Open Access Article
Open Access ArticleA novel yttrium stabilized zirconia and ceria composite electrolyte lowering solid oxide fuel cells working temperature to 400 °C†
Yu Liua,
Liwen Zuoa,
Yulian Yea,
Cong Jianga,
Dan Zhengb,
Chunlei Liu*a,
Baoyuan Wang *ab and
Xunying Wang
*ab and
Xunying Wang *ab
*ab
aSchool of Microelectronics, Hubei University, Wuhan, Hubei 430062, PR China. E-mail: wangxunying@hubu.edu.cn; baoyuanw@163.com; liuchunlei7788945@163.com
bHubei Yangtze Memory Laboratories, Wuhan, 430205, China
First published on 14th November 2023
Abstract
Reducing the working temperature and improving the ionic conductivity of electrolytes have been the critical challenges for the gradual development of solid oxide fuel cells (SOFCs) in practical applications. The researchers all over the world attempt to develop alternative electrolyte materials with sufficient ionic conductivity. In this work, YSZ–CeO2 composite material was used as electrolytes in the construction of symmetrical SOFCs. The maximum power densities (Pmax) of YSZ–CeO2 based fuel cell can reach 680 mW cm−2 at 450 °C, 510 mW cm−2 at 430 °C, 330 mW cm−2 at 410 °C and even 200 mW cm−2 as the operational temperature was reduced to 390 °C. A series of characterizations indicates that the activation energy of the YSZ–CeO2 composite is significantly decreased, and the enhancement effect for ion conduction comes from interface transport. Our findings indicate the YSZ–CeO2 composite material can be a highly promising candidate for advanced low-temperature SOFC.
1 Introduction
Energy is the basic driving force of world development, and is also one of the three major substances for human survival in modern society. Unfortunately, the non-renewable nature of fossil energy and the excessive exploitation, use and waste of fossil energy have brought it to the brink of exhaustion. Besides, the excessive exploitation also caused serious environmental problems. Therefore, renewable energy must be developed to realize the sustainable development of human society.1–3 Solid oxide fuel cells (SOFCs) can directly convert chemical energy into electricity. Compared with traditional energy conversion systems, SOFCs, as a clean energy conversion technology, have high energy conversion efficiency4–6 and fuel flexibility. However, high working temperature (800–1000 °C) is the key technical problem for SOFCs, which greatly limits the commercial development.7,8 Therefore, reducing the working temperature of SOFCs meanwhile maintaining high performance of fuel cells has been the research direction that researchers are interested in.In previous studies, various ionic conductors such as yttrium stabilized zirconia (YSZ), gadolinium doped cerium (GDC) and La1−xSrxGa1−yMgyO3−δ (LSGM) have been widely used as electrolytes for SOFCs.9–11 Among them, YSZ is considered as the most successful electrolyte material so far, but its poor ionic conductivity at low temperature limits its application in low temperature SOFC (LT-SOFC).12,13 One common way to increase the low temperature performance of SOFC with YSZ based electrolyte is to decrease the thickness of the electrolyte layer.14 However, due to the thickness of thin-film electrolyte has to be restricted by the requirement of gas densification and mechanical strength for the electrolyte layer, the SOFC with YSZ thin-film electrolyte is still hard to work at temperature lower than 500 °C.15–17 Recently, it has been discovered that heterointerfaces can provide fast ionic conduction route, and constructing hetero-structure is an effective way to increase the ionic conductivity.18,19
Ceria (CeO2) has been shown to be multifunctional in many fields, such as lithium batteries, fuel cells and a series of energy-related devices, which has attracted extensive interest from researchers.20,21 The most important feature of CeO2 is the capacity for storing and releasing oxygen through Ce4+/Ce3+ redox cycles, which largely depends on the type and concentration of oxygen vacancies in the lattice and surface structure. Unique physical properties are associated with Ce3+ ions and oxygen vacancies.22 According to relevant researches, the formed CeO2−δ@CeO2 core–shell heterostructure may contributes to the development of electrolytes with high ionic conductivity under low temperature, and the hypoxic layer on the surface provides fast ion transport pathways.23,24
On the basis of these ideas, in our work, a novel YSZ–CeO2 composite material is fabricated from nitrate (Ce(NO3)3)-yttrium stabilized zirconia (YSZ) composite and was adopted as electrolyte in the symmetric fuel cell assembled with Ni-NCAL (Ni0.8Co0.15Al0.05LiO2−δ) as the electrodes. Study results revealed that the fabricated fuel cell possesses excellent output power densities at around 400 °C. Various characterizations have been used to systematically study the characteristics of YSZ–CeO2 composite material, and the results validate the great potential application value of YSZ–CeO2 composite electrolytes in LT-SOFCs.
2 Experimental
2.1 Materials fabrication
In our study, the cerium nitrate hexahydrate (Ce(NO3)3·6H2O) with purity ≥99.5% was purchased from Macklin company. YSZ powder was directly purchased from Inframat company. The YSZ–CeO2 composite material was prepared using the following procedure. Firstly, Ce(NO3)3·6H2O powder was mixed with 8YSZ (8 mol% Y2O3) powder together. The mixture was thoroughly blended after being ground for 2 hours, and then placed in muffle furnace to calcine at 600 °C for 3 hours. Finally, the homogeneous YSZ–CeO2 composite powder materials with different mass ratios (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 70
40, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 80
30, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 85
20 and 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) were obtained after being fully ground.
15) were obtained after being fully ground.
