Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metabolic profiling and biological activity of two Livistona species: L. chinensis and L. australis†
Seham S. El-hawary‡
a,
Ahlam Elwekeel‡ *b,
Sara O. Abo El-Elab,
Usama Ramadan Abdelmohsen
*b,
Sara O. Abo El-Elab,
Usama Ramadan Abdelmohsen cd and
Asmaa I. Owisbe
cd and
Asmaa I. Owisbe
aDepartment of Pharmacognosy, Faculty of Pharmacy, Cairo University, Cairo, Egypt
bDepartment of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt. E-mail: Ahlam_hasham2000@yahoo.com; Ahlam.hassanain@pharm.bsu.edu.eg
cDepartment of Pharmacognosy, Faculty of Pharmacy, Minia University, Minia, Egypt
dDepartment of Pharmacognosy, Faculty of Pharmacy, Deraya University, New Minia, Egypt
eDepartment of Pharmacognosy, Faculty of Pharmacy, Heliopolis University for Sustainable Development, Cairo, Egypt
First published on 15th May 2023
Abstract
Livistona is a genus of family Arecaceae and widely grown in tropical areas. The phytochemical analysis of the leaves and fruits of two Livistona species, L. chinensis and L. australis were carried out using UPLC/MS and determination of the total phenolic and total flavonoid contents, in addition to the isolation and identification of five phenolic compounds and one fatty acid from L. australis fruits. The total phenolic compounds varied from 19.72 to 78.87 mg GAE g−1 dry plant, while the total flavonoid contents were in the range of 4.82–17.75 mg RE g−1 dry plant. The UPLC/MS analysis of the two species led to the characterization of forty-four metabolites belonging mainly to the different classes of flavonoids and phenolic acids, while the compounds isolated from L. australis fruits were identified as gallic acid, vanillic acid, protocatechuic acid, hyperoside, quercetin 3-O-α-D-arabinopyranoside and dodecanoic acid. The in vitro biological evaluation of L. australis leaves and fruits were estimated as anticholinesterase, telomerase reverse transcriptase (TERT) potentiation and anti-diabetic through measuring the capacity of the extracts to inhibit dipeptidyl peptidase (DPP-IV). The results revealed that the leaves showed remarkable anticholinesterase and antidiabetic activity compared to fruits with IC50 values of 65.55 ± 3.75 ng mL−1 and 90.8 ± 4.48 ng mL−1, respectively. In the TERT enzyme assay, the leaves extract triggered a 1.49-fold increase in telomerase activity. This work showed that the Livistona species are a good source for flavonoids and phenolics, which play an important role in anti-aging and the treatment of chronic diseases, such as diabetes and Alzheimer's.
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder and is considered to be the main cause of dementia. Worldwide, about 40 million people are expected to suffer from dementia, and this number is expected to be doubled by 2050.1 A low level of acetylcholine in the brain is the key marker of the disease. Inhibition of the acetylcholinesterase (AChE) enzyme, which is responsible for the degradation of acetylcholine, is the most promising approach for the treatment of AD. Many natural compounds, such as terpenoids, coumarins, flavonoids, alkaloids and other phenolic compounds, have shown anticholinesterase activity.2Another sign associated with aging is telomere attrition. At the ends of linear chromosomes are DNA repeats called telomeres, which ensure chromosome stability throughout replication. Every time a cell divides, as well as when there is oxidative stress, the telomere length is decreased. When telomeres significantly shorten in length, cells can no longer divide, causing cell death. There is an enzyme called telomerase, which creates the particular DNA sequence at the telomeres.3–7 The substances that potentiate telomerase are called telomerase activators and are used as antiageing sources.
Another heath problem that has an impact on human health is T2DM. The number of patients expected to suffer from diabetes in 2040 will be 642 million worldwide, according to the International Diabetes Federation.8 Lowering the blood glucose level is the primary treatment of T2DM, which can be done through α-glucosidase inhibitors or the stimulation of insulin secretion.9 Incretins are hormones that are released from the small intestine in response to dietary intake, and have an impact similar to glucagon. The main incretins are a glucagon-like peptide (GLP-1) and a glucose-dependent insulinotropic peptide (GIP). These incretins potentiate insulin secretion from pancreatic B-cells.10 Dipeptidyl peptidase-IV is responsible for the degradation of these incretins. Thus, DPP-IV inhibitors prevent the degradation of these incretins, which led to an increase in the insulin level and lowering of the blood glucose level.11
Livistona is a genus belonging to the family Arecaceae and it is widely distributed in tropical areas, with about 36 species reported in the genus. Phytochemical studies on Livistona have reported on many compounds, such as flavonoids, phenolics, ceramides and glycerides,12–14 pyranone derivatives15 and sulfated flavonoids.16 Biologically, L. chinensis has been reported to have anticancer17 and cardioprotective18 properties, while L. decipiens has been reported to have antioxidant and cytotoxic activities.19 A recent report on the leaves and fruit extracts of the plant also showed inhibitory activity against coronavirus.20 The fruits of L. chinensis are edible in eastern Asia, they are a component of prepared soups used in the treatment of liver cancer and chronic hepatitis.21 The nutritional evaluation of the leaves and fruits of L. australis also showed that the fruits are a good source of calcium and potassium.22 This work aims to identify the secondary metabolites in the leaves and fruits of L. chinensis and L. australis using UPLC coupled with high-resolution electron spray ionization mass (HRESIMS), and evaluate their anticholinesterase, DPP-IV inhibitory potential, and telomerase activation potential.
2. Experimental
2.1. Plant material
The fruits and leaves of Livistona australis and Livistona chinensis were supplied from El-Orman Botanical Garden, Giza, Egypt on Jan. 2018. The samples were authenticated by Eng. Trease Labib (El Orman Botanical Garden) and Dr Reem Samir Hamdy (Plant Taxonomy, Botany Department, Faculty of Science, Cairo University, Giza, Egypt). Voucher specimens (BUPD-71-2018 for L. australis and BUPD-73-2018 for L. chinensis) were kept in the Pharmacognosy Department, Faculty of Pharmacy, Beni-Suef University, Egypt. The plants were cleaned, dried and finely powdered for the study.2.2. Chemicals and reagents
Solvents such as n-hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc), ethanol (EtOH), and methanol (MeOH), were obtained from the El-Nasr Company for Pharmaceuticals and Chemicals, Egypt. Acetonitrile, methanol (HPLC grade) and DMSO were purchased from (Sigma-Aldrich, St. Louis, MO, USA). Acetyl-cholinesterase inhibitor screening kits (cat. # IACE-100) were purchased from BioAssay Systems (Hayward, CA, USA). Donepezil (Cat # sc-218265) was purchased from Santa Cruz Biotechnology (Texas, USA). TERT ELISA kit (cat. # K4187-100) was purchased from Biovision incorporated (Milpitas, CA, USA), The curcumin standard for the TERT assay was obtained from Sigma-Aldrich, Germany.2.3. Apparatus
The NMR analysis for the isolated compounds was carried out using Bruker Avance III 400 MHz (Bruker, USA). LC/MS analysis of the extracts was done on an Acquity Ultra Performance Liquid Chromatography (UPLC) system connected with a Synapt G2 HDMS quadrupole time-of-flight hybrid mass spectrometer (Waters, USA). Spectrophotometric determination of the total phenolic and flavonoid contents was measured using a Jenway 6305 spectrophotometer.2.4. Metabolomics study
Three grams of each sample were exhaustively defatted using n-hexane (3 × 15 mL). The marc was then extracted with 70% ethanol, filtered with Whatman filter paper, and evaporated under vacuum to afford the dry residue. One mg of each extract was dissolved in 1 mL methanol and analyzed using LC/MS, as reported by Owis et al., 2020.23 Briefly, the samples were analyzed using a C-18 column (2.1 × 100 mm, 1.7 μm particle size; Waters, Milford, MA, USA). A binary solvent was used for separation; solvent A was 0.1% formic acid in water, while solvent B was acetonitrile, and a gradient elution from 0–100% of solvent B in 6 min with flow rate 0.3 mL min−1 was followed. Electron spray ionization mass spectrometry was used to detect the metabolites; the processed data set was subjected to molecular formula prediction, as well as peak identification. The positive and negative ionization mode data sets from the plant extract were dereplicated against the Dictionary of Natural Products (DNP) database.2.5. Determination of total phenolics and total flavonoids contents
The fruits and leaves of L. australis and L. chinensis at 1 g each were extracted with 50 mL of 80% methanol for 2 h on an orbital shaker set at 200 rpm. Each mixture was separately centrifuged for 20 min, and the supernatants were decanted. The residues were re-extracted under the same conditions. Supernatants were separately combined. The volume was adjusted to 100 mL; these extracts were used for total phenolic and total flavonoid determination. The total phenolic content was assessed using Folin–Ciocalteu reagent as follows.24 A volume of 300 μL of each extract was mixed with 2.25 mL Folin reagent. After 5 minutes, 2.25 mL of 6% sodium carbonate was added. The mixture was rested for 90 min, and the blue color that developed was measured at 725 nm. The results were expressed as mg gallic acid equivalents per g dried sample (mg GAE g−1). Meanwhile, the flavonoid content was assessed by colorimetric method.24 Briefly, 0.5 mL of each extract was diluted with 2.25 mL distilled water, and then 150 μL of 5% NaNO2 was added. Six min later, 300 μL of 10% AlCl3 was mixed and the reaction was rested for 6 min. Finally, 1 mL of 1 M NaOH was added. The mixture was mixed well and the absorbance was measured at 510 nm. Results of the total flavonoids were expressed as mg rutin equivalents per g of dried sample (mg RE g−1).2.6. Extraction and fractionation
Air-dried, powdered pericarp of L. australis (1.75 kg) was extracted with 70% ethanol (5 L) by cold maceration for 72 h, and then filtered through filter paper. The marc was re-extracted again until exhaustion, and the collected extracts was dried under vacuum at 45 °C to afford the crude extract (340 g). A part of the dried extract (200 g) was fractionated using n-hexane, dichloromethane and ethyl acetate to give 22, 14 and 6 g, respectively. The n-hexane fraction (6 g) was chromatographed on a silica gel (E-Merck, Germany) column using hexane with 10% ethyl acetate increments to afford one major compound 1 (500 mg). The ethyl acetate fraction (5 g) was chromatographed on a polyamide (E-Merck, Germany) column starting with 100% water as the eluent, then with decreasing polarity by 20% MeOH to afford five sub-fractions. The sub-fraction eluted with 20% water (500 mg) was re-chromatographed on a Sephadex LH-20 column using methanol to afford compounds 2 and 3 (25, 10 mg, respectively). The sub-fraction eluted with 60% water (100 mg) was re-chromatographed on a Sephadex LH-20 column (Sigma-Aldrich, USA) using methanol as an eluent to afford three compounds 4–6 (15, 12, 20 mg, respectively).2.7. Biological studies
2.8. Statistical analysis
Data were presented as the mean ± standard deviation (SD). All analyses were done in triplicate. Statistical and graphical evaluations were done using Graph Pad Prism 7 and Microsoft Excel 2010.3. Results and discussion
3.1. Metabolic analysis
Metabolic profiling of the secondary metabolites in L. chinensis and L. australis leaves and fruits were conducted using LC-HRESIMS (chromatograms in ESI data, Fig. S1–S8†) for dereplication/detection of the putative compounds responsible for the various activities assigned to Livistona. It led to the characterization of forty-four compounds (Table 1, Fig. 1) belonging to different classes of flavonoids and phenolic acids.| Metabolites name | L. chinensis | L. australis | MF | m/z | ||
|---|---|---|---|---|---|---|
| L | F | L | F | |||
| a L: leaves, F: fruits, MF: molecular formula, ND: not detected. | ||||||
| Syringic acid | + | + | + | + | C9H10O5 | 197.0447 |
| 4-Hydroxybenzoic acid | ND | + | ND | + | C7H6O3 | 137.0238 |
| Protocatechuic acid | + | + | + | + | C7H6O4 | 153.0189 |
| Rutin | ND | ND | ND | + | C27H30O16 | 609.1455 |
| Chlorogenic acid | + | + | + | + | C16H18O9 | 353.0881 |
| Cryptochlorogenic acid | + | + | + | + | C16H18O9 | 353.0881 |
| Neochlorogenic acid | + | + | + | + | C16H18O9 | 353.0881 |
| Vanillic acid | + | + | + | + | C8H8O4 | 167.0342 |
| Isovanillic acid | + | + | + | + | C8H8O4 | 167.0342 |
| Vicenin II | + | + | ND | ND | C27H30O15 | 593.1514 |
| Isoorientin-7-O-sulfate | + | + | + | ND | C21H20O14S | 527.0501 |
| Orientin-7-O-sulfate | + | + | + | ND | C21H20O14S | 527.0501 |
| Orientin | + | + | + | + | C21H20O11 | 447.0931 |
| Isoorientin | + | + | + | + | C21H20O11 | 447.0931 |
| Cynaroside | + | + | + | + | C21H20O11 | 447.0932 |
| Caffeic acid | + | + | + | + | C9H8O4 | 179.0346 |
| 5-O-Caffeoylshikimic acid | + | + | ND | + | C16H16O8 | 335.0769 |
| 3-O-Caffeoylshikimic acid | + | + | ND | + | C16H16O8 | 335.0769 |
| Schaftoside | + | + | + | ND | C26H28O14 | 563.1405 |
| Isoschaftoside | + | + | + | ND | C26H28O14 | 563.1405 |
| (+)-Catechin | ND | + | + | + | C15H14O6 | 289.0713 |
| (−)-Catechin | ND | + | + | + | C15H14O6 | 289.0713 |
| Epicatechin | ND | + | + | + | C15H14O6 | 289.0713 |
| Quercitrin | + | + | + | + | C21H20O11 | 447.0929 |
| Isovitexin 7-O-sulfate | + | + | + | ND | C21H20O13S | 511.0558 |
| Vitexin 7-O-sulfate | + | + | + | ND | C21H20O13S | 511.0558 |
| Isovitexin | + | + | ND | ND | C21H20O10 | 431.0974 |
| Vitexin | + | + | ND | ND | C21H20O10 | 431.0974 |
| Hyperoside | ND | + | + | + | C21H20O12 | 463.0876 |
| Isoquercetin | ND | + | + | + | C21H20O12 | 463.0876 |
| Quercetin | ND | ND | ND | + | C15H10O7 | 301.0351 |
| Avicularin | ND | ND | ND | + | C20H18O11 | 433.0977 |
| Quercetin 3-O-α-D-arabinopyranoside | ND | ND | ND | + | C20H18O11 | 433.0977 |
| Tricin 7-O-β-glucopyranoside-2′′-sulphate sodium salt | + | ND | + | ND | C23H24O15S | 571.0759 |
| Isorhamnetin-3-O-β-D-glucopyranoside | ND | + | ND | + | C22H22O12 | 477.1032 |
| cis-3,5,3′,5′-Tetrahydroxy-4′-methoxystilbene | ND | + | ND | + | C15H14O5 | 273.0766 |
| trans-3,5,3′,5′-Tetrahydroxy-4′-methoxystilbene | ND | + | ND | + | C15H14O5 | 273.0766 |
| (−)-Epiafzelechin | ND | + | ND | + | C15H14O5 | 273.0766 |
| Luteolin | + | + | + | + | C15H10O6 | 285.0395 |
| Homovanillic acid | + | + | + | + | C9H10O4 | 181.0498 |
| Naringenin | ND | + | ND | + | C15H12O5 | 271.0599 |
