 Open Access Article
Open Access ArticleSelf-healing hydrogels for bone defect repair
Weiwei Lia,
Yanting Wua,
Xu Zhanga,
Tingkui Wub,
Kangkang Huangb,
Beiyu Wang*b and
Jinfeng Liao *a
*a
aState Key Laboratory of Oral Diseases, National Clinical Research Centre for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, 610041, China. E-mail: liaojinfeng.762@163.com
bDepartment of Orthopedics, Orthopedic Research Institute, West China Hospital, Sichuan University, Chengdu, 610041, China. E-mail: dove-baker@126.com
First published on 5th June 2023
Abstract
Severe bone defects can be caused by various factors, such as tumor resection, severe trauma, and infection. However, bone regeneration capacity is limited up to a critical-size defect, and further intervention is required. Currently, the most common clinical method to repair bone defects is bone grafting, where autografts are the “gold standard.” However, the disadvantages of autografts, including inflammation, secondary trauma and chronic disease, limit their application. Bone tissue engineering (BTE) is an attractive strategy for repairing bone defects and has been widely researched. In particular, hydrogels with a three-dimensional network can be used as scaffolds for BTE owing to their hydrophilicity, biocompatibility, and large porosity. Self-healing hydrogels respond rapidly, autonomously, and repeatedly to induced damage and can maintain their original properties (i.e., mechanical properties, fluidity, and biocompatibility) following self-healing. This review focuses on self-healing hydrogels and their applications in bone defect repair. Moreover, we discussed the recent progress in this research field. Despite the significant existing research achievements, there are still challenges that need to be addressed to promote clinical research of self-healing hydrogels in bone defect repair and increase the market penetration.
Introduction
Bone tissue is a hard connective tissue of the human body and is constantly reshaped throughout the life of an individual.1,2 Bone tissue exerts both supporting and protective effects and can protect fragile organs within the body.3 When bone defect areas are small, most bones can undergo self-healing without any treatment.4,5 However, when bone tissue suffers damage beyond its ability to repair itself, bone damage can occur. Many factors affect the ability of bone tissue to regenerate and repair itself.6 Severe bone defects can be caused by tumor resection,7,8 severe trauma,9 infection,10 congenital malformation, osteogenesis imperfecta,11 rheumatoid arthritis,12 and osteoporosis.13,14 Bone tissue healing involves a complex signaling cascade, as shown in Fig. 1.15 However, when the defect size is extremely large (i.e., ≥2.5 cm), the natural healing mechanism is insufficient to fully repair the defect.14,16 Such defects persist for the remainder of the patient's life and are known as “critical-size defects” (CSDs).17,18 When the size of a bone defect exceeds the CSD threshold, it cannot repair itself, thus requiring clinical intervention.19 However, despite the high incidence of bone injury, treatment selection remains controversial.20 Bone defect repair involves a complex process of regeneration and reconstruction and includes structural and functional repair.21 Bone healing is a dynamic and continuous process that is accompanied by an alternating metabolic model. Currently, the most common clinical treatment for severe bone repair is bone grafting.22 Bone grafts usually refer to natural or artificial materials that have positive therapeutic effects on bone regeneration.23 They include autografts, allografts, xenografts, and grafts of synthetic bone materials.24 In general, autografts are considered to be the gold standard for bone transplantation owing to their excellent osteointegration, biocompatibility, osteoinductivity, osteoconductivity and osteogenic properties.25,26 However, autograft transplantation is an expensive procedure that requires a second operation, which can lead to new trauma as well as infection, inflammation, and chronic symptoms.27,28 In addition, autografts are limited by scarcity of bone sources.21 Allografts are most commonly used as an alternative to autografts, but they also have certain drawbacks, such asdonor scarcity, immune rejection, poor osseointegration, and spread of infectious disease.29,30 In addition, long-term use of immunosuppressant drugs is required when immune rejection occurs in allografts; this can lead to several life-threatening complications.31 Therefore, the natural bone supply used for grafts cannot meet the increasing patient demand.32 Numerous bone scaffolds have been developed via bone tissue engineering (BTE), and their application has shown great promise for bone repair.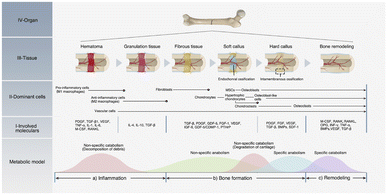 | ||
| Fig. 1 Process and mechanism of bone healing. (a) Inflammation phase; (b) bone formation phase; (c) remodeling phase. Bone healing is a dynamic and continuous process accompanied by an alternating metabolic model. In each phase, different cells and cytokines play the dominant roles.19 Reproduced from ref. 19 with permission from Elsevier, copyright 2021. | ||
Due to the limitations of bone grafts, BTE has been widely used for repairing bone tissue defects.33 Langer and Vacanti reported that BTE technologies are the result of an interdisciplinary approach that utilizes biological and engineering principles to develop viable alternatives for repairing, maintaining, or improving bone tissue function.34 BTE has the advantages of high modifiability, low risk of infection, good biocompatibility and no evident complications.1,35 BTE has been used to develop biomaterials with excellent properties that promote bone regeneration and maintain and improve tissue function.36 In addition, engineered bone provides a suitable environment for cell adhesion, migration, proliferation, and differentiation.37 BTE represents a new strategy for inducing bone regeneration by combining biological technologies and biomaterials, which is a challenging and complicated process.38 In general, BTE involves repairing bone defects by grafting scaffolds into bone defects and then gradually replacing scaffold materials with newly formed bone.39 BTE approaches mainly consist of three basic elements: bone scaffolds, bone cells and growth factors, as shown in Fig. 2.40,41 Scaffold materials can be divided into inorganic materials, natural or synthetic polymers, and composites.42 Among all biomaterials, hydrogels are widely used as BTE scaffolds owing to their desirable properties.43–45
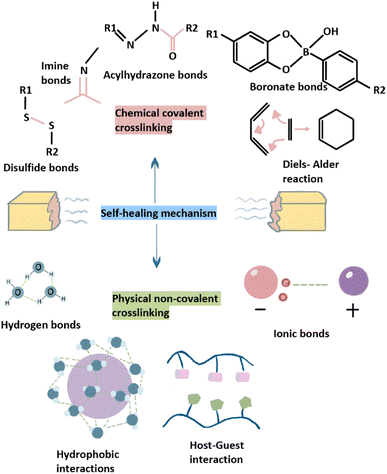 | ||
| Fig. 2 The mechanisms of SHHs include chemical covalent crosslinking and physical non-covalent crosslinking. | ||
Hydrogels are three-dimensional (3D) networks composed of hydrophilic polymeric chains or low-molecular-mass gelators with high water content. This composition indicates that hydrogels can absorb large amounts of water, causing swelling without dissolving, and can therefore be used to fabricate extracellular matrix (ECM) simulation scaffolds.46–48 High water content facilitates the diffusion of intercellular molecules and supports the growth, proliferation, and migration of bone cells.49 In addition to their excellent biological properties and high water content, hydrogels exhibit good biocompatibility and facilitate inherent cell-to-cell interactions.43,50 The porous structure of hydrogels allows fluid flow and the reconstruction of new blood vessels, which facilitates the diffusion of oxygen, nutrients, and metabolic waste.51–53 Moreover, hydrogels can be designed in any size or shape and can be injected into bone defects to accommodate irregular shapes.54,55 Owing to these excellent biological properties, hydrogels have been widely studied in tissue engineering, drug transport, and medical device research.56–62 In addition, hydrogels are applied in wound dressings,63–65 electronic equipment,66 biosensor development,67–69 cell imaging,70 waste disposal,71 and artificial skin-like materials.72–74 However, a lack of toughness, low mechanical strength and batch variation have limited the application of hydrogels in the medical field.75–77 Adding inorganic and/or organic fillers to hydrogels can improve their properties and broaden their application range.78 Furthermore, they cannot undergo self-healing, making it difficult to resist damage caused by fatigue or corrosion of scaffolds in human tissue.79 Therefore, the integration of self-healing behavior into scaffold materials is an innovative strategy for bone defect repair.80
A self-healing hydrogel (SHH) is a special type of hydrogel that can repair itself following external damage. SHHs exhibit better fatigue resistance, reusability, hydrophilicity and responsiveness to environmental stimuli than traditional hydrogels and are therefore more suitable for regenerative medicine.81 Originally, the concept of self-healing was inspired by the healing processes of natural tissues, such as the formation of bone callus, repair of broken ends after fracture, and formation of small wounds during skin surface healing.82 Self-healing is a unique feature of biological systems that can restore integrity and prolong life after being damaged,83 and most scaffolds used for BTE cannot self-heal, especially in wet environments. However, an ideal self-healing polymer system should repair damage autonomously in any location.83 The self-healing ability of hydrogels mainly depends on the reversibility of crosslinking.84 The type and number of bonds involved in crosslinking between molecules determines the properties of SHHs (such as high mechanical strength and self-healing). Some hydrogels can add a self-healing agent to a pre-existing microporous structure. The self-healing agent is then released in response to damage to initiate self-healing at the defect site.85 Moreover, SHHs can be divided into chemical covalent crosslinking and physical noncovalent crosslinking based on the specific self-healing mechanisms involved.86 The dynamic covalent interactions in SHHs usually require the application of external stimuli, such as pH, alternating current, or ultraviolet light to induce hydrogel self-repair.87,88 In contrast, autonomous SHHs usually use noncovalent interactions, either alone or in combination with other interactions, and do not require external stimuli, such as ionic bonding, hydrogen-bonding, supramolecular interactions, hydrophobic bonding, molecular diffusion, and chain entanglement.89 Regardless of the mechanism, SHHs should be designed to match their intended application. Ideally, SHHs should respond autonomously, rapidly, and repeatedly to induce damage under mild conditions, thereby ensuring long-term use and stable function.90,91 In other words, SHHs can convert irreversible damage to reversible damage.92 Moreover, SHHs can maintain their original properties, such as mechanical properties, fluidity, biocompatibility, after self-healing.93 Compared with hydrogels based on dynamic covalent crosslinking, SHHs based on physical noncovalent crosslinking exhibit poor mechanical properties due to weak and reversible noncovalent interactions. The self-healing ability of hydrogels is usually inversely proportional to their mechanical strength, which is generally achieved via enhanced crosslinking.94 The mechanical properties of SHHs with multiple crosslinking, such as (nano) composites and hybrid and interpenetrating polymer network hydrogels, can be improved to some extent.65 However, traditional hydrogels are generally not sensitive to external stimuli and can break without recovery. In such cases, the lifetime of hydrogels is greatly shortened, consumption of raw materials is increased, and hydrogel durability is reduced. Consequently, the use of SHHs can reduce replacement costs, and improve system safety.95 Therefore, the application of traditional hydrogels in biomedicine and other fields is limited, whereas SHHsshow great potential for future applications.96
At the beginning of this review, we explained how the self-healing ability of bone tissue is affected by various risk factors. Artificial surgical intervention is needed when the size of a particular bone defect exceeds the CSD threshold. Autografts are the “gold standard method;” however, owing to their limited application, BTE has attracted increasing attention. Because of their good biocompatibility and hydrophilicity, hydrogels have been widely used in studies on bone tissue repair. Although hydrogels have many advantages over other scaffold materials, they also have limitations such as insufficient toughness and low mechanical strength.97 Thus, this review demonstrates that the development of SHHs is promising in the field of bone tissue repair. This review aimed to systematically discusses the wide application of SHHs in bone tissue and broaden our understanding of their prospects and challenges.
