated chemical vapor deposition (iCVD), followed by in situ polymerization on the top position without disrupting the vacuum. The propagating chains on the prime coating surface served as the initiating sites for the subsequent grafting of PVP. The resulting coating thus consisted of a cross-linked prime layer and a top-grafted PVP layer.45 Liposomes are self-assembled vesicles of amphiphilic lipid molecules, and they are treated as cells, model of cells, or tools for drug delivery systems. Modifications of polymer chains on the surfaces of liposomes are typically installed using a “grafting to” approach,47 as shown in Fig. 4b. Abdel-Rahman et al. prepared a 3D hybrid scaffold based on a collagen-grafted-chitosan–glucan fiber (CO-g-CGF–HBS) by a freeze-drying technique. The swelling percentage, hydrolytic stability, and modulus of elasticity of HBS were enhanced after the chemical modification of CO with CGF. The chemical modification of CO with different ratios of CGF significantly improved the physicochemical and antibacterial properties of HBS.46
The initial attachment of bacteria to the surface of implanted medical devices is the first stage in biofilm formation. Thus, the inhibition of bacterial colonization using antibacterial coatings, particularly during the most susceptible first 6 h period after implantation, is a fundamental strategy to prevent biofilm formation.48–51 To achieve ultralow fouling surfaces, hydrophilic polymer coatings such as polyethylene glycol (PEG)52–55 and zwitterionic polymers56,57 have been extensively explored. The grafted chains generate a bound hydration layer that prevents the contact between foulants and the surface and induces a “cushion effect” repelling the approaching foulants.55 Zhao et al. developed a PEG- and ACH11-functionalized surface.58 Hydrophilic poly(ethylene glycol) (PEG) and antithrombotic peptide ACH11 were co-immobilized onto Au to develop a multifunctional surface with remarkable hemocompatibility, protein adsorption, antiplatelet aggregation, anticoagulant properties, and good histocompatibility (Fig. 4c). Chou et al. reported that a facile, effective and economic grafting-to method, which forms a stable chemisorption coating on versatile surfaces, was developed by the original design in the zwitterionic copolymer formulation of poly(glycidyl methacrylate-co-sulfobetaine methacrylate) (poly(GMA-co-SBMA)).59 The epoxide functional groups of GMA segments could provide strong reactivity with nucleophiles via the ring-opening reaction. Thus, poly(GMA-co-SBMA) is capable of forming covalent bonding with versatile surfaces including polymer, ceramic, and metal substrates with hydroxyl groups after surface pre-treatment using UV and ozone, as shown in Fig. 4d. Masuda et al. hypothesized that ARGET ATRP could be used to modify a liposome surface by the grafting-from method.60 The molecular weight of the grafted polymer chain was systematically controlled by changing the monomer concentrations in the “grafting from” polymerization, as shown in Fig. 4e. Zhou et al. reported that ethyl ketones (AAEK) were covalently grafted onto cellulose films (CF) via a copper-catalyzed azide–alkyne 1,3-dipolar cycloaddition click reaction.61 The purpose of this study is to explore the effectiveness of surface-decorated aryl(β-amino) AAEK, a promised enzyme A (SrtA) inhibitor of Staphylococcus aureus, to improve the anti-adhesion ability of biomaterials. Du et al. synthesized and grafted AACA onto the surface of PIB by plasma pre-treatment and UV-induced graft polymerization.62 The hydrophilicity and hemocompatibility of PIB were largely improved by surface modification of aminocaproic acid (AACA), which were confirmed by water contact angle and platelet adhesion, respectively. Alvarez-Paino et al. reported a new approach for surface functionalization of poly(lactic acid) (PLA) microparticles that allows the decoration of the outer shell of the polyesters with additional functionalized poly(poly(ethylene glycol) methacrylate) and poly[N-(3-aminopropyl)methacrylamide] brushes, chosen for their potential abilities to mediate cell adhesion,63 as shown in Fig. 4f.
In summary, the surface grafting modification technology significantly enhances the surface properties of biomaterials such as cell adhesion, hydrophilicity, biocompatibility, and stain resistance, while maintaining the same characteristics of the substrate material. Surface coating has the disadvantage of instability and easy shedding, while surface grafting can enhance its stability in the physiological environment, and the grafted chain can prevent dirt from contacting the surface of biomaterials. Obviously, the traditional organic solvent-based polymer grafting method is lengthy and complicated, which is easy to contaminate the material surface. The solvent-free grafting method can obtain better surface properties to a certain extent. Surface grafting requires less energy and is more environmentally friendly than plasma surface modification techniques. The functionalization of spherical particles cannot be controlled uniformly by plasma treatment, and the morphology of the underlying material can also be altered. However, the limitation of surface grafting may lead to inefficient antifouling properties, and the precise control of the synthetic process on the surface of biomaterials remains flawed and needs to be improved.
