Open Access Article
Open Access ArticleRecent advances in synthesis of polymers based on palm oil and its fatty acids
Erythrina Stavila
 a,
Frita Yuliati
a,
Frita Yuliati
 a,
Azis Adharis
a,
Azis Adharis
 b,
Joddy Arya Laksmono
b,
Joddy Arya Laksmono
 a and
Muhammad Iqbal
a and
Muhammad Iqbal
 *c
*c
aResearch Center for Polymer Technology, Research Organization for Nanotechnology and Material (ORNM), National Research and Innovation Agency (BRIN), Gedung 460 KST B. J. Habibie/Puspiptek, Jl. Raya Puspiptek, Tangerang Selatan 15315, Banten, Indonesia
bDepartment of Chemistry, Faculty of Science and Computer Science, Universitas Pertamina (UPER), Jl. Teuku Nyak Arief, RT.7/RW.8, Simprug, Jakarta Selatan 12220, Daerah Khusus Ibukota Jakarta, Indonesia
cDepartment of Chemistry, Faculty of Mathematics and Science, Institut Teknologi Bandung (ITB), Jl. Ganesha No. 10, Bandung 40135, Jawa Barat, Indonesia. E-mail: m.iqbal@itb.ac.id
First published on 15th May 2023
Abstract
Palm oil is a versatile bio-renewable resource for consumer products, oleochemicals, and biofuels. The utilization of palm oil in polymer production as a bio-based polymer is considered a promising alternative to conventional petrochemical-based polymers due to its non-toxicity, biodegradability, and vast obtainability. Triglycerides and fatty acids in palm oil and their derivatives can be utilized as bio-based monomers for synthesizing polymers. This review summarizes the recent advancement in using palm oil and its fatty acids for polymer synthesis and their applications. Moreover, this review will overview the most commonly used synthesis pathways for producing palm oil-based polymers. Therefore, this review can be used as a reference for designing a new approach to synthesizing palm oil-based polymers with desired properties.
1. Introduction
Polymers are a vastly used material in our daily life, mainly used as plastics, foams, elastomers, fibers, coatings, epoxy resin, and other specialty materials.1 Each year, a massive demand for polymers increases polymer production. This immense use of polymer has caused a rising demand for fossil resources as its main raw material. However, there are growing apprehensions about the rapid depletion of fossil resources. It has led to increasing research on using bio-renewable and non-toxic resources as polymer raw materials.1,2 Moreover, emissions and hazardous waste are generated from this fossil resource consumption, which would lead to severe environmental issues such as pollution and global climate change.1 Therefore, developing more sustainable polymers from bio-renewable and non-toxic resources is appealing to reduce the current dependency on fossil resources.Bio-based polymers are environmentally friendly materials and potential alternatives to conventional petrochemical-based polymers. By definition, bio-based polymers are sustainable polymeric materials that can be synthesized from renewable feedstocks and are preferably produced via biological and biochemical methods.3 Bio-based polymers are classified into two categories: naturally obtained polymer and polymer synthesized from bio-derived substances.3 Protein, carbohydrates, terpenes, lignin, vegetable oils and their derivatives are examples of renewable feedstocks that can be utilized to produce bio-based materials.4,5 Among other renewable resources, vegetable oils have been considered as potential resources for sustainable chemicals and polymeric materials because of their low toxicity, biodegradability, and vast obtainability.6
Vegetable oils are extracted from various plants and termed after their plant origin, for instance, palm oil, olive oil, rapeseed oil, sesame oil, sunflower oil, cottonseed oil, corn oil, soybean oil, linseed oil, etc.6 These vegetable oils and their derivatives can be used in industrial applications such as in the synthesis of polymers such as polyols, polyesters, polyurethanes, polyamides, epoxides, etc. The uses of various types of vegetable oils for monomers preparation, polymer synthesis, and their applications have been reviewed elsewhere.6–12 However, to the best of our knowledge, there is no available review that focuses only on the use of palm oil as raw bio-renewable feedstock for polymer synthesis. Therefore, in this review, we will focus on and discuss the recent advance in the utilization of palm oil and its fatty acids for polymer synthesis and its application.
2. Palm oil
Palm oil is isolated from the mesocarp part of the fruit of the oil palm trees (Elaeis guineensis Jacquin), which is known as a tropical, perennial crop that is primarily cultivated for its vegetable oil.13 Besides palm oil, the oil palm tree also produces kernel oil extracted from the kernel or the endosperm. Both palm oil and kernel oil are used in many applications, such as oleochemicals, ingredients of many foods, health and household products, industrial goods, and biodiesel.13 In Southeast Asia, the initial commercial plantings of oil palm were introduced in 1911 (in Bogor, Indonesia) from the offspring of four seeds that originated from West Africa and were planted in 1848.13 Indonesia is well-known as the world's biggest producer and supplier of palm oil since 2005.14 Since then up until 2020, oil palm plantation in Indonesia spreads up to 16.3 million hectares. It produces more than 35 million tons of palm oil annually.15 In 2021, crude palm oil (CPO) production reached 46.89 million tons, which was 0.31% lower than in 2020 at 47.03 million tons yearly.16 Regarding contribution to national revenue, oil palm plantation has been an essential source of a commodity in the Indonesian agricultural sector.15 Thus, in Indonesia, access to palm oil and its derivatives products is expected to be relatively easy and come with a low cost.The growing request for edible oil led to increase palm oil production since the oil palm tree is a highly profitable crop, thus significantly influencing Indonesia's oil palm venture expansion.17 Areas with oil palm cultivation in Indonesia, namely North Sumatera, Riau, and Central Kalimantan, show lower average rural poverty and higher gross regional product (GRP).18 The area with oil palm plantations benefits in their regional economic development and poverty alleviation. However, the positive aspect regarding the oil palm expansion is also followed by the negative impact on the environment, especially concerning deforestation and climate change.18 In some cases, deforestation somehow was involved as an irresponsible act on plantation expansion. Deforestation and land conversion contribute to global carbon emissions,19 which consequently play a major role in global climate change.15 Unfortunately, this land conversion and deforestation for oil palm plantations development in Indonesia and Malaysia have emitted CO2 of ∼500 million tons every year,20 which deems the oil palm industry unsustainable. Due to environmental concerns, the EU, one of the world's main importers of palm oil, insists on reducing and stopping the usage of palm oil-based biofuels altogether. They believe that the palm oil industry does not fulfill the standards for EU's biofuel raw material production, i.e., without deforestation, generating low carbon emission, and avoiding conversion of areas full of biodiversity.15,21 Based on these developments, further research and study on sustainability in the palm oil industry has to be improved and established to eliminate the downside of this industry to the environment. The growth in regional economic and poverty alleviation cannot be used to justify the environmental damage due to the irresponsible practice. Thus, it is important to make this industry sustainable and more environmentally friendly. Especially in Indonesia, a lot of people's lives depend on this industry. One of the efforts that can be done is utilizing the oil palm plantation product as a renewable material feedstock for polymer production, which also give significant contribution in polymer chemistry field. Therefore, this article will review the research that have been performed in synthesis of polymer from palm oil and its fatty acids, so then it can be used as a reference for designing new approach in polymer synthesis from palm oil and exploring new possibilities of application of the obtained polymers.
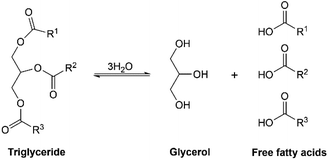
Palm oil mainly consists of triglyceride with its unique fatty acid profile (see Fig. 1) and minor components like tocotrienols and tocopherols (vitamin E), carotenoids (precursor for vitamin A), phytosterols, squalene, chlorophyll, and low levels of phenolic compounds.22 The fatty acids in palm oil consist of about 44% palmitic acid (saturated), 5% stearic acid (saturated), 39% oleic acid (unsaturated), and 10% linoleic acid (unsaturated).23,24 The composition of saturated fatty acids (palmitic and stearic acid) may differ from each country.22 The stereospecific distribution of the fatty acyl residues in triglyceride of palm oil is shown in Table 1. The low linoleic acid content is beneficial for oil stability towards oxidative deterioration.24 The double bond content from the unsaturated fatty acids moiety of palm oil can be determined by iodine value (IV) which is 50–55 mg per 100 g.25 The higher IV specifies more double bonds or unsaturation content in the vegetable oils. In terms of IV, vegetable oils can be categorized into three types: drying oils (IV > 130), semi-drying oils (IV 90–130), and non-drying oils (IV < 90).25,26 Thus, palm oil with low iodine value of 50–55 mg per 100 g is considered as a non-drying oil.
Palm oil is known as the most fractionated oil, and its fraction products have been used in many different applications.27 Fractionation is a selective separation of multi-component mixtures into two or more fractions with different chemical and physical properties; by physical and thermomechanical processes.27 By utilizing the distinction in the crystallization performance of triglycerides, palm oil fractionation produces a liquid of palm olein (65–70%) and a solid fraction of palm stearin (30–35%).22,27 Palm olein has melting points of 18–20 °C and contains a higher amount of oleic acid (39–45%) and linoleic acid (10–13%) than palm oil, which is used as a deep frying oil.24 Whereas, palm stearin has melting points of 48–50 °C and is composed of a higher portion of saturated fatty acids (49–68% palmitic acid) and triglycerides, which is utilized as vanaspati.24 Further fractionation from palm olein (deep frying oil) or palm stearin (vanaspati) can be done to obtain more fraction products, such as super olein (cooking oil), top olein (salad oil), super stearin (animal feed), soft palm middle fraction (margarine), and hard palm middle fraction (confectionery).22,27 In the following section, we will discuss selected articles that reported the application of epoxidized palm olein and other fractionation products as sources of palm oil-based monomers.
3. Conversion of triglyceride and fatty acid from palm oil to bio-based monomers
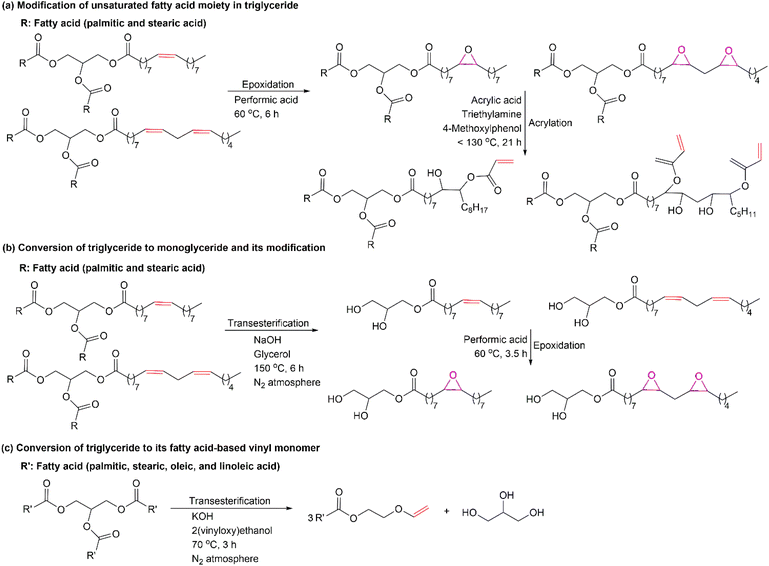
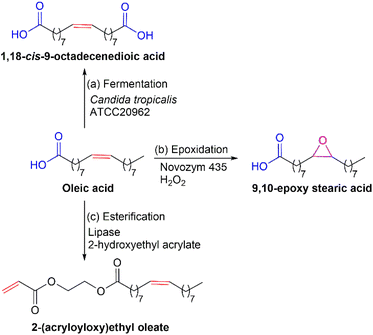
Palm oil, fractionated products, and fatty acids can be used as renewable feedstocks for polymer production. Several methods for triglyceride modification are presented in Fig. 2. The utilization of different modification processes of triglyceride will result in bio-based monomers with particular functional groups for further polymerization. Fig. 2 displays examples of the synthetic routes that can be used to modify triglyceride into bio-based monomers. In Fig. 2(a), the unsaturated fatty acid moiety in a triglyceride can be modified via epoxidation of the double bonds.28 The epoxidized product can be further modified by acrylation to attach the vinyl functional group to the triglyceride.29 Another option for preparing bio-based monomers from triglyceride is converting triglyceride to its monoglyceride via transesterification using glycerol, as shown in Fig. 2(b).30 The double bond of the unsaturated fatty acid chain in monoglyceride can be modified through epoxidation to produce oxirane rings that will be available for further modification. The other alternative in preparing bio-based monomer from triglyceride is conversion to its fatty acids, as shown in Fig. 2(c). The reaction between triglyceride and 2-(vinyloxy)ethanol using a base catalyst produce fatty acid-based vinyl monomers.31 These vinyl monomers are suitable to be polymerized by radical polymerization technique. Based on the three possibilities synthetic routes, by fragmenting triglyceride to its fatty acids will increase the accessibility to the double bond in the unsaturated fatty acids chains for further modification. It is because triglyceride is bulkier than fatty acid. Therefore, direct reaction to triglyceride will be less effective as compared to fatty acids.Besides directly using palm oil, fatty acids derived from palm oil may also be utilized as a monomer for polymer synthesis. Fig. 3 shows several options for enzymatic modification of fatty acids to make them suitable as bio-based monomers for polymer synthesis. In Fig. 3, oleic acid is used as a model compound for bio-based monomer formation from fatty acids. In Fig. 3(a), conversion of oleic acid to its α,ω diacid functional monomer can be carried out via whole-cell bio-transformation using Candida tropicalis ATCC20962.32 This diacid is an appropriate monomer in polycondensation for producing polyester, polyesteramide, etc. In Fig. 3(b), the double bond in oleic acid can be modified via chemo-enzymatic epoxidation to produce a bio-based monomer with the oxirane ring.33 This oxirane ring will be available for further functionalization. Another alternative for preparing bio-based monomers from fatty acids is shown in Fig. 3(c). Esterification of oleic acid with 2-hydroxyethyl acrylate using lipase from Candida antarctica immobilized on an acrylic resin as a catalyst.34 The obtained monomer will have a vinyl functional group suitable for radical polymerization. These three enzymatic synthetic routes can be used to prepare fatty acid-based monomers. The utilization of enzymes as a catalyst will be an added value to the green process.
There are advantages to using palm oil or fatty acids in polymer production, as summarized in Table 2. Information from Table 2 can be considered in choosing between palm oil or fatty acids as raw material feedstock for making bio-based monomers. The decision to use palm oil or fatty acid will affect the synthetic pathways needed to prepare bio-based monomers and, consequently, the properties of the resulting polymers. Depending on the type of reactive functional groups available in the palm oil or fatty acid-based monomers, the polymerization techniques can be achieved via polycondensation, free radical polymerization, atom transfer radical polymerization (ATRP), reversible addition–fragmentation chain transfer polymerization (RAFT), or cationic polymerization. The following section will discuss the type of polymers synthesized from palm oil or its derivatives and their applications. Polymers based on palm oil and its fatty acids consist of polymers from fatty acid-based vinyl monomers, polyester, polyols, polyurethanes, polyepoxides, composites, polyamides, polyesteramides, etc.
| Palm oil | Fatty acids |
|---|---|
| Cost efficient | Effective formulation |
| • Vastly abundant and easy to get. | • Effective modification to only saturated or unsaturated fatty acids35 |
| • Palm oil is cheaper than fatty acids, so it might decrease polymer production expenses35 | • Lessen unwanted by-products or impurities |
| • Oil is non-corrosive compared to fatty acids; therefore, no anticorrosion equipment is required35 | • Easier to achieve desired polymer products with specific properties |
| • Oil showed better resistance to discoloration during storage than fatty acids35 |
4. Polymers from palm oil or fatty acid-based vinyl monomers
This section will discuss the modification of triglyceride and fatty acid from palm oil to vinyl monomers that later is utilized for polymer production. As reported by Yuan and coworkers on the conversion of triglycerides from high oleic soybean oil to N-hydroxyalkyl fatty amides and further modification to form non-cyclic methacrylate, imino ether, and cyclic norbornene monomers.36 This report explained that the double bonds group in the fatty acids was unchanged during the free radical polymerization of non-cyclic methacrylate monomers. Therefore, this study indicated the importance of adding reactive vinyl groups to the fatty acid moiety of triglycerides from vegetable/plant oil for radical polymerization. The following explanation in this section shows reports on the modification of palm oil triglyceride to attach vinyl functional.As for adding vinyl groups to fatty acids, various conversion methods of fatty acids from vegetable oils and their derivatives into functionalized monomers for effective radical polymerization have been thoroughly reviewed.37 The development of radical polymerization techniques of fatty acid-(meth)acrylate monomers and their polymer applications are also discussed.37 Another review is reported on plant oil-based acrylic monomers (POBMs) made from modification of triglycerides from various plant oils such as high oleic soybean, olive, sunflower, canola, soybean, corn, high oleic sunflower, and linseed oil.38 Moreover, Since reviews on fatty acid-vinyl monomers from vegetable oil have been available, thus in our review, we present only selected reports on fatty acid-based vinyl monomers specially derived from palm oil. Especially fatty acids such as palmitic, stearic, oleic, and linoleic acids are part of the component in palm oil triglycerides. So, we can have a general idea of how palm oil and its fatty acid have been utilized in producing palm oil-based polymer. Finally, this information can be used to design a new synthetic approach to the following practical step for utilizing palm oil and its fatty acids in polymer synthesis.