| 7-O-Methylluteolin | + | + | ND | + | C16H12O6 | 299.0558 |
| Chrysoeriol | + | + | ND | + | C16H12O6 | 299.0558 |
| Tricin | + | + | + | + | C17H14O7 | 329.0658 |
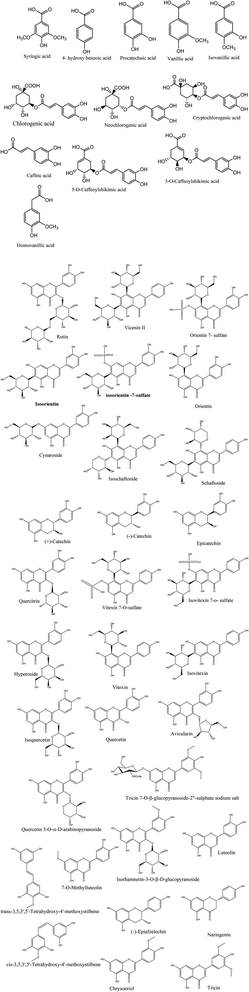 | ||
| Fig. 1 Structures of the dereplicated metabolites from the alcoholic extract of L. chinensis and L. australis leaves and fruits. | ||
3.2. Total phenolic and total flavonoids content
Folin–Ciocalteu reagent was used to determine the total phenolic content in the fruits and leaves of L. australis and L. chinensis extracts. The Folin–Ciocalteu reagent consists of complex polymeric ions (yellow solution) formed from phosphotungstic and phosphomolybdic heteropolyacids. This reagent produces complex molybdenum-tungsten blue from the oxidation of phenolates, which can be spectrophotometrically detected at 725 nm.24 As shown in Table 2, the order of the phenolic content was L. australis leaves > L. chinensis fruit > L. australis fruits > L. chinensis leaves with the values of 78.87, 44.50, 32.82 and 19.72 mg GAE g−1, respectively.| Samples | Total phenolic (mg GAE g−1) | Total flavonoid (mg RE g−1) |
|---|---|---|
| a Values are presented in mean ± SD (n = 3), expressed as mg gallic acid equivalent per g of dry sample (mg GAE g−1) for total phenolics and as mg rutin equivalent per g of dry sample (mg RE g−1) for total flavonoids. | ||
| L. chinensis leaves | 19.72 ± 3.49 | 4.82 ± 0.29 |
| L. chinensis fruits | 44.50 ± 1.60 | 17.75 ± 1.80 |
| L. australis leaves | 78.87 ± 0.29 | 8.16 ± 0.35 |
| L. australis fruits | 32.82 ± 0.77 | 12.81 ± 0.612 |
Flavonoids are the widely distributed and most common group of plant phenolics, which are characterized by the presence of a benzo-γ-pyrone structure.33 The total flavonoids content was determined in the assigned extracts by reaction with sodium nitrite, followed by the formation of a colored complex of flavonoid-aluminum using aluminum chloride that was spectrophotometrically monitored at 510 nm.24 For both Livistona species, the samples containing the highest flavonoid content was L. chinensis fruit (17.75 mg RE g−1), followed by the L. australis fruit (12.81 mg RE g−1), L. australis leaves (8.16 mg RE g−1) and L. chinensis leaves (4.82 mg RE g−1), as indicated in Table 2.
The total flavonoid and phenolic contents for L. chinensis previously reported and the results were found to be 139.51 ± 5.90 μg g−1 dry plant and 112.07 ± 2.24 μg g−1 dry plant, respectively28 while the L. speciosa seed extract showed a total phenolic content of 2.35 mg of gallic acid/1 g of sample, and the total flavonoid was found 39.27 mg of rutin/1 g of sample.34 In our study, the L. australis leaves had the highest phenolic content, while the L. chinensis fruits showed a high flavonoid content.
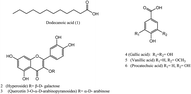
3.3. Identification of the isolated compounds
Six known compounds were isolated and identified from the pericarp of L. australis: one fatty acid from the hexane fraction and five phenolic compounds from the ethyl acetate fraction. The chemical structures of the pure isolated compounds were elucidated using NMR spectrometry, and confirmed by comparing the obtained data with those previously reported in the literature (Fig. 2). The identified compounds were:(1) dodecanoic acid; (lauric acid,C12H24O2),35 (2) hyperoside (quercetin 3-O-β-D-galactopyranoside, C21H20O12),36 (3) quercetin 3-O-α-D-arabinopyranoside (C20H18O11),37 (4) gallic acid (C7H6O5),38 (5) vanillic acid (C8H8O4) and (6) procatechuic acid (C7H6O4).39 These compounds have been isolated and characterized for the first time from L. australis fruits (spectroscopic data in ESI data, Fig. S9–S20†).
3.4. Biological studies
Phenolic compounds have been reported to lower the incidence of many chronic diseases, such as cancer, cardiovascular diseases (CVD), chronic respiratory diseases, diabetes and neurodegenerative diseases.40 In this work, extracts of L. australis showed a higher content of phenolic compounds than L. chinensis, so the biological evaluation was done for L. australis regarding anticholinesterase, and antiaging and antidiabetic properties.| Extract/control | Anticholinesterase IC50 (ng ml−1) | Telomerase activation conc (ng ml−1) | DPP4 inhibition IC50 (ng ml−1) |
|---|---|---|---|
| L. australis leaves | 65.55 ± 3.75 | 12.23 ± 0.59 (1.49) | 90.8 ± 4.48 |
| L. australis fruits | 122 ± 6.97 | 11.5 ± 0.65 (1.41) | 359 ± 17.7 |
| Donepezil | 49.44 ± 2.82 | — | — |
| Curcumin | — | 12.67 ± 0.49 (1.54) | — |
| Sitagliptin | — | — | 63.8 ± 3.15 |
| Control | — | 8.206 ± 0.33 (1) | — |
4. Conclusion
This research focused on the phytochemical characterization of the leaves and fruits of Livistona chinensis and L. australis, and the biological activities of L. australis leaves and fruits. The extracts were rich with phenolic compounds, especially flavonoids. Biological evaluation showed that the leaves extract displayed the best inhibitory effects against acetyl cholinesterase and DPP-IV, and good potentiation for telomerase activity. However, further experimental studies, such as in vivo animal studies and in silico study, could be planned depending on the present results.Ethical statement
Ethics approval was not required for this research.Author contributions
Collection, drying, and extraction of the plant: S. O. A., LC/MS analysis: U. R. A., compound isolation and identification: A. E., conceptualization, and methodology: S. S. E. and A. I. O., all authors contributed to the writing, reviewing, revising and editing of the manuscript.Conflicts of interest
The authors declare that there is no conflict of interest.References
- K. G. Yiannopoulou and S. G. Papageorgiou, J. Cent. Nerv. Syst. Dis., 2020, 12, 1179573520907397 CrossRef PubMed.
- A. N. Tamfu, S. Kucukaydin, B. Yeskaliyeva, M. Ozturk and R. M. Dinica, Molecules, 2021, 26, 5582 CrossRef CAS PubMed.
- E. H. Blackburn, C. W. Greider and J. W. Szostak, Nat. Med., 2006, 12, 1133–1138 CrossRef CAS PubMed.
- C. B. Harley, Curr. Mol. Med., 2005, 5, 205–211 CrossRef CAS PubMed.
- M. Lulkiewicz, J. Bajsert, P. Kopczynski, W. Barczak and B. Rubis, Mol. Biol. Rep., 2020, 47, 7181–7188 CrossRef CAS PubMed.
- Y. Aydin, in Molecular Basis and Emerging Strategies for Anti-aging Interventions, ed. S. I. Rizvi and U. Çakatay, Springer Singapore, Singapore, 2018, pp. 97–109, DOI: DOI:10.1007/978-981-13-1699-9_7.
- C. López-Otín, M. A. Blasco, L. Partridge, M. Serrano and G. Kroemer, Cell, 2013, 153, 1194–1217 CrossRef PubMed.
- F. Gao, Y. Fu, J. Yi, A. Gao, Y. Jia and S. Cai, J. Agric. Food Chem., 2020, 68, 12141–12151 CrossRef CAS PubMed.
- J. Kaur, R. Singla and V. Jaitak, Lett. Drug Des. Discovery, 2018, 15, 634–642 CrossRef CAS.
- T. Wu, C. K. Rayner and M. Horowitz, Metab. Control, 2015, 137–171 CAS.
- A. K. Langley, T. J. Suffoletta and H. R. Jennings, Pharmacotherapy, 2007, 27, 1163–1180 CrossRef CAS PubMed.