Self-assembled monolayers as a surface modification strategy
Self-assembled monolayers (SAMs) are nano-thick ordered molecular coatings formed by molecular components adsorbed from solution or gas phase to solid surface and finally arranged in a regular manner.64 The molecules from SAMs are well bound to the surface of the substrate material and have specific functional groups. A summary of the common surface modification strategies is given in Table 3.| Reference | Surface functional groups | Substrates | Cell experiments | Biocompatibility/bioactivity |
|---|---|---|---|---|
| 68 | Amine (NH2), octyl (CH3) | Ti6Al4V | Mouse fibroblast L929 cells, Staphylococcus aureus, Escherichia coli | Hydrophobic octyl surfaces (1035 ± 38 ng cm−2) showed the maximum adsorption of bovine serum albumin (BSA) |
| Hydrophilic COOH surfaces (647 ± 38 ng cm−2) showed the maximum adsorption of fibronectin (FN) | ||||
| Hybrid surfaces showed the maximum cell adhesion (%) and proliferation, larger nuclei area and the least cell circularity | ||||
| Cells exhibited higher proliferation rate on all the modified surfaces as compared to unmodified Ti6Al4V, suggesting good cytocompatibility | ||||
| 69 | Silanization | Silicon wafers | Serum, saliva | The modified materials that span a broad range of physicochemical properties, from hydrophilic to hydrophobic surfaces (water contact angles from 15° to 115°), negative to positive surface charge (zeta potentials from −120 to +40 mV at physiologic pH) |
| The chemical surface functionalities exerted a substantial effect on the total amounts of proteins adsorbed | ||||
| 71 | Different chemical groups (–OH, –OEG, –COOH, –NH2, and –PO3H2) | Gold slides | Recombinant mouse osteopontin (OPN), mouse bonemarrow mesenchymal stem cells (mBMSCs) | The amount of adsorbed OPN was highest on SAMs-NH2 (89.01 ± 13.62 ng cm−2) and lowest on SAMs-OEG (3.39 ± 0.63 ng cm−2) |
| Cells on SAMs-COOH, SAMs-NH2, and SAMs-PO3H2 with pre-adsorbed OPN showed larger spreading, better viabilities, and higher expression levels of αv/β3 genes | ||||
| OPN on SAMs-COOH, SAMs-NH2, and SAMs-PO3H2 exhibited higher bioactivity | ||||
| 77 | Thioctic acid-functionalized dendritic polyglycerol sulfate (dPGS) | Gold-coated sensors | Blood proteins albumin (Alb) and fibrinogen (Fib) | Compared to non-sulfated dPG, dPGS showed enhanced protein adsorption driven by ionic interactions and enhanced cellular uptake via the formed protein corona |
| The formation of densely packed protein layers in case of Fib and a more loosely packed protein layer in case of Alb | ||||
| 80 | Self-assembled monolayer of phosphonates (SAMPs) | Silicon dioxide (SiO2) | Chondrocytes | Chondrocytes attach to the modified surface, without substantial changes in gene expression SAMPs modification to SiO2 increased chondrocyte adhesion by 3× after 4 h and 4.5× after 24 h |
| 81 | [(3-Aminopropyl)triethoxysilae (APTES), and octadecyltrimethoxysilane (OTS)], amino acid (histidine and leucine)-conjugated | Poly(dimethylsiloxane) (PDMS) | Induced pluripotent stem cells (iPSCs) | Modified surfaces were found to be hydrophilic |
| PDMS surface chemical properties were enhanced for the differentiation of iPSCs into cardiomyocytes | ||||
| All SAM-modified surfaces increased the number of viable iPSCs, when compared to native PDMS | ||||
| 84 | ω-(Ethylene glycol)2–4- and ω-(GRGDS)-, α-benzamidinobolaamphiphiles | Gold | MC3T3-E1 cells | Modified surfaces can be used to reverse cell adhesion in a noninvasive manner |
| A versatile tool to study and control cell adhesion and differentiation | ||||
| 85 | Antimicrobial peptides (AMPs), elastin-like recombinamers (ELRs) | Gold sputtered cover glasses | S. aureus ATCC 25923, S. epidermidis ATCC 35984 | The multifunctional SAMs exhibit protein anti-fouling activity |
| Strong anti-biofilm activity and cytocompatibility of these coatings was demonstrated | ||||
| 86 | Chitosan layer | Polyetheretherketone (PEEK) | MC3T3-E1, Gram-negative P. gingivalis, Gram-positive S. mutans | The inclusion of chitosan on the surface of PEEK-CS increased fibronectin adherence, enhancing the adhesion, proliferation, and differentiation of MC3T3-E1 subclone 14 cells substantially |
| Modified PEEK surfaces demonstrated decreased adhesion force to P. gingivalis, and less initial bacterial adhesion to P. gingivalis and S. mutans |
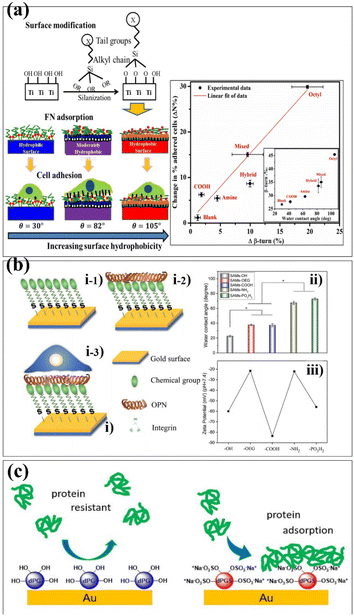
The SAM surface modification technology has been widely used in protein adhesion, cell adhesion, antibacterial and antifouling. The properties of biomaterials are mainly determined by the proteins adsorbed on their surfaces.65,66 These proteins play a role in regulating cell adhesion, migration, proliferation and differentiation.67 Therefore, it is essential to regulate the adhesion of proteins on the surface of biomaterials. Hasan et al. investigated the effect of SAM-functionalized biomaterials on protein adsorption.68 The ability of Ti6Al4V to adsorb proteins and adhere to cells was tested by forming a hydrophilic, hydrophobic, and moderately hydrophobic monolayer on its surface. Similar as surface coating works, researchers controlled the surface properties of Ti6Al4V using a technique called silanization, which forms a covalent bond between the surface molecules linkages. It makes the biomaterials more stable and robust in performance (Fig. 5a). Lehnfeld et al. developed an in vitro model system based on silica surface in order to investigate the effect of adsorbed protein layers on the chemical modification of biomaterials.69 It was modified by seven silanized SAMs. The results show that the chemical surface function of biomaterials greatly affects the total amounts of proteins adsorbed. Osteopontin (OPN) can mediate the cell behaviour of materials.70 Chen et al. reported SAMs with different surface chemistries to investigate the behaviour of OPN on these SAMs.71 The results indicated that the adsorption capacity of SAMs-NH2 to OPN was stronger and the protein content was the highest. Meanwhile, in vitro cell experiments indicated that SAMs-COOH, SAMs-NH2 and SAMs-PO3H2 adsorbed with OPN can promote cell behaviour. It also proved that the SAM adsorbed with OPN has higher bioactivity, providing a new idea for the surface modification of biomaterials (Fig. 5b). Biomaterials often come into contact with blood when implanted in the body and it is essential to avoid the adsorption of non-specific proteins on the surface.72–74 Previous studies have shown that dendritic polyglycerin (dPG) protects biomaterial surfaces from non-specific protein adsorption and effectively reduces the adhesion of undiluted serum proteins.75 In addition, a polyvalent form of dendritic polyglycerol sulfates (dPGS), which can effectively inhibit and bind to inflammation in vivo, was introduced.76 Stöbener et al. obtained a stable dPGS self-assembled monolayer on a gold substrate surface, which was acid-functionalized thioctic.77 Compared with unsulfated dPGS, there are many negatively charged sulfate groups on the surface of dPGS, which enhance the adsorption of proteins under ionic interactions and lead to the partial rearrangement of the protein structures (Fig. 5c).