2.2 Physical characterizations
X-ray diffractometer (Bruker AXS D8 Advance) was used to obtain X-ray diffraction (XRD) patterns. Field emission scanning electron microscopy (FESEM, JSM7100F) equipped with Oxford energy-dispersive spectrometer (EDS) was used to examine the surface morphology and element distribution of samples. The detailed morphology at interface region between two phases was further investigated by high revolution transmission electron microscopy (HR-TEM, JEOL JE-2100F). The valence states of the elements were examined by X-ray photoelectron spectroscopy (XPS, Escalab 250Xi, Thermo Fisher Scientific). Raman analysis of the material was conducted on Renishao via China with wavelength of 532 nm. To detect the electrical conductivity, YSZ–CeO2 powder was compressed under a pressure of 500 MPa to obtain pellets with diameter of 13 mm, followed by being brushed Pt paste for ionic conductivity test or Ag paste for electronic conductivity test. Linear scan voltage (LSV) method was adopted to characterize the conductivity of the materials, which was conducted on digital source instrument (Keithley 2460).2.3 SOFC fabrication and electrochemical characterization
The symmetrical electrode pieces were fabricated as follows: NCAL slurry was prepared by mixing commercial NCAL powder with turpentine permeable alcohol in a mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then the slurry was painted evenly with a brush on the nickel foam, finally dried the NCAL coated nickel foam at 110 °C for 7–10 minutes to finish the preparation of NCAL electrode pieces. Wherein the nickel foam acts as current collector and gas diffusion layer, while NCAL is responsible for catalytical reaction. The YSZ–CeO2 composite powder was sandwiched by two pieces of Ni–NCAL to construct Ni–NCAL/YSZ–CeO2/Ni–NCAL configuration, which was pressed under a loading of 500 MPa for 1–2 minutes to complete fuel cell fabrication. Before the electrochemical performance testing, the cell was pre-treated by H2 at 450 °C for 30 minutes. During the test, hydrogen was supplied as the fuel and ambient air served as the oxidant. The current density–voltage (I–V) and current density–power (I–P) curves of the assembled cells were recorded by electronic load (IT8511, ITECH). The electrochemical impedance spectroscopy (EIS) was characterized by Gamry Reference 3000 electrochemical workstation in open-circuit voltage mode, with an AC signal amplitude of 10 mV and frequency range of 0.1–106 Hz.
1, and then the slurry was painted evenly with a brush on the nickel foam, finally dried the NCAL coated nickel foam at 110 °C for 7–10 minutes to finish the preparation of NCAL electrode pieces. Wherein the nickel foam acts as current collector and gas diffusion layer, while NCAL is responsible for catalytical reaction. The YSZ–CeO2 composite powder was sandwiched by two pieces of Ni–NCAL to construct Ni–NCAL/YSZ–CeO2/Ni–NCAL configuration, which was pressed under a loading of 500 MPa for 1–2 minutes to complete fuel cell fabrication. Before the electrochemical performance testing, the cell was pre-treated by H2 at 450 °C for 30 minutes. During the test, hydrogen was supplied as the fuel and ambient air served as the oxidant. The current density–voltage (I–V) and current density–power (I–P) curves of the assembled cells were recorded by electronic load (IT8511, ITECH). The electrochemical impedance spectroscopy (EIS) was characterized by Gamry Reference 3000 electrochemical workstation in open-circuit voltage mode, with an AC signal amplitude of 10 mV and frequency range of 0.1–106 Hz.
3 Results and discussion
Fig. 1(a) presented the SEM images of the as-prepared YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) powder. Obviously, the particle size of YSZ–CeO2 composite material was in nanometer level with irregular shape. Fig. 1(b) showed the XRD pattern of the original YSZ–CeO2 material. The diffraction peaks located at 30.1°, 34.9°, 50.1°, 59.6°, 62.5°, 73.6°, 81.6° and 84.1° can be indexed to (1 0 1), (0 0 2), (1 1 2), (1 0 3), (2 0 2), (0 0 4), (2 1 3) and (1 1 4) planes of fluorite YSZ (JCPDS No. 82-1244), respectively; these peaks at 28.7°, 33.2°, 47.7°, 56.6°, 69.7°, 77.1°, 79.5° and 88.9° are corresponded to the (1 1 1), (2 0 0), (2 2 0), (3 1 1), (4 0 0), (3 3 1), (4 2 0), (4 2 2) planes of fluorite cubic phase of CeO2 (JCPDS No. 75-0076), respectively. There are no other stray peaks in the pattern, indicating that there is no impurity in the YSZ–CeO2 (70
30) powder. Obviously, the particle size of YSZ–CeO2 composite material was in nanometer level with irregular shape. Fig. 1(b) showed the XRD pattern of the original YSZ–CeO2 material. The diffraction peaks located at 30.1°, 34.9°, 50.1°, 59.6°, 62.5°, 73.6°, 81.6° and 84.1° can be indexed to (1 0 1), (0 0 2), (1 1 2), (1 0 3), (2 0 2), (0 0 4), (2 1 3) and (1 1 4) planes of fluorite YSZ (JCPDS No. 82-1244), respectively; these peaks at 28.7°, 33.2°, 47.7°, 56.6°, 69.7°, 77.1°, 79.5° and 88.9° are corresponded to the (1 1 1), (2 0 0), (2 2 0), (3 1 1), (4 0 0), (3 3 1), (4 2 0), (4 2 2) planes of fluorite cubic phase of CeO2 (JCPDS No. 75-0076), respectively. There are no other stray peaks in the pattern, indicating that there is no impurity in the YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) composite. Fig. 1(c) displays the typical Raman spectrum of the hydrogen treated YSZ–CeO2 (70
30) composite. Fig. 1(c) displays the typical Raman spectrum of the hydrogen treated YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) material. The strong Raman peak centered at about 465 cm−1 is corresponding to the F2g symmetric stretching mode of the Ce–8O vibrational unit.25–27 The weak peak located at about 260 cm−1 is assigned to the second-order mode of CeO2.28 And the Raman peaks at approximately 623 cm−1 and 150 cm−1 are indexed to YSZ.29 This indicates that the chemical state of the YSZ–CeO2 (70
30) material. The strong Raman peak centered at about 465 cm−1 is corresponding to the F2g symmetric stretching mode of the Ce–8O vibrational unit.25–27 The weak peak located at about 260 cm−1 is assigned to the second-order mode of CeO2.28 And the Raman peaks at approximately 623 cm−1 and 150 cm−1 are indexed to YSZ.29 This indicates that the chemical state of the YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) material under H2 atmosphere is stable.