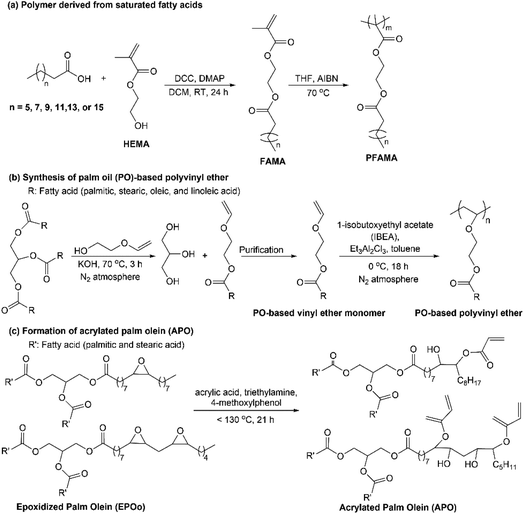
Here are examples of the synthesis of fatty acid/ester-based vinyl monomers from palm oil, as shown in Fig. 4. Maiti et al. reported on the synthesis of a series of fatty acid-based methacrylate monomers (FAMA) via Steglich esterification of 2-hydroxyethyl methacrylate (HEMA) with saturated fatty acids, see Fig. 4(a).39 They obtained high-yield monomers of 74–85% indicating an efficient way to produce novel fatty acid-based vinyl monomers. Polymerization of FAMA via RAFT resulted in fatty acid-based polymers (PFAMA) with controlled molecular weights, as shown in Fig. 4(a). However, in the Steglich esterification for FAMA formation, they used dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP), known for its toxicity toward humans and the environment, thus limiting the scale-up probability.37 Using a more environmentally friendly catalyst, transesterification of methyl laurate and methyl oleate with HEMA was performed with Candida antarctica lipase B immobilized within a porous polymethacrylate (CAL-B, Novozymes A/S) at optimal reaction condition of 60 °C for 24 h in toluene.40 Interestingly, they also reused the CAL-B for ten batches of the same reaction, which revealed that the enzyme retained its activity at the yield of 88% for methyl laurate with HEMA and yield of 76% for methyl oleate and HEMA. Another report on the use of enzymes as bio-catalyst is in the preparation of oleic acid-based epoxy monomer. The reaction was performed between 2-hydroxyethyl acrylate and epoxy stearic acid using lipase from immobilized Candida antarctica on an acrylic resin as a catalyst in toluene at 37 °C for 24 h that resulted in a yield of 87%.34 Oleic acid-based epoxy monomer was subsequently polymerized by radical polymerization using AIBN in toluene for 24 h. Moreover, palm oil methyl ester (from unsaturated fatty esters) is reported as a monomer for polymeric surfactant preparation. Palm oil methyl ester was modified to methyl ester sulfonate (MES) via a reaction of the methyl ester with NaHSO3, and then, aqueous radical polymerization of MES and acrylamide was initiated by potassium persulfate.41 The obtained polymeric methyl ester sulfonate (PMES) can be used as a substitute surfactant for enhanced oil recovery (EOR) since it can decrease the interfacial tension (IFT) and improve the viscosity control, two beneficial features for EOR application.
Kalita and coworkers synthesized palm oil (PO)-based vinyl ether monomer via transesterification of triglyceride with 2-(vinyloxy)ethanol, see Fig. 4(b).31 Through cationic polymerization, only the vinyl ether group from the monomer was selectively polymerized for producing its PO-based polyvinyl ether with number average molecular weight (Mn) of ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mole−1. Kalita et al. also reported the preparation of plant oil-based polyvinyl ethers from linseed, soybean, and palm oil to be compared with commonly used drying oil as binders for artist paints.42 Plant oil was converted to plant oil-based vinyl ether monomers that consisted of 2-vinyloxy ethyl soyate (2-VOES), 2-vinyloxy ethyl palmitate (2-VOEP), and 2-vinyloxy ethyl linseedate (2-VOEL). These vinyl ether monomers from plant oil were further polymerized to produce homo- and copolymers. Applying these polymers in paint binders showed excellent mechanical properties and reduced cure shrinkage better than drying oils.
000 g mole−1. Kalita et al. also reported the preparation of plant oil-based polyvinyl ethers from linseed, soybean, and palm oil to be compared with commonly used drying oil as binders for artist paints.42 Plant oil was converted to plant oil-based vinyl ether monomers that consisted of 2-vinyloxy ethyl soyate (2-VOES), 2-vinyloxy ethyl palmitate (2-VOEP), and 2-vinyloxy ethyl linseedate (2-VOEL). These vinyl ether monomers from plant oil were further polymerized to produce homo- and copolymers. Applying these polymers in paint binders showed excellent mechanical properties and reduced cure shrinkage better than drying oils.
Preparation of vinyl monomer by acrylation can also be done directly to palm oil triglycerides. Synthesis of acrylated palm olein (APO) was conducted through ring-opening oxirane groups of the epoxidized palm olein (EPOo) using acrylic acid, which resulted in APO with a high yield of 86% and molecular weight of 1750 Da as shown in Fig. 4(c).29 APO has hydroxy groups that are beneficial for surface modification to increase the drug transport performance to the targeted organ. Su et al. reported on the acrylation of different types of vegetable oils for UV-curable coatings application.43 Vegetable oils, including palm, peanut, olive, canola, corn, rapeseed, and grapeseed oil, were reacted with acrylic acid and boron trifluoride ether to form vegetable oil-based acrylate prepolymers. The cured film derived from grapeseed oil showed the best mechanical and thermal properties among all the prepared cured films. Furthermore, Wu et al. reported on the preparation of a vinyl and UV-responsive monomer via modification of palm oil to palm oil fatty acid-ethyl acrylamide (POFA-EA).44 The obtained POFA-EA was used to formulate palm oil-based inks by blending them with acrylic acid (AA) at different weight ratios. To produce Zn2+-containing elastomers, ZnO or ZnCl2 at different weight content and a photo-initiator was added to the blend solution of POFA-EA/AA and then used as palm oil-based inks for photocuring 3D printing. A series of thermoplastic elastomers from palm oil with dynamic physical crosslinks (noncovalent interaction, see Fig. 5) were produced by photocuring 3D printing. Noncovalent interactions improved tensile strength up to 4.2 MPa and fracture elongation of 851% of the elastomers. Moreover, these interactions promote more abilities in the elastomers, like self-healing, recovery, and reconfiguration. This approach of physical crosslinking-enable printability might propose a new method for producing thermoplastic polymers via photocuring 3D printing.
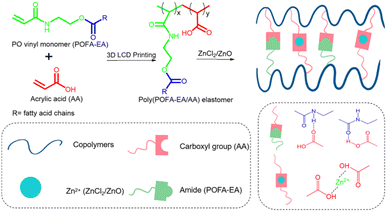 | ||
| Fig. 5 Illustration of plausible noncovalent (hydrogen bond) interactions between AA and POFA-EA in palm oil-based thermoplastic elastomers. Reproduced with permission from ref. 44 Copyright 2021 Elsevier. | ||
As shown in the abovementioned paragraph, we have selected reports showing fatty acids or palm oil as starting materials for polymer production. As for fatty acids as a renewable feedstock for polymer synthesis, there are options for chemical reaction or enzymatic synthesis. Based on the reported studies, enzymatic synthesis in monomer preparation of fatty acid-vinyl monomers was highly effective. Moreover, the immobilized enzyme can be reused for several reactions, and the reaction results in a good yield. However, as far as we know, there are no reports on the enzymatic conversion of triglyceride to fatty acid-based vinyl monomers. So, we suggest that researchers in this field try this triglyceride transesterification using an enzyme as a catalyst to attach vinyl functional groups to fatty acids moieties. As a consideration, since triglyceride is a bigger molecule than a fatty acid, perhaps enzymatic reaction using triglyceride will be more complex and less effective than fatty acid as feedstock.
5. Polyesters
Polyesters are polymeric materials with ester linkages in every repeating unit of their main polymer chain. Synthesis of polyesters can be performed via alcoholysis and esterification process, ring-opening polymerization of ring monomers, and polycondensation of AA and BB or AB-type monomers. Polyesters are valuable polymeric materials that have been used as plastics, fibers, elastomers, plasticizers, coatings, and in biomedical applications.45 There have been several examples of reported polyesters prepared from biomass and bio-renewable feedstock.10,45 In this section, we will discuss several reports on the synthesis and application of palm oil and its fatty acid-based polyesters.5.1. Alcoholysis and esterification method
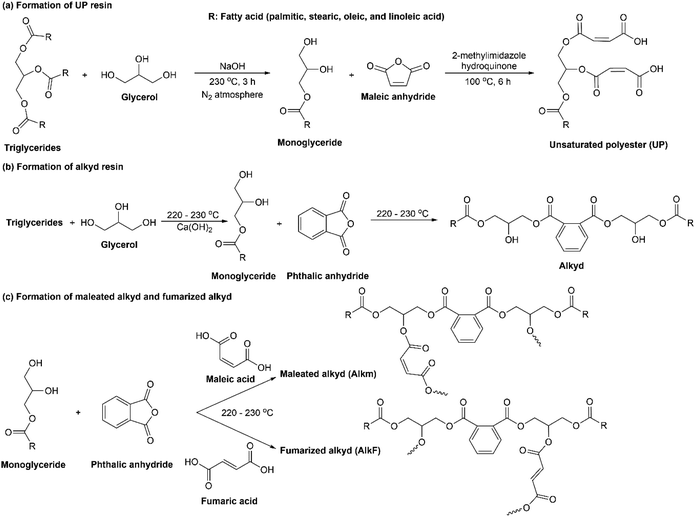
Alcoholysis can be done by reaction of palm oil with glycerol to form palm oil monoglyceride for subsequently esterified with maleic anhydride (MA) to obtain unsaturated polyester (UP), as shown in Fig. 6(a). Lai and coworkers reported a study on this UP's activation energy and swelling properties.46 The cured-UP resins with higher MA content exhibited lower penetration of solvents. Another report on the utilization of palm oil for alkyd preparation using nanoparticles as a catalyst is available. Ong et al. reported on the synthesis of palm oil-based alkyd via alcoholysis and polyesterification process using a homogeneous base catalyst combined with copper oxide (CuO) nanoparticles.47 Incorporating CuO nano-catalyst into the conventional NaOH catalysis system improved the aimed reactions' catalytic process, reducing the reaction time for both processes. Another advantage of using CuO nanoparticles in alkyd preparation was that the obtained resin possessed better antimicrobial activity than neat alkyd resin. Moreover, a lower molecular weight of alkyd resin from the CuO–NaOH system was better suited for surface coating application.Palm oil-based alkyds for UV-curable coatings were prepared from palm stearin.48 Alkyds were prepared via alcoholysis using Ca(OH)2 and glycerol at 230 °C to form monoglycerides. Further esterification between monoglycerides and phthalic anhydride was performed to produce alkyds, as shown in Fig. 6(b). A further modification was carried out by combining phthalic acid and maleic acid during polyesterification, see Fig. 6(c). The incorporation of maleic acid increased the unsaturation in the alkyds chain and made it more UV-curable. Increasing maleic acid content in alkyds resulted in coatings with better film properties that were shown by better water and alkali resistance, higher pencil hardness, as well as improved thermal stability. Moreover, adding a small amount of trimethylolpropane triacrylate (TMPTA) as a trifunctional crosslinker into the coating formulation enhanced the film properties significantly. The same trend was also observed from alkyds prepared by combining phthalic anhydride and different maleic anhydride content during polyesterification.35 Furthermore, alkyds modification was carried out by incorporating fumaric acid or maleic acid during alkyds formation, which produced fumarized alkyd (AlkF) or maleated alkyd (AlkM), respectively, as shown in Fig. 6(c).49 AlkF produced coating with better film hardness and higher Tg than AlkM, which meant that the fumarization process was a better approach for increasing alkyd unsaturation groups. Moreover, to improve the coating performance of alkyd resins, there are reports on combining two different oils (palm stearin/dehydrated castor oil or palm stearin/linseed oil)50 or blending palm stearin-based alkyd resin with commercial ketone resin.51
Ang et al. reported on the utilization of palm oil-based alkyd as a polymeric plasticizer in polyvinyl chloride (PVC).52 By incorporating 20 wt% palm oil-based alkyd to PVC, Tg value reduced from 85.3 °C to 66.7 °C, indicating a successful plasticizing process. Moreover, PVC contained 20 wt% palm oil-based polyester showed better migration resistance than PVCs with the addition of two commercially available plasticizers, acetyl triethyl citrate (ATEC) and diethylhexylphthalate (DEHP). Furthermore, the obtained palm oil-based alkyd was used as a co-plasticizer to di-octyl phthalate (DOP) or di-isononyl phthalate (DiNP) as the primary plasticizer for flexible PVC.53 The incorporation of palm oil-based alkyd into plasticized-PVC resulted in an improvement of mechanical and thermal properties. Further studies on the effect of different molecular weights of palm oil-based alkyds for PVC plasticizer was performed.54 Blending 20 wt% of palm oil-based alkyds to PVC resulted in reduced Tg value. The smaller size plasticizer showed better plasticizer ability than the high molecular weight plasticizer because the small size plasticizer penetrates better to PVC chains. Meanwhile, in n-hexane and water/ethanol as solvent, films of PVC plasticized with high molecular weight alkyd displayed better migration and leaching resistance as compared to that of PVC plasticized with low molecular alkyds and commercially available plasticizer of DEHP; thus, high molecular weight alkyd showed less toxicity compared to DEHP. Moreover, a significant improvement in tensile strength and elongation at break was observed on plasticized-PVC film with high molecular weight palm oil-based alkyds.
Ramli and coworkers reported on the incorporation of palm oil-based alkyd resin in polyaniline (PANI) to prepare films of PANI/Alkyd with good adhesion properties on fluoride-doped tin oxide (FTO) for solar panel application.55 Three alkyds with different acid numbers were blended with PANI at various ratios of PANI/Alkyd and then cured on the FTO glass. PANI/Alkyd film with the lowest acid number of alkyd displayed the shortest curing time compared to those with a high acid number. All PANI/Alkyd films exhibited good adhesion, while the PANI film showed poor adhesion performance. Unfortunately, blending PANI with palm oil-based alkyd decreased the film's conductivity since alkyd was not a conducting polymer. Furthermore, the blending process of PANI and alkyd was carried out during coating preparations by mixing various alkyd concentrations with acrylate monomers, M-cresol, and different loading of PANI, then followed by benzophenone for UV curing. The alkyd/PANI coating films of 20% PANI presented the highest conductivity of 1.467 × 10−1 S cm−1.56
Palm fatty acid distillate (PFAD) is a side product from the physical refining process of crude palm oil (CPO), which mostly consist of free fatty acids (∼45% palmitic acid and 33% oleic acid) with small numbers of glycerides, bioactive compounds like phytosterols, tocopherols, squalene, tocotrienols, other hydrocarbons.57,58 There are reports on the use of PFAD for alkyd resins preparation. Chiplunkar et al. prepared alkyd resins from palm oil and PFAD as an additive in liquid detergent.57 The results showed that the PFAD-based alkyd resin was comparable to palm oil-based alkyd resin and commercial liquid detergent. Moreover, Teo and coworkers reported utilizing PFAD in synthesizing hydroxyl-terminated macromer to produce UV-curable urethane acrylate resin for wood-coating applications.58 The highest Mw observed at the PFAD content of 45% was considered the optimal concentration of PFAD. These macromers were further modified to make PFAD urethane acrylate.
From the abovementioned reported articles, palm oil-based alkyds have the potential to be applied as surface coating, plasticizers, and additives. For coating application, further modification by adding more unsaturated groups to alkyd resins is necessary to increase the UV-curable ability of the resins. However, no additional modification is needed to prepare alkyds for plasticizer and additives application. Thus, it is more beneficial from an economic point of view—significantly, the utilization of PFAD as the raw material feedstock for producing alkyd liquid detergent additive. Since PFAD is a by-product of the CPO refining process, producing PFAD-based alkyd will be more environmentally friendly and economically beneficial. As for the alkyd for plasticizers, this application demonstrated more agreement with the nature of the alkyd resins. Palm oil-based alkyd resins have more saturated fatty acids moieties that serve as side chains in the polymer backbone. This saturated fatty acid contributed to more flexibility in the polymer, which is consequently suitable for decreasing glass transition temperature (Tg).
5.2. Ring-opening polymerization of epoxidized palm olein
Synthesis of palm oil-based polyester polyol can be performed via ring-opening polymerization of epoxidized palm olein (EPOo), as shown in Fig. 7. The epoxidized palm olein mainly consists of monoepoxidized that resulted from oleic acid epoxidation and diepoxidized that obtained from linoleic acid epoxidation, see Fig. 7(a) and (b). The ring-opening of EPOo was done using phthalic acid at the optimal ratio of EPOo/phthalic acid of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reaction condition at 175 °C for 5 h, which resulted in a clear bright yellowish liquid polyol at room temperature.59,60 The synthesized polyester polyols exhibited high molecular weight and had secondary hydroxyl that was useful for sufficient gel time of polyurethane in the adhesive application. Yeoh and coworkers reported on the preparation of polyester polyols via a one-pot reaction of epoxidized palm olein (EPOo) and malonic acid under catalyst-free and solvent-free conditions.61 At optimal reaction conditions resulted in a bright yellowish clear liquid polyester polyol with a could point of 12, pour point of 10 °C, Mn of 5201 Da, a hydroxyl value (OHV) of 98.19 and an acid value of 1.44 mg KOH per g sample. From the same author, instead of using malonic acid (see Fig. 7(c)), they used glutaric acid for making palm oil-based polyester polyol, which led to palm oil-based polyester polyol with an acid value of 1.95 and OHV of 84.50 mg KOH per g sample, Mn of 6698, pour point of 12 °C, and cloud point of 12 °C.62 All the resulted polyester polyols were in their liquid form, which is beneficial for the next step of modification like in preparation of polyurethane since there will be no heating involves to melt the polyester polyols.
1 and reaction condition at 175 °C for 5 h, which resulted in a clear bright yellowish liquid polyol at room temperature.59,60 The synthesized polyester polyols exhibited high molecular weight and had secondary hydroxyl that was useful for sufficient gel time of polyurethane in the adhesive application. Yeoh and coworkers reported on the preparation of polyester polyols via a one-pot reaction of epoxidized palm olein (EPOo) and malonic acid under catalyst-free and solvent-free conditions.61 At optimal reaction conditions resulted in a bright yellowish clear liquid polyester polyol with a could point of 12, pour point of 10 °C, Mn of 5201 Da, a hydroxyl value (OHV) of 98.19 and an acid value of 1.44 mg KOH per g sample. From the same author, instead of using malonic acid (see Fig. 7(c)), they used glutaric acid for making palm oil-based polyester polyol, which led to palm oil-based polyester polyol with an acid value of 1.95 and OHV of 84.50 mg KOH per g sample, Mn of 6698, pour point of 12 °C, and cloud point of 12 °C.62 All the resulted polyester polyols were in their liquid form, which is beneficial for the next step of modification like in preparation of polyurethane since there will be no heating involves to melt the polyester polyols.