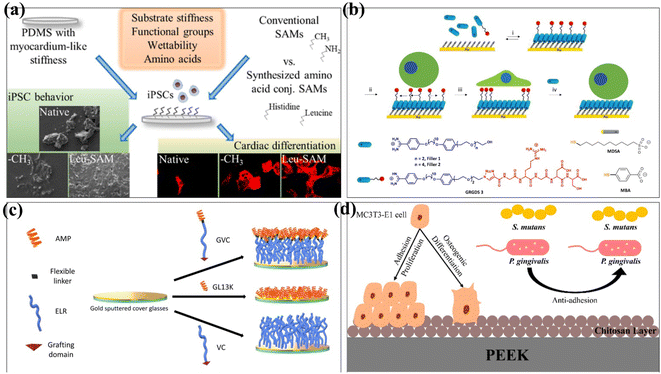
Cells attached to the surface of biomaterials can perceive and respond to chemical and physical surface features.78,79 Therefore, the control of cell adhesion behaviour should be considered in the design of biomaterials to influence cell migration, proliferation and differentiation. Donnelly et al. investigated the effect of the self-assembled monolayer of phosphonates (SAMPs) on chondrocyte adhesion on silica (SiO2) and polyvinyl alcohol (PVA) materials.80 The experimental results showed that chondrocytes attached to SAMP–SiO2 increased 3 times after 4 hours and 4.5 times after 24 hours. At the same time, the modified PVA material increased the number of chondrocytes by at least 31 times in the same time. In conclusion, the SAMP surface modification technology can improve chondrocyte adhesion and diffusion on the material without altering gene expression. Functional groups on the SAMs have been found to enhance cell interactions with biomaterials and induce stem cell differentiation.81 For example, amino (–NH3)-conjugated SAMs promote osteogenic differentiation,82 while methyl (–CH3) supports chondrogenesis in stem cells.83 Öztürk-Öncel et al. modified different functional groups on the surface of poly(dimethylsiloxane) (PDMS) substrate using amino acid-conjugated SAMs.81 Amino acid-conjugated SAM-modified materials improved induced pluripotent stem cell (iPSCs) behaviour, and these functional groups with different surface functions had synergistic effects on iPSCs attachment, viability, and cardiomyocyte differentiation (Fig. 6a). Yeung et al. reported a reversible self-assembled monolayer (rSAMs) that achieves the dynamic control of surface composition and adjustable lateral fluidity by adding inert filler amphiphiles to the tripeptide RGD-functionalized rSAMs, so as to achieve the purpose of regulating cell adhesion behaviour84 (Fig. 6b).
SAM-modified biomaterials have also been widely used in antibacterial and antifouling fields. In 2019, Acosta et al. used antimicrobial peptides (AMPs) and elastin-like recombinamers (ELRs) to design self-assembled monolayers.85 The antibacterial properties of GL13K peptide and the low pollution activity of ELRs were combined and fixed on the surface of gold substrates by covalent grafting. The recombinant AMP/peptide SAM was evaluated to provide anti-biofilm properties against Staphylococcus epidermidis and Staphylococcus aureus (Fig. 6c). Qiu et al. used a self-assembly method to covalently graft the chitosan layer onto the surface of PEEK by hydroxylation, introducing amino groups and glutaraldehyde crosslinking.86 The method changes the surface morphology as PEEK increased the surface roughness and decreased the contact angle. The rough PEEK-CS surfaces are more likely to osteogenic than smooth PEEK-CS surfaces, which is attributed to the higher osteogenic activity of rough implant surfaces and in favour of cell adhesion, cell proliferation, and calcium nodule deposition.87 The adhesion of bacteria on PEEK-CS to Porphyromonas gingivalis and Streptococcus mutans was decreased. Chitosan modification significantly improved the osteogenic ability and antibacterial adhesion of polyether ether ketone in vitro (Fig. 6d).
Different from surface coating modification, self-assembled monolayers can form coatings on various metal surfaces with high flexibility. At the same time, some SAMs also exhibit good thermal and hydrolytic stability. SAMs are controllable for cell adhesion and do not change the overall mechanical properties or surface roughness of the biomaterials, resulting in better biocompatibility of the materials. The different functional groups of the self-assembled monolayer make the interaction between cells and biomaterials different. The fabrication of self-assembled monolayers is simple and does not require expensive and sophisticated equipment. Although self-assembled monolayer-modified biomaterials have shown good antibacterial and osteogenic properties in vitro, in vivo antibacterial experiments are still insufficient. The long-term stability of functionalized biomaterials needs to be verified, especially for orthopedic and dental applications.
Plasma strategies in surface modification processes
Plasma is a substance that exists in the fourth state, which can be regarded as a collection of electrons, single- or multiple charged positive and negative ions accompanied by neutral atoms, excited particles, electromagnetic radiation, molecules, and molecular fragments which are in charge balance.88,89 In most experimental situations, the plasma is generated by discharge.88 It can generate the energy and produce plasma by applying electric and magnetic fields or even nuclear reactions.90 This method has the advantage of only changing the surface properties of the material, such as surface chemistry, roughness, surface charge, and also enhancing the biocompatibility of the material, while the bulk properties of the material remain unchanged.90,91 Gas plasmas can be generated under both atmospheric pressure and low-pressure conditions.92 A large number of gases can be used in plasma, including argon, nitrogen, and argon–oxygen mixture, which are used to generate functional groups or free radicals on the surface of materials. These free radicals can improve the adhesion of the surface of biomaterials or graft other polymers on it to induce surface hydrophilicity.93,94 Conversely, conventional gas plasma treatments and plasma immersion ion implantation can be conformal modified around the material, allowing treatment of 3D structures with high surface area and volume ratios with a wide range of internal porosity.95 According to the interaction between the plasma and the surface of biomaterials, it can be divided into three categories: plasma polymerization, plasma treatment, and plasma graft etching.94 Plasma surface modification techniques can be used directly or in combination with other surface modification techniques to achieve the purpose of enhancing the surface properties of biomaterials.93 This section reviews the gas plasma strategy and plasma immersion ion implantation strategy in the literature in recent years, discussing and summarizing several studies on plasma treatment of different biomaterials to illustrate the importance of plasma technology in the surface modification of biomaterials. A summary of the common surface modification strategies is given in Table 4.| Reference | Methods | Substrate | Cell experiments | Biocompatibility/bioactivity |
|---|---|---|---|---|
| 96 | Oxygen plasma | Quartz | Bone marrow stromal cells (BMSCs) MC3T3-E1 cells | Cell density increased from 34 ± 1 cells per mm2 to 78 ± 3 cells per mm2, obvious graded distribution, oriented migration |
| 99 | Argon plasma | Poly(lactic-co-glycolic acid) (PLGA) | N/A | Water contact angle dropped from 70° to 42° |