30) material under H2 atmosphere is stable.
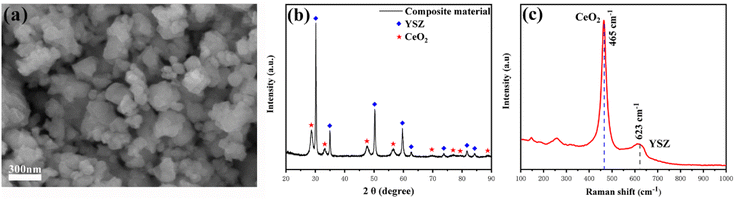 | ||
Fig. 1 (a) The SEM images of the as-prepared YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) powders, (b) the XRD patterns of YSZ–CeO2 (70 30) powders, (b) the XRD patterns of YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) and (c) Raman spectrum of hydrogen treated YSZ–CeO2 (70 30) and (c) Raman spectrum of hydrogen treated YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) powder. 30) powder. | ||
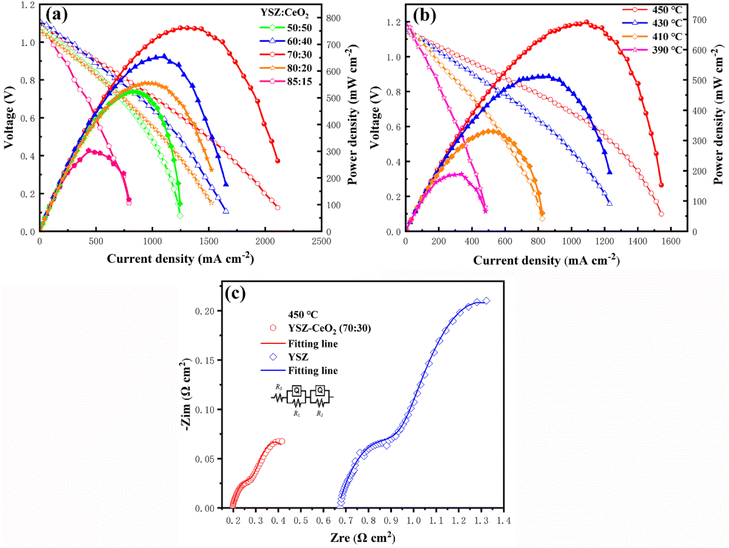
To assess the potential of YSZ–CeO2 composite electrolyte for SOFC, typical current density–voltage (I–V) and current–power density (I–P) curves of Ni–NCAL/YSZ–CeO2/Ni–NCAL fuel cells with different compositions of YSZ to CeO2 were tested using H2 as fuel and air as oxidant at 550 °C. Obviously, as Fig. 2(a) depicts, the composition of YSZ–CeO2 electrolyte significantly influences the electrochemical performance of assembled cell. The peak power density (Pmax) initially enhanced with the increase of CeO2 content, and then decreased as the CeO2 content further increased. The Pmax of 760 mW cm−2 and open-circuit voltages (OCV) of 1.08 V were obtained for the fuel cell with YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, wt/wt) composite electrolyte. Fig. 2(b) further gives the electrochemical performance of the SOFC with the YSZ-CeO2 (70
30, wt/wt) composite electrolyte. Fig. 2(b) further gives the electrochemical performance of the SOFC with the YSZ-CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, wt/wt) composite at various temperatures. It was discovered that the fuel cell delivered exciting electrochemical performance in low temperature range, 680 mW cm−2, 510 mW cm−2, and 330 mW cm−2 at 450 °C, 430 °C and 410 °C, respectively, and can even maintain at 200 mW cm−2 as the operational temperature reduced to 390 °C. The Pmax of this fuel cell device under 390–450 °C is much higher than those reported in the ref. 30–32.
30, wt/wt) composite at various temperatures. It was discovered that the fuel cell delivered exciting electrochemical performance in low temperature range, 680 mW cm−2, 510 mW cm−2, and 330 mW cm−2 at 450 °C, 430 °C and 410 °C, respectively, and can even maintain at 200 mW cm−2 as the operational temperature reduced to 390 °C. The Pmax of this fuel cell device under 390–450 °C is much higher than those reported in the ref. 30–32.
The unexpected cell performance at low temperature indicated that the YSZ–CeO2 composite developed in this work has enormous advantage in electrolyte application for low temperature SOFC. To further study the reason for excellent performance of the YSZ–CeO2 composite at low temperature, the corresponding EIS for the fuel cell working at 450 °C was conducted and analyzed. Besides, for comparison, the EIS of the SOFC with YSZ electrolyte was also tested under the same conditions. The tested spectra were shown in Fig. 2(c), and the inset presented the equivalent circuit of R0(R1Q1)(R2Q2) for simulation. Table 1 lists the corresponding fitting results. Q represents constant phase element (CPE) which describe the electric double layer at the interface between electrode and electrolyte.33,34 R0 reveals the high frequency intercept on the real axis and represents the ohmic resistance, which includes the bulk ionic conduction resistance of the electrolyte, the electronic conduction resistance of the electrodes, and the contact resistance associated with the interface between electrodes and electrolyte layer. R1 corresponds to the first arc at the intermediate frequency and is designated as the grain boundary ionic conduction resistance.35 R2 represents the low-frequency arc and belongs to the charge transfer process.36 It can be seen that both R0 and R1 of the SOFC with YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, wt/wt) composite electrolyte are lower than those of the SOFC with YSZ electrolyte. The electrodes of the two fuel cells are the same, therefore it indicates that the YSZ–CeO2 composite possesses both lower bulk ionic conduction resistance and lower grain boundary ionic conduction resistance. Moreover, lower R2 of the YSZ–CeO2 based fuel cell indicates it also possesses lower charge transfer resistance. Based on the above discussions, the excellent ionic conductivity of the composite electrolyte leads to more tri-phase interfaces at the interface between electrodes and electrolyte layer, which is beneficial to improve the catalyst utilization, and thus decreases the charge transfer resistance. Moreover, it is well known that Ce4+ in CeO2 can be reduced to Ce3+ by H2 in the SOFC working environment, which can increase the electrolyte electronic conductivity.37 Therefore, the stability of the Ce element in the YSZ–CeO2 (70
30, wt/wt) composite electrolyte are lower than those of the SOFC with YSZ electrolyte. The electrodes of the two fuel cells are the same, therefore it indicates that the YSZ–CeO2 composite possesses both lower bulk ionic conduction resistance and lower grain boundary ionic conduction resistance. Moreover, lower R2 of the YSZ–CeO2 based fuel cell indicates it also possesses lower charge transfer resistance. Based on the above discussions, the excellent ionic conductivity of the composite electrolyte leads to more tri-phase interfaces at the interface between electrodes and electrolyte layer, which is beneficial to improve the catalyst utilization, and thus decreases the charge transfer resistance. Moreover, it is well known that Ce4+ in CeO2 can be reduced to Ce3+ by H2 in the SOFC working environment, which can increase the electrolyte electronic conductivity.37 Therefore, the stability of the Ce element in the YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, wt/wt) composite electrolyte was characterized by XPS (Fig. S1†). As shown in Fig. S1,† the ratio of Ce3+/Ce4+ in the original electrolyte is about 3
30, wt/wt) composite electrolyte was characterized by XPS (Fig. S1†). As shown in Fig. S1,† the ratio of Ce3+/Ce4+ in the original electrolyte is about 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, while it increased to about 4
7, while it increased to about 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 for the electrolyte which had experienced fuel cell test. Thus the electron conductivity of the H2 treated electrolyte was further investigated (Fig. S2†). The calculated results showed that the electron conductivity of the H2 treated electrolyte was too low, and thus the effect of electronic leakage of the electrolyte layer on the SOFC performance can be ignored.
6 for the electrolyte which had experienced fuel cell test. Thus the electron conductivity of the H2 treated electrolyte was further investigated (Fig. S2†). The calculated results showed that the electron conductivity of the H2 treated electrolyte was too low, and thus the effect of electronic leakage of the electrolyte layer on the SOFC performance can be ignored.
| T (450 °C) | R0 | Q1 | n1 | R1 | Q2 | n2 | R2 |
|---|---|---|---|---|---|---|---|
| YSZ–CeO2 | 0.1958 | 0.3733 | 0.5211 | 0.1044 | 2.4040 | 0.7347 | 0.1903 |
| YSZ | 0.6648 | 0.1283 | 0.4438 | 0.3193 | 0.9026 | 0.6764 | 0.6729 |
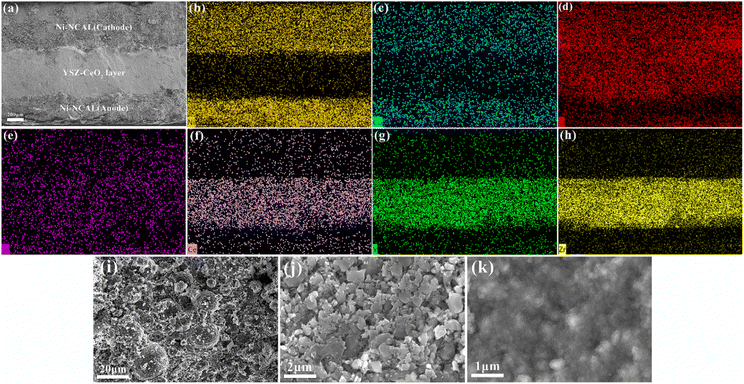
Fig. 3(a) gives the cross-sectional SEM image of the fuel cell using the YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, wt/wt) electrolyte after performance testing in H2/Air at 450 °C. It clearly shows a dense and uniform morphology without stratification, which indicates the well matching of thermal expansion coefficient between electrodes and electrolyte. The EDS mappings of cell obtained from cross-sectional SEM image are shown in Fig. 3(b)–(h). Ni, Co and O elements can be obviously detected on both electrode sides, which identified the composition of the electrode. Besides, due to the content of Al element in NCAL electrode is too small (1.3%), the it is hard to distinguish the distribution of Al in electrodes and electrolyte layer. The atomic mass of Li is too light to be detected by EDS detector. Elements such as Ce, Y, Zr and O can be observed in the electrolyte layer. Moreover, the uniform distribution of these elements in the electrolyte layer indicates that the two-phase materials are mixed evenly, which is conducive to the establishment of continuous and uniform ion conduction network. And a high-magnification image of the electrolyte layer in Fig. 3(k) shows that YSZ–CeO2 composite layer is dense and no through-holes, which is beneficial to prevent gas leakage. The detailed morphology of cathode and anode are showed in Fig. 3(i) and (j), respectively. The porous nature of the NCAL electrode makes it easier for air or H2 to diffuse through the electrodes. In addition, after performance testing the anode exhibited significantly different morphology compared with cathode. The NCAL on cathode side presented as micrometer sphere, whereas the NCAL material in anode side is small particles. It is due to that the NCAL in the anode are reduced by H2, and thus the sphere appearance changed.38
30, wt/wt) electrolyte after performance testing in H2/Air at 450 °C. It clearly shows a dense and uniform morphology without stratification, which indicates the well matching of thermal expansion coefficient between electrodes and electrolyte. The EDS mappings of cell obtained from cross-sectional SEM image are shown in Fig. 3(b)–(h). Ni, Co and O elements can be obviously detected on both electrode sides, which identified the composition of the electrode. Besides, due to the content of Al element in NCAL electrode is too small (1.3%), the it is hard to distinguish the distribution of Al in electrodes and electrolyte layer. The atomic mass of Li is too light to be detected by EDS detector. Elements such as Ce, Y, Zr and O can be observed in the electrolyte layer. Moreover, the uniform distribution of these elements in the electrolyte layer indicates that the two-phase materials are mixed evenly, which is conducive to the establishment of continuous and uniform ion conduction network. And a high-magnification image of the electrolyte layer in Fig. 3(k) shows that YSZ–CeO2 composite layer is dense and no through-holes, which is beneficial to prevent gas leakage. The detailed morphology of cathode and anode are showed in Fig. 3(i) and (j), respectively. The porous nature of the NCAL electrode makes it easier for air or H2 to diffuse through the electrodes. In addition, after performance testing the anode exhibited significantly different morphology compared with cathode. The NCAL on cathode side presented as micrometer sphere, whereas the NCAL material in anode side is small particles. It is due to that the NCAL in the anode are reduced by H2, and thus the sphere appearance changed.38
To investigate the detailed information of the YSZ–CeO2 composite morphology and phase distribution, the YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, wt/wt) composite was further observed by HR-TEM. Fig. 4(b)–(e) depict the associated TEM-EDS mapping images. The agglomeration of Ce element in the edge indicates the YSZ–CeO2 composite material is core–shell structure with YSZ as core and CeO2 as shell. As depicted in Fig. 4(f), the TEM image enlarged at low magnification shows that the particle size distribution of YSZ–CeO2 composite materials ranges from tens to hundreds of nanometers. The corresponding HR-TEM image is shown in Fig. 4(g). The lattice fringes at 0.296 nm and 0.270 nm correspond to the (1 1 1) plane of YSZ and the (2 0 0) plane of CeO2, respectively. The relative unordered elemental distribution in the interface between YSZ and CeO2 is beneficial to provide fast ion conduction path, and thus improve the ionic conductivity of the material.39,40 This also explains the reason for the lower ionic conduction resistance of the YSZ–CeO2 composite compared with YSZ electrolyte (Fig. 2(c)).
30, wt/wt) composite was further observed by HR-TEM. Fig. 4(b)–(e) depict the associated TEM-EDS mapping images. The agglomeration of Ce element in the edge indicates the YSZ–CeO2 composite material is core–shell structure with YSZ as core and CeO2 as shell. As depicted in Fig. 4(f), the TEM image enlarged at low magnification shows that the particle size distribution of YSZ–CeO2 composite materials ranges from tens to hundreds of nanometers. The corresponding HR-TEM image is shown in Fig. 4(g). The lattice fringes at 0.296 nm and 0.270 nm correspond to the (1 1 1) plane of YSZ and the (2 0 0) plane of CeO2, respectively. The relative unordered elemental distribution in the interface between YSZ and CeO2 is beneficial to provide fast ion conduction path, and thus improve the ionic conductivity of the material.39,40 This also explains the reason for the lower ionic conduction resistance of the YSZ–CeO2 composite compared with YSZ electrolyte (Fig. 2(c)).