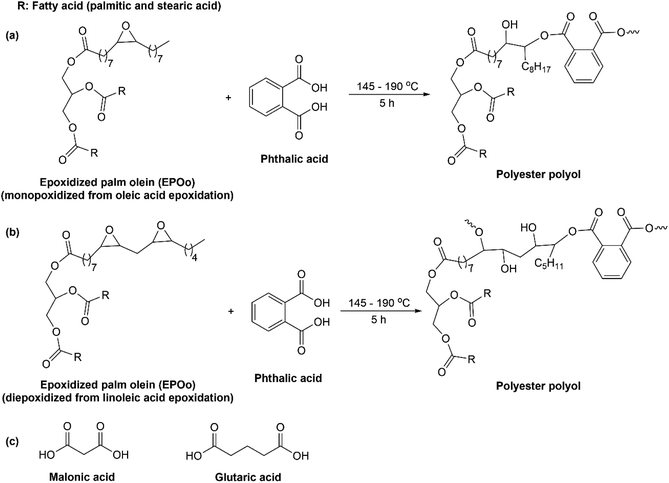 | ||
| Fig. 7 (a) Ring-opening of oxirane group from monoepoxidized and (b) diepoxidized palm olein using phthalic acid, and (c) other diacids that were used to ring-opening epoxidized palm olein. | ||
Oxirane rings in triglyceride are valuable functional groups for further modification. This section discussed the ring-opening polymerization of oxirane functional groups to produce polyester. In a few reports mentioned above, catalysts and solvents are unnecessary in the reactions. However, high-temperature reactions (>100 °C) are needed to perform ring-opening reactions from oxirane rings. This high-temperature reaction is in line with high energy consumption. The ring-opening polymerization of oxirane rings will be preferable if it can be done at a low temperature and short reaction times by adding an environmentally friendly and non-toxic catalyst.
5.3. Polycondensation of AA and BB or AB-type monomers
Palm oil-based polyester can also be synthesized via polycondensation of AA and BB or AB-type monomers. Herein several reports on the synthesis of palm oil-based polyester using these methods. Palm oil-based polyester polyol was prepared via polycondensation of deacidified glycerol monostearate (DGSM) and glutaric acid in the molar ratio of functionality OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COOH of 1
COOH of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.06 in non-catalyzed and solvent-free condition at 200 °C for 12 h.63 The obtained palm oil-based polyester polyol had a light yellowish appearance with a molecular weight (Mw) of 3270 Da, an acid value of 3.30, and a hydroxyl value of 115 mg KOH per g sample. Palmitic acid-based polyester was also synthesized via polycondensation of AA and BB-type monomers. Palmitic acid was converted to methyl palmitate and subsequently changed to malonate derivative (diester). Palmitic acid-based polyester was synthesized using malonate diester, diol (1,6-hexanediol or 1,12-dodecanediol), and Ti(OiPr)4 as a catalyst at 120 °C for 23 h.64 The resulted polyesters of poly(dodecyl 2-tetradecylmalonate) (PDTDM) showed better yield and thermal properties than poly(hexyl 2-tetradecylmalonate) (PHTDM). Bahadi et al. synthesized palm oleic acid-based polyester (POABP) for precursor in the plastic industry, which was prepared from polycondensation of AB-type monomer of epoxidized oleic acid.65 Ester linkages in the POABP were formed from the reaction between the carboxyl and oxirane functional groups in the epoxidized oleic acid. POABP had molecular weights of 1963–5501 g mol−1, glass transition (Tg) of −19 °C, and thermal degradation of 328 °C with no melting temperature observed.
1.06 in non-catalyzed and solvent-free condition at 200 °C for 12 h.63 The obtained palm oil-based polyester polyol had a light yellowish appearance with a molecular weight (Mw) of 3270 Da, an acid value of 3.30, and a hydroxyl value of 115 mg KOH per g sample. Palmitic acid-based polyester was also synthesized via polycondensation of AA and BB-type monomers. Palmitic acid was converted to methyl palmitate and subsequently changed to malonate derivative (diester). Palmitic acid-based polyester was synthesized using malonate diester, diol (1,6-hexanediol or 1,12-dodecanediol), and Ti(OiPr)4 as a catalyst at 120 °C for 23 h.64 The resulted polyesters of poly(dodecyl 2-tetradecylmalonate) (PDTDM) showed better yield and thermal properties than poly(hexyl 2-tetradecylmalonate) (PHTDM). Bahadi et al. synthesized palm oleic acid-based polyester (POABP) for precursor in the plastic industry, which was prepared from polycondensation of AB-type monomer of epoxidized oleic acid.65 Ester linkages in the POABP were formed from the reaction between the carboxyl and oxirane functional groups in the epoxidized oleic acid. POABP had molecular weights of 1963–5501 g mol−1, glass transition (Tg) of −19 °C, and thermal degradation of 328 °C with no melting temperature observed.
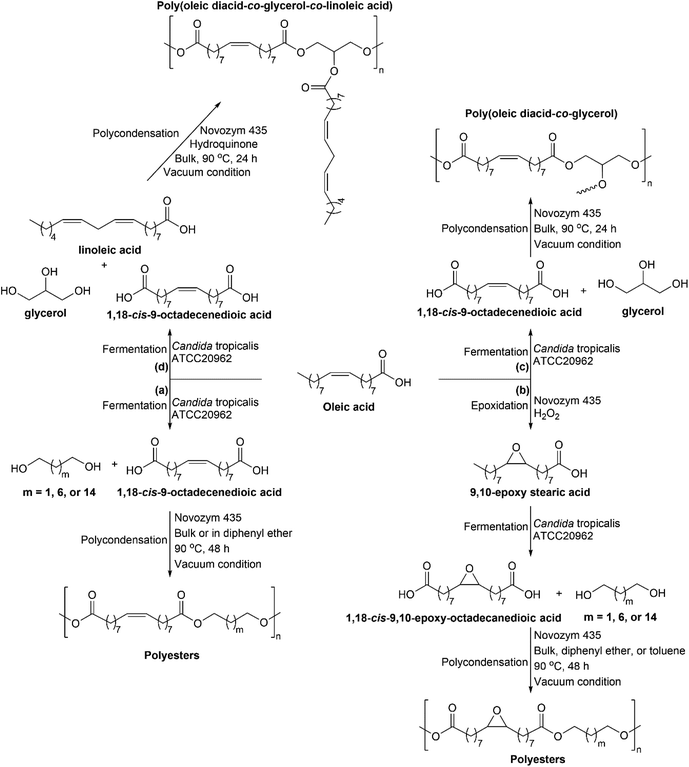
Several reports have been available on the enzymatic modification of fatty acids to fatty diacids and their polymerization. Yang and coworkers reported on the conversion of oleic, erucic, and 9,10-epoxy stearic acids to their corresponding α,ω-diacids functional monomers of 1,18-cis-9-octadecenedioic, 1,22-cis-9-docosenedioic, and 1,18-cis-9,10-epoxy-octadecanedioic acids by whole-cell bio-transformation using Candida tropicalis ATCC20962 as the catalyst that in good yield and with intact double bond or oxirane group, as shown in Fig. 8.32 Modification of oleic acid to 9,10-epoxy stearic acid was done by a chemo-enzymatic method using oleic acid, hydrogen peroxide, and lipase Novozym 435 as the catalyst, see Fig. 8(b). This method was adopted from the previously reported article on lipase-mediated chemo-enzymatic epoxidation of linoleic acid.33 The obtained α,ω-diacids functional monomers were polymerized via enzymatic polycondensation of α,ω-diacids monomer with diols (1,3-propanediol, 1,8-octanediol, or 1,16-hexadecanediol) using Novozym 435 as a catalyst in bulk or diphenyl ether at 90 °C, as shown in Fig. 8(a) and (b), which led to the formation of low melting points (23–40 °C) polyesters with molecular weight (Mw) ranging from 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–57
000–57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.32 Yang and coworkers also reported on the polycondensation of 1,18-cis-9-octadecenedioic acid and glycerol using either enzymatic (Novozym 435 at 90 °C) or chemical (dibutyltin oxide, DBTO at 150 °C) catalyst and without solvent addition.66 Novozym 435-catalyzed polycondensation of oleic diacid and glycerol with a ratio of 1
000 g mol−1.32 Yang and coworkers also reported on the polycondensation of 1,18-cis-9-octadecenedioic acid and glycerol using either enzymatic (Novozym 435 at 90 °C) or chemical (dibutyltin oxide, DBTO at 150 °C) catalyst and without solvent addition.66 Novozym 435-catalyzed polycondensation of oleic diacid and glycerol with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 produced polyester with Mn value of 9000 g mol−1 after 24 h reaction, see Fig. 8(c), whereas using DBTO after 6 h reaction resulted in polyester with Mn value of 1750 g mol−1 and increasing reaction time to 8 h led to gel formation that was due to the formation of crosslinking product. Using Novozym 435 as the catalyst, crosslinking and gel formation were not observed. The reason was a steric hindrance at the CAL B's active site that limits the branching formation and crosslinking reactions.66 Another study on oleic diacids reported the copolymerization of oleic diacids, glycerol, and crude linoleic acid using Novozym 435 as the catalyst at 90 °C and bulk reaction condition to produce polymeric triglyceride mimetic structures, as shown in Fig. 8(d).67 By using a monomer ratio of 1
1 produced polyester with Mn value of 9000 g mol−1 after 24 h reaction, see Fig. 8(c), whereas using DBTO after 6 h reaction resulted in polyester with Mn value of 1750 g mol−1 and increasing reaction time to 8 h led to gel formation that was due to the formation of crosslinking product. Using Novozym 435 as the catalyst, crosslinking and gel formation were not observed. The reason was a steric hindrance at the CAL B's active site that limits the branching formation and crosslinking reactions.66 Another study on oleic diacids reported the copolymerization of oleic diacids, glycerol, and crude linoleic acid using Novozym 435 as the catalyst at 90 °C and bulk reaction condition to produce polymeric triglyceride mimetic structures, as shown in Fig. 8(d).67 By using a monomer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.67 for oleic diacids, glycerol, and crude linoleic acid, resulting in a product with Mn ∼9500 g mol−1 and 64% trisubstituted glycerol unit after 8 h reaction. The trisubstituted glycerol unit increased by nearly 100% when the proportion of crude linoleic acid increased to 1.33.
0.67 for oleic diacids, glycerol, and crude linoleic acid, resulting in a product with Mn ∼9500 g mol−1 and 64% trisubstituted glycerol unit after 8 h reaction. The trisubstituted glycerol unit increased by nearly 100% when the proportion of crude linoleic acid increased to 1.33.
From the reports mentioned above, using an enzyme as a catalyst benefited in milder reaction conditions and lower reaction temperature than using a chemical catalyst. Moreover, in enzyme catalysis, unwanted side products such as gel formation and crosslinking can be avoided since the pocket of the active site in the enzyme has specificity on the type of substrate and substrate acceptance limitation due to the steric hindrance. The synthesis of bio-based polymers will be more environmentally friendly, not only through the use of bio-renewable resources as feedstocks but also by using green processes, like using an enzyme as the catalyst.68 However, there is also a limitation in using an enzyme as a catalyst in polymerization, which is the relatively long reaction time (up to days of reaction) to produce a high molecular weight of polymers. It will be better if, soon, the enzyme can be used as a catalyst in a short reaction time (less than one day) to produce high molecular weight polymers.
6. Polyols
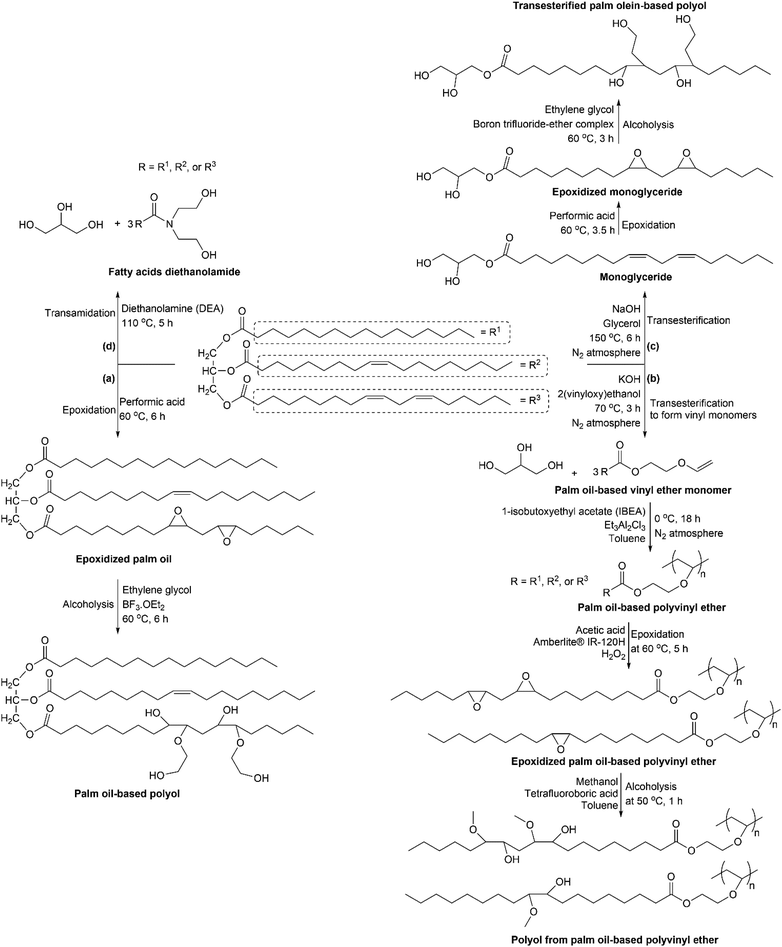
Polyols are polymeric materials containing multiple hydroxyl groups. Fig. 9 shows several methods that can be used to prepare palm oil-based polyols. In Fig. 9(a), the palm oil-based polyol was synthesized via epoxidation and ring-opening of oxirane groups.28 There are several reports on the epoxidation of palm oil using performic acid,28,69 peracetic acid,31,70,71 or a combination of performic acid, sulfuric acid, and other catalysts.72 Subsequently, the ring-opening of oxirane groups can be carried out via alcoholyisis or hydroxylation using methanol and tetrafluoroboric acid,31 H2O and sulfuric acid,69 ethylene glycol and BF3·OEt2,28 diethylene glycol,70 methanol and HCl,71 or a combination of methanol, water, and formic acid.72 Interestingly, Liu et al. utilized used-frying palm oil as triglyceride feedstock to prepare polyol.71 Moreover, Pillai and coworkers reported on the preparation of polyol from a modified palm oil triacylglycerol (PMTAG) via epoxidation and hydroxylation method.73 This PMTAG was obtained as a side product from a cross-metathesis reaction of 1-butene and palm oil for commercially producing 1-decene and 4,4-dodecene. A report on the alcoholysis of epoxidized palm olein (EPOo) was performed using isobutanol and K10 montmorillonite as a catalyst at different reaction temperatures and times.74 All the palm oil-based polyols resulted in a high yield of more than 90%. The produced polyols had low acid value, viscosity, molecular weight distribution, and polydispersity index with functionality in the range of 2–3. This low molecular weight polyol with a functionality of 2 had the potential to be used in coating application.Kalita and coworkers reported that triglycerides in palm oil were modified to fatty acid-based vinyl monomers before epoxidation and alcoholysis, as shown in Fig. 9(b).31 Double bonds from the vinyl group were polymerized by cationic polymerization. The obtained polymer had unsaturated and saturated fatty acids as side chains. The double bond in the unsaturated fatty acids was further modified via epoxidation to form oxirane rings. Subsequently, the oxirane group was ring-opened via an alcoholysis reaction with methanol to form polyols.
Slightly different methods in polyol preparation, as mentioned above, transesterification was performed prior to the epoxidation process, see Fig. 9(c). Arniza et al. prepared transesterified palm oil-based polyol via three-step reactions involving: (1) transesterification of palm olein using glycerol and catalyst, (2) epoxidation of transesterified palm olein using performic acid, and (3) ring-opening of oxirane group from the epoxidized product using ethylene glycol and boron trifluoride as shown in Fig. 9(c).30 Mohammed et al. reported the preparation of polyols from palm oil and soybean oil by transesterifying triglycerides using glycerol and CaO as a catalyst to form monoglycerides.75 The produced monoglycerides act as a polyol to prepare thermoplastic and thermosetting polyurethanes. Glycerol and CaO were also used in the transesterification of palm olein to produce palm oil-based polyol, as reported by Lumcharoen and coworkers.76 Moreover, Mohd Tahir and coworkers reported on the synthesis of polyol from purified-waste cooking oil (WCO).77 The purification of waste cooking oil was performed by filtration and further adsorption using sugarcane bagasse (SCB) activated carbon. WCO-based polyol was prepared by first making a polyhydric solution, which contained a mixture of diethanolamine, monoethylene glycol, and potassium acetate with a ratio of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
3.