| 100 | Argon and argon–oxygen plasma | Nanofibers | Fibroblast cells | Further increase in the number of fibroblast cells (145 ± 9) |
| 101 | CH4, C2F6, oxygen | General-purpose polystyrene (GPPS) | S. aureus, E. coli | Antibacterial activity R = 4.1146, has antibacterial effect |
| 102 | Diethyl phosphite (DEP) | Titanium (Ti) | C. Albicans and S. aureus L929 fibroblast cells | 72 h of antifungal assay, 13 cfu mL−1 of S. aureus and no live C. albicans was observed, increased cell viability of L929 fibroblast cells (93%) |
| 105 | Nitrogen ion (PIII) | Silk films | Bovine arterial endothelial cells (BAECs) | Cell adhesion on PIII treated silk films was 2.5 s fold higher than on untreated silk films, PIII-treated silk supported significantly higher cell proliferation |
| 106 | C, nitrogen (PIII) | Zirconia | BMSCs | The number of cells on zirconia disks was significantly increased, the number of cells attached on surface was highest among the four groups (P < 0.05), C and N2-PIII promoted cell proliferation activity |
| 107 | Nitrogen ion (PIII) | Silk films | Plasma proteins | Recombinantly expressed domain V of the human the rate and the amount of basement membrane proteoglycan perlecan (rDV) binding were greater on PIII silk, rDV bioactivity was retained following covalent immobilization on silk, rDV-functionalized PIII silk inhibits thrombogenic activity in whole blood systems |
Xue et al. grafted a layer of α-bromoisobutyryl bromide (initiator) on the surface of quartz substrates treated with oxygen plasma.96 Subsequently, to protect the initiator from oxygen plasma corrosion in inclined reactive ion etching (RIE), a polymer film was coated as a protective layer.97,98 The geometric gradient was introduced into the prepared polystyrene (PS) microspheres array by inclining RIE. Since the polymer film is etched away, the gaps between the initiators are filled with silica hydroxyl groups. Gradient polyhydroxyethyl methacrylate (PHEMA)/polyethylene glycol nanopatterned arrays were fabricated from gradient initiator/PEG nanopatterned arrays by surface-initiated atom-transfer radical polymerization (SI-ATAP) on quartz substrates. Finally, the protein was covalently immobilized on PHEMA nanodots, resulting in graded and ordered gradient protein/PEG nanopattern arrays (Fig. 7a). Gradient biomaterials based on plasma preparation can affect three basic behaviours of cells: adhesive density, polarization and migration. Phat et al. performed the surface modification of polylactic acid–glycolic acid (PLGA), collagen, and PLGA-collagen using a PDC-001 Expanded Plasma Cleaner.99 The contact angles of water and diiodomethane on PLGA films treated by argon plasma were significantly decreased (p < 0.05), but had little effects on the thermal degradation of collagen and PLGA-collagen at high temperatures. The results of the bicinchoninic acid assay showed that the argon-plasma treated scaffolds released less collagen to PBS++ (p < 0.05). Mozaffari et al. used a PF-200 plasma DBD device to plasmonic electrospun nanofiber scaffolds with argon and an argon–oxygen mixture.100 The surface of the untreated nanofibers was smooth, while the surface roughness of the nanofibers treated with argon and argon oxygen plasma was greatly increased, which was related to the bombardment of high-energy particles when the samples were treated with plasma. At the same time, the argon–oxygen plasma-treated nanofibers exhibited a high degree of hydrophilicity, and the water droplets were completely absorbed into the scaffold, which was related to the introduction of polar groups. In contrast to air plasma modification, Onodera et al. formed fluorine-containing diamond-like carbon (DLC) films on a General-Purpose Polystyrene (GPPS) substrate using CH4 and C2F6 as gas sources.101 Subsequently, the film was subjected to atmospheric plasma treatment with oxygen to improve the biocompatibility of the film. The film has antibacterial properties, and fluorine has an inhibitory effect on glucose metabolism for bacterial energy production. Physical changes have also occurred on the film surface including increased hydrophilicity and decreased surface wettability to achieve the purpose of antibacterial activity. Kaleli-Can et al. used the plasma polymerization technique for surface modification with titanium using diethyl phosphite (DEP) as the gas source in an RF/low-pressure plasma device under 0.15 mbar.102 The water contact angle of the DSP-coated Ti is significantly smaller, and the surface energy is about two times higher than that of the unmodified Ti surface. The surface roughness was increased, and it had an inhibitory effect on C. albicans, which showed good antibacterial activity. Amphotropic plasmonic polymer prepared from DEP can effectively prevent biofilm formation on the titanium surface.
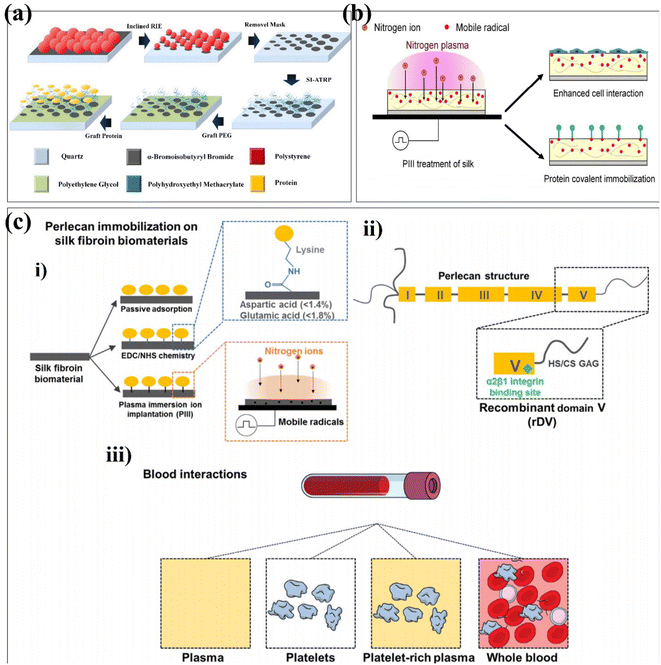
Ion implantation is the process by which positively charged ions in plasma are accelerated into the surface of a material.103 Generally, the energy of the accelerated ion is much higher than the bond energy of the surface layer of the modified material, resulting in bond breaking.104 Kondyurin et al. reported the silk fibroin biomaterials using plasma immersion ion implantation (Fig. 7b).105 PIII transforms the surface layer of the biomaterial into a compact carbon-rich structure with no significant effect on its roughness. Due to the presence of free radicals during PIII treatment, the surface exposed to the atmosphere undergoes oxidation. PIII enhances silk interactions with proteins and cells. This provides a research direction for the application of silk fibroin biomaterials in tissue engineering and regenerative medicine in the future. It has been found that the biological activity can be improved by building functional groups. Guo et al. randomly divided the zirconia into four groups and used the corresponding parameters to modify the zirconia surface using plasma immersion ion implantation.106 The surface roughness of the material was unchanged, the nitrogen-containing functional groups were introduced, and the stability of zirconia was not affected. In vitro data showed that PIII-treated zirconia promoted the adhesion, proliferation, and osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BMSCs). Lau et al. used the PIII treatment technique to covalently immobilize recombinantly expressed domain V (rDV) on silk biomaterials, independent of immobilized proteins modified by specific amino acids in the silk protein chain.107 The silk biomaterials biofunctionalized with rDV using PIII treatment show blood compatibility in terms of platelets (Fig. 7c). Different from plasma immersion ion implantation, researchers have also proposed ion implantation surface modification to improve properties such as biocompatibility, corrosion resistance and antibacterial properties.