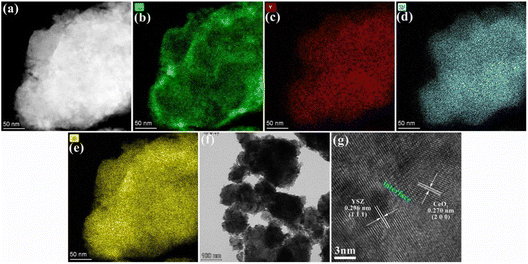 | ||
| Fig. 4 (a) TEM and corresponding EDS images of (b) Ce, (c) Y, (d) Zr and (e) O elements, (f and g) HR-TEM images of the tested YSZ–CeO2 electrolyte. | ||
The conductivities of the original YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, wt/wt) electrolyte were further characterized in different environments. The charge carriers are different in different environments. Electrons are the main charge carriers when the SOFC device is in N2 environment; O2− and electrons are the main charge carriers when the SOFC device is in air environment; H+ and electrons are the main charge carriers when the SOFC device is in H2 environment. The proton conductivities obtained in hydrogen atmosphere as a function of temperature were expressed in Fig. 5(a). The activation energy of proton motion of the original YSZ–CeO2 electrolyte is found to be 0.47 eV at 400–500 °C according to the Arrhenius curve, which is much lower than those of reported for YSZ (1.16 eV at 300 °C–600 °C).41–43 The activation energy is the average difference between the energies of activated molecules and ordinary molecules. The much lower activation energy indicating that within this temperature range, the energy needed for proton transport from its normal state to an active state is relatively low, thus proton transport is easier for the YSZ–CeO2 electrolyte than YSZ. Fig. 5(b) gives the Arrhenius curve of the tested YSZ–CeO2 electrolyte obtained in air atmosphere, it can be inferred that the activation energy of the composite material under air atmosphere is 0.69 eV, which is higher than that under H2 atmosphere. Moreover, it can be seen that the conductivity of electrolyte in H2 is much higher than in air. Namely the conductivity of the YSZ–CeO2 electrolyte membrane was mainly attributed by proton conduction, which facilitates the outstanding low temperature performance due to the easier transportation of proton than O2−.44,45 As shown in Fig. 5(c), the electronic conductivity obtained in N2 environment is relatively low in the 400–500 °C temperature range, which is favorable for cell performance.
30, wt/wt) electrolyte were further characterized in different environments. The charge carriers are different in different environments. Electrons are the main charge carriers when the SOFC device is in N2 environment; O2− and electrons are the main charge carriers when the SOFC device is in air environment; H+ and electrons are the main charge carriers when the SOFC device is in H2 environment. The proton conductivities obtained in hydrogen atmosphere as a function of temperature were expressed in Fig. 5(a). The activation energy of proton motion of the original YSZ–CeO2 electrolyte is found to be 0.47 eV at 400–500 °C according to the Arrhenius curve, which is much lower than those of reported for YSZ (1.16 eV at 300 °C–600 °C).41–43 The activation energy is the average difference between the energies of activated molecules and ordinary molecules. The much lower activation energy indicating that within this temperature range, the energy needed for proton transport from its normal state to an active state is relatively low, thus proton transport is easier for the YSZ–CeO2 electrolyte than YSZ. Fig. 5(b) gives the Arrhenius curve of the tested YSZ–CeO2 electrolyte obtained in air atmosphere, it can be inferred that the activation energy of the composite material under air atmosphere is 0.69 eV, which is higher than that under H2 atmosphere. Moreover, it can be seen that the conductivity of electrolyte in H2 is much higher than in air. Namely the conductivity of the YSZ–CeO2 electrolyte membrane was mainly attributed by proton conduction, which facilitates the outstanding low temperature performance due to the easier transportation of proton than O2−.44,45 As shown in Fig. 5(c), the electronic conductivity obtained in N2 environment is relatively low in the 400–500 °C temperature range, which is favorable for cell performance.
 | ||
| Fig. 5 Arrhenius curves of the original YSZ–CeO2 electrolyte obtained in different atmospheres: (a) H2, (b) air and (c) N2. | ||
XPS characterizations were used to analyze the chemical state of oxygen element in YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, wt/wt) electrolyte before and after performance test. The spectra are decomposed into several peaks that are assigned to different symmetric signals.46 Lattice oxygen (OL) in YSZ or CeO2 is belong to the peak centered at about 529 eV, while the higher binding energy peak at about 531 eV is attributed to the oxygen atoms in the deficiency regions (OV).47 As can be seen in Fig. 6, after the performance test of fuel cells, the area ratio of OV increases from 26.35% to 56.17%, thus it can be inferred that more oxygen vacancies were generated in situ in the electrolyte during fuel cell operation. This may be due to the reduction of Ce4+ to Ce3+ during the fuel cell operation. The increase of oxygen vacancy content is beneficial to increase the ionic conductivity of the fuel cell under low temperature, which is also one reason for the excellent low temperature performance of the SOFC.
30, wt/wt) electrolyte before and after performance test. The spectra are decomposed into several peaks that are assigned to different symmetric signals.46 Lattice oxygen (OL) in YSZ or CeO2 is belong to the peak centered at about 529 eV, while the higher binding energy peak at about 531 eV is attributed to the oxygen atoms in the deficiency regions (OV).47 As can be seen in Fig. 6, after the performance test of fuel cells, the area ratio of OV increases from 26.35% to 56.17%, thus it can be inferred that more oxygen vacancies were generated in situ in the electrolyte during fuel cell operation. This may be due to the reduction of Ce4+ to Ce3+ during the fuel cell operation. The increase of oxygen vacancy content is beneficial to increase the ionic conductivity of the fuel cell under low temperature, which is also one reason for the excellent low temperature performance of the SOFC.
4 Conclusions
The output power of the SOFC with YSZ–CeO2 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, wt/wt) composite electrolyte at 450 °C can reach 680 mW cm−2. Evenly, the Pmax can maintain at 200 mW cm−2 as the operational temperature reduced to 390 °C. The excellent performance indicates that the YSZ–CeO2 composite electrolyte can still work normally at around 400 °C. The enhanced ionic conductivity of the YSZ–CeO2 composite electrolyte compared with YSZ electrolyte provides the basis for higher cell performance. Moreover, the activation energy of proton movement in YSZ–CeO2 composite electrolyte is lower than that of oxygen ion movement, indicating that the composite electrolyte is a kind of proton conduction material, which is more suited to working under low temperature. The hetero-interface formed in the composite helped to improve ionic conductivity. These study results demonstrate the high potential of the YSZ–CeO2 composite electrolyte in low-temperature SOFCs.
30, wt/wt) composite electrolyte at 450 °C can reach 680 mW cm−2. Evenly, the Pmax can maintain at 200 mW cm−2 as the operational temperature reduced to 390 °C. The excellent performance indicates that the YSZ–CeO2 composite electrolyte can still work normally at around 400 °C. The enhanced ionic conductivity of the YSZ–CeO2 composite electrolyte compared with YSZ electrolyte provides the basis for higher cell performance. Moreover, the activation energy of proton movement in YSZ–CeO2 composite electrolyte is lower than that of oxygen ion movement, indicating that the composite electrolyte is a kind of proton conduction material, which is more suited to working under low temperature. The hetero-interface formed in the composite helped to improve ionic conductivity. These study results demonstrate the high potential of the YSZ–CeO2 composite electrolyte in low-temperature SOFCs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51872080), Wuhan Science and Technology Bureau (No. 2020010601012293).Notes and references
- B. Zhu, P. D. Lund, R. Raza, Y. Ma, L. Fan, M. Afzal, J. Patakangas, Y. He, Y. Zhao, W. Tan, Q.-A. Huang, J. Zhang and H. Wang, Adv. Energy Mater., 2015, 5, 1401895 CrossRef.
- C. Xia, Z. Qiao, C. Feng, J. S. Kim, B. Wang and B. Zhu, Materials, 2017, 11, 40 CrossRef.
- C. Xia, Z. Qiao, L. Shen, X. Liu, Y. Cai, Y. Xu, J. Qiao and H. Wang, Int. J. Hydrogen Energy, 2018, 43, 12825–12834 CrossRef CAS.
- A. Gilane, S. Fop, F. Sher, R. I. Smith and A. C. McLaughlin, J. Mater. Chem. A, 2020, 8, 16506–16514 RSC.
- C. Xia, Y. Mi, B. Wang, B. Lin, G. Chen and B. Zhu, Nat. Commun., 2019, 10, 1707 CrossRef PubMed.
- X. Liu, W. Dong, Y. Tong, L. Wei, M. Yuan, X. Wang, B. Wang and B. Zhu, Electrochim. Acta, 2019, 295, 325–332 CrossRef CAS.
- Z. Gao, L. V. Mogni, E. C. Miller, J. G. Railsback and S. A. Barnett, Energy Environ. Sci., 2016, 9, 1602–1644 RSC.
- A. Rafique, R. Raza, N. Akram, M. Kaleem Ullah, A. Ali, M. Irshad, K. Siraj, M. A. Khan, B. Zhu and R. Dawson, RSC Adv., 2015, 5, 86322–86329 RSC.
- B. Yadian, R. Chen, H. Liu, H. Sun, Q. Liu, C. L. Gan, Z. Kun, C. Zhao, B. Zhu and Y. Huang, Nano Res., 2015, 8, 1857–1864 CrossRef CAS.
- W. Lee, S.-Y. Chen, Y.-S. Chen, C.-L. Dong, H.-J. Lin, C.-T. Chen and A. Gloter, J. Phys. Chem. C, 2014, 118, 26359–26367 CrossRef CAS.
- Y. Cai, C. Xia, B. Wang, W. Zhang, Y. Wang and B. Zhu, ACS Sustain. Chem. Eng., 2017, 5, 10387–10395 CrossRef CAS.
- S. Fop, K. S. McCombie, E. J. Wildman, J. M. S. Skakle, J. T. S. Irvine, P. A. Connor, C. Savaniu, C. Ritter and A. C. McLaughlin, Nat. Mater., 2020, 19, 752–757 CrossRef CAS PubMed.
- W. Dong, Y. Tong, B. Zhu, H. Xiao, L. Wei, C. Huang, B. Wang, X. Wang, J.-S. Kim and H. Wang, J. Mater. Chem. A, 2019, 7, 16728–16734 RSC.
- Z. Qiao, C. Xia, Y. Cai, M. Afzal, H. Wang, J. Qiao and B. Zhu, J. Power Sources, 2018, 392, 33–40 CrossRef CAS.
- B. Wang, Y. Wang, L. Fan, Y. Cai, C. Xia, Y. Liu, R. Raza, P. A. van Aken, H. Wang and B. zhu, J. Mater. Chem. A, 2016, 4, 15426–15436 RSC.
- Y. Lin, R. Ran and Z. Shao, Int. J. Hydrogen Energy, 2010, 35, 8281–8288 CrossRef CAS.
- A. D. Liyanage, S. D. Perera, K. Tan, Y. Chabal and K. J. Balkus, ACS Catal., 2014, 4, 577–584 CrossRef CAS.
- H. You, C. Zhao, B. Qu, G. Guan and A. Abudula, J. Alloys Compd., 2016, 669, 46–54 CrossRef CAS.
- T. Hong, K. S. Brinkman and C. Xia, ChemElectroChem, 2016, 3, 805–813 CrossRef CAS.