The transamidation process also can be used to prepare polyol from palm oil, see Fig. 9(d). Transamidation of refined bleached deodorized palm oil (RBDPO) using diethanolamine without the addition of catalyst and solvent free resulted in the formation of diethanolamide.78 Using the amidation method, palm oil-based diethanolamide was prepared from the reaction of palm oil with diethanolamine and sodium methoxide as the catalyst.79 Transesterification-alcoholysis reaction using palm oil-based diol, triethanolamine, and lithium hydroxide was carried out to synthesize polyol ester for polymeric nanoparticles in drug delivery system application.80
Instead of using whole palm oil as feedstock, epoxidation and alcoholysis can also be performed on fatty acid methyl ester (FAME) to produce fatty-acid methyl ester-based polyol (PolyFAME-EG).81 FAME (biodiesel) was derived from palm oil, which contains C12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (0.1%), C14
0 (0.1%), C14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (0.6%), C16
0 (0.6%), C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (5.7%), C18
0 (5.7%), C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (3.6%), C18
0 (3.6%), C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (72.7%), and C18
1 (72.7%), and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (17.1%). Moreover, empty fruit bunch (EFB) from oil palm trees can also be used as a source for polyol preparation. Amran et al. reported on the liquefaction of oil palm EFB and EFB pulp sheets (cellulose) via the microwave-assisted method for polyols production.82 Further studies on the liquefaction of oil palm EFB and EFB cellulose to produce polyols were carried out in sulfuric acid and polyethylene glycol-glycerol as co-solvent at different designated temperatures and reaction times.83
2 (17.1%). Moreover, empty fruit bunch (EFB) from oil palm trees can also be used as a source for polyol preparation. Amran et al. reported on the liquefaction of oil palm EFB and EFB pulp sheets (cellulose) via the microwave-assisted method for polyols production.82 Further studies on the liquefaction of oil palm EFB and EFB cellulose to produce polyols were carried out in sulfuric acid and polyethylene glycol-glycerol as co-solvent at different designated temperatures and reaction times.83
Several methods are selected to prepare polyols and may result in different types of polyols in terms of their hydroxyl value. OHV of polyols prepared via various methods is summarized in Table 3. The OHV may vary from 78 to 749 mg KOH per g sample. We can conclude that using more reaction steps in fragmenting the triglycerides into its fatty acid or fatty ester will result in better access to the double bonds in the fatty acid moieties. The direct use of palm oil for epoxidation and alcoholysis resulted in low OHV (<100 mg KOH per g sample). We can assume that it will be much harder to access double bonds in a fatty acid moiety of triglyceride than in the fatty acid moiety of monoglyceride, or even better if the source of the double bond is from free fatty acid or fatty ester. Using conventional reaction as shown in Fig. 9, the reaction scheme (c) produced polyol with an OHV of 330 mg KOH per g sample,30 which was the highest compared to other palm oil-based polyols. Moreover, a microwave-assisted method can achieve OHV up to 749.22 mg KOH per g from liquefaction oil palm EFB.82 This information on hydroxyl value is important whether the polyols are further used for producing polyurethanes (PUs). The low and high hydroxyl values of polyols do not align with the quality of polyols but with the number of hydroxyl groups involved in making urethane linkages. A low hydroxyl value of polyol meant fewer hydroxyl groups for making urethane linkages than the high hydroxyl value of polyol. There are specifications for polyols in the polyurethane industry, such as polyols used in the production of flexible foam/elastomers PU should have a molecular weight of 1000–6500, OHV of 28–160 mg KOH per g sample, and functionality of 2–3, whereas, polyols utilized in the production of rigid/structural foam PU should have molecular weights of 400–1200, OHV of 250–1000 mg KOH per g sample, and functionality of 3–8.84 Therefore, PUs from polyol with low hydroxyl value will have different properties with PUs from polyol high hydroxyl value. The next section will discuss the utilization of palm oil-based polyols for PUs production.
| Source of triglyceride or fatty acid | Methods in polyol preparation | Hydroxyl value (OHV) mg KOH per g sample | Ref. |
|---|---|---|---|
| a This OHV had a unit in mL NaOH per g sample. | |||
| • Palm oil | • Epoxidation using performic acid | 102 | 69 |
| • Hydroxylation using H2O and H2SO4 | |||
| • Palm oil | • Epoxidation using peracetic acid | 147.1 | 70 |
| • Alcoholysis using diethylene glycol | |||
| • Residual palm oil (RPO) | • Epoxidation using performic acid, sulfuric acid, and catalyst | 78.525 | 72 |
| • Alcoholysis using methanol and water | |||
| • PMTAG | • Epoxidation using perchloric acid | 155 | 73 |
| • Hydroxylation using H2O and HClO4 | |||
| • EPOo | • Alcoholysis using isobutanol and K10 montmorillonite as a catalyst | 124.71 | 74 |
| • Palm olein | • Transesterification using glycerol and catalyst | 330 | 30 |
| • Epoxidation using performic acid | |||
| • Alcoholysis using ethylene glycol and BF3 | |||
| • Palm oil | • Transesterification of palm oil | 233 | 75 |
| • Soya oil | • Transesterification of soya oil | 251 | |
| • Palm olein | • Transesterification using glycerol and CaO | 140 | 76 |
| • Purified waste cooking oil (WCO) | • Formation of polyhydric solution | 148.79 | 77 |
| • Transesterification between WCO and polyhydric solution | |||
| • RBDPO | • Transamidation using diethanolamide | 254.20a | 78 |
| • Palm oil-based diol | • Transesterification and alcoholysis using triethanolamine and LiOH | 182.51 | 80 |
| • Biodiesel (FAME) | • Epoxidation using performic acid | 166.5 | 81 |
| • Alcoholysis using ethylene glycol and BF3 | |||
| • Oil palm EFB | • Microwave liquefaction using ethylene glycol and sulfuric acid | 749.22 | 82 |
| • EFB cellulose | 639.91 | ||
| • Oil palm EFB | • Liquefaction using sulfuric acid and polyethylene glycol-glycerol at different temperature | 228.08 | 83 |
| • EFB cellulose | 270.49 | ||
7. Polyurethanes (PUs)
Synthesis of polyurethanes can be performed through the reaction of the compounds containing two or more hydroxyl functional groups (diol/polyol) with compounds containing two or three isocyanate functional groups to form urethane (–NHCOO–) linkages, as shown in Fig. 10(a). Fig. 10(b) displays commonly used isocyanates for producing polyurethanes. Depending on the synthetic route and applications, there are several types of PUs, for example, foams (low-density flexible, semi-rigid, or rigid foams), elastomers, and thermoplastic.85 Typically, PUs have segmented structures consisting of soft and hard segments. The diol/polyols contribute to the soft segment formation, whereas isocyanates provide the hard segment structure. A combination of monomers with low molecular weight will result in very concentrated urethane bonds, increasing the number of hard segments in the PUs structure and consequently leading to hard and stiff PUs. Conversely, using a long and high molecular weight of monomers will result in more soft segments in the PUs structure, which subsequently yields elastomeric and flexible PUs. Thus, the primary factor in determining PUs properties is the NCO/OH ratio.85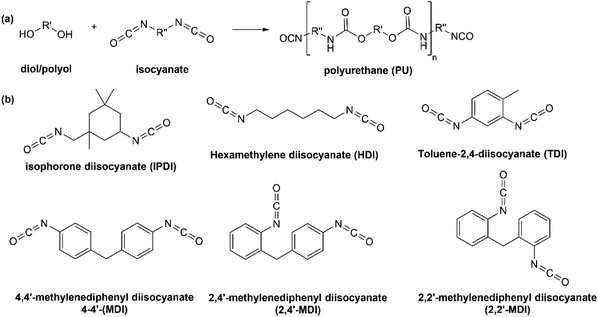 | ||
| Fig. 10 (a) Synthesis of polyurethane between diol/polyol with isocyanate and (b) different types of isocyanates. | ||
Synthesis of PUs can be performed by the one-shot or prepolymer method. The one-shot process is the mixing directly of all components for making PUs. In the prepolymer method, a polyurethane prepolymer with –NCO end group is produced via the reaction of diols/polyols with isocyanates, which is subsequently chain extended to form PUs.85 Since polyols as the source of hydroxyl groups and isocyanates for making polyurethanes can be synthesized from vegetable oil. Thus, the synthesis of PUs may also be prepared from raw renewable feedstock such as vegetable oil. A review of the synthesis, characterization, and properties of polyurethanes from various vegetable oils has been reported.9,25,84 This section discusses the recent advancement of palm oil-based polyurethanes.
7.1. PUs foams
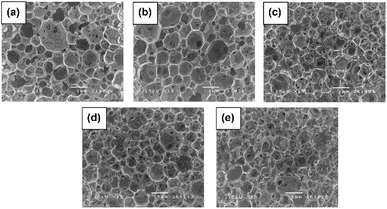 | ||
| Fig. 11 Flexible PU foams SEM micrographs of (a) reference foam from 100% petrochemical-based polyether polyol; foams from blends of the petrochemical-based polyol with (b) 10%, (c) 20%, (d) 30%, and (d) 40% palm oil-based polyol. Reproduced with permission from ref. 76 Copyright 2014 Trans Tech Publications. | ||
A high hydroxyl value (OHV) of polyols can be used for producing PU rigid foam. However, by using blended polyol of transesterified palm olein-based polyol (OHV of 330 mg KOH per g sample) and petrochemical-based polyol (polyether polyol with OHV of 430–470 mg KOH per g sample) as the source of polyols, resulted in semi-rigid PU foams with lower reactivity of the foaming profile, lower density, and compressive strength than that of 100% petrochemical-based polyol.30 Semi-rigid PU foams were also produced by blended palm oil-based polyol with OHV of 102 mg KOH per g sample up to 70 wt% with commercially available polyether polyol (OHV of 449 mg KOH per g sample).69 PU foam was also prepared from 100% palm oil-based polyol. However, the obtained PU foam morphology had a heterogenous sample with big pores (collapsed cells) zones and others with smaller cells, as shown in Fig. 12(a). Moreover, rigid PU foams were obtained by blending palm oil-based polyol with OHV of 147.1 mg KOH per g sample up to 70 wt% with commercially available polyether polyol (OHV of 449 mg KOH per g sample).70 Incorporation of palm oil-based polyol resulted in deceleration of the foaming and gelling reactions, decreased density, mechanical, and dynamic mechanical performance with the increased palm oil-based content in PU foams. Furthermore, Septevani et al. reported on a study of the preparation of rigid polyurethane by substituting petrochemical-based polyether polyol with palm kernel oil-based polyester polyol and still maintaining the thermal and mechanical performances comparable to PUs prepared from petrochemical-based polyether polyol.88 Rigid PU foams incorporated with palm oil-based polyol up to 30% displayed comparable mechanical and thermal performances as well as dimensional stability as compared to that of reference PU foam from petrochemical-based polyether polyol.
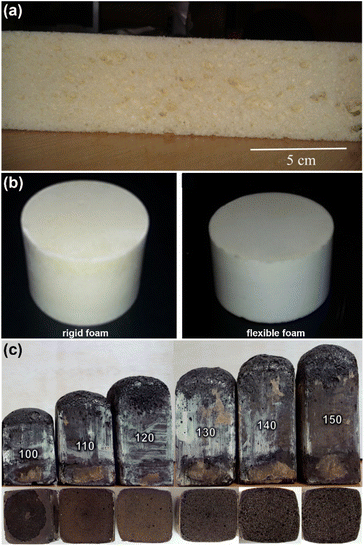 | ||
| Fig. 12 PU foams picture of (a) cross-section from semi-rigid PU foam derived from palm oil-based polyol (reproduced with permission from ref. 69 Copyright 2017 Elsevier) (b) rigid and flexible PU foams produced from PMTAG polyol (reproduced with permission from ref. 93 Copyright 2016 Elsevier), and (c) rigid PU foams prepared from liquefied oil palm EFB polyol at various NCO indexes (reproduced with permission from ref. 95 Copyright 2021 MDPI). | ||
Hydroxy telechelic natural rubber (HTNR) was used as a polyol source with the combination of palm oil-based polyol to produce flexible PU foams. A series of PU foams (PUFs) were prepared by varying the mixture ratios of bio-based polyols (palm oil-based polyol and HTNR), 1,4-butanediol (BDO) as chain extender, catalysts, surfactant, H2O as a blowing agent, dichloromethane (DCM), and diisocyanate (TDI or p-MDI).28 All the PUFs displayed single Tg, which indicated good miscibility between palm oil-based polyol and HTNR. p-MDI-based PUFs exhibited higher storage modulus (E′), hardness, and compressive strength than that TDI-based PUFs.28 Rigid and flexible PU foams in Fig. 12(b) were prepared from polyol derived from PMTAG polyol.93 The rigid and flexible PU foams had small-sized closed cells with cell sizes of 270 ± 40 μm and 386 ± 55 μm, respectively. The closed cell structure and the thermal stability of these rigid and flexible foams indicated the appropriateness for application in thermal insulation. Regarding the structure and physical properties of the foams, the PMTAG polyol might be able to compete with the commercially available bio-based and petrochemical based-polyol for producing industrial-scale PU foams. Moreover, Mohd Tahir et al. reported on the preparation of rigid PU foam waste cooking oil (WCO)-based polyol.77 Chavarro Gomez and coworkers studied the characterization of rigid PU foam prepared from polyol of mixtures of various ratios of residual palm oil (RPO) and algae oil (AO).94 The rigid PU foam with 50% AO content showed improvement in homogeneous cell structure, slightly lower in thermal stability and conductivity than that of industrial insulation materials. Conversely, bio-based PU foams with higher RPO content demonstrated better flexural stress, which could be used in applications requiring abrasion resistance.
Polyols produced by the liquefaction of oil palm empty fruit bunch (EFB) and EFB cellulose were used to produce rigid PU foams.83 The PU foam from oil palm EFB-based polyol displayed larger cell sizes and distribution than from EFB cellulose-based polyol. Thus, PU foam from EFB cellulose-based polyol displayed better thermal and mechanical performance than PU foam from oil palm EFB-based polyol. Further studies on the preparation of rigid PU foams from oil palm EFB-based polyol and p-MDI with the variation of isocyanate indexes (100, 110, 120, 130, 140, and 150) as shown in Fig. 12(c).95 The normalized compressive strength of EFB-based PU foams with a 120 NCO index was comparable to PU foam from petrochemical-based polyol (as reference PU foam). Moreover, pictures of PU foams prepared using oil palm-based polyols are shown in Fig. 12(a)–(c). The appearance of PU foams was also affected by the polyol's color's physical state. Thus, depending on PU applications, in PU formulation, it is crucial to consider the type of polyol, isocyanate (aliphatic or aromatic), the isocyanate index, the amount of water (blowing agent), and other chemicals needed to prepare the PU foams with desired chemical, mechanical, and thermal properties.
7.2. Adhesives and coatings
Polyurethane (PU) for wood adhesive application was prepared via the reaction between palm oil-based polyester polyol with polymeric 4,4′-MDI (p-MDI) at NCO/OH ratio of 1.3 and the addition of glycerol as a crosslinker, which resulted in PU adhesive with good spread-ability on the wood surface and low gel time (∼90 min) and had a lap shear of twice higher than the commercially available adhesives (Titebond™ and Weldbond™).59 Further studies from the same author on the variation of glycerol concentrations and the use of different isocyanates (p-MDI and TDI) in the production of PU adhesives from palm oil polyester polyol were also reported.60 p-MDI-based PU adhesives displayed better lap shear strength and thermal stability than TDI-based PU adhesives. PU adhesive prepared by p-MDI or TDI, both of the products showed superior mechanical strength that was two times higher in lap shear strength than that of commercially available wood adhesives polyvinyl acetate adhesive (Titebond™ and Weldbond™).60Velayutham et al. reported on the properties of palm oil-based PU coating influenced by oleic acid and chemical crosslinks content.96 PU film with 65% oleic acid content displayed the highest thermal stability at 213 °C. All the PU films showed constant Tg values, regardless of the NCO/OH ratio increase. PU films with 28% oleic acid content demonstrated the highest rupture strength of 36 MPa and possibly a very robust coating. Variations content of oleic acid and NCO/OH ratios led to a high-quality coating with superior performance and unfailing long-term application. Moreover, the emulsion type of paint was prepared using palm oleic acid-based polyol (Mw of 950) with 4,4′-MDI.97
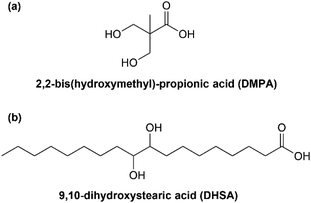
PU films were prepared via the pre-polymerization method using palm kernel oil-based monoester polyol (PKO-p) and 2,4′-MDI with different ratios of NCO/OH.98 Prior to the addition of MDI, diethylene glycol (DEG) as a chain extender was mixed with PKO-p. All PUs before the curing process appeared as sticky film. However, after curing, the film with a higher NCO ratio had a rigid physical state, while the film with a higher polyol ratio had a flexible physical form. The PU film from PKO-p was clear and semiflexible, which can be utilized in coating applications, particularly onto the fiberboard, since PU was hydrophobic. Moreover, Teo and coworkers reported on UV-curable urethane acrylate derived from palm fatty acid distillate (PFAD). Five different concentrations of PFAD (15, 25, 45, 55, and 70% w/w) were used to prepare PFAD hydroxyl-terminated macromers.58 Each obtained macromer was reacted with 2-hydroxyethyl acrylate (HEA) and TDI to form PFAD-based urethane acrylate resins with acrylate as side chains. UV-cured PFAD-based urethane acrylate resins with 15–55% PFAD resulted in hard films with Tg values range of 35–62 °C. UV-cured PFAD-based urethane acrylate resin containing 70% PFAD formed a soft film with Tg < 30 °C. However, the UV-cured of PFAD-based urethane acrylate resins with 45% PFAD film displayed the best chemical and physical properties, which would be useful for wood coating applications. In another study, Kosheeladevi et al. presented the application of 9,10-dihydroxystearic acid (DHSA) from palm oil as an internal emulsifier or isocyanate-reactive compound for waterborne polyurethane dispersions (WPUDs) productions.99 DHSA is a promising candidate to fully or partially substitute 2,2-bis(hydroxymethyl)-propionic acid (DMPA) in the preparation of WPUDs. The lower melting temperature of DHSA than DMPA benefited in reducing the reaction time and temperature in the early phase of the synthesis of producing a homogenous mixture with polyol. In terms of film hardness, the WPUDs film with a higher DMPA content had a better hardness value than those DHSA-contained films. The reason was due to the hard property of DMPA as a smaller compound compared to DSHA, a big compound with a C-18 carbon chain length, as shown in Fig. 13. Consequently, DHSA contributes to softness and flexibility to the films. The WPUDs films and coatings with the mixture of 0.5/0.5 of DMPA/DHSA content exhibited very good properties in tensile strength, hardness, elongation at break, and water resistance.
The reported articles show that PUs can be used in adhesive and coating applications. For adhesive applications, palm oil-based PUs demonstrated better mechanical strength than commercially available wood adhesives Titebond™ and Weldbond™.59,60 These results showed the potential of palm oil-based PUs to replace petrochemical-based PUs for adhesive application. Moreover, the utilization of PFAD as raw material feedstock and DHSA as an isocyanate compound for waterborne polyurethane dispersions (WPUDs) production will increase the sustainability and environmentally friendly process.
7.3. Thermoplastic, thermosetting, and elastomer PUs
Thermoplastic and thermosetting PUs were prepared from palm and soybean oil-based polyols.75 Monoglycerides (act as polyol) from palm and soybean oil had OHV of 233 and 251 mg KOH per g, respectively. Due to high crosslink, thermosetting polyurethanes (TSPUs) displayed better thermal properties than those thermoplastic polyurethanes (TPPUs). TSPUs performed well in adhesion and pencil hardness but were poorer in impact strength and flexural than TPPUs. Soybean oil-based PU showed better performances in thermal, pencil hardness, and impact strength than that palm oil-based PU. However, soybean and palm oil-based PU showed similar physical resistance and adhesion test properties. Moreover, Kalita and coworkers reported on the utilization of palm oil-based polyvinyl ether [HO-poly(2-VOEP)] and palm oil-based polyols in producing polyurethane thermoset network using hexamethylene diisocyanate trimer (HDT) as the isocyanate-functional component.31 Their studies showed that the urethane network from [HO-poly(2-VOEP)] polyol cured faster and had significantly higher modulus, hardness, tensile strength, gel content, and chemical resistance than that of the analogous urethane network from palm oil-based polyols. The conversion of palm oil to palm oil-based vinyl ether monomer and subsequently to palm oil-based polyvinyl ether provide advantages to the fatty acid ester pendant group in making the urethane network. Furthermore, Zeng et al. studied the preparation of palm oil-based thermoplastic polyurethane (TPU) elastomers that was performed by reaction of palm oil-based diethanolamide (POEA) with 1,4-butanediol (BDO) and 1,6-diisocyanatohexane.79 Soft to hard segments ratio controls the toughness and mechanical strength of the elastomers, which was tunable by adjusting the ratio of BDO and POEA. Increasing the BDO content improved the toughness and tensile strength of the elastomers. The obtained elastomers had a high stretchability (up to a strain of 831%), restore ability, and self-healing ability (∼87%).Somarathna et al. studied palm-based PU elastomers properties for potentially being applied as a protective coating for strengthening structures of concrete and from impact loading.100 The palm-based PU elastomers were prepared via the pre-polymerization method from a reaction of palm-based polyol and 4,4′-MDI with the addition of different content of PEG as a plasticizer at ambient temperature and free catalyst. The PEG content in PU elastomers significantly influenced the mechanical performances of PUs. Palm-based PU elastomers with 2–8% w/w PEG potentially be used for strengthening concrete applications in regards to their overall performances of high strain characteristics, good moduli, and strength properties. Moreover, Mohd Norhisham et al. reported on the preparation of soft PU elastomers with adhesion properties from polyols of palm olein and palm oil fatty acid methyl ester.81 Bio-based polyols were used as the source of polyols for PUs preparation, which consists of a polyol of palm oil fatty acid methyl ester-ethylene glycol (PolyFAME-EG) and commercially available palm olein polyols (Pioneer M-60 and Pioneer E-135). The obtained PU elastomers had high renewable content of more than 70%. PU elastomers with an NCO/OH ratio of 0.73 displayed adhesion properties with peel adhesion in the range of 1.98–2.27 N per inch, which was potentially used as a pressure-sensitive adhesive with high renewable content. Furthermore, Liu and coworkers reported on the study of bio-based elastic PU (EPU) for controlled-release urea fertilizer produced from the waste of frying palm oil.71 Production of PU was performed using polyol, MDI, DBTL, and acetone. EPU was prepared using a similar formulation for making PU with the addition of acrylonitrile (AN as a second monomer). The acrylonitrile created an interpenetrating polymer network for EPU via physical crosslinking. The PU and EPU were further coated with various concentrations of urea fertilizer and subsequently cured, which resulted in palm oil-based PU-coated urea fertilizer (PCU) and EPU-coated urea fertilizer (ECU). The surface of ECU displayed more hydrophobicity than PCU. In terms of the cumulative N release rates, the ECU displayed slower release than PCU, which was excellent for controlled-release fertilizer characteristics. Therefore, the modification of PU using AN was crucial to make the coatings denser and more hydrophobic. Due to its elasticity, the ECU exhibited different swelling volumes on diverse release days as compared to PCU, as shown in Fig. 14(a) and (b). The volume of ECU was twice bigger than the initial volume on day 49th and shrank on day 77th when close to the complete release of nutrients, as shown in Fig. 14(c).
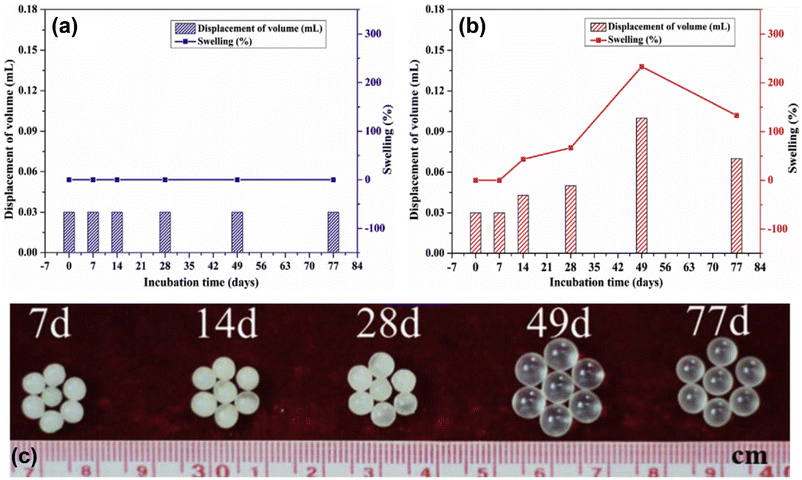 | ||
| Fig. 14 Swelling rates of (a) PCU, (b) ECU, and (c) picture of swollen ECU coated with 7% urea fertilizer. Reproduced with permission from ref. 71 Copyright 2019 Elsevier. | ||
Gomez et al. reported on the synthesis of PUs from residual palm oil (RPO) polyol for biodegradable polyurethanes.72 RPO has a much lower iodine value (IV) number than that refined palm oil but a higher number of free fatty acid (FFA) contents. Also, 10% of jatropha oil (JO) or algae oil (AO) was added to the RPO. Among all the synthesized RPO-based PUs, thermal properties of RPO-based PU with the addition of AO > RPO-based PU with the addition of JO > RPO-based PU. Moreover, Rasli Rosli et al. reported on the synthesis of shape-memory PU (SMPU) via two-step polymerization of polycaprolactone (PCL), isocyanates, and palm kernel oil-based polyol.101 Different ratios of 4,4-methylenebis (cyclohexyl isocyanate) (HMDI) and IPDI were used to prepare SMPU. The SMPU with high IPDI content resulted in better shape-memory behavior; meanwhile, SMPU with high HMDI content led to SMPU with better chain flexibility.
The natural behavior of palm oil-based polymer will be more flexible due to the higher saturated fatty acid content in palm oil. Thus, the production of thermoplastic and elastomers materials will be in accord with the natural properties of palm oil-based polymers. In terms of preparing thermosetting PUs, it will be easier to choose vegetable oils with more unsaturated fatty acids content. Therefore, less modification will involve in the preparation of PUs thermosetting.
7.4. Polymer electrolytes
Polyurethanes are a promising candidate to be utilized as a polymeric host for application in polymer electrolytes. However, the low conductivity and high Tg of PUs limited the use of PUs in polymer electrolytes application. Efforts to increase PUs conductivity can be carried out by incorporating ionic salt as a charge carrier. Moreover, the incorporation of ionic salt not only improves the conductivity of PUs but also reduces the crystallinity of PUs and leads to a decrease in the Tg value. In order to further lower the Tg of PUs, the addition of a chain extender, such as polyethylene glycol (PEG), in PUs formulation and plasticizer is another possible option that can be performed.Table 4 summarizes the types of polyols, isocyanates, chain extenders, and charge carriers that have been used in the preparation of PUs for electrolyte applications. Based on Table 4, the incorporation of ionic salt as a charge carrier significantly improves the conductivity properties of PUs. Among all the ionic salt, the inclusion of 25 wt% MPII to palm-based PU presented the highest conductivity of 9.07 × 10−4 S cm−1. From these reported studies, the conductivity properties of PUs are affected by the type and concentration of charge carriers, the isocyanate to polyol ratio, and the type of isocyanate selected in PU formulation.
| Type of polyol and isocyanate | Chain extender | Charge carrier | Tg (°C) | The highest conductivity (S cm−1) | Ref. |
|---|---|---|---|---|---|
| a NA: not available. The data was not available in the reference. | |||||
| Palm kernel oil-based polyol (PKO-p) and 2,4′-MDI | PEG | Iodopropane | 17 | 2.38 × 10−9 | 102 |
| PKO-p and 4,4′-MDI | PEG | LiClO4 | 89.65 | 1.19 × 10−7 at 298 K | 103 |
| 5.01 × 10−5 at 373 K | |||||
| PKO-p and 2,4′-MDI | — | LiI | 30 | 5.33 × 10−5 | 104 |
| PKO-p and 2,4′-MDI | — | LiCF3SO3 | NAa | 1.6 × 10−5 | 105 |
| Waste cooking oil-based polyol and 4,4′-MDI | — | LiTFSI | 361.3 | 5.76 × 10−6 | 106 |
| Waste cooking oil-based polyol and HDI | — | LiTFSI | 313.6 | 6.03 × 10−7 | 106 |
| PKO-p and 2,4′-MDI | — | LiI | NAa | 7.6 × 10−4 | 107 |
| PKO-p and 2,4′-MDI | — | 1-methyl-3-propylimidazolium iodide (MPII) | 58 | 9.07 × 10−4 | 108 |
| PKO-p and 4,4′-MDI | PEG | — | 78.1 | NAa | 109 |
| PKO-p and 4,4′-MDI | PEG | LiClO4 | NAa | NAa | 110 |
Interestingly, this palm kernel oil-based polyurethane for screen-printed electrode (SPE) was applied for histamine detection in mackerel fish.110 Histamine is an essential chemical compound that can be used as a parameter in allergic reactions and the freshness of food. The PU-LiClO4 film on the SPE surface had pores to entrap histamine and produce an anodic peak at the specific potential. A reasonable limit of quantification (LoQ) and limit of detection (LoD) was observed in the histamine concentrations range of 0.015–1 mmol L−1. The interfering compounds, i.e., cadaverine and putrescine, did not disrupt the signal from histamine. This method was validated for histamine detection in mackerel fish, which resulted in good accuracy with acceptable relative standard deviation (RSD). SPE-PU-LiClO4 demonstrated a simple yet good selectivity, posing the promising potential for histamine determination in mackerel fish.
8. Polyepoxides
Epoxy resins (polyepoxides) are a member of monomeric or oligomeric materials that possess two or more ring-like epoxy/oxirane groups in the monomer to be further reacted to form thermoset.111,112 The formation of crosslinking of the epoxide group is through a condensation mechanism with secondary amine and acid anhydride and with or without a suitable catalyst.112 Epoxies have been used in many applications, such as casting, protective coating in industrial, tooling, bonding and adhesives, laminating, additives for molding, and materials for construction, which are due to their superior resistance to corrosion and chemical, low creeping performances, high modulus and stiffness properties.112,113 A review that focused on epoxy resins prepared from vegetable oil and their composites with the addition of bio-based hardeners incorporated with fiber or filler has been reported.114 However, this section will discuss the literature on epoxy resins that utilized palm or its fatty acid in their preparation process.A study on the effect on morphology and properties of blending 10 wt% epoxidized palm oil (EPO) with epoxy resin (based on DGEBA) was reported by Sarwono et al.115 Incorporating 10 wt% of EPO into epoxy resin resulted in lower Tg and mechanical properties due to the decrease in crosslinking density and the plasticizer effect from EPO. Fernandes and coworkers reported on blends of EPO and DEGBA-based resin.116 A series of epoxy resin blends of commercially available epoxy resin diglycidyl ether of bisphenol-A (DEGBA-based resin) and epoxidized vegetable oil-based resin (epoxidized neat, waste, and purified vegetable oils) with various loading (5–30 wt%) was prepared.116 The vegetable oil was a blend of palm and rapeseed oil with an approximate ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The waste vegetable oil (WVO) was used in deep fat fryers for 4 days. WVO was purified by single117 and multiple extractions and subsequently purified with activated carbon.118 Moreover, Issam et al. reported on the study of properties of blending various ratios of palm oil-based alkyd to epoxy resins.113 In general, alkyd resin and epoxy resin showed good compatibility. The higher composition of epoxy resin resulted in smoother surface morphology due to more effective crosslinking between oxirane functional groups of epoxy resin to the hydroxyl functional groups of alkyd resin. In terms of hardness and surface smoothness, the optimal blending alkyd/epoxy resin ratio was 30
1. The waste vegetable oil (WVO) was used in deep fat fryers for 4 days. WVO was purified by single117 and multiple extractions and subsequently purified with activated carbon.118 Moreover, Issam et al. reported on the study of properties of blending various ratios of palm oil-based alkyd to epoxy resins.113 In general, alkyd resin and epoxy resin showed good compatibility. The higher composition of epoxy resin resulted in smoother surface morphology due to more effective crosslinking between oxirane functional groups of epoxy resin to the hydroxyl functional groups of alkyd resin. In terms of hardness and surface smoothness, the optimal blending alkyd/epoxy resin ratio was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70. The blended resins of higher epoxy resins content displayed better water and chemical resistance and better adhesion compared to the sole alkyd resin.
70. The blended resins of higher epoxy resins content displayed better water and chemical resistance and better adhesion compared to the sole alkyd resin.
Thermoset from the bio-based resin was prepared from modification of epoxidized palm oil via acrylation and then maleinization reaction, as shown in Fig. 15.119 The acrylation reaction of epoxidized palm oil added a reactive double bond onto triglyceride, as shown in Fig. 15(a).120 Meanwhile, the maleinization reaction was done to introduce acid functionality onto triglyceride (see Fig. 15(b)), thus resulting in more crosslink sites.119 Further studies on the acrylated epoxidized palm oil (AEPO) were carried out in preparation for hybrid thermosets derived from vinyl ester resin and AEPO.121 Variations of content loading of 5–20 wt% of AEPO were used to replace vinyl ester petrochemical-based resin. Increasing the content of AEPO in the hybrid thermosets resins led to lower flexural modulus and strength, whereas the flexural strain increased. The incorporation of 5 wt% AEPO displayed a proper balance of stiffness/toughness, which was considered a suitable matrix resin for further use in composite applications. Moreover, a study on the degree of conversion (DOC) of maleinated acrylated epoxidized palm oil (MAEPO) with isobornyl methacrylate (ISBMA) for UV-curable pressure-sensitive adhesives (PSA) was reported by Tugiman et al.122 Furthermore, Salih and coworkers reported on the synthesis of epoxidized palm oil acrylate (EPOLA) for coating applications.120 The EPOLA-cured film with Irg-187 displayed higher conversion, hardness percentages, and crosslinking density, accompanied by better thermal and mechanical properties compared to EPOLA film cured with D-1173. Meanwhile, a comparative study of acrylated epoxidized jatropha oil and acrylated epoxidized palm oil was reported by Aung et al.123 The acrylated epoxidized jatropha oil displayed better scratch resistance, glossiness, hardness, and shorter UV curing time than that acrylated epoxidized palm oil. This observation was due to jatropha oil having higher unsaturated content in its triglyceride than palm oil. Moreover, Sofian Alias and coworkers reported on the preparation of palm oil-based urethane acrylate (POBUA) as a green anticorrosion coating.124 The acrylic groups in POBUA provide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkages to be crosslinked by UV irradiation, which acts as a barrier coating to avoid attacks from corrosive agents on the surface of the mild steel surface. More studies on radiation-curable palm oil-based products have been reviewed by Tajau et al.125
C linkages to be crosslinked by UV irradiation, which acts as a barrier coating to avoid attacks from corrosive agents on the surface of the mild steel surface. More studies on radiation-curable palm oil-based products have been reviewed by Tajau et al.125
 | ||
| Fig. 15 Modification of epoxidized palm oil (EPO) via (a) acrylation using acrylic acid to form AEPO or EPOLA and (b) maleinization using maleic anhydride to produce MAEPO. | ||
Mu and coworkers reported on the preparation of palm oil-based epoxy resins with recyclability, high mechanical strength, and reconfigurable shape-memory properties.126 Initially, palm oil was converted to palm oil-based methyl methacrylate (PMA) monomer by the amidation and esterification method. The PMA monomer was modified into epoxy monomer (EPMA) and further free polymerized. The produced polymer was cured with citric acid (CA) in various feed ratios of acid/epoxy at 90 °C without using a catalyst to form palm oil-based epoxy resins (EP-CA). The EP-CA resins had ester and hydroxyl functional groups in the oxirane crosslinking network that can perform the transesterification process at elevated temperatures, which leads to rearrangement capability. Therefore, EP-CA resins could be reshaped, recycled, and welded without losing the resin's mechanical properties. Furthermore, this dynamic chemical crosslinking contributed to EP-CA resins for shape-memory properties. Both temporary and permanent shapes can be controlled at different temperatures of ≥120 °C, as shown in Fig. 16.
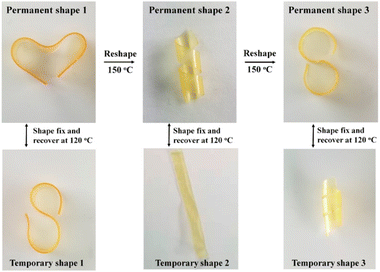 | ||
| Fig. 16 Pictures of EP-CA with a ratio acid/epoxy of 40 undergoing permanent and temporary shape reconfigurations. Reprinted with permission from ref. 126 Copyright 2020 American Chemical Society. | ||
In summary, using palm oil as a feedstock for polyepoxide synthesis has shown promising results based on the product's general thermal and mechanical properties and potential applications. The reported derivatizations reactions to obtain compounds with more oxirane ring functional groups, which are essential for the formation of the resin, are relatively straightforward. In addition, the polymerization reaction (curing process) is relatively simple. Therefore, further exploration of palm oil-based epoxy resins would be an engaging research route, especially for the upscaling means.