In short, plasma-based surface modification techniques have great potential for biomaterial implants. Surface coating, self-assembling monolayers and ion implantation are limited to certain biological material chemistry or surface pretreatment. These chemical processes are time-consuming and complex, and require the addition of organic reagents, which may damage the implant. However, plasma surface modification technology has the advantages of short reaction time, environmental safety, and the ability to change the surface properties of the material without affecting the overall properties of the material. The interaction between plasma and some biomaterials can enhance their biocompatibility with biological cells, which can be used in tissue engineering. In addition, the films prepared by plasma surface modification have the characteristics of thermal stability, chemical inertness, and enhanced toughness. Plasma surface modification can improve the surface wettability and cell attachment of biomaterials, and also improve their osteogenic and antibacterial properties. However, during the plasma process, the choice of the precursor can affect the properties of the biomaterial, leading to results that differ from the desirable properties. If the antibacterial layer on the surface of biomaterials lacks the killing and release mechanism, its antibacterial function will be weakened or even disappeared.
Other surface modification strategies
Appeared in healing of bone engineering, it lacks osteogenic activity of the implanted fixation material and the infection of bacteria.108,109 It is an important topic to pursue osteogenic activity and antibacterial properties. The previous reports have found that active ionic components play an important role in bone formation, development and repair, and that some metal ions can act as antibacterial agents.110 Yang et al. prepared a protective coating containing Zn and Sr ions by a one-pot hydrothermal method.111 After surface modification, a cluster of crystalline structures was formed on the surface of magnesium alloys. At the same time, the corrosion resistance of the magnesium alloy was significantly improved, and the cells grew well on the surface. The combined use of Zn and Sr ions promoted the osteogenic differentiation of the cells with antibacterial effects. Kazimierczak et al. have synthesized magnesium (HA-Mg) and (HA-Zn) ion-substituted nano-hydroxyapatite (HA) synthesized to prepare a more biocompatible chitosan-agarose-hydroxyapatite (HA) scaffold (chit/aga/HA).112 These two above-mentioned metal ions have been found to have synergistic effects on the biological response in cell tests. The addition of Mg2+ to this biomaterial structure can promote osteoblast spreading, promote cell proliferation on the scaffold surface, and promote osteocalcin production by mesenchymal stem cells. Moreover, the addition of Zn2+ can promote the production of type I collagen by MSCs and extracellular matrix calcification. It has been found that the nanostructure on the surface of biomaterials has an important effect on cell activity and tissue formation.113 Wen et al. added polydo and the physical properties were not changed. Shuai et al. reduced silver nanoparticles on carbon nanotubes (CNTs) in situ.114 The CNT@Ag powder was then prepared by laser powder bed fusion (LPBF) to prepare the Zn-CNTs@Ag scaffold implant, which had an orthogonal porous structure that facilitates cell migration, tissue formation, and nutrient transportation. The scaffold exhibited favourable antibacterial activity and biocompatibility.Conclusions and future prospects
As implanted in the human body, biomaterials have been widely used in the biological applications and clinical treatment. Especially in microbial infection, it is not easy to evitable in surgical implantation and resulted inflammation, biofilm harmful. Therefore, the surface modification could change the physical, chemical and biological properties of implant materials in order to improve the biocompatibility, antibacterial and antifouling properties. In this review, the main surface modification strategies and methods are commonly illustrated. Among this, it is an effective way to enhance the antibacterial activity and reduce the adhesion of non-specific proteins and bacteria via surface modification of medical biomaterials. Towards hydrophilic contact angle, surface roughness, and surface functional groups, they ensured target material acceptable and biocompatible. Although the existing surface modification results are satisfactory, there are still many deficiencies that need further investigations. First of all, the coating on the surface of the base material for a long time will cause different degrees of shedding, causing further infection. Second, surface modification increases the economic cost of biomaterials, and the modification process may pollute the environment. Lastly, higher complexity of the modification process also needs to be solved. In different strategies, there are differences in the interactions of human microenvironments. In fact, in vivo studies of surface-modified biomaterials are still lacking to further demonstrate their antibacterial properties and stability. The testing environment for the antimicrobial activity of a biological material should be relevant to the strains present in its application scenario. In order to guarantee the long-term stability of implants applied in orthopedics and dentistry under functional conditions, it is essential to test the mechanical properties of materials. In addition, the antibacterial properties, biocompatibility and other mechanisms of surface coatings or films in physiological environments need to be investigated. Surface-modified biomaterials have a good prospect for clinical applications, but there are still difficulties in the preparation and processing of materials in large-scale production and development, and the realization of clinical translation and large-scale production needs to be optimized in future research. Therefore, future recommendations in this field include reducing the additional cost of surface modification strategies, improving the stability of modified biomaterials, using facile approaches for further modifying biomaterials reasonably and achieving clinical translation.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Chongqing Municipal Natural Science Foundation: CSTB2022NSCQ-MSX0113; Scientific Foundation Project of Chongqing Education Commission: KJQN201900436; National Natural Science Foundation of China: 81127901, 12074051.References
- S. H. Rao, B. Harini, R. P. K. Shadamarshan, K. Balagangadharan and N. Selvamurugan, Int. J. Biol. Macromol., 2018, 110, 88–96 CrossRef CAS PubMed.
- C. Kang and F. Fang, Adv. Manuf., 2018, 6, 137–154 CrossRef CAS.
- M. Xiao, Y. M. Chen, M. N. Biao, X. D. Zhang and B. C. Yang, Mater. Sci. Eng., C, 2017, 70, 1057–1070 CrossRef CAS PubMed.
- S. Acharya, S. Suwas and K. Chatterjee, Nanoscale, 2021, 13, 2286–2301 RSC.
- H. Zhang, J. Han, Y. Sun, Y. Huang and M. Zhou, Mater. Sci. Eng., C, 2015, 56, 22–29 CrossRef CAS PubMed.
- M. Saini, Y. Singh, P. Arora, V. Arora and K. Jain, World J. Clin. Cases, 2015, 3, 52–57 CrossRef PubMed.
- S. Lee, S. Kim, J. Park and J. Y. Lee, Int. J. Biol. Macromol., 2020, 151, 1314–1321 CrossRef CAS PubMed.
- J. M. Morais, F. Papadimitrakopoulos and D. J. Burgess, AAPS J., 2010, 12, 188–196 CrossRef CAS PubMed.