- P. Chen and J. Yang, Chin. Tradit. Herb Drugs, 2007, 8, 665–667 Search PubMed.
- T. Yuan, S.-P. Yang, H.-Y. Zhang, S.-G. Liao, W. Wang, Y. Wu, X.-C. Tang and J.-M. Yue, J. Asian Nat. Prod. Res., 2009, 11, 243–249 CrossRef CAS PubMed.
- X. Zeng, Q. Qiu, C. Jiang, Y. Jing, G. Qiu and X. He, Fitoterapia, 2011, 82, 609–614 CrossRef CAS PubMed.
- S. K. El-Desouky, M. E. Kassem, Z. I. A. Fifi and A. M. G. El-Deen, Nat. Prod. Commun., 2009, 4, 499–500 CrossRef CAS PubMed.
- M. E. Kassem, S. Shoela, M. M. Marzouk and A. A. Sleem, Nat. Prod. Res., 2012, 26, 1381–1387 CrossRef CAS PubMed.
- W. Lin, J. Zhao, Z. Cao, Q. Zhuang, L. Zheng, Q. Cai, D. Chen, L. Wang, Z. Hong and J. Peng, Oncol. Rep., 2013, 29, 1859–1866 CrossRef PubMed.
- S. Li, S. Luo, H. Chen, Y. Zheng, L. Lin, H. Yao and X. Lin, Drug Des., Dev. Ther., 2019, 13, 1555 CrossRef CAS PubMed.
- H. Ibrahim, F. Elshaarawy and E. Haggag, J. Adv. Pharm. Educ. Res., 2018, 2, 256–268 Search PubMed.
- S. S. El-Hawary, T. F. Ali, S. O. A. El-Ela, A. Elwekeel, U. R. Abdelmohsen and A. I. Owis, RSC Adv., 2022, 12, 19505–19511 RSC.
- Y. Wang, J. Zhai, D. Yang, N. Han, Z. Liu, Z. Liu, S. Li and J. Yin, Oxid. Med. Cell. Longevity, 2021, 2021, 7807046 Search PubMed.
- S. S. El-Hawary, A. I. Owis, S. O. A. El-Ela and A. Elwekeel, Egypt. J. Chem., 2022, 65(5), 291–295 Search PubMed.
- A. I. Owis, M. S. El-Hawary, D. El Amir, O. M. Aly, U. R. Abdelmohsen and M. S. Kamel, RSC Adv., 2020, 10, 19570–19575 RSC.
- M. F. A. Bakar, M. Mohamed, A. Rahmat and J. Fry, Food Chem., 2009, 113, 479–483 CrossRef.
- S. Dhanasekaran, P. Perumal and M. Palayan, J. Appl. Pharm. Sci., 2015, 5, 12–016 CrossRef.
- M. S. Refaey, R. A. Abdelhamid, H. Elimam, Y. A. Elshaier, A. Ali and M. A. Orabi, Bioorg. Chem., 2021, 108, 104643 CrossRef CAS PubMed.
- P. K. Kempegowda, F. Zameer, C. K. Narasimashetty, S. P. Kollur and S. K. Murari, Afr. J. Tradit., Complementary Altern. Med., 2018, 15, 11–25 CrossRef CAS.
- R. Ahmed, E. Elkhrisy, W. A. H. EL-kashak, M. El Raey, M. Nassar and E.-S. A. Aboutabl, J. Adv. Pharm. Educ. Res., 2019, 3, 23–29 Search PubMed.
- X. Zeng, Y. Wang, Q. Qiu, C. Jiang, Y. Jing, G. Qiu and X. He, Fitoterapia, 2012, 83, 104–109 CrossRef CAS PubMed.
- M. Wu, C. Wang, C. Mai, J. Chen, X. Lai, L. He, S. Huang and X. Zhang, J. Funct. Foods, 2019, 61, 103460 CrossRef CAS.
- D. Olennikov, I. Zilfikarov and S. Khodakova, Chem. Nat. Compd., 2013, 49, 526–529 CrossRef CAS.