9. Polymer composites
Polymer composites are polymeric materials containing a reinforcement, wherein the polymer serves as a matrix mixed with the reinforcements or bonds to the reinforcements, mainly used in automotive and aerospace applications.127 Preparation and properties studies of polymeric nanocomposites derived from vegetable oils with carbon nanomaterials as reinforcements have been reported.25,128 Oil palm products (palm oil and palm kernel oil) have been used as raw material feedstocks for polymer synthesis, as described in this review. Moreover, there is also lignocellulosic biomass produced from the oil palm tree, which includes oil palm trunk (OPT), palm pressed fiber, fronds, empty fruit bunch (EFB), and shells.129 This lignocellulosic fiber from oil palm is also known as oil palm fiber (OPF), and it can be utilized as reinforcement/filler in the composite. A comprehensive review of the mechanical performances of polymer composites reinforced with fiber from oil palm trees has recently been available.129 It is interesting that researchers have utilized not only products but also waste from oil palm plantations to make bio-based polymer and reduce waste by reusing it in various applications. Whereas for this review, we will focus on palm oil-based polymer composites regarding the preparation and characterization of palm oil-based polymer composites.Mohd Sari and coworkers reported on the preparation of lightweight concrete incorporated with palm kernel oil (PKO)-based polyurethane for non-load bearing structural application.130,131 Lightweight-concretes were prepared by two different methods: mixing fresh concrete (mixture of cement, water, and fine sand) with PKO-based polyol and crude MDI130 or by adding ground PKO-based PU to fresh mix concrete.131 At the optimum loading of PKO-based PU, the produced lightweight concrete showed a density of 1600 kg m−3 with compressive strength of 7.8 (ref. 130) and 17.5 (ref. 131) MPa, respectively. Moreover, Alamawi et al. reported on the use of PKO-based polyol (PKO-p) as a partial replacement for bituminous binder.132 They described that the incorporation of PKO-p as a bio-binder (BIB) resulted in similar chemical and thermal properties compared to conventional binders, and the BIB can be adjusted for suitable applications. Another report on bitumen modification was done by Vural kök and coworkers.133 Modifying bitumen by adding 7 wt% of palm oil-based PU with an NCO/OH ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 resulted in the same or even better performance in elasticity as well as high and low-temperature properties compared to bitumen modified with 4 wt% SBS.
1 resulted in the same or even better performance in elasticity as well as high and low-temperature properties compared to bitumen modified with 4 wt% SBS.
Thermosets polymer can also be applied as a polymeric matrix in the production of polymer composites. Nik Salleh and coworkers reported on radiation-curable hybrid coatings that used silico-organic nanoparticles as filler and epoxy acrylates or epoxidized palm oil acrylate (EPOLA) as a polymeric matrix, which resulted in polymer nanocomposites with excellent resistance to abrasion properties as well as scratch performance compared to that of pure acrylates.134 Moreover, Wu et al. prepared a series of palm oil-based thermosets for fiber-reinforced polymer composites that were synthesized by free radical copolymerization of a palm oil fatty acid-ethyl acrylamide (POFA-EA) and natural phenolic (NP) crosslinkers.135 NPs consist of methyl gallate-methacrylate (GM), eugenol-methacrylate (EM), and tannic acid-methacrylate (TM). The thermosets of POFA-EA/GM and POFA-EA/TM with high bio-based content displayed superior mechanical properties and Tgs, better than other vegetable oil-based analogous. The obtained thermosets were then used as the matrix for producing bamboo fibers-reinforced bio-composites via compression molding technique, which displayed comparable mechanical properties and Tgs to their petrochemical-based composite counterparts. Further studies on these palm oil (PO)-based thermosets for fiber-reinforced polymer composites were carried out by preparing copolymer from POFA-EA with isosorbide-methacylate (IM).136 The composites of palm oil-based thermosets reinforced with kenaf, glass, bamboo, and carbon fibers displayed higher Tgs of 136–193 °C compared to their petrochemical-based counterparts. Moreover, the PO-based thermosets matrix also exhibited chemical degradation in an alkali solution that benefited from recycling high-performance reinforcement like carbon fibers. Improvement from their previous studies, Wu et al. reported on the utilization of the photo-curing 3D printing method to prepare highly-customizable PO-based thermoset composites reinforced with micro-scale bamboo fibers (MBFs).137 The PO-based composite inks were formulated by mixing POFA-EA/EM, various loading of MBFs (neat or treated with methacrylic anhydride), and Irgacure 819 (a photo-initiator). Treating MBFs with methacrylic anhydride increased interfacial adhesion between matrix and fibers, improving composites' mechanical properties. This photo-curing 3D printing method provided a more straightforward technique for customizing the shape of polymer composite, as shown in Fig. 17(a). Moreover, PO-based composites fully degraded in alkaline solution and then MBFs were recovered by filtration and drying process, see Fig. 17(b). In Fig. 17(c), the mechanical properties of PO-based composites of original and reprinted using recycled MBFs demonstrated recovery efficiencies of tensile and flexural strength of 68.7% and 80%, respectively. Reprinted PO-based composite using recycled MBFs preserved high shape reliability, as shown in Fig. 17(d).
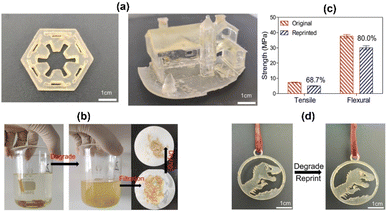 | ||
| Fig. 17 (a) PO-based composites printed in the shape of a hexagon and a house, (b) complete degradation of printed PO-based composite in alkaline solution (3 wt%) after 3 h for MBFs recovery, (c) recovery efficiencies of the tensile and flexural strength of PO-based composites original and reprinted using recycled MBFs after alkaline degradation, and (d) reprint of PO-based composite reinforced with recycled MBFs. Reproduced with permission from ref. 137 Copyright 2022 Elsevier. | ||
Koay et al. compared the properties of composites from cocoa pod husk (CPH)-filled polypropylene (PP) with the addition of different green coupling agents (GCA) from coconut oil (GCA-C) and palm oil (GCA-P), respectively.138 The variation loadings of GCA-C and GCA-P were added in PP/CPH composites to enhance adhesion between matrix surfaces and filler. At the optimum loading of GCA-C or GCA-P at 3 wt%, the PP/CPH composites displayed significant increases in tensile strength and tensile modulus. GCA-C performed better in increasing interfacial adhesion between the PP matrix and CPH than GCA-P. Furthermore, GCA-P of 3 wt% was further used in the study on the torque rheological performance of PP/CPH composites.139 The PP/CPH composite's rheological behavior depended on the change in viscosity from the variation loading of filler and adhesion between the polymer matrix and CPH. At higher filler loading, higher melt viscosity was observed due to CPH agglomeration and better adhesion of matrix filler in the addition of GCA-P.
Islam et al. reported on the preparation of various cured composite films from palm oil-based polyalkyds with multiwalled carbon nanotubes (MWCNTs),140 montmorillonite nanoclays (MNCs),141 or a combination of MWCNTs with or without ZnO.142 The polyalkyds were prepared by alcoholysis using glycerol and CaO, and subsequently esterification using phthalic anhydride or maleic anhydride.140–142 The addition of nanomaterials with or without ZnO was carried out during sonication or in situ during the esterification reaction. The concentration of 1.0 wt% was the optimal concentration for all the cured films. The addition of nanomaterial via in situ was the best way to incorporate it into the polyalkyds matrix since it resulted in better interfacial interaction and entanglement between the nanoparticles and the polymeric chains. As we compared the mechanical properties of the polyalkyd composites films, the film incorporated with MNCs141 showed better mechanical properties than the film incorporated with MWCNTs140 or a combination of MWCNTs with or without ZnO.142 However, polyalkyd composites film incorporated with MWCNTs140 displayed the highest Tmax at 392 °C during thermal degradation.
There are several reports on the preparation of polymer composites from palm oil-based polyurethane with various types of reinforcements or fillers. In polymer composite, polyurethane act as a polymer matrix. There are several types of reinforcements or fillers, such as multi-walled carbon nanotubes (MWCNTs),143 diaminopropane-montmorillonite (DAP-MMT) nanoclay,144 organoclay montmorillonite (OMMT),145 clay nanocomposites,146 cellulose nanocrystal (CNC),147 aluminum (Al) powder,148 bentonites and chitosan,149 halloysite nanotubes (HNTs),150 empty fruit bunch (EFB) from the oil palm tree,151 and reclaimed rockwool fibers from discarded industrial piping insulation wastes.152 Moreover, Jasmi et al. reported on the polyurethane-graphene nanocomposite for application as an optical fiber Bragg grating (FBG) temperature sensor.153 PU-graphene nanocomposite displayed better thermal stability at 217 °C and conductivity of 1.39 × 10−9 S cm−1 than neat PU at 204 °C and 9.43 × 10−11 S cm−1. The incorporation of reinforcement/filler into the PU matrix influenced the PU morphology and the polymer composite's mechanical and thermal properties.
Based on the reported work of palm oil-based polymer composites discussed above, it is revealed that most of the explored works are intended to improve mechanical properties, thermal stability, chemical resistance, and physical resistance. Indeed, the composites showed improvements compared to their precursor materials counterparts. These composites should be promising materials for structural application, e.g., constructions, coatings, containers, etc. On the other hand, research for different types of applications is still yet to be explored. For instance, it is already widely known that polymer composite has been developed for biomedical applications, separation and purification technology, and catalysis. Therefore, with a similar approach, it is expected that those fields of applications are also possible for palm oil-based polymer composites.
10. Miscellaneous polymers
This section will discuss more polymers derived from palm oil or fatty acids that do not fall into the polymers categories in the previous sections. The classification of polymers will be divided into two, which are palm oil fatty acid-based polymers and palm oil-based polymers. Based on the information shown in this section, there are many promising options to explore, from the utilization of palm oil and its derivatives for polymer synthesis to be used in various applications.10.1. Palm oil fatty acid-based polymers
Zeimaran et al. reported on the synthesis of polyacids that were prepared from palm acid or sunflower oil via the addition reaction technique.154 The polyacids had branch structures that mainly consisted of triacids. Palm acid oil is a side product from the palm oil alkaline refining process, which consists of primarily free fatty acids up to 62.6% and neutral oil with moisture content < 2%.155 Moreover, fatty acids have a higher number of carbon atoms (C > 10), such as lauric, myristic, palmitic, and stearic acids from palm oil, which are known as non-toxic, environmentally friendly, and renewable materials.156 Thotakura et al. reported on the preparation and properties studies of polymeric micelles from chitosan-palmitic acid for tamoxifen drug delivery.157 The utilization of chitosan enhanced biodistribution and bio-adhesion, and the presence of palmitic acid enhanced drug loading. Moreover, Akbari Javar and coworkers prepared palmitic acid-based polyamide as a biodegradable polymer for drug delivery application that was synthesized by the polycondensation method.158 Palmitic acid served to improve polymer hydrophobicity, flexibility, and degradability. The authors claim that this palmitic acid-based polyamide for methylprednisolone delivery displayed an acceptable profile for drug release and good physicochemical characteristics. Also, Wong et al. reported the preparation of a series of polyesteramides from palm stearin and steric acid to develop an amorphous solid dispersion (SD) for mefenamic acid.159 More on the utilization of fatty acid, palmitic acid was used as a co-monomer to synthesize carbamoylethyl pullulan-grafted-palmitic acid (CP-g-PA) diblock copolymer for application of delivering the raloxifene (RA) to breast tumor.160 Furthermore, Zhang et al. reported on the synthesis of comb-like copolymers from higher fatty acids, glycidyl methacrylate, and para(p)-anisic acid for polymeric cold flow improver in the blend of biodiesel-diesel application.161 The higher fatty acid as the copolymer branch chain increased the matching ability between parafilm and higher fatty acid methyl esters. In contrast, the anisic acid side chain increased the rigidity of the copolymers. The addition of copolymers in diesel-biodiesel blends displayed satisfying performance on lowering cloud point, pour point, and cold filter plugging point.10.2. Palm oil-based polymers
Lee and coworkers reported a reaction between epoxidized natural rubber (ENR) and palm oil-based alkyd with various –OH to –COOH ratios at ambient temperature (27–29 °C).162 Alkyd with higher carboxylic content was more reactive toward rubber epoxide than lower carboxylic content alkyds since the hydroxyl functional group did not react with ENR. More studies on alkyd regarding the utilization of palm oil-based alkyd in microcapsules were reported by Shahabudin et al.163 The microcapsules were prepared via in situ polymerization in an oil-in-water emulsion using two different methods with encapsulation of alkyd during the preparation of microcapsules, which resulted in poly(urea-melamine-formaldehyde) (PUMF) and poly(urea-formaldehyde) (PUF) shells in the range of 300–500 micron that entrapped a core of palm oil-based alkyd resin. Further studies on the microencapsulation of palm oil-alkyd in PUFM shells as a healing agent in the epoxy matrix were reported by the same authors.164 spherical and free-flowing PUMF microcapsules with encapsulated palm oil-based alkyd produced a good yield of 65%, as shown in Fig. 18. As compared to PUF microcapsules, the presence of melamine in PUMF provides more crosslinking reactions that lead to an increase in the strength of the shell. Microcapsules PUMF exhibited good dispersion in the epoxy matrix and remained embedded during the reaction of epoxy with amine hardener.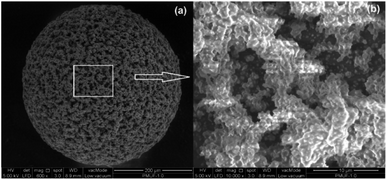 | ||
Fig. 18 FESEM picture of PMUF microcapsule at (a) 500× and (b) 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00× magnifications. Reproduced with permission from ref. 164 Copyright 2016 MDPI. 00× magnifications. Reproduced with permission from ref. 164 Copyright 2016 MDPI. | ||
Ling and coworkers reported on the synthesis and characterization of poly(alkyd-urethane)s (PAUs) from various vegetable oils.165 Five types of poly(alkyd-urethane) were consist of palm oil-based poly(alkyd-urethane) (POPAU), sunflower oil-based poly(alkyd-urethane) (SFPAU), palm-sunflower oil-based poly(alkyd-urethane) (POSFPAU), soy oil-based poly(alkyd-urethane) (SOPAU), and palm-soy oil-based poly(alkyd-urethane) (POSOPAU). SFPAU exhibited drying superiority with the highest pencil hardness and impact resistance results, following this order: SFPAU > SOPAU > POSFPAU > POSOPAU > POPAU. There are also acrylated palm oil-based polyurethanes. Physical properties of acrylated palm oil-based polyurethanes using different reactive diluents were reported by Oon et al.166 Acrylated palm oil-based PU was formulated with various reactive diluents (monoacrylate, diacrylate, and triacrylate) with various content in the presence of photo-initiator (Irgacure) and film formation agent (NMP). PU film using monoacrylate displayed better properties than other films using diacrylate or triacrylate. Moreover, Mas'ud et al. reported on the direct polymerization of double bonds from the unsaturated fatty acids in palm oil triglycerides without any triglyceride modification.167 Palm oil-based polymer was prepared by cationic polymerization of waste-used cooking palm oil using boron trifluoride-etherate as a catalyst under microwave irradiation methods with various power settings and reaction times. Polymerization was considered complete by treating the reaction mixture under 600 or 800 watts for 5 minutes of microwave irradiation since a solid product was formed.
Hoong et al. reported on the preparation of high molecular weight polyols by cationic ring-opening copolymerization of epoxidized natural oils (ENO) with tetrahydrofuran (THF).168 Epoxidation of the respective natural oils resulted in epoxidized methyl oleate (EMO), epoxidized soyabean oil (ESO), epoxidized cocoa butter (ECB), and epoxidized palm oil (EPO). Polyols were produced from the copolymerization of the respective epoxidized natural oil with THF. Moreover, modification of triglycerides from red palm olein was carried out by reaction of epoxidized palm olein with 5-norbornene-2-carboxylic acid to produce norbornene palm olein (NPO) with one norbornene (NBE) unit per NPO.169 The copolymerization of NPO and NBE was performed via ring-opening metathesis polymerization (ROMP) with 2nd generation Grubbs (G2) catalyst at 30 °C. The produced copolymer also had crosslinked structure since it had poor solubility in acetic acid, water, and many organic solvents. Ring-opening copolymerization of norbornene-based palm olein (NPO) monomer with norbornene was monitored in real-time by time-domain 1H NMR (TD-NMR).170 Another study about copolymers from palm oil was the copolymerization of palm oil with sulfur via the inverse vulcanization method.171 The elemental sulfur has an octet ring structure that will be opened if it is heated higher than its floor temperature (>159 °C) to form sulfur atoms with a biradical linear chain, accompanied by color changes from yellow to orange. The biradical linear chain of sulfur will homopolymerize to form polysulfide, which is not stable and subsequently depolymerize leading to the reformation of elemental structure crystals. Therefore, by adding palm oil that has unsaturated fatty acids to the biradical linear chain at 170 °C resulted in poly(S-Palm oil). This poly(S-Palm oil) can potentially be applied in oil spill removal, mercury removal, palladium capture, and slow-release fertilizers applications.
11. Applications of polymers based on palm oil and its derivatives
Polymers have been part of our daily life. The ongoing development research on palm oil-based polymers showed the potential of these polymers as alternatives to petrochemical-based polymers. Table 5 summarizes numerous applications of polymers based on palm oil and its derivatives. These polymers demonstrated promising applications in several fields, such as in industrial, construction, and biomedical applications, as shown in Table 5. Moreover, based on the reported article, there are commercially available palm oil-based polyols named Pioneer M-60 and Pioneer E-135.81 These polyols can be used in polyurethanes production. The presence of these commercially available bio-based polyols hopefully would encourage the polymer industry to produce polyurethane with high bio-based content. Consequently, it will produce less toxic and more environmentally friendly polyurethanes. Another possibility to urge this bio-based polymer production is for government to implement a policy in the polymer industry to use a certain percent of bio-renewable feedstock in their polymer production. In Indonesia, the bio-renewable feedstock source can be from oil palm plantation products or waste.| Type of polymers | Application | Ref. |
|---|---|---|
| Polymeric methyl ester sulfonate (PMES) | Polymeric surfactant for enhanced oil recovery (EOR) | 41 |
| Plant oil-based polyvinyl ethers | Paint binders | 42 |
| Acrylated palm olein (APO) | Drug delivery application | 29 |
| Vegetable oil-based acrylate prepolymer | UV-curable coating | 43 |
| Palm oil fatty acid-ethyl acrylamide (POFA-EA) | Thermoplastic polymer | 44 |
| Polyesters/alkyds | Coatings | 47, 50 and 51 |
| UV-curable resin/coating | 35, 48 and 49 | |
| Plasticizer in polyvinyl chloride (PVC) | 52–54 | |
| Additive in polyaniline (PANI) | 55 and 56 | |
| Additive in liquid detergent | 57 | |
| Polyol ester | Polymeric nanoparticles for drug carrier | 80 |
| Polyurethanes (PUs) | PU foams for tissue engineering in biomedical application | 61–63 |
| PU foams | 28, 77, 83, 93–95 | |
| Adhesives and coatings | 58–60, 96–98 | |
| Thermosetting | 31 and 75 | |
| Thermoplastic or elastomers | 75, 79, 81, 100 and 101 | |
| Controlled-release urea fertilizer | 71 | |
| Polymer electrolytes | 102–110 | |
| Polyepoxides | Polymeric matric for bio-based composite | 119 and 121 |
| UV-curable pressure-sensitive adhesives (PSA) | 122 | |
| Coatings | 120, 123 and 124 | |
| Thermoset with reconfigurable shape-memory properties | 126 | |
| Polymer composites | Lightweight-concrete | 130 and 131 |
| Bitumen binder and modified bitumen | 132 and 133 | |
| Coatings | 134, 140–142 | |
| Fiber-reinforced polymer (FRP) composites | 135–137 | |
| Green coupling agent | 138 and 139 | |
| PU foams reinforced with nanoparticle | 143–145, 147 and 150 | |
| PU foam with aluminum filler | 148 | |
| PU foam with reclaimed rockwool fibers for thermal-insulating application | 152 | |
| PU reinforced with nanoparticles or filler for surface-coating application | 146 and 149 | |
| Optical fiber Bragg grating (FBG) temperature sensor | 153 | |
| Polyacid | Elastomers or hydrogels for biomedical application | 154 |
| Chitosan-palmitic acid copolymer | Polymeric micelles for drug delivery of tamoxifen | 157 |
| Palmitic acid-based polyamide | Drug delivery of methylprednisolone | 158 |
| Stearic acid-based polyesteramide | Polymer carrier in solid dispersion for mefenamic acid | 159 |
| Carbamoylethyl pullulan-grafted-palmitic acid diblock copolymer | Drug delivery of raloxifene to breast tumor | 160 |
| Copolymer of higher fatty acids, glycidyl methacrylate, and p-anisic acid | Polymeric cold flow improver in blends of bio-diesel and diesel | 161 |
| Alkyd microencapsulation | Palm oil-based alkyd as a self-healing agent for epoxy matrix | 163 and 164 |
| Poly(alkyd-urethane)s | Surface coating or binder in composites | 165 |
| Acrylated palm oil-based PU | Coating | 166 |
| Poly(S-Palm oil) | Mercury removal, palladium capture, slow-release fertilizers, and oil spill removal | 171 |
Palm oil and its fatty acids are known as non-toxic, biodegradable, and bio-renewable resources.156 These environmentally friendly characteristics are essential properties for materials in biomedical applications. These should be a good enough reason to urge more research on developing polymers derived from palm oil or its fatty acids for biomedical applications. Moreover, this bio-based polymer should be prepared via a non-toxic and green process, such as using water as a dispersant or enzyme as a catalyst. The use of bio-renewable resources and the green process will guarantee the sustainability of the preparation of this bio-based polymer. Therefore, environmental damage and toxic waste can be avoided as far away as possible.
12. Summary
Palm oil and its fatty acids are bio-renewable resources with biodegradability and vast obtainability. This review has shown the recent progress of research on the utilization of palm oil and its fatty acids in producing polymers like fatty acid-based vinyl polymers, polyesters, polyols, polyurethanes, polyepoxides, polymer composites, polyacids, polyamides, polyesteramides, and various types of palm oil-based or fatty acid-based copolymers. Besides palm oil and its fatty acids, research incorporating palm oil fractionation products like palm stearin, palm olein, and palm fatty acid distillate is also available. Moreover, other oil palm plantation products and waste such as palm kernel oil, palm acid oil, residual palm oil, empty fruit bunch (EFB), and waste from used cooking oil can also be used in polymer production. These palm oil-based monomers and polymers are considered environmentally friendly materials and promising alternatives to conventional petrochemical-based monomers and polymers. Several of these palm-based polymers have been commercially available such as polyols with different OHV values, which can be used for polyurethanes production. Moreover, studies on the mechanical and thermal properties of the material from palm oil-based polymers have also been performed and reported. Based on this knowledge, we can design studies to obtain palm oil-based polymers with desired thermal and mechanical properties.13. Outlook
Palm oil and its fatty acids have great potential to be used as raw materials for producing bio-based polymers. These bio-based polymers should have mechanical and thermal properties comparable to or even better than commercially available petrochemical-based polymer counterparts. Thus, it is very likely that in the future, bio-based polymers can replace petrochemical-based polymers. In addition, bio-based polymers are derived from bio-renewable resources and preferably have biodegradability properties. Thus, it may lessen the negative impact of polymer waste on the environment. Moreover, green processes should be performed in preparing bio-based polymers, such as using enzymes as catalysts. To this day, most enzyme-catalyzed polymer synthesis only results in low molecular weight polymers, which are undesirable in industrial applications. However, this low molecular weight bio-based polymer, depending on the type of the polymers and their properties, especially if they have non-toxic, biocompatibility, and biodegradability properties, can be used, for example, in drug delivery system applications. Furthermore, to overcome the generated waste problem, more utilization of waste from oil palm plantations (residual palm oil and EFB) or used cooking oil is also a viable option for bio-renewable feedstock for polymer production. Therefore, there will be no direct competition with the use of palm oil as a consumer product.Author contributions
Conceptualization, E. S., F. Y., and M. I.; investigation and methodology, E. S., F. Y., A. A., J. A. L., and M. I.; writing—original draft preparation, E. S., F. Y., and M. I.; writing—review and editing, E. S., A. A., and J. A. L.; funding acquisition, J. A. L. and M. I.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge Manajemen Talenta BRIN (National Research and Innovation Agency) Indonesia (SK No. 151/II/HK/2022) for the financial support provided during this visiting researcher program. The author also would like to thank Prawistin Noorlaily for her help during the literature acquisition process.References
- Y. Jiang and K. Loos, Polymers, 2016, 8, 243 CrossRef PubMed.
- T. Cai, J. Liu, H. Cao and C. Cui, Ind. Crops Prod., 2020, 145, 112155 CrossRef CAS.
- K. Masutani and Y. Kimura, in Encyclopedia of Polymeric Nanomaterials, ed. S. Kobayashi and K. Müllen, Springer Berlin Heidelberg, Berlin, Heidelberg, 2021, pp. 1–7, DOI:10.1007/978-3-642-36199-9_390-1.
- S. Molina-Gutiérrez, V. Ladmiral, R. Bongiovanni, S. Caillol and P. Lacroix-Desmazes, Green Chem., 2019, 21, 36–53 RSC.
- Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354–362 CrossRef CAS PubMed.
- Y. Xia and R. C. Larock, Green Chem., 2010, 12, 1893–1909 RSC.
- M. R. Islam, M. D. H. Beg and S. S. Jamari, J. Appl. Polym. Sci., 2014, 131 Search PubMed.
- U. Biermann, U. T. Bornscheuer, I. Feussner, M. A. R. Meier and J. O. Metzger, Angew. Chem., Int. Ed., 2021, 60, 20144–20165 CrossRef CAS PubMed.
- D. P. Pfister, Y. Xia and R. C. Larock, ChemSusChem, 2011, 4, 703–717 CrossRef CAS PubMed.
- S. Miao, P. Wang, Z. Su and S. Zhang, Acta Biomater., 2014, 10, 1692–1704 CrossRef CAS PubMed.
- E. Nekhavhambe, H. E. Mukaya and D. B. Nkazi, J. Adv. Manuf. Process., 2019, 1, e10030 CAS.
- K. F. Adekunle, Open J. Polym. Chem., 2015, 05, 34–40 CrossRef CAS.
- K. J. Goh, C. K. Wong and P. H. C. Ng, in Encyclopedia of Applied Plant Sciences, ed. B. Thomas, B. G. Murray and D. J. Murphy, Academic Press, Oxford, Second Edition, 2017, pp. 382–390, DOI:10.1016/B978-0-12-394807-6.00176-3.
- S. J. Santosa, Clean: Soil, Air, Water, 2008, 36, 453–465 CAS.
- W. Indriyadi, Salus Cultura, 2022, 2, 1–10 CrossRef.
- Indonesian Palm Oil Association (IPOA), Palm oil performance in 2021 and prospect in 2022, https://gapki.id/en/news/21136/palm-oil-performance-in-2021-and-prospect-in-2022, accessed 20 Oct, 2022.
- J. Sayer, J. Ghazoul, P. Nelson and A. Klintuni Boedhihartono, Global Food Secur., 2012, 1, 114–119 CrossRef.
- M. A. Shahputra and Z. Zen, IOP Conf. Ser.: Earth Environ. Sci., 2018, 122, 012008 CrossRef.
- K. Obidzinski, R. Andriani, H. Komarudin and A. Andrianto, Ecol. Soc., 2012, 17, 25 Search PubMed.
- S. Searle, Palm oil is the elephant in the greenhouse, https://theicct.org/palm-oil-is-the-elephant-in-the-greenhouse/, accessed 26 Dec, 2022 Search PubMed.
- A. D. Pratama, Politica, 2019, 10, 95–112 Search PubMed.
- G. Pande, C. C. Akoh and O.-M. Lai, in Palm Oil, ed. O.-M. Lai, C.-P. Tan and C. C. Akoh, AOCS Press, 2012, pp. 561–586, DOI:10.1016/B978-0-9818936-9-3.50022-8.
- R. Sambanthamurthi, K. Sundram and Y.-A. Tan, Prog. Lipid Res., 2000, 39, 507–558 CrossRef CAS PubMed.
- S. W. Lin, in Vegetable Oils in Food Technology, 2011, pp. 25–58, DOI:10.1002/9781444339925.ch2.
- C. Zhang, T. F. Garrison, S. A. Madbouly and M. R. Kessler, Prog. Polym. Sci., 2017, 71, 91–143 CrossRef CAS.
- J. M. Raquez, M. Deléglise, M. F. Lacrampe and P. Krawczak, Prog. Polym. Sci, 2010, 35, 487–509 CrossRef CAS.
- M. Kellens, V. Gibon, M. Hendrix and W. De Greyt, Eur. J. Lipid Sci. Technol., 2007, 109, 336–349 CrossRef CAS.
- R. Jaratrotkamjorn and V. Tanrattanakul, J. Appl. Polym. Sci., 2020, 137, 49310 CrossRef CAS.
- R. Tajau, R. Rohani and M. Z. Salleh, J. Polym. Environ., 2020, 28, 2734–2748 CrossRef CAS.
- M. Z. Arniza, S. S. Hoong, Z. Idris, S. K. Yeong, H. A. Hassan, A. K. Din and Y. M. Choo, J. Am. Oil Chem. Soc., 2015, 92, 243–255 CrossRef CAS PubMed.
- H. Kalita, A. Jayasooriyamu, S. Fernando and B. J. Chisholm, J. Oil Palm Res., 2015, 27, 39–56 CAS.
- Y. Yang, W. Lu, X. Zhang, W. Xie, M. Cai and R. A. Gross, Biomacromolecules, 2010, 11, 259–268 CrossRef CAS PubMed.
- C. Orellana-Coca, S. Camocho, D. Adlercreutz, B. Mattiasson and R. Hatti-Kaul, Eur. J. Lipid Sci. Technol., 2005, 107, 864–870 CrossRef CAS.
- S. Miao, N. Callow, S. Zhang, Z. Su, P. Wang and Y. Liu, Biotechnol. Lett., 2013, 35, 887–890 CrossRef CAS PubMed.
- D. T. C. Ang and S. N. Gan, Prog. Org. Coat., 2012, 73, 409–414 CrossRef CAS.
- L. Yuan, Z. Wang, N. M. Trenor and C. Tang, Macromolecules, 2015, 48, 1320–1328 CrossRef CAS.
- J. Lomège, V. Lapinte, C. Negrell, J.-J. Robin and S. Caillol, Biomacromolecules, 2019, 20, 4–26 CrossRef PubMed.
- A. Kohut, S. Voronov, Z. Demchuk, V. Kirianchuk, K. Kingsley, O. Shevchuk, S. Caillol and A. Voronov, Molecules, 2020, 25, 2990 CrossRef CAS PubMed.
- B. Maiti and P. De, RSC Adv., 2013, 3, 24983–24990 RSC.
- Y.-W. Kim, G. T. Eom, J.-S. Hong and K.-W. Chung, J. Am. Oil Chem. Soc., 2011, 88, 1727–1736 CrossRef CAS.
- A. D. K. Wibowo, L. A. Yoshi, A. S. Handayani and Joelianingsih, Colloid Polym. Sci., 2021, 299, 81–92 CrossRef CAS.
- D. Kalita, I. Tarnavchyk, D. C. Webster and B. J. Chisholm, Prog. Org. Coat., 2022, 163, 106607 CrossRef CAS.
- Y. Su, H. Lin, S. Zhang, Z. Yang and T. Yuan, Polymers, 2020, 12, 1165 CrossRef CAS PubMed.
- Y. Wu, M. Fei, T. Chen, C. Li, T. Fu, R. Qiu and W. Liu, Addit. Manuf., 2021, 47, 102268 CAS.
- Q. Zhang, M. Song, Y. Xu, W. Wang, Z. Wang and L. Zhang, Prog. Polym. Sci., 2021, 120, 101430 CrossRef CAS.
- C.-M. Lai, H. D. Rozman and G.-S. Tay, Polym. Eng. Sci., 2013, 53, 1138–1145 CrossRef CAS.
- H. R. Ong, M. M. R. Khan, R. Ramli, M. W. Rahman and R. M. Yunus, RSC Adv., 2015, 5, 95894–95902 RSC.
- D. T. C. Ang and S. Neon Gan, Pigm. Resin Technol., 2012, 41, 302–310 CrossRef CAS.
- D. T. C. Ang and S. N. Gan, J. Appl. Polym. Sci., 2012, 125, E306–E313 CrossRef CAS.
- A. A. Nanvaee, R. Yahya and S.-N. Gan, J. Clean. Prod., 2013, 54, 307–314 CrossRef CAS.
- A. R. N. Azimi, R. Yahya and S.-N. Gan, Prog. Org. Coat., 2013, 76, 712–719 CrossRef CAS.
- D. T.-C. Ang, Y. K. Khong and S. N. Gan, J. Vinyl Addit. Technol., 2016, 22, 80–87 CrossRef CAS.
- K. M. Lim, Y. C. Ching and S. N. Gan, Polymers, 2015, 7, 2031–2043 CrossRef CAS.
- N. Z. Rozaki, S.-N. Gan and D. T.-C. Ang, J. Polym. Environ., 2017, 25, 286–295 CrossRef CAS.
- M. F. Ramli, S. N. Gan, W. H. Lim and S. W. Phang, Polym. Polym. Compos., 2017, 25, 537–544 CAS.
- S. N. A. Ramlan, W. J. Basirun, S.-W. Phang and D. T.-C. Ang, Prog. Org. Coat., 2017, 112, 9–17 CrossRef CAS.
- P. P. Chiplunkar, V. V. Shinde and A. P. Pratap, J. Surfactants Deterg., 2017, 20, 137–149 CrossRef CAS.
- K. T. Teo, A. Hassan and S. N. Gan, Polymers, 2018, 10, E1374 CrossRef PubMed.
- A. Khoon Poh, L. Choy Sin, C. Sit Foon and C. Cheng Hock, J. Appl. Polym. Sci., 2014, 131, 39967 Search PubMed.
- A. Khoon Poh, L. Choy Sin, C. Sit Foon, C. Cheng Hock and J. Adhes, Sci. Technol., 2014, 28, 1020–1033 Search PubMed.
- F. H. Yeoh, C. S. Lee, Y. B. Kang, S. F. Wong and S. F. Cheng, J. Appl. Polym. Sci., 2018, 135, 46861 CrossRef.
- F. H. Yeoh, C. S. Lee, Y. B. Kang, S. F. Wong, S. F. Cheng and W. S. Ng, Polymers, 2020, 12, 1842 CrossRef CAS PubMed.
- W. S. Ng, C. S. Lee, C. H. Chuah and S.-F. Cheng, Ind. Crops Prod., 2017, 97, 65–78 CrossRef CAS.
- A. A. W. Japir, N. Salih and J. Salimon, Turk. J. Chem., 2021, 45, 585–599 CrossRef CAS PubMed.
- M. Bahadi, N. Salih and J. Salimon, Biointerface Res. Appl. Chem., 2021, 11, 14359–14371 CAS.
- Y. Yang, W. Lu, J. Cai, Y. Hou, S. Ouyang, W. Xie and R. A. Gross, Macromolecules, 2011, 44, 1977–1985 CrossRef CAS.
- Y.-R. Zhang, S. Spinella, W. Xie, J. Cai, Y. Yang, Y.-Z. Wang and R. A. Gross, Eur. Polym. J., 2013, 49, 793–803 CrossRef CAS.
- E. Stavila and K. Loos, J. Renewable Mater., 2015, 3, 268–280 CrossRef CAS.
- N. E. Marcovich, M. Kurańska, A. Prociak, E. Malewska and K. Kulpa, Ind. Crops Prod., 2017, 102, 88–96 CrossRef CAS.
- N. E. Marcovich, M. Kurańska, A. Prociak, E. Malewska and S. Bujok, Polym. Int., 2017, 66, 1522–1529 CrossRef CAS.
- J. Liu, Y. Yang, B. Gao, Y. C. Li and J. Xie, J. Clean. Prod., 2019, 209, 528–537 CrossRef CAS.
- J. C. Gomez, R. Zakaria, M. M. Aung, M. N. Mokhtar and R. Yunus, Polymers, 2021, 13, 4214 CrossRef CAS PubMed.
- P. K. S. Pillai, S. Li, L. Bouzidi and S. S. Narine, Ind. Crops Prod., 2016, 84, 205–223 CrossRef CAS.
- N. M. Noor, T. Ismail, Y. S. Kian and H. A. Hassan, J. Oil Palm Res., 2013, 25, 92–99 Search PubMed.
- I. A. Mohammed, E. A. J. Al-Mulla, N. K. A. Kadar and M. Ibrahim, J. Oleo Sci., 2013, 62, 1059–1072 CrossRef CAS PubMed.
- D. Lumcharoen and O. Saravari, Adv. Mater. Res., 2014, 911, 352–356 Search PubMed.
- S. Mohd Tahir, W. N. Wan Salleh, N. S. Nor Hadid, N. F. Enderus and N. A. Ismail, Mater. Sci. Forum, 2016, 846, 690–696 Search PubMed.
- R. Fauzi, R. A. Majid, D. K. Sohami, B. Fauzi, N. R. Mohamed and S. N. L. Mamauod, J. Eng. Appl. Sci., 2019, 14, 1469–1474 CrossRef CAS.
- Y. Zeng, Y. Chen, D. Sha, Y. Wu, R. Qiu and W. Liu, ACS Sustainable Chem. Eng., 2022, 10, 11524–11532 CrossRef CAS.
- R. Tajau, R. Rohani, W. N. R. Wan Isahak, M. Z. Salleh and Z. Ghazali, Adv. Polym. Technol., 2018, 37, 3552–3560 CrossRef CAS.
- S. Mohd Norhisham, T. I. Tuan Noor Maznee, H. Nurul Ain, P. P. Kosheela Devi, A. Srihanum, M. N. Norhayati, S. K. Yeong, A. H. Hazimah, C. M. Schiffman, A. Sendijarevic, V. Sendijarevic and I. Sendijarevic, Int. J. Adhes. Adhes., 2017, 73, 38–44 CrossRef CAS.
- U. A. Amran, S. Zakaria, C. H. Chia, Z. Fang and M. Z. Masli, Energy Fuels, 2017, 31, 10975–10982 CrossRef CAS.
- U. A. Amran, S. Zakaria, C. H. Chia, R. Roslan, S. N. S. Jaafar and K. M. Salleh, Cellulose, 2019, 26, 3231–3246 CrossRef CAS.
- K. H. Badri, in Polyurethane, ed. Z. Fahmina and S. Eram, IntechOpen, Rijeka, 2012, ch. 20, DOI:10.5772/47966.
- A. Reghunadhan and S. Thomas, in Polyurethane Polymers, ed. S. Thomas, J. Datta, J. T. Haponiuk and A. Reghunadhan, Elsevier, Amsterdam, 2017, pp. 1–16, DOI:10.1016/B978-0-12-804039-3.00001-4.
- H. Pawlik and A. Prociak, J. Polym. Environ., 2012, 20, 438–445 CrossRef CAS.
- A. Prociak, E. Malewska, M. Kurańska, S. Bąk and P. Budny, Ind. Crops Prod., 2018, 115, 69–77 CrossRef CAS.
- A. A. Septevani, D. A. C. Evans, C. Chaleat, D. J. Martin and P. K. Annamalai, Ind. Crops Prod., 2015, 66, 16–26 CrossRef CAS.
- S. Eram and Z. Fahmina, in Polyurethane, ed. Z. Fahmina and S. Eram, IntechOpen, Rijeka, 2012, ch. 1, DOI:10.5772/51663.
- J. Konieczny and K. Loos, Polymers, 2019, 11, 256 CrossRef PubMed.
- R. Morales-Cerrada, R. Tavernier and S. Caillol, Polymers, 2021, 13, 1255 CrossRef CAS PubMed.
- T. Calvo-Correas, A. Santamaria-Echart, A. Saralegi, L. Martin, Á. Valea, M. A. Corcuera and A. Eceiza, Eur. Polym. J., 2015, 70, 173–185 CrossRef CAS.
- P. K. S. Pillai, S. Li, L. Bouzidi and S. S. Narine, Ind. Crops Prod., 2016, 83, 568–576 CrossRef CAS.
- J. Chavarro Gomez, R. Zakaria, M. M. Aung, M. N. Mokhtar and R. B. Yunus, J. Mater. Res. Technol., 2020, 9, 16303–16316 CrossRef CAS.
- U. A. Amran, K. M. Salleh, S. Zakaria, R. Roslan, C. H. Chia, S. N. S. Jaafar, M. S. Sajab and M. Mostapha, Polymers, 2021, 13, 3072 CrossRef CAS PubMed.
- T. S. Velayutham, W. H. Abd Majid, B. K. Ng and S. N. Gan, J. Appl. Polym. Sci., 2013, 129, 415–421 CrossRef CAS.
- S. Suryani and Z. Zaimahwati, Appl. Mech. Mater., 2014, 525, 162–165 Search PubMed.
- C. S. Wong and K. H. Badri, Mater. Sci. Appl., 2012, 03, 78–86 CAS.
- P. P. KosheelaDevi, T. I. Tuan Noor Maznee, S. S. Hoong, H. Nurul 'Ain, S. M. Norhisham, M. N. Norhayati, A. Srihanum, S. K. Yeong, A. H. Hazimah, V. Sendijarevic and A. Sendijarevic, J. Appl. Polym. Sci., 2016, 133 Search PubMed.
- H. M. C. C. Somarathna, S. N. Raman, K. H. Badri, A. A. Mutalib, D. Mohotti and S. D. Ravana, Polymers, 2016, 8, 202 CrossRef PubMed.
- N. A. Rasli Rosli and S. Ahmad Zubir, Mater. Sci. Forum, 2020, 1010, 142–147 Search PubMed.
- N. I. Adam, H. Hanibah, R. H. Y. Subban, M. Kassim, N. N. Mobarak, A. Ahmad, K. H. Badri and M. S. Su'ait, Ind. Crops Prod., 2020, 155, 112757 CrossRef CAS.
- C. S. Wong, K. H. Badri, N. Ataollahi, K. P. Law, M. S. Su’ait and N. I. Hassan, IJCME, 2014, 8, 1243–1250 Search PubMed.
- M. S. Su'ait, P. I. Z. Syed Mahadzir, K. H. Badri and A. Ahmad, Medziagotyra, 2022, 28, 322–332 Search PubMed.
- F. N. Daud, A. Ahmad and K. Haji Badri, Int. J. Polym. Sci., 2014, 2014, 326716 Search PubMed.
- N. A. Kamarulzaman and S. M. Tahir, AIP Conf. Proc., 2017, 1885, 020247 CrossRef.
- M. S. Su'ait, A. Ahmad, K. H. Badri, N. S. Mohamed, M. Y. A. Rahman, C. L. A. Ricardo and P. Scardi, Int. J. Hydrogen Energy, 2014, 39, 3005–3017 CrossRef.
- M. S. Su'ait, F. N. Jumaah, H. M. Faizzi, S. Mamat, N. A. Ludin, W. A. Farhan, A. Haron, N. Atifah, M. N. Latif, K. H. Badri and A. Ahmad, Ind. Crops Prod., 2018, 113, 406–413 CrossRef.
- M. A. Munir, K. H. Badri, L. Y. Heng, A. Inayatullah, H. A. Badrul, E. Emelda, E. Dwinta, N. Kusumawardani, A. S. Wulandari, V. Aprilia and R. B. Y. Supriyono, Int. J. Polym. Sci., 2022, 2022, 6815187 Search PubMed.
- M. A. Munir, K. H. Badri, L. Y. Heng, A. Inayatullah, E. Nurinda, D. Estiningsih, A. Fatmawati, V. Aprilia and N. Syafitri, ACS Omega, 2022, 7, 5982–5991 CrossRef CAS PubMed.
- J. L. Massingill and R. S. Bauer, in Applied Polymer Science: 21st Century, ed. C. D. Craver and C. E. Carraher, Pergamon, Oxford, 2000, pp. 393–424, DOI:10.1016/B978-008043417-9/50023-4.
- G. Gibson, in Brydson's Plastics Materials, ed. M. Gilbert, Butterworth-Heinemann, Eighth Edition, 2017, pp. 773–797, DOI:10.1016/B978-0-323-35824-8.00027-X.
- A. M. Issam, A. K. N. Khizrien and I. Mazlan, Polym.-Plast. Technol. Eng., 2011, 50, 1256–1261 CrossRef CAS.
- R. Mustapha, A. R. Rahmat, R. Abdul Majid and S. N. H. Mustapha, Polym.-Plast. Technol. Mater., 2019, 58, 1311–1326 CAS.
- A. Sarwono, Z. Man and M. A. Bustam, J. Polym. Environ., 2012, 20, 540–549 CrossRef CAS.
- F. C. Fernandes, K. Kirwan, D. Lehane and S. R. Coles, Eur. Polym. J., 2017, 89, 449–460 CrossRef CAS.
- P. Felizardo, M. J. Neiva Correia, I. Raposo, J. F. Mendes, R. Berkemeier and J. M. Bordado, Waste Manage., 2006, 26, 487–494 CrossRef CAS PubMed.
- É. de Castro Vasques, C. R. Granhen Tavares, C. Itsuo Yamamoto, M. Rogério Mafra and L. Igarashi-Mafra, Environ. Technol., 2013, 34, 2361–2369 CrossRef PubMed.
- A. Fakhari, A. R. Rahmat, M. U. Wahit and Y. Shoot Kian, Adv. Mater. Res., 2014, 931–932, 78–82 CAS.
- A. M. Salih, M. B. Ahmad, N. A. Ibrahim, K. Z. H. M. Dahlan, R. Tajau, M. H. Mahmood and W. M. Z. W. Yunus, Molecules, 2015, 20, 14191–14211 CrossRef CAS PubMed.
- A. Fakhari, A. R. Rahmat, M. U. Wahit, S. N. H. Mustapha and W. N. Wan Tajulruddin, Appl. Mech. Mater., 2015, 695, 73–76 Search PubMed.
- N. Tugiman, A. R. Rahmat, J. Jamaluddin, W. W. Tajulruddin and R. Mustapha, Chem. Eng. Trans., 2017, 56, 223–228 Search PubMed.
- M. M. Aung, Z. Yaakob, L. C. Abdullah, M. Rayung and W. J. Li, Ind. Crops Prod., 2015, 77, 1047–1052 CrossRef CAS.
- M. Sofian Alias, N. K. Othman, S. Radiah Mohd Kamarudin, H. Harun, M. Mohamed, S. Fatahiyah Mohamad, N. Ubaidah Saidin, R. Tajau and K. Azhar Abdul Halim, Mater. Today: Proc., 2022, 66, 3993–3999 CAS.
- R. Tajau, R. Rohani, M. S. Alias, N. H. Mudri, K. A. Abdul Halim, M. H. Harun, N. Mat Isa, R. Che Ismail, S. Muhammad Faisal, M. Talib, M. Rawi Mohamed Zin, I. Izni Yusoff, N. Khairul Zaman and I. Asyila Ilias, Polymers, 2021, 13, 1865 CrossRef CAS PubMed.
- S. Mu, Y. Zhang, J. Zhou, B. Wang and Z. Wang, ACS Sustainable Chem. Eng., 2020, 8, 5296–5304 CrossRef CAS.
- J. P. Greene, in Automotive Plastics and Composites, ed. J. P. Greene, William Andrew Publishing, 2021, pp. 191–222, DOI:10.1016/B978-0-12-818008-2.00007-6.
- A. M. Díez-Pascual and A. Rahdar, Macromol, 2021, 1, 276–292 Search PubMed.
- M. R. M. Asyraf, M. R. Ishak, A. Syamsir, N. M. Nurazzi, F. A. Sabaruddin, S. S. Shazleen, M. N. F. Norrrahim, M. Rafidah, R. A. Ilyas, M. Z. A. Rashid and M. R. Razman, J. Mater. Res. Technol., 2022, 17, 33–65 CrossRef CAS.
- K. A. Mohd Sari, S. Mat and K. H. Badri, J. Sustainable Cem.-Based Mater., 2012, 1, 192–201 CrossRef.
- K. A. Mohd Sari, S. Mat, K. Haji Badri and M. F. Mohd Zain, Appl. Mech. Mater., 2013, 357–360, 1082–1085 Search PubMed.
- M. Y. Alamawi, F. H. Khairuddin, N. I. M. Yusoff, K. Badri and H. Ceylan, Constr. Build. Mater., 2019, 204, 122–131 CrossRef CAS.
- B. Vural Kök, E. Aydoğmuş, M. Yilmaz and M. Akpolat, Constr. Build. Mater., 2021, 289, 123152 CrossRef.
- N. G. Nik Salleh, M. Sofian Alias, H. J. Gläsel and R. Mehnert, Radiat. Phys. Chem., 2013, 84, 70–73 CrossRef CAS.
- Y. Wu, M. Fei, T. Chen, R. Qiu and W. Liu, Compos. Commun., 2020, 22, 100489 CrossRef.
- Y. Wu, M. Fei, T. Chen, R. Qiu and W. Liu, Ind. Crops Prod., 2021, 170, 113744 CrossRef CAS.
- Y. Wu, C. Li, T. Chen, R. Qiu and W. Liu, Composites, Part A, 2022, 152, 106676 CrossRef CAS.
- S. C. Koay and S. Husseinsyah, J. Thermoplast. Compos. Mater., 2017, 30, 938–949 CrossRef.
- S. C. Koay, M. Y. Chan, M. M. Pang and K. Y. Tshai, Adv. Polym. Technol., 2018, 37, 2246–2252 CrossRef CAS.
- M. R. Islam, M. D. H. Beg and S. S. Jamari, J. Appl. Polym. Sci., 2016, 133 Search PubMed.
- M. R. Islam, M. D. H. Beg and S. S. Jamari, Prog. Org. Coat., 2016, 91, 17–24 CrossRef CAS.
- M. R. Islam, A. N. Yahaya and N. Isa, J. Appl. Polym. Sci., 2017, 134 CAS.
- A. A. Sinar, Z. Firuz, M. A. Nur Azni, N. H. Ahmad Zaidi, M. A. Hazizan and H. A. Sahrim, Mater. Sci. Forum, 2015, 819, 246–250 Search PubMed.
- N. N. P. Nik Pauzi, R. A. Majid, M. H. Dzulkifli and M. Y. Yahya, Composites, Part B, 2014, 67, 521–526 CrossRef CAS.
- S. Adnan, T. N. M. Tuan Ismail, N. Mohd Noor, N. S. M. Nek Mat Din, N. A. Hanzah, Y. Shoot Kian and H. Abu Hassan, Adv. Mater. Sci. Eng., 2016, 2016, 4316424 Search PubMed.
- M. R. Satriananda, S. Mulyati and F. Mulana, Polym. Renewable Resour., 2018, 9, 103–110 Search PubMed.
- A. A. Septevani, D. A. C. Evans, D. J. Martin and P. K. Annamalai, Ind. Crops Prod., 2018, 112, 378–388 CrossRef CAS.
- A. Sinar Arzuria, F. Zainuddin, N. H. A. Zaidi, Y. M. Daud, H. W. Soon, Z. Nur Afikah and M. F. F. Muhiyadin, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 429, 012044 Search PubMed.
- T. Rihayat, Suryani, Satriananda, M. Yunus, Sariadi and Fitria, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 536, 012035 CAS.
- A. Alis, R. A. Majid and Z. Mohamad, Chem. Eng. Trans., 2019, 72, 415–420 Search PubMed.
- S. N. Syed Mahmud, M. A. Jusoh, Y. K. Yeow and F. Esa, Mater. Sci. Forum, 2020, 981, 169–175 Search PubMed.
- M. H. Dzulkifli, R. A. Majid and M. Y. Yahya, J. Mater. Cycles Waste Manage., 2022, 24, 2416–2425 CrossRef CAS.
- F. Jasmi, N. H. Azeman, A. A. A. Bakar, M. S. D. Zan, K. H. Badri and M. S. Su'ait, IEEE Access, 2018, 6, 47355–47363 Search PubMed.
- E. Zeimaran, M. R. A. Kadir, H. M. Nor, T. Kamarul and I. Djordjevic, Bioorg. Med. Chem. Lett., 2013, 23, 6616–6619 CrossRef CAS PubMed.
- A. Kuntom, W.-L. Siew and Y.-A. Tan, J. Am. Oil Chem. Soc., 1994, 71, 525–528 CrossRef CAS.
- W. N. Lee, N. M. Salleh and S.-F. Cheng, Ind. Crops Prod., 2021, 170, 113808 CrossRef CAS.
- N. Thotakura, M. Dadarwal, R. Kumar, B. Singh, G. Sharma, P. Kumar, O. P. Katare and K. Raza, Int. J. Biol. Macromol., 2017, 102, 1220–1225 CrossRef CAS PubMed.
- R. Akbari Javar, M. I. Bin Noordin, M. Khoobi and A. Ghaedi, J. Inorg. Organomet. Polym. Mater., 2020, 30, 2520–2532 CrossRef CAS.
- W. S. Wong, C. S. Lee, H. M. Er and W. H. Lim, J. Pharm. Innovation, 2017, 12, 76–89 CrossRef.
- S. Sethi, S. Bhatia, S. Kamboj and V. Rana, Int. J. Pharm., 2021, 603, 120720 CrossRef CAS PubMed.
- X. Zhang, X. An, B. Sun, H. Shui, H. Lin and S. Han, Ind. Crops Prod., 2022, 188, 115471 CrossRef CAS.
- S. Y. Lee, S. N. Gan, A. Hassan, K. Terakawa, T. Hattori, N. Ichikawa and D. H. Choong, J. Appl. Polym. Sci., 2011, 120, 1503–1509 CrossRef CAS.
- N. Shahabudin, R. Yahya and S. N. Gan, Macromol. Symp., 2015, 354, 305–313 CrossRef CAS.
- N. Shahabudin, R. Yahya and S. N. Gan, Polymers, 2016, 8, 125 CrossRef PubMed.
- J. S. Ling, I. Ahmed Mohammed, A. Ghazali and M. Khairuddean, Ind. Crops Prod., 2014, 52, 74–84 CrossRef CAS.
- M. Onn, A. F. Mohd and M. F. Yhaya, MATEC Web Conf., 2016, 47, 05014 CrossRef.
- Z. A. Mas'ud, M. Farid, H. Surya and S. Bambang, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 509, 012083 Search PubMed.
- S. S. Hoong, S. K. Yeong, H. A. Hassan, A. K. Din and Y. M. Choo, J. Oleo Sci., 2015, 64, 101–115 CrossRef CAS PubMed.
- H. Fernandes, R. M. Souza Filho, J. L. Silva Sá and B. S. Lima-Neto, RSC Adv., 2016, 6, 75104–75110 RSC.
- H. Fernandes, J. G. Filgueiras, E. R. de Azevedo and B. S. Lima-Neto, Eur. Polym. J., 2020, 140, 110048 CrossRef CAS.
- A. Abbasi, W. Z. N. Yahya, M. M. Nasef, M. Moniruzzaman and A. S. M. Ghumman, Polym. Polym. Compos., 2021, 29, S1446–S1456 CAS.
| This journal is © The Royal Society of Chemistry 2023 |