- A. Josyula, K. S. Parikh, I. Pitha and L. M. Ensign, Drug Delivery Transl. Res., 2021, 11, 1675–1688 CrossRef CAS PubMed.
- D. Campoccia, L. Montanaro and C. R. Arciola, Biomaterials, 2006, 27, 2331–2339 CrossRef CAS PubMed.
- H. Chouirfa, H. Bouloussa, V. Migonney and C. Falentin-Daudré, Acta Biomater., 2019, 83, 37–54 CrossRef CAS PubMed.
- F. Batool, H. Özçelik, C. Stutz, P. Y. Gegout, N. Benkirane-Jessel, C. Petit and O. Huck, J. Tissue Eng., 2021, 12, 20417314211041428 Search PubMed.
- M. Benčina, M. Resnik, P. Starič and I. Junkar, Molecules, 2021, 26, 1418 CrossRef PubMed.
- E. Xiang, M. N. Gómez-Cerezo, Y. Ali, S. S. Ramachandra, N. Yang, M. Dargusch, C. S. Moran, S. Ivanovski and A. Abdal-hay, ACS Appl. Mater. Interfaces, 2022, 14, 22554–22569 CrossRef CAS PubMed.
- M. S. Abesekara and Y. Chau, Front. Bioeng. Biotechnol., 2022, 10, 972790 CrossRef PubMed.
- W. L. Murphy, T. C. McDevitt and A. J. Engler, Nat. Mater., 2014, 13, 547–557 CrossRef CAS PubMed.
- S. Chen, C. Wu and H. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 31882–31891 CrossRef CAS PubMed.
- Y. Liu, B. Rath, M. Tingart and J. Eschweiler, J. Biomed. Mater. Res., Part A, 2020, 108, 470–484 CrossRef CAS PubMed.
- A. Lijnev, J. Elango, V. M. Gómez-López, C. P. Martínez, J. M. G. Marín and J. E. M. S. D. Val, Pharmaceutics, 2022, 15, 98 CrossRef PubMed.
- S. Sharma, A. Mandhani and B. Basu, ACS Biomater. Sci. Eng., 2022, 8, 4497–4523 CrossRef CAS PubMed.
- L. R. S. Hill, J. W. J. Craft, P. Chinwangso, H. Tran, M. D Marquez and T. R. Lee, ACS Appl. Bio Mater., 2021, 4, 1563–1572 CrossRef PubMed.
- M. M. Omrani, H. Kumar, M. G. A. Mohamed, K. Golovin, A. S Milani, A. Hadjizadeh and K. Kim, J. Biomed. Mater. Res., Part B, 2021, 109, 622–629 CrossRef PubMed.
- D. G. Castner, Nature, 2003, 422, 129–130 CrossRef CAS PubMed.
- W. L. Murphy, T. C. McDevitt and A. J. Engler, Nat. Mater., 2014, 13, 547–557 CrossRef CAS PubMed.
- L. L. Hench, Biomaterials, 1998, 19, 1419–1423 CrossRef CAS PubMed.
- J. V. Rau, M. Curcio, M. G. Raucci, K. Barbaro, I. Fasolino, R. Teghil, L. Ambrosio, A. D. Bonis and A. R. Boccaccini, ACS Appl. Mater. Interfaces, 2019, 11, 5812–5820 CrossRef CAS PubMed.
- S. T. Chen, C. Y. Wu and H. Y. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 31882–31891 CrossRef CAS PubMed.
- D. Ding, Y. Xie, K. Li, L. Huang and X. Zheng, Materials, 2018, 11, 546 CrossRef PubMed.
- A. Richter, Y. Li, C. Rehbock, S. Barcikowski, A. Haverich, M. Wilhelmi and U. Böer, Adv. Mater. Interfaces, 2021, 8, 2002015 CrossRef CAS.
- M. Dulski, K. Dudek, D. Chalon, J. Kubacki, S. Sulowicz, Z. Piotrowska-Seget, A. Mrozek-Wilczkiewicz, R. Gawecki and A. Nowak, ACS Appl. Bio Mater., 2019, 2, 987–998 CrossRef CAS PubMed.
- S. Liu, X. Zhao, J. Tang, Y. Han and Q. Lin, ACS Biomater. Sci. Eng., 2021, 7, 1065–1073 CrossRef CAS PubMed.
- X. Zhao, G. Wang, H. Zheng, Z. Lu, X. Zhong, X. Cheng and H. Zreiqat, ACS Appl. Mater. Interfaces, 2013, 5, 8203–8209 CrossRef CAS PubMed.
- A. Stepulane, A. K. Rajasekharan and M. Andersson, ACS Appl. Bio Mater., 2022, 5, 5289–5301 CrossRef CAS PubMed.
- Y. Xiang, R. Jin, Y. Zhang, K. Li, G. Liu, X. Song, Y. Wang and Y. Nie, Biomacromolecules, 2021, 22, 3510–3521 CrossRef CAS PubMed.
- J. Tang, S. Liu, Y. Han, R. Wang, J. Xia, H. Chen and Q. Lin, J. Mater. Chem. B, 2021, 9, 1546–1556 RSC.
- T. I. Croll, A. J. O'Connor, G. W. Stevens and J. J. Cooper-White, Biomacromolecules, 2004, 5, 463–473 CrossRef CAS PubMed.
- W. Sun, W. Liu, Z. Wu and H. Chen, Macromol. Rapid Commun., 2020, 41, e1900430 CrossRef PubMed.
- M. Henze, D. Mädge, O. Prucker and J. Rühe, Macromolecules, 2014, 47, 2929–2937 CrossRef CAS.
- Z. Bogdan and L. Igor, Macromol. Rapid Commun., 2011, 32, 859–869 CrossRef PubMed.
- H. Roghani-Mamaqani, V. Haddadi-Asl, K. Khezri and M. Salami-Kalajahi, RSC Adv., 2014, 4, 24439–24452 RSC.
- D. Kumar, J. Pandey, V. Raj and P. Kumar, Open Med. Chem. J., 2017, 11, 109–126 CrossRef CAS PubMed.
- A. Abu Naim, A. Umar, M. M. Sanagi and N. Basaruddin, Carbohydr. Polym., 2013, 98, 1618–1623 CrossRef CAS PubMed.
- B. Yu, C. Tang and C. Yin, Biomaterials, 2014, 35, 6369–6378 CrossRef CAS PubMed.
- Y. Chen, X. Liu, R. Liu, Y. Gong, M. Wang, Q. Huang, Q. Feng and B. Yu, Theranostics, 2017, 7, 1072–1087 CrossRef CAS PubMed.