Characteristics of SHHs
The properties of hydrogels vary greatly depending on the source of raw materials, crosslinking method, polymerization method, electric charge, environmental response, and degradability. Herein, we discussed the common properties of hydrogels and their evaluation.Pores and porosity
The porosity of hydrogels is beneficial for ECM secretion, cell binding, migration, and inward growth, which play an important role in directing tissue formation and function.98 Currently, many techniques, including solvent casting/particle leaching, freeze–drying, gas foaming, and electrospinning technologies, can accurately control the aperture size.99 Moreover, recent studies have reported optimum pore size ranges for different cells or tissue types. For example, pore sizes of ∼5 μm are used for neovascularization, 5–15 μm for fibroblast ingrowth, ∼20 μm for hepatocyte ingrowth, 20–125 μm for skin regeneration, 70–120 μm for chondrocyte ingrowth, 40–150 μm for fibroblast binding, 45–150 μm for liver tissue regeneration, 60–150 μm for vascular smooth muscle cell binding, 100–300 μm for bladder smooth muscle cell adhesion and ingrowth, 100–400 μm for bone regeneration, and 200–350 μm for osteoconduction, depending on the porosity and scaffold material used.100 Lan et al. constructed a high-porosity polyethylene glycol diacrylate (PEGDA) hydrogel foam and varied its porosity from 25% to 75% by adjusting the initial air-to-solution volume ratio.101 The hydrogel foam with the highest porosity (∼75%) showed the greatest water uptake along with good water absorption, moisture retention, and exudate management.Swelling properties
The osmotic driving force generated by the pores in a hydrogel (through capillary forces) allows it to absorb up to thousands of times of its dry weight; thus, it can expand without dissolving until equilibrium is reached. The amount of free and bound water can be determined using various methods, such as nuclear magnetic resonance, differential scanning calorimetry, X-ray powder diffraction, dielectric relaxation spectroscopy, quasielastic neutron scattering, infrared spectroscopy, and diffusion or adsorption.102 The swelling of hydrogels allows them to change their volume in response to different physical, chemical, or biological stimuli, which is critical for their self-healing behavior. Moreover, the swelling rate indicates the rate of exchange of nutrients and metabolites.Mechanical properties
The elastic modulus measurements of the brain, muscle, and bone tissues have been reported as 1, 10, and 100 kPa, respectively.103 As a scaffold structure to simulate the ECM, the mechanical properties of hydrogels should meet the demands of practical biomedical applications and support cell activities and functions. An increase in porosity will decrease the load-bearing material per unit volume, which in turn affects the mechanical properties of hydrogels. Gerecht et al. conducted a series of tensile and compression tests in poly(glycerol-co-sebacate)-acrylate (PGSA) and revealed that increased porosity had a substantial impact on the mechanical properties of PGSA but did not reduce ultimate strain.104 In addition, the mechanical strength of hydrogels can be controlled by the nature of the network. The elastic modulus strongly depends on the type of structure (i.e., crosslinking, association, entanglements) or interactions present in the network. Hydrogels with good mechanical properties can be induced by helicoidal structures and the presence of glassy nodules or crystalline domains. In contrast, hydrogels formed via hydrogen bonding; molecular specific binding; metal–ligand coordination; ionic, hydrophobic, host–guest, and antigen–antibody interactions; π–π stacking; chain entanglements, thermal- or pH-induced gelation; and protein or polypeptide interactions have weak mechanical strength.105 The mechanical properties of hydrogel biomaterials are primarily defined using theoretical frameworks of time-independent rubber elasticity and time-dependent viscoelasticity to analyze the structure of hydrogels and estimate effective crosslinking density. Currently, the most common evaluations of mechanical properties involve tensile testing for the characterization of elastic behavior and dynamic mechanical analysis for evaluating viscoelastic behavior.106 Further, the less common mechanical tests include bulge testing, spherical ball inclusion, and micropipette aspiration.107Biological properties
The goal of evaluating the biocompatibility of any material is to determine whether it has any toxic effects on the body.108 Biocompatibility is an indispensable property of hydrogels and can be defined as the ability of a material to remain in contact with organs without causing damage to surrounding tissues and without triggering any undesirable response.109 A recent study evaluated the biocompatibility of a Man/BSA MeHA hydrogel using the resazurin cell viability test, alkaline phosphatase activity assay, and immunohistochemistry test; they revealed that it showed good biocompatibility with primary human alveolar bone cells.110 In another study, the researchers investigated the biocompatibility of a hydrogel by evaluating the cytotoxicity of different O-HACC/polyvinyl alcohol (PVA) compound ratios and GO dosage hydrogels in normal murine fibroblasts. Their results showed that GO and chitosan (CS) quaternary ammonium salt had good biocompatibility with murine fibroblasts and were nontoxic.111Biodegradability
Biodegradability is another property that is essential for the design and application of hydrogels. In addition to facilitating cell proliferation and vascular infiltration, biodegradability can reduce the possibility of inflammatory reactions caused by degradation products. The degradation rate of a hydrogel should match the growth rate of the corresponding target tissue. In vitro cell studies have shown that if hydrogels cannot degrade over time, the morphology, proliferation, and migration of cells will be inhibited.107 However, biomaterials that biodegrade rapidly may not be able to serve as a space-filling scaffold capable of supporting new tissue formation.112 Raza et al. discussed the influence of hydrolytic degradation in thiol-norbornene hydrogels synthesized via thiol-norbornene photo-click reactions on cell function by monitoring the viability of human mesenchymal stem cells (hMSCs). Their results showed that cell viability was more strongly promoted in the presence of nonhydrolytically labile hydrogels.113,114 In addition, Khetan et al. demonstrated that the differentiation of hMSCs in covalently crosslinked hydroxyapatite (HA) hydrogels is regulated by the generation of degradation-mediated cellular traction, independent of cell morphology or matrix mechanics.115The self-healing ability of SHHs
In addition to the abovementioned general properties, SHHs applied under physiological conditions exhibit rapid self-healing ability.116 The self-healing ability of hydrogels can be evaluated using both qualitative and quantitative methods. Qualitative methods mainly refer visual or microscopic examination to determine the healing of hydrogel wound systems. These methods involve the assessment of variables such as the degree of crack closure and the degree of broken end connection. Currently, the procedure of the commonly used method for qualitatively evaluating the self-healing ability of hydrogels is as follows. Briefly, two hydrogels each of the same size are stained with methyl orange and methyl blue. They are then sliced into two equal halves.117 Next, two differently stained halves are combined in a single mold and placed at a constant temperature for a predetermined time. The self-healing process is then recorded using a digital camera.118 During this process, the hydrogel is sealed in a storage bag to prevent the interference of evaporation with healing.119 The self-healing ability is assessed by determining whether key properties (such as mechanical strength and elastic modulus) are restored to original levels following hydrogel repair. These measurements may include the results of compressive, tensile, or three-point bending tests. The degree of self-healing ability is represented by the healing efficiency (HE), which is calculated as follows: HE = (properties of hydrogels after healing)/(properties of the original hydrogel) × 100% (here, measurements of the properties must be performed under identical conditions). The self-healing ability of hydrogels damaged via shear force can be measured using cyclic rheological tests of low and high oscillatory strains. In addition, the self-healing ability may be related to the time of material in the defect. A faster healing speed is more conducive to good clinical prognoses, but different tissues possess different intracellular environments, causing variations in hydrogel performance. It is therefore necessary to select appropriate methods to quantify self-healing ability. For example, self-healing materials in dentistry should be subjected to dynamic testing conditions to simulate mastication, which would have practical clinical significance.120 Moreover, SHHs should be injectable, and shear-thinning behavior is the main determinant of the injectability of hydrogels.121,122 In addition to rapid healing ability, biocompatibility, and good injectability, SHHs should possess adjustable mechanical properties.123 For example, sufficient tensile and compressive strength is required to withstand stress and deformation in the environment conditions.124Mechanisms of self-healing hydrogels
In SHHs, self-healing can either be induced by external stimuli or interactions within the hydrogel (such as dynamic chemical bonds and noncovalent interactions).125 Externa stimulus-induced self-healing mechanism is caused by the release of self-healing agents in microcontainers.126 The main disadvantage of this mechanism is that the self-healing agent is gradually consumed during the self-healing process. Thus, it can be used for a limited number of times and cannot be repeated indefinitely.127 Accordingly, autonomous self-healing has become a hot research topic in recent years owing to the diversity of potential autonomic interactions.128,129 Early SHHs mainly release healing agents via microcapsules or microtubules, but these methods can only achieve sufficient healing once or twice.130 To address these limitations, constitutionally dynamic chemistries, including noncovalent and dynamic covalent chemistries, have recently been used to construct SHHs that are capable of multiple rounds of reversible healing.131 Thus, SHH design is divided into two main categories: dynamic covalent and noncovalent interactions.132 The mechanism of the dynamic covalent interaction is to restore the original state by triggering a reversible process through external stimulation or a healing agent. The noncovalently bonded system is the most effective form of SHH as it can undergo self-repair without external stimulation and can fully return to the original structure and function. Noncovalent interactions are based on weak sacrificial links (such as hydrogen, ionic, or hydrophobic bonds).133 These two common forms of crosslinking bonds in SHHs are shown in Fig. 2.The “click chemistry,” also referred to as “link chemistry,” “dynamic group chemistry” or “rapid combinatorial chemistry,” has many salient advantages, including simple reaction conditions, fast reaction speed, nontoxic and harmless secondary products, low generation of impurities, and high yield.151 The DA reaction, which belongs to a class of biocompatible reactions designed to bind substrates to specific biomolecules, is also important for click chemistry.181 DA reactions have also been used to construct SHHs that react with diene and dienophile reagents.182 However, DA reactions require high temperatures (>100 °C) to induce reversibility. This high temperature requirement limits the biomedical application of DA reactions.
Based on the above discussion, we can clearly understand the mechanism and importance of the self-healing effect of hydrogels. According to different intramolecular interactions involved in SHHs, their mechanisms of action can be classified as noncovalent or covalent interactions. As different SHHs act in different ways and have varying self-healing mechanisms, all of them are not perfect (Table 1).
| Classification | Self-healing mechanisms | Advantages | Disadvantages | Properties | Examples | Applications | References |
|---|---|---|---|---|---|---|---|
| Chemical covalent cross-linking | Imine bonds (Schiff base) | Excellent mechanical strength | Amadori rearrangement | Injectability, fast gelation process, super hydrophilicity, excellent biocompatibility, excellent neutral stability and ultrasensitive pH-responsive behavior | AHA/cystamine dihydrochloride (AHA/Cys) hydrogels, the double-network GMO hydrogels (DN-GMO), QCS/OHA–PEDOT–BBH–EGF hydrogels | Cancer treatment, controlled drug release, biosensors, wound dressings | 128 and 153–155 |
| Disulfide bonds | Room temperature interaction | Produce reaction by-products (mercaptan, toxic) | Enhanced mechanical properties, good biocompatibility, exceptional stretchability, complete and rapid degradation | Dynamically crosslinked gold nanoparticle-hyaluronan hydrogels, OSA–HPCS hydrogel, PVA/ce-MoS2 hydrogel | Controlled drug and gene delivery | 156–159 | |
| Boronate ester bonds | Nonspecific chemical bonding in situ formation | Bond cannot be formed under extreme pH conditions | Good mechanical properties, excellent biocompatibility and conductivity | Cellulose based hydrogels from CMC–B(OH)2 and PVA, CNC-g-PGMA/PDMA-stat-PAPBA NC hydrogels, glucose-responsive hydrogel electrode, poly(NIPAAm-co-APBA-co-AAm) and PVAd | Tissue engineering, wound repairing, controlled drug release, detection of glucose | 160–162 | |
| DA “click chemistry” | One-step reaction, thermally reversible reaction | Requires high temperature and a long time | Good biocompatibility, biodegradability, excellent gel-forming properties | F127@ChS–PEG–OChS hydrogel, self-assembly and core crosslinking of PFMA-b-PSS and PFMA-b-PMTAC and formation of a hydrogel | Drug delivery, cartilage tissue engineering | 163–165 | |
| Physical noncovalent cross-linking | Hydrogen bond | Nontoxic chemical bonding | Poor mechanical strength | High stability, biocompatibility and poor mechanical strength | Polydopamine–polyacrylamide (PDA–PAM) single network hydrogel, TTA–UC hydrogel, the urea–PA hydrogel | Detection of drug release, antibacterial activity | 135 and 166 |
| Hydrophobic interaction | Widely formed in every water-containing system | Poor bonding strength | Conductive, enhanced mechanical properties tunable mechanical properties and thermal response behavior | Stimuli-responsive graphene-based hydrogel, the MA–UPy–PEO–UPy–MA hydrogel, SHH based on the hydrophobic interaction of a biocompatible four-arms star polymer, poly(ethylene glycol)-b-poly(γ-o-nitrobenzyl-L-glutamate) | Drug release, tissue engineering stents, wound dressings, and biosensors | 167–169 | |
| Host–guest interaction | Convenient to adjust the molecular properties of the guest species through a wide range of stimuli | Specific in some cases, binding to each other temporarily | Superior self-healing, injectability, flexibility, stimuli-responsiveness and biocompatibility | Injectable polypseudorotaxane hydrogels, the cellulose-derived hydrogels (CAAs), the injectable Tet-Ada/poly (β-CD) hydrogels | Injectable drug depots, wound dressings, advanced inks for 3D printing of cell scaffolds | 170 and 171 | |
| Ionic bonds | High toughness, anti-fatigue abilities, antifreezing capability, and stretchability | Slow healing process, good thixotropic property | Biodegradability, non-toxicity, biocompatibility, renewable and easy accessibility | Ionic alginate hydrogels, Phos-cycC6/BPmoc-F3/SOx/Ca2+ hydrogel, mesoporous silica/polyacrylate hybrid hydrogel, chitosan/polyzwitterion-based double network hydrogel | Drug delivery capacitive, resistive bimodal sensors | 172–176 |
Application of self-healing hydrogels for bone defect repair
Many recent studies have reported the preparation of high-performance, SHHs that possess good histocompatibility, adjustability, and nontoxicity. The use of SHHs in bone defect repair is therefore an area of active research. For example, Wu et al. performed thiol modification by introducing disulfide crosslinking to NIPAAm-g-CS (NC) hydrogels and assessed their toxicity and bioactive effect using in vitro cell experiments.156 They revealed that the hydrogel had no cytotoxicity. In addition, they solved the problem of poor biocompatibility and biodegradability by copolymerizing NC with NIPAAm during hydrogel formation. The original NC hydrogel has been shown to be a suitable cell carrier scaffold owing to its good biocompatibility and biodegradability. However, its use for biomedical applications was limited by its weak mechanical properties. Wu et al. artificially enhanced the mechanical properties of NC hydrogel, incorporated thiol side chains into CS, and formed disulfide bonds through thioloxidation, thus completing the modification of the NC hydrogel. This hydrogel is therefore expected to be a cell-bearing tissue regeneration biomaterial. In another study, Lu et al. used acylhydrazone-based crosslinking with or without in vivo DA cross-linking to produce dynamic SHHs. These hydrogels were loaded with bone morphogenetic protein-4 and injected into bone defects to promote bone regeneration. Ma et al. prepared an injectable hydrogel consisting of alginate oxide hybrid HA nanoparticles (NPs) and carboxymethyl CS via Schiff base reaction. The self-healing properties of these hydrogels were verified via splicing and rheological experiments (Fig. 3). These hydrogels have broad application prospects for BTE.183 Pan et al. prepared a novel biocompatible injectable and self-healing nanohybrid hydrogel via reversible Schiff base reaction between –HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of oxidized sodium alginate (OSA) and –NH2 of glycol CS mixed with calcium phosphate (CaP) NPs. The results of their experiments showed that this novel hydrogel is an ideal candidate for BTE applications and drug delivery.184 Bai et al. prepared a self-healing dual crosslinked injectable hydrogel using DA click chemistry for repairing skull defects in rats.185 In their experiment, they first used maleimido terminated F127 (F127-AMI) and furfurylamine-grafted chondroitin sulfate (ChS–furan) to synthesize F127-crosslinked ChS (F127@ChS) via DA click chemistry. Next, the dual crosslinked hydrogels were prepared based on F127@ChS and PEG–AMI. Shi et al. proposed a strategy for the assembly of silk fibroin (SF)-based hydrogels under physiological conditions based on dynamic metal-bisphosphonate (BP) coordination bonds between SF microfibers (mSF) and a polysaccharide binder.125 They used biomineralization to generate mSF coated with CaP and chelated by a bisphosphonate ligand of the binder to form a reversible crosslink (Fig. 4). Based on the reversibility of the coordination bond between CaP and BP ligands, the SF-based hydrogels exhibited self-healing properties and did not require external stimulation during healing. In addition, these hydrogels had shear thinning properties that allow them to fill irregularly shaped tissue defects without breaking. However, these hydrogels are characterized by poor mechanical properties and insufficient stability under physiological conditions. To overcome this limitation, photosensitive polyacrylate groups were introduced into the adhesive to improve the mechanical properties of self-healing SF-based hydrogels when exposed to UV light. To demonstrate this phenomenon, the authors implanted a composite scaffold prepared using SF into rat skulls with a severe defect (diameter = 8 mm) and examined the animals 4 and 8 weeks after implantation. They concluded that the hydrogel stimulated the formation of new bone in the CSD rat skull model. In addition to single covalent crosslinking, two different covalent crosslinking methods have been used to form high-performance SHHs. In a previous study, Lu et al. attempted to overcome the limitations of hydrogels based on ChS (which show insufficient strength and inaccurate mechanical tunability and are non-self-healing and noninjectable) using DA click chemistry and dynamic hydrazone bond crosslinking to form hydrogels with excellent performance. Compared with hydrogels formed via single crosslinking, the abovementioned hydrogels showed increased viability and reduced apoptosis in rat MSCs as well as excellent tissue adhesive ability in vivo.186 These experimental results demonstrated that the hydrogels were suitable for use as scaffolds in rat skull tissue engineering. In this experiment, new bone tissue formation was detected in the skull defect area.
O of oxidized sodium alginate (OSA) and –NH2 of glycol CS mixed with calcium phosphate (CaP) NPs. The results of their experiments showed that this novel hydrogel is an ideal candidate for BTE applications and drug delivery.184 Bai et al. prepared a self-healing dual crosslinked injectable hydrogel using DA click chemistry for repairing skull defects in rats.185 In their experiment, they first used maleimido terminated F127 (F127-AMI) and furfurylamine-grafted chondroitin sulfate (ChS–furan) to synthesize F127-crosslinked ChS (F127@ChS) via DA click chemistry. Next, the dual crosslinked hydrogels were prepared based on F127@ChS and PEG–AMI. Shi et al. proposed a strategy for the assembly of silk fibroin (SF)-based hydrogels under physiological conditions based on dynamic metal-bisphosphonate (BP) coordination bonds between SF microfibers (mSF) and a polysaccharide binder.125 They used biomineralization to generate mSF coated with CaP and chelated by a bisphosphonate ligand of the binder to form a reversible crosslink (Fig. 4). Based on the reversibility of the coordination bond between CaP and BP ligands, the SF-based hydrogels exhibited self-healing properties and did not require external stimulation during healing. In addition, these hydrogels had shear thinning properties that allow them to fill irregularly shaped tissue defects without breaking. However, these hydrogels are characterized by poor mechanical properties and insufficient stability under physiological conditions. To overcome this limitation, photosensitive polyacrylate groups were introduced into the adhesive to improve the mechanical properties of self-healing SF-based hydrogels when exposed to UV light. To demonstrate this phenomenon, the authors implanted a composite scaffold prepared using SF into rat skulls with a severe defect (diameter = 8 mm) and examined the animals 4 and 8 weeks after implantation. They concluded that the hydrogel stimulated the formation of new bone in the CSD rat skull model. In addition to single covalent crosslinking, two different covalent crosslinking methods have been used to form high-performance SHHs. In a previous study, Lu et al. attempted to overcome the limitations of hydrogels based on ChS (which show insufficient strength and inaccurate mechanical tunability and are non-self-healing and noninjectable) using DA click chemistry and dynamic hydrazone bond crosslinking to form hydrogels with excellent performance. Compared with hydrogels formed via single crosslinking, the abovementioned hydrogels showed increased viability and reduced apoptosis in rat MSCs as well as excellent tissue adhesive ability in vivo.186 These experimental results demonstrated that the hydrogels were suitable for use as scaffolds in rat skull tissue engineering. In this experiment, new bone tissue formation was detected in the skull defect area.
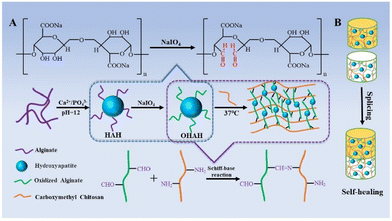 | ||
| Fig. 3 Schematic illustration (A) of the preparation of injectable hydrogels via Schiff base reaction. (B) The self-healing property of the hydrogels.183 Reproduced from ref. 183 with permission from Elsevier, copyright 2020. | ||
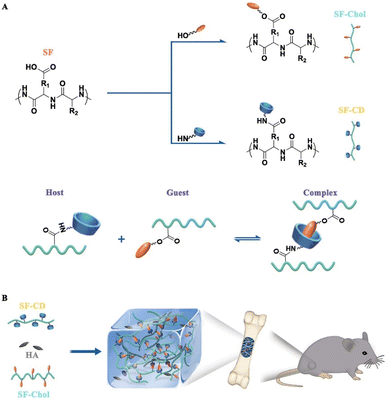 | ||
| Fig. 4 Construction of the novel bone grafts (SF@HG@HA) with self-healing capability. (A) Synthesis of specific host (SF–CD) and guest (SF–Chol) macromers. Interaction of β-cyclodextrin (CD, host) and cholesterol (Chol, guest) in formation of a reversible host–guest (HG) complex crosslink. (B) Schematic of supramolecular hydrogel formation through host–guest complexation and its application as bone graft for promoting bone regeneration.91 Reproduced from ref. 91 with permission from Elsevier, copyright 2021. | ||
In recent years, hydrogels based on noncovalent interaction have been widely investigated. Some experimental methods for preparing high-performance hydrogels via noncovalent crosslinking are listed below. Bai et al. fabricated a novel inorganic–organic hybrid hydrogel with self-healing ability based on SF using dynamic host–guest interactions.91 Herein, SF was combined with β-CD and cholesterol molecules via standard amidation and esterification reactions to assemble supramolecular hydrogels with self-healing behavior. Next, HA NPs were added to the hydrogel to construct inorganic–organic hybrid composites (known as SF@HG@HA). Finally, a CSD model was used to evaluate the ability of this hydrogel to promote the formation of new bone. It was concluded that the hydrogel showed good biocompatibility and biodegradation and could promote the formation of new bone in an in vitro cell experiment. To enhance the mechanical properties of hydrogels, many studies have focused on the preparation of multinetwork SHHs. For example, Bi et al. prepared a CS–PVA double-network (DN) hydrogel that exhibited both high strength and toughness. This hydrogel was based on multihydrogen bond interactions using a freezing–heating alternating treatment, which was applied to an alkaline solution of CS–PVA.187 Their experiments showed that the hydrogel preparation process was simple, nontoxic, and harmless and may be suitable for use in tissue engineering repair. In addition, the adhesion of macrophages to the material matrix plays a crucial role in the implantation of biomaterials into the human body and the application of biomaterials in specific biomedical processes. Xu et al. enhanced the adhesion of macrophages by improving the hydrophobicity of the surface of a methyl-gellan gum hydrogel.188 It has been demonstrated that the hydrophobicity of a substrate can be used to regulate the macrophage response; this property can be beneficial for wound healing or repair. Feng et al. prepared gelatin macromolecules via host–guest interactions between aromatic residues of gelatin and free-diffused photocrosslinked acrylic β-CD monomers.189 Subsequent macromolecules were crosslinked with each other to produce highly elastic supramolecular gelatin hydrogels that were only crosslinked via weak host–guest interactions (Fig. 4). The hydrogel thus obtained showed the following advantages. First, it maintained excessive compression and tensile strength. Second, it showed fast self-healing after mechanical damage. Third, it could injected in a gel state and remolded to the target geometry. Fourth, it could promote cell infiltration and migration into the hydrogel. Fifth, excessive β-CD makes the hydrogel adhere tissue and enhances the loading capacity and delivery of hydrophobic drugs. The researchers implanted this hydrogel in rats with skull defects and conducted cell and animal studies 10 weeks later. Finally, they concluded that the hydrogel supported cell recruitment, differentiation, and bone regeneration, making it a useful biomaterial carrier for therapeutic cells and drugs via a minimally invasive procedure.
SHHs for cell loading
Early cell cultures were performed using stiff materials such as polystyrene and glass (conventional 2D culture). The 2D cultures are simple to perform but cannot mimic the in vivo structure, and cells cultured in this environment tend to behave abnormally.190 This led to the development of sophisticated 3D systems in which cultured cells are embedded in hydrogels rather than on top of substrates. Hydrogels have biological and mechanical properties similar to those of biological tissue and can mimic the native ECM.191 Moreover, the degradation of hydrogels is inhibited during the growth, migration, and proliferation of cells. Yang et al. prepared cellulose-based SHHs via dynamic covalent hydrazone bonding and found that L929 cells could readily proliferate in 3D hydrogel environments.192To balance the advantages and disadvantages of covalent and noncovalent crosslinking, many studies have focused on the preparation of DN SHHs based on two forms of crosslinking. Hydrogels are emerging as carriers to encapsulate cells and drugs.193 Initially, conventional hydrogels commonly used for cell encapsulation were based on static chemical covalent crosslinking, but the resulting hydrogels lacked dynamic properties (such as injectability or self-healing).194 Subsequent studies have shown that dynamic hydrogels based on physical noncovalent crosslinking can heal themselves and are suitable for injection, which compensates for the limitations of chemically crosslinked hydrogels. However, these hydrogels are less stable and biocompatible. Feng et al. developed unique cell-infiltratable and injectable (Ci–I) gelatin hydrogels, which are largely stabilized by physical crosslinking caused by host–guest interactions and are further reinforced by limited chemical crosslinking.195 They further evaluated the efficacy of Ci–I gelation hydrogels as cellular carriers for treating enclosed bone abnormality in steroid-associated osteonecrosis (SAON) of the femoral head. In vivo animal studies have shown that these hydrogels can retain their original mechanical properties after injection, promote the regeneration of bone in situ, facilitate cell chemotaxis and aggregation, and accelerate the healing of SAON. In addition, these hydrogels are easy to prepare and can continuously deliver hydrophobic drugs and cells. This is the first study demonstrating the feasibility of using injectable hydrogels to encapsulate stem cells and small molecules to treat bone disorders in deep and enclosed anatomical locations (e.g., SAON in the hip).195 Another combined physical–chemical crosslinking method has been used to prepare hydrogels with desirable properties. Demineralized bone matrix (DBM) powder is known to be a potential alternative bone graft material because of its similar composition and structure to autologous bone and its ability to promote bone regeneration.196 However, the limitations of DBM, such as easy inactivation of growth factors during the preparation process, and lack of bone regeneration cells, hinder its wide application in bone transplantation. Li et al. introduced hypoxic-pretreated bone marrow stromal cells (BMSCs) to provide growth factors and bone regeneration cells for bone formation. Furthermore, they prepared an injectable SHH based on a double crosslinking structure, in which a dynamically crosslinked Schiff base network acted as a self-healing component that allows injection of payloads into the defect site. Moreover, a borax ion cross-linked physical network strengthened its mechanical properties.13 This was used to transport DBM powder and hypoxia-pretreated BMSCs to the bone defect site. Finally, the experimental results showed that the bone defects of rabbits were almost completely healed after 12 weeks of applying hydrogel/DBM/BMSC. In addition, Deng et al. constructed a novel DN biocompatible hydrogel using PEGDA and short-chain CS via ionic–covalent crosslinking.197 The CS-based ionic network and PEGDA-based covalent network as well as the hydrogen bonds between them together provide excellent mechanical properties. Good mechanical properties are one of the advantages of hydrogels in 3D printing technology. Moreover, this modified hydrogel has potential for tissue engineering because of its stronger, printable properties.
With technological development and improvements in tissue regeneration, increasing number of studies have focused on replicating or imitating the complex structure and function of tissues and organs. The application of 3D printing technology has become a research hotspot due to the high degree of formability and controllability of the printed hydrogels. The 3D printing technology is currently a promising biotechnology tool for tissue engineering.198 Recently, hydrogels based on reversible noncovalent interactions have been gradually developed using 3D bioprinting. However, most noncovalent crosslinking hydrogels are fragile and can be easily damaged. Although they have self-healing ability, this process takes a long time; therefore, none of these hydrogels are suitable for 3D bioprinting. Hydrogels that can be used in 3D printing technology should show shear thinning properties, good mechanical properties, appropriate yield strength, and fidelity. Therefore, to overcome these limitations, Zhang et al. constructed biomimetic scaffolds using hydrogel microparticulates as 3D bioprinting ink, which showed excellent mechanical properties and rapid self-healing under ambient conditions.199 CS methacrylate (CHMA) and PVA, both known as biocompatible polymers, are hybridized to prepare hydrogels with mechanical properties that are tunable via the chemical crosslinking of CHMA and physical crosslinking of PVA through freeze–thawing (Fig. 5). However, the obtained CHMA/PVA hydrogels were rigid and not suitable for 3D printing. Therefore, the authors pressed the hydrogel through a nozzle of a specific diameter, and the gels were transformed into a slurry of microparticles, which became thixotropic. After centrifugal degassing, hydrogels were formed via hydrogen bonds between the particles. Therefore, they exhibited a typical shear-induced reversible gel–fluid transition. Such printed hydrogel scaffolds are conducive to cell adhesion and growth, and can thereby induce spheroid BMSC formation. This has broad future prospects for bone regeneration in tissue engineering in the future. Furthermore, Zhao et al. prepared a biomaterial of 3D printed porous metal scaffolds and infliximab-based hydrogels. This promising biomaterial had self-healing, histocompatible, and anti-inflammatory properties. Experimental results showed that the composite scaffold can repair bone defects in a rabbit model of severe rheumatoid arthritis.200 It is easy to speculate that SHHs suitable for 3D printing have higher performance requirements, and when these difficulties are overcome, they will have great potential for bone repair.
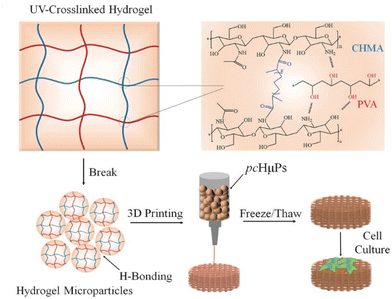 | ||
| Fig. 5 Schematic illustration to the preparation of the self-healing pre-cross-linked hydrogel microparticles (pcHμPs) by 3D printing for cell spheroid growth.199 Reproduced from ref. 199 with permission from Wiley, copyright 2020. | ||
Recently, the application of photothermal therapy (PTT) in bone regeneration has attracted considerable attention. PTT is a new hyperthermia method that uses the photothermal effect of different types of photothermal agents to convert absorbed light energy into heat energy, which helps facilitate efficient and noninvasive treatment of various diseases.201 PTT has great potential for both wound healing and bone regeneration by promoting MSC differentiation and osteoblast maturation.202 For example, gold nanorods/nano-HA (nHA) were shown to exert photothermal therapeutic effects on postoperative tumor and bone defect repair in a mouse model of tibial osteosarcoma.203 In another study using a rat skull defect model, a GelMA/PMMA/PDA hydrogel with mild PTT showed a stronger bone repair effect than pure hydrogel and control.204 Luo et al. prepared the OSA–CS–polydopamine-decorated nHA (PHA)–DDP (cisplatin) bifunctional hydrogel with photothermal effect, which promoted the adhesion and proliferation of bone MSCs in vitro and further induced bone regeneration in vivo.205 Thus, the combination of PTT with chemotherapy, photodynamic therapy, or immunotherapy may improve efficacy and reduce side effects.206 Matheny performed a prospective, randomized, controlled, blinded clinical trial to evaluate the safety and efficacy of a novel self-crosslinked HA hydrogel. This hydrogel was compared with carboxymethylcellulose viscous foam in terms of promoting healing after ethmoidectomy. This study concluded that the hydrogel provided superior wound healing in this experiment.207 Twelve patients requiring extraction of premolars and implants were selected for randomized controlled trials. Patients in the control group received a glass-reinforced HA synthetic bone substitute, Bonelike by Biosckin® (BL®), and those in the experimental group received DEXGEL bone. The stability of primary implants was analyzed using the implant stability coefficient method; the study finally concluded that the hydrogel reinforcement material was easy to handle and showed good defect healing effect.208 Currently, only a few clinical trials have examined the application of hydrogels in bone repair, which poses a challenge for future clinical transformation.
SHHs for drug delivery
In addition to the abovementioned applications, SHHs can be used for drug delivery, wound dressings, cell culture, and diagnostic applications (such as bioassays and bioimaging). In terms of drug delivery, hydrogel is a 3D network structure that can carry and deliver small drug molecules and absorb exudates to promote wound healing. For example, injectable hydrogels usually have a pore size of 50–300 μm.209 The speed of drug absorption is different from that of drug delivery, and using hydrogels can help overcome the common limitations of slow absorption and low efficiency of the traditional drug delivery method.210 However, when the hydrogel is damaged, the concentration of drug rapidly increases to the peak concentration, which can induce toxicity in local tissues. SHHs may be a better alternative to avoid this risk because they have a rapid self-healing ability and can deliver therapeutic drugs and cells in a controllable manner.211 Therefore, the effectiveness of SHHs depends on the specific drugs and cells delivered.SHHs for DNA delivery
Currently, the application of gene therapy in bone regeneration is an important research topic. One area of research focuses on the development of polymer substrates that can deliver DNA. Among them, the most common polymer is poly(lactate-glycolic acid) (PLGA), but its degradation products are known to damage DNA.212 Hydrophilic porous hydrogels that carry drugs or cells can compensate for the lack of PLGA with their high delivery efficiency. Adding DNA to hydrogels that are already widely used in bone and cartilage tissue engineering to deliver drugs or cells can help alter cell behavior and thereby enhance tissue formation.213 The encapsulation of DNA via polymerization can control the release of DNA both spatially and temporally, thereby sustaining the local delivery of therapeutic factors for tissue regeneration.214 Dadsetan et al. demonstrated the potential of an oligo (PEG) fumarate (OPF) hydrogel for sustained delivery of DNA complexes by using OPF hydrogels to transport DNA and bone cells.215 Komatsu et al. found that gelatin hydrogel as a substrate for local gene delivery was more capable of inducing bone regeneration than atelocollagen.216 Localized gene delivery is a promising alternative therapy as it may allow the sustained expression of specific osteoinductive growth factors in cells near the damaged site.217Prospective and challenges
Bone is the second most transplanted tissue in the world after blood, and traditional bone transplantation surgery has significant disadvantages, such as pain, high cost, and susceptibility to infection.218 As one of the key materials in BTE, scaffolds should have many specific properties. However, the osteoinductivity of synthetic materials is lower than that of allograft materials and has therefore not been widely adopted.219 However, hydrogels have been widely used as scaffolds in BTE due to their good biocompatibility, nontoxicity, and injectability. Because of the great prospects hydrogels for bone repair, many researchers have prepared synthetic hydrogels with better properties than natural hydrogels using interdisciplinary methods.In terms of clinical translation, many facial corrections and esthetic hydrogel-based products have been approved by the US Food and Drug Administration.103 Based on the clinicaltrials.gov database (https://clinicaltrials.gov/), 514 completed and recruiting clinical trials have been performed in various application areas, including cancer treatment, esthetic correction, spinal fusion, tissue regeneration, and incontinence.220 Despite the fact that SHHs are a hot topic in the field of bone repair, market penetration is low due to the lack of clinical studies demonstrating their effectiveness. This review discusses the unsolved challenges and clinical translational potential of hydrogels to facilitate the adoption of self-healing hydrogels in clinical settings. We focused on the following aspects. First, the potential side effects and long-term efficacy of hydrogel injection/implantation are uncertain. The immune response to foreign implanted biomaterials generally consists of inflammatory events and wound healing processes that lead to fibrosis. Vegas et al. found that the distribution of triazole modification creates a unique hydrogel surface that inhibits recognition by macrophages and fibrous deposition.221 Second, hydrogels for specific diseases require considerable care regarding the appropriate route of implantation and lowest effective dose. For instance, an inappropriate implantation route may lead to postoperative complications such as swelling, nodules, and pain. Moreover, most drugs are dose-dependent, and drug delivery-related hydrogels must balance benefits and side effects when releasing the drug. Third, self-healing hydrogels are difficult to prepare and are not yet ready for mass production. For hydrogels embedded with active substances or living cells, preservation is another challenge for large-scale clinical applications. Fourth, studies have shown that the intracellular stromal microenvironment is dynamic, and cellular behavior and the associated signaling cascades remain unexplored. When cell-loaded hydrogels are implanted into the complex microenvironment, various cells in the body as well as those in the hydrogel will interact with the surrounding cells via paracrine crosstalk, resulting in negative effects.103 Finally, the long-standing incompatibility of SHH toughness and rapid self-repair has not yet been fully addressed.222 Although some strategies have been formulated to deal with these problems, it is necessary to continue to innovate and optimize the performance of hydrogels.
The prospect of combining hydrogels with advanced biotechnology is also notable. For example, when SHHs with good tensile properties are combined with 3D printing technology, the hydrogels obtained can be arbitrarily printed into target shapes to adapt to different sizes and shapes of bone defects. Furthermore, mechanical self-healing properties can be improved by adding reinforcement materials (such as inorganic or organic fillers). Moreover, printed hydrogels can be dynamically adjusted. Dynamic adjustment involves the introduction of the fourth dimension into the 3D structure therefore, it is known as “4D printing” technology. The combination of SHHs and 4D printing technology enables hydrogels to be controlled in both time and space. Introducing the dimension of time also means that the morphology of hydrogels can change over time. This morphological adjustment property enables hydrogels to morphologically adjust according to various stimuli in the bone tissue healing process, which involves different stages of bone repair. However, even if 4D printing has superior features, it is not yet perfect. As the mechanisms involved in 4D printing technology are extremely complex, simulation of the complex dynamic deformation of the original tissue is a difficult problem, which needs further investigation on 4D printing technology.
The first market appearance of a hydrogel was reported in 1949, wherein PVA was crosslinked with formaldehyde. This hydrogel was marketed under the trade name Ivalon and was used in biomedical implants.223 However, the real turning point in the production and use of hydrogels was the synthesis of poly(2-hydroxyethyl methacrylate) gels, which were invented and studied by Otto Wichterle and Drahoslav Lim during the development of modern soft hydrogel contact lenses in 1960.224 This represented the starting point for the spread of a flourishing hydrogel market. Until 2016, the hydrogel market was valued at USD 10.87 billion and was projected to reach USD 15.33 billion by 2022. This shows a compound annual growth rate of 6.04% from 2017 to 2022.225 Finally, biomedical research is advancing rapidly. With the continuous creation of new advanced technologies, research on SHHs can be further refined, and the performance of SHHs will be more aligned with human needs. Over time, the unique advantages of SHHs can compensate for the limitations of autografts, thereby becoming a widely used bone repair material showing consistently good performance. However, considerable effort is required for improving SHH performance.
Conclusions
Herein, we conducted a systematic review of available research related to the self-healing behavior, self-healing mechanism, main performance requirements and application of hydrogels in bone repair. Depending on the biomaterial from which hydrogels are prepared, their self-healing mechanisms also differ, and the properties of hydrogels vary accordingly. Moreover, there are many factors that cause bone defects, and bone repair remains a major challenge. Currently, SHHs are considered a promising scaffold material in tissue engineering, only SHHs with adequate performance requirements have been designed, owing to the differences in bone defects among clinical cases. Thus, the design and preparation of SHHs are promising but challenging, and additional research is warranted to further improve them. However, we speculate that SHHs should be used in clinical trials as soon as possible to repair bone defects in patients.Conflicts of interest
There is no conflict of interest for all the authors.Acknowledgements
This work was financially supported by the National Natural Science Foundation (32171354, 31972925, 31700839), the Fundamental Research Funds for Central Universities.References
- E. J. Sheehy, D. J. Kelly and F. J. O’Brien, Mater. Today Bio, 2019, 3, 100009 CrossRef CAS PubMed
.
- F. M. Klenke and K. A. Siebenrock, in Reference Module in Biomedical Sciences, Elsevier, 2016, DOI:10.1016/B978-0-12-801238-3.99488-1
.
- A. A. El-Rashidy, J. A. Roether, L. Harhaus, U. Kneser and A. R. Boccaccini, Acta Biomater., 2017, 62, 1–28 CrossRef CAS PubMed
.
- C. Li, J. Sun, K. Shi, J. Long, L. Li, Y. Lai and L. Qin, J. Mater. Chem. B, 2020, 8, 4575–4586 RSC
.
- E. Mancuso, L. Shah, S. Jindal, C. Serenelli, Z. M. Tsikriteas, H. Khanbareh and A. Tirella, Mater. Sci. Eng., C, 2021, 126, 112192 CrossRef CAS PubMed
.
- J. Raphel, M. Holodniy, S. B. Goodman and S. C. Heilshorn, Biomaterials, 2016, 84, 301–314 CrossRef CAS PubMed
.
- Z. Yang, H. Tao, Z. Ye, L. Jin, N. Lin and D. Yang, J. Int. Med. Res., 2018, 46, 3219–3225 CrossRef PubMed
.
- J. F. Liao, K. Shi, Y. P. Jia, Y. T. Wu and Z. Y. Qian, Bioact. Mater., 2021, 6, 2221–2230 CrossRef CAS PubMed
.
- P. N. Soucacos, Z. T. Kokkalis, M. Piagkou and E. O. Johnson, Injury, 2013, 44, S70–S75 CrossRef PubMed
.
- V. Chadayammuri, M. Hake and C. Mauffrey, Patient Safety in Surgery, 2015, 9, 32 CrossRef PubMed
.
- N. V. Shrivas, A. K. Tiwari, R. Kumar, S. Patil, D. Tripathi and S. Badhyal, J. Biomech. Eng., 2021, 143, 081011 CrossRef PubMed
.
- R. Fleischmann, R. Landewe and J. S. Smolen, Semin. Arthritis Rheum., 2016, 46, 279–285 CrossRef PubMed
.
- D. Li, Z. Yang, X. Zhao, Y. Luo, Y. Ou, P. Kang and M. Tian, J. Mater. Chem. B, 2021, 9, 479–493 RSC
.
- E. H. Schemitsch, J. Orthop. Trauma, 2017, 31, S20–S22 CrossRef PubMed
.
- M. B. Sordi, A. Cruz, M. C. Fredel, R. Magini and P. T. Sharpe, Mater. Sci. Eng., C, 2021, 124, 112055 CrossRef CAS PubMed
.
- D. Li, Q. Hu, P. Kang, J. Yang, Z. Zhou, B. Shen and F. Pei, International Orthopaedics, 2018, 42, 2787–2795 CrossRef PubMed
.
- D. Li, X. Xie, P. Kang, B. Shen, F. Pei and C. Wang, J. Orthop. Sci., 2017, 22, 1060–1065 CrossRef PubMed
.
- L. Yang, I. Ullah, K. Yu, W. Zhang, J. Zhou, T. Sun, L. Shi, S. Yao, K. Chen, X. Zhang and X. Guo, Biofabrication, 2021, 13(3), 035007 CrossRef CAS PubMed
.
- G. Zhu, T. Zhang, M. Chen, K. Yao, X. Huang, B. Zhang, Y. Li, J. Liu, Y. Wang and Z. Zhao, Bioact. Mater., 2021, 6, 4110–4140 CrossRef CAS PubMed
.
- J. F. Liao, R. X. Han, Y. Z. Wu and Z. Y. Qian, Bone Res., 2021, 9(1), 18 CrossRef CAS PubMed
.
- Q. Wang, J. Yan, J. Yang and B. Li, Mater. Today, 2016, 19, 451–463 CrossRef CAS
.
- T. Zhang, Q. Wei, H. Zhou, Z. Jing, X. Liu, Y. Zheng, H. Cai, F. Wei, L. Jiang, M. Yu, Y. Cheng, D. Fan, W. Zhou, X. Lin, H. Leng, J. Li, X. Li, C. Wang, Y. Tian and Z. Liu, Bioact. Mater., 2021, 6, 3659–3670 CrossRef CAS PubMed
.
- W. J. Basirun, B. Nasiri-Tabrizi and S. Baradaran, Crit. Rev. Solid State Mater. Sci., 2018, 43, 177–212 CrossRef CAS
.
- N. Wang, S. Thameem Dheen, J. Y. H. Fuh and A. Senthil Kumar, Bioprinting, 2021, 23, e00146 CrossRef
.
- I. A. Urban, E. Montero, A. Monje and I. Sanz-Sanchez, J. Clin. Periodontol., 2019, 46, 319–339 CrossRef PubMed
.
- C. A. de Sousa, C. A. Araujo Lemos, J. F. Santiago-Junior, L. P. Faverani and E. P. Pellizzer, J. Dent., 2018, 76, 1–8 CrossRef PubMed
.
- A. Mostafavi, T. Abudula, C. S. Russell, E. Mostafavi, T. J. Williams, N. Salah, A. Alshahrie, S. Harris, S. M. M. Basri, Y. K. Mishra, T. J. Webster, A. Memic and A. Tamayol, Acta Biomater., 2021, 127, 313–326 CrossRef CAS PubMed
.
- D. Li, L. Deng, Z. Yang, X. Xie, P. Kang and Z. Tan, J. Biomater. Appl., 2016, 30, 1322–1333 CrossRef CAS PubMed
.
- W. Wang and K. W. K. Yeung, Bioact. Mater., 2017, 2, 224–247 CrossRef PubMed
.
- Q. Zhang, K. Huang, J. Tan, X. Lei, L. Huang, Y. Song, Q. Li, C. Zou and H. Xie, Chin. Chem. Lett., 2022, 33, 1623–1626 CrossRef CAS
.
- J. A. Lenis, P. Rico, J. L. G. Ribelles, M. A. Pacha-Olivenza, M. L. González-Martín and F. J. Bolívar, Mater. Sci. Eng., C, 2020, 116, 111268 CrossRef CAS PubMed
.
- Y. Chen, N. Kawazoe and G. Chen, Acta Biomater., 2018, 67, 341–353 CrossRef CAS PubMed
.
- I. Erezuma, I. Lukin, M. Desimone, Y. S. Zhang, A. Dolatshahi-Pirouz and G. Orive, Biomater. Adv., 2023, 146, 213274 CrossRef CAS PubMed
.
- T. Liu, X. Zhang, Y. Luo, Y. Huang and G. Wu, Stem Cells Int., 2016, 2016, 1416047 Search PubMed
.
- J. Liu, L. Yang, K. Liu and F. Gao, Front. Pharmacol., 2023, 14, 1050954 CrossRef CAS PubMed
.
- A. Shaabani and R. Sedghi, Carbohydr. Polym., 2021, 264, 118045 CrossRef CAS PubMed
.
- B. W. Tan, Q. Tang, Y. J. Zhong, Y. L. Wei, L. F. He, Y. T. Wu, J. B. Wu and J. F. Liao, Int. J. Oral Sci., 2021, 13(1), 9 CrossRef CAS PubMed
.
- Y. Yu, B. Sun, C. Yi and X. Mo, Front. Mater. Sci., 2017, 11, 93–105 CrossRef
.
- X. Su, T. Wang and S. Guo, Regener. Ther., 2021, 16, 63–72 CrossRef CAS PubMed
.
- W.-C. Ji, X.-W. Zhang and Y.-S. Qiu, World J. Exp. Med., 2016, 6, 58–62 CrossRef PubMed
.
- J. R. Bush, H. Liang, M. Dickinson and E. A. Botchwey, Polym. Adv. Technol., 2016, 27, 1050–1055 CrossRef CAS PubMed
.
- E. Quinlan, A. López-Noriega, E. Thompson, H. M. Kelly, S. A. Cryan and F. J. O'Brien, J. Controlled Release, 2015, 198, 71–79 CrossRef CAS PubMed
.
- J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS PubMed
.
- Y. T. Wu, X. Zhang, Q. Zhao, B. W. Tan, X. Y. Chen and J. F. Liao, J. Biomed. Nanotechnol., 2020, 16, 1667–1686 CrossRef CAS PubMed
.
- Y. Miao, Y. Chen, J. Luo, X. Liu, Q. Yang, X. Shi and Y. Wang, Bioact. Mater., 2023, 21, 97–109 CrossRef CAS PubMed
.
- X. Lv, Y. Liu, S. Song, C. Tong, X. Shi, Y. Zhao, J. Zhang and M. Hou, Carbohydr. Polym., 2019, 205, 312–321 CrossRef CAS PubMed
.
- R. Kadri, J. Bacharouch, K. Elkhoury, G. Ben Messaoud, C. Kahn, S. Desobry, M. Linder, A. Tamayol, G. Francius, J. F. Mano, L. Sanchez-Gonzalez and E. Arab-Tehrany, Mater. Today Bio, 2020, 6, 100046 CrossRef CAS PubMed
.
- I. Antoniuk, D. Kaczmarek, A. Kardos, I. Varga and C. Amiel, Polymers, 2018, 10, 566 CrossRef PubMed
.
- S. R. Shin, C. Shin, A. Memic, S. Shadmehr, M. Miscuglio, H. Y. Jung, S. M. Jung, H. Bae, A. Khademhosseini, X. Tang and M. R. Dokmeci, Adv. Funct. Mater., 2015, 25, 4486–4495 CrossRef CAS PubMed
.
- W. Bonani, N. Cagol and D. Maniglio, in Biomimicked Biomaterials: Advances in Tissue Engineering and Regenerative Medicine, ed. H. J. Chun, R. L. Reis, A. Motta and G. Khang, 2020, vol. 1250, pp. 49–61 Search PubMed
.
- K. Elkhoury, C. S. Russell, L. Sanchez-Gonzalez, A. Mostafavi, T. J. Williams, C. Kahn, N. A. Peppas, E. Arab-Tehrany and A. Tamayol, Adv. Healthcare Mater., 2019, 8(18), 1900506 CrossRef PubMed
.
- C. Zhang, Q. Dong, K. Liang, D. Zhou, H. Yang, X. Liu, W. Xu, Y. Zhou and P. Xiao, Int. J. Biol. Macromol., 2018, 119, 270–277 CrossRef CAS PubMed
.
- C. D. Spicer, Polym. Chem., 2020, 11, 184–219 RSC
.
- H. D. N. Tran, K. D. Park, Y. C. Ching, H. Cong and N. Dai Hai, J. Ind. Eng. Chem., 2020, 89, 58–82 CrossRef CAS
.
- Q. Zhang, B. Shi, J. Ding, L. Yan, J. P. Thawani, C. Fu and X. Chen, Acta Biomater., 2019, 88, 57–77 CrossRef CAS PubMed
.
- S. Sun, L.-B. Mao, Z. Lei, S.-H. Yu and H. Coelfen, Angew. Chem., Int. Ed., 2016, 55, 11765–11769 CrossRef CAS PubMed
.
- Q. Zeng, M. S. Desai, H.-E. Jin, J. H. Lee, J. Chang and S.-W. Lee, Biomacromolecules, 2016, 17, 2619–2625 CrossRef CAS PubMed
.
- J. Qu, X. Zhao, Y. Liang, T. Zhang, P. X. Ma and B. Guo, Biomaterials, 2018, 183, 185–199 CrossRef CAS PubMed
.
- R. Narayanaswamy and V. P. Torchilin, Molecules, 2019, 24(3), 603 CrossRef PubMed
.
- Z. Bao, P. Gao, G. Xia, Z. Wang, M. Kong, C. Feng, X. Cheng, Y. Liu and X. Chen, J. Mater. Chem. B, 2016, 4, 3936–3944 RSC
.
- M. Grosjean, L. Gangolphe and B. Nottelet, Adv. Funct. Mater., 2023, 33, 2205315 CrossRef CAS
.
- Y. Y. Tang, X. M. Sun, J. C. Ma and Q. S. Yan, J. Biomater. Sci., Polym. Ed., 2023, 1–22 CrossRef PubMed
.
- H. Hamedi, S. Moradi, S. M. Hudson and A. E. Tonelli, Carbohydr. Polym., 2018, 199, 445–460 CrossRef CAS PubMed
.
- G. Xia, Y. Liu, M. Tian, P. Gao, Z. Bao, X. Bai, X. Yu, X. Lang, S. Hu and X. Chen, J. Mater. Chem. B, 2017, 5, 3172–3185 RSC
.
- M. M. Qin, Y. Q. Guo, F. F. Su, X. P. Huang, Q. P. Qian, Y. L. Zhou and J. Y. Pan, Chem. Eng. J., 2023, 455, 140854 CrossRef CAS
.
- X. Jing, H.-Y. Mi, Y.-J. Lin, E. Enriquez, X.-F. Peng and L.-S. Turng, ACS Appl. Mater. Interfaces, 2018, 10, 20897–20909 CrossRef CAS PubMed
.
- J. Tavakoli and Y. Tang, Polymers, 2017, 9(8), 364 CrossRef PubMed
.
- S. G. Son, H. J. Park, S. M. Kim, S. J. Kim, M. S. Kil, J. M. Jeong, Y. E. Lee, Y. Eom, S. Y. Hwang, J. Park and B. G. Choi, Chem. Eng. J., 2023, 454, 140443 CrossRef
.
- Q. Ling, X. Fan, M. Ling, J. Liu, L. Zhao and H. Gu, ACS Appl. Mater. Interfaces, 2023, 15, 12350–12362 CrossRef CAS PubMed
.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105–121 CrossRef CAS PubMed
.
- N. N. Dil and M. Sadeghi, J. Hazard. Mater., 2018, 351, 38–53 CrossRef CAS PubMed
.
- C. Shao, M. Wang, L. Meng, H. Chang, B. Wang, F. Xu, J. Yang and P. Wan, Chem. Mater., 2018, 30, 3110–3121 CrossRef CAS
.
- X. Liu, C. Tang, X. Du, S. Xiong, S. Xi, Y. Liu, X. Shen, Q. Zheng, Z. Wang, Y. Wu, A. Horner and J.-K. Kim, Mater. Horiz., 2017, 4, 477–486 RSC
.
- Z. Lei, Q. Wang, S. Sun, W. Zhu and P. Wu, Adv. Mater., 2017, 29(22) DOI:10.1002/adma.201700321
.
- C. Pascual-Garrido, F. Rodriguez-Fontan, E. A. Aisenbrey, K. A. Payne, J. Chahla, L. R. Goodrich and S. J. Bryant, J. Orthop. Res., 2018, 36, 64–75 CrossRef CAS PubMed
.
- M. Mehrali, A. Thakur, C. P. Pennisi, S. Talebian, A. Arpanaei, M. Nikkhah and A. Dolatshahi-Pirouz, Adv. Mater., 2017, 29(8) DOI:10.1002/adma.201603612
.
- I. M. Tayler and R. S. Stowers, Acta Biomater., 2021, 132, 4–22 CrossRef CAS PubMed
.
- H. T. Liao, M.-J. Tsai, M. Brahmayya and J.-P. Chen, Int. J. Mol. Sci., 2018, 19(9), 2537 CrossRef PubMed
.
- C. Acevedo, V. A. Stadelmann, D. P. Pioletti, T. Alliston and R. O. Ritchie, Nat. Biomed. Eng., 2018, 2, 62–71 CrossRef PubMed
.
- L. Shi, P. Ding, Y. Wang, Y. Zhang, D. Ossipov and J. Hilborn, Macromol. Rapid Commun., 2019, 40(7), 1800837 CrossRef PubMed
.
- W. Zhu, J. Zhang, Z. Wei, B. Zhang and X. Weng, Materials, 2023, 16(3), 1215 CrossRef CAS PubMed
.
- M. Diba, S. Spaans, K. Ning, B. D. Ippel, F. Yang, B. Loomans, P. Y. W. Dankers and S. C. G. Leeuwenburgh, Adv. Mater. Interfaces, 2018, 5(17), 1800118 CrossRef
.
- J. F. Patrick, M. J. Robb, N. R. Sottos, J. S. Moore and S. R. White, Nature, 2016, 540, 363–370 CrossRef CAS PubMed
.
- Z. B. Liu, J. Li, Z. P. Zhang, J. Z. Liu, C. Y. Wu and Y. Q. Yu, Eur. Polym. J., 2023, 182, 111728 CrossRef CAS
.
- Y. L. Fang, X. S. Du, Z. L. Du, H. B. Wang and X. Cheng, J. Mater. Chem. A, 2017, 5, 8010–8017 RSC
.
- Y. Wang, S. Zhang and J. Wang, Chin. Chem. Lett., 2021, 32, 1603–1614 CrossRef CAS
.
- A. Sun, X. He, X. Ji, D. Hu, M. Pan, L. Zhang and Z. Qian, Chin. Chem. Lett., 2021, 32, 2117–2126 CrossRef CAS
.
- A. Sreedevi Madhavikutty, A. K. Singh Chandel, C.-C. Tsai, N. F. Inagaki, S. Ohta and T. Ito, Sci. Technol. Adv. Mater., 2023, 24, 2175586 CrossRef PubMed
.
- W. Zhu, J. Y. Zhang, Z. Q. Wei, B. Z. Zhang and X. S. Weng, Materials, 2023, 16(3), 1215 CrossRef CAS PubMed
.
- L. Zhang, Z. Liu, X. Wu, Q. Guan, S. Chen, L. Sun, Y. Guo, S. Wang, J. Song, E. M. Jeffries, C. He, F.-L. Qing, X. Bao and Z. You, Adv. Mater., 2019, 31(23), e1901402 CrossRef PubMed
.
- S. Bai, M. Zhang, X. Huang, X. Zhang, C. Lu, J. Song and H. Yang, Chem. Eng. J., 2021, 413, 127512 CrossRef CAS
.
- Y. Y. Yang, L. F. Xu, J. F. Wang, Q. Y. Meng, S. L. Zhong, Y. Gao and X. J. Cui, Carbohydr. Polym., 2022, 283, 119161 CrossRef CAS PubMed
.
- S. Yue, H. He, B. Li and T. Hou, Nanomaterials, 2020, 10, 1511 CrossRef CAS PubMed
.
- X. Lin, M. Wang, J. Zhao, X. Wu, J. Xie and J. Yang, Carbohydr. Polym., 2023, 304, 120502 CrossRef CAS PubMed
.
- C. E. Diesendruck, N. R. Sottos, J. S. Moore and S. R. White, Angew. Chem., Int. Ed., 2015, 54, 10428–10447 CrossRef CAS PubMed
.
- C. Bao, Y.-J. Jiang, H. Zhang, X. Lu and J. Sun, Adv. Funct. Mater., 2018, 28(23), 1800560 CrossRef
.
- M. F. Zhang, X. Chen, K. D. Yang, Q. Dong, H. J. Yang, S. J. Gu, W. L. Xu and Y. S. Zhou, Carbohydr. Polym., 2023, 301, 120372 CrossRef CAS PubMed
.
- M. Verhulsel, M. Vignes, S. Descroix, L. Malaquin, D. M. Vignjevic and J.-L. Viovy, Biomaterials, 2014, 35, 1816–1832 CrossRef CAS PubMed
.
- N. Annabi, J. W. Nichol, X. Zhong, C. Ji, S. Koshy, A. Khademhosseini and F. Dehghani, Tissue Eng., Part B, 2010, 16, 371–383 CrossRef CAS PubMed
.
- S. H. Oh, I. K. Park, J. M. Kim and J. H. Lee, Biomaterials, 2007, 28, 1664–1671 CrossRef CAS PubMed
.
- Z. Lan, R. Kar, M. Chwatko, E. Shoga and E. Cosgriff-Hernandez, J. Biomed. Mater. Res., Part A, 2023, 111, 465–477 CrossRef CAS PubMed
.
- M. Bercea, Polymers, 2022, 14(12), 2365 CrossRef CAS PubMed
.
- H. Cao, L. Duan, Y. Zhang, J. Cao and K. Zhang, Signal Transduction Targeted Ther., 2021, 6, 426 CrossRef CAS PubMed
.
- S. Gerecht, S. A. Townsend, H. Pressler, H. Zhu, C. L. E. Nijst, J. P. Bruggeman, J. W. Nichol and R. Langer, Biomaterials, 2007, 28, 4826–4835 CrossRef CAS PubMed
.
- M. Bercea, Polymers, 2022, 14(12), 2365 CrossRef CAS PubMed
.
- Y. Xiao, E. A. Friis, S. H. Gehrke and M. S. Detamore, Tissue Eng., Part B, 2013, 19, 403–412 CrossRef CAS PubMed
.
- A. Vedadghavami, F. Minooei, M. H. Mohammadi, S. Khetani, A. Rezaei Kolahchi, S. Mashayekhan and A. Sanati-Nezhad, Acta Biomater., 2017, 62, 42–63 CrossRef CAS PubMed
.