- S. Sengodan, R. Lan, J. Humphreys, D. Du, W. Xu, H. Wang and S. Tao, Renewable Sustainable Energy Rev., 2018, 82, 761–780 CrossRef CAS.
- H. Li, A. Petz, H. Yan, J. C. Nie and S. Kunsági-Máté, J. Phys. Chem. C, 2011, 115, 1480–1483 CrossRef CAS.
- B. Xu, Q. Zhang, S. Yuan, S. Liu, M. Zhang and T. Ohno, Catal. Today, 2017, 281, 135–143 CrossRef CAS.
- Y. Xing, Y. Wu, L. Li, Q. Shi, J. Shi, S. Yun, M. Akbar, B. Wang, J.-S. Kim and B. Zhu, ACS Energy Lett., 2019, 4, 2601–2607 CrossRef CAS.
- B. Wang, B. Zhu, S. Yun, W. Zhang, C. Xia, M. Afzal, Y. Cai, Y. Liu, Y. Wang and H. Wang, NPG Asia Mater., 2019, 11, 15 CrossRef.
- M. Valášková, J. Kupková, G. S. Martynková, J. Seidlerová, V. Tomášek, M. Ritz, K. Kočí, V. Klemm and D. Rafaja, Appl. Clay Sci., 2018, 151, 164–174 CrossRef.
- F. Meng, L. Wang and J. Cui, J. Alloys Compd., 2013, 556, 102–108 CrossRef CAS.
- Z. Wu, M. Li, J. Howe, H. M. Meyer 3rd and S. H. Overbury, Langmuir, 2010, 26, 16595–16606 CrossRef CAS PubMed.
- F. Liang, Y. Yu, W. Zhou, X. Xu and Z. Zhu, J. Mater. Chem. A, 2015, 3, 634–640 RSC.
- T. Li, M. Zhang, Y. Yuan, S. Muhammad, H. Nishijima and W. Pan, Solid State Ionics, 2018, 326, 11–17 CrossRef CAS.
- F. He, S. Liu, T. Wu, M. Yang, W. Li, G. Yang, F. Zhu, H. Zhang, K. Pei, Y. Chen, W. Zhou and Z. Shao, Adv. Funct. Mater., 2022, 32, 2206756 CrossRef CAS.
- M. Liang, Y. Song, D. Liu, L. Xu, M. Xu, G. Yang, W. Wang, W. Zhou, R. Ran and Z. Shao, Appl. Catal., B, 2022, 318, 121868 CrossRef CAS.
- C. Duan, J. Tong, M. Shang, S. Nikodemski, M. Sanders, S. Ricote, A. Almansoori and R. O’Hayre, Science, 2015, 349, 1321–1326 CrossRef CAS PubMed.
- Z. He, J. Nie, K. Liu, K. Sivajee Ganesh, M. Akbar, C. Xia, X. Wang, W. Dong, J. Huang and B. Wang, Int. J. Hydrogen Energy, 2021, 46, 9799–9808 CrossRef CAS.
- L. Fan, H. Zhang, M. Chen, C. Wang, H. Wang, M. Singh and B. Zhu, Int. J. Hydrogen Energy, 2013, 38, 11398–11405 CrossRef CAS.
- H. Hu, Q. Lin, Z. Zhu, X. Liu, M. Afzal, Y. He and B. Zhu, J. Power Sources, 2015, 275, 476–482 CrossRef CAS.
- H. Hu, Q. Lin, A. Muhammad and B. Zhu, J. Power Sources, 2015, 286, 388–393 CrossRef CAS.
- P. Vinchhi, M. Khandla, K. Chaudhary and R. Pati, Inorg. Chem. Commun., 2023, 152, 110724 CrossRef CAS.
- Y. Meng, W. Zhang, Z. He, C. Liu, J. Gao, M. Akbar, R. Guo, S. Zhou, Y. Ji, X. Wang and Y. Yang, Int. J. Hydrogen Energy, 2021, 46, 9874–9881 CrossRef CAS.
- A. Gayen, K. R. Priolkar, P. R. Sarode, V. Jayaram and S. Emura, Chem. Mater., 2004, 16, 2317–2328 CrossRef CAS.
- S. S. Liu, S. Toh, T. Daio, M. Koyama and S. Matsumura, ECS Trans., 2013, 57, 1401–1405 CrossRef.
- O. O. Agbede, K. Hellgardt and G. H. Kelsall, Mater. Today Chem., 2020, 16, 100252 CrossRef CAS.
- O. Kwon and G. Choi, Solid State Ionics, 2006, 177, 3057–3062 CrossRef CAS.
- C.-C. T. Yang, W.-C. J. Wei and A. Roosen, Mater. Chem. Phys., 2003, 81, 134–142 CrossRef CAS.
- T. Shimonosono, Y. Hirata, Y. Ehira, S. Sameshima, T. Horita and H. Yokokawa, Solid State Ionics, 2004, 174, 27–33 CrossRef CAS.
- S. J. Litzelman and H. L. Tuller, Solid State Ionics, 2009, 180, 1190–1197 CrossRef CAS.
- R. C. Rabelo-Neto, H. B. E. Sales, C. V. M. Inocêncio, E. Varga, A. Oszko, A. Erdohelyi, F. B. Noronha and L. V. Mattos, Appl. Catal., B, 2018, 221, 349–361 CrossRef CAS.
- E. Paparazzo, J. Phys.: Condens.Matter, 2018, 30, 343003 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01507f |
| This journal is © The Royal Society of Chemistry 2023 |