- M. Sun, H. Qiu, C. Su, X. Shi, Z. Wang, Y. Ye and Y. Zhu, ACS Appl. Bio Mater., 2019, 2, 3983–3991 CrossRef CAS PubMed.
- R. M. Abdel-Rahman, V. Vishakha, I. Kelna, J. Jancar and A. M. Abdel-Mohsen, Int. J. Biol. Macromol., 2022, 202, 671–680 CrossRef CAS PubMed.
- D. Takahashi, Y. Koda, Y. Sasaki and K. Akiyoshi, Polym. J., 2018, 50, 789–797 CrossRef.
- K. Yu, J. C. Lo, M. Yan, X. Yang, D. E. Brooks, R. E. Hancock, D. Lange and J. N. Kizhakkedathu, Biomaterials, 2017, 116, 69–81 CrossRef CAS PubMed.
- Z. Sadrearhami, T. K. Nguyen, R. Namivandi-Zangeneh, K. Jung, E. H. H. Wong and C. Boyer, J. Mater. Chem. B, 2018, 6, 2945–2959 RSC.
- I. Francolini, G. Donelli, F. Crisante, V. Taresco and A. Piozzi, Adv. Exp. Med. Biol., 2015, 831, 93–117 CrossRef PubMed.
- E. M. Hetrick and M. H. Schoenfisch, Chem. Soc. Rev., 2006, 35, 780–789 RSC.
- H. Keum, J. Y. Kim, B. Yu, S. J. Yu, J. Kim, H. Jeon, D. Y. Lee, S. G. Im and S. Jon, ACS Appl. Mater. Interfaces, 2017, 9, 19736–19745 CrossRef CAS PubMed.
- K. L. Prime and G. M. Whitesides, Science, 1991, 252, 1164–1167 CrossRef CAS PubMed.
- Q. Yu, Z. Wu and H. Chen, Acta Biomater., 2015, 16, 1–13 CrossRef CAS PubMed.
- X. Liu, L. Yuan, D. Li, Z. Tang, Y. Wang, G. Chen, H. Chen and J. L. Brash, J. Mater. Chem. B, 2014, 2, 5718–5738 RSC.
- L. R. Carr, H. Xue and S. Jiang, Biomaterials, 2011, 32, 961–968 CrossRef CAS PubMed.
- S. Jiang and Z. Cao, Adv. Mater., 2010, 22, 920–932 CrossRef CAS PubMed.
- J. Zhao, L. Bai, K. Muhammad, X. K. Ren, J. Guo, S. Xia, W. Zhang and Y. Feng, ACS Biomater. Sci. Eng., 2019, 5, 2846–2857 CrossRef CAS PubMed.
- Y. N. Chou, T. C. Wen and Y. Chang, Acta Biomater., 2016, 40, 78–91 CrossRef CAS PubMed.
- T. Masuda, N. Shimada and A. Maruyama, Langmuir, 2019, 35, 5581–5586 CrossRef CAS PubMed.
- Z. Zhou, L. Wang, Y. Hu, R. Song, N. Mei, T. Chen and S. Tang, Int. J. Biol. Macromol., 2021, 167, 66–75 CrossRef CAS PubMed.
- Y. Du, C. Li, J. Jin, C. Li and W. Jiang, Colloids Surf., B, 2018, 161, 73–82 CrossRef CAS PubMed.
- M. Alvarez-Paino, M. H. Amer, A. Nasir, V. Cuzzucoli Crucitti, J. Thorpe, L. Burroughs, D. Needham, C. Denning, M. R. Alexander, C. Alexander and F. R. A. J. Rose, ACS Appl. Mater. Interfaces, 2019, 11, 34560–34574 CrossRef CAS PubMed.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1169 CrossRef CAS PubMed.
- K. L. Christman, Science, 2019, 363, 340–341 CrossRef CAS PubMed.
- T. Hoshiba, C. Yoshikawa and K. Sakakibara, Langmuir, 2018, 34, 4043–4051 CrossRef CAS PubMed.
- A. A. Khalili and M. R. Ahmad, Int. J. Mol. Sci., 2015, 16, 18149–18184 CrossRef CAS PubMed.
- A. Hasan, V. Saxena and L. M. Pandey, Langmuir, 2018, 34, 3494–3506 CrossRef CAS PubMed.
- J. Lehnfeld, Y. Dukashin, J. Mark, G. D. White, S. Wu, V. Katzur, R. Müller and S. Ruhl, J. Dent. Res., 2021, 100, 1047–1054 CrossRef CAS PubMed.
- L. Liu, S. Chen, C. M. Giachelli, B. D. Ratner and S. Jiang, J. Biomed. Mater. Res., Part A, 2005, 74, 23–31 CrossRef PubMed.
- Z. Chen, Y. Fan, L. Wang, Z. Bian and L. Hao, RSC Adv., 2021, 11, 36360–36366 RSC.
- J. Ladd, Z. Zhang, S. Chen, J. C. Hower and S. Jiang, Biomacromolecules, 2008, 9, 1357–1361 CrossRef CAS PubMed.
- Z. Zhang, M. Zhang, S. Chen, T. A. Horbett, B. D. Ratner and S. Jiang, Biomaterials, 2008, 29, 4285–4291 CrossRef CAS PubMed.
- C. Blaszykowski, S. Sheikh and M. Thompson, Chem. Soc. Rev., 2012, 41, 5599–5612 RSC.
- C. Siegers, M. Biesalski and R. Haag, Chemistry, 2004, 10, 2831–2838 CrossRef CAS PubMed.
- J. Dernedde, A. Rausch, M. Weinhart, S. Enders, R. Tauber, K. Licha, M. Schirner, U. Zügel, A. vonBonin and R. Haag, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 19679–19684 CrossRef CAS PubMed.
- D. D. Stöbener, F. Paulus, A. Welle, C. Wöll and R. Haag, Langmuir, 2018, 34, 10302–10308 CrossRef PubMed.
- N. Huettner, T. R. Dargaville and A. Forget, Trends Biotechnol., 2018, 36, 372–383 CrossRef CAS PubMed.
- J. Ivaska, Nat. Cell Biol., 2012, 14, 981–983 CrossRef CAS PubMed.
- P. E. Donnelly, L. Imbert, K. L. Culley, R. F. Warren, T. Chen and S. A. Maher, J. Biomater. Sci., Polym. Ed., 2019, 30, 215–232 CrossRef CAS PubMed.
- M. Ö. Öztürk-Öncel, C. O. Heras-Bautista, L. Uzun, D. Hür, J. Hescheler, K. Pfannkuche and B. Garipcan, ACS Biomater. Sci. Eng., 2021, 7, 1539–1551 CrossRef PubMed.
- J. M. Curran, R. Chen and J. A. Hunt, Biomaterials, 2006, 27, 4783–4793 CrossRef CAS PubMed.
- M. Chen, Y. Zhang, Y. Zhou, Y. Zhang, M. Lang, Z. Ye and W. S. Tan, Colloids Surf., B, 2015, 134, 322–331 CrossRef CAS PubMed.
- S. Y. Yeung, Y. Sergeeva, G. Pan, S. Mittler, T. Ederth, T. Dam, P. Jönsson, Z. El-Schich, A. G. Wingren, A. Tillo, S. Hsiung Mattisson, B. Holmqvist, M. M. Stollenwerk and B. Sellergren, ACS Appl. Mater. Interfaces, 2022, 14, 41790–41799 CrossRef CAS PubMed.
- S. Acosta, L. Quintanilla, M. Alonso, C. Aparicio and J. C. Rodríguez-Cabello, ACS Biomater. Sci. Eng., 2019, 5, 4708–4716 CrossRef CAS PubMed.
- P. Qiu, L. Feng, Q. Fu, T. Dai, M. Liu, P. Wang and Y. Lan, Langmuir, 2022, 38, 14712–14724 CrossRef CAS PubMed.
- S. B. de Souza Ferreira, B. R. de Assis Dias, C. S. Obregón, C. C. Gomes, R. R. de Araújo Pereira, J. S. Ribeiro Godoy, T. I. Estivalet Svidzinski and M. L. Bruschi, Pharm. Dev. Technol., 2014, 19, 173–180 CrossRef PubMed.
- T. Lu, Y. Qiao and X. Liu, Interface Focus, 2012, 2, 325–336 CrossRef PubMed.
- W. Wang and K. Yeung, Surf. Innovations, 2019, 8, 1–12 Search PubMed.
- H. T. Sasmazel, M. Alazzawi and N. Alsahib, Molecules, 2021, 26, 1665 CrossRef CAS PubMed.
- K. Bazaka, M. Jacob, W. Chrzanowski and K. Ostrikov, RSC Adv., 2015, 5, 48739–48759 RSC.
- P. K. Chu, J. Y. Chen, L. P. Wang and N. Huang, Mater. Sci. Eng., R, 2002, 36, 143–206 CrossRef.
- E. Akdoan and H. T. Irin, Mater. Sci. Eng., C, 2021, 131, 112474 CrossRef PubMed.
- T. Desmet, R. Morent, N. De Geyter, C. Leys, E. Schacht and P. Dubruel, Biomacromolecules, 2009, 10, 2351–2378 CrossRef CAS PubMed.
- W. Liu, Y. Li, T. Wang, D. Li, L. Fang, S. Zhu, H. Shen, J. Zhang, H. Sun and B. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 12587–12593 CrossRef CAS PubMed.
- P. Xue, W. Liu, Z. Gu, X. Chen, J. Nan, J. Zhang, H. Sun, Z. Cui and B. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 1595–1603 CrossRef CAS PubMed.
- P. T. Vu, J. P. Conroy and A. M. Yousefi, Biomimetics, 2022, 7, 218 CrossRef CAS PubMed.
- P. Xue, J. Nan, T. Wang, S. Wang, S. Ye, J. Zhang, Z. Cui and B. Yang, Small, 2017, 13, 1601807 CrossRef PubMed.
- P. T. Vu, J. P. Conroy and A. M. Yousefi, Biomimetics, 2022, 7, 218 CrossRef CAS PubMed.
- A. Mozaffari, M. Parvinzadeh Gashti, M. Mirjalili and M. Parsania, Membranes, 2021, 11, 31 CrossRef CAS PubMed.
- S. Onodera, S. Fujii, H. Moriguchi, M. Tsujioka and K. Hirakuri, Diamond Relat. Mater., 2020, 107, 107835 CrossRef CAS.
- G. Kaleli-Can, H. F. Özgüzar, S. Kahriman, M. Türkal, J. S. Göçmen, E. Yurtçu and M. Mutlu, Mater. Today Commun., 2020, 25, 101565 CrossRef CAS.
- E. A. Wakelin, A. Fathi, M. Kracica, G. C. Yeo, S. G. Wise, A. S. Weiss, D. G. McCulloch, F. Dehghani, D. R. Mckenzie and M. M. Bilek, ACS Appl. Mater. Interfaces, 2015, 7, 23029–23040 CrossRef CAS PubMed.
- M. Chen, L. Ouyang, T. Lu, H. Wang, F. Meng, Y. Yang, C. Ning, J. Ma and X. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 16824–16833 CrossRef CAS PubMed.
- A. Kondyurin, K. Lau, F. Tang, B. Akhavan, W. Chrzanowski, M. S. Lord, J. Rnjak-Kovacina and M. M. Bilek, ACS Appl. Mater. Interfaces, 2018, 10, 17605–17616 CrossRef CAS PubMed.
- S. Guo, N. Liu, K. Liu, Y. Li, W. Zhang, B. Zhu, B. Gu and N. Wen, RSC Adv., 2020, 10, 35917–35929 RSC.
- K. Lau, A. Waterhouse, B. Akhavan, L. Gao, H. N. Kim, F. Tang, J. M. Whitelock, M. M. Bilek, M. S. Lord and J. Rnjak-Kovacina, Acta Biomater., 2021, 132, 162–175 CrossRef CAS PubMed.
- K. W. Lo, B. D. Ulery, K. M. Ashe and C. T. Laurencin, Adv. Drug Delivery Rev., 2012, 64, 1277–1291 CrossRef CAS PubMed.
- Y. Liu, Z. Zheng, J. N. Zara, C. Hsu, D. E. Soofer, K. S. Lee, R. K. Siu, L. S. Miller, X. Zhang, D. Carpenter, C. Wang, K. Ting and C. Soo, Biomaterials, 2012, 33, 8745–8756 CrossRef CAS PubMed.
- A. Hoppe, N. S. Güldal and A. R. Boccaccini, Biomaterials, 2011, 32, 2757–2774 CrossRef CAS PubMed.
- G. Yang, H. Yang, L. Shi, T. Wang, W. Zhou, T. Zhou, W. Han, Z. Zhang, W. Lu and J. Hu, ACS Biomater. Sci. Eng., 2018, 4, 4289–4298 CrossRef CAS PubMed.
- P. Kazimierczak, J. Kolmas and A. Przekora, Int. J. Mol. Sci., 2019, 20, 3835 CrossRef CAS PubMed.
- R. Kane and P. X. Ma, Mater. Today, 2013, 16, 418–423 CrossRef CAS PubMed.
- C. Shuai, Z. Dong, W. Yang, C. He, Y. Yang and S. Peng, ACS Biomater. Sci. Eng., 2021, 7, 5484–5496 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: