Open Access Article
Open Access ArticleEach side chain of cyclosporin A is not essential for high passive permeability across lipid bilayers†
Takahiro Ono a,
Kazuhito V. Tabata
a,
Kazuhito V. Tabata b,
Hiroyuki Noji
b,
Hiroyuki Noji bc,
Jumpei Morimoto
bc,
Jumpei Morimoto *a and
Shinsuke Sando
*a and
Shinsuke Sando *ac
*ac
aDepartment of Chemistry & Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: jmorimoto@chembio.t.u-tokyo.ac.jp; ssando@chembio.t.u-tokyo.ac.jp
bDepartment of Applied Chemistry, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
cDepartment of Bioengineering, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
First published on 13th March 2023
Abstract
We compared the passive permeability of cyclosporin A (CsA) derivatives with side chain deletions across lipid bilayers. CsA maintained passive permeability after losing any one of the side chains, which suggests that the propensity of the backbone of CsA is an important component for high passive permeability.
Cyclic peptides can interact with proteins specifically and strongly using their broad surface area, making them attractive drug candidates.1–4 However, most cyclic peptides have low passive permeability across biological membranes. Low membrane permeability is an obstacle to accessing the targets inside cells and oral absorption of cyclic peptides. Therefore, design principles are required to produce highly permeable cyclic peptides.
Cyclosporin A (CsA) is a prominent cyclic peptide and a lodestar in the research field of cyclic peptide permeability due to its high passive permeability despite its high molecular weight.5 Investigations into the structural determinants for the high permeability of CsA would significantly contribute to the establishment of the design principles of highly permeable cyclic peptides. The previous studies using model cyclic peptides other than CsA suggest that the differences in the backbone, i.e., the pattern of amide N-methylation and stereochemistry, can primarily change the conformations and passive permeability of cyclic peptides.6,7 Therefore, the position of seven amide N-methylations and the presence of a D-isomer in the backbone of CsA probably have a predominant contribution to the passive permeability. On the other hand, the importance of each residue to the high passive permeability of CsA is not clear. Each of the 11 residues of CsA is supposed to play a role in the binding to the target proteins, cyclophilins and calcineurin, and/or the high membrane permeability. There are a few previous studies investigating structure–permeability relationships of CsA, but they have not reached a definitive answer (Fig. 1 top).8–11 For example, the necessity of the 1st residue (4R)-4-[(E)-2-butenyl]-4,N-dimethyl-L-threonine (MeBmt), a nonproteinogenic amino acid characterizing the sequence of CsA, for the high passive permeability is still controversial. J. Seo's group conducted a comparison of CsA and cyclosporin O (CsO), which has Leu at the 1st residue and norvaline (Nva) at 2nd residue, and concluded that Bmt is essential for the passive permeability due to the lower permeability of CsO than CsA in PAMPA (parallel artificial membrane permeability assay) and Caco-2 assay.11 On the other hand, in a report by R. S. Lokey's group, Bmt to Leu substitution did not decrease the permeability of CsA in MDCK-LE assay.10
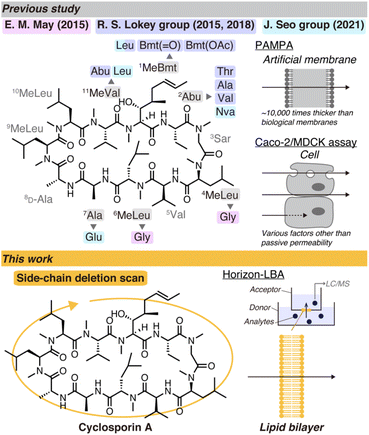 | ||
| Fig. 1 Schematic illustration of studies of cyclosporin A derivatives with modified side chains in previous studies (top) and this work (bottom). | ||
An issue in the previous studies of the structure–permeability relationship of CsA is that the passive permeability was measured by PAMPA or cell monolayer-based permeability assays like Caco-2 and MDCK assays, which cannot measure pure passive permeability across lipid bilayers.12–15 The artificial membrane of PAMPA is about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times thicker than lipid bilayers, and its main component is an organic solvent. In cell-based assays, various factors, such as degradation and adsorption to proteins, prohibit a fair comparison of passive permeability between different compounds. Permeability assays using lipid bilayers are desirable to evaluate passive permeability. Investigations into the correlation between passive permeability across lipid bilayers and the structure of CsA derivatives would provide explicit insights into the necessity of each residue for the passive permeability of CsA. The knowledge about the importance of each side chain is expected to be valuable for designing permeable cyclic peptides based on the CsA backbone.
000 times thicker than lipid bilayers, and its main component is an organic solvent. In cell-based assays, various factors, such as degradation and adsorption to proteins, prohibit a fair comparison of passive permeability between different compounds. Permeability assays using lipid bilayers are desirable to evaluate passive permeability. Investigations into the correlation between passive permeability across lipid bilayers and the structure of CsA derivatives would provide explicit insights into the necessity of each residue for the passive permeability of CsA. The knowledge about the importance of each side chain is expected to be valuable for designing permeable cyclic peptides based on the CsA backbone.
To draw conclusions about the importance of each side chain of CsA for passive permeability, we here report a comparison of lipid bilayer permeability of the CsA derivatives with a side-chain deletion scan (Fig. 1 bottom). We used a lipid bilayer-based permeability assay developed by our group named Horizon-LBA (horizontal lipid bilayer permeability assay), by which we successfully evaluated the lipid bilayer permeability of CsA and CsO in a previous report.16
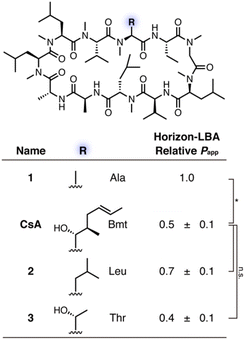
First, to define the necessity of MeBmt residue in CsA for its passive permeability, for which different suggestions have been presented,10,11 three CsA derivatives that have Ala, Leu, or Thr at the 1st position of CsA were designed and synthesized (Fig. 2, see Schemes S1 and S2† for synthetic procedures). Ala, Leu, and Thr are substructures of Bmt, and a comparison of their permeability would provide insights about whether each part in MeBmt, such as β-OH and the long hydrocarbon chain, is required for passive permeability. The permeability of CsA derivatives was evaluated by Horizon-LBA (Fig. 2). For a fair comparison of the permeabilities, the relative permeabilities of each peptide were measured using the mixture of peptides as analytes, and the relative Papp of each peptide was determined with the Papp of peptide 1 in each experiment as 1.0. As a result, 1 showed higher permeability than CsA, and the significant differences in the relative permeability of 2 and 3 from CsA were not observed. Although the permeability coefficient of 3 was slightly lower than CsA, the passive permeability was maintained. The reduction of lipophilicity by the substitution of Bmt to Thr is considered to be a reason for the lower permeability of 3. On the other hand, the fact that 1 (R = Ala) maintained permeability suggests that the Bmt residue, including the β-OH and long hydrocarbon chain, is not necessary at the 1st position of CsA for the high passive permeability.
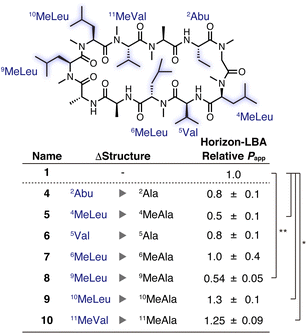
Subsequently, a side-chain deletion scan was conducted based on 1, which had the highest permeability in the synthesized CsA derivatives, to evaluate whether the CsA derivative can maintain the passive permeability after the further loss of a side chain. We synthesized CsA derivatives 4–10, in which each residue of 1 was substituted to alanine/N-methylalanine residue (see the ESI† for synthetic procedures). The relative permeability of all the derivatives was measured by Horizon-LBA (Fig. 3). As a result, none of the CsA derivatives showed a substantial decrease in passive permeability than the parental CsA derivative (1). Although 5 and 8 showed lower permeability coefficients than 1, the degree of decrease was less than 50%, and they maintained passive permeability. These results suggest that the substituted side chains are not necessary for the high passive permeability of CsA. The calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (A
P values (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) of the CsA derivatives were determined to understand the reasons for the permeability difference (Table S3†); however, no correlation between permeability and lipophilicity AlogP was found.
P) of the CsA derivatives were determined to understand the reasons for the permeability difference (Table S3†); however, no correlation between permeability and lipophilicity AlogP was found.
Finally, we conducted a structure-based analysis to clarify the reason for the maintained passive permeability of the CsA derivatives 1–10. The number of solvent-exposed hydrogen bond donors was measured by NMR. One of the hypotheses about the efficient passive membrane permeation of CsA is that the formation of a conformation called the “closed form” with a low number of solvent-exposed hydrogen bond donors in lipid membranes contributes to the passive permeability.5,17 Since the substitutions to alanine/N-methylalanine did not lead to the fatal loss of passive permeability of CsA, we hypothesized that the intramolecular hydrogen bonds of CsA could be maintained without each of the side chains. Amide temperature coefficients (ATC) of 1–10 were measured in chloroform-d (Fig. 4a). ATC is useful for measuring the number of solvent-exposed hydrogen bond donors.18 All the amide NHs of 1–10 had Δδ/ΔT more positive than −4.6 ppb K−1, suggesting that these CsA derivatives maintain the intramolecular hydrogen bonds in a non-polar solvent when a residue is substituted to alanine/N-methylalanine residue. The results of permeability experiments and ATC suggest that CsA can maintain the intramolecular hydrogen bonds even when it loses a side chain, and the maintenance of the “closed conformation” is an important structural determinant for high membrane permeability (Fig. 4b). In the future, in addition to ATC, more detailed conformational studies, such as NOEs (nuclear Overhauser effect) measurements and in silico simulations, will further clarify the relationships between the conformations and permeability of the CsA derivatives.
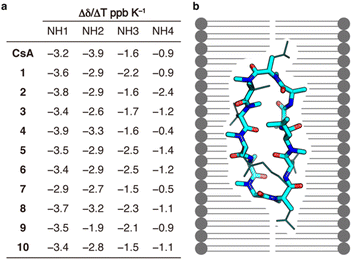 | ||
| Fig. 4 Amide temperature coefficients of the CsA derivatives. (a) Δδ/ΔT ppb K−1 of 1–10, which was calculated from 1H NMR and COSY in chloroform-d. (b) Schematic illustration showing one example of the “closed conformation” constructed by the dominant contribution of the backbone of CsA. The backbone and side chains are shown in stick model and line model, respectively. The source of the 3D-structure is CCDC ID: DEKSAN.15 | ||
In this study, we compared the permeability of CsA derivatives directly using a lipid bilayer. The substitution of Bmt in CsA and the following side-chain deletion scan showed that, even when one of any side chains is replaced with (Me)Ala, CsA maintains passive permeability across lipid bilayers. Especially the necessity of Bmt for the permeability of CsA has been controversial, but the fact that the substitution of Bmt to Ala did not reduce the lipid bilayer permeability is evidence of the nonessentiality of Bmt for passive permeation. These results suggest that the specific side chains of CsA are not essential for the permeability, and the backbone structure, e.g., stereochemistries at α-carbons and the positions of amide N-methylations, dominantly contributes to the passive permeability. Therefore, the backbone of CsA can be useful as a scaffold with high permeability, and a library of CsA derivatives with various side chains, as long as satisfying appropriate lipophilicity requirements, would be useful for drug discovery for intracellular target proteins. In the permeability experiments, we used a single lipid composition (DOPC) and buffer (PBS). Considering the existence of effects of lipid compositions on the passive permeability of compounds and the plausible interactions of CsA with some ions,19–21 lipid bilayer permeability measurements of CsA and its derivatives using various lipids and ions are important research subjects to understand the structural determinants of the permeation of CsA in more detail. In the future, investigations into the effects of the substitution to various side chains other than Ala on physicochemical properties, such as lipophilicity and surface area, and the permeability of CsA will provide further insights into the permeation mechanism of CsA.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
T. O. was supported by a Program for Leading Graduate Schools (MERIT). This work was supported by CREST, Japan Science and Technology Agency, Grant Numbers JPMJCR21N5 (S. S.), JPMJCR18S6 (K. V. T.), PRESTO, Japan Science and Technology Agency, Grant Number JPMJPR21AF (J. M.), and JSPS Grant Numbers JP17H06355 (K. V. T.).Notes and references
- A. Zorzi, K. Deyle and C. Heinis, Curr. Opin. Chem. Biol., 2017, 38, 24–29 CrossRef CAS PubMed.
- D. S. Nielsen, N. E. Shepherd, W. Xu, A. J. Lucke, M. J. Stoermer and D. P. Fairlie, Chem. Rev., 2017, 117, 8094–8128 CrossRef CAS PubMed.
- A. A. Vinogradov, Y. Yin and H. Suga, J. Am. Chem. Soc., 2019, 141, 4167–4181 CrossRef CAS PubMed.
- L. K. Buckton, M. N. Rahimi and S. R. McAlpine, Chem.–Eur. J., 2021, 27, 1487–1513 CrossRef CAS PubMed.
- K. M. Corbett, L. Ford, D. B. Warren, C. W. Pouton and D. K. Chalmers, J. Med. Chem., 2021, 64, 13131–13151 CrossRef CAS PubMed.
- T. Rezai, B. Yu, G. L. Millhauser, M. P. Jacobson and R. S. Lokey, J. Am. Chem. Soc., 2006, 128, 2510–2511 CrossRef CAS PubMed.
- E. Biron, J. Chatterjee, O. Ovadia, D. Langenegger, J. Brueggen, D. Hoyer, H. A. Schmid, R. Jelinek, C. Gilon, A. Hoffman and H. Kessler, Angew. Chem., Int. Ed., 2008, 47, 2595–2599 CrossRef CAS PubMed.
- E. M. May, Doctral dissertation, Harvard Univ., Cambridge, MA., 2015.
- C. L. Ahlbach, K. W. Lexa, A. T. Bockus, V. Chen, P. Crews, M. P. Jacobson and R. S. Lokey, Future Med. Chem., 2015, 7, 2121–2130 CrossRef CAS PubMed.
- M. R. Naylor, A. M. Ly, M. J. Handford, D. P. Ramos, C. R. Pye, A. Furukawa, V. G. Klein, R. P. Noland, Q. Edmondson, A. C. Turmon, W. M. Hewitt, J. Schwochert, C. E. Townsend, C. N. Kelly, M. J. Blanco and R. S. Lokey, J. Med. Chem., 2018, 61, 11169–11182 CrossRef CAS PubMed.
- D. Lee, S. Lee, J. Choi, Y.-K. Song, M. J. Kim, D.-S. Shin, M. A. Bae, Y.-C. Kim, C.-J. Park, K.-R. Lee, J.-H. Choi and J. Seo, J. Med. Chem., 2021, 64, 8272–8286 CrossRef CAS PubMed.
- M. Kansy, F. Senner and K. Gubernator, J. Med. Chem., 1998, 41, 1007–1010 CrossRef CAS PubMed.
- P. Artursson and J. Karlsson, Biochem. Biophys. Res. Commun., 1991, 175, 880–885 CrossRef CAS PubMed.
- J. D. Irvine, L. Takahashi, K. Lockhart, J. Cheong, J. W. Tolan, H. E. Selick and J. R. Grove, J. Pharm. Sci., 1999, 88, 28–33 CrossRef CAS PubMed.
- L. Di, C. Whitney-Pickett, J. P. Umland, H. Zhang, X. Zhang, D. F. Gebhard, Y. Lai, J. J. Federico III, R. E. Davidson, R. Smith, E. L. Reyner, C. Lee, B. Feng, C. Rotter, M. V. Varma, S. Kemphall, K. Fenner, A. F. El-Kattan, T. E. Liston and M. D. Troutman, J. Pharm. Sci., 2011, 100, 4974–4985 CrossRef CAS PubMed.
- T. Ono, K. V Tabata, Y. Goto, Y. Saito, H. Suga, H. Noji, J. Morimoto and S. Sando, Chem. Sci., 2023, 14, 345–349 RSC.
- H. Loosli, H. Kessler, H. Oschkinat, H. Weber, T. J. Petcher and A. Widmer, Helv. Chim. Acta, 1985, 68, 682–704 CrossRef CAS.
- C. K. Wang, S. E. Northfield, B. Colless, S. Chaousis, I. Hamernig, R. Lohman, D. S. Nielsen, C. I. Schroeder, S. Liras, D. A. Price, D. P. Fairlie and D. J. Craik, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 17504–17509 CrossRef CAS PubMed.
- S. Purushothaman, J. Cama and U. F. Keyser, Soft Matter, 2016, 12, 2135–2144 RSC.
- J. A. Carver, N. H. Rees, D. L. Turner, S. J. Senior and B. Z. Chowdhry, J. Chem. Soc., Chem. Commun., 1992, 1682–1684 RSC.
- M. Köck, H. Kessler, D. Seebach and A. Thaler, J. Am. Chem. Soc., 1992, 114, 2676–2686 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01358h |
| This journal is © The Royal Society of Chemistry 2023 |