- F. S Elshaarawy, S. A Mina, N. M Gabr, S. M Abdelkhalik, R. Kamel, H. A Ibrahim and E. G. Haggag, J. Adv. Pharm. Educ. Res., 2018, 2, 201–211 Search PubMed.
- T. Mabry, K. R. Markham and M. B. Thomas, The systematic identification of flavonoids, Springer Science & Business Media, 2012 Search PubMed.
- P. Takolpuckdee, Thai J. Pharm. Sci., 2016, 40 Search PubMed.
- E. S. El-ghaly, Al-Azhar J. Pharm. Sci., 2014, 49, 259–268 CrossRef.
- Z. Xiao, H. Wu, T. Wu, H. Shi and B. Hang, Chem. Nat. Compd., 2006, 42, 736–737 CrossRef CAS.
- A. De Almeida, M. Miranda, I. Simoni, M. Wigg, M. Lagrota and S. Costa, Phytother. Res., 1998, 12, 562–567 CrossRef CAS.
- Y. A. Lim, M. C. Mei, I. T. Kusumoto, H. Miyashiro, M. Hattori, M. P. Gupta and M. J. P. R. Correa, Phytother. Res., 1997, 11, 22–27 CrossRef CAS.
- Y. Yu, H. Gao, Z. Tang, X. Song and L. J. Wu, Asian J. Tradit. Med., 2006, 1, 101–104 CAS.
- C. W. Haminiuk, G. M. Maciel, M. S. Plata-Oviedo and R. M. Peralta, Int. J. Food Sci. Technol., 2012, 47, 2023–2044 CrossRef CAS.
- V. Zarotsky, J. J. Sramek and N. R. Cutler, Am. J. Health-Syst. Pharm., 2003, 60, 446–452 CrossRef CAS PubMed.
- J. Sun, B. Wang, Y. Niu, Y. Tan, C. Fan, N. Zhang, J. Xue, J. Wei and J. Xiang, Entropy, 2020, 22, 239 CrossRef CAS PubMed.
- S. E. Arnold and A. Kumar, Med. Clin. North Am., 1993, 77, 215–230 CrossRef CAS PubMed.
- J. A. Schneider, Z. Arvanitakis, W. Bang and D. A. Bennett, Neurology, 2007, 69, 2197–2204 CrossRef PubMed.
- I. Bakoyiannis, A. Daskalopoulou, V. Pergialiotis and D. Perrea, Biomed. Pharmacother., 2019, 109, 1488–1497 CrossRef CAS PubMed.
- S. S. El-Hawwary, H. M. Abd Almaksoud, F. R. Saber, H. Elimam, A. M. Sayed, M. A. El Raey and U. R. Abdelmohsen, RSC Adv., 2021, 11, 18009–18025 RSC.
- Y.-S. Cong, W. E. Wright and J. W. Shay, Microbiol. Mol. Biol. Rev., 2002, 66, 407–425 CrossRef CAS PubMed.
- B. Molgora, R. Bateman, G. Sweeney, D. Finger, T. Dimler, R. B. Effros and H. F. Valenzuela, Cells, 2013, 2, 57–66 CrossRef CAS PubMed.
- C. B. Harley, W. Liu, M. Blasco, E. Vera, W. H. Andrews, L. A. Briggs and J. M. Raffaele, Rejuvenation Res., 2011, 14, 45–56 CrossRef CAS PubMed.
- K. Fujiwara, T. Inoue, N. Yorifuji, M. Iguchi, T. Sakanaka, K. Narabayashi, K. Kakimoto, S. Nouda, T. Okada and K. Ishida, J. Clin. Biochem. Nutr., 2015, 56, 155–162 CrossRef CAS PubMed.
- S. Shaikh, E.-J. Lee, K. Ahmad, S.-S. Ahmad, J.-H. Lim and I. Choi, Pharmaceuticals, 2021, 14, 591 CrossRef CAS PubMed.
- B. T. Zhao, D. D. Le, P. H. Nguyen, M. Y. Ali, J.-S. Choi, B. S. Min, H. M. Shin, H. I. Rhee and M. H. Woo, Chem.-Biol. Interact., 2016, 253, 27–37 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01229h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |