Open Access Article
Open Access ArticleDevelopment of validated methods for the simultaneous quantification of Finasteride and Tadalafil in newly launched FDA-approved therapeutic combination: greenness assessment using AGP, analytical eco-scale, and GAPI tools†
Ahmed K. Kammoun a,
Maan T. Khayata,
Ahmad J. Almalkia and
Rasha M. Youssef
a,
Maan T. Khayata,
Ahmad J. Almalkia and
Rasha M. Youssef *b
*b
aDepartment of Pharmaceutical Chemistry, Faculty of Pharmacy, King Abdulaziz University, P.O. Box 80260, Jeddah 21589, Saudi Arabia
bDepartment of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Alexandria University, El, -Messalah, Alexandria 21521, Egypt. E-mail: rasha.youssef@alexu.edu.eg; Fax: +20 3 4873273; Tel: +20 3 4871317
First published on 17th April 2023
Abstract
The primary objectives of green chemistry are the reduction of generation and use of hazardous substances. In healthcare, the most active areas of research in green chemistry are medication manufacturing and analysis. Analysts take serious steps for converting traditional analytical methods to eco-friendly ones that minimize the negative effects of solvents and chemicals on the environment and improve the healthcare. In the proposed work, two analytical methods are presented for the quantification of Finasteride (FIN) and Tadalafil (TAD) simultaneously in newly launched FDA-approved dosage form without prior separation. The first method is derivative spectrophotometry, which is based on measuring the amplitudes of first derivative spectrophotometric peaks of FIN and TAD in ethanolic solution at 221 nm and 293 nm, respectively. On the other hand, measuring the peak-to-peak amplitudes of second derivative spectrum of TAD solution at 291–299 nm was also performed. Regression equations show good linearity for FIN and TAD in the ranges of 10–60 μg mL−1 and 5–50 μg mL−1, respectively. The second method is the RP-HPLC method, where the chromatographic separation was achieved using the XBridgeTM C18 (150 × 4.6 mm, 5 μm) column. The eluent was the mixture of acetonitrile:phosphate buffer with triethylamine, 1% (v/v) adjusted to pH = 7 in the ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (by volume). The flow rate was 1.0 mL min−1 with DAD-detection at 225 nm. This analytical procedure was linear over the ranges of 10–60 μg mL−1 and 2.5–40 μg mL−1 for FIN and TAD, respectively. The presented methods were validated (regarding ICH guidelines) and statistically compared by applying the t-test and F-test with the reported method. The greenness appraisal was performed using three different tools. The proposed validated methods were found to be green, sensitive, selective, and can be successfully used for quality control test.
50 (by volume). The flow rate was 1.0 mL min−1 with DAD-detection at 225 nm. This analytical procedure was linear over the ranges of 10–60 μg mL−1 and 2.5–40 μg mL−1 for FIN and TAD, respectively. The presented methods were validated (regarding ICH guidelines) and statistically compared by applying the t-test and F-test with the reported method. The greenness appraisal was performed using three different tools. The proposed validated methods were found to be green, sensitive, selective, and can be successfully used for quality control test.
1. Introduction
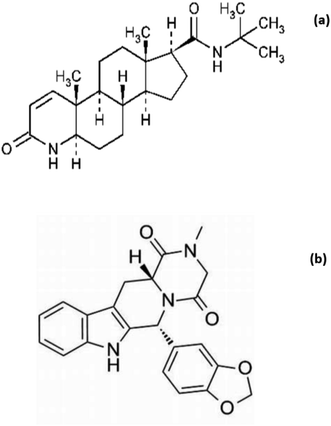
The most common diseases among males around the world are benign prostatic hyperplasia (BPH) and its associated lower urinary tract symptoms.1,2 Finasteride (FIN) (Fig. 1) is one of the 5α-reductase inhibitors available for the treatment of BPH. FIN inhibits the type II isoenzyme, which has prostate higher activity level than the type I isoform.3,4 In October 2011, one of the most famous phosphodiesterase-5 inhibitors drugs, Tadalafil (TAD) (Fig. 1) was used daily for treating BPH. This drug represented a new action for the treatment of BPH symptoms.The treatment of men who were at risk of BPH progression by FIN combination therapy represented an innovation in therapeutics. In December 2021, a combination of FIN and TAD was approved by FDA for the treatment of urinary tract symptoms caused by BPH in men after oral administration under the trade name Entadfi™.
Many analytical methods were reported for the analysis of FIN and TAD in their pure forms, dosage forms, and in different biological fluid including spectroscopic techniques and different chromatographic techniques.5–15
As a latterly approved combination, there is an urgent need for the development of selective analytical methods for their quantitative determination in bulk or dosage forms. In the literature, two analytical techniques were developed for the simultaneous determination of FIN and TAD, one of these methods is the chromatographic method using the LC-MS/MS technique for the determination of the studied drugs in human plasma.16 Although LC-MS/MS is a specific and highly sensitive technique, it is considered a sophisticated technique and affects the speed and cost of the analysis of the compounds of interest. In July 2022, a spectroscopic technique was developed for the determination of FIN and TAD in a capsule.17 Literature data lack any chromatographic method for the assaying of the proposed combination in its dosage form.
Since 1999, the term “green analytical chemistry” was suggested for consideration. Most actions in making chemical procedures greener emphasize the need for using more benign solvents, reagents reduction, and lowering the energy consumption. Anastas, the father of green chemistry, shed light on the need to develop green analytical methods. Since that time, the concern in applying the principles of green analytical chemistry has grown.18–22
One of the challenges in green analytical chemistry is the assessment of the greenness of analytical processes. It is renowned that the process that cannot be measured cannot be managed. Thus, the goal of the proposed work is to design, develop, and optimize green analytical methods for the simultaneous quantitation of FIN and TAD in their recent FDA-approved capsules. In addition, the greenness evaluation of the proposed techniques was performed by applying analytical greenness profile (AGP), eco-scale penalty points approach, and green analytical procedure index (GAPI). All developed methods were fully validated.
2. Experimental
2.1. Materials and reagents
Pharmaceutical grade of Finasteride and Tadalafil were supplied from Sigma-Aldrich (GmbH, Steinheim, Germany). Ethanol, TEA, and buffer components are from Sigma-Aldrich®, St. Louis, MO, United States. HPLC grade acetonitrile is from Tedia, Ohio, USA.2.2. Pharmaceutical formulations
A commercial product (Entadfi™ labeled to contain 5 mg FIN and 5 mg TAD per capsule) was studied.2.3. Instrumentation
The eluent consisted of acetonitrile and phosphate buffer adjusted to pH 7 with 0.1% TEA (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). Then, it was degassed and filtered using a 0.45 μm pore size membrane filter. The samples were filtered using 0.45 μm disposable filters. The flow rate was adjusted to be 1 mL min−1.
50). Then, it was degassed and filtered using a 0.45 μm pore size membrane filter. The samples were filtered using 0.45 μm disposable filters. The flow rate was adjusted to be 1 mL min−1.
2.4. Standard solutions
1 mg mL−1 stock standard solutions of FIN and TAD were prepared separately in ethanol. Then, dilution was performed by ethanol to 100 μg mL−1 (working standard solutions).2.5. Construction of calibration graphs
| Parameters | FIN | TAD | |||
|---|---|---|---|---|---|
| Derivative spectrophotometric method | HPLC method | Derivative spectrophotometric method | HPLC method | ||
| 1D at 221 nm | 1D at 293 nm | 2D from peak to peak (291–299 nm) | |||
| a *Calculated as mean% recovery from the analysis of synthetic mixtures of different ratios (FIN/TAD: 30/10, 10/10, 10/20 μg mL−1); n = 9. ** Calculated as mean RSD% from the analysis of the synthetic mixtures; n = 9. *** in μg mL−1 r is correlation coefficient, Sa is standard deviation of intercept, Sb is standard deviation of slope, and Sy/x is standard deviation of residuals. | |||||
| Accuracy* (mean ± RSD%) | 101.87 ± 1.5 | 98.9 ± 1.1 | 100.31 ± 1.5 | 100.14 ± 0.8 | 98.2 ± 0.7 |
| Precision** (RSD%) | |||||
| Intraday | 1.80 | 0.99 | 0.50 | 0.91 | 0.55 |
| Interday | 1.14 | 1.25 | 1.89 | 1.54 | 0.89 |
| LOD*** | 2.03 | 2.00 | 1.32 | 1.29 | 0.88 |
| LOQ*** | 6.16 | 6.5 | 4.01 | 3.91 | 2.9 |
| Linearity range*** | 10–60 | 10–60 | 5–60 | 5–60 | 2.5–40 |
| Intercept (a) | 1.788 × 10−3 | −7.81 | 4.45 × 10−3 | 1.65 × 10−2 | 7.58 |
| Slope (b) | 3.48 × 10−3 | 8.31 | 1.14 × 10−2 | 2.61 × 10−2 | 44.95 |
| r | 0.9995 | 0.9995 | 0.9998 | 0.9998 | 0.9998 |
| Sa | 1.71 × 10−3 | 5.01 | 2.99 × 10−3 | 6.66 × 10−3 | 8.85 |
| Sb | 4.69 × 10−5 | 0.13 | 8.76 × 10−5 | 1.95 × 10−4 | 0.39 |
| Sy/x | 2.15 × 10−3 | 5.38 | 4.58 × 10−3 | 1.02 × 10−2 | 13.10 |
| Sb% | 1.35 | 1.56 | 0.77 | 0.75 | 0.87 |
| F | 5514.54 | 4168.66 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040.37 040.37 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898.21 898.21 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021.60 021.60 |
| Significance F | 8.39 × 10−9 | 3.40 × 10−7 | 1.36 × 10−11 | 1.18 × 10−11 | 3.54 × 10−8 |
2.6. Analysis of synthetic mixture
Adjusted volumes of each of FIN and TAD working stock solutions were transferred into a set of 10 mL volumetric flasks. The content of each flask was diluted to volume with ethanol (for derivative spectrophotometric method) and mobile phase (for HPLC method) such that the drugs' concentrations were within the ranges mentioned in Table 1.2.7. Procedure for capsule analysis
The developed methods were applied for the quantification of FIN and TAD in capsules. Ten capsules were emptied and their content weighed. A measured quantity, corresponding to 25 mg FIN and 25 mg TAD, was transferred to a 25 mL volumetric flask. 15 mL ethanol was added. Sonication was done for 20 min. After completing the volume with ethanol, the solution was filtered. 1 mL filtrate was diluted to 10 mL with ethanol to obtain the final capsules extract containing FIN/TAD of 100/100 μg mL−1 for FIN and TAD analysis. Steps were followed as under Section 2.5.3. Results and discussion
3.1. The derivative spectrophotometric method
Spectrophotometry is a recognized technique for routine drug testing in quality control laboratories around the world. In addition, spectrophotometry has also gained an essential role toward green analytical chemistry. FIN and TAD are combined in capsule formulation in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
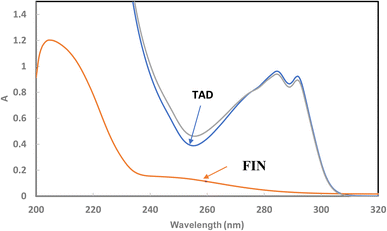
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The UV absorption spectra for FIN and TAD solutions in ethanol were scanned (Fig. 2). The spectra showed complete overlap between the investigated drugs in the region of 200–300 nm. It appeared from Fig. 2 that FIN is a weakly absorbing compound in comparison with TAD, which shows high UV absorbance. The challenge is to simultaneously determine the two drugs that are combined in a 1
1. The UV absorption spectra for FIN and TAD solutions in ethanol were scanned (Fig. 2). The spectra showed complete overlap between the investigated drugs in the region of 200–300 nm. It appeared from Fig. 2 that FIN is a weakly absorbing compound in comparison with TAD, which shows high UV absorbance. The challenge is to simultaneously determine the two drugs that are combined in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in the dosage form. Thus, it subsequently necessitates an effective mathematical separation procedure. The first derivative of the absorption spectra (1D) was applied successfully for the determination of both FIN and TAD while the second derivative of absorption spectra (2D) was applied only for the quantification of TAD.
1 ratio in the dosage form. Thus, it subsequently necessitates an effective mathematical separation procedure. The first derivative of the absorption spectra (1D) was applied successfully for the determination of both FIN and TAD while the second derivative of absorption spectra (2D) was applied only for the quantification of TAD.
 | ||
| Fig. 2 Absorption spectra of 30 μg mL−1 FIN, 30 μg mL−1 TAD and their synthetic mixture (30/30 μg mL−1). | ||
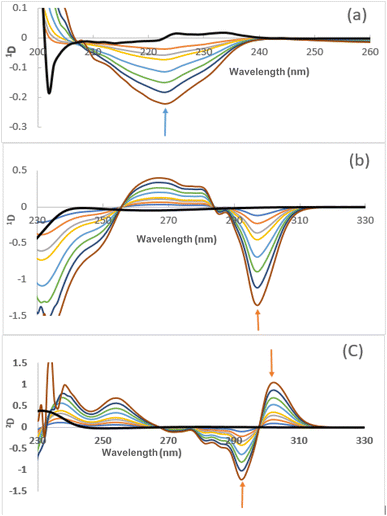
Fig. 3 shows the 1D and 2D spectra of both drugs. FIN can be successfully estimated by measuring its 1D value at 221 nm, which corresponds to a zero-crossing for TAD, whereas TAD can be determined by measuring its 1D value at 293 nm and its 2D values from peak-to-peak at 291–199 nm (corresponding to no contribution from FIN). The influence of several values of Δλ on the 1D and 2D spectra was tested. Δλ = 3 nm was selected for the 1D determination of FIN, and Δλ = 9 nm was chosen for the 1D and 2D determination of TAD as the optimal condition to obtain a good signal-to-noise ratio.
Generally, the characteristics of the derivative spectra can constitute a specialized fingerprint for drug identification as the amplitude ratios at the chosen wavelength can be considered to be suitable parameters for the confirmation of drug purity.24 The ratio of absolute 2D values in the range of 291–299 nm for TAD standard solutions and capsules was calculated to detect the presence of any interferences. The obtained results show that the ratio was 2.085 with RSD% of 0.34 (n = 8). These RSD% value are less than 2%. Therefore, the 2D method is specific for TAD and can be used to examine its identity and purity.
3.2. The HPLC method
The proposed HPLC method aimed to develop a chromatographic system capable of eluting and separating FIN and TAD from one another. In addition, its target is to fulfill the system's general requirements.3.2.1.1. Stationary phase. Different stationary phases were tried. Using both C8 and C18 (250 × 4.6 mm) columns, the two peaks of FIN and TAD were eluted at very high retention times. The C18 (150 × 4.6 mm) column succeeded efficiently in separating the two drugs within acceptable retention times.
3.2.1.2. Organic modifier. The type of organic modifier greatly affects the peak shape of both investigated drugs. Different organic modifiers have been tried including ethanol, methanol, and acetonitrile. Using ethanol and methanol in mobile phase led to peak broadening, decreased efficiency, and caused distortion of the shape of FIN and TAD peaks. On the other hand, the use of acetonitrile allowed the elution of FIN and TAD with acceptable peak shape.
Binary mixtures of drugs were injected with the mobile phase containing different percentages of acetonitrile. Fig. S1† shows the retention times obtained for the two compounds as a function of acetonitrile percentage in the eluent. 50% acetonitrile was selected to give optimum separation. At lower acetonitrile proportions, separation occurred but with the excess tailing of the TAD peak and increasing retention time for the FIN peak. Using a higher ratio of acetonitrile succeeded in separating the two drugs with reasonable retention times but with decreasing greenness of the method.
3.2.1.3. pH of the aqueous part of the mobile phase. The effect of pH of the aqueous component of the eluent was studied. Various pH values (from 2.0 to 7.0) using phosphate buffer (adjusted by orthophosphoric acid or sodium hydroxide) together with acetonitrile in a ratio of 50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v were tried. The best pH value for the optimum resolution of the components under analysis was 7. Below this value, the asymmetry of the FIN peak appeared and the forked tailed TAD peak was obtained. Triethylamine (TEA) was also added to the aqueous part not only to provide the required pH but also to prevent peak tailing of FIN and TAD.
50 v/v were tried. The best pH value for the optimum resolution of the components under analysis was 7. Below this value, the asymmetry of the FIN peak appeared and the forked tailed TAD peak was obtained. Triethylamine (TEA) was also added to the aqueous part not only to provide the required pH but also to prevent peak tailing of FIN and TAD.
3.2.1.4. Detection wavelength. To increase the selectivity and sensitivity of the method, different wavelengths were tried for the quantification of proposed drugs. It was found that the detection at 225 nm for both FIN and TAD gave the best selectivity in addition to sensitivity.
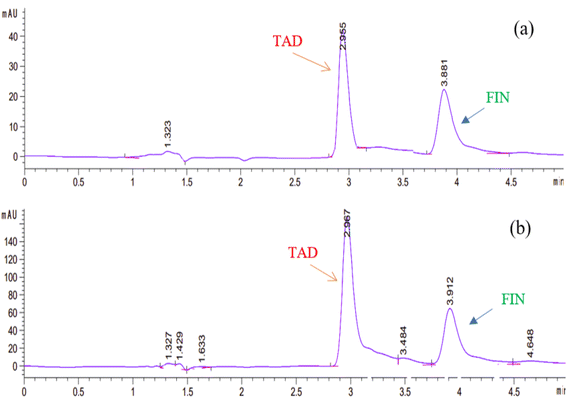
3.2.1.5. Flow rate. The impact of flow rate was tested in the range of 0.5–1.5 mL min−1. A flow rate of 1 mL min−1 was selected as it was associated with reasonable retention times and the highest theoretical plates. A flow rate of more than 1 mL min−1 resulted in low resolution between FIN and TAD, whilst a flow rate less than 1 mL min−1 resulted in high retention times of both the drugs. Thus, the optimum chromatographic conditions stated previously were adopted for all measurements. Fig. 4 shows the significant separation of FIN and TAD with optimum run time, peak sharpness, and resolution.
3.3. Validation of the proposed methods
To guarantee the analytical validity of the proposed methods, different parameters of ICH guidelines were evaluated.| Parameters | FIN* | TAD* | ||||
|---|---|---|---|---|---|---|
| Derivative spectrophotometric method | SD of 1D at 221 nm | RSD% | SD of 1D at 293 nm | RSD% | SD of 2D from peak to peak (291–299 nm) | RSD% |
| a * Average of three concentrations 10, 30, 50 μg mL−1 and 5, 30, 60.0 μg mL−1 for FIN and TAD, respectively. ** PerkinElmer Lambda EZ201 UV-visible spectrophotometer and Thermo-Spectronic UV-vis spectrophotometer connected to a Harvest computer system. | ||||||
| (1) Spectrophotometer of different models** | 0.006 | 1.14 | 0.019 | 1.57 | 0.012 | 1.67 |
| (2) Ethanol of different lots | 0.021 | 1.73 | 0.014 | 1.95 | 0.023 | 1.82 |
| Parameters | FIN* | TAD* | ||
|---|---|---|---|---|
| HPLC method | RSD% of peak areas | k/ ± SD | RSD% of peak areas | k/ ± SD |
| (1) Acetonitrile percentage in mobile phase (45, 50, and 55%) | 1.80 | 1.21 ± 0.015 | 0.98 | 1.93 ± 0.009 |
| (2) pH of the aqueous phase (6.2, 6.6, 7, 7.2, and 7.5) | 1.56 | 1.20 ± 0.010 | 1.55 | 1.93 ± 0.008 |
| (3) Flow rate of the mobile phase (0.8, 1, and 1.2 mL min−1) | 1.10 | 1.19 ± 0.006 | 1.64 | 1.90 ± 0.006 |
| (4) Wavelength of detection (220, 222, 225, 227, and 230) | 1.95 | 1.21 ± 0.005 | 1.75 | 1.93 ± 0.003 |
It was found that slight intended changes in the parameters had no significant influence on the determination of FIN and TAD using the proposed methods. Acceptable and satisfied robustness was indicated by the low RSD% of 1D and 2D in the derivative spectrophotometric method and the nearly unchanged capacity factor (k/) values of both drugs in the HPLC method (Table 2).
| Entafi® capsulesa | FIN | TAD | |||||
|---|---|---|---|---|---|---|---|
| Derivative spectrophotometric method | HPLC method | Reported method17 | Derivative spectrophotometric method | HPLC method | Reported method17 | ||
| 1D at 221 nm | 1D at 293 nm | 2D from peak to peak (291–299 nm) | |||||
| a Labeled to contain 5 mg FIN and 5 mg TAD/capsule.b Mean ± standard deviation of five determinations.c Theoretical values of t and F are 2.31 and 6.39, respectively, at 95% confidence limit. | |||||||
| Mean% recovery ± SDb | 98.80 ± 0.751 | 100.36 ± 1.181 | 99.90 ± 1.560 | 99.62 ± 0.804 | 99.91 ± 0.515 | 100.05 ± 0.915 | 99.32 ± 0.484 |
| Er (%) | −1.20 | 0.36 | −0.10 | −0.38 | −0.09 | 0.15 | −0.68 |
| tc | 0.67 | 0.53 | — | 0.71 | 1.87 | 1.68 | — |
| Fc | 4.31 | 1.71 | — | 2.76 | 1.13 | 3.57 | — |
Moreover, the derivative spectrophotometric method, the ratios of 2D values between (292–299 nm), as shown in Fig. 3, were measured to test the presence of any interferences. The results for several concentrations of TAD standard solutions and capsules are indicated in Table S2.† The obtained results show RSD% values less than 2%, indicating that the derivative spectrophotometric method is specific for TAD and can be used to test its purity.
The peak purity of FIN and TAD was tested using a G1315D PDA detector for the HPLC method. The purity angle was within the purity threshold limit for both the drugs in the capsule sample solution, indicating the ability of the HPLC method to determine FIN and TAD without interferences from co-formulation adjuvants.
3.4. Application on dosage form (Entafi® capsules)
The proposed methods were performed to assay FIN and TAD in Entafi® capsules. Five determinations of capsule sample solutions were measured. The results in Table 3 provided reasonable RSD% and recovery% values for both the investigated drugs. Thus, FIN and TAD were found to match the label claims. As shown in Table 3, the results of the proposed methods were statistically compared with that of the reported one.17 The values of t and F tests do not surpass the theoretical values, thus advocating that there is no significant variation between the developed methods in the proposed study and the reported one.3.5. Greenness appraisal
The second assessment tool is the eco-scale penalty points.18,26 The eco-scale penalty points were calculated for the proposed methods. All calculations are cited in Table 4. It was observed that the derivative spectrophotometric and HPLC methods are highly eco-friendly. This is confirmed by the calculated values of analytical eco-scale score, which is more than 75 (Table 4). Thus, the proposed methods are excellent in greenness assessment.
| Reagents/instruments | Spectrophotometric method | HPLC method |
|---|---|---|
| (a) Analytical Greenness Profile (AGP) | ||
| Health hazard | Moderately toxic, NFPA = 2 | Moderately toxic, NFPA = 2 and 3 |
| Safety hazard | Highest NFPA flammability and instability score = 3 and 0 | Highest NFPA flammability and instability score = 3 and 0 |
| Environmental hazard | <50 mL | <50 mL |
| Energy | Very little solvent evaporation | Energy of HPLC |
| Waste amount | ≤50 mL | ≤50 mL |
| Health hazard | Moderately toxic, NFPA = 2 | Moderately toxic, NFPA = 2 and 3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (b) Analytical eco-scale penalty points | ||
| Reagents | ||
| Ethanol | 12 | — |
| Acetonitrile | — | 8 |
| Phosphate buffer pH 7 | — | Not hazardous |
| TEA | — | 8 |
| Instruments | ||
| Spectrophotometry | 0 | — |
| HPLC | — | 1 |
| Occupational hazard | 0 | 0 |
| Waste | 3 | 5 |
| Total penalty points | 15 | 22 |
| Analytical | 85 | 78 |
| Eco-scale total score | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| (c) Green analytical procedure index (GAPI) | ||
| Sample preparation | ||
| Collection (1) | At-line | At-line |
| Preservation (2) | None | None |
| Transport (3) | None | None |
| Storage (4) | Under normal conditions | Under normal conditions |
| Type of method: direct or indirect (5) | Filtration | Filtration |
| Reagent and solvents | ||
| Amount (9) | <10 mL | 10–100 mL |
| Health hazard (10) | NFPA health hazard score = 2 | NFPA health hazard score = 1, 2, and 3 |
| Safety hazard (11) | Instability score = 0 | Instability score = 0 |
| Flammability score = 3 | Flammability score = 3 | |
| Instrumentation | ||
| Energy (12) | ≤ 0.1 kW h per sample | ≤ 1.5 kW h per sample |
| Occupational hazard (13) | — | — |
| Waste (14) | 1–10 mL | > 10 mL |
| Waste treatment (15) | No treatment | No treatment |
| Quantification | Yes | Yes |
Finally, the third assessment tool is the GAPI method,21 which is also illustrated by a pictogram. This colored pictogram is divided into 15 segments. Each segment can take green or yellow or red color regarding the level of greenness. The proposed methods' GAPI pictograms are shown in Fig. S3.† The GAPI pictogram of the derivative spectrophotometric method demonstrates four green segments, seven yellow segments, and only one red segment, while the GAPI pictogram of the HPLC method shows two green segments, eight yellow segments, and two red segments. These results provide that the proposed methods are green and eco-friendly. As shown in Fig. S3,† few number of red batches are observed in case of the derivative spectrophotometric method, which is due to the use of only one solvent, using direct method for analysis and production of few amounts of waste. This approach indicates that the developed derivative spectrophotometric analysis is relatively greener than the chromatographic one.
| Methods | AGP | Analytical eco-scale total score | GAPI |
|---|---|---|---|
| Proposed derivative spectrophotometric method | 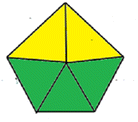 |
85 | 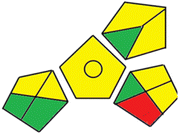 |
| Proposed HPLC method | 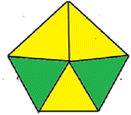 |
78 | 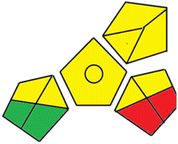 |
| Reported method17 | 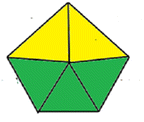 |
85 | 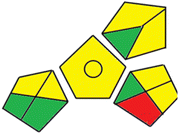 |
4. Conclusion
The present study proposes two fully validated analytical techniques for the simultaneous determination of FIN and TAD in bulk and capsules. Both proposed methods are sensitive, selective, and can be used for routine quality control investigation. Also, they were ecologically assessed using three greenness assessment tools. The findings indicate that they are environmentally benign. The present derivative spectrophotometric method has advantages of low cost of operation and greenness when compared to HPLC. The suggested HPLC method has advantages over the reported method as it is straight forward, does not need further mathematical treatment of measured data, having higher selectivity and taking a short time as the analysis time is only 5 min. The main advantage of the proposed derivative spectrophotometric method in comparison with the reported method is the greenness as it used ethanol instead of methanol.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, Saudi Arabia has funded this project under grant no. (G: 306-166-1443).References
- J. K. Parsons, Curr. Bladder Dysfunct. Rep., 2010, 5, 212–218 CrossRef PubMed.
- D. E. Irwin, Z. S. Kopp, B. Agatep, I. Milsom and P. Abrams, BJU Int., 2011, 108, 1132–1138 CrossRef PubMed.
- K. T. McVary, C. G. Roehrborn, A. L. Avins, M. J. Barry, R. C. Bruskewitz, R. F. Donnell, H. E. Foster Jr, C. M. Gonzalez, S. A. Kaplan, D. F. Penson, J. C. Ulchaker and J. T. Wei, J. Urol., 2011, 185, 1793–1803 CrossRef PubMed.
- F. Giuliano, S. Uckert, M. Maggi, L. Birder, J. Kissel and L. Viktrup, Eur. Urol., 2013, 63, 506–516 CrossRef CAS PubMed.
- H. Berniati Tampubolon, E. Sumarlik, S. Dwi Saputra, S. Cholifah, W. Farina Kartinasari and G. Indrayanto, J. Liq. Chromatogr. Relat. Technol., 2006, 29, 2753–2765 CrossRef.
- N. A. Zambianco, V. A. O. P. da Silva, L. O. Orzari, E. J. Corat, H. G. Zanin, T. A. Silva, G. A. Buller, E. M. Keefe, C. E. Banks and B. C. Janegitz, J. Electroanal. Chem., 2020, 877 Search PubMed.
- S. Saglik and S. Tatar Ulu, Anal. Biochem., 2006, 352, 260–264 CrossRef CAS PubMed.
- O. Salih Hassan and A. I. Khaleel, Mater. Today: Proc., 2021, 45, 5569–5574 CAS.
- M. A. Magdy, B. H. Anwar, I. A. Naguib and N. S. Abdelhamid, Spectrochim. Acta, Part A, 2020, 226, 117611 CrossRef CAS PubMed.
- E. R. Sartori, D. N. Clausen, I. M. R. Pires and C. A. R. Salamanca-Neto, Diamond Relat. Mater., 2017, 77, 153–158 CrossRef CAS.
- M. R. Rezk, M. A. Tantawy, M. Wadie and S. A. Weshahy, Spectrochim. Acta, Part A, 2020, 227, 117547 CrossRef CAS PubMed.
- K. A. Al and A. A. Gouda, Chem. Ind. Chem. Eng. Q., 2011, 17, 125–132 CrossRef.
- M. Yunoos, D. G. Sankar, B. P. Kumar and S. Hameed, E-J. Chem., 2010, 7, 833–836 CrossRef CAS.
- M. A. Abu El-Enin, S. Al-Ghaffar Hammouda Mel, D. T. El-Sherbiny, D. R. El-Wasseef and S. M. El-Ashry, Luminescence, 2016, 31, 173–178 CrossRef CAS PubMed.
- A. Alvarez-Lueje, S. Brain-Isasi, L. J. Núñez-Vergara and J. A. Squella, Talanta, 2008, 75, 691–696 CrossRef CAS PubMed.
- N. Pappula, B. Kodali and P. V. Datla, J. Pharm. Biomed. Anal., 2018, 152, 215–223 CrossRef CAS PubMed.
- A. H. Abdelazim and S. Ramzy, BMC Chem., 2022, 16, 55 CrossRef CAS PubMed.
- A. Gałuszka, Z. M. Migaszewski, P. Konieczka and J. Namieśnik, TrAC, Trends Anal. Chem., 2012, 37, 61–72 CrossRef.
- A. F. El-Yazbi, F. M. Aboukhalil, E. F. Khamis, R. M. Youssef and M. A. El-Sayed, J. Planar Chromatogr.–Mod. TLC, 2021, 34, 455–466 CrossRef CAS.
- A. F. El-Yazbi, F. M. Aboukhalil, E. F. Khamis, R. M. Youssef and M. A. El-Sayed, Beni-Suef Univ. J. Basic Appl. Sci., 2022, 11, 69 CrossRef.
- J. Plotka-Wasylka, Talanta, 2018, 181, 204–209 CrossRef CAS PubMed.
- A. F. El-Yazbi, F. M. Aboukhalil, E. F. Khamis, R. M. Youssef and M. A. El-Sayed, Microchem. J., 2021, 163, 105900 CrossRef CAS.
- R. M. Youssef, J. AOAC Int., 2008, 91, 73–82 CrossRef CAS PubMed.
- R. M. Youssef, Saudi Pharm. J., 2010, 18, 45–49 CrossRef CAS PubMed.
- M. A. Magdy, N. F. Farid, B. H. Anwar and N. S. Abdelhamid, Chromatographia, 2022, 85, 1075–1086 CrossRef CAS.
- A. F. El-Yazbi, E. F. Khamis, R. M. Youssef, M. A. El-Sayed and F. M. Aboukhalil, Heliyon, 2020, 6, e04819 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01437a |
| This journal is © The Royal Society of Chemistry 2023 |