- S. Naahidi, M. Jafari, M. Logan, Y. Wang, Y. Yuan, H. Bae, B. Dixon and P. Chen, Biotechnol. Adv., 2017, 35, 530–544 CrossRef CAS PubMed
.
- E. Caló and V. V. Khutoryanskiy, Eur. Polym. J., 2015, 65, 252–267 CrossRef
.
- K. Areevijit, N. Dhanesuan, J. A. Luckanagul and S. Rungsiyanont, J. Biomater. Appl., 2020, 35, 1294–1303 CrossRef PubMed
.
- J. Cao, G. He, X. Ning, X. Chen, L. Fan, M. Yang, Y. Yin and W. Cai, Carbohydr. Polym., 2022, 287, 119318 CrossRef CAS PubMed
.
- E. Alsberg, H. J. Kong, Y. Hirano, M. K. Smith, A. Albeiruti and D. J. Mooney, J. Dent. Res., 2003, 82, 903–908 CrossRef CAS PubMed
.
- Q. Huang, Y. Zou, M. C. Arno, S. Chen, T. Wang, J. Gao, A. P. Dove and J. Du, Chem. Soc. Rev., 2017, 46, 6255–6275 RSC
.
- A. Raza and C. C. Lin, Macromol. Biosci., 2013, 13, 1048–1058 CrossRef CAS PubMed
.
- S. Khetan, M. Guvendiren, W. R. Legant, D. M. Cohen, C. S. Chen and J. A. Burdick, Nat. Mater., 2013, 12, 458–465 CrossRef CAS PubMed
.
- X. Zhang and R. M. Waymouth, J. Am. Chem. Soc., 2017, 139, 3822–3833 CrossRef CAS PubMed
.
- S. Gupta, A. Sharma, J. V. Kumar, V. Sharma, P. K. Gupta and R. S. Verma, Int. J. Biol. Macromol., 2020, 162, 1358–1371 CrossRef CAS PubMed
.
- X. Yang, H. Yang, X. Jiang, B. Yang, K. Zhu, N. C.-H. Lai, C. Huang, C. Chang, L. Bian and L. Zhang, Carbohydr. Polym., 2021, 256, 117574 CrossRef CAS PubMed
.
- S. Chen, F. Tang, L. Tang and L. Li, ACS Appl. Mater. Interfaces, 2017, 9, 20895–20903 CrossRef CAS PubMed
.
- J. L. Ruben, F. J. M. Roeters, A. F. Montagner and M. C. D. N. J. M. Huysmans, J. Mech. Behav. Biomed. Mater., 2014, 30, 75–82 CrossRef CAS PubMed
.
- H. Wang, M. Bongio, K. Farbod, A. W. G. Nijhuis, J. van den Beucken, O. C. Boerman, J. C. M. van Hest, Y. Li, J. A. Jansen and S. C. G. Leeuwenburgh, Acta Biomater., 2014, 10, 508–519 CrossRef CAS PubMed
.
- X. Zhang, B. Tan, Y. Wu, M. Zhang, X. Xie and J. Liao, Carbohydr. Polym., 2022, 293, 119722 CrossRef CAS PubMed
.
- L. Cai, S. Liu, J. Guo and Y.-G. Jia, Acta Biomater., 2020, 113, 84–100 CrossRef CAS PubMed
.
- W. Zhang, R. Wang, Z. Sun, X. Zhu, Q. Zhao, T. Zhang, A. Cholewinski, F. Yang, B. Zhao, R. Pinnaratip, P. K. Forooshani and B. P. Lee, Chem. Soc. Rev., 2020, 49, 433–464 RSC
.
- L. Shi, F. Wang, W. Zhu, Z. Xu, S. Fuchs, J. Hilborn, L. Zhu, Q. Ma, Y. Wang, X. Weng and D. A. Ossipov, Adv. Funct. Mater., 2017, 27(37), 1700591 CrossRef
.
- J. C. Cremaldi and B. Bhushan, Beilstein J. Nanotechnol., 2018, 9, 907–935 CrossRef CAS PubMed
.
- J. Li, W. Li, M. Kong, Z. Li, T. Yang, Q. Wang and W. Teng, J. Nanobiotechnol., 2023, 21, 62 CrossRef CAS PubMed
.
- J. Qu, X. Zhao, P. X. Ma and B. Guo, Acta Biomater., 2017, 58, 168–180 CrossRef CAS PubMed
.
- Z. Wang, Y. Ren, Y. Zhu, L. Hao, Y. Chen, G. An, H. Wu, X. Shi and C. Mao, Angew. Chem., Int. Ed., 2018, 57, 9008–9012 CrossRef CAS PubMed
.
- N. Pathan and P. Shende, Mater. Sci. Eng., C, 2021, 125, 112099 CrossRef CAS PubMed
.
- S. Y. An, D. Arunbabu, S. M. Noh, Y. K. Song and J. K. Oh, Chem. Commun., 2015, 51, 13058–13070 RSC
.
- L. Yue, X. Zhang, Y. Wang, W. Li, Y. Tang and Y. Bai, Eur. Polym. J., 2021, 146, 110258 CrossRef CAS
.
- M. I. Shekh, G. M. Zhu, W. Xiong, W. L. Wu, F. J. Stadler, D. Patel and C. T. Zhu, Int. J. Biol. Macromol., 2023, 224, 604–620 CrossRef CAS PubMed
.
- M. F. Akhtar, M. Hanif and N. M. Ranjha, Saudi Pharm. J., 2016, 24, 554–559 CrossRef PubMed
.
- J. Y. C. Lim, Q. Lin, K. Xue and X. J. Loh, Mater. Today Adv., 2019, 3, 100021 CrossRef
.
- B. D. Rabideau and A. E. Ismail, Phys. Chem. Chem. Phys., 2015, 17, 5767–5775 RSC
.
- Y. Wang, Biomaterials, 2018, 178, 663–680 CrossRef CAS PubMed
.
- W. Wang, Y. Zhang and W. Liu, Prog. Polym. Sci., 2017, 71, 1–25 CrossRef
.
- J. Chen, X. Xu, M. Liu, Y. Li, D. Yu, Y. Lu, M. Xiong, I. Wyman, X. Xu and X. Wu, Carbohydr. Polym., 2021, 264, 117978 CrossRef CAS PubMed
.
- D. Wang, M. Wagner, H.-J. Butt and S. Wu, Soft Matter, 2015, 11, 7656–7662 RSC
.
- K. Miyamae, M. Nakahata, Y. Takashima and A. Harada, Angew. Chem., Int. Ed., 2015, 54, 8984–8987 CrossRef CAS PubMed
.
- Q. Feng, K. Wei, S. Lin, Z. Xu, Y. Sun, P. Shi, G. Li and L. Bian, Biomaterials, 2017, 112, 346–347 CrossRef CAS PubMed
.
- N. Ding, X. Cai, P. Zhang, S. Dong, B. Du, J. Nie and P. Yu, ACS Appl. Polym. Mater., 2021, 3, 2709–2721 CrossRef CAS
.
- J. Ye, S. Fu, S. Zhou, M. Li, K. Li, W. Sun and Y. Zhai, Eur. Polym. J., 2020, 139, 110024 CrossRef CAS
.
- M. Chen, X. Ren, L. Dong, X. Li and H. Cheng, Int. J. Biol. Macromol., 2021, 182, 1259–1267 CrossRef CAS PubMed
.
- C.-H. Li and J.-L. Zuo, Adv. Mater., 2020, 32, 1903762 CAS
.
- A. Alam, Q. Meng, G. Shi, S. Arabi, J. Ma, N. Zhao and H.-C. Kuan, Compos. Sci. Technol., 2016, 127, 119–126 CrossRef CAS
.
- Y. Wu, X. Zhang, B. Tan, Y. Shan, X. Zhao and J. Liao, Biomater. Adv., 2022, 133, 112641 CrossRef PubMed
.
- D. Mawad, A. Artzy-Schnirman, J. Tonkin, J. Ramos, S. Inal, M. M. Mahat, N. Darwish, L. Zwi-Dantsis, G. G. Malliaras, J. J. Gooding, A. Lauto and M. M. Stevens, Chem. Mater., 2016, 28, 6080–6088 CrossRef CAS PubMed
.
- Y. Xu, Y. Li, Q. Chen, L. Fu, L. Tao and Y. Wei, Int. J. Mol. Sci., 2018, 19(8), 2198 CrossRef PubMed
.
- Y. Tu, N. Chen, C. Li, H. Liu, R. Zhu, S. Chen, Q. Xiao, J. Liu, S. Ramakrishna and L. He, Acta Biomater., 2019, 90, 1–20 CrossRef CAS PubMed
.
- B. Chen, Y. Liang, J. Zhang, L. Bai, M. Xu, Q. Han, X. Han, J. Xiu, M. Li, X. Zhou, B. Guo and Z. Yin, Theranostics, 2021, 11, 5911–5925 CrossRef CAS PubMed
.
- M. Wu, J. Chen, W. Huang, B. Yan, Q. Peng, J. Liu, L. Chen and H. Zeng, Biomacromolecules, 2020, 21, 2409–2420 CrossRef CAS PubMed
.
- H. L. Hou, L. Cardo, D. Mancino, B. Arnaiz, A. Criado and M. Prato, Nanoscale, 2020, 12, 23824–23830 RSC
.
- J. Lei, X. Li, S. Wang, L. Yuan, L. Ge, D. Li and C. Mu, ACS Appl. Polym. Mater., 2019, 1, 1350–1358 CrossRef CAS
.
- S.-W. Wu, X. Liu, A. L. Miller II, Y.-S. Cheng, M.-L. Yeh and L. Lu, Carbohydr. Polym., 2018, 192, 308–316 CrossRef CAS PubMed
.
- V. T. Tran, M. T. I. Mredha, J. Y. Na, J.-K. Seon, J. Cui and I. Jeon, Chem. Eng. J., 2020, 394, 124941 CrossRef CAS
.
- Y. Cao, Y. Yao, Y. Li, X. Yang, Z. Cao and G. Yang, J. Colloid Interface Sci., 2019, 544, 121–129 CrossRef CAS PubMed
.
- A. Skardal, J. Zhang, L. McCoard, S. Oottamasathien and G. D. Prestwich, Adv. Mater., 2010, 22, 4736–4740 CrossRef CAS PubMed
.
- H. An, Y. Bo, D. Chen, Y. Wang, H. Wang, Y. He and J. Qin, RSC Adv., 2020, 10, 11300–11310 RSC
.
- W. Du, A. Deng, J. Guo, J. Chen, H. Li and Y. Gao, Carbohydr. Polym., 2019, 223, 115084 CrossRef CAS PubMed
.
- T. Kajisa and T. Sakata, Sci. Technol. Adv. Mater., 2017, 18, 26–33 CrossRef CAS PubMed
.
- M. Montiel-Herrera, A. Gandini, F. M. Goycoolea, N. E. Jacobsen, J. Lizardi-Mendoza, M. Recillas-Mota and W. M. Argüelles-Monal, Carbohydr. Polym., 2015, 128, 220–227 CrossRef CAS PubMed
.
- F. Yu, X. Cao, L. Zeng, Q. Zhang and X. Chen, Carbohydr. Polym., 2013, 97, 188–195 CrossRef CAS PubMed
.
- O. Guaresti, C. García-Astrain, R. H. Aguirresarobe, A. Eceiza and N. Gabilondo, Carbohydr. Polym., 2018, 183, 278–286 CrossRef CAS PubMed
.
- G. R. Bardajee, F. Mizani and S. S. Hosseini, J. Polym. Res., 2017, 24(3), 48 CrossRef
.
- Y. Deng, I. Hussain, M. Kang, K. Li, F. Yao, S. Liu and G. Fu, Chem. Eng. J., 2018, 353, 900–910 CrossRef CAS
.
- T. Takei, R. Yoshihara, S. Danjo, Y. Fukuhara, C. Evans, R. Tomimatsu, Y. Ohzuno and M. Yoshida, Int. J. Biol. Macromol., 2020, 149, 140–147 CrossRef CAS PubMed
.
- H. Jiang, L. Duan, X. Ren and G. Gao, Eur. Polym. J., 2019, 112, 660–669 CrossRef CAS
.
- S. M. Mantooth, B. G. Munoz-Robles and M. J. Webber, Macromol. Biosci., 2019, 19, 1800281 CrossRef PubMed
.
- R. Yu, Y. Yang, J. He, M. Li and B. Guo, Chem. Eng. J., 2021, 417, 128278 CrossRef CAS
.
- H.-H. Lee, Y.-W. Kim, J. Woo, H. Park, K. Hur, Z. Suo and J.-Y. Sun, Extreme Mechanics Letters, 2019, 33, 100572 CrossRef
.
- T. Kopač, M. Krajnc and A. Ručigaj, Int. J. Biol. Macromol., 2021, 168, 695–707 CrossRef PubMed
.
- A. Wang, Y. Wang, B. Zhang, K. Wan, J. Zhu, J. Xu, C. Zhang and T. Liu, Chem. Eng. J., 2021, 411, 128506 CrossRef CAS
.
- J. Cao, Y. Wang, C. He, Y. Kang and J. Zhou, Carbohydr. Polym., 2020, 242, 116420 CrossRef CAS PubMed
.
- W. Tanaka, H. Shigemitsu, T. Fujisaku, R. Kubota, S. Minami, K. Urayama and I. Hamachi, J. Am. Chem. Soc., 2019, 141, 4997–5004 CrossRef CAS PubMed
.
- V. K. Anupama Devi, R. Shyam, A. Palaniappan, A. K. Jaiswal, T.-H. Oh and A. J. Nathanael, Polymers, 2021, 13(21), 3782 CrossRef PubMed
.
- S. Talebian, M. Mehrali, N. Taebnia, C. P. Pennisi, F. B. Kadumudi, J. Foroughi, M. Hasany, M. Nikkhah, M. Akbari, G. Orive and A. Dolatshahi-Pirouz, Adv. Sci., 2019, 6, 1801664 CrossRef PubMed
.
- N. Lagneau, L. Terriac, P. Tournier, J. J. Helesbeux, G. Viault, D. Séraphin, B. Halgand, F. Loll, C. Garnier, C. Jonchère, M. Rivière, A. Tessier, J. Lebreton, Y. Maugars, J. Guicheux, C. Le Visage and V. Delplace, Biomater. Sci., 2023, 11, 2033–2045 RSC
.
- C. C. Deng, W. L. A. Brooks, K. A. Abboud and B. S. Sumerlin, ACS Macro Lett., 2015, 4, 220–224 CrossRef CAS PubMed
.
- D.-q. Li, S.-y. Wang, Y.-j. Meng, Z.-w. Guo, M.-m. Cheng and J. Li, Carbohydr. Polym., 2021, 268, 118244 CrossRef CAS PubMed
.
- A. E. G. Baker, R. Y. Tam and M. S. Shoichet, Biomacromolecules, 2017, 18, 4373–4384 CrossRef CAS PubMed
.
- L. Ma, W. Su, Y. Ran, X. Ma, Z. Yi, G. Chen, X. Chen, Z. Deng, Q. Tong, X. Wang and X. Li, Int. J. Biol. Macromol., 2020, 165, 1164–1174 CrossRef CAS PubMed
.
- P. Pan, X. Chen, H. Xing, Y. Deng, J. Chen, F. A. Alharthi, A. A. Alghamdi and J. Su, Chin. Chem. Lett., 2021, 32, 2159–2163 CrossRef CAS
.
- X. Bai, S. Lü, Z. Cao, B. Ni, X. Wang, P. Ning, D. Ma, H. Wei and M. Liu, Carbohydr. Polym., 2017, 166, 123–130 CrossRef CAS PubMed
.
- S. Lu, X. Bai, H. Liu, P. Ning, Z. Wang, C. Gao, B. Ni and M. Liu, J. Mater. Chem. B, 2017, 5, 3739–3748 RSC
.
- S. Bi, J. Pang, L. Huang, M. Sun, X. Cheng and X. Chen, Int. J. Biol. Macromol., 2020, 146, 99–109 CrossRef CAS PubMed
.
- Z. Xu, D.-G. Hwang, M. D. Bartlett, S. Jiang and K. M. Bratlie, Biochem. Eng. J., 2021, 165, 107821 CrossRef CAS
.
- Q. Feng, K. Wei, S. Lin, Z. Xu, Y. Sun, P. Shi, G. Li and L. Bian, Biomaterials, 2016, 101, 217–228 CrossRef CAS PubMed
.
- S. R. Caliari and J. A. Burdick, Nat. Methods, 2016, 13, 405–414 CrossRef CAS PubMed
.
- A. M. Rosales and K. S. Anseth, Nat. Rev. Mater., 2016, 1(2), 15012 CrossRef CAS PubMed
.
- X. Yang, G. Liu, L. Peng, J. Guo, L. Tao, J. Yuan, C. Chang, Y. Wei and L. Zhang, Adv. Funct. Mater., 2017, 27(40), 1703174 CrossRef
.
- M. H. Turabee, T. Thambi, D. Huu Thuy Trang, J. H. Jeong and D. S. Lee, Biomater. Sci., 2018, 6, 661–671 RSC
.
- L. Bian, C. Hou, E. Tous, R. Rai, R. L. Mauck and J. A. Burdick, Biomaterials, 2013, 34, 413–421 CrossRef CAS PubMed
.
- Q. Feng, J. Xu, K. Zhang, H. Yao, N. Zheng, L. Zheng, J. Wang, K. Wei, X. Xiao, L. Qin and L. Bian, ACS Cent. Sci., 2019, 5, 440–450 CrossRef CAS PubMed
.
- C. I. A. van Houdt, D. A. Cardoso, B. A. J. A. van Oirschot, D. J. O. Ulrich, J. A. Jansen, S. C. G. Leeuwenburgh and J. J. J. P. van den Beucken, J. Tissue Eng. Regener. Med., 2017, 11, 2537–2548 CrossRef CAS PubMed
.
- Z. W. Deng, T. B. Qian and F. Hang, ACS Biomater. Sci. Eng., 2020, 6, 7061–7070 CrossRef CAS PubMed
.
- K. Huang, J. Huang, J. Zhao, Z. Gu and J. Wu, Chin. Chem. Lett., 2022, 33, 1941–1945 CrossRef CAS
.
- H. Zhang, Y. Cong, A. R. Osi, Y. Zhou, F. Huang, R. P. Zaccaria, J. Chen, R. Wang and J. Fu, Adv. Funct. Mater., 2020, 30(13), 1910573 CrossRef CAS
.
- Y. Zhao, C. Gao, H. Liu, H. Liu, Y. Feng, Z. Li, H. Liu, J. Wang, B. Yang and Q. Lin, Acta Biomater., 2021, 121, 653–664 CrossRef CAS PubMed
.
- L. Zhao, X. Zhang, X. Wang, X. Guan, W. Zhang and J. Ma, J. Nanobiotechnol., 2021, 19, 335 CrossRef CAS PubMed
.
- Z. Wan, P. Zhang, L. Lv and Y. Zhou, Theranostics, 2020, 10, 11837–11861 CrossRef CAS PubMed
.
- J. Liao, K. Shi, Y. Jia, Y. Wu and Z. Qian, Bioact. Mater., 2021, 6, 2221–2230 CrossRef CAS PubMed
.
- Y. Wu, X. Zhang, B. Tan, Y. Shan, X. Zhao and J. Liao, Biomater. Adv., 2022, 133, 112641 CrossRef PubMed
.
- S. Luo, J. Wu, Z. Jia, P. Tang, J. Sheng, C. Xie, C. Liu, D. Gan, D. Hu, W. Zheng and X. Lu, Macromol. Biosci., 2019, 19, e1900047 CrossRef PubMed
.
- N. Xu, X. Zhang, T. Qi, Y. Wu, X. Xie, F. Chen, D. Shao and J. Liao, MedComm, 2022, 1, e25 Search PubMed
.
- K. E. Matheny, E. Y. Tseng, K. B. Carter Jr, W. B. Cobb and K. J. Fong, American Journal of Rhinology & Allergy, 2014, 28, 508–513 Search PubMed
.
- A. Machado, I. Pereira, F. Costa, A. Brandão, J. E. Pereira, A. C. Maurício, J. D. Santos, I. Amaro, R. Falacho, R. Coelho, N. Cruz and M. Gama, Clin. Oral Investig., 2023, 27, 979–994 CrossRef PubMed
.
- K. Flegeau, R. Pace, H. Gautier, G. Rethore, J. Guicheuex, C. Le Visage and P. Weiss, Adv. Colloid Interface Sci., 2017, 247, 589–609 CrossRef CAS PubMed
.
- G. Chang, Y. Chen, Y. Li, S. Li, F. Huang, Y. Shen and A. Xie, Carbohydr. Polym., 2015, 122, 336–342 CrossRef CAS PubMed
.
- A. Zhang, Y. Liu, D. Qin, M. Sun, T. Wang and X. Chen, Int. J. Biol. Macromol., 2020, 164, 2108–2123 CrossRef CAS PubMed
.
- D. J. Quick and K. S. Anseth, J. Controlled Release, 2004, 96, 341–351 CrossRef CAS PubMed
.
- D. Costa, A. J. Valente, M. G. Miguel and J. Queiroz, Adv. Colloid Interface Sci., 2014, 205, 257–264 CrossRef CAS PubMed
.
- H. Storrie and D. J. Mooney, Adv. Drug Delivery Rev., 2006, 58, 500–514 CrossRef CAS PubMed
.
- M. Dadsetan, J. P. Szatkowski, K. L. Shogren, M. J. Yaszemski and A. Maran, J. Biomed. Mater. Res., Part A, 2009, 91, 1170–1177 CrossRef CAS PubMed
.
- K. Komatsu, T. Shibata, A. Shimada, H. Ideno, K. Nakashima, Y. Tabata and A. Nifuji, J. Biomater. Sci., Polym. Ed., 2016, 27, 419–430 CrossRef CAS PubMed
.
- M. D. Krebs, E. Salter, E. Chen, K. A. Sutter and E. Alsberg, J. Biomed. Mater. Res., Part A, 2010, 92, 1131–1138 Search PubMed
.
- H. J. Haugen, S. P. Lyngstadaas, F. Rossi and G. Perale, J. Clin. Periodontol., 2019, 46, 92–102 CrossRef PubMed
.
- R. J. Miron, Q. Zhang, A. Sculean, D. Buser, B. E. Pippenger, M. Dard, Y. Shirakata, F. Chandad and Y. Zhang, Clin. Oral Investig., 2016, 20, 2259–2265 CrossRef PubMed
.
- A. Mandal, J. R. Clegg, A. C. Anselmo and S. Mitragotri, Bioeng. Transl. Med., 2020, 5, e10158 CAS
.
- A. J. Vegas, O. Veiseh, J. C. Doloff, M. Ma, H. H. Tam, K. Bratlie, J. Li, A. R. Bader, E. Langan, K. Olejnik, P. Fenton, J. W. Kang, J. Hollister-Locke, M. A. Bochenek, A. Chiu, S. Siebert, K. Tang, S. Jhunjhunwala, S. Aresta-Dasilva, N. Dholakia, R. Thakrar, T. Vietti, M. Chen, J. Cohen, K. Siniakowicz, M. Qi, J. McGarrigle, A. C. Graham, S. Lyle, D. M. Harlan, D. L. Greiner, J. Oberholzer, G. C. Weir, R. Langer and D. G. Anderson, Nat. Biotechnol., 2016, 34, 345–352 CrossRef CAS PubMed
.
- S. Talebian, M. Mehrali, N. Taebnia, C. P. Pennisi, F. B. Kadumudi, J. Foroughi, M. Hasany, M. Nikkhah, M. Akbari, G. Orive and A. Dolatshahi-Pirouz, Adv. Sci., 2019, 6(16), 1801664 CrossRef PubMed
.
- A. Rafique, K. Mahmood Zia, M. Zuber, S. Tabasum and S. Rehman, Int. J. Biol. Macromol., 2016, 87, 141–154 CrossRef CAS PubMed
.
- M. Zare, A. Bigham, M. Zare, H. Luo, E. Rezvani Ghomi and S. Ramakrishna, Int. J. Mol. Sci., 2021, 22(12), 6376 CrossRef CAS PubMed
.
- S. Cascone and G. Lamberti, Int. J. Pharm., 2020, 573, 118803